- 1GW Institute for Neurosciences, Department of Pharmacology and Physiology, The George Washington University School of Medicine and Health Sciences, Washington, DC, United States
- 2Autism and Neurodevelopmental Disorders Institute, The George Washington University, Washington, DC, United States
Hundreds of genes are mutated in non-syndromic intellectual disability (ID) and autism spectrum disorder (ASD), with each gene often involved in only a handful of cases. Such heterogeneity can be daunting, but rare recessive loss of function (LOF) mutations can be a good starting point to provide insight into the mechanisms of neurodevelopmental disease. Biallelic LOF mutations in the signaling scaffold CC2D1A cause a rare form of autosomal recessive ID, sometimes associated with ASD and seizures. In parallel, we recently reported that Cc2d1a-deficient mice present with cognitive and social deficits, hyperactivity and anxiety. In Drosophila, loss of the only ortholog of Cc2d1a, lgd, is embryonically lethal, while in vertebrates, Cc2d1a has a homolog Cc2d1b which appears to be compensating, indicating that Cc2d1a and Cc2d1b have a redundant function in humans and mice. Here, we generate an allelic series of Cc2d1a and Cc2d1b LOF to determine the relative role of these genes during behavioral development. We generated Cc2d1b knockout (KO), Cc2d1a/1b double heterozygous and double KO mice, then performed behavioral studies to analyze learning and memory, social interactions, anxiety, and hyperactivity. We found that Cc2d1a and Cc2d1b have partially overlapping roles. Overall, loss of Cc2d1b is less severe than loss of Cc2d1a, only leading to cognitive deficits, while Cc2d1a/1b double heterozygous animals are similar to Cc2d1a-deficient mice. These results will help us better understand the deficits in individuals with CC2D1A mutations, suggesting that recessive CC2D1B mutations and trans-heterozygous CC2D1A and CC2D1B mutations could also contribute to the genetics of ID.
Introduction
Autosomal recessive loss of function (LOF) of the signaling scaffold Coiled-coil and C2 Domain containing 1A (CC2D1A) causes a spectrum of neurodevelopmental conditions including fully penetrant intellectual disability (ID), and variably penetrant autism spectrum disorder (ASD), seizures, and aggressive behavior (Basel-Vanagaite et al., 2006; Manzini et al., 2014; Reuter et al., 2017). In Drosophila, where only one CC2D1 homolog, lethal giant discs lgd, is present, removal of lgd is lethal during the larval stage (Gallagher and Knoblich, 2006; Jaekel and Klein, 2006). Expression of either human CC2D1A or CC2D1B can rescue the phenotypes observed in Drosophila (Drusenheimer et al., 2015), suggesting that CC2D1A and CC2D1B act redundantly. Despite wide expression of CC2D1A and its binding to multiple proteins involved in the immune response (Chang et al., 2011; Chen et al., 2012), CC2D1A LOF in humans appears to only affect the brain, leading to a spectrum of behavioral deficits. While this indicates that CC2D1B is not fully able to compensate in the brain leading to the human presentation, it is unclear whether CC2D1B itself could have a role in neurodevelopmental disorders.
Studies on the genetic causes of ID and ASD, in particular, are identifying a large contribution of de novo and hypomorphic mutations to these diseases (Sanders et al., 2012; Lim et al., 2013; Yu et al., 2013; Musante and Ropers, 2014). Many of the mutated genes would have greater impact on development if completely lost, leading to multi-system disorders and/or brain malformations, while the heterozygous and hypomorphic mutations found in ASD/ID affect neurons more mildly, leading to a grossly normal brain, but with cognitive and social deficits (Yu et al., 2013). We wondered whether a similar mechanism is at play in patients with CC2D1A LOF mutations, where CC2D1B can only partially compensate. If this was the case, removal of both CC2D1 genes would be incompatible with embryogenesis, indicating that these proteins together have a critical developmental role. Nothing is known about the role of CC2D1B in brain development. By comparing how individual loss of each gene affects cognitive, social, and affective function we have studied the relative role of CC2D1A and CC2D1B in the brain and defined whether CC2D1B should also be considered as a candidate gene for ID.
Mice deficient for Cc2d1a develop normally in utero, but die soon after birth because of breathing and swallowing deficits (Zhao et al., 2011; Al-Tawashi et al., 2012; Oaks et al., 2017). By conditionally removing Cc2d1a in the forebrain, we have previously shown that Cc2d1a LOF recapitulates features of ID and ASD in adult animals (Oaks et al., 2017). Cc2d1a conditional knockout (1a-cKO) mice show learning and memory deficits, social deficits, hyperactivity, anxiety, and repetitive behaviors (Oaks et al., 2017).
To define how CC2D1B compensates for loss of CC2D1A and contributes to these phenotypes, we generated a Cc2d1b knockout (1b-KO) line and developed an allelic series of Cc2d1a and Cc2d1b LOF, including Cc2d1a/1b double heterozygote (1a/1b-dHET) and double KO (1a/1b-KO) animals. Removal of both CC2D1 proteins causes early embryonic lethality, showing that CC2D1 function has an essential developmental role as in Drosophila. 1b-KO and 1a/1b-dHET animals are viable and fertile suggesting that Cc2d1a and Cc2d1b are not fully redundant, and that Cc2d1a has a critical role in respiration in the mouse.
When we tested the behavioral performance of 1b-KOs we found that Cc2d1b LOF caused only cognitive deficits, which are partially overlapping with those observed in Cc2d1a conditional LOF. Since direct comparison with a global Cc2d1a KO is not possible because of postnatal mortality, we also tested 1a/1b-dHETs which showed a combination of deficits with features of both 1b-KO and 1a-cKO animals, including delayed memory acquisition and retention, as well as increased anxiety and hyperactivity, mostly in males. Our findings indicate that CC2D1 function is critical for embryonic development and that the CC2D1 proteins regulate multiple behaviors with some sex-specificity for males. Both CC2D1A and CC2D1B are involved in learning and memory, while CC2D1A alone appears to contribute to anxiety and hyperactivity.
Materials and Methods
Animals
This study was carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee of The George Washington University. A Cc2d1b null mouse line (1b-KO) was generated by the Knockout Mouse Project Repository (Project ID CDS 34981) at the University of California Davis, with the allele Cc2d1btm1a(KOMP)Wtsi. Cc2d1b null mice carry an engrailed 2 splice acceptor (En2SA) gene-trap allele with bicistronic expression of β-galactosidase as well as a neomycin resistance cassette, flanked by FRT (flippase recombinase target) recombination sites, in the genomic region between exons 2 and 3 of Cc2d1b (Figure 1A). Cc2d1a/1b double heterozygous (1a/1b-dHET) mice were generated by crossing Cc2d1a heterozygotes (1a-HET) with Cc2d1b heterozygotes (1b-HET). 1a-HET mice were bred from a Cc2d1a null mouse line (KO) generated by the Knockout Mouse Project Repository (Project Design ID 49663) at the University of California as was previously described by Oaks et al. (2017). All lines are maintained on a C57BL/6 background. For genotyping, polymerase chain reaction (PCR) amplifications were performed on 1 μL of proteinase K (New England Biolabs, Ipswich, MA, United States) digested tail DNA samples. PCR reactions (50 μL) consisted of GoTaq Flexi buffer (Promega, Madison, WI, United States), 100 μM dNTPs, 50 μM each of forward and reverse primers (sequence available upon request), 1 mM MgCl2, and 1.25 U GoTaq Flexi DNA polymerase (Promega, Madison WI, United States), and were run with optimized reaction profiles determined for each genotype. A 25-μL aliquot from each reaction was analyzed by gel electrophoresis on a 1.0% agarose gel for the presence of the desired band.
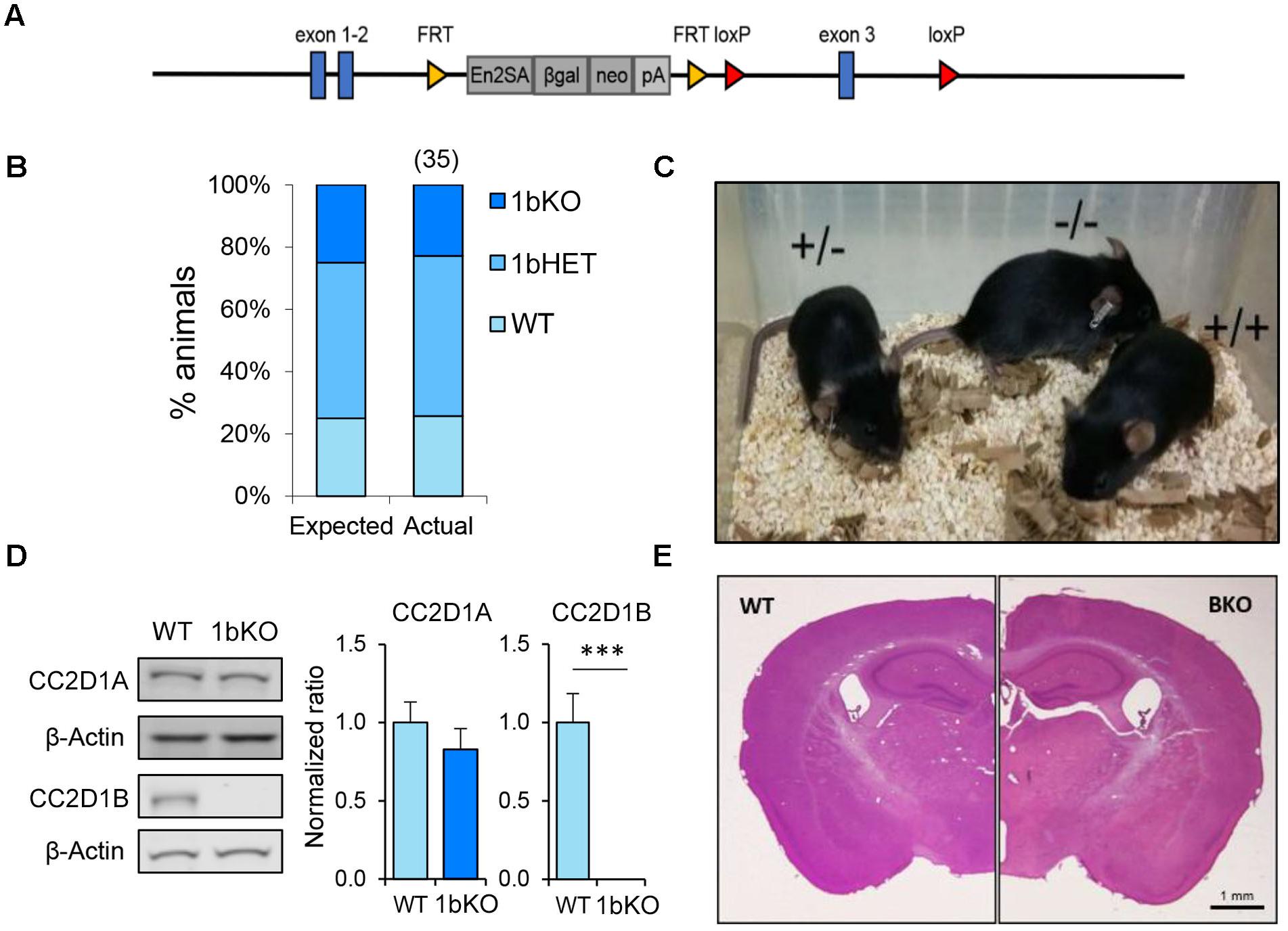
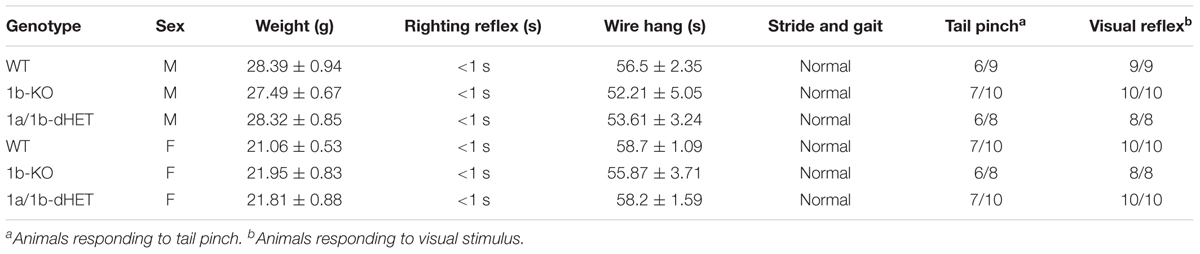
FIGURE 1. Cc2d1b KO mice are viable and fertile and present normal anatomical development of the brain. (A) Gene trap containing engrailed two splice acceptor (En2SA) sequence followed by a β-Galactosidase cassette (βgal) and a neomycin resistance cassette (neo) is flanked by flippase recognition target (FRT) sites between exons 2 and 3. LoxP sites for Cre recombinase targeting flank exon 3. (B) 1bKO mice are born in predicted Mendelian ratios (number of pups indicated above in parentheses; data from 5 litters) and (C) are indistinguishable from WT and 1bHET mice. (D) Immunoblot analysis of CC2D1A and CC2D1B expression in WT and 1bKO mice. Normal levels of CC2D1A and a complete absence of CC2D1B are shown in the 1bKO mice. (E) The size and organization of the adult 1bKO brain is indistinguishable from WT brain stained with hematoxylin and eosin. Scale bar: 1 mm.
Histological Preparation and Microscopy
To prepare tissue for histological analysis, deeply anesthetized mice were transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were removed and post-fixed in PFA. Cryosections from adult mouse brains were prepared by mounting in Neg-50 (Thermo Fisher Scientific, Waltham, MA, United States) and cut at 40 μm on a Cryostar NX50 cryostat (Thermo Fisher Scientific, Waltham, MA, United States), then stained with Hematoxylin and Eosin (H&E, VWR International, Radnor, PA, United States) to visualize tissue architecture. Imaging of H&E stained sections was performed on a Leica M165 FC stereo microscope (Leica Microsystems, Buffalo Grove, IL, United States).
Behavioral Tests
A standardized battery of behavioral testing was applied to each cohort of animals, 1b-KO and 1a/1b-dHET male and female mice, at 3–4 months of age. As both 1b-KOs and 1a/1b-dHETs were generated from the same 1a/1b-dHET, breeding the wild-type (WT) controls were littermates shared by both cohorts and all behavioral tests were performed at the same time for WT, 1b-KOs, and 1a/1b-dHETs. Behavioral tests were performed in the Manzini lab behavioral suite in the George Washington University Animal Research Facility following a 60 min period of acclimatization. Initial characterization to analyze any neurological abnormalities including the analysis of basic motor and somatosensory function was performed on a subset of the behavioral cohort as described by Rogers et al. (1997): righting reflex, wire hang, gait analysis, tail pinch, and visual reach. Cognitive and social function and other behaviors were tested in the open field test, novel object recognition test (NORT) (Bevins and Besheer, 2006), Morris water maze (MWM) (Vorhees and Williams, 2006), and 3-chamber social interaction test (Nadler et al., 2004). Behavioral analysis was performed via automated animal tracking using ANY-maze (Stoelting, Wood Dale, IL, United States).
Righting Reflex
Coordination, motor strength, and vestibular function were tested by placing each mouse on its back and timing its ability to return to an upright position.
Wire Hang
Motor strength was tested by timing the latency to fall to a mouse cage containing bedding while the mouse was hanging from a wire cage-top not higher than 18 cm.
Gait Analysis
Motor coordination and strength were assessed by painting the paws of each mouse with red non-toxic tempera paint and making them walk through a narrow tunnel over white paper. Abnormalities of paw placement and stride length were noted or indicated as normal.
Tail Pinch
The ability of each mouse to respond to mild pain was tested by pinching the tip of the tail with fine, ethanol-cleaned forceps. Reactions were categorized as either response or no response.
Visual Reach
Vision was tested by measuring the latency to the first attempt to reach for a nearby wire cage-top while the mouse was being held by the base of the tail at a height of 18 cm over an open cage.
Open Field Test
The open field test was performed in an unfamiliar 50 cm × 50 cm plastic box (Stoelting, Wood Dale, IL, United States). Animals were placed in the center of the arena and ambulatory activity was monitored by digital video for 15 min. The arena was divided into two areas, an outer zone and a center zone (25 cm ×25 cm; 25% of total area). Total distance traveled and time spent in each area was measured.
Novel Object Recognition Test
The NORT (Bevins and Besheer, 2006; Oaks et al., 2017) was performed in the same apparatus described for the open field test. The test consisted of three different phases: habituation, training, and test. The habituation phase lasted for 30 min while the animals were exposed to the box and then returned to the home cage while the box was cleaned. During the training phase, the animal was placed in the same box with two identical objects located in opposite corners, at a distance of 5 cm from the walls. To assess short-term memory, the animal was returned to the home cage during an interval of 15 min. During the test, a familiar object, identical to those used in the habituation phase, was placed in one corner, while in the opposite corner an unfamiliar object was placed. Exploration activity was monitored for 10 min at each phase, with exploration defined as time spent actively observing or touching the object from within a radius of 5 cm. Cumulative time spent with each object was measured by video analysis using ANY-maze to determine the location of the animal’s nose relative to the objects in the enclosure. Preference for the novel object was defined as the ratio of the time spent with the novel object to the time spent with the familiar object. Animals that did not interact with the object and stopped in a corner of the cage were removed from the analysis.
Morris Water Maze
The MWM (Vorhees and Williams, 2006; Oaks et al., 2017) apparatus was a 120 cm ×120 cm round metal tub (Stoelting, Wood Dale, IL, United States) where distinct visual cues were placed at the cardinal points. White non-toxic paint was added to the water to make the surface opaque for the hidden trials and it was maintained at 24°C. Each trial consisted of four independent drops, one at each cardinal point around the tub, with the mouse facing the wall of the tub. Each drop lasted 60 s, or until the mouse found the platform, whichever occurred first. Each animal completed two trials (four drops each) with a visible platform, five trials with a platform hidden under the water surface, and two reversal trials where the location of the hidden platform (HP) was changed. The sequence of nine trials was performed over 9 days, with one trial per day. A 60-s probe trial was also performed the day after the HP series was completed, by removing the platform from the water before proceeding to the reversal phase on the following day.
Three-Chamber Social Interaction Test
The social interaction test (Nadler et al., 2004; Kaidanovich-Beilin et al., 2011) was performed in a clear rectangular acrylic box (60 cm × 40 cm) divided into three chambers (40 cm × 20 cm) with small openings (10 cm × 5 cm) in the adjoining walls (Everything Plastic, Philadelphia, PA, United States). The test consisted of two phases, the habituation phase and the sociability phase. During the habituation phase, empty inverted wire cups (10 cm in diameter) were placed in the center of the chambers at the ends. Each mouse was placed in the center chamber of the apparatus and allowed to explore the different chambers for 5 min. During the second phase, an unfamiliar mouse of the same sex as the tested mouse was placed under the wire cup in one of the side chambers. The experimental mice were allowed to explore for 10 min during the sociability phase. Total time spent in the Object (containing empty cup) and Mouse (with unfamiliar mouse under the cup) chambers was used to determine the social preference of each mouse tested, while the time sniffing within a 2-cm radius of the mouse-containing cup were recorded as measures of social approach and social interaction.
Results
CC2D1A and CC2D1B Have Partially Redundant Function in Development
Loss of CC2D1A in humans causes a variable spectrum of ID, ASD, and seizures and the removal of Cc2d1a in the murine forebrain leads to several cognitive, social, and affective behavioral phenotypes (Manzini et al., 2014; Oaks et al., 2017). As no human mutations in CC2D1B have been identified to date, we asked whether loss of Cc2d1b in the mouse would lead to similar phenotypes as loss of Cc2d1a. A Cc2d1b-deficient line (1b-KO) had been generated from the Knockout Mouse Project (KOMP) as a gene-trap allele inserted in intron 2 of Cc2d1b (Figure 1A). We obtained heterozygous animals and bred them to homozygosity, finding that 1b-KO mice are born in Mendelian ratios (Figure 1B). Differently from Cc2d1a KO (1a-KO) pups, which die shortly after birth (Zhao et al., 2011; Al-Tawashi et al., 2012; Drusenheimer et al., 2015; Oaks et al., 2017), 1b-KO mice are viable, fertile, and indistinguishable from WT littermates (Figure 1C). Basic behavioral functions were tested in adult WT and 1b-KO males and females: coordination (righting reflex), strength (wire hang), locomotion (stride and gait), pain sensitivity (tail pinch), and vision (visual reflex). No differences were observed in basic sensory and motor function (Table 1). We confirmed via western blot analysis of cortical protein lysates that CC2D1B was completely absent in these animals and that CC2D1A was expressed at normal levels (Figure 1D). Cryosections generated from the adult brain of 1b-KO animals and stained using hematoxylin and eosin (H&E) showed no differences in brain size and organization from WT littermates (Figure 1E). In summary, loss of Cc2d1b does not affect respiratory function and deglutition in the infant as observed in 1a-KOs, and 1b-KO adult mice are indistinguishable from WT littermates.
CC2D1A and CC2D1B contain very similar protein domains and are thought to have redundant functions in endocytic traffic and gene transcription (Hadjighassem et al., 2009; Usami et al., 2012; Drusenheimer et al., 2015). Because CC2D1B LOF did not result in postnatal lethality, we wondered whether the two proteins would only be partially redundant. To test this hypothesis, we crossed 1b-KOs and 1a-KOs to generate Cc2d1a/Cc2d1b double heterozygous (1a/1b-dHET) and double KO mice (1a/1b-KO). As 1aKO pups die soon after birth (Zhao et al., 2011; Al-Tawashi et al., 2012; Oaks et al., 2017), we did not expect 1a/1b-KO animals to survive and we genotyped litters at postnatal day (P)0, collecting tissue from both live and dead pups. However, while dead 1a-KO and 1a-KO/1b-HET were found in the expected ratios, 1a/1b-dKO pups were never retrieved (Figure 2A), suggesting that double knockouts may die earlier during embryonic development. Examination of prenatal litters only identified 1a/1b-dKO tissue mid-gestation at E11.5, but the embryo was almost entirely absent, leaving only a hypomorphic and largely empty yolk sac (Figure 2B). These results indicate that removal of both CC2D1 proteins leads to early embryonic lethality.
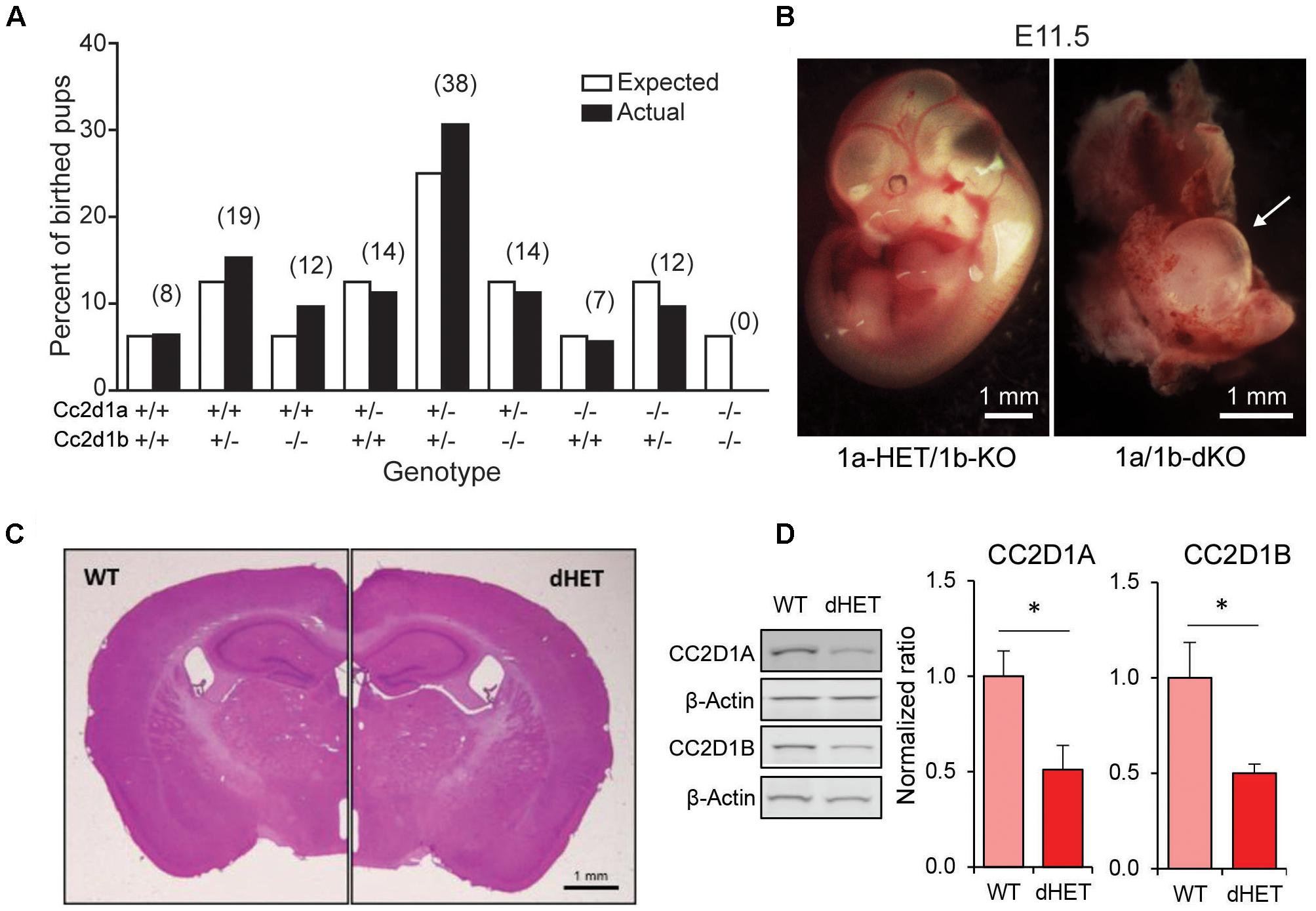
FIGURE 2. Cc2d1a/Cc2d1b double LOF is embryonic lethal, while double heterozygotes are viable. (A) Genotypes at postnatal day 0 (P0) of 124 pups resulting from 22 double heterozygous (1a/1b-dHET) crosses (number of pups for each genotype indicated above in parentheses). The 1a/1b double knockout (A–/– B–/–) pups were never found at P0. (B) Representative images of normal embryonic day 11.5 (E11.5) embryo with a single intact Cc2d1 allele (Left) and a double KO embryo (Right; arrow indicates empty yolk sac). Scale bars: 1 mm. (C) The size and organization of the adult 1a/1b-dHET brain is indistinguishable from wild-type mice stained with hematoxylin and eosin. Scale bar: 1 mm. (D) Immunoblot analysis of CC2D1A and CC2D1B expression in wild-type and 1a/1b-dHET mice. A half dose of each CC2D1 protein was found. Results expressed as mean ± SEM. ∗p < 0.05 (two tailed t-test).
1a/1b-dHETs were viable, fertile, and indistinguishable from WT littermates with normal gross brain anatomy (Figure 2C) and normal basic motor and sensory function (Table 1). We tested the expression levels of CC2D1A and CC2D1B in 1a/1b-dHET mice and found that as expected, only a half dose of each CC2D1 protein was present (Figure 2D). Thus, combined CC2D1 function is necessary for embryonic morphogenesis, but 1b-KO or 1a/1b-dHET animals develop normally, indicating that CC2D1A and CC2D1B have similar functions as it pertains to gross anatomical development and survival.
Both CC2D1A and CC2D1B Are Important for Cognitive Function
We have previously found that loss of Cc2d1a leads to a constellation of behavioral deficits: cognitive and social impairment, anxiety, hyperactivity, and repetitive behaviors (Oaks et al., 2017). We generated a cohort of 1b-KO and 1a/1b-HET male and female mice for behavioral analysis by crossing 1a/1b-HETs, so that we could compare behavioral performance in both lines at the same time. In the short-term memory version of the NORT (Bevins and Besheer, 2006) mice are placed in an arena with two identical objects that they are free to explore. After being removed back to their cages for 15 min, they are put in the arena where one of the now known objects has been substituted for a novel object (Figure 3A). In this test, WT male and female mice spend roughly four times longer exploring the novel object, while 1b-KOs and 1a/1b-dHETs show no difference (Figures 3B,C) (Males: WT, T2/T1 = 1.21 ± 0.32, T4/T3 = 3.90 ± 0.75, n = 10, p = 0.004∗∗; 1b-KO, T2/T1 = 1.05 ± 0.24, T4/T3 = 1.60 ± 0.46, n = 11, p = 0.309; 1a/1b-dHET, T2/T1 = 1.08 ± 0.23, T4/T3 = 1.62 ± 0.46, n = 12, p = 0.307. Females: WT, T2/T1 = 1.20 ± 0.25, T4/T3 = 4.39 ± 1.40, n = 10, p = 0.038 ∗; 1b-KO, T2/T1 = 0.84 ± 0.16, T4/T3 = 0.93 ± 0.24, n = 10, p = 0.757; 1a/1b-dHET, T2/T1 = 1.34 ± 0.48, T4/T3 = 1.46 ± 0.28, n = 10, p = 0.824). This deficit was not due to reduced interest in the objects, as animals spent similar amounts of time in exploratory behaviors, with 1a/1b-dHET males showing significantly more exploration (Figure 3D T1+T2 – Males: WT, t = 26.97 ± 5.75s, n = 10; 1b-KO, t = 23.17 ± 3.65s, n = 11, p = 0.999; 1a/1b-dHET, t = 65.27 ± 15.93s, n = 12, p = 0.167; Females: WT, t = 40.56 ± 5.19s, n = 10; 1b-KO, t = 71.67 ± 17.47s, n = 10, p = 0.423; 1a/1b-dHET, t = 54.30 ± 10.56s, n = 10, p = 0.960. Figure 3E T3+T4 – Males: WT, t = 21.93 ± 5.54s; 1b-KO, t = 17.91 ± 3.57s, p = 0.999; 1a/1b-dHET, t = 50.83 ± 16.0s, p = 0.640. Females: WT, t = 15.39 ± 2.12s; 1b-KO, t = 68.38 ± 26.04s, p = 0.090; 1a/1b-dHET, t = 31.86 ± 10.61s, p = 0.959. Figure 3F SUM T1,2,3,4 – Males: WT, t = 48.90 ± 9.35s; 1b-KO, t = 41.08 ± 6.20s, p = 0.942; 1a/1b-dHET, t = 116.1 ± 28.24s, p = 0.033 ∗; Females: WT, t = 55.95 ± 6.62s, 1b-KO; t = 140.1 ± 42.64s, p = 0.073; 1a/1b-dHET, t = 86.16 ± 20.52s, p = 0.660).
FIGURE 3. CC2D1A and CC2D1B are both involved in object memory. (A) Schematic design of short-term novel object recognition test (NORT), with a novel object replacing a familiar object after a 15 min interval. (B,C) In contrast to WT, 1bKO, and 1a/1bHET male (B) and female (C) mice showed no preference for the novel object relative to a familiar object. Results expressed as mean ± SEM, ∗∗p < 0.01 (two-tailed t-test with equal variance). (D–F) Exploration time divided by initial exploration of training objects 1 and 2 (D), test objects 3 and 4 (E) and total exploration across the two phases of the NORT (F). 1a-1bHET males show a trend toward increased exploration in each test phase which reaches significance when both phases are combined. Results expressed as mean ± SEM, one-way ANOVA with Dunnett’s multiple comparison test, ∗p < 0.05, ∗∗p < 0.01.
To further assess cognitive function, the 1b-KO mice were tested using the MWM paradigm which probes spatial memory acquisition, retention, and flexibility, by testing the ability of a mouse to learn, remember, and relearn the location of a platform hidden under opaque water (Morris, 1984). After the mice are trained using a visible platform to escape from the water, the platform is hidden under the surface in a different location and the animals undergo training on five consecutive days to learn the location of the platform. On the following day, memory retention is tested by removing the platform and measuring the amount of time the mouse spends in the area where the platform was previously located (probe trial). Finally, the position of the platform is changed and the animal must display flexibility by learning a new location (reversal). 1a-cKO animals show a delay in initial acquisition of the location of the HP, but after they learn, they can retain the memory in the probe trial, and learn a new location in the reversal (Oaks et al., 2017). Both 1b-KO and 1a/1b-dHET males and females presented deficits in this test (Figure 4). 1b-KO males and females and 1a/1b-dHET males were delayed in the HP acquisition showing significant differences in day 2 or 3 of the test (HP2 and HP3 in Figures 4B,F) (Males HP3: WT, t = 6.82 ± 0.69s, n = 11; 1b-KO, t = 10.97 ± 1.85s, n = 10, p = 0.042 ∗; 1a/1b-dHET, t = 11.99 ± 1.28s, n = 13, p = 0.0027 ∗∗. Females HP2: WT, t = 12.30 ± 1.32s, n = 13; 1b-KO, t = 19.62 ± 1.74s, n = 10, p = 0.0025 ∗∗; 1a/1b-dHET, t = 14.66 ± 1.64s, n = 11, p = 0.247). 1a/1bHET males and females were also affected in the probe trial where they spent less time in the platform quadrant during the first 15 s of the 60-s trial (Figures 4D,H) (Probe 15 s – Males: WT, t = 9.51 ± 0.83s, n = 11; 1b-KO, t = 6.13 ± 0.50s, n = 10, p = 0.0029 ∗∗; 1a/1b-dHET, t = 5.48 ± 0.80s, n = 13, p = 0.0021 ∗∗. Females: WT, t = 7.18 ± 0.80s, n = 13; 1b-KO, t = 5.77 ± 0.65s, n = 10, p = 0.203; 1a/1b-dHET, t = 4.36 ± 0.82s, n = 11, p = 0.022 ∗). Finally, 1b-KO males, but not females, were affected throughout the 60-s probe trial and spent less time exploring the correct quadrant in the probe trial testing memory retention (Figure 4D) (Probe 60 s – Males: WT, t = 25.40 ± 1.78s, n = 11; 1b-KO, t = 19.58 ± 1.30s, n = 10, p = 0.018 ∗; 1a/1b-dHET, t = 22.74 ± 2.63s, n = 13, p = 0.428. Females: WT, t = 21.19 ± 1.85s, n = 13; 1b-KO, t = 20.57 ± 1.54s, n = 10, p = 0.809; 1a/1b-dHET, t = 18.43 ± 2.62s, n = 11, p = 0.389). Animals heterozygous for loss of Cc2d1a or Cc2d1b alone showed normal behavioral performance (Supplementary Figures 1, 2 and Supplementary Table 1). In summary, loss of CC2D1B leads to cognitive deficits in both memory acquisition and retention. In general, males appear more severely affected than females in both 1bKO and 1a/1bHET lines, suggesting that CC2D1A and CC2D1B have overlapping roles in cognitive function.
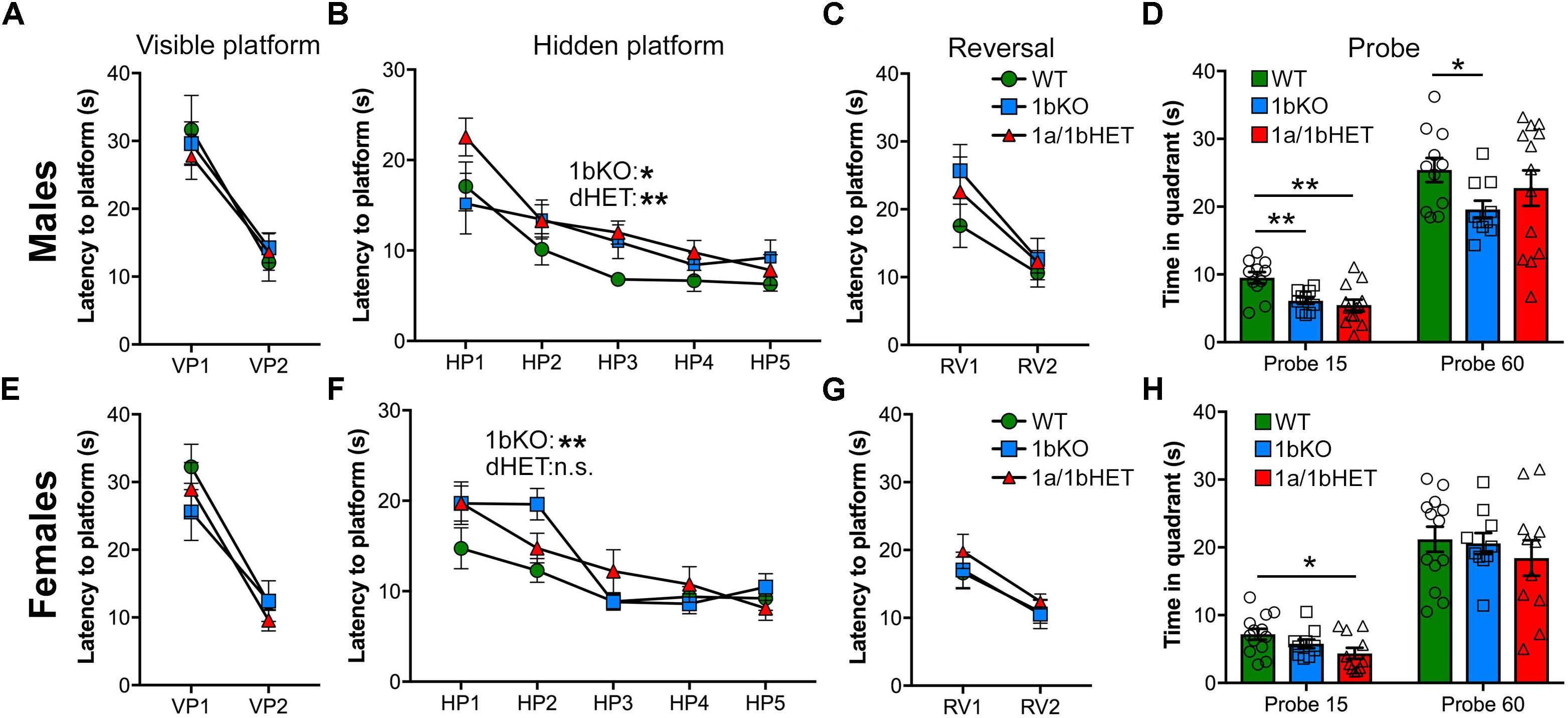
FIGURE 4. CC2D1B is involved in spatial memory formation and retention with mild male-specificity. Hippocampus-dependent spatial memory was assessed in 1bKO and 1a/1bdHET mice via the Morris Water Maze test. Spatial learning was measured as latency to escape in three different stages, visible platform (VP), hidden platform (HP), or the reversal (RV) of the HP position. No deficits were shown by males (A) or females (E) of any genotype in identifying the platform in the VP trial. (B) Both 1bKO and 1a/1bHET males showed a delay in learning the location of the HP, and a similar deficit was present in 1bKO females (F). (C,G) No differences were found in the RV during the test. (D,H) Spatial memory retention was measured between the HP and RV trials by the time spent swimming in the quadrant where the platform was previously located. Significant spatial memory impairment was found in the 1bKO male mice compared to WT both during the first 15 s and at the end of the trial after 60 s, while female 1bKO mice showed no deficit. 1a/1bHET males and females spent less time looking for the platform during the first 15 s, but subsequently recovered. Two-way ANOVA with repeated measures was used for analysis of the HP phase. Multiple t-tests with equal variance were uses for individual timepoints and probe analysis ∗p < 0.05, ∗∗p < 0.01.
Only CC2D1A Is Involved in Anxiety and Hyperactivity
1A-cKO animals showed increased mobility and reduced entry into the center of the open field arena, indicating hyperactivity and anxiety (Oaks et al., 2017). In addition, removal of Cc2d1a in the forebrain also leads to ulcerative dermatitis due to obsessive grooming and social interaction deficits (Oaks et al., 2017). 1b-KO males and females performed similarly to WT littermates in the open field test and showed no signs of hyperactivity or anxiety (Figure 5) (Distance – Males: WT, d = 25.16 ± 2.29m, n = 11; 1b-KO, d = 29.63 ± 1.96m, n = 11, p = 0.498; Females: WT, d = 34.65 ± 1.36m, n = 13; 1b-KO, d = 42.37 ± 3.28m, n = 11, p = 0.097. Time in center – Males: WT, t = 78.13 ± 5.23s, n = 11; 1b-KO, t = 83.17 ± 14.26s, n = 11, p = 0.988; Females: WT, t = 77.45 ± 11.78s, n = 10; 1b-KO, t = 87.75 ± 17.65s, n = 10, p = 0.969). Interestingly, 1a/1b-dHETs showed increased locomotion and avoidance of open spaces, as previously observed for the 1a-cKOs, but only in males, similar to the exploration in the NORT where increased exploratory behavior was only observed in 1a/1b-dHET males (Figures 5A,B) (Distance – Males: WT, d = 25.16 ± 2.29m, n = 11; 1a/1b-dHET, d = 35.85 ± 2.94m, n = 13, p = 0.0076 ∗∗; Females: WT, d = 34.65 ± 1.36m, n = 13; 1a/1b-dHET, d = 35.35 ± 2.51m, n = 11, p = 0.999. Time in center – Males: WT, t = 78.13 ± 5.23s, n = 11; 1a/1b-dHET, t = 41.32 ± 3.71s, n = 13, p = 0.0198 ∗; Females: WT, t = 77.45 ± 11.78s, n = 10; 1b-KO, t = 121.90 ± 15.19s, n = 11, p = 0.1225). No ulcerative dermatitis or obsessive grooming was observed in any of these mouse lines.
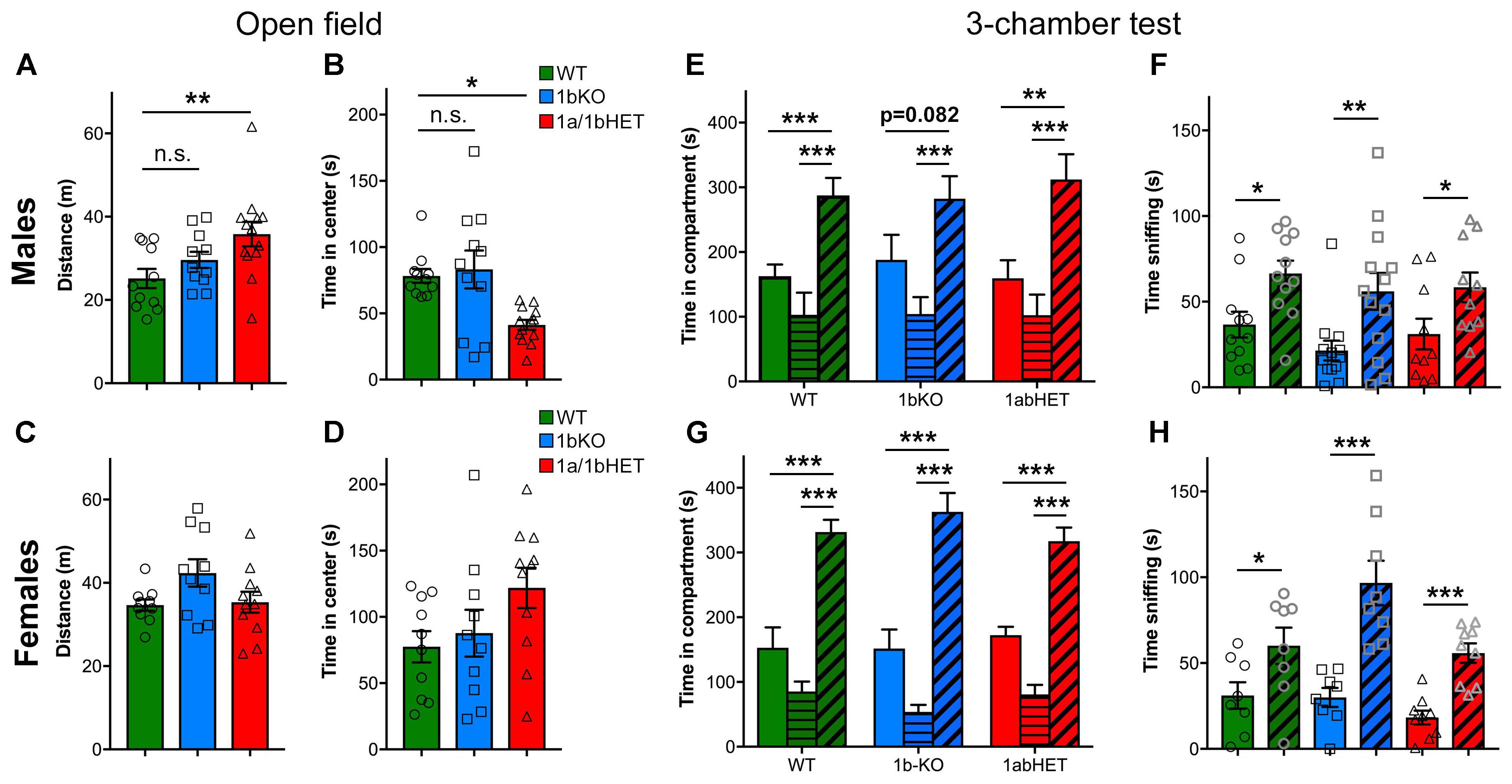
FIGURE 5. CC2D1A contributes to anxiety and hyperactivity. (A–D) Exploratory and general locomotor activity in a novel environment was assessed on the open field test. Total path length (A) and time spent in the center zone (B) are only affected in 1a/1bHET male mice, while females show no difference (C,D). Results expressed as mean ± SEM, one-way ANOVA with Dunnett’s multiple comparison test, ∗p < 0.05, ∗∗p < 0.01. (E–H) Social interaction behavior was assessed by the three-chamber test, presented as time spent in each chamber (E,G) and time spent sniffing the novel mouse vs. the empty cup (F,H). 1bKO male mice showed significantly increased sniffing of the novel mouse vs. the cup (F), but did not show a significant increase in time in the compartment indicating that they may display less interest for the mouse (E). 1a/1bHET males and females and 1bKO females showed no difference from WT littermates (E–H). Results expressed as mean ± SEM. Two-tailed t-test with equal variance ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Finally, all mice were tested in the social approach version of the three-chambered test. In this test, the mouse is placed in an apparatus with three communicating chambers. In the left chamber, there is a novel mouse of the same sex under a wire cup, while in the right chamber there is an empty wire cup. Mice spend more time exploring and sniffing the stranger mouse than the object and this is considered a social action (Nadler et al., 2004; Kaidanovich-Beilin et al., 2011). The 1a-cKO showed no preference for the conspecific both as in the time spent around the mouse enclosure and the time spent sniffing the stranger mouse (Oaks et al., 2017). 1a/1b-dHET males and females and 1b-KO females behaved like WT mice in this test (Figures 5E–H). 1b-KO males were moderately affected showing non-significant difference between the empty cup and the stranger (Figure 5E) [Males: WT, time with mouse (tm) = 287.65 ± 26.81s, time with object (to) = 162.74 ± 18.15s, n = 11, p = 0.00098 ∗∗∗; 1b-KO, tm = 282.37 ± 34.83s, to = 187.98 ± 28.63s, n = 13, p = 0.082; 1a/1b-dHET, tm = 312.05 ± 39.03s, to = 159.39 ± 28.11s, n = 10, p = 0.0052 ∗∗. Females: WT, tm = 331.50 ± 19.14s, to = 152.70 ± 31.59s, n = 8, p = 0.00026 ∗∗∗; 1b-KO, tm = 362.93 ± 29.06s, to = 151.53 ± 29.39s, n = 8, p = 0.00016 ∗∗∗; 1a/1b-dHET, tm = 317.62 ± 20.89s, to = 172.47 ± 12.91s, n = 9, p = 0.00002 ∗∗∗]. The deficit in 1b-KO males was primarily due to a subset of animals showing preference for the object (Supplementary Figure 3). All genotypes showed significantly increased time spent sniffing the stranger mouse, indicating that once in the chamber the 1b-KO animals interact with the other animal (Figures 5F,H) [Males: WT, time sniffing mouse (tsm) = 66.47 ± 7.44s, time sniffing object (tso) = 36.61 ± 7.51s, n = 11, p = 0.0105 ∗; 1b-KO, tsm = 56.04 ± 10.78s, tso = 21.47 ± 5.78s, n = 13, p = 0.009 ∗∗; 1a/1b-dHET, tsm = 58.40 ± 8.65s, tso = 31.11 ± 8.95s, n = 10, p = 0.042 ∗. Females: WT, tsm = 60.11 ± 10.60s, tso = 31.15 ± 7.71s, n = 8, p = 0.044 ∗; 1b-KO, tsm = 96.68 ± 13.00s, tso = 29.93 ± 5.55s, n = 8, p = 0.00033 ∗∗∗; 1a/1b-dHET, tsm = 55.80 ± 5.66s, to = 18.26 ± 4.02s, n = 9, p = 0.00005 ∗∗∗].
In conclusion, 1b-KO and 1a/1b-dHET animals show only partially overlapping behavioral profiles in anxiety, hyperactivity, and sociability. 1b-KO mice of either sex do not appear anxious or hyperactive and only males show a mild sociability deficit in the three-chamber test. 1a/1b-dHET males are more similar to 1a-cKO mice, with increased locomotion and decreased time in the center of the open field. These results show that CC2D1A and CC2D1B only have partially redundant roles in cognitive and social function. Each of the Cc2d1 genes contributes to aspects of learning and memory and sociability, but Cc2d1a appears to be more critical for hyperactivity and anxiety. Interestingly, both lines display sexually dimorphic phenotypes with males being mildly more affected than females.
Discussion
Cognitive development is controlled by a multitude of mechanisms regulating synaptic transmission and neuronal function. Hundreds of genes have been found mutated in patients with ID and ASD and the generation of mouse models has deepened our understanding of how each gene contributes to disease and behavior (Nestler and Hyman, 2010; Ey et al., 2011; Kazdoba et al., 2015). Mutations in the gene encoding CC2D1A cause a rare form of ID and ASD in humans, and this protein is emerging as a critical regulator of intracellular signaling with roles in cognitive function (Basel-Vanagaite et al., 2006; Manzini et al., 2014), immunity (Zhao et al., 2010; Chang et al., 2011) and cancer (Yamada et al., 2015). Removal of the only CC2D1 homolog in Drosophila, lgd, causes early lethality and severe deficits in morphogenesis, and both human proteins can rescue lgd LOF phenotypes, suggesting that the vertebrate CC2D1 proteins have redundant functions (Drusenheimer et al., 2015). In fact, deficits in lgd mutant flies are more severe than in 1a-KO and 1b-KO mice (Drusenheimer et al., 2015). We hypothesized that the neuropsychiatric phenotypes observed in humans carrying CC2D1A LOF mutations are likely due to the inability of CC2D1B to fully substitute for CC2D1A.
Initial evidence to support our hypothesis was provided by the fact that 1a-KO mice are anatomically normal but die soon after birth due to breathing and swallowing deficits (Zhao et al., 2011; Al-Tawashi et al., 2012; Chen et al., 2012; Oaks et al., 2017), while 1b-KOs are viable and fertile (Drusenheimer et al., 2015). No respiratory deficits have been reported in humans with CC2D1A mutations and these findings indicated that Cc2d1a has an essential role in breathing regulation in the brain stem in the mouse where CC2D1B cannot complement CC2D1A function. We do not know whether this difference between mice and humans is due to the timing of birth which is at an earlier stage of neural development in mice, or to differences in CC2D1A and CC2D1B expression in the brain stem in the two species.
The current study provides further evidence that Cc2d1a LOF is more severe than Cc2d1b LOF through behavioral studies. Forebrain-specific Cc2d1a-deficient mice 1a-cKO display an array of cognitive and social deficits, in addition to anxiety and hyperactivity (Oaks et al., 2017). 1b-KO mice only display cognitive deficits, with object-recognition impairment in the NORT and reduced memory acquisition and retention in the MWM test, but no other phenotypes. Interestingly, the MWM test results reveal different roles for the CC2D1 proteins in spatial learning and memory. 1a-cKO animals showed delayed learning, but no deficit in remembering the location of the platform once it was learned (Oaks et al., 2017), while 1b-KO mice also displayed reduced memory retention in the probe, especially in males. Parallel studies in the 1a/1b-dHET line confirm this difference observing deficits in both spatial memory acquisition and retention. In comparing cognitive performance in 1b-KOs with 1a/1b-dHET and previously published 1a-cKOs, all lines were equally deficient in the NORT, indicating that object recognition circuits in the cortex and hippocampus are affected (Antunes and Biala, 2012).
Cc2d1b also differs from Cc2d1a, as it appears to have no role in social behavior, hyperactivity, and anxiety. Results from the 1a/1b-dHETs suggest that partial loss of Cc2d1a in combination with a half dosage of Cc2d1b is sufficient to generate hyperactivity and anxiety. Interestingly, only complete loss of Cc2d1a leads to social deficits. Taken together, our results indicate that Cc2d1a and Cc2d1b have roles in behavioral function that are only partially redundant. Behavior is regulated by a multitude of molecular and cellular mechanisms, but it is interesting to note how each of these two homologous proteins may contribute to specific sets of behaviors. These effects could be due to their role in controlling a variety of intracellular signaling processes and thereby affecting multiple cellular functions.
CC2D1A and CC2D1B were reported to regulate endocytosis and gene transcription (Hadjighassem et al., 2011; Martinelli et al., 2012; Usami et al., 2012; Drusenheimer et al., 2015), but CC2D1A has been the most studied to date. Many of the pathways regulated by CC2D1A, such as Akt, CREB, and NF-κB, are important for learning and memory (Bourtchuladze et al., 1994; Meffert et al., 2003; Lai et al., 2006; Majumdar et al., 2011). Initial findings in Cc2d1a-deficient cells showed an imbalance in signaling activation (Al-Tawashi et al., 2012; Manzini et al., 2014) and mild disruptions in endosome size (Drusenheimer et al., 2015), again demonstrating how CC2D1B is not fully able to compensate for CC2D1A. Our results in the 1a/1b-dHET also imply that there is a balance in CC2D1A and CC2D1B activity, and experiments in Drosophila and mammalian cells suggest that Cc2d1a and lgd expression and subcellular localization must be finely regulated to control endosomal trafficking and signaling through recruitment to specific signaling complexes (Gallagher and Knoblich, 2006; Jaekel and Klein, 2006; Manzini et al., 2014; Drusenheimer et al., 2015). This could be explained by a critical role for the CC2D1 proteins in the maintenance of signaling homeostasis. Homeostasis is broadly defined as the ability of a cell to return to a set point and maintain equilibrium. Many genes mutated in ASD and ID control homeostatic mechanisms in synaptic transmission, transcription, and signaling (De Rubeis et al., 2014; Pinto et al., 2014), and genomic deletions and duplications may show similar neurodevelopmental phenotypes leading to the hypothesis that the pathogenesis of neurodevelopmental disorders is linked to homeostatic imbalance (Ramocki and Zoghbi, 2008). Behavioral impairments in cognitive and social function could then be caused by subtle disruptions in multiple cellular processes limiting the ability of individual neurons and/or neuronal circuits to respond to stimuli, including environmental changes or stressors. In this respect, defining the role of CC2D1A and CC2D1B in homeostatic signaling of multiple pathways disrupted in ASD and ID could be important to dissect whether different signaling pathways contribute to distinct behavioral deficits.
Finally, in light of the cognitive defects in the 1b-KO mice, it may be worthwhile to search for CC2D1B mutations in patients with cognitive deficits, and to also consider the possibility of trans-heterozygous cases where CC2D1A and CC2D1B mutations are both present in heterozygosity. While complete loss of both CC2D1 genes is embryonic lethal, haploinsufficiency of both CC2D1A and CC2D1B may lead to ID and ASD as CC2D1A LOF does. In the Genome Aggregation Database browser, which collects allele frequency data from more than 100,000 individuals in different populations, there are 43 likely gene disrupting (stop codon, frameshift, or splice site) alleles for CC2D1A and 89 for CC2D1B. These variants alone or in combination may further contribute to the genetic burden of ID.
Author Contributions
MZ and MM designed the study and wrote the manuscript. MZ, AO, HP, and JA conducted the experiments and analyzed the data.
Funding
This work was supported by NIH grant R00HD067379, a Pilot Award from the Intellectual and Developmental Disabilities Research Center (IDDRC) at The Children’s Research Institute (P30HD040677), and institutional start-up funds from The George Washington University to MM. NIH grants to VelociGene at Regeneron Inc. (U01HG004085) and the CSD Consortium (U01HG004080) funded the generation of gene-targeted ES cells for 8500 genes in the KOMP Program and archived and distributed by the KOMP Repository at UC Davis and CHORI (U42RR024244). For more information or to obtain KOMP products go to www.komp.org or email service@komp.org.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Tom Maynard and Irene Zohn for advice on mouse line generation and the analysis of the double knockouts; Anthony LaMantia, Judy Liu, Maria Chahrour, Emanuela Santini, and Sally Till for their helpful discussion on experimental design. The Cc2d1a and Cc2d1b KO mouse strains used for this research project were generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP Repository (www.komp.org).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00065/full#supplementary-material
References
Al-Tawashi, A., Jung, S. Y., Liu, D., Su, B., and Qin, J. (2012). Protein implicated in nonsyndromic mental retardation regulates protein kinase A (PKA) activity. J. Biol. Chem. 287, 14644–14658. doi: 10.1074/jbc.M111.261875
Antunes, M., and Biala, G. (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. doi: 10.1007/s10339-011-0430-z
Basel-Vanagaite, L., Attia, R., Yahav, M., Ferland, R. J., Anteki, L., Walsh, C. A., et al. (2006). The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation. J. Med. Genet. 43, 203–210. doi: 10.1136/jmg.2005.035709
Bevins, R. A., and Besheer, J. (2006). Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 1, 1306–1311. doi: 10.1038/nprot.2006.205
Bourtchuladze, R., Frenguelli, B., Blendy, J., Cioffi, D., Schütz, G., and Silva, A. J. (1994). Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79, 59–68. doi: 10.1016/0092-8674(94)90400-6
Chang, C.-H., Lai, L.-C., Cheng, H.-C., Chen, K.-R., Syue, Y.-Z., Lu, H.-C., et al. (2011). TBK1-associated protein in endolysosomes (TAPE) is an innate immune regulator modulating the TLR3 and TLR4 signaling pathways. J. Biol. Chem. 286, 7043–7051. doi: 10.1074/jbc.M110.164632
Chen, K.-R., Chang, C.-H., Huang, C.-Y., Lin, C.-Y., Lin, W.-Y., Lo, Y.-C., et al. (2012). TBK1-associated protein in endolysosomes (TAPE)/CC2D1A is a key regulator linking RIG-I-like receptors to antiviral immunity. J. Biol. Chem. 287, 32216–32221. doi: 10.1074/jbc.C112.394346
De Rubeis, S., He, X., Goldberg, A. P., Poultney, C. S., Samocha, K., Ercument Cicek, A., et al. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215. doi: 10.1038/nature13772
Drusenheimer, N., Migdal, B., Jäckel, S., Tveriakhina, L., Scheider, K., Schulz, K., et al. (2015). The mammalian orthologs of Drosophila Lgd, CC2D1A and CC2D1B, function in the endocytic pathway, but their individual loss of function does not affect notch signalling. PLoS Genet. 11:e1005749. doi: 10.1371/journal.pgen.1005749
Ey, E., Leblond, C. S., and Bourgeron, T. (2011). Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 4, 5–16. doi: 10.1002/aur.175
Gallagher, C. M., and Knoblich, J. A. (2006). The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev. Cell 11, 641–653. doi: 10.1016/j.devcel.2006.09.014
Hadjighassem, M. R., Austin, M. C., Szewczyk, B., Daigle, M., Stockmeier, C. A., and Albert, P. R. (2009). Human Freud-2/CC2D1B: a novel repressor of postsynaptic serotonin-1A receptor expression. Biol. Psychiatry 66, 214–222. doi: 10.1016/j.biopsych.2009.02.033
Hadjighassem, M. R., Galaraga, K., and Albert, P. R. (2011). Freud-2/CC2D1B mediates dual repression of the serotonin-1A receptor gene. Eur. J. Neurosci. 33, 214–223. doi: 10.1111/j.1460-9568.2010.07498.x
Jaekel, R., and Klein, T. (2006). The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev. Cell 11, 655–669. doi: 10.1016/j.devcel.2006.09.019
Kaidanovich-Beilin, O., Lipina, T., Vukobradovic, I., Roder, J., and Woodgett, J. R. (2011). Assessment of social interaction behaviors. J. Vis. Exp. 48:e2473. doi: 10.3791/2473
Kazdoba, T. M., Leach, P. T., and Crawley, J. N. (2015). Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 15, 7–26. doi: 10.1111/gbb.12256
Lai, W.-S., Xu, B., Westphal, K. G. C., Paterlini, M., Olivier, B., Pavlidis, P., et al. (2006). Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc. Natl. Acad. Sci. U.S.A. 103, 16906–16911. doi: 10.1073/pnas.0604994103
Lim, E. T., Raychaudhuri, S., Sanders, S. J., Stevens, C., Sabo, A., Macarthur, D. G., et al. (2013). Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron 77, 235–242. doi: 10.1016/j.neuron.2012.12.029
Majumdar, D., Nebhan, C. A., Hu, L., Anderson, B., and Webb, D. J. (2011). An APPL1/Akt signaling complex regulates dendritic spine and synapse formation in hippocampal neurons. Mol. Cell. Neurosci. 46, 633–644. doi: 10.1016/j.mcn.2011.01.003
Manzini, M. C., Xiong, L., Shaheen, R., Tambunan, D. E., Di Costanzo, S., Mitisalis, V., et al. (2014). CC2D1A regulates human intellectual and social function as well as NF-κB signaling homeostasis. Cell Rep. 8, 647–655. doi: 10.1016/j.celrep.2014.06.039
Martinelli, N., Hartlieb, B., Usami, Y., Sabin, C., Dordor, A., Miguet, N., et al. (2012). CC2D1A is a regulator of ESCRT-III CHMP4B. J. Mol. Biol. 419, 75–88. doi: 10.1016/j.jmb.2012.02.044
Meffert, M. K., Chang, J. M., Wiltgen, B. J., Fanselow, M. S., and Baltimore, D. (2003). NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 6, 1072–1078. doi: 10.1038/nn1110
Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60. doi: 10.1016/0165-0270(84)90007-4
Musante, L., and Ropers, H. H. (2014). Genetics of recessive cognitive disorders. Trends Genet. 30, 32–39. doi: 10.1016/j.tig.2013.09.008
Nadler, J. J., Moy, S. S., Dold, G., Trang, D., Simmons, N., Perez, A., et al. (2004). Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3, 303–314. doi: 10.1111/j.1601-183X.2004.00071.x
Nestler, E. J., and Hyman, S. E. (2010). Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169. doi: 10.1038/nn.2647
Oaks, A. W., Zamarbide, M., Tambunan, D. E., Santini, E., Di Costanzo, S., Pond, H. L., et al. (2017). Cc2d1a loss of function disrupts functional and morphological development in forebrain neurons leading to cognitive and social deficits. Cereb. Cortex 27, 1670–1685. doi: 10.1093/cercor/bhw009
Pinto, D., Delaby, E., Merico, D., Barbosa, M., Merikangas, A., Klei, L., et al. (2014). Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 94, 677–694. doi: 10.1016/j.ajhg.2014.03.018
Ramocki, M. B. M., and Zoghbi, H. Y. H. (2008). Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 455, 912–918. doi: 10.1038/nature07457
Reuter, M. S., Tawamie, H., Buchert, R., Hosny Gebril, O., Froukh, T., Thiel, C., et al. (2017). Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry 74, 293–299. doi: 10.1001/jamapsychiatry.2016.3798
Rogers, D. C., Fisher, E. M., Brown, S. D., Peters, J., Hunter, A. J., and Martin, J. E. (1997). Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome 8, 711–713. doi: 10.1007/s003359900551
Sanders, S. J., Murtha, M. T., Gupta, A. R., Murdoch, J. D., Raubeson, M. J., Willsey, A. J., et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241. doi: 10.1038/nature10945
Usami, Y., Popov, S., Weiss, E. R., Vriesema-Magnuson, C., Calistri, A., and Göttlinger, H. G. (2012). Regulation of CHMP4/ESCRT-III function in human immunodeficiency virus type 1 budding by CC2D1A. J. Virol. 86, 3746–3756. doi: 10.1128/JVI.06539-11
Vorhees, C. V., and Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858. doi: 10.1038/nprot.2006.116
Yamada, T., Amann, J. M., Fukuda, K., Takeuchi, S., Fujita, N., Uehara, H., et al. (2015). Akt kinase-interacting protein Aki1 signals through CREB to drive diffuse malignant mesothelioma. Cancer Res. 75, 4188–4197. doi: 10.1158/0008-5472.CAN-15-0858
Yu, T. W., Chahrour, M. H., Coulter, M. E., Jiralerspong, S., Okamura-Ikeda, K., Ataman, B., et al. (2013). Using whole-exome sequencing to identify inherited causes of autism. Neuron 77, 259–273. doi: 10.1016/j.neuron.2012.11.002
Zhao, M., Li, X.-D., and Chen, Z. (2010). CC2D1A, a DM14 and C2 domain protein, activates NF-kappaB through the canonical pathway. J. Biol. Chem. 285, 24372–24380. doi: 10.1074/jbc.M109.100057
Keywords: intellectual disability, learning, social function, anxiety, hyperactivity, rare diseases, mouse models
Citation: Zamarbide M, Oaks AW, Pond HL, Adelman JS and Manzini MC (2018) Loss of the Intellectual Disability and Autism Gene Cc2d1a and Its Homolog Cc2d1b Differentially Affect Spatial Memory, Anxiety, and Hyperactivity. Front. Genet. 9:65. doi: 10.3389/fgene.2018.00065
Received: 11 October 2017; Accepted: 15 February 2018;
Published: 02 March 2018.
Edited by:
Amritha Jaishankar, Rare Genomics Institute, United StatesReviewed by:
Theodora Katsila, University of Patras, GreeceNelson L. S. Tang, The Chinese University of Hong Kong, Hong Kong
Copyright © 2018 Zamarbide, Oaks, Pond, Adelman and Manzini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Chiara Manzini, Y21hbnppbmlAZ3d1LmVkdQ==; Y2hpYXJhLm1hbnppbmlAZ21haWwuY29t
 Marta Zamarbide
Marta Zamarbide Adam W. Oaks
Adam W. Oaks Heather L. Pond1
Heather L. Pond1 M. Chiara Manzini
M. Chiara Manzini