- 1College of Medicine, University of Central Florida, Orlando, FL, United States
- 2Department of Medicine, University of Chicago, Chicago, IL, United States
- 3Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia
- 4Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Staphylococcus aureus is the most commonly identified airway colonizer of cystic fibrosis (CF) patients, and infections with methicillin-resistant S. aureus (MRSA) are associated with poor outcomes. Yet, little is known about the intrahost evolution of S. aureus among CF patients. We investigated convergent evolution and adaptation of MRSA among four CF patients with long-term respiratory carriage. For each patient, we performed whole-genome sequencing on an average of 21 isolates (range: 19–23) carried for a mean of 1,403 days (range: 903–1,679), including 25 pairs of isolates collected on the same day. We assessed intrahost diversity, population structure, evolutionary history, evidence of switched intergenic regions (IGRs), and signatures of adaptation in the context of patient age, antibiotic treatment, and co-colonizing microbes. Phylogenetic analysis delineated distinct multilocus sequence type ST5 (n = 3) and ST72 (n = 1) clonal populations in addition to sporadic, non-clonal isolates, and uncovered a putative transmission event. Variation in antibiotic resistance was observed within clonal populations, even among isolates collected on the same day. Rates of molecular evolution ranged from 2.21 to 8.64 nucleotide polymorphisms per year, and lineage ages were consistent with acquisition of colonization in early childhood followed by subsequent persistence of multiple sub-populations. Selection analysis of 1,622 core genes present in all four clonal populations (n = 79) found 11 genes variable in three subjects – most notably, ATP-dependent protease clpX, 2-oxoglutarate dehydrogenase odhA, fmtC, and transcription-repair coupling factor mfd. Only one gene, staphylococcal protein A (spa), was found to have evidence of gene-wide diversifying selection. We identified three instances of intrahost IGR switching events, two of which flanked genes related to quorum sensing. The complex microbial ecology of the CF airway poses challenges for management. We illustrate appreciable intrahost diversity as well as persistence of a dominant lineage. We also show that intrahost adaptation is a continual process, despite purifying selective pressure, and provide targets that should be investigated further for their function in CF adaptation.
Introduction
Cystic fibrosis (CF) is an autosomal recessive, debilitating disease that is caused by mutations in the gene encoding the CF transmembrane conductance regulator protein (CFTR), which occurs at an incidence of 1/2,500 live births. CF manifests as a clinical syndrome characterized by insufficient mucociliary clearance with chronic pulmonary infections that ultimately leads to a progressive decline in lung function and shortened life span. A key component of CF patient treatment is management of bacterial respiratory pathogens, including Staphylococcus aureus, which may colonize the CF lung for years (Branger et al., 1996). This often requires recurrent and prolonged periods of antimicrobial use (Schwerdt et al., 2018). Together with the frequent occurrence of polymicrobial carriage and infection, host immunity, and the challenges of nutrient acquisition, the evolutionary forces shaping intrahost microbial populations colonizing the CF lung are complex (McAdam et al., 2011). Identifying important genotypes of pathogenic bacterial species that are associated with adaptation to persistence and deciphering which genotypes represent bystanders, protective commensals, or pathogens, are central to long-term clinical management.
Among persistent colonizers of the CF lung, S. aureus may cause recurrent infections (Harrison, 2007; Kahl, 2010). However, in comparison to Pseudomonas aeruginosa (Feliziani et al., 2014; Marvig et al., 2015) or even Burkholderia spp. (Lieberman et al., 2011, 2014; Price et al., 2013; Silva et al., 2016), less is known about S. aureus intrahost evolution in the CF lung (Rolain et al., 2009; McAdam et al., 2011; Ankrum and Hall, 2017; Schwerdt et al., 2018). The various species inhabiting the CF lung share several evolutionary strategies relating to persistent microbial carriage. Persistent colonization usually involves a single clonal population with the sporadic appearance of non-clonal isolates. After initial colonization there is a rapid increase in population size as the microbe adapts to the lung environment (Lieberman et al., 2011). This adaptation usually involves resistance to antimicrobials through target site modification or gain of mobile genetic elements carrying antibiotic resistance associated genes as well as substitutions in metabolic loci and the formation of small colony variants or hypermutators (Feliziani et al., 2014). Local adaptation may also occur in various compartments of the respiratory system, resulting in intrahost sub-population structure (Lieberman et al., 2014). Drift, population structure, and relaxed purifying selection often lead to evolutionary rates that are higher than those observed in studies of interhost transmission (Didelot et al., 2016). Yet, despite these shared evolutionary strategies of bacteria in the CF lung, there remains tremendous variability in observations across pathogens and studies.
Here we investigate the intrahost diversity, host adaptation, and convergent evolution of methicillin-resistant S. aureus (MRSA) among four CF patients with long-term respiratory carriage. For each patient, we performed whole-genome sequencing on an average of 21 isolates (range: 19–23) carried for a mean of 1,403 days (range: 903–1,679). We sought to identify trends in population dynamics within and between patients, specifically assessing diversifying selection in the same genes across clonal intrahost populations, which would provide the strongest evidence for convergent evolution. We further consider variation in intergenic regions (IGR) and the impact of within-host IGR switching events associated with regulatory modification.
Materials and Methods
Bacterial Strains, Genome Sequencing, and Assembly
We investigated S. aureus isolates collected from four patients who were seen at the CF clinic at the University of Chicago from 2003 to 2009 (see Supplementary Table S1 in the Supplementary Material). Clinical, laboratory, and demographic data were abstracted from the medical records. This included the antibiogram for each isolate as determined by Vitek, age, recent hospitalizations, presence of indwelling devices, recent surgery, antibiotic treatment, identification of other respiratory organisms, and lung function tests, when available. Informed consent was obtained from participants 18 years of age or older; for participants between 7 and 17 years of age, consent was obtained from the parent or guardian as well as assent from the child. The study was approved by the Institutional Review Board of the Biological Sciences Division of the University of Chicago.
Genomic DNA was extracted from clinical isolates using the Qiagen blood and tissue kit (Qiagen). WGS libraries were constructed using the Nextera XT kit, which were subsequently sequenced on an Illumina HiSeq to produce 2 × 150 paired end reads with a target coverage of 100×. De novo assembly was performed with Spades v3.11.1 followed by scaffolding with SSPACE v3.0. Assembly refinement (gap filling and error correction) was carried out using three iterations of Pilon v1.12. Final assemblies were filtered to remove contigs shorter than 250 bp and censor sites with < 5 read depth, base quality < 20, and mapping quality < 20. Genome annotation was then performed using Prokka v1.13. Multilocus sequence types (MLST) were assigned using the Center for Genomic Epidemiology web server1, which also identified the closest matching reference strain in NCBI (Zankari et al., 2013). Antibiotic resistance associated genes were identified using ARIBA v2.12.1 and the CARD database (Hunt et al., 2017). Pan-genome analysis was performed on the entire sample and multiple references using Roary v3.12.0 (Page et al., 2015). A single-nucleotide polymorphism (SNP) alignment was extracted from the core-genome alignment using snp-sites v2.4.0 and a maximum likelihood (ML) phylogeny was inferred with RAxML v8.2.11 using the ASC_GTRGAMMA substitution model with 100 bootstrap replicates (Stamatakis, 2014).
Inter- and Intrahost Population Structure
The ML phylogeny was used to assess inter- and intrahost population structure to determine the clonality of each patient’s S. aureus population and identify possible epidemiologic linkages (i.e., transmission events). Mean pairwise SNP distances were calculated for each intrahost population. Isolates with divergent MLST types, as compared to the major frequency ST, were excluded, as well as isolates belonging to the major frequency ST with mean pairwise SNP distances greater that three standard deviations of the mean. These later isolates were indicative of independent acquisitions of S. aureus unrelated to the dominant carried population. For clonal inter- and intra-host S. aureus populations, pan-genome analysis was repeated, core-genome alignments were obtained, and ML phylogenies inferred. To test for recombination in the core genome, a network was inferred for each intrahost clonal sample using SplitsTree4 and analyzed with the PHI test (Bruen et al., 2006; Huson and Bryant, 2006). SNPs in the core genome were then used to assess intrahost population structure with RhierBAPS (Cheng et al., 2013; Tonkin-Hill et al., 2018). Last, Piggy v1.4 was used to assess IGR diversity and identify “switched” IGRs upstream of genes, which could impact gene expression (Thorpe et al., 2018).
Intrahost Diversity and Coalescent Analysis
Intrahost variation was explored through assessment of allele frequency and SNP diversity compared to collection time point. For each intrahost population, the temporal signal, indicative of a measurably evolving population, was investigated by regressing the root-to-tip genetic distance against sampling times. To maintain confidentiality, all dates have been scaled as years from collection of the first isolate in the study sample (i.e., Year 0). We used BacDating v1.0 to estimate the intrahost evolutionary rates and date the most recent common ancestor (MRCA) (Didelot et al., 2018). MCMC chain lengths were run for 100–800 million cycles until effective sample sizes (ESS) over 100 were observed for mu, sigma, and alpha parameters, as suggested by the authors. MRCAs were then compared to clinical and demographic characteristics such as age and time of follow-up.
Selection Analysis
We investigated signatures of selection among inter- and intra-host populations, focusing specifically on core genes present among all isolates in the clonal intrahost sample. First, for each core gene, we calculated mean pairwise SNP diversity and Watterson’s Θ at 0-fold and 4-fold degenerate sites, i.e., those nucleotide sites which all changes are synonymous and non-synonymous, respectively. We then assessed gene-by-gene diversity across all intrahost populations and focused subsequent analysis on genes that demonstrated nucleotide variation in at least two patients’ intrahost populations. For those genes, we used the tool BUSTED (Branch-Site Unrestricted Statistical Test for Episodic Diversification) implemented in HyPhy v2.3.14 to test for gene-wide diversifying selection on internal branches of the genealogy (Pond et al., 2005; Murrell et al., 2015). BUSTED analysis was carried out on the entire interhost sample as well as each clonal intrahost population. This allowed for the identification of genes potentially experiencing diversifying selection during the duration of intrahost carriage as well as at a population level. Functional annotations for genes experiencing selection were assessed using Kegg Orthologies2.
Results
Patient age at the start of follow-up ranged from 4 to 26 years of age with a duration of follow-up ranging from 903 to 1,679 days (mean = 1,403 [3.8 years]) (Table 1). For patients one through four, we analyzed 21, 23, 22, and 19 S. aureus isolates, respectively, of which 18, 22, 21, and 18 were identified as clonal based on MLST typing, SNP divergence, and interhost phylogeny (Figure 1). Duration of time between isolates ranged from 0 days (collected on the same day, n = 25 pairs) to 547 days, with the majority of isolates collected during routine quarterly visits (average time between isolate pairs = 69.3, standard deviation = 87.0, median = 49, Supplementary Figure S1). Three patients (1, 2, and 4) were colonized with a dominant ST5 population while Patient 3 was colonized with ST72. Clonal ST5 strains were most closely related to reference genome JH1 (Accession CP000736.1), a SCCmec II, PFGE type USA100, spa type t002 strain, which falls into Clonal Complex (CC) 5 clade II-B based on recent analysis by Challagundla et al. (2018). This strongly suggests that these isolates are also USA100, spa type t002 (Driebe et al., 2015; Challagundla et al., 2018). Non-clonal isolates often belonged to ST8, the most prevalent community-associated MRSA lineage in the United States (Otter and French, 2012). In each instance, the ST8 isolate was only observed at a single time point. In general, there was little evidence of recent transmission or intermixing of clonal populations. However, in one instance of a putative epidemiological linkage, two ST5 isolates belonging to Patients 1 and 2 appeared divergent from their clonal populations yet identical to each other (0 core genome SNP differences). Closer inspection of the collection dates revealed that these isolates were collected 37 days apart. No other instances of suspected transmission events were observed.
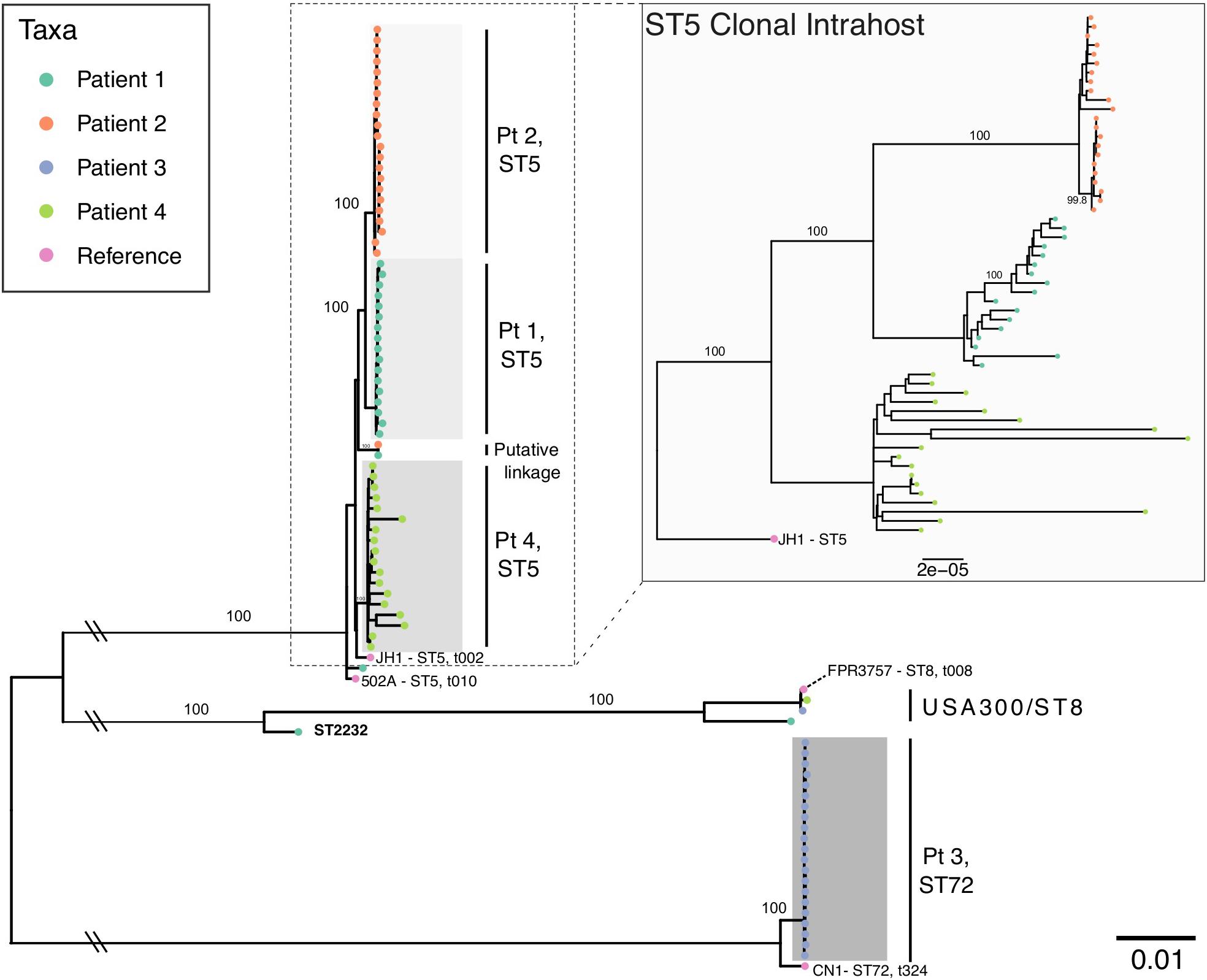
Figure 1. Maximum likelihood phylogeny of all intrahost isolates from four patients with S. aureus reference genomes. The main phylogeny was inferred using RAxML on a core genome alignment of all intrahost isolates with four S. aureus reference genomes (JH1, 502A, FPR3757, and CN1), which are annotated with multilocus sequence type (ST) and spa type. Tip points are colored by patient and bootstrap values for major clades are annotated. Clades are shaded to identify clonal intrahost populations, which are labeled to the right. Double slashes indicate where the basal branches separating the ST5 and ST72 clades have been truncated. The inlayed phylogeny shows ST5 clonal intrahost isolates only and was inferred from a core gene alignment of 2,164 COGs (1,966,902 bp) rooted using the JH1 ST5 reference.
After testing for recombination, we assessed nucleotide diversity in the core genome and IGRs of each clonal intrahost population. For each intrahost clonal sample, the PHI test did not identify significant evidence of recombination (p > 0.01). Intrahost nucleotide diversity varied significantly among subjects, regardless of duration of follow-up and core genome size (Table 2). In particular, Patient 3 with the ST72 population had the lowest diversity in both core and IGR despite the longest duration of follow-up (4.8 years), while Patient 4, who had the shortest duration of follow-up (2.5 years), had the highest core and IGR nucleotide diversity. Variation was also observed in the distribution of SNP frequencies (Supplementary Figure S2). At opposite extremes, Patient 2 had a higher density of intermediate frequency SNPs, while Patient 4 had a higher density of singletons. In general, pairwise SNP distances generally increased with time separating the collection date of isolates (Supplementary Figure S1). This is consistent with our observation when we assessed root-to-tip phylogenetic distance to determine temporal signal (see coalescent analysis below). We found a significant range in intrahost diversity between 25 pairs of isolates collected on the same day (Supplementary Figure S3). The amount of diversity among isolates collected on the same day could in part be explained by the observation that these pairs were often collected from patients later in their follow-up (Mean = 904.2 days), reflecting larger intrahost population sizes during persistent colonization or the existence of hypermutator strains that evolved later in carriage. Overall, there was a significant range in intrahost diversity among same-day isolates from each patient. Last, assessment of clonal intrahost population structure identified three to four ‘sequence clusters’ within each population (see phylogenies and coalescent analysis below); however, due to the low diversity of each intrahost population, we are cautious about the interpretation here.
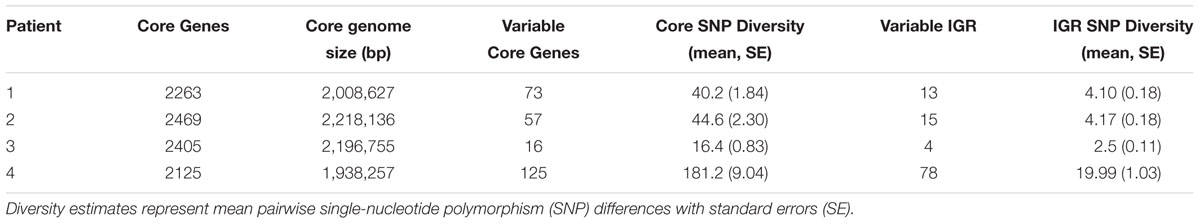
Table 2. Intrahost core-genome and intergenic (IGR) diversity of clonal Staphylococcus aureus isolates.
Intrahost Coalescent Analysis
We sought to estimate intrahost evolutionary rates and date the MRCA for each patient’s clonal S. aureus population. First, temporal signal was evaluated by assessing the root-to-tip collection using maximum likelihood phylogenies and the day of isolation. For all clonal intrahost populations, root-to-tip correlations were significant; however, correlation coeffecients were relatively low for Patients 2 and 4 (Supplementary Figure S4). This was likely the result of outliers in the root-to-tip analysis as well as the existence of intrahost sub-population structure. Evolutionary rates ranged from 2.21 [95% highest posterior densities (HPD): 1.16, 3.52] to 8.64 [2.09, 17.6] SNPs per year in the core-genome (Figure 2). We further investigated the gene sequence of DNA mismatch repair protein mutS and mutL, which have previously been associated with hypermutator phenotypes (Prunier and Leclercq, 2005). We identified non-synonymous mutations in both genes among patient 4 isolates; however, while this suggests a possible role in elevated intrahost diversity, the phenotypic effects are unknown.
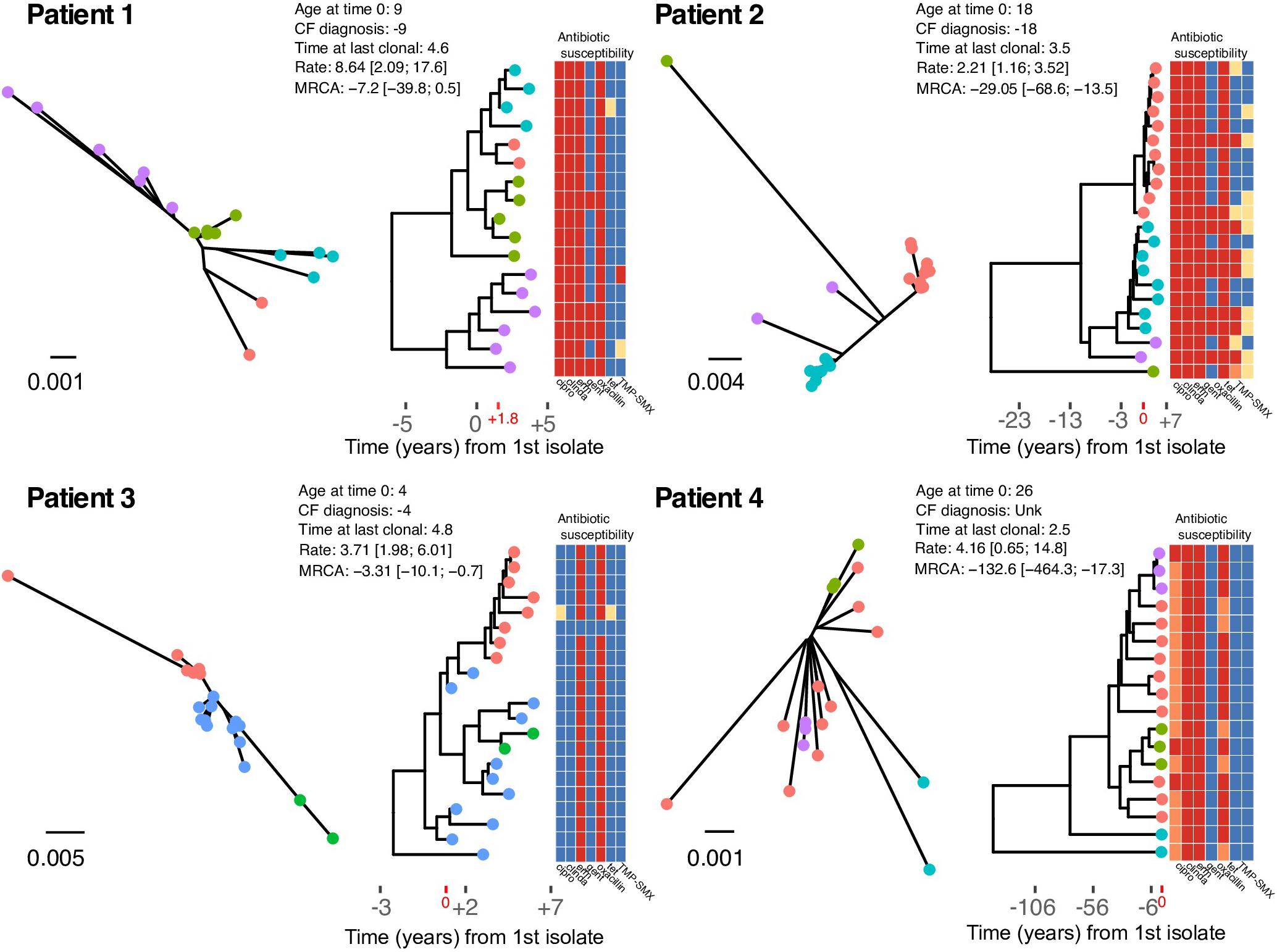
Figure 2. Un-rooted maximum likelihood phylogenies (left), and time-scaled Bayesian coalescent phylogenies with heatmap of phenotypic antibiotic susceptibilities (right) for each patient. Tip labels are colored by sequence cluster as identified by rhierBAPS and illustrate inferred intrahost sub-population structure. Time is scaled as years from the date of the first isolate in our study sample (Year 0), and times are provided for the CF diagnosis, age at time 0, and time at the date of the last clonal isolate (i.e., years of follow-up). Evolutionary rates scaled as SNPs/core-genome/year and estimates of time to the most recent common ancestor (years) are annotated on the phylogeny. The heatmap shows the phenotypic susceptibility results (resistant – red, intermediate – orange, susceptible – blue, and not tested – yellow) for ciprofloxacin (cipro), clindamycin (clinda), erythromycin (erm), gentamicin (gent), oxacillin, tetracycline (tet), and trimethoprim-sulfamethoxazole (TMP-SMX).
In general, younger individuals (Patients 1 and 3) had MRCAs that were consistent with primary colonization by a clonal population at a young age. In the case of Patient 3, S. aureus was likely acquired during infancy. Older individuals (Patients 2 and 4) had MRCAs that predated their biological age. Specifically, Patient 1 was diagnosed in infancy and had the first positive S. aureus isolate 5 years latter. The earliest isolate in our sample (Year 0) was collected 4 years after the first S. aureus isolate, and the first clonal isolate was observed nearly 2 years later (+1.8) (Figure 2). Coalescent analysis dates the MRCA of the clonal population near the time of diagnosis. Patient 2 was 18 years of age when the first isolate in our sample was obtained (Year 0), with the first positive S. aureus respiratory culture documented one-year prior. The MRCA for the clonal population, scaled as years from the date of the first isolate in our study sample (Year 0), was estimated prior to their date of CF diagnosis. Patient 3 was diagnosed with CF in infancy and had the first positive S. aureus respiratory culture 2 years later, 3 years prior to the first isolates in our sample (Year 0), which was collected at 4 years of age. Coalescent analysis dated the MRCA for the clonal population as -3.3 [95% HPD: -10.1, -0.7] years prior to the patient’s first collected clonal isolate. Patient 4, an adult CF patient, has an unknown diagnosis date and had the first positive S. aureus respiratory culture at the age of 26, with the first isolate in our sample collected that year. Our follow-up for Patient 4 began with two isolates that were collected on the same day, which were separated by 131 SNPs, demonstrating appreciable intrahost diversity within a clearly defined clonal population. Consistent with the high intrahost diversity, coalescent analysis dated the MRCA at over a 100 years, with two basal isolates driving the estimate (Figure 2). Overall, despite evolutionary rates with over-lapping HPDs (i.e., not significantly different), intrahost diversity and corresponding MRCA estimates varied among patients and was associated with age. This may reflect differences in mutation rates or the amount of selective pressure on pathogen populations among patients and the time those forces were acting on the populations. To investigate this further, we searched for signals of diversifying selection within and among intrahost populations.
Antibiotic Resistance
Within clonal intrahost populations, we found sporadic variation in phenotypic antibiotic susceptibility. ST5 populations in patients were generally resistant to fluoroquinolones, lincosamides, macrolides, and penicillin, while Patient 4’s ST72 population was only resistant to macrolides and penicillin. Variation in aminoglycoside (i.e., gentamicin) and tetracycline resistance was the most common, occurring in Patients 1 and 2 and not isolated to specific clades of the population (Figure 2). We found that gentamicin resistance was conferred by AAC(6′)-Ie-APH(2″)-Ia aminoglycoside acetyltransferase (ARO:3002597), which was harbored on a 3 kb transposon. In at least one instance in an isolate from Patient 2 (CF_Pt2_3_166), we found that the transposon was inserted in a 2.2 kb plasmid. However, it is difficult to conclude from the genome assemblies if this was consistent across all isolates possessing the transposon. During their clinical management, Patients 1 and 2 routinely received the aminoglycoside tobramycin, which could have driven resistance although in Patient 2, the clade that predominantly harbored gentamicin resistance (lower clade with teal-colored tip points in Figure 2) was later replaced by a predominantly susceptible clade. Unfortunately, the absence of detailed antibiotic treatment data for Patients 3 and 4 prevents a more in-depth analysis of the impact of antibiotic use on intrahost population structure.
Selection Analysis
Selection analysis primarily focused on 1,622 core genes (1,484,432 bp) shared by all four clonal intrahost populations as well as two lineage specific references, JH1 for ST5 and CN1 for ST72, which allowed for annotation of functional pathways. First, we assessed nucleotide diversity (Watterson’s Θ) at 0-fold (strictly non-synonymous) and 4-fold (synonymous) degenerate sites in each clonal intrahost population, finding low 0-fold/4-fold values for the few genes for which this statistic could be calculated. Only three of 71 estimates were above one (Supplementary Figure S4).
Supplementary Table S2 lists the clusters of orthologous groups (COGs) variable in two or more patients with annotations and population genetic statistics, i.e., those genes in which mutations arose during intrahost colonization. Within the core genome of the clonal intrahost sample (n = 79), eleven COGs were variable in three subjects (Table 3) and 119 were variable in at least two isolates. There were no COGs that were variable among all four patients, and most often, variable COGs were observed among ST5 isolates belonging to Patients 1, 2, and 4. Among these genes were ATP-dependent protease clpX, 2-oxoglutarate dehydrogenase E1 odhA, fmtC, and transcription-repair coupling factor mfd.
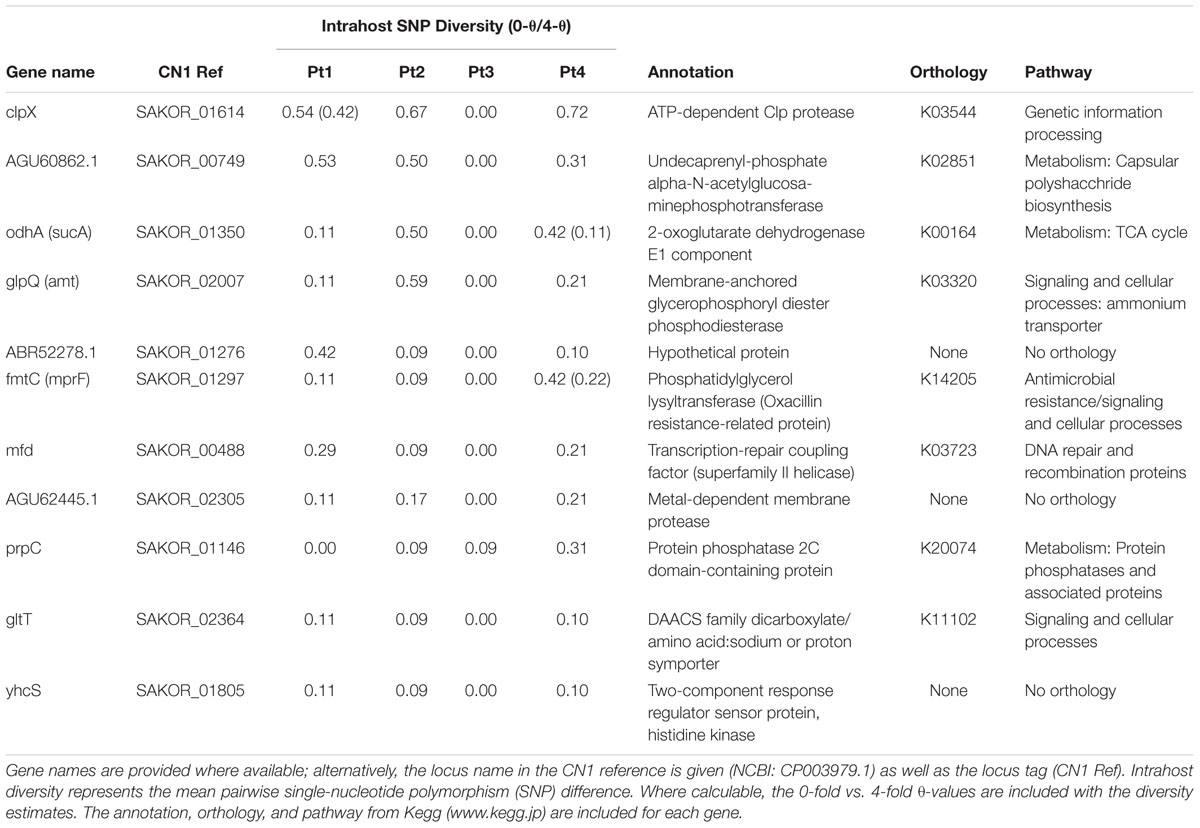
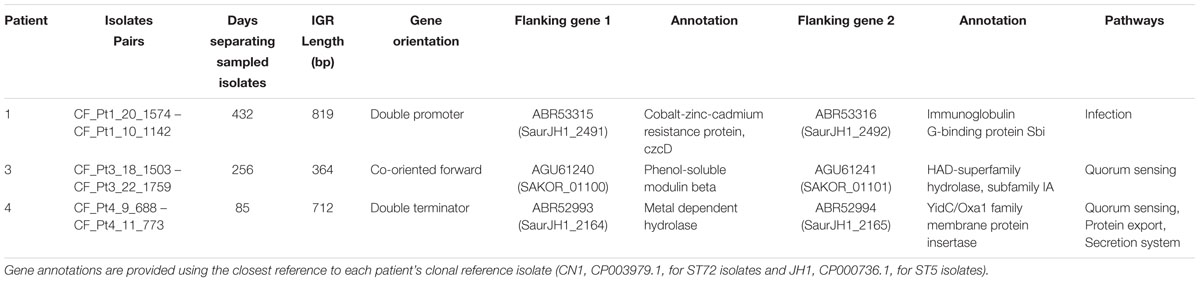
Table 3. Core genome clusters of orthologous groups (COGs) that contained nucleotide diversity among three clonal intrahost populations.
Selection analysis performed on all (ST5 and ST72) clonal isolates using BUSTED identified seven COGs that were significantly associated (p-value < 0.05) with gene-wide episodic diversifying selection among internal branches in the phylogeny (Supplementary Table S2). BUSTED analysis was then run using alignments from each patient’s clonal population as well as on only the patients with ST5 clonal populations. This represented the strictest test for diversifying selection, requiring substitutions to have occurred intrahost and selection to act upon those amino acid changes. Only one gene, staphylococcal protein A (spa), was found to have evidence of gene-wide diversifying selection in a patient (Patient 4, p-value = 0.025) and the ST5 sub-sample (Patients 1/2/4, p-value = 0.0002).
Last, we examined intrahost IGR diversity and occurrences of intrahost IGR switching events, which have recently been shown to correlate with changes in gene expression (Thorpe et al., 2018). We identified 611 core IGR (303,529 bp) found among all four clonal intrahost populations and lineage specific references. Of those, six were diverged in two or more intrahost samples. Three of these instances were the result of divergence between ST5 and ST72; however, in one instance we observed the same mutation arise intrahost. This occurred in the IGR flanking the coding sequences for phenol soluble modulin β (PSMb) (SaurJH1_1257 and SaurJH1_1258) in Patients 1 and 4, who shared the C- > T mutation at position 40 of the IGR. There were three instances of intrahost IGR switching events, one each occurring in three patients (Table 4). While the phenotypic impact and significance of these switches remains to be determined, two switches flanked genes related to quorum sensing.
Discussion
Staphylococcus aureus overtook P. aeruginosa as the most prevalent respiratory organism among American CF patients in 2003 (Cystic Fibrosis Foundation Patient Registry, 2017). Since then, the prevalence of S. aureus and MRSA has continued to increase, with more than half of all CF patients having at least one positive S. aureus culture in 2017. This trend is concerning as MRSA been independently associated with decreased lung function and increased mortality among CF patients (Dasenbrook et al., 2008, 2010). S. aureus is also one of the earliest colonizers of the CF airway, and acquired strains can persist for years, often dominated by a single intrahost lineage (Kahl et al., 2003; Al-Zubeidi et al., 2014; Schwerdt et al., 2018). However, trajectories of S. aureus carriage vary by patient (Kahl et al., 2013; Schwerdt et al., 2018), reflecting the complexities of patient-specific CF management and complicating our understanding of pathogen adaptation. Despite its high prevalence, arguably far less is known about S. aureus genomic epidemiology and intrahost evolution than other CF pathogens such a P. aeruginosa or Burkholderia spp. Here, we studied the intrahost evolution of four patients with persistent MRSA carriage to identify trends in pathogen adaptation. We illustrate persistence of a dominant lineage as well as appreciable intrahost genomic diversity, including variation in antibiotic resistance, even among isolates collected on the same day. We also show that intrahost adaptation is a continual process, despite purifying selective pressure, and provide targets that should be investigated further for their function in CF adaptation.
We found that each patient studied here possessed a persistent clonal S. aureus population characterized by considerable genomic and phenotypic diversity as well as intrahost population structure. Within-host nucleotide diversity varied considerably among patients while intrahost evolutionary rates were consistent, ranging from 1.91 × 10-6 to 9.96 × 10-7 SNPs per site per year. These rate estimates fall within the range of 10-7 to 10-5 detailed in previous intrahost studies, generally higher than interhost rates calculated over longer timescales but in line with the recent estimate of 1.55 × 10-6 SNPs/site/year for CC5 by Biek et al. (2015), Didelot et al. (2016), Challagundla et al. (2018). We find that Patients 2 and 4, who were older at the start of the study than Patients 1 and 3, had significantly higher overall diversity estimates. Snapshot estimates of intrahost diversity gleaned from isolates collected on the same day also showed higher values for Patients 2 and 4. As a result, the MRCAs for their populations are estimated to be considerably older, and while these estimates were driven in both instances by phylogenetically basal outliers, they suggest that Patients 2 and 4 have likely been persistently colonized since early childhood or even infancy. Indeed, the MRCA estimates from Patients 1 and 3 are consistent with early colonization, matching epidemiological data from the general CF population that identifies S. aureus as one of the earliest colonizers (Cystic Fibrosis Foundation Patient Registry, 2017).
Variation in intrahost recombination rates may also explain differences observed evolutionary history (Castillo-Ramírez et al., 2011; Lapierre et al., 2016). In S. aureus, recombination largely involves exchange of MGEs (Lindsay, 2014; McCarthy et al., 2014). Here we observe variation in MGE, specifically involving transposons, and subsequently excluded those loci from coalescent analysis. Homologous recombination may also occur in the core genome (Everitt et al., 2014); however, previous studies of CC5 clade II-B have found a relatively low recombination rate (ρ/𝜃 = 0.0031) compared to other clones (Challagundla et al., 2018), and homologous recombination within low-diversity intrahost samples would likely have little effect on nucleotide variation. While we did not identify significant evidence for homologous recombination, residual recombination may be have persisted. To enhance the ability to detect intrahost recombination events, future studies should consider using long-read sequencing data to generate a closed intrahost reference genome.
Intrahost population dynamics of S. aureus are likely to differ by patient due to a number of clinical and epidemiological factors that exert varied selective pressure. Here, further consideration of Patient 4’s clonal population in context of other ST5 isolates (Figure 1) suggests differences in the underlying population dynamics. Phylogenetic branch lengths are longer, consistent with higher diversity, and the unrooted ML phylogeny is more star-like (Figure 2). This contrasts all other patients, which have well-defined intrahost population structure - each with at least two extant lineages. While Patient 4 possessed mutations in genes previously associated with hypermutator phenotypes, it is more likely that a longer carriage duration and higher intrahost population size are responsible for the observed diversity since the estimated evolutionary rate was not significantly different. It is unclear whether Patient 4’s clonal population represents a single dominant intrahost lineage; however, the population structure within other patients reinforces previous findings that mutations rarely fix in intrahost pathogen populations. This suggests that intrahost clonal interference – whereby lineages are unable to reach fixation due to competition between multiple beneficial genotypes – is a common theme in intrahost population dynamics (Gerrish and Lenski, 1998; Fogle et al., 2008; Lieberman et al., 2014). Indeed, the distribution of SNP densities largely shows a high density of intermediate mutations that correspond to the population structure observed within each patient’s clonal population. Continued surveillance of intrahost populations in cases of chronic carriage may reveal whether these sub-populations persist or are removed in a bottleneck event.
In addition to each patient’s dominant clonal population, transient S. aureus strains were also identified during their course of follow-up. This finding was consistent with our previous study on intrahost evolution of S. aureus ST8 among individuals with recurrent skin and soft tissue infections, which found that non-ST8 strains often followed antibiotic treatment and preceded strain replacement (Azarian et al., 2016). Here, we observed that invading strains, which were often ST8 – the most common community-associated MRSA strain in the United States – did not supplant the resident population. The same was also true for the two non-clonal ST5 isolates collected from Patients 1 and 2, which appeared to be epidemiologically linked. These strains were not phenotypically more susceptible to antibiotics, as may be expected, suggesting other dynamics at work. The resident population may be better adapted to persistence – for example, have attenuated virulence compared to invading ST8 stains – or other competitive advantage as has recently been described in asymmetric owner-intruder competitive strategies among pneumococci (Lees et al., 2018). In the latter, quorum-sensing mediated fratricide is employed by resident bacteria to exude “ownership” of a host, resisting challenge by intruders. In staphylococci, the quorum-sensing accessory gene regulator (agr) system, which plays a role in biofilm formation and bacterial persistence, may fill a similar role and warrants further investigation (Painter et al., 2014). Overall, frequent healthcare contact increases exposure of CF patients to pathogens, and understanding why these sporadic strains fail to persist would assist in ascribing risk as well as further our knowledge or microbial interactions.
Persistence of S. aureus among CF patients is well recognized, and long-term carriage is often associated with specific phenotypes including small-colony variants, antibiotic resistance, and biofilm-formation (Branger et al., 1996; Kahl et al., 1998, 2003; Dasenbrook et al., 2008). Persistence generally involves the ability to grow in nutrient poor conditions, resist chronic antibiotic use, evade host defenses, tolerate stress, and attenuate virulence (Richards et al., 2015). Here were characterize persistence and assess the selective pressures shaping genomic variation associated with these traits, specifically investigating convergent evolution events among loci across multiple patients, which has previously been shown in intrahost studies of Burkholderia pseudomallei (Price et al., 2013), P. aeruginosa (Marvig et al., 2015), and Burkholderia dolosa (Lieberman et al., 2011). We hierarchically considered increasing levels of evidence of convergent adaptation as (i) mutations arising intrahost in the same loci, including IGR, among multiple patients, (ii) non-synonymous mutations occurring on internal branches of the intrahost phylogeny and/or fixing in a sub-population/clade, and (iii) significant tests for gene-wide positive selection. Further, we assessed the occurrence of intrahost IGR switching events for their potential role in host adaptation.
We found that mutations had arisen among three intrahost population in 11 genes, three of which, odhA, mprF, and clpX, have previously been associated with persistence, and one, transcription-repair coupling factor (mfd) is associated with differences in mutation frequencies among strains (Han et al., 2008). In particular, 2-oxoglutarate dehydrogenase (odhA), which is a component of the tricarboxylic acid (TCA) cycle, has been shown to be up-regulated in biofilms (Resch et al., 2005), and odhA mutants have been shown to demonstrate attenuated virulence and increased survival in oxygen-limited conditions (Gaupp et al., 2010). Here, we found that in addition to nucleotide variation in odhA in three patients, a non-synonymous mutation (S622G) was fixed in the dominant lineage of Patient 2’s intrahost population; the same lineage had a fixed non-synonymous mutation in clpX (A82V), providing evidence for genotypic differences involved in clonal interference in at least one patient. Functionally, ATP-dependent protease ATP-binding subunit ClpX is essential for stress tolerance, replication, and biofilm formation, and S. aureus ClpX mutants have attenuated virulence (Frees et al., 2004; Chen et al., 2015). Unfortunately, we are unable to assess the phenotypic affect of these mutations, which have not been previously documented. Last, multiple peptide resistance factor (mprF/fmtC) is a membrane protein that regulates the charge of the cell membrane, and mutations in mprF are associated with daptomycin resistance and persistence phenotypes through increased host evasion and adhesion (Yang et al., 2009; Richards et al., 2015). Mutations occurred in mprF among three patients; however, non-synonymous mutations were limited to terminal branches of the tree. Overall, the identification of nucleotide and amino acid variation in these loci, combined with the findings implicating their role in persistence, provides good evidence that there is strong selective pressure in vivo.
While identifying loci in which mutations commonly arise intrahost can provide candidates for recent adaptation, assessing evidence of gene-wide diversifying selection on internal phylogenetic branches is a stringent test for convergence. Seven loci were significant in the sample including all intrahost clonal isolates; however, further examination showed that these genes were significantly diverged between ST5 and ST72 taxa and may not be associated with recent adaptation. Comparing ST5 and intrahost samples only, a number of genes had an appreciable proportion of sites with ω-values (i.e., the fixation ratio of selected vs. neutral mutants) greater than one. However, only immunoglobulin G binding protein A (SpA), a cell-wall associated protein that functions in host immune evasion (Atkins et al., 2008), was significant among ST5 isolates and Patient 4’s intrahost sample. At least one other study has shown within-host variation of the Protein A in longitudinal samples from CF patients (Kahl et al., 2005). Overall, clear evidence of diversifying selection is scant, and in fact, few genes possessed 0-fold vs. 4-fold Θ-values (equivalent to dN/dS) greater than one (Supplementary Figure S5). The majority of tested loci had values less than one (mean = 0.32), consistent with previous findings from multiple organisms including S. aureus (Lieberman et al., 2011; Golubchik et al., 2013). While purifying selection is likely relaxed within a host on shorter timescales (Rocha et al., 2006), the scarcity of non-synonymous substitutions found here suggests that chronic S. aureus carriage in CF patients is sufficient to purge deleterious mutations. There is likely also tremendous selective pressure on strongly deleterious mutations that would impact the function of proteins associated with the aspects of persistence detailed above. Therefore, the amino acid substitutions we find here that have become fixed in dominant intrahost sub-populations likely confer a phenotypic advantage and should be explored further. Last, as three of the four patients studied here possessed relatively closely related ST5 populations, it is likely that the founding populations were already pre-adapted to persistence. This may have broad implications for MRSA epidemiology as the ST5 USA100 strain is the predominant healthcare associated strain of MRSA in the United States. In future studies, comparing transiently to persistently carried S. aureus strains may identify ancestrally acquired traits important for persistence.
Selection has historically been thought to act largely on amino acid changes in coding sequences; however, recently it has been shown that IGRs, which account for ∼15% of microbial genomes, may also be acted upon by selection (Thorpe et al., 2017). Further, parallel evolution has also been observed among longitudinal intrahost samples of P. aeruginosa from CF infection (Khademi et al., 2019). In our sample, we observe at least one instance of the same mutation occurring in the IGR flanking PSMb genes. While these loci are involved in pathogenesis (Wang et al., 2007), we do not attempt to interpret the phenotypic affects, if any, which both occurred on terminal branches on the phylogeny. In addition to nucleotide variation in IGRs, switching events have been shown to act as a mechanism for regulating expression in S. aureus (Thorpe et al., 2018). Here, we find evidence of three intrahost switching events, two of which occurred between genes associated with quorum sensing. Without expression data, we are unable to gauge the importance of these events; however, this approach could be used in larger datasets combined with RNA-seq data to investigate the frequency and evolutionary impact of intrahost IGR switches. There is currently limited understanding of IGR variation; however, we find evidence that it may important during persistent S. aureus carriage.
Antibiotics exert a strong selective pressure on microbial populations, and CF management often requires prolonged use of broad-spectrum antibiotics based empirically on clinical history and microbiological data (Döring et al., 2012). As antibiotic therapy can vary significantly among patients, it is necessary to assess microbial adaptation in the context of differential resistance profiles among intrahost samples as well as the presence of other important co-colonizing organisms. The patients studied here demonstrated common clinical CF trajectories characterized by the consistent isolation of multiple organisms and subsequent treatment. The ST5 MRSA populations identified in Patients 1, 2, and 4 were nearly all universally resistant to fluoroquinolones, macrolides, and lincosamides, well-adapted to persisting through frequent antibiotic treatments targeting other organisms. Acquired resistance to gentamicin, trimethoprim/sulfamethoxazole, and tetracycline was observed; yet, despite chronic treatment with inhaled tobramycin and frequent treatment with bactrim, resistance did not sweep in the intrahost populations of Patients 1 and 2. In fact, there were instances in each patient where gentamicin resistant and susceptible isolates were cultured on the same day (Figure 3). Further, the transposon harboring the resistance conferring gene, AAC(6′)-Ie-APH(2″)-Ia, which was most likely Tn4001 as has previously been observed among CC5 isolates, appeared mobile in both patients, as it was contemporaneously identified in isolates from different clades (Challagundla et al., 2018). This finding has implications for treatment, as the overlap in time of extant resistant and susceptible lineages may complicate the choice of a fully active therapeutic regimen.
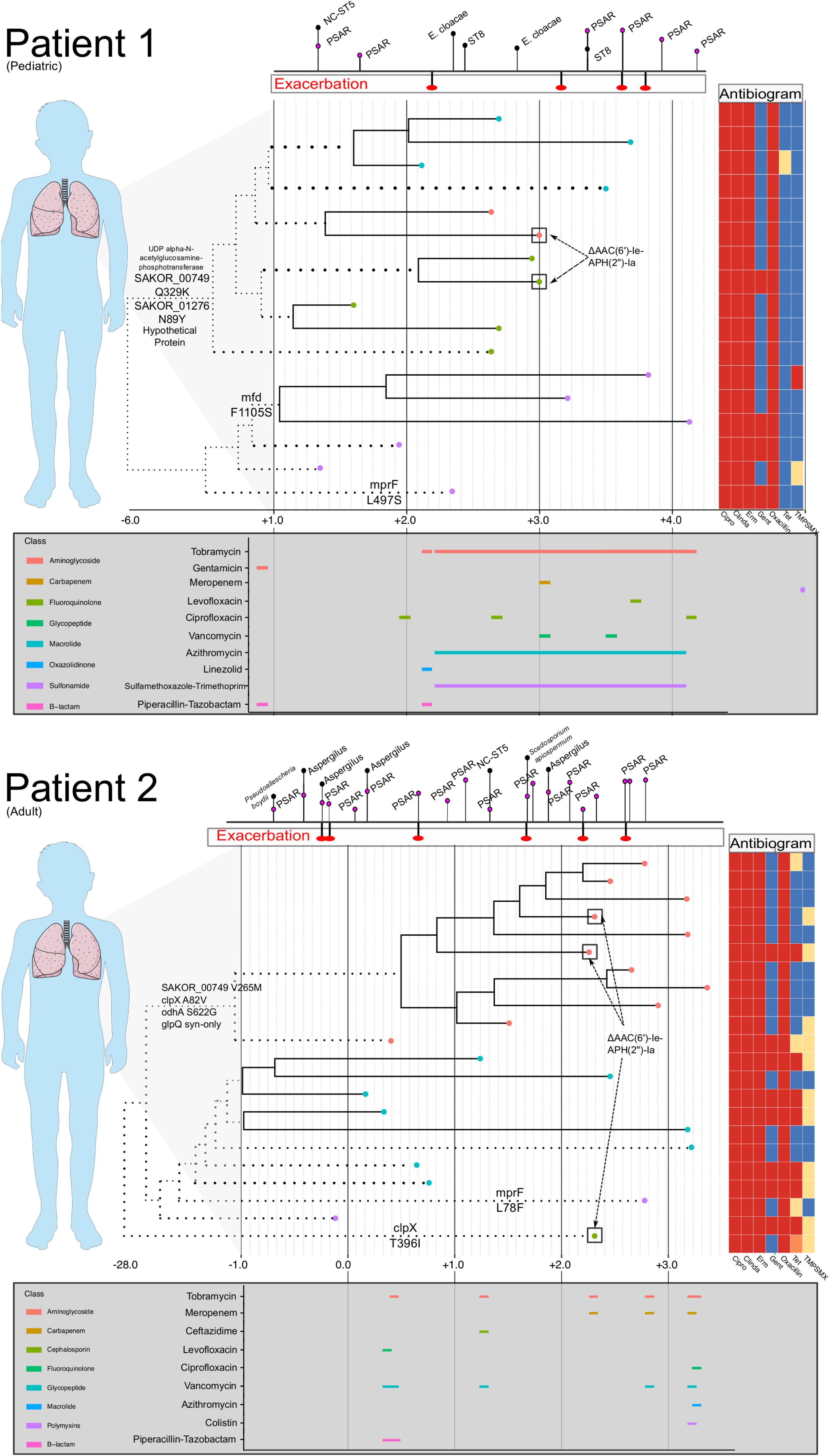
Figure 3. Intrahost genomic epidemiology of Patients 1 and 2. For Patients 1 and 2, a time-scaled phylogeny (as shown in Figure 2) is shown, annotated with phenotypic antibiogram, antibiotic treatment, and notable genomic events. Time is scaled as years from the date of the first isolate in our study sample (Year 0). The phylogeny has been compressed to focus on events occurring during the study period and branches that have been scaled are dotted. Tip labels are colored based on intrahost sub-population structure. Above each time-series is a timeline dating CF exacerbations and identification of other respiratory organisms including Pseudomonas aeruginosa (PSAR) and non-clonal (NC) Staphylococcus aureus. Below each time series is a timeline showing antibiotic usage and drug class (legend to the left of the timeline) during the study period. For Patient 1, chronic azithromycin and trimethoprim/sulfamethoxazole was prescribed three times a week. Inhaled tobramycin was prescribed every other month (every 12 h, 28 days on, 28 days off). Instances where antibiograms varied among isolates collected on the same day or within 14 days are annotated on the tree. Last, notable substitutions among loci listed in Table 3 are annotated on the branches of the phylogeny, specifically focusing on substitutions that occurred on basal internal branches that bifurcate dominant clades of the intrahost population. Patients 3 and 4 were not included because of lack of completeness of their treatment and clinical history.
The ecology of microbes in the airways of CF patients is complex, posing challenges for treatment and overall management. Chief among those challenges is parsing which cultured organisms pose a clinical threat or may in fact be protective. This requires detailed knowledge of pathogen adaptation during prolonged carriage in the context of treatment and polymicrobial interactions. Even with these data, teasing apart the relative contribution of patient-specific selective pressures – age, antibiotic exposure, pathogen and host genotype, immunity, and microbiome – may be arduous. To further resolve these intrahost dynamics, we carried out a detailed analysis of long-term MRSA carriage. Our findings support previous studies illustrating appreciable intrahost diversity as well as persistence of a dominant lineage. We also show that intrahost adaptation is a continual process, despite purifying selective pressure, and provide targets that should be investigated further for their function in CF adaptation. The role of previously CF adapted S. aureus lineages and how those lineages, once established in the CF airways, resist intruders requires further analysis. The ultimate goal is to identify key patient and pathogen predictors of long-term CF outcomes that can be used to optimize therapy to eliminate pathogenic bacteria while protecting the resident protective flora in an effort to improve quality of life.
Data Availability
Whole-genome sequencing data have been deposited in NCBI Sequence Read Archive (SRA) under BioProject PRJNA527261.
Ethics Statement
The study was approved by the Institutional Review Board of the Biological Sciences Division of The University of Chicago.
Author Contributions
TA performed the analysis, constructed figures, drafted the initial manuscript, and revised the final draft. JR abstracted data from the patient medical records and approved the final draft. ZY performed laboratory analysis and microbiological processing of the isolates and approved the final draft. MD conceived the study, provided funding for whole genome sequencing, assisted in drafting the initial manuscript, and revised the final draft.
Funding
The work was supported in part by NIH NIAID grant K23 AI095361 (MD).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Xavier Didelot for technical assistance with BactDating and Sergei L. Kosakovsky Pond for technical assistance with HyPhy. It is a noble deed when creators of analytical tools take the time to assist end-users with analysis questions. We would also like to thank Brian J. Arnold for his guidance, distilled from hours of stimulating discussions about bacterial population genomics.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00546/full#supplementary-material
FIGURE S1 |(A) Distribution of days separating pairs of sampled isolates stratified into seven categories; (B) Pairwise SNP distances stratified by categorized duration of time separating collection date of isolates. As expected, pairwise SNP distances generally increased with time separating the collection date of isolates. This is consistent with what is observed when assessing root-to-tip phylogenetic distance to determine temporal signal. Note the significant range in intrahost diversity observed between pairs of isolates collected on the same day.
FIGURE S2 | Distribution of allele frequencies among clonal intrahost populations. The plots are symmetrical and only include the intrahost isolates without reference to illustrate mutations that were intermediate or fixed in the population. For example, Patient 2 has a higher density of intermediate frequency mutations than Patient 4, the effects of which can be seen in the maximum likelihood phylogenies (Figure 2).
FIGURE S3 | Mean pairwise SNP differences among isolates collected on the same day grouped by patient. The number same-day isolate pairs, mean, estimates, and standard errors (SE) are given for each patient. Overall, Patient 4 (Pt4) had significantly higher diversity among same-day pairs then other patients.
FIGURE S4 | Maximum likelihood core genome phylogenies (left) with best-fit root based on root-to-tip correlation (right) of collection date and genetic distance. Raw mutation rate in SNPs/genome/year (Rate), most recent common ancestor in years (MRCA), correlation coefficient (R2), and significance test (p) are given at the top of each figure. Isolates are dated based on the day of collection relative to the collection date of the first isolate (day 0). Therefore, clonal populations may have MRCA dates > 0 (e.g., Patient 1).
FIGURE S5 | Diversity (Watterson’s Θ) at 0-fold and 4-fold degenerate sites for each patient. The boxplot shows the distribution of 0-fold vs. 4-fold Θ values by patient, among clusters of orthologous groups (COGs) which Θ could be calculated at 0-fold (strictly non-synonymous) and 4-fold (synonymous) degenerate sites.
TABLE S1 | List of isolates, BioSample accession numbers, metadata, molecular typing, and genome assembly statistics.
TABLE S2 | List of clusters of orthologous groups (COGs) variable in two or more patients. Each COG is annotated using the NCBI locus tag for reference strain CN1 (CP003979.1). The intrahost mean pairwise SNP distance is specified for each COG by patient as well as the mean p-distance. The results from HyPhy BUSTED analysis are included for COGs for which the test statistic could be calculated.
Footnotes
References
Al-Zubeidi, D., Hogan, P. G., Boyle, M., Burnham, C. A. D., and Fritz, S. A. (2014). Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolated in serial cultures from the respiratory tract of children with cystic fibrosis. Pediatr. Infect. Dis. J. 33, 549–553. doi: 10.1097/INF.0000000000000204
Ankrum, A., and Hall, B. G. (2017). Population dynamics of Staphylococcus aureus in cystic fibrosis patients to determine transmission events by use of whole-genome sequencing. J. Clin. Microbiol. 55, 2143–2152. doi: 10.1128/jcm.00164-17
Atkins, K. L., Burman, J. D., Chamberlain, E. S., Cooper, J. E., Poutrel, B., Bagby, S., et al. (2008). Staphylococcus aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol. Immunol. 45, 1600–1611. doi: 10.1016/J.MOLIMM.2007.10.021
Azarian, T., Daum, R. S., Petty, L. A., Steinbeck, J. L., Yin, Z., Nolan, D., et al. (2016). Intrahost evolution of methicillin-resistant Staphylococcus aureus USA300 among individuals with reoccurring skin and soft-tissue infections. J. Infect. Dis. 214, 895–905. doi: 10.1093/infdis/jiw242
Biek, R., Pybus, O. G., Lloyd-Smith, J. O., and Didelot, X. (2015). Measurably evolving pathogens in the genomic era. Trends Ecol. Evol. 30, 306–313. doi: 10.1016/j.tree.2015.03.009
Branger, C., Gardye, C., and Lambert-Zechovsky, N. (1996). Persistence of Staphylococcus aureus strains among cystic fibrosis patients over extended periods of time. J. Med. Microbiol. 45, 294–301. doi: 10.1099/00222615-45-4-294
Bruen, T. C., Philippe, H., and Bryant, D. (2006). A simple and robust statistical test for detecting the presence of recombination. Genetics 172, 2665–2681. doi: 10.1534/genetics.105.048975
Castillo-Ramírez, S., Harris, S. R., Holden, M. T. G., He, M., Parkhill, J., Bentley, S. D., et al. (2011). The impact of recombination on dN/dS within recently emerged bacterial clones. PLoS Pathog. 7:e1002129. doi: 10.1371/journal.ppat.1002129
Challagundla, L., Reyes, J., Rafiqullah, I., Sordelli, D. O., Echaniz-Aviles, G., Velazquez-Meza, M. E., et al. (2018). Phylogenomic classification and the evolution of clonal complex 5 methicillin-resistant Staphylococcus aureus in the Western Hemisphere. Front. Microbiol. 9:1901. doi: 10.3389/fmicb.2018.01901
Chen, J., Du, X., Wu, J., Zhang, Y., Cui, P., Zhang, W., et al. (2015). Transposon mutagenesis identifies novel genes associated with Staphylococcus aureus persister formation. Front. Microbiol. 6:1437. doi: 10.3389/fmicb.2015.01437
Cheng, L., Connor, T. R., Sirén, J., Aanensen, D. M., and Corander, J. (2013). Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol. Biol. Evol. 30, 1224–1228. doi: 10.1093/molbev/mst028
Cystic Fibrosis Foundation Patient Registry (2017). Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation.
Dasenbrook, E. C., Checkley, W., Merlo, C. A., Konstan, M. W., Lechtzin, N., and Boyle, M. P. (2010). Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. J. Am. Med. Assoc. 303, 2386–2393. doi: 10.1001/jama.2010.791
Dasenbrook, E. C., Merlo, C. A., Diener-West, M., Lechtzin, N., and Boyle, M. P. (2008). Persistent methicillin-resistant Staphylococcus aureus and rate of FEV 1 decline in cystic fibrosis. Am. J. Respir. Crit. Care Med. 178, 814–821. doi: 10.1164/rccm.200802-327OC
Didelot, X., Croucher, N. J., Bentley, S. D., Harris, S. R., and Wilson, D. J. (2018). Bayesian inference of ancestral dates on bacterial phylogenetic trees. Nucleic Acids Res. 46:e134. doi: 10.1093/nar/gky783
Didelot, X., Walker, A. S., Peto, T. E., Crook, D. W., and Wilson, D. J. (2016). Within-host evolution of bacterial pathogens. Nat. Rev. Microbiol. 14, 150–162. doi: 10.1038/nrmicro.2015.13
Döring, G., Flume, P., Heijerman, H., and Elborn, J. S. (2012). Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J. Cyst. Fibros. 11, 461–479. doi: 10.1016/J.JCF.2012.10.004
Driebe, E. M., Sahl, J. W., Roe, C., Bowers, J. R., Schupp, J. M., Gillece, J. D., et al. (2015). Using whole genome analysis to examine recombination across diverse sequence types of Staphylococcus aureus. PLoS One 10:e0130955. doi: 10.1371/journal.pone.0130955
Everitt, R. G., Didelot, X., Batty, E. M., Miller, R. R., Knox, K., Young, B. C., et al. (2014). Mobile elements drive recombination hotspots in the core genome of Staphylococcus aureus. Nat. Commun. 5:3956. doi: 10.1038/ncomms4956
Feliziani, S., Marvig, R. L., Luján, A. M., Moyano, A. J., Di Rienzo, J. A., Krogh Johansen, H., et al. (2014). Coexistence and within-host evolution of diversified lineages of hypermutable pseudomonas aeruginosa in long-term cystic fibrosis infections. PLoS Genet. 10:e1004651. doi: 10.1371/journal.pgen.1004651
Fogle, C. A., Nagle, J. L., and Desai, M. M. (2008). Clonal interference, multiple mutations and adaptation in large asexual populations. Genetics 180, 2163–2173. doi: 10.1534/genetics.108.090019
Frees, D., Chastanet, A., Qazi, S., Sørensen, K., Hill, P., Msadek, T., et al. (2004). Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 54, 1445–1462. doi: 10.1111/j.1365-2958.2004.04368.x
Gaupp, R., Schlag, S., Liebeke, M., Lalk, M., and Götz, F. (2010). Advantage of upregulation of succinate dehydrogenase in Staphylococcus aureus biofilms. J. Bacteriol. 192, 2385–2394. doi: 10.1128/JB.01472-09
Gerrish, P. J., and Lenski, R. E. (1998). The fate of competing beneficial mutations in an asexual population. Genetica 10, 127–144. doi: 10.1023/A:1017067816551
Golubchik, T., Batty, E. M., Miller, R. R., Farr, H., Young, B. C., Larner-Svensson, H., et al. (2013). Within-host evolution of Staphylococcus aureus during asymptomatic carriage. PLoS One 8:e61319. doi: 10.1371/journal.pone.0061319
Han, J., Sahin, O., Barton, Y.-W., and Zhang, Q. (2008). Key role of mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog. 4:e1000083. doi: 10.1371/journal.ppat.1000083
Harrison, F. (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153, 917–923. doi: 10.1099/mic.0.2006/004077-0
Hunt, M., Mather, A. E., Sánchez-Busó, L., Page, A. J., Parkhill, J., Keane, J. A., et al. (2017). ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb. Genomics 3:e000131. doi: 10.1099/mgen.0.000131
Huson, D. H., and Bryant, D. (2006). Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267. doi: 10.1093/molbev/msj030
Kahl, B., Herrmann, M., Everding, A. S., Koch, H. G., Becker, K., Harms, E., et al. (1998). Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177, 1023–1029. doi: 10.1086/515238
Kahl, B. C. (2010). Impact of Staphylococcus aureus on the pathogenesis of chronic cystic fibrosis lung disease. Int. J. Med. Microbiol. 300, 514–519. doi: 10.1016/j.ijmm.2010.08.002
Kahl, B. C., Duebbers, A., Lubritz, G., Haeberle, J., Koch, H. G., Ritzerfeld, B., et al. (2003). Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41, 4424–4427. doi: 10.1128/JCM.41.9.4424-4427.2003
Kahl, B. C., Hirschhausen, N., Peters, G., Küster, P., Birtel, J., Bianconi, I., et al. (2013). Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int. J. Med. Microbiol. 303, 685–692. doi: 10.1016/j.ijmm.2013.09.012
Kahl, B. C., Mellmann, A., Deiwick, S., Peters, G., and Harmsen, D. (2005). Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J. Clin. Microbiol. 43, 502–505. doi: 10.1128/JCM.43.1.502-505.2005
Khademi, S. M. H., Sazinas, P., and Jelsbak, L. (2019). Within-host adaptation mediated by intergenic evolution in Pseudomonas aeruginosa. Genome Biol. Evol. 11, 1385–1397. doi: 10.1093/gbe/evz083.
Lapierre, M., Blin, C., Lambert, A., Achaz, G., and Rocha, E. P. C. (2016). The impact of selection, gene conversion, and biased sampling on the assessment of microbial demography. Mol. Biol. Evol. 33, 1711–1725. doi: 10.1093/molbev/msw048
Lees, J. A., Shen, P., Weiser, J. N., Bee, G. C. W., and Brown, S. P. (2018). Pneumococcal quorum sensing drives an asymmetric owner–intruder competitive strategy during carriage via the competence regulon. Nat. Microbiol. 4, 198–208. doi: 10.1038/s41564-018-0314-4
Lieberman, T. D., Flett, K. B., Yelin, I., Martin, T. R., McAdam, A. J., Priebe, G. P., et al. (2014). Genetic variation of a bacterial pathogen within individuals with cystic fibrosis provides a record of selective pressures. Nat. Genet. 46, 82–87. doi: 10.1038/ng.2848
Lieberman, T. D., Michel, J. B., Aingaran, M., Potter-Bynoe, G., Roux, D., Davis, M. R., et al. (2011). Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43, 1275–1280. doi: 10.1038/ng.997
Lindsay, J. A. (2014). Staphylococcus aureus genomics and the impact of horizontal gene transfer. Int. J. Med. Microbiol. 304, 103–109. doi: 10.1016/J.IJMM.2013.11.010
Marvig, R. L., Sommer, L. M., Molin, S., and Johansen, H. K. (2015). Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 47, 57–64. doi: 10.1038/ng.3148
McAdam, P. R., Holmes, A., Templeton, K. E., and Fitzgerald, J. R. (2011). Adaptive evolution of Staphylococcus aureus during chronic endobronchial infection of a cystic fibrosis patient. PLoS One 6:e24301. doi: 10.1371/journal.pone.0024301
McCarthy, A. J., Loeffler, A., Witney, A. A., Gould, K. A., Lloyd, D. H., and Lindsay, J. A. (2014). Extensive horizontal gene transfer during Staphylococcus aureus co-colonization in vivo. Genome Biol. Evol. 6, 2697–2708. doi: 10.1093/gbe/evu214
Murrell, B., Weaver, S., Smith, M. D., Wertheim, J. O., Murrell, S., Aylward, A., et al. (2015). Gene-wide identification of episodic selection. Mol. Biol. Evol. 32, 1365–1371. doi: 10.1093/molbev/msv035
Otter, J. A., and French, G. L. (2012). Community-associated meticillin-resistant Staphylococcus aureus: the case for a genotypic definition. J. Hosp. Infect. 81, 143–148. doi: 10.1016/j.jhin.2012.04.009
Page, A. J., Cummins, C. A., Hunt, M., Wong, V. K., Reuter, S., Holden, M. T. G., et al. (2015). Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:btv421. doi: 10.1093/bioinformatics/btv421
Painter, K. L., Krishna, A., Wigneshweraraj, S., and Edwards, A. M. (2014). What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol. 22, 676–685. doi: 10.1016/J.TIM.2014.09.002
Pond, S. L. K., Frost, S. D. W., and Muse, S. V. (2005). HyPhy: hypothesis testing using phylogenies. Bioinformatics 21, 676–679. doi: 10.1093/bioinformatics/bti079
Price, E. P., Sarovich, D. S., Mayo, M., Tuanyok, A., Drees, K. P., Kaestli, M., et al. (2013). Within-host evolution of Burkholderia pseudomallei over a twelve-year chronic carriage infection. mBio 4:e00388-13. doi: 10.1128/mBio.00388-13
Prunier, A.-L., and Leclercq, R. (2005). Role of mutS and mutL genes in hypermutability and recombination in Staphylococcus aureus. J. Bacteriol. 187, 3455–3464. doi: 10.1128/JB.187.10.3455-3464.2005
Resch, A., Rosenstein, R., Nerz, C., and Götz, F. (2005). Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 71, 2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005
Richards, R. L., Haigh, R. D., Pascoe, B., Sheppard, S. K., Price, F., Jenkins, D., et al. (2015). Persistent Staphylococcus aureus isolates from two independent cases of bacteremia display increased bacterial fitness and novel immune evasion phenotypes. Infect. Immun. 83, 3311–3324. doi: 10.1128/IAI.00255-15
Rocha, E. P. C., Smith, J. M., Hurst, L. D., Holden, M. T. G., Cooper, J. E., Smith, N. H., et al. (2006). Comparisons of dN/dS are time dependent for closely related bacterial genomes. J. Theor. Biol. 239, 226–235. doi: 10.1016/j.jtbi.2005.08.037
Rolain, J. M., François, P., Hernandez, D., Bittar, F., Richet, H., Fournous, G., et al. (2009). Genomic analysis of an emerging multiresistant Staphylococcus aureus strain rapidly spreading in cystic fibrosis patients revealed the presence of an antibiotic inducible bacteriophage. Biol. Direct 4, 1–15. doi: 10.1186/1745-6150-4-1
Schwerdt, M., Neumann, C., Schwartbeck, B., Kampmeier, S., Herzog, S., Görlich, D., et al. (2018). Staphylococcus aureus in the airways of cystic fibrosis patients - A retrospective long-term study. Int. J. Med. Microbiol. 308, 631–639. doi: 10.1016/j.ijmm.2018.02.003
Silva, I. N. I. N., Santos, P. M., Santos, M. R. M. R., Zlosnik, J. E. A., Speert, D. P., Buskirk, S. W., et al. (2016). Long-term evolution of Burkholderia multivorans during a chronic cystic fibrosis infection reveals shifting forces of selection. mSystems 1:e00029-16. doi: 10.1128/mSystems.00029-16
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Thorpe, H. A., Bayliss, S. C., Hurst, L. D., and Feil, E. J. (2017). Comparative analyses of selection operating on nontranslated intergenic regions of diverse bacterial species. Genetics 206, 363–376. doi: 10.1534/genetics.116.195784
Thorpe, H. A., Bayliss, S. C., Sheppard, S. K., and Feil, E. J. (2018). Piggy: a rapid, large-scale pan-genome analysis tool for intergenic regions in bacteria. Gigascience 7, 1–11. doi: 10.1093/gigascience/giy015
Tonkin-Hill, G., Lees, J. A., Bentley, S. D., Frost, S. D. W., and Corander, J. (2018). RhierBAPS: An R implementation of the population clustering algorithm hierBAPS. Wellcome Open Res. 3:93. doi: 10.12688/wellcomeopenres.14694.1
Wang, R., Braughton, K. R., Kretschmer, D., Bach, T. H. L., Queck, S. Y., Li, M., et al. (2007). Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514. doi: 10.1038/nm1656
Yang, S.-J., Xiong, Y. Q., Dunman, P. M., Schrenzel, J., François, P., Peschel, A., et al. (2009). Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus Strains. Antimicrob. Agents Chemother. 53, 2636–2637. doi: 10.1128/AAC.01415-08
Keywords: intrahost evolution, Staphylococcus aureus, cystic fibrosis, MRSA, selection
Citation: Azarian T, Ridgway JP, Yin Z and David MZ (2019) Long-Term Intrahost Evolution of Methicillin Resistant Staphylococcus aureus Among Cystic Fibrosis Patients With Respiratory Carriage. Front. Genet. 10:546. doi: 10.3389/fgene.2019.00546
Received: 15 March 2019; Accepted: 22 May 2019;
Published: 12 June 2019.
Edited by:
Jesse Shapiro, Université de Montréal, CanadaReviewed by:
Ben Pascoe, University of Bath, United KingdomSantiago Castillo Ramírez, National Autonomous University of Mexico, Mexico
Copyright © 2019 Azarian, Ridgway, Yin and David. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taj Azarian, VGFqLkF6YXJpYW5AdWNmLmVkdQ==
 Taj Azarian
Taj Azarian Jessica P. Ridgway2
Jessica P. Ridgway2 Michael Z. David
Michael Z. David