
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 22 August 2018
Sec. Genetics of Common and Rare Diseases
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00302
This article is part of the Research Topic Proceedings of the ‘Third International Conference of FMR1 Premutation: Basic Mechanisms and Clinical Involvement’ View all 20 articles
 Reem R. Al Olaby1
Reem R. Al Olaby1 Hiu-Tung Tang1
Hiu-Tung Tang1 Blythe Durbin-Johnson2
Blythe Durbin-Johnson2 Andrea Schneider3,4
Andrea Schneider3,4 David Hessl4,5
David Hessl4,5 Susan M. Rivera5,6
Susan M. Rivera5,6 Flora Tassone1,3*
Flora Tassone1,3*Approximately 30–40% of male and 8–16% of female carriers of the Fragile X premutation will develop a neurodegenerative movement disorder characterized by intentional tremor, gait ataxia, autonomic dysfunction, cognitive decline, and Parkinsonism during their lifetime. At the molecular level, premutation carriers have increased expression levels of the FMR1 and the antisense FMR1 (ASFMR1) mRNAs. Both genes undergo alternative splicing giving rise to a number of different transcripts. Alteration in the alternative splicing process might be associated with FXTAS. In this study, we have investigated the correlation between objective measures of movement (balance and tremor using the CATSYS battery) and the expression of both the FMR1 and the ASFMR1 genes. In addition, we investigated whether their expression level and that of the ASFMR1 131 bp splice isoform could distinguish between premutation carriers with FXTAS and non-FXTAS premutation carriers. Confirming previous findings, the expression levels of transcripts at the FMR1 locus positively correlated with the CGG repeat number and significantly differentiated the premutation carriers from the control groups. Furthermore, premutation carriers with and without FXTAS, showed a significant difference in the expression level of the ASFMR1 131 bp splice isoform when compared to age and gender matched controls. However, there was no significant difference in the ASFMR1 131 bp splice isoform expression level when comparing premutation carriers with and without FXTAS. Finally, our results indicate significant group differences in CATSYS dominant hand reaction time and postural sway with eyes closed in premutation carriers without FXTAS compared to controls. In addition, a significant inverse association between the tremor intensity and the expression level of ASFMR1 131 bp splice isoform in premutation carriers compared to controls, was observed, suggesting a potential role in the pathogenesis of FXTAS.
Individual carriers of a premutation allele in the FMR1 gene (55–200 CGG repeats) are at risk of developing Fragile X-associated tremor/ataxia syndrome (FXTAS) a late onset neurodegenerative disorder characterized by intentional tremor, gait ataxia, autonomic dysfunction, and Parkinsonism (Tassone and Hall, 2016). Cognitive decline, particularly frontal executive dysfunction, is also very common (Allen et al., 2008; Grigsby et al., 2008). Approximately 46% of males and 17% of females will develop and be affected by FXTAS during their lifetime. The onset of the motor signs in men is typically in the early 60s (Tassone et al., 2007) and the penetrance is age-related, such that 75% of men ≥80 years of age are affected (Jacquemont et al., 2004). Patients with FXTAS have several types of motor dysfunction, hence they are considered within the combinational movement disorders (Hall et al., 2014).
A higher frequency of a number of phenotypes including psychiatric conditions, dysautonomia, sleep apnea, hypertension, migraine, rheumatologic conditions, endocrine diseases, seizures, peripheral neuropathy, immune mediated conditions (such as fibromyalgia and hypothyroidism) has also been described in premutation carriers (reviewed in Hagerman and Hagerman, 2016).
Several molecular mechanisms have been proposed to contribute to the different phenotypes observed in the FMR1- associated disorders. One of the main mechanisms proposed to lead to the premutation pathogenesis is RNA toxicity due to the 2 to 8-fold increase in the FMR1 mRNA expression levels observed in premutation carriers (Tassone et al., 2000; Kenneson et al., 2001; Allen et al., 2005). RNA toxicity leads to protein sequestration where the expanded CGG repeat sequesters a number of CGG binding proteins, hence, partially or fully impairing their normal function in the cell (Hagerman and Hagerman, 2015). Recently, a non-AUG initiated (RAN) translation, where a polyglycine-containing protein, FMRpolyG is generated by initiating at non-AUG codons located upstream of the CGG-repeat region (Todd et al., 2013), has been proposed as a key mechanism contributing to neurodegeneration. Further, DNA damage has been proposed as a pathological model due to the formation of co-transcriptional R-loops which trigger a DNA damage response (DDR) leadings to neuronal death (Ginno et al., 2013; Hamperl and Cimprich, 2014; Loomis et al., 2014; Julie and Karlene, 2015).
In an effort to elucidate other mechanistic pathways that might be involved in FXTAS, Ladd et al. (2007) identified a novel gene, the antisense FMR1 (ASFMR1), the main identified non-coding RNA (ncRNA) at the FMR1 locus. The gene includes the CGG repeat region of the FMR1 gene in the antisense orientation. Its expression is driven by two promoters that are flanked by CTCF-binding sites, the FMR1 bidirectional promoter and a second one located in the second intron of the FMR1 gene (Ladd et al., 2007). Similarly, to the FMR1 gene, the levels of expression of ASFMR1 mRNA are elevated in the cells derived from premutation carriers compared to controls (Ladd et al., 2007; Loesch et al., 2011) while no expression is detected in patients with Fragile X syndrome. Furthermore, the ASFMR1 transcript undergoes to premutation-specific alternative splicing which might be potentially associated with FXTAS and other FMR1 associated disorders (Ladd et al., 2007; Hall et al., 2017).
Loesch et al. (2011) showed that the elevated sense/antisense FMR1 transcript levels in the gray zone (40–54 CGG repeats) and in premutation carriers might contribute to the development of a parkinsonian phenotype due to mitochondrial dysfunction that leads to progressive neurodegeneration. A recent study by Hall et al. (2017) showed that both male and female premutation carriers had higher expression levels of ASFMR1 splice isoform corresponding to the isoform described by Ladd et al. (2007), compared to controls after adjusting for age, confirming previous finding Ladd et al. (2007). Although the authors suggested ASFMR1 splice isoform as a predictor of FXTAS, they reported that there was no significant difference in the expression levels between non-FXTAS premutation carriers and FXTAS patients (Hall et al., 2017).
Besides the molecular measures, clinical measures have a significant role in monitoring premutation carriers with and without FXTAS. A computerized coordination-tremor-balance test system (CATSYS) is one of the important clinical assessments for FXTAS. It is a quantitative neurological test battery used to quantify movement abnormalities (tremor and ataxia) recording five main neuromotor control measures. Previous studies reported on the sensitivity of the test in identifying preclinical symptoms of FXTAS (Allen et al., 2008) and on its ability to differentiate between premutation carriers with FXTAS and controls (Aguilar et al., 2008; Narcisa et al., 2011).
In this study, we investigated the correlation between levels of mRNA expression of the FMR1 locus (mRNA expression levels of the FMR1 and of the ASFMR1 genes and of the ASFMR1 131 bp splice isoform) and a selected group of measures in the CATSYS associated with the core symptoms of FXTAS (tremor and balance) in the non-FXTAS premutation carriers compared to controls. In addition, we investigated whether there was a differential transcript profile at the FMR1 locus expression between premutation carriers with and without FXTAS compared to controls. The main aim of the study was to determine whether a combination of neurological and molecular measures could help predict the prognosis of non-FXTAS male premutation carriers.
Individuals were recruited through the MIND Institute Fragile X Research and Treatment Center, by postings in the community, from flyers posted through the National Fragile X Foundation, from over 1,200 extended pedigrees of probands with Fragile X-associated disorders seen for clinical care or for being participants in research studies involving non-FXTAS premutation carriers, carried out at the MIND Institute. Participants provided informed consent according to protocols approved by the UC Davis Institutional Review Board. Biological samples were collected under protocols approved by the UC Davis Institutional Review Board.
Participants included 52 non-FXTAS male premutation carriers (mean age = 59.3, SD = 8.3) and 26 healthy controls (mean age = 55.6, SD = 8.7) (Table 1).
In addition, we investigated the difference in expression levels of transcripts at the FMR1 locus in 15 trios. Each trio was age-matched, and included a male premutation carrier with FXTAS (FXTAS stage 4 or 5) (n = 15), a non-FXTAS male premutation carrier (FXTAS stage 0 or 1) (n = 15) and a healthy control (n = 15) (total of 45 participants).
Group status for each participant was confirmed through DNA testing as having 55–200 CGG repeats (carriers of the FMR1 premutation), or having 5–44 CGG repeats (normal range, comparison group). Individuals who had a gray zone allele (45–54 CGG repeats) or a full mutation (<200 CGG repeats) were not included in this study.
CATSYS included various tasks grouped into five existing categories (Després et al., 2000): postural tremor, intentional tremor, postural sway, manual coordination, and reaction time. All tasks were appended to the standard CATSYS protocol and analyzed accordingly.
The CATSYS measures were administered as described in Aguilar et al. (2008). Postural tremor was measured per the CATSYS protocol. For both dominant and non-dominant hands, the patient was asked to grasp a Tremor Pen® (it contains a biaxial micro-accelerometer to measure the movement in plane perpendicular to the axis of the pen) and hold it as steadily as possible four inches in front of the navel. For the intention tremor performance task, the patient was asked to grip the pen in the same way as in the postural tremor task, yet not in a steady position, but to alternately tap the centers of two points located on either side of the computer screen, designated as points A and B, for each hand.
As for the postural sway protocol, patients were asked to stand on the force plate for 30 and 10 s, once with the eyes open and once closed. Manual hand coordination was measured by asking patients to rhythmically tap the drum in time with sounds generated by the CATSYS program. The testing using this category was completed with assessing the finger coordination, which included the rhythmic tapping of the right and left index finger. Finally, the reaction time for response, which was an auditory stimulus, in this case was recorded using the reaction handle of the CATSYS system. The duration for testing each hand was 40 s where the auditory stimuli were triggered at random intervals.
Genomic DNA was isolated from peripheral blood lymphocytes (5 ml of whole blood using standard methods; Qiagen, Valencia, CA, United States). The CGG size of the premutation and normal alleles were obtained using a combination of Southern Blot and PCR analysis. For Southern blot analysis, 5–10 μg of isolated genomic DNA was digested with EcoRI and NruI. Probe hybridization used the FMR1-specific dig-labeled StB12.3. Details are as previously described (Tassone et al., 2008). PCR analysis was performed using the AmplideX PCR/CE FMR1 Reagents (Asuragen, Inc.) as described in Filipovic-Sadic et al. (2010). Total RNA was isolated from 2.5 ml of peripheral blood collected in PAXgene Blood RNA tubes using the PAXgene Blood RNA Kit (Qiagen, Valencia, CA, United States) and quantified using NanoDrop. cDNA synthesis reaction was as described by Tassone et al. (2000).
qRT-PCR was performed using both Assays-on-Demand from Applied Biosystem (Applied Biosystems, Foster City, CA, United States) and custom designed TaqMan primers and probe assays to measure transcripts expression levels. Custom designed primers and probe were used for normalization (Tassone et al., 2000). Custom designed primers and probe were also designed to quantify the ASFMR1 gene and the ASFMR1 splice isoform (Ladd et al., 2007).
Left- and right-handed clinical measures were converted to dominant and non-dominant based on the patient’s handedness; if handedness information was missing measures from the right hand were used for the dominant side. A patient with a CGG repeat ranging from 110 to 130 was assigned a repeat length of 120 for analysis purposes.
By visual inspection, CATSYS data deviated substantially from a normal distribution both on the original scale and on the log scale (other transformations were not considered, as model coefficients estimated based on these are rather less interpretable than the aforementioned scales). Therefore, non-parametric methods were used throughout for analysis of these data.
Age, clinical measures, and molecular measures were compared between groups using Wilcoxon rank sum tests. Distributions of categorical demographic characteristics were compared between groups using Fisher’s exact test. Correlations were estimated and tested using the Spearman (non-parametric) correlation. Clinical measures were compared between visits using the Wilcoxon signed-rank test.
In the trios study, expression levels of ASFMR1, the splice isoform, and FMR1 were compared among premutation participants with FXTAS, premutation participants with non-FXTAS, and control subjects using linear models. Age and CGG repeat number were adjusted for by including these as covariates in each model. Post hoc pairwise comparisons between groups were conducted using the Tukey HSD method. Analyses were conducted using R, version 3.4.2 (R Core Team, 2017).
p-values were adjusted for multiple testing within each table using the Benjamini–Hochberg method.
In this study, among the total 78 subjects, 82.1% (n = 64) were Caucasian and 38.5% (n = 30) had a higher degree education (MA/MS/PhD/MD). In addition, 83.3% (n = 65) were right hand dominant. The demographics data showed no statistical significant difference between any of the groups (Table 1).
As for the trio study, participants were individually matched for CGG repeats and age; where the mean age (SD) for the 15 controls was 63 (±8.9), for the 15 FXTAS premutation carriers was 65 (±8), and for the 15 non-FXTAS premutation carriers was 62 (±8). The mean (SD) for the CGG repeat number was 98.47 (±15.8) in male premutation carriers with FXTAS, 90.27 (±22.8) in non-FXTAS premutation carriers and 29.67 (±4.4) in controls (Table 2). The mean (SD) of the FMR1 mRNA was 2.9 (±0.6) in the premutation with FXTAS, 1.9 (±1.1) in the non-FXTAS premutation and 1.4 (±0.4) in controls. The mean of the ASFMR1 mRNA was 0.51 (±0.3) in the premutation with FXTAS, 0.53 (±0.3) in the non-FXTAS premutation and 0.3 (±0.1) in controls. The mean of the ASFMR1 131 bp splice isoform mRNA was 1.9 (±1.6) in the premutation with FXTAS, 1.7 (±2.1) in the non-FXTAS premutation and 0.1 (±0.1) in controls.
Three CATSYS performance measures were found to be significantly different between non-FXTAS premutation carriers and controls; the reaction time test for dominant hand (Average reaction time) (p = 0.0094), the 30 s postural sway test with eyes open (p = 0.0176), and the 10 s postural sway test with eyes closed (p = 0.0094) (Table 3). This means that both the reaction time and the sway area are significantly increased in non-FXTAS premutation carriers compared to controls.
On the other hand, a subset of measures lost their significance upon correction for multiple testing; reaction time test for non-dominant hand (average reaction time) (p = 0.0463), dominant foot (sway area) (p = 0.0394) and non-dominant foot (sway area) (p = 0.0287).
A significant correlation between the non-dominant hand tremor intensity and the ASFMR1 131 bp splicing isoform was observed in non-FXTAS premutation carriers (p = 0.0304) but not in controls (p = 0.3363) (Figure 1A). The expression level of ASFMR1 131 bp splicing isoform showed a significant inverse correlation with the non-dominant hand tremor intensity (-0.44) with the higher splicing isoform expression levels of this being associated with lower non-dominant hand tremor intensity score.

FIGURE 1. Plots showing the correlation between the expression level of the ASFMR1 spicing isoform 131 bp mRNA and tremor intensity in the non-dominant hand (A), tremor intensity in the dominant hand (B), and tremor intensity while writing with dominant hand (C) in controls compared to premutations without FXTAS.
Correlation between ASFMR1 131 bp splicing and two CATSYS performance tasks, tremor intensity of dominant hand (p = 0.0489) (Figure 1B), and tremor intensity while writing with dominant hand (p = 0.0268) (Figure 1C) lost significance after correction for multiple testing.
None of the CATSYS measures showed any statistically significant association with age, CGG repeat number, expression levels of FMR1, or ASFMR1 mRNAs.
Premutation carriers (with and without FXTAS) showed significantly elevated expression of both FMR1 and ASFMR1 131 bp splicing isoform mRNA compared to controls with a p < 0.0001 in both cases (Figures 2A,C and Table 4). No significant elevation was observed for the ASFMR1 mRNA (Figure 2B and Table 4).
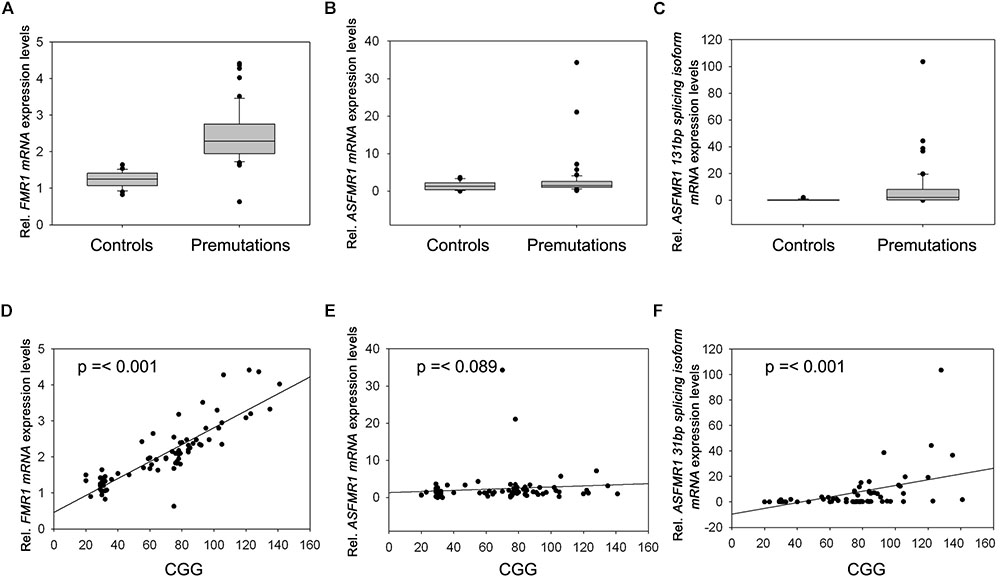
FIGURE 2. Box plots showing the expression of FMR1 (A), ASFMR1 (B), and ASFMR1 131 bp splicing isoform (C) in premutation carriers compared to controls. Correlation between CGG repeat number and the expression levels of (D) FMR1, (E) ASFMR1, and (F) ASFMR1 131 bp splicing isoform mRNAs.
None of the molecular measures were significantly correlated with age. As expected, the CGG repeat number was significantly correlated with the expression of both FMR1 (p < 0.0001) and ASFMR1 131 bp splicing isoform mRNAs (p = 0.001) (Table 5 and Figures 2D–F) but not for the ASFMR1 mRNA (Figure 2E and Table 5).
When analyzing the trios, as expected we observed a significant higher expression of FMR1 mRNA in the premutation carriers with FXTAS and without FXTAS compared to control subjects (Figure 3A; p < 0.001 and p < 0.001, respectively, Sellier et al., 2014). In addition, we observed significantly higher expression level of ASFMR1 131 bp splicing isoform mRNA in both non-FXTAS premutation subjects [p = 0.003, Mean (SD) = 1.7 (2.1)] and subjects with FXTAS [p = 0.004, Mean (SD) = 1.9 (1.6)] compared to control subjects [Mean (SD) = 0.1 (0.1)] (Figure 3C). However, the ASFMR1 expression levels between groups did not reach statistical significance (Figure 3B). These three molecular measures were significantly correlated with the number of CGG repeats (Figures 3D–F).
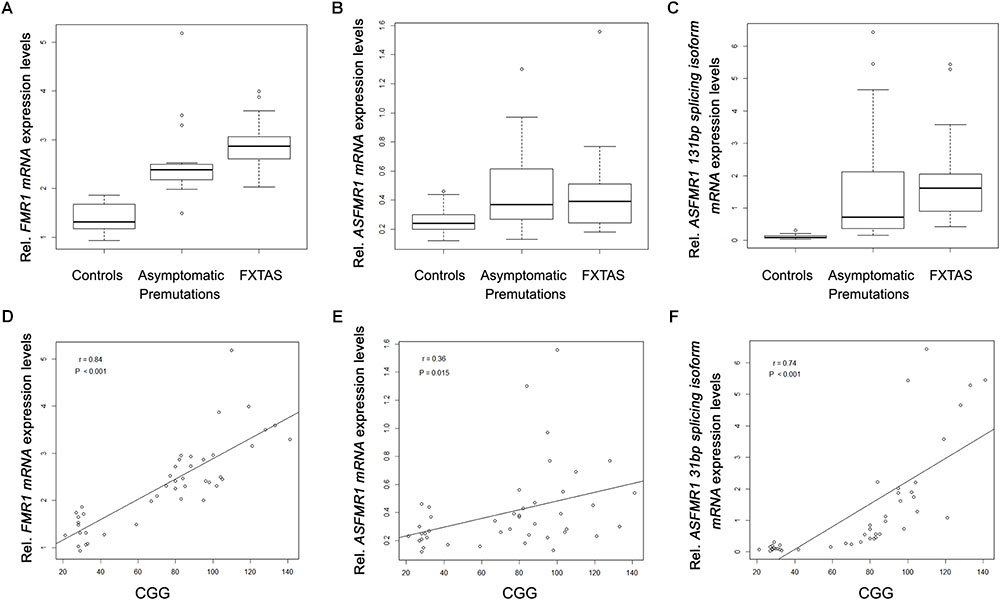
FIGURE 3. Box plots showing differential expression levels of FMR1 (A), ASFMR1 (B) and ASFMR1 131 bp splicing isoform (C) mRNAs in controls compared to non-FXTAS premutation carriers and FXTAS premutation carriers. FMR1, ASFMR1, and ASFMR1 131 bp splicing isoform mRNAs expression levels, as function of the CGG repeat number, are shown in D, E and F respectively.
This study aimed to identify clinical and molecular measures that might associate with development of FXTAS in male Fragile X premutation carriers. CATSYS is among the helpful tools to distinguish between premutation carriers with and without FXTAS and controls because it allows for quantitative documentation of neuromotor and deficits with minimal operating training and time (Aguilar et al., 2008). Specifically, among the measures found useful, are those that are considered components of the key FXTAS phenotypic characteristics such as tremor intensity, postural tremors, and postural sway (Aguilar et al., 2008; Narcisa et al., 2011). Our previous reports (Aguilar et al., 2008; Narcisa et al., 2011), looking specifically at the CATSYS measure in FMR1 premutation carriers with and without FXTAS and, unaffected controls, found the method to be sufficiently accurate for the cause. In addition, the 2008 study by Allen colleagues showed a high clinical usefulness of the battery.
In this study, we showed that three CATSYS task performance measures were significantly different in non-FXTAS male premutation carriers compared to controls. Two of them were measures of postural sway, which are associated with ataxia. Our results appear to be consistent with those of Narcisa et al. (2011), as both showed that non-FXTAS premutation carriers had higher postural sway compared to controls. However the significance of this effect was lost when correcting for multiple testing. Several factors could explain these findings including the age group of the non-FXTAS participants (mean = 59.3) compared to the mean of 52.89 in the Narcisa et al. (2011) study. Further, gender might be another contributing factor, where Narcisa et al. (2011) included females only, whereas, our study had males only. Hence, a subset of our subjects might be showing signs of ataxia because of being older males.
Importantly, this study aimed to identify molecular biomarkers at the FMR1 locus (FMR1, ASFMR1, and ASFMR1 131 bp splicing isoform) that may correlate with early emergence of movement abnormalities in premutation carriers without FXTAS and might be helpful as potential biomarkers in longitudinal studies. We found an inverse correlation between both ASFMR1 131 bp splice isoform and tremor intensity for the non-dominant hand. Thus, using a combination of molecular and clinical phenotypic measures could help to identify changes in premutation carriers most at risk for developing FXTAS.
Interestingly, the expression of ASFMR1 131 bp splice isoform mRNA was higher in premutation carriers overall compared to controls, but it did not distinguish between non-FXTAS and FXTAS premutation carrier groups. The function, if any, of this splicing isoform is currently unknown and the dysregulation of the alternative splicing process and the levels of expression of the ASFMR1 could be part of the pathogenesis of FXTAS as could be the case for the FMR1 gene (Tseng et al., 2017). Moreover, it was previously found that RAN translation, which is one of the mechanisms proposed to explain the pathogenesis of FXTAS, also occurs in the antisense direction generating novel proteins that accumulate in ubiquitinated inclusions in FXTAS patients (Krans et al., 2016) further supporting the potential role of the antisense transcript in FXTAS. Further studies are needed to shed lights on the significance of increased expression levels of this isoforms in premutation carriers.
This study demonstrates that sensitive postural sway and tremor tests might be used in early identification of premutation carriers at risk for FXTAS, however, future longitudinal analyses with larger sample sizes are needed to confirm this hypothesis. Objective and sensitive movement measures should also be useful for monitoring disease progression, severity, and response to intervention. Further studies are warranted to further assess the correlation between the clinical and molecular measures to confirm our observations in this study.
RAO drafted the manuscript and contributed to the data analysis. FT designed the study, contributed to the data analysis, and writing the manuscript. DH and SR contributed to the design, implementation of the clinical assessments, and writing the manuscript. H-TT carried out the expression experiments and provided figures and methods for the manuscripts. AS contributed to the clinical analysis and writing the manuscript. BD-J carried out the statistical analysis and contributed to writing the manuscript.
This study was supported by Grant Nos. R01MH078041, 2R01HD036071, and U54HD079125. The work was also supported by Award No. T32MH073124 from the National Institute of Mental Health, IDDRC Grant No. U54 HD079125 and the CTSC Grant No. UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was dedicated to the memory of Matteo.
FT received funding from Asuragen, Inc. and Zynerba.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aguilar, D., Sigford, K. E., Soontarapornchai, K., Nguyen, D. V., Adams, P. E., Yuhas, J. M., et al. (2008). A quantitative assessment of tremor and ataxia in FMR1 premutation carriers using CATSYS. Am. J. Med. Genet. A 146, 629–635. doi: 10.1002/ajmg.a.32211
Allen, E. G., Juncos, J., Letz, R., Rusin, M., Hamilton, D., Novak, G., et al. (2008). Detection of early FXTAS motor symptoms using the CATSYS computerised neuromotor test battery. Med. Genet. 45, 290–297. doi: 10.1136/jmg.2007.054676
Allen, E. G., Sherman, S., Abramowitz, A., Leslie, M., Novak, G., Rusin, M., et al. (2005). Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behav. Genet. 35, 435–445. doi: 10.1007/s10519-005-2792-4
Després, C., Lamoureux, D., and Beuter, A. (2000). Standardization of a neuromotor test battery: the CATSYS system. Neurotoxicology 21, 725–735.
Filipovic-Sadic, S., Sah, S., Chen, L., Krosting, J., Sekinger, E., Zhang, W., et al. (2010). A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 56, 399–408. doi: 10.1373/clinchem.2009.136101
Ginno, P. A., Lim, Y. W., Lott, P. L., Korf, I., and Chédin, F. (2013). GC skew at the 5’ and 3’ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 23, 1590–1600. doi: 10.1101/gr.158436.113
Grigsby, J., Brega, A. G., Engle, K., Leehey, M. A., Hagerman, R. J., Tassone, F., et al. (2008). Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology 22, 48–60. doi: 10.1037/0894-4105.22.1.48
Hagerman, P. J., and Hagerman, R. J. (2015). Fragile X-associated tremor/ataxia syndrome. Ann. N. Y. Acad. Sci. 1338, 58–70. doi: 10.1038/nbt.3121.ChIP-nexus
Hagerman, R. J., and Hagerman, P. (2016). Fragile X - associated tremor / ataxia syndrome — features, mechanisms and management. Nat. Publ. Group 12, 403–412. doi: 10.1038/nrneurol.2016.82
Hall, D., Hall, P., Pandya, S., Sharp, K., Zhou, L., et al. (2017). “Antisense FMR1 splice variant and loss of AGG interruptions are predictors of Fragile X-associated tremor/ataxia syndrome (FXTAS),” in Proceedings of the 3rd International Conference on FMR1 Premutation: Basic Mechanisms and Clinical Involvement, Jerusalem.
Hall, D. A., Birch, R. C., Anheim, M., Jønch, A. E., Pintado, E., O’Keefe, J., et al. (2014). Emerging topics in FXTAS. J. Neurodev. Disord. 6:31. doi: 10.1186/1866-1955-6-31
Hamperl, S., and Cimprich, K. A. (2014). The contribution of co-transcriptional RNA: DNA hybrid structures to DNA damage and genome instability. DNA Repair 19, 84–94. doi: 10.1016/j.dnarep.2014.03.023
Jacquemont, S., Farzin, F., Hall, D., Leehey, M., Tassone, F., Gane, L., et al. (2004). Aging in individuals with the FMR1 mutation. Am. J. Ment. Retard. 109, 154–164. doi: 10.1016/j.pestbp.2011.02.012.Investigations
Julie, S., and Karlene, C. (2015). R-loops breaking bad. Trends Cell Biol. 25, 514–522. doi: 10.1016/j.tcb.2015.05.003.R-Loops
Kenneson, A., Zhang, F., Hagedorn, C. H., and Warren, S. T. (2001). Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 10, 1449–1454. doi: 10.1093/hmg/10.14.1449
Krans, A., Kearse, M. G., and Todd, P. K. (2016). RAN translation from antisense CCG repeats in Fragile X Tremor/ Ataxia Syndrome. Ann. Neurol. 80, 871–881. doi: 10.1111/obr.12065.Variation
Ladd, P. D., Smith, L. E., Rabaia, N. A., Moore, J. M., Georges, S. A., Hansen, S. R., et al. (2007). An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum. Mol. Genet. 16, 3174–3187. doi: 10.1093/hmg/ddm293
Loesch, D. Z., Godler, D. E., Evans, A., Bui, Q. M., Gehling, F., Kotschet, K. E., et al. (2011). Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet. Med. 13, 392–399. doi: 10.1097/GIM.0b013e3182064362
Loomis, E. W., Sanz, L. A., Chédin, F., and Hagerman, P. J. (2014). Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 10:e1004294. doi: 10.1371/journal.pgen.1004294
Narcisa, V., Aguilar, D., Nguyen, D. V., Campos, L., Brodovsky, J., White, S., et al. (2011). A Quantitative assessment of tremor and ataxia in female FMR1 premutation carriers using CATSYS. Curr. Gerontol. Geriatr. Res. 2011, 1–7. doi: 10.1155/2011/484713
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Sellier, C., Usdin, K., Pastori, C., Peschansky, V. J., Tassone, F., and Charlet-Berguerand, N. (2014). The multiple molecular facets of fragile X-associated tremor/ataxia syndrome. J. Neurodev.Disord. 6, 1–10. doi: 10.1186/1866-1955-6-23
Tassone, F., Beilina, A., Carosi, C., Albertosi, S., Bagni, C., Li, L., et al. (2007). Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13, 555–562. doi: 10.1261/rna.280807
Tassone, F., Hagerman, R. J., Taylor, A. K., Gane, L. W., Godfrey, T. E., and Hagerman, P. J. (2000). Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15. doi: 10.1086/302720
Tassone, F., and Hall, D. A. (eds). (2016). FXTAS, FXPOI, and Other Premutation Disorders. Basel: Springer International Publishing.
Tassone, F., Pan, R., Amiri, K., Taylor, A. K., and Hagerman, P. J. (2008). A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagn. 10, 43–49. doi: 10.2353/jmoldx.2008.070073
Todd, P. K., Oh, S. Y., Krans, A., He, F., Sellier, C., Frazer, M., et al. (2013). Article CGG repeat-associated translation mediates neurodegeneration in Fragile X Tremor Ataxia Syndrome. Neuron 78, 440–455. doi: 10.1016/j.neuron.2013.03.026
Keywords: FMR1, ASFMR1, transcription, premutation, FXTAS, CATSYS, splicing isoforms
Citation: Al Olaby RR, Tang H-T, Durbin-Johnson B, Schneider A, Hessl D, Rivera SM and Tassone F (2018) Assessment of Molecular Measures in Non-FXTAS Male Premutation Carriers. Front. Genet. 9:302. doi: 10.3389/fgene.2018.00302
Received: 22 January 2018; Accepted: 17 July 2018;
Published: 22 August 2018.
Edited by:
Prashant Kumar Verma, King Abdulaziz University, Saudi ArabiaReviewed by:
Ranhui Duan, Central South University, ChinaCopyright © 2018 Al Olaby, Tang, Durbin-Johnson, Schneider, Hessl, Rivera and Tassone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flora Tassone, ZnRhc3NvbmVAdWNkYXZpcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.