- 1Research Division of Natural Resources, Hormozgan Agricultural and Natural Resources Research and Education Center, Agricultural Research, Education and Extension Organization (AREEO), Bandar Abbas, Iran
- 2Research Division of Natural Resources, Golestan Agricultural and Natural Resources Research and Education Center, Agricultural Research, Education and Extension Organization (AREEO), Gorgan, Iran
- 3Department of Natural Resource Sciences, Thompson Rivers University, Kamloops, BC, Canada
- 4Department of Environmental Sciences and Engineering, Islamic Azad University, Bandar Abbas, Iran
- 5Research Institute of Forests and Rangelands, Agricultural Research, Education and Extension Organization, Tehran, Iran
- 6Faculty of Technology, Department of Forestry and Wood Technology, Linnaeus University, Växjö, Sweden
This study aims to explore the salt tolerance mechanisms of Avicennia marina from the Persian Gulf, addressing the gap in understanding whether mangroves are facultative or obligatory halophytes. Seedlings were exposed to freshwater (control), low salinity (25% seawater), medium salinity (50% seawater), and high salinity (100% seawater) treatments for six months. The results revealed that medium salinity promoted optimal growth, with the highest values for plant height, root and shoot biomass, leaf area, and seedling vigor (2.86%). The non-salinity treatment showed lower vigor (1.96%) and greater leaf loss (17.96%). Medium salinity increased nitrogen and potassium levels, while high salinity elevated chloride and sodium content. Photosynthetic pigments were higher in moderate and low salinity treatments, and catalase activity peaked in freshwater, with peroxidase activity highest in both freshwater and high salinity treatments. These findings provide evidence that A. marina from the Persian Gulf is a facultative halophyte, capable of thriving in both saline and non-saline conditions, with salinity enhancing its growth and health.
Introduction
Mangrove ecosystems, located at the interface of terrestrial and marine environments, represent a unique and essential component of coastal biodiversity. These remarkable trees thrive in saline environments where few other plants can survive, embodying nature’s resilience to one of the most challenging environmental stressors—salinity (Gilman et al., 2008). Salinity imposes significant physiological and biochemical challenges on plants, such as reduced water uptake, ion imbalances, and potential toxicity, all of which hinder growth and productivity (Parihar et al., 2015). While the resilience of mangroves to salinity has been widely recognized, critical questions remain about the mechanisms driving this resilience and their variability across different populations and environmental contexts.
As discussed by Flowers and Colmer (2008), halophytes, including mangroves, demonstrate diverse growth responses to salinity, with optimal growth often occurring at moderate salinity levels. These conditions enable energy-intensive mechanisms like ion compartmentation and osmotic adjustment, which support rapid biomass production and distinguish mangroves from glycophytes and drought-tolerant xerophytes. However, a central debate in mangrove ecology concerns whether mangroves are facultative or obligate halophytes. While some studies suggest that mangroves can survive without salt and grow best at moderate salinity levels (Krauss and Ball, 2013), others argue that mangroves require salinity for survival and development.
The study by Nguyen et al. (2015) on Avicennia marina highlighted the importance of salinity for developing hydraulic systems that support water use and carbon gain. Seedlings of A. marina did not grow in 0–5% seawater, with optimal growth observed in 50–75% seawater. Growth rates were influenced by leaf area and net assimilation rate, with gas exchange being highest in moderate salinities. Although they concluded that A. marina is an obligate halophyte, requiring saline conditions for proper development, their findings also indicated that salinity tolerance in A. marina varies by location and species. For instance, necrotic lesions were observed under high salinity conditions, with the optimal salinity for growth ranging from 10–50% seawater (Nguyen et al., 2015). These findings underscore the complexity of mangrove responses to salinity and the importance of considering geographic and environmental variability.
Despite the growing body of research, significant gaps persist in understanding the salinity tolerance mechanisms of mangroves across different geographic regions, particularly in the Persian Gulf, where environmental conditions are extreme, with high salinity levels and temperatures. Studies on A. marina, a dominant mangrove species in the Indo-Pacific region, have largely focused on populations from tropical and subtropical zones (Win, 2019). However, the Persian Gulf populations of A. marina face unique challenges, including hypersaline waters, frequent droughts, and anthropogenic pressures, making them an invaluable model for studying extreme environmental adaptation. Yet, few studies have explored the biochemical, physiological, and morphological responses of these populations to varying salinity levels, leaving critical knowledge gaps regarding their resilience and ecological function.
In this study, we focus on the growth reaction and biochemical and nutrient-level responses of Avicennia marina seedlings from Persian Gulf populations under different salinity regimes. By analyzing parameters such as ion accumulation, osmotic adjustment, and key nutrient levels, we hypothesize that A. marina seedlings optimize nutrient uptake and employ specific biochemical strategies to adapt to moderate salinity levels, while extreme salinity induces stress responses that impair growth and physiological function. This study aims to test these hypotheses and provide insights essential for understanding the resilience mechanisms of A. marina in some of the world’s most challenging coastal environments. Our work addresses critical gaps in knowledge regarding the role of nutrient management and biochemical strategies in conferring resilience to extreme salinity conditions. By focusing on these aspects, this study not only contributes to the broader understanding of mangrove adaptation but also provides insights essential for the conservation and sustainable management of A. marina in some of the world’s most challenging coastal environments.
Materials and methods
Propagule collection and salinity treatments
Thirty healthy A. marina trees at each of four locations in Southern Iran were randomly selected for the collection of propagules Khore-Azini (26° 31′ 34.20″ N and 57° 6′ 13.22″ E), Sirik port, Gheshm Island (26° 53′ 46.94″ N and 55° 45′ 37″ E), Kamir port (26°, 57′ 10.84″ N and 55° 45′ 37″ E) (Figure 1). Mangrove forests in the coastal areas of Hormozgan Province have an average annual rainfall of about 160 mm and an average maximum temperature of 35 to 41°C in the hottest month of the year while the absolute maximum temperature ever recorded is 50°C.
The propagules were planted in plastic pots (25 × 10 cm) containing a mixture of clay, sand, and organic matter (mangrove humus) at a 1:1:1 volume ratio. These seedlings were nurtured in an open nursery under natural environmental conditions, including temperature, light, and moisture. After a two-week growth period, we implemented four distinct salinity treatments: a freshwater group (tap water), a low salinity group (EC = 8,500 μm/cm), a moderate salinity group (EC: 29000 μm/cm), and a high salinity group (EC: 57000 μm/cm). All the treatments involved 200 propagules, divided into 4 treatments (with 10 propagules in each plastic pot, totaling n = 50 for each treatment), utilizing a randomized design as described by Khan and Aziz (2001). The salinity treatments were prepared by blending natural seawater and freshwater, where the non-salinity group was irrigated exclusively with freshwater, low salinity consisted of 25% seawater and 75% freshwater, moderate salinity had a 50:50 mix of seawater and freshwater, and high salinity was composed of 100% seawater. To ensure optimal growth conditions, the plants were irrigated twice daily until flooding for a duration of 6 months (Kodikara et al., 2018).
Growth reaction and biomass allocation
Growth characteristics [plant vigor, number of leaves, root and shoot length, and stem base diameter (collar)] and biomass (fresh and dry biomass of leaves and roots) were quantified by randomly selecting 6 seedlings from each pot (n = 30 for each treatment). Plant height was measured with a ruler and collar diameter was recorded with a digital caliper (Wang et al., 2024). Plant vigor was classified by observational evaluation by the same researcher. The plant were accessed using the following criteria: (i) poor quality: observation of pest and disease (lower than 50%), yellow color and severe drying in leaves (higher than 50%), an abundance of small leaves (higher than 50%), and a very bent stem; (ii) medium quality: the beginning of disease and pest in the seedling (up to 50%), seeing the signs and beginning of drying and yellowing in the leaf (up to 50%), small leaves (up to 50%) and curved stem and low and reversible bending, and finally, (iii) high-quality grade: seedlings without any sign of pests and diseases, green color, without drying, big leaves and straight and cylindrical stems. Plants classified as poor, medium, and excellent classification was given a score of 1, 3, and 5, respectively (weighting) to be comparable (Moslehi et al., 2021). Finally, the mangrove seedlings were harvested and separated into shoot, roots and leaves. Then, they were weighed and dried in an oven at 75° for 48 h (Basyuni et al., 2007). The data has been shown as aboveground (shoot+ leaf) and belowground (root) biomass.
Biochemical variables
Antioxidase enzyme activities in three plant organs (root, shoot, and leaf), as well as the content of chlorophyll and carotenoids in leaf, were measured at the end of the experiment. Four seedlings were randomly selected from each pot. The plants were separated into leaves, roots, and shoots, with each plant part combined from each pot. This resulted in one combined sample of each plant part for each pot and 5 samples for each treatment.
To measure enzyme activity, proteins were separately extracted from different plant tissue samples. First, 1 g of leaves was ground in a porcelain mortar containing 3 mL of 50 mM phosphate buffer (pH = 7.2). The phosphate buffer contained ethylene di-amine tetra acetic acid (EDTA) (1 mM), phenylmethanesulfonyl fluoride (PMSF) (1 mM) and polyvinyl pyrrolidone 1% (PVP). After being ground, the extract was placed in a centrifuge at 1400 rpm for 15 min. The supernatant solution was used to quantify enzyme activity.
Catalase activity was measured using the method described by Dhindsa (1981). First, 1% PVP was added to the protein extract. 100 microliters of this solution were added to a solution of 2.87 mL of 50 mM potassium phosphate buffer (pH = 7) and 30 microliters of 15 mM hydrogen peroxide that were previously mixed together in an ice bath. The decrease in hydrogen peroxide was quantified by measuring the decline in A240 using a spectrophotometer. The activity was expressed in units where one unit of catalase converts one μmole of hydrogen peroxide per minute. Also, 50 μL of the extract was added to the 2.5 mL buffer (Tris 100 mM, H2O2 5 mM, and Pyrogallol 10 mM) and absorbance was recorded at 425 nm by spectrophotometry for determining peroxidase activity. Photosynthetic pigments, including chlorophyll a and b, and carotenoids was determined using the methods outlined in Arnon (1967). Fresh leaves (0.5 gr) were placed in a mortar, mixed with liquid nitrogen, and then crushed. 20 mL of acetone 80% was then added to the sample prior to placement in a centrifuge (6,000 rpm, 4,020 × g.) for 10 min. The upper portion of the separated extract from the centrifuge was transferred to a glass flask. The sample was analyzed using a spectrophotometer (Spectronic Genesys 5, Thermo Fisher Scientific, USA) at 663 nm for chlorophyll a, 645 nm for chlorophyll b, and 470 nm for carotenoids. Finally, the amount of chlorophyll a and b, as well as carotenoids, was obtained in terms of mgr/ wet weight of the sample using the following equations: Chla = (19.3 A663-0.86 A645) V/100w.
where, v is the volume of strained solution (upper solution obtained from centrifugation), A is the absorption of light 663, 645 and 470 nm and W is the fresh weight of the sample (g).
Plant nutrients
Besides soil nitrogen content, concentration of ions such as K+ (potassium), Na + (sodium), and Cl- (chloride) in the samples of the leaves and roots of 30 seedlings were examined by the dry ash method (Ehyaee and Behbahanizadeh 1993). Briefly, hydrochloric acid (2 M) was used to dissolve ground biomass after drying and grinding leaves and roots (Chapman and Pratt 1961). The sodium and potassium concentrations were determined using flame photometers (PFP7, Jenway, Staffordshire, UK) (Mountousis et al., 2009). Ion analyzers were used to analyze the ground biomass after immersing it in distilled water and for chloride analysis (Jenway PCLM3). Nitrogen was measured using the wet ashing method (H2O2 and H2SO4) and using the Kjeldahl method (Model Vab20, Germany) (Ehyaee and Behbahanizadeh 1993).
Statistical analysis
Statistical analysis was performed using Excel and Jamovi (The Jamovi Project, 2024) employing a one-way ANOVA to assess differences between groups (Zhao et al., 2024). Post-hoc mean comparisons were conducted using the Tukey test to determine which specific groups differed significantly, with a p-value threshold of 0.05 for statistical significance.
Results
Growth reaction and biomass allocation
The one-way ANOVA revealed that all the growth and biomass variables of the mangrove seedlings, except for seedling diameter, root length, and root/shoot ratio, were significantly affected by the different treatments of saline intensity (Table 1). In our experiment, seedlings cultivated under the moderate salinity treatment exhibited significant growth and biomass attributes, such as enhanced height (mean 16.08 cm), increased fresh mass (mean 5.726 g), dry root mass (mean 2.052 g), and shoot mass (mean 6.62 g for fresh and 1.726 g for dry) (Figures 2, 3). In the case of all the mentioned variables, no significant differences were observed among the remaining treatments. The mean comparison results demonstrated that seedlings under low and medium salinity levels exhibited higher vigor (means 2.83 and 2.86, respectively) when compared to those under the freshwater treatment and high levels of salinity (Figure 2). The highest leaf area value was observed in seedlings under the moderate saline condition (mean 21.28 cm2), with no significant difference noted among the remaining treatments. Interestingly, seedlings under moderate salinity levels not only produced more new leaves throughout the experiment but also exhibited a lower leaf loss (mean 2.94%). In terms of leaf shedding, the highest values were observed for the seedlings belonging to the freshwater treatment (mean 14.03%) and high salinity (mean 11.58%) stress level.
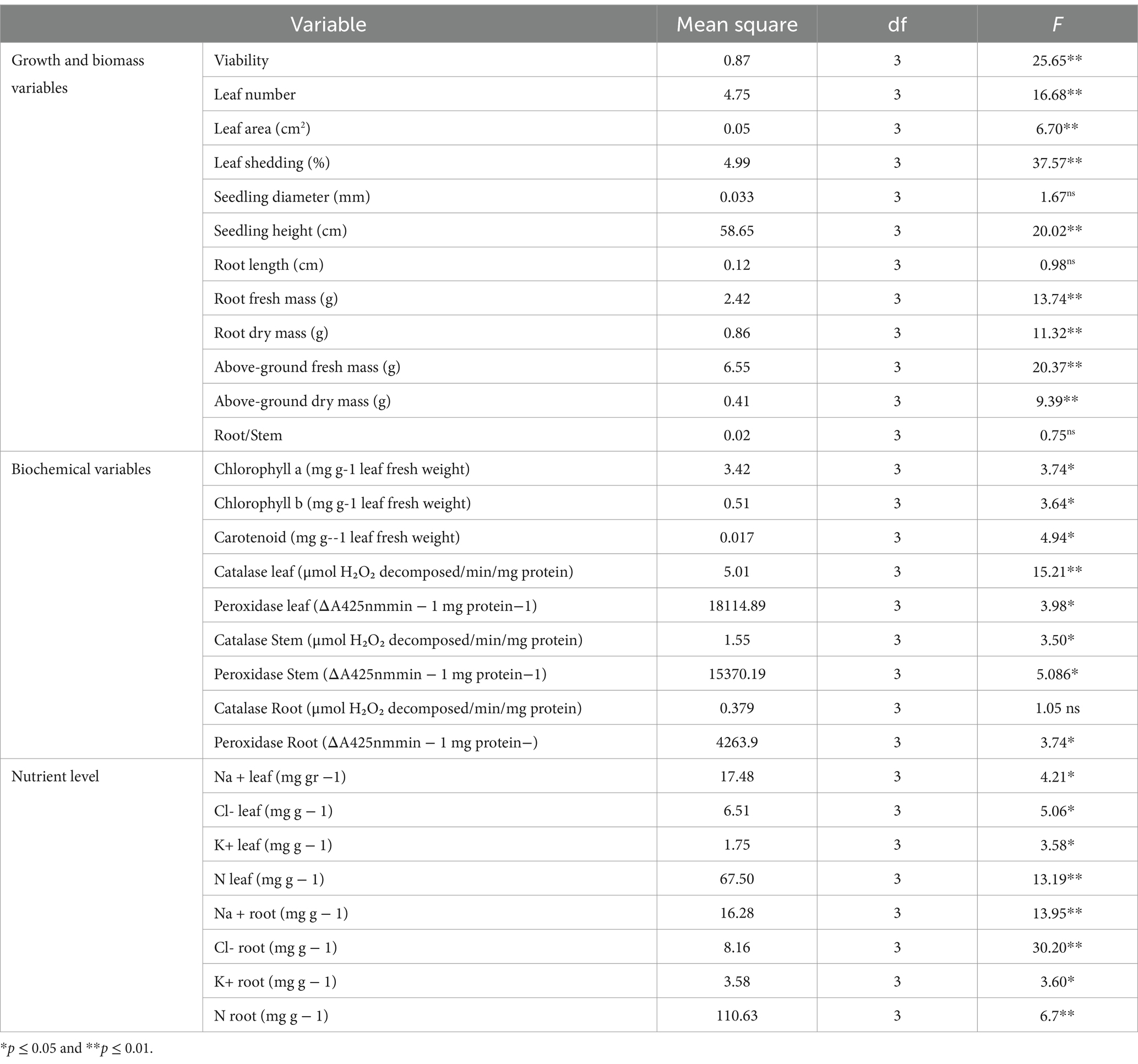
Table 1. Variance analysis of growth, biochemical, and ion content characteristics of mangrove seedlings in different salinity treatments.
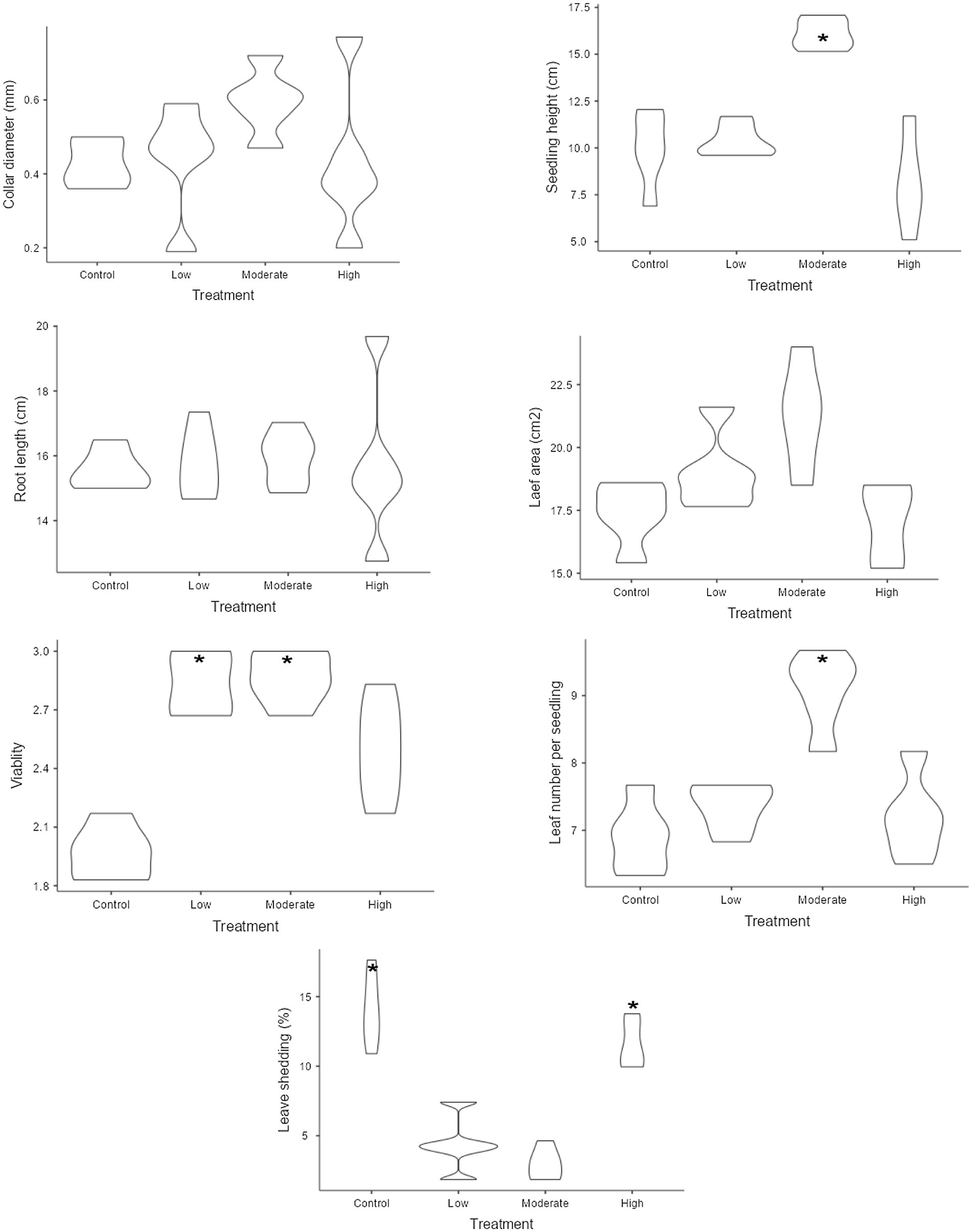
Figure 2. Variation in plant growth variables under different levels of salinity. Control refers to seedlings watered with freshwater, while the low, moderate, and high salinity levels correspond to seedlings watered with 25, 50, and 100% seawater, respectively. The violin plots show plant growth distribution under different salinity levels. Wider plots indicate more variation, while narrower ones show less variability. Stars denote statistically significant differences between groups (significant at the 5% level).
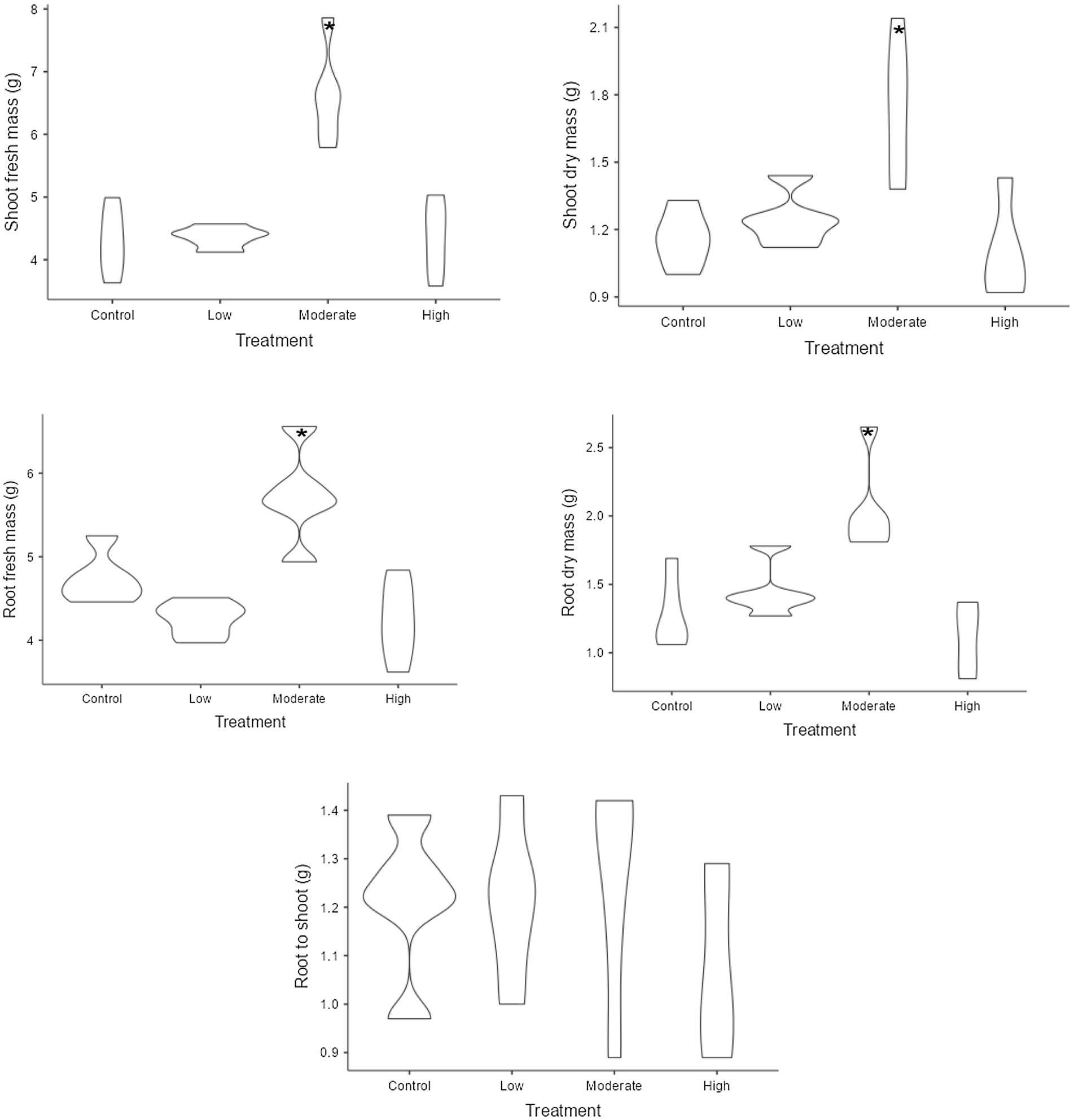
Figure 3. The variation of biomass variables under different level of salinity. Control refers to seedlings watered with freshwater, while the low, moderate, and high salinity levels correspond to seedlings watered with 25, 50, and 100% seawater, respectively. The violin plots show biomass distribution under different salinity levels. Wider plots indicate more variation, while narrower ones show less variability. Stars denote statistically significant differences between groups (significant at the 5% level).
Plant nutrients
The nitrogen content in both leaves and roots were consistently higher in seedlings subjected to low and moderate salinity conditions (Figure 4). In contrast, the lowest amounts of nitrogen in both tissues were observed in plants exposed to freshwater (means 37.69 mg g −1 for leaves and 28.8 mg g −1 for roots) and pure seawater conditions (means 37.36 mg g −1 for leaves and 27.76 mg g −1 for roots). The leaves of seedlings subjected to high salinity condition exhibited a partial reduction in the concentration of potassium cations in their tissues, with a more pronounced change observed in the leaves compared to the roots. On the other hand, mangrove seedlings under moderate saline condition still exhibited the higher values (mean 13.46 mg g −1 for leaves and 9.82 mg g −1 for roots). As predicted, the concentration of sodium cations was lower after being irrigated with freshwater while we could not observe any significant shift under different intensities of salinity. The seedlings under high salinity levels exhibited the highest concentration of chlorine in their tissues (8.8 mg g −1 for leaves and 8.4 mg g −1 for roots), with no significant changes recorded among the remaining treatments. In general, fresh-watered seedlings tended to show lower values (6.28 mg g −1 for leaves and 4.94 mg g −1 for roots).
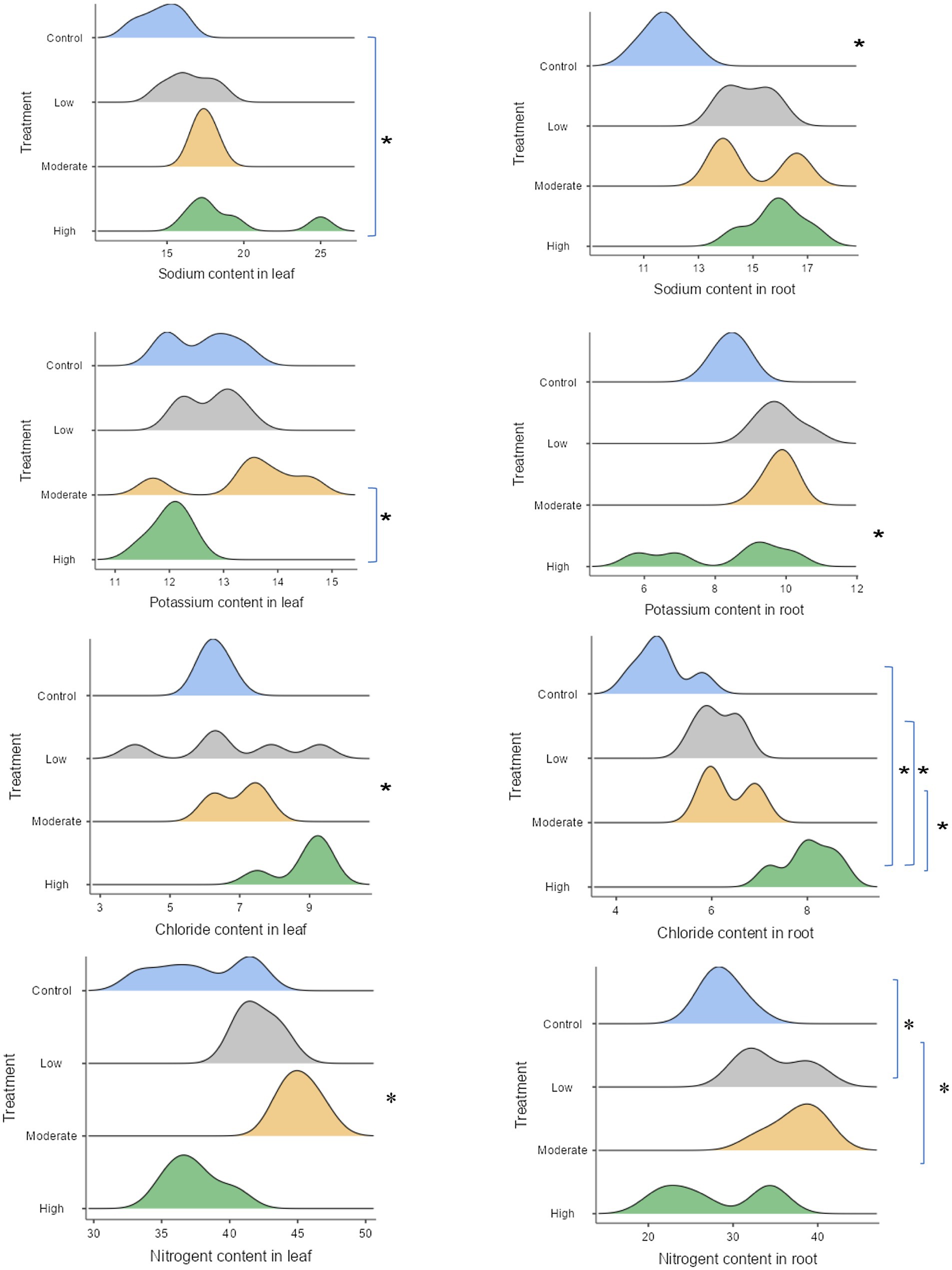
Figure 4. Changes in nutrient levels in seedling tissues after 6 months of saline stress, with statistical significance indicated by stars (significant at the 5% level), as shown in the ridgeline plot. Wider ridges indicate more concentrated data, while narrower ones show less variability. Control refers to seedlings watered with freshwater, while the low, moderate, and high salinity levels correspond to seedlings watered with 25, 50, and 100% seawater, respectively.
Biochemical variables
The chlorophyll content in the leaves was predominantly higher in seedlings subjected to the moderate salinity condition (Figure 5). In contrast, seedlings exposed to both low (mean 0.32 mg g−1 leaf fresh weight) and medium (mean 0.312 mg g−1 leaf fresh weight) saline treatments exhibited lower levels of carotenoids in their leaves, while carotenoid content remained consistently higher in the non-salinity (mean 0.412 mg g−1 leaf fresh weight) and high salinity (mean 0.376 mg g−1 leaf fresh weight) conditions.
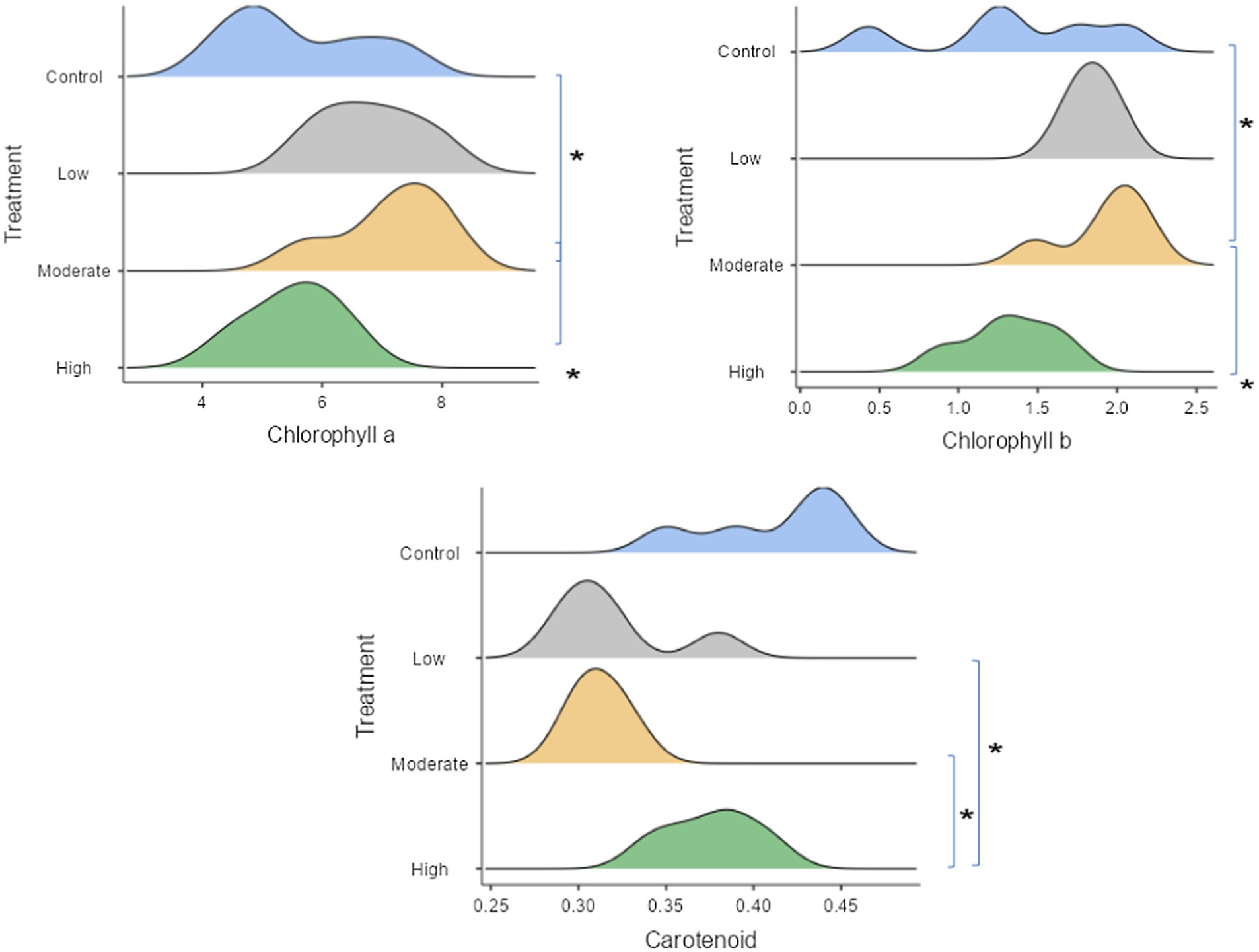
Figure 5. Plant pigments responsible to saline stress, with statistical significance indicated by stars (significant at the 5% level), as shown in the ridgeline plot. Wider ridges indicate more concentrated data, while narrower ones show less variability. Control refers to seedlings watered with freshwater, while the low, moderate, and high salinity levels correspond to seedlings watered with 25, 50, and 100% seawater, respectively.
Antioxidant enzyme activities were assessed in three distinct seedling tissues. The findings revealed a significant increase in catalase activity in the leaf and stem tissues under both non- salinity (means 6.32 μmol H₂O₂ decomposed/min/mg protein for leaves and 4.06 μmol H₂O₂ decomposed/min/mg protein for stems) and high salinity conditions (means 5.26 μmol H₂O₂ decomposed/min/mg protein for leaves and 4.8 μmol H₂O₂ decomposed/min/mg protein for stems) (Figure 6).
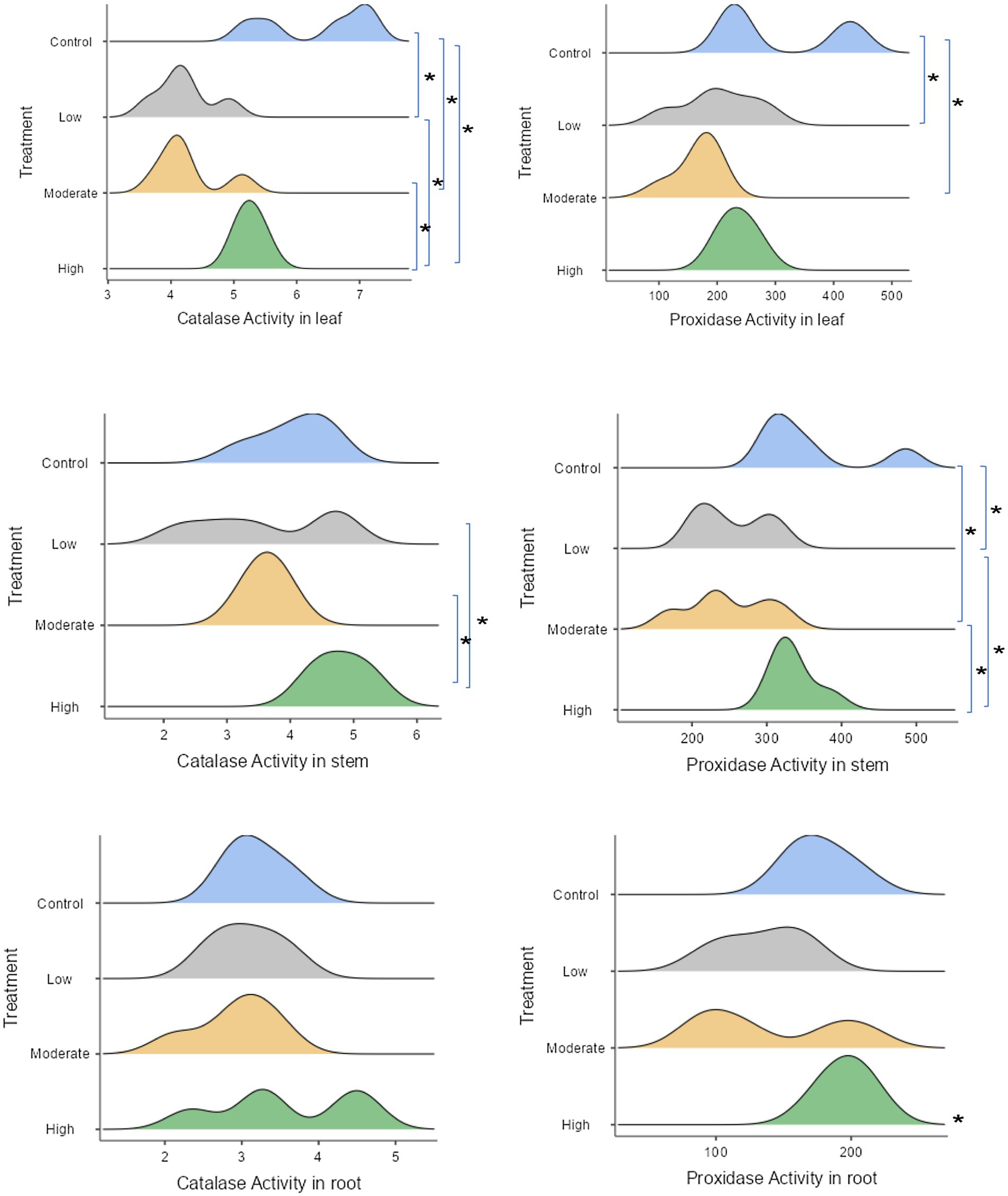
Figure 6. The response of plant antioxidant enzyme defense to saline stress, with statistical significance indicated by stars (significant at the 5% level), as shown in the ridgeline plot. Wider ridges indicate more concentrated data, while narrower ones show less variability. Control refers to seedlings watered with freshwater, while the low, moderate, and high salinity levels correspond to seedlings watered with 25, 50, and 100% seawater, respectively.
In contrast, catalase activity remained stable in the root tissue and did not respond to our experimental treatments. A similar pattern was observed for peroxidase enzymes (Figure 6). In fresh-watered seedlings, there was a tendency for an increase in peroxidase enzyme levels in their leaves (mean 306.02 ΔA425nmmin−1mg protein−1) and shoots (mean 356.46 ΔA425nm min−1mg protein−1). Conversely, peroxidase enzyme expression was significantly higher in the roots of seedlings exposed to high salinity condition (mean 196.06 ΔA425nm min−1mg protein−1).
Discussion
In our study, we investigated the response of Avicennia marina (grey mangrove) seedlings to varying levels of saline condition, focusing on growth, biomass, and biochemical variables to gain deeper insights into their adaptive mechanisms. Our results contribute to the growing body of literature highlighting the resilience of mangroves, particularly A. marina, in coastal ecosystems. The findings of this study are consistent with the idea that mangroves are facultative halophytes, as proposed by (Krauss and Ball, 2013). While A. marina can survive in a wide range of salinities, it demonstrates optimal growth under moderate salinity conditions. This challenges the commonly held view that mangroves require salt for survival, presenting an opportunity to reconsider their ecological strategy in coastal environments.ur results align with the work of Flowers and Colmer (2008), who noted that halophytes, including mangroves, exhibit diverse growth responses to salinity. These plants typically show optimal growth at moderate salinity, where energy-intensive mechanisms like ion compartmentalization and osmotic adjustment allow for enhanced biomass production. In our study, moderate saline conditions were shown to significantly promote growth, leading to increased height, fresh biomass, and dry biomass in both roots and shoots. These observations support the idea that moderate salinity levels are not only tolerable but beneficial for mangrove growth, enhancing their biomass production capacity.
Further analysis revealed that the vigor rate of the seedlings was highest under low and moderate salinity conditions. This suggests that there is a specific salinity threshold at which A. marina seedlings thrive. This finding is consistent with the concept that mangroves are particularly adapted to conditions with salinity levels that support their physiological processes without overwhelming their tolerance mechanisms (Alongi, 2008). These results reinforce the view that A. marina and other mangrove species are most productive in habitats with moderate salinity, which likely explains their distribution patterns along coastal gradients, where they encounter fluctuating salt concentrations in tidal zones.
In contrast, we observed that extreme salinity conditions—both too low (freshwater) and too high (pure seawater)—hindered seedling growth and nutrient uptake. This aligns with the findings of Nguyen et al. (2015), who reported that seedlings of A. marina showed optimal growth in 50–75% seawater, with significant reductions in growth under extreme saline conditions. The study by Nguyen et al. also indicated that high salinity levels led to necrotic lesions, suggesting that while A. marina can tolerate saline conditions, excessive salinity can still cause damage and limit growth. Our study similarly observed a decline in growth under both non-salinity and high salinity treatments, further supporting the idea that extremes of salinity can be detrimental to mangrove development. Recent studies have highlighted the importance of freshwater availability in the growth and productivity of A. marina. Santini et al. (2015) and (Hayes et al., 2019) demonstrated that A. marina, despite being a salt-tolerant species, actively utilizes freshwater sources such as groundwater and rainwater in natural conditions. Their findings suggest that access to freshwater can significantly enhance growth rates, making it a key limiting factor for mangrove development. In our study, we accounted for this ecological aspect by supplementing seawater with freshwater to examine the plant’s physiological responses. Our results align with these previous findings, reinforcing the idea that while A. marina is highly adapted to saline environments, its growth performance improves when freshwater sources are available. This highlights the species’ capacity to optimize water use under variable conditions, further contributing to our understanding of mangrove adaptation strategies.
Regarding nutrient dynamics, our study found that nitrogen content in both roots and leaves increased under the moderate salinity condition, supporting the idea that moderate salinity may trigger physiological responses that optimize nutrient uptake and water conservation. These findings are in line with the results of Munns and Tester (2008), who reported that moderate salinity levels can stimulate nutrient uptake in halophytes by promoting physiological adaptations to cope with salt stress. In contrast, the lowest nitrogen content was recorded under extreme freshwater and high salinity conditions. This suggests that nutrient leaching in freshwater or osmotic stress in high salinity may hinder nutrient availability, leading to reduced growth and lower nutrient content in the tissues.
Further investigation of ion regulation revealed that A. marina seedlings accumulated higher levels of potassium in response to moderate salinity, while extreme salinity led to a reduction in potassium content. This observation aligns with the findings of Maathuis (2009) and Patel et al. (2010), who noted that halophytes actively regulate potassium and sodium ion concentrations to maintain cellular homeostasis. Potassium plays a key role in osmotic regulation, and its accumulation under moderate salinity may help mangroves adapt to salt stress. At the same time, high salinity conditions likely lead to competition between ions, reducing potassium levels and affecting overall plant health.
In addition to ion regulation, our study also examined the role of antioxidants in protecting A. marina seedlings from oxidative damage induced by salinity stress. We observed that catalase activity was elevated under both high salinity and non-salinity conditions, indicating that A. marina uses antioxidants to mitigate oxidative stress. Elevated catalase activity is a common response to salinity-induced oxidative stress, as this enzyme plays a key role in breaking down hydrogen peroxide, a reactive oxygen species (ROS) that can cause cellular damage (Gill and Tuteja, 2010). Similarly, peroxidase activity increased under high salinity conditions, suggesting that A. marina seedlings activate multiple antioxidant defenses to cope with oxidative stress. These findings support the idea that mangroves possess robust antioxidant systems to protect their tissues from the harmful effects of salt-induced oxidative damage.
Chlorophyll content was another key indicator of physiological performance in our study. Seedlings exposed to moderate salinity exhibited higher chlorophyll content compared to those in non-salinity or high salinity treatments. This suggests that moderate salinity may enhance photosynthetic efficiency, contributing to better growth and biomass production. Chlorophyll is essential for photosynthesis, and the higher chlorophyll content in seedlings under moderate salinity conditions indicates that photosynthetic activity was optimized for growth under these conditions. On the other hand, carotenoid content, which is important for photoprotection, was found to be lower in seedlings exposed to low and medium salinity levels. This may reflect a trade- off between optimizing energy capture for growth and minimizing photodamage under different salinity stresses.
Conclusion
In summary, our study supports the idea that A. marina, like other mangroves, is a facultative halophyte that thrives under moderate salinity conditions. The optimal salinity range for growth allows for efficient nutrient uptake, ion regulation, and photosynthetic activity. These results provide new insights into the ecological strategies of mangroves, suggesting that while they are capable of surviving in a range of saline conditions, their growth and development are most robust in environments with moderate salinity. Understanding the factors that influence mangrove growth and survival is crucial for the conservation and management of coastal ecosystems, where mangroves play a key role in mitigating coastal erosion, enhancing biodiversity, and providing critical ecosystem services.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
MM: Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft. AA: Writing – original draft. TP: Writing – review & editing. MD: Writing – original draft. MH: Writing – original draft. MZ: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Office of Natural Recourses and Watershed Management of Hormozgan Province. Maryam Moslehi has received research support from this organization.
Acknowledgments
The first author sincerely thanks the General Office of Natural Resources and Watershed Management of Hormozgan Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alongi, D. M. (2008). Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 76, 1–13. doi: 10.1016/j.ecss.2007.08.024
Basyuni, M., Oku, H., Tsujimoto, E., Kinjo, K., Baba, S., and Takara, K. (2007). Triterpene synthases from the Okinawan mangrove tribe, Rhizophoraceae. FEBS J. 274. doi: 10.1111/j.1742-4658.2007.06025.x
Chapman, H. D., and Pratt, P. F. (1961). Methods of analysis for soils, plants and waters. University of California, Los Angeles, 60-61, 150–179.
Dhindsa, R. S., and Motowe, W. (1981). Drought tolerance in two mosses: correlation with enzymatic defense against lipid peroxidation. J. Exp. Bot. 32, 79–91.
Ehyaee, M., and Behbahanizadeh, A. A. (1993). Description of soil chemical analysis methods. Soil and Water Research Institute, 2.
Flowers, T. J., and Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytol. 179, 945–963. doi: 10.1111/j.1469-8137.2008.02531.x
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gilman, E. L., Ellison, J., Duke, N. C., and Field, C. (2008). Threats to mangroves from climate change and adaptation options: a review. Aquat. Bot. 89, 237–250. doi: 10.1016/j.aquabot.2007.12.009
Hayes, M. A., Jesse, A., Welti, N., Tabet, B., Lockington, D., and Lovelock, C. E. (2019). Groundwater enhances above-ground growth in mangroves. J. Ecol. 107, 1120–1128. doi: 10.1111/1365-2745.13105
Khan, M. A., and Aziz, I. (2001). Salinity tolerance in some mangrove species from Pakistan. Wetl. Ecol. Manag. 9, 219–223. doi: 10.1023/A:1011112908069
Kodikara, K. A. S., Jayatissa, L. P., Huxham, M., Dahdouh-Guebas, F., and Koedam, N. (2018). The effects of salinity on growth and survival of mangrove seedlings changes with age. Acta Bot Brasilica 32, 37–46. doi: 10.1590/0102-33062017abb0100
Krauss, K. W., and Ball, M. C. (2013). On the halophytic nature of mangroves. Trees Structure Function 27, 7–11. doi: 10.1007/s00468-012-0767-7
Maathuis, F. J. (2009). Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12:397398, 250–258. doi: 10.1016/j.pbi.2009.04.003
Moslehi, M., Pypker, T., Bijani, A., Ahmadi, A., and Hallaj, M. H. S. (2021). Effect of salinity on the vegetative characteristics, biomass and chemical content of red mangrove seedlings in the south of Iran. Scientia Forestalis 49:e3748. doi: 10.18671/scifor.v49n132.16
Mountousis, I., Papanikolaou, K., Stanogias, G., Roukos, Ch., Chatzitheodoridis, F., and Papazafiriou, A. (2009). Mineral content of the herbage material in pastures of Mt. Varnoudas NW Greece. Agron. Res. 7, 837–846.
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nguyen, H. T., Stanton, D. E., Schmitz, N., Farquhar, G. D., and Ball, M. C. (2015). Growth responses of the mangrove Avicennia marina to salinity: development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 115, 397–407. doi: 10.1093/aob/mcu257
Parihar, P., Singh, S., Singh, R., Singh, V. P., and Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. Pollut. Res. 22, 4056–4075. doi: 10.1007/s11356-014-3739-1
Patel, N. T., Gupta, A., and Pandey, A. N. (2010). Strong positive growth responses to salinity by Ceriopstagal, a commonly occurring mangrove of the Gujarat coast of India. AoB Plants 2010:plq011. doi: 10.1093/aobpla/plq011
Santini, N. S., Reef, R., Lockington, D. A., and Lovelock, C. E. (2015). The use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiologia 745, 59–68. doi: 10.1007/s10750-014-2091-2
The Jamovi Project (2024). Jamovi (version 2.5) [computer software]. Available online at: https://www.jamovi.org
Wang, R., Lu, X., Han, H., Zhang, X., Ma, Y., Liu, Q., et al. (2024). Eco-physiological characteristics of Tetracentron sinense Oliv. Saplings in response to different light intensities. J For Res 35:46. doi: 10.1007/s11676-023-01693-4
Win, T.-Z.-N. (2019). Diversity and distribution of true mangroves in Myeik coastal areas, Myanmar. J Aquacult Marine Biol 8, 154–161. doi: 10.15406/jamb.2019.08.00256
Keywords: antioxidant enzymes, growth reaction, mangrove ecosystem, salinity, tolerant mechanism, Avicennia marina
Citation: Moslehi M, Ahmadi A, Pypker T, Dehghani Ghanatghestani M, Hassani M and Zarafshar M (2025) Halophyte adaptations in gray mangrove seedlings to salinity on the Persian Gulf coastline. Front. For. Glob. Change. 8:1523229. doi: 10.3389/ffgc.2025.1523229
Edited by:
Ned Fetcher, Wilkes University, United StatesReviewed by:
Ko Hinokidani, Tokyo University of Agriculture, JapanMarilyn C. Ball, Australian National University, Australia
Copyright © 2025 Moslehi, Ahmadi, Pypker, Dehghani Ghanatghestani, Hassani and Zarafshar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Moslehi, bWFyeWFtLm1vc2xlaGk1MDhAZ21haWwuY29t; Mehrdad Zarafshar, bWVocmRhZC56YXJhZnNoYXJAbG51LnNl
 Maryam Moslehi1*
Maryam Moslehi1* Tom Pypker
Tom Pypker Mehrdad Zarafshar
Mehrdad Zarafshar