
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 29 May 2017
Sec. Systems Biology Archive
Volume 8 - 2017 | https://doi.org/10.3389/fgene.2017.00065
This article is part of the Research Topic DNA Damage and Inflammation under stress View all 6 articles
 Evagelia Spanou1†
Evagelia Spanou1† Polyxeni Kalisperati1†
Polyxeni Kalisperati1† Ioannis S. Pateras2
Ioannis S. Pateras2 Alexandros Papalampros3
Alexandros Papalampros3 Alexandra Barbouti4
Alexandra Barbouti4 Athanasios G. Tzioufas5
Athanasios G. Tzioufas5 Athanassios Kotsinas2*
Athanassios Kotsinas2* Stavros Sougioultzis1
Stavros Sougioultzis1The fundamental role of human Toll-like receptors (TLRs) and NOD-like receptors (NLRs), the two most studied pathogen recognition receptors (PRRs), is the protection against pathogens and excessive tissue injury. Recent evidence supports the association between TLR/NLR gene mutations and susceptibility to inflammatory, autoimmune, and malignant diseases. PRRs also interfere with several cellular processes, such as cell growth, apoptosis, cell proliferation, differentiation, autophagy, angiogenesis, cell motility and migration, and DNA repair mechanisms. We briefly review the impact of TLR4 and NOD1/NOD2 and their genetic variability in the process of inflammation, tumorigenesis and DNA repair, focusing in the gastrointestinal tract. We also review the available data on new therapeutic strategies utilizing TLR/NLR agonists and antagonists for cancer, allergic diseases, viral infections and vaccine development against both infectious diseases and cancer.
The human innate immune system is activated when pathogen recognition receptors (PRRs) recognize either pathogen-associated molecular patterns (PAMPs), or danger-associated molecular patterns (DAMPs) (Akira et al., 2006; Kawai and Akira, 2011). PRRs are in both cell membranes and cytosol of macrophages, fibroblasts, mast cells, dendritic cells, and circulating leucocytes (Newton and Dixit, 2012). They include members of the Toll-like receptors (TLRs), nucleotide-binding, and oligomerization domain containing receptors (NOD-like receptors, NLRs), retinoic acid-inducible gene(RIG) I-like RNA helicases, C-type lectins, and AIM2-like receptors(ALRs) (Saxena and Yeretssian, 2014) (Figure 1).
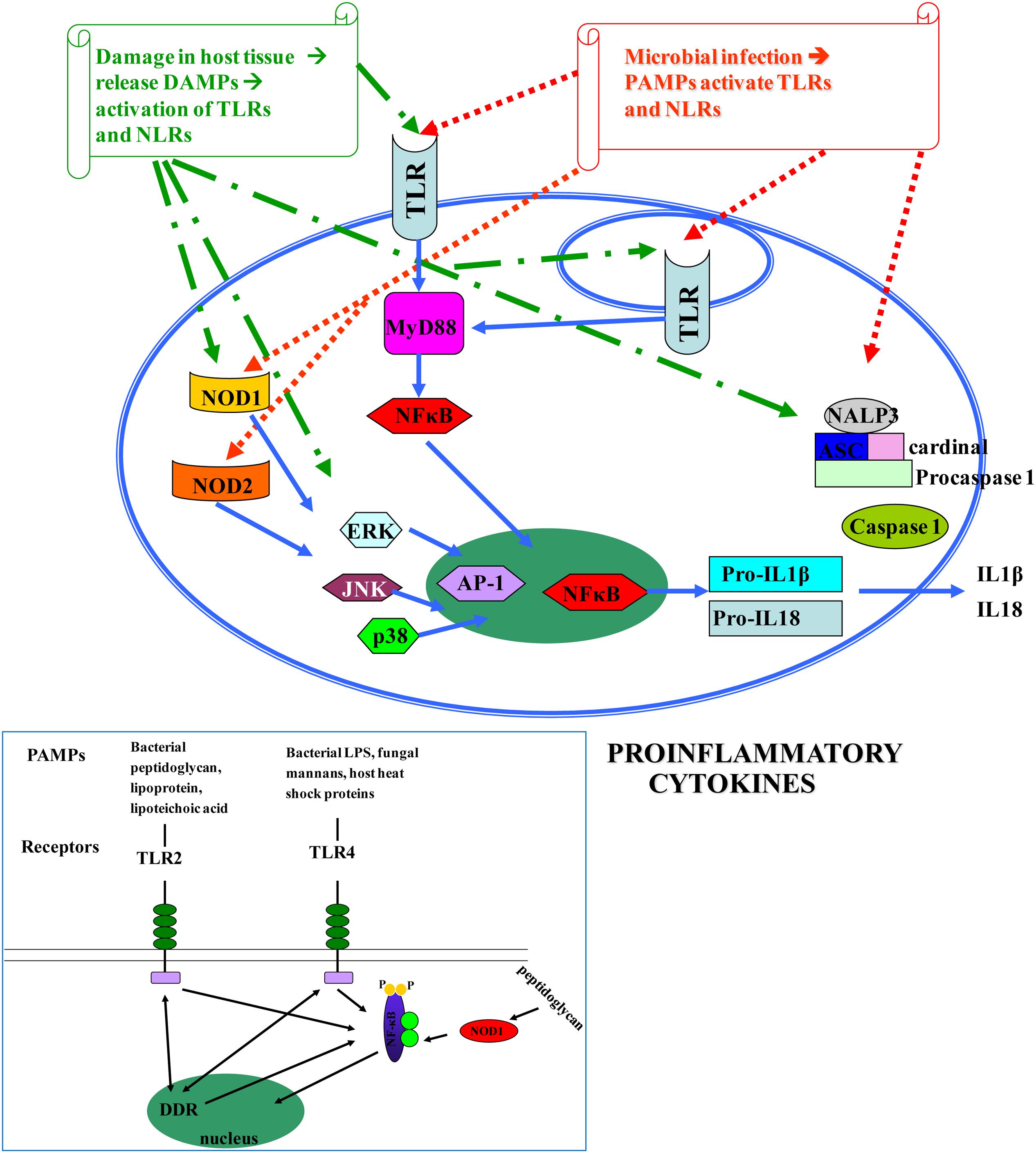
FIGURE 1. Pathogen-associated molecular patterns (PAMPs) [microbial nucleic acids, including DNA (e.g., unmethylated CpG motifs), double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), 5′-triphosphate RNA, lipoproteins, surface glycoproteins, and membrane components such as peptidoglycans, lipoteichoic acid, lipopolysaccharide (LPS), and glycosylphosphatidylinositol] and DAMPs [endogenous molecules normally found in cells and released during cell death, such as ATP, uric acid, the cytokine IL1a, heparin sulfate, RNA, and DNA] (Kawai and Akira, 2010; Tang et al., 2012) bind to TLRs and NLRs, activate NF-κB and AP-1 transcription factors and lead to the production of pro-inflammatory cytokines that perpetuate inflammation and induce tissue damage. DAMPs are localized within the nucleus and cytoplasm (HMGB1), cytoplasm alone (S100 proteins), exosomes (heat shock proteins, HSP), the extracellular matrix (hyaluronic acid), and in plasma components such as complement (C3a, C4a, and C5a), but also, they can be mimicked by release of intracellular mitochondria, consisting of formyl peptides and mitochondrial DNA (with CpG DNA repeats) (Tang et al., 2012). Different TLRs serve as receptors for diverse ligands (Mitchell et al., 2007).
Available data have shown that genetic variability influences the susceptibility and evolution of several human diseases, like autoimmune diseases or infections, by affecting numerous cellular processes hence modulating the response to environmental and intrinsic factors (Orr and Chanock, 2008). Diseases associated with deficiencies in a single gene are not common in the population, therefore many epidemiological studies are now focused on the diversity of the contributing factors of complex illnesses (Orr and Chanock, 2008), responsible for most of the human morbidity and mortality. It is generally accepted that multiple genetic defects contribute to the phenotype of complex diseases, while the effects of single polymorphisms are usually veiled. Powerful tools such as high throughput expression profile analysis and genome-wide association studies (GWAS) are currently implemented to investigate the different polymorphisms and their interactions that culminate to disease development (Mayerle et al., 2013; Kim et al., 2014).
In this review, we highlight the impact of genetic diversity encoded in the TLR4 and NOD1/NOD2 loci to the progression of inflammation, tumorigenesis and the process of DNA repair, focusing in the gastrointestinal tract.
There are 10 members of TLRs, type I transmembrane glycoproteins, in humans (TLR1–TLR10) (Janssens and Beyaert, 2003). Their extracellular domain contains leucine-rich repeats (LRRs) expressed by cells of the innate immune system, which are involved in ligand binding (Bowie and O’Neill, 2000), while the intracellular tail contains a Toll/interleukin (IL)-1 receptor (TIR), that mediates downstream signaling. TLRs are well conserved across species and were first described in Drosophila (Medzhitov et al., 1997). They recognize bacterial and viral PAMPs in the extracellular environment (TLR1, TLR2, TLR4, TLR5, TLR6) or endolysosomes (TLR3, TLR7, TLR8, TLR9, TLR10) (O’Neill, 2006). Different TLRs serve as receptors for diverse ligands (Mitchell et al., 2007). TLRs are essential for the initiation of protective immunity against infections. Nevertheless, aberrant TLR responses may contribute to inappropriate acute and chronic inflammation and to systematic autoimmune diseases. In addition, it has become apparent that endogenous molecules released by dying cells or by some pathological conditions activate TLRs, further promoting inflammatory or autoimmune diseases (Kawai and Akira, 2010; Koberlin et al., 2016) (Figure 1). Despite the extensive study of TLRs in the gastrointestinal tract, the exact location and function of individual TLRs in various disease states is still evolving (Fukata and Abreu, 2008).
TLR4 is an essential member of the TLR family, which responds to bacterial lipopolysaccharide(LPS), a component of the outer membrane of Gram(–) bacteria(Akira et al., 2006).
Recent studies, conducted in several populations, have shown associations between TLR polymorphisms and the risk of gastric cancer (GC) (Table 1A). Some of these polymorphisms, such as TLR4rs4986790 (Asp299Gly), TLR4rs4986791 (Thr399Ile), TLR4 rs10759932, CD14 -260C/T, and TLR2-196to-174del appear to be associated with gastric precancerous lesions which may lead to intestinal type GC (Castano-Rodriguez et al., 2013). Especially two of the above polymorphisms, TLR4rs4986790 and TLR4rs4986791, disrupt the normal structure of the extracellular domain of TLR4, resulting in a protein with reduced binding affinity to the ligands of Helicobacter pylori (El-Omar et al., 2008).
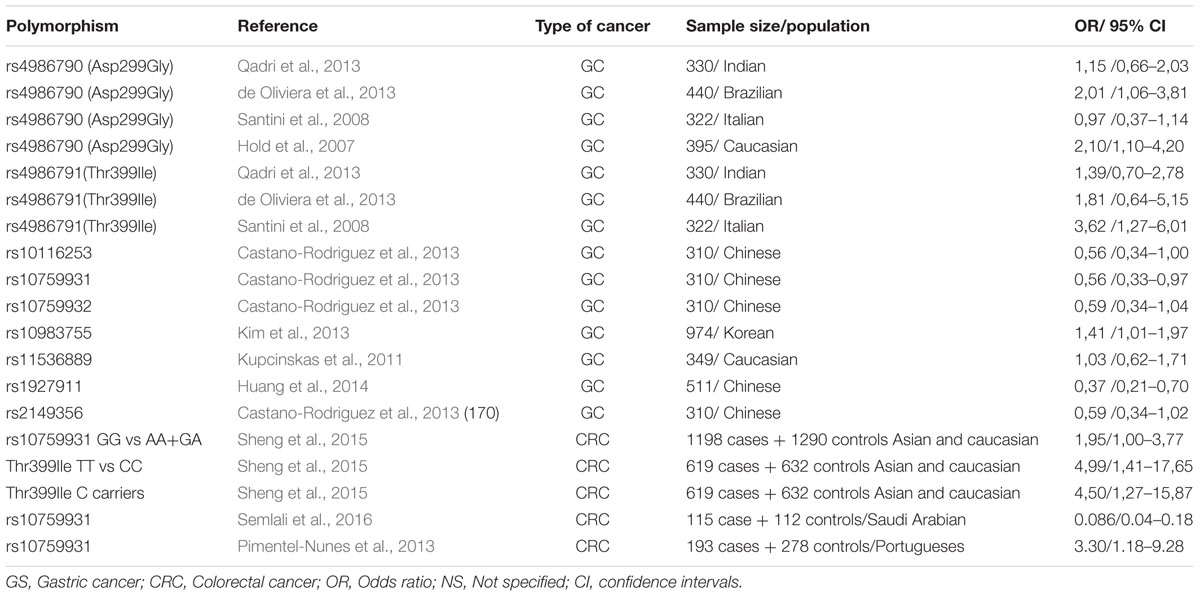
TABLE 1A. Genetic polymorphisms in the TRL4 signaling pathway that have been studied in relation to gastric cancer and CRC.
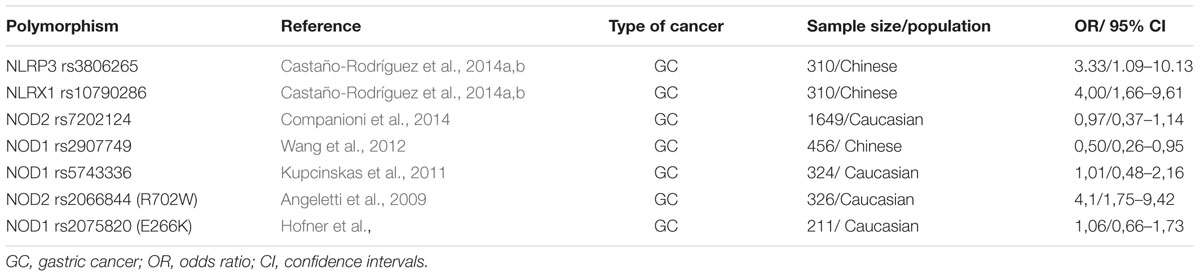
TABLE 1B. Genetic polymorphisms in the NOD-like receptor signaling pathway that have been studied in relation to gastric cancer.
Data are few regarding TLR4 polymorphisms and H. pylori– associated diseases. Analyzing a population from Northern India, Achyut et al. (2007) concluded that TLR4rs4986791 substitution may be a risk factor for gastritis and precancerous lesions, while they reported a significant association between TLR4rs4986790 and neutrophil infiltration. In another study from Hungary, Hofner et al. (2007) found no association of TLR4 polymorphisms between H. pylori positive patients with or without gastritis or duodenal ulcers. Two studies in children by Tseng et al. (2006) and Moura et al. (2008), reported no association between TLR4rs4986790 and risk of infection. Based on the current evidence, it seems likely that these polymorphisms have a marginal or no impact in H. pylori acquisition risk and associated inflammation. However, a blunt IgA antibody production against H. pylori infection was observed in Greek patients with TLR4 polymorphisms, suggesting that a defect or dysregulation of humoral mucosal defense may be present (Manolakis et al., 2010).
The lack of significant effects of the TLR4 polymorphisms in infections is not uncommon among Europeans. Indo-European populations are frequently (6–14%) double heterozygous for both polymorphisms (Ferwerda et al., 2007), and TLR4rs4986790/TLR4rs4986791 haplotype may not functionally differ from wild type TLR4. Conversely, TLR4rs4986790 was frequently found (10–18%) among African populations, with only 2% having TLR4rs4986791 co-segregation (Bochud et al., 2009; Bhuvanendran et al., 2011). Disparities between Europeans (co-segregation) compared to Asian and African populations (lack of co-segregation) may explain the significant associations noted for endemic diseases in Asia and Africa.
Sato et al. (2012) reported that the genetic variation TLR4rs11536889 (+3725G/C) may contribute to the translational regulation of TLR4 and influences the response to LPS. According to Liu et al. (2011), TLR4rs10759932 decreases the expression of FOXP3, a marker for regulatory T (Treg) cells that are increased in H. pylori gastritis and probably contribute to H. pylori persistence (Jang, 2010).
Regarding colorectal cancer (CRC), Abuli et al. (2013) reported 20 susceptible SNPs in 18 risk loci for CRC, among which were TLR gene polymorphisms. The GG genotype of TLR4rs4986790 and the TT genotype of TLR4rs4986791 polymorphisms might be correlated with an increased risk of CRC, and may serve as biomarkers (Pimentel-Nunes et al., 2013; Sheng et al., 2015; Semlali et al., 2016) (Table 1A). In addition, a study by Wang et al. (2010) suggested that high immunohistochemical expression of TLR4 in colorectal tumors is associated with liver metastases and poor prognosis. In contrast, Nihon-Yanagi et al. (2012), support that TLR2 is mainly involved in colon tumorigenesis. Similar, apparently controversial results have been reported for other factors involved in gastrointestinal carcinogenesis (Evangelou et al., 2014). Taken together, we assume that TLRs are involved in colon cancer development and further work is needed to clarify their exact role.
The NLR family includes NODs, NLRPs (also called NALPs), IL-1β-converting enzyme (ICE)-protease activating factor (IPAF), neuronal apoptosis inhibitor factors (NAIPs), and MHC class II transactivator (CIITA)(Ting et al., 2006). These molecules are in the cytoplasm and survey for the presence of intracellular pathogens. In humans, there are 22 known NLRs associated with many diseases (Zhong et al., 2013; Kim et al., 2015). There are four distinct domains in every NLR: a central NACHT (NAIP, CIITA, HET-E, and TP-2), an N-terminal domain that facilitates oligomerization, the ligand sensing LRRs on the C-terminal and the effector domain, which may be pyrin domain (PYD), caspase recruitment domain family (CARD), or baculoviral IAP repeat (BIR). Each NLR contains different effector domain which mediates signal transduction to downstream targets leading to activation of inflammatory caspases by inflammasomes or NF-κB by NODs. NAIP contains BIR domain, IPAF, while some of the NALP family contain CARD domain and most NALPs contain PYD domain (Saxena and Yeretssian, 2014).
NOD1 (NLRC1) and NOD2 (NLRC2) were the first NLRs reported. NODs initiate the activation of MAPKs and NF-κB via interaction with serine-threonine kinase RICK and activation of kinase TAK1 (Inohara et al., 2005). These two molecules (NOD1-NOD2) are essential for tissue homeostasis and host defense against bacterial pathogens (Philpott et al., 2014). Interestingly, single-nucleotide polymorphisms (SNPs) in the NOD2 (CARD15) gene are considered as a significant risk factor in Crohn’s disease (Ogura et al., 2001). NOD1 is expressed in both hematopoietic and non-hematopoietic cells, while NOD2 is restricted to hematopoietic and some specialized epithelial cells, like Paneth cells of the small intestine (Ogura et al., 2003).
In addition, NODs seem to be essential for host defense against non-invasive Gram (-) bacteria, such as H. pylori (Viala et al., 2004). Upon activation, both NOD1 and NOD2 self-oligomerize and, through homotypic CARD-CARD interactions, recruit the CARD containing adaptor receptor-interacting protein kinase 2 (RIP2 or RIPK2), leading to the formation of a ‘Nodosome’, a multi-protein signaling complex that results in NF-κB and MAPK-mediated inflammatory and antimicrobial response (Magalhaes et al., 2011; Keestra et al., 2013). In addition, NLR activation leads to formation of a molecular scaffold complex termed inflammasome. Three human inflammasomes have been described based on the involved NLR protein: the NLP1, the NLP3 and the IPAF. All of them activate caspase-1, a protein essential for the transformation of the pro-IL-1β and pro-IL-18 to the mature cytokines IL-1β and IL-18, which play central role in inflammatory processes (Fukata et al., 2009) (Figure 1).
The four most studied polymorphisms of NOD2 are: rs2066842C/T, rs2066844C/T, rs2066845C/G, rs2066847insC (Table 1B). As they are in coding region, they affect the function of NOD2, by altering the primary amino acid sequence (Liu et al., 2014). These four polymorphisms were initially associated with increased risk of Crohn’s disease (Hugot et al., 2001) and ulcerative colitis (Gazouli et al., 2005). Kurzawski et al. (2004) first linked NOD2 polymorphisms with CRC. Subsequent studies were inconsistent regarding the association of the NOD2 polymorphisms with risk of multiple cancers such as gastric, endometrial, breast, ovarian and laryngeal. A meta-analysis by Liu et al. (2014) suggested that NOD2rs2066844C/T, rs2066845C/G, and rs2066847insC polymorphisms may be associated with increased cancer risk, especially gastrointestinal (Table 1B). NOD2 polymorphisms have been correlated with dysplastic changes of gastric mucosa in the presence of H. pylori (Hnatyszyn et al., 2010); carriers also have increased prevalence of early onset breast and lung cancer (Lener et al., 2006).
On the other hand, no mutations in the NOD1 gene have been associated with intestinal inflammation or CRC. Oppositely, a study by Chen et al. (2008) in a murine model of colitis-associated colon cancer revealed a basic anti-tumorigenic function of intact NOD1. Nevertheless, NOD1 polymorphisms have been associated with the development of atopic eczema, asthma and increased serum IgE concentration (Hysi et al., 2005), while polymorphisms in the intronic region of NOD1 have been linked with the age of IBD onset (McGovern et al., 2005).
It is vital for every cell to protect the integrity of all the encoded information it hosts and enable the accurate transfer of genetic material during cell division. Given that all human cells are exposed to a multitude of genotoxic insults, endogenous and exogenous (Jackson and Bartek, 2009), a highly conserved and advanced DNA recognition and repair network, against a variety of DNA lesions is in operation. The DDR is a complex network of molecular mechanisms, which identifies the genetic damage and induces biochemical pathways which cause cell cycle arrest (so-called control points, checkpoints), promotes repair of lesions in the genetic material, or, alternatively, proceeds to the activation of anti-tumor barriers, apoptosis and senescence (Halazonetis et al., 2008; Gorgoulis and Halazonetis, 2010; Evangelou et al., 2013; Velimezi et al., 2013).
Among all types of genetic damage, the double-stranded breaks (DSBs) constitute the greatest threat to the cell. The presence of DSBs results in the DDR activation having as a key effector the tumour-suppressor protein p53 (Rodier et al., 2007). DSBs can be induced by various stimuli such as ionizing radiation, activated oncogenes, or defective telomeres and are very harmful, even fatal, for the cell. Early activation of DDR in human precancerous lesions highlights the importance of this network in preventing cancer progression (Gorgoulis et al., 2005; Bartkova et al., 2006; Halazonetis et al., 2008; Gorgoulis and Halazonetis, 2010). However, continuous activation of DDR constitutes a sustained “pressure” eventually leading to the mutation of the TP53 gene and loss of the anti-tumor barriers elicited by DDR, providing an explanation for the extremely high rate of TP53 mutations in sporadic solid tumors and initiation of DDR in advanced cancers (Halazonetis et al., 2008; Negrini et al., 2010).
Pathogen recognition receptors are major sensors of innate immunity but they also affect adaptive immune responses. In addition, many PRRs seem to interfere with several cellular processes such as cell growth, apoptosis, cell proliferation, differentiation, autophagy, angiogenesis, cell motility and migration (Kutikhin et al., 2014). Currently there is a strong interest in investigating the impact of PRRs in the process of DNA repair. Undoubtedly, DDR and the immune system are parts of the same protective mechanism aiming to maintain cellular integrity against endogenous and exogenous threats. DAMPs or PAMPs engagement to TLRs leads to DDR activation, by induction of activator protein-1(AP-1) and inflammatory mediators such as IL-12, IL-18, and IL-23, known downstream effectors of TLR signaling (Harberts and Gaspari, 2013). Nevertheless, aberrant activation of the protective mechanism can be harmful not only for the cellular, but also for the whole organismal systemic homeostasis resulting in chronic, even fatal, diseases. Indeed, the state of chronic inflammation observed in many pathologies, such as neoplasia and autoimmunity, may be partially attributed to persistent DDR stimulation (Pateras et al., 2015). From all the above it is conceivable that a common initiating point is potentially shared between malignancies, connective tissue diseases and infectious diseases.
The role of DDR in the pathogenesis of autoimmune diseases is well established (Solier and Pommier, 2014; Gunther et al., 2015; Souliotis et al., 2016). According to a recent report (Funabiki et al., 2014), lupus-like features were developed spontaneously in a mutant mouse line bearing MDA5 (RIG-I-like receptor) gain of function mutation in the absence of the appropriate viral ligand, thus providing direct evidence connecting dysregulation of PRRs with autoimmunity. Furthermore, it is well-established that chronic infection or chronic inflammation is a major driving force in 20% of human cancer and TLR/NLR signaling pathways serve as a link between chronic inflammation and cancer such as colorectal and other tumors (Wang et al., 2006; El-Omar et al., 2008; Goto et al., 2008; Lowe et al., 2010; Yang et al., 2010; Cui et al., 2014).
Based on recent reports (Wang et al., 2013; Ahmad et al., 2014), TLR4 may both upregulate and downregulate specific DNA repair proteins, in various ways in a cell specific manner. Intracellular TLRs, such as TLR7, TLR8, TLR9 stimulated by imidazoquinolines, ssRNA, anti-phospholipid antibodies, bacteria, viral CpG-DNA, and IgG-chromatin complexes (Kutikhin, 2011), signal via the protein encoded by myeloid differentiation primary response gene 88 (MyD88) and also modulate DNA repair in a specific manner. Regarding the NLRs, Licandro et al. (2013) reported that the ectodomain of NLRP3 recognizes certain DAMPs, leading to inflammasome formation and to the development of aseptic inflammation. Taken together, the above presented data imply that PRRs, and especially TLR4, TLR7, TLR8, TLR9, and NLRP3, may be important regulators of DNA repair machinery.
On the other hand, it is worth mentioning that DDR in turn, controls human TLR gene expression. Menendez et al. (2011) studied p53 responsiveness in primary human lymphocytes from healthy volunteers and found that most of the TLR genes respond to p53 via canonical as well as non-canonical promoter binding sites. They also observed considerable inter-individual variability suggesting that DNA and p53 metabolic stresses can diversely modulate the innate immune system as well as downstream cytokines.
Toll-like receptor (TLR) agonists are being developed for the treatment of cancer, allergic diseases, viral infections, but also as adjuvants for vaccines against infectious diseases and cancer (Romagne, 2007) with considerable success. For example, BCG (Bacillus Calmette-Guerin) and Imiquimod, used as treatment for bladder cancer and basal cell carcinoma, respectively, contain several TLR agonists that contribute to their antineoplastic efficacy (Uehori et al., 2003; Geisse et al., 2004; Dunne et al., 2011; Vacchelli et al., 2012).
Monophosphoryl lipid A (MPL), a TLR4 agonist purified from Salmonella Minnesota LPS is used as an adjuvant, to enhance adaptive immune responses, in human licensed vaccines against papillomavirus (HPV) and hepatitis B virus (HBV) infections (Maisonneuve et al., 2014). Moreover, promising ongoing research in this field investigates the potential of other TLR agonists, either alone or in combination, as adjuvants in vaccines against bacterial, viral and neoplastic diseases (Cooper et al., 2008; Maisonneuve et al., 2014).
Agonists to TLR7/8/9, have been successfully tested in adults as novel therapeutics for allergies, asthma and allergic rhinitis, because they induce a strong Th1 response (Hennessy et al., 2010; Aryan et al., 2014). A single-stranded DNA-based synthetic oligodeoxynucleotide that activates TLR-9 in intestinal immunocytes, and induces the production of anti-inflammatory cytokines has been administered topically during lower GI endoscopy in patients with ulcerative colitis, refractory to standard therapy, with promising results (Atreya et al., 2016).
On the other hand, inappropriate TLR stimulation is observed in chronic idiopathic inflammatory and autoimmune diseases. Thus, TLRs antagonists aiming to attenuate the exaggerated inflammatory response have been tested for potential clinical benefit in acute and chronic infections, including sepsis, with variable success (Rossignol and Lynn, 2005; Opal et al., 2013; Savva and Roger, 2013).
TLR antagonists may also prove to be of benefit in treatment of autoimmune diseases, especially Systemic Lupus Erythematosus (SLE), although clinical data are not yet available (Kanzler et al., 2007; Wu et al., 2015). It is worth mentioning that hydroxychloroquine, an anti-malarial agent with acknowledged anti-inflammatory properties used for years as treatment of SLE and rheumatoid arthritis (RA), has been recently found that is a potent TLR inhibitor. TLR blockage has also been studied in acute respiratory distress syndrome (ARDS), acute lung injury, RA, asthma, myocardial ischemia reperfusion injury, inflammatory bowel diseases, and pain management (Dunne et al., 2011; Connolly and O’Neill, 2012).
In contrast to TLRs, the effect of NLR agonists or antagonists has not yet been tested in humans. Nevertheless, data from basic research show that manipulation of the NLR associated molecular pathways holds promise as future therapeutic target for the treatment of inflammation and cancer.
Nonetheless, the pleiotropic actions, redundancy, complex interactions, and the possibility of functional mutations of the involved molecules should always be kept in mind when interpreting the outcome of any therapeutic attempt. Intuitively, augmenting a weak or attenuating an excessive inflammatory reaction, by targeted therapeutic interventions may fine-tune host’s response and control disease progression. As briefly outlined above, TLRs and NLRs are key molecules involved in the inflammatory process and suitable candidates for therapeutic manipulation. Available data thus far point out that their therapeutic potential has been only partially exploited.
Nonetheless, it must always be kept in mind the pleiotropic actions, redundancy, complex interactions and the possibility of functional mutations of the involved molecules, in order to interpret the outcome of any therapeutic attempt. Future research should shed more light on the complex evolving operation of the PRRs and the associated molecular pathways in various disease states, in order to timely select the appropriate targets for therapeutic intervention.
ES, PK, ISP, AP, AB, AGT, AK, SS designed the manuscript. ES, PK, ISP, AP, AB collected data, reviewed literature and generated tables and figures. AGT, AK and SS wrote and supervised the manuscript.
This work was supported by NKUA SARG grants 12128, 8916.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abuli, A., Lozano, J. J., Rodriguez-Soler, M., Jover, R., Bessa, X., Muñoz, J., et al. (2013). Genetic susceptibility variants associated with colorectal cancer prognosis. Carcinogenesis 34, 2286–2291. doi: 10.1093/carcin/bgt179
Achyut, B. R., Ghoshal, U. C., Moorchung, N., and Mittal, B. (2007). Association of Toll-like receptor-4 (Asp299Gly and Thr399Ileu) gene polymorphisms with gastritis and precancerous lesions. Hum. Immunol. 68, 901–907. doi: 10.1016/j.humimm.2007.10.006
Ahmad, I., Simanyi, E., Guroji, P., Tamimi, I. A., delaRosa, H. J., Nagar, A., et al. (2014). Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. J. Invest. Dermatol. 134, 1710–1717. doi: 10.1038/jid.2013.530
Akira, S., Uematsu, S., and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. doi: 10.1016/j.cell.2006.02.015
Angeletti, S., Galluzzo, S., Santini, D., Ruzzo, A., Vincenzi, B., Ferraro, E., et al. (2009). NOD2/CARD15 polymorphisms impair innate immunity and increase susceptibility to gastric cancer in an Italian population. Hum. Immunol. 70, 729–732. doi: 10.1016/j.humimm.2009.04.026
Aryan, Z., Holgate, S. T., Radzioch, D., and Rezaei, N. (2014). A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. Int. Arch. Allergy Immunol. 164, 46–63. doi: 10.1159/000362553
Atreya, R., Bloom, S., Scaldaferri, F., Gerardi, V., Admyre, C., Karlsson, A., et al. (2016). Clinical effects of a topically applied toll-like receptor 9 agonist in active moderate-to-severe ulcerative colitis. J. Crohn’s Colitis 10, 1294–1302. doi: 10.1093/ecco-jcc/jjw103
Bartkova, J., Rezaei, N., Liontos, M., Karakaidos, P., Kletsas, D., Issaeva, N., et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637. doi: 10.1038/nature05268
Bhuvanendran, S., Hussin, H. M., Meran, L. P., Anthony, A. A., Zhang, L., Burch, L. H., et al. (2011). Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and typhoid susceptibility in asian malay population in Malaysia. Microbes Infect. 13, 844–851. doi: 10.1016/j.micinf.2011.04.007
Bochud, P. Y., Sinsimer, D., Aderem, A., Siddiqui, M. R., Saunderson, P., Britton, S., et al. (2009). Polymorphisms in Toll-like receptor 4 (TLR4) are associated with protection against leprosy. Eur. J. Clin. Microbiol. Infect. Dis. 28, 1055–1065. doi: 10.1007/s10096-009-0746-0
Bowie, A., and O’Neill, L. A. (2000). The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 67, 508–514.
Castano-Rodriguez, N., Kaakoush, N. O., Goh, K. L., Fock, K. M., and Mitchell, H. M. (2013). The role of TLR2, TLR4 and CD14 genetic polymorphisms in gastric carcinogenesis: a case-control study and meta-analysis. PLoS ONE 8:e60327. doi: 10.1371/journal.pone.0060327
Castaño-Rodríguez, N., Kaakoush, N. O., Goh, K. L., Fock, K. M., and Mitchell, H. M. (2014a). The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: a case-control study and gene expression analyses. PLoS ONE 9:e98899. doi: 10.1371/journal.pone.0117870
Castaño-Rodríguez, N., Kaakoush, N. O., and Mitchell, H. M. (2014b). Pattern-recognition receptors and gastric cancer. Front. Immunol. 22, 336. doi: 10.3389/fimmu.2014.00336
Chen, G. Y., Redondo, G., and Nunez, G. (2008). The innate immune receptor NOD1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 68, 10060–10067. doi: 10.1158/0008-5472.CAN-08-2061
Companioni, O., Bonet, C., Munoz, X., Weiderpass, E., Panico, S., Tumino, R., et al. (2014). Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European Prospective Investigation into Cancer-Eurgast cohort. Int. J. Cancer 134, 92–1011. doi: 10.1002/ijc.28357
Connolly, D. J., and O’Neill, L. A. (2012). New developments in toll-like receptor targeted therapeutics. Curr. Opin. Pharmacol. 12, 510–518. doi: 10.1016/j.coph.2012.06.002
Cooper, C. L., Angel, J. B., Seguin, I., Davis, H. L., and Cameron, D. W. (2008). CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin. Infect. Dis. 46, 1310–1314. doi: 10.1086/533467
Cui, J., Chen, Y., Wang, H. Y., and Wang, R. F. (2014). Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin. Immunother. 10, 3270–3285. doi: 10.4161/21645515.2014.979640
de Oliviera, J. G., Rossi, A. F., Nizato, D. M., Miyasaki, K., and Silva, A. E. (2013). Profiles of gene polymorphisms in cytokines and Toll-like receptors with higher for gastric cancer. Dig. Dis. Sci. 58, 978–988. doi: 10.1007/s10620-012-2460-5
Dunne, A., Marshall, N. A., and Mills, K. H. (2011). TLR based therapeutics. Curr. Opin. Pharmacol. 11, 404–411. doi: 10.1016/j.coph.2011.03.004
El-Omar, E. M., Ng, M. T., and Hold, G. L. (2008). Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene 27, 244–252. doi: 10.1038/sj.onc.1210912
Evangelou, K., Bartkova, J., Kotsinas, A., Pateras, I. S., Liontos, M., Velimezi, G., et al. (2013). The DNA damage checkpoint precedes activation of ARF in response to escalating oncogenic stress during tumorigenesis. Cell Death. Differ. 20, 1485–1497. doi: 10.1038/cdd.2013.76
Evangelou, K., Havaki, S., and Kotsinas, A. (2014). E2F transcription factors and digestive system malignancies: how much do we know? World J. Gastroenterol. 20, 10212–10216. doi: 10.3748/wjg.v20.i29.10212
Ferwerda, B., McCall, M. B., Alonso, S., Giamarellos-Bourboulis, E. J., Mouktaroudi, M., Izagirre, N., et al. (2007). TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. U.S.A. 104, 16645–16650. doi: 10.1073/pnas.0704828104
Fukata, M., and Abreu, M. T. (2008). Role of Toll-like receptors in gastrointestinal malignancies. Oncogene 27, 234–243. doi: 10.1038/sj.onc.1210908
Fukata, M., Vamadevan, A. S., and Abreu, M. T. (2009). Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin. Immunol. 21, 242–253. doi: 10.1016/j.smim.2009.06.005
Funabiki, M., Kato, H., Miyachi, Y., Toki, H., Motegi, H., Inoue, M., et al. (2014). Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40, 199–212. doi: 10.1016/j.immuni.2013.12.014
Gazouli, M., Mantzaris, G., Kotsinas, A., Zacharatos, P., Papalambros, E., Archimandritis, A., et al. (2005). Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J. Gastroenterol. 11, 681–685. doi: 10.3748/wjg.v11.i5.681
Geisse, J., Caro, I., Lindholm, J., Golitz, L., Stampone, P., and Owens, M. (2004). Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 50, 722–733. doi: 10.1016/j.jaad.2003.11.066
Gorgoulis, V. G., and Halazonetis, T. D. (2010). Oncogene-induced senescence: the bright and dark side of the response. Curr. Opin. Cell Biol. 22, 816–827. doi: 10.1016/j.ceb.2010.07.013
Gorgoulis, V. G., Vassiliou, L. V., Karakaidos, P., Zacharatos, P., Kotsinas, A., Liloglou, T., et al. (2005). Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913. doi: 10.1038/nature03485
Goto, Y., Arigami, T., Kitago, M., Nguyen, S. L., Narita, N., Ferrone, S., et al. (2008). Activation of toll-like receptors 2,3, and 4 on human melanoma cells induces inflammatory factors. Mol. Cancer Ther. 7, 3642–3653. doi: 10.1158/1535-7163.MCT-08-0582
Gunther, C., Kind, B., Reijns, M. A., Berndt, N., Martinez-Bueno, M., Wolf, C., et al. (2015). Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J. Clin. Invest. 125, 413–424. doi: 10.1172/JCI78001
Halazonetis, T. D., Gorgoulis, V. G., and Bartek, J. (2008). An oncogene-induced DNA damage model for cancer development. Science (New York, NY) 319, 1352–1355. doi: 10.1126/science.1140735
Harberts, E., and Gaspari, A. A. (2013). TLR signaling and DNA repair: are they associated? J. Investig. Dermatol. 133, 296–302. doi: 10.1038/jid.2012.288
Hennessy, E. J., Parker, A. E., and O’Neill, L. A. (2010). Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307. doi: 10.1038/nrd3203
Hnatyszyn, A., Szalata, M., Stanczyk, J., Cichy, W., and Slomski, R. (2010). Association of c.802C> polymorphism of NOD2/CARD15 gene with the chronic gastritis and predisposition to cancer in H. pylori infected patients. Exp. Mol. Pathol. 88, 388–393. doi: 10.1016/j.yexmp.2010.03.003
Hofner, P., Gyulai, Z., Kiss, Z. F., Tiszai, A., Tiszlavicz, L., Toth, G., et al. (2007). Genetic polymorphisms of NOD1 and IL-8, but not polymorphisms of TLR4 genes, are associated with Helicobacter pylori-induced duodenal ulcer and gastritis. Helicobacter 12, 124–131. doi: 10.1111/j.1523-5378.2007.00481.x
Hold, G. L., Rabkin, C. S., Chow, W. H., Smith, M. G., Gammon, M. D., Risch, H. A., et al. (2007). Afunctional polymorphism of toll-like receptor 4 gene increases risk of gastriccarcinoma and its precursors. Gastroenterology 132, 905–912. doi: 10.1053/j.gastro.2006.12.026
Huang, L., Yuan, K., Liu, J., Ren, X., Dong, X., and Tian, W. (2014). Polymorphisms of TLR4 gene and risk of gastric cancer. Gene 537, 46–50. doi: 10.1016/j.gene.2013.12.030
Hugot, J. P., Chamaillard, M., Zouali, H., Lesage, S., Cezard, J. P., Belaiche, J., et al. (2001). Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603. doi: 10.1038/35079107
Hysi, P., Kabesch, M., Moffatt, M. F., Schedel, M., Carr, D., Zhang, Y., et al. (2005). NOD1 variation, immunoglobulin E and asthma. Hum. Mol. Genet. 14, 935–941. doi: 10.1093/hmg/ddi087
Inohara, N., Chamaillard, M., McDonald, C., and Nunez, G. (2005). NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383. doi: 10.1146/annurev.biochem.74.082803.133347
Jackson, S. P., and Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature 461, 1071–1078. doi: 10.1038/nature08467
Jang, T. J. (2010). The number of Foxp3-positive regulatory T cells is increased in Helicobacter pylori gastritis and gastric cancer. Pathol. Res. Pract. 206, 34–38. doi: 10.1016/j.prp.2009.07.019
Janssens, S., and Beyaert, R. (2003). Role of toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16, 637–646. doi: 10.1128/CMR.16.4.637-646.2003
Kanzler, H., Barrat, F. J., Hessel, E. M., and Coffman, R. L. (2007). Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 13, 552–559. doi: 10.1038/nm1589
Kawai, T., and Akira, S. (2010). Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. doi: 10.1016/j.immuni.2011.05.006
Kawai, T., and Akira, S. (2011). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384. doi: 10.1038/ni.1863
Keestra, A. M., Winter, M. G., Auberger, J. J., Frassle, S. P., Xavier, M. V., and Winter, S. E. (2013). Manipulation of small Rho GTPases in pathogen-induced process detected by NOD1. Nature 496, 233–237. doi: 10.1038/nature12025
Kim, J., Cho, Y. A., Choi, I. J., Lee, Y. S., Kim, S. Y., and Hwang, J. A. (2013). Effects of polymorphisms of innate immunity genes and environmental factors on the risk of noncardia gastric cancer. Cancer Res. Treat. 45, 313–324. doi: 10.4143/crt.2013.45.4.313
Kim, S., Becker, J., Bechheim, M., Kaiser, V., Noursadeghi, M., Fricker, N., et al. (2014). Characterizing the genetic basis of innate immune response in TLR4-activated human monocytes. Nature Commun. 5:5236. doi: 10.1038/ncomms6236
Kim, Y. K., Shin, J. S., and Nahm, M. H. (2015). NOD-Like receptors in infection, immunity, and diseases. Yonsei Med. J. 57, 5–14. doi: 10.3349/ymj.2016.57.1.5
Koberlin, M. S., Heinz, L. X., and Superti-Furga, G. (2016). Functional crosstalk between membrane lipids and TLR biology. Curr. Opin. Cell Biol. 39, 28–36. doi: 10.1016/j.ceb.2016.01.010
Kupcinskas, J., Wex, T., Bornschein, J., Selgrad, M., Leja, M., Juozaityte, E., et al. (2011). Lack ofassociation between gene polymorphisms of Angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med. Genet. 12:112. doi: 10.1186/1471-2350-12-112
Kurzawski, G., Suchy, J., Kładny, J., Grabowska, E., Mierzejewski, M., Jakubowska, A., et al. (2004). The NOD2 3020insC mutation and the risk of colorectal cancer. Cancer Res. 64, 1604–1606. doi: 10.1158/0008-5472.CAN-03-3791
Kutikhin, A. G. (2011). Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum. Immunol. 72, 1095–1116. doi: 10.1016/j.humimm.2011.07.307
Kutikhin, A. G., Yuzhalin, A. E., Tsitko, E. A., and Brusina, E. B. (2014). Pattern recognition receptors and DNA repair: starting to put a jigsaw puzzle together. Front. Immunol. 5:343. doi: 10.3389/fimmu.2014.00343
Lener, M. R., Oszutowska, D., Castaneda, J., Kurzawski, G., Suchy, J., Nej-Wolosiak, K., et al. (2006). Prevalence of the NOD2 3020insC mutation in aggregations of breast and lung cancer. Breast Cancer Res. Treat. 95, 141–145. doi: 10.1007/s10549-005-9057-z
Licandro, G., Ling Khor, H., Beretta, O., Lai, J., Derks, H., and Laudisi, F. (2013). The NLRP3 inflammasome affects DNA damage responses after oxidative and genotoxic stress in dendritic cells. Eur. J. Immunol. 43, 2126–2137. doi: 10.1002/eji.201242918
Liu, J., He, C., Xu, Q., Xing, C., and Yuan, Y. (2014). NOD2 polymorphisms associated with cancer risk: a meta-analysis. PLoS ONE 9:e89340. doi: 10.1371/journal.pone.0089340
Liu, J., Radler, D., Illi, S., Krucker, E., Turan, E., and Von Motius, E. (2011). TLR2 polymorphisms influence neonatal regulatory T cells depending on maternal atopy. Allergy 66, 1020–1025. doi: 10.1111/j.1398-9995.2011.02573.x
Lowe, E. L., Crother, T. R., Rabizadeh, S., Hu, B., Wang, H., Chen, S., et al. (2010). Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE 5:e13027. doi: 10.1371/journal.pone.0013027
Magalhaes, J. G., Lee, J., Geddes, K., Rubino, S., Philpott, D. J., and Girardin, S. E. (2011). Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur. J. Immunol. 41, 1445–1455. doi: 10.1002/eji.201040827
Maisonneuve, C., Bertholet, S., Philpott, D. J., and De Gregorio, E. (2014). Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 111, 12294–12299. doi: 10.1073/pnas.1400478111
Manolakis, A. C., Kapsoritakis, A. N., Tiaka, E. K., Satra, M., Gerovassili, A., Tsiompanidis, I., et al. (2010). Impact of TLR-4 polymorphisms on circulating levels of antibodies against Helicobacter pylori. Helicobacter 15, 481–482. doi: 10.1111/j.1523-5378.2010.00785.x
Mayerle, J., den Hoed, C. M., Schurmann, C., Stolk, L., Homuth, G., Peters, M. J., et al. (2013). Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 309, 1912–1920. doi: 10.1001/jama.2013.4350
McGovern, D. P., Hysi, P., Ahmad, T., van Heel, D. A., Moffatt, M. F., Carey, A., et al. (2005). Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum. Mol. Genet. 14, 1245–1250. doi: 10.1093/hmg/ddi135
Medzhitov, R., Preston-Hurlburt, P., and Janeway, C. A. Jr. (1997). A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397. doi: 10.1038/41131
Menendez, D., Shatz, M., Azzam, K., Garantziotis, S., Fessler, M. B., and Resnick, M. A. (2011). The toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 7:e1001360. doi: 10.1371/journal.pgen.1001360
Mitchell, J. A., Paul-Clark, M. J., Clarke, G. W., McMaster, S. K., and Cartwright, N. (2007). Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J. Endocrinol. 193, 323–330. doi: 10.1677/JOE-07-0067
Moura, S. B., Almeida, L. R., Guerra, J. B., Rocha, G. A., Camargos Rocha, A. M., Melo, F. F., et al. (2008). Toll-like receptor (TLR2, TLR4 and TLR5) gene polymorphisms and Helicobacter pylori infection in children with and without duodenal ulcer. Microbes Infect. 10, 1477–1483. doi: 10.1016/j.micinf.2008.08.009
Negrini, S., Gorgoulis, V. G., and Halazonetis, T. D. (2010). Genomic instability–an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228. doi: 10.1038/nrm2858
Newton, K., and Dixit, V. M. (2012). Signaling in innate immunity and inflammation. Cold. Spring. Harb. Perspect. Biol. 4:a006049. doi: 10.1101/cshperspect.a006049
Nihon-Yanagi, Y., Terai, K., Murano, T., Matsumoto, T., and Okazumi, S. (2012). Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol. Immunother. 61, 71–77. doi: 10.1007/s00262-011-1085-4
Ogura, Y., Bonen, D. K., Inohara, N., Nicolae, D. L., Chen, F. F., Ramos, R., et al. (2001). A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606. doi: 10.1038/35079114
Ogura, Y., Lala, S., Xin, W., Smith, E., Dowds, T. A., Chen, F. F., et al. (2003). Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52, 1591–1597. doi: 10.1136/gut.52.11.1591
O’Neill, L. A. (2006). How Toll-like receptors signal: what we know and what we don’t know. Curr. Opin. Immunol 18, 3–9. doi: 10.1016/j.coi.2005.11.012
Opal, S. M., Laterre, P. F., Francois, B., LaRosa, S. P., Angus, D. C., Mira, J. P., et al. (2013). Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis the ACCESS randomized trial. JAMA 309, 1154–1162. doi: 10.1001/jama.2013.2194
Orr, N., and Chanock, S. (2008). Common genetic variation and human disease. Adv. Genet. 62, 1–32. doi: 10.1016/S0065-2660(08)00601-9
Pateras, I. S., Havaki, S., Nikitopoulou, X., Vougas, K., Townsend, P. A., Panayiotidis, M. I., et al. (2015). The DNA damage response and immune signaling alliance: is it good or bad? Nature decides when and where. Pharmacol. Therapeut. 154, 36–56. doi: 10.1016/j.pharmthera.2015.06.011
Philpott, D. J., Sorbara, M. T., Robertson, S. J., Croitoru, K., and Girardin, S. E. (2014). NOD proteins: regulators of inflammation in health and disease. Nat. Rev. 14, 9–23. doi: 10.1038/nri3565
Pimentel-Nunes, P., Teixeira, A. L., Pereira, C., Gomes, M., Brandao, C., Rodrigues, C., et al. (2013). Functional polymorphisms of Toll-like receptors 2 and 4 alter the risk for colorectal carcinoma in Europeans. Dig. Liver Dis. 45, 63–69. doi: 10.1016/j.dld.2012.08.006
Qadri, Q., Rasool, R., Afroze, D., Naqash, S., Gulzar, G. M., Yousuf, A., et al. (2013). Study ofTLR4 and IL-8 Gene Polymorphisms in H. pylori-Induced Inflammation in gastric cancer in an ethnic kashmiri population. Immunol. Invest. 43, 324–336. doi: 10.3109/08820139.2013.854378
Rodier, F., Campisi, J., and Bhaumik, D. (2007). Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 35, 7475–7484. doi: 10.1093/nar/gkm744
Romagne, F. (2007). Current and future drugs targeting one class of innate immunity receptors: the Toll-like receptors. Drug Discov. Today 12, 80–87. doi: 10.1016/j.drudis.2006.11.007
Rossignol, D. P., and Lynn, M. (2005). TLR4 antagonists for endotoxemia and beyond. Curr. Opin. Investig. Drugs 6, 496–502.
Santini, D., Angeletti, S., Ruzzo, A., Dicuonzo, G., Galluzzo, S., and Vincenzi, B. (2008). Toll-like receptor-4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypes. Clin. Exp. Immunol. 154, 360–364. doi: 10.1111/j.1365-2249.2008.03776.x
Sato, K., Yoshimura, A., Kaneko, T., Ukai, T., Ozaki, Y., Nakamura, H., et al. (2012). A single nucleotide polymorphism in 3’-untranslated region contributes to the regulation of Toll-like receptor 4 translation. J. Biol. Chem. 287, 163–172. doi: 10.1074/jbc.M111.338426
Savva, A., and Roger, T. (2013). Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 4:387. doi: 10.3389/fimmu.2013.00387
Saxena, M., and Yeretssian, G. (2014). NOD-Like receptors: master regulators of inflammation and cancer. Front. Immunol. 5:327. doi: 10.3389/fimmu.2014.00327
Semlali, A., Reddy Parine, N., Arafah, M., Mansour, L., Azzi, A., Al Shahrani, O., et al. (2016). Expression and polymorphism of toll-like receptor 4 and effect on nf-kappab mediated inflammation in colon cancer patients. PLoS ONE 11:e0146333. doi: 10.1371/journal.pone.0146333
Sheng, W. Y., Yong, Z., Yun, Z., Hong, H., and Hai, L. L. (2015). Toll-like receptor 4 gene polymorphisms and susceptibility to colorectal cancer: a meta-analysis and review. Arch. Med. Sci. 11, 699–707. doi: 10.5114/aoms.2015.53288
Solier, S., and Pommier, Y. (2014). The nuclear gamma-H2AX apoptotic ring: implications for cancers and autoimmune diseases. Cell. Mol. Life. Sci. 71, 2289–2297. doi: 10.1007/s00018-013-1555-2
Souliotis, V. L., Vougas, K., Gorgoulis, V. G., and Sfikakis, P. P. (2016). Defective DNA repair and chromatin organization in patients with quiescent systematic lupus erythematosus. Arthr. Res. Ther. 18, 182. doi: 10.1186/s13075-016-1081-3
Tang, D., Kang, R., Coyne, C. B., Zeh, H. J., and Lotze, M. T. (2012). PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 249, 158–175. doi: 10.1111/j.1600-065X.2012.01146.x
Ting, J. P., Kastner, D. L., and Hoffman, H. M. (2006). CATERPILLERs, pyrin and hereditary immunological disorders. Nat. Rev. 6, 183–195. doi: 10.1038/nri1788
Tseng, F. C., Brown, E. E., Maiese, E. M., Yeager, M., Welch, R., Gold, B. D., et al. (2006). Polymorphisms in cytokine genes and risk of Helicobacter pylori infection among Jamaican children. Helicobacter 11, 425–430. doi: 10.1111/j.1523-5378.2006.00433.x
Uehori, J., Matsumoto, M., Tsuji, S., Akazawa, T., Takeuchi, O., Akira, S., et al. (2003). Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus calmette-guerin peptidoglycan. Infect. Immun. 71, 4238–4249. doi: 10.1128/IAI.71.8.4238-4249.2003
Vacchelli, E., Galluzzi, L., Eggermont, A., Fridman, W. H., Galon, J., Sautes-Fridman, C., et al. (2012). Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 1, 894–907. doi: 10.4161/onci.20931
Velimezi, G., Liontos, M., Vougas, K., Roumeliotis, T., Bartkova, J., Sideridou, M., et al. (2013). Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat. Cell Biol. 15, 967–977. doi: 10.1038/ncb2795
Viala, J., Chaput, C., Boneca, I. G., Cardona, A., Girardin, S. E., Moran, A. P., et al. (2004). Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5, 1166–1174. doi: 10.1038/ni1131
Wang, E. L., Qian, Z. R., Nakasono, M., Tanahashi, T., Yoshimoto, K., Bando, Y., et al. (2010). High expression of Toll-like receptor 4/myelioddifferentation factor 88 signals correlates with poor prognosis in colorectal cancer. Cancer Immunol. Immunother. 102, 908–915. doi: 10.1038/sj.bjc.6605558
Wang, H., Rayburn, E. R., Wang, W., Kandimalla, E. R., Agrawal, S., and Zhang, R. (2006). Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol. Cancer Ther. 5, 1585–1592. doi: 10.1158/1535-7163.MCT-06-0094
Wang, P., Zhang, L., Jiang, J. M., Ma, D., Tao, H. X., Yuan, S. L., et al. (2012). Association of NOD1 and NOD2 genes polymorphisms with Helicobacter pylori related gastric cancer in a Chinese population. World J. Gastroenterol. 18, 2112–2120. doi: 10.3748/wjg.v18.i17.2112
Wang, Z., Yan, J., Lin, H., Hua, F., Wang, X., Liu, H., et al. (2013). Toll-like receptor 4 activity protects against hepatocellular tumorigenesis and progression by regulating expression of DNA repair protein Ku70 in mice. Hepatology 57, 1869–1881. doi: 10.1002/hep.26234
Wu, Y. W., Tang, W., and Zuo, J. P. (2015). Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol. Sin. 36, 1395–1407. doi: 10.1038/aps.2015.91
Yang, H., Zhou, H., Feng, P., Zhou, X., Wen, H., Xie, X., et al. (2010). Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J. Exp. Clin. Cancer Res. 29:92. doi: 10.1186/1756-9966-29-92
Keywords: toll-like receptors (TLRs), nod-like receptors (NLRs), DNA damage response (DDR), single nucleotide polymorphism (SNP), mutation, inflammation and tumorigenesis
Citation: Spanou E, Kalisperati P, Pateras IS, Papalampros A, Barbouti A, Tzioufas AG, Kotsinas A and Sougioultzis S (2017) Genetic Variability as a Regulator of TLR4 and NOD Signaling in Response to Bacterial Driven DNA Damage Response (DDR) and Inflammation: Focus on the Gastrointestinal (GI) Tract. Front. Genet. 8:65. doi: 10.3389/fgene.2017.00065
Received: 25 November 2016; Accepted: 09 May 2017;
Published: 29 May 2017.
Edited by:
Linda Pattini, Politecnico di Milano, ItalyReviewed by:
David McMillen, University of Toronto Mississauga, CanadaCopyright © 2017 Spanou, Kalisperati, Pateras, Papalampros, Barbouti, Tzioufas, Kotsinas and Sougioultzis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athanassios Kotsinas, YWtvdHNpbkBtZWQudW9hLmdy
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.