
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Fungal Biol. , 16 April 2021
Sec. Fungal Genomics and Evolution
Volume 2 - 2021 | https://doi.org/10.3389/ffunb.2021.656751
 Hiroki Takahashi1,2,3†
Hiroki Takahashi1,2,3† Maiko Umemura4†
Maiko Umemura4† Akihiro Ninomiya5
Akihiro Ninomiya5 Yoko Kusuya1
Yoko Kusuya1 Masaaki Shimizu6
Masaaki Shimizu6 Syun-ichi Urayama5,7
Syun-ichi Urayama5,7 Akira Watanabe1†
Akira Watanabe1† Katsuhiko Kamei1
Katsuhiko Kamei1 Takashi Yaguchi1
Takashi Yaguchi1 Daisuke Hagiwara1,5,7*†
Daisuke Hagiwara1,5,7*†Filamentous fungi produce various bioactive compounds that are biosynthesized by sets of proteins encoded in biosynthesis gene clusters (BGCs). For an unknown reason, many BGCs are transcriptionally silent in laboratory conditions, which has hampered the discovery of novel fungal compounds. The transcriptional reactiveness of fungal secondary metabolism is not fully understood. To gain the comprehensive view, we conducted comparative genomic and transcriptomic analyses of nine closely-related species of Aspergillus section Fumigati (A. fumigatus, A. fumigatiaffinis, A. novofumigatus, A. thermomutatus, A. viridinutans, A. pseudoviridinutans, A. lentulus, A. udagawae, and Neosartorya fischeri). For expanding our knowledge, we newly sequenced genomes of A. viridinutans and A. pseudoviridinutans, and reassembled and reannotated the previously released genomes of A. lentulus and A. udagawae. Between 34 and 84 secondary metabolite (SM) backbone genes were identified in the genomes of these nine respective species, with 8.7–51.2% being unique to the species. A total of 247 SM backbone gene types were identified in the nine fungi. Ten BGCs are shared by all nine species. Transcriptomic analysis using A. fumigatus, A. lentulus, A. udagawae, A. viridinutans, and N. fischeri was conducted to compare expression levels of all SM backbone genes in four different culture conditions; 32–83% of SM backbone genes in these species were not expressed in the tested conditions, which reconfirmed that large part of fungal SM genes are hard to be expressed. The species-unique SM genes of the five species were expressed with lower frequency (18.8% in total) than the SM genes that are conserved in all five species (56%). These results suggest that the expression tendency of BGCs is correlated with their interspecies distribution pattern. Our findings increase understanding of the evolutionary processes associated with the regulation of fungal secondary metabolism.
Filamentous fungi produce various small molecules known as secondary metabolites (SMs; also called natural products) that are thought to contribute to their survival in environmental niches (Keller, 2015; Künzler, 2018). Fungal SMs are biosynthesized by enzyme sets, which include backbone-producing and tailoring enzymes. The backbone-producing enzymes are represented by non-ribosomal peptide synthetases (NRPSs) and polyketide synthases (PKSs), whereas terpene cyclases and dimethylallyl tryptophan synthases are also involved (Keller, 2019). Genes encoding backbone-producing and tailoring enzymes, transcriptional regulators, and efflux pumps are often arrayed in a biosynthesis gene cluster (BGC). Fungi, including phytopathogens and human pathogens, possess large numbers of SM-producing gene clusters in their genomes, which indicates an ability to produce myriad metabolites that could be used to impact humans (Sanchez et al., 2012; Hansen et al., 2015; Nielsen et al., 2017; Vesth et al., 2018; Kjærbølling et al., 2020).
Fungal SM gene clusters are, in general, transcriptionally silent in tested lab conditions, which makes it difficult for us to comprehensively explore fungal SMs and to understand the ecological roles of the SMs (Brakhage and Schroeckh, 2011). For example, genomic study revealed that >30 genes encoding enzymes to produce SM backbones were found in Aspergillus fumigatus, A. niger, and A. oryzae, 74.2–91.4% of which were not expressed or were expressed at a very low level in any of the cell types (hyphae, resting conidia, or germinating conidia; Hagiwara et al., 2016). One explanation for the low expression of SM-related genes in laboratory-controlled conditions is that unknown ecological cues trigger fungal SM production but cannot be reproduced in the laboratory. Many researchers have attempted to artificially activate such silent SM genes by coculturing multiple microorganisms (Netzker et al., 2018), adding inhibitors for the histone deacetylases that regulate epigenetic status (Pfannenstiel and Keller, 2019), or treatment with plant hormones (Morishita et al., 2019). The molecular mechanisms underlying artificial activation of SM-related genes remain to be investigated.
Recent progress in DNA sequencing technology has advanced our understanding of fungal SM-related gene distribution across species. Comparative genomics has shown that SM-related genes (BGCs) are species-unique or narrowly taxonomically distributed. For example, among four representatives of Aspergillus fungi—A. fumigatus, A. nidulans, A. niger, and A. oryzae—no BGCs are shared by all species, and 91.6–96.1% of the BGCs are species-unique (Lind et al., 2015). This is in sharp contrast to primary metabolic genes, where only 7.5–15.4% of the genes are species-unique. However, when focusing on more closely related species that belong to same section, a taxonomic group below genus but above species, several BGCs are shared in common among species of Aspergillus section Nigri or Aspergillus section Flavi (Vesth et al., 2018; Kjærbølling et al., 2020). These reports provide an evolutionary insight into how secondary metabolic pathways evolve and degenerate in filamentous fungi.
A. fumigatus is a life-threatening pathogenic fungus of humans and is a representative member of Aspergillus section Fumigati (Rokas et al., 2020). This group also includes nonpathogenic fungi, such as Neosartorya fischeri, which has been well-studied by comparison with A. fumigatus in terms of genome structure, pathogenicity, drug resistance, toxin production, and SMs (Fedorova et al., 2008; Mead et al., 2019; Knowles et al., 2020, Steenwyk et al., 2020). Only a few extrolites of A. fumigatus and N. fischeri are overlapped even though 30.3% of A. fumigatus SM-related genes are also found in N. fischeri (Mead et al., 2019), which suggests that transcriptional or/and translational regulation of the SM genes are different in each species. Similarly, A. novofumigatus, which also belongs to Aspergillus section Fumigati, was reported to share 70.5% of A. fumigatus SM-related genes (Kjærbølling et al., 2018), and few metabolites were reported to be shared with A. fumigatus.
In the present study, we compared the SM-related genes among closely-related fungal species of Aspergillus section Fumigati to determine how many SM genes are shared or unique among this group of species. Transcriptome analysis was then performed for five species to gain an overview of how many of the SM-related genes are transcriptionally silent, and insight into the relationships between the tendency for expression and the interspecies distribution of SM genes. The expression profiles of the common BGCs were diverse among the species, whereas species-unique SM-related genes were frequently not expressed in the tested culture conditions.
To compare more genomes of species in Aspergillus section Fumigati, A. viridinutans IFM 47045 and A. pseudoviridinutans IFM 55266 were newly sequenced using the Illumina short read system. The sequenced reads were assembled into 47 and 24 scaffolds, with total length 34.88 and 33.33 Mb, respectively (Table 1). The numbers of predicted proteins were 10,039 and 11,281, and the quality of the genome assembly was confirmed (presence of BUSCO genes: 99.7 and 99.6%), respectively. Our group has reported genome sequences of A. lentulus IFM 54703 and A. udagawae IFM 46973 (Kusuya et al., 2015, 2016). To improve the genome datasets, we here reassembled the sequence of A. udagawae IFM 46973 using ALLPATHS-LG (ver. R52488) and reannotated the genes of A. lentulus IFM 54703 and A. udagawae IFM 46973 (the updated gene IDs are designated Alt_000001-T1 and Aud_000001-T1, respectively). As a result, the sequence assembly was much improved; the genomes of both species were composed of 17 scaffolds. The four abovementioned genome datasets were deposited and updated in the NCBI (https://www.ncbi.nlm.nih.gov/).
In addition to these genomes, genome information for A. fumigatus Af293 (Nierman et al., 2005), N. fischeri NRRL 181 (Fedorova et al., 2008), A. fumigatiaffinis CNM-CM6805 (Dos Santos et al., 2020), A. novofumigatus IBT 16806 (Kjærbølling et al., 2018), and A. thermomutatus HMR AF 39 (Parent-Michaud et al., 2019) was available at the NCBI and was retrieved for this study. Consequently, a phylogenetic tree was constructed using conserved genes in these nine closely-related species in Aspergillus section Fumigati. N. fischeri was the closest relative to A. fumigatus (Figure 1A).
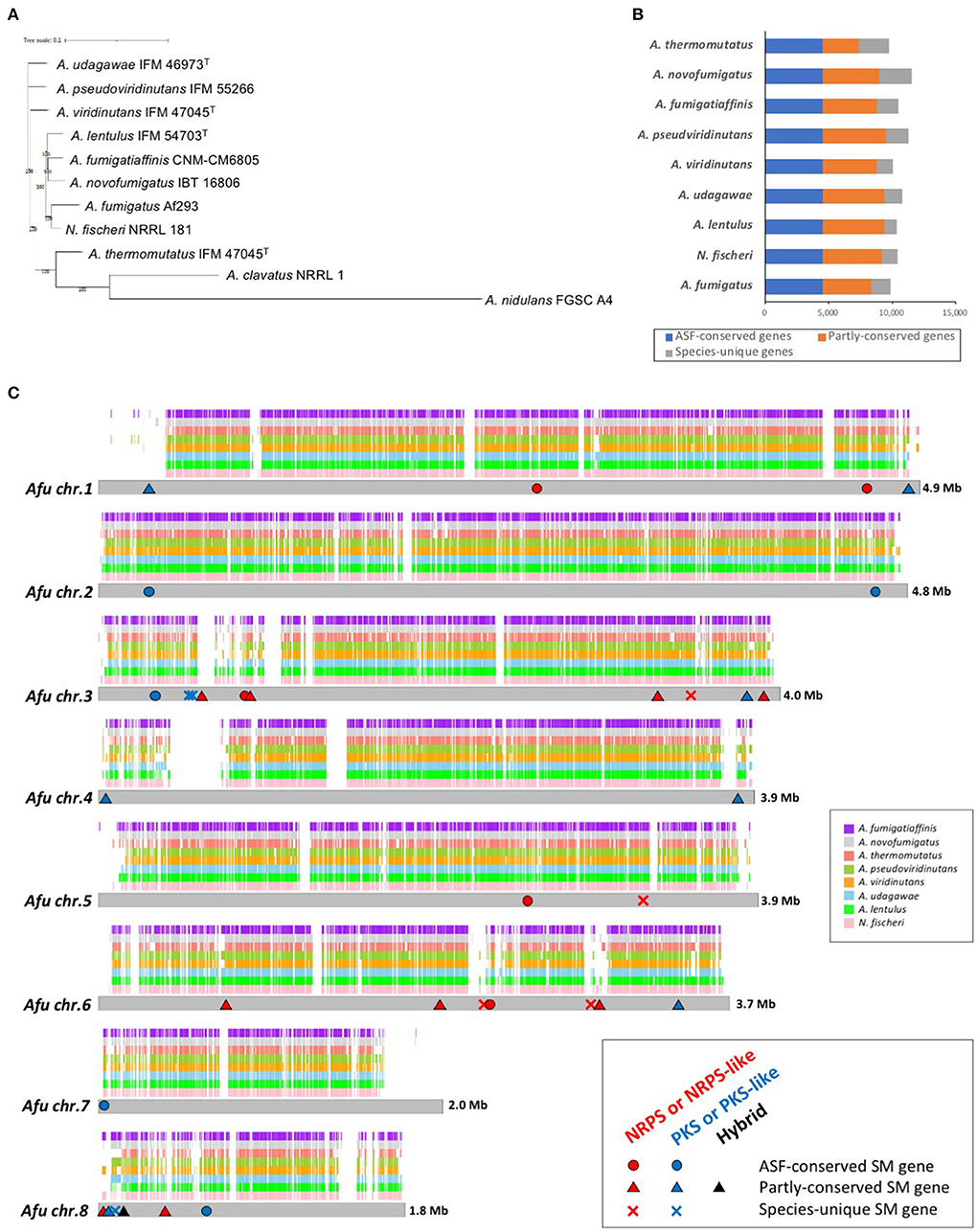
Figure 1. Genome characteristics of Aspergillus section Fumigati strains. (A) Phylogenetic tree of the nine species of Aspergillus section Fumigati used here in addition to A. clavatus and A. nidulans as the outgroup. The tree was constructed using iTOL. (B) The numbers of genes that are conserved across the section (“ASF-conserved genes”), partly conserved, or species-unique are shown. (C) Whole-genome synteny plot based on A. fumigatus chromosomes. The syntenic genes are mapped to A. fumigatus chromosomes. The positions of the ASF-conserved, partly-conserved, and species-unique SM backbone genes of A. fumigatus are indicated with circles, triangles, and crosses, respectively. Genes encoding non-ribosomal peptide synthetase (NRPS) or NRPS-like proteins are colored red, polyketide synthase (PKS) or PKS-like proteins in blue, and PKS-NRPS hybrid proteins in black.
The numbers of predicted proteins in the fungi ranged from 9,702 to 11,534 and are summarized in Table 1. By reciprocal best hit (RBH) search with strict criteria (>80% identity and >80% of length covered), 4,493 genes were determined to be orthologs conserved in the nine Aspergillus section Fumigati species (referred to hereafter as “ASF-conserved genes”), which represented the core-genome (Figure 1B). Among the nine species, there were 948–2,618 species-unique genes (i.e., having no orthologs in other species) (Table 1; Figure 1B).
Synteny analysis of the genomes revealed that most of the A. fumigatus genome is covered by the other species, but that there are several regions unique to A. fumigatus (Figure 1C). The number of A. fumigatus genes that reside in the syntenic region to each other species was 6,874 (A. thermomutatus) to 8,266 (N. fischeri) (69.9–84.0% of A. fumigatus genes). This further suggested that N. fischeri is the closest relative to A. fumigatus among the Aspergillus section Fumigati species.
SM backbone genes encoding PKSs, NRPSs, and PKS-NRPS hybrids were identified from the genome data using antiSMASH software with manual refinement. In total, 34–84 such genes were identified in the nine species (Table 2; Supplementary Table 1). Orthologous SM-related proteins that showed identities of >80% in >80% of the protein were identified. Twenty-seven of the 34 SM backbone genes in A. fumigatus have orthologs in other species, and seven genes are unique to A. fumigatus (Figure 2A). The other species share 17–22 of the SM backbone genes with A. fumigatus. N. fischeri had the most SM genes in common with A. fumigatus (Figure 2A). Notably, 10 genes were shared among all nine studied species of Aspergillus section Fumigati (referred to hereafter as “ASF-conserved SM genes”) (Figure 2B). Meanwhile, there were 18 and 57 genes that were conserved in 5–8 species and 2–4 species, respectively. The species-unique SM backbone genes were identified in each species. A. lentulus has 4 (the fewest), and A. pseudoviridinutans has 41 species-unique SM backbone genes (the most). In total, 510 SM backbone genes were identified and grouped into 247 orthologous types (Figure 2B). A cladogram was generated based on a binary matrix (presence/absence of the SM backbone genes), which revealed that N. fischeri is the most closely related species to A. fumigatus based on the SM backbone protein distribution across species (Figure 2C).

Table 2. The numbers of SM backbone genes predicted in the genome of strains of Aspergillus section Fumigati.
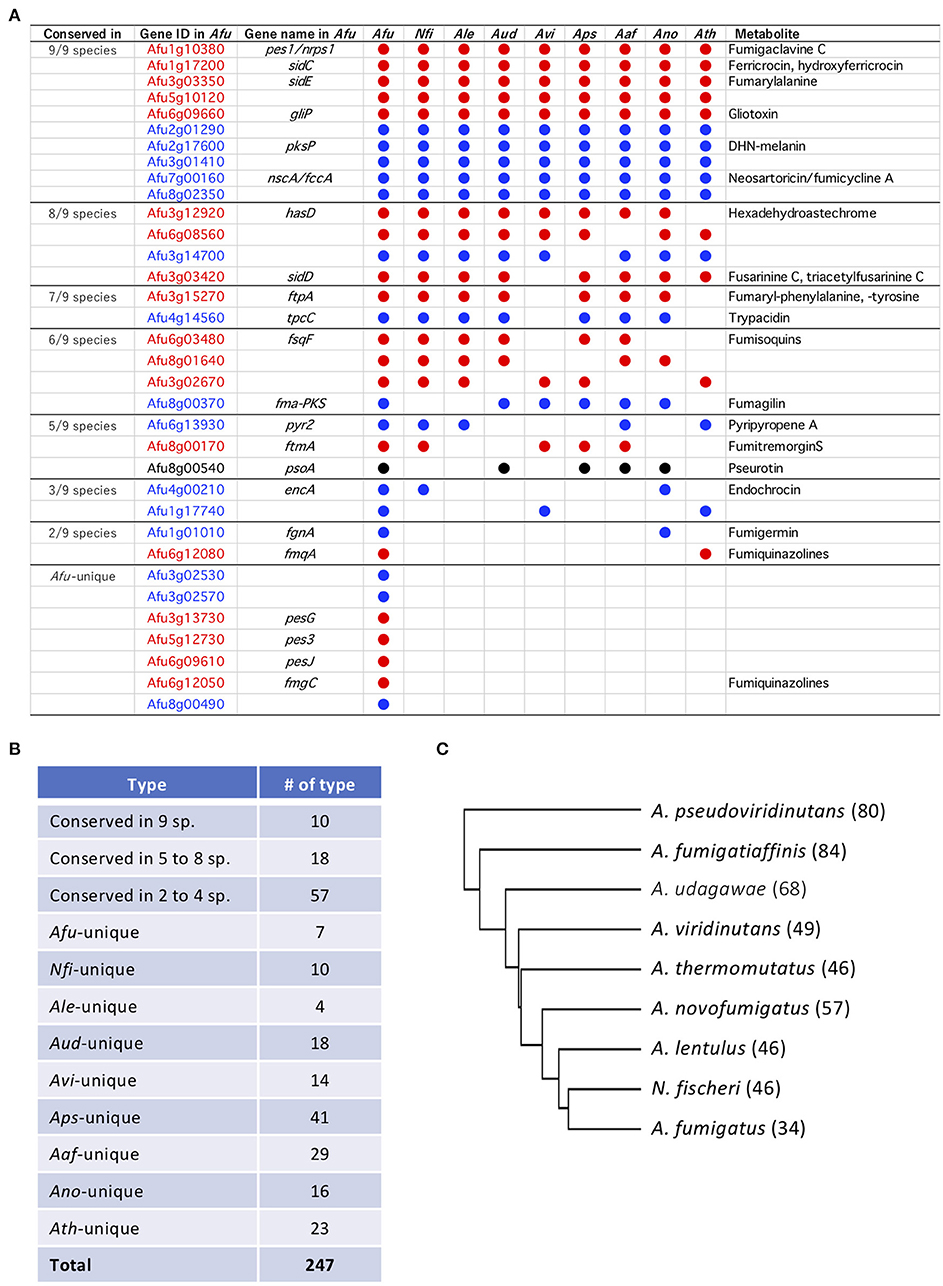
Figure 2. Secondary metabolite (SM) backbone genes conserved across Aspergillus section Fumigati. (A) Summary of A. fumigatus SM backbone genes. Some are conserved in other Fumigati species. Afu, A. fumigatus; Nfi, Neosartorya fischeri; Ale, A. lentulus; Aud, A. udagawae; Avi, A. viridinutans; Aps, A. pseudoviridinutans; Aaf, A. fumigatiaffinis; Ano, A. novofumigatus; Ath, A. thermomutatus. (B) Summary of the numbers of SM gene types. (C) A cladogram was constructed using a binary matrix (presence/absence of the PKSs and NRPSs) with Cluster 3.0. The tree was constructed and drawn using Tree View. The numbers of SM backbone genes are shown in parentheses behind the species names.
The 10 ASF-conserved SM backbone genes include five NRPSs and five PKSs, among which six were previously characterized in A. fumigatus as being involved in the biosynthesis of fumigaclavine C (O'Hanlon et al., 2012), ferricrocin (Schrettl et al., 2007), fumarylalanine (Steinchen et al., 2013), gliotoxin (Cramer et al., 2006), 1,8-dihydroxynaphthalene (DHN)-melanin (Langfelder et al., 1998), and neosartoricin/fumicyclines (Chooi et al., 2013; König et al., 2013) (Figure 2A). The BGCs containing the ASF-conserved SM backbone genes (hereafter designated BGC1 to BGC10) were compared among the nine Aspergillus section Fumigati species (Supplementary Table 2). BGC1 and BGC3 were almost perfectly conserved across the species in terms of gene composition, gene order, and the similarity of the encoded proteins (Supplementary Figure 1). Although there were a few examples of gene loss or low protein similarity, BGC2, BGC4, BGC5, BGC8, and BGC9 were also well-conserved among the species. Meanwhile, BGC6, BGC7, and BGC10 were diverse due to extensive gene loss and low protein similarity in several components. In particular, BGC7 of A. fumigatus was unique because four of the 10 component genes have no orthologs in the other species (Supplementary Figure 1). BGC2, BGC7, BGC8, and BGC9 contain one or two genes encoding a transcription factor (TF), which are conserved in all nine species (Supplementary Table 2; Supplementary Figure 1). Interestingly, most of the TFs are located at the ends of gene clusters.
To gain insights into SM gene expression, transcriptomic analysis was conducted using five representative species of Aspergillus section Fumigati: A. fumigatus, N. fischeri, A. lentulus, A. udagawae, and A. viridinutans. The fungal strains were cultivated in four different media: potato dextrose broth (PDB), Czapek-Dox medium (CD), Sabouraud broth (SB), and potato dextrose agar (PDA). In A. fumigatus, the median numbers of Transcripts Per Kilobase Millions (TPMs) of ASF-conserved genes (n = 4,493) were 44.6, 28.3, 17.5, and 52.9 for culture on PDB, CD, SB, and PDA, respectively, much higher than the values for A. fumigatus-unique genes (3.6, 1.5, 2.2, and 5.0; n = 1,507) (Figure 3A). This was also the case for the other species (Figures 3B–E). These data indicated that a set of genes that was well-conserved among aspergilli was transcriptionally more active than species-unique genes in all the tested species.
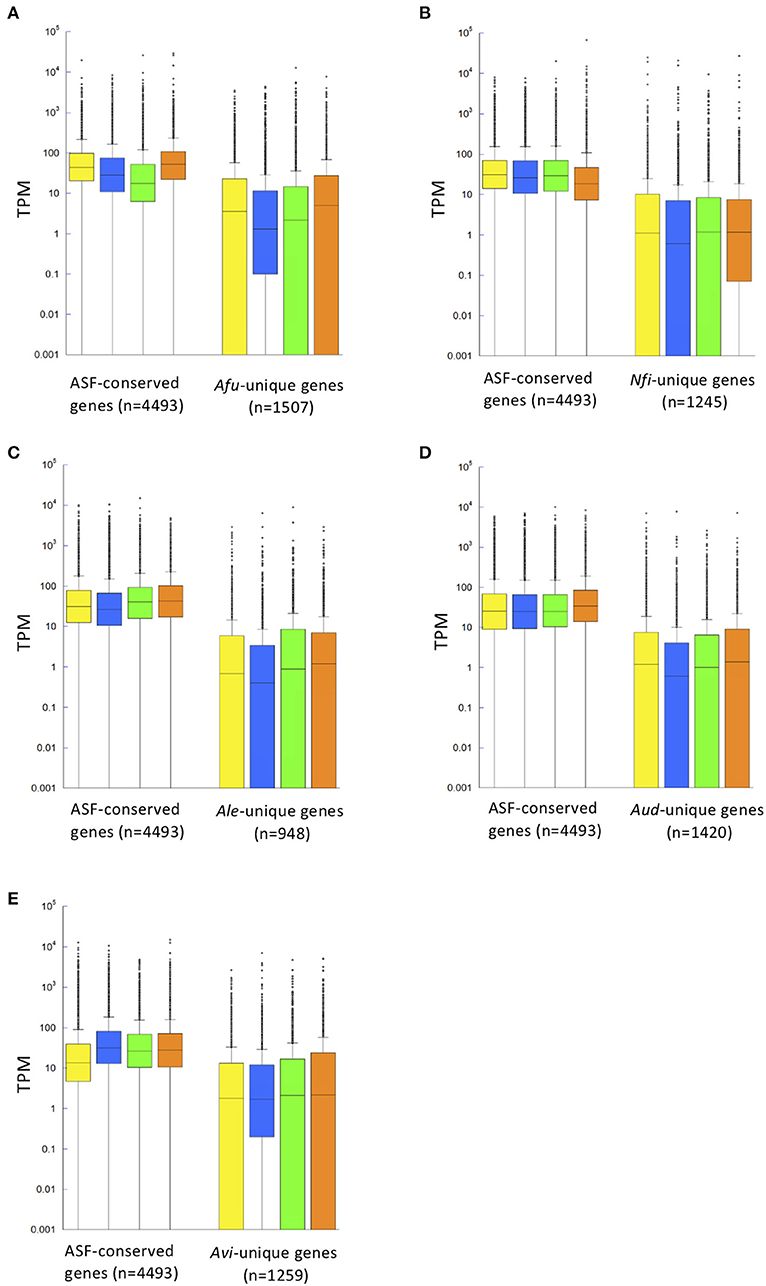
Figure 3. Distribution of gene expression levels shown in a box plot. Gene expression patterns of ASF-conserved genes as the core genome (n = 4,493) and species-unique genes are shown for A. fumigatus (A), N. fischeri (B), A. lentulus (C), A. udagawae (D), and A. viridinutans (E) in four different conditions [potato dextrose broth (PDB), Czapek-Dox medium (CD), Sabouraud broth (SB), and potato dextrose agar (PDA)], indicated by yellow, blue, green, and orange boxes, respectively. Gene expression levels are described in Transcripts Per Kilobase Millions (TPM) obtained in RNA-sequencing analysis. The box plot graph is drawn using PlotsOfData (https://huygens.science.uva.nl/PlotsOfData/) (Postma and Goedhart, 2019).
When a gene with an expression level >1/ of the mean TPM was considered as being expressed, 12.8% of A. fumigatus genes were not expressed in any of the conditions tested (Figure 4A), and were thus considered to be silent genes. Only 3.5% of the ASF-conserved genes were silent in A. fumigatus. In contrast, 37.7% of species-unique genes were silent. This tendency was also observed in the other species (Figure 4A). With regard to SM backbone genes, 32.3% of the genes were silent in A. fumigatus, and the proportion of silent SM backbone genes was 71.7, 76.0, 83.8, and 55.1% in N. fischeri, A. lentulus, A. udagawae, and A. viridinutans, respectively (Figure 4A). These data highlight that the proportion of SM backbone genes that were expressed was quite low in species of Aspergillus section Fumigati.
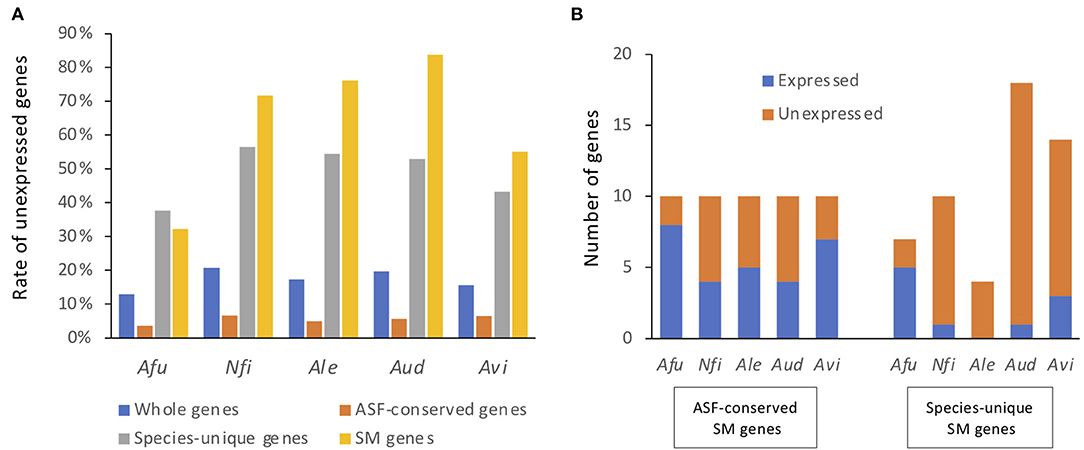
Figure 4. Proportions and numbers of unexpressed SM backbone genes. Genes with TPMs > 5% of the mean TPM in either condition were regarded as expressed genes, whereas the remainder were regarded as unexpressed genes. (A) The proportion of genes that were not expressed in any culture condition are shown for all genes, ASF-conserved genes, species-unique genes, and SM backbone genes. Afu, A. fumigatus; Nfi, N. fischeri; Ale, A. lentulus; Aud, A. udagawae; Avi, A. viridinutans. (B) The numbers of SM backbone genes that were expressed and not expressed in any culture condition.
For ASF-conserved SM genes, the proportion of silent genes ranged from 20 to 60% in the species of Aspergillus section Fumigati (44% in total) (Figure 4B). In contrast, 28.5–100% of the species-unique SM genes were not expressed in any of the culture conditions tested (81.1% in total) (Figure 4B). These results suggest that the ASF-conserved SM genes are transcriptionally more active than the less conserved SM genes. Lists of SM backbone genes with expression values are shown in Supplementary Tables 3–7.
The SM gene clusters that were activated in (a) specific condition(s) were further analyzed. We sought gene clusters whose component genes were coordinately regulated using the MIDDAS-M program (Umemura et al., 2013). Consequently, 14, 13, 12, 12, and 19 sets of genes were found to be expressed as clustered genes in A. fumigatus, N. fischeri, A. lentulus, A. udagawae, and A. viridinutans, respectively (Supplementary Table 8). Of these, 4, 6, 3, 5, and 9 expressed clusters contained SM backbone genes, respectively (Table 3). All these clusters belonged to the ASF-conserved or partly conserved SM genes, and no cluster with species-unique SM genes was found by the MIDDAS-M analysis. Interestingly, most of the expressed clusters have been characterized and are predicted to produce known metabolites. The gliotoxin (gliP) cluster was expressed in A. fumigatus, A. lentulus, and A. viridinutans, and the DHN-melanin (pksP) cluster in A. fumigatus and A. viridinutans. Furthermore, the fusarinine C (sidD), ferricrocin (sidC), hexadehydroastechrome (hasD), and fumitremorgin (ftmA) clusters was expressed in multiple species studied here. In addition to the metabolites whose BGCs are not present in A. fumigatus, the clusters for terrein and viriditoxin were coordinately expressed in A. lentulus and A. viridinutans, respectively.
To gain more insight into variations in the expression patterns of the clusters across the species, expression levels of the component genes in BGCs were depicted using a heat map (Figure 5). This confirmed the result from MIDDAS-M analysis that BGC4 (for pksP) was exclusively expressed in PDA in A. fumigatus and A. viridinutans, and that BGC8 (for gliP) was expressed in A. fumigatus in CD and SB, in A. lentulus in PDA, and in A. udagawae in CD. BGC1 was transcriptionally active in a cluster-wide manner in all conditions in all species.
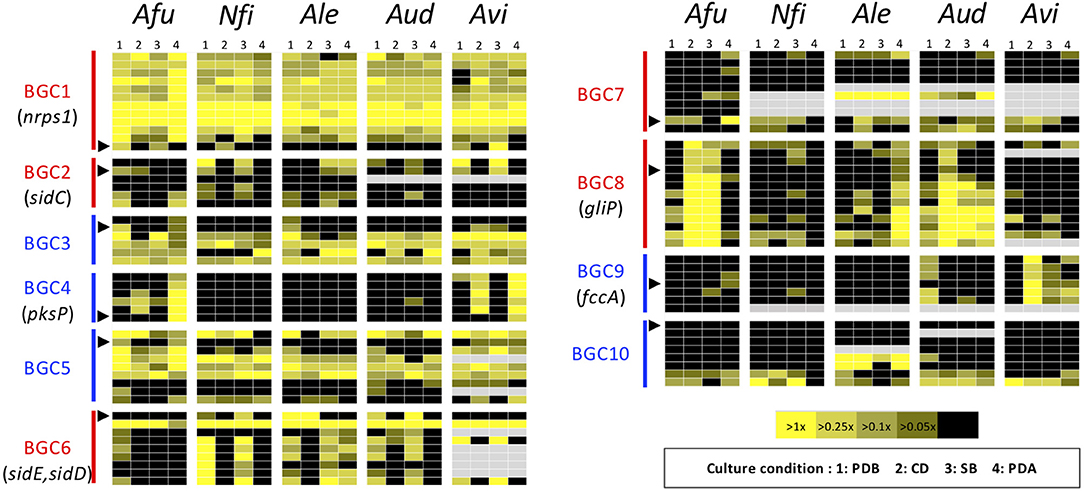
Figure 5. Heat map revealing expression profiles of the genes in ASF-conserved biosynthesis gene clusters. The color of the bar between the biosynthesis gene cluster (BGC) ID and the panels indicates the type of SM backbone gene, as follows: red: NRPS or NRPS-like; blue: PKS or PKS-like. A black triangle indicates the SM backbone gene in the BGC. Gray panels indicate the absence of the corresponding gene. Afu, A. fumigatus; Nfi, N. fischeri; Alu, A. lentulus; Aud, A. udagawae; Avi, A. viridinutans.
The transcriptome data revealed coordinate expression of some BGCs, suggesting productions of some metabolites, which have not been reported in the species. First, we examined whether A. lentulus and A. udagawae are capable of producing gliotoxin in CD. No production of gliotoxin was detectable in these species, although A. fumigatus produced the metabolite (Supplementary Figure 2).
The MIDDAS-M analysis also highlighted that the cluster for terrein, containing two PKSs, Alt_007763-T1 and Alt_007763-T2, was markedly activated in A. lentulus. This cluster is very similar to the ter cluster of A. terreus in terms of gene content, order, and directions (Figure 6A; Supplementary Table 9) (Zaehle et al., 2014). The cluster in A. lentulus was coordinately expressed, with the highest expression level observed in PDB (Figure 6B). Culture extracts were examined by comparison with the standard compound terrein, revealing that A. lentulus produced terrein in PDB and on PDA (Figure 6C).
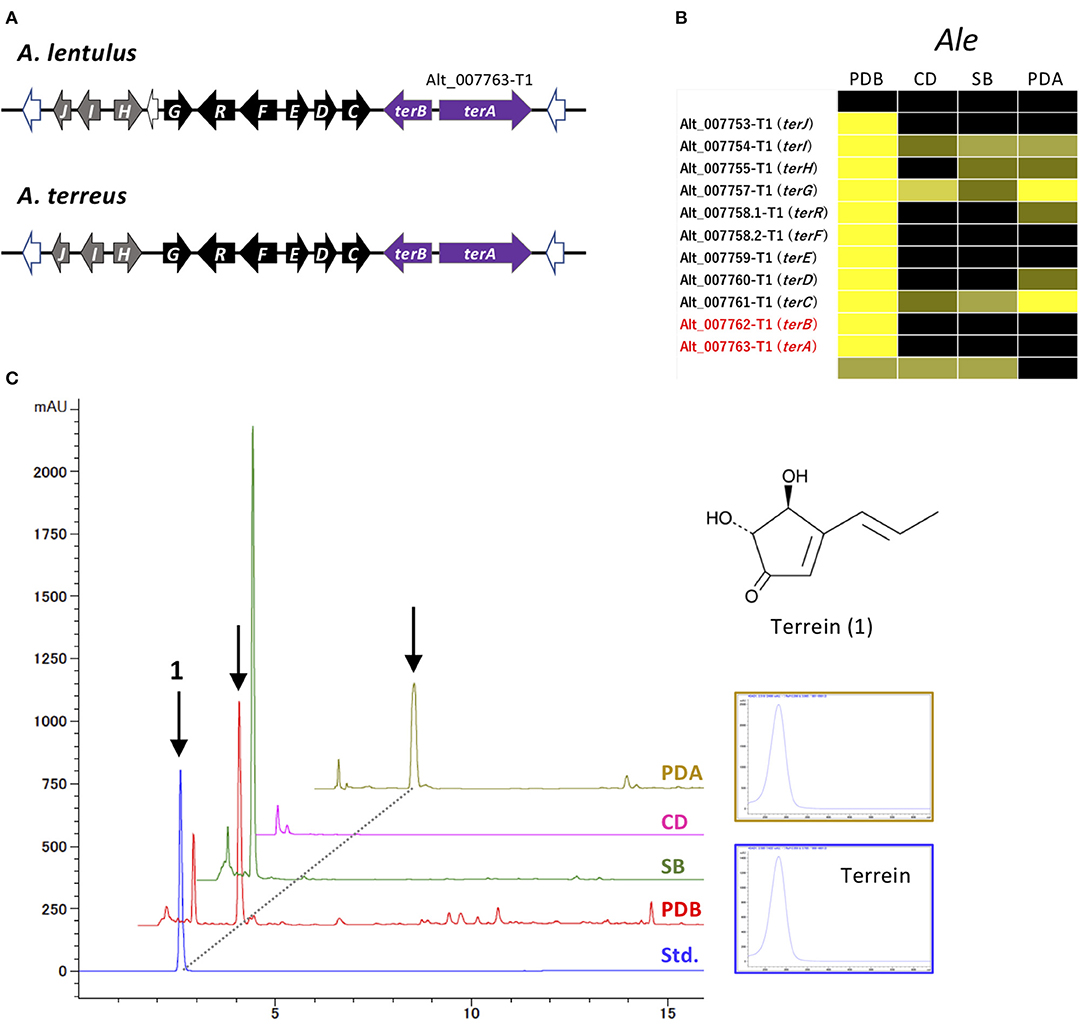
Figure 6. Characterization of the ter cluster and terrein production in A. lentulus. (A) Schematic structure of the ter cluster in A. lentulus and A. terreus. The SM backbone genes (PKSs) are indicated by purple arrows. Genes (terG, terH, terI, and terJ) whose involvement in terrein production remains obscure in A. terreus are indicated by gray arrows. (B) The expression profiles of ter genes in A. lentulus shown using a heat map. (C) The production of terrein in A. lentulus. The strains were cultivated in PDB, SB, CD, and PDA, and ethyl acetate-derived culture extracts were analyzed using high-performance liquid chromatography. Terrein (1) production was identified by comparison with the standard compound (Std.). The corresponding peaks are indicated by arrows, and the UV spectrum from a PDA culture is compared with that of the standard compound.
The vdt cluster for viriditoxin, which contains eight genes, has been reported in A. viridinutans (Urquhart et al., 2019) (Figure 7A). In A. viridinutans, the cluster was highly expressed in a coordinated manner in PDB, SB, and PDA (Figure 7B). On the basis of genome comparison, this cluster is also present in A. udagawae and A. pseudoviridinutans, and it was found that the genetic content of the clusters is well-conserved between these species (Figure 7A). It was of interest that no genes of the vdt cluster were expressed in A. udagawae in any of the studied conditions (Figure 7B). This suggested that the vdt cluster was active in A. viridinutans, but silent in A. udagawae.
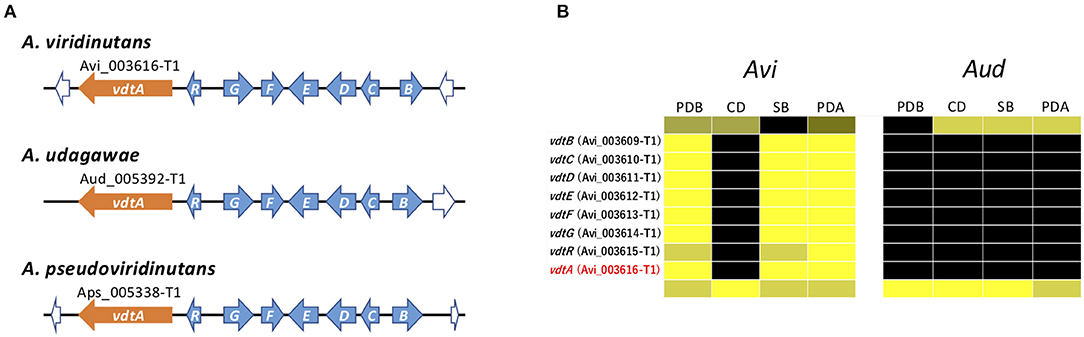
Figure 7. Characterization of vdt cluster. (A) Schematic structure of the vdt cluster from A. viridinutans, A. udagawae, and A. pseudoviridinutans. The SM backbone genes (PKSs) are indicated by orange arrows. The other component genes are indicated by light blue arrows. (B) The expression profiles of vdt genes in A. viridinutans and A. udagawae shown using a heat map.
To gain deeper insight into interspecies variations in the transcriptional regulation of the clusters for gliotoxin, terrein, and viriditoxin biosynthesis, we investigated whether there are cis-elements in the promoter regions of each component gene in each cluster. Each cluster contains a gene encoding a C6-type transcription factor (GliZ, TerR, and VdtR) that may function as a cluster-specific transcriptional regulator (Supplementary Figure 1H, Figures 6A, 7A). Because C6-type transcription factors reportedly bind to inverted CGG triplets spaced by several nucleotides, such as CGG(Nx)CCG (MacPherson et al., 2006), we sought to identify such bipartite motifs conserved in the promoter regions of component genes in the gli, ter, and vdt clusters using BioProspector (Liu et al., 2001). As binding site 5′-TCGG(N3)CCGA-3′ has been reported for the cluster-specific transcription factor GliZ (Schoberle et al., 2014), a palindromic sequence was sought in the gli clusters of A. fumigatus, N. fischeri, A. lentulus, A. udagawae, and A. viridinutans, setting the gap as three bases (Figure 8A). The consensus sequence was found to be highly conserved among the five closely-related Aspergillus section Fumigati species in terms of position in the promoter region of each gene. No consensus sequence was detected for the gliZ gene in any of the five species; this has been reported previously for A. fumigatus (Schoberle et al., 2014).
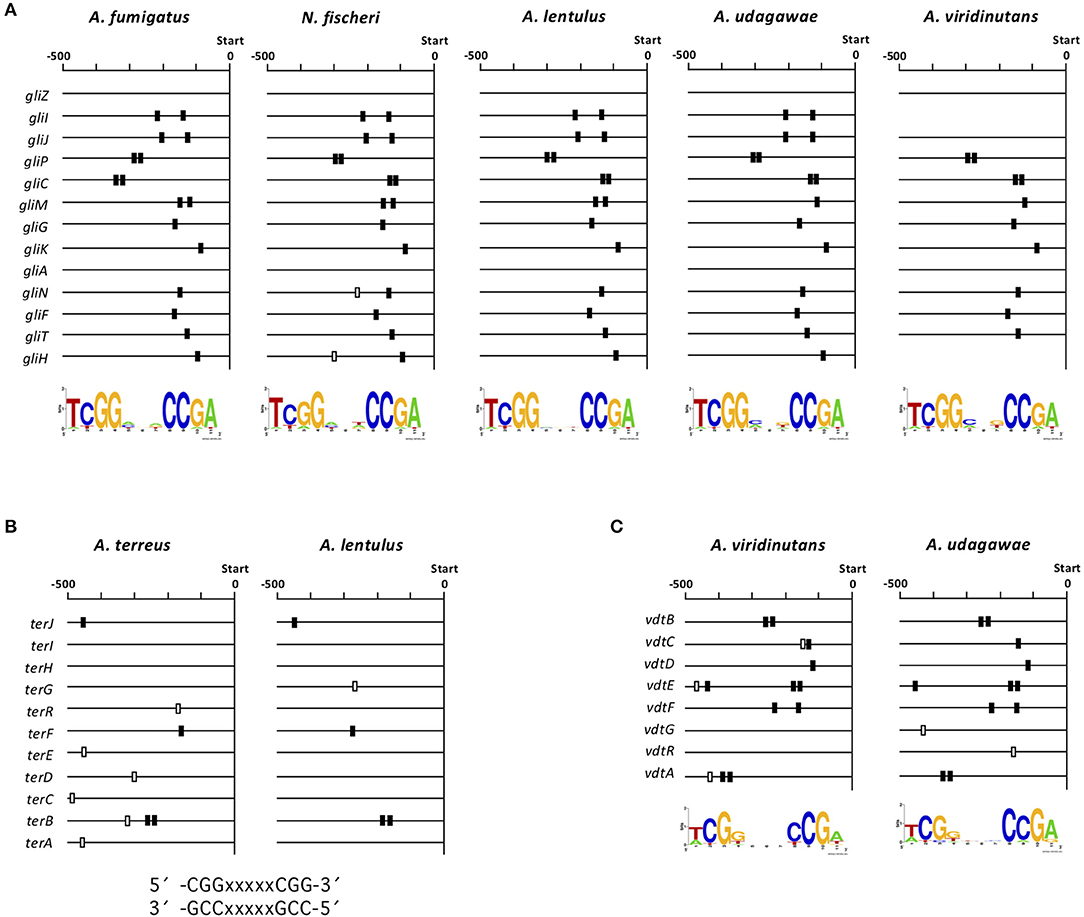
Figure 8. Consensus sequences in the promoter regions of gli, ter, and vdt genes. (A) The consensus sequences were detected in the 13 gli genes in each species using BioProspector (Release 2), and the top hit motifs are shown under the map. The position of the sequence (TCGGNNNCCGA) is indicated by a box. The black boxes indicate consensus sequences that were considered to correspond to those in the other species tested. (B) Consensus sequences were sought in the 11 ter genes in A. lentulus and A. terreus by manual inspection. The positions of the reported consensus sequences (CCGNNNNNCCG and CGGNNNNNCGG) in the cluster is indicated by black or white boxes. (C) Consensus sequences were detected in the eight vdt genes in A. viridinutans and A. udagawae using BioProspector (Release 2), and the top hit motifs are shown under the map. Positions of the sequence (TCGGNNNCCGA) are indicated by black or white boxes. −500, 500-bp upstream from the translational initiation site of each gene.
For the ter cluster of A. terreus, the consensus sequence for binding of TerR, a cluster specific transcription factor, was proposed to be 5′-TCGGHHWYHCGG-3′ (Gressler et al., 2015). Thus, similar sequences (5′-CGGXXXXXCGG-3′ and 5′-CCGXXXXXCCG-3′) were sought in the ter cluster of A. lentulus. Four ter genes contained either of the sequences in the promoter region, and four of the five sequences corresponded to those in A. terreus (Figure 8B). No consensus sequence has been reported for the vdt cluster in any fungal species. Thus, we sought such a sequence using several combinations of parameters. When the gap was set as three bases, the well-conserved sequence 5′-TCGG(N3)CCGA-3′ was found in all component genes of this cluster in both A. viridinutans and A. udagawae (Figure 8C). The positions of the consensus sequence were highly conserved between the species. Collectively, cis-elements for the SM gene clusters with a C6-type transcription factor were well-conserved in an interspecies manner.
Comparative genomics studies regarding fungal SMs have been conducted intensively during the last decade, revealing the genetic diversity, universality, and plasticity of SM gene clusters in fungal genomes (Hansen et al., 2015; Nielsen et al., 2017; Vesth et al., 2018; Kjærbølling et al., 2020). Our genomic study provides a comprehensive catalog of SM backbone genes in A. lentulus, A. udagawae, A. viridinutans, A. pseudoviridinutans, A. fumigatiaffinis, A. novofumigatus, and A. thermomutatus, whereas those of A. fumigatus and N. fischeri have been well-investigated previously. Larsen et al. reported auranthine, cyclopiazonic acid, neosartorin, pyripyropene A, and terrein as major metabolites of A. lentulus (Larsen et al., 2007), which is supported by the presence of the corresponding backbone genes (cpaA for cyclopiazonic acid, nsrB for neosartorin, pyr2 for pyripyropene A, and terAB for terrein) in the genome (Supplementary Table 5). Integrating studies of natural products and genomics will be a good way to further expand our understanding of fungal SM production.
A. fumigatus was estimated to have the largest number of characterized SM gene clusters among fungi even though the number of signature genes predicted by antiSMASH or other research was relatively small (~34 genes) (Sanchez et al., 2012; Mead et al., 2019). However, in comparison, the other species of Aspergillus section Fumigati possess 46–86 SM backbone genes. In the present study, we identified 10 SM backbone genes that are conserved in all the tested species of Aspergillus section Fumigati. Interestingly, the ASF-conserved SM gene clusters included the clusters responsible for the pathogenicity-related metabolites siderophores, DHN-melanin, and gliotoxin. This genomic signature indicated that these species have considerable potential for opportunistic pathogenicity, although some of the species have not been reported as pathogens. Transcriptome analysis of N. fischeri, A. lentulus, A. udagawae, and A. viridinutans as well as A. fumigatus revealed that expression profiles for the pathogenicity-related metabolite clusters were different among the species. For instance, the cluster for DHN-melanin was only activated in A. fumigatus and A. viridinutans on culture on PDA. This is consistent with what is known about spatiotemporal production of DHN-melanin. DHN-melanin is reported to be produced during the conidiation stage and exists on the surface of conidia in A. fumigatus (Chang et al., 2020). It is noteworthy that in the present study A. fumigatus and A. viridinutans produced conidia during culture on PDA, whereas conidiation was not observed for N. fischeri, A. lentulus, or A udagawae. If they were placed in conditions where conidia are produced, DHN-melanin may be biosynthesized and cover the conidial surface. Coordinate induced expression of the gli genes of A. fumigatus, A. lentulus, and A. udagawae was observed in certain conditions (Figure 5; Table 3). However, besides A. fumigatus, none of the species of Aspergillus section Fumigati tested were able to synthesize gliotoxin in CD culture (Supplementary Figure 2). It is possible that medium-dependent post-transcriptional regulation affects the production of fungal metabolites. Notably, N. fischeri was recently reported to produce gliotoxin when grown on CD agar or 5% blood agar plates at 37°C for 4 d (Knowles et al., 2020). This finding also supported the hypothesis that widely conserved BGCs tend to be more active in each species.
One of the important results presented here is that the ASF-conserved SM backbone genes were transcriptionally more active than species-unique genes in species of Aspergillus section Fumigati (Figure 4B). This tendency was also observed among all genes. Only a small fraction (3.5–6.7%) of ASF-conserved genes were silent, whereas the proportion of unexpressed species-unique genes was 37–56% (Figure 4A). Accordingly, the magnitude of expressed species-unique genes was lower than that of ASF-conserved genes (Figure 3). This significant difference can be explained by the presence of genes related to housekeeping machinery and primary metabolism among the ASF-conserved genes, which are in general kept highly activated throughout cell growth. With regard to secondary metabolism, the narrowly distributed SM genes, in particular the species-unique SM genes, tended to be less preferentially expressed. During the course of evolution, transcriptional regulation may be a potential target for the degeneration of secondary metabolism, although further clarification is required.
Transcriptome analysis of five species in four different conditions allowed us to comprehensively explore conditions for activation of SM gene clusters. For example, the backbone genes for DHN-melanin and trypacidin were highly expressed in A. fumigatus when grown on PDA (Supplementary Table 3). In N. fischeri, the NRPS genes for fusarinine C and triacetylfusarinine C were highly expressed in PDB (Supplementary Table 4). In A. lentulus, the backbone genes for fumarylalanine and terrein were highly expressed in PDB (Supplementary Table 5). The genes for fumarylalanine and hexadehydroastechrome were markedly expressed in SB and on PDA, respectively in A. udagawae, whereas genes for fumigaclavine C, neosartoricin/fumicycline A, and viriditoxin showed high expression levels in A. viridinutans in preferential conditions (Supplementary Tables 6, 7). Notably, fumicycline A production is induced in A. fumigatus when it is cocultured with Streptomyces rapamycinicus (König et al., 2013). Given that A. viridinutans produces this metabolite in monoculture, the molecular mechanisms underlying the transcriptional activation are likely to be different between the two species. Viriditoxin production was previously reported in A. viridinutans as well as Paecilomyces variotii (Urquhart et al., 2019). This metabolite shows a variety of biological activities such as potent inhibition of the bacterial cell division-related protein FtsZ (Wang et al., 2003), suggesting a protective role in competition against other microorganisms. Biosynthesis genes for terrein were identified in A. terreus, and terrein was produced in PDB and on PDA (Zaehle et al., 2014). Intriguingly, we showed that A. lentulus also produced large amounts of terrein in these conditions (Figure 6C). The consistency in the conditions required for terrein production suggests that the ter cluster is regulated in a similar manner in A. lentulus and A. terreus. This in turn suggests that the fungal SM gene clusters retain their transcriptional regulatory mechanism in different hosts.
Coordinated expression of the genes in SM BGCs involves a cluster-specific transcription factor or/and epigenetic regulator. Some BGCs contains transcription factors that play a pivotal role in activation of the cluster. The gli, ter, and vdt clusters have been investigated in A. fumigatus, A. terreus, and P. variotii, respectively. The transcription factors (GliZ, TerR, and VdtR) of each cluster were demonstrated to play an essential role in activation of the cluster and metabolite production (Schoberle et al., 2014; Gressler et al., 2015; Urquhart et al., 2019). Comparative studies here showed that the consensus transcription factor binding sequences for the genes in the BGCs are highly conserved among different species. However, different expression patterns of the BGCs were observed in different species, resulting in different metabolite production (e.g., of gliotoxin) (Supplementary Figure 2). One explanation for this inconsistency is that variations in the sequences of the promoter regions excepting the consensus sequences might affect the efficiency of RNA polymerase binding. Alternatively, other regulators might participate in regulating the expression of the clusters, which could lead to changes in the environmental cues required for cluster activation and metabolite production. As a consequence, transcriptional regulation of secondary metabolism might be diversified in the course of adaptation to host-specific environments.
In conclusion, we combined comparative genomic and transcriptomic analyses to study variations in the transcriptional activities of fungal BGCs in closely related species. This research provides a perspective on how the BGC distribution may be correlated with the tendency for transcriptomic silent SM-related genes. On the basis of our findings, diversification of transcriptional regulation might occur in the course of the evolution and degeneration of SM gene clusters. Further efforts to characterize such transcriptional diversity will expand our understanding of the evolutionary processes affecting fungal secondary metabolism.
The strains A. fumigatus Af293, A. lentulus IFM 54703, A. udagawae IFM 46973, A. viridinutans IFM 47045, A. pseudoviridinutans IFM 55266, and N. fischeri NRRL 181 were provided through the National Bio-Resource Project, Japan (http://www.nbrp.jp/) and are preserved at the Medical Mycology Research Center, Chiba University. The genomes of A. lentulus IFM 54703 (Kusuya et al., 2016) and A. udagawae IFM 46973 (Kusuya et al., 2015) were previously sequenced, and the data were retrieved from the NCBI database. A. viridinutans strain IFM 47045 (NRRL 4365) was isolated from rabbit dung in Australia. A. pseudoviridinutans strain IFM 55266 was isolated from a patient in Japan and identified using tubulin and calmodulin partial gene sequences (Lyskova et al., 2018).
Strains were grown in liquid PDB (BD Difco, Franklin Lakes, NJ, USA), SB (BD Difco), or CD (BD Difco) at 37°C for 5 d by inoculating each culture with three 0.5-cm2 agar plugs. For asexual stage culture, mycelia cultured in PDB at 37°C for 3 d were harvested using a Miracloth, washed with distilled water, and then placed onto PDA plates (BD Difco) for another 2 d of culture at 37°C.
The genomic DNAs of A. viridinutans and A. pseudoviridinutans were extracted from a 2-d-old culture using phenol-chloroform and NucleoBond buffer set III (TaKaRa, Shiga, Japan). The DNA was fragmented in an S2 sonicator (Covaris, MA, USA), and then purified using a QIAquick gel extraction kit (Qiagen, CA, USA). A paired-end library was constructed using an NEBNext Ultra DNA Library Prep Kit (New England BioLabs, MA, USA) and NEBNext Multiplex Oligos (New England BioLabs) in accordance with the manufacturer's instructions. Mate-paired libraries with insert sizes of 3.5–4.5, 5–7, and 8–11 kb were generated using the gel selection-based protocol of the Nextera Mate Pair Kit (Illumina, San Diego, CA, USA) and a 0.6% agarose gel, in accordance with the manufacturer's instructions. The quality of the libraries was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Paired-end sequencing (100 bp) was performed by HiSeq 1500 (Illumina) using HiSeq reagent kit v1, in accordance with the manufacturer's instructions; 150-bp paired-end sequencing on a HiSeq X system (Illumina) was carried out by GENEWIZ (South Plainfield, NJ, USA).
Adapters and low-quality bases from Illumina reads were trimmed by Trim Galore (ver. 0.6.4) (Krueger, 2015) with default settings (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Mitochondrial genomes were assembled using GetOrganelle (ver. 1.6.4) (Jin et al., 2020) from the trimmed reads. To filter the mitochondrial reads, the trimmed reads were aligned against mitochondrial genomes by BWA (ver. 0.7.17-r1188) (Li and Durbin, 2009), and the mapped reads were filtered by SAMtools (ver. 1.9) (Li et al., 2009) and SeqKit (Shen et al., 2016). The assembly of nuclear genomes of A. udagawae, A. viridinutans, and A. pseudoviridinutans was carried out by ALLPATHS-LG (ver. R52488) (Gnerre et al., 2011).
The annotation of assembled genomes of A. lentulus, A. udagawae, A. viridinutans, and A. pseudoviridinutans was performed by Funannotate pipeline (ver. 1.7.4) (https://funannotate.readthedocs.io/en/latest/). Following identification of repeat sequences by RepeatModeler (ver. 1.0.11) (http://www.repeatmasker.org/RepeatModeler.html) and RepeatMasker (ver. 4.0.7) (https://www.repeatmasker.org), Funannotate ab initio prediction was performed with the option “–busco_seed_species=aspergillus_fumigatus” by Augustus (ver. 3.3.3) (Stanke et al., 2006), GeneMark-ES (ver. 4.38) (Ter-Hovhannisyan et al., 2008), GlimmerHMM (ver. 3.0.4) (Majoros et al., 2004), and SNAP (ver. 2006-07-28) (Ian, 2004) using exon hints from the proteins of A. fumigatus Af293 and N. fischeri NRRL 181 downloaded from the Aspergillus Genome Database (Cerqueira et al., 2014). Functional annotation of predicted genes was performed by using the SwissPROT (Bairoch and Apweiler, 2000), InterPro (ver. 5.42-78.0) (Jones et al., 2014; Mitchell et al., 2019), eggNOG (ver. 4.5.1) (Buchfink et al., 2015; Huerta-Cepas et al., 2016), MEROPS (ver. 12.0) (Rawlings et al., 2018), and dbCAN (ver. 8.0) (Yin et al., 2012) databases. SM gene clusters were predicted by antiSMASH (ver. 4.2.0) (Blin et al., 2019). The completeness of draft genomes and predicted proteins was evaluated by BUSCO (ver. 4.0.6) (Seppey et al., 2019) with the database eurotiales_odb10. Genome synteny analysis between A. fumigatus and other species was conducted per Kjærbølling et al. (2020). Most tools were obtained through Bioconda (Grüning et al., 2018).
A phylogenetic tree of A. fumigatus and related species was constructed using orthologs common in 11 species including A. clavatus NRRL 1 (Fedorova et al., 2008) and A. nidulans FGSC A4 (Galagan et al., 2005) as the outgroup. Orthologous genes among the 11 species were identified by OrthoFinder (ver. 2.3.12) (Emms and Kelly, 2019). Then, 4,465 single copy ortholog genes were aligned by using MAFFT (ver. 7.471) (Katoh and Standley, 2013), and well-aligned regions were extracted using Gblocks (ver. 0.91b) (Talavera and Castresana, 2007) with the options “-t=p, -b4=5, -b5=h.” A total of 4,465 aligned sequences of each strain were concentrated into one long protein sequence. A phylogenetic tree was constructed using multithreaded RAxML (ver. 8.2.12) (Stamatakis, 2014), the PROTGAMMAWAG model, and 100 bootstrap replicates, and visualized by iTOL (Letunic and Bork, 2019).
Identification of SM genes was performed with antiSMASH (Blin et al., 2019). NRPS, PKS, and PKS-NRPS hybrid genes were extracted from the results. The orthologous relationships were determined by RBH with the criteria of BLASTp (ver. 2.7.1+) coverage >80% and identity >80% (Camacho et al., 2009). A cladogram was constructed based on a binary matrix (presence/absence of the PKSs and NRPSs) using Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm#ctv). A tree and heat map were constructed using Tree View (Saldanha, 2004).
Each strain was cultured, harvested, and cells were ground into a fine powder using a mortar. Total RNA was isolated using Sepazol-RNA Super G (Nacalai, Kyoto, Japan) in accordance with the manufacturer's instructions. The RNA isolation was carried out with two biological replicates, and equivalent quantities of the total RNA were pooled for preparation of the RNA-Seq libraries. The RNA-Seq libraries were constructed using a KAPA mRNA Hyper Prep Kit (Nippon Genetics, Tokyo, Japan), in which mRNA was purified by poly-A selection, second-strand cDNA was synthesized from the mRNA, the cDNA ends were blunted and polyAs added at the 3′- ends, and appropriate indexes were ligated to the ends. The libraries were PCR amplified, and the quantity and quality were assessed using a Bioanalyzer (Agilent Technologies). Each pooled library was sequenced using Illumina HiSeq 1500 apparatus. The gene expression levels were estimated using a previously described method (Takahashi et al., 2017). Briefly, the sequencing reads were mapped to the reference genomes using STAR (ver. 2.4.2a) (Dobin et al., 2013). A raw read count was conducted using HTSeq (ver. 0.5.3p3) (Anders et al., 2015), and transcript abundances were estimated as TPMs (Conesa et al., 2016). The expressed PKS and NRPS genes were identified using the 5% mean TPM criterion.
Binary logarithms of TPM values generated in the four culture conditions were analyzed using the MIDDAS-M algorithm to detect gene clusters in which certain condition(s) coordinately expressed or repressed genes (Umemura et al., 2013). Briefly, the induction ratio of each gene was evaluated for every pairwise combination of the four culture conditions. After Z-score normalization, the gene cluster expression scores were calculated for each gene using the algorithm. The maximum cluster size was set as 30. The threshold to detect clusters was set to the value corresponding to a false positive rate of 0, which was evaluated from data in which the original gene order was randomly shuffled. The threshold values were 2.1E5, 2.3E5, 2.4E5, 1.3E5, and 3.5E5 in A. fumigatus, A. lentulus, A. udagawae, A. viridinutans, and N. fischeri, respectively.
For gliotoxin detection, 5 mL of culture supernatant was extracted with an equivalent volume of ethyl acetate. The organic layer was concentrated in vacuo, and the precipitate was dissolved in 2 mL of dimethylsulfoxide (DMSO). The DMSO solution (5 μL) was subjected to HPLC analysis, which was performed using an Infinity1260 modular system (Agilent Technologies, Santa Clara, CA) consisting of an autosampler, high-pressure pumps, a column oven, and a photodiode-array detector with InfinityLab Poroshell 120 EC-C18 column (particle size: 2.7 μM; length: 100 mm; internal diameter: 3.0 mm; Agilent Technologies). Running conditions were gradient elution, 5–40% acetonitrile in water over 18 min; flow rate, 0.8 mL min−1; and detection at 254 nm. Gliotoxin production was identified by comparing the retention times and the UV spectra of natural products with those of a gliotoxin standard that was purchased from Sigma-Aldrich (St. Louis, MO).
For terrein detection, 5 μL of culture supernatant was subjected to HPLC analysis, which was performed using the same HPLC system as described above. Running conditions were gradient elution, 5–100% acetonitrile in water over 30 min; flow rate, 0.8 mL min−1; and detection at 254 nm. Terrein production was identified in the same manner as gliotoxin production. The authentic standard was purchased from Cayman Chemical Company (Ann Arbor, MI).
Sequence 500 bp upstream of each gene encoded in gli and vdt clusters was retrieved as the promoter sequence. Two-block motifs were sought by BioProspector (Release 2) (Liu et al., 2001) with the options “-d 1 -W 4 -w 4 -g 3 -G 3.” The sequence logos were depicted by WebLogo (Crooks et al., 2004).
The whole-genome sequences of A. viridinutans IFM 47045 and A. pseudoviridinutans IFM 55266 have been deposited at DDBJ/EMBL/GenBank under accession numbers BOPL01000001-47 and BHVY01000001-24, respectively. Re-assembled sequence data of A. lentulus IFM 54703 and A. udagawae IFM 47045 have been also placed under accession numbers BCLY01000001, BCLY01000004-BCLY01000009, BCLY01000011-BCLY01000013, and BCLY01000016-BCLY01000017, and BBXM02000001-17, respectively. The raw RNA-Seq data from this work have been submitted to the DDBJ Short Read Archive under accession number PRJDB7496.
HT, MU, and DH designed the research. HT, MU, AN, MS, YK, S-iU, TY, and DH performed experiments and analyzed data. HT, AW, KK, and TY contributed new materials/tools. HT, MU, AN, TY, and DH wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Bioresource Project (to HT and TY), by AMED under grant numbers JP19fm0208024 (to HT, AW, and DH) and 20jm0110015 (to HT, AW, KK, TY, and DH), and by a grant from the Institute for Fermentation, Osaka (to DH). HT was partly supported by MEXT KAKENHI (16H06279) and the National Bioscience Database Center (NBDC) of the Japan Science and Technology Agency (JST).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Atsushi Iwama, Dr. Motohiko Ohshima, and Dr. Atsunori Saraya (Chiba University) for technical support with the Illumina HiSeq 1500, and Dr. Teigo Asai (The University of Tokyo) for fruitful discussions on potential metabolites in the strains. We thank James Allen, DPhil, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffunb.2021.656751/full#supplementary-material
Supplementary Figure 1. ASF-conserved BGCs of nine species of Aspergillus section Fumigati. The genes predicted to be cluster components are indicated with blue arrows, whereas those indicated with white arrows are outside the cluster. Orange arrows indicate SM backbone genes, and red arrows indicate transcription factors. Black arrows indicate genes that show identity <80% compared with the corresponding gene in A. fumigatus.
Supplementary Figure 2. Production of gliotoxin. The strains were cultivated in CD medium, and the culture extracts obtained using ethyl acetate were analyzed by HPLC. Gliotoxin (1) production was identified by reference to the standard compound (Std.). The corresponding peak from A. fumigatus is indicated by an arrow, and the UV spectrum is shown in the red box. Afu, A. fumigatus; Nfi, N. fischeri; Ale, A. lentulus; Aud, A. udagawae; Avi, A. viridinutans.
Supplementary Table 1. List of SM backbone genes in the nine Aspergillus section Fumigati species.
Supplementary Table 2. The ASF-conserved BGCs.
Supplementary Tables 3–7. The SM backbone genes with expression values (TPM) in A. fumigatus, N. fischeri, A. lentulus, A. udagawae, and A. viridinutans.
Supplementary Table 8. Coordinately expressed gene clusters identified by MIDDAS-M analysis.
Supplementary Table 9. Comparison of ter cluster between A. lentulus and A. terreus.
Anders, S., Pyl, P. T., and Huber, W. (2015). HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. doi: 10.1093/bioinformatics/btu638
Bairoch, A., and Apweiler, R. (2000). The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucl. Acids Res. 28, 45–48. doi: 10.1093/nar/28.1.45
Blin, K., Pascal Andreu, V., de Los Santos, E. L. C., Del Carratore, F., Lee, S. Y., Medema, M. H., et al. (2019). The antiSMASH database version 2: a comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucl. Acids Res. 47, D625–D630. doi: 10.1093/nar/gky1060
Brakhage, A. A., and Schroeckh, V. (2011). Fungal secondary metabolites - strategies to activate silent gene clusters. Fungal Genet. Biol. 48, 15–22. doi: 10.1016/j.fgb.2010.04.004
Buchfink, B., Xie, C., and Huson, D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60. doi: 10.1038/nmeth.3176
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al. (2009). BLAST+: architecture and applications. BMC Bioinformat. 10:421. doi: 10.1186/1471-2105-10-421
Cerqueira, G. C., Arnaud, M. B., Inglis, D. O., Skrzypek, M. S., Binkley, G., Simison, M., et al. (2014). The Aspergillus Genome Database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucl. Acids Res. 42, D705–D710. doi: 10.1093/nar/gkt1029
Chang, P. K., Cary, J. W., and Lebar, M. D. (2020). Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotechnol. 104, 2277–2286. doi: 10.1007/s00253-020-10347-y
Chooi, Y. H., Fang, J., Liu, H., Filler, S. G., Wang, P., and Tang, Y. (2013). Genome mining of a prenylated and immunosuppressive polyketide from pathogenic fungi. Org. Lett. 15, 780–783. doi: 10.1021/ol303435y
Conesa, A., Madrigal, P., Tarazona, S., Gomez-Cabrero, D., Cervera, A., McPherson, A., et al. (2016). A survey of best practices for RNA-seq data analysis. Genome Biol. 17:13. doi: 10.1186/s13059-016-1047-4
Cramer, R. A., Gamcsik, M. P., Brooking, R. M., Najvar, L. K., Kirkpatrick, W. R., Patterson, T. F., et al. (2006). Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5, 972–980. doi: 10.1128/EC.00049-06
Crooks, G. E., Hon, G., Chandonia, J. M., and Brenner, S. E. (2004). WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190. doi: 10.1101/gr.849004
Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., et al. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. doi: 10.1093/bioinformatics/bts635
Dos Santos, R. A. C., Steenwyk, J. L., Rivero-Menendez, O., Mead, M. E., Silva, L. P., Bastos, R. W., et al. (2020). Genomic and phenotypic heterogeneity of clinical isolates of the human pathogens. Front. Genet. 11:459. doi: 10.3389/fgene.2020.00459
Emms, D. M., and Kelly, S. (2019). OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20:238. doi: 10.1186/s13059-019-1832-y
Fedorova, N. D., Khaldi, N., Joardar, V. S., Maiti, R., Amedeo, P., Anderson, M. J., et al. (2008). Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. doi: 10.1371/journal.pgen.1000046
Galagan, J. E., Calvo, S. E., Cuomo, C., Ma, L. J., Wortman, J. R., Batzoglou, S., et al. (2005). Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438, 1105–1115. doi: 10.1038/nature04341
Gnerre, S., Maccallum, I., Przybylski, D., Ribeiro, F. J., Burton, J. N., Walker, B. J., et al. (2011). High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. U. S. A. 108, 1513–1518. doi: 10.1073/pnas.1017351108
Gressler, M., Hortschansky, P., Geib, E., and Brock, M. (2015). A new high-performance heterologous fungal expression system based on regulatory elements from the Aspergillus terreus terrein gene cluster. Front. Microbiol. 6:184. doi: 10.3389/fmicb.2015.00184
Grüning, B., Dale, R., Sjödin, A., Chapman, B. A., Rowe, J., Tomkins-Tinch, C. H., et al. (2018). Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat. Methods 15, 475–476. doi: 10.1038/s41592-018-0046-7
Hagiwara, D., Takahashi, H., Kusuya, Y., Kawamoto, S., Kamei, K., and Gonoi, T. (2016). Comparative transcriptome analysis revealing dormant conidia and germination associated genes in Aspergillus species: an essential role for AtfA in conidial dormancy. BMC Genomics 17:358. doi: 10.1186/s12864-016-2689-z
Hansen, F. T., Gardiner, D. M., Lysøe, E., Fuertes, P. R., Tudzynski, B., Wiemann, P., et al. (2015). An update to polyketide synthase and non-ribosomal synthetase genes and nomenclature in Fusarium. Fungal Genet. Biol. 75, 20–29. doi: 10.1016/j.fgb.2014.12.004
Huerta-Cepas, J., Szklarczyk, D., Forslund, K., Cook, H., Heller, D., Walter, M. C., et al. (2016). eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucl. Acids Res. 44, D286–D293. doi: 10.1093/nar/gkv1248
Jin, J. J., Yu, W. B., Yang, J. B., Song, Y., dePamphilis, C. W., Yi, T. S., et al. (2020). GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21:241. doi: 10.1186/s13059-020-02154-5
Jones, P., Binns, D., Chang, H. Y., Fraser, M., Li, W., McAnulla, C., et al. (2014). InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. doi: 10.1093/bioinformatics/btu031
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Keller, N. P. (2015). Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 11, 671–677. doi: 10.1038/nchembio.1897
Keller, N. P. (2019). Fungal secondary metabolism: regulation, function and drug discovery. Nat. Rev. Microbiol. 17, 167–180. doi: 10.1038/s41579-018-0121-1
Kjærbølling, I., Vesth, T., Frisvad, J. C., Nybo, J. L., Theobald, S., Kildgaard, S., et al. (2020). A comparative genomics study of 23 Aspergillus species from section Flavi. Nat. Commun. 11:1106. doi: 10.1038/s41467-019-14051-y
Kjærbølling, I., Vesth, T. C., Frisvad, J. C., Nybo, J. L., Theobald, S., Kuo, A., et al. (2018). Linking secondary metabolites to gene clusters through genome sequencing of six diverse Aspergillus species. Proc. Natl. Acad. Sci. U. S. A. 115, E753–E761. doi: 10.1073/pnas.1715954115
Knowles, S. L., Mead, M. E., Silva, L. P., Raja, H. A., Steenwyk, J. L., Goldman, G. H., et al. (2020). Gliotoxin, a known virulence factor in the major human pathogen Aspergillus fumigatus, is also biosynthesized by its nonpathogenic relative. mBio 11, e03361–e03319. doi: 10.1128/mBio.03361-19
König, C. C., Scherlach, K., Schroeckh, V., Horn, F., Nietzsche, S., Brakhage, A. A., et al. (2013). Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. Chembiochem 14, 938–942 doi: 10.1002/cbic.201300070
Krueger, F. (2015). Trim Galore!: A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files. Babraham Inst. Available online at: https://www.bioinformatics.babraham.ac.uk/projects (accessed January 21, 2021).
Künzler, M. (2018). How fungi defend themselves against microbial competitors and animal predators. PLoS Pathog. 14:e1007184. doi: 10.1371/journal.ppat.1007184
Kusuya, Y., Sakai, K., Kamei, K., Takahashi, H., and Yaguchi, T. (2016). Draft genome sequence of the pathogenic filamentous fungus Aspergillus lentulus IFM 54703T. Genome Announc. 4, e01568–e01515. doi: 10.1128/genomeA.01568-15
Kusuya, Y., Takahashi-Nakaguchi, A., Takahashi, H., and Yaguchi, T. (2015). Draft genome sequence of the pathogenic filamentous fungus Aspergillus udagawae strain IFM 46973T. Genome Announc. 3, e00834–e00815. doi: 10.1128/genomeA.00834-15
Langfelder, K., Jahn, B., Gehringer, H., Schmidt, A., Wanner, G., and Brakhage, A. A. (1998). Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 187, 79–89. doi: 10.1007/s004300050077
Larsen, T. O., Smedsgaard, J., Nielsen, K. F., Hansen, M. A., Samson, R. A., and Frisvad, J. C. (2007). Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med. Mycol. 45, 225–232. doi: 10.1080/13693780601185939
Letunic, I., and Bork, P. (2019). Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucl. Acids Res. 47, W256–W259. doi: 10.1093/nar/gkz239
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Lind, A. L., Wisecaver, J. H., Smith, T. D., Feng, X., Calvo, A. M., and Rokas, A. (2015). Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus. PLoS Genet. 11:e1005096. doi: 10.1371/journal.pgen.1005096
Liu, X., Brutlag, D. L., and Liu, J. S. (2001). BioProspector: discovering conserved DNA motifs in upstream regulatory regions of co-expressed genes. Pac. Symp. Biocomput. 6:127−138.
Lyskova, P., Hubka, V., Svobodova, L., Barrs, V., Dhand, N. K., Yaguchi, T., et al. (2018). antifungal susceptibility of the Aspergillus viridinutans complex: comparison of two in vitro methods. Antimicrob. Agents Chemother. 62, e01927–e01917. doi: 10.1128/AAC.01927-17
MacPherson, S., Larochelle, M., and Turcotte, B. (2006). A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70, 583–604. doi: 10.1128/MMBR.00015-06
Majoros, W. H., Pertea, M., and Salzberg, S. L. (2004). TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics 20, 2878–2879. doi: 10.1093/bioinformatics/bth315
Mead, M. E., Knowles, S. L., Raja, H. A., Beattie, S. R., Kowalski, C. H., Steenwyk, J. L., et al. (2019). Characterizing the pathogenic, genomic, and chemical traits of Aspergillus fischeri, a close relative of the major human fungal pathogen Aspergillus fumigatus. mSphere 4, e00018–e00019. doi: 10.1101/430728
Mitchell, A. L., Attwood, T. K., Babbitt, P. C., Blum, M., Bork, P., Bridge, A., et al. (2019). InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucl. Acids Res. 47, D351–D360. doi: 10.1093/nar/gky1100
Morishita, Y., Okazaki, Y., Luo, Y. Y., Nunoki, J., Taniguchi, T., Oshima, Y., et al. (2019). Use of plant hormones to activate silent polyketide biosynthetic pathways in Arthrinium sacchari, a fungus isolated from a spider. Org. Biomol. Chem. 17, 780–784. doi: 10.1039/C8OB02837K
Netzker, T., Flak, M., Krespach, M. K., Stroe, M. C., Weber, J., Schroeckh, V., et al. (2018). Microbial interactions trigger the production of antibiotics. Curr. Opin. Microbiol. 45, 117–123. doi: 10.1016/j.mib.2018.04.002
Nielsen, J. C., Grijseels, S., Prigent, S., Ji, B., Dainat, J., Nielsen, K. F., et al. (2017). Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2:17044. doi: 10.1038/nmicrobiol.2017.44
Nierman, W. C., Pain, A., Anderson, M. J., Wortman, J. R., Kim, H. S., Arroyo, J., et al. (2005). Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438, 1151–1156. doi: 10.1038/nature04332
O'Hanlon, K. A., Gallagher, L., Schrettl, M., Jöchl, C., Kavanagh, K., Larsen, T. O., et al. (2012). Nonribosomal peptide synthetase genes pesL and pes1 are essential for Fumigaclavine C production in Aspergillus fumigatus. Appl. Environ. Microbiol. 78, 3166–3176. doi: 10.1128/AEM.07249-11
Parent-Michaud, M., Dufresne, P. J., Fournier, É., Martineau, C., Moreira, S., Perkins, V., et al. (2019). Draft genome sequence of azole-resistant Aspergillus thermomutatus (Neosartorya pseudofischeri) strain HMR-AF-39, isolated from a human nasal septum abscess aspirate. Microbiol. Resour. Announc. 8, e01444–e01418. doi: 10.1128/MRA.01444-18
Pfannenstiel, B. T., and Keller, N. P. (2019). On top of biosynthetic gene clusters: how epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 37:107345. doi: 10.1016/j.biotechadv.2019.02.001
Postma, M., and Goedhart, J. (2019). PlotsOfData-A web app for visualizing data together with their summaries. PLoS Biol. 17:e3000202. doi: 10.1371/journal.pbio.3000202
Rawlings, N. D., Barrett, A. J., Thomas, P. D., Huang, X., Bateman, A., and Finn, R. D. (2018). The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucl. Acids Res. 46, D624–D632. doi: 10.1093/nar/gkx1134
Rokas, A., Mead, M. E., Steenwyk, J. L., Oberlies, N. H., and Goldman, G. H. (2020). Evolving moldy murderers: Aspergillus section Fumigati as a model for studying the repeated evolution of fungal pathogenicity. PLoS Pathog. 16:e1008315. doi: 10.1371/journal.ppat.1008315
Saldanha, A. J. (2004). Java Treeview–extensible visualization of microarray data. Bioinformatics 20, 3246–3248. doi: 10.1093/bioinformatics/bth349
Sanchez, J. F., Somoza, A. D., Keller, N. P., and Wang, C. C. (2012). Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 29, 351–371. doi: 10.1039/c2np00084a
Schoberle, T. J., Nguyen-Coleman, C. K., Herold, J., Yang, A., Weirauch, M., Hughes, T. R., et al. (2014). A novel C2H2 transcription factor that regulates gliA expression interdependently with GliZ in Aspergillus fumigatus. PLoS Genet. 10:e1004336. doi: 10.1371/journal.pgen.1004336
Schrettl, M., Bignell, E., Kragl, C., Sabiha, Y., Loss, O., Eisendle, M., et al. (2007). Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 3, 1195–1207. doi: 10.1371/journal.ppat.0030128
Seppey, M., Manni, M., and Zdobnov, E. M. (2019). BUSCO: assessing genome assembly and annotation completeness. Methods Mol. Biol. 1962, 227–245. doi: 10.1007/978-1-4939-9173-0_14
Shen, W., Le, S., Li, Y., and Hu, F. (2016). SeqKit: A cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11:e0163962. doi: 10.1371/journal.pone.0163962
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stanke, M., Schöffmann, O., Morgenstern, B., and Waack, S. (2006). Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics 7:62. doi: 10.1186/1471-2105-7-62
Steenwyk, J. L., Mead, M. E., Knowles, S. L., Raja, H. A., Roberts, C. D., Bader, O., et al. (2020). Variation among biosynthetic gene clusters, secondary metabolite profiles, and cards of virulence across Aspergillus species. Genetics 216, 481–497. doi: 10.1534/genetics.120.303549
Steinchen, W., Lackner, G., Yasmin, S., Schrettl, M., Dahse, H. M., Haas, H., et al. (2013). Bimodular peptide synthetase SidE produces fumarylalanine in the human pathogen Aspergillus fumigatus. Appl. Environ. Microbiol. 79, 6670–6676. doi: 10.1128/AEM.02642-13
Takahashi, H., Kusuya, Y., Hagiwara, D., Takahashi-Nakaguchi, A., Sakai, K., and Gonoi, T. (2017). Global gene expression reveals stress-responsive genes in Aspergillus fumigatus mycelia. BMC Genomics 18:942. doi: 10.1186/s12864-017-4316-z
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577. doi: 10.1080/10635150701472164
Ter-Hovhannisyan, V., Lomsadze, A., Chernoff, Y. O., and Borodovsky, M. (2008). Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 18, 1979–1990. doi: 10.1101/gr.081612.108
Umemura, M., Koike, H., Nagano, N., Ishii, T., Kawano, J., Yamane, N., et al. (2013). MIDDAS-M: motif-independent de novo detection of secondary metabolite gene clusters through the integration of genome sequencing and transcriptome data. PLoS ONE 8:e84028. doi: 10.1371/journal.pone.0084028
Urquhart, A. S., Hu, J., Chooi, Y. H., and Idnurm, A. (2019). The fungal gene cluster for biosynthesis of the antibacterial agent viriditoxin. Fungal Biol. Biotechnol. 6:2. doi: 10.1186/s40694-019-0072-y
Vesth, T. C., Nybo, J. L., Theobald, S., Frisvad, J. C., Larsen, T. O., Nielsen, K. F., et al. (2018). Investigation of inter- and intraspecies variation through genome sequencing of Aspergillus section Nigri. Nat. Genet. 50, 1688–1695. doi: 10.1038/s41588-018-0246-1
Wang, J., Galgoci, A., Kodali, S., Herath, K. B., Jayasuriya, H., Dorso, K., et al. (2003). Discovery of a small molecule that inhibits cell division by blocking FtsZ, a novel therapeutic target of antibiotics. J. Biol. Chem. 278, 44424–44428. doi: 10.1074/jbc.M307625200
Yin, Y., Mao, X., Yang, J., Chen, X., Mao, F., and Xu, Y. (2012). dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucl. Acids Res. 40, W445–W451. doi: 10.1093/nar/gks479
Keywords: comparative genomic analysis, Aspergillus fumigatus, gliotoxin, terrein, viriditoxin, Aspergillus section Fumigati, comparative transcriptomic analysis, secondary metabolic gene
Citation: Takahashi H, Umemura M, Ninomiya A, Kusuya Y, Shimizu M, Urayama S, Watanabe A, Kamei K, Yaguchi T and Hagiwara D (2021) Interspecies Genomic Variation and Transcriptional Activeness of Secondary Metabolism-Related Genes in Aspergillus Section Fumigati. Front. Fungal Biol. 2:656751. doi: 10.3389/ffunb.2021.656751
Received: 21 January 2021; Accepted: 23 March 2021;
Published: 16 April 2021.
Edited by:
Daniel Croll, Université de Neuchâtel, SwitzerlandReviewed by:
Elisha Thynne, University of Kiel, GermanyCopyright © 2021 Takahashi, Umemura, Ninomiya, Kusuya, Shimizu, Urayama, Watanabe, Kamei, Yaguchi and Hagiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Hagiwara, aGFnaXdhcmEuZGFpc3VrZS5nYkB1LnRzdWt1YmEuYWMuanA=
†ORCID: Daisuke Hagiwara orcid.org/0000-0003-1382-3914
Hiroki Takahashi orcid.org/0000-0001-5627-1035
Maiko Umemura orcid.org/0000-0001-8730-1380
Akira Watanabe orcid.org/0000-0002-3057-2937
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.