
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 12 November 2018
Sec. Applied Genetic Epidemiology
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00525
 Amy Elizabeth Howell1*
Amy Elizabeth Howell1* Jie Zheng2
Jie Zheng2 Philip C. Haycock2
Philip C. Haycock2 Alexandra McAleenan3
Alexandra McAleenan3 Caroline Relton2
Caroline Relton2 Richard M. Martin2
Richard M. Martin2 Kathreena M. Kurian1
Kathreena M. Kurian1Gliomas are a group of primary brain tumors, the most common and aggressive subtype of which is glioblastoma. Glioblastoma has a median survival of just 15 months after diagnosis. Only previous exposure to ionizing radiation and particular inherited genetic syndromes are accepted risk factors for glioma; the vast majority of cases are thought to occur spontaneously. Previous observational studies have described associations between several risk factors and glioma, but studies are often conflicting and whether these associations reflect true casual relationships is unclear because observational studies may be susceptible to confounding, measurement error and reverse causation. Mendelian randomization (MR) is a form of instrumental variable analysis that can be used to provide supporting evidence for causal relationships between exposures (e.g., risk factors) and outcomes (e.g., disease onset). MR utilizes genetic variants, such as single nucleotide polymorphisms (SNPs), that are robustly associated with an exposure to determine whether there is a causal effect of the exposure on the outcome. MR is less susceptible to confounding, reverse causation and measurement errors as it is based on the random inheritance during conception of genetic variants that can be relatively accurately measured. In previous studies, MR has implicated a genetically predicted increase in telomere length with an increased risk of glioma, and found little evidence that obesity related factors, vitamin D or atopy are causal in glioma risk. In this review, we describe MR and its potential use to discover and validate novel risk factors, mechanistic factors, and therapeutic targets in glioma.
Malignant gliomas are responsible for approximately 80% of all malignant brain tumors, with glioblastoma being the most prevalent histological subtype (Ostrom et al., 2014) (∼45% of all gliomas Ostrom et al., 2014; Visser et al., 2015). Although glioma is a relatively rare cancer, with ∼9,200 cases diagnosed each year in the United Kingdom (Cancer Research United Kingdom, 2015), the disease poses a serious health burden owing to its poor prognosis. The heterogeneous nature of the tumor cells makes the vast majority of gliomas surgically incurable (Kelly, 2010). Additionally, difficulty is faced as therapeutic agents need to penetrate the blood brain barrier (Azad et al., 2015). As a result the median survival rate of grade III gliomas is two to 5 years (Wen and Kesari, 2008) and just 15 months for glioblastoma (WHO grade IV) (Stupp et al., 2009). The 5-year survival for glioma varies, from approximately 58% for ependymoma patients to approximately 5% for glioblastoma patients (Ostrom et al., 2014; Visser et al., 2015; Cancer Research United Kingdom, 2016).
The only environmental factor consistently associated with glioma risk is moderate to high exposure to ionizing radiation, accounting for only a small proportion of cases (Bondy et al., 2008; Braganza et al., 2012; Urbanska et al., 2014). Evidence was first provided from the Israeli Tinea Capitus cohort of children who had undergone radiation therapy for a benign medical condition (Sadetzki et al., 2005). This was supported by data from the Childhood Cancer Survivor Study that followed-up 14,361 children and adolescents (aged < 21 at initial diagnosis) who had survived for 5 years (Neglia et al., 2006). During follow-up, 40 gliomas were diagnosed, compared to an anticipated incidence of 4.62 (standardized incidence ratios (SIR) = 8.66, 95% confidence interval (CI) 6.24–11.6). These gliomas arose at a median of 9 years after original diagnosis. In a case-control analysis (with 4 controls per case, matched on age at diagnosis, sex and time since diagnosis, and the analysis adjusted for original cancer diagnosis) the odds ratio (OR) for glioma amongst children who underwent radiation therapy vs. those who did not was 6.78 (95% CI 1.54–29.7) (Neglia et al., 2006). The authors found that the risk of glioma per Gray of radiation was greatest among children who received radiation therapy at less than 5 years of age. After adjustment for radiation dose, neither original cancer diagnosis nor chemotherapy was associated with risk (Neglia et al., 2006). Taylor et al. (2010) carried out a study of 17,980 participants who had survived at least 5 years after diagnosis of childhood cancer. In this study the risk of glioma increased linearly with dose of radiation (Taylor et al., 2010).
Rarely, glioma occurs in more than one family member, indicating a genetic susceptibility. This susceptibility is most often described within cases where inherited tumor syndromes are present, such as Li-Fraumeni syndrome, Turcot syndrome and neurofibromatosis type 1 (Louis et al., 2016). Kinnersley et al. (2018) reviewed glioma genome wide association study (GWAS) and summarized reported associations at the 27 glioma-risk SNPs (Kinnersley et al., 2018); genetic susceptibility loci are summarized in Table 1. These risk variants contribute to an increase in glioma risk; however, additional somatic mutations are a requisite for tumorigenesis in individuals with these germline variants or familial syndromes (Rice et al., 2016).
There have been several risk factors that have been linked to the occurrence of glioma, though results from these investigations may be spurious because of the biases that pervade observational studies (Louis et al., 2016). A recently published systematic review presents risk factors for glioma onset that are shown to increase, decrease or have a null association with glioma risk (Quach et al., 2017).
Observational studies suggest that allergies (asthma, eczema, hay fever) are associated with lower glioma risk (Wigertz et al., 2007; Berg-Beckhoff et al., 2009; Amirian et al., 2016; Wang et al., 2016) and, consistent with this, asthma-susceptibility genotypes are associated with a reduced risk of glioma (Schwartzbaum et al., 2005). Short term use of anti-inflammatory medicine has also been reported to reduce glioma risk (Scheurer et al., 2011); although other studies have found conflicting results (Daugherty et al., 2011; Gaist et al., 2013). The possible role of allergies in decreasing the risk of glioma, including glioblastoma, may be due to an increase in immune surveillance, which in turn may destroy damaged, pro-cancerous cells earlier (Scheurer et al., 2011; Safaeian et al., 2013; Zhao et al., 2014). This hypothesis is supported by reports of a higher occurrence of glioma in HIV and AIDS patients (Blumenthal et al., 1999; Jukich et al., 2001; Hall and Short, 2009); as this is based on the result from a small number of studies with small sample sizes the estimate may be biased.
Brain tumors are observed to occur more often in Europeans compared with individuals of an African or Asian origin (McLendon et al., 1985; Kuratsu et al., 2001; Darefsky and Dubrow, 2009; Ostrom et al., 2013), an observation that has also been reported within children. Robertson et al. (2002) investigated ethnic variation in the incidence of adult brain cancer in 994,725 individuals over 10.5 years of follow-up. The authors identified 373 people who developed brain cancer (232 glioblastomas, 106 astrocytomas and 35 oligodendrogliomas) of whom 50 were of African ancestry and 323 of European ancestry. Age adjusted incidence rates (per 100,000 race specific-population/year) were 0.11 and 0.46 (p = 0.003) in the African and European populations, respectively. The authors report a significant difference in incidence rates for the three most common gliomas and suggest that glioma is more common in individuals of European ancestry than in individuals of African ancestry (Robertson et al., 2002). Other studies have reported that glioma occurs 3.5 times more often in Europeans compared to African Americans (Davis et al., 1999). The explanation for this observed ethnic discrepancy remains unclear and while it is possible that a genetic difference exists between the two groups (Mochizuki et al., 1999; Chen et al., 2001; Das et al., 2002), detection bias cannot be ruled out (Dubrow and Darefsky, 2011).
Certain occupations are reported to be linked with a higher risk of glioma, including physicians (Carozza et al., 2000; Krishnan et al., 2003; Pukkala et al., 2009), firefighters (Carozza et al., 2000; Krishnan et al., 2003) and farmers (Khuder et al., 1998; Zheng et al., 2001). Occupational exposure to metals such as arsenic and lead has attracted attention with respect to brain tumors as they are able to penetrate the blood brain barrier (Sunderman, 2001; Wang and Du, 2013; Liao et al., 2016). Exposure to lead has been associated with glioma risk (Anttila et al., 1996; van Wijngaarden and Dosemeci, 2006) and brain cancer mortality (Cocco et al., 1998; van Wijngaarden and Dosemeci, 2006). In a cohort study of 1,779,646 men and 1,066,346 women aged 25–64 years at baseline and subsequently followed for 19 years, an increased glioma risk was observed amongst men exposed to arsenic, mercury, and petroleum products (Navas-Acien et al., 2002). However, no relationship of lead, cadmium, nickel, chromium and iron with glioma risk was reported in a study of 1856 cases and 5189 controls (Parent et al., 2017). Other studies investigating the relationship between glioma and occupational exposure to metal (Samkange-Zeeb et al., 2010) or lead (Rajaraman et al., 2006; Bhatti et al., 2009), and between brain cancer more generally and lead (Lam et al., 2007) reported no strong evidence of a causal association.
There has been speculation that certain lifestyle choices, including alcohol intake, the use of drugs, or dietary exposure to nitrous compounds affect the risk of glioma; however, to date the evidence is inconclusive (Giles et al., 1994; Michaud et al., 2009; Kyritsis et al., 2011; Little et al., 2013; Shao et al., 2016; Tamimi and Juweid, 2017).
Mobile phone use has been speculated to be associated with brain tumor risk (Schüz et al., 2006). However, conflicting finding have also been reported (Frei et al., 2011). In a nationwide study involving Danish citizens aged 30 years or older (born after 1925), there was no evidence that mobile phone use increased brain tumor risk (Frei et al., 2011).
Other risk factors that are not discussed here have been investigated in relation to glioma risk, including but not limited to: Type 1 and type 2 diabetes, body mass index, birth weight, hypertension, height, birth weight, menarche (age at onset), menopause (age at onset), coffee/caffeine consumption, low-density lipoprotein cholesterol, insulin-like growth factor 1, insulin-like growth factor binding protein, triglycerides, high-density lipoprotein cholesterol, pesticide exposure, extremely low frequency magnetic fields, vitamin E, A and C levels (Preston-Martin and Mack, 1991; Kaplan et al., 1997; Houben et al., 2004; Linos et al., 2007; Holick et al., 2010; Kabat et al., 2011; Little et al., 2013; Malerba et al., 2013; Lee et al., 2014; Andersen et al., 2015; Li et al., 2015; Zhou et al., 2015; Seliger et al., 2016a,b; Zhao et al., 2016; Wiedmann et al., 2017).
As described above, and in common with many other diseases, the search for risk factors for glioma has largely been based on observational cohort, case-control and cross-sectional studies (Lawlor et al., 2004). Numerous cases exist of seemingly robust observational associations between putative risk factors and disease outcomes; however, interventions to modify these risk factors do not produce the anticipated benefits under randomized controlled trial (RCT) conditions (Davey Smith and Hemani, 2014). One of the postulated reasons for this is the susceptibility of observational (non-experimental) studies to several biases (specifically, confounding, measurement error and reverse causation) that can generate spurious associations and which can be difficult to eradicate even through statistical adjustment (Davey Smith and Hemani, 2014).
A confounder is a factor that is a common cause of both the disease under consideration and the exposure of interest. Importantly, a confounder is not on the causal pathway between the exposure and outcome (Hammer et al., 2009). For instance, in 2002 an association had been established between alcohol intake and the incidence of 3.6% of all cancers (Boffetta et al., 2006; Testino, 2011) but it is still uncertain whether an association exists between any class of glioma and alcohol intake (Braganza et al., 2014; Qi et al., 2014). An observed association between glioma incidence and alcohol intake could be because individuals who consume more alcohol are more likely to smoke (Hart et al., 2010) and to adhere to an unhealthy life-style; (Sayon-Orea et al., 2011; Bendsen et al., 2013) thus, it could be these other factors that influence the risk of glioma rather than alcohol consumption per se (Sergentanis et al., 2015).
Reverse causation occurs when the disease outcome precedes, and leads to, the exposure rather than being a consequence of the exposure (Flegal et al., 2011). For example a higher level of blood glucose has been reported to be protective against glioma (Kitahara et al., 2014); however, an alternative explanation is that tumors take-up glucose, leading to low glucose levels (Schwartzbaum et al., 2017).
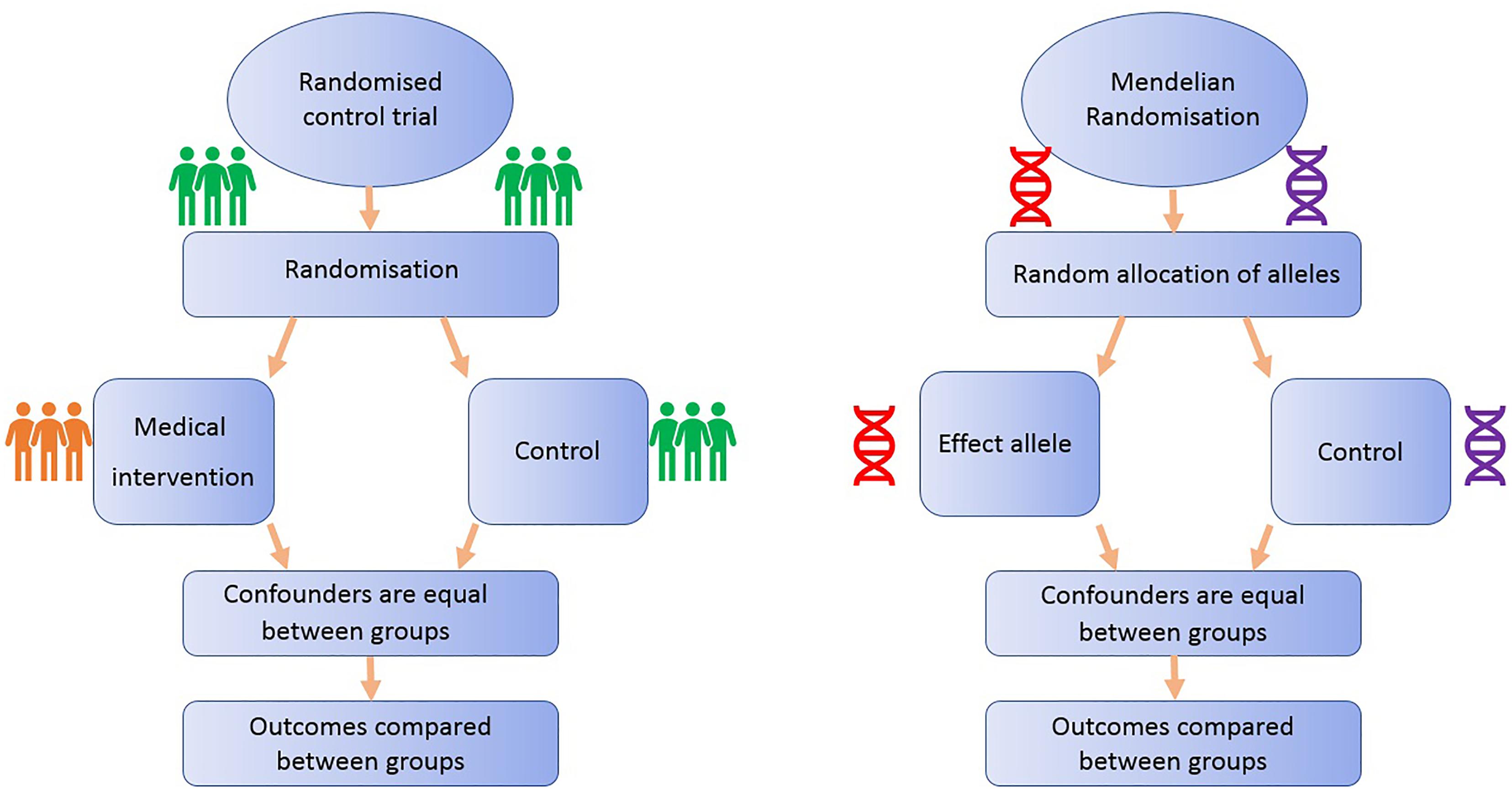
FIGURE 1. Comparison of Mendelian randomization (MR) with randomized control trial. This demonstrates the analogy between a randomized control trial and a Mendelian randomized study.
Randomized controlled trials are considered the gold standard study design for inferring causality, as successful randomization, adequately blinded implementation of the intervention, high rates of follow-up and intention-to-treat analysis should yield results that are relatively free from the biases afflicting observational studies (Iturrieta-Zuazo and Walter, 2015). On the other hand, RCTs often reflect short-term exposures at one time point in life, with limited follow-up, and participants are usually not representative of general populations, a particularly important issue if the priority is to identify primary prevention targets (Meyer, 2010). Additionally, due to ethical, practical and financial reasons, it is not feasible to randomize people to every risk factor (Bochud and Rousson, 2010): e.g., exposure to power lines, mobile phone use or breastfeeding.
One method to appraise causality within observational epidemiology is the use of Mendelian randomization (MR). MR is a type of “instrumental variable” analysis that utilizes genetic variants, such as SNPs, that are robustly associated with an exposure as proxies for the risk factor of interest. The aim of MR is to strengthen causal inference in observational studies of associations between risk factors and disease (Lawlor et al., 2008).
All MR studies make use of germline genetic data as opposed to somatic data. Germline genetic variants tend to be randomly distributed with respect to most human traits in the general population. This is because of Mendel’s laws of inheritance (segregation, independent assortment) and the fixed nature of germline genotypes (Castle, 1903). Thus, germline genetic variants are less likely to be affected by the sorts of confounding factors that typically bias observational findings (Qi, 2009). Additionally, as germline genotype cannot be affected by the presence of disease, the generation of spurious results through reverse causation is avoided (Larsson et al., 2017). Germline genetic variants can thus be regarded as randomized proxies for an exposure of interest, in the same way that the allocation group in an RCT is a proxy for an intervention of interest (Figure 1). MR can exploit SNPs that are associated with modifiable risk factors to strengthen causal inference about the nature of relationships between risk factors and disease (Larsson et al., 2017).
The application of MR involves three assumptions (Figure 2): (1) the genetic variants (“instruments”) are reliably associated with the risk factor of interest; (2) the genetic variants are independent of confounding factors (Didelez and Sheehan, 2007; VanderWeele et al., 2014); and (3) the genetic variants are only associated with the disease outcome through the risk factor of interest (Greenland, 2000; Lawlor et al., 2008). Within the constraints of these assumptions, genetic instruments (SNPs) can be used as proxies for a large range of cancer-related modifiable exposures. One-sample MR is the standard application of MR. There is one data set that contains all the data on the SNPs, exposure, and outcome for all participants (Haycock et al., 2016). Due to the rare nature of glioma, one-sample MR is likely to be statistically underpowered. As a result, MR techniques have been developed to allow analysis when genetic association studies are conducted in two separate samples sets: one set for the exposure of interest and one for the outcome (Inoue and Solon, 2010). This method is referred to as two-sample MR (Hartwig et al., 2016).
Like most diseases, glioma GWAS to date have examined genetic variation in relation to the causes of disease risk, using case-control study designs, as opposed to disease progression (Melin et al., 2017). The primary application of MR in glioma research has, therefore, focused primarily on causal effects of environmental exposures on disease risk (Walsh et al., 2015; Haycock et al., 2017; Disney-Hogg et al., 2018a; Takahashi et al., 2018), as opposed to survival. There are some instances where factors are involved in both disease incidence and progression, such as low-density lipoprotein cholesterol levels for heart disease risk and recurrence (Ference et al., 2017), although such instances may be exceptional. Cases do exist where a risk factor for a disease is not implicated in progression, as has been proposed for the relationship between folate consumption and colon cancer (Kim, 2003). Thus, current case-control GWAS of glioma risk have the potential to inform on the underlying causal mechanisms of disease onset but (at the current time) may be less informative for discovering drug targets to improve glioma survival (Paternoster et al., 2017). The latter requires case-only GWAS that examine genetic variation in relation to disease progression, but such studies are currently rare (Melin et al., 2017). The most probable explanation for this is due to a research focus to determine mechanisms that cause disease incidence and because of the challenges inherent in collecting progression data (see section “Future of MR in research” below). At present, a few MR studies have been conducted that investigate progression of disease (Brunner et al., 2017) but none in glioma progression, which is required for the discovery of targets for improving glioma survival (Paternoster et al., 2017).
Mendelian randomization can be used to identify and investigate potential drug targets (Mokry et al., 2015; Zheng et al., 2017). A quarter of the drugs that enter clinical development fail due to their ineffectiveness (Ashburn and Thor, 2004; Arrowsmith and Miller, 2013). Current drug targets are authenticated using in-vitro and animal models, but these can fail to predict the potential benefits (or harms) in humans (Mokry et al., 2015; Zheng et al., 2017). Nelson et al. (2015) aimed to establish whether current genetic evidence could predict drug mechanisms. The authors reported that opting for targets that are genetically supported may result in twice the success rate in clinical development (Nelson et al., 2015). MR could substantially augment these methods (Mokry et al., 2015; Zheng et al., 2017). The theory is that specific genetic variants can be utilized to imitate the effects of targeting a protein pharmacologically. If the variant codes for a potential drug target that causes an alteration in activity of the encoded protein, the causal effect of the drug on disease can be assessed by MR (Sofat et al., 2010; Evans and Smith, 2015). Additionally, MR can be used to examine all pairwise associations between serum protein levels and disease risk (Sun et al., 2018). If a variant is identified that is robustly associated with levels of a serum protein that display a putative causal relationship with disease risk, methods can be employed to search for available drugs that cause an alteration in the levels of that protein (Corsello et al., 2017). As discussed, only case-control GWAS exist at present for glioma which may be less informative for the discovery of drug targets to improve survival (Paternoster et al., 2017).
Table 2 provides a summary of some of the different methods used to obtain MR estimates (Hemani et al., 2018).
Mendelian randomization has widely recognized limitations (Glynn, 2010). For some exposures there is a lack of genetic variants (SNPs) available for instrumentation (Smith and Ebrahim, 2004). For example, ionizing radiation emitted by mobile phones has been suggested as a risk factor for glioma (Yang et al., 2017). However, currently no genetic variants have been associated with exposure (or response) to ionizing radiation and therefore MR analysis cannot be performed for this particular risk factor (Smith, 2010).
A key limitation of MR is pleiotropy (Sheehan et al., 2008). Pleiotropy occurs when a genetic variant has more than one effect. If one or more of these effects influence the outcome through pathways other than the exposure of interest (so called horizontal pleiotropy) a core MR assumption is violated, i.e., that variants only exert their effect on the outcome via their influence on the exposure of interest (Evans et al., 2013; Burgess, 2014; Bennett and Holmes, 2017; Yarmolinsky et al., 2017). Techniques have been developed, such as MR-Egger regression, that can quantify the amount of bias caused by horizontal pleiotropy, as well as providing a valid causal estimate despite the presence of horizontal pleiotropy (Bowden et al., 2015). Another type of pleiotropy that exists is vertical pleiotropy. This is where the genetic variants have associations with biomarkers that are downstream of the biomarker of interest (Bennett and Holmes, 2017). Thus, they are on the causal pathway and should be considered as intermediates of the relationship between an exposure and an outcome, not as confounding factors.
Mendelian randomization studies typically require large sample sizes, an issue that can be compounded by the rare nature of glioma. One way to increase power is to develop genetic risk scores that contain multiple alleles to explain more of the variance in the exposure of interest. This runs the risk of including invalid variants, such as those that do not exert their effect on the outcome via the exposure of interest (Evans et al., 2013; Burgess, 2014; Yarmolinsky et al., 2017), although such potential violations of the MR assumption can be formally tested using MR-Egger regression. Power can also be increased by using a two-sample approach, where large case-control GWAS can be used even if they have not measured the exposure of interest.
Limitations of MR have been discussed in detail in several published papers (Davey Smith and Ebrahim, 2003; Smith and Ebrahim, 2004; Lawlor et al., 2008; Sheehan et al., 2008; Bochud and Rousson, 2010; Davey Smith, 2011b; VanderWeele et al., 2014).
Two-sample MR is a method that can harness information from GWAS summary statistics and has been applied to the context of glioma to look at several risk factors. We discuss key studies that have used two-sample MR to investigate associations between previously reported risk factors and glioma (Table 3).
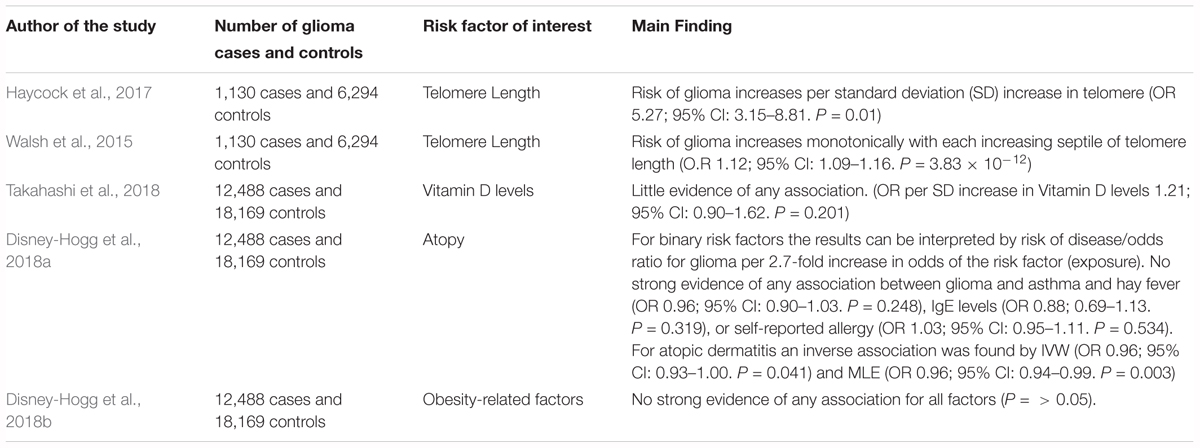
TABLE 3. Description of MR studies that have investigated the causal association between a factor and glioma risk.
An MR study to evaluate the causal relevance of telomere length on the risk of cancer and non-neoplastic diseases found that genetically predicted longer telomeres increased the risk of glioma, while being protective for certain non-neoplastic diseases, such as cardiovascular diseases (Zheng et al., 2017). The analysis employed summary genetic data for 35 cancers and 45 non-neoplastic diseases, including 1,130 glioma cases and 6,294 controls. The strongest association was for glioma (OR per SD increase in genetically predicted telomere length was 5.27; 95% CI: 3.15–8.81) (Zheng et al., 2017). A possible explanation for this observation is that telomere shortening may act as a tumor suppressor, restricting the proliferative potential of cells. Therefore, those with longer telomeres have a greater probability of obtaining somatic mutations due to an increased proliferative potential (Hanahan and Weinberg, 2011).
Walsh et al. (2015) also used an MR approach to establish whether a genotypically estimated longer or shorter telomere length was linked with an increased risk of glioma and whether inheritance of SNPs associated with telomere length are indicators of glioma risk. The authors accessed differences in genotypically estimated relative telomere length in a total of 1,130 glioma patients and 6,294 controls. The average approximated telomere length was 31bp (5.7%) longer in glioma cases compared with controls in discovery analyses (P = 7.82 × 10-8). This finding was supported in the replication analysis as the mean telomere length was 27 bp (5.0%) longer in glioma cases than controls (1.48 × 10-3). The authors reported that the risk of glioma increases monotonically with each increasing septile of telomere length (O.R 1.12; 95% CI: 0.90–1.62). Additionally, the authors reported that four telomere length-associated SNPs were significantly related with glioma risk in pooled analyses, including those in the telomerase component genes TERC (O.R 1.14; 95% C.I. = 1.03–1.28) and TERT (O.R 1.39; 95% C.I. = 1.27–1.52), and those in the CST complex genes OBFC1 (O.R 1.18; 95% C.I. = 1.05–1.33) and CTC1 (O.R 1.14; 95% C.I. = 1.02–1.28). The indication of risk alleles for glioma close to TERC and TERT that are also related with telomere length suggests that telomerase is important in glioma formation (Walsh et al., 2014).
Takahashi et al. (2018) used two-sample MR to investigate whether a causal relationship exists between circulating vitamin D and glioma risk, involving 12,488 glioma cases and 18,169 controls. The authors reported no strong evidence of a causal relationship between vitamin D and glioma when either the inverse-variance weighted (IVW) method (OR per SD increase 1.21, 95% CI: 0.90–1.62, P = 0.201) or the maximum likelihood estimation (MLE) method (OR per SD increase 1.20, 95% CI: 0.98–1.48, P = 0.083) was used (Takahashi et al., 2018).
Disney-Hogg et al. (2018a) used an MR approach to evaluate the observed inverse relationship between allergies and glioma risk. The instrumental variables were SNPs robustly associated with atopic dermatitis, asthma and hay fever, IgE levels, and self-reported allergy. The study involved 12,488 cases and 18,169 controls. The authors found no significant association between glioma and asthma, hay fever, IgE levels, or self-reported allergy. For atopic dermatitis an inverse association was found (OR per 2.7-fold increase in odds of atopic dermatitis) by the IVW (OR 0.96, 95% CI 0.93–1.00, P = 0.041) and MLE methods (OR 0.96, 95% CI 0.94–0.99, P = 0.003), but not for weighted median estimate (WME) and mode-based estimate (MBE) methods (Disney-Hogg et al., 2018a), suggesting that having atopic dermatitis reduces the risk of glioma.
Disney-Hogg et al. (2018b) carried out an MR analysis to interrogate the observed association between obesity-related factors and risk of glioma. The authors identified variants that were robustly associated with 10 key obesity-related factors: 2-h post-challenge glucose, BMI, fasting glucose, fasting insulin, HDL cholesterol, LDL cholesterol, type-2 diabetes, total cholesterol, triglycerides and waist-hip ratio. This study encompassed 12,488 cases and 18,169 controls. This study found little evidence that indicated that obesity-related factors contribute to glioma (Disney-Hogg et al., 2018b).
There are several different design strategies for MR that have been discussed in detail by Zheng et al. (2017). The potential application of these different MR study designs in glioma research are outlined below.
Improved knowledge of signaling pathways that are causally associated glioma incidence can be helpful to design preventative strategies and effective therapeutic targets (Wang et al., 2015). A useful MR strategy to establish whether a molecular intermediate plays a role in the causal pathway between a risk factor and disease is the use of two-step MR (Relton and Davey Smith, 2012). An improved understanding of the molecular changes that drive glioma formation will allow for opportunities to modify disease causing factors.
Bidirectional MR involves using instruments for both the exposure and the outcome to assess the direction of causality: i.e., does the exposure cause the outcome or does the outcome cause the exposure (Timpson et al., 2010). For instance, observational studies have suggested that there is an inverse association between allergies and glioma risk, but the direction and causality of the association remains uncertain: it is not clear whether allergies decrease the risk of glioma or whether the inverse association arises because of suppression of the immune system by glioma itself (Schoemaker et al., 2006).
There are cases in which genetic variants are related to numerous correlated phenotypes (Low, 2001), for example, genetic variants that associate with lipoprotein metabolism tend not to correlate with just one specific lipid fraction (Wurtz et al., 2013). As a result assessing the causal association of one specific intermediate phenotype with disease can be challenging (Davey Smith and Hemani, 2014). Multi-phenotype MR can be used in these cases (Davey Smith and Hemani, 2014; Burgess et al., 2015; Burgess and Thompson, 2015; Kemp et al., 2016). Multivariable MR can be applied to glioma research when testing the effect of lipids on glioma to identify the independent effect of each lipid subtypes on glioma.
Hypothesis-driven MR has huge potential in glioma research. Hypothesis-driven MR can validate the relationship between a risk factor and glioma for which a causal association has previously been reported.
In addition, hypothesis-free MR has the potential to identify novel causal associations. Hypothesis-free MR can be used to examine causality in complex frameworks in glioma, as a well as a method to data mine high-dimensional studies (Evans and Davey Smith, 2015). Haycock et al. (2017) implemented a mixture of hypothesis-driven and hypothesis-free MR to investigate the relationship between telomere length and 22 cancers and 32 primary non-neoplastic diseases.
Mendelian randomization-Base is a tool that improves the accessibility of GWAS summary data for MR research (Hemani et al., 2016). MR-Base can assist hypothesis-free testing as it allows researchers to examine all pairwise associations to data mine for causal relationships of interest (Davey Smith, 2011a). Where novel associations are identified, these associations can then be subjected to formal and extensive hypothesis-testing studies (Evans and Smith, 2015).
Factorial MR can be used to develop therapeutic strategies to improve glioma survival. Factorial RCT is where a participant is either assigned to a group that obtains neither intervention, one of the interventions, or both (Montgomery et al., 2003). In a factorial trial the separate effects of each intervention can be considered, as well as, the benefits of obtaining both interventions together (Montgomery et al., 2003). Similarly, factorial MR can be performed by using combinations of genetic variants to attain unconfounded estimates of the effect of co-occurrence of the two drug targets on disease (Davey Smith and Hemani, 2014). In glioma research if we have two drug targets and we want to know the combined effects of these two drugs on glioma, then we can apply factorial MR. Factorial MR can assess the antitumor efficacy of drug targets on glioma by investigating the combination of different targeted drugs (Reardon and Wen, 2006).
For GWAS and MR of glioma progression to be successful for the development of drug targets to improve glioma survival, large scale case-only studies will be required with both progression and germline genetic data. RCTs offer a potential reservoir of data for such studies; however, due to the rare nature of glioma, sample size is limited (Vuorinen et al., 2003; Gehring et al., 2009; U.S.National Library of Medicine, 2018). A limitation of progression studies is the introduction of collider bias, discussed in detail in Paternoster et al. (2017). Collider bias is problematic in MR of disease progression as a risk factor of interest that causes the disease may be correlated with other risk factors involved in incidence, and any association between the index risk factor and progression can be confounded by these correlated risk factors. If the problems of sample size and collider bias can be adequately overcome, GWAS and MR of disease progression offer a promising opportunity to identify new treatments for glioma that could enhance survival (Davey Smith et al., 2017). Additionally, an improved understanding of the molecular changes that drive glioma progression will allow for opportunities to develop targeted molecular therapies. At present, although there are some examples where targeted therapy responses have been recorded in glioma patients, no targeted therapy has been approved as an effective treatment in clinical trials (Touat et al., 2017).
Future research will involve hypothesis free MR, which make use of omics data. There is a growing body of evidence showing that epigenetic biomarkers of glioma can be used for prediction and prognosis. Notably in neuro-oncology the O6-methylguanine-DNA methyltransferase promoter methylation can act both a prognostic and predictive biomarker for glioblastoma (Esteller et al., 2000; Olson et al., 2011; Reifenberger et al., 2012; Wick et al., 2012). As genetic variants associated with DNA methylation seem to overlap with expression quantitative trait loci (eQTLs) at many loci throughout the genome (Bell et al., 2012; Shi et al., 2014), both DNA methylation and gene expression may exist on the causal pathway between genetic variation and disease. The ability to identify epigenetic and transcriptomic markers for glioma risk and progression could be important in understanding the underlying mechanisms of glioma. Using an MR approach, the causal chain between DNA methylation, gene expression and glioma onset/progression can be investigated (Relton and Davey Smith, 2012).
Given the lack of large-scale case-only studies with data on progression and germline genetic data, a priority of research in the near term should be to identify causes of glioma onset. The findings from such studies will be informative for the design of primary and secondary prevention strategies. The latter could be particularly valuable for glioma prevention in high risk populations, such as childhood cancer survivors (who received radiation therapy), people with genetic syndromes known to increase risk of glioma and people exposed to known causal factors because of their occupations. For example, if a specific dietary factor is found to be causally associated with a decrease in glioma risk, high risk populations could be advised to increase their consumption of that specific dietary factor.
Mendelian randomization offers a promising, novel way to identify risk factors and drug targets for glioma to both inform public health policy for prevention, as well as, allowing the development of therapeutic approaches to improve prognosis. The latter will require the development of large-scale case-only studies with data on progression and germline genetic data.
AH contributed to the manuscript research and writing. KK, PH, RM, CR, JZ, and AM reviewed and revised the manuscript.
This research was supported by Brain Tumour Bank and Research Fund and Southmead Hospital Charity. Charity registration number:1055900. RM, AM, and CR are supported by a Cancer Research United Kingdom programme grant, the Integrative Cancer Epidemiology Programme (Grant no. C18281/A19169). Dr. Haycock is supported by CRUK Population Research Postdoctoral Fellowship C52724/A20138.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Amirian, E. S., Zhou, R., Wrensch, M. R., Olson, S. H., Scheurer, M. E., Il’yasova, D., et al. (2016). Approaching a scientific consensus on the association between allergies and Glioma risk: a report from the glioma international case-control study. Cancer Epidemiol. Biomark. Prev. 25, 282–290. doi: 10.1158/1055-9965.epi-15-0847
Andersen, L., Friis, S., Hallas, J., Ravn, P., Kristensen, B. W., and Gaist, D. (2015). Hormonal contraceptive use and risk of glioma among younger women: a nationwide case-control study. Br. J. Clin. Pharmacol. 79, 677–684. doi: 10.1111/bcp.12535
Anttila, A., Heikkila, P., Nykyri, E., Kauppinen, T., Pukkala, E., Hernberg, S., et al. (1996). Risk of nervous system cancer among workers exposed to lead. J. Occup. Environ. Med. 38, 131–136. doi: 10.1097/00043764-199602000-00010
Arrowsmith, J., and Miller, P. (2013). Trial watch: phase II and phase III attrition rates 2011-2012. Nat. Rev. Drug Discov. 12:569. doi: 10.1038/nrd4090
Ashburn, T. T., and Thor, K. B. (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683. doi: 10.1038/nrd1468
Azad, T. D., Pan, J., Connolly, I. D., Remington, A., Wilson, C. M., and Grant, G. A. (2015). Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg. Focus 38:E9. doi: 10.3171/2014.12.Focus14758
Bell, J. T., Tsai, P. C., Yang, T. P., Pidsley, R., Nisbet, J., Glass, D., et al. (2012). Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 8:e1002629. doi: 10.1371/journal.pgen.1002629
Bendsen, N. T., Christensen, R., Bartels, E. M., Kok, F. J., Sierksma, A., Raben, A., et al. (2013). Is beer consumption related to measures of abdominal and general obesity? A systematic review and meta-analysis. Nutr. Rev. 71, 67–87. doi: 10.1111/j.1753-4887.2012.00548.x
Bennett, D. A., and Holmes, M. V. (2017). Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart 103, 1400–1407. doi: 10.1136/heartjnl-2016-310605
Berg-Beckhoff, G., Schuz, J., Blettner, M., Munster, E., Schlaefer, K., Wahrendorf, J., et al. (2009). History of allergic disease and epilepsy and risk of glioma and meningioma (INTERPHONE study group, Germany). Eur. J. Epidemiol. 24, 433–440. doi: 10.1007/s10654-009-9355-6
Bhatti, P., Stewart, P. A., Hutchinson, A., Rothman, N., Linet, M. S., Inskip, P. D., et al. (2009). Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol. Biomarkers. Prev. 18, 1841–1848. doi: 10.1158/1055-9965.epi-09-0197
Blumenthal, D. T., Raizer, J. J., Rosenblum, M. K., Bilsky, M. H., Hariharan, S., and Abrey, L. E. (1999). Primary intracranial neoplasms in patients with HIV. Neurology 52, 1648–1651. doi: 10.1212/WNL.52.8.1648
Bochud, M., and Rousson, V. (2010). Usefulness of Mendelian randomization in observational∗ epidemiology. Int. J. Environ. Res. Public Health 7, 711–728. doi: 10.3390/ijerph7030711
Boffetta, P., Hashibe, M., La Vecchia, C., Zatonski, W., and Rehm, J. (2006). The burden of cancer attributable to alcohol drinking. Int. J. Cancer 119, 884–887. doi: 10.1002/ijc.21903
Bondy, M. L., Scheurer, M. E., Malmer, B., Barnholtz-Sloan, J. S., Davis, F. G., Il’yasova, D., et al. (2008). Brain tumor epidemiology: consensus from the brain tumor epidemiology consortium. Cancer 113 7(Suppl.), 1953–1968. doi: 10.1002/cncr.23741
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Braganza, M. Z., Kitahara, C. M., Berrington de Gonzalez, A., Inskip, P. D., Johnson, K. J., and Rajaraman, P. (2012). Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 14, 1316–1324. doi: 10.1093/neuonc/nos208
Braganza, M. Z., Rajaraman, P., Park, Y., Inskip, P. D., Freedman, N. D., Hollenbeck, A. R., et al. (2014). Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br. J. Cancer 110, 242–248. doi: 10.1038/bjc.2013.611
Brunner, C., Davies, N. M., Martin, R. M., Eeles, R., Easton, D., Kote-Jarai, Z., et al. (2017). Alcohol consumption and prostate cancer incidence and progression: a mendelian randomisation study. Int. J. Cancer 140, 75–85. doi: 10.1002/ijc.30436
Burgess, S. (2014). Sample size and power calculations in mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43, 922–929. doi: 10.1093/ije/dyu005
Burgess, S., Dudbridge, F., and Thompson, S. G. (2015). Re: ”Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects”. Am. J. Epidemiol. 181, 290–291. doi: 10.1093/aje/kwv017
Burgess, S., and Thompson, S. G. (2015). Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260. doi: 10.1093/aje/kwu283
Cancer Research United Kingdom (2015). Brain, Other CNS and Intracranial Tumours Incidence Statistics. Available: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/brain-other-cns-and-intracranial-tumours/incidence#collapseTen [accessed April, 18 2018].
Cancer Research United Kingdom (2016). Survival for All Types of Brain Tumour. Available: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/brain-other-cns-and-intracranial-tumours/incidence#collapseTen [accessed 09, 2018].
Carozza, S. E., Wrensch, M., Miike, R., Newman, B., Olshan, A. F., Savitz, D. A., et al. (2000). Occupation and adult Gliomas. Am. J. Epidemiol. 152, 838–846. doi: 10.1093/aje/152.9.838
Castle, W. E. (1903). Mendel’s law of heredity. Science 18, 396–406. doi: 10.1126/science.18.456.396
Chen, P., Aldape, K., Wiencke, J. K., Kelsey, K. T., Miike, R., Davis, R. L., et al. (2001). Ethnicity delineates different genetic pathways in malignant Glioma. Cancer Res. 61, 3949–3954.
Cocco, P., Dosemeci, M., and Heineman, E. F. (1998). Brain cancer and occupational exposure to lead. J. Occup. Environ. Med. 40, 937–942. doi: 10.1097/00043764-199811000-00001
Corsello, S. M., Bittker, J. A., Liu, Z., Gould, J., McCarren, P., Hirschman, J. E., et al. (2017). The drug repurposing hub: a next-generation drug library and information resource. Nat. Med. 23, 405–408. doi: 10.1038/nm.4306
Darefsky, A. S., and Dubrow, R. (2009). International variation in the incidence of adult primary malignant neoplasms of the brain and central nervous system. Cancer Causes Control 20, 1593–1604. doi: 10.1007/s10552-009-9404-1
Das, A., Tan, W. L., Teo, J., and Smith, D. R. (2002). Glioblastoma multiforme in an Asian population: evidence for a distinct genetic pathway. J. Neurooncol. 60, 117–125. doi: 10.1023/A:1020622415786
Daugherty, S. E., Moore, S. C., Pfeiffer, R. M., Inskip, P. D., Park, Y., Hollenbeck, A., et al. (2011). Nonsteroidal anti-inflammatory drugs and glioma in the NIH-AARP diet and health study cohort. Cancer Prev. Res. 4, 2027–2034. doi: 10.1158/1940-6207.capr-11-0274
Davey Smith, G. (2011a). Random allocation in observational data: how small but robust effects could facilitate hypothesis-free causal inference. Epidemiology 22, 460–463; discussion 467–468. doi: 10.1097/EDE.0b013e31821d0426
Davey Smith, G. (2011b). Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 6, 27–43. doi: 10.1007/s12263-010-0181-y
Davey Smith, G., and Ebrahim, S. (2003). ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22. doi: 10.1093/ije/dyg070
Davey Smith, G., and Hemani, G. (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98. doi: 10.1093/hmg/ddu328
Davey Smith, G., Paternoster, L., and Relton, C. (2017). When will mendelian randomization become relevant for clinical practice and public health? JAMA 317, 589–591. doi: 10.1001/jama.2016.21189
Davis, F. G., McCarthy, B., and Jukich, P. (1999). The descriptive epidemiology of brain tumors. Neuroimaging Clin. N. Am. 9, 581–594.
Didelez, V., and Sheehan, N. (2007). Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 16, 309–330. doi: 10.1177/0962280206077743
Disney-Hogg, L., Cornish, A. J., Sud, A., Law, P. J., Kinnersley, B., Jacobs, D. I., et al. (2018a). Impact of atopy on risk of glioma: a Mendelian randomisation study. BMC Med. 16:42. doi: 10.1186/s12916-018-1027-5
Disney-Hogg, L., Sud, A., Law, P. J., Cornish, A. J., Kinnersley, B., Ostrom, Q. T., et al. (2018b). Influence of obesity-related risk factors in the aetiology of Glioma. Br. J. Cancer 118, 1020–1027. doi: 10.1038/s41416-018-0009-x
Dubrow, R., and Darefsky, A. S. (2011). Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer 11:325. doi: 10.1186/1471-2407-11-325
Esteller, M., Garcia-Foncillas, J., Andion, E., Goodman, S. N., Hidalgo, O. F., Vanaclocha, V., et al. (2000). Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 343, 1350–1354. doi: 10.1056/nejm200011093431901
Evans, D. M., Brion, M. J., Paternoster, L., Kemp, J. P., McMahon, G., Munafo, M., et al. (2013). Mining the human phenome using allelic scores that index biological intermediates. PLoS Genet. 9:e1003919. doi: 10.1371/journal.pgen.1003919
Evans, D. M., and Davey Smith, G. (2015). Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu. Rev. Genomics Hum. Genet. 16, 327–350. doi: 10.1146/annurev-genom-090314-050016
Evans, D. M., and Smith, G. D. (2015). Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu. Rev. Genomics Hum. Genet. 16, 327–350. doi: 10.1146/annurev-genom-090314-050016
Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., et al. (2017). Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2472. doi: 10.1093/eurheartj/ehx144
Flegal, K. M., Graubard, B. I., Williamson, D. F., and Cooper, R. S. (2011). Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am. J. Epidemiol. 173, 1–9. doi: 10.1093/aje/kwq341
Frei, P., Poulsen, A. H., Johansen, C., Olsen, J. H., Steding-Jessen, M., and Schüz, J. (2011). Use of mobile phones and risk of brain tumours: update of Danish cohort study. BMJ 343:3. doi: 10.1136/bmj.d6387
Gaist, D., García-Rodríguez, L. A., Sørensen, H. T., Hallas, J., and Friis, S. (2013). Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of Glioma: a case–control study. Br. J. Cancer 108, 1189–1194. doi: 10.1038/bjc.2013.87
Gehring, K., Sitskoorn, M. M., Gundy, C. M., Sikkes, S. A., Klein, M., Postma, T. J., et al. (2009). Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J. Clin. Oncol. 27, 3712–3722. doi: 10.1200/jco.2008.20.5765
Giles, G. G., McNeil, J. J., Donnan, G., Webley, C., Staples, M. P., Ireland, P. D., et al. (1994). Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne. Australia. Int. J. Cancer 59, 357–362. doi: 10.1002/ijc.2910590311
Glynn, R. J. (2010). Promises and limitations of mendelian randomization for evaluation of biomarkers. Clin. Chem. 56, 388–390. doi: 10.1373/clinchem.2009.142513
Greenland, S. (2000). An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 29, 722–729. doi: 10.1093/ije/29.4.722
Hall, J. R., and Short, S. C. (2009). Management of glioblastoma multiforme in HIV patients: a case series and review of published studies. Clin. Oncol. 21, 591–597. doi: 10.1016/j.clon.2009.04.006
Hammer, G. P., du Prel, J. B., and Blettner, M. (2009). Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl. Int. 106, 664–668. doi: 10.3238/arztebl.2009.0664
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Hart, C. L., Davey Smith, G., Gruer, L., and Watt, G. C. (2010). The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: a 30 year cohort study. BMC Public Health 10:789. doi: 10.1186/1471-2458-10-789
Hartwig, F. P., Davies, N. M., Hemani, G., and Davey Smith, G. (2016). Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 45, 1717–1726. doi: 10.1093/ije/dyx028
Haycock, P. C., Burgess, S., Nounu, A., Zheng, J., Okoli, G. N., Bowden, J., et al. (2017). Association between telomere length and risk of cancer and non-neoplastic diseases: a mendelian randomization study. JAMA Oncol. 3, 636–651. doi: 10.1001/jamaoncol.2016.5945
Haycock, P. C., Burgess, S., Wade, K. H., Bowden, J., Relton, C., and Davey Smith, G. (2016). Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 103, 965–978. doi: 10.3945/ajcn.115.118216
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base platform supports systematic causal inference across the human phenome. eLife 7:e34408. doi: 10.7554/eLife.34408
Hemani, G., Zheng, J., Wade, K. H., Laurin, C., Elsworth, B., Burgess, S., et al. (2016). MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. bioRxiv [Preprint]. doi: 10.1101/078972
Holick, C. N., Smith, S. G., Giovannucci, E., and Michaud, D. S. (2010). Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol. Biomark. Prev. 19, 39–47. doi: 10.1158/1055-9965.Epi-09-0732
Houben, M. P., Louwman, W. J., Tijssen, C. C., Teepen, J. L., van Duijn, C. M., and Coebergh, J. W. (2004). Hypertension as a risk factor for glioma? Evidence from a population-based study of comorbidity in glioma patients. Ann. Oncol. 15, 1256–1260. doi: 10.1093/annonc/mdh306
Inoue, A., and Solon, G. (2010). Two-sample instrumental variables estimators. Rev. Econ. Stat. 92, 557–561. doi: 10.1162/REST_a_00011
Iturrieta-Zuazo, I., and Walter, S. (2015). Mendelian randomization: present and future of epidemiological studies in cardiology. Revista Española Cardiología 68, 87–91. doi: 10.1016/j.recesp.2014.06.026
Jukich, P. J., McCarthy, B. J., Surawicz, T. S., Freels, S., and Davis, F. G. (2001). Trends in incidence of primary brain tumors in the United States, 1985-1994. Neuro Oncol. 3, 141–151. doi: 10.1093/neuonc/3.3.141
Kabat, G. C., Park, Y., Hollenbeck, A. R., Schatzkin, A., and Rohan, T. E. (2011). Reproductive factors and exogenous hormone use and risk of adult glioma in women in the NIH-AARP diet and health study. Int. J. Cancer 128, 944–950. doi: 10.1002/ijc.25413
Kaplan, S., Novikov, I., and Modan, B. (1997). Nutritional factors in the etiology of brain tumors: potential role of nitrosamines, fat, and cholesterol. Am. J. Epidemiol. 146, 832–841. doi: 10.1093/oxfordjournals.aje.a009201
Kelly, P. J. (2010). Gliomas: survival, origin and early detection. Surg. Neurol. Int. 1:96. doi: 10.4103/2152-7806.74243
Kemp, J. P., Sayers, A., Smith, G. D., Tobias, J. H., and Evans, D. M. (2016). Using Mendelian randomization to investigate a possible causal relationship between adiposity and increased bone mineral density at different skeletal sites in children. Int. J. Epidemiol. 45, 1560–1572. doi: 10.1093/ije/dyw079
Khuder, S. A., Mutgi, A. B., and Schaub, E. A. (1998). Meta-analyses of brain cancer and farming. Am. J. Ind. Med. 34, 252–260. doi: 10.1002/(SICI)1097-0274(199809)34:3<252::AID-AJIM7>3.0.CO;2-X
Kim, Y. I. (2003). Role of folate in colon cancer development and progression. J. Nutr. 133(11 Suppl. 1), 3731s–3739s. doi: 10.1093/jn/133.11.3731S
Kinnersley, B., Houlston, R. S., and Bondy, M. L. (2018). Genome-wide association studies in Glioma. Cancer Epidemiol. Biomark. Prev. 27, 418–428. doi: 10.1158/1055-9965.epi-17-1080
Kitahara, C. M., Linet, M. S., Brenner, A. V., Wang, S. S., Melin, B. S., Wang, Z., et al. (2014). Personal history of diabetes, genetic susceptibility to diabetes, and risk of brain glioma: a pooled analysis of observational studies. Cancer Epidemiol. Biomarkers. Prev. 23, 47–54. doi: 10.1158/1055-9965.epi-13-0913
Krishnan, G., Felini, M., Carozza, S. E., Miike, R., Chew, T., and Wrensch, M. (2003). Occupation and adult Gliomas in the San Francisco Bay Area. J. Occup. Environ. Med. 45, 639–647. doi: 10.1097/01.jom.0000069245.06498.48
Kuratsu, J., Takeshima, H., and Ushio, Y. (2001). Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int. J. Clin. Oncol. 6, 183–191. doi: 10.1007/pl00023928
Kyritsis, A. P., Bondy, M. L., and Levin, V. A. (2011). Modulation of glioma risk and progression by dietary nutrients and antiinflammatory agents. Nutr. Cancer 63, 174–184. doi: 10.1080/01635581.2011.523807
Lam, T. V., Agovino, P., Niu, X., and Roche, L. (2007). Linkage study of cancer risk among lead-exposed workers in New Jersey. Sci. Total Environ. 372, 455–462. doi: 10.1016/j.scitotenv.2006.10.018
Larsson, S. C., Traylor, M., Malik, R., Dichgans, M., Burgess, S., and Markus, H. S. (2017). Modifiable pathways in Alzheimer’s disease: mendelian randomisation analysis. BMJ 359:j5375. doi: 10.1136/bmj.j5375
Lawlor, D. A., Davey Smith, G., Bruckdorfer, K. R., Kundu, D., and Ebrahim, S. (2004). Observational versus randomised trial evidence. Lancet 364, 755–756. doi: 10.1016/s0140-6736(04)16926-2
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N., and Davey Smith, G. (2008). Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163. doi: 10.1002/sim.3034
Lee, S. T., Bracci, P., Zhou, M., Rice, T., Wiencke, J., Wrensch, M., et al. (2014). Interaction of allergy history and antibodies to specific varicella-zoster virus proteins on glioma risk. Int. J. Cancer 134, 2199–2210. doi: 10.1002/ijc.28535
Li, H. X., Meng, H. Y., Peng, X. X., Zong, Q., Zhang, K., and Han, G. L. (2015). A meta-analysis of association between pesticides exposure and glioma risk in adults. J. Craniofac. Surg. 26, e672–e673. doi: 10.1097/scs.0000000000001707
Liao, L. M., Friesen, M. C., Xiang, Y. B., Cai, H., Koh, D. H., Ji, B. T., et al. (2016). Occupational lead exposure and associations with selected cancers: the shanghai men’s and women’s health study cohorts. Environ. Health Perspect. 124, 97–103. doi: 10.1289/ehp.1408171
Linos, E., Raine, T., Alonso, A., and Michaud, D. (2007). Atopy and risk of brain tumors: a meta-analysis. J. Natl. Cancer Inst. 99, 1544–1550. doi: 10.1093/jnci/djm170
Little, R. B., Madden, M. H., Thompson, R. C., Olson, J. J., Larocca, R. V., Pan, E., et al. (2013). Anthropometric factors in relation to risk of glioma. Cancer Causes Control 24, 1025–1031. doi: 10.1007/s10552-013-0178-0
Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., et al. (2016). The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820. doi: 10.1007/s00401-016-1545-1
Low, K. B. (2001). “Pleiotropy A2 - brenner, sydney,” in Encyclopedia of Genetics, ed. J. H. Miller (New York, NY: Academic Press), 1490–1491.
Malerba, S., Galeone, C., Pelucchi, C., Turati, F., Hashibe, M., La Vecchia, C., et al. (2013). A meta-analysis of coffee and tea consumption and the risk of glioma in adults. Cancer Causes Control 24, 267–276. doi: 10.1007/s10552-012-0126-4
McLendon, R. E., Robinson, J. S. Jr., Chambers, D. B., Grufferman, S., and Burger, P. C. (1985). The glioblastoma multiforme in georgia, 1977-1981. Cancer 56, 894–897. doi: 10.1002/1097-0142(19850815)56:4<894::AID-CNCR2820560432>3.0.CO;2-#
Melin, B. S., Barnholtz-Sloan, J. S., Wrensch, M. R., Johansen, C., Il’yasova, D., Kinnersley, B., et al. (2017). Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat. Genet. 49, 789–794. doi: 10.1038/ng.3823
Meyer, R. M. (2010). Generalizing the results of cancer clinical trials. J. Clin. Oncol. 28, 187–189. doi: 10.1200/jco.2009.25.8608
Michaud, D. S., Holick, C. N., Batchelor, T. T., Giovannucci, E., and Hunter, D. J. (2009). Prospective study of meat intake and dietary nitrates, nitrites, and nitrosamines and risk of adult glioma. Am. J. Clin. Nutr. 90, 570–577. doi: 10.3945/ajcn.2008.27199
Mochizuki, S., Iwadate, Y., Namba, H., Yoshida, Y., Yamaura, A., Sakiyama, S., et al. (1999). Homozygous deletion of the p16/MTS-1/CDKN2 gene in malignant gliomas is infrequent among Japanese patients. Int. J. Oncol. 15, 983–989. doi: 10.3892/ijo.15.5.983
Mokry, L. E., Ahmad, O., Forgetta, V., Thanassoulis, G., and Richards, J. B. (2015). Mendelian randomisation applied to drug development in cardiovascular disease: a review. J. Med. Genet. 52, 71–79. doi: 10.1136/jmedgenet-2014-102438
Montgomery, A. A., Peters, T. J., and Little, P. (2003). Design, analysis and presentation of factorial randomised controlled trials. BMC Med. Res. Methodol. 3:26. doi: 10.1186/1471-2288-3-26
Navas-Acien, A., Pollan, M., Gustavsson, P., and Plato, N. (2002). Occupation, exposure to chemicals and risk of gliomas and meningiomas in Sweden. Am. J. Ind. Med. 42, 214–227. doi: 10.1002/ajim.10107
Neglia, J. P., Robison, L. L., Stovall, M., Liu, Y., Packer, R. J., Hammond, S., et al. (2006). New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the childhood cancer survivor study. J. Natl. Cancer Ins. 98, 1528–1537. doi: 10.1093/jnci/djj411
Nelson, M. R., Tipney, H., Painter, J. L., Shen, J., Nicoletti, P., Shen, Y., et al. (2015). The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860. doi: 10.1038/ng.3314
Olson, R. A., Brastianos, P. K., and Palma, D. A. (2011). Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J. Neurooncol. 105, 325–335. doi: 10.1007/s11060-011-0594-5
Ostrom, Q. T., Bauchet, L., Davis, F. G., Deltour, I., Fisher, J. L., Langer, C. E., et al. (2014). The epidemiology of glioma in adults: a ”state of the science” review. Neuro Oncol. 16, 896–913. doi: 10.1093/neuonc/nou087
Ostrom, Q. T., Gittleman, H., Farah, P., Ondracek, A., Chen, Y., Wolinsky, Y., et al. (2013). CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 15(Suppl. 2), ii1–ii56. doi: 10.1093/neuonc/not151
Parent, M. E., Turner, M. C., Lavoue, J., Richard, H., Figuerola, J., Kincl, L., et al. (2017). Lifetime occupational exposure to metals and welding fumes, and risk of glioma: a 7-country population-based case-control study. Environ. Health 16:90. doi: 10.1186/s12940-017-0300-y
Paternoster, L., Tilling, K., and Davey Smith, G. (2017). Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet. 13:e1006944. doi: 10.1371/journal.pgen.1006944
Preston-Martin, S., and Mack, W. (1991). Gliomas and meningiomas in men in Los Angeles county: investigation of exposures to N-nitroso compounds. IARC Sci. Publ. 105, 197–203.
Pukkala, E., Martinsen, J. I., Lynge, E., Gunnarsdottir, H. K., Sparén, P., Tryggvadottir, L., et al. (2009). Occupation and cancer – follow-up of 15 million people in five Nordic countries. Acta Oncol. 48, 646–790. doi: 10.1080/02841860902913546
Qi, L. (2009). Mendelian randomization in nutritional epidemiology. Nutr. Rev. 67, 439–450. doi: 10.1111/j.1753-4887.2009.00218.x
Qi, Z. Y., Shao, C., Yang, C., Wang, Z., and Hui, G. Z. (2014). Alcohol consumption and risk of glioma: a meta-analysis of 19 observational studies. Nutrients 6, 504–516. doi: 10.3390/nu6020504
Quach, P., El Sherif, R., Gomes, J., and Krewksi, D. (2017). A systematic review of the risk factors associated with the onset and progression of primary brain tumours. Neurotoxicology 61, 214–232. doi: 10.1016/j.neuro.2016.05.009
Rajaraman, P., Stewart, P. A., Samet, J. M., Schwartz, B. S., Linet, M. S., Zahm, S. H., et al. (2006). Lead, genetic susceptibility, and risk of adult brain tumors. Cancer Epidemiol. Biomark. Prev. 15, 2514–2520. doi: 10.1158/1055-9965.epi-06-0482
Reardon, D. A., and Wen, P. Y. (2006). Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist 11, 152–164. doi: 10.1634/theoncologist.11-2-152
Reifenberger, G., Hentschel, B., Felsberg, J., Schackert, G., Simon, M., Schnell, O., et al. (2012). Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int. J. Cancer 131, 1342–1350. doi: 10.1002/ijc.27385
Relton, C. L., and Davey Smith, G. (2012). Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int. J. Epidemiol. 41, 161–176. doi: 10.1093/ije/dyr233
Rice, T., Lachance, D. H., Molinaro, A. M., Eckel-Passow, J. E., Walsh, K. M., Barnholtz-Sloan, J., et al. (2016). Understanding inherited genetic risk of adult glioma – a review. Neuro Oncol. Pract. 3, 10–16. doi: 10.1093/nop/npv026
Robertson, J. T., Gunter, B. C., and Somes, G. W. (2002). Racial differences in the incidence of gliomas: a retrospective study from Memphis, Tennessee. Br. J. Neurosurg. 16, 562–566. doi: 10.1080/02688690209168361
Sadetzki, S., Chetrit, A., Freedman, L., Stovall, M., Modan, B., and Novikov, I. (2005). Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat. Res. 163, 424–432. doi: 10.1667/RR3329
Safaeian, M., Rajaraman, P., Hartge, P., Yeager, M., Linet, M., Butler, M. A., et al. (2013). Joint effects between five identified risk variants, allergy, and autoimmune conditions on glioma risk. Cancer Causes Control 24, 1885–1891. doi: 10.1007/s10552-013-0244-7
Samkange-Zeeb, F., Schlehofer, B., Schuz, J., Schlaefer, K., Berg-Beckhoff, G., Wahrendorf, J., et al. (2010). Occupation and risk of glioma, meningioma and acoustic neuroma: results from a German case-control study (interphone study group, Germany). Cancer Epidemiol. 34, 55–61. doi: 10.1016/j.canep.2009.12.003
Sayon-Orea, C., Martinez-Gonzalez, M. A., and Bes-Rastrollo, M. (2011). Alcohol consumption and body weight: a systematic review. Nutr. Rev. 69, 419–431. doi: 10.1111/j.1753-4887.2011.00403.x
Scheurer, M. E., Amirian, E. S., Davlin, S. L., Rice, T., Wrensch, M., and Bondy, M. L. (2011). Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. Int. J. Cancer 129, 2290–2296. doi: 10.1002/ijc.25883
Schoemaker, M. J., Swerdlow, A. J., Hepworth, S. J., McKinney, P. A., van Tongeren, M., and Muir, K. R. (2006). History of allergies and risk of glioma in adults. Int. J. Cancer 119, 2165–2172. doi: 10.1002/ijc.22091
Schüz, J., Jacobsen, R., Olsen, J. H., Boice, J. J. D., McLaughlin, J. K., and Johansen, C. (2006). Cellular telephone use and cancer risk: update of a nationwide danish cohort. J. Natl. Cancer Inst. 98, 1707–1713. doi: 10.1093/jnci/djj464
Schwartzbaum, J., Ahlbom, A., Malmer, B., Lonn, S., Brookes, A. J., Doss, H., et al. (2005). Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 65, 6459–6465. doi: 10.1158/0008-5472.can-04-3728
Schwartzbaum, J., Edlinger, M., Zigmont, V., Stattin, P., Rempala, G. A., Nagel, G., et al. (2017). Associations between prediagnostic blood glucose levels, diabetes, and glioma. Sci. Rep. 7:1436. doi: 10.1038/s41598-017-01553-2
Seliger, C., Meier, C. R., Becker, C., Jick, S. S., Bogdahn, U., Hau, P., et al. (2016a). Statin use and risk of glioma: population-based case-control analysis. Eur. J. Epidemiol. 31, 947–952. doi: 10.1007/s10654-016-0145-7
Seliger, C., Ricci, C., Meier, C. R., Bodmer, M., Jick, S. S., Bogdahn, U., et al. (2016b). Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro Oncol. 18, 340–349. doi: 10.1093/neuonc/nov100
Sergentanis, T. N., Tsivgoulis, G., Perlepe, C., Ntanasis-Stathopoulos, I., Tzanninis, I. G., Sergentanis, I. N., et al. (2015). Obesity and risk for brain/CNS tumors, gliomas and meningiomas: a meta-analysis. PLoS One 10:e0136974. doi: 10.1371/journal.pone.0136974
Shao, C., Zhao, W., Qi, Z., and He, J. (2016). Smoking and glioma risk: evidence from a meta-analysis of 25 observational studies. Medicine 95:e2447. doi: 10.1097/md.0000000000002447
Sheehan, N. A., Didelez, V., Burton, P. R., and Tobin, M. D. (2008). Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 5:e177. doi: 10.1371/journal.pmed.0050177
Shi, J., Marconett, C. N., Duan, J., Hyland, P. L., Li, P., Wang, Z., et al. (2014). Characterizing the genetic basis of methylome diversity in histologically normal human lung tissue. Nat. Commun. 5:3365. doi: 10.1038/ncomms4365
Smith, G. D. (2010). Mendelian randomization for strengthening causal inference in observational studies:application to gene × environment interactions. Perspect. Psychol. Sci. 5, 527–545. doi: 10.1177/1745691610383505
Smith, G. D., and Ebrahim, S. (2004). Mendelian randomization: prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42. doi: 10.1093/ije/dyh132
Sofat, R., Hingorani, A. D., Smeeth, L., Humphries, S. E., Talmud, P. J., Cooper, J., et al. (2010). Separating the mechanism-based and off-target actions of cholesteryl ester transfer protein inhibitors with CETP gene polymorphisms. Circulation 121, 52–62. doi: 10.1161/circulationaha.109.865444
Stupp, R., Hegi, M. E., Mason, W. P., van den Bent, M. J., Taphoorn, M. J., Janzer, R. C., et al. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466. doi: 10.1016/s1470-2045(09)70025-7
Sun, B. B., Maranville, J. C., Peters, J. E., Stacey, D., Staley, J. R., Blackshaw, J., et al. (2018). Genomic atlas of the human plasma proteome. Nature 558, 73–79. doi: 10.1038/s41586-018-0175-2
Sunderman, F. W. Jr. (2001). Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 31, 3–24.
Takahashi, H., Cornish, A. J., Sud, A., Law, P. J., Kinnersley, B., Ostrom, Q. T., et al. (2018). Mendelian randomisation study of the relationship between vitamin D and risk of glioma. Sci. Rep. 8:2339. doi: 10.1038/s41598-018-20844-w
Tamimi, A. F., and Juweid, M. (2017). “Epidemiology and outcome of glioblastoma,” in Glioblastoma, ed. S. De Vleeschouwer (Brisbane, QLD: Codon Publications Copyright).
Taylor, A. J., Little, M. P., Winter, D. L., Sugden, E., Ellison, D. W., Stiller, C. A., et al. (2010). Population-based risks of CNS tumors in survivors of childhood cancer: the British childhood cancer survivor study. J. Clin. Oncol. 28, 5287–5293. doi: 10.1200/jco.2009.27.0090
Timpson, N. J., Nordestgaard, B. G., Harbord, R. M., Zacho, J., Frayling, T. M., Tybjærg-Hansen, A., et al. (2010). C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. 35, 300. doi: 10.1038/ijo.2010.137
Touat, M., Idbaih, A., Sanson, M., and Ligon, K. L. (2017). Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann. Oncol. 28, 1457–1472. doi: 10.1093/annonc/mdx106
U.S.National Library of Medicine (2018). Intraoperative Ultrasound Guided Glioma Surgery; a Randomised, Controlled Trial. Available at: https://clinicaltrials.gov/ct2/show/NCT03531333?id=NCT03531333&rank=1&load=cart
Urbanska, K., Sokolowska, J., Szmidt, M., and Sysa, P. (2014). Glioblastoma multiforme - an overview. Contemp. Oncol. 18, 307–312. doi: 10.5114/wo.2014.40559
van Wijngaarden, E., and Dosemeci, M. (2006). Brain cancer mortality and potential occupational exposure to lead: findings from the national longitudinal mortality study, 1979-1989. Int. J. Cancer 119, 1136–1144. doi: 10.1002/ijc.21947
VanderWeele, T. J., Tchetgen Tchetgen, E. J., Cornelis, M., and Kraft, P. (2014). Methodological challenges in mendelian randomization. Epidemiology 25, 427–435. doi: 10.1097/ede.0000000000000081
Visser, O., Ardanaz, E., Botta, L., Sant, M., Tavilla, A., Minicozzi, P., et al. (2015). Survival of adults with primary malignant brain tumours in Europe; results of the EUROCARE-5 study. Eur. J. Cancer 51, 2231–2241. doi: 10.1016/j.ejca.2015.07.032
Vuorinen, V., Hinkka, S., Farkkila, M., and Jaaskelainen, J. (2003). Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir. 145, 5–10. doi: 10.1007/s00701-002-1030-6
Wald, A. (1940). The fitting of straight lines if both variables are subject to error. Ann. Math. Stat. 11, 284–300. doi: 10.1214/aoms/1177731868
Walsh, K. M., Codd, V., Rice, T., Nelson, C. P., Smirnov, I. V., McCoy, L. S., et al. (2015). Longer genotypically-estimated leukocyte telomere length is associated with increased adult glioma risk. Oncotarget 6, 42468–42477. doi: 10.18632/oncotarget.6468
Walsh, K. M., Codd, V., Smirnov, I. V., Rice, T., Decker, P. A., Hansen, H. M., et al. (2014). Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat. Genet. 46, 731–735. doi: 10.1038/ng.3004
Wang, B., and Du, Y. (2013). Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013:898034. doi: 10.1155/2013/898034
Wang, G., Xu, S., Cao, C., Dong, J., Chu, Y., He, G., et al. (2016). Evidence from a large-scale meta-analysis indicates eczema reduces the incidence of glioma. Oncotarget 7, 62598–62606. doi: 10.18632/oncotarget.11545
Wang, H., Xu, T., Jiang, Y., Xu, H., Yan, Y., Fu, D., et al. (2015). The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia 17, 239–255. doi: 10.1016/j.neo.2015.02.002
Wen, P. Y., and Kesari, S. (2008). Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507. doi: 10.1056/NEJMra0708126
Wick, W., Platten, M., Meisner, C., Felsberg, J., Tabatabai, G., Simon, M., et al. (2012). Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 13, 707–715. doi: 10.1016/s1470-2045(12)70164-x
Wiedmann, M. K. H., Brunborg, C., Di Ieva, A., Lindemann, K., Johannesen, T. B., Vatten, L., et al. (2017). The impact of body mass index and height on the risk for glioblastoma and other glioma subgroups: a large prospective cohort study. Neuro Oncol. 19, 976–985. doi: 10.1093/neuonc/now272
Wigertz, A., Lonn, S., Schwartzbaum, J., Hall, P., Auvinen, A., Christensen, H. C., et al. (2007). Allergic conditions and brain tumor risk. Am. J. Epidemiol. 166, 941–950. doi: 10.1093/aje/kwm203
Wurtz, P., Kangas, A. J., Soininen, P., Lehtimaki, T., Kahonen, M., Viikari, J. S., et al. (2013). Lipoprotein subclass profiling reveals pleiotropy in the genetic variants of lipid risk factors for coronary heart disease: a note on Mendelian randomization studies. J. Am. Coll. Cardiol. 62, 1906–1908. doi: 10.1016/j.jacc.2013.07.085
Yang, M., Guo, W., Yang, C., Tang, J., Huang, Q., Feng, S., et al. (2017). Mobile phone use and glioma risk: a systematic review and meta-analysis. PLoS One 12:e0175136. doi: 10.1371/journal.pone.0175136
Yarmolinsky, J., Wade, K. H., Richmond, R. C., Langdon, R. J., Bull, C. J., Tilling, K. M., et al. (2017). Causal inference in cancer epidemiology: what is the role of Mendelian randomization? bioRxiv [Preprint]. doi: 10.1101/223966
Zhao, H., Cai, W., Su, S., Zhi, D., Lu, J., and Liu, S. (2014). Allergic conditions reduce the risk of glioma: a meta-analysis based on 128,936 subjects. Tumour Biol. 35, 3875–3880. doi: 10.1007/s13277-013-1514-4
Zhao, L., Zheng, Z., and Huang, P. (2016). Diabetes mellitus and the risk of glioma: a meta-analysis. Oncotarget 7, 4483–4489. doi: 10.18632/oncotarget.6605
Zheng, J., Baird, D., Borges, M.-C., Bowden, J., Hemani, G., Haycock, P., et al. (2017). Recent developments in mendelian randomization studies. Curr. Epidemiol. Rep. 4, 330–345. doi: 10.1007/s40471-017-0128-6
Zheng, T., Cantor, K. P., Zhang, Y., Keim, S., and Lynch, C. F. (2001). Occupational risk factors for brain cancer: a population-based case-control study in Iowa. J. Occup. Environ. Med. 43, 317–324. doi: 10.1097/00043764-200104000-00005
Keywords: Mendelian randomization, glioma, risk factors, genetic variant, causal inference, SNP, causal association
Citation: Howell AE, Zheng J, Haycock PC, McAleenan A, Relton C, Martin RM and Kurian KM (2018) Use of Mendelian Randomization for Identifying Risk Factors for Brain Tumors. Front. Genet. 9:525. doi: 10.3389/fgene.2018.00525
Received: 04 June 2018; Accepted: 19 October 2018;
Published: 12 November 2018.
Edited by:
Brian L. Yaspan, Genentech, Inc., United StatesReviewed by:
Jing Dong, Baylor College of Medicine, United StatesCopyright © 2018 Howell, Zheng, Haycock, McAleenan, Relton, Martin and Kurian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Elizabeth Howell, YWgxODU3OEBicmlzdG9sLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.