- 1Institute of Human Genetics, Technical University of Munich, Munich, Germany
- 2Institute of Human Genetics, Helmholtz Zentrum München, Neuherberg, Germany
- 3Department of Pediatrics, Technical University of Munich, Munich, Germany
Introduction: Microdeletions in the chromosomal region 2q34 and its neighboring regions lead to a phenotypic spectrum including autism, intellectual disability, and epilepsy. Up to now, only few affected patients have been reported. Therefore, the genetic pathogenesis is not completely understood. One of the most discussed candidate genes in this context is MAP2, a gene responsible for microtubule polymerization and neurite outgrowth.
Materials and Methods: We present a 4.5-year-old male patient with epilepsy, mild developmental delay, and behavioral abnormalities. SNP-Array analysis was performed to search for pathogenic copy number variations.
Results: SNP-Array analysis revealed a 1.5 Mb de novo microdeletion on the long arm of chromosome 2 (2q34). The identified microdeletion included the candidate genes UNC80, LANCL1, and most importantly MAP2.
Discussion: The reported microdeletion identified in this patient is the smallest one described in the literature so far spanning MAP2 next to UNC80 and LANCL1. In this context MAP2 is the most important candidate gene concerning neuronal development and its function should be further examined.
Introduction
A number of genes that are relevant for neurodevelopment and neuronal function are located in the chromosomal region 2q34. Examples for this are LANCL1 (Online Mendelian Inheritance in Man, OMIM, ∗602070), encoding for lanthionine synthetase C-like protein 1 that is involved in nerve growth factor (NGF)-induced nerve growth (Zhang et al., 2009), and MAP2 (OMIM ∗157130), encoding for microtubule-associated protein-2, a neurosteroid-receptor mediating microtubule polymerization, which is also taking part in NGF-induced neurite outgrowth (Fontaine-Lenoir et al., 2006). In 2003 the role of chromosomal region 2q34 in the context of neurodevelopment was emphasized by a single case described by Pescucci et al. (2003). They reported a 14-year-old patient with a microdeletion 2q34 who showed autistic traits and Rett-like features. MAP2 was postulated as one of the possible syndrome-inducing candidate genes amongst others (Pescucci et al., 2003). Up to now, a few additional cases were reported concerning this chromosomal region. One of the most recent publications described a 4.5-year-old girl with autistic traits, Rett-like features, and a microdeletion 2q33.3q34. The authors concluded that NRP2, ADAM23, KLF7, CREB1, MAP2, UNC80, and LANCL1, all taking part in neuronal function, are possible candidate genes for this phenotype (Jang et al., 2015). Furthermore, in a genome-wide linkage meta-analysis the chromosomal region 2q34 was proposed as susceptibility locus (non-parametric logarithm of odds score 3.43) for juvenile myoclonic epilepsy (Consortium et al., 2012). In conclusion, this region is of high interest regarding neuronal development and function although all relevant factors have not been elucidated so far.
We present a 4.5-year-old male patient with the smallest microdeletion 2q34 reported so far. One of the genes in this 1.5 Mb spanning region is the candidate gene MAP2. The patient’s clinical features include mild developmental delay, behavioral abnormalities as well as epilepsy.
Materials and Methods
The study was approved by the local Ethics Committee of the Technical University of Munich and performed according to the standard of the Helsinki Declaration of 1975. Blood samples for molecular karyotyping were collected from the patient and the parents after written informed consent. Written informed consent concerning publication of this case report was also obtained from the parents of the participant.
DNA was extracted from peripheral blood using the Gentra Puregene Blood Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA samples of the patient and his parents were analyzed using the single nucleotide polymorphism (SNP)-Array Affymetrix® CytoScanTM 750K Array (Affymetrix® Inc., Santa Clara, CA, United States) with an average space of 4 kb between two oligonucleotides. Scanning was performed by the Affymetrix® GeneChip Scanner 3000 7G (resolution 0.51–2.5 μm). The data analysis was conducted using the Affymetrix® Chromosome Analysis Suite Software (ChAS), version 3.0, hg19.
Case Report and Results
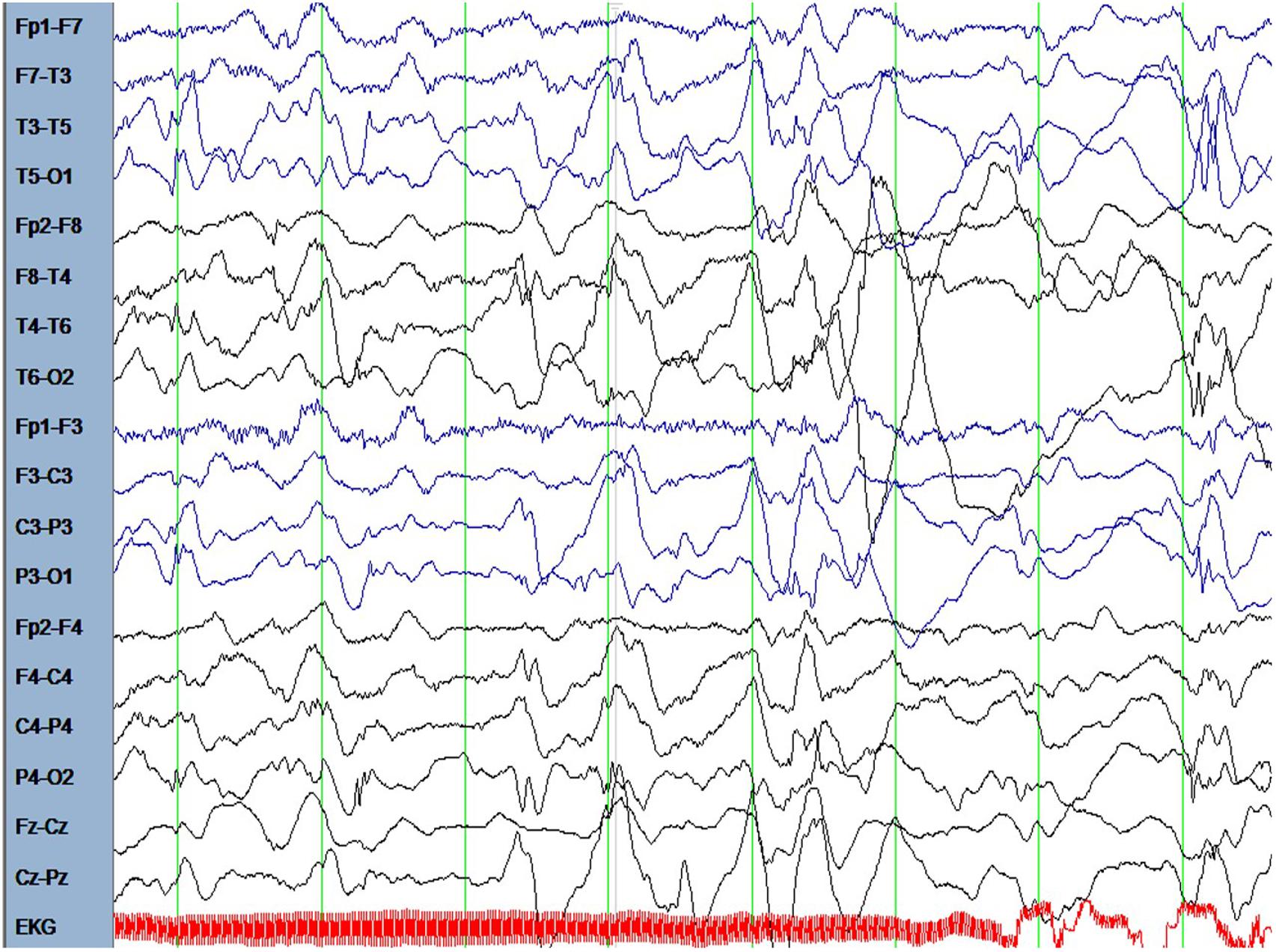
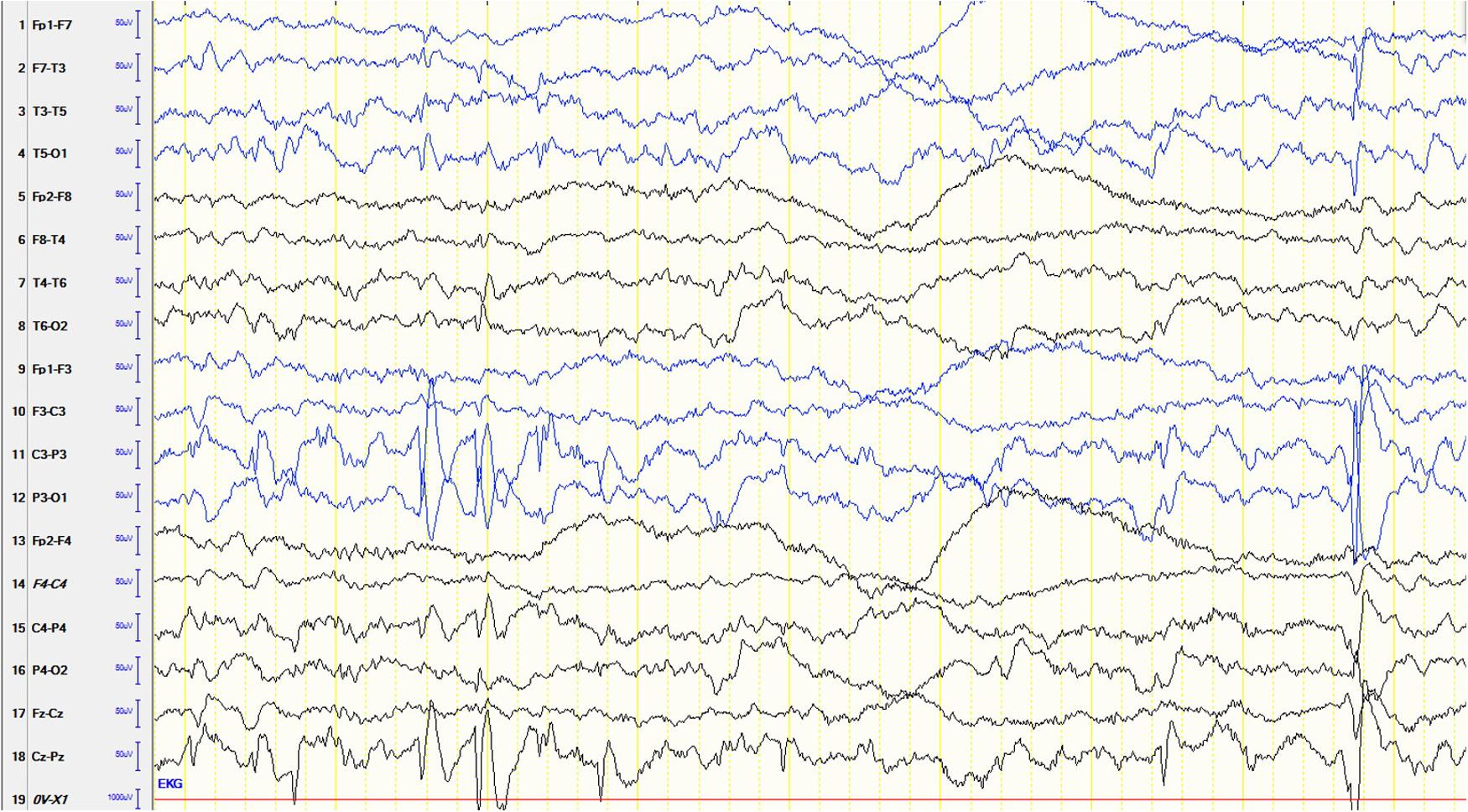
The male patient is the first and only child of healthy parents. He was born at gestational age of 37 weeks. His birth measurements were as follows: birth weight 2730 g (-1.5 standard deviations, SD), birth length 49 cm (-0.68 SD), head circumference at birth 34 cm (-0.43 SD). There were no dysmorphological signs seen at birth. From the beginning, the boy had difficulties falling asleep and suffered from insomnia until the age of 15 months. He was presented at the children’s hospital at the age of 6.5 months because of weight loss and recurrent emesis. One month later the mother noted several episodes of typical Blitz-Nick-Salaam (BNS) seizures. An electroencephalogram (EEG) was performed and showed hypsarrhythmia (Figure 1). Consequently, the patient was diagnosed as having West syndrome. A medication with vitamin B6 and sulthiame was started. After 5 days the seizures disappeared. A motoric developmental delay had also been noted before seizure onset which worsened in the course of the disease. The patient was not capable of rolling from supine to prone position anymore and showed partly limited head control when erected from supine position. A magnetic resonance imaging (MRI) of the brain performed at the age of 9 months was normal. Vitamin B6 was stopped at that time. At the age of 14 months his developmental skills were at the level of a 10-month-old infant. A study of his behavior showed that he was seeking for attention and stimuli to stabilize and regulate himself even when being tired and exhausted. Sulthiame was reduced at the age of 19 months and stopped at the age of 22 months as there were no more seizures and the EEG was normal. At the age of 26 months his mother described a behavior that was in line with a lack of impulse control. He also had problems in closeness-distance regulation, showed short spans of concentration, and was distracted easily. In an EEG performed at the age of 3.5 years rolandic spikes located left centrotemporally were detected (Figure 2). Sleep activation was seen in another EEG without clinical seizures. Therefore, a medication with sulthiame was started again. The pathological findings in the EEG improved in the further course. A MRI of the brain reconfirmed that there were no pathological signs present. Although he improved his psychomotor skills and was able to run and speak, his fine motor skills at the age of 4.5 years were comparable to a 2.5-year-old child. At that age he was presented at our institute for genetic counseling.
SNP-Array analysis revealed a 1.5 Mb microdeletion in the chromosomal region 2q34 including eight OMIM genes (MAP2, UNC80, RPE, KANSL1L, ACADL, MYL1, LANCL1, and CPS1) out of nine Reference Sequence (RefSeq) genes (genome position: chr2:210,440,530-211,955,369, GRCh37/hg19). Three transcript variants of MAP2 (NM_002374.3, NM_031847.2, and NM_031845.2) were completely included in this microdeletion. One transcript variant of MAP2 (NM_001039538.1) was deleted except for the two first exons. Subsequent SNP-Array analysis of the parents revealed the de novo status of the patient’s microdeletion (Figure 3). A comparison of the identified microdeletion 2q34 with previously reported microdeletions in the chromosomal region 2q33.2-q35 including MAP2 (Figure 4) showed that the microdeletion in our patient is the shortest reported so far. MAP2, UNC80 and LANCL1 are amongst the proposed candidate genes by Jang et al. (2015) for the phenotype in patients with a microdeletion 2q33.3q34 (Figure 4).
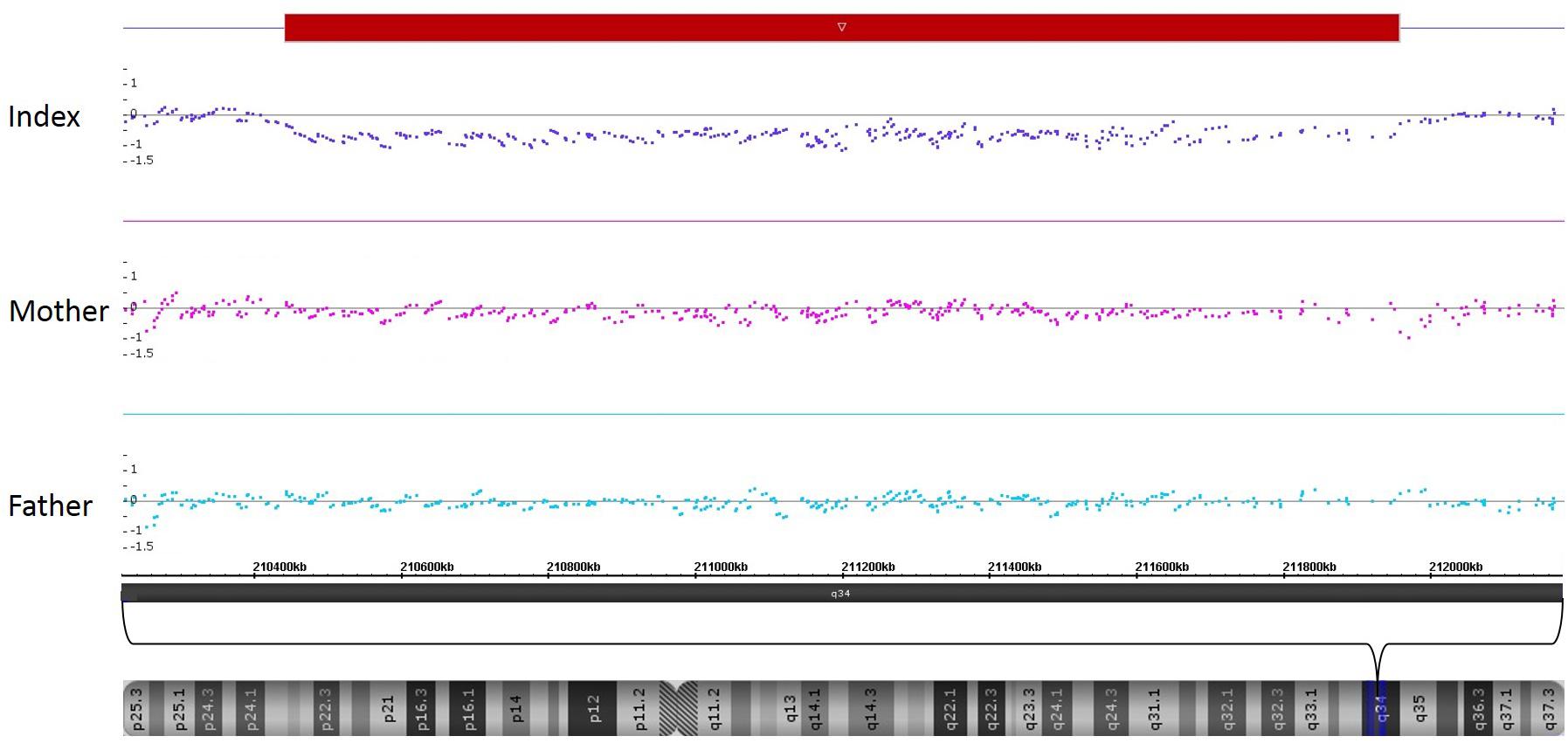
FIGURE 3. SNP-Array-analysis of the patient identified a microdeletion in the chromosomal region 2q34 (red bar, genome position: chr2:210,440,530-211,955,369, GRCh37/hg19). Comparison with the parents’ results revealed the de novo status of this microdeletion.
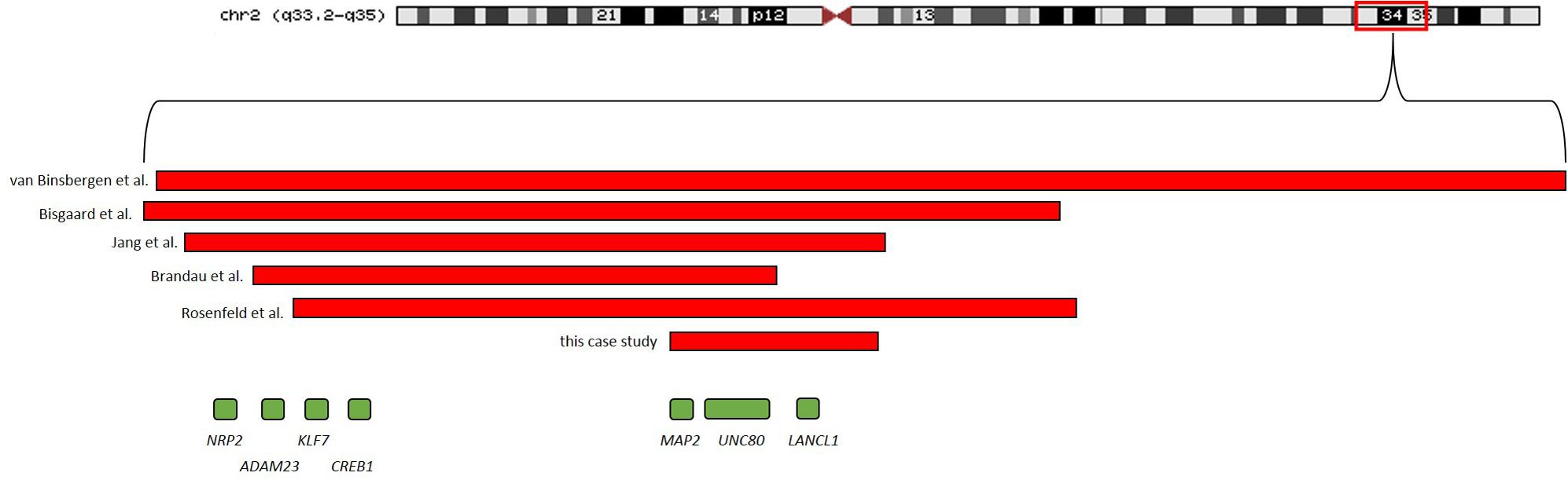
FIGURE 4. Microdeletions including MAP2 in previous reported patients as well as the microdeletion identified in the patient of this study are shown.
Discussion
There are several genes located in the chromosomal region 2q34 that have a part in neuronal development and function. This region has already been associated with juvenile myoclonic epilepsy and Alzheimer’s disease by linkage analysis (Scott et al., 2003; Consortium et al., 2012). Up to now, only a few cases have been reported that describe neurological abnormalities due to a loss of this critical region. Thus, especially concerning developmental delay and autistic traits in behavior, there is more than one candidate gene to be expected. One of the most promising genes causing this symptomatic is MAP2 (Pescucci et al., 2003; Mukaetova-Ladinska et al., 2004; Rosenfeld et al., 2010; van Binsbergen et al., 2014; Jang et al., 2015). The case of our patient affected by developmental delay, epilepsy, neurocognitive, and behavioral abnormalities is fitting to the hypothesis that MAP2 is one of the candidate genes, as our patient’s microdeletion is the smallest one described in the literature so far spanning MAP2 amongst other genes. Although one transcript is not completely deleted it can be expected that there is a loss of function of MAP2 because only the first two exons remain.
MAP2 is a neurosteroid receptor important for the microtubule polymerization in the outgrowth of neurites (Fontaine-Lenoir et al., 2006). The reason for MAP2 being the most likely candidate gene responsible for the patient’s phenotype is its high probability of loss of function intolerance (pLI) generated by >120,000 control alleles of the Exome Aggregation Consortium (ExAC) browser. It’s the only pLI of the deleted genes in our patient that reaches the amount of 1.0. Furthermore, in ExAC there is no deletion listed concerning MAP2. The pLI was calculated as previously described (Lek et al., 2016). In 1997 evidence was already present that MAP2 expression is reduced in patients with Rett syndrome while being upregulated in Down syndrome (Kaufmann et al., 1997). Mukaetova-Ladinska et al. (2004) examined postmortem the MAP2 expression in the dorsolateral prefrontal cortex in two individuals with autistic features, one with recorded epileptic seizures from birth. They reported a depletion of the MAP2 expression in neurons and dendrites, particular in the cortical domain, as well as changes in the cytoarchitecture of the cortex (Mukaetova-Ladinska et al., 2004). Van Binsbergen et al. (2014) identified a 14.79 Mb de novo deletion on 2q33.2q35 in a fetus with brain developmental anomalies (i.e., delayed gyral formation, neuronal heterotopia of the white matter, small cerebellum) amongst other syndromic features and emphasized MAP2’s role in neurodevelopment. Our patient showed no abnormalities in the MRI of the brain consistent with other MAP2-deficient patients reported so far (Bisgaard et al., 2006; Brandau et al., 2008; Jang et al., 2015). Also noteworthy is a recent publication in Nature in which out of 8,361 reported candidate de novo non-sense variants in patients with developmental disorder four were located in MAP2 (Deciphering Developmental Disorders Study, 2017).
Another two candidate genes for the 2q34 phenotype, spanned by the microdeletion in our patient, are UNC80 and LANCL1. These had also already been proposed as candidate genes by Jang et al. (2015), UNC80 seems to be involved in the Ca2+ dependent excitability of neurons (Lu et al., 2010). It has been associated with the clinical phenotype of autosomal-recessive inherited infantile hypotonia with psychomotor retardation and characteristic facies 2 (IHPRF2; OMIM ∗612636). With a pLI of 0.14 it is unlikely that a possible haploinsufficiency of this gene might contribute to the phenotype of our patient. However, it should be noted that the symptoms resulting from a microdeletion of a single gene are not comparable with the symptoms caused by a contiguous gene syndrome. Therefore, UNC80 might also contribute to the phenotype of our patient. The other candidate gene already mentioned is LANCL1, taking part in NGF-induced nerve growth (Zhang et al., 2009). A more recent publication by Huang et al. (2014) showed that the transcribed protein LANCL1 is important for neuronal protection from reactive oxygen species (ROS). The authors reported postnatal neuronal degeneration in LANCL1 knockout mice (LANCL1 -/-) (Huang et al., 2014). Like in the case of UNC80 it is not very likely that a heterozygous loss of LANCL1 leads to clinical symptoms because of a pLI value of 0. But taken together it has to be emphasized that this region and their genes seems to be important for the proper neuronal development and function.
In the latest publication concerning microdeletion 2q34 and 2q33.3, respectively, the authors additionally mentioned CREB1, KLF7, ADAM23, and NRP2 (Jang et al., 2015). These genes are not spanned by the microdeletion in our patient. Their role in contributing to the phenotype of patients with a microdeletion 2q33q34 remains still to be elucidated as the clinical symptoms of our patient seem to be considerably more moderate. There are a few patients whose microdeletion do not affect the now discussed region in our patient, like the microdeletion spanning ERBB4 that is located more distally in 2q34 in a patient with intellectual disability and hyperactivity (Kasnauskiene et al., 2013) or patients with the proximal microdeletion 2q32q33 and severe mental retardation (Van Buggenhout et al., 2005).
Conclusion
The 1.5 Mb microdeletion on 2q34 in our patient is the shortest microdeletion spanning MAP2, a top candidate gene responsible for autism and developmental delay, reported so far. Although the phenotype composed of epilepsy, developmental delay, and behavioral abnormalities was mild in our patient in contrast to other patients with a microdeletion on 2q33q34, our case might help to elucidate the contribution of MAP2 to this exceedingly interesting region in context of neuronal development and function. The patient has the unusual presentation of a BNS epilepsy with rapid response to treatment with pyridoxine and sulthiame and an EEG with rolandic spikes 3 years later without having seizures.
Author Contributions
DW: wrote the manuscript and did the genetic counseling. SA: performed the SNP-Array analysis. CM: pediatrician who performed the EEG and did the medical treatment of the patient. TM: head of the institute, provided the material, methods, and education. JH: supervised the genetic counseling and the whole process of the making of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the family for participation. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
References
Bisgaard, A. M., Kirchhoff, M., Tumer, Z., Jepsen, B., Brondum-Nielsen, K., Cohen, M., et al. (2006). Additional chromosomal abnormalities in patients with a previously detected abnormal karyotype, mental retardation, and dysmorphic features. Am. J. Med. Genet. A 140, 2180–2187. doi: 10.1002/ajmg.a.31425
Brandau, D. T., Lund, M., Cooley, L. D., Sanger, W. G., and Butler, M. G. (2008). Autistic and dysmorphic features associated with a submicroscopic 2q33.3-q34 interstitial deletion detected by array comparative genomic hybridization. Am. J. Med. Genet. A 146A, 521–524. doi: 10.1002/ajmg.a.32153
Consortium, E., Leu, C., De Kovel, C. G., Zara, F., Striano, P., Pezzella, M., et al. (2012). Genome-wide linkage meta-analysis identifies susceptibility loci at 2q34 and 13q31.3 for genetic generalized epilepsies. Epilepsia 53, 308–318. doi: 10.1111/j.1528-1167.2011.03379.x
Deciphering Developmental Disorders Study (2017). Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433–438. doi: 10.1038/nature21062
Fontaine-Lenoir, V., Chambraud, B., Fellous, A., David, S., Duchossoy, Y., Baulieu, E. E., et al. (2006). Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 4711–4716. doi: 10.1073/pnas.0600113103
Huang, C., Chen, M., Pang, D., Bi, D., Zou, Y., Xia, X., et al. (2014). Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required for neuronal survival. Dev. Cell 30, 479–487. doi: 10.1016/j.devcel.2014.06.011
Jang, D. H., Chae, H., and Kim, M. (2015). Autistic and Rett-like features associated with 2q33.3-q34 interstitial deletion. Am. J. Med. Genet. A 167A, 2213–2218. doi: 10.1002/ajmg.a.37119
Kasnauskiene, J., Ciuladaite, Z., Preiksaitiene, E., Utkus, A., Peciulyte, A., and Kucinskas, V. (2013). A new single gene deletion on 2q34: ERBB4 is associated with intellectual disability. Am. J. Med. Genet. A 161A, 1487–1490. doi: 10.1002/ajmg.a.35911
Kaufmann, W. E., Taylor, C. V., Hohmann, C. F., Sanwal, I. B., and Naidu, S. (1997). Abnormalities in neuronal maturation in Rett syndrome neocortex: preliminary molecular correlates. Eur. Child Adolesc. Psychiatry 6(Suppl. 1), 75–77.
Lek, M., Karczewski, K. J., Minikel, E. V., Samocha, K. E., Banks, E., Fennell, T., et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. doi: 10.1038/nature19057
Lu, B., Zhang, Q., Wang, H., Wang, Y., Nakayama, M., and Ren, D. (2010). Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron 68, 488–499. doi: 10.1016/j.neuron.2010.09.014
Mukaetova-Ladinska, E. B., Arnold, H., Jaros, E., Perry, R., and Perry, E. (2004). Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathol. Appl. Neurobiol. 30, 615–623. doi: 10.1111/j.1365-2990.2004.00574.x
Pescucci, C., Meloni, I., Bruttini, M., Ariani, F., Longo, I., Mari, F., et al. (2003). Chromosome 2 deletion encompassing the MAP2 gene in a patient with autism and Rett-like features. Clin. Genet. 64, 497–501. doi: 10.1046/j.1399-0004.2003.00176.x
Rosenfeld, J. A., Ballif, B. C., Torchia, B. S., Sahoo, T., Ravnan, J. B., Schultz, R., et al. (2010). Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet. Med. 12, 694–702. doi: 10.1097/GIM.0b013e3181f0c5f3
Scott, W. K., Hauser, E. R., Schmechel, D. E., Welsh-Bohmer, K. A., Small, G. W., Roses, A. D., et al. (2003). Ordered-subsets linkage analysis detects novel Alzheimer disease loci on chromosomes 2q34 and 15q22. Am. J. Hum. Genet. 73, 1041–1051. doi: 10.1086/379083
van Binsbergen, E., Ellis, R. J., Abdelmalik, N., Jarvis, J., Randhawa, K., Wyatt-Ashmead, J., et al. (2014). A fetus with de novo 2q33.2q35 deletion including MAP2 with brain anomalies, esophageal atresia, and laryngeal stenosis. Am. J. Med. Genet. A 164A, 194–198. doi: 10.1002/ajmg.a.36202
Van Buggenhout, G., Van Ravenswaaij-Arts, C., Mc Maas, N., Thoelen, R., Vogels, A., Smeets, D., et al. (2005). The del(2)(q32.2q33) deletion syndrome defined by clinical and molecular characterization of four patients. Eur. J. Med. Genet. 48, 276–289. doi: 10.1016/j.ejmg.2005.05.005
Keywords: 2q34 deletion, behavioral abnormalities, seizure, developmental delay, MAP2
Citation: Westphal DS, Andres S, Makowski C, Meitinger T and Hoefele J (2018) MAP2 – A Candidate Gene for Epilepsy, Developmental Delay and Behavioral Abnormalities in a Patient With Microdeletion 2q34. Front. Genet. 9:99. doi: 10.3389/fgene.2018.00099
Received: 10 November 2017; Accepted: 09 March 2018;
Published: 26 March 2018.
Edited by:
Noor Ahmad Shaik, King Abdulaziz University, Saudi ArabiaReviewed by:
Vita Dolzan, University of Ljubljana, SloveniaSireesha Movva, Medgenome Pvt. Ltd., India
Copyright © 2018 Westphal, Andres, Makowski, Meitinger and Hoefele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominik S. Westphal, ZG9taW5pay53ZXN0cGhhbEBtcmkudHVtLmRl
 Dominik S. Westphal
Dominik S. Westphal Stephanie Andres1
Stephanie Andres1 Thomas Meitinger
Thomas Meitinger Julia Hoefele
Julia Hoefele