- 1Key Laboratory of Buffalo Genetics, Breeding and Reproduction Technology, Ministry of Agriculture and Guangxi Buffalo Research Institute, Chinese Academy of Agricultural Sciences, Nanning, China
- 2Institute of Animal and Dairy Sciences, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 3Faculty of Veterinary Sciences, Bahauddin Zakariya University, Multan, Pakistan
- 4Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, Faisalabad, Pakistan
Curcumin (a polyphenolic compound in turmeric) is famous for its potent anti-inflammatory, anti-oxidant, and anti-cancer properties, and has a great potential to act as an epigenetic modulator. The epigenetic regulatory roles of curcumin include the inhibition of DNA methyltransferases (DNMTs), regulation of histone modifications via the regulation of histone acetyltransferases (HATs) and histone deacetylases (HDACs), regulation of microRNAs (miRNA), action as a DNA binding agent and interaction with transcription factors. These mechanisms are interconnected and play a vital role in tumor progression. The recent research has demonstrated the role of epigenetic inactivation of pivotal genes that regulate human pathologies such as cancers. Epigenetics helps to understand the mechanism of chemoprevention of cancer through different therapeutic agents. In this regard, dietary phytochemicals, such as curcumin, have emerged as a potential source to reverse epigenetic modifications and efficiently regulate the expression of genes and molecular targets that are involved in the promotion of tumorigenesis. The curcumin may also act as an epigenetic regulator in neurological disorders, inflammation, and diabetes. Moreover, curcumin can induce the modifications of histones (acetylation/deacetylation), which are among the most important epigenetic changes responsible for altered expression of genes leading to modulating the risks of cancers. Curcumin is an effective medicinal agent, as it regulates several important molecular signaling pathways that modulate survival, govern anti-oxidative properties like nuclear factor E2-related factor 2 (Nrf2) and inflammation pathways, e.g., nuclear factor kappa B (NF-κB). Curcumin is a potent proteasome inhibitor that increases p-53 level and induces apoptosis through caspase activation. Moreover, the disruption of 26S proteasome activity induced by curcumin through inhibiting DYRK2 in different cancerous cells resulting in the inhibition of cell proliferation opens up a new horizon for using curcumin as a potential preventive and treatment approach in proteasome-linked cancers. This review presents a brief summary of knowledge about the mechanism of epigenetic changes induced by curcumin and the potential effects of curcumin such as anti-oxidant activity, enhancement of wound healing, modulation of angiogenesis and its interaction with inflammatory cytokines. The development of curcumin as a clinical molecule for successful chemo-prevention and alternate therapeutic approach needs further mechanistic insights.
Introduction
Curcumin, scientifically known as diferuloylmethane, is a yellow polyphenol and the active component of the perennial herb Curcuma longa, usually known as turmeric (Aggarwal and Sung, 2009). It contains 80% curcuminoid complex, 17% dimethoxy-curcumin, and 3% bisdemethoxy-curcumin (Lao et al., 2006). Curcumin is well known for its potent anticancer activities which have been systematically examined through elucidation of a number of potential mechanisms of actions. Even though, pharmacokinetic studies revealed that curcumin is found in quite lesser plasma concentration in human beings as compared to in vitro, yet many preclinical investigations have confirmed the anticancer activities of curcumin (Cheng et al., 2001; Sharma et al., 2004). Curcumin can provoke the apoptosis but slow down the proliferation of cancer cell lines (Huminiecki et al., 2017). The active biological activity of curcumin at slightly low concentrations in humans might be due to epigenetic modulation of different pathways. Epigenetics leads to heritable alterations in the expression of genes while maintaining their coding DNA sequences. Moreover, it provides an effective approach to discriminately inactivate or activate the expression of genes by endogenous and exogenous substances (Davis and Ross, 2007). Mechanisms of epigenetics include changes in DNA methylation, histone modification, and alteration in the expression of miRNAs (Yoo and Jones, 2006; Winter et al., 2009). Natural complexes like resveratrol, epigallocatechin gallate (EGCG) and curcumin have been known to induce epigenetic changes that may enhance the sensitivity of cancerous cells to usual chemo-therapeutic agents and consequently suppress tumor growth (Li et al., 2010).
Curcumin is an effective therapeutic compound, because it controls many important pathways of molecular signaling which in turn modulate the survival and pathways that govern anti-oxidative factors (such as nuclear factor E2-related factor 2, Nrf2) and inflammatory responses like nuclear factor kappa B (Hatcher et al., 2008). Other than its role in controlling Nrf2 in various kinds of cancers, curcumin harmonizes Nrf2 expression in various human ailments such as neurocognitive disorders, diabetes, and renal disorders. Curcumin is well known as an anti-inflammatory mediator because it controls the anti-inflammatory reaction by decreasing the activities of cyclooxygenase (COX-2) and inducible nitric oxide synthase (iNOS) via withholding the transcription of nuclear factor kappa B (NF-κB), ultimately leading to the cessation of tumorigenesis (Surh et al., 2001). Curcumin also reduces the expression of genes being regulated by NF-κB, including 5-lipoxygenase (5-LOX), tumor necrosis factor (TNF), adhesion molecules, interleukins (IL-1, IL-6, IL-8), chemokine receptor type 4 (CXCR-4), and C-reactive protein (Rao, 2007; Skommer et al., 2007).
Studies conducted on small intestine and liver of mice have shown that curcumin can suppress or induce the expression of many genes associated with the regulation of cell cycle, apoptosis, cell adhesion, phosphatases, and kinases. The changes in several phase II antioxidant/detoxification enzymes that are controlled by Nrf2 indicate the possible roles of curcumin and Nrf2 in lowering the risk of cancer (Shen et al., 2006). A recent study in mice revealed that curcumin is a neuro-protectant with respect to hemin-induced damage among foremost cultures of cerebellar granule neurons. These neuroprotective effects were mediated through glutathione (GSH) synthesis or inhibition of the heme oxygenase system by means of buthionine sulfoximine and tin mesoporphyrin, respectively. Moreover, the activities of glutathione S-transferase, glutathione reductase and superoxide dismutase were enhanced by 2.3-, 1.4-, and 5.2-fold, respectively, after a 24-h incubation with curcumin. These findings suggested that an antioxidant response and Nrf2 activation may have contributed significantly to the protective effect of curcumin against neuronal death induced by hemin (González-Reyes et al., 2013).
Curcumin is a unique molecule with diverse biological activities, including its beneficial effects on diabetes, particularly regarding insulin sensitivity. Studies have reported that curcumin reduced glucose intolerance without influencing weight gain by inducing the nuclear translocation of Nrf2 along with its downstream target of heme oxygenase-1, which was decreased by a high-fat diet (He et al., 2012). Nrf2 is recognized as a major stress responsive factor that ameliorates adverse effects of different stressors like xenobiotics, inflammation, excessive metabolites, and misfolded proteins. Investigations on the molecular mechanisms responsible for the apparent anti-oxidant potential of curcumin proved the ability of curcumin to protect nerve cells against ischemic abrasion by involving the Akt/Nrf2 pathway (Wu et al., 2013). Investigation of ischemia-reperfusion injury in Nrf2−/− mice revealed noticeably poorer vascular permeability, kidney function, and survival in relation to wild-type mice. Diabetic nephropathy model induced by streptozotocin (STZ) revealed that Nrf2−/− mice had severely injured kidney and damaged DNA in comparison with normal mice. Treatment by cyclosporine A resulted in relatively higher interstitial fibrosis and kidney damage in Nrf2−/− mice (Liu M. et al., 2009; Jiang et al., 2010; Shin et al., 2010). It was further noticed that the provision of curcumin (100 mg/kg) greatly reduced the infiltration of macrophages into kidney and the expression of pro-inflammatory cytokines, including TNF-α and IL-1β accompanied with the inhibition of NF-κB in animals suffering from STZ diabetes (Soetikno et al., 2011). Moreover, curcumin also ameliorated adverse effects of arsenic induced hepatotoxicity and oxidative damage by reducing elevated serum levels of liver enzymes (AST and ALT) and the inflation of hepatic malondialdehyde (MDA). It also decreased hepatic and blood GSH levels through the activation of Nrf2 (Gao et al., 2013). Additionally, curcumin also catalyzes the expression of genes that transcribe anti-oxidant enzymes (Rogers et al., 2012; Trujillo et al., 2013). Above mentioned diverse array of biological functions mediated by curcumin provides a glimpse of its therapeutic potential in different human diseases.
Epigenetic Modulation by Curcumin
Different studies have clearly described the potent role of curcumin as an epigenetic modulator. Most significant activities of curcumin are summarized in following aspects.
Histone Deacetylation/Acetylation
Histone acetylation and deacetylation are two major histone modifications which are considered as significant epigenetic changes to alter the expression of genes. Their imbalance can lead to the risk of cancer (Gibbons, 2005). Histone deacetylases (HDACs) are the enzymes that interact with DNA by means of multiprotein compounds, including co-activators and co-repressors. HDACs eliminate the acetyl group from histone proteins which are associated with gene silencing while histone acetyltransferases (HATs) cause acetylation relevant to gene transcription. The balance between acetylation and deacetylation is important for the regulation of gene function. Irregular activities of HDACs and HATs have been associated with the onset of cancer. Around 18 HDACs have been identified which are grouped into four classes based on their similarities with yeast deacetylases (Xu et al., 2007).
The inhibition of HDACs by different substances, is being considered as a cancer therapeutic approach owing to their potential for regulating many cellular activities (Ceccacci and Minucci, 2016; Li and Seto, 2016). Potential effects of curcumin on the activities of HDACs/HATs proved that curcumin is the most potent inhibitor of HDACs as presented in Table 1 (Bora-Tatar et al., 2009). Curcumin has also been proven to be more effective when compared to sodium butyrate and valproic acid, which are considered as popular inhibitors of HDACs. Furthermore, the use of curcumin significantly reduced the levels of class I HDACs, leading to an increased level of acetylation (Liu et al., 2005; Chen et al., 2007, 2013). Curcumin has shown inhibition of ∼50% HDAC activity at a very high concentration of 500 μM in HeLa nuclear extracts (with IC50 value of 115 μM). It has also been reported that curcumin induced global inhibition of HDAC activity and reduced HDAC8 isoform activity while increasing the expression of suppressors of cytokine signaling, SOCS1 and SOCS3 in the leukemic cell (Chen et al., 2013). Moreover, curcumin exhibited increased acetylation of histone H4 by decreasing levels of HDAC1, 3 and 8 in the Raji cells (Liu et al., 2005). Direct inhibition of HDAC4’s transcription leading to reduced overall HDAC activity, has been revealed by treatment of the medulloblastoma cells with curcumin (Lee et al., 2011). These findings proved that curcumin is a potent inhibitor of HDAC activity with free binding energy and inhibition constant (for HDAC8) comparable to trichostatin A and vorinostat (Bora-Tatar et al., 2009). Conversely, the restoration of HDAC2 level in affected lungs in chronic respiratory disorders has also been observed by the use of curcumin. These contradictive findings revealed that the effect of curcumin on HDAC activity is variable and probably cell line specific (Meja et al., 2008).
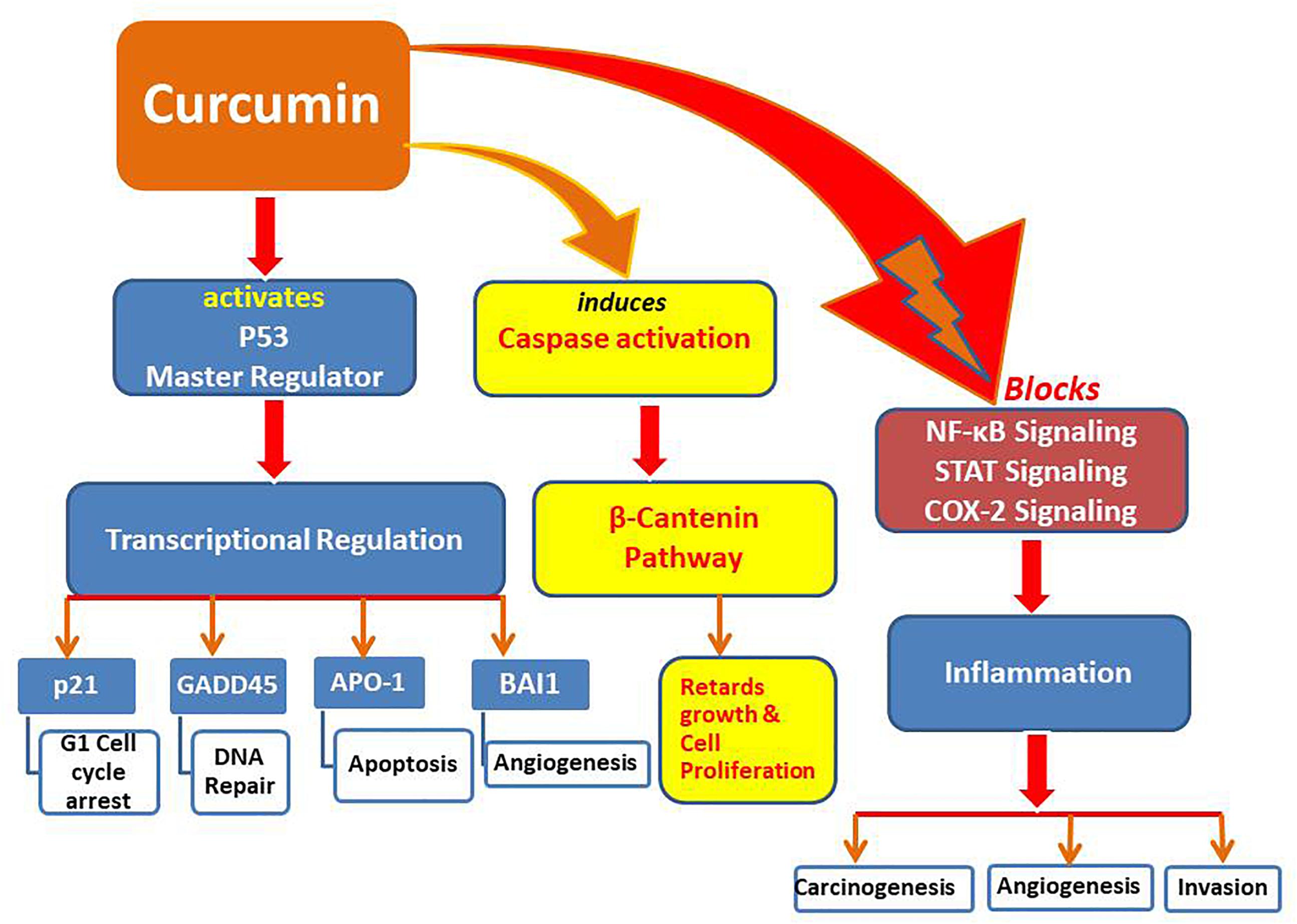
Potential therapeutic role of tumor suppressor gene p53 is well established in human cancer pathogenesis, as up-regulation of p53 could inhibit cellular proliferation through inducing cell cycle arrest and apoptosis in cancer cells (Kruse and Gu, 2009; Gong et al., 2018; Su et al., 2018). P53 is an important tumor suppression protein with dual activity as transcription activator and repressor being associated with cell proliferation, DNA repair and cell malignancy. Mutated or non-functional p-53 have been found in more than 50% human cancers leading to apoptotic resistance and continued proliferation (Kim and An, 2016). Pharmacological re-activation of p53 in treating cancers is envisioned as an effective therapeutic strategy as restoration of p53 results in apoptosis in lymphoma while suspends cell growth and senescence in sarcoma (Ventura et al., 2007). Activation of p53 by acetylation leads to its binding with DNA that ultimately mediates transcription of downstream targets (like GADD45 and p21) to arrest cell cycle and induce apoptosis (Gu and Roeder, 1997). Curcumin has shown acetylation of p53 leading to activation of p53 signaling pathway (Fu et al., 2018). The acetylated p53 recruits HATs like p300, CBP, etc., for further histone (H3 and H4) acetylation at the acetylated p53 binding sites leading to enhanced transcription of specific genes. Moreover, acetylation of p53 is also responsible for sustaining the combination of HATs and p53 along with maintenance of activity of p53 (Barlev et al., 2001; Labuschagne et al., 2018).
It has also been reported that HDAC1 induces deacetylation of p53 leading to its degradation (Ito et al., 2002). Curcumin has shown inhibition of HDAC1 leading to upregulation of the acetylated H3 and p53, that ultimately mediates tumor suppression and apoptosis (Balasubramanyam et al., 2004). In addition to acetylation, curcumin has also shown cytoplasmic activation, nuclear translocation and phosphorylation of p53 protein (on serine 15 moiety) which increases its level in cancer cells (Liontas and Yeger, 2004; Pan et al., 2008). P53 protein plays a vital role in cellular adaptive response to environmental stress. Positive correlation between p53 levels and treatment response in cancer therapy has been observed while decrease in p53 levels showed chemo-resistance in cancer cells. Moreover, mutated p53 have exhibited oncogenic properties and negative chemo-resistance effects as tumors with mutated p53 showed poor response to cancer therapy in lung and prostate cancers (Robles and Harris, 2009). Curcumin has also shown induction of ROS synthesis that increases p53 levels and its downstream proteins p21 and Bax (Thayyullathil et al., 2008). Moreover, curcumin has shown apoptosis independently of p53 particularly in the cells without functional p53 protein by downregulating Bcl-2 and p38 MAPK (Watson et al., 2010). Alternatively, curcumin can activate PPARγ which in turn transactivates p53 leading to mediation of cell senescence (Jin et al., 2016). Curcumin alone can induce the expression of p53 gene (Trp53) restoring the level as well as function of p53 (Das and Vinayak, 2015). It is reported that level of p53 in cells is controlled through many routes but major route is p53-murine double minute 2 (MDM2) pathway through an auto-regulatory feedback loop. Curcumin has shown downregulation of MDM2 leading to upregulation of the expression of p53 and Bax in multiple myeloma cancerous cells (Li et al., 2015). In conclusion, it is revealed that curcumin is effective for activation and restoration of p53 levels especially in the cells with an abnormal p53 expression or function. For example, curcumin has shown cell cycle arrest (at G2 phase) in p53 deficient breast cancer cells.
Abnormal activity of both HDACs and HATs is associated with the cancer pathogenesis, as a balance between histone acetylation and deacetylation is required for normal cellular physiology. Molecules like curcumin that modulate both HDACs and HATs to restore this balance possess significant anti-tumor potential. Curcumin has shown inhibition of the activity of certain isoforms of HAT and considered as a first natural selective HAT inhibitor (Devipriya and Kumaradhas, 2013). Recently, an in vitro study revealed partial inhibition of acetylation of histone H3K9 along with reversal of upregulation of the caspase activity (Caspase-3 and 8) and downregulation of Bcl-2 by curcumin in alcohol induced apoptosis in cardiac cells (Yan et al., 2017). Curcumin has also shown inhibition of acetylation of non-histone proteins like HIV-Tat protein leading to obstruct viral proliferation, exhibiting its potential as an adjuvant in HIV therapy (Morimoto et al., 2008).
Curcumin has been found to be specifically linked with the suppression of acetylation (of histone and p53) through inhibiting p300/CREB binding protein HAT activity from chromatin but not a DNA template. Curcumin is specific inhibitor of p300/CBP family with no effects on other HATs like PCAF/GCN5 (Balasubramanyam et al., 2004; Marcu et al., 2006). This selective inhibitory effect of curcumin is mediated by its Michael reaction acceptor function and makes curcumin a better pharmacological molecule with an anticancer therapeutic potential. The inhibition of p300/CBP can lead to degradation of p53 but it was not observed to a greater extent because p53 can be simultaneously acetylated by other HATs (not inhibited by curcumin) which restore its acetylation status in physiological range even after curcumin treatment. Moreover, curcumin triggered caspase-3 and poly (ADP-ribose) polymerase-mediated apoptosis among glioma cells by histone hypo-acetylation (Kang et al., 2006). The acetylation/deacetylation of molecular chaperones, transcription factors, cytoskeletal, and effector proteins is being focused as an important epigenetic regulatory approach (Glozak et al., 2005). NF-κB is a pro-inflammatory transcription agent which undergoes acetylation prior to the activation of hundreds of genes concerned with varied cellular processes (Gupta et al., 2010). NF-κB is acetylated at numerous lysine residues by means of p300/CBP acetyltransferases. Curcumin has also been reported to suppress the acetylation of RelA mediated by p300 (Chen et al., 2001).
Similarly, curcumin greatly decreased the expression of acetylated CBP/p300 HAT, leading to the inhibition of NF-κB binding (Yun et al., 2010). Additionally, curcumin suppressed the acetylation resulting from hypertrophy and binding of GATA4 that is a hypertrophy-responsive transcription agent in rat cardiomyocytes. It signifies that the suppression of activity of p300 HAT by curcumin might serve as a potential therapeutic intervention for heart failure in humans (Morimoto et al., 2008). Lastly, curcumin also incited the re-controlling fates of neural stem cell through the reduction of intensities of acetylation of H3 and H4 histone proteins (Kang et al., 2006).
Curcumin can modify both HATs and HDACs through similar mechanisms. For instance, oxidative pressure can stimulate NF-κB by the activation of natural HAT activity which results in the expression of pro-inflammatory mediators; on the other hand, it may suppress the activity of HDACs (Rahman et al., 2004). Consequently, curcumin may control acetylation and deacetylation by modulating oxidative stress. Curcumin modifies N-terminal tail regions of histones (H3, H4, and H2A) that ultimately affect many cell signaling pathways, leading to altered expression of many genes (Fu and Kurzrock, 2010; Azad et al., 2013). This modification can influence a diverse array of cellular processes like transcription, cell cycle, differentiation, DNA repair and recombination, etc. (Strahl and Allis, 2000; Duncan et al., 2008; Scully, 2010). Recently, treatment with curcumin revealed the inhibition of p300 HAT activity, leading to a decreased acetylation of pro-nociceptive proteins (BDNF and Cox-2) in neuropathic pain in mice model (Zhu et al., 2014). Moreover, curcumin has also been shown to modulate DNA damage response pathways by inhibiting HAT activities (Ogiwara et al., 2013). Histone modifications mediated by curcumin are not well defined except its function in acetylation, and thus require further investigations to provide insights into its potential mechanism of action.
DNA Methylation
Methylation has a vital role in managing normal biological activities in living systems (Esteller, 2007). Methylation of DNA is a type of transmissible change in the DNA that does not alter coding nucleotide sequence, however, it can directly suppress the expression of a gene (Das and Singal, 2004). Both hypo-methylation and hyper-methylation of DNA have been observed within cancer cells. Hypo-methylation can assist the expression of pro-metastatic genes and quiescent proto-oncogenes, and then enhance the progression of tumor. Localized hyper-methylation at particular CpG islands in promoter sections of particular genes (e.g., genes linked with tumor suppression) may lead to the silencing of transcription and a failure to control tumor development (Ehrlich, 2009).
S-adenosyl-methionine functions as a donor of methyl group for DNA methylation in the presence of DNA methyl-transferases, including DNMT1, DNMT3a, and DNMT3b, to produce 5-methylcytosine (Herman and Baylin, 2003). Studies exploring the influence of curcumin on DNA methylation are summarized in Table 1. Curcumin has been shown to inhibit the activities of DNMTs and then significantly modify the pattern of DNA methylation in different tumor cells (Liu Z. et al., 2009; Jha et al., 2010; Kuck et al., 2010; Link et al., 2013). Molecular docking studies revealed covalent blockage of catalytic thiolate of DNMT1 by curcumin, leading to hypo-methylation (Liu Z. et al., 2009). Curcumin induced reversal of DNA methylation in Leukemia cells, an action comparable to decitabine (a potent hypo-methylating agent) in global DNA methylation studies. Moreover, it also induced demethylation and expression of Neurog1 in LNCaP prostate cancer cells (Shu et al., 2011). The effect of curcumin on global hypo-methylation of DNA was not observed as it did not modify the methylation pattern of long interspersed nuclear elements-1 (LINE-1) in human colon cancer cells. However, it reduced the methylation of genes related to NF-κB pathway. Interestingly, it has been observed that hypo-methylating activity of curcumin was dependent on the density of methylation owing to selective demethylation of partially methylated CpG sites other than fully methylated genes (Link et al., 2013). Moreover, treatment with curcumin exhibited reactivation of silenced tumor suppressive genes by inducing demethylation of promoters of these genes (e.g., RARβ2 in human cervical cancer cell lines and p15INK4B in acute myeloid leukemia), leading to a remarkable tumor suppression (Jha et al., 2010; Yu et al., 2013). Similarly, it also induced the reversal of methylation of Nrf2 promoter in prostate cancer cells (Khor et al., 2011).
Contrarily, some studies reported no demethylation activity by curcumin as no significant global DNA hypo-methylation was observed in both leukemia and colorectal cancer following the treatments with curcumin (Medina-Franco et al., 2010; Link et al., 2013). Later on, these contradictive findings were confirmed by Hassan et al. (2015) based on the analysis of global DNA methylation by DNA pyrosequencing. They reported that both curcumin and its structural analog dimethoxycurcumin (DMC) did not reveal any significant hypo-methylation activity even at very high concentrations. Surprisingly, they observed the induction of expression of promoter-methylated genes by DMC without reversing DNA methylation. Previous studies also supported these findings as the induction of expression in respective genes has been observed in methylated promoters (Pruitt et al., 2006; Raynal et al., 2012).
FDA has approved two hypo-methylating agents namely 5-azacytidine and decitabine for curing myelodysplastic syndrome (MDS). Both of these have potential to make cancer cells more sensitive toward chemotherapeutic agents. It would be valuable to discover how the variable hypo-methylation induced by curcumin can trigger chemo-sensitization in cancers. Importantly, an initial trial using docetaxel after treatment with curcumin in patients of metastatic breast cancer ensured temporary recoveries in five patients while disease remained constant in three out of eight patients (Bayet-Robert et al., 2010). Such unpredicted responses may be due to release of these two agents in a sequential way. The sequential release of both of these hypo-methylating agents maximized epigenetic activity of curcumin for the treatment of cancer. Additionally, use of curcumin in various cancer models has proved that it could be utilized as a chemosensitizer for treatment of cancer.
miRNA Expression
miRNAs are described as tiny non-coding regulatory RNAs consisting of 17–25 nucleotides (Croce, 2009). They reduce the rate of translation and/or enhance the destruction of mRNAs when they are expressed abnormally. They also play significant roles in cell differentiation, cell cycle, apoptosis, metastasis, angiogenesis, invasion, and development of tumors (Negrini et al., 2007). Further, 50 miRNA genes have been recognized in humans and it is hypothesized that about 500 human miRNA genes have yet to be discovered. The actual functional role of many miRNAs is still unknown in mammals (Bentwich et al., 2005). However, it is considered that in humans about 30% of the genome could be regulated by miRNAs (Bartel, 2004).
Instability of miRNA expression, processing of precursors of miRNA, changes in miRNA sequence and its target mRNA, may impart negative influences on cellular activities and is considered to be linked with cancer (Davis and Ross, 2008). Formation of cancerous stem cells along with typically drug resistant epithelial-mesenchymal transition (EMT) phenotype of cancerous cell lines is controlled by a few miRNAs (Li et al., 2010). Moreover, genes responsible mainly for signaling pathways including Akt, NF-κB, and MAPK are regulated by curcumin (Mukhopadhyay et al., 2001; Sarkar and Li, 2004). Similarly, miRNAs are also capable of regulating these cellular signaling pathways. Therefore, functional modulation of miRNA is considered as a rational therapeutic approach for the treatment of different types of cancers.
Curcumin has been reported to modulate the expression of miRNA within human pancreatic cancer cells. Based on curcumin incubation for a period of 72 h, a considerable up-regulation was noticed in 11 miRNAs while 18 miRNAs were down-regulated, of which miRNA-22 was most up-regulated while miRNA-199a was most down-regulated. Curcumin induced up-regulation of miRNA-22 inhibited the expression of target genes, including estrogen receptor 1 and Sp1 (Sun et al., 2008). These findings suggested that the regulation of particular miRNAs through curcumin can suppress the growth of pancreatic cancerous cells. Moreover, programmed cell death among A549/DDP multidrug-resistant human lung adenocarcinomic cells may be prompted through curcumin by miRNA-based signaling pathway. Curcumin mainly showed the down-regulation of miRNA-186 expression in these cells (Zhang et al., 2010).
Epigenetic manipulation of miRNAs by curcumin has been observed in pancreatic cancer cells in a study with identification of around 50 candidate genes targeted by miRNA-22 (Sun et al., 2008). Additionally, curcumin has also shown to induce gemcitabine sensitivity in pancreatic cancer cells by altering the expression of miR-21 and miR-200 (Ali et al., 2010). The miRNA-200 has potential to suppress EMT, the introductory step of metastasis, through regulation of the epithelial cellular network by directly targeting transcriptional inhibitors of E-cadherin, ZEB1, and ZEB2 (Korpal et al., 2008). Thus, it would be a significant therapeutic approach to target particular miRNAs for cancer treatment. It could be done by eradicating drug tolerant EMT-type cells or cancer stem cells.
In contrast, miRNA-21 is overexpressed in various tumors and it facilitates invasion, metastasis, and cancer. Therefore, it is considered as an oncomiR. Moreover, an increased concentration of miRNA-21 has been observed in chemotherapy resistant colorectal cancer patients (Yu et al., 2013). Treatment with curcumin reduces the activity of miRNA-21 promoter. It also decreases the expression of miRNA-21 in primary tumors by suppressing the binding of AP-1 to the promoter while initiating the expression of the tumor inhibitors such as Pdcd4 which is a target of miRNA-21 (Mudduluru et al., 2011).
Treatment with curcumin revealed the up-regulation of miRNA-203 expression but a down-regulation of its target genes (Akt2 and Src), ultimately leading to a reduced proliferation but an increased apoptosis in bladder cancer cells (Saini et al., 2011). These effects were mediated by the hypo-methylation of the promoter region of miRNA-203 induced by curcumin. Similarly, curcumin up-regulated the expression of miRNA-15a and miRNA-16 while suppressing anti-apoptotic protein (Bcl-2) in breast cancer cells (Yang et al., 2010). The up-regulation of tumor suppressive miRNAs (let-7a,b,c,d, miR-26a, miR-101, miR-146a, and miR-200b, c) has also been mediated by curcumin in pancreatic carcinomas (Bao et al., 2012). A higher expression of miRNA-130a has been associated with chemo-resistance in patients with colon cancers, resulting in poor clinical outcome (Kara et al., 2015). Recently, it is reported that curcumin mediated a down-regulation of miR-130a, leading to the activation of Wnt/β-Catenin in colon cancer (Dou et al., 2017). Similarly, the down-regulation of miR-27a (oncogenic) but the upregulation of miR-34a were induced by curcumin accompanied by modulation of downstream targets, leading to cell cycle arrest and apoptosis in colorectal cancer cells (Toden et al., 2015).
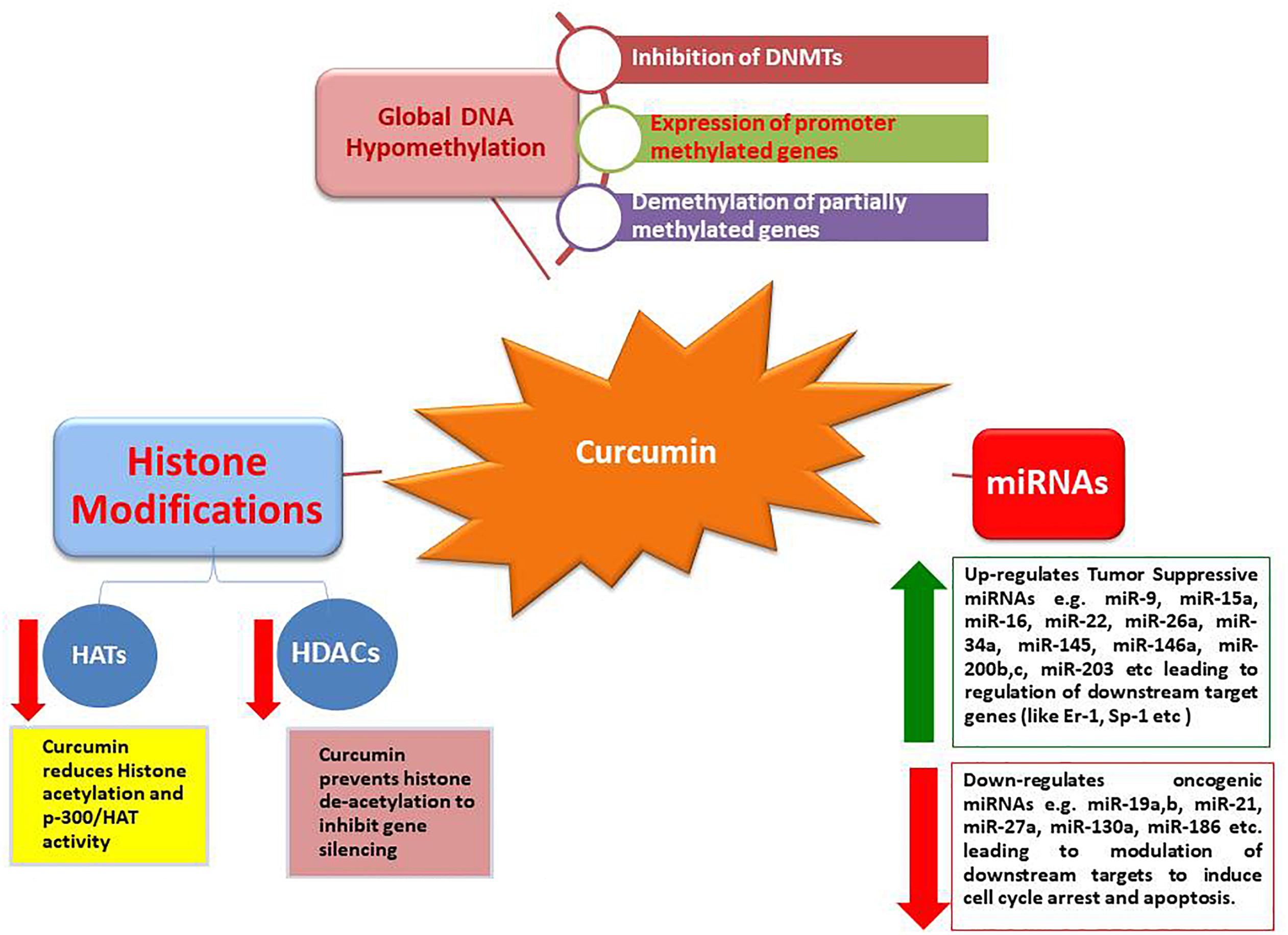
These results provide strong evidence that bioactive compounds like curcumin could be utilized as a successful remedy against cancers, particularly when traditional therapeutics are combined with natural chemo-preventive compounds which are generally safe for humans (Li et al., 2010). Synergistic effects of epigenetic changes (de-methylation and histone acetylation/deacetylation) induced by curcumin make it the most promising anticancer molecule that can also selectively up-regulate tumor suppressive miRNAs while down-regulate oncogenic miRNAs, leading to arrest cancer progression (Figures 1, 2).
Curcumin and Binding of DNA
The molecular foundations for various therapeutic modes of actions of curcumin are not well known due probably to the reason that so far most of the research work has mainly focused on the potent macromolecular targets of curcumin, i.e., proteins. Whereas, less attention has been paid to its potential to directly bind with DNA and/or to directly regulate epigenetic processes as an agent of DNA binding. In the year 2004, techniques of absorption spectroscopy and circular dichroism were used to demonstrate a direct communication among curcumin and both synthetic and natural duplexes of DNA. Analysis of molecular modeling estimations and spectral data inferred the DNA binding ability of curcumin. Curcumin is also a capable molecular probe to investigate naturally significant conformational polymorphisms induced by cations and pH within DNA/RNA (Zsila et al., 2004). Therefore, it could be recognized as a novel phenolic minor groove-binding factor with potent anticancer ability and therapeutic potential. Similarly, by using the techniques of UV analysis and Fourier transform infrared (FTIR), it was found that curcumin has the ability to bind with minor and major grooves of the DNA duplex, with the backbone phosphate group and also with the RNA base (Nafisi et al., 2009). Interface between these biopolymers and curcumin showed no change in conformation. However, Nafisi et al. (2009) reported that thymine O2 attaches curcumin to the minor groove of DNA while guanine and adenine N7 facilitate curcumin to bind not only to the major groove but also to the backbone phosphate group. Further, uracil O2 causes RNA binding whereas guanine and adenine N7 atoms facilitate the binding to the backbone phosphate group. Interestingly, the binding of curcumin with DNA was stronger as compared its binding to RNA.
Pentamidine is basically a diarylamidine antibiotic used for treating leishmaniasis, pneumonia, and trypanosomiasis (Fairlamb, 2003). It functions by interacting with the genome of pathogens through selective binding with the DNA’s minor groove, just like curcumin, and disrupts regular activities of the pathogenic topo-isomerases (Neidle, 2001; Bischoff and Hoffmann, 2002). Curcumin has also been recommended for the treatment of trypanosomiasis (Araujo and Leon, 2001; Saleheen et al., 2002).
Influence of Curcumin on Transcriptional Factors
Transcriptional factors are proteins which can selectively bind to enhancer or promoter regions (possibly at histone tails) to regulate the expression of many genes. A number of transcriptional factors have been discovered and characterized with functionally diverse DNA binding and activation domains in many organisms. Many transcriptional factors possess therapeutic potential owing to their involvement in key regulatory pathways (Shishodia et al., 2007). For instance, transcription factors like NF-κB, signal transducer and transcription activator (STAT) and activator protein-1 (AP-1) regulate the expression of the genes which control cellular proliferation, survival, metastasis, invasion, transformation, programmed cell death, adhesion, and angiogenesis (Aggarwal, 2004; Aggarwal et al., 2009; Gupta et al., 2010).
Transcriptional factors which play significant roles in carcinogenesis include electrophile response element (EpRE), β-catenin, early growth response-1 (Egr-1), androgen receptor (AR), peroxisome proliferator-activated receptor-c (PPAR-c), and NF-E2-related factor 2 (Nrf2). Curcumin has been shown to inhibit NF-κB activation leading to suppression of cigarette smoke induced NF-κB-dependent cyclin D1, cyclooxygenase-2, and matrix metalloproteinase-9 expression through mediating IκBα kinase pathway in human lung carcinoma (Shishodia et al., 2003). Modulation of NF-κB (down-regulation) by curcumin was suggested to be reduced by the inhibition of IκB kinase (IKK). Curcumin also has the potential to repress active NF-κB within mantle cell lymphoma by suppressing the activity of IKK (Shishodia et al., 2005). Consequently, the levels of matrix metalloproteinase-9, cyclooxygenase-2, and cyclin D1 were down-regulated. Moreover, curcumin caused the inhibition of NF-κB pathway induced by paclitaxel within breast cancerous cells as well as the suppression of human breast cancerous cells in nude mice (Aggarwal et al., 2005).
The activator proteins play a key role in the process of tumorigenesis owing to their ability to transform cancer cells (Karin et al., 1997). The inhibition of tumorigenic factors that can activate AP-1 and JNK has been observed in response to curcumin treatment (Huang et al., 1991; Chen and Tan, 1998). Curcumin induced inhibition of AP-1 was a result of its direct interface with AP-1 DNA binding motif (Bierhaus et al., 1997). The activation of both STAT3 and NF-κB was inhibited by curcumin, leading to the down-regulations of genes involved in apoptosis and cellular proliferation (Mackenzie et al., 2008). Curcumin has also exhibited the inhibition of STAT3 phosphorylation, leading to the induction of apoptosis in cellular myeloma (Bharti et al., 2003, 2004).
It is well established that curcumin is a proteasome inhibitor that increases p53 and induces apoptosis through caspase activation (Bech-Otschir et al., 2001; Jana et al., 2004). Many studies have described curcumin mediated effects on proteasome, but the exact mechanism of proteasome inhibition has not been clearly validated. Recently, Banerjee et al. (2018) showed that curcumin is a highly effective inhibitor of dual-specificity tyrosine-regulated kinase 2 (DYRK2). They reported that curcumin disrupts 26S proteasome activity by inhibiting DYRK2 in different cancerous cells, leading to the inhibition of cell proliferation in mice. This finding opens up a new horizon for using curcumin as a potential preventive and treatment approach in proteasome-linked cancers (e.g., triple negative breast cancer and myeloma). The interaction of curcumin with a variety of cellular molecules is due to its adaptable chemical structure. Such interactions lead to diversified biological effects, including regulation of the cell cycle, growth suppression, the induction of developmental distinction, the scavenge of reactive oxygen species (ROS), chemo-prevention and the up-regulation of pro-apoptotic factors (Surh, 1999; Chauhan, 2002; Miquel et al., 2002; Saleheen et al., 2002; Taher et al., 2003; Itokawa et al., 2008).
Other Potential Effects of Curcumin
Numerous studies have reported multiple potential effects induced by curcumin which basically stem from the interaction of curcumin with DNA, RNA, and proteins. Most important effects can be categorized as antioxidant activity, wound healing, modulation of angiogenesis and inflammatory cytokines.
Anti-oxidant Effects of Curcumin
Oxidative stress plays significant contributing roles in many diseases such as cerebral ischemia, myocardial ischemia, shock and hemorrhage, hypoxia, cancer and the injury of neuron cells. Curcumin possesses more potent antioxidant activities than recognized antioxidants (e.g., vitamin E and C), making it a potent therapeutic agent in many inflammatory diseases (Toda et al., 1985). Curcumin is an ideal scavenger of a wide range of reactive oxygen species (Reddy and Lokesh, 1994; Unnikrishnan and Rao, 1995; Sreejayan and Rao, 1997). Moreover, curcumin had capacity for the suppression of lipid peroxidation in various animal models (Sreejayan and Rao, 1994). Curcumin induced the inhibition of lipid peroxidation, leading to the protection of renal cells (LLC-PK1) from oxidative injury (Cohly et al., 1998). It also mediated biochemical modifications induced by ischemia in the heart in a feline model (Dikshit et al., 1995).
Treatment of vascular endothelial cells with curcumin increased the expression of heme oxygenase, leading to the alleviation of oxidative damage (Motterlini et al., 2000). Curcumin has also proven its efficacy in protecting rat myocardium from myocardial ischemic injury induced by isoprenaline (Nirmala and Puvanakrishnan, 1996; Manikandan et al., 2004). These beneficial effects of curcumin were mainly associated with its ability in ROS scavenging and the inhibition of lysosomal enzymes (Nirmala et al., 1999). Treatment with curcumin also exhibited beneficial effects in kidney damage by limiting the expression of Fas-L and Fas (Jones et al., 2000).
Curcumin mediated subcellular redistribution by modulating protein kinase pathways in hypoxic rabbit hearts and exhibited the translocation of Hsp70i from the particulate to the cytosolic fraction (Rafiee et al., 2003). Supplementation of curcumin in diet has shown desirable effects in neurodegenerative disorders like Alzheimer’s disease (Calabrese et al., 2003; Yang et al., 2005). Neuroprotection by curcumin was mediated through preventing lipid peroxidation, decreasing peroxynitrite formation but increasing endogenous antioxidant enzymes within a cerebral ischemia model of rats (Thiyagarajan and Sharma, 2004). Conclusively, it has been revealed that curcumin is valuable in alleviating many ailments that originate due to oxidative stresses. Such defensive properties of curcumin are chiefly due to its antioxidant potential and thus it should be exploited to produce new therapeutic options to fight against fatal diseases.
Improving Wound Healing by Curcumin
Repairing tissues and healing wounds are complex mechanisms which include processes like soreness, granulation and remodeling of tissues. A complicated sequence of the processes is initiated after injury, including interface between various cytokines, proteins of extra-cellular matrix (ECM), growth factors and their regulators. Previous knowledge about the potential of curcumin in injury healing prompted for the evaluation of curcumin’s effect in improving wound healing. Biopsies of wounds treated by curcumin indicated multiple infiltrating cells like fibroblasts, neutrophils, and macrophage in comparison with untreated injuries. Wound contraction was accelerated due to increased migration of myofibroblasts, fibroblasts, and macrophages within wounds treated by curcumin (Sidhu et al., 1998). Relocation of different cells serves as an effective source of growth factors needed for the maintenance of numerous natural processes of wound healing. Fibronectin (FN) and collagen expression are stimulated by transforming growth factor beta 1 (TGF-β1) along with the increase of in vivo development of granulation tissue during wound healing (Quaglino et al., 1990). Treatment with curcumin enhanced the expression of collagen and FN (Sidhu et al., 1998).
Further, curcumin enhanced the creation of granulation tissue, including rapid re-epithelialization, greater cell content, and neo-vascularization of wound impaired with hydrocortisone as well as diabetes (Sidhu et al., 1999) by controlling the expression of TGF-β1, its receptors and nitric oxide synthase during wound healing (Mani et al., 2002). Modulation of NF-κB activity by curcumin exerts advantageous effects by increasing the regeneration of muscles soon after trauma (Thaloor et al., 1999). Many reports suggest an antioxidant role of curcumin in wound healing by indicating its ability to prevent damage resulting from hydrogen peroxide in fibroblasts and keratinocytes in humans (Phan et al., 2001). Similarly, treatment of collagen matrix with curcumin exhibited rapid wound repair, better cell proliferation, and effective foraging of free radicals when compared with collagen treated and control rats (Gopinath et al., 2004). Pre-treatment with curcumin improved the formation of nucleotides, collagen, hexosamine, and nitrite. Histological investigation of injury biopsies revealed a better deposition of collagen and increased vascular and fibroblast levels, indicating the potential role of curcumin in improving radiation-induced delay in wound healing (Jagetia and Rajanikant, 2005).
Anti-oxidative potential of curcumin also makes it a promising anti-ulcer agent owing to its defensive ability against lipid peroxidation, depletion of glutathione and oxidation of proteins. Curcumin not only accelerated wound repair but also protected against gastric ulcer by improvement in MMP-2 activity and reduction of MMP-9 activity (Swarnakar et al., 2005). These findings evidently recommended the effectiveness of curcumin in wound healing, improved the control with respect to the formation of granulation tissue along with the stimulation of growth factors. It is evident that curcumin supplements wound healing at various levels. Recently, nano-curcumin showed mobilization of fibroblasts at wound site by activating the Wnt signaling pathway partly mediated through Dickkopf-related protein-1. Moreover, it also exhibited persistent inhibition of the inflammatory response through decreasing monocyte chemoattractant protein-1 (Dai et al., 2017).
Angiogenesis Modulation by Curcumin
Angiogenesis is of vital importance in many physiological activities, including reproductive process, embryo development, bone repair, and wound healing. Contrarily, irregular angiogenesis has been found to be pathological and linked with the growth of tumors, retinopathy of diabetes, hemangiomas, and rheumatoid arthritis. The role of angiogenesis regarding to the growth of primary tumors along with their metastasis to remote organs is well established (Folkman, 1995). Studies have revealed the potent anti-angiogenic ability of curcumin (Thaloor et al., 1998). Moreover, curcumin has also shown to inhibit corneal neo-vascularization in the mouse cornea (Arbiser et al., 1998). Such therapeutic effectiveness in the cornea has also been suggested after the provision of dietary curcuminoids in mice (Mohan et al., 2000). Reports have shown that certain analogs of curcumin may exhibit angiostatic abilities (Ahn et al., 2002; Shim et al., 2002). Similar consequences of curcumin analogs were also documented for genes of MMP-9 and VEGF (Hahm et al., 2004). It is well recognized that MMP genes and related particular suppressors have a key role in regulating the re-organization of matrix and initiating angiogenesis (Schnaper et al., 1993). The modulation of MMPs (accountable for the reduction of angiogenic activities) has been shown by curcumin and its analogs (Thaloor et al., 1998; Kim et al., 2002; Hahm et al., 2004).
Mediation of Inflammatory Cytokines
Curcumin has shown the repression of downstream pro-neoplastic and pro-inflammatory mediators, including reduced expression of IL-8 and IL-6 as a reaction to acidic contact in human oesophageal epithelial cell lines (Rafiee et al., 2009). It also decreased impulsive expression of IL-8 and IL-6 within four varied squamous carcinoma cell lines of the head and neck. These results revealed curcumin-induced inhibition of intermediary signaling pathways like NF-κB. Furthermore, curcumin also suppresses the production of interleukin 8 and 6 in neck and head cancerous cells by inhibiting Iκ β kinase in humans (Cohen et al., 2009).
Pretreatment by curcumin restored hepatic cytokines, including IL-1α and β, IL-2, IL-6, and IL-10 to normal intensities after injuries. Moreover, NF-κB and AP-1 were activated differentially at 2 and 24 h after hemorrhage. Liver injury was reduced by serum aspartate transaminase in animals pretreated by curcumin which were suffering from severe hemorrhage. Such consequences showed that the defense provided by curcumin against resuscitation/hemorrhage damage may be due to the inactivation of transcriptional factors responsible for cytokine regulation (Gaddipati et al., 2003). Under different experimental conditions, curcumin is recognized as a capable anti-inflammatory factor (Pari et al., 2008). Curcumin mediated the expression of ultraviolet or TNF-α-induced inflammatory cytokines in human cells (Jiang et al., 2009; Reuter et al., 2009; Wang et al., 2009). All these molecular modulations induced by curcumin with vital health benefits could be exploited in alternative therapeutics to manage severe diseases in humans (Figure 2).
Conclusion
Evidence provided by diversified experiments conducted in recent years support the argument that dietary phytochemicals like curcumin have significant strengths as epigenetic modulators. Epigenetic alterations may be modulated through nutritional, pharmacological and environmental interference. This feature has stimulated the insight for development of therapeutic approaches focusing on different epigenetic factors, including HAT, DNMTs, miRNAs, and HDAC, by addition of dietary polyphenols like curcumin. Curcumin is a versatile molecule having adaptable structure with diverse biological functions. Curcumin is a potent proteasome inhibitor that increases p53 level and induces apoptosis by mitochondrial caspase activation. Curcumin also disrupts 26S proteasome activity by inhibiting DYRK2 in different cancerous cells, resulting in the inhibition of cell proliferation. However, further research work is required to explore the full epigenetic potential of curcumin for preventing and curing lethal diseases like cancers.
Author Contributions
FH conceived the idea and drafted the outline. MAA, AJ, and AN collected the literature and wrote the different sections of manuscript. FH, MSR, and MSK edited and revised the manuscript. FH and CY made final changes, edited the manuscript, and finalized table and figures.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31460613 and 31560649) and National Key Research and Development Program of China (2016YFD0500507 and 2018YFD0501600).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abusnina, A., Keravis, T., Yougbaré, I., Bronner, C., and Lugnier, C. (2011). Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 55, 1677–1689. doi: 10.1002/mnfr.201100307
Aggarwal, B. B., Shishodia, S., Takada, Y., Banerjee, S., Newman, R. A., and Bueso-Ramos, C. E. (2005). Curcumin suppresses the paclitaxel-induced nuclear factor-kappa B pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 11, 7490–7498. doi: 10.1158/1078-0432.ccr-05-1192
Aggarwal, B. B., and Sung, B. (2009). Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 30, 85–94. doi: 10.1016/j.tips.2008.11.002
Aggarwal, B. B., Van Kuiken, M. E., Iyer, L. H., Harikumar, K. B., and Sung, B. (2009). Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. 234, 825–849. doi: 10.3181/0902-MR-78
Ahn, J. W., Yoo, J. S., Rho, J. R., Shin, J., and Kwon, H. J. (2002). Hydrazinocurcumin, a novel synthetic curcumin derivative, is a potent inhibitor of endothelial cell proliferation. Bio. Medi. Chem. 10, 2987–2992. doi: 10.1016/s0968-0896(02)00129-3
Ali, S., Ahmad, A., Banerjee, S., Padhye, S., Dominiak, K., and Schaffert, J. M. (2010). Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 70, 3606–3617. doi: 10.1158/0008-5472.CAN-09-4598
Araujo, C. C., and Leon, L. L. (2001). Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 96, 723–728. doi: 10.1590/s0074-02762001000500026
Arbiser, J. L., Klauber, N., Rohan, M. T., Leeuwen, R., Huang, R. V., Fisher, C., et al. (1998). Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 4, 376–383. doi: 10.1007/bf03401744
Azad, G. K., Singh, V., Golla, U., and Tomar, R. S. (2013). Depletion of cellular iron by curcumin leads to alteration in histone acetylation and degradation of Sml1p in Saccharomyces cerevisiae. PLoS One 8:e59003. doi: 10.1371/journal.pone.0059003
Balasubramanyam, K., Varier, R. A., Altaf, M., Swaminathan, V., Siddappa, N. B., and Ranga, U. (2004). Curcumin, a novel p300/CREBbinding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 279, 51163–51171. doi: 10.1074/jbc.m409024200
Banerjee, S., Jib, C., Mayfielda, J. E., Goelc, A., Xiaob, J., Dixona, J. E., et al. (2018). Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase. Proc. Nat. Acad. Sci. U.S.A. 115, 8155–8160. doi: 10.1073/pnas.1806797115
Bao, B., Ali, S., Banerjee, S., Wang, Z., Logna, F., Azmi, A. S., et al. (2012). Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 72, 335–345. doi: 10.1158/0008-5472.CAN-11-2182
Barlev, N. A., Liu, L., Chehab, N. H., Mansfield, K., Harris, K. G., Halazonetis, T. D., et al. (2001). Acetylation of p53 activates transcription through recruitment of Coactivators/histone acetyltransferases. J. Mol. cell 8, 1243–1254. doi: 10.1016/s1097-2765(01)00414-2
Barnes, P. J. (2009). Role of HDAC2 in the pathophysiology of COPD. Annu. Rev. Physiol. 71, 451–464. doi: 10.1146/annurev.physiol.010908.163257
Bayet-Robert, M., Kwiatkowski, F., Leheurteur, M., Gachon, F., Planchat, E., and Abrial, C. (2010). Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 9, 8–14. doi: 10.4161/cbt.9.1.10392
Bech-Otschir, D., Kraft, R., Huang, X., Henklein, P., Kapelari, B., Pollmann, C., et al. (2001). COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 20, 1630–1639. doi: 10.1093/emboj/20.7.1630
Bentwich, I., Avniel, A., Karov, Y., Aharonov, R., Gilad, S., and Barad, O. (2005). Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 37, 766–770. doi: 10.1038/ng1590
Bharti, A. C., Donato, N., and Aggarwal, B. B. (2003). Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 171, 3863–3871. doi: 10.4049/jimmunol.171.7.3863
Bharti, A. C., Shishodia, S., Reuben, J. M., Weber, D., Alexanian, R., and Raj-Vadhan, S. (2004). Nuclear factor-kappa B and STAT3 are constitutively active in CD138 ?cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 103, 3175–3184. doi: 10.1182/blood-2003-06-2151
Bierhaus, A., Zhang, Y., Quehenberger, P., Luther, T., Haase, M., and Muller, M. (1997). The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb. Haemost. 77, 772–782. doi: 10.1055/s-0038-1656049
Bischoff, G., and Hoffmann, S. (2002). DNA-binding of drugs used in medicinal therapies. Curr. Med. Chem. 9, 312–348.
Bora-Tatar, G., Dayangac-Erden, D., Demir, A. S., Dalkara, S., Yelekci, K., and Erdem-Yurter, H. (2009). Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: activity and docking studies. Bioorg. Med. Chem. 17, 5219–5228. doi: 10.1016/j.bmc.2009.05.042
Calabrese, V., Butterfield, D. A., and Stella, A. M. (2003). Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer’s disease. Ital. J. Biochem. 52, 177–181.
Ceccacci, E., and Minucci, S. (2016). Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br. J. Cancer 114, 605–611. doi: 10.1038/bjc.2016.36
Chauhan, D. P. (2002). Chemotherapeutic potential of curcumin for colorectal cancer. Curr. Pharm. Des. 8, 1695–1706. doi: 10.2174/1381612023394016
Chen, C. Q., Chen, Y., Li, X. G., Liu, H. L., and Wu, Q. (2004). Effects of curcumin on proliferation of NB4 cells and acetylation of histone H3 and p53. Chin. J. Cancer Res. 16, 256–259. doi: 10.1007/s11670-004-0038-2
Chen, C. Q., Yu, K., Yan, Q. X., Xing, C. Y., Chen, Y., Yan, Z., et al. (2013). Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis 24, 1442–1449. doi: 10.1093/carcin/bgt070
Chen, L., Fischle, C., Verdin, E., and Greene, W. C. (2001). Duration of nuclear NF-kappa B action regulated by reversible acetylation. Science 293, 1653–1657. doi: 10.1126/science.1062374
Chen, Y., Shu, W., Chen, W., Wu, Q., Liu, H., and Cui, G. (2007). Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin. Pharmacol. Toxicol. 101, 427–433. doi: 10.1111/j.1742-7843.2007.00142.x
Chen, Y. R., and Tan, T. H. (1998). Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17, 173–178. doi: 10.1038/sj.onc.1201941
Cheng, A. L., Hsu, C. H., Lin, J. K., Hsu, M. M., Ho, Y. F., Shen, T. S., et al. (2001). Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 21, 2895–2900.
Cohen, A. N., Veena, M. S., Srivatsan, E. S., and Wang, M. B. (2009). Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Ikappa beta kinase. Arch. Otolaryngol. Head Neck Surg. 135, 190–197. doi: 10.1001/archotol.135.2.190
Cohly, H. H., Taylor, A., Angel, M. F., and Salahudeen, A. K. (1998). Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLCPK1) cell injury. Free Radi. Biol. Med. 24, 49–54. doi: 10.1016/s0891-5849(97)00140-8
Croce, C. M. (2009). Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10, 704–714. doi: 10.1038/nrg2634
Cui, L., Miao, J., and Cui, L. (2007). Cytotoxic effect of curcumin on malaria parasite plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicro. Agents Chemo 51, 488–494. doi: 10.1128/AAC.01238-06
Dai, X., Liu, J., Zheng, H., Wichmann, J., Hopfner, U., Sudhop, S., et al. (2017). Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 9, e368. doi: 10.1038/am.2017.31
Das, L., and Vinayak, M. (2015). Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer. PLoS One 10:e0124000. doi: 10.1371/journal.pone.0124000
Davis, C. D., and Ross, S. A. (2007). Dietary components impact histone modifications and cancer risk. Nutr. Rev. 65, 88–94. doi: 10.1111/j.1753-4887.2007.tb00285.x
Davis, C. D., and Ross, S. A. (2008). Evidence for dietary regulation of microRNA expression in cancer cells. Nutr. Rev. 2008, 477–482. doi: 10.1111/j.1753-4887.2008.00080.x
Devipriya, B., and Kumaradhas, P. (2013). Molecular flexibility and the electrostatic moments of curcumin and its derivatives in the active site of p300: a theoretical charge density study. Chem. Biol. Interact. 204, 153–165. doi: 10.1016/j.cbi.2013.05.002
Dikshit, M., Rastogi, L., Shukla, R., and Srimal, R. C. (1995). Prevention of ischaemia-induced biochemical changes by curcumin and quinidine in the cat heart. Indian J. Med. Res. 101, 31–35.
Dou, H., Shen, R., Tao, J., Huang, L., Shi, H., Chen, H., et al. (2017). Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/β-Catenin pathways via miR-130a. Front. Pharmacol. 8:877. doi: 10.3389/fphar.2017.00877
Du, L., Xie, Z., Wu, L. C., Chiu, M., Lin, J., Chan, K. K., et al. (2012). Reactivation of RASSF1A in breast cancer cells by curcumin. Nutr. Cancer 64, 1228–1235. doi: 10.1080/01635581.2012.717682
Duncan, E. M., Muratore-Schroeder, T. L., Cook, R. G., Garcia, B. A., Shabanowitz, J., Hunt, D. F., et al. (2008). Cathepsin L proteolytically processes Histone H3 during mouse embryonic stem cell differentiation. Cell 135, 284–294. doi: 10.1016/j.cell.2008.09.055
Ehrlich, M. (2009). DNA hypomethylation in cancer cells. Epigenomics 1, 239–259. doi: 10.2217/epi.09.33
Esteller, M. (2007). Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 8, 286–298. doi: 10.1038/nrg2005
Fairlamb, A. H. (2003). Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol. 19, 488–494. doi: 10.1016/j.pt.2003.09.002
Folkman, J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31.
Fu, H., Wang, C., Yang, D., Wei, Z., Xu, J., Hu, Z., et al. (2018). Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 233, 4634–4642. doi: 10.1002/jcp.26190
Fu, S., and Kurzrock, R. (2010). Development of curcumin as an epigenetic agent. Cancer 116, 4670–4676. doi: 10.1002/cncr.25414
Gaddipati, J. P., Sundar, S. V., Calemine, J., Seth, P., Sidhu, G. S., and Maheshwari, R. K. (2003). Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock 19, 150–156. doi: 10.1097/00024382-200302000-00011
Gao, S., Duan, X., Wang, X., Dong, D., Liu, D., and Li, X. (2013). Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem. Toxicol. 59, 739–747. doi: 10.1016/j.fct.2013.07.032
Gao, W., Chan, J. Y., and Wong, T. S. (2014). Curcumin exerts inhibitory effects on undifferentiated nasopharyngeal carcinoma by inhibiting the expression of miR-125a-5p. Clin. Sci. 127, 571–579. doi: 10.1042/CS20140010
Gibbons, R. J. (2005). Histone modifying and chromatin remodeling enzymes in cancer and dysplastic syndromes. Hum. Mol. Genet. 14, R85–R92.
Glozak, M. A., Sengupta, N., Zhang, X., and Seto, E. (2005). Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23. doi: 10.1016/j.gene.2005.09.010
Gong, C., Yang, Z., Zhang, L., Wang, Y., Gong, W., and Liu, Y. (2018). Quercetin suppresses DNA double-strand break repair and enhances the radio-sensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. Onco Targets Ther. 11, 17–27. doi: 10.2147/ott.s147316
González-Reyes, S., Guzman-Beltran, C., Medina-Campos, O. N., and Pedraza-Chaverri, J. (2013). Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid. Med. Cell. Longev. 2013, 801418. doi: 10.1155/2013/801418
Gopinath, D., Ahmed, M. R., Gomathi, K., Chitra, K., Sehgal, P. K., and Jayakumar, R. (2004). Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 25, 1911–1917. doi: 10.1016/s0142-9612(03)00625-2
Gu, W., and Roeder, R. G. (1997). Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606. doi: 10.1016/s0092-8674(00)80521-8
Gupta, S. C., Sundaram, C., Reuter, S., and Aggarwal, B. B. (2010). Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–787. doi: 10.1016/j.bbagrm.2010.05.004
Hahm, E. R., Gho, Y. S., Park, S., Park, C., Kim, K. W., and Yang, C. H. (2004). Synthetic curcumin analogs inhibit activator protein-1 transcription and tumor-induced angiogenesis. Biochem. Biophy. Res. Comm. 321, 337–344. doi: 10.1016/j.bbrc.2004.06.119
Hassan, H. E., Carlson, S., Abdallah, I., Buttolph, T., Glass, K. C., and Fandy, T. E. (2015). Curcumin and dimethoxycurcumin induced epigenetic changes in leukemia cells. Pharm. Res. 32, 863–875. doi: 10.1007/s11095-014-1502-4
Hatcher, H., Planalp, R., Cho, J., Torti, F. M., and Torti, S. V. (2008). Curcumin: from ancient medicine to current clinical trials. Cell Mol. Life Sci. 65, 1631–1652. doi: 10.1007/s00018-008-7452-4
He, H. J., Wang, G. Y., Gao, Y., Ling, W. H., Yu, Z. W., and Jin, T. R. (2012). Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 3, 94–104. doi: 10.4239/wjd.v3.i5.94
Herman, J. G., and Baylin, S. B. (2003). Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 349, 2042–2054. doi: 10.1056/nejmra023075
Hu, J., Wang, Y., and Chen, Y. (2009). Curcumin-induced histone acetylation in malignant hematologic cells. J. Huazhong. Univ. Sci. Technolog. Med. Sci. 29, 25–28. doi: 10.1007/s11596-009-0105-5
Huang, M. T., Lysz, T., Ferraro, T., Abidi, T. F., Laskin, J. D., and Conney, A. H. (1991). Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 51, 813–819.
Huminiecki, L., Horbańczuka, J., and Atanasovab, A. G. (2017). The functional genomic studies of curcumin. Semin. Cancer Biol. 46, 107–118. doi: 10.1016/j.semcancer.2017.04.002
Ito, A., Kawaguehi, Y., Lai, C. H., Kovacs, J. J., Higashimoto, Y., Appella, E., et al. (2002). MDM2-HDAC1 mediated deacetylation of p53 is required for its degradation. EMBO J. 21, 6236–6245. doi: 10.1093/emboj/cdf616
Itokawa, H., Shi, Q., Akiyama, T., Morris-Natschke, S. L., and Lee, K. H. (2008). Recent advances in the investigation of curcuminoids. Chin. Med. 3:11. doi: 10.1186/1749-8546-3-11
Jagetia, G. C., and Rajanikant, G. K. (2005). Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemi body gamma irradiation. Plast. Reconst. Sur. 115, 515–528. doi: 10.1097/01.prs.0000148372.75342.d9
Jana, N. R., Dikshit, P., Goswami, A., and Nukina, N. (2004). Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J. Biol. Chem. 279, 11680–11685. doi: 10.1074/jbc.m310369200
Jha, A. K., Nikbakht, M., Parashar, G., Shrivastava, A., Capalash, N., and Kaur, J. (2010). Reversal of hypermethylation and reactivation of the RARβ2 gene by natural compounds in cervical cancer cell lines. Folia Biol. 56, 195–200.
Jiang, A., Wang, X., Shan, X., Li, Y., Wang, P., Jiang, P., et al. (2015). Curcumin reactivates silenced tumor suppressor gene RARβ by reducing DNA methylation. Phytother. Res. 29, 1237–1245. doi: 10.1002/ptr.5373
Jiang, T., Huang, Z., Lin, Y., Zhang, Z., Fang, D., and Zhang, D. D. (2010). The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes Metab. Res. Rev. 59, 850–860. doi: 10.2337/db09-1342
Jiang, Z. Y., Zou, L., Shi, S. S., Lu, Y. R., Dong, J., Yang, C. H., et al. (2009). Effects of curcumin on TNF-alpha and TGF-beta1 in serum and lung tissue of SiO(2)-induced fibrosis in mice. Xi Bao Yu Fen ZiMian Yi XueZaZhi 25, 399–401.
Jin, H., Lian, N., Zhang, F., Chen, L., Chen, Q., Lu, C., et al. (2016). Activation of PPARγ/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 7:e2189. doi: 10.1038/cddis.2016.92
Jones, E. A., Shahed, A., and Shoskes, D. A. (2000). Modulation of apoptotic and inflammatory genes by bioflavonoids and angiotensin II inhibition in ureteral obstruction. Urology 56, 346–351. doi: 10.1016/s0090-4295(00)00608-7
Kang, S. K., Cha, S. H., and Jeon, H. G. (2006). Curcumin-induced histone hypo-acetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 15, 165–174. doi: 10.1089/scd.2006.15.165
Kara, M., Yumrutas, O., Ozcan, O., Celik, O. I., Bozgeyik, E., Bozgeyik, I., et al. (2015). Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma. Gene 567, 81–86. doi: 10.1016/j.gene.2015.04.065
Karin, M., Liu, Z., and Zandi, E. (1997). AP-1 function and regulation. Curr. Opin. Cell Biol. 9, 240–246. doi: 10.1016/s0955-0674(97)80068-3
Khor, T. O., Huang, Y., Wu, T. W., Shu, L., Lee, J., and Kong, A. N. (2011). Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 82, 1073–1078. doi: 10.1016/j.bcp.2011.07.065
Kim, J. H., Shim, J. S., Lee, S. K., Kim, K. W., Rha, S. Y., Chung, H. C., et al. (2002). Microarray-based analysis of anti-angiogenic activity of demethoxycurcumin on human umbilical vein endothelial cells: crucial involvement of the down-regulation of matrix metalloproteinase. Jpn. J. Cancer Res. 93, 1378–1385. doi: 10.1111/j.1349-7006.2002.tb01247.x
Kim, S., and An, S. S. (2016). Role of p53 isoforms and aggregations in cancer. Medicine 95:e3993. doi: 10.1097/MD.0000000000003993
Korpal, M., Lee, E. S., Hu, G., and Kang, Y. (2008). The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1andZEB2. J. Biol. Chem. 283, 14910–14914. doi: 10.1074/jbc.C800074200
Kronski, E., Fiori, M. E., Barbieri, O., Astigiano, S., Mirisola, V., and Killian, P. H. (2014). miR1 81b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 8, 581–595. doi: 10.1016/j.molonc.2014.01.005
Kruse, J. P., and Gu, W. (2009). Modes of p53 regulation. Cell 137, 609–622. doi: 10.1016/j.cell.2009.04.050
Kuck, D., Singh, N., Lyko, F., and Medina-Franco, J. L. (2010). Novel and selective DNA methyltransferase inhibitors: docking-based virtual screening and experimental evaluation. Bioorg. Med. Chem. 18, 822–829. doi: 10.1016/j.bmc.2009.11.050
Labuschagne, C. F., Zani, F., and Vousden, K. H. (2018). Control of metabolism by p53 - cancer and beyond. Biochim. Biophys. Acta Rev. Cancer 1870, 32–42. doi: 10.1016/j.bbcan.2018.06.001
Lao, C. D., Ruffin, M. T., Normolle, D., Heath, D. D., Murray, S. I., and Bailey, J. M. (2006). Dose escalation of a curcuminoid formulation. BMC Compl. Altern. Med. 6:10. doi: 10.1186/1472-6882-6-10
Lee, S. J., Krauthauser, C., Maduskuie, V., Fawcett, P. T., Olson, J. M., and Rajasekaran, S. A. (2011). Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 11:144. doi: 10.1186/1471-2407-11-144
Li, W., Wang, Y., Song, Y., Xu, L., Zhao, J., and Fang, B. (2015). A preliminary study of the effect of curcumin on the expression of p53 protein in a human multiple myeloma cell line. Oncol. Lett. 9, 1719–1724. doi: 10.3892/ol.2015.2946
Li, X., Xie, W., Xie, C., Huang, C., Zhu, J., and Liang, Z. (2014). Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A- induced MCF-7 breast cancer cell proliferation. Phytother. Res. 28, 1553–1560. doi: 10.1002/ptr.5167
Li, Y., Kong, D., Wang, Z., and Sarkar, F. H. (2010). Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm. Res. 27, 1027–1041. doi: 10.1007/s11095-010-0105-y
Li, Y., and Seto, E. (2016). HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 6:a026831. doi: 10.1101/cshperspect.a026831
Link, A., Balaguer, F., Shen, Y., Lozano, J. J., Leung, H. C., Boland, C. R., et al. (2013). Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One 8:e57709. doi: 10.1371/journal.pone.0057709
Liontas, A., and Yeger, H. (2004). Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 24, 987–998.
Liu, H. L., Chen, Y., Cui, G. H., and Zhou, F. (2005). Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol. Sin. 26, 603–609. doi: 10.1111/j.1745-7254.2005.00081.x
Liu, M., Grigoryev, D. N., Crow, M. T., Haas, M., Yamamoto, M., and Reddy, S. P. (2009). Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 76, 277–285. doi: 10.1038/ki.2009.157
Liu, Y. L., Yang, H. P., Gong, L., Tang, C. L., and Wang, H. J. (2011). Hypomethylation effects of curcumin, demethoxycurcumin and bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung cancer cell lines. Mol. Med. Rep. 4, 675–679. doi: 10.3892/mmr.2011.473
Liu, Z., Xie, Z., and Jones, W. (2009). Curcumin is a potent DNA hypomethylation agent. Bioorg. Med. Chem. Lett. 19, 706–709. doi: 10.1016/j.bmcl.2008.12.041
Mackenzie, G. G., Queisser, N., Wolfson, M. L., Fraga, C. G., Adamo, A. M., and Oteiza, P. I. (2008). Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF kappa B and STAT3 pathways in Hodgkin’s lymphoma cells. Int. J. Cancer 123, 56–65. doi: 10.1002/ijc.23477
Mani, H., Sidhu, G. S., Kumari, R., Gaddipati, J. P., Seth, P., and Maheshwari, R. K. (2002). Curcumin differentially regulates TGF-β1, its receptors and nitric oxide synthase during impaired wound healing. Bio. Fact. 16, 29–43. doi: 10.1002/biof.5520160104
Manikandan, P., Sumitra, M., Aishwarya, S., Manohar, B. M., Lokanadam, B., and Puvanakrishnan, R. (2004). Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int. J. Biochem. Cell Bio. 36, 1967–1980. doi: 10.1016/j.biocel.2004.01.030
Marcu, M. G., Jung, Y. J., Lee, S., Chung, E. J., Lee, M. J., and Trepel, J. (2006). Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2, 169–174. doi: 10.2174/157340606776056133
Medina-Franco, J. L., Lopez-Vallejo, F., Kuck, D., and Lyko, F. (2010). Natural products as DNA methyltransferase inhibitors: a computer-aided discovery approach. Mol. Divers. 15, 293–304. doi: 10.1007/s11030-010-9262-5
Meja, K. K., Rajendrasozhan, S., Adenuga, D., Biswas, S. K., Sundar, I. K., and Spooner, G. (2008). Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 39, 312–323. doi: 10.1165/rcmb.2008-0012OC
Miquel, J., Bernd, A., Sempere, J. M., Diaz-Alperi, J., and Ramirez, A. (2002). The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A Review. Arch. Gerontol. Geriatr. 34, 37–46. doi: 10.1016/s0167-4943(01)00194-7
Mohan, R., Sivak, J., Ashton, P., Russo, L. A., Pham, B. Q., Kasahara, N., et al. (2000). Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J. Biol. Chem. 275, 10405–10412. doi: 10.1074/jbc.275.14.10405
Morimoto, T., Sunagawa, Y., Kawamura, T., Takaya, T., Wada, H., and Nagasawa, A. (2008). The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest. 118, 868–878. doi: 10.1172/JCI33160
Motterlini, R., Foresti, R., Bassi, R., and Green, C. J. (2000). Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Rad. Biol. Med. 28, 1303–1312. doi: 10.1016/s0891-5849(00)00294-x
Mudduluru, G., George-William, J. N., Muppala, S., Asangani, A. I., Regalla, K., and Nelson, L. D. (2011). Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 31, 185–197. doi: 10.1042/BSR20100065
Mukhopadhyay, A., Bueso-Ramos, C., Chatterjee, D., Pantazis, P., and Aggarwal, B. B. (2001). Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 20, 7597–7609. doi: 10.1038/sj.onc.1204997
Nafisi, S., Adelzadeh, M., Norouzi, Z., and Sarbolouki, M. N. (2009). Curcumin binding to DNA and RNA. DNA Cell Biol. 28, 201–208. doi: 10.1089/dna.2008.0840
Negrini, M., Ferracin, M., Sabbioni, S., and Croce, C. M. (2007). MicroRNAs in human cancer: from research to therapy. J. Cell Sci. 120, 1833–1840. doi: 10.1242/jcs.03450
Neidle, S. (2001). DNA minor-groove recognition by small molecules. Nat. Prod. Rep. 18, 291–309. doi: 10.1039/a705982e
Nirmala, C., Anand, S., and Puvanakrishnan, R. (1999). Curcumin treatment modulates collagen metabolism in isoproterenol induced myocardial necrosis in rats. Mol. Cell. Biochem. 197, 31–37.
Nirmala, C., and Puvanakrishnan, R. (1996). Effect of curcumin on certain lysosomal hydrolases in isoproterenol-induced myocardial infarction in rats. Biochem. Pharm. 51, 47–51. doi: 10.1016/0006-2952(95)02118-3
Ogiwara, H., Ui, A., Shiotani, B., Zou, L., Yasui, A., and Kohno, T. (2013). Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis 34, 2486–2497. doi: 10.1093/carcin/bgt240
Pan, W., Yang, H., Cao, C., Song, X., Wallin, B., Kivlin, R., et al. (2008). AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol. Rep. 20, 1553–1559.
Parashar, G., Parashar, N. C., and Capalash, N. (2012). Curcumin causes promoter hypomethylation and increased expression of FANCF gene in SiHa cell line. Mol. Cell Biochem. 365, 29. doi: 10.1007/s11010-012-1240-z
Pari, L., Tewas, D., and Eckel, J. (2008). Role of curcumin in health and disease. Arch. Physiol. Biochem. 114, 127–149. doi: 10.1080/13813450802033958
Phan, T. T., See, P., Lee, S. T., and Chan, S. Y. (2001). Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J. Trauma 51, 927–931. doi: 10.1097/00005373-200111000-00017
Pruitt, K., Zinn, R. L., Ohm, J. E., McGarvey, K. M., Kang, S. H., Watkins, D. N., et al. (2006). Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2:e40. doi: 10.1371/journal.pgen.0020040.eor
Quaglino, D., Nanney, L. B., Kennedy, R., and Davidson, J. M. (1990). Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. J. Lab. Invest. 63, 307–319.
Rafiee, P., Nelson, V. M., and Manley, S. (2009). Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am. J. Physiol. 296, 388–398. doi: 10.1152/ajpgi.90428.2008
Rafiee, P., Shi, Y., Pritchard, K. A., Ogawa, H., Eis, A. L., Komorowski, R. A., et al. (2003). Cellular redistribution of inducible Hsp70 protein in the human and rabbit heart in response to the stress of chronic hypoxia: role of protein kinases. J. Biol. Chem. 278, 43636–43644. doi: 10.1074/jbc.m212993200
Rahman, I., Marwick, J., and Kirkham, P. (2004). Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem. Pharmacol. 68, 1255–1267. doi: 10.1016/j.bcp.2004.05.042
Rao, C. V. (2007). Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 595, 213–226. doi: 10.1007/978-0-387-46401-5_9
Raynal, N. J., Si, J., Taby, R. F., Gharibyan, V., Ahmed, S., Jelinek, J., et al. (2012). DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res. 72, 1170–1181. doi: 10.1158/0008-5472.CAN-11-3248
Reddy, A. C., and Lokesh, B. R. (1994). Studies on the inhibitory effects of curcumin and 1916 eugenol on the formation of reactive oxygen species and the oxidation of 1917 ferrous iron. Mol. Cell. Biochem. 137, 1–8. doi: 10.1007/bf00926033
Reuter, S., Charlet, J., Juncker, T., Teiten, M. H., Dicato, M., and Diederich, M. (2009). Effect of curcumin on nuclear factor kappaB signaling pathways in human chronic myelogenous K562 leukemia cells. Ann. N. Y. Acad. Sci. 1171, 436–447. doi: 10.1111/j.1749-6632.2009.04731.x
Robles, A. I., and Harris, C. C. (2009). Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb. Perspect. Biol. 2:a001016. doi: 10.1101/cshperspect.a001016
Rogers, N. M., Stephenson, M. D., Kitching, A. R., Horowitz, J. D., and Coates, P. T. (2012). Amelioration of renal ischaemia-reperfusion injury by liposomal delivery of curcumin to renal tubular epithelial and antigenpresenting cells. Br. J. Pharmacol. 166, 194–209. doi: 10.1111/j.1476-5381.2011.01590.x
Ryu, H., Smith, K., and Camelo, S. A. (2005). Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. 93, 1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x
Sadoul, K., Boyault, C., Pabion, M., and Khochbin, S. (2008). Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie 90, 306–312. doi: 10.1016/j.biochi.2007.06.009
Saini, S., Arora, S., Majid, S., Shahryari, V., Chen, Y., Deng, G., et al. (2011). Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev. Res. 4, 1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267
Saleheen, D., Ali, S. A., Ashfaq, K., Siddiqui, A. A., Agha, A., and Yasinzai, M. M. (2002). Latent activity of curcumin against leishmaniasis in vitro. Biol. Pharm. Bull. 25, 386–389. doi: 10.1248/bpb.25.386
Sarkar, F. H., and Li, Y. (2004). Cell signaling pathways altered by natural chemo-preventive agents. Mutat. Res. 555, 53–64. doi: 10.1016/j.redox.2017.03.025
Schnaper, H. W., Grant, D. S., Stetler-Stevenson, W. G., Friedman, R., D’orazi, G., Murphy, A. N., et al. (1993). Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J. Cell. Phy. 156, 235–246. doi: 10.1002/jcp.1041560204
Scully, R. (2010). A histone code for DNA repair. Nat. Rev. Mol. Cell Biol. 11, 164–164. doi: 10.1038/nrm2855
Shankar, S., and Srivastava, R. K. (2007). Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 30, 905–918.
Sharma, R. A., Euden, S. A., Platton, S. L., Cooke, D. N., Shafayat, A., Hewitt, H. R., et al. (2004). Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 10, 6847–6854. doi: 10.1158/1078-0432.CCR-04-0744
Shen, G., Xu, C., Hu, R., Jain, M. R., Gopalkrishnan, A., and Nair, S. (2006). Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer Ther. 5, 39–51. doi: 10.1158/1535-7163.mct-05-0293
Shim, J. S., Kim, D. H., Jung, H. J., Kim, J. H., Lim, D., Lee, S. K., et al. (2002). Hydrazinocurcumin, a novel synthetic curcumin derivative, is a potent inhibitor of endothelial cell proliferation. Bioorg. Med. Chem. 10, 2439–2444. doi: 10.1016/S0968-0896(02)00116-5
Shin, D. H., Park, H. M., Jung, K. A., Choi, H. J., Kim, A., and Kim, D. D. (2010). The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic. Biol. Med. 48, 1051–1063. doi: 10.1016/j.freeradbiomed.2010.01.021
Shishodia, S., Amin, H. M., Lai, R., and Aggarwal, B. B. (2005). Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem. Pharmacol. 70, 700–713. doi: 10.1016/j.bcp.2005.04.043
Shishodia, S., Potdar, P., Gairola, C. G., and Aggarwal, B. B. (2003). Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 24, 1269–1279. doi: 10.1093/carcin/bgg078
Shishodia, S., Singh, T., and Chaturvedi, M. M. (2007). Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 595, 127–148. doi: 10.1007/978-0-387-46401-5_4
Shu, L., Khor, T. O., Lee, J. H., Boyanapalli, S. S., Huang, Y., and Wu, T. Y. (2011). Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurogl by curcumin in prostate LNCaP cells. AAPS J. 13, 606–614. doi: 10.1208/s12248-011-9300-y
Sidhu, G. S., Mani, H., Gaddipati, J. P., Singh, A. K., Seth, P., Banaudha, K. K., et al. (1999). Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 7, 362–374. doi: 10.1046/j.1524-475x.1999.00362.x
Sidhu, G. S., Singh, A. K., Thaloor, D., Banaudha, K. K., Patnaik, G. K., Srimal, R. C., et al. (1998). Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 6, 167–177. doi: 10.1046/j.1524-475x.1998.60211.x
Skommer, J., Wlodkowic, D., and Pelkonen, J. (2007). Gene-expression profiling during curcumin-induced apoptosis reveals downregulation of CXCR4. Exp. Hematol. 35, 84–100.
Soetikno, V., Sari, V. R., Veeraveedu, P. T., Thandavarayan, R. A., Harima, H., and Sukumaran, V. (2011). Curcumin ameliorates macrophage infiltration by inhibiting NF-kappa B activation and pro-inflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 8:35. doi: 10.1186/1743-7075-8-35
Sreejayan, M., and Rao, M. N. A. (1997). Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmac. 49, 105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x
Sreejayan, X., and Rao, M. N. (1994). Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharm. Pharmacol. 46, 1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x
Strahl, B. D., and Allis, C. D. (2000). The language of covalent histone modifications. Nature 403, 41–45. doi: 10.1038/47412
Su, P., Yang, Y., Wang, G., Chen, X., and Ju, Y. (2018). Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. Int. J. Oncol. 53, 1343–1353. doi: 10.3892/ijo.2018.4461
Sun, M., Estrov, Z., Ji, Y., Coombes, K. R., Harris, D. H., and Kurzrock, R. (2008). Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 7, 464–473. doi: 10.1158/1535-7163.MCT-07-2272
Surh, Y. (1999). Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 428, 305–327. doi: 10.1016/s1383-5742(99)00057-5
Surh, Y. J., Chun, K. S., Cha, H. H., Han, S. S., Keum, Y. S., and Park, K. K. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 480-481, 243–268. doi: 10.1016/s0027-5107(01)00183-x
Swarnakar, S., Ganguly, K., Kundu, P., Banerjee, A., Maity, P., and Sharma, A. V. (2005). Curcumin regulates expression and activity of matrix metalloproteinases- 9 and -2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 280, 9409–9415. doi: 10.1074/jbc.m413398200
Taher, M. M., Lammering, G., Hershey, C., and Valerie, K. (2003). Curcumin inhibits ultraviolet light induced human immunodeficiency virus gene expression. Mol. Cell Biochem. 254, 289–297.
Thaloor, D., Miller, K. J., Gephart, J., Mitchell, P. O., and Pavlath, G. K. (1999). Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. Cell Physiol. 277, C320–C329. doi: 10.1152/ajpcell.1999.277.2.C320
Thaloor, D., Singh, A. K., Sidhu, G. S., Prasad, P. V., Kleinman, H. K., and Maheshwari, R. K. (1998). Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 9, 305–312.
Thayyullathil, F., Chathoth, S., Hago, A., Patel, M., and Galadari, S. (2008). Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 45, 1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014
Thiyagarajan, M., and Sharma, S. S. (2004). Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 74, 969–985. doi: 10.1016/j.lfs.2003.06.042
Toda, S., Miyase, T., Arichi, H., Tanizawa, H., and Takino, Y. (1985). Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chemi. Pharma. Bull. 33, 1725–1728. doi: 10.1248/cpb.33.1725
Toden, S., Okugawa, Y., Buhrmann, C., Nattamai, D., Anguiano, E., Baldwin, N., et al. (2015). Novel Evidence for curcumin and boswellic acid- induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. 8, 431–443. doi: 10.1158/1940-6207.CAPR-14-0354
Trujillo, J., Chirinio, Y., Molina-Jijón, E., Andérica-Romero, C., Tapia, E., and Pedraza-Chaverrí, J. (2013). Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 1, 448–456. doi: 10.1016/j.redox.2013.09.003
Unnikrishnan, M. K., and Rao, M. N. A. (1995). Curcumin inhibits nitrogen dioxide induced oxidation of hemoglobin. Mol. Cell. Biochem. 146, 35–37. doi: 10.1007/bf00926878
Ventura, A., Kirsch, D. G., McLaughlin, M. E., Tuveson, D. A., Grimm, J., Lintault, L., et al. (2007). Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665. doi: 10.1038/nature05541
Wang, S. L., Li, Y., Wen, Y. F., Chen, Y. F., Na, L. X., Li, S. T., et al. (2009). Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappa B and JNK pathway. Biomed. Environ. Sci. 22, 32–39. doi: 10.1016/S0895-3988(09)60019-2
Watson, J. L., Greenshields, A., Hill, R., Hilchie, A., Lee, P. W., Giacomantonio, C. A., et al. (2010). Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogenactivated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol. Carcinog. 49, 13–24.
Winter, J., Jung, S., Keller, S., Gregory, R. I., and Diederichs, S. (2009). Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11, 228–234. doi: 10.1038/ncb0309-228
Wu, J., Li, Q., Wang, X., Yu, S., Li, L., and Wu, X. (2013). Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 8:e59843. doi: 10.1371/journal.pone.0059843
Xu, W. S., Parmigiani, R. B., and Marks, P. A. (2007). Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26, 5541–5552. doi: 10.1038/sj.onc.1210620
Yan, X., Pan, B., Lv, T., Liu, L., Zhu, J., Shen, W., et al. (2017). Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. J. Biomed. Sci. 24:1. doi: 10.1186/s12929-016-0310-z
Yang, C. H., Yue, J., Sims, M., and Pfeffer, L. M. (2013). The curcumin analog EF24 targets NF-kappaB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One 8:e71130. doi: 10.1371/journal.pone.0071130
Yang, F., Lim, G. P., Begum, A. N., Ubeda, O. J., Simmons, M. R., Ambegaokar, S. S., et al. (2005). Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 280, 5892–5901. doi: 10.1074/jbc.m404751200
Yang, J., Cao, Y., Sun, J., and Zhang, Y. (2010). Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med. Oncol. 27, 1114–1118.
Yoo, C. B., and Jones, P. A. (2006). Epigenetic therapy of cancer: past, present and future. Nat. Rev. Drug Discov. 5, 37–50. doi: 10.1038/nrd1930
Yu, J., Peng, Y., Wu, L. C., Xie, Z., Deng, Y., Hughes, T., et al. (2013). Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS One 8:e55934. doi: 10.1371/journal.pone.0055934
Yun, J. M., Jialal, I., and Devaraj, S. (2010). Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 22, 450–458. doi: 10.1016/j.jnutbio.2010.03.014
Yun, J. M., Jialal, I., and Devaraj, S. (2011). Epigenetic regulation of high glucose-induced pro-inflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 22, 450–458. doi: 10.1016/j.jnutbio.2010.03.014
Zhang, J., Zhang, T., Ti, X., Shi, J., Wu, C., and Ren, X. (2010). Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem. Biophys. Res. Commun. 399, 1–6. doi: 10.1016/j.bbrc.2010.07.013
Zhao, S. F., Zhang, X., Zhang, X. J., Shi, X. Q., Yu, Z. J., and Kan, Q. C. (2014). Induction of micro RNA-9 mediates cytotoxicity of curcumin against SKOV3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 15, 3363–3368. doi: 10.7314/apjcp.2014.15.8.3363
Zheng, J., Wu, C., Lin, Z., Guo, Y., Shi, L., and Dong, P. (2014). Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation—a novel mechanism suppressing liver fibrosis. FEBS J. 281, 88–103. doi: 10.1111/febs.12574
Zhu, X., Li, Q., Chang, R., Yang, D., Song, Z., Guo, Q., et al. (2014). Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One 9:e91303. doi: 10.1371/journal.pone.0091303
Keywords: curcumin, cancer, epigenetic modulation, alternative treatment, angiogenesis
Citation: Hassan F, Rehman MS, Khan MS, Ali MA, Javed A, Nawaz A and Yang C (2019) Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 10:514. doi: 10.3389/fgene.2019.00514
Received: 05 December 2018; Accepted: 10 May 2019;
Published: 04 June 2019.
Edited by:
Asma Amleh, The American University in Cairo, EgyptReviewed by:
Stella Gagliardi, Fondazione Istituto Neurologico Nazionale Casimiro Mondino (IRCCS), ItalyTomas J. Ekström, Karolinska Institute (KI), Sweden
Copyright © 2019 Hassan, Rehman, Khan, Ali, Javed, Nawaz and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengjian Yang, eWNqMDc0NkBzaW5hLmNvbQ==
 Faiz-ul Hassan
Faiz-ul Hassan Muhammad Saif-ur Rehman2
Muhammad Saif-ur Rehman2 Muhammad Amjad Ali
Muhammad Amjad Ali Chengjian Yang
Chengjian Yang