- 1Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 2Pharmacy Unit, Udon Thani Hospital, Udon Thani, Thailand
- 3School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 4Department of Pathology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 5Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand
Severe cutaneous adverse drug reactions (SCARs) including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reactions with eosinophilia and systemic symptoms (DRESS) are potentially life-threatening cutaneous reactions caused by several drugs. Recently, a number of genes encoding for human antigen presenting proteins, HLA alleles, have been discovered as valid pharmacogenetic markers for prediction of these life-threatening reactions. This study was aimed to determine the distribution of HLA alleles including the HLA class I and class II genes in 183 unrelated individuals of a Thai population using high resolution HLA genotyping in order to obtain 2-field data (4-digit resolution) and compare the frequencies of the HLA alleles that have been proposed as markers of SCARs with other ethnics. Results revealed a high prevalence of pharmacogenetic markers of drug-induced SCARs e.g., B*13:01 for dapsone; B*15:02 for carbamazepine and oxcarbazepine; B*58:01, A*33:03 and C*03:02 for allopurinol; C*08:01, C*14:02 and DRB1*12:02 for co-trimoxazole. Whereas, low prevalence of pharmacogenetic markers of SCARs induced by abacavir, B*57:01 and phenytoin, B*56:02/B*56:04 were noticed. The allele frequencies of B*13:01, B*15:02, and B*58:01 observed in a Thai population were significantly higher than those reported in Japanese and Caucasian populations. Similar to those observed in other Southeast Asian populations, low frequencies of A*31:01 and B*57:01 alleles were noted in the study population. Based on the frequencies of HLA pharmacogenetic markers, Thai and other Southeast Asian populations may at higher risk of drug-induced SCARs compared with Caucasian population.
Introduction
Adverse drug reactions are generally classified into two major types, type A and type B. Type A adverse drug reactions are generally related to the mechanism of action and dose of the drugs. Whereas, type B adverse drug reactions are unpredictable reactions occurring only in susceptible individuals and generally not related to the mechanism of action of the drugs (Aronson and Ferner, 2005). Although type B adverse drug reactions occur less frequently, they are relatively more severe than type A adverse drug reactions. Among type B adverse drug reactions, cutaneous adverse drug reactions are the most common reactions. Phenotypes of cutaneous reactions caused by drugs may range from mild cutaneous reactions such as maculopapular rash, urticaria to life-threatening severe cutaneous adverse reactions (SCARs) such as Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and drug reactions with eosinophilia and systemic symptoms (DRESS) (Roujeau, 2005). SJS and TEN are cutaneous reactions with the same etiology but differ only to the extent of skin detachment relative to the body surface area (BSA) which is limited to less than 10% of BSA in SJS, and widespread with more than 30% of BSA in TEN. DRESS is characterized by a generalized skin rash with fever, hematologic abnormalities e.g., eosinophilia or atypical lymphocytes, as well as multiple organ involvement may be present. Mortality of SCARs ranges from 5 to 10% in SJS or DRESS and up to 30% in TEN (Roujeau, 2005). Moreover, the patients who recover from SCARs episodes may be left with sequelae or long-lasting disabilities such as blindness. Therefore, identification of factors that are involved in the individual susceptibility to these SCARs may significantly decrease the mortality rate and healthcare costs as well as providing increased safety for drug therapy.
Several lines of evidence have shown that genetic polymorphisms of human leukocyte antigen (HLA) genes may play important roles in the susceptibility of an individual to these life-threatening SCARs. To date, several HLA alleles have been discovered to be strongly associated with SCARs and some of them have been proposed as valid genetic markers for prediction of these life-threatening reactions. These include B*57:01 for abacavir-induced drug hypersensitivity (Mallal et al., 2002, 2008); B*15:02 for carbamazepine-induced SJS/TEN (Chung et al., 2004; Hung et al., 2006; Tassaneeyakul et al., 2010; Genin et al., 2014); B*58:01 (Hung et al., 2005; Tassaneeyakul et al., 2009), A*33:03, and C*03:02 (Hung et al., 2005) for allopurinol-induced SCARs; B*13:01 for dapsone hypersensitivity (Zhang et al., 2013). In addition, other HLA alleles have been reported to be associated with drug-induced SCARs such as B*15:02, B*51:01, B*56:02/B*56:04, C*14:02 for phenytoin-induced SCARs (Chung et al., 2014; Tassaneeyakul et al., 2016); B*15:02, C*06:02, C*08:01, DRB1*12:02 for co-trimoxazole-induced SJS/TEN (Kongpan et al., 2015); B*35:05 for nevirapine-induced rash (Chantarangsu et al., 2009) or SJS/TEN (Carr et al., 2013); A*02:06 and B*44:03 for cold medication-induced SJS/TEN (Ueta et al., 2014) and B*59:01 for methazolamide-induced SJS/TEN (Yang et al., 2016). Apart from drug-induced SCARs, several of type B adverse drug reactions including drug-induced agranulocytosis (Cheung et al., 2016) and pure red cell aplasia (Praditpornsilpa et al., 2009) have also been reported to be associated with certain HLA alleles. Given the serious and life-threatening consequences of SCARs and their strong association with HLA alleles, the regulatory agencies as well as the Clinical Pharmacogenetics Implementation Consortium (CPIC) suggest physicians to perform HLA screening tests in individual patients prior to initiation of some drug prescriptions (Leckband et al., 2013; Saito et al., 2016).
It should be noted that the associations between SCARs and HLA alleles are specific to certain alleles of HLA gene, therefore high resolution DNA typing is an essential tool for determination of these pharmacogenetic markers. Information about the frequency of these pharmacogenetic HLA alleles, particularly the 2-field data (4-digit resolution) are important parameters necessary for estimating the size of population at risk for drug-induced SCARs. In this study, the distribution of the HLA class I and class II alleles in unrelated individuals of a Thai population was investigated using high resolution DNA typing technique. In addition, the frequencies of HLA alleles which have been proposed as pharmacogenetic markers of drug hypersensitivity were compared between Thais and other ethnic groups.
Materials and Methods
Subjects
A total of 183 unrelated native Thais were recruited in the study and all of them were classified as native Northeastern Thais according to family history of their parents and grandparents. Study population was recruited from subjects who underwent for annual health checkup program in hospitals located in the Northeastern region of Thailand and all subjects had no history of drug allergy. Written informed consent was obtained from each subject. The study protocol was approved by the Ethics Committee for Human Research, Khon Kaen University, Thailand (HE510837).
Genomic DNA Preparation
Peripheral blood samples were collected into EDTA-coated tubes. Leukocytes were separated by centrifugation at 2500 rpm for 15 min. Genomic DNA was then isolated from leukocytes using a QIAamp® DNA Blood mini kits (QIAGEN® GmbH, Hilden, Germany).
HLA Genotyping
The 4-digit resolution of HLA alleles of both Class I (e.g. HLA-A, HLA-B, HLA-C), and Class II (e.g., HLA-DRB1) genes were genotyped by using the WAKFLOW® HLA typing kits (Wakunaga Pharmaceutical Co. Ltd, Hiroshima, Japan), which is based on the reverse sequence-specific oligonucleotide probes (SSO) method coupled with xMAP technology designed to use with the Luminex® system. In brief, the target DNA was first amplified by polymerase chain reaction (PCR) with biotinylated primers specifically designed for each HLA locus. The PCR product was subsequently denatured and hybridized to the complementary oligonucleotide probes immobilized on fluorescently coded microsphere beads. The biotinylated PCR product was labeled with phycoerythrin-conjugated streptavidin to allow it to be detected by the Luminex® 100 system (Luminex Corporation, Austin, Texas, USA). The HLA alleles were analyzed using WAKFLOW® HLA Software version 3.2 based on IPD-IMGT/HLA Database release 3.20.0 (https://www.ebi.ac.uk/ipd/imgt/hla/). In case of genotyping ambiguities, the most common alleles in Thai population were assigned based on The Allele Frequency Net Database (www.allelefrequencies.net).
Statistical Analysis
The allele frequencies and genotype frequencies of the HLA alleles were determined by direct counting. The samples were tested for the Hardy-Weinberg equilibrium using the Chi-square or Fisher's exact test using SPSS Statistics 17.0 (SPSS Inc., Chicago, USA). The haplotype frequencies were also carried out using the haplo.em function in haplo.stats packages (version 1.7.7) operated in the R language version 3.3.1 (https://CRAN.R-project.org/package=haplo.stats). The linkage disequilibrium of individual HLA alleles at each of two loci (D'ij) and their correlation coefficient (r2) were calculated using the PLINK V1.07 program (http://zzz.bwh.harvard.edu/plink/). The difference in the frequencies of HLA alleles which have been proposed as pharmacogenetic markers of drug hypersensitivity between this study population and other ethnic groups was tested using the chi-square method and a P-value of less than 0.05 was considered as statistical significance.
Results
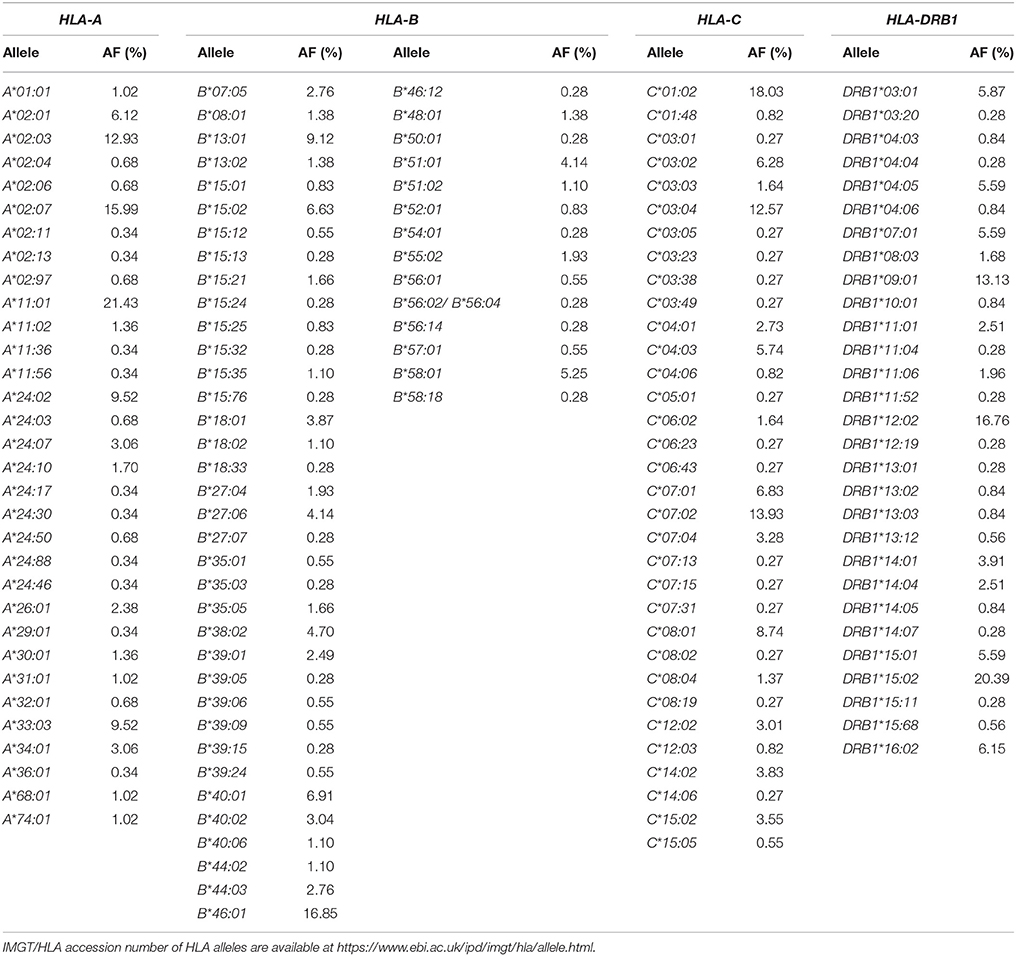
One hundred and eighty-three unrelated Thais consisting of 77 women (42.08%) and 106 men (57.92%) were recruited for the study. Of the HLA Class I and Class II alleles, 32 HLA-A alleles, 50 HLA-B alleles, 33 HLA-C alleles, and 29 HLA-DRB1 alleles were identified in the study population as shown in Table 1. The frequencies of HLA class I and II alleles observed in this study did not significantly deviate from the Hardy-Weinberg equilibrium (P > 0.05). Among the HLA class I, the common alleles of HLA-A allele were A*11:01 (21.43%), A*02:07 (15.99%), A*02:03 (12.93%), A*24:02 (9.52%), and A*33:03 (9.52%) (Table 1). The common alleles of HLA-B were B*46:01 (16.85%), B*13:01 (9.12%), B*40:01 (6.91%), B*15:02 (6.63%), and B*58:01 (5.25%) (Table 1). While the common alleles of HLA-C were C*01:02 (18.03%), C*07:02 (13.93%), C*03:04 (12.57%), C*08:01 (8.74%), and C*07:01 (6.83%) (Table 1). For the HLA class II, only HLA-DRB1 genotypes were determined in the present study. Of the HLA-DRB1 alleles, the common alleles were DRB1*15:02 (20.39%), DRB1*12:02 (16.76%), DRB1*09:01 (13.13%), DRB1*16:02 (6.15%), and DRB1*03:01 (5.87%) (Table 1). The allele frequencies of HLA alleles that have been proposed as pharmacogenetic markers of drug hypersensitivity were compared with other ethnics (Table 2).
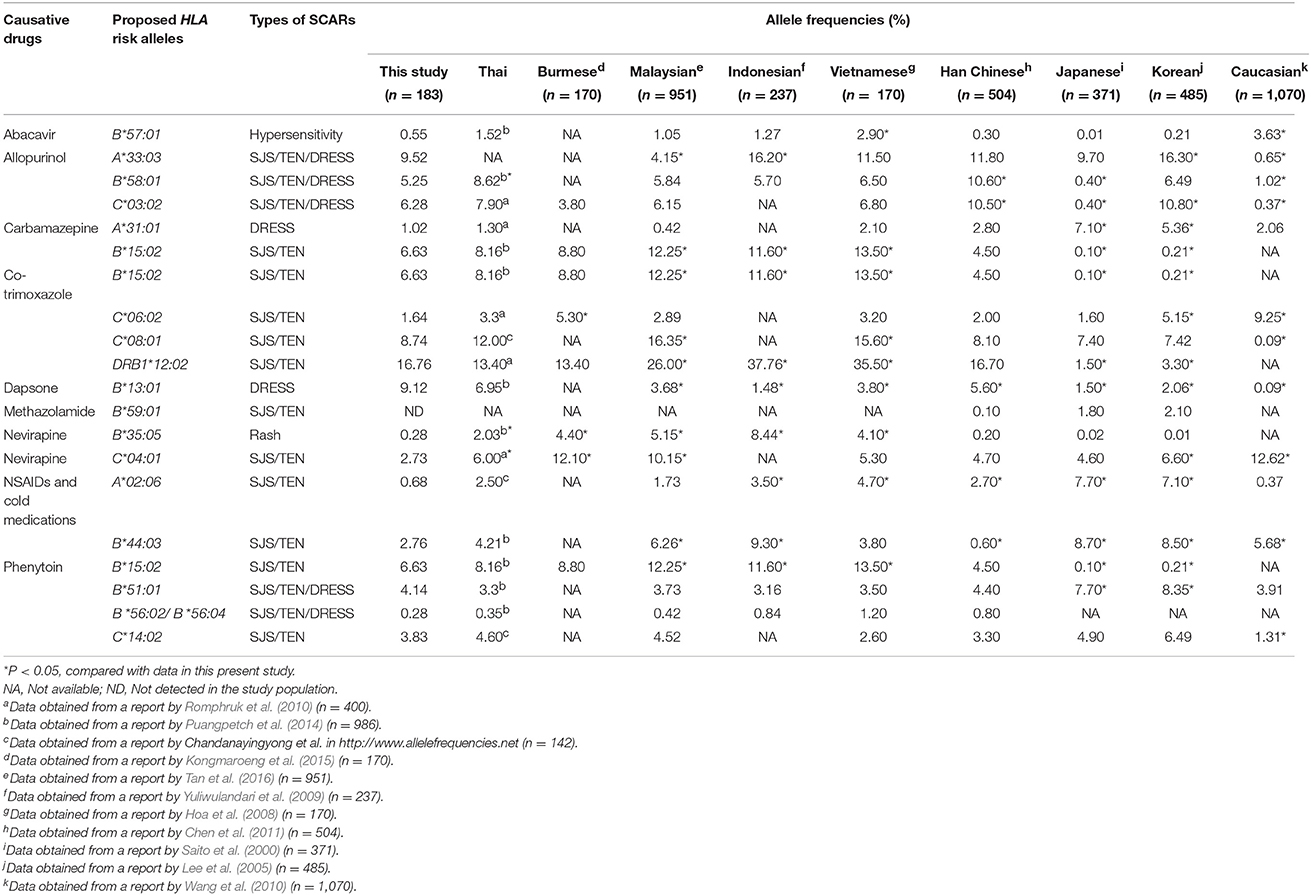
Table 2. Comparison of the frequencies of HLA alleles which have been proposed as pharmacogenetic markers of drug hypersensitivity in several ethnic populations.
Among the HLA-A genotypes, the common genotypes observed in this study population were A*02:07+A*11:01 (7.48%), A*11:01+A*11:01 (6.12%), A*11:01+A*24:02 (5.44%) whereas those of HLA-B genotypes were B*13:01+B*46:01 (4.42%), B*15:02+B*46:01 (3.87%), B*40:01+B*46:01 (3.31%). For HLA-C genotypes, the common genotypes were C*01:02+C*03:04 (6.01%), C*01:02+C*07:02 (4.37%), C*03:04+C*07:02 (3.83%). Among the HLA-DRB1 genotypes, the common genotypes were DRB1*12:02+DRB1*15:02 (6.15%), DRB1*09:01+DRB1*12:02 (5.03%), and DRB*09:01+DRB1*15:02 (5.03%). In addition, the common frequencies of the two-locus HLA haplotypes were B*46:01~C*01:02 (14.34%), A*02:07~B*46:01 (10.25%), A*02:07~C*01:02 (10.18%), B*58:01~C*03:02 (5.59%), A*33:03~C*03:02 (5.59%), and B*13:01~C*03:04 (5.24%). For the three-locus HLA haplotypes, the predominant haplotypes were A*02:07~B*46:01~C*01:02 (9.40%), A*33:03~B*58:01~C*03:02 (4.53%), and A*11:01~B*46:01~C*01:02 (3.11%). While the common haplotypes of the four-locus HLA haplotypes were A*02:07~B*46:01~C*01:02~DRB1*09:01 (5.92%) and A*33:03~B*58:01~C*03:02~DRB1*03:01 (3.50%). The HLA genotypes and HLA haplotypes that were found in more than 1% of the study population are presented in Tables S1, S2.
Discussion
The allele, genotype, and haplotype frequencies of both HLA class I and class II genes in a Thai population were obtained from high-resolution HLA typing and presented in 2-field data (4-digit resolution). Large variations at both HLA class I and class II loci in which 32 alleles for HLA-A, 50 alleles for HLA-B, 33 alleles for HLA-C and 29 alleles for HLA-DRB1 were observed in the study population. Higher frequencies of B*13:01, B*15:02, and B*58:01 alleles which have been proposed as a genetic markers of SCARs induced by dapsone, carbamazepine and allopurinol were observed in this study population compared with those reported in Japanese and Caucasian populations. Whereas, the frequency of A*31:01 allele, a genetic marker of carbamazepine-induced DRESS, was significantly lower than those reported in Japanese and Korean populations. Higher frequencies of HLA risk alleles suggested that Thai and other Southeast Asian populations may at higher risk of drug-induced SCARs compared with Japanese and Caucasian populations.
It is now well recognized that HLA molecules play key role in the immunopathogenesis of drug-induced SCARs and at least three models have been proposed to explain how a drug elicits a HLA-dependent T cell reactions and leads to immunoresponse (Chung et al., 2016). The hapten/prohapten model proposes that the offending drug or its metabolite covalently binds to an endogenous peptide to form a HLA-peptide-drug complex in the antigen-presenting cells (APC), then these modified peptides are recognized as foreign by T-cells and stimulate an immune response. Whereas, the p-i model proposes that drug/its metabolite may directly and non-covalently bind to the HLA and/or TCR protein in a peptide independent manner to directly activate T-cells. The altered peptide repertoire model proposes that the offending drug occupies a specific site in the peptide binding groove of the HLA molecules, changing the chemistry of the binding cleft and the repertoire of peptides that are recognized by HLA molecules (Chung et al., 2016). High frequencies of some HLA alleles which have been proposed as valid marker for drug-induced SCARs observed in a Thai population suggesting a high number of Thai patients may be at a higher risk of drug-induced SCARs. Screening of such HLA alleles prior to prescribing a drug in order to predict or avoid drug-induced SCARs in this population need to be considered.
For HLA-A alleles, the A*11:01 was the allele with the highest frequency (21.43%.) in the study population (Table 1). This result was consistent with the 1-field data of the HLA-A allele (23.3%) previously reported in a Northeastern Thai population (Romphruk et al., 2010). To date, only two alleles of HLA-A are reported to be associated with drug hypersensitivity, including A*31:01 related with carbamazepine-induced DRESS (Genin et al., 2014) and A*33:03 related allopurinol-induced SCARs (Hung et al., 2005). Recent studies have demonstrated that the A*31:01 allele was strongly associated with carbamazepine-induced DRESS in several ethnicities including European and Asian (Genin et al., 2014) populations with the odds ratios ranging from 6.4 to 57.6. In contrast, the association between A*31:01 and carbamazepine-induced SJS/TEN was not significant in European nor Chinese populations (Genin et al., 2014). The A*31:01 allele is now proposed as a genetic marker for DRESS caused by carbamazepine in multiethnic societies (Genin et al., 2014). Compared with other ethnic groups, the allele frequency of A*31:01 in this study population (1.02%) was about 2- to 7- fold lower than those reported in Japanese (Saito et al., 2000), Korean (Lee et al., 2005), Europeans (Wang et al., 2010). Similar to that observed in this study population, the frequency of A*31:01 was also low in other South-East Asians populations (Table 2).
The markedly strong association between B*15:02 allele and SJS/TEN caused by carbamazepine has been discovered in Han Chinese (Chung et al., 2004; Hung et al., 2006), Indian (Mehta et al., 2009) and South-East Asians including Thai (Tassaneeyakul et al., 2010) and Malaysian (Chang et al., 2011) populations. It should be noted that the association of B*15:02 and carbamazepine-induced SJS/TEN is ethnicity specific, with the association relevant in Asian populations but not in Japanese (Kaniwa et al., 2010), Korean (Kim et al., 2011) and Europeans (Lonjou et al., 2008). Moreover, this allele has recently been shown to be strongly associated with oxcarbazepine-induced SJS/TEN in Han Chinese and Thai populations (Chen et al., 2017). Furthermore, a significant association between B*15:02 and lamotrigine-induced SJS/TEN in Han Chinese has been reported (Cheung et al., 2013). Compared with Japanese, Korean and European populations, the frequency of the B*15:02 allele in Thai and other Southeast Asian populations was much higher (Table 2). A high frequency of the B*15:02 allele in Southeast Asian populations suggests that these populations may have a higher risk of SJS/TEN induced by carbamazepine or oxcarbazepine. This hypothesis was supported by the data from the World Health Organization Uppsala Monitoring Center (WHO-UMC) showing that carbamazepine-was associated with SJS and TEN from the two Southeast Asian countries, Thailand and Malaysia that was far in excess of that from the predominantly European countries. It has also been demonstrated that B*15:02 screening prior to carbamazepine treatment is cost-effective for prevention of carbamazepine-induced SJS/TEN in Thailand (Rattanavipapong et al., 2013) and Singapore (Dong et al., 2012).
Although previous study in Chinese population has reported a significant association between the B*15:02 allele and phenytoin-induced SJS/TEN (Chung et al., 2014), this association was not significant in the Thai population (Tassaneeyakul et al., 2016). In addition, several HLA alleles including B*51:01, B*56:02/B*56:04 and C*14:02 have been reported to be associated with phenytoin-induced SCARs in Thai population (Chung et al., 2014; Tassaneeyakul et al., 2016. The allele frequencies of B*51:01, B*56:02/B*56:04 and C*14:02 in Thai population found in the present study were 4.14, 0.28, and 3.83%, respectively (Table 2). Moreover, previous study has shown that the risk of phenytoin-induced SCARs in patients who carried B*51:01~C*14:02 haplotype was almost 6-fold compared with those who did not has this haplotype (Tassaneeyakul et al., 2016). Results from haplotype analysis among HLA risk alleles of phenytoin-induced SCARs in the study reveal that the B*51:01~C*14:02 haplotype is the only haplotype that exhibit frequency higher than 1% in the study population (Table S2). In addition, the linkage disequilibrium between B*51:01~C*14:02 in the study population was also noticed (HF = 3.85%, D'ij = 0.83, r2 = 0.69).
For the B*58:01 allele, a proposed pharmacogenetic marker of allopurinol-induced SCARs, the frequency of this allele in this study population was 5.25%. The allele frequency observed in a Thai population was close to those reports in several Southeast Asians including Malaysian, Indonesian and Vietnamese populations (Table 2). The high frequency of this allele has also been reported in Han Chinese and Korean populations (Table 2). The high frequency of B*58:01 alleles observed in this study may suggest that Thai patients who use allopurinol may be at a high risk of allopurinol-induced SCARs. According to the data from the spontaneous reports by the Health Product Vigilance Center of Thailand, allopurinol is second ranked of common culprit drugs, with at least 1,488 patients suffering from SJS/TEN during the last 20 years (unpublished data, available at: http://thaihpvc.fda.moph.go.th/thaihvc/Public/News/uploads/hpvc_5_13_0_100526.pdf). Screening for the B*58:01 allele prior to allopurinol administration has been shown to be a cost-effective intervention for prevention of allopurinol-induced SJS/TEN in Thailand (Saokaew et al., 2014) may partly due to high frequency of this allele in Thai population as observed in the present study. In addition, high frequencies of A*33:03 (9.52%) and C*03:02 (6.28%) alleles were observed in this study. Linkage disequilibrium among B*58:01~C*03:02 (HF = 5.59%, D'ij = 0.94, r2 = 0.78), B*58:01~A*33:03 (HF = 4.90%, D'ij = 0.79, r2 = 0.39), A*33:03~C*03:02 (HF = 5.59%, D'ij = 0.75, r2 = 0.40) was noticed in the study population. Previous studies have reported the significant association between A*33:03 and C*03:02 and allopurinol-induced SCARs (Hung et al., 2005). Results from the present study suggest that it is likely that the association between A*33:03 and C*03:02 alleles and allopurinol-induced SCARs may partly due to linkage disequilibrium with B*58:01 allele.
According to the B*13:01, the frequency of this allele found in this study population was quite high (9.12%, Table 2). The B*13:01 allele has been reported to be strongly linked with the dapsone-induced hypersensitivity syndrome in Han Chinese (Zhang et al., 2013). A significant association between this allele and dapsone-induced SCARs was also revealed in Thai patients (Tempark et al., 2017). A high frequency of the B*13:01 allele observed in this study and its significant association with dapsone-induced SCARs suggests that although the use of dapsone is not common in Thailand, a patient who receives this drug may need to be closely monitored in order to prevent the dapsone-induced drug hypersensitivity.
The B*57:01 allele is considered as a rare HLA-B allele in the Thai population with the frequency of only 0.55% (Table 2). Compared with Caucasians, the frequency of the B*57:01 in Thai and other Southeast Asians as well as East Asian populations was much lower (Table 2). The strong association between the B*57:01 and abacavir hypersensitivity has been demonstrated particularly in Caucasian populations both in retrospective and prospective studies (Mallal et al., 2002, 2008), however, there is still no clear evidence for such an association in Asian populations. The rare frequency of the B*57:01 allele found in this study suggests that B*57:01 screening may not be a cost-effective intervention for prevention of abacavir-induced SCARs in Thailand.
Although the sample size of this study was not so large when compared with other previous reports in Thai population, only low resolution data of HLA alleles (2-digit resolution) (Kupatawintu et al., 2010; Romphruk et al., 2010) and no complete data of HLA alleles of Class I and Class II (Puangpetch et al., 2014), were demonstrated in most of those previous reports. As mentioned in previous association studies, only specific HLA alleles have been demonstrated to be associated with drug-induced SCARs. It should be noticed that although there are more than 400 and 90 alleles of 2-digit resolution of B*15 and B*58 in a worldwide population, higher risks of carbamazepine-induced SJS/TEN and allopurinol-induced SCARs are observed only in patients who carried B*15:02 allele (Hung et al., 2006; Tassaneeyakul et al., 2010) and B*58:01 allele (Hung et al., 2005; Tassaneeyakul et al., 2009). Thus, genotyping of 4-digit resolution of HLA alleles prior prescription of these causative drugs is necessary to identify individuals who may at higher risk of these life-threatening adverse drug reactions.
However, it should be noted that detecting the 4-digit resolution of HLA alleles requires the medium to high throughput HLA genotyping technology and this technology are quite expensive and may not be available in small hospitals. In addition, data from cost-effectiveness analysis may be necessary whether implementation of HLA risk allele screening is justifiable and valuable in preventing drug-induced SCARs in nationwide. Higher frequencies of B*1502 and B*58:01 observed in a Thai population as observed in the present study suggest that B*1502 and B*58:01 screening may be cost-effectiveness tools for prevention of carbamazepine- and allopurinol-induced SJS/TEN in a Thai population whereas screening of the low frequencies HLA risk alleles may not be warranty cost-effectiveness.
In conclusion, the 2-field data or 4-digit resolution of allele, genotype and haplotype frequencies both of HLA Class I and Class II that have been reported as genetic markers of drug hypersensitivity in a Thai population are reported in this study. The results revealed high frequency of A*33:03, B*13:01, B*15:02, B*58:01, C*03:02 C*08:01, C*14:02, and DRB1*12:02 alleles but a low frequency of A*31:01, B*57:01, and B*56:02/B*56:04 in this study population. The information obtained from this study provides essential parameters for estimating the size of population who may at higher risk of drug-induced SCARs and useful for cost-effective analysis of these HLA alleles screening prior drug administration.
Author Contributions
WiT and NN: Study design; PK, UK, NS, and KK: Subject enrollment; NN, TK, NS, AD, and KK: Perform experiment; WiT, NN, WT, SK, and NS: Data collection and analysis; WiT, NN, WT, SK, NS, PK, UK, AD, KK, and TK: Manuscript drafting.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ET and handling Editor declared their shared affiliation.
Acknowledgments
This work was supported by grants from the Invitation Research, Faculty of Medicine, Khon Kaen University. The authors thank Professor James A. Will, University of Wisconsin-Madison, for his valuable comments and critical review of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00277/full#supplementary-material
References
Aronson, J. K., and Ferner, R. E. (2005). Clarification of terminology in drug safety. Drug Saf. 28, 851–870. doi: 10.2165/00002018-200528100-00003
Carr, D. F., Chaponda, M., Jorgensen, A. L., Castro, E. C., van Oosterhout, J. J., Khoo, S. H., et al. (2013). Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin. Infect. Dis. 56, 1330–1339. doi: 10.1093/cid/cit021
Chang, C. C., Too, C. L., Murad, S., and Hussein, S. H. (2011). Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int. J. Dermatol. 50, 221–224. doi: 10.1111/j.1365-4632.2010.04745.x
Chantarangsu, S., Mushiroda, T., Mahasirimongkol, S., Kiertiburanakul, S., Sungkanuparph, S., Manosuthi, W., et al. (2009). HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet. Genom. 19, 139–146. doi: 10.1097/FPC.0b013e32831d0faf
Chen, C. B., Hsiao, Y. H., Wu, T., Hsih, M. S., Tassaneeyakul, W., Jorns, T. P., et al. (2017). Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology 88, 78–86. doi: 10.1212/WNL.0000000000003453
Chen, P. L., Fann, C. S., Chu, C. C., Chang, C. C., Chang, S. W., Hsieh, H. Y., et al. (2011). Comprehensive genotyping in two homogeneous Graves' disease samples reveals major and novel HLA association alleles. PLoS ONE 6:e16635. doi: 10.1371/journal.pone.0016635
Cheung, C. L., Sing, C. W., Tang, C. S., Cheng, V. K., Pirmohamed, M., Choi, C. H., et al. (2016). HLA-B*38:02:01 predicts carbimazole/methimazole-induced agranulocytosis. Clin. Pharmacol. Ther. 99, 555–561. doi: 10.1002/cpt.309
Cheung, Y. K., Cheng, S. H., Chan, E. J., Lo, S. V., Ng, M. H., and Kwan, P. (2013). HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia 54, 1307–1314. doi: 10.1111/epi.12217
Chung, W. H., Chang, W. C., Lee, Y. S., Wu, Y. Y., Yang, C. H., Ho, H. C., et al. (2014). Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA 312, 525–534. doi: 10.1001/jama.2014.7859
Chung, W. H., Hung, S. I., Hong, H. S., Hsih, M. S., Yang, L. C., Ho, H. C., et al. (2004). Medical genetics: a marker for Stevens-Johnson syndrome. Nature 428:486. doi: 10.1038/428486a
Chung, W. H., Wang, C. W., and Dao, R. L. (2016). Severe cutaneous adverse drug reactions. J. Dermatol. 43, 758–766. doi: 10.1111/1346-8138.13430
Dong, D., Sung, C., and Finkelstein, E. A. (2012). Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 79, 1259–1267. doi: 10.1212/WNL.0b013e31826aac73
Genin, E., Chen, D. P., Hung, S. I., Sekula, P., Schumacher, M., Chang, P. Y., et al. (2014). HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenom. J. 14, 281–288. doi: 10.1038/tpj.2013.40
Hoa, B. K., Hang, N. T., Kashiwase, K., Ohashi, J., Lien, L. T., Horie, T., et al. (2008). HLA-A, -B, -C, -DRB1 and -DQB1 alleles and haplotypes in the Kinh population in Vietnam. Tissue Antigens 71, 127–134. doi: 10.1111/j.1399-0039.2007.00982.x
Hung, S. I., Chung, W. H., Jee, S. H., Chen, W. C., Chang, Y. T., Lee, W. R., et al. (2006). Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet. Genom. 16, 297–306. doi: 10.1097/01.fpc.0000199500.46842.4a
Hung, S. I., Chung, W. H., Liou, L. B., Chu, C. C., Lin, M., Huang, H. P., et al. (2005). HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. U.S.A. 102, 4134–4139. doi: 10.1073/pnas.0409500102
Kaniwa, N., Saito, Y., Aihara, M., Matsunaga, K., Tohkin, M., Kurose, K., et al. (2010). HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 51, 2461–2465. doi: 10.1111/j.1528-1167.2010.02766.x
Kim, S. H., Lee, K. W., Song, W. J., Jee, Y. K., Lee, S. M., Kang, H. R., et al. (2011). Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 97, 190–197. doi: 10.1016/j.eplepsyres.2011.08.010
Kongmaroeng, C., Romphruk, A., Puapairoj, C., Leelayuwat, C., Kulski, J. K., Inoko, H., et al. (2015). HLA alleles and haplotypes in Burmese (Myanmarese) and Karen in Thailand. Tissue Antigens 86, 199–204. doi: 10.1111/tan.12637
Kongpan, T., Mahasirimongkol, S., Konyoung, P., Kanjanawart, S., Chumworathayi, P., Wichukchinda, N., et al. (2015). Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet. Genom. 25, 402–411. doi: 10.1097/FPC.0000000000000153
Kupatawintu, P., Pheancharoen, S., Srisuddee, A., Tanaka, H., Tadokoro, K., and Nathalang, O. (2010). HLA-A, -B, -DR haplotype frequencies in the Thai Stem Cell Donor Registry. Tissue Antigens 75, 730–736. doi: 10.1111/j.1399-0039.2010.01450.x
Leckband, S. G., Kelsoe, J. R., Dunnenberger, H. M., George, A. L. Jr., Tran, E., Berger, R., et al. (2013). Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin. Pharmacol. Ther. 94, 324–328. doi: 10.1038/clpt.2013.103
Lee, K. W., Oh, D. H., Lee, C., and Yang, S. Y. (2005). Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens 65, 437–447. doi: 10.1111/j.1399-0039.2005.00386.x
Lonjou, C., Borot, N., Sekula, P., Ledger, N., Thomas, L., Halevy, S., et al. (2008). A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet. Genom. 18, 99–107. doi: 10.1097/FPC.0b013e3282f3ef9c
Mallal, S., Nolan, D., Witt, C., Masel, G., Martin, A. M., Moore, C., et al. (2002). Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359, 727–732. doi: 10.1016/S0140-6736(02)07873-X
Mallal, S., Phillips, E., Carosi, G., Molina, J. M., Workman, C., Tomazic, J., et al. (2008). HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 358, 568–579. doi: 10.1056/NEJMoa0706135
Mehta, T. Y., Prajapati, L. M., Mittal, B., Joshi, C. G., Sheth, J. J., Patel, D. B., et al. (2009). Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J. Dermatol. Venereol. Leprol. 75, 579–582. doi: 10.4103/0378-6323.57718
Praditpornsilpa, K., Kupatawintu, P., Mongkonsritagoon, W., Supasyndh, O., Jootar, S., Intarakumthornchai, T., et al. (2009). The association of anti-r-HuEpo-associated pure red cell aplasia with HLA-DRB1*09-DQB1*0309. Nephrol. Dial. Transplant 24, 1545–1549. doi: 10.1093/ndt/gfn450
Puangpetch, A., Koomdee, N., Chamnanphol, M., Jantararoungtong, T., Santon, S., Prommas, S., et al. (2014). HLA-B allele and haplotype diversity among Thai patients identified by PCR-SSOP: evidence for high risk of drug-induced hypersensitivity. Front. Genet. 5:478. doi: 10.3389/fgene.2014.00478
Rattanavipapong, W., Koopitakkajorn, T., Praditsitthikorn, N., Mahasirimongkol, S., and Teerawattananon, Y. (2013). Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia 54, 1628–1638. doi: 10.1111/epi.12325
Romphruk, A. V., Romphruk, A., Kongmaroeng, C., Klumkrathok, K., Paupairoj, C., and Leelayuwat, C. (2010). HLA class I and II alleles and haplotypes in ethnic Northeast Thais. Tissue Antigens 75, 701–711. doi: 10.1111/j.1399-0039.2010.01448.x
Roujeau, J. C. (2005). Clinical heterogeneity of drug hypersensitivity. Toxicology 209, 123–129. doi: 10.1016/j.tox.2004.12.022
Saito, S., Ota, S., Yamada, E., Inoko, H., and Ota, M. (2000). Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens 56, 522–529. doi: 10.1034/j.1399-0039.2000.560606.x
Saito, Y., Stamp, L. K., Caudle, K. E., Hershfield, M. S., McDonagh, E. M., Callaghan, J. T., et al. (2016). Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin. Pharmacol. Ther. 99, 36–37. doi: 10.1002/cpt.161
Saokaew, S., Tassaneeyakul, W., Maenthaisong, R., and Chaiyakunapruk, N. (2014). Cost-Effectiveness Analysis of HLA-B*5801 Testing in Preventing Allopurinol-Induced SJS/TEN in Thai Population. PLoS ONE 9:e94294. doi: 10.1371/journal.pone.0094294
Tan, L. K., Mohd-Farid, B., Salsabil, S., Heselynn, H., Wahinuddin, S., Lau, I. S., et al. (2016). HLA-A, -B, -C, -DRB1 and -DQB1 alleles and haplotypes in 951 Southeast Asia Malays from Peninsular Malaysia. Hum. Immunol. 77, 818–819. doi: 10.1016/j.humimm.2016.06.022
Tassaneeyakul, W., Jantararoungtong, T., Chen, P., Lin, P. Y., Tiamkao, S., Khunarkornsiri, U., et al. (2009). Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet. Genom. 19, 704–709. doi: 10.1097/FPC.0b013e328330a3b8
Tassaneeyakul, W., Prabmeechai, N., Sukasem, C., Kongpan, T., Konyoung, P., Chumworathayi, P., et al. (2016). Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharmacogenet. Genom. 26, 225–234. doi: 10.1097/FPC.0000000000000211
Tassaneeyakul, W., Tiamkao, S., Jantararoungtong, T., Chen, P., Lin, S. Y., Chen, W. H., et al. (2010). Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 51, 926–930. doi: 10.1111/j.1528-1167.2010.02533.x
Tempark, T., Satapornpong, P., Rerknimitr, P., Nakkam, N., Saksit, N., Wattanakrai, P., et al. (2017). Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharmacogenet. Genom. 27, 429–437.doi: 10.1097/FPC.0000000000000306
Ueta, M., Kaniwa, N., Sotozono, C., Tokunaga, K., Saito, Y., Sawai, H., et al. (2014). Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe mucosal involvement. Sci. Rep. 4:4862. doi: 10.1038/srep04862
Wang, S. S., Abdou, A. M., Morton, L. M., Thomas, R., Cerhan, J. R., Gao, X., et al. (2010). Human leukocyte antigen class I and II alleles in non-Hodgkin lymphoma etiology. Blood 115, 4820–4823. doi: 10.1182/blood-2010-01-266775
Yang, F., Xuan, J., Chen, J., Zhong, H., Luo, H., Zhou, P., et al. (2016). HLA-B*59:01: a marker for Stevens-Johnson syndrome/toxic epidermal necrolysis caused by methazolamide in Han Chinese. Pharmacogenom. J. 16, 83–87. doi: 10.1038/tpj.2015.25
Yuliwulandari, R., Kashiwase, K., Nakajima, H., Uddin, J., Susmiarsih, T. P., Sofro, A. S., et al. (2009). Polymorphisms of HLA genes in Western Javanese (Indonesia): close affinities to Southeast Asian populations. Tissue Antigens 73, 46–53. doi: 10.1111/j.1399-0039.2008.01178.x
Keywords: HLA allele frequency, high-resolution, drug hypersensitivity, genetic marker, Thai
Citation: Nakkam N, Konyoung P, Kanjanawart S, Saksit N, Kongpan T, Khaeso K, Khunarkornsiri U, Dornsena A, Tassaneeyakul W and Tassaneeyakul W (2018) HLA Pharmacogenetic Markers of Drug Hypersensitivity in a Thai Population. Front. Genet. 9:277. doi: 10.3389/fgene.2018.00277
Received: 08 May 2018; Accepted: 06 July 2018;
Published: 06 August 2018.
Edited by:
George P. Patrinos, University of Patras, GreeceReviewed by:
Evangelia Eirini Tsermpini, University of Patras, GreeceAlessio Squassina, Università degli studi di Cagliari, Italy
Copyright © 2018 Nakkam, Konyoung, Kanjanawart, Saksit, Kongpan, Khaeso, Khunarkornsiri, Dornsena, Tassaneeyakul and Tassaneeyakul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wichittra Tassaneeyakul, d2ljaGl0dHJhLnRhc3NhbmVleWFrdWxAZ21haWwuY29t; d2ljaGl0dEBra3UuYWMudGg=
 Nontaya Nakkam
Nontaya Nakkam Parinya Konyoung2
Parinya Konyoung2 Sirimas Kanjanawart
Sirimas Kanjanawart Wichittra Tassaneeyakul
Wichittra Tassaneeyakul