
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 24 April 2019
Sec. Computational Genomics
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.00351
This article is part of the Research Topic Deep Learning for Toxicity and Disease Prediction View all 12 articles
Chronic diseases are one of the biggest threats to human life. It is clinically significant to predict the chronic disease prior to diagnosis time and take effective therapy as early as possible. In this work, we use problem transform methods to convert the chronic diseases prediction into a multi-label classification problem and propose a novel convolutional neural network (CNN) architecture named GroupNet to solve the multi-label chronic disease classification problem. Binary Relevance (BR) and Label Powerset (LP) methods are adopted to transform multiple chronic disease labels. We present the correlated loss as the loss function used in the GroupNet, which integrates the correlation coefficient between different diseases. The experiments are conducted on the physical examination datasets collected from a local medical center. In the experiments, we compare GroupNet with other methods and models. GroupNet outperforms others and achieves the best accuracy of 81.13%.
Chronic diseases account for a majority of healthcare costs and they have been the main cause of mortality in the worldwide (Lehnert et al., 2011; Shanthi et al., 2015). With the development of preventive medicine, it is very important to predict chronic diseases as early as possible. However, it is difficult for clinicians to make useful diagnosis in advance, because the pathogeny of chronic disease is fugacious and complex. In general, clinicians firstly form the diagnostic results of chronic disease according to the physical examination records based on their expertise and experience. Nevertheless, with more and more physical examination records produced, clinicians would have difficulty forming accurate diagnosis in limited time. Artificial intelligence technology has brought enormous reform in medical domain, and it can help doctor diagnose by forming the diagnostic results automatically based on the prediction models. In clinical practice, a symptom is always associated with multiple chronic diseases based on the physical examination records. Hence, the diagnosis or prediction of multiple chronic diseases could be transformed into a multi-label classification problem.
Multi-label classification problem is one of the supervised learning problems where an instance may be associated with multiple labels simultaneously. Currently, Multi-label classification problems have appeared in more and more applications, such as diseases prediction, semantic analysis, object tracking, and image classification, etc. Many successful multi-label algorithms have been obtained by the problem transformation methods. Problem transformation methods firstly convert the multi-label classification problems into several binary classification problems or a multi-class classification problem, and then apply original machine learning algorithms to handle them. The binary relevance (BR) method and label powerset (LP) method (Zhang and Zhou, 2014) are two representative label transformation methods. Plenty of competitive machine learning algorithms have been proposed based on problem transformation methods in the literatures, such as support vector machines (SVM) (Gu et al., 2015; Khan et al., 2018), decision tree (DT) (Hong et al., 2018), random forest (RF) (Murphy, 2018), etc.
Currently, deep learning technique is applied to various fields successfully since it provides a more efficient learning mechanism for classification problems than classical machine learning methods. For medical data analysis, numerous machine learning methods have been applied to analyze various medical data. BPMLL (Zhang and Zhou, 2006) is a back-propagation neural network for multi-label functional genomics classification, and it addresses correctly predicted labels that should be ranked higher than those mistakenly predicted labels by modifying the loss function. Lipton et al. (2015) utilized the LSTM to analyze time-series clinical data to diagnose 128 different diseases. In order to reduce over-fitting and improve the classification performance of the LSTM architecture, label replication and auxiliary outputs strategies were applied in their work. Maxwell et al. (2017) used a 2-layer deep neural network to classify three chronic diseases based on physical examination records and found combine deep learning algorithms with RAkEL (Tsoumakas and Vlahavas, 2007) method that could improve multi-label classification performance. Miotto et al. (2016) combined a 3-layer autoencoder (AE) and logistic regression classifiers to predict ICD 9-based disease diagnosis using a prediction window. Liang et al. (2014) used a Deep Belief Network (DBN) to generate patient vectors, and then applied a support vector machine (SVM) to classify these generated patient vectors for general disease diagnoses. Jin et al. (2018) made hospital mortality prediction with medical named entities and multimodal learning based on the Long Short-Term Memory (LSTM) architecture, and they outperformed the benchmark by 2% AUC. However, applying deep learning technique to the medical data is still challenging because medical data are sparse, heterogeneous and unstructured.
In this work, we apply the convolutional neural network (CNN) to handle the classification of multiple chronic diseases based on the physical examination records. Because the CNN is the most widely used deep learning method, and it usually gets the desirable classification performance in various classification problems (such as medical image analysis, medical text analysis, and disease prediction). For multiple chronic diseases label transformation, we use two common problem transformation methods: binary relevance (BR) and label powerset (LP) methods in the data preprocessing phase, in order to get expected performance. BR converts multiple chronic disease classification problem into several binary chronic disease classification problems while LP transforms multiple chronic disease classification in a single-label multi-class classification problem.
The main contributions of this work can be summarized as following. Firstly, we devise the convolution block named group block, which both decreases the number of convolution parameter and enhances the overall classification performance. Secondly, a novel CNN architecture named GroupNet using group block is proposed for the classification of multiple chronic diseases based on the physical examination dataset. Thirdly, we devise the correlated loss (CL) to improve the classification performance used in the proposed GroupNet. The proposed GroupNet achieves the best accuracy of 81.13% and increases the overall classification results by at least 2.57% than any other state-of-art deep learning and machine learning methods.
The rest of this work is organized as follows. Section Dataset and Data Preprocessing introduces dataset and data preprocessing. Section Problem Formulation provides definition of the multi-label chronic disease prediction problem. The group convolution strategy, group block and GroupNet architecture are presented in Section Methods. Correlation loss and optimization strategies are elucidated in Section Loss Function and Optimization. Section Experiments and Evaluation describes experiment setup and evaluation measures. Results and Discussion are illuminated in Section Results and Discussion. Finally, Conclusions concludes this work along with future work.
In the work, we mainly focus on multiple chronic disease classification. It can be formulated into a multi-label classification problem. There are three common chronic diseases are selected from the physical examination records: hypertension (H), diabetes (D), and fatty liver (FL).
In the experiments, the physical examination datasets are collected from a local medical center, which contain 110,300 physical examination records from about 80,000 anonymous patients (Li et al., 2017a,b). Sixty-two feature items are selected from over 100 examination items based on medical expert experience and related literature in every physical examination record. These feature items contain 4 basic physical examination items, 26 blood routine items, 12 urine routine items, and 20 items from liver function.
Two multi-label transformation methods consisting of binary relevance (BR) and label powerset (LP) method are used in this work. For BR method, the diagnosis of a given patient can be one of three possible results: all three chronic diseases, different combination of the chronic diseases, or no signs of any three chronic diseases, which means that there are totally eight different sets of diagnoses {000, 100, 010, 001, 110, 101, 011, 111}. Based on Label Powerset (LP) method, we get eight different prediction labels and can be represented by {0, 1, 2, 3, 4, 5, 6, 7}.
In order to understand dataset better and receive expected results, we do some data analysis in the stage of data preprocessing as shown in Figure 1. Figure 1A presents the multi-label distribution of chronic diseases, and single-label distribution of three chronic diseases is shown in Figure 1B. The results demonstrate that the multi-label distribution of chronic diseases is highly skewed, 62.5% of physical examination records is occupied by normal and HFL, and while independent diabetes (D) only hold 1% of physical examination records according to Figure 1A. The single label distribution of fatty liver is a balanced proportion, while the single label distributions of hypertension and diabetes are both imbalanced as you can see from Figure 1B. The correlation coefficient analysis can indicate the label dependencies, and it can be calculated by Pearson product-moment correlation coefficient (PMMC) (Mohamad Asri et al., 2018; Weber and Immink, 2018). Figure 1C shows that the correlation coefficient value between hypertension and flatty liver is maximum among three chronic disease pairs, but the correlation coefficient value is only 0.24. According to the theory of correlation coefficient, we can infer that the correlation between three chronic diseases are not strong.
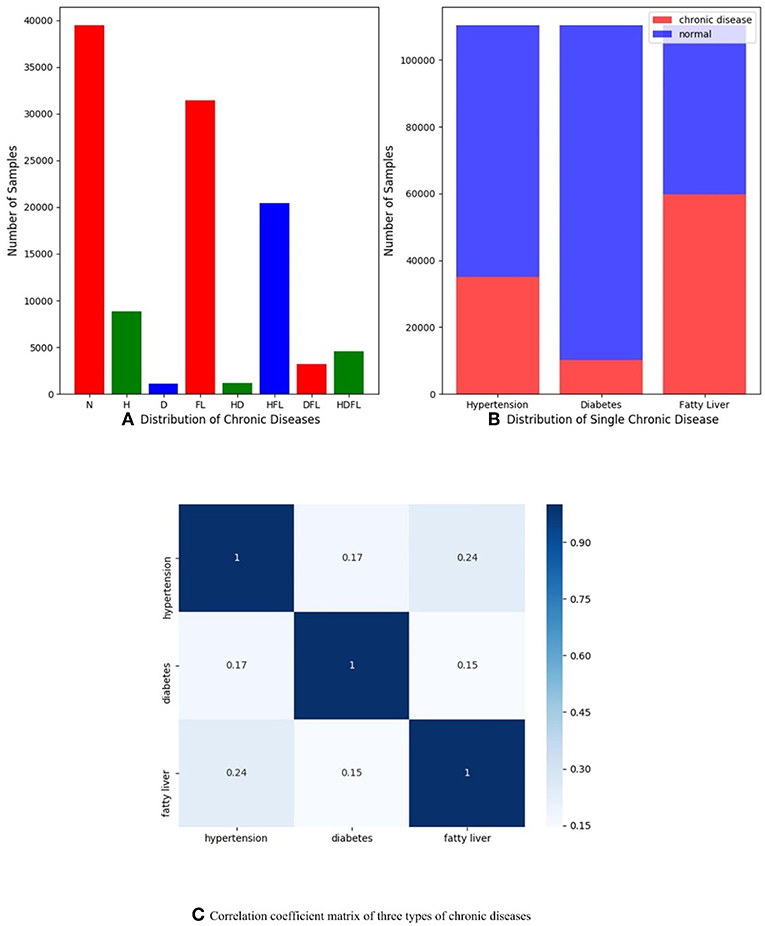
Figure 1. (A) Distribution of multiple chronic diseases; (B) Distribution of single-label of three chronic diseases dependencies; (C) Correlation coefficient matrix of three types of chronic diseases (hypertension, diabetes, and fatty liver), and they are computed by Pearson product-moment correlation coefficient.
We firstly use simple data augmentation method to handle label imbalance problem. However, this method does not work as we expected likely due to the fact that correlation coefficient value among diseases is small as you can see in Figure 1C. Focal loss (Lin et al., 2017) strategy is utilized to relieve label imbalance problem in this work. Furthermore, a cost-sensitive loss learning algorithm called correlated loss (CL) would be described in Group Convolution Strategy in detail and correlation coefficient values between chronic diseases is used as hyper-parameters in the correlated loss. The correlation loss is mainly proposed for improving overall classification performance. Physical examination data are split into two parts, 70% of the data for training and 30% of the data for testing in the experiments.
In medicine filed, the goal of multiple chronic diseases prediction is to predict onset of chronic diseases in advance based on disease prediction model. To this end, we solve multiple chronic diseases prediction problem based on the physical examination dataset. It can be formulated into a multi-label classification problem in computer science. Firstly, we use problem transform methods to transform multiple chronic disease classification problem into multi-label problem classification. Secondly we construct CNN architectures to resolve the multi-label classification.
To improve the performance of a convolutional neural network (CNN) architecture. It is easy to be adopted that we increase the number of convolution kernel in every convolution layer simply. However, it would increase the number of convolution parameter and weaken the classification results. Some well-known and successful convolutional neural network architectures have been proposed to handle this problem, such as IGCNets (Zhang et al., 2017; Sun et al., 2018; Xie et al., 2018), and ShuffleNet (Ma et al., 2018). One common ground for these CNN architectures is that they are implemented based on group convolution strategy (Krizhevsky et al., 2012).
In the implementation of the group convolution strategy, there are being two continuous convolution layers at least. The number of convolution kernel in every convolution layer is split into several independent group convolution partitions. An example of group convolution strategy is shown in Figure 2. A CNN model consists of two continuous convolution layers, in which m and n convolution kernels are set respectively. By the group convolution strategy, we split every convolution layer into two partition convolution units and the number of convolution kernels is the half. The reduction of convolution parameters is shown in Equation 1.
Inspired by group convolution strategy, we propose the group block in this work. Group block consists of two parts, which are group convolution and cluster convolution. The architecture of group block is shown in Figure 3.
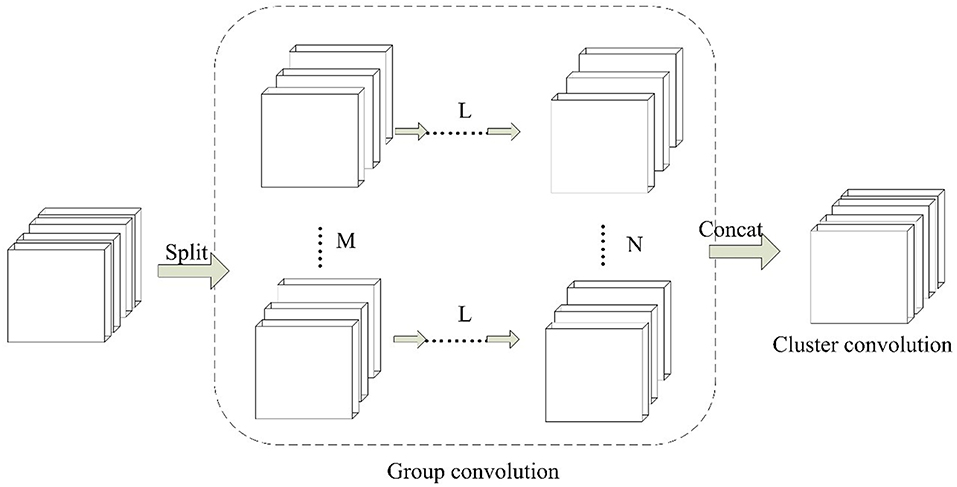
Figure 3. The paradigm of Group Block. For L continuous convolution layers, M and N denotes the number of independent partition convolution units.
In the group convolution part, it splits one convolution unit to multiple partition convolution units. The number of partition convolution units can be set randomly for different convolution layers L. For example, it can be set to split M or N convolution units. In cluster convolution part, a 1 × 1 convolution layer is designed after the group convolution part. It is implemented to cluster the correlated feature maps and enhances discriminability for local patches within the receptive field.
The parameters of group block are described by (L, Ni (i = 1, … m), j). Here L denotes the number of continuous convolution layers. Ni (i = 1, … m) shows the number of partition convolution units in the ith convolutional layer. j is the number of cluster convolution layers.
In this work, we construct the CNN architecture based on the proposed group block named the GroupNet, shown in Figure 4. The proposed group block is the core part of the GroupNet, which is a variant of group convolution. The main difference between the proposed group block and the traditional group convolution is that we add a cluster convolution part after group convolution part in group block. Hence, the GroupNet architecture built on the group block improves the classification performance efficiently when comparing to several advanced CNN architectures.
The GroupNet architecture contains six layers: input layer, group block, max-pooling layer, dropout layer, fully-connected layer and softmax layer. The detail parameters of GroupNet architecture is listed in Figure 5. Small convolution kernels always are used to reduce the computation burden and improve the classification performance (Huang et al., 2016; Iandola et al., 2016; Sandler et al., 2018). In this work, we use 1 × 3 as the convolution kernel size. Because convolution kernel size 1 × 3 achieves better performance than other convolution kernel sizes in the experiments. Because physical examination data are one-dimensional. Hence, one-dimensional convolution kernel is adopted. Furthermore, softmax function is used as classifier, because it is standard to use the softmax as classifier in deep learning.
Well-known dropout (Srivastava et al., 2014; Bouthillier et al., 2015) technique is available to alleviate over-fitting for CNN. In this work, we set a dropout layer between the max-pooling layer and the fully-connected layer and the drop rate is 0.5 which is set experimentally.
In this work, LP and BP are adopted to resolve the multi-label classification, respectively. LP method is to transform multiple chronic disease classification into the single-label multi-class classification, while BR method converts the multi-label chronic disease classification into three binary classifications. Correspondingly, LP-GroupNet and BR-GroupNet are named in experiments.
Focal loss (FL) (Lin et al., 2017) is a variant of standard cross entropy loss, and it alleviates loss of correctly classified examples domain the gradient in the training and can be computed as following.
Here p is the probability for predicted label. (1 − p)γ is modulating factor and γ is a focusing parameter. γ is set manually. When γ = 0, focal loss is equal to standard cross entropy loss. The cross entropy loss is described as following.
CE (p, q) is a cross entropy loss, p and q represent the expected output and actual output, respectively.
In the BR-GroupNet architecture, each binary classifier is independent of each other, in order to enhance connection between independent classifiers and each classifier can learn useful information from each other. Hence, we propose a cost-sensitive learning algorithm named correlated loss (CL) for the BR-GroupNet to enhance classification performance by learning loss information from each other. In the BR-GroupNet architecture, the correlated loss of each binary classifier consists of two parts: main loss and auxiliary loss. Main loss can be computed by the classifier itself and auxiliary loss is the sum of product associated classifier loss and correlation coefficient value. In this work, correlation coefficient value between two chronic diseases is chosen as a hyper parameter in auxiliary loss, because correlation coefficient value between two diseases is small and it also indicates disease dependencies between two diseases. Therefore, correlated loss (CL) of an independent binary classifier in BR-GroupNet can be computed as follows.
Here α is a correlation coefficient value between every two labels, which is calculated by Pearson product-moment correlation coefficient (PMMC). In this work, we choose three chronic diseases as multi-label chronic disease prediction targets and only three independent binary classifiers are required. For the correlated loss of each independent classifier, the loss of each classifier itself as main loss, and the sum of product of two associated classifier losses and correlation coefficient values as the auxiliary loss. Hence, we set the range of parameter i from 1 to 2 in this work.
In this work, we use two different methods to calculate the correlated loss based on CE and FL, respectively, and named CL1 and CL2 as seen in Equations 5, 6. In order to validate whether selecting correlation coefficient value between two chronic diseases as hyper parameters of CL can work as we expected. The GroupNet architecture with correlated loss named BR-GroupNet-CL.
In the training of CNN models, back-propagation method is carried out for the gradient. There are many hyper parameters of CNN models that need to be optimized. It is experimental, time-consuming and difficult to choose best hyper parameters. To initialize hyper parameters with less tuning in the training phase, Adam (Kingma and Ba, 2014; Chen et al., 2018; Reddi et al., 2018) optimizer is used for the gradient. It is a first-order gradient-based descent optimizer of stochastic objective function. Adam is based on adaptive estimates of lower-order moments and computes individual learning rates for different hyper parameter from estimates of first and second moments of the gradients. Comparing to stochastic gradient descent optimization (SGD) (Orr and Müller, 2003), Adam is more efficient, which requires less memory and training time.
The proper activation function also improves classification performance. There are several popular activation functions for neural networks, such as sigmoid, tanh, rectified linear unit (ReLU) (Nair and Hinton, 2010), Leaky ReLU (LeakyReLU) (Maas et al., 2013), Exponential Linear Units (ELU) (Clevert et al., 2015), Self-Normalizing Linear Units (SELU) (Klambauer et al., 2017), and so on. In this work, we test and compare all different activation functions in our datasets and choose the preferable one in all CNN models.
We implement all experiments based on the Scikit-learn library, WEKA software and Tensorflow platform. Scikit-learn library and WEKA are used to implement several machine learning methods, such as SVM, SMO, DT, Multilayer Perceptron (MLP). Tensorflow platform is used to implement deep learning methods, such as the proposed GroupNet architectures, IGCNet, GoogleNet (Szegedy et al., 2015), VGGNet (Simonyan and Zisserman, 2014), AlexNet, and deep neural network (DNN), Long Short-Term Memory (LSTM), and Gated Recurrent Unit (GRU) (Shickel et al., 2018). The experiments run on a machine with Intel (R) 3.20 GHz CPU (i5-6500) and 8 GB RAM.
Furthermore, several experiments are conducted to select proper parameters based on the LP-GroupNet, such as batch size, learning rate, epochs, convolution kernel size, dropout rate, activation function, and focusing parameter γ in focal loss. In order to select preferable number of convolution units of group block for the GroupNet, we deploy three GroupNet architectures based on three different group blocks. The detail parameter setting of three different group blocks are {2, 2, 2, 1}, {2, 3, 3, 1} and {2, 4, 4, 1}, and Figure 5 gives concrete CNN architectures of the three different GroupNet architectures, namely LP-GroupNet (Figure 5A), LP-GroupNet-3 (Figure 5B), and LP-GroupNet-4 (Figure 5C).
Since multi-label classification can be converted into single-label multi-class classification and so the measures to evaluate single-label multi-class classification also can be used for this work. We adopt four common evaluation measures: F-score, accuracy, recall and precision measures to compare the performance of different methods for multi-label chronic disease classification. The accuracy is a measure to ensure that ratio of the prediction of true labels is correct. Precision is a measure system that is related to reproducibility, or how many predictions are correct. Recall is the fraction of true labels that were predicted correctly. F-score (F1) measure is the harmonic mean of precision and recall, and is a popular evaluation measure in the research area of data mining. Because the label distribution of chronic disease is skewed as described in Dataset and Data Preprocessing, weighted recall, weighted precision, weighted F-score are used to evaluate the classification performance of different methods. F1 evaluates the overall performance of the method better than accuracy, precision and recall according to related works (Tsoumakas and Katakis, 2007; Zhang and Zhou, 2014). Recall is an important evaluation measure in clinical. Different to normal F-score, the value of weighted F-score is not between weighted precision and weighed recall, instead it is smaller than both weighted precision and weighed recall. The following equations show how to calculate these values. TP, TN, FP, and FN are true positive, true negative, false positive, and false negative, respectively.
Accuracy, Precisionweighted, Recallweighted, andF1weightedcan be computed by Equations (7–10). kidenotes the single labels accounted for the proportion of all labels, lis equal to 8 and i ranges 1–8.
In this section, we present results of hyper parameter selection in both Figures 6, 7. Figure 6A shows how accuracy changes with epochs, and epochs are set 1, 5, 10, 15, 20, 25, 30, 40, 50, and 100, respectively in experiments. When epochs is above certain epochs like 20, the performance of the LP-GroupNet actually decreases drastically due to over-fitting. It is evident that the LP-GroupNet achieves the best performance when the epochs is 20 as you can see from Figure 6A.
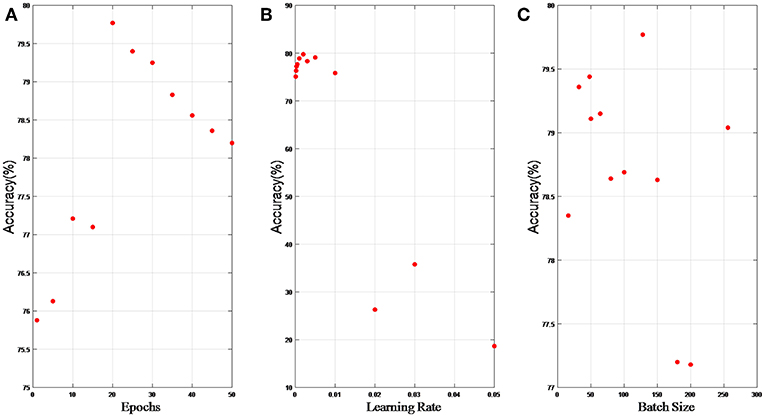
Figure 6. (A) relationship between accuracy and epochs; (B) relationship between accuracy and learning rate; (C) relationship between accuracy and batch size.
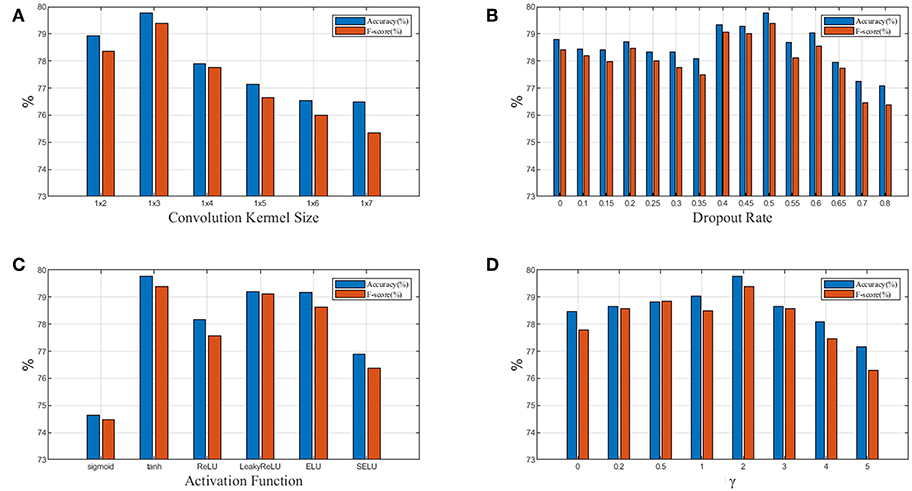
Figure 7. (A) Relationship between performance and convolution kernel size; (B) Relationship between performance and dropout rate; (C) Relationship between performance and activation function; (D) Relationship between performance and focusing parameter γ in focal loss. Blue denotes accuracy and red denotesF1weighted.
Figure 6B shows the relationship between accuracy with learning rate, respectively. We set the learning rate 0.05, 0.03, 0.02, 0.01, 0.005, 0.003, 0.002, 0.001, 0.0005, 0.0003, 0.0002, and 0.0001, respectively in the experiments. It is clear that the LP-GroupNet obtains the best performance when the learning rate is 0.002 according to Figure 6B.
Figure 6C shows how batch size affects the LP-GroupNet performance, and we set batch size to 16, 32, 48, 50, 64, 80, 100, 128, 150, 180, 200, and 256, respectively. Accuracy changes with batch size quite significantly as you can see from Figure 6C. Results from the experiments show that the GroupNet achieves the best performance when batch size reaches 128.
In Figure 7A, we test 6 different convolution kernel sizes. The LP-GroupNet achieves best performance when convolution kernel size is 1 × 3. Furthermore, we also conclude that smaller convolution kernel works better than larger convolution kernel in previous works. Figure 7B presents how dropout rates influence the classification performance. It is shown that the LP-GroupNet gets the better performance when dropout rate is 0.5. It is difficult to find considerate dropout rate in the experiments as you can see from Figure 7B, because there is not a good way to find the best dropout rate theoretically except by experiments.
Figure 7C shows a performance comparison among six different activation functions: tanh, sigmoid, ReLU, LeakyReLU, ELU, and SELU. The tanh receives the best performance with 79.77% based on the GroupNet, while sigmoid receives the worst performance with 74.65%. It is noticeable that LeakyReLU and ELU both get accuracy over 79%. In order to achieve considerable performance, the tanh function is more adaptive as activation function than others in this work. Figure 7D shows how focusing parameter γ in focal loss affects the LP-GroupNet performance and γ is set 0, 0.2, 0.5, 1.0, 2.0, 3.0, 4.0, and 5.0, respectively. When focusing parameter is 0, focal loss is equivalent to standard cross entropy loss. It is clear that it results in the best performance with the accuracy of 79.77% when focusing parameter γ is 2.
Table 1 gives a comparison between Adam optimizer and SGD optimizer. It is apparent that Adam optimizer outperforms SGD optimizer. Furthermore, SGD optimizer requires 160 epochs to achieve the accuracy at 75.09%, while Adam optimizer uses 20 epochs to achieve the accuracy 79.77%. With trading-off on training time and accuracy, Adam is selected as optimizer.
Table 2 presents the results for LP-GroupNet, LP-GroupNet-3, and LP-GroupNet-4. The results illuminate that the LP-GroupNet gets better performance than LP-GroupNet-3 and LP-GroupNet-4 models. It confirms that when the number of partition convolution units is 2 in group block, the GroupNet is able to handle the data more effectively and achieves the performance as we expected.
Table 3 lists the final optimal hyper-parameter settings.
Table 4 presents comparison results of the GroupNet and other CNN models based on LP method. The GroupNet achieves the best performance and increases 1.21% at least than other four CNN models on all evaluation measures.
It is observed that the BR-GroupNet model provides the accuracy with 80.54% in Table 5. It increases over 0.77% than LP-GroupNet on all evaluation measures and F1weighted receives the best improvement with 0.95%, which demonstrates that BR-GroupNet model is more suitable for this work than LP-GroupNet.
Table 6 presents a comparison among correlated loss and other loss functions based on the BR-GroupNet architecture. For convenience, cross entropy loss is named as CE in short, focal loss as FL, correlated loss based on cross entropy loss as CL1, and correlated loss based on focal loss as CL2.
It is obvious that CL2 gets the best accuracy with 81.13%. The results also demonstrate that CL works better than FL and CE based on the BR-GroupNet in this work, which increases approximately 0.6% on all metrics. The results from CL1 and CL2 demonstrate that correlation coefficient value between two chronic diseases is selected as hyper parameter of CL can work as we expected. Furthermore, FL achieves better performance than CE, which confirms that FL can improve classification performance by reducing the proportion of correctly classified instance loss in all loss in the training phase.
Table 7 presents the results for the BR-GroupNet-CL, four state-of-art CNN architectures, two RNN architectures (LSTM and GRU) and seven classical machine learning methods. According to these results, deep learning methods get better performance than classic machine learning methods generally, which show deep learning methods have great potentials in disease prediction. It is apparent that the BR-GroupNet-CL architecture provides the best performance among all of them on all metrics, while the SVM receives the worst performance. IGCNet, GoogleNet, AlexNet, VGGNet, LSTM, GRU, and BPMLL show similar performance and they all receive over 75% on all evaluation measures. According to the Table 7, BR-GroupNet-CL gets the best accuracy and F1weighted with 81.13 and 81.02%, respectively, and it increases 2.61% than other comparative methods which confirms that the proposed BR-GroupNet-CL is more able to receive considerable performance for multi-label chronic disease classification. Particularly, BR-GroupNet-CL model achieves Recallweighted with 81.13% and increases at least 2.57% comparing to other methods, which is a considerable improvement for disease classification clinically.
We propose a novel group block inspired by group convolution strategy to reduce the number of convolution parameters and improve the classification performance. Furthermore, we develop the GroupNet based on group block, then combine GroupNet with BR and LP methods for multi-label classification of chronic diseases, respectively. We present a cost sensitive learning algorithm named correlated loss to improve the performance. The results indicate that the proposed GroupNet gets the best accuracy with 81.13%, which is nearly 2.6% higher than all other comparison methods.
In the future work, we will focus on enhancing the learning ability of the CNN model and reduce over-fitting in the training. The transfer learning and adversarial learning methods will be applied to the model.
RL conceived the project. XZ implemented the algorithm and performed the computational analysis. SZ and HZ supervised the experiments. XZ, RL, SZ, and HZ drafted the manuscript. All authors revised and approved the final version of the manuscript.
The project is supported by the Program of Scientific and Technological Research of Henan Province, China, under Contract 152102210249, 192102310215, and Program of Key Science and Technological Research of Henan Province (18A520049).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the Collaborative Innovation Center on Internet Healthcare and Health Service of Henan Province, Zhengzhou University, for providing the medical records for analysis. The physical examination dataset used in this study is located at http://pinfish.cs.usm.edu/dnn/. There are two versions of the data available for download: a simple text file and an ARFF file for use with WEKA. Details about the format of the data are located on the webpage. The personally identifiable information from this dataset has been removed to ensure patient anonymity.
Bouthillier, X., Konda, K., Vincent, P., and Memisevic, R. (2015). Dropout as data augmentation. arXiv:1506.08700.
Chen, X., Liu, S., Sun, R., and Hong, M. (2018). On the convergence of a class of adam-type algorithms for non-convex optimization. arXiv:1808.02941.
Clevert, D.-A., Unterthiner, T., and Hochreiter, S. (2015). Fast and accurate deep network learning by exponential linear units (ELUs). CoRR abs/1511.07289. Available online at: http://arxiv.org/abs/1511.07289
Gu, B., Sheng, V. S., and Li, S. (2015). “Bi-parameter space partition for cost-sensitive SVM,” in Proceedings of the 24th International Conference on Artificial Intelligence IJCAI'15 (Buenos Aires: AAAI Press), 3532–3539.
Hong, H., Liu, J., Bui, D. T., Pradhan, B., Acharya, T. D., Pham, B. T., et al. (2018). Landslide susceptibility mapping using J48 decision tree with adaboost, bagging and rotation forest ensembles in the guangchang area (China). Catena 163, 399–413. doi: 10.1016/j.catena.2018.01.005
Huang, G., Liu, Z., and Weinberger, K. Q. (2016). Densely connected convolutional networks. CoRR abs/1608.06993. Available online at: http://arxiv.org/abs/1608.06993
Iandola, F. N., Moskewicz, M. W., Ashraf, K., Han, S., Dally, W. J., and Keutzer, K. (2016). SqueezeNet: AlexNet-level accuracy with 50x fewer parameters and < 0.5mb model size. CoRR abs/1602.07360. Available online at: http://arxiv.org/abs/1602.07360
Jin, M., Bahadori, M. T., Colak, A., Bhatia, P., Celikkaya, B., Bhakta, R., et al. (2018). Improving hospital mortality prediction with medical named entities and multimodal learning. arXiv:1811.12276.
Khan, S. H., Hayat, M., Bennamoun, M., Sohel, F. A., and Togneri, R. (2018). Cost-sensitive learning of deep feature representations from imbalanced data. IEEE Trans Neural Netw Learn Syst. 29, 3573–3587. doi: 10.1109/TNNLS.2017.2732482
Klambauer, G., Unterthiner, T., Mayr, A., and Hochreiter, S. (2017). Self-normalizing neural networks. CoRR abs/1706.02515. Available online at: http://arxiv.org/abs/1706.02515
Krizhevsky, A., Sutskever, I., and Hinton, G. E. (2012). “ImageNet Classification with Deep Convolutional Neural Networks,” in Advances in Neural Information Processing Systems 25, 1097–1105. doi: 10.1145/3065386
Lehnert, T., Heider, D., Leicht, H., Heinrich, S., Corrieri, S., Luppa, M., et al. (2011). Health care utilization and costs of elderly persons with multiple chronic conditions. Med. Care Res. Rev. 68, 387–420. doi: 10.1177/1077558711399580
Li, R., Liu, W., Lin, Y., Zhao, H., and Zhang, C. (2017a). An ensemble multilabel classification for disease risk prediction. J Health Eng. 2017:8051673. doi: 10.1155/2017/8051673
Li, R., Zhao, H., Lin, Y., Maxwell, A., and Zhang, C. (2017b). “Multi-label classification for intelligent health risk prediction,” in IEEE International Conference on Bioinformatics and Biomedicine (Shenzhen: IEEE), 986–993.
Liang, Z., Zhang, G., Huang, J. X., and Hu, Q. V. (2014). “Deep learning for healthcare decision making with EMRs,” in Bioinformatics and Biomedicine (BIBM), 2014 IEEE International Conference on (IEEE), 556–559. doi: 10.1109/BIBM.2014.6999219
Lin, T.-Y., Goyal, P., Girshick, R., He, K., and Dollár, P. (2017). Focal loss for dense object detection. arXiv:1708.02002.
Lipton, Z. C., Kale, D. C., Elkan, C., and Wetzel, R. (2015). Learning to diagnose with LSTM recurrent neural networks. arXiv:1511.03677.
Ma, N., Zhang, X., Zheng, H.-T., and Sun, J. (2018). Shufflenet v2: practical guidelines for efficient cnn architecture design. arXiv:1807.111645.
Maas, A. L., Hannun, A. Y., and Ng, A. Y. (2013). “Rectifier nonlinearities improve neural network acoustic models,” in Proceedings of the International Conference on Machine (Atlanta, GA), 3.
Maxwell, A., Li, R., Yang, B., Weng, H., Ou, A., Hong, H., et al. (2017). Deep learning architectures for multi-label classification of intelligent health risk prediction. BMC Bioinform. 18:523. doi: 10.1186/s12859-017-1898-z
Miotto, R., Li, L., Kidd, B. A., and Dudley, J. T. (2016). Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci. Rep. 6:26094. doi: 10.1038/srep26094
Mohamad Asri, M. N., Hashim, N. H., Mat Desa, W. N. S., and Ismail, D. (2018). Pearson Product Moment Correlation (PPMC) and Principal Component Analysis (PCA) for objective comparison and source determination of unbranded black ballpoint pen inks. Austr. J. Forensic Sci. 50, 323–340. doi: 10.1080/00450618.2016.1236292
Murphy, K. P. (2018). Machine Learning: A Probabilistic Perspective (Adaptive Computation and Machine Learning Series). London, UK: The MIT Press.
Nair, V., and Hinton, G. E. (2010). “Rectified Linear Units Improve Restricted Boltzmann Machines,” in Proceedings of the 27th International Conference on International Conference on Machine Learning ICML'10 (Haifa: Omnipress), 807–814.
Reddi, S. J., Kale, S., and Kumar, S. (2018). “On the convergence of Adam and Beyond,” in International Conference on Learning Representations (Vancouver, BC: Vancouver Convention Center).
Sandler, M., Howard, A. G., Zhu, M., Zhmoginov, A., and Chen, L.-C. (2018). Inverted residuals and linear bottlenecks: mobile networks for classification, detection and segmentation. CoRR abs/1801.04381. Available online at: http://arxiv.org/abs/1801.04381
Shanthi, M., Stephen, D., and Bo, N. (2015). Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46:e121. doi: 10.1161/STROKEAHA.115.008097
Shickel, B., Tighe, P. J., Bihorac, A., and Rashidi, P. (2018). Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J. Biomed. Health Inform. 22, 1589–1604. doi: 10.1109/JBHI.2017.2767063
Simonyan, K., and Zisserman, A. (2014). Very deep convolutional networks for large-scale image recognition. arXiv:1409.1556.
Srivastava, N., Hinton, G., Krizhevsky, A., Sutskever, I., and Salakhutdinov, R. (2014). Dropout: a simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 15, 1929–1958. Available online at: http://jmlr.org/papers/v15/srivastava14a.html
Sun, K., Li, M., Liu, D., and Wang, J. (2018). IGCV3: interleaved low-rank group convolutions for efficient deep neural networks. CoRR abs/1806.00178. Available online at: http://arxiv.org/abs/1806.00178
Szegedy, C., Sermanet, P., Reed, S., Anguelov, D., Erhan, D., et al. (2015). “Going deeper with convolutions,” in 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 1–9. doi: 10.1109/CVPR.2015.7298594
Tsoumakas, G., and Katakis, I. (2007). Multi-label classification: an overview. Int. J. Data Warehousing Mining 3, 1–13. doi: 10.4018/jdwm.2007070101
Tsoumakas, G., and Vlahavas, I. (2007). “Random k-labelsets: an ensemble method for multilabel classification,” in European Conference on Machine Learning (Springer), 406–417.
Weber, J. H., and Immink, K. A. S. (2018). Maximum likelihood decoding for gaussian noise channels with gain or offset mismatch. IEEE Commun. Lett. 22, 1128–1131. doi: 10.1109/LCOMM.2018.2809749
Xie, G., Wang, J., Zhang, T., Lai, J., Hong, R., and Qi, G.-J. (2018). IGCV2: interleaved structured sparse convolutional neural networks. CVPR abs/1804.06202. Available online at: http://arxiv.org/abs/1804.06202
Zhang, M.-L., and Zhou, Z.-H. (2006). Multilabel neural networks with applications to functional genomics and text categorization. IEEE Transac. Knowl. Data Eng. 18, 1338–1351. doi: 10.1109/TKDE.2006.162
Zhang, M.-L., and Zhou, Z.-H. (2014). A review on multi-label learning algorithms. IEEE Transac. Knowl. Data Eng. 26, 1819–1837. doi: 10.1109/TKDE.2013.39
Keywords: multi-label classification, chronic disease, group block, GroupNet, correlated loss
Citation: Zhang X, Zhao H, Zhang S and Li R (2019) A Novel Deep Neural Network Model for Multi-Label Chronic Disease Prediction. Front. Genet. 10:351. doi: 10.3389/fgene.2019.00351
Received: 29 September 2018; Accepted: 01 April 2019;
Published: 24 April 2019.
Edited by:
Minjun Chen, National Center for Toxicological Research (FDA), United StatesReviewed by:
Harinder Singh, J. Craig Venter Institute, United StatesCopyright © 2019 Zhang, Zhao, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Runzhi Li, cnpsaUBoYS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.