- Department of Ophthalmology and Vision Science, University of California, Davis, Sacramento, CA, United States
Among genome engineering tools, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-based approaches have been widely adopted for translational studies due to their robustness, precision, and ease of use. When delivered to diseased tissues with a viral vector such as adeno-associated virus, direct genome editing can be efficiently achieved in vivo to treat different ophthalmic conditions. While CRISPR has been actively explored as a strategy for treating inherited retinal diseases, with the first human trial recently initiated, its applications for complex, multifactorial conditions such as ocular angiogenesis has been relatively limited. Currently, neovascular retinal diseases such as retinopathy of prematurity, proliferative diabetic retinopathy, and neovascular age-related macular degeneration, which together constitute the majority of blindness in developed countries, are managed with frequent and costly injections of anti-vascular endothelial growth factor (anti-VEGF) agents that are short-lived and burdensome for patients. By contrast, CRISPR technology has the potential to suppress angiogenesis permanently, with the added benefit of targeting intracellular signals or regulatory elements, cell-specific delivery, and multiplexing to disrupt different pro-angiogenic factors simultaneously. However, the prospect of permanently suppressing physiologic pathways, the unpredictability of gene editing efficacy, and concerns for off-target effects have limited enthusiasm for these approaches. Here, we review the evolution of gene therapy and advances in adapting CRISPR platforms to suppress retinal angiogenesis. We discuss different Cas9 orthologs, delivery strategies, and different genomic targets including VEGF, VEGF receptor, and HIF-1α, as well as the advantages and disadvantages of genome editing vs. conventional gene therapies for multifactorial disease processes as compared to inherited monogenic retinal disorders. Lastly, we describe barriers that must be overcome to enable effective adoption of CRISPR-based strategies for the management of ocular angiogenesis.
Introduction
Ocular angiogenesis, which is characterized by the formation of new blood vessels from pre-existing vasculature in the eye, underlies the leading causes of blindness across different age groups, including retinopathy of prematurity (ROP), proliferative diabetic retinopathy (PDR), and neovascular age-related macular degeneration (nAMD) (Dreyfuss et al., 2015). Current management of these conditions involve intraocular pharmacotherapies that target vascular endothelial growth factor (VEGF), but are constrained by the variable efficacy and limited durability of these agents. Gene therapies may provide longer term suppression of ocular angiogenesis by hijacking cells in the eye to serve as “biofactories” to produce VEGF antagonists, but their effectiveness remain unclear. Here we discuss the potential for genome editing using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology for management of ocular angiogenic conditions.
Ocular Angiogenic Diseases and Therapies
Retinal and Choroidal Neovascularization
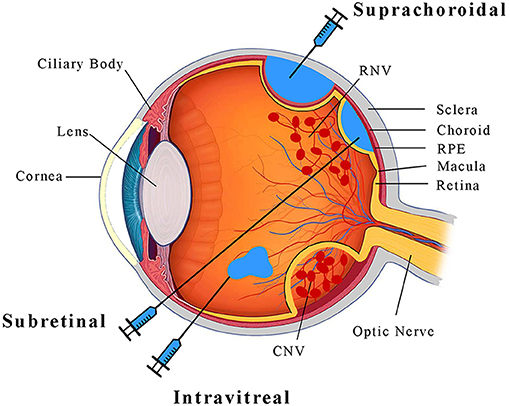
Pathologic ocular angiogenesis occurs in various parts of the eye, but the two vascular supplies most commonly affected are the retinal and choroidal vasculatures (Figure 1). Retinal vessels arise from the central retinal artery, and supply the innermost layers of the neurosensory retina, such as retinal ganglion cells. Due to their small caliber, retinal vessels are highly susceptible to microvascular diseases such as diabetes mellitus. Diabetic retinopathy is the leading cause of blindness in working-age adults in the United States (Bermea et al., 2018), particularly due to the development of neovascularization in PDR and/or exudation in diabetic macular edema (DME) (Ellis et al., 2019). Another important cause of pathologic retinal neovascularization (RNV) is ROP (Hellström et al., 2013), where early postnatal exposure of premature infants to oxygen delays maturation of the retinal vasculature and leads to retinal ischemia. In both PDR and ROP, the development of RNV leads to retinal and vitreous hemorrhage, fibrovascular proliferation and scarring, and eventually, retinal detachment.
Unlike retinal vessels, the choroidal vasculature, also known simply as the choroid (Figure 1), supplies the outer retinal layers closer to the eye wall. The choroid consists of a spongy meshwork of different caliber vessels that is separated from the retina by the retinal pigment epithelium (RPE) and Bruch's membrane, which together regulate the exchange of nutrients and waste between outer retinal photoreceptors and choroidal vessels, and also serve as part of the blood-retinal barrier. The choroid has the highest blood flow of any organ in the body (Nickla and Wallman, 2010), and creates an oxygen-rich environment which combined with the redox-sensitive lipids of photoreceptors leads to the accumulation of reactive oxygen species with age. In patients with nAMD, this chronic oxidative damage and accumulation of lipid-rich deposits called drusen (Yiu et al., 2020b) can trigger breakdown of the RPE-Bruch's membrane complex leading to choroidal neovascularization (CNV), which can cause subretinal hemorrhage and fibrosis that lead to photoreceptor demise. In pathologic myopia, axial elongation of the globe can similarly lead to breakdown of the RPE-Bruch's membrane barrier to result in CNV and vision loss.
Molecular Pathways of Ocular Angiogenesis
Pathologic ocular angiogenesis is regulated by multiple angiogenic factors including the VEGF family, platelet-derived growth factors (PDGFs), fibroblast growth factors (FGFs), insulin-like growth factors (IGFs), transforming growth factor- β (TGFβ) superfamily, endothelins, galectins, and integrins (Cabral et al., 2017). Among these, VEGF is considered as the most potent pro-angiogenic factor, as multiple pivotal trials have shown the effectiveness of anti-VEGF therapies in nAMD (Rosenfeld et al., 2006; Brown et al., 2009), DME (Brown et al., 2013), retinal vein occlusions (Brown et al., 2010; Campochiaro et al., 2010; Yiu et al., 2020c), and ROP (Stahl et al., 2019). VEGF is primarily an endothelial cell mitogen. Among its 5 family members (VEGFa-e), VEGFa is the dominant form and considered to be most pathologic, but inhibition of VEGFa alone may trigger compensatory mechanisms from other VEGF isoforms and/or proangiogenic factors (Singh et al., 2015; Cabral et al., 2018). VEGF is secreted by multiple cell types, including endothelial cells, pericytes, RPE, Muller glia, macrophages, and astrocytes (Stone et al., 1996; Miller, 1997; Robbins et al., 1997; Ida et al., 2003). VEGF from Muller cells has been implicated as the major pathologic source in RNV (Wang et al., 2010; Jiang et al., 2014), whereas VEGF from RPEs plays a more significant role in CNV pathogenesis (Kurihara et al., 2012). Thus, although intravitreal agents that globally suppress VEGF are effective in treating a range of neovascular conditions in the eye, more targeted, cell-specific therapies may be more efficacious while minimizing adverse effects on physiologic angiogenesis.
Current Treatments for Ocular Angiogenesis
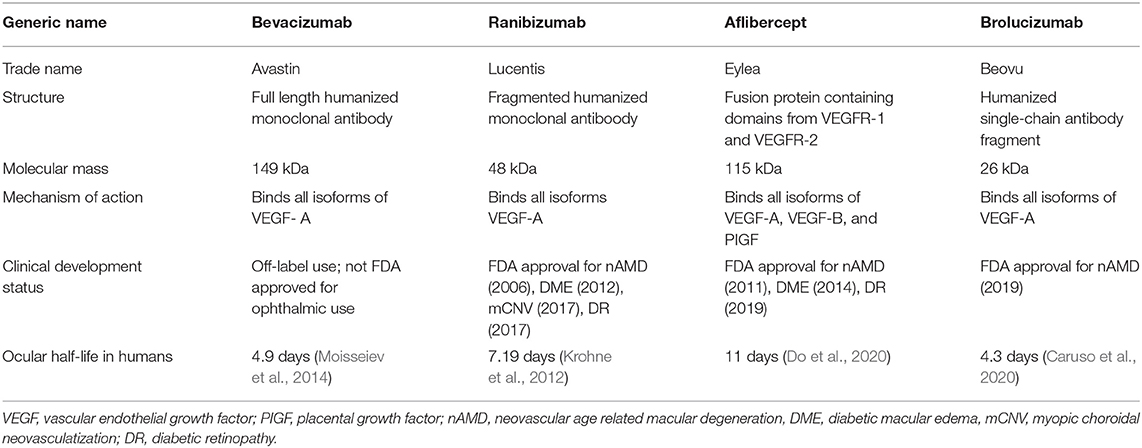
Current anti-VEGF pharmacotherapies include humanized full-length monoclonal antibodies (bevacizumab), antibody fragments (ranibizumab, brolucizumab), and recombinant decoy receptors (aflibercept) (Table 1) (Todorich et al., 2014). Intravitreal injections are usually given in outpatients settings, but with frequencies as often as every 4–12 weeks, these treatments are costly and burdensome for patients (Suzuki et al., 2014; Cabral et al., 2017). Early success with off-label use of intravitreal bevacizumab led to the development of ranibizumab, which was designed to better penetrate the neurosensory retina and reduce systemic exposure based on its smaller size (48 kDa) (Heier et al., 2006), and received FDA approval for nAMD in 2006 following its success in phase 3 clinical trials (Rosenfeld et al., 2006; Kaiser et al., 2007; Brown et al., 2009). Since then, it has expanded its applications to diabetic macular edema, retinal vein occlusion-related macular edema, myopic CNV, and diabetic retinopathy, although it has not demonstrated superiority over the lower cost bevacizumab in randomized, prospective studies (CATT Research Group et al., 2011). Later studies led to the approval of aflibercept, which has demonstrated non-inferiority to ranibizumab for most of these indications, with the exception of DME in which aflibercept showed better short-term visual outcomes in eyes with poor baseline vision (Heier et al., 2014; Korobelnik et al., 2014a,b; Clark et al., 2016). The most recently-approved brolucizumab is the smallest (28 kDa) in size, and may exhibit greater durability due to the higher achievable therapeutic molar dose, but may also trigger more intraocular inflammation that could limits its wide adoption (Baumal et al., 2020; Dugel et al., 2020). Other anti-angiogenic pharmacotherapies under investigation include pegylated anti-VEGF designed ankyrin repeat proteins (abicipar pegol), antibodies against Tie-2 receptor ligands (faricimab, nesvacumab, ARP-1536), and PDGF antagonists (ranucumab, X-82) (Shen et al., 2014; Frye et al., 2015; Callanan et al., 2018; Sahni et al., 2019; Cohen et al., 2020; Heier et al., 2020; Khanani et al., 2020).
Another mode of therapy for ocular angiogenesis is photodynamic therapy (PDT), which combines intravenous delivery of a porphyrin-based photosensitizer (verteporfin) with focal low-intensity light exposure to trigger singlet oxygen release within the CNV, causing vascular occlusion and ablation of the lesion. In clinical trials, PDT with verteporfin reduces vision loss and CNV in eyes with nAMD [Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group, 1999; Verteporfin in Photodynamic Therapy Study Group, 2001], but did not show benefit over ranibizumab monotherapy (Cartwright et al., 1990). Although largely supplanted by anti-VEGF therapy today, PDT remains an important treatment modality for chronic central serous chorioretinopathy (Fujita et al., 2015), choroidal hemangiomas (Tsipursky et al., 2011), and polypoidal choroidal vasculopathy (Koh et al., 2012).
Gene Therapies for Ocular Angiogenesis
Vectors for Ocular Gene Therapy
The pursuit of gene therapies for ocular angiogenesis was born out of a need for more sustained treatments to overcome the burden of repeated injections. Ocular gene therapy has gained renewed interest since the approval of the first retinal gene therapy using an AAV2 vector to express the RPE65 gene encoding a retinal isomerase for patients with type 2 Leber Congenital Amaurosis (Bainbridge et al., 2008, 2015; Maguire et al., 2008). Unlike adenoviruses, which has been largely abandoned due to its immunogenicity (Walther and Stein, 2000), AAVs are well-suited for human applications because they are non-pathogenic, replication-deficient, and exhibit low immunogenicity. Different serotypes of AAV combined with cell-specific promoters can target distinct retinal cell types. In murine and non-human primate retina, ganglion cells are mainly transduced with AAV2 and AAV8, while photoreceptors and RPE can be efficiently transduced with AAV2, AAV5, AAV7, AAV8, AAV9 (Auricchio et al., 2001; Hori et al., 2019). As the viral tropism can differ between species, however, pre-clinical animal studies may not directly translate directly to human trials. In human retina, Wiley et al. reported that AAV4 and 5 are most efficient at transducing photoreceptors, AAV4 for transducing ganglion cells and the inner nuclear layer, and AAV4 and AAV6 for RPE cells, although the authors acknowledged donor-to-donor and age-dependent difference in transduction efficiency (Wiley et al., 2018). AAVs have a limited packaging capacity (4.7 kb). Larger genes are more suitably transduced with lentiviral vectors (Yáñez-Muñoz et al., 2006), which has a larger carrying capacity (10 kb), but has a greater risk of insertional mutagenesis as it integrates into the host genome (Walther and Stein, 2000). Synthetic delivery platforms such as poly (lactic-co-glycolide) (PLGA) nanoparticles have good biocompatibility and low immunogenicity (Kapoor et al., 2015; Mir et al., 2017), but are typically less efficient at transducing retinal cells compared with viral vectors.
Modes of Vector Delivery
Most current retinal gene therapies employ a subretinal injection to deliver the viral vector (Figure 1). This method involves a vitrectomy surgery during which a thin cannula is inserted through the retina to create subretinal bleb in which viral particles can interface directly with photoreceptors and RPE. This method enables efficient gene transfer (Stieger et al., 2009) and exhibits minimal immunogenicity due to retinal immune privilege (Peng et al., 2017). However, the surgical procedure is invasive and the therapeutic effect is limited to the focal area of the bleb (Stout and Francis, 2011). Intravitreal injections can be performed in outpatient clinic settings, and the injected agent can diffuse across the entire globe (Figure 1), but efficacy is limited by the internal limiting membrane (ILM) which serves as a barrier on the inner surface of the retina (Stout and Francis, 2011). Newer generations of AAV derived by directed evolution, such as the AAV2-7m8 serotype (Dalkara et al., 2013), may be required to enable efficient transgene expression after intravitreal delivery. More recently, our research team and others have demonstrated the effective delivery of AAV into the suprachoroidal space—a potential space between the choroid and the scleral wall of the eye (Figure 1) (Ding et al., 2019; Yiu et al., 2020a). Although suprachoroidal drug delivery using microneedles has shown some promise in human studies (Willoughby et al., 2018; Yeh et al., 2020), its utility for viral gene therapy remains unclear.
Gene Therapy Strategies for Ocular Angiogenesis
Most current anti-angiogenesis gene therapy strategies employ a biofactory approach of transducing retinal cells with viral vectors to produce VEGF antagonists. Early studies using AAV expression of the soluble VEGF receptor sFlt-1 demonstrated long-term expression of sFlt-1 and reduction in CNV size without significant immune response in non-human primates (MacLachlan et al., 2011; Lai et al., 2012) and in phase 1 human studies (Heier et al., 2014; Rakoczy et al., 2015a), but showed no clear functional benefit in a phase IIa trial of 32 patients (Constable et al., 2016). Many of the enrolled patients in this study had previously been treated with multiple anti-VEGF treatments, so it was unclear if there was a ceiling effect where additional visual gains would have been limited. Also, among the 21 patients that received the treatment, 12 of them had pre-existing neutralizing antibodies against AAV2, although the authors found no clear correlation between these antibodies and therapeutic efficacy (Constable et al., 2016). It is worth noting that the impact of pre-existing neutralizing antibodies against the viral vector may depend on the delivery route. While the effectiveness of subretinal AAV2 did not appear correlated with pre-existing antibody titers in several studies (Bennett et al., 2012; Rakoczy et al., 2015b; Constable et al., 2016), intravitreal injections of AAV2 showed lower therapeutic efficacy when serum neutralizing antibodies were present (Heier et al., 2014), perhaps due to the greater immune privilege of the subretinal space. Nevertheless, intravitreal injections using the newer generation AAV2-7m8 vector (ADVM-022) has shown sustained expression of aflibercept for more than 12 months in non-human primates (Grishanin et al., 2019), and stabilized visual acuity and retinal anatomy in 10 of 12 human patients without rescue anti-VEGF treatments over 24 weeks in a phase I study (NCT03748784), although transient intraocular inflammation was noted (Boyer, 2020). More recently, interim analysis of the phase II study found that 9 of 12 patients did not require rescue injection for 54 weeks, and 6 of these 9 patients maintained their vision for 74 weeks without additional injections (http://investors.adverum.com/news-releases/news-release-details/adverum-biotechnologies-announces-positive-interim-data-cohorts). Another strategy employing subretinal AAV8 to express a monoclonal anti-VEGF antibody fragment (RGX-314) has been found to be comparable to anti-VEGF Fab expression (Liu et al., 2018), and interim assessment of the phase I/IIa trial in nAMD patients also showed sustained expression (NCT03066258).
Crispr-Based Approaches for Ocular Angiogenesis
Genome Editing Using CRISPR-Cas9 Endonucleases
Most current anti-angiogenic gene therapy strategies only mimic pharmacologic VEGF inhibition. Thus, they do not affect intracellular targets and do not distinguish pathologic from physiologic cellular sources. Rather than targeting angiogenic factors at the protein or RNA level, which require transgene expression for sustained activity, genome editing using CRISPR-based systems enables modifications at the DNA level, providing (1) permanent suppression of angiogenic signals, (2) potential disruption of both extracellular and intracellular targets, and (3) possible cell-specific delivery aimed at more pathologically relevant sources.
Derived from prokaryotic adaptive immune systems, CRISPR-associated Cas9 endonucleases can induce a site-specific cleavage in the target DNA with programmable guide RNAs (gRNAs), creating double-strand breaks (DSBs) that can be repaired by error-prone non-homologous end-joining (NHEJ) or homology directed repair (HDR) when paired with donor DNA template (Yiu, 2018; Rodríguez-Rodríguez et al., 2019). The fast-moving technology now includes a compendium of different Cas orthologs from various bacterial and archaeal species, including engineered CRISPR-Cas proteins that enable gene repression without modifying DNA. For example, fusion of deactivated Cas9 lacking its catalytic domain with Kruppel Associated Box transcription repressor domains (CRISPRi) enables RNA-guided gene repression (Gilbert et al., 2013; Mandegar et al., 2016; Cox et al., 2017; Kim et al., 2017a). Cas13 is another CRISPR endonuclease that differs from Cas9 in that it targets RNA instead of DNA, and can knockdown mRNA transcripts with similar efficacy and fewer off-target effects than RNA interference (Abudayyeh et al., 2017, 2019). Fusion of catalytically deactivated Cas13 (dCas13) with Adenosine Deaminase Acting on RNA (ADAR) enables more precise RNA base editing (Cox et al., 2017; Abudayyeh et al., 2019). While these systems showed effective repression of target genes in mammalian cells without modulating DNA (Cox et al., 2017; Thakore et al., 2018; Abudayyeh et al., 2019; Chung et al., 2019; Truong et al., 2019), they have not yet been extensively applied to ocular angiogenesis.
CRISPR Delivery to Ocular Tissues
While the Cas9 from Streptococcus pyogenes (SpCas9) has been the most well-characterized ortholog, its larger size (4.2 kb) limits its ability to be packaged with gRNAs into a single AAV vector for in vivo use. Strategies to circumvent this limitation include packaging in lentiviral vectors, employing dual AAV vectors to express full-length SpCas9 separately from the gRNAs, or using “split-Cas9” by dividing the expression of SpCas9 at its disordered linker (V713–D718) and reconstituting the full-length protein by split-intein protein trans-splicing (Chew et al., 2016). In addition, smaller Cas9 orthologs from Staphylococcus aureus (SaCas9) and Campylobacter jejuni (CjCas9) can be packaged along with gRNAs in an “all-in-one” AAV vector (Kim et al., 2017; Chung et al., 2020). The first human clinical trial utilizing CRISPR technology in the eye commenced in late 2019 and evaluates subretinal AAV-mediated delivery of SaCas9 with a pair of gRNAs to target a deep intronic mutation in the CEP290 gene for the treatment of type 10 Leber congenital amaurosis (NCT03872479) (Maeder et al., 2019). Interestingly, despite the theoretical advantage of using a single viral vector for clinical translation, various groups including ours have found that genome editing efficiency of these smaller Cas9 variants are inferior to dual-vector delivery of SpCas9 and gRNAs (Chung et al., 2020; Li, F. et al., 2020).
Although viral vectors allow efficient transfer of genome editing tools to retinal cells, sustained viral expression of the Cas9 endonuclease can lead to off-target effects. Unlike conventional gene augmentation or biofactory strategies, CRISPR systems do not require long-term transgene expression, where the sustained presence of Cas9 can potentially trigger non-specific mutations. An alternative strategy for clinical application is to directly deliver recombinant Cas9 proteins and gRNAs as ribonucleoprotein complexes (RNPs) to the eye, which can induced DNA cleavage almost immediately and degrade rapidly in cells, helping to minimize off-target effects and cellular toxicity (Kim et al., 2014; Jo et al., 2015). Direct application of RNPs to human cells demonstrated more efficient gene cleavage than plasmid transfection, with up to 79% on-target mutation and minimum off-target effects (Kim et al., 2014), and when delivered subretinally into mouse eyes to target VEGF, results in up to 40% reduction in a laser-induced model of CNV (Kim et al., 2017b). Despite the benefits of RNP, nuclear delivery of Cas9 proteins is still challenging, mainly due to endosomal entrapment in cytosol. Synthetic vehicles such as cell penetrating peptides (CPPs) may increase delivery efficiency by up to 80% (Zuris et al., 2015).
Genomic Targets for Ocular Angiogenesis
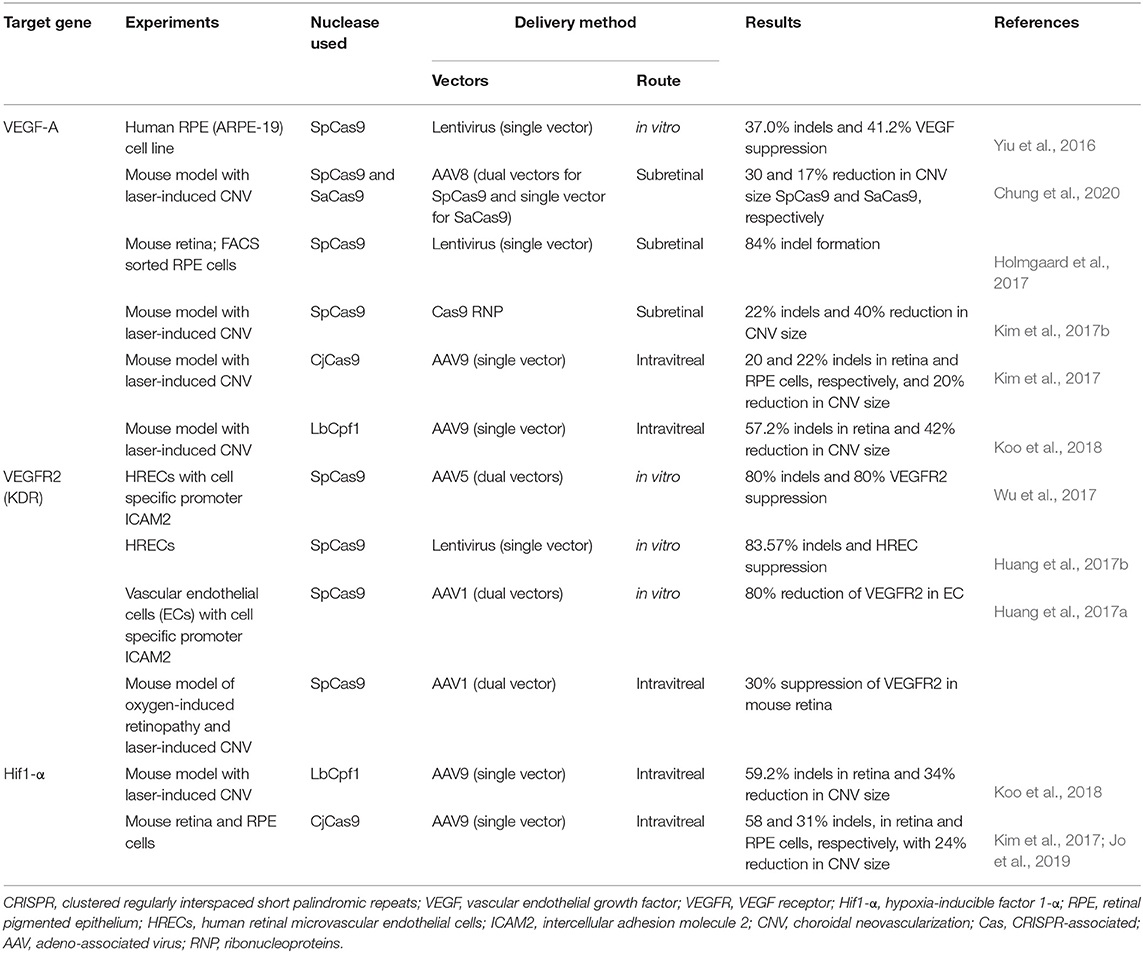
As mentioned before, an advantage of using CRISPR technology over current anti-angiogenesis gene therapy approaches is the ability to target both extracellular cytokines and intracellular mediators including trans- and cis-regulatory elements. Beside targeting VEGF, for example, genome editing strategies could be designed to target the hypoxia-inducible factor 1α (Hif-1α) transcription factor, or the hypoxia response element (HRE) in the VEGF promoter to which Hif-1α binds. CRISPR-Cas9 may also target VEGF receptors or downstream signals. Finally, CRISPR-based strategies enable simultaneous and multiplexed targeting of several factors using an array of gRNAs (Cong et al., 2013; Mali et al., 2013b; Zhang et al., 2019), which can potentially make genome editing particularly well-suited for multifactorial conditions such as ocular angiogenesis. Thus, the design of CRISPR-based anti-angiogenesis therapies can be more sophisticated, more effective, and more specific than pharmacologic and conventional gene therapies. Here, we summarize efforts using genome editing to target various pro-angiogenic signals in preclinical models of ocular angiogenesis (Table 2).
VEGF
Our group successfully employed SpCas9 to target exon 1 of VEGF-A in human RPE cells using lentiviral vectors in vitro, with up to 37% indel formation, 41% reduction in VEGF-A protein, and 39% reduction in endothelial cell tube formation (Yiu et al., 2016). Holmgaard et al. later tested lentiviral-mediated SpCas9 delivery in the mouse retina, targeting exon 3 instead of exon 1, and achieved up to 84% VEGFa knockdown in mouse RPE cells (Holmgaard et al., 2017). Due to safety concerns related to the use of lentiviral vectors, Kim and colleagues subretinally injected preassembled Cas9 RNPs directly, and reduced VEGFa in RPE and laser-induced CNV by ~40% (Kim et al., 2017b). The same group also performed intravitreal AAV9 injections to deliver the smaller CjCas9 ortholog (Kim et al., 2017) and the type V endonuclease Cpf1 (also known as Cas12a) from Lachnospiracea bacterium (LbCpf1) which creates staggered DNA cuts (Koo et al., 2018), reducing laser-induced CNV area in mouse eyes by 24 and 42%, respectively. The mechanism by which intravitreal AAV in these studies effectively penetrated the retina to suppress CNV is unclear, although laser injury may have disrupted the ILM barrier. Interestingly, although some reports suggest that co-delivery of two AAV vectors may be less efficient than a single AAV (Trapani et al., 2014), our group found that dual AAV8 delivery of SpCas9 and gRNAs resulted in higher on-target editing rates in vivo, greater VEGF protein reduction, and more effective CNV suppression in mouse eyes than SaCas9 expression using a single AAV vector (Chung et al., 2020). In addition to DNA editing, Zhou et al. (2020) also utilized CasRx (RfxCas13d) to target VEGFa mRNA in the mouse retina demonstrating efficient VEGF knock down and CNV suppression using a paired gRNA system. Due to differences in CRISPR nucleases, gRNA design, mode of ocular delivery, and methods for quantifying efficacy, comparisons between studies are difficult. Nevertheless, these results show that VEGF protein reduction does not scale linearly with functional CNV suppression, and that despite varying levels of genomic VEGF disruption, CNV suppression in rodent models rarely exceeds 50%. This is supported by the fact that widely-used and clinically-effective pharmacologic anti-VEGF agents such as aflibercept achieve similar efficacies in laser CNV animal models (Koo et al., 2018; Chung et al., 2020). Thus, despite concerns that genome editing may not achieve the same high levels of VEGF inhibition as pharmacologic agents, they may still be effective in clinically settings.
VEGF Receptors
VEGF-A regulates angiogenesis through the tyrosine kinase receptors VEGFR-1 (Flt-1) and VEGFR-2 (KDR). Although VEGFR-1 has higher binding affinity, VEGFR-2 has greater kinase activity and mediates most of the pro-angiogenic signal (Shibuya, 2011). Early gene therapies employed AAV to express soluble VEGFR-1 (sFlt-1) as a decoy receptor, but showed limited efficacy in human trials (MacLachlan et al., 2011; Lai et al., 2012; Rakoczy et al., 2015; Constable et al., 2016). For genome editing strategies, VEGFR-2 has been the target of choice. But unlike VEGF which is secreted by multiple cell types, VEGF receptor knockdown must be targeted to endothelial cells. A dual AAV5 system expressing SpCas9 under the endothelial cell-specific promoter ICAM2 successfully depleted VEGFR-2 by 80% and reduced in vitro angiogenesis from human retinal microvascular endothelial cells (HREC) (Wu et al., 2017). A lentiviral vector carrying SpCas9 to target VEGFR2 also showed over 80% indel formation and HREC suppression in vitro (Huang et al., 2017b). The same group later demonstrated intravitreal AAV1-mediated suppression of VEGFR2 in both an oxygen-induced retinopathy (OIR) model of retinal neovascularization and laser-induced CNV (Huang et al., 2017a). Although these data support the potential benefit of targeting VEGF receptors, the pathway for clinical translation remains unclear. AAVs have natural tropism for neurons, skeletal muscle, and hepatocytes but not for vascular endothelium, although viral capsid modifications may enable greater transduction efficiency in these cells (Nicklin et al., 2001; Körbelin et al., 2016). Subretinal AAV also lack access to choroidal endothelial cells due to the RPE and Bruch's membrane, although disruption of this barrier by CNV pathology may overcome this limitation.
Hif-1α
Hif-1α is a major regulator of the cellular hypoxic response (Zimna and Kurpisz, 2015), and transcriptionally activates a variety of pro-angiogenic factors, chemokines, and receptors including VEGF, PDGF-B, and angiopoietins 1/2 (Semenza, 2003; Ceradini et al., 2004; Greijer et al., 2005; Manalo et al., 2005). AAV9 delivery of CjCas9 with gRNAs to target Hif-1α demonstrated ~20% CNV suppression in mouse eyes (Kim et al., 2017), with no detectable toxicity or off-target effects up to 14 months after treatment (Jo et al., 2019). Interestingly, the authors noted cone dysfunction in eyes that underwent genomic disruption of VEGF but not Hif-1α, implicating the latter as a safer therapeutic target. The same team also utilized LbCpf1 to knockdown Hif-1α in mice, with up to 34% reduction of laser-induced CNV (Koo et al., 2018). While the ability to target upstream regulatory factors such as Hif-1α appears attractive, there are also potential risks. Hif-1α mediates various pathways involved in physiologic, as well as pathologic, angiogenesis. Thus, permanent suppression of high-level regulators such as Hif-1α may have unintended adverse consequences, and may explain the limited use of pharmacologic Hif-1α antagonists such as doxorubicin in clinical ophthalmic practice.
Limitations of Crispr for Ocular Angiogenesis
Therapeutic Thresholds of Pro-Angiogenic Targets
Genome editing strategies face a unique set of challenges when applied to the treatment of angiogenic conditions. In inherited retinal diseases where gradual photoreceptor loss leads to eventual blindness, only 10% of photoreceptors need to be preserved or rescued to restore useful vision (Geller and Sieving, 1993; Ratnam et al., 2013). However, the therapeutic threshold for VEGF or other pro-angiogenic factors are unknown, and likely varies significantly between disease processes and individual patients. In humans, VEGF levels in the aqueous humor poorly predict disease severity in eyes with diabetic macular edema (Kwon and Jee, 2018). Variable responses to anti-VEGF pharmacotherapies are evidenced by many patients who do not respond to treatment. Because the laser-induced CNV model is notoriously unreliable as a predictor of clinical efficacy in humans, the relative effectiveness of genome editing strategies cannot be reliably compared with anti-VEGF pharmacotherapies in these animal models.
Additionally, the stochastic nature of CRISPR-based approaches limits our ability to carefully titrate the degree of angiogenic suppression. For example, Cas9 cleavage generates a mosaic of biallelic null mutants, haploinsufficient, and unedited wild-type cells, thus causing variable and incomplete VEGF suppression. The use of multiple gRNAs can increase the amount of gene knockdown (Tsai et al., 2018), but over-suppression may also be detrimental as VEGF is required for the normal, physiologic maintenance of vascular and neural tissues (Kurihara et al., 2012), and chronic anti-VEGF treatments have been linked to geographic or choroidal atrophy in patients with AMD and DME (Yiu et al., 2014; Grunwald et al., 2015). Thus, partial suppression of angiogenic pathways over the long-term may actually be preferred for clinical applications. For example, lentiviral delivery of VEGF-A-shRNA which reduces VEGF-A to physiologic levels rescues OIR in rats without interfering with retinal vascular development (Wang et al., 2013). Also, while current anti-VEGF therapies involve repeated, pulsatile treatments that result in fluctuations in VEGF suppression; continuous, stable VEGF suppression using sustained delivery systems appear effective even at lower therapeutic doses (Campochiaro et al., 2019).
Finally, it is important to note that pathologic angiogenesis involves the complex interaction of many pro- and anti-angiogenic factors (Cabral et al., 2017). Although pharmacologic VEGF inhibition appears to be effective across a spectrum of neovascular retinal diseases, genome editing produces a cellular mosaic of homozygous null mutants which could be impacted by compensatory paracrine effects from neighboring cells or upregulation of other proangiogenic pathways.
Cell Specificity of CRISPR-Based Therapies
Unlike gene therapies that employ a biofactory approach, CRISPR-mediated strategies must be appropriately directed at pathologic cell types. For example, genomic VEGF ablation in Muller glia may be more suitable for treating retinal neovascularization, while VEGF suppression from RPE or choroidal endothelial cells may be more appropriate for CNV. Similarly, genomic disruption of the VEGF receptor must be targeted to retinal or choroidal endothelial cells depending on the nature of the disease, although recent evidence suggests that retinal vessels may also contribute to CNV pathogenesis (Snyder et al., 2018; Yiu et al., 2019; Lee et al., 2020). Target cell specificity may be conferred by cell-specific promoters, AAV serotypes with different cellular tropisms, or distinct modes of vector delivery. For example, subretinal injections are more likely to effectively treat a CNV lesion, while intravitreal injections may be better suited for widespread retinal neovascular diseases. Although cellular targeting may be particularly challenging for certain cell types in the retina, these considerations could enable greater precision and safety profile of CRISPR-based treatment platforms.
Minimization of Off-Target Activity
Because genome editing therapies are optimally prescribed prior to the onset of severe vision loss, the prospect of potentially inducing unintended mutations is of particular concern. Strategies to minimize off-target genome editing activity include truncated gRNAs, high fidelity Cas9 variants, anti-CRISPR proteins, and self-destructing CRISPR systems. Several studies have shown that truncated gRNAs (<20 nt in length) reduce off-target activity without compromising on-target specificity by minimizing their binding affinity to DNA (Pattanayak et al., 2013; Fu et al., 2014; Tsai et al., 2015). Utilizing Cas9 nickases with paired gRNAs also limit off-target effects, as individual off-target sites of single-stranded nicks are rapidly repaired by base excision mechanisms (Mali et al., 2013a; Ran et al., 2013; Cho et al., 2014). Newly-engineered high fidelity Cas9 variants such as SpCas9-HF1 (Kleinstiver et al., 2016) and high fidelity SaCas9 (Tan et al., 2019; Xie et al., 2020) also showed promise in reducing non-specific mutations. By binding to SpCas9's protospacer adjacent motif (PAM) recognition sites, small anti-CRISPR proteins (Acr) less than 200 amino acids can be delivered via AAV vectors to suppress genome editing activity (Shin et al., 2017). In vivo AAV8 delivery of AcrIIC3 with Nme2Cas9 in mice inhibited genome editing without demonstrating cytotoxicity (Lee et al., 2019), although the optimal timing of Acr delivery remain to be determined. Finally, self-destructing “kamikaze” CRISPR systems employ gRNAs that target the Cas9 endonuclease itself to limit prolonged genome editing activity (Li et al., 2018). Although dual AAV2-mediated expression of SpCas9 and gRNA targeting SpCas9 successfully reduced SpCas9 mRNA after intravitreal delivery (Li et al., 2019), concerns of incomplete Cas9 removal may limit the usefulness of these approaches (Li et al., 2018). Given the unique optical system of the eye as an organ, optogenetically-controlled nanoCRISPR technology could enable spatial and temporal control of Cas9 endonuclease activity to improve safety and precision in future studies (Chen et al., 2020).
Immune Responses to CRISPR Components
The foreign nature of bacterial-derived CRISPR-Cas9 proteins has the potential to elicit unwanted host immune responses that may limit therapeutic efficacy. In mice, AAV-mediated Cas9 delivery triggered both humoral and cellular response against Cas9 (Chew et al., 2016). Moreover, mice with preexisting antibodies against SaCas9 evoked CD8+ cytotoxic T cell activity which eliminated the edited cell population despite still showing efficient genome editing (Li et al., 2020). In humans, pre-existing anti-Cas9 antibodies and antigen-reactive T cells are prevalent due to the ubiquitous nature of S. pyogenes and S. aureus from which the most commonly-used SpCas9 and SaCas9 endonucleases are derived (Attenello, 2019; Charlesworth et al., 2019; Wagner et al., 2019). Studies in human blood donors revealed pre-existing antibodies against SaCas9 and SpCas9 ranging from 78–79 to 58–65%, respectively (Attenello, 2019) (Charlesworth et al., 2019). Interestingly, effector T cell responses have been found in donor peripheral blood monocytes (PBMCs) upon restimulating with recombinant Cas9s, suggesting that adaptive immunity may lead to diminishing therapeutic efficacy (Wagner et al., 2019). Although antibodies against intracellular proteins may not directly lead to immune-mediated elimination of Cas9-expressing cells (Crudele and Chamberlain, 2018), these pre-existing antibodies can limit the efficacy of the CRISPR gene therapy.
Conclusion
The application of genome editing for retinal diseases has received significant attention since the recent initiation of the first human trial using CRISPR technology. Genome editing pose unique advantages as well as challenges when compared to anti-VEGF pharmacotherapies or current gene therapy approaches. Genomic ablation can permanently suppress pro-angiogenic pathways, providing a true cure for ocular angiogenic conditions. Viral-mediated delivery of CRISPR components also enables efficient targeting of not only secreted factors such as VEGF, but also intracellular targets including upstream transcription factors and regulatory elements, as well as downstream signal mediators. Yet, the needs to overcome a high therapeutic threshold and maximize cellular specificity, while minimizing off-target activity and host immune responses, must be adequately addressed to facilitate more rapid translation of research outcomes into real world therapies.
Author Contributions
SC and GY conceived the project. SC, T-NS, and TN collected literature. SC, T-NS, and GY wrote the manuscript. GY oversaw the project. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Alcon Research Institute, the Macula Society, the BrightFocus Foundation and the grants NIH K08 EY026101 and NIH R21 EY031108.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abudayyeh, O. O., Gootenberg, J. S., Essletzbichler, P., Han, S., Joung, J., Belanto, J. J., et al. (2017). RNA targeting with CRISPR–Cas13. Nature 550, 280–284. doi: 10.1038/nature24049
Abudayyeh, O. O., Gootenberg, J. S., Franklin, B., Koob, J., Kellner, M. J., Ladha, A., et al. (2019). A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386. doi: 10.1126/science.aax7063
Attenello, F. J. (2019). Immunity to CRISPR-Cas9. Sci. Transl. Med. 11:eaaw5328. doi: 10.1126/scitranslmed.aaw5328
Auricchio, A., Kobinger, G., Anand, V., Hildinger, M., O'Connor, E., Maguire, A. M., et al. (2001). Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10, 3075–3081. doi: 10.1093/hmg/10.26.3075
Bainbridge, J. W. B., Mehat, M. S., Sundaram, V., Robbie, S. J., Barker, S. E., Ripamonti, C., et al. (2015). Long-term effect of gene therapy on leber's congenital amaurosis. N. Engl. J. Med. 372, 1887–1897. doi: 10.1056/NEJMoa1414221
Bainbridge, J. W. B., Smith, A. J., Barker, S. S., Robbie, S., Henderson, R., Balaggan, K., et al. (2008). Effect of gene therapy on visual function in leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239. doi: 10.1056/NEJMoa0802268
Baumal, C. R., Spaide, R. F., Vajzovic, L., Freund, K. B., Walter, S. D., John, V., et al. (2020). Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology 127, 1345–1359. doi: 10.1016/j.ophtha.2020.04.017
Bennett, J., Ashtari, M., Wellman, J., Marshall, K. A., Cyckowski, L. L., Chung, D. C., et al. (2012). AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 4:120ra15. doi: 10.1126/scitranslmed.3002865
Bermea, K. C., Rodríguez-García, A., Tsin, A., and Barrera-Saldaña, H. A. (2018). Somatolactogens and diabetic retinopathy. Growth Horm. IGF Res. 41, 42–47. doi: 10.1016/j.ghir.2018.02.002
Boyer, D. S. (2020). Phase 1 study of intravitreal gene therapy with ADVM-022 for neovascular AMD (OPTIC trial). Presented at the Angiogenesis, Exudation, and Degeneration 2020 (Miami, FL).
Brown, D. M., Campochiaro, P. A., Singh, R. P., Li, Z., Gray, S., Saroj, N., et al. (2010). Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117, 1124–1133.e1. doi: 10.1016/j.ophtha.2010.02.022
Brown, D. M., Michels, M., Kaiser, P. K., Heier, J. S., Sy, J. P., and Ianchulev, T. (2009). Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116, 57–65.e5. doi: 10.1016/j.ophtha.2008.10.018
Brown, D. M., Nguyen, Q. D., Marcus, D. M., Boyer, D. S., Patel, S., Feiner, L., et al. (2013). Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120, 2013–2022. doi: 10.1016/j.ophtha.2013.02.034
Cabral, T., Lima, L. H. M., Mello, L. G., Polido, J. P., Correa, E. P., Oshima, A., et al. (2018). Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol. Retina 2, 31–37. doi: 10.1016/j.oret.2017.04.004
Cabral, T., Mello, L. G. M., Lima, L. H., Polido, J., Regatieri, C. V., Belfort, R., et al. (2017). Retinal and choroidal angiogenesis: a review of new targets. Int. J. Retina Vitr. 3:31. doi: 10.1186/s40942-017-0084-9
Callanan, D., Kunimoto, D., Maturi, R. K., Patel, S. S., Staurenghi, G., Wolf, S., et al. (2018). Double-masked, randomized, phase 2 evaluation of abicipar pegol (an anti-VEGF DARPin therapeutic) in neovascular age-related macular degeneration. J. Ocul. Pharmacol. Ther. 34, 700–709. doi: 10.1089/jop.2018.0062
Campochiaro, P. A., Heier, J. S., Feiner, L., Gray, S., Saroj, N., Rundle, A. C., et al. (2010). Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117, 1102–1112.e1. doi: 10.1016/j.ophtha.2010.02.021
Campochiaro, P. A., Marcus, D. M., Awh, C. C., Regillo, C., Adamis, A. P., Bantseev, V., et al. (2019). The port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology 126, 1141–1154. doi: 10.1016/j.ophtha.2019.03.036
Cartwright, M. J., King, M. H., Weinberg, R. S., and Guerry, R. K. (1990). Micrococcus endophthalmitis. Arch. Ophthalmol. Chic. 108, 1523–1524. doi: 10.1001/archopht.1990.01070130025012
Caruso, A., Füth, M., Alvarez-Sánchez, R., Belli, S., Diack, C., Maass, K. F., et al. (2020). Ocular half-life of intravitreal biologics in humans and other species: meta-analysis and model-based prediction. Mol. Pharm. 17, 695–709. doi: 10.1021/acs.molpharmaceut.9b,01191
CATT Research Group, Martin, D. F., Maguire, M. G., Ying, G., Grunwald, J. E., Fine, S. L., et al. (2011). Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897–1908. doi: 10.1056/NEJMoa1102673
Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., et al. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 10, 858–864. doi: 10.1038/nm1075
Charlesworth, C. T., Deshpande, P. S., Dever, D. P., Camarena, J., Lemgart, V. T., Cromer, M. K., et al. (2019). Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254. doi: 10.1038/s41591-018-0326-x
Chen, X., Chen, Y., Xin, H., Wan, T., and Ping, Y. (2020). Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing. Proc. Natl. Acad. Sci. U.S.A. 117, 2395–2405. doi: 10.1073/pnas.1912220117
Chew, W. L., Tabebordbar, M., Cheng, J. K. W., Mali, P., Wu, E. Y., Ng, A. H. M., et al. (2016). A multifunctional AAV–CRISPR–Cas9 and its host response. Nat. Methods 13, 868–874. doi: 10.1038/nmeth.3993
Cho, S. W., Kim, S., Kim, Y., Kweon, J., Kim, H. S., Bae, S., et al. (2014). Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24, 132–141. doi: 10.1101/gr.162339.113
Chung, J. Y., Ain, Q. U., Song, Y., Yong, S.-B., and Kim, Y.-H. (2019). Targeted delivery of CRISPR interference system against Fabp4 to white adipocytes ameliorates obesity, inflammation, hepatic steatosis, and insulin resistance. Genome Res. 29, 1442–1452. doi: 10.1101/gr.246900.118
Chung, S. H., Mollhoff, I. N., Nguyen, U., Nguyen, A., Stucka, N., Tieu, E., et al. (2020). Factors impacting efficacy of AAV-mediated CRISPR-based genome editing for treatment of choroidal neovascularization. Mol. Ther. Methods Clin. Dev. 17, 409–417. doi: 10.1016/j.omtm.2020.01.006
Clark, W. L., Boyer, D. S., Heier, J. S., Brown, D. M., Haller, J. A., Vitti, R., et al. (2016). Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 123, 330–336. doi: 10.1016/j.ophtha.2015.09.035
Cohen, M. N., O'Shaughnessy, D., Fisher, K., Cerami, J., Awh, C. C., Salazar, D. E., et al. (2020). APEX: a phase II randomised clinical trial evaluating the safety and preliminary efficacy of oral X-82 to treat exudative age-related macular degeneration. Br. J. Ophthalmol. 1–7. doi: 10.1136/bjophthalmol-2020-316511
Cong, L., Ran, F. A., Cox, D., Lin, S., Barretto, R., Habib, N., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. doi: 10.1126/science.1231143
Constable, I. J., Pierce, C. M., Lai, C.-M., Magno, A. L., Degli-Esposti, M. A., French, M. A., et al. (2016). Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine 14, 168–175. doi: 10.1016/j.ebiom.2016.11.016
Cox, D. B. T., Gootenberg, J. S., Abudayyeh, O. O., Franklin, B., Kellner, M. J., Joung, J., et al. (2017). RNA editing with CRISPR-Cas13. Science 358, 1019–1027. doi: 10.1126/science.aaq0180
Crudele, J. M., and Chamberlain, J. S. (2018). Cas9 immunity creates challenges for CRISPR gene editing therapies. Nat. Commun. 9:3497. doi: 10.1038/s41467-018-05843-9
Dalkara, D., Byrne, L. C., Klimczak, R. R., Visel, M., Yin, L., Merigan, W. H., et al. (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5:189ra76. doi: 10.1126/scitranslmed.3005708
Ding, K., Shen, J., Hafiz, Z., Hackett, S. F., Silva, R. L. E., Khan, M., et al. (2019). AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J. Clin. Invest. 130, 4901–4911. doi: 10.1172/JCI129085
Do, D. V., Rhoades, W., and Nguyen, Q. D. (2020). Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina 40, 643–647. doi: 10.1097/IAE.0000000000002566
Dreyfuss, J. L., Giordano, R. J., and Regatieri, C. V. (2015). Ocular angiogenesis. J. Ophthalmol. 2015:892043. doi: 10.1155/2015/892043
Dugel, P. U., Singh, R. P., Koh, A., Ogura, Y., Weissgerber, G., Gedif, K., et al. (2020). HAWK and HARRIER: 96-Week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. doi: 10.1016/j.ophtha.2020.06.028
Ellis, M. P., Lent-Schochet, D., Lo, T., and Yiu, G. (2019). Emerging concepts in the treatment of diabetic retinopathy. Curr. Diab. Rep. 19:137. doi: 10.1007/s11892-019-1276-5
Frye, M., Dierkes, M., Küppers, V., Vockel, M., Tomm, J., Zeuschner, D., et al. (2015). Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 212, 2267–2287. doi: 10.1084/jem.20150718
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M., and Joung, J. K. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284. doi: 10.1038/nbt.2808
Fujita, K., Imamura, Y., Shinoda, K., Matsumoto, C. S., Mizutani, Y., Hashizume, K., et al. (2015). One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 122, 555–561. doi: 10.1016/j.ophtha.2014.09.034
Geller, A. M., and Sieving, P. A. (1993). Assessment of foveal cone photoreceptors in stargardt's macular dystrophy using a small dot detection task. Vision Res. 33, 1509–1524. doi: 10.1016/0042-6989(93)90144-l
Gilbert, L. A., Larson, M. H., Morsut, L., Liu, Z., Brar, G. A., Torres, S. E., et al. (2013). CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451. doi: 10.1016/j.cell.2013.06.044
Greijer, A. E., van der Groep, P., Kemming, D., Shvarts, A., Semenza, G. L., Meijer, G. A., et al. (2005). Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J. Pathol. 206, 291–304. doi: 10.1002/path.1778
Grishanin, R., Vuillemenot, B., Sharma, P., Keravala, A., Greengard, J., Gelfman, C., et al. (2019). Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol. Ther. 27, 118–129. doi: 10.1016/j.ymthe.2018.11.003
Grunwald, J. E., Pistilli, M., Ying, G.-S., Maguire, M. G., Daniel, E., Martin, D. F., et al. (2015). Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 122, 809–816. doi: 10.1016/j.ophtha.2014.11.007
Heier, J. S., Antoszyk, A. N., Pavan, P. R., Leff, S. R., Rosenfeld, P. J., Ciulla, T. A., et al. (2006). Ranibizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 113, 633–642.e4. doi: 10.1016/j.ophtha.2005.10.052
Heier, J. S., Clark, W. L., Boyer, D. S., Brown, D. M., Vitti, R., Berliner, A. J., et al. (2014). Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology 121, 1414–1420.e1. doi: 10.1016/j.ophtha.2014.01.027
Heier, J. S., Wykoff, C. C., Waheed, N. K., Kitchens, J. W., Patel, S. S., Vitti, R., et al. (2020). Intravitreal combined aflibercept + anti–platelet-derived growth factor receptor β for neovascular age-related macular degeneration. Ophthalmology 127, 211–220. doi: 10.1016/j.ophtha.2019.09.021
Hellström, A., Smith, L. E., and Dammann, O. (2013). Retinopathy of prematurity. Lancet 382, 1445–1457. doi: 10.1016/S0140-6736(13)60178-6
Holmgaard, A., Askou, A. L., Benckendorff, J. N. E., Thomsen, E. A., Cai, Y., Bek, T., et al. (2017). In vivo knockout of the vegfa gene by lentiviral delivery of CRISPR/Cas9 in mouse retinal pigment epithelium cells. Mol. Ther. Nucleic Acids 9, 89–99. doi: 10.1016/j.omtn.2017.08.016
Hori, T., Fukutome, M., and Koike, C. (2019). Adeno associated virus (AAV) as a tool for clinical and experimental delivery of target genes into the mammalian retina. Biol. Pharm. Bull. 42, 343–347. doi: 10.1248/bpb.b18-00913
Huang, X., Zhou, G., Wu, W., Duan, Y., Ma, G., Song, J., et al. (2017a). Genome editing abrogates angiogenesis in vivo. Nat. Commun. 8:112. doi: 10.1038/s41467-017-00140-3
Huang, X., Zhou, G., Wu, W., Ma, G., D'Amore, P. A., Mukai, S., et al. (2017b). Editing VEGFR2 blocks VEGF-induced activation of akt and tube formation. Invest. Ophthalmol. Vis. Sci. 58, 1228–1236. doi: 10.1167/iovs.16-20537
Ida, H., Tobe, T., Nambu, H., Matsumura, M., Uyama, M., and Campochiaro, P. A. (2003). RPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGF. Invest. Ophthalmol. Vis. Sci. 44, 5430–5437. doi: 10.1167/iovs.03-0609
Jiang, Y., Wang, H., Culp, D., Yang, Z., Fotheringham, L., Flannery, J., et al. (2014). Targeting Müller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 55, 824–831. doi: 10.1167/iovs.13-13755
Jo, D. H., Koo, T., Cho, C. S., Kim, J. H., Kim, J.-S., and Kim, J. H. (2019). Long-term effects of in vivo genome editing in the mouse retina using campylobacter jejuni cas9 expressed via adeno-associated virus. Mol. Ther. 27, 130–136. doi: 10.1016/j.ymthe.2018.10.009
Jo, Y.-I., Suresh, B., Kim, H., and Ramakrishna, S. (2015). CRISPR/Cas9 system as an innovative genetic engineering tool: enhancements in sequence specificity and delivery methods. Biochim. Biophys. Acta 1856, 234–43. doi: 10.1016/j.bbcan.2015.09.003
Kaiser, P. K., Brown, D. M., Zhang, K., Hudson, H. L., Holz, F. G., Shapiro, H., et al. (2007). Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am. J. Ophthalmol. 144, 850–857. doi: 10.1016/j.ajo.2007.08.012
Kapoor, D. N., Bhatia, A., Kaur, R., Sharma, R., Kaur, G., and Dhawan, S. (2015). PLGA: a unique polymer for drug delivery. Ther. Deliv. 6, 41–58. doi: 10.4155/tde.14.91
Khanani, A. M., Patel, S. S., Ferrone, P. J., Osborne, A., Sahni, J., Grzeschik, S., et al. (2020). Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 138, 964–972. doi: 10.1001/jamaophthalmol.2020.2699
Kim, E., Koo, T., Park, S. W., Kim, D., Kim, K., Cho, H.-Y., et al. (2017). In vivo genome editing with a small Cas9 orthologue derived from campylobacter jejuni. Nat. Commun. 8:14500. doi: 10.1038/ncomms14500
Kim, K., Park, S. W., Kim, J. H., Lee, S. H., Kim, D., Koo, T., et al. (2017b). Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 27, 419–426. doi: 10.1101/gr.219089.116
Kim, K., Ryu, S.-M., Kim, S.-T., Baek, G., Kim, D., Lim, K., et al. (2017a). Highly efficient RNA-guided base editing in mouse embryos. Nat. Biotechnol. 35, 435–437. doi: 10.1038/nbt.3816
Kim, S., Kim, D., Cho, S. W., Kim, J., and Kim, J.-S. (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019. doi: 10.1101/gr.171322.113
Kleinstiver, B. P., Pattanayak, V., Prew, M. S., Tsai, S. Q., Nguyen, N., Zheng, Z., et al. (2016). High-fidelity CRISPR-Cas9 variants with undetectable genome-wide off-targets. Nature 529, 490–495. doi: 10.1038/nature16526
Koh, A., Lee, W. K., Chen, L.-J., Chen, S.-J., Hashad, Y., Kim, H., et al. (2012). EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32, 1453–1464. doi: 10.1097/IAE.0b013e31824f91e8
Koo, T., Park, S. W., Jo, D. H., Kim, D., Kim, J. H., Cho, H.-Y., et al. (2018). CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat. Commun. 9:1855. doi: 10.1038/s41467-018-04175-y
Körbelin, J., Dogbevia, G., Michelfelder, S., Ridder, D. A., Hunger, A., Wenzel, J., et al. (2016). A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med. 8, 609–625. doi: 10.15252/emmm.201506078
Korobelnik, J.-F., Do, D. V., Schmidt-Erfurth, U., Boyer, D. S., Holz, F. G., Heier, J. S., et al. (2014a). Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121, 2247–2254. doi: 10.1016/j.ophtha.2014.05.006
Korobelnik, J.-F., Holz, F. G., Roider, J., Ogura, Y., Simader, C., Schmidt-Erfurth, U., et al. (2014b). Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology 121, 202–208. doi: 10.1016/j.ophtha.2013.08.012
Krohne, T. U., Liu, Z., Holz, F. G., and Meyer, C. H. (2012). Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 154, 682–686.e2. doi: 10.1016/j.ajo.2012.03.047
Kurihara, T., Westenskow, P. D., Bravo, S., Aguilar, E., and Friedlander, M. (2012). Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 122, 4213–4217. doi: 10.1172/JCI65157
Kwon, J.-W., and Jee, D. (2018). Correction: aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS ONE 13:e0207902. doi: 10.1371/journal.pone.0207902
Lai, C.-M., Estcourt, M. J., Himbeck, R. P., Lee, S.-Y., Yew-San Yeo, I., Luu, C., et al. (2012). Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 19, 999–1009. doi: 10.1038/gt.2011.169
Lee, J., Mou, H., Ibraheim, R., Liang, S.-Q., Liu, P., Xue, W., et al. (2019). Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins. RNA 25, 1421–1431. doi: 10.1261/rna.071704.119
Lee, S. C., Tran, S., Amin, A., Morse, L. S., Moshiri, A., Park, S. S., et al. (2020). Retinal vessel density in exudative and nonexudative age-related macular degeneration on optical coherence tomography angiography. Am. J. Ophthalmol. 212, 7–16. doi: 10.1016/j.ajo.2019.11.031
Li, A., Lee, C. M., Hurley, A. E., Jarrett, K. E., De Giorgi, M., Lu, W., et al. (2018). A self-deleting AAV-CRISPR system for in vivo genome editing. Mol. Ther. Methods Clin. Dev. 12, 111–122. doi: 10.1016/j.omtm.2018.11.009
Li, A., Tanner, M. R., Lee, C. M., Hurley, A. E., Giorgi, M. D., Jarrett, K. E., et al. (2020). AAV-CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol. Ther. 28, 1432–1441. doi: 10.1016/j.ymthe.2020.04.017
Li, F., Hung, S. S. C., Mohd Khalid, M. K. N., Wang, J.-H., Chrysostomou, V., Wong, V. H. Y., et al. (2019). Utility of self-destructing CRISPR/cas constructs for targeted gene editing in the retina. Hum. Gene Ther. 30, 1349–1360. doi: 10.1089/hum.2019.021
Li, F., Wing, K., Wang, J.-H., Luu, C. D., Bender, J. A., Chen, J., et al. (2020). Comparison of CRISPR/cas endonucleases for in vivo retinal gene editing. Front. Cell. Neurosci. 14:570917. doi: 10.3389/fncel.2020.570917
Liu, Y., Fortmann, S. D., Shen, J., Wielechowski, E., Tretiakova, A., Yoo, S., et al. (2018). AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol. Ther. 26, 542–549. doi: 10.1016/j.ymthe.2017.12.002
MacLachlan, T. K., Lukason, M., Collins, M., Munger, R., Isenberger, E., Rogers, C., et al. (2011). Preclinical safety evaluation of AAV2-sFLT01— a gene therapy for age-related macular degeneration. Mol. Ther. 19, 326–334. doi: 10.1038/mt.2010.258
Maeder, M. L., Stefanidakis, M., Wilson, C. J., Baral, R., Barrera, L. A., Bounoutas, G. S., et al. (2019). Development of a gene-editing approach to restore vision loss in leber congenital amaurosis type 10. Nat. Med. 25, 229–233. doi: 10.1038/s41591-018-0327-9
Maguire, A. M., Simonelli, F., Pierce, E. A., Pugh, E. N., Mingozzi, F., Bennicelli, J., et al. (2008). Safety and efficacy of gene transfer for leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248. doi: 10.1056/NEJMoa0802315
Mali, P., Aach, J., Stranges, P. B., Esvelt, K. M., Moosburner, M., Kosuri, S., et al. (2013a). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838. doi: 10.1038/nbt.2675
Mali, P., Yang, L., Esvelt, K. M., Aach, J., Guell, M., DiCarlo, J. E., et al. (2013b). RNA-Guided human genome engineering via Cas9. Science 339, 823–826. doi: 10.1126/science.1232033
Manalo, D. J., Rowan, A., Lavoie, T., Natarajan, L., Kelly, B. D., Ye, S. Q., et al. (2005). Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669. doi: 10.1182/blood-2004-07-2958
Mandegar, M. A., Huebsch, N., Frolov, E. B., Shin, E., Truong, A., Olvera, M. P., et al. (2016). CRISPR interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell 18, 541–553. doi: 10.1016/j.stem.2016.01.022
Miller, J. W. (1997). Vascular endothelial growth factor and ocular neovascularization. Am. J. Pathol. 151, 13–23.
Mir, M., Ahmed, N., and Rehman, A. U. (2017). Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 159, 217–231. doi: 10.1016/j.colsurfb.2017.07.038
Moisseiev, E., Waisbourd, M., Ben-Artsi, E., Levinger, E., Barak, A., Daniels, T., et al. (2014). Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 252, 331–337. doi: 10.1007/s00417-013-2495-0
Nickla, D. L., and Wallman, J. (2010). The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168. doi: 10.1016/j.preteyeres.2009.12.002
Nicklin, S. A., Buening, H., Dishart, K. L., de Alwis, M., Girod, A., Hacker, U., et al. (2001). Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol. Ther. J. Am. Soc. Gene Ther. 4, 174–181. doi: 10.1006/mthe.2001.0424
Pattanayak, V., Lin, S., Guilinger, J. P., Ma, E., Doudna, J. A., and Liu, D. R. (2013). High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 31, 839–843. doi: 10.1038/nbt.2673
Peng, Y., Tang, L., and Zhou, Y. (2017). Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 58, 217–226. doi: 10.1159/000479157
Rakoczy, E. P., Lai, C.-M., Magno, A. L., Wikstrom, M. E., French, M. A., Pierce, C. M., et al. (2015). Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet Lond. Engl. 386, 2395–2403. doi: 10.1016/S0140-6736(15)00345-1
Ran, F. A., Hsu, P. D., Lin, C.-Y., Gootenberg, J. S., Konermann, S., Trevino, A. E., et al. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389. doi: 10.1016/j.cell.2013.08.021
Ratnam, K., Carroll, J., Porco, T. C., Duncan, J. L., and Roorda, A. (2013). Relationship between foveal cone structure and clinical measures of visual function in patients with inherited retinal degenerations. Invest. Ophthalmol. Vis. Sci. 54, 5836–5847. doi: 10.1167/iovs.13-12557
Robbins, S. G., Conaway, J. R., Ford, B. L., Roberto, K. A., and Penn, J. S. (1997). Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors Chur Switz. 14, 229–241. doi: 10.3109/08977199709021522
Rodríguez-Rodríguez, D. R., Ramírez-Solís, R., Garza-Elizondo, M. A., De Lourdes Garza-Rodríguez, M., and Barrera-Saldaña, H. A. (2019). Genome editing: a perspective on the application of CRISPR/Cas9 to study human diseases (review). Int. J. Mol. Med. 43, 1559–1574. doi: 10.3892/ijmm.2019.4112
Rosenfeld, P. J., Brown, D. M., Heier, J. S., Boyer, D. S., Kaiser, P. K., Chung, C. Y., et al. (2006). Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419–1431. doi: 10.1056/NEJMoa054481
Sahni, J., Patel, S. S., Dugel, P. U., Khanani, A. M., Jhaveri, C. D., Wykoff, C. C., et al. (2019). Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema. Ophthalmology 126, 1155–1170. doi: 10.1016/j.ophtha.2019.03.023
Semenza, G. L. (2003). Angiogenesis in ischemic and neoplastic disorders. Annu. Rev. Med. 54, 17–28. doi: 10.1146/annurev.med.54.101601.152418
Shen, J., Frye, M., Lee, B. L., Reinardy, J. L., McClung, J. M., Ding, K., et al. (2014). Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J. Clin. Invest. 124, 4564–4576. doi: 10.1172/JCI74527
Shibuya, M. (2011). Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis. Genes Cancer 2, 1097–1105. doi: 10.1177/1947601911423031
Shin, J., Jiang, F., Liu, J.-J., Bray, N. L., Rauch, B. J., Baik, S. H., et al. (2017). Disabling Cas9 by an anti-CRISPR DNA mimic. Sci. Adv. 3:e1701620. doi: 10.1126/sciadv.1701620
Singh, N. K., Kotla, S., Kumar, R., and Rao, G. N. (2015). Cyclic AMP response element binding protein mediates pathological retinal neovascularization via modulating DLL4-NOTCH1 signaling. EBioMedicine 2, 1767–1784. doi: 10.1016/j.ebiom.2015.09.042
Snyder, K., Yazdanyar, A., Mahajan, A., and Yiu, G. (2018). Association between the cilioretinal artery and choroidal neovascularization in age-related macular degeneration: a secondary analysis from the age-related eye disease study. JAMA Ophthalmol. 136, 1008–1014. doi: 10.1001/jamaophthalmol.2018.2650
Stahl, A., Lepore, D., Fielder, A., Fleck, B., Reynolds, J. D., Chiang, M. F., et al. (2019). Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet Lond. Engl. 394, 1551–1559. doi: 10.1016/S0140-6736(19)31344-3
Stieger, K., Schroeder, J., Provost, N., Mendes-Madeira, A., Belbellaa, B., Meur, G. L., et al. (2009). Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol. Ther. J. Am. Soc. Gene Ther. 17, 516–523. doi: 10.1038/mt.2008.283
Stone, J., Chan-Ling, T., Pe'er, J., Itin, A., Gnessin, H., and Keshet, E. (1996). Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 37, 290–299.
Stout, J. T., and Francis, P. J. (2011). Surgical approaches to gene and stem cell therapy for retinal disease. Hum. Gene Ther. 22, 531–535. doi: 10.1089/hum.2011.060
Suzuki, M., Nagai, N., Izumi-Nagai, K., Shinoda, H., Koto, T., Uchida, A., et al. (2014). Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br. J. Ophthalmol. 98, 1186–1191. doi: 10.1136/bjophthalmol-2013-304670
Tan, Y., Chu, A. H. Y., Bao, S., Hoang, D. A., Kebede, F. T., Xiong, W., et al. (2019). Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc. Natl. Acad. Sci. U.S.A. 116, 20969–20976. doi: 10.1073/pnas.1906843116
Thakore, P. I., Kwon, J. B., Nelson, C. E., Rouse, D. C., Gemberling, M. P., Oliver, M. L., et al. (2018). RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat. Commun. 9:1674. doi: 10.1038/s41467-018-04048-4
Todorich, B., Yiu, G., and Hahn, P. (2014). Current and investigational pharmacotherapeutic approaches for modulating retinal angiogenesis. Expert Rev. Clin. Pharmacol. 7, 375–391. doi: 10.1586/17512433.2014.890047
Trapani, I., Colella, P., Sommella, A., Iodice, C., Cesi, G., de Simone, S., et al. (2014). Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 6, 194–211. doi: 10.1002/emmm.201302948
Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group (1999). Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch. Ophthalmol. 117, 1329–1345.
Truong, V. A., Hsu, M.-N., Kieu Nguyen, N. T., Lin, M.-W., Shen, C.-C., Lin, C.-Y., et al. (2019). CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucl. Acids Res. 47:e74. doi: 10.1093/nar/gkz267
Tsai, S. Q., Zheng, Z., Nguyen, N. T., Liebers, M., Topkar, V. V., Thapar, V., et al. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33, 187–197. doi: 10.1038/nbt.3117
Tsai, Y.-T., Wu, W.-H., Lee, T.-T., Wu, W.-P., Xu, C. L., Park, K. S., et al. (2018). Clustered regularly interspaced short palindromic repeats-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology 125, 1421–1430. doi: 10.1016/j.ophtha.2018.04.001
Tsipursky, M. S., Golchet, P. R., and Jampol, L. M. (2011). Photodynamic therapy of choroidal hemangioma in sturge-weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv. Ophthalmol. 56, 68–85. doi: 10.1016/j.survophthal.2010.08.002
Verteporfin in Photodynamic Therapy Study Group (2001). Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Am. J. Ophthalmol. 131, 541–560. doi: 10.1016/s0002-9394(01)00967-9
Wagner, D. L., Amini, L., Wendering, D. J., Burkhardt, L.-M., Akyüz, L., Reinke, P., et al. (2019). High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25, 242–248. doi: 10.1038/s41591-018-0204-6
Walther, W., and Stein, U. (2000). Viral vectors for gene transfer. Drugs 60, 249–271. doi: 10.2165/00003495-200060020-00002
Wang, H., Smith, G. W., Yang, Z., Jiang, Y., McCloskey, M., Greenberg, K., et al. (2013). Short hairpin RNA-mediated knockdown of VEGFA in Müller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity. Am. J. Pathol. 183, 964–974. doi: 10.1016/j.ajpath.2013.05.011
Wang, J., Xu, X., Elliott, M. H., Zhu, M., and Le, Y.-Z. (2010). Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 59, 2297–2305. doi: 10.2337/db09-1420
Wiley, L. A., Burnight, E. R., Kaalberg, E. E., Jiao, C., Riker, M. J., Halder, J. A., et al. (2018). Assessment of adeno-associated virus serotype tropism in human retinal explants. Hum. Gene Ther. 29, 424–436. doi: 10.1089/hum.2017.179
Willoughby, A. S., Vuong, V. S., Cunefare, D., Farsiu, S., Noronha, G., Danis, R. P., et al. (2018). Choroidal changes after suprachoroidal injection of triamcinolone acetonide in eyes with macular edema secondary to retinal vein occlusion. Am. J. Ophthalmol. 186, 144–151. doi: 10.1016/j.ajo.2017.11.020
Wu, W., Duan, Y., Ma, G., Zhou, G., Park-Windhol, C., D'Amore, P. A., et al. (2017). AAV-CRISPR/Cas9–mediated depletion of VEGFR2 blocks angiogenesis in vitro. Invest. Ophthalmol. Vis. Sci. 58, 6082–6090. doi: 10.1167/iovs.17-21902
Xie, H., Ge, X., Yang, F., Wang, B., Li, S., Duan, J., et al. (2020). High-fidelity SaCas9 identified by directional screening in human cells. PLoS Biol. 18:e3000747. doi: 10.1371/journal.pbio.3000747
Yáñez-Muñoz, R. J., Balaggan, K. S., MacNeil, A., Howe, S. J., Schmidt, M., Smith, A. J., et al. (2006). Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12, 348–353. doi: 10.1038/nm1365
Yeh, S., Khurana, R. N., Shah, M., Henry, C. R., Wang, R. C., Kissner, J. M., et al. (2020). Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis. Ophthalmology 127, 948–955. doi: 10.1016/j.ophtha.2020.01.006
Yiu, G. (2018). Genome editing in retinal diseases using CRISPR technology. Ophthalmol. Retina 2, 1–3. doi: 10.1016/j.oret.2017.09.015
Yiu, G., Chung, S. H., Mollhoff, I. N., Nguyen, U. T., Thomasy, S. M., Yoo, J., et al. (2020a). Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol. Ther. Methods Clin. Dev. 16, 179–191. doi: 10.1016/j.omtm.2020.01.002
Yiu, G., Chung, S. H., Mollhoff, I. N., Wang, Y., Nguyen, U. T., Shibata, B., et al. (2020b). Long-term evolution and remodeling of soft drusen in rhesus macaques. Invest. Ophthalmol. Vis. Sci. 61:32. doi: 10.1167/iovs.61.2.32
Yiu, G., Manjunath, V., Chiu, S. J., Farsiu, S., and Mahmoud, T. H. (2014). Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am. J. Ophthalmol. 158, 745–75.e2. doi: 10.1016/j.ajo.2014.06.006
Yiu, G., Tieu, E., Nguyen, A. T., Wong, B., and Smit-McBride, Z. (2016). Genomic disruption of VEGF-a expression in human retinal pigment epithelial cells using CRISPR-Cas9 endonuclease. Invest. Ophthalmol. Vis. Sci. 57, 5490–5497. doi: 10.1167/iovs.16-20296
Yiu, G., Vuong, V. S., Tran, S., Migacz, J., Cunefare, D., Farsiu, S., et al. (2019). Vascular response to sildenafil citrate in aging and age-related macular degeneration. Sci. Rep. 9:5049. doi: 10.1038/s41598-019-41509-2
Yiu, G., Welch, R. J., Wang, Y., Wang, Z., Wang, P.-W., and Haskova, Z. (2020c). Spectral-domain OCT predictors of visual outcomes after ranibizumab treatment for macular edema resulting from retinal vein occlusion. Ophthalmol. Retina 4, 67–76. doi: 10.1016/j.oret.2019.08.009
Zhang, Y., Wang, J., Wang, Z., Zhang, Y., Shi, S., Nielsen, J., et al. (2019). A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in saccharomyces cerevisiae. Nat. Commun. 10:1053. doi: 10.1038/s41467-019-09005-3
Zhou, C., Hu, X., Tang, C., Liu, W., Wang, S., Zhou, Y., et al. (2020). CasRx-mediated RNA targeting prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Natl. Sci. Rev. 7, 835–837. doi: 10.1093/nsr/nwaa033
Zimna, A., and Kurpisz, M. (2015). Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. BioMed Res. Int. 2015:549412. doi: 10.1155/2015/549412
Keywords: CRISPR, genome editing, retina, angiogenesis, choroidal neovascularization, retinal neovascularization, VEGF, anti-VEGF
Citation: Chung SH, Sin T-N, Ngo T and Yiu G (2020) CRISPR Technology for Ocular Angiogenesis. Front. Genome Ed. 2:594984. doi: 10.3389/fgeed.2020.594984
Received: 14 August 2020; Accepted: 01 December 2020;
Published: 22 December 2020.
Edited by:
Stephen Tsang, Columbia University, United StatesReviewed by:
Bence Gyorgy, University Hospital of Basel, SwitzerlandGuei-Sheung Liu, University of Tasmania, Australia
Copyright © 2020 Chung, Sin, Ngo and Yiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Glenn Yiu, Z3lpdSYjeDAwMDQwO3VjZGF2aXMuZWR1
 Sook Hyun Chung
Sook Hyun Chung Tzu-Ni Sin
Tzu-Ni Sin Taylor Ngo
Taylor Ngo Glenn Yiu
Glenn Yiu