- 1Department of Health Sciences (DISSAL), University of Genoa, Genoa, Italy
- 2Clinica Malattie Infettive, IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 3Department of Surgical Sciences and Integrated Diagnostics (DISC), University of Genoa, Genoa, Italy
- 4Unità di Microbiologia, IRCCS Ospedale Policlinico San Martino, Genoa, Italy
Introduction
Candida infections have been increasingly recognized as a serious concern for critically ill patients and/or immunocompromised individuals with serious underlying diseases (Pappas et al., 2018). Although C. albicans accounts for most of invasive infections, non-albicans species are becoming more frequent (Giacobbe et al., 2020). Among these, C. auris garnered major attention due to its variable antifungal resistance profiles and ability to disseminate within the health-care setting (Jeffery-Smith et al., 2018; Giacobbe et al., 2021).
Although many sites of infection have been reported (Choi et al., 2017; Heath et al., 2019; Khatamzas et al., 2019; Roberts et al., 2019; Shenoy et al., 2019; Supreeth et al., 2020; Shaukat et al., 2021; Mirhendi et al., 2022), candidemia remains the most common invasive infection caused by C. auris. Supported by the available evidence, in this article we share our view on the current challenges in the diagnosis and treatment of candidemia by C. auris, in particular when caused by multidrug-resistant (MDR) isolates.
Identification of C. auris and assessment of resistance patterns
The emergence of C. auris posed important challenges regarding laboratory detection and infection control measures (ECDC, 2018; Keighley et al., 2021). Indeed, the common misidentification by diagnostic platforms available in clinical microbiology and public health laboratories, a poor understanding of resistance to antifungal drugs and disinfectants, together with the ability to persistently colonize abiotic and biotic surfaces, were deemed among major factors contributing to the rapid spread of C. auris within the healthcare system (Desoubeaux et al., 2022).
Standard culture-based approaches do not permit differentiation of C. auris from other common Candida spp. as it lacks a distinctive traits, although newly formulated chromogenic media gave promising results for its rapid presumptively identification based on colony color and appearance (Borman et al., 2021). Furthermore, the tolerance to growth temperature up to 42°C (unlike many other Candida species), as well as the inability to make hyphae or pseudohyphae on Corn Meal agar plates (as generally observed for C. guilliermondii, C. lusitaniae, and C. parapsilosis), could be of some help to differentiate between C. auris and related species, although none of the former methods is ideal for an accurate species confirmation. On the other hand, the use of salt/dulcitol selective media (i.e., characterized by a high salinity - 10% NaCl - and by a carbon source based on dulcitol rather than glucose) could represent a useful strategy to selectively screen for C. auris in non-sterile body sites (CDC, 2022).
The use of biochemistry-based methods similarly poses major problem in an accurate identification of C. auris, since its biochemical assimilation profile is very similar to that of other closely related species, most commonly belonging to the C. haemulonii complex (but also C. famata, C. sake, Rhodotorula glutinis, R. mucilaginosa, and Saccharomyces spp.), leading to a great deal of misidentification (Jeffery-Smith et al., 2018; Lockhart et al., 2022). Some incremental improvements have been achieved by updating databases of most common biochemical platforms (i.e., Vitek 2, MicroScan Walkaway, BD Phoenix), but a suboptimal Candida spp. identification has been reported and discrepancies can arise. To overcome these issues, multiple guidance algorithms have been proposed to identify C. auris based on phenotypic laboratory methods and initial species identification (CDC, 2019).
At present, a reliable recognition of C. auris can be definitively achieved by mass spectrometry MALDI-TOF (i.e., bioMérieux Vitek MS, Bruker Biotyper 2.0 Microflex LT), provided that an up-to-date spectra database is used (Keighley et al., 2021). Considering the recent global emergence of this fungal pathogen, diagnostic microbiology laboratories should check with the manufacturer concerning the presence of C. auris in the database of their identification platforms and, if not available, referral of non-albicans Candida spp. invasive isolates to a reference laboratory is advisable (ECDC, 2018). To help fill this gap, molecular PCR-based assays relying on amplification and sequencing of D1/D2, RPB1/RPB2 and ITS loci have been increasingly adopted as complementary identification tests (Jeffery-Smith et al., 2018), whereas the superior discriminative power of WGS proved extremely useful to resolve new introduction vs. local transmission events in outbreak settings (Lockhart et al., 2017; Di Pilato et al., 2021; Salah et al., 2021). Noteworthy, among the currently marketed syndromic tests employed for the rapid diagnosis of bloodstream infections, which are the most powerful molecular tools that may assist in a timely manner diagnosis of infections, very few tests (e.g. the GenMark ePlex Blood Culture Identification Fungal Pathogen Panel and BioFire FilmArray BCID2 panels) include C. auris within the target organisms (Dumkow et al., 2021).
Although misidentification is potentially problematic, the reduced susceptibility to some antifungal agents (Keighley et al., 2021), as well as the rapid emergence of MDR isolates (i.e., exhibiting resistance to at least two antifungal classes) upon antifungal treatment (Jacobs et al., 2022; Rybak et al., 2022), are among the major causes of concern from C. auris infections.
Further concerns are related to antifungal susceptibility testing (AFST), which is complicated by the lack of C. auris-specific interpretative breakpoints, yet to be reported by both the Clinical Laboratory Standards Institute (CLSI) and the European Committee for Antimicrobial Susceptibility Testing (EUCAST). Strikingly, there are not likely to be established breakpoints in the near future either, since clinical trials of currently available antifungal agents against C. auris are missing. Nevertheless, based on available pharmacokinetic/pharmacodynamic data, tentative interpretative criteria have been proposed by the CDC (i.e., to be used with the reference broth microdilution method endorsed by CLSI) (CDC, 2020).
According to the CDC breakpoints, a high frequency of fluconazole- and amphotericin B-resistant isolates was observed (surpassing 90% and 30%, respectively), representing a specific phenotypic signature of C. auris since its emergence in 2009. On the other hand, a reduced susceptibility to other triazole antifungals (i.e., voriconazole, itraconazole, and isavuconazole) and echinocandins was observed less frequently (Garcia-Bustos et al., 2021; Sanyaolu et al., 2022).
An additional challenge diagnostic laboratories should be aware of involves determination of echinocandins MICs, mostly caspofungin, for which AFST may be impacted by the paradoxical growth effect (i.e., also known as Eagle effect), a phenomenon that could lead to major errors in predicting echinocandin resistance in a susceptible isolate (Briano et al., 2022; Kordalewska and Perlin, 2022). As such, confirmation of AFST results by a reference laboratory is advisable.
Treatment in real-life experiences
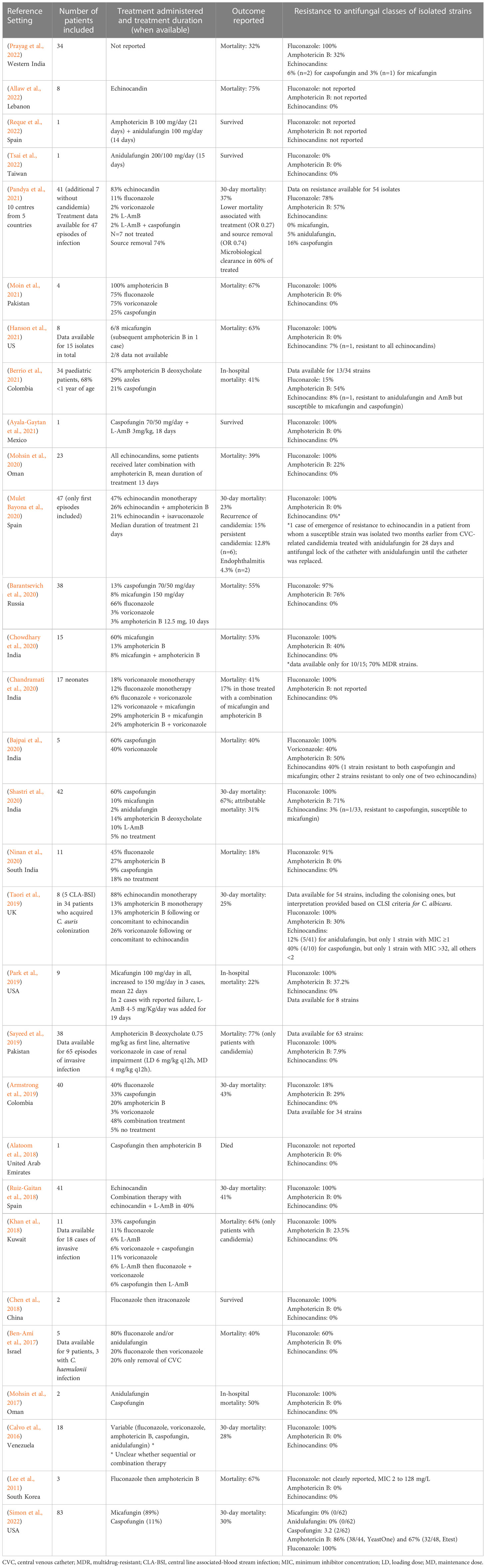
Previous studies report a prevalence of candidemia among colonized patients of around 17% (Schelenz et al., 2016; Garcia-Bustos et al., 2020), with an estimated cumulative incidence up to over 25% with increasing length of stay in critically ill subjects (Briano et al., 2022). Despite many reports of C. auris candidemia, coherent data on efficacy of active antifungal therapy are scanty, and hampered by small sample sizes, heterogeneity of treatment, and lack of adjustment for confounding factors (see Table 1). For example, many studies reported the use of antifungals with no in vitro activity. In two large Spanish studies, in which all strains were susceptible to echinocandins and echinocandins were included in the treatment regimen in all 41 and 47 patients, 30-day mortality was, respectively, 41% and 23% (Ruiz-Gaitan et al., 2018; Mulet Bayona et al., 2020).
A necessary premise is that the prompt identification of C. auris colonization with the implementation of screening protocols in high-risk areas, and the subsequent put in place of adequate infection control measures to prevent cross-transmission of this pathogen among health-care facilities (such as contact precautions, cohorting or isolation of colonized patients) remain pivotal in the prevention of invasive C. auris infections, and thus of the consequent impact of C. auris on patients’ outcome. However, when C. auris candidemia develops, antifungal therapy should be promptly initiated. To date, echinocandins are the mainstay of C. auris candidemia treatment. Indeed, while resistance to fluconazole and polyenes may reach over 90% and 30%, respectively, resistance to echinocandins is around 5-7%, although important differences exist across studies (see Table 1) (Garcia-Bustos et al., 2021; Sanyaolu et al., 2022). High rates of MDR (intended as resistance to at least two classes of antifungals and reaching up to 25-50%) do jeopardize the possibility of second line treatments in patients who either fail or develop complications to first-line treatment with echinocandins (Garcia-Bustos et al., 2021; Sanyaolu et al., 2022; Vinayagamoorthy et al., 2022). In addition, biofilm production contributes significantly to hamper treatment of C. auris infections, through contributing to resistance to antifungals by efflux pumps or inhibition of drug diffusion, and by increasing the probability of persistent infection in case of inadequate source control (Sanyaolu et al., 2022).
There are worrisome reports of emerging resistance to echinocandins after a first treatment course with these antifungals, particularly in case of catheter-related infections (Biagi et al., 2019; Al-Obaid et al., 2022; Briano et al., 2022; Mulet-Bayona et al., 2022). These reports highlight how adequate source control is an essential intervention to improve treatment success and possibly prevent induction or selection of resistance (Biagi et al., 2019; Mulet-Bayona et al., 2022). Once again, however, a clear report of the number of patients who needed to switch to second line treatments is missing, although much needed to better understand the impact of antifungal resistance in clinical practice. For example, combinations of antifungals have been tested mostly in in vitro. Synergism was noted for echinocandins and azoles combinations, in particular for anidulafungin or micafungin and isavuconazole (Caballero et al., 2021), anidulafungin and isavuconazole (but not anidulafungin and voriconazole) (Caballero et al., 2021), and micafungin and voriconazole, while no synergy was observed for voriconazole and caspofungin (Fakhim et al., 2017). In vitro evidence of non-fungicidal activity of echinocandins monotherapy was also reported, supporting the interest in drug combinations (Caballero et al., 2021). No antagonism, but also no synergy (except for one strain), was reported for flucytosine-based combinations, including amphotericin B, micafungin and voriconazole (Bidaud et al., 2019; O'Brien et al., 2020). Synergy between amphotericin B and micafungin was demonstrated in 8 among 10 tested strains (Jaggavarapu et al., 2020). Of note, in addition to known limitations of in vitro synergy models, activity of drug combinations may be not only species-specific but also strain-specific (Caballero et al., 2021).
Novel drugs in clinical development
Some novel antifungal agents are in advanced phases of clinical development that, for the first time in years, could improve/expand spectrum of activity, route of administration, drug-drug interactions, tolerability of currently available antifungals (Rauseo et al., 2020; Hoenigl et al., 2021; Jacobs et al., 2021).
Rezafungin is a second generation echinocandin, administered intravenously once weekly thanks to its prolonged half-life (Sandison et al., 2017). Its activity against C. auris was investigated in two invasive candidiasis models of immunocompromised mice, in which rezafungin exhibited potent in vivo activity (Lepak et al., 2018) and performed better than amphotericin B or micafungin in terms of kidney tissue penetration (Hager et al., 2018b). A phase II randomized double-blind study (STRIVE), including 207 patients (none with C. auris infection), safety and efficacy of rezafungin was similar to caspofungin for treating candidemia and/or invasive candidiasis (Thompson et al., 2021). Very recently, the results of a noninferiority, double-blind study (RESTORE), comparing rezafungin vs. caspofungin for treating invasive candidiasis and/or candidemia have been released (Thompson et al., 2022). The 14-day overall cure (clinical cure, microbiological cure and radiological cure) in the modified ITT population was 60.6% (57/94) and 59.1% (55/93) in caspofungin-treated and rezafungin-treated patients, respectively (95% CI for difference -14.9 to 12.7).
Fosmanogepix (APX001), the prodrug of manogepix (APX001A, E1211), inhibits the inositol acyltransferase enzyme (Gtw1) involved in the trafficking and anchoring of mannoproteins on the fungal wall (Shaw and Ibrahim, 2020). It displays potent fungistatic activity against most pathogenic Candida spp., including C. auris (MIC90 ≤0.12 mg/ml), although not against C. krusei and C. kefyr (Miyazaki et al., 2011). The drug is available in both oral and intravenous formulations (Shaw and Ibrahim, 2020), distributes well to many difficult-to-treat body sites and shows a favorable drug-drug interaction profile. In a murine model of disseminated C. auris infection, survival was 80-100% and 50% in fosmanogepix-treated and anidulafungin-treated animals, respectively (Hager et al., 2018a). In a phase II, single arm study, including 21 non-neutropenic patients with candidemia treated with fosmanogepix, success rate was 80% (16/20) (Pappas et al., 2020). Among nine critically ill subjects with C. auris candidemia, treatment success at end of fosmanogepix treatment and 30-day survival were both 89% (Kullberg et al., 2021).
Ibrexafungerp (SCY-078 or MK-3118) is a 1,3-beta-D-glucan synthase inhibitor with fungicidal activity against Candida spp., including C. auris (Ghannoum et al., 2018). Compared to echinocandins, ibrexafungerp binds a different site of the same target, so cross-resistance is limited (Jimenez-Ortigosa et al., 2017). Differently from echinocandins, ibrexafungerp is administered orally. An intravenous formulation has also completed phase 1 of clinical development (SCYNEXIS, 2021). In preclinical models, ibrexafungerp was shown to improve survival of neutropenic mice with C. auris invasive candidiasis (Wiederhold et al., 2021). In recently published phase II study, oral ibrexafungerp following initial echinocandin therapy was compared to standard of care (step-down to fluconazole) in non-neutropenic patients with invasive candidiasis. Favorable clinical response was 71%, 86%, and 71% in patients receiving ibrexafungerp 500 mg, ibrexafungerp 750 mg, and fluconazole (Spec et al., 2019). One phase III open-label study (CARES, NCT03363841) specifically evaluating the safety and efficacy of ibrexafungerp for C. auris infection is currently ongoing. In the first ten patients enrolled, complete response was 80% (Juneja et al., 2021). Other studies assessing the safety and efficacy of oral ibrexafungerp for invasive candidiasis are currently ongoing (FURI, NCT03059992 and MARIO, NCT 05178862).
Conclusion
Despite various factors (e.g., concomitant infectious and non-infectious diseases) very likely contribute to the prognosis of patients with C. auris candidemia, especially if critically ill, proper diagnosis and antifungal treatment remain pivotal to improve cure rates. While some crucial improvements in the diagnosis of C. auris candidemia have been observed in the last decade, there is still uncertainty about the best approach for treating MDR C. auris candidemia when echinocandins are unavailable due to resistance or other reasons, leading to wide heterogeneity of approaches across studies and likely relying on the current lack of high certainty data. The availability of novel antifungal agents could provide both additional (possibly first line) options and additional clinical data for both reducing heterogeneity and improving treatment of MDR C. auris candidemia in daily practice.
Author contributions
Conceptualization, DG, MM, AM, and MB. writing—original draft preparation, DG, LM, VDP, MM, and AV. writing—review and editing, DG, LM, VDP, MM, AV, AM, and MB. supervision, DG, MM, AM, and MB. All authors contributed to the article and approved the submitted version.
Conflict of interest
Outside the submitted work, MB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from BioMérieux, Cidara, Gilead, Menarini, MSD, Pfizer, and Shionogi. Outside the submitted work, DG reports investigator-initiated grants from Pfizer, Shionogi, and Gilead Italia, and speaker and/or advisor fees from Pfizer and Tillotts Pharma. Outside the submitted work, AM reports investigator-initiated grant from Gilead Italia. Outside the submitted work, VDP reports research grant from Seegene Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alatoom A., Sartawi M., Lawlor K., AbdelWareth L., Thomsen J., Nusair A., et al. (2018). Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the united Arab Emirates. Int. J. Infect. Dis. 70, 36–37. doi: 10.1016/j.ijid.2018.02.005
Allaw F., Haddad S. F., Habib N., Moukarzel P., Naji N. S., Kanafani Z. A., et al. (2022). COVID-19 and C. auris: A case-control study from a tertiary care center in Lebanon. Microorganisms 10 (5), 1011. doi: 10.3390/microorganisms10051011
Al-Obaid I., Asadzadeh M., Ahmad S., Alobaid K., Alfouzan W., Bafna R., et al. (2022). Fatal breakthrough candidemia in an immunocompromised patient in Kuwait due to Candida auris exhibiting reduced susceptibility to echinocandins and carrying a novel mutation in hotspot-1 of FKS1. J. Fungi (Basel) 8 (3), 267. doi: 10.3390/jof8030267
Armstrong P. A., Rivera S. M., Escandon P., Caceres D. H., Chow N., Stuckey M. J., et al. (2019). Hospital-associated multicenter outbreak of emerging fungus Candida auris, Colombia 2016. Emerg. Infect. Dis. 25 (7), 1339-1346. doi: 10.3201/eid2507.180491
Ayala-Gaytan J. J., Montoya A. M., Martinez-Resendez M. F., Guajardo-Lara C. E., de J.T.-R.R., Salazar-Cavazos L., et al. (2021). First case of Candida auris isolated from the bloodstream of a Mexican patient with serious gastrointestinal complications from severe endometriosis. Infection 49 (3), 523–525. doi: 10.1007/s15010-020-01525-1
Bajpai V., Govindaswamy A., Sagar S., Kumar S., Garg P., Xess I., et al. (2020). Multidrug-resistant Candida auris fungemia in critical care units: Experience from a tertiary care hospital in India. Microb. Drug Resist. 26 (2), 145–149. doi: 10.1089/mdr.2019.0021
Barantsevich N. E., Vetokhina A. V., Ayushinova N. I., Orlova O. E., Barantsevich E. P. (2020). Candida auris bloodstream infections in Russia. Antibiotics (Basel) 9 (9), 557. doi: 10.3390/antibiotics9090557
Ben-Ami R., Berman J., Novikov A., Bash E., Shachor-Meyouhas Y., Zakin S., et al. (2017). Multidrug-resistant candida haemulonii and C. auris, tel Aviv, Israel. Emerg. Infect. Dis. 23 (1), 195-203. doi: 10.3201/eid2302.161486
Berrio I., Caceres D. H., Coronell R. W., Salcedo S., Mora L., Marin A., et al. (2021). Bloodstream infections with Candida auris among children in Colombia: Clinical characteristics and outcomes of 34 cases. J. Pediatr. Infect. Dis. Soc. 10 (2), 151–154. doi: 10.1093/jpids/piaa038
Biagi M. J., Wiederhold N. P., Gibas C., Wickes B. L., Lozano V., Bleasdale S. C., et al. (2019). Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect. Dis. 6 (7), ofz262. doi: 10.1093/ofid/ofz262
Bidaud A. L., Botterel F., Chowdhary A., Dannaoui E. (2019). In vitro antifungal combination of flucytosine with amphotericin b, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob. Agents Chemother. doi: 10.1128/AAC.01393-19
Borman A. M., Fraser M., Johnson E. M. (2021). CHROMagarTM candida plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol 59 (3), 253–258. doi: 10.1093/mmy/myaa049
Briano F., Magnasco L., Sepulcri C., Dettori S., Dentone C., Mikulska M., et al. (2022). Candida auris candidemia in critically ill, colonized patients: Cumulative incidence and risk factors. Infect. Dis. Ther. 11 (3), 1149–1160. doi: 10.1007/s40121-022-00625-9
Caballero U., Kim S., Eraso E., Quindos G., Vozmediano V., Schmidt S., et al. (2021). In vitro synergistic interactions of isavuconazole and echinocandins against Candida auris. Antibiotics (Basel) 10 (4), 355. doi: 10.3390/antibiotics10040355
Calvo B., Melo A. S., Perozo-Mena A., Hernandez M., Francisco E. C., Hagen F., et al. (2016). First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 73 (4), 369–374. doi: 10.1016/j.jinf.2016.07.008
CDC (2019) Algorithm to identify Candida auris based on phenotypic laboratory method and initial species identification. Available at: https://www.cdc.gov/fungal/candida-auris/pdf/Testing-algorithm_by-Method_508.pdf (Accessed 7 Jan 2023).
CDC (2020) Cnadida auris. antifungal susceptibility testing and interpretation. centers for disease control and prevention (CDC). Available at: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (Accessed 28 September 2022).
CDC (2022) Identification of Candida auris. Available at: https://www.cdc.gov/fungal/candida-auris/identification.html (Accessed 7 Jan 2023).
Chandramati J., Sadanandan L., Kumar A., Ponthenkandath S. (2020). Neonatal Candida auris infection: Management and prevention strategies - a single centre experience. J. Paediatr. Child Health 56 (10), 1565–1569. doi: 10.1111/jpc.15019
Chen Y., Zhao J., Han L., Qi L., Fan W., Liu J., et al. (2018). Emergency of fungemia cases caused by fluconazole-resistant Candida auris in Beijing, China. J. Infect. 77 (6), 561–571. doi: 10.1016/j.jinf.2018.09.002
Choi H. I., An J., Hwang J. J., Moon S. Y., Son J. S. (2017). Otomastoiditis caused by Candida auris: Case report and literature review. Mycoses 60 (8), 488–492. doi: 10.1111/myc.12617
Chowdhary A., Tarai B., Singh A., Sharma A. (2020). Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg. Infect. Dis. 26 (11), 2694–2696. doi: 10.3201/eid2611.203504
Desoubeaux G., Coste A. T., Imbert C., Hennequin C. (2022). Overview about Candida auris: What's up 12 years after its first description? J. Mycol Med. 32 (2), 101248. doi: 10.1016/j.mycmed.2022.101248
Di Pilato V., Codda G., Ball L., Giacobbe D. R., Willison E., Mikulska M., et al. (2021). Molecular epidemiological investigation of a nosocomial cluster of C. auris: Evidence of recent emergence in Italy and ease of transmission during the COVID-19 pandemic. J. Fungi (Basel) 7 (2), 140. doi: 10.3390/jof7020140
Dumkow L. E., Worden L. J., Rao S. N. (2021). Syndromic diagnostic testing: a new way to approach patient care in the treatment of infectious diseases. J. Antimicrob. Chemother. 76 (Suppl 3), iii4–iii11. doi: 10.1093/jac/dkab245
ECDC (2018). Candida auris in healthcare settings – Europe – first update, 23 April 2018. Stockholm: European centre for disease prevention and control (ECDC). (Stockholm: European Centre for Disease Prevention and Control)
Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J. F., et al. (2017). In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob. Agents Chemother. 61 (11). doi: 10.1128/AAC.01056-17
Garcia-Bustos V., Cabanero-Navalon M. D., Ruiz-Sauri A., Ruiz-Gaitan A. C., Salavert M., Tormo M. A., et al. (2021). What do we know about Candida auris? State of the art, knowledge gaps, and future directions. Microorganisms 9 (10), 2177. doi: 10.3390/microorganisms9102177
Garcia-Bustos V., Salavert M., Ruiz-Gaitan A. C., Cabanero-Navalon M. D., Sigona-Giangreco I. A., Peman J. (2020). A clinical predictive model of candidaemia by Candida auris in previously colonized critically ill patients. Clin. Microbiol. Infect. 26 (11), 1507–1513. doi: 10.1016/j.cmi.2020.02.001
Ghannoum M., Long L., Larkin E. L., Isham N., Sherif R., Borroto-Esoda K., et al. (2018). Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrob. Agents Chemother. 62 (6). doi: 10.1128/AAC.00244-18
Giacobbe D. R., Magnasco L., Sepulcri C., Mikulska M., Koehler P., Cornely O. A., et al. (2021). Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expert Rev. Clin. Pharmacol. 14 (10), 1205–1220. doi: 10.1080/17512433.2021.1949285
Giacobbe D. R., Maraolo A. E., Simeon V., Magne F., Pace M. C., Gentile I., et al. (2020). Changes in the relative prevalence of candidaemia due to non-albicans candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses 63 (4), 334–342. doi: 10.1111/myc.13054
Hager C. L., Larkin E. L., Long L. A., Ghannoum M. A. (2018b). Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J. Antimicrob. Chemother. 73 (8), 2085–2088. doi: 10.1093/jac/dky153
Hager C. L., Larkin E. L., Long L., Zohra Abidi F., Shaw K. J., Ghannoum M. A. (2018a). In vitro and In vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob. Agents Chemother. 62 (3). doi: 10.1128/AAC.02319-17
Hanson B. M., Dinh A. Q., Tran T. T., Arenas S., Pronty D., Gershengorn H. B., et al. (2021). Candida auris invasive infections during a COVID-19 case surge. Antimicrob. Agents Chemother. 65 (10), e0114621. doi: 10.1128/AAC.01146-21
Heath C. H., Dyer J. R., Pang S., Coombs G. W., Gardam D. J. (2019). Candida auris sternal osteomyelitis in a man from Kenya visiting Australia 2015. Emerg. Infect. Dis. 25 (1), 192–194. doi: 10.3201/eid2501.181321
Hoenigl M., Sprute R., Egger M., Arastehfar A., Cornely O. A., Krause R., et al. (2021). The antifungal pipeline: Fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81 (15), 1703–1729. doi: 10.1007/s40265-021-01611-0
Jacobs S. E., Jacobs J. L., Dennis E. K., Taimur S., Rana M., Patel D., et al. (2022). Candida auris pan-Drug-Resistant to four classes of antifungal agents. Antimicrob. Agents Chemother. 66 (7), e0005322. doi: 10.1128/aac.00053-22
Jacobs S. E., Zagaliotis P., Walsh T. J. (2021). Novel antifungal agents in clinical trials. F1000Res 10, 507. doi: 10.12688/f1000research.28327.2
Jaggavarapu S., Burd E. M., Weiss D. S. (2020). Micafungin and amphotericin b synergy against Candida auris. Lancet Microbe 1 (8), e314–e315. doi: 10.1016/S2666-5247(20)30194-4
Jeffery-Smith A., Taori S. K., Schelenz S., Jeffery K., Johnson E. M., Borman A., et al. (2018). Candida auris: A review of the literature. Clin. Microbiol. Rev. 31 (1). doi: 10.1128/CMR.00029-17
Jimenez-Ortigosa C., Perez W. B., Angulo D., Borroto-Esoda K., Perlin D. S. (2017). De novo acquisition of resistance to SCY-078 in candida glabrata involves FKS mutations that both overlap and are distinct from those conferring echinocandin resistance. Antimicrob. Agents Chemother. 61 (9). doi: 10.1128/AAC.00833-17
Juneja D., Ross C., Breedt J., Juneja D., Ross C., Breedt J., et al. (2021). “Outcomes of oral ibrexafungerp in the treatment of ten patients with Candida auris infections, from the CARES study,” in 31st ECCMID; 2021, Vienna.
Keighley C., Garnham K., Harch S. A. J., Robertson M., Chaw K., Teng J. C., et al. (2021). Candida auris: Diagnostic challenges and emerging opportunities for the clinical microbiology laboratory. Curr. Fungal Infect. Rep. 15 (3), 116–126. doi: 10.1007/s12281-021-00420-y
Khan Z., Ahmad S., Benwan K., Purohit P., Al-Obaid I., Bafna R., et al. (2018). Invasive Candida auris infections in Kuwait hospitals: epidemiology, antifungal treatment and outcome. Infection 46 (5), 641–650. doi: 10.1007/s15010-018-1164-y
Khatamzas E., Madder H., Jeffery K. (2019). Neurosurgical device-associated infections due to Candida auris - three cases from a single tertiary center. J. Infect. 78 (5), 409–421. doi: 10.1016/j.jinf.2019.02.004
Kordalewska M., Perlin D. S. (2022). Deciphering Candida auris paradoxical growth effect (Eagle effect) in response to echinocandins. Methods Mol. Biol. 2517, 73–85. doi: 10.1007/978-1-0716-2417-3_6
Kullberg B. J., K. B., Pappas P. J., Kullberg B. J., Boffard K., Pappas P. J., et al. (2021). “Clinical efficacy and safety of the novel antifungal fosmanogepix in patients with candidaemia and/or invasive candidiasis caused by Candida auris: Results from a phase II proof of concept trial,” in 31st ECCMID; 2021, Vienna.
Lee W. G., Shin J. H., Uh Y., Kang M. G., Kim S. H., Park K. H., et al. (2011). First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 49 (9), 3139–3142. doi: 10.1128/JCM.00319-11
Lepak A. J., Zhao M., Andes D. R. (2018). Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob. Agents Chemother. 62 (11). doi: 10.1128/AAC.01572-18
Lockhart S. R., Etienne K. A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N. P., et al. (2017). Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 64 (2), 134–140. doi: 10.1093/cid/ciw691
Lockhart S. R., Lyman M. M., Sexton D. J. (2022). Tools for detecting a "Superbug": Updates on Candida auris testing. J. Clin. Microbiol. 60 (5), e0080821. doi: 10.1128/jcm.00808-21
Mirhendi H., Charsizadeh A., Aboutalebian S., Mohammadpour M., Nikmanesh B., de Groot T., et al. (2022). South Asian (Clade I) Candida auris meningitis in a paediatric patient in Iran with a review of the literature. Mycoses 65 (2), 134–139. doi: 10.1111/myc.13396
Miyazaki M., Horii T., Hata K., Watanabe N. A., Nakamoto K., Tanaka K., et al. (2011). In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55 (10), 4652–4658. doi: 10.1128/AAC.00291-11
Mohsin J., Hagen F., Al-Balushi Z. A. M., de Hoog G. S., Chowdhary A., Meis J. F., et al. (2017). The first cases of Candida auris candidaemia in Oman. Mycoses 60 (9), –575. doi: 10.1111/myc.12647
Mohsin J., Weerakoon S., Ahmed S., Puts Y., Al Balushi Z., Meis J. F., et al. (2020). A cluster of Candida auris blood stream infections in a tertiary care hospital in Oman from 2016 to 2019. Antibiotics (Basel) 9 (10). doi: 10.3390/antibiotics9100638
Moin S., Farooqi J., Rattani S., Nasir N., Zaka S., Jabeen K. (2021). C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol 59 (12), 1238–1242. doi: 10.1093/mmy/myab057
Mulet-Bayona J. V., Salvador-Garcia C., Tormo-Palop N., Gimeno-Cardona C. (2022). Recurrent candidemia and isolation of echinocandin-resistant Candida auris in a patient with a long-term central catheter. Enferm Infecc Microbiol. Clin. (Engl Ed) 40 (6), 334–335. doi: 10.1016/j.eimce.2022.03.011
Mulet Bayona J. V., Tormo Palop N., Salvador Garcia C., Herrero Rodriguez P., Abril Lopez de Medrano V., Ferrer Gomez C., et al. (2020). Characteristics and management of candidaemia episodes in an established Candida auris outbreak. Antibiotics (Basel) 9 (9), 558. doi: 10.3390/antibiotics9090558
Ninan M. M., Sahni R. D., Chacko B., Balaji V., Michael J. S. (2020). Candida auris: Clinical profile, diagnostic challenge and susceptibility pattern: Experience from a tertiary-care centre in south India. J. Glob Antimicrob. Resist. 21, 181–185. doi: 10.1016/j.jgar.2019.10.018
O'Brien B., Chaturvedi S., Chaturvedi V. (2020). In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from new York outbreak. Antimicrob. Agents Chemother. 64 (4). doi: 10.1128/AAC.02195-19
Pandya N., Cag Y., Pandak N., Pekok A. U., Poojary A., Ayoade F., et al. (2021). International multicentre study of Candida auris infections. J. Fungi (Basel) 7 (10), 878. doi: 10.3390/jof7100878
Pappas P. G., Kullberg B. J., Vazquez J. A., Pappas P. G., Kullberg B. J., Vazquez J. A., et al. (2020). “Clinical safety and efficacy of novel antifungal, fosmanogepix, in the treatment of candidemia: Results from a phase 2 proof of concept trial. IDWeek 2020,” in Programs and abstracts of the 55th annual infectious diseases society of America (IDSA) meeting; October 2020(Philadelphia (PA).
Pappas P. G., Lionakis M. S., Arendrup M. C., Ostrosky-Zeichner L., Kullberg B. J. (2018). Invasive candidiasis. Nat. Rev. Dis. Primers 4, 18026. doi: 10.1038/nrdp.2018.26
Park J. Y., Bradley N., Brooks S., Burney S., Wassner C. (2019). Management of patients with Candida auris fungemia at community hospital, brooklyn, new York, USA 2016-2018(1). Emerg. Infect. Dis. 25 (3), 601–602. doi: 10.3201/eid2503.180927
Prayag P. S., Patwardhan S., Panchakshari S., Rajhans P. A., Prayag A. (2022). The dominance of Candida auris: A single-center experience of 79 episodes of candidemia from Western India. Indian J. Crit. Care Med. 26 (5), 560–563. doi: 10.5005/jp-journals-10071-24152
Rauseo A. M., Coler-Reilly A., Larson L., Spec A. (2020). Hope on the horizon: Novel fungal treatments in development. Open Forum Infect. Dis. 7 (2), ofaa016. doi: 10.1093/ofid/ofaa016
Reque J., Arlandis R., Panizo N., Pascual M. J., Perez-Alba A. (2022). Candida auris invasive infection after kidney transplantation. Case Rep. Nephrol. 2022, 6007607. doi: 10.1155/2022/6007607
Roberts S. C., Zembower T. R., Bolon M. K., Kadakia A. R., Gilley J. H., Ko J. H., et al. (2019). Successful treatment of a Candida auris intra-articular infection. Emerg. Microbes Infect. 8 (1), 866–868. doi: 10.1080/22221751.2019.1625287
Ruiz-Gaitan A., Moret A. M., Tasias-Pitarch M., Aleixandre-Lopez A. I., Martinez-Morel H., Calabuig E., et al. (2018). An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 61 (7), 498–505. doi: 10.1111/myc.12781
Rybak J. M., Barker K. S., Munoz J. F., Parker J. E., Ahmad S., Mokaddas E., et al. (2022). In vivo emergence of high-level resistance during treatment reveals the first identified mechanism of amphotericin b resistance in Candida auris. Clin. Microbiol. Infect. 28 (6), 838–843. doi: 10.1016/j.cmi.2021.11.024
Salah H., Sundararaju S., Dalil L., Salameh S., Al-Wali W., Tang P., et al. (2021). Genomic epidemiology of Candida auris in Qatar reveals hospital transmission dynamics and a south Asian origin. J. Fungi (Basel) 7 (3), 240. doi: 10.3390/jof7030240
Sandison T., Ong V., Lee J., Thye D. (2017). Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob. Agents Chemother. 61 (2). doi: 10.1128/AAC.01627-16
Sanyaolu A., Okorie C., Marinkovic A., Abbasi A. F., Prakash S., Mangat J., et al. (2022). Candida auris: An overview of the emerging drug-resistant fungal infection. Infect. Chemother. 54 (2), 236–246. doi: 10.3947/ic.2022.0008
Sayeed M. A., Farooqi J., Jabeen K., Awan S., Mahmood S. F. (2019). Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan. BMC Infect. Dis. 19 (1), 384. doi: 10.1186/s12879-019-3999-y
Schelenz S., Hagen F., Rhodes J. L., Abdolrasouli A., Chowdhary A., Hall A., et al. (2016). First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 5, 35. doi: 10.1186/s13756-016-0132-5
SCYNEXIS (2021) SCYNEXIS announces successful completion of phase 1 trial evaluating intravenous (IV) formulation of ibrexafungerp: SCYNEXIS, Inc. (SCYX). Available at: https://www.scynexis.com/news-media/press-releases/detail/260/scynexis-announces-successful-completion-of-phase-1-trial.
Shastri P. S., Shankarnarayan S. A., Oberoi J., Rudramurthy S. M., Wattal C., Chakrabarti A. (2020). Candida auris candidaemia in an intensive care unit - prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 57, 42–48. doi: 10.1016/j.jcrc.2020.01.004
Shaukat A., Al Ansari N., Al Wali W., Karic E., El Madhoun I., Mitwally H., et al. (2021). Experience of treating Candida auris cases at a general hospital in the state of Qatar. IDCases 23, e01007. doi: 10.1016/j.idcr.2020.e01007
Shaw K. J., Ibrahim A. S. (2020). Fosmanogepix: A review of the first-in-Class broad spectrum agent for the treatment of invasive fungal infections. J. Fungi (Basel) 6 (4), 239. doi: 10.3390/jof6040239
Shenoy V., Ballenberger M., Prince A., Maslak S. (2019). Panophthalmitis from Candida auris. Ann. Intern. Med. 171 (12), 941–943. doi: 10.7326/L19-0323
Simon S. P., Li R., Silver M., Andrade J., Tharian B., Fu L., et al. (2022). Comparative outcomes of Candida auris bloodstream infections: A multicenter retrospective case-control study. Clin. Infect. Dis. ciac735. doi: 10.1093/cid/ciac735
Spec A., Pullman J., Thompson G. R., Powderly W. G., Tobin E. H., Vazquez J., et al. (2019). MSG-10: a phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J. Antimicrob. Chemother. 74 (10), 3056–3062. doi: 10.1093/jac/dkz277
Supreeth S., Al Ghafri K. A., Jayachandra R. K., Al Balushi Z. Y. (2020). First report of Candida auris spondylodiscitis in Oman: A rare presentation. World Neurosurg. 135, 335–338. doi: 10.1016/j.wneu.2019.09.021
Taori S. K., Khonyongwa K., Hayden I., Athukorala G. D. A., Letters A., Fife A., et al. (2019). Candida auris outbreak: Mortality, interventions and cost of sustaining control. J. Infect. 79 (6), 601–611. doi: 10.1016/j.jinf.2019.09.007
Thompson G. R., Soriano A., Cornely O. A., Thompson G. R., Soriano A., Cornely O. A., et al. (2022) ReSTORE: Efficacy and safety results of the phase 3, noninferiority trial of rezafungin in the treatment of candidemia and/or invasive candidiasis (IC) cidara poster #L0244. Available at: https://www.cidara.com/wp-content/uploads/2022/04/ReSTORE-Paper-Poster_FINAL.pdf (Accessed 30 September 2022).
Thompson G. R., Soriano A., Skoutelis A., Vazquez J. A., Honore P. M., Horcajada J. P., et al. (2021). Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: The STRIVE trial. Clin. Infect. Dis. 73 (11), e3647–e3655. doi: 10.1093/cid/ciaa1380
Tsai Y. T., Lu P. L., Tang H. J., Huang C. H., Hung W. C., Tseng Y. T., et al. (2022). The first invasive Candida auris infection in Taiwan. Emerg. Microbes Infect. 11 (1), 1867–1875. doi: 10.1080/22221751.2022.2100280
Vinayagamoorthy K., Pentapati K. C., Prakash H. (2022). Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in coronavirus disease (COVID-19) patients: A systematic review. Mycoses 65 (6), 613–624. doi: 10.1111/myc.13447
Wiederhold N. P., Najvar L. K., Olivo M., Morris K. N., Patterson H. P., Catano G., et al. (2021). Ibrexafungerp demonstrates In vitro activity against fluconazole-resistant Candida auris and In vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob. Agents Chemother. 65 (6). doi: 10.1128/AAC.02694-20
Keywords: Candida auris, candidemia, diagnosis, treatment, MDR
Citation: Giacobbe DR, Mikulska M, Vena A, Di Pilato V, Magnasco L, Marchese A and Bassetti M (2023) Challenges in the diagnosis and treatment of candidemia due to multidrug-resistant Candida auris. Front. Fungal Biol. 4:1061150. doi: 10.3389/ffunb.2023.1061150
Received: 04 October 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
João Nobrega De Almeida Júnior, Clinical Hospital of University of São Paulo, BrazilReviewed by:
Vijeta Bajpai, Tata Memorial Hospital, IndiaElizabeth L. Berkow, Centers for Disease Control and Prevention (CDC), United States
Libera Maria Dalla Costa, Pelé Pequeno Príncipe Research Institute, Brazil
Copyright © 2023 Giacobbe, Mikulska, Vena, Di Pilato, Magnasco, Marchese and Bassetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Roberto Giacobbe, ZGFuaWVsZXJvYmVydG8uZ2lhY29iYmVAdW5pZ2UuaXQ=
 Daniele Roberto Giacobbe
Daniele Roberto Giacobbe Malgorzata Mikulska1,2
Malgorzata Mikulska1,2 Antonio Vena
Antonio Vena Vincenzo Di Pilato
Vincenzo Di Pilato Matteo Bassetti
Matteo Bassetti