- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Infectious Disease, Taizhou People’s Hospital, Taizhou, China
- 3State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, China
- 4NHC Key Laboratory of Antibody Technique, Nanjing Medical University, Nanjing, China
- 5Department of Immunology, Nanjing Medical University, Nanjing, China
Background and Aims: Although long non-coding RNAs (lncRNAs) have been linked to many diseases including asthma, little is known about lncRNA transcriptomes of CD4+ T cells in asthma. The present study aimed to explore the lncRNAs profile in the CD4+T cells from the mouse model of acute asthma.
Methods: Next generation sequencing for lncRNAs and mRNAs was performed on CD4+ T cells from asthma and control mice. Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) pathway analyses were performed to predict the functions and signal pathways for the aberrant lncRNAs. The selected lncRNAs were further measured using quantitative real-time PCR (polymerase chain reaction) and observed in the fluorescence in situ hybridization (FISH). The lncRNA–mRNA co-expression network was constructed via Pearson’s correlation coefficient and Cytoscape 3.6.
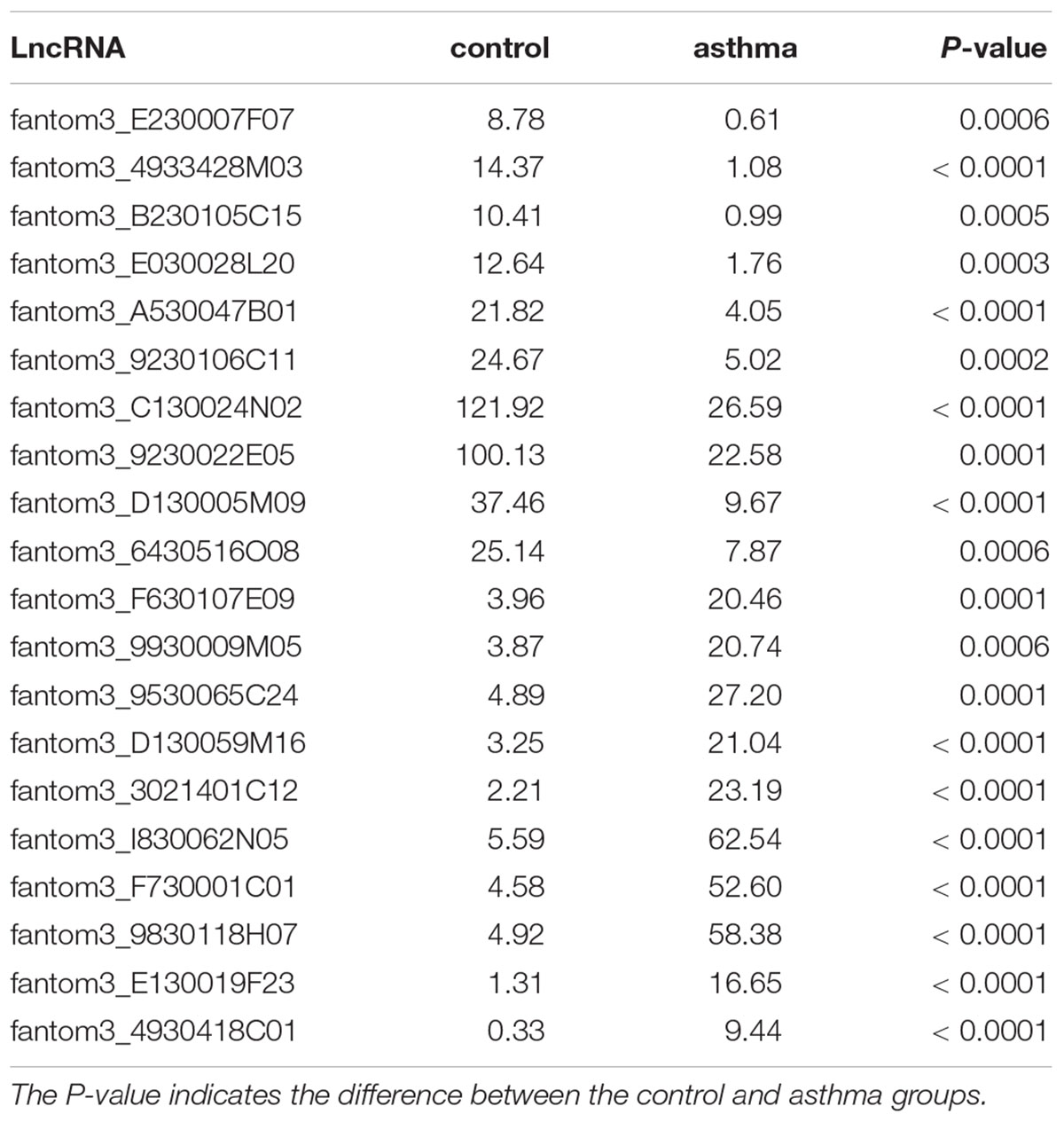
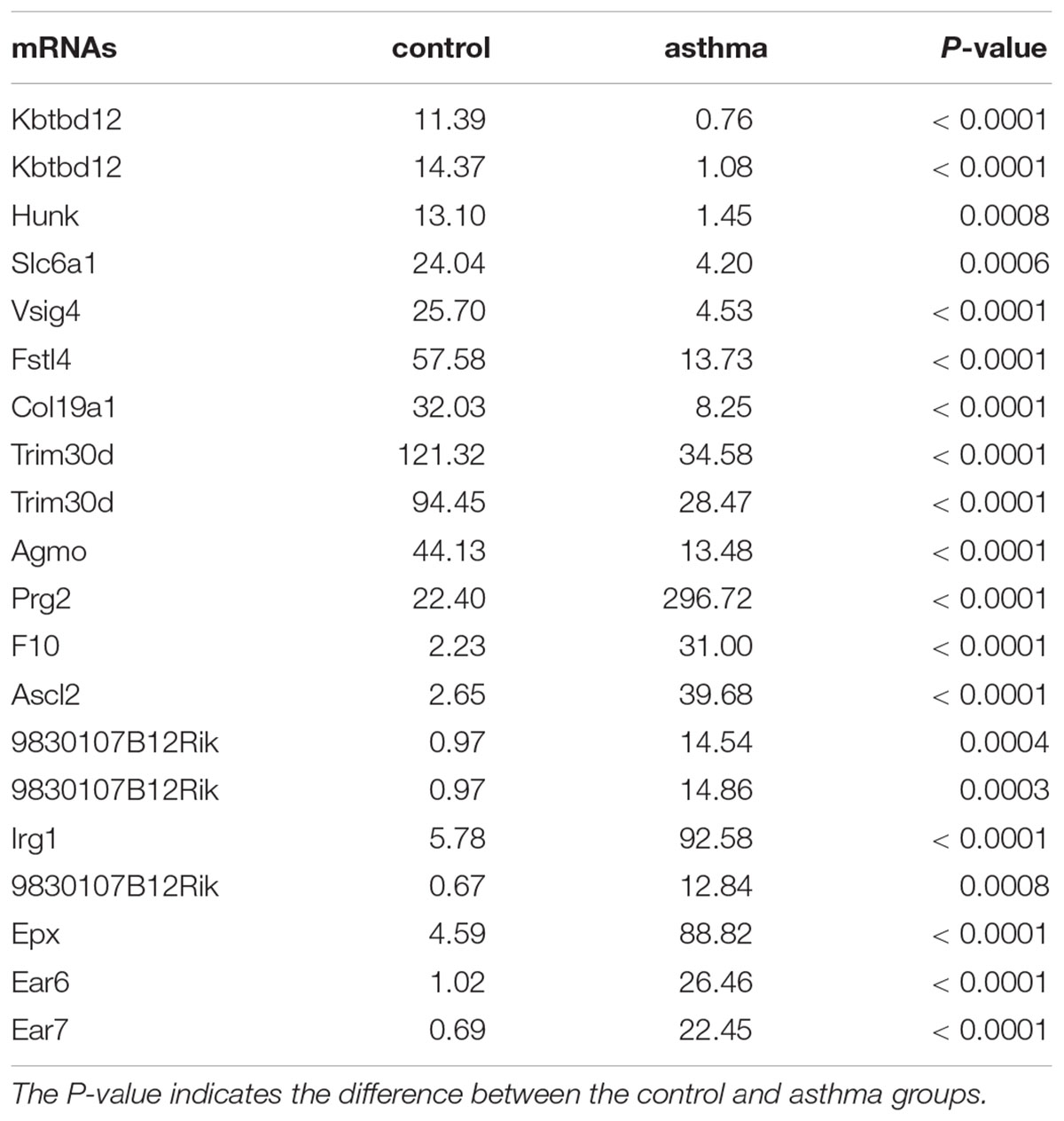
Results: Next generation sequencing revealed 36 up-regulated lncRNAs and 98 down-regulated lncRNAs in acute asthma compared with controls. KEGG pathway analysis showed that cytokine-cytokine receptor interaction had the highest enrichment scores. A co-expression network was constructed in which 23 lncRNAs and 301 mRNAs altered formed a total of 12424 lncRNA and mRNA pairs. To validate the RNA sequencing results, we measured the 4 different lncRNAs using qPCR. The lncRNA fantom3_9230106C11 was significantly reduced in CD4+ T cells of asthma. Bioinformatics analysis showed that lncRNA fantom3_9230106C11 had the potential to interact with many miRNAs and transcription factors related to Th2 differentiation.
Conclusion: This study provided the first evidence for different expression of lncRNAs of CD4+T cells in asthma and may serve as a template for further, larger functional in-depth analyses regarding asthma molecular lncRNAs.
Introduction
Allergic asthma is a type of asthma provoked by allergens, including pollen, animal dander, fungal spores, or house dust mites (HDM) (Takyar et al., 2013; Hondowicz et al., 2016). Once processed and presented by dendritic cells, allergens promote the differentiation and expansion of Th2 cells, releasing type 2 cytokines (IL-4, IL-5, IL-13) and expressing the master transcription factor GATA-3. Th2 cells orchestrate eosinophil maturation and survival, airway hyperresponsiveness, and B cell isotype switching to IgE (Holgate, 2012). Consequently, Th2 cells play crucial roles in the cascade reactions of allergic asthma.
Non-coding RNAs emerged as essential players in Th2 cell differentiation and allergic asthma. Recently, we reported the aberrant microRNA (miRNA) profile in CD4+ T cells from a murine model of acute asthma (Liu et al., 2018). In addition to miRNAs, long non-coding RNAs (lncRNAs), which comprise 200 nucleotides lacking putative open reading frames, may regulate CD4+ T cell differentiation in asthma (Zhang F. et al., 2017). In allergic asthma, the lncRNA profile has been documented in primary airway smooth muscle cells, CD8+ T cells or blood samples (Tsitsiou et al., 2012; Austin et al., 2017; Zhu et al., 2018). To our knowledge, lncRNA expression in the CD4+ T cells in asthma remains elusive.
In this study, we performed next-generation sequencing and data mining to investigate the whole spectrum of transcriptional signatures (mRNAs and lncRNAs) of CD4+ T cells in the murine model of acute asthma. Some lncRNAs were measured in CD4+ cells ex vivo or in Th2 cells in vitro using real-time quantitative reverse transcription PCR (qRT-PCR) and fluorescence in situ hybridization (FISH). Moreover, cross-talk between mRNAs and lncRNAs associated with CD4+ T cell differentiation was revealed.
Materials and Methods
Establishment of the Model of Acute Asthma
Specific pathogen-free female C57BL/6J mice (18 to 22 g) aged 6 to 8 weeks were obtained from the College of Veterinary Medicine Yangzhou University (Yangzhou, China). All experiments that involved animal and tissue samples were performed in accordance with the guidelines and procedures approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC-1709011).
The model of acute asthma was established as described previously (Liu et al., 2018). Briefly, the asthmatic mice (n = 3) were sensitized on days 0 and 14 by the intraperitoneal injection of 20 μg ovalbumin (OVA) (Grade V, Sigma-Aldrich) emulsified in 2 mg aluminum hydroxide gel (InvivoGen, San Diego, CA, United States) in a total volume of 200 μl. These sensitized mice were exposed to aerosolized 1% OVA in sterile saline for 30 min from day 20 to day 22, consecutively. The control subjects (n = 3) were sensitized and challenged using the same protocol as used for saline alone. All mice were monitored daily and were alive before sacrifice.
Twenty-four hours after the final challenge, lung function was evaluated by the direct measurement of lung resistance and dynamic compliance in restrained, tracheostomized, mechanically ventilated mice via the FinePointe RC System (Buxco Research Systems, Wilmington, NC, United States) under general anesthesia as described previously (Kerzerho et al., 2013). The sera were collected to measure total IgE using an ELISA kit according to the manufacturer’s instructions (eBioscience, Thermo Fisher Scientific, United States). To determine lung tissue inflammation, the right upper lung lobe was removed, fixed, dehydrated and embedded in paraffin. The fixed embedded tissues were cut into 5 μm sections on a Leica model 2165 rotary microtome (Leica, Nussloch, Germany), and the tissue slides were stained with hematoxylin and eosin (H&E).
CD4+ T Cell Purification From the Spleen
Mice were anesthetized by i.p. injection of a mixture of 10 mg/kg xylazine (MTC Pharmaceuticals, Cambridge, ON, Canada) and 200 mg/kg ketamine hydrochloride (Rogar/STB, London, ON, Canada). The anaesthetized mice were sacrificed with cervical dissociation. The spleen was removed, ground and prepared into single cell suspensions. CD4+ T cells in the spleen were sorted using CD4 (L3T4) micro beads (130-049-201, Miltenyi Biotec, United States). Briefly, the single cell suspensions were incubated with CD4 (L3T4) micro beads. The magnetically labeled cells were flushed and collected. Finally, the purity of CD4+ T cells was quantified using anti-CD4-APC antibodies (17-0041-81, eBioscience).
RNA Isolation, Library Preparation, and Sequencing
Total RNA was isolated using the miRNeasy Mini Kit (Qiagen, Germany) and stored at -80°C until use. RNA purity was assessed using the ND-1000 Nanodrop. Each RNA sample had an A260:A280 ratio above 1.8 and A260:A230 ratio above 2.0. RNA integrity was evaluated using the Agilent 2200 TapeStation (Agilent Technologies, United States) and each sample had the RIN above 7.0. Briefly, rRNAs were removed from Total RNA using Epicenter Ribo-Zero rRNA Removal Kit (illumina, United States) and fragmented to approximately 200bp. Subsequently, the purified RNAs were subjected to first strand and second strand cDNA synthesis following by adaptor ligation and enrichment with a low-cycle according to instructions of NEBNext® UltraTM RNA Library Prep Kit for Illumina (NEB, United States).
The purified library products were evaluated using the Agilent 2200 TapeStation and Qubit® 2.0 (Life Technologies, United States) and then diluted to 10 pM for cluster generation in situ on the pair-end flow cell followed by sequencing (2 × 150 bp) on HiSeq 3000. The clean reads were obtained after removal of reads containing adapter, ploy-N and at low quality from raw data. Sequence data were mapped to mouse reference genome mm10 with TopHat v2.0.13. gfold v1.1.2 was subsequently employed to count the number of reads mapped to each gene. Differential expression was assessed by DEGseq using RPKM as input. Differentially expressed genes were chosen according to the criteria of fold change >2 and adjusted P-value <0.05. All the differentially expressed genes were used for heat map analysis and KEGG enrichment analyses. For KEGG enrichment analysis, a P-value <0.05 was used as the threshold to determine significant enrichment of the gene sets.
Bioinformatics Data Analysis and Data Mining
The transcriptome was assembled using Cufflinks and Scripture based on the reads mapped to the reference genome. The assembled transcripts were annotated using the Cuff compare program from the Cufflinks package. The unknown transcripts were used to screen for putative lncRNAs. Three computational approaches, including CPC/CNCI/Pfam, were combined to sort non-coding RNA candidates from putative protein-coding RNAs in the unknown transcripts. Putative protein-coding RNAs were filtered out using a minimum length and exon number threshold. Transcripts with lengths greater than 200 nt and with more than two exons were selected as lncRNA candidates and further screened using CPC/CNCI/Pfam, which distinguished protein-coding genes from non-coding genes.
The UCSC genome browser was used to locate lncRNA fantom3_9230106C11. The predicted potential target genes whose loci were within a 10-kb window upstream or downstream of the given aberrantly expressed lncRNA were considered cis-regulated genes. Other genes in the co-expression network were identified as trans-regulated according to complementary base pairing by LncTar (Li et al., 2015). In addition, Bibiserv (Sczyrba et al., 2003) and RNA22 (Miranda et al., 2006) were used to predict the interactive miRNAs. Different mRNAs in the our NGS were compared with known genes about asthma in the human disease database Malacards associated with asthma1 and asthma-associated genes2 using Wayne chart3.
CD4+ T Cell Differentiation in vitro
Naive CD4+ T cells in the spleen were sorted using a Naive CD4+ T Cell isolation kit (130-104-453, Miltenyi Biotec, United States). Briefly, the single cell suspensions were incubated with biotin-antibody cocktails, which depleted the non-T cells and memory CD4+ T cells. The untouched naive CD4+ T lymphocytes were seeded onto anti-CD3 (85-16-0031-85, eBioscience, 1 mg/ml) precoated 96-well plates. For Th1 polarization, naive CD4+ T cells were further stimulated with anti-CD28 (85-16-0281-85, eBioscience, 2 μg/ml), IL-2 (212-12, PeproTech, 20 ng/ml), IL-12 (210-12, PeproTech, 50 ng/ml) and anti-IL-4 (85-16-7041-85, eBioscience, 10 μg/ml). For Th2 polarization, naive CD4+ T cells were further stimulated with anti-CD28 (85-16-0281-85, eBioscience, 2 μg/ml), IL-2 (212-12, PeproTech, 20 ng/ml), IL-4 (214-14, PeproTech, 200 ng/ml), anti-IFN-γ (85-16-7311-85, eBioscience, 10 μg/ml), and anti-IL-12 (85-16-7123-85, eBioscience, 10 μg/ml). Half of the complete RPMI-1640 medium (16000-044, Gibco, United States) with 10% FBS (Gibco, United States) was replaced with fresh medium to maintain the cytokine environments. The CD4+ T cells were harvested and analyzed on day 5.
Flow Cytometry Analysis ex vivo and in vitro
For nuclear protein assays, spleens from animal experiments were prepared as single-cell suspensions. After RBC (red blood cell) lysis, the cells were stained with CD16/CD32 FcR (Fc Receptor) blocking antibody, Fixable Viability Dye eFluorTM 506 and anti-CD4-APC (eBioscience, 17-0041-83). For intracellular staining, the cells were fixed and permeabilized (eBioscience, 00-5523-00) according to the manufacturer’s instructions, and then intracellular products were stained. Flow cytometry was performed with anti-Gata3-PE-Cy7 (BD Biosciences, 560405), anti-T-bet-PE-Cy7 (eBioscience, 25-5825-80) and isotype controls.
For cytokine assays, T cells were restimulated on day 5 of culture for 5 h with 20 nM PMA (70-CS1001, Multiscience, China) and blocked with BFA (70-CS1002, Multiscience, China). After 5 h, the cells were harvested, gently washed with PBS containing 1% bovine serum albumin (BSA) and fixed in IC fixation buffer (00-822-49, eBioscience, United States). The fixed cells were permeabilized with permeabilization buffer (00-8333-56, eBioscience, United States) and stained with PE-anti-IFN-γ (85-12-7311-84, eBioscience, United States), PE-anti-IL4 (85-12-7041-83, eBioscience, United States) or the isotype control. The stained CD4+ T lymphocytes were analyzed on the BD FACSCalibur, and the data were analyzed by FlowJo software (TreeStar).
Real-Time Quantitative Polymerase Chain Reaction
RNAs from cells or tissues were isolated using a miRNeasy mini kit. The cDNA was synthesized using the Prime Script RT Reagent Kit (TaKaRa, Kyoto, Japan) following the manufacturer’s instructions. The quantitative PCR analysis was performed using the CFX96 system (Bio-Rad laboratories) in conjunction with ready-to-use fast-start SYBR Premix Ex Taq II (TaKaRa, Kyoto, Japan). The cycling conditions were 95°C for 30 s, followed by 95°C for 5 s and 60°C for 30 s for up to 40 cycles and dissociation at 95°C for 15 s, 60°C for 30 s and a final extension at 95°C for 15 s. The relative abundance of gene targets was determined by the comparative CT (cycle threshold) number normalized against tested β-actin comparative CT. The primers used are shown in Table 1, which were designed by RiboBio Institute (Guangzhou, China) or from primerbank by using primer5.
Correlation and Co-expression Analysis
The co-expression analysis was based on Pearson’s correlation coefficient. Considering the influence of random factors, it is found that using Pearson correlation coefficient only is not strict. Therefore, when the number of samples is less than eight, due to the small number of samples, we use the mixed washing method to calculate the P-value to further screen.
The co-expression relationship was screened according to Pearson correlation coefficient and P-value. Differentially expressed lncRNAs and mRNAs with fold changes ≥2 and p < 0.05 were analyzed. For each lncRNA-mRNA pair, the Pearson correlation (COR) was calculated to identify significantly correlated pairs. The Pearson correlation value cutoff was 0.95 and p < 0.05. To create a visual representation, a lncRNA-mRNA regulatory network was constructed using Cytoscape 3.6.
Fluorescence in situ Hybridization (FISH)
Fluorescence in situ hybridization assays were performed using the RiboTM Fluorescent in situ Hybridization Kit and the RiboTM lncRNA FISH Probe Mix (Ribo, Guangzhou, China) according to the manufacturer’s protocols. Briefly, the cells were fixed in 4% formaldehyde for 10 min and then washed with PBS. The cells were incubated with 20 μmol/L lncRNA FISH probe mix at 37°C overnight. After washing, the FISH preparations were counterstained with DAPI observed in confocal microscopy for appropriate fluorescence filter sets (Zeiss, Oberkochen, Germany). The lncRNA probe labeled with Cy3 was designed and synthesized by RiboBio Co., Ltd., RiboTM U6 and RiboTM 18S were used as reference controls for the subcellular localization of lncRNA.
Statistical Analysis
The statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad, San Diego, CA, United States). The results for variables that were normally distributed are displayed as the means ± SEM. An ANOVA was performed to establish equal variance, and a 2-tailed Student’s t-test with Bonferroni correction was applied to determine statistical significance, which was defined as P < 0.05.
Results
Establishment of an Acute Model of Asthma
As the well-established murine model of asthma, OVA challenge provoked evident pulmonary inflammation, airway hypersensitivity and higher IgE in serum (Figures 1A–C). CD4+ T cells were magnetically sorted from the spleen, and the purity was validated by flow cytometry. As shown in Figure 1D, 98.5% of the cells were CD4 positive (Figure 1D).
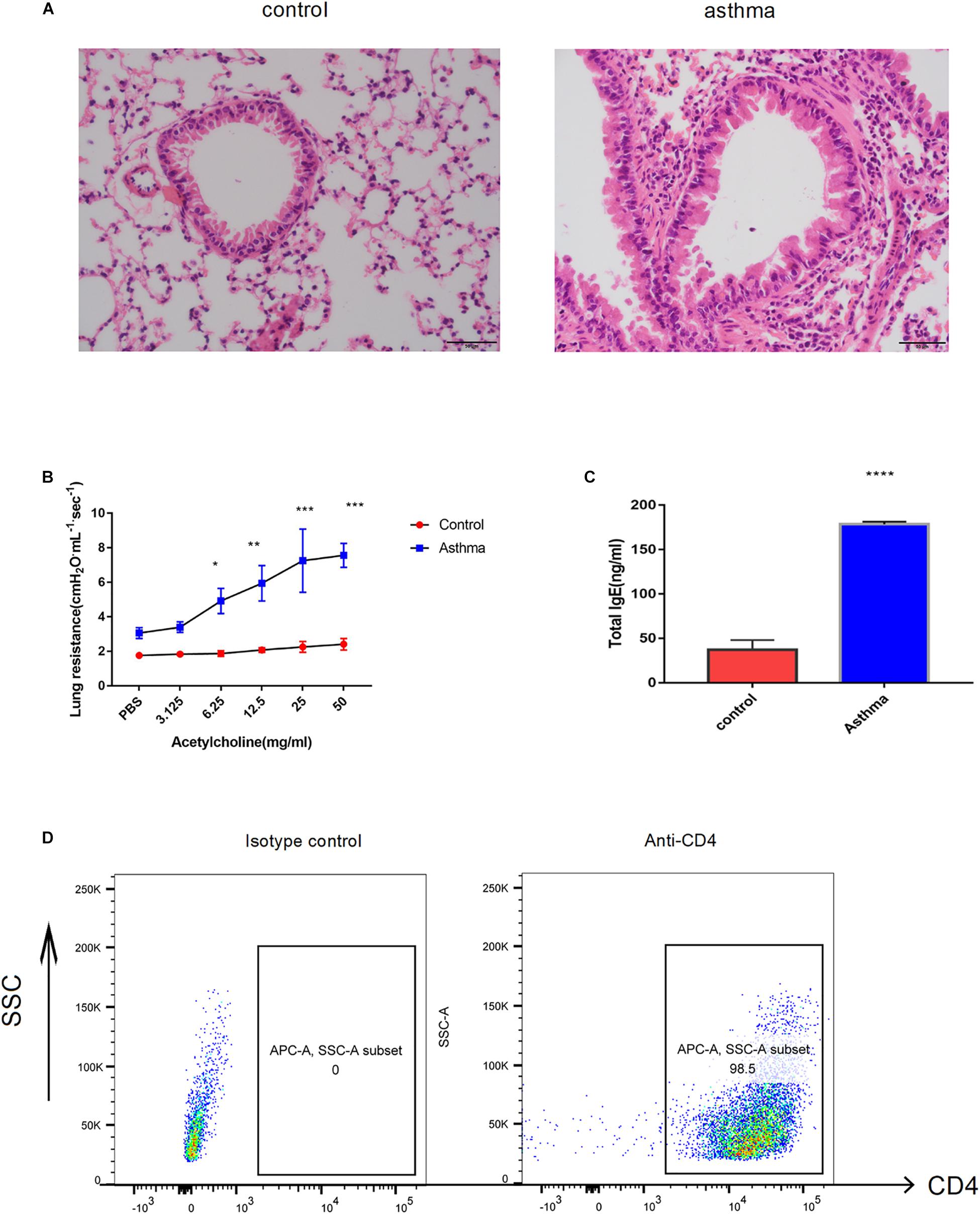
Figure 1. Ovalbumin (OVA)-induced acute model of asthma. (A) Pulmonary inflammatory response was increased in the mice challenged with OVA, bar = 20 μm. (B) The airway hyperresponse was elevated in the mice from the asthma group, ∗p < 0.05 (C). Sera IgE was significantly increased in the mice from the asthma group, ∗p < 0.05. (D) CD4+ T cells magnificently sorted from the spleen were validated by flow cytometry. Representative results of two independent experiments with five mice in each group, ∗∗p < 0.01, ∗∗∗p < 0.01, ∗∗∗∗p < 0.0001.
LncRNA Profile of CD4+ T Cells in Asthma
To obtain a global overview of CD4+ T cells in the asthma transcriptome, we constructed and sequenced 6 RNA-Seq libraries, including controls (n = 3) and asthma mice (n = 3). All of the data have been submitted to Sequence Read Archive (SRA4) with the accession number of PRJNA540404. In total, 134 lncRNAs were significantly altered in expression, including 98 downregulated lncRNAs and 36 upregulated lncRNAs (Figure 2). The top 10 decreased lncRNAs and top 10 increased were listed in Table 2.
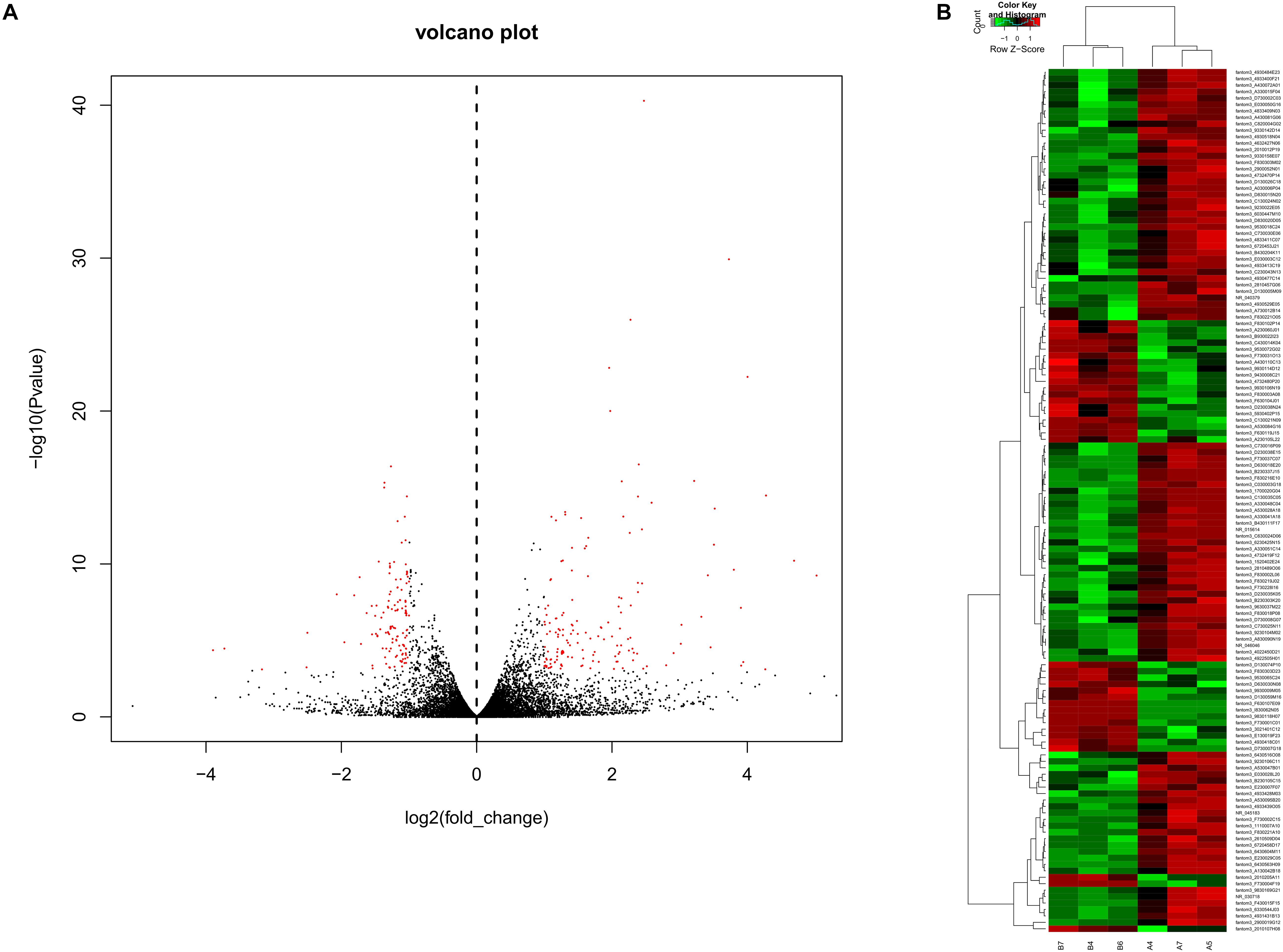
Figure 2. Volcano plots and heat maps of lncRNA expression between the asthma and control groups. (A) Volcano plot assessment of lncRNA expression in CD4+ T cells between the asthma and control groups. Red dots represent different lncRNAs (p < 0.05) showing fold changes >=2 or <= -2. (B) Heat map analysis of differentially expressed lncRNAs between the asthma and control groups. Green indicates low expression, and red indicates high expression. A4, A5, and A7 were the control group; B4, B6, and B7 were CD4+ T cells from the asthma group.
The mRNA Profile of CD4+ T Cells in Asthma
In RNA sequencing, we explored not only the lncRNAs but also the mRNAs from the same samples. With respect to mRNAs, 141 mRNAs were significantly downregulated and 160 mRNAs were significantly upregulated in the CD4+ T cells from asthma (Figure 3). The top 10 decreased and top 10 increased mRNAs are listed in Table 3, including reduced Kbtbd12, Hunk, and Slc6a1 and elevated Ear7, Ear6, and Epx. Among of the 301 differently expressed mRNAs, 17 mRNAs were in the human disease database Malacards associated with asthma (see text footnote 1), 28 mRNAs were in the asthma-associated genes5. More importantly, 11 mRNAs were overlapped in our sequencing data, MalaCards database and asthma-associated genes, including IL4, IL-10, MMP9, VCAM-1, Il1rl1, Alox5, Il1rn, Ccr3, Cysltr1, Epx, and Ccl24.
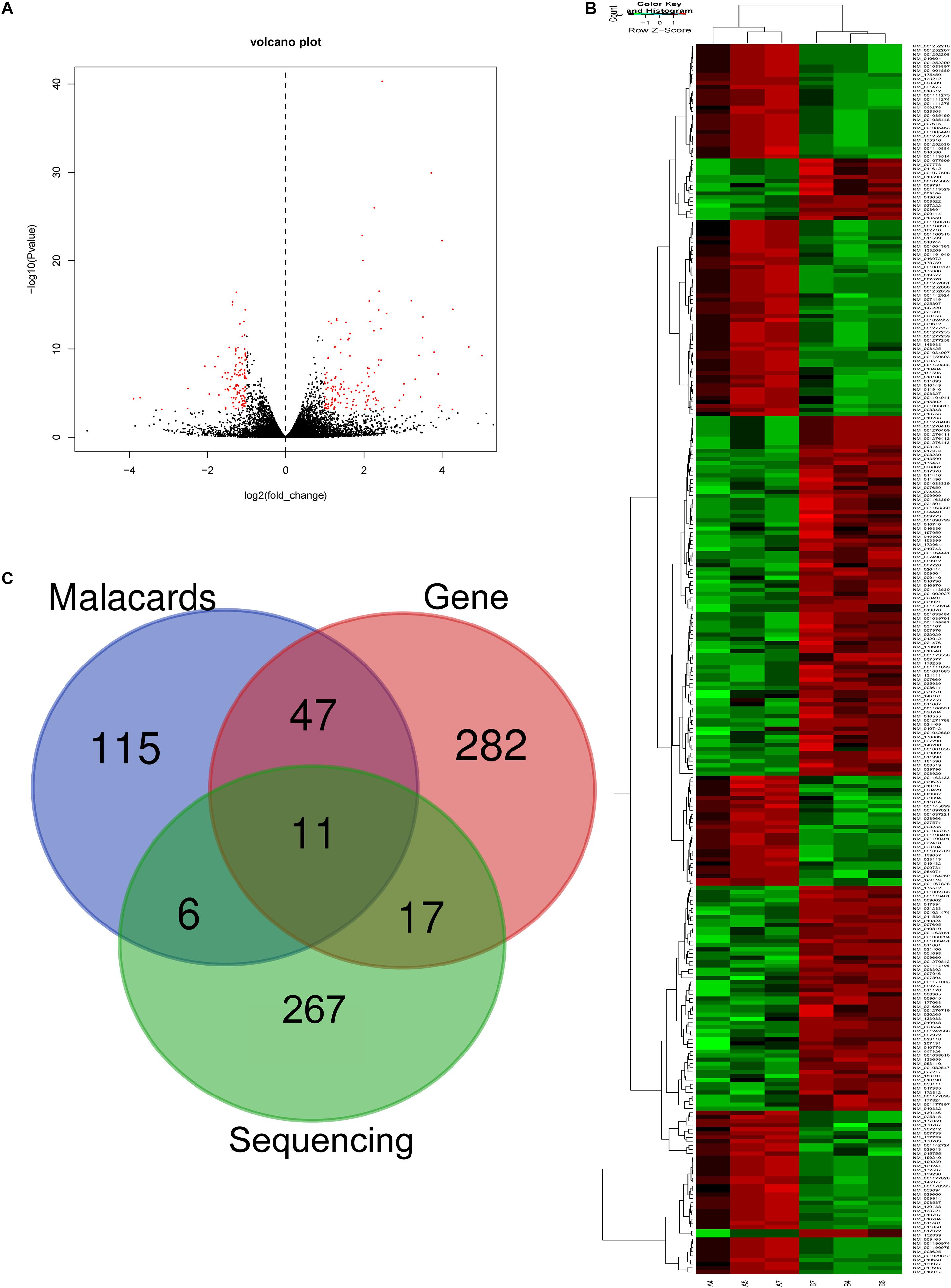
Figure 3. Volcano plots and heat map of mRNA expression between asthma and control groups. (A) Volcano plot assessment of gene expression in CD4+ T cells between asthma and control groups. Red dots represent different mRNAs (p < 0.05) showing fold changes >=2 or <= -2. (B) Heat map analysis of differentially expressed mRNAs between the asthma and control groups. Green indicates low expression, and red indicates high expression. A4, A5, and A7 were the control groups; B4, B6, and B7 were CD4+ T cells from the asthma group. (C) The Venn diagram of genes about asthma from sequencing compared with gene database and malacards database.
The lncRNA-mRNA Co-expression Network
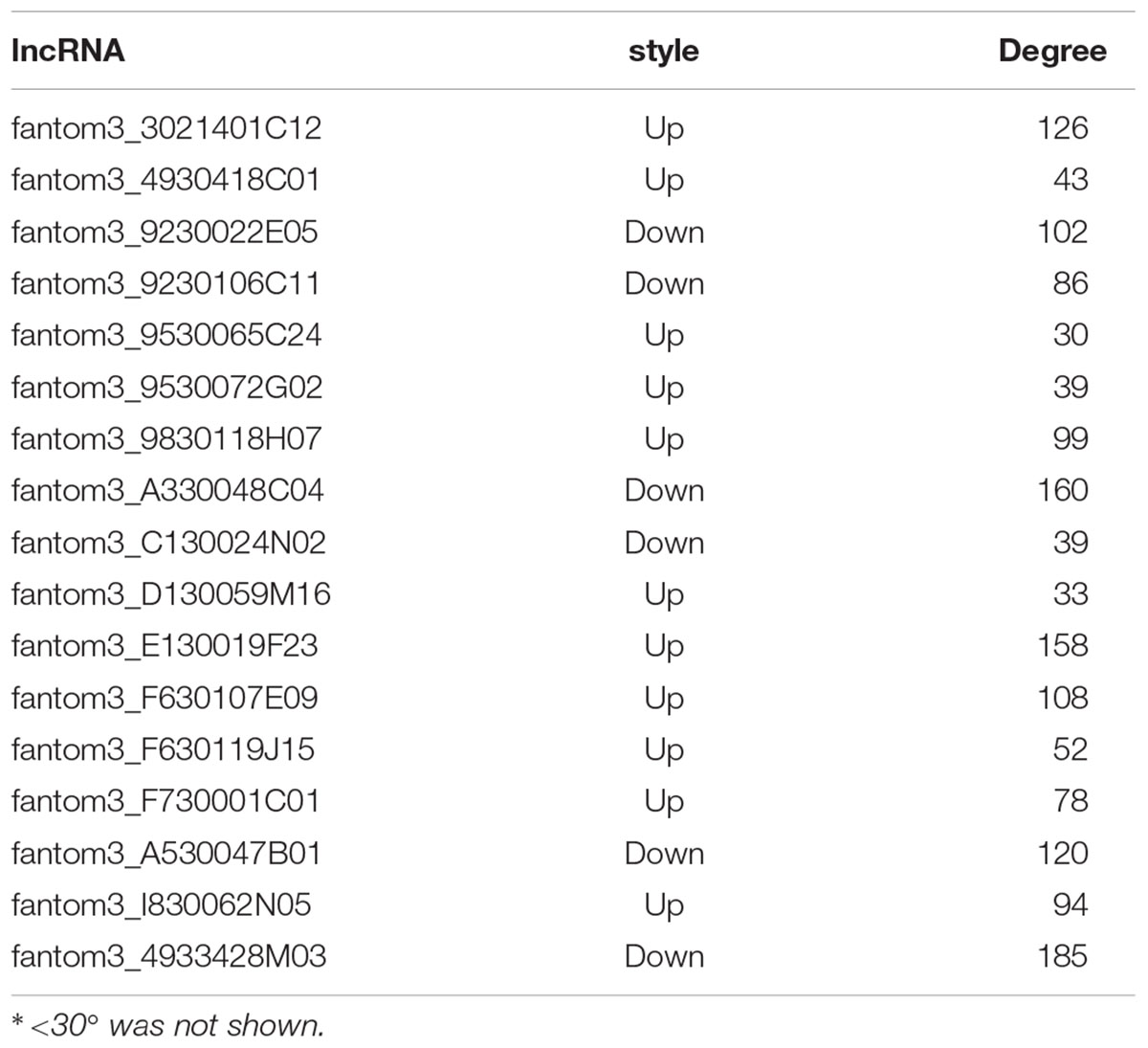
To further examine the function of these differentially expressed lncRNAs in CD4+ T cells in asthma, we constructed an lncRNA-mRNA co-expression network between 301 differentially expressed mRNAs and 23 differentially expressed lncRNAs. The results showed that the co-expression network comprised 12424 connections between lncRNAs and mRNAs. Notably, fantom3_4933428M03 and fantom3_9230106C11 exhibited a high degree of connectivity, suggesting that these two lncRNAs may play key roles (Figure 4 and Table 4). Moreover, our results highlighted the potential internal adjustment correlations between the differentially expressed lncRNAs and mRNAs in CD4+ T cells between the asthma and control groups.
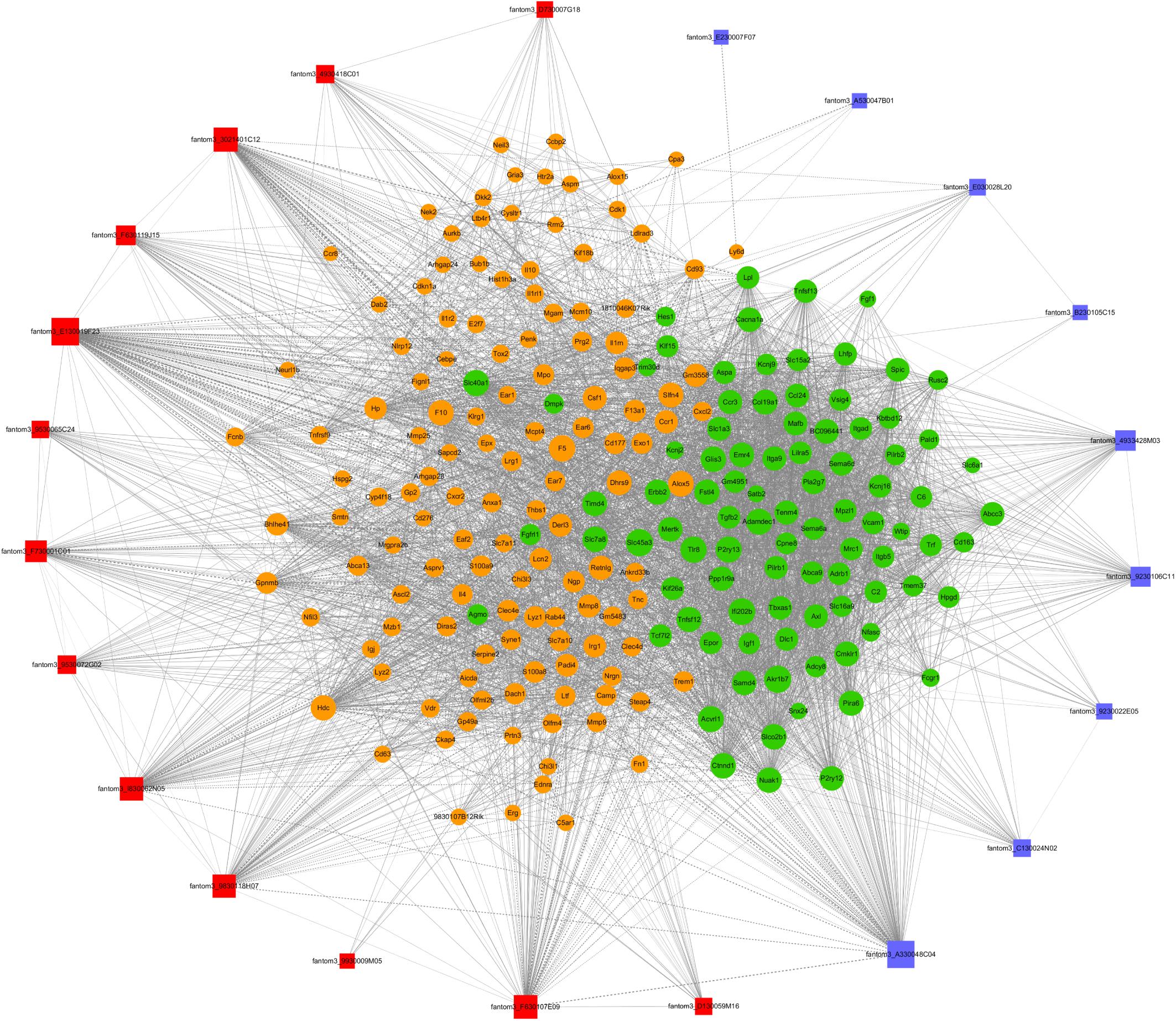
Figure 4. LncRNA–mRNA co-expression networks. Twenty-three lncRNAs and 301 mRNAs were included in the co-expression network | COR| >0.95; p < 0.05). The red boxes indicate upregulated lncRNAs, and the blue boxes indicate downregulated lncRNAs. The green nodes indicate downregulated mRNAs, and the yellow nodes indicate upregulated mRNA. The value of “degree” indicates the size of the boxes or nodes. The width of the lines denotes the Pearson correlation coefficient, where the full line indicates a positive correlation and the imaginary line indicates a negative correlation.
Validation of RNA-Seq Data With Real-Time PCR
To confirm the differentially expressed gene data, we further analyzed some dysregulated lncRNAs using qRT-PCR ex vivo and in vitro. In OVA-induced asthma, Th2 cells orchestrate the pathological cascades reactions. As shown in Figure 5A, CD4+ T cells from the asthma group showed higher expression of the Th2 master transcription factor Gata-3 and decreased expression of the Th1 master transcription factor T-bet. Different from the sequencing data, the expression of LncRNA fantom3_4933428M03 or fantom3_F630107E09 was unexpectedly similar in the CD4+ T cells from either the asthma or the control group. However, lncRNA fantom3_9230106C11 was in accordance with sequencing data, which was significantly decreased in the asthma CD4+ T cells (Figure 5B). To further explore the data reliability, we induced Th1 or Th2 cells in vitro (Figures 6A,B). As expected, the expression of lncRNA fantom3_9230106C11 was significantly decreased in Th2 cells (Figure 6C). FISH assays indicated that lncRNA fantom3_9230106C11 was localized in the cytoplasm of CD4+ T cells (Figure 7).
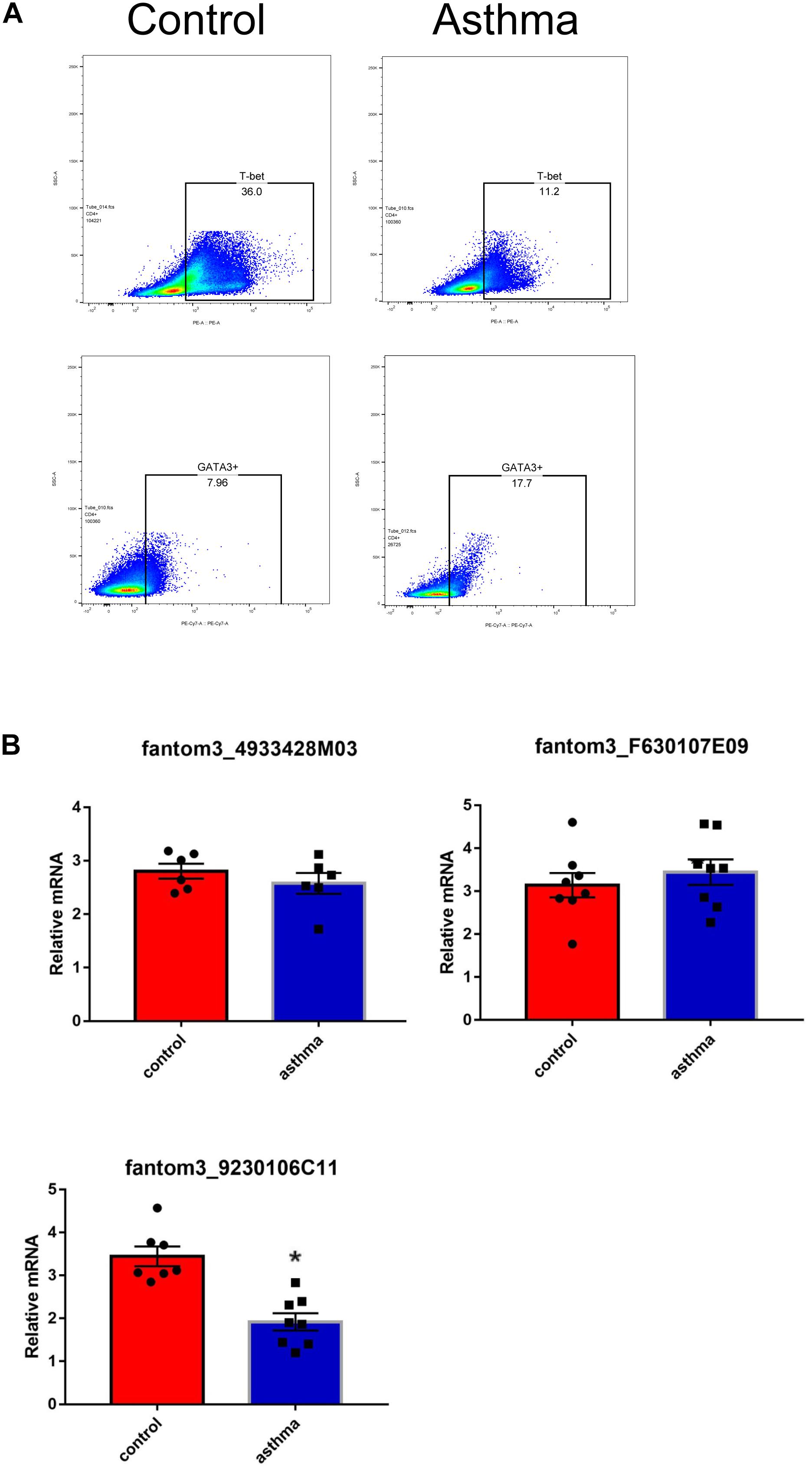
Figure 5. Measurement of selected lncRNAs using qRT-PCR ex vivo. (A) CD4+ T cells in the asthma had lower T-bet and increased Gata-3 expression. Representative result for 3 independent experiments. (B) The expression of randomly selected lncRNAs in CD4+ T cells between the asthma and control groups were quantified ex vivo. ∗p < 0.05, 6∼8 samples were analyzed in each group.
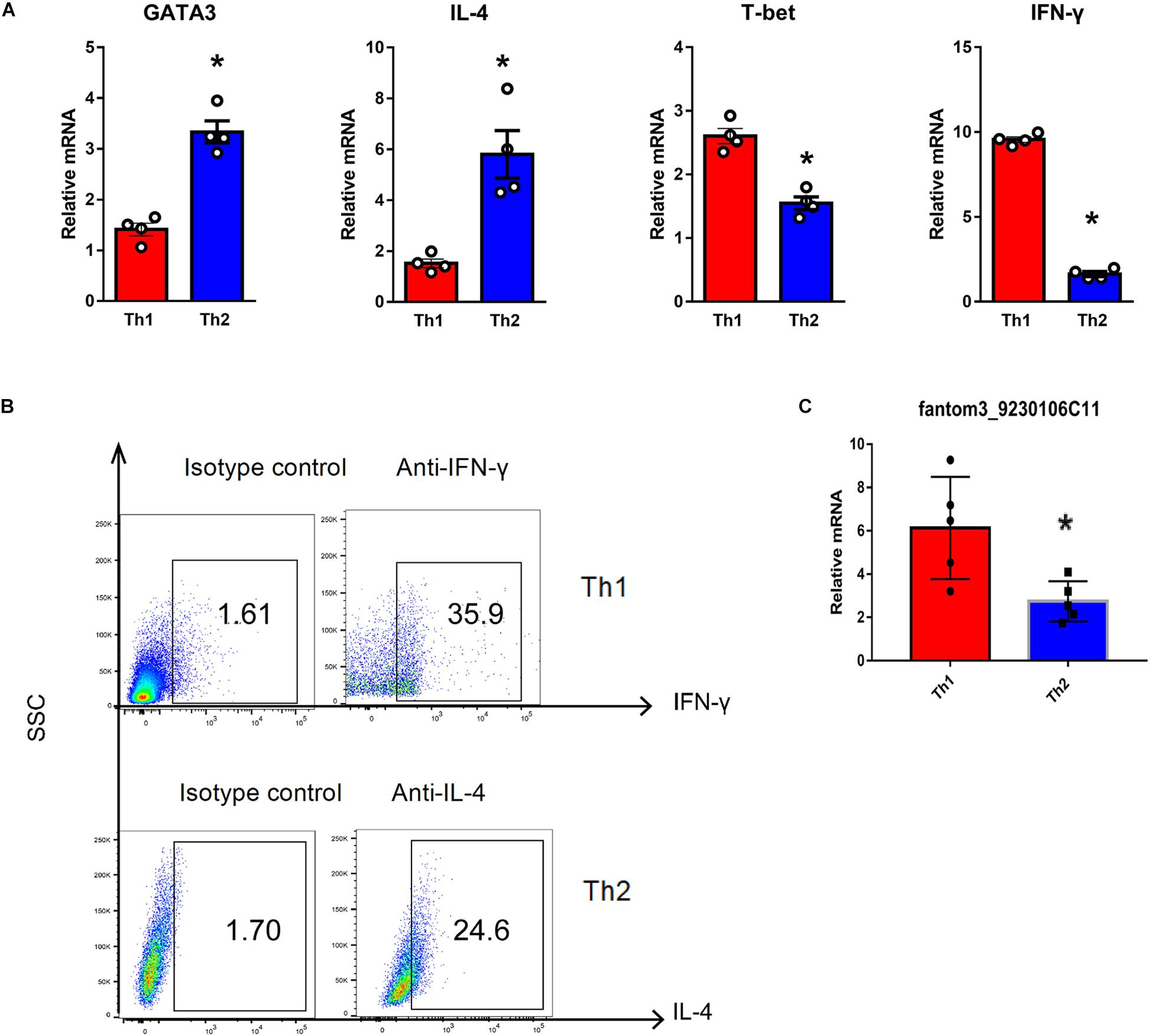
Figure 6. Quantification of LncRNA in Th1 and Th2 cells in vitro. (A) Typical cytokines and transcription factors for Th1 or Th2 were measured using qRT-PCR. Th1 cells exhibited elevated T-bet and IFN-γ levels. In contrast, Th2 cells expressed higher levels of Gata-3 and IL-4. In each group, 4 samples were analyzed. (B) Intracellular cytokines in Th1 and Th2 cells were measured by flow cytometry. Representative result for 3 independent experiments. (C) LncRNA fantom3_9230106C11 was measured in Th1 and Th2 cells in vitro using qRT-PCR. In each group, 5 samples were analyzed. ∗p < 0.05.
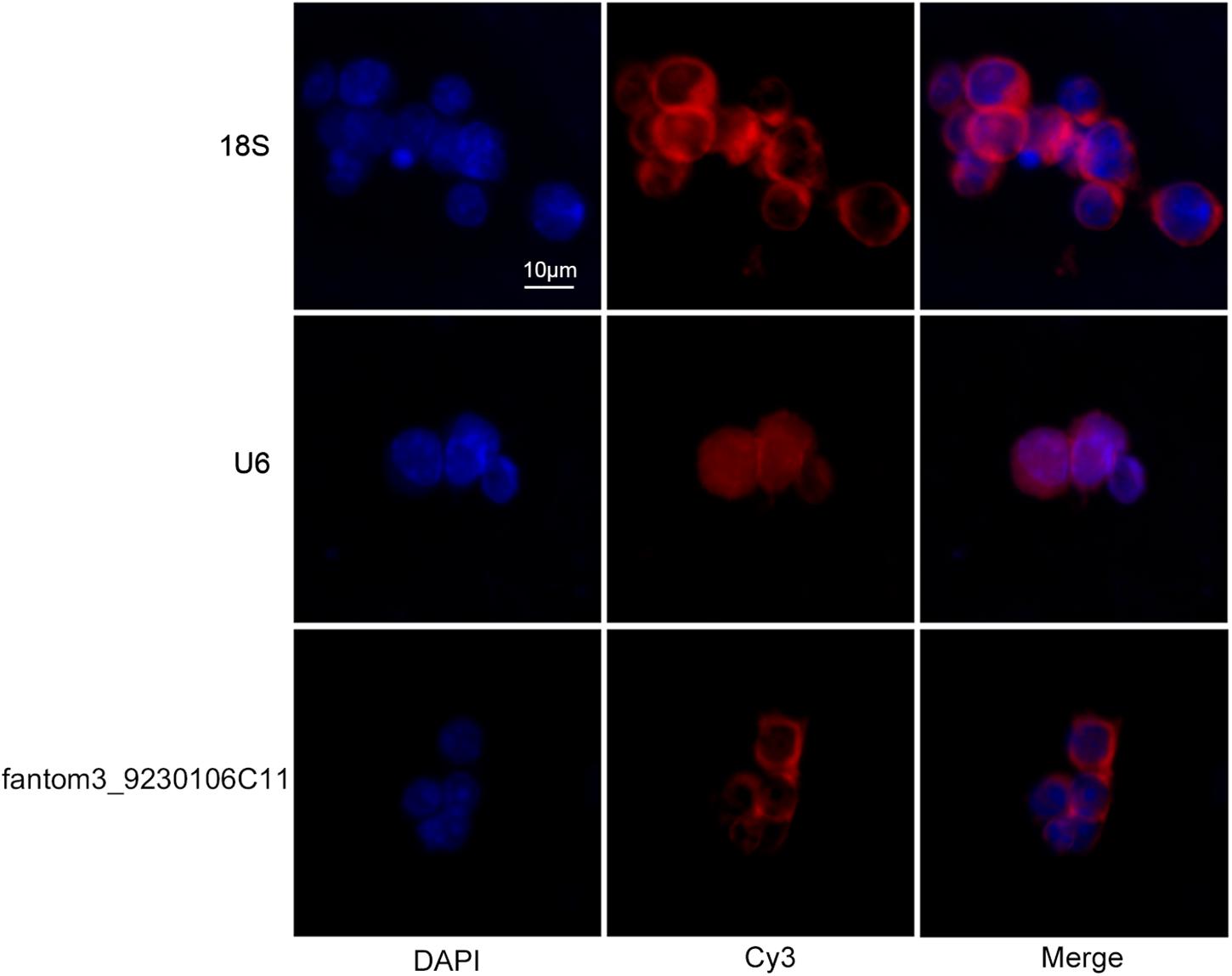
Figure 7. Intracellular location of fantom3_9230106C11 expression in CD4+ T cells by FISH. The nucleus was stained with DAPI, and lncRNA was stained with probe-cyt3. RiboTM U6 and RiboTM 18S were used as reference controls for subcellular localization.
Prediction of lncRNA fantom3_9230106C11 Targets
The above results indicated that lncRNA fantom3_9230106C11 may be involved in Th2 cell differentiation in asthma. LncRNA fantom3_9230106C11 was located at chr6:34412743–34415062 (GenBank: AK033773.1), overlapping with intron 3, exon 4, and intron 4 of Akr1b7 (chr6: 34412362–34423137). Sequence blast analysis shows that lncRNA fantom3_9230106C11 is 99.8% similar with Mus musculus lncRNA URS00009B6C6F6. In the NONCODE (current version v5.0) http://www.noncode.org/, Mus musculus lncRNA URS00009B6C6F is renamed with NONMMUT056297.2, which is highly expressed in the hippocampus (Data Source:ERP000591).
We further explored the potential candidates that may interact with the lncRNA. Diverse transcription factors, such as T-bet, Gata-3, c-maf, stat4, stat5, stat6, jun-b, Dec-2, IRF4, Notch, Gfi-1, and YY1, may be involved in Th2 differentiation (Hwang et al., 2013; Lee, 2014). We used LncTar (Li et al., 2015) and found that lncRNA fantom3_9230106C11 had no effects on the mRNAs of these transcription factors. The Consite Predication Database (Mahmood et al., 2018) showed that transcription factors Gata1, Gklf, SOX17, S8, and Ahr-ARNT may be bound to the promoter of LncRNA fantom3_9230106C11. In addition to transcription factors, a wide range of miRNAs contribute to Th2 differentiation, i.e., miR-17∼92, miR-29, miR-126, miR-132-3p, miR-148a, mir-24, mir-27, mir-19, and mir-155 were confirmed to regulate Th2 differentiation (Istomine et al., 2016; Pua et al., 2016). In the LncTar, Bibiserv, and RNA 22 analysis, lncRNA fantom3_9230106C11 was predicted to bind with miR-19 and other miRNAs. Collectively, the potential candidates of LncRNA fantom3_9230106C11 may include Akr1b7, Gata1 and miRNAs (Figure 8).
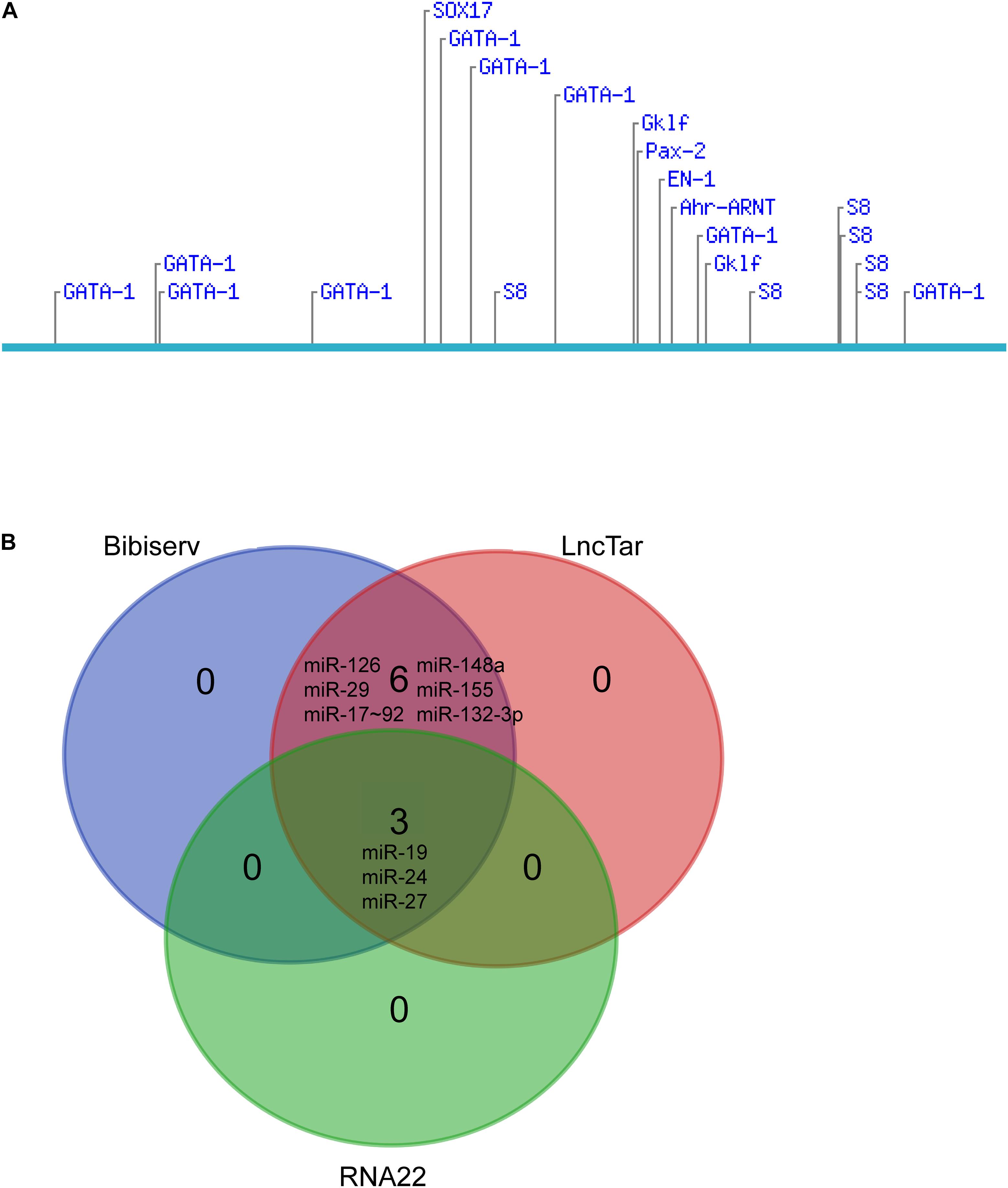
Figure 8. Bioinformatics analysis of lncRNA fantom3_9230106C11. (A) Putative transcription factor binding sites found along the lncRNA fantom3_9230106C11 using Consite and TF scores >95%. (B) The Venn diagram of potential miRNA candidates using LncTar, Bibiserv, and RNA22.
Discussion
Allergic bronchial asthma is a common chronic inflammation with well-defined pathological features, including intermittent airway hyperresponsiveness, pulmonary eosinophil infiltration, and excessive mucus secretion (Chang et al., 2015). Numerous studies have demonstrated that allergic asthma is driven predominantly by a Th2 type of immune response in both human and mouse models of asthma (Kaiko and Foster, 2011). LncRNAs have been linked with airway smooth muscle cells in asthma (Zhang et al., 2016; Austin et al., 2017; Yu et al., 2017; Zhang X.-Y. et al., 2017). However, to our knowledge, there is no report on lncRNA expression in CD4+ T cells in asthma. Therefore, we constructed a mouse model of acute asthma and screened RNA transcripts (mRNAs, lncRNAs) from CD4+ T cells via next-generation sequencing.
In our study, we identified 134 lncRNAs and 301 mRNAs abnormally expressed in CD4+ T cells of asthma compared with controls. In the mRNA expression pattern analysis, elevated Ear7, Ear6 and Epx were closely associated with eosinophils and type 2 inflammations (Ochkur et al., 2017). IL-4, IL-13, and IL-21 (Lajoie et al., 2014), which promoted Th2 differentiation and allergic asthma, were also significantly increased in the CD4+ T cells from the asthma mouse model. However, the Th1 master transcription factor T-bet and the Th2 master transcription factor Gata3 were comparable in CD4+ T cells from both control and asthma mice, suggesting that Th2 differentiation in asthma may be independent of Gata3 (O’Shea and Paul, 2010). In the lncRNA profile analysis, the roles of a wide range of lncRNAs with varied expression in asthma CD4+ T cells were largely unknown. In the lncRNA and mRNA co-expression network, lncRNAs may regulate diverse mRNAs, including Ear6 and Epx, which are required for Th2 differentiation and asthma etiology.
Using real-time qRT-PCR, we demonstrated that lncRNA fantom3_9230106C11 was decreased in CD4+ T cells from asthma mice ex vivo and in Th2 cells in vitro. By filtering the aberrantly expressed genes located near the lncRNA fantom3_9230106C11s, we found that lncRNAs might regulate the transcription of Akr1b7 in cis. lncRNA fantom3_9230106C11s covered intron 3, exon 4, and intron 4 of Akr1b7. Akr1b7 encodes aldose reductase-related protein 1, which may catalyze xenobiotic aromatic aldehydes (Liu et al., 2009). The roles of Akr1b7 in Th2 differentiation or asthma, however, remain uncertain. The bioinformation prediction indicated that the transcription factors Gata1, Gklf, and Ahr-ARNT may be potential candidates for lncRNA fantom3_9230106C11. Gata1 served as a surrogate for Gata3 in its canonic role of programming Th2 gene expression (Sundrud et al., 2005). Gklf (gut-enriched Krüppel-like factor; or Kruppel-like factor 4, KLF4) was required in Th2 cell responses in vivo (Tussiwand et al., 2015). Ahr-ARNT, which may regulate IL-33, IL-25, and TSLP, was closely associated with allergic severe asthma (Weng et al., 2018). Moreover, considering that lncRNA fantom3_9230106C11 resides in the cytoplasm, it may interact with miR-19 and other miRNAs, which are closely associated with Th2 differentiation.
Our study was not without limitations. First, BALB/c and C57BL/6 mouse strains are the two most commonly used in asthma models. Compared with the C57BL/6 mice used in this study, BALB/c mice were more prone to a Th2 immune response (Gueders et al., 2009). Although allergic asthma was Th2-dominated, Th1 and other CD4+ T subpopulations were also involved in the initiation and aggravation of disease. Therefore, the C57BL/6 mouse model was well recognized in the recapitulation of disease features in asthma. Second, spleen CD4+ T cells rather than lung CD4+ T cells were analyzed in the present study. We tried to sort CD4+ T cells from lung parenchyma and pulmonary lymph nodes. However, there were not enough purified CD4+ T cells (5∗106 from each mouse) to complete the NGS experiment. Previously, adoptive transfer experiments demonstrated that peripheral CD4+ T lymphocytes regulate asthma pathogenesis (Cohn et al., 2004; Hubeau et al., 2006). Therefore, we postulated that spleen CD4+ T cells may be surrogates for peripheral CD4+ T lymphocytes. Third, we have not validated our observations in the clinical samples. LncRNAs are considered poorly conserved across different species (Ma et al., 2013). However, the conservation may be multidimensional (Diederichs, 2014). The expression of aberrant lncRNAs (lncRNA fantom3_9230106C11) in the clinical samples should be evaluated.
Conclusion
In conclusion, we conducted a comprehensive analysis of lncRNA profiles in CD4+ T cells from an asthma model using next-generation sequencing. The co-expression network of lncRNAs and mRNAs was constructed. The present study provided a platform for elucidating the roles of lncRNAs in Th2 differentiation and asthma pathogenesis.
Ethics Statement
All experiments that involved animal and tissue samples were performed in accordance with the guidelines and procedures approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC-1709011).
Author Contributions
MH and MZ conceived the idea and designed the research. ZW, NJ, ZC, and CW conducted the animal and in vitro experiments. ZS, WY, and MZ analyzed the results. FH performed the flow cytometry. ZW and MZ wrote the manuscript. All the authors reviewed and approved of the manuscript.
Funding
This research was supported by the Precision Medicine Research of The National Key Research and Development Plan of China (2016YFC0905800), National Natural Science Foundation of China (81671563, 81770031, and 81700028), Natural Science Foundation of Jiangsu Province (BK20171501, BK20171080, and BK20181497), Jiangsu Province’s Young Medical Talent Program, China (QNRC2016600), and Jiangsu Provincial Health and Family Planning Commission Foundation (Q2017001).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^https://www.malacards.org/
- ^https://www.ncbi.nlm.nih.gov/gene/?term=asthma
- ^http://bioinformatics.psb.ugent.be/webtools/Venn/
- ^www.ncbi.nlm.nih.gov/sra
- ^https://www.ncbi.nlm.nih.gov/gene
- ^https://rnacentral.org/rna/URS00009B6C6F
References
Austin, P. J., Tsitsiou, E., Boardman, C., Jones, S. W., Lindsay, M. A., Adcock, I. M., et al. (2017). Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J. Allergy Clin. Immunol. 139, 780–789. doi: 10.1016/j.jaci.2016.06.014
Chang, P. J., Michaeloudes, C., Zhu, J., Shaikh, N., Baker, J., Chung, K. F., et al. (2015). Impaired nuclear translocation of the glucocorticoid receptor in corticosteroid-insensitive airway smooth muscle in severe asthma. Am. J. Respir. Criti. Care Med. 191, 54–62. doi: 10.1164/rccm.201402-0314OC
Cohn, L., Elias, J. A., and Chupp, G. L. (2004). Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 22, 789–815. doi: 10.1146/annurev.immunol.22.012703.104716
Diederichs, S. (2014). The four dimensions of noncoding RNA conservation. Trends Genet. 30, 121–123. doi: 10.1016/j.tig.2014.01.004
Gueders, M. M., Paulissen, G., Crahay, C., Quesada-Calvo, F., Hacha, J., Van Hove, C., et al. (2009). Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 58, 845–854. doi: 10.1007/s00011-009-0054-2
Holgate, S. T. (2012). Innate and adaptive immune responses in asthma. Nat. Med. 18, 673–683. doi: 10.1038/nm.2731
Hondowicz, B. D., An, D., Schenkel, J. M., Kim, K. S., Steach, H. R., Krishnamurty, A. T., et al. (2016). Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity 44, 155–166. doi: 10.1016/j.immuni.2015.11.004
Hubeau, C., Apostolou, I., and Kobzik, L. (2006). Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am. J. Pathol. 168, 1931–1939. doi: 10.2353/ajpath.2006.051231
Hwang, S. S., Kim, Y. U., Lee, S., Jang, S. W., Kim, M. K., Koh, B. H., et al. (2013). Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 110, 276–281. doi: 10.1073/pnas.1214682110
Istomine, R., Pavey, N., and Piccirillo, C. A. (2016). Posttranscriptional and translational control of gene regulation in CD4+ T cell subsets. J. Immunol. 196, 533–540. doi: 10.4049/jimmunol.1501337
Kaiko, G. E., and Foster, P. S. (2011). New insights into the generation of Th2 immunity and potential therapeutic targets for the treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 11, 39–45. doi: 10.1097/ACI.0b013e328342322f
Kerzerho, J., Maazi, H., Speak, A. O., Szely, N., Lombardi, V., Khoo, B., et al. (2013). Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J. Allergy Clin. Immunol. 131, 1048–1057, 1057.e1-2. doi: 10.1016/j.jaci.2012.09.027
Lajoie, S., Lewkowich, I., Herman, N. S., Sproles, A., Pesce, J. T., Wynn, T. A., et al. (2014). IL-21 receptor signalling partially mediates Th2-mediated allergic airway responses. Clin. Exp. Allergy 44, 976–985. doi: 10.1111/cea.12341
Lee, G. R. (2014). Transcriptional regulation of T helper type 2 differentiation. Immunology 141, 498–505. doi: 10.1111/imm.12216
Li, J., Ma, W., Zeng, P., Wang, J., Geng, B., Yang, J., et al. (2015). LncTar: a tool for predicting the RNA targets of long noncoding RNAs. Brief. Bioinform. 16, 806–812. doi: 10.1093/bib/bbu048
Liu, M. J., Takahashi, Y., Wada, T., He, J., Gao, J., Tian, Y., et al. (2009). The aldo-keto reductase Akr1b7 gene is a common transcriptional. Mol. Pharmacol. 76, 604–611. doi: 10.1124/mol.109.057455
Liu, Y., Chen, Z., Xu, K., Wang, Z., Wu, C., Sun, Z., et al. (2018). Next generation sequencing for miRNA profile of spleen CD4(+) T cells in the murine model of acute asthma. Epigenomics 10, 1071–1083. doi: 10.2217/epi-2018-0043
Ma, L., Bajic, V. B., and Zhang, Z. (2013). On the classification of long non-coding RNAs. RNA Biol. 10, 925–933. doi: 10.4161/rna.24604
Mahmood, T., Tahir, T., Munir, F., and Shinwari, Z. K. (2018). Characterization of regulatory elements in OsRGLP2 gene promoter from different rice accessions through sequencing and in silico evaluation. Comput. Biol. Chem. 73, 206–212. doi: 10.1016/j.compbiolchem.2018.02.015
Miranda, K. C., Huynh, T., Tay, Y., Ang, Y. S., Tam, W. L., Thomson, A. M., et al. (2006). A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126, 1203–1217. doi: 10.1016/j.cell.2006.07.031
Ochkur, S. I., Doyle, A. D., Jacobsen, E. A., LeSuer, W. E., Li, W., Protheroe, C. A., et al. (2017). Frontline science: eosinophil-deficient MBP-1 and EPX double-knockout mice link pulmonary remodeling and airway dysfunction with type 2 inflammation. J. Leukoc. Biol. 102, 589–599. doi: 10.1189/jlb.3HI1116-488RR
O’Shea, J. J., and Paul, W. E. (2010). Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102. doi: 10.1126/science.1178334
Pua, H. H., Steiner, D. F., Patel, S., Gonzalez, J. R., Ortiz-Carpena, J. F., Kageyama, R., et al. (2016). MicroRNAs 24 and 27 suppress allergic inflammation and target a network of regulators of T helper 2 cell-associated cytokine production. Immunity 44, 821–832. doi: 10.1016/j.immuni.2016.01.003
Sczyrba, A., Kruger, J., Mersch, H., Kurtz, S., and Giegerich, R. (2003). RNA-related tools on the bielefeld bioinformatics server. Nucleic Acids Res. 31, 3767–3770. doi: 10.1093/nar/gkg576
Sundrud, M. S., Vancompernolle, S. E., Eger, K. A., Bruno, T. C., Subramaniam, A., Mummidi, S., et al. (2005). Transcription factor GATA-1 potently represses the expression of the HIV-1 coreceptor CCR5 in human T cells and dendritic cells. Blood 106, 3440–3448. doi: 10.1182/blood-2005-03-0857
Takyar, S., Vasavada, H., Zhang, J. G., Ahangari, F., Niu, N., Liu, Q., et al. (2013). VEGF controls lung Th2 inflammation via the miR-1-Mpl (myeloproliferative leukemia virus oncogene)-P-selectin axis. J. Exp. Med. 210, 1993–2010. doi: 10.1084/jem.20121200
Tsitsiou, E., Williams, A. E., Moschos, S. A., Patel, K., Rossios, C., Jiang, X., et al. (2012). Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 129, 95–103. doi: 10.1016/j.jaci.2011.08.011
Tussiwand, R., Everts, B., Grajales-Reyes, G. E., Kretzer, N. M., Iwata, A., Bagaitkar, J., et al. (2015). Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 42, 916–928. doi: 10.1016/j.immuni.2015.04.017
Weng, C. M., Wang, C. H., Lee, M. J., He, J. R., Huang, H. Y., Chao, M. W., et al. (2018). Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy 73, 2192–2204. doi: 10.1111/all.13462
Yu, X., Zhe, Z., Tang, B., Li, S., Tang, L., Wu, Y., et al. (2017). α-Asarone suppresses the proliferation and migration of ASMCs through targeting the lncRNA-PVT1/miR-203a/E2F3 signal pathway in RSV-infected rats. Acta Biochim. Biophys. Sin. 49, 598–608. doi: 10.1093/abbs/gmx048
Zhang, F., Liu, G., Wei, C., Gao, C., and Hao, J. (2017). Linc-MAF-4 regulates Th1/Th2 differentiation and is associated with the pathogenesis of multiple sclerosis by targeting MAF. FASEB J. 31, 519–525. doi: 10.1096/fj.201600838R
Zhang, X.-Y., Tang, X.-Y., Ma, L.-J., Guo, Y.-L., Li, X.-S., Zhao, L.-M., et al. (2017). Schisandrin B down-regulated lncRNA BCYRN1 expression of airway smooth muscle cells by improving miR-150 expression to inhibit the proliferation and migration of ASMC in asthmatic rats. Cell Proliferation 50:e12382. doi: 10.1111/cpr.12382
Zhang, X. Y., Zhang, L. X., Tian, C. J., Tang, X. Y., Zhao, L. M., Guo, Y. L., et al. (2016). LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am. J. Transl. Res. 8, 3409–3418.
Keywords: asthma, CD4+ T lymphocyte, long non-coding RNA, mRNA, next-generation sequencing
Citation: Wang Z, Ji N, Chen Z, Wu C, Sun Z, Yu W, Hu F, Huang M and Zhang M (2019) Next Generation Sequencing for Long Non-coding RNAs Profile for CD4+ T Cells in the Mouse Model of Acute Asthma. Front. Genet. 10:545. doi: 10.3389/fgene.2019.00545
Received: 04 February 2019; Accepted: 22 May 2019;
Published: 07 June 2019.
Edited by:
Yun Zheng, Kunming University of Science and Technology, ChinaReviewed by:
Changning Liu, Xishuangbanna Tropical Botanical Garden (CAS), ChinaFei Li, Zhejiang University, China
Copyright © 2019 Wang, Ji, Chen, Wu, Sun, Yu, Hu, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mao Huang, aG02MTE0QDE2My5jb20=; Mingshun Zhang, bWluZ3NodW56aGFuZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhengxia Wang1†
Zhengxia Wang1† Mingshun Zhang
Mingshun Zhang