- 1College of Animal Science and Technology, Northwest A&F University, Yangling, China
- 2College of Information Engineering, Northwest A&F University, Yangling, China
- 3College of Veterinary Medicine, Northwest A&F University, Yangling, China
- 4Guilin Medical University, Guilin, China
- 5Ningxia Tianyuan Tan Sheep Farm, Hongsibu, China
- 6Institute of Farm Animal Genetics, Friedrich-Loeffler-Institut, Neustadt, Germany
- 7Recombinetics, Saint Paul, MN, United States
Since its emergence, CRISPR/Cas9-mediated base editors (BEs) with cytosine deaminase activity have been used to precisely and efficiently introduce single-base mutations in genomes, including those of human cells, mice, and crop species. Most production traits in livestock are induced by point mutations, and genome editing using BEs without homology-directed repair of double-strand breaks can directly alter single nucleotides. The p.96R > C variant of Suppressor cytokine signaling 2 (SOCS2) has profound effects on body weight, body size, and milk production in sheep. In the present study, we successfully obtained lambs with defined point mutations resulting in a p.96R > C substitution in SOCS2 by the co-injection of BE3 mRNA and a single guide RNA (sgRNA) into sheep zygotes. The observed efficiency of the single nucleotide exchange in newborn animals was as high as 25%. Observations of body size and body weight in the edited group showed that gene modification contributes to enhanced growth traits in sheep. Moreover, targeted deep sequencing and unbiased family trio-based whole genome sequencing revealed undetectable off-target mutations in the edited animals. This study demonstrates the potential for the application of BE-mediated point mutations in large animals for the improvement of production traits in livestock species.
Introduction
Clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9 is widely used to establish site-specific genome-edited cell lines and animal models (Sander and Joung, 2014). The Cas9 protein, under the guidance of single guide (sg) RNA, cleaves DNA at sequence-specific sites in the genome and produces a double-strand break (DSB). To response DSB, cellular DNA repair pathways generated more abundant insertion and deletions (indels) by non-homologous end-joining (NHEJ) than that of homology-directed repair (HDR)-mediated gene correction (Chu et al., 2015; Paquet et al., 2016; Sakuma et al., 2016). Therefore, developing alternative approaches to correct point mutations that do not need DSBs is highly expected. Rat cytidine deaminase (rAPOBEC1) linked to nCas9 (Cas9 nickase) was reported to efficiently convert C→T at target sites without introducing DSBs (Komor et al., 2016). After several generations of modification, base editor 3 (BE3) including rAPOBEC1, nCas9 (A840H), and uracil DNA glycosylase inhibitor (UGI) was developed; the mutation efficiency was up to 74.9% in mammalian cells (Komor et al., 2016). To further optimize BE3, previous studies have been conducted to improve target specificity (Kim D. et al., 2017), editing efficiency and product purity (Komor et al., 2017), expand the genome-targeting scope (Kim K. et al., 2017), and reduce off-target effects (Kim D. et al., 2017). To prove that BE3 has a high efficiency for converting C:G to T:A base pairs, several groups have used BE3 to silence genes by introducing nonsense mutations (Billon et al., 2017; Kuscu et al., 2017).
Sheep are a phenotypically diverse livestock species that are raised globally for meat, milk, and fiber production. Suppressor of cytokine signaling 2 (SOCS2), a member of the SOCS protein family, is a negative regulator of biological processes mediated by various cytokines, such as metabolism, skeletal muscle development, and the response to infection (Inagaki-Ohara et al., 2014; Letellier and Haan, 2016). The most important of these processes is the regulation of GH signaling during growth and development (Yang et al., 2012; Dobie et al., 2018). SOCS2 is the major gene involved in the promotion of bone development in mice, and it plays a vital role in the control of bone mass and body weight (Metcalf et al., 2000; Dobie et al., 2018). A point mutation g.C1901T (p.R96C) in SOCS2 that completely abrogates SOCS2 binding affinity for the phosphopeptide of growth hormone receptor (GHR) is highly associated with an increased body weight and size in sheep (Rupp et al., 2015). We recently reported the usage of the BE3 system to induce nonsense mutations in the goat FGF5 gene, to generate animals with longer hair fibers (Li G. et al., 2018). It was the first base editing study in large animals and further inspired us to examine the feasibility of induce amino acid exchanges in sheep. In the present study, we obtained BE3-mediated lambs by co-injection of a BE3 mRNA and guide RNA target the p.R96C variant in SOCS2. In addition, we used a parent-progeny whole genome sequencing (WGS) approach to show that no off-target mutations were detected and the mutation frequency in edited animals is equivalent to that in control groups.
Materials and Methods
Animals
Tan sheep were maintained at the Ningxia Tianyuan Sheep Farm, Hongsibu, Ningxia Autonomous Region, China. All experimental animals were provided water and standard feed ad libitum, consistent with normal sheep, and were treated according to Guidelines for the Care and Use of Laboratory Animals formulated by the College of Animal Science and Technology, Northwest A&F University. The experimental study was approved by the Northwest A&F University Animal Care and Use Committee (Approval ID: 2016NXTS001).
Design of sgRNA
The sequences target the g.C1901T (p.R96C) variant in the ovine SOCS2 gene is listed in Supplementary Table S1. Two oligonucleotides (Supplementary Table S2) used for the transcription of sgRNA in vitro were precisely synthesized and annealed to form double-stranded oligos. These double-stranded oligos were subcloned into the pUC57-T7-gRNA vector as described previously (Shen et al., 2013). The clones containing the desired sequence were selected, expanded by cultivation, and the plasmid was extracted using a plasmid extraction kit (AP-MN-P-250G; Axygen, Union City, CA, United States), sgRNA was transcribed in vitro using the MEGAshortscript Kit (AM1354; Ambion, Foster City, CA, United States) and purified using the MEGAclear Kit (AM1908; Ambion). Subsequently, the BE3 mRNA in vitro transcription vector (No. 44758; Addgene, Cambridge, MA, United States) was used as a template to produce BE3 mRNAs following a previously published protocol (Shen et al., 2013).
Production of Single-Nucleotide Mutation Sheep
Healthy ewes (3–5 years old) with regular estrous cycles were selected as donors for zygote collection. The superovulation treatment of donors and the procedures for zygote collection were as described previously (Wang et al., 2015). Briefly, an EAZI-BREED controlled internal drug release (CIDR) Sheep and Goat Device (containing 300 mg of progesterone) was inserted into the vagina of the donor sheep for 12 days and superovulation was performed 60 h before CIDR Device removal. Zygotes at the 1-cell stage were surgically collected and immediately transferred to TCM-199 medium (Gibco, Gaithersburg, MD, United States). A mixture of BE3 mRNA (25 ng μL-1) and sgRNA (10 ng μL-1) was co-injected into the cytoplasm of 1-cell stage zygotes using an Eppendorf FemtoJet system. The injection pressure, injection time, and compensatory pressure were 45 kPa, 0.1 s, and 7 kPa, respectively. Microinjections were performed on the heated stage of the Olympus ON3 micromanipulation system. Injected embryos were cultured in Quinn’s Advantage Cleavage Medium and Blastocyst Medium (Sage BioPharma, Toronto, ON, Canada) for ∼24 h and were then transferred into surrogates, as reported previously (Wang et al., 2016). Pregnancy was determined by observed estrous behaviors of surrogates at every ovulation cycle. After 150 days of pregnancy, newborn lambs were delivered and genotyped.
Genotyping of Delivered Animals
Peripheral venous blood samples were collected from newborn lambs at day 15 after birth for genomic DNA extraction. Polymerase chain reaction (PCR) amplification-based Sanger sequencing was conducted using KOD-NEO-Plus enzyme (DR010A; TOYOBA, Osaka, Japan) and primers are listed in Supplementary Table S3.
Prediction of Off-Target Sites
Potential off-target sites with up to three mismatches were predicted using the openly available tool SeqMap (Jiang and Wong, 2008). The process for searching for off-target sites was implemented as previously described (Wang et al., 2015; Niu et al., 2017). The primers used for amplifying off-target sites by captured deep sequencing are given in Supplementary Table S4.
Captured Deep-Sequencing
On-target and potential off-target mutations were amplified using a KAPA HiFi HotStart PCR Kit (#KK2501; KAPA Biosystems, Wilmington, MA, United States) for deep sequencing library generation. Pooled PCR amplicons were sequenced using the MiSeq with TruSeq HT Dual Index system (Illumina, San Diego, CA, United States).
Whole Genome Sequencing
Genomic DNA of nine animals from three edited families were used for WGS. Nine DNA libraries with insert sizes of approximately 300 bp were constructed following the manufacturer’s instructions, and 150-bp paired-end reads were generated using the Illumina HiSeq XTen PE150 platform. The qualified reads were mapped to the sheep reference genome (Jiang et al., 2014) using the BWA (v0.7.13) tool (Li and Durbin, 2009). Local realignment and base quality recalibration were assessed with the Genome Analysis Toolkit (GATK) (McKenna et al., 2010). Single-nucleotide polymorphisms (SNPs) and small indels (<50 bp) were called using GATK (McKenna et al., 2010) and SAMtools (Li et al., 2009).
Identification of Off-Target Mutations
The called SNPs were filtered according to the following criteria: (1) SNPs that were identified by both GATK and SAMtools; (2) excluding SNPs that exist in NCBI sheep SNP database (>59 million SNPs); (3) excluding SNPs that exist in our sheep SNP database (n = 294, >79 million SNPs1); (4) within the remaining SNPs, SNPs with C and G converted to other base types were selected. The potential off-target sites were predicted using Cas-OFFinder (Bae et al., 2014) by consider allowing up to five mismatches. SNPs within the predicted off-target sites were identified as off-target mutations.
Identification of de novo Mutations
Putative de novo SNPs and indels were identified according to our recent report (Wang et al., 2018). Briefly, the SNPs/indels were identified by both GATK and SAMtools, and SNPs/indels that were found in the NCBI and our own sheep SNP databases were removed. Next, the SNPs/indels inherited from parents were excluded. Additional SNPs/indels were filtered based on parameters including read depth and Phred-scaled likelihood (PL) scores (Wang et al., 2018). Finally, the mis-aligned or miscalled SNPs/indels were removed manually. Genome-wide structure variations (SVs) were called using BreakDancer (Chen et al., 2009), then the SVs specific in the edited animals were remained. To identify the de novo SVs, common SVs in every two founders, and the read depth <50%, as well as the scaffolds were removed.
Results and Discussion
Generation of Edited Animals
To obtain lambs comprising the precise g.1901C > T mutation in SOCS2, we micro-injected the BE3 mRNA and sgRNA into the cytoplasm of 1-cell-stage embryos. The sgRNA was designed to encompass the target point mutation p.R96C in SOCS2 (Figure 1A). Five mated Tan sheep that were treated for superovulation received 54 one-cell fertilized oocytes; after 53 embryos were subjected to micro-injection, 20 developing embryos were transplanted into eight recipients. Three recipient sheep were confirmed with pregnancy. After ∼150 days of gestation, four lambs (#28, #34, #41, and #42) were obtained (Table 1).
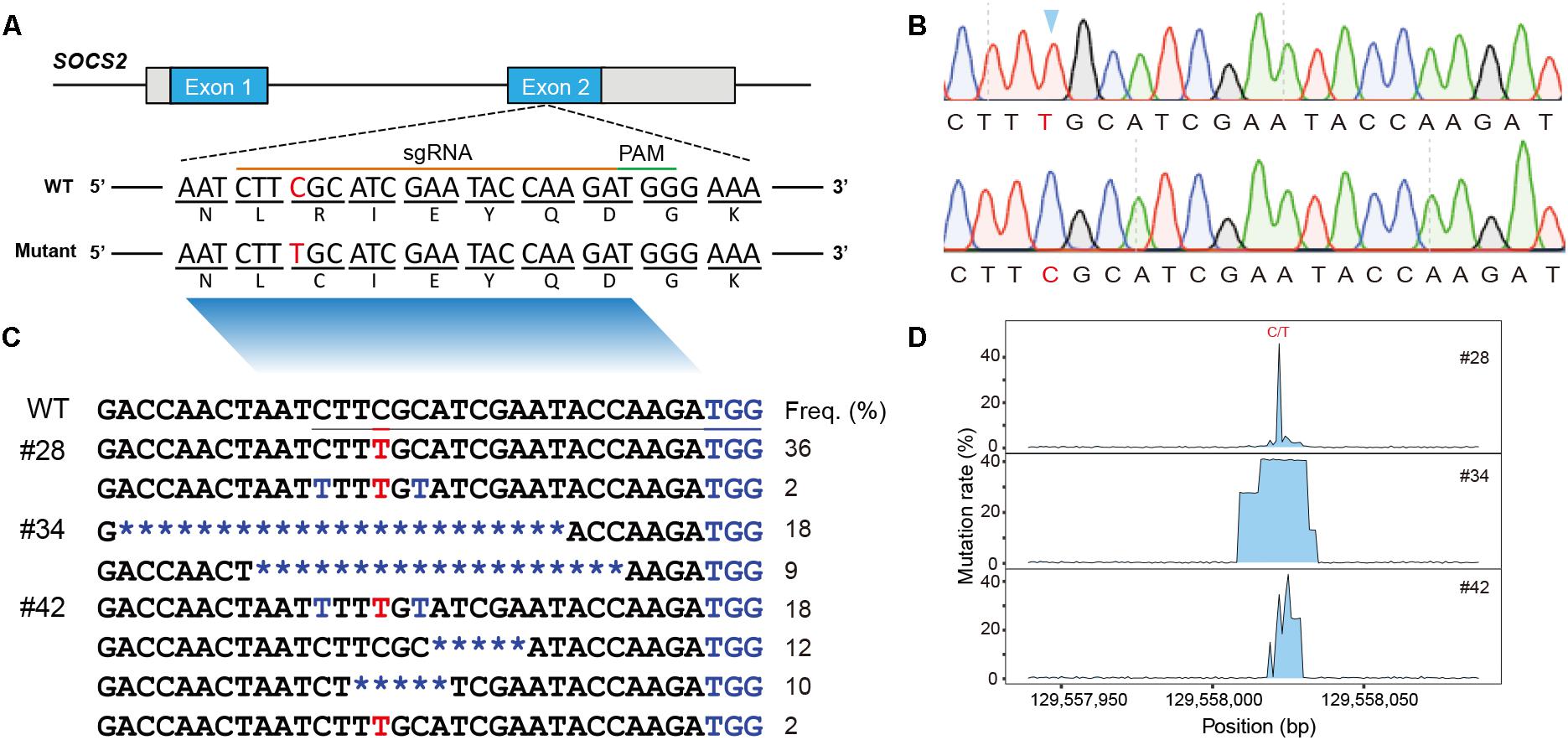
Figure 1. Animals and mutational spectra in the edited goat genome. (A) Scheme showing the target site in the sheep SOCS2 gene. sgRNA sequences are presented in yellow. PAM sequences are highlighted in green. The BE3-mediated nucleotide substitutions (g.C1901T, p.R96C) are highlighted. (B) Sanger sequencing chromatogram of intended mutations directed by the BE3 system. (C) Genotypes of target sites derived from deep sequencing in the three founder animals. (D) Mutation rate at the targeted region.
Genomic DNA was isolated from the blood samples of the four lambs (#28, #34, #41, and #42) and the targeted region was evaluated by PCR-based Sanger sequencing; this analysis confirmed that three animals (#28, #34, and #42) were edited at the target site (Supplementary Figure S1A). We then used TA cloning and sequencing to further validate the genotypes of the three edited animals, and a nucleotide substitution at the p.R96C mutation site was found in #28 and #42 (Supplementary Figure S1B). TA-cloned sequencing further revealed short indels in the edited animals, for example, the founder animal #34 had 19- and 23-bp deletions and #42 was mosaic with the defined point mutation and a 5-bp deletion (Supplementary Figure S1B). To fully screen the genotypes in the edited animals, these three edited animals were subject to targeted deep sequencing, which confirmed the TA-sequencing results and identified additional low incidence of C-T genotypes within the editing window (Figures 1B,C; Gehrke et al., 2018). We demonstrated that BE3-medicated modification in sheep led to a gene knockout animal (#34), apparent mosaics, and a low incidence of short indels in edited animals (Figure 1D). To further investigate the mosaicism in the BE-edited animals, we sequenced the modified loci in additionally biopsied tissues (tail, muscle, and skin) of the three animals (#28, #34, and #42). We identified same heterozygous genotypes in these tissues as observed in whole blood in #28 and #42 (Supplementary Figure S1A), indicating the genetic modification occurred during early embryogenesis. The non-specificity of the programmable deaminase BE3 often results in short indels and mosaicism (Kim D. et al., 2017; Park et al., 2017; Sasaguri et al., 2018). Efforts have been made to optimize DNA specificity and minimize bystander effects of BEs (Rees et al., 2017; Gehrke et al., 2018) or to develop advanced cytidine and adenine BEs with high efficiency (e.g., BE4max, AncBE4max, and ABEmax; Koblan et al., 2018).
Although the editing efficiency in this study was as high as 75.0% (3/4), we only generated one animal with the precise point substitution (#28, 25%, 1/4) (Table 1). The efficiency of precise single-base substitution was equivalent to that observed in goats (24%) (Niu et al., 2018) and sheep (Zhou et al., 2018), and was significantly higher than that in zebrafish (4%) (Armstrong et al., 2016). We expect to use a variety of newly invented BEs to improve the DNA specificity and diminish bystander effects in the editing window.
Phenotypes of Edited Animals
Subsequently, we analyzed the growth curve and body size of mutant and control lambs to assess whether the p.R96C mutation impaired the function of the SOCS2 protein associated with morphology. The body weight of three edited sheep (#28, #34, and #42) was higher than that in the control group on D0, D30, and D60; body length and height in modified sheep were higher than those in the control group (Figure 2). We did not observe clear phenotypic differences in the three edited animals that were related to their genotypes (substitutions, deletions, or both), even the body parameters of #42 was higher than in the other two animals (Figure 2). Considering the mosaicism in the edited animals, more phenotypic data from a long period is needed to address the correlation of mutation types to phenotypes. Nevertheless, these results are consistent with a spontaneous mutation in SOCS2 causing a 30–50% increase in the postnatal growth of mice (Horvat and Medrano, 2001), and the SOCS2 p.R96C mutation in sheep led to an increase body weight, body size, and milk yield (Rupp et al., 2015). Moreover, SOCS2 deletion protects bone heath in inflammatory bowel disease and causes a high-growth phenotype in mice (Horvat and Medrano, 2001; Dobie et al., 2018). We found that two animals (#34 and #42) with SOCS2 indels were as healthy as normal sheep, and we did not observe any health issues.
Figure 2. Observed morphological phenotypes in edited animals and controls. (A) Body weight. (B) Body height. (C) Body length.
Off-Target Mutations in Edited Animals
To characterize off-target effects induced by the BE system, a deep sequencing assay was used to amplify predicted off-target sites in all the three edited animals. Five off-target sites (OT1–OT5) were predicted using the SeqMap tool (Jiang and Wong, 2008) (Supplementary Table S5). Targeted deep sequencing revealed that the frequency of BE3-induced point mutations is low at all predicted sites in all three founder animals (#28, #34, and #42) (Figure 3), indicating that the incidence of BE3-induced off-target mutations is rare.
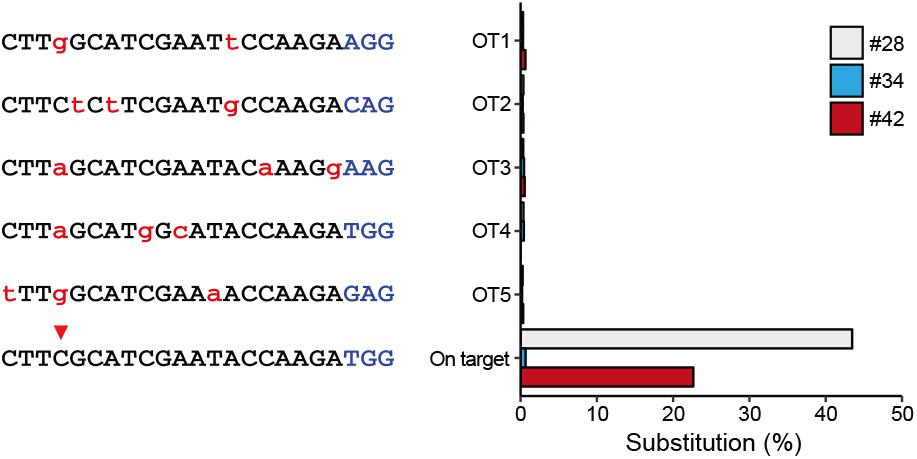
Figure 3. Detection of potential off-targeted sites by deep sequencing. Five potential off-targeted sites (OT1–OT5) were predicted by Cas-OFFinder. Deep sequencing was used to determine substitution frequencies at predicted target sites for the three founder animals. Mismatched nucleotide and PAM sequences are indicated in red and in blue, respectively.
To further characterize off-target mutations at the whole-genome scale, we conducted family trio-based WGS to assess off-targets and de novo mutations in the three edited animals (#28, #34, and #42) (Figure 4A). We calculated the kinship coefficient for pair-wised animals to guarantee the pedigree information (Supplementary Table S6). The WGS yielded an average sequence coverage of 37.3× per individual, within a range of 34- to 41-fold, and generated 12–13 million SNPs for each animal (Supplementary Table S7). SNPs were first called by both GATK and SAMtools, and an average of 16 million SNPs were identified for each founder. Of the SNPs we were able to map in this study, we next removed naturally occurring variants in the NCBI sheep SNP database (>59 million SNPs) and in our own sheep SNP database (>79 million SNPs from 294 individuals) and filtered out SNPs that were inherited from parents, resulting in ∼37,000 remaining SNPs for each founder animal. We then excluded base substitutions including SNP types C to T/A/G and their antisense type G to A/T/C according to a recent study (Kim D. et al., 2017). Subsequently, we assessed the remaining SNPs that were within the predicted off-target sites (tolerant to five mismatches) (Supplementary Table S8) using Cas-OFFinder (Bae et al., 2014), and no single variants were identified (Figure 4B), indicating that no off-target mutations were induced by BE3 in the present study. The detailed filtering procedure is summarized in Supplementary Table S9.
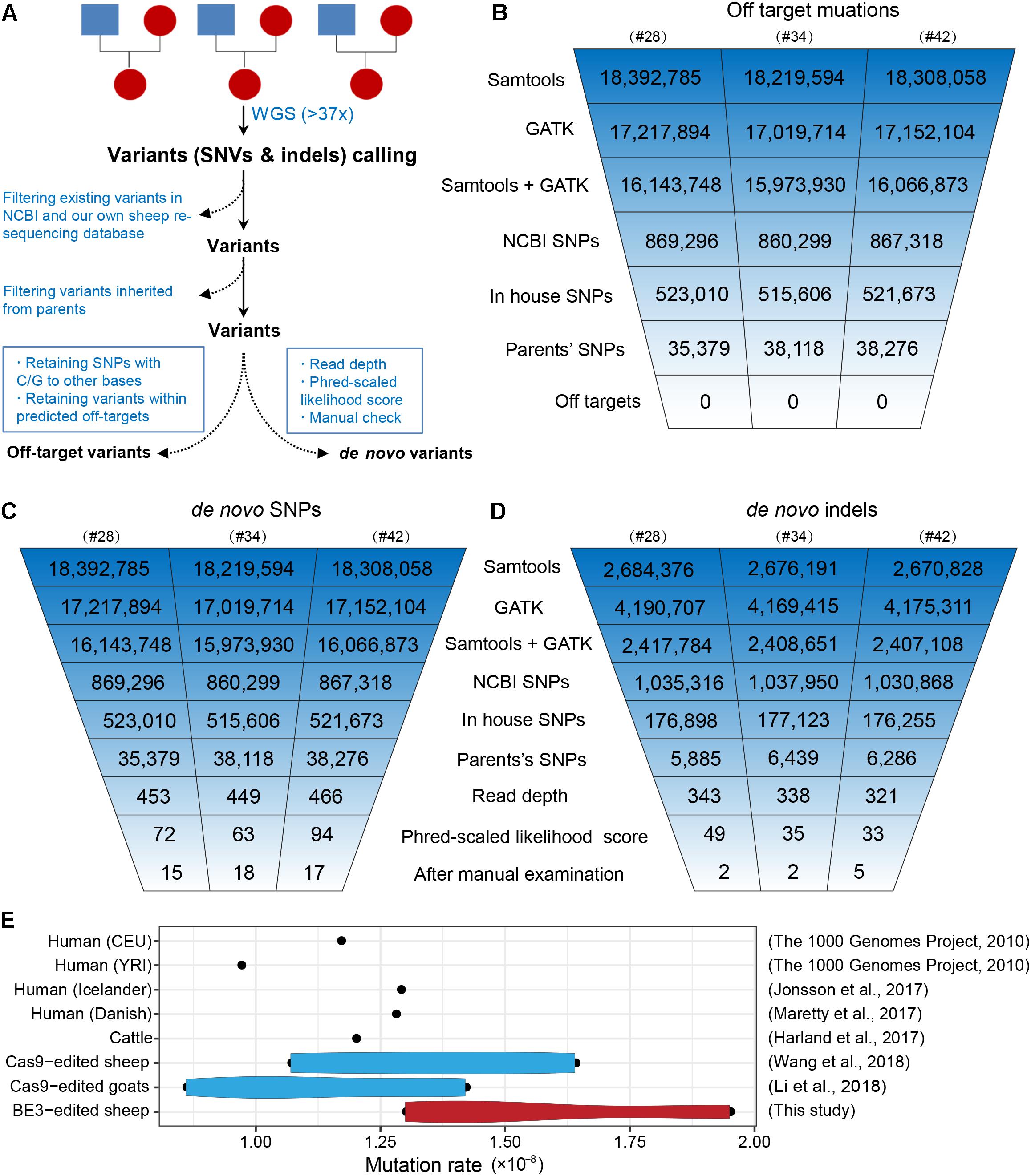
Figure 4. Identification of the genome-wide off-targets and de novo mutations by trio-based WGS. (A) Schematic representation of the study design for the identification of genome-wide off-target variants and de novo mutations. Summary of the filtering process of off-target mutations (B), de novo SNPs (C), and de novo indels (D). (E) Mutation rates in BE3-edited sheep and other populations.
To identify de novo mutations (SNPs and indels) in the edited animals, we used a stringent pipeline for variant filtering, as previously described (Li C. et al., 2018; Wang et al., 2018). Briefly, we selected SNPs that were identified by both GATK and SAMtools and removed existing SNPs in both the NCBI SNP database and our own sheep SNP database as well as the SNPs found in their parents. Next, we filtered out SNPs according to sequence read depth, PL scores, and manual examination of the FASTQ files (Wang et al., 2018). The remaining 15, 18, and 17 SNPs in individuals #28, #34, and #42 were identified as de novo SNPs for each progeny (Figure 4C). We next validated these de novo SNPs with Sanger sequencing. Of the 46 successfully amplified and sequenced SNPs, 44 of them were determined as true variants (Supplementary Figure S2 and Supplementary Table S10), indicating the pipeline for identification of de novo SNPs was robust. Similarly, we were able to identify 5, 5, and 10 de novo indels in individuals #28, #34, and #42, respectively (Figure 4D and Supplementary Table S11). To further characterize the large-scale genomic alterations induced by base editing, we called the SVs by BreakDancer (Chen et al., 2009), and identified a total of ten de novo SVs in the three BE-mediated animals (Supplementary Tables S12, S13), none of these variants were adjacent to the SOSC2 site.
Additionally, we estimated the mutation rates per base pair per generation (Li C. et al., 2018), and found that no apparent differences between the BE3 and control animals in term of frequency of de novo SNPs in the present study and former studies (Figure 4E). Albeit only three trios were analyzed in this study, the mutation rate in base-edited sheep was equivalent to that in human populations (1000 Genomes Project Consortium et al., 2010; Maretty et al., 2017), cattle (Harland et al., 2017), as well as our previously generated CRISPR/Cas9-edited sheep and goat populations (Li C. et al., 2018; Wang et al., 2018). Along with our previous studies reporting the de novo mutations in edited animals and their offspring (Li C. et al., 2018; Li G. et al., 2018; Wang et al., 2018) and recently two trio-based studies in mice (Iyer et al., 2018; Willi et al., 2018), we demonstrate that the mutation frequency does not differ in Cas9-mediated or BE-mediated animals, thereby providing evidence to support the reliability of genome editing in large animals for biomedicine and agriculture.
Conclusion
In summary, a single sheep carrying the SOCS2 p.R96C mutation was successfully generated using programmable deaminases BE3. We confirmed that BE3 did not induce unintended off-target mutations at the genome-wide scale, and the mutation frequency in BE-mediated animals was equivalent to those in Cas9-edited animals and in natural populations. This study facilitates gene correction and genetic improvement of large animals caused by single base mutations.
Data Availability
All relevant results are within the paper and its Supplementary Data Files. The raw WGS was available at NCBI SRA database under BioProject ID: PRJNA505205.
Author Contributions
SZ, XZ, XW, BM, and YC conceived the study. BC, YW, QD, XZ, JL, YL, YD, GL, HY, and BM performed the experiments. CH, CL, and XW analyzed the dataset. QK and WN provided samples. XW, TS, and BP wrote the article.
Funding
This work was supported by the National Natural Science Foundation of China (31772571), and Local Grants (NXTS20118-001, 2017NY-072, and 2018KJXX-009). XW is a Tang Scholar at Northwest A&F University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00215/full#supplementary-material
Footnotes
References
1000 Genomes Project Consortium, Durbin, R. M., Altshuler, D., Durbin, R. M., Abecasis, G. R., Bentley, D. R., et al. (2010). A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073. doi: 10.1038/nature09534
Armstrong, G. A. B., Liao, M., You, Z., Lissouba, A., Chen, B. E., and Drapeau, P. (2016). Homology directed knockin of point mutations in the zebrafish tardbp and fus genes in ALS using the CRISPR/Cas9 System. PLoS One 11:e0150188. doi: 10.1371/journal.pone.0150188
Bae, S., Park, J., and Kim, J.-S. (2014). Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475. doi: 10.1093/bioinformatics/btu048
Billon, P., Bryant, E. E., Joseph, S. A., Nambiar, T. S., Hayward, S. B., Rothstein, R., et al. (2017). CRISPR-mediated base editing enables efficient disruption of eukaryotic genes through induction of STOP codons. Mol. Cell 67, 1068–1079. doi: 10.1016/j.molcel.2017.08.008
Chen, K., Wallis, J. W., McLellan, M. D., Larson, D. E., Kalicki, J. M., Pohl, C. S., et al. (2009). Breakdancer: an algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 6, 677–681. doi: 10.1038/nmeth.1363
Chu, V. T., Weber, T., Wefers, B., Wurst, W., Sander, S., Rajewsky, K., et al. (2015). Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548. doi: 10.1038/nbt.3198
Dobie, R., MacRae, V. E., Pass, C., Milne, E. M., Ahmed, S. F., and Farquharson, C. (2018). Suppressor of cytokine signaling 2 (Socs2) deletion protects bone health of mice with DSS-induced inflammatory bowel disease. Dis. Model. Mech. 11:dmm028456. doi: 10.1242/dmm.028456
Gehrke, J. M., Cervantes, O., Clement, M. K., Wu, Y., Jing, Z., Bauer, D. E., et al. (2018). An APOBEC3A-Cas9 base editor with minimized bystander and off-target activities. Nat. Biotechnol. 36, 977–982. doi: 10.1038/nbt.4199
Harland, C., Charlier, C., Karim, L., Cambisano, N., Deckers, M., Mni, M., et al. (2017). Frequency of mosaicism points towards mutation-prone early cleavage cell divisions. bioRxiv [Preprint]. doi: 10.1101/079863
Horvat, S., and Medrano, J. F. (2001). Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics 72, 209–212. doi: 10.1006/geno.2000.6441
Inagaki-Ohara, K., Mayuzumi, H., Kato, S., Minokoshi, Y., Otsubo, T., Kawamura, Y. I., et al. (2014). Enhancement of leptin receptor signaling by SOCS3 deficiency induces development of gastric tumors in mice. Oncogene 33, 74–84. doi: 10.1038/onc.2012.540
Iyer, V., Boroviak, K., Thomas, M., Doe, B., Riva, L., Ryder, E., et al. (2018). No unexpected CRISPR-Cas9 off-target activity revealed by trio sequencing of gene-edited mice. PLoS Genet. 14:e1007503. doi: 10.1371/journal.pgen.1007503
Jiang, H., and Wong, W. H. (2008). SeqMap: mapping massive amount of oligonucleotides to the genome. Bioinformatics 24, 2395–2396. doi: 10.1093/bioinformatics/btn429
Jiang, Y., Xie, M., Chen, W., Talbot, R., Maddox, J. F., Faraut, T., et al. (2014). The sheep genome illuminates biology of the rumen and lipid metabolism. Science 344, 1168–1173. doi: 10.1126/science.1252806
Kim, D., Lim, K., Kim, S. T., Yoon, S. H., Kim, K., Ryu, S. M., et al. (2017). Genome-wide target specificities of CRISPR RNA-guided programmable deaminases. Nat. Biotechnol. 35, 475–480. doi: 10.1038/nbt.3852
Kim, K., Ryu, S. M., Kim, S. T., Baek, G., Kim, D., Lim, K., et al. (2017). Highly efficient RNA-guided base editing in mouse embryos. Nat. Biotechnol. 35, 435–437. doi: 10.1038/nbt.3816
Koblan, L. W., Doman, J. L., Wilson, C., Levy, J. M., Tay, T., Newby, G. A., et al. (2018). Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846. doi: 10.1038/nbt.4172
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A., and Liu, D. R. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. doi: 10.1038/nature17946
Komor, A. C., Zhao, K. T., Packer, M. S., Gaudelli, N. M., Waterbury, A. L., Koblan, L. W., et al. (2017). Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: a base editors with higher efficiency and product purity. Sci. Adv. 3:eaao4774. doi: 10.1126/sciadv.aao4774
Kuscu, C., Parlak, M., Tufan, T., Yang, J. K., Szlachta, K., Wei, X. L., et al. (2017). CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat. Methods 14, 710–712. doi: 10.1038/nmeth.4327
Letellier, E., and Haan, S. (2016). SOCS2: physiological and pathological functions. Front. Biosci. 8, 189–204. doi: 10.2741/e760
Li, C., Zhou, S., Li, Y., Li, G., Ding, Y., Li, L., et al. (2018). Trio-based deep sequencing reveals a low incidence of off-target mutations in the offspring of genetically edited goats. Front. Genet. 9:449. doi: 10.3389/fgene.2018.00449
Li, G., Zhou, S., Li, C., Cai, B., Yu, H., Ma, B., et al. (2018). Base pair editing of goat embryos: nonsense codon introgression into FGF5 to improve cashmere yield. bioRxiv [Preprint]. doi: 10.1101/348441
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with burrows–wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Maretty, L., Jensen, J. M., Petersen, B., Sibbesen, J. A., Liu, S., Villesen, P., et al. (2017). Sequencing and de novo assembly of 150 genomes from Denmark as a population reference. Nature 548, 87–91. doi: 10.1038/nature23264
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., et al. (2010). The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. doi: 10.1101/gr.107524.110
Metcalf, D., Greenhalgh, C. J., Viney, E., Willson, T. A., Starr, R., Nicola, N. A., et al. (2000). Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 405, 1069–1073. doi: 10.1038/35016611
Niu, Y., Jin, M., Li, Y., Li, P., Zhou, J., Wang, X., et al. (2017). Biallelic beta-carotene oxygenase 2 knockout results in yellow fat in sheep via CRISPR/Cas9. Anim. Genet. 48, 242–244. doi: 10.1111/age.12515
Niu, Y., Zhao, X., Zhou, J., Li, Y., Huang, Y., Cai, B., et al. (2018). Efficient generation of goats with defined point mutation (I397V) in GDF9 through CRISPR/Cas9. Reprod. Fertil. Dev. 30, 307–312. doi: 10.1071/rd17068
Paquet, D., Kwart, D., Chen, A., Sproul, A., Jacob, S., Teo, S., et al. (2016). Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125–129. doi: 10.1038/nature17664
Park, D. S., Yoon, M., Kweon, J., Jang, A. H., Kim, Y., and Choi, S. C. (2017). Targeted base editing via RNA-guided cytidine deaminases in xenopus laevis embryos. Mol. Cells 40, 823–827. doi: 10.14348/molcells.2017.0262
Rees, H. A., Komor, A. C., Yeh, W.-H., Caetano-Lopes, J., Warman, M., Edge, A. S. B., et al. (2017). Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun. 8:15790. doi: 10.1038/ncomms15790
Rupp, R., Senin, P., Sarry, J., Allain, C., Tasca, C., Ligat, L., et al. (2015). A point mutation in suppressor of cytokine signalling 2 (Socs2) increases the susceptibility to inflammation of the mammary gland while associated with higher body weight and size and higher milk production in a sheep model. PLoS Genet. 11:e1005629. doi: 10.1371/journal.pgen.1005629
Sakuma, T., Nakade, S., Sakane, Y., Suzuki, K. T., and Yamamoto, T. (2016). MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat. Protoc. 11, 118–133. doi: 10.1038/nprot.2015.140
Sander, J. D., and Joung, J. K. (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. doi: 10.1038/nbt.2842
Sasaguri, H., Nagata, K., Sekiguchi, M., Fujioka, R., Matsuba, Y., Hashimoto, S., et al. (2018). Introduction of pathogenic mutations into the mouse Psen1 gene by Base Editor and Target-AID. Nat. Commun. 9:2892. doi: 10.1038/s41467-018-05262-w
Shen, B., Zhang, J., Wu, H., Wang, J., Ma, K., Li, Z., et al. (2013). Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723. doi: 10.1038/cr.2013.46
Wang, X., Liu, J., Niu, Y., Li, Y., Zhou, S., Li, C., et al. (2018). Low incidence of SNVs and indels in trio genomes of Cas9-mediated multiplex edited sheep. BMC Genome 19:397. doi: 10.1186/s12864-018-4712-z
Wang, X., Niu, Y., Zhou, J., Yu, H., Kou, Q., Lei, A., et al. (2016). Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Sci. Rep. 6:32271. doi: 10.1038/srep32271
Wang, X., Yu, H., Lei, A., Zhou, J., Zeng, W., Zhu, H., et al. (2015). Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Sci. Rep. 5:13878. doi: 10.1038/srep13878
Willi, M., Smith, H. E., Wang, C., Liu, C., and Hennighausen, L. (2018). Mutation frequency is not increased in CRISPR-Cas9-edited mice. Nat. Methods 15, 756–758. doi: 10.1038/s41592-018-0148-2
Yang, H. L., Sun, C., Sun, C., and Qi, R. L. (2012). Effect of suppressor of cytokine signaling 2 (SOCS2) on fat metabolism induced by growth hormone (GH) in porcine primary adipocyte. Mol. Biol. Rep. 39, 9113–9122. doi: 10.1007/s11033-012-1783-9
Zhou, S., Yu, H., Zhao, X., Cai, B., Ding, Q., Huang, Y., et al. (2018). Generation of gene-edited sheep with a defined Booroola fecundity gene (FecBB) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9. Reprod. Fertili. Dev. 30, 1616–1621. doi: 10.1071/RD18086
Keywords: base editing, genome editing, point mutation, whole genome sequencing, off-target mutation
Citation: Zhou S, Cai B, He C, Wang Y, Ding Q, Liu J, Liu Y, Ding Y, Zhao X, Li G, Li C, Yu H, Kou Q, Niu W, Petersen B, Sonstegard T, Ma B, Chen Y and Wang X (2019) Programmable Base Editing of the Sheep Genome Revealed No Genome-Wide Off-Target Mutations. Front. Genet. 10:215. doi: 10.3389/fgene.2019.00215
Received: 16 November 2018; Accepted: 27 February 2019;
Published: 15 March 2019.
Edited by:
David Jay Segal, University of California, Davis, United StatesReviewed by:
Pavel Georgiev, Institute of Gene Biology (RAS), RussiaNathan Ellis, The University of Arizona, United States
Copyright © 2019 Zhou, Cai, He, Wang, Ding, Liu, Liu, Ding, Zhao, Li, Li, Yu, Kou, Niu, Petersen, Sonstegard, Ma, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohua Ma, bWFiaEBud2FmdS5lZHUuY24= Yulin Chen, Y2hlbnl1bGluQG53YWZ1LmVkdS5jbg== Xiaolong Wang, eGlhb2xvbmd3YW5nQG53YWZ1LmVkdS5jbg==
†Co-first authors
 Shiwei Zhou1†
Shiwei Zhou1† Chong He
Chong He Honghao Yu
Honghao Yu Bjoern Petersen
Bjoern Petersen Tad Sonstegard
Tad Sonstegard Xiaolong Wang
Xiaolong Wang