
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 19 February 2025
Sec. Forest Disturbance
Volume 8 - 2025 | https://doi.org/10.3389/ffgc.2025.1394664
Human activities have significantly altered over three-quarters of the Earth’s land surface, intensifying in the last century and threatening remaining ecosystems with ongoing land use changes and climate change. In Chile’s Mediterranean zone, habitat degradation and climate change pose severe threats to biodiversity, particularly affecting endemic tree species with recalcitrant seeds, such as Beilschmiedia miersii, which can face recruitment limitations due to changing environmental conditions and prolonged droughts. This study aims to determine if soil and site-specific conditions at B. miersii population sites limit seed germination and establishment. The study used Lens culinaris as a surrogate bioindicator species to assess soil conditions and their effects on germination and growth. We used laboratory-based experiments and statistical models to analyze the influence of biotic and abiotic factors, including soil nutrients, vegetation cover, and climatic changes. The study’s findings indicate that soil conditions at the examined sites do not pose significant limitations to germination and plant growth. This suggests that the studied sites retain the potential for recruitment, despite the observed variations in soil and site conditions. The results imply that other factors, such as historical climate changes and herbivory, may be impeding recruitment success. While further research is needed to identify the specific factors hindering recruitment and develop effective conservation strategies, this study provides valuable insights into the potential limitations affecting B. miersii recruitment. These findings highlight the importance of considering multiple environmental factors beyond soil conditions when addressing recruitment challenges in threatened species.
Humans have severely altered ecosystems worldwide (Ellis, 2015). Currently, it is estimated that more than three quarters of the Earth’s land surface has been directly altered by anthropogenic activities. These modifications have intensified in the last century, with almost a third of global land surface changes occurring during the last 60 years (Winkler et al., 2021). The remaining original ecosystems are increasingly threatened by ongoing land use change processes (Newbold et al., 2015), and for those habitats that persist, by increasing stress from climate change (Mantyka-Pringle et al., 2015; Segan et al., 2016). Climate change can be a severe threat to the long-term viability of species, particularly due to modifications of temperature and precipitation regimes (Urban, 2015; Román-Palacios and Wiens, 2020; Reynaert et al., 2021). Furthermore, habitat degradation and fragmentation induced by land use change processes can have synergistic effects with climate change, which may increase the current rates of biodiversity loss (García-Valdés et al., 2015; Selwood et al., 2015; Northrup et al., 2019). In the case of long-lived organisms with limited dispersal and long generation times, such as many tree species, viability of species will largely depend on the capacity of the remaining populations to reproduce on-site, which will be contingent climatic conditions within the area (Selwood et al., 2015). There is evidence that climate change is already affecting crucial processes in the life cycle of trees, namely seedling, germination, recruitment, and establishment (e.g., Walck et al., 2011; Yukich et al., 2023).
However, besides climatic conditions, other environmental factors at site level, such as vegetation cover, community composition, litter depth, and soil conditions, may also play a key role controlling the reproduction process of tree species (e.g., Dupuy and Chazdon, 2008; Montgomery et al., 2010; Promis and Allen, 2017). Therefore, small changes at site level during the establishment stage, which is one of the most vulnerable life stages of plants, can greatly influence seedling survivorship (e.g., Lloret et al., 2004). This, in combination with the inherent characteristic of seeds, can make reproduction success even more difficult. For example, plants with recalcitrant seeds (desiccation sensitive seeds) have a short viability span window (few weeks to a few months) for germination because of seed sensitivity to changes in site temperature and humidity (De Vitis et al., 2020; Brock et al., 2023). In addition, some species may present mast seeding strategies in response to climatic cues, meaning that changes in local climatic conditions may reduce the frequency of favorable signals, severely limiting the productions of seeds, leading to negative consequences for tree recruitment and forest dynamics (Bogdziewicz, 2022). Under these circumstances, site environmental conditions will have a pivotal effect on seeds germination once favorable climatic signals trigger the production of seeds.
The Mediterranean zone of Chile has been severely degraded due to the historical change in land use to agriculture, forestry, and urban development (Schulz et al., 2010), leading to large expanses of habitat loss and fragmentation (Fuentealba et al., 2021). Furthermore, models predict that this area could experience a temperature increase of 2°C and a decrease of precipitation (Araya-Osses et al., 2020; Vicuña et al., 2021). These factors combined (i.e., degradation and climate change) are becoming a threat to local biodiversity due to the synergetic effects on species extinction, which in turn can negatively impact the long-term viability of current ecosystems (Muñoz-Sáez et al., 2021). This is of particular concern because this part of the country is considered a global biodiversity hotspot due to high endemism of plant species (Martinez-Harms et al., 2021). Specifically, this hotspot has several endemic tree species with recalcitrant seeds such as Pouteria splendens (A.DC.) Kuntze, Beilschmiedia berteroana (Gay) Kosterm, Beilschmiedia miersii (Gay) Kosterm, Citronella mucronata (Ruiz et Pavón) D.Don, and Cryptocarya alba (Molina) Looser (Figueroa and Jaksic, 2004, Henriquez et al., 2012, Magni et al., 2023), which are already showing signs of recruitment limitations (e.g., Sotes et al., 2018; Magni et al., 2022, 2023).
The genus Beilschmiedia, within the Lauraceae family, is of particular interest because it is mostly distributed throughout the tropics (Nishida, 1999). However, in Chile this genus is distributed in a subtropical zone that corresponds to a Mediterranean climate, where climatic conditions are harsher, with lower water availability and a warmer and dryer summer season (Fernández and Morales, 2016). Beilschmiedia miersii, which is the species from the genus that has the most northern distribution in Chile, is a shade tolerant tree with a maximum height of approximately 25 meters (Henríquez and Simonetti, 2001). Previous dendroecology studies by Venegas-González et al. (2023) show that the age at different populations of B. mierssi can range from 34 to 348 years, with an average age among all sites of 114 years. Fruits are drupes (4 cm long, 2–3 cm wide), recalcitrant, with an unknown disperser (Henríquez and Simonetti, 2001; Marticorena and Rodríguez, 2001). B. miersii is usually associated with other species with recalcitrant seeds such as C. alba. It has been reported that B. miersii shows no seed production and a lack of seedlings in several sites during the last few years, a phenomenon that is not observed with the species Cryptocarya alba (although low germination in site is observed) (Magni et al., 2022; Fernández et al., 2023). Beilschmiedia species are known to show mast seeding processes; however, this has been hardly studied in Chile due to the lack of long-term data on seed fall (Yukich et al., 2023). Thus, B. miersii could be experiencing seeding and recruitment limitations due to the current megadrought, 13 consecutive years with rainfall deficits between 25 to 45%, affecting the central area of Chile (Venegas-González et al., 2023).
Although it is expected that the El Niño Southern Oscillation may still produce wetter years over central Chile (Cai et al., 2020), potentially triggering seeding production by B. miersii, there is scarce information on the current capacity of soil conditions for seed germination and recruitment, or other additional site-scale factors that may impede the first stages of plants establishment. Previous studies have shown that B. miersii germination and survival rates respond positively to leaf litter depth, litter water content, and canopy cover (Kremer et al., 2019), and also that herbivory and seed predations by small mammals and free-range cattle are prevalent limiting factors on the species recruitment (Morales, 2015a). Nevertheless, no studies have analyzed potential soil restriction induced by climate change on this species. Droughts can modify soil physical and biological process, leading to mineral stress, soil toxicity and accumulation of allelopathic compounds (Lynch and St Clair, 2004; Brevik, 2013; Zhong et al., 2023), which may restrict seed germinations and growth even if precipitations regimes are adequate 180 mm to 419 mm for B. miersii according to Becerra et al. (2004).
However, assessing potential soil restrictions for B. miersii establishment is very challenging due to the lack of recent seeds on-site and the short-term viability of seeds collected from previous years. Indeed, this is a common issue for long-lived laurifolious tree species that share their habitat with B. miersii, as they also produce recalcitrant seeds with limited seasonal availability, which has been exacerbated by increasing droughts driven by climate change (Magni et al., 2022). An alternative approach is the use of a surrogate species to evaluate if the soils of current B. miersii populations present unfavorable conditions for seed germination and growth. Surrogate species are selected substitutes used to represent the ecological responses of one or more species to environmental variables, particularly when direct assessment of the target species is impractical or constrained (Caro and O’Doherty, 1999). For the case of forest soil assessment, surrogate species should be able to quickly respond to several chemical and physical characteristics, including pH, salinity, organic matter, toxic elements, nutrients and texture (Schoenholtz et al., 2000).
Lens culinaris is an annual legume species belonging to the Fabaceae family, originally domesticated in the Eastern Mediterranean, but currently widely grown as a crop in Mediterranean, sub-tropical and temperate climates (Khazaei et al., 2016). Lens culinaris has been increasingly used as a bioindicator species for environmental analysis over the last decade. For example, Ostroumov (2016) used L. culinaris seedlings to test the toxic effects of surfactants on plant growth. Other authors have used L. culinaris as a bioindicator to assess metabolic toxicity induced by pesticides (Salazar and Maldonado, 2019; Salazar and Quintero, 2020; Salazar and Quintero, 2021). Salazar and Maldonado (2020) analyzed the effects of a widely used disinfectant on L. culinaris to evaluate its potential as a bioindicator for toxicology assessments. De Silva et al. (2022) used L. culinaris to evaluate the effects of microplastics on germination and seedling growth. Salazar and Vega (2023), evaluated the response of L. culinaris to the widely used medication acetaminophen, finding inhibitions of root development and cell abnormalities. Belasri et al. (2024) used L. culinaris to assess the effect of landfill leachate on vegetation, finding evidence of metabolic effects that reduced germination rates of the bioindicator species. These studies show that L. culinaris is highly sensitive and responds quickly to environmental factors, making it a suitable surrogate to evaluate potential limiting factors of soils for seed germinations. Nevertheless, as many legumes, L. culinaris has nitrogen-fixing capabilities making its seeds more tolerant to growth in nitrogen-restricted environments, which should be accounted for when interpreting the results.
In this work, we aim to test if the soil and other specific conditions from the sites currently holding populations of B. miersii have any limitations for germination and establishment of seeds, using L. culinaris as a bioindicator surrogate species. In particular, we are interested in assessing the direct effects of soil nutrients, and indirect effects of vegetation structure and historic changes of climatic patterns on the capacity of soils to provide suitable conditions for seed germination and growth. To determine if there is any restriction for germination in sites dominated by B. miersii we collected field data and soil samples from 15 B. miersii population sites, using these samples to run germination tests under controlled laboratory conditions. We hypothesize significant differences in germination and seedling growth rates between soil origins, which will be mainly explained by differences in soil available nutrients associated with changes in climatic patterns depending on site regions.
The field data and soil collection were conducted in the Mediterranean climate ecosystems of the Metropolitan and Valparaíso administrative regions, where most of the B. miersii populations are located (Novoa, 2004). We selected a total of 15 sites on a latitudinal gradient, from south to north, and with different degrees of habitat alteration due to urbanization-related processes (Figure 1). The climatic conditions of the area vary slightly between the Valparaíso and Metropolitan regions, with the oceanic western influence being the main factor resulting in these differences. Because of the importance of the oceanic influence on sites, we decided to select around half of the sites in coastal areas and the remaining half in central (inland) areas. The annual mean temperature in the area is 15°C and the precipitation varies between 150 and 450 mm (Araya-Osses et al., 2020). However, these conditions are changing due to climate change, with an increase of maximum temperatures and a decrease of precipitation (Quintana, 2012; Araya-Osses et al., 2020; Vicuña et al., 2021).
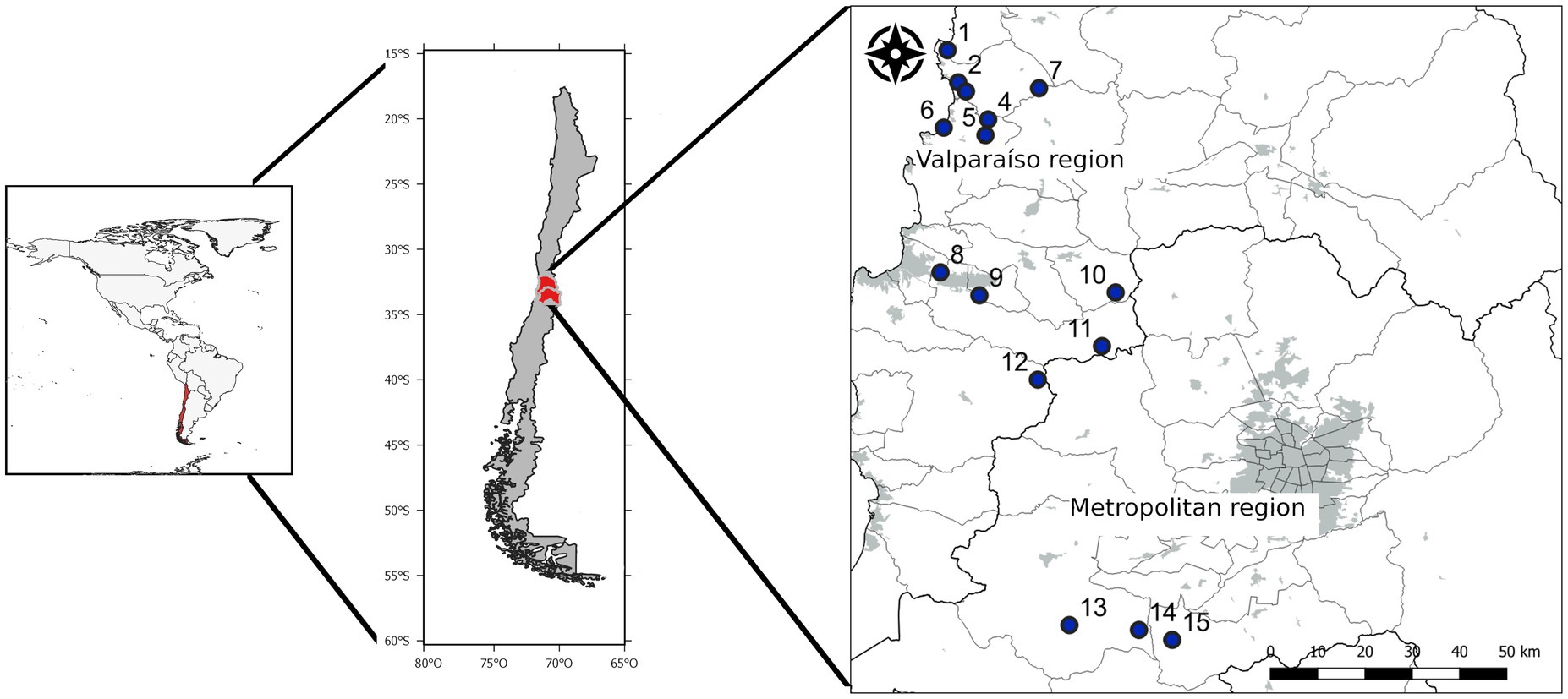
Figure 1. Map showing the study area within the central zone of Chile. Numbers indicate the different study sites: (1) Frances, (2) Aguas Claras, (3) Catapilco, (4) Canela, (5) Quirilluca, (6) Rincon, (7) Melon*, (8), Belloto, (9) Recreo, (10) Alvarado*, (11) Colliguay*, (12) Pangue*, (13) Tantehue*, (14) Arbol and (15) Patagual. Gray zones in the right map represent the extent of current urban areas and green areas the distribution of the B. miersii. * Represents sites located at central (inland) areas.
Most of the soils of the sites analyzed correspond to the alfisol orders and have a mixed loam surface texture, with soil use class VI and VII, mainly due to the slope or depth of the soils (Reyes Calvo et al., 2015). All sampled sites have populations of adult individuals and were selected to have at least three adult individuals incorporated within a 30×30 meter plot, which was then used for vegetation and soil sampling.
We placed a 30×30 meters plot at each site, which was then subdivided into nine subplots of 10×10 meters, made up of a matrix of eight equidistant (10 m) transects of 30 meters of length, four East–West and four North–South. These eight transects were used to estimate total and B. miersii specific vegetation cover with a resolution of 10 cm by using long-distance measuring tapes (line intercept method). Only woody species and individuals intercepting the measuring tape were considered. The space occupied by each individual, including their crown, was recorded. We selected the center and four corner plots from the nine-subplot matrix. In each selected plot, we collected one core sample per B. miersii individual to estimate the site-level age structure of the species. In each of the five subplots we measured the diameter at breast height (DBH) for all present B. miersii trees. To determine the age distribution and the last establishment of new individuals for each site, increment cores were taken. Also, the diameter at core height and the height of core extraction was recorded. Some sites only have a limited number of individuals (4 to 5) within the sampling plots. For these cases, cores were taken from every individual present in the site. Two sites were not part of core sampling because of permission issues as they were in protected conservation areas (i.e., Arbol and Patagual sites). Cores were mounted and then sanded. The age of the tree was estimated by visually counting the annual growth rings in a core sample using a stereo microscope, and adding the number of years it took for the tree to reach the coring height.
To uncover factors influencing germination and growth, we analyzed soil and measured leaf litter depth. Soil samples at the site level were taken from the first 20 cm at the center of each of the five subplots used for estimating age structure. Samples were poured into a bucket, homogeneously mixed and then placed within a large airtight bag of 25×25 cm. After each field trip, soil samples were stored in a dark controlled environment, and half of each sample were sent for laboratory analysis, where soil electrical conductivity, organic matter, and available nitrogen, phosphorus, and potassium were quantified. We used the same five subplots to measure litter depth at site level with a resolution of 0.5 centimeter for each measurement. In addition, we estimated the below-canopy leaf litter depth by taking measurements under the canopy of five individual B. miersii selected at random.
To evaluate the potential effect of site environmental conditions on soil and how this could affect germination, we employed a surrogate bioindicator species. This approach addressed three key challenges associated with B. miersii seeds. First, herbivory by small mammals (e.g., rats) and large mammals (e.g., cows) limited seed availability at the study sites (Morales et al., 2015). Second, potential variability in seed production across sites could have resulted in underrepresentation in certain areas, introducing unwanted variation into the study. Finally, the recalcitrant nature of B. miersii seeds, with their short viability, presented logistical difficulties. Collecting enough seeds from all sites within a timeframe that ensured consistent viability was challenging, potentially biasing germination results.
Bioindicator species are widely used to assess environmental issues like limitations on contaminated soils, pesticide toxicity (in both crop and non-crop plants), and water quality (OECD, 2006, Sinnett et al., 2011). However, their application in predicting effects on native species is often limited (Boutin et al., 2004). This can be due to logistical challenges associated with testing a large number of native species, because the target native species may be threatened or rare or for statistical reasons (Wach et al., 2016). Because of these reasons more generalist and sensitive species have been used. For instance, Morales (2015a) evaluated the potential autotoxicity of B. tawa by first testing the effects of leaf leachate on a bioindicator species, Lactuca sativa L. Then experiments were performed using B. tawa seeds themselves. Interestingly, the study found that L. sativa germination was more affected by the leachate than B. tawa.
The surrogate species approach allowed us to identify and exclude the influence of obvious soil limitations affecting germination and growth at the site level, enabling us to focus on other potential abiotic factors. Given the range of potential suitable bioindicator species, we first determined the soil pH range to narrow down our options based on germination compatibility with the observed soil conditions.
We measured the soil pH of all stored collected samples using the Kalra and Maynard (1991) methodology for organic soils using distilled water as suspension medium. Three replicates of each soil sample were measured with a CE-PH20S meter (PCE Instruments®, Meschede, Germany). The average of these three replicates was calculated, representing the pH of each site. The objective of this process was to select a surrogate bioindicator species that could be used to indicate problems in soil samples that may hinder germination and growth processes. According to the pH range obtained (5.6 to 7.3), we searched for bioindicator species that could thrive under those conditions and that were easily available. After this process we decided to use Lens culinaris, as this species grows well within this pH range. It has been described as a bioindicator species and has been used in phytoremediation experiments (e.g., Jebara et al., 2015; Montejano-Ramírez and Valencia-Cantero, 2024). Lens culinaris has a Mediterranean origin and is a member of the Fabaceae family, which has several native herbs and woody species frequently present in the ecosystems where B. miersii naturally grows. Given the limited existing literature, we selected the ‘Campo lindo’ cultivar., the only one documented for our specific research objectives (Pino-Palma et al., 2021). Although originating in Canada, its genetic composition aligns with cultivars better adapted to the Mediterranean climate (Khazaei et al., 2016).
To evaluate differences in germination and seedling growth rates between soil origins, we used a laboratory-based experiment. We used petri dishes half filled with substrate from the different soil origins, with three replicates per each of the 15 sites (n = 45). In each dish, 20 seeds of L. culinaris were sown, totalling 900 seeds for the experiment. The dishes were randomly placed in two germination chambers (BJPX-A300II, BIOBASE®) and maintained there for 6 days until the end of the experiment. The temperature and humidity were set at 22°C and 70%, respectively. In addition, a light and night cycle of 16/8 h was set. Seed germination was recorded daily in each plate until the hypocotyl appeared. After this step, the observed mortality for seeds and seedlings was recorded daily. At the end of the experiment petri dishes were taken out of the chambers and all seeds were measured with a millimeter graduated rule in relation to two variables: hypocotyl and radicular length. Germination percentage, mortality percentage, and germination speed were calculated (Chiapusio et al., 1997; Morales, 2015b).
To evaluate the potential effect of site environmental conditions on soil that could affect germination and growth, we used two different regression models approaches. Germination data was non-normally distributed; therefore, we fitted a quasi-Poisson generalized model to the data. The remaining data were normally distributed; thus, we used linear models to analyze the effects of site conditions on hypocotyl and radicle growth, and on germination speed.
All regressions (non-linear and linear) were performed using R 4.2.1 and the “Stats” package (R Core Team, 2023). To detect significant differences among soil origins, a post hoc test was performed using a Tukey HSD with the package “emmeans” (Lenth, 2025). Effect size calculations were done using the packages “countES” (Coxe, 2023) for non-linear models and “effectsize” for linear models (Ben-Shachar et al., 2020).
To evaluate which biotic and abiotic variables have an effect on germination and growth, we used a generalized linear mixed model for germination and linear mixed models for the other response variable (Speed germination index (S), hypocotyl length and radicle length). The models included a set of independent variables representing site conditions. These variables were soil pH, electric conductivity, organic matter, available nitrogen, phosphorus, potassium, vegetation cover, depth of leaf litter at site level, depth of leaf litter below canopy of B. miersii individuals, vegetation richness, and changes in precipitation and temperatures (maximum and minimum) in the last 20 years. In all the fitted models, region was used as a random factor.
To estimate changes in climatic variables across the 15 sampled sites we used data from the global climate model CHELSA (Karger et al., 2017). The CHELSA model provides continuous coverage of global climate based on a rescaling of a reanalysis of global circulation data at high resolution (30 arcsec, ~1 km) (Brun et al., 2022). To perform this task, the centroid of each sampling site was intersected with the data at the monthly level for the period 1990–2018 for the following variables: precipitation, minimum T°, and maximum T°. Subsequently, we computed the mean decadal historical changes (last 20 years) for these variables using the last decade (i.e., 2010–2018) as a reference. For calculations and spatial processing, we used the R “stats” (R Core Team, 2023) and the “raster” package (Hijmans, 2023).
To avoid multicollinearity, a correlation matrix was built using the package “stats” (R Core Team, 2023) to determine the correlation between the variables analyzed (Supplementary Table S1). All pairs of variables with a correlation greater than 0.6 were identified, and one of them discarded, which was done automatically using the “findCorrelation” function from the package “Caret” (Kuhn and Max, 2008). The variables that were discarded after this analysis were the difference in maximum temperature, leaf litter depth at site level, leaf litter below individuals, nitrogen, and species richness.
All the fitted mixed models (non-linear and linear) were fitted using the package “lme4” (Bates et al., 2015). We selected the best model for germination data by identifying the model with the lowest “quasi-AIC” (QAIC) based on Bolker and R Development Core Team (2022), using the package “Bbmle” (Bolker and R Development Core Team, 2022). For the remaining models, we selected the best models by identifying those with the lower AIC. This methodology fits a complete model and then compares it against simpler models using a stepwise algorithm included in the package “stats” (R Core Team, 2023). Comparisons between site regions were done using a Mann–Whitney test using the package “stats” (R Core Team, 2023).
Table 1 shows a descriptive summary of the data collected in the 15 analyzed sites, including canopy cover, soil characteristics, plant species richness and climatic variability. All sites corresponded to forest patches, with an average canopy cover of 75.6% and 14.3 species per site. However, both the canopy cover and species richness show important variances between sites. The site showing the lowest canopy cover was Rincón, with 49.3%, while the one showing the highest cover was Árbol, with 91.1%. Species richness ranges from six species in Catapilco and Recreo, to 26 in Pangue. Our data revealed that the oldest trees were concentrated at Tantehue (center) and Aguas Claras (coast), with average ages of 186.8 and 152.9 years, respectively. The youngest trees on average were located in the center at the Colliguay and Pangue sites with an average age of 48.8 and 49.5 years, respectively. Interestingly, there was little variation in ages at site level in the center regions in comparison with the sites located toward the coast (Figure 2). In terms of sites region, we only find significant differences for species richness and precipitations (Wilcoxon rank-sum test p = 0.012 and p = 0.018, respectively, Table 1).
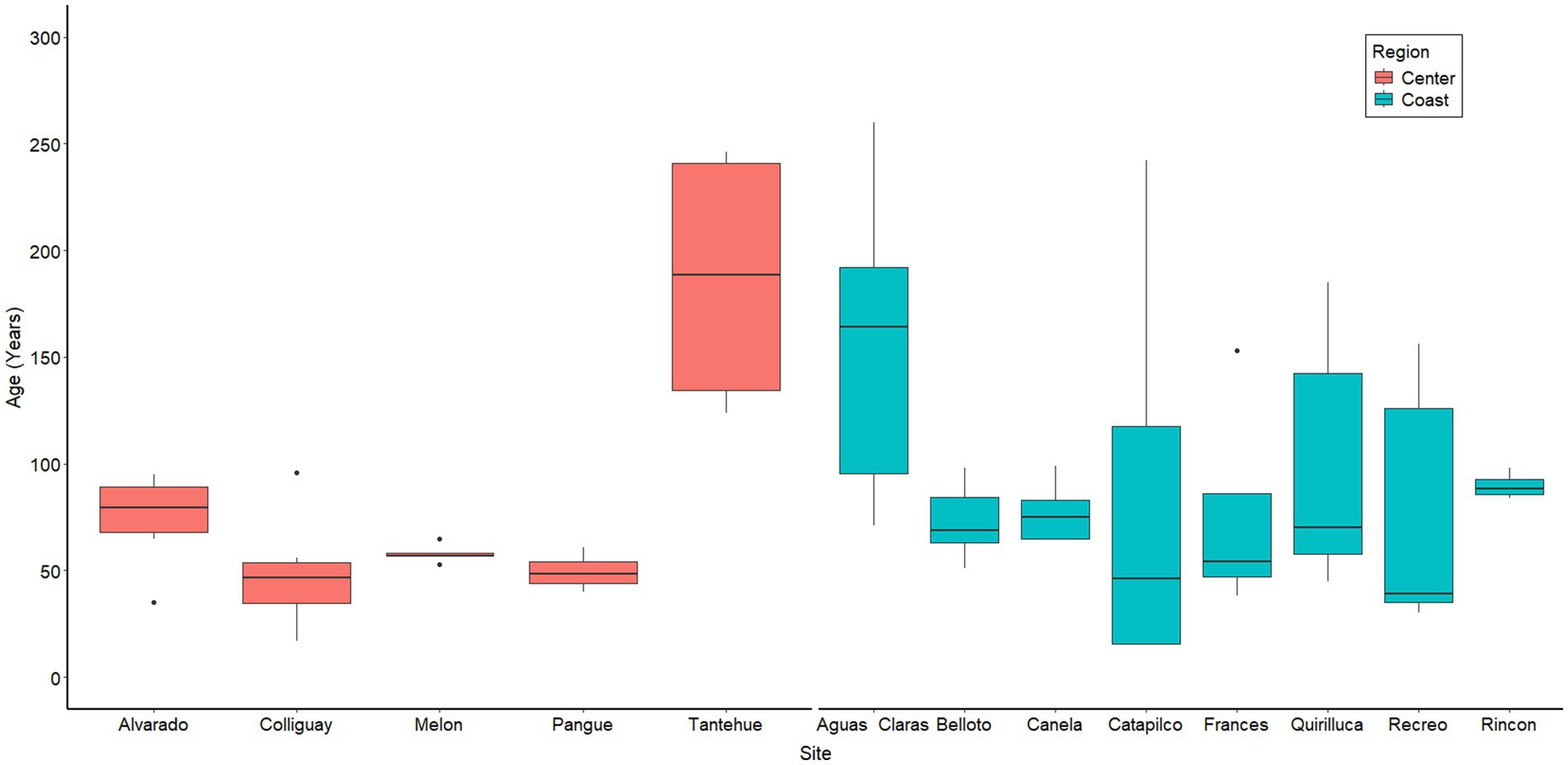
Figure 2. Plot illustrating the age distribution of trees across different sites. The ages were determined using increment core samples.
The results from a quasi-poisson model, using germination as a response variable and the soil origin as a predictor variable, showed that there were significant differences in seed germination among soil origins (D = 32.93, d.f = 14, p = 0.000). The soil origin with the lowest germination rate was Patagual with 75%. Three sites showed an intermediate rate of germination, Aguas Claras (85%), Catapilco (80%), and Quirilluca (85%). The rest of the soil origins ranged from 95 to 100% (Figure 3). A post hoc test indicated significant differences between the Patagual soil origin and 10 other soil origins. The effect size of this analysis was relatively large according to Cohen (2013) (d = −1.35, d > 0.8). We did not find any difference in germination among coastal and central regions (W = 0.06, p = 0.051; Figure 4).
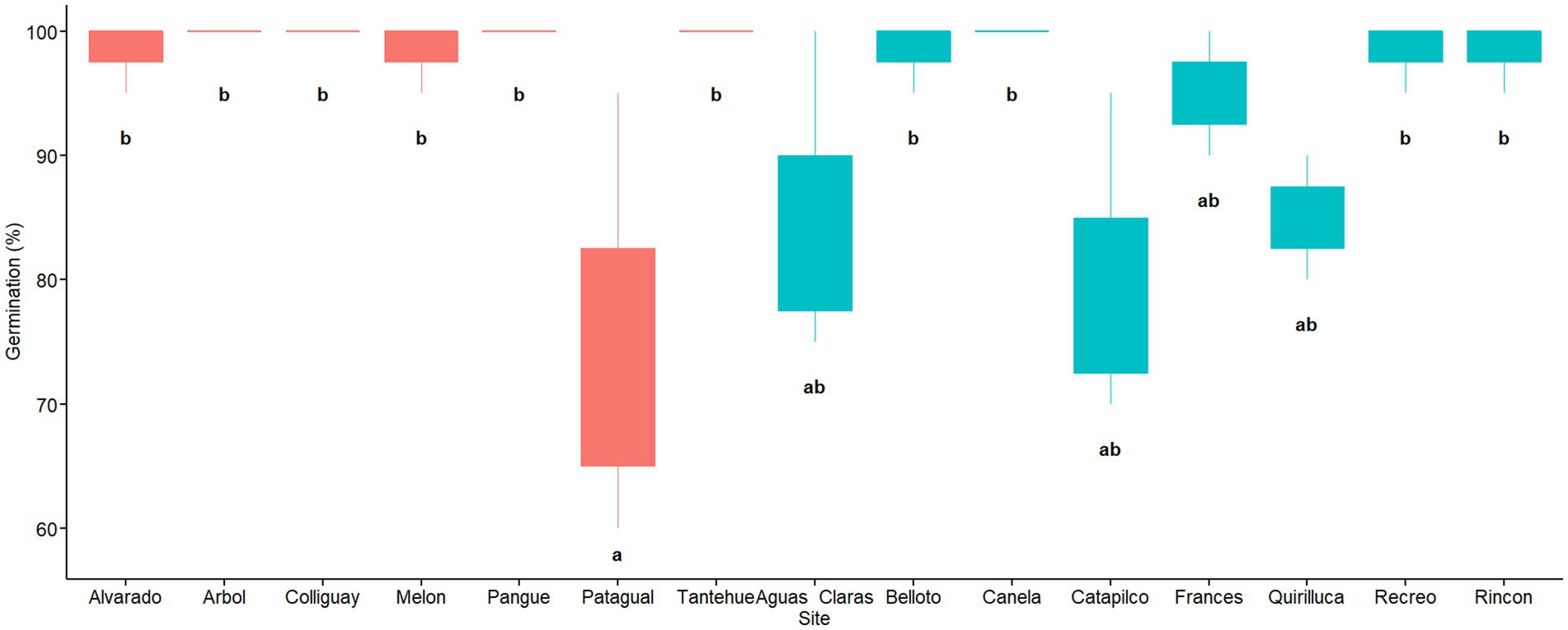
Figure 3. Germination rate (%) per soil origin. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Different letters indicate statistically significant differences at p ≤ 0.05.
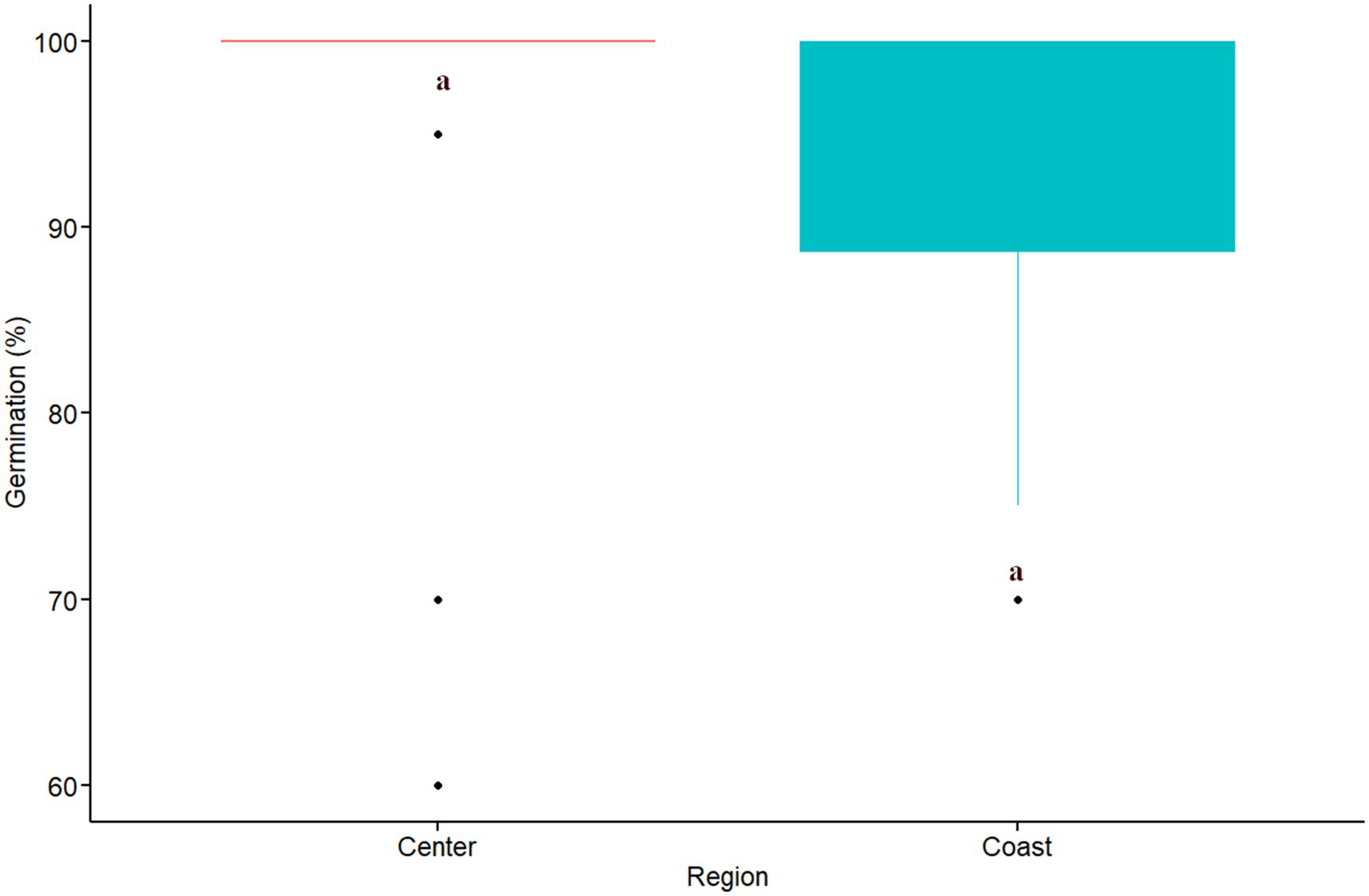
Figure 4. Germination rate (%) per region. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Equivalent letters indicate no statistically significant differences at p ≤ 0.05.
A linear model was used to evaluate the effect of the soil origin on the germination speed (GSI) showing that the soil origin has an effect on the germination speed (F14,30 = 14.21, MSE = 40.9, p = 0.000). The soil of the Catapilco site had the lowest GSI with 2.86, being 5.7 times lower than the maximum value of GSI at the Melon site (GSI = 16.41). Following this low GSI value were Patagual and Aguas Claras with a value of 5.14 and 5.8, respectively. Then there is a group of soil origins with intermediate values such as Frances, Rincon, Recreo, Arbol, and Canela that range from 8.44 to 9.5. Finally, there were a group of six sites showing relatively higher GSI values, which ranged from 10.76 to 16.41. The sites within this group were Alvarado, Pangue, Tantehue, Belloto, Colliguay, and Melon (Figure 5). A post hoc test revealed that the GSI varied among sites and that these differences were statistically significant (Figure 6). We also found differences in GSI among locations (W = 393, p = 0.001; Figure 5).
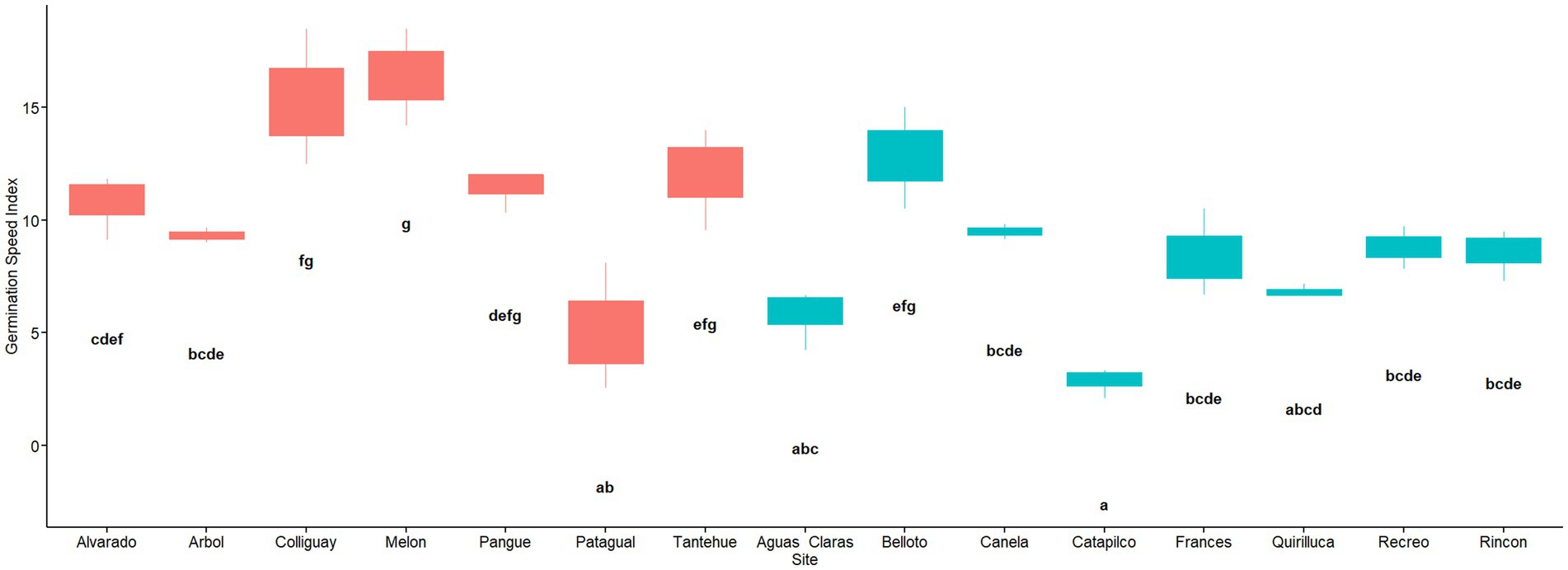
Figure 5. Germination Speed Index (GSI) per soil origin. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Different letters indicate statistically significant differences at p ≤ 0.05.

Figure 6. Germination Speed Index (GSI) per region. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Different letters indicate statistically significant differences at p ≤ 0.05.
The effect of different soil origins (sites) on hypocotyl length was significant (F14,30 = 9.98, MSE = 831.3, p = 0.000) and the omega effect size was large (ω2 = 0.74). The soil origins with the lowest and highest hypocotyl mean length were Catapilco and Melon, with a value of 10.8 and 71.3 mm respectively; a difference of 6.6 times. Data show that there are three groups with similar hypocotyl length values. Among these groups the one with the highest hypocotyl growth is composed of the soil origins of Belloto, Canela, Colliguay, Frances and Rincon ranging from 43.4 to 52.2 mm. A group with intermediate hypocotyl length (from 31.7 to 40.5 mm) was represented by the soil origins Pangue, Quirilluca, Recreo and Tantehue. The group of soil origins with the lowest hypocotyl length (less than 30 mm) was represented by five soil origins (Aguas Claras, Alvarado, Arbol, Catapilco and Patagual). Among these soil origins, some had an extremely poor hypocotyl development that did not exceed the 16 mm mark (Catapilco and Aguas claras). A post hoc test showed that some of these groups differed significantly between them (Figure 7). We did not find differences in the hypocotyl mean length among regions (W = 230, p = 0.620; Figure 8).
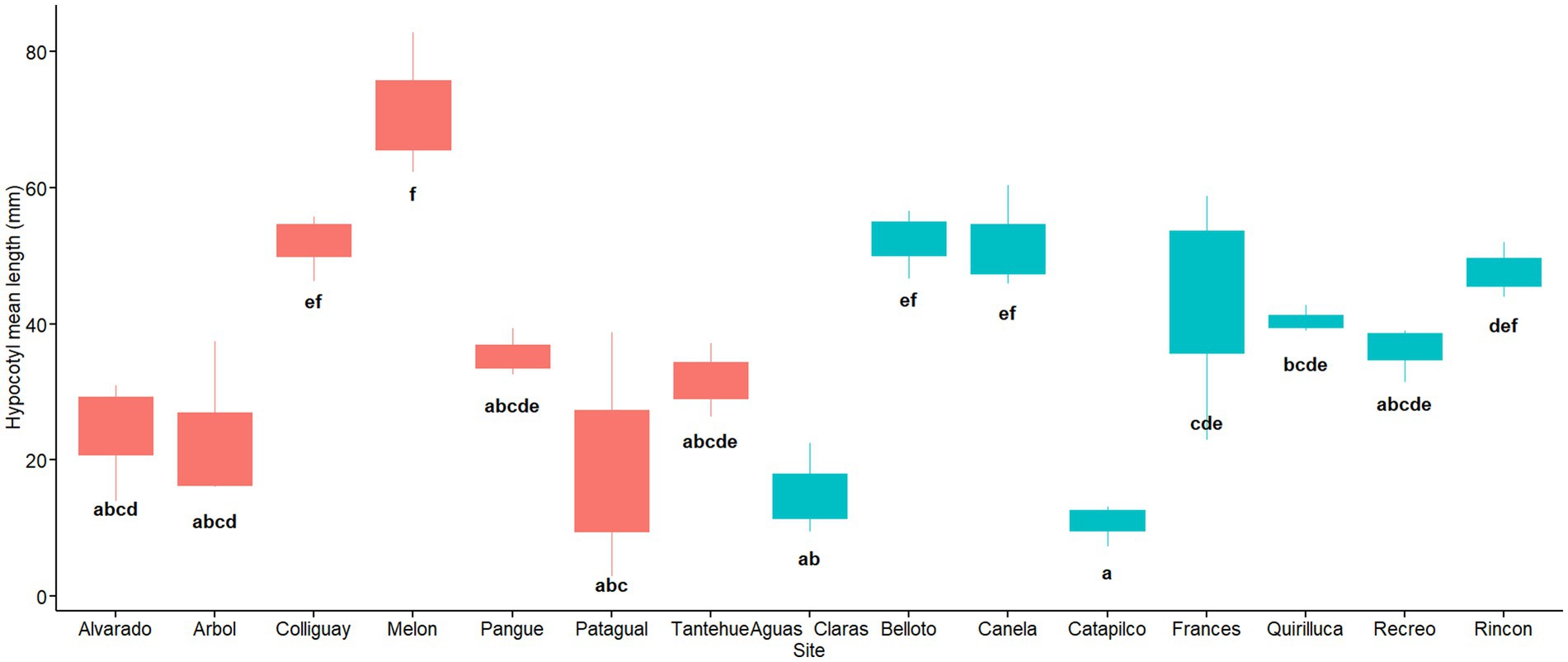
Figure 7. Hypocotyl length per soil origin. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Different letters indicate statistically significant differences at p ≤ 0.05.
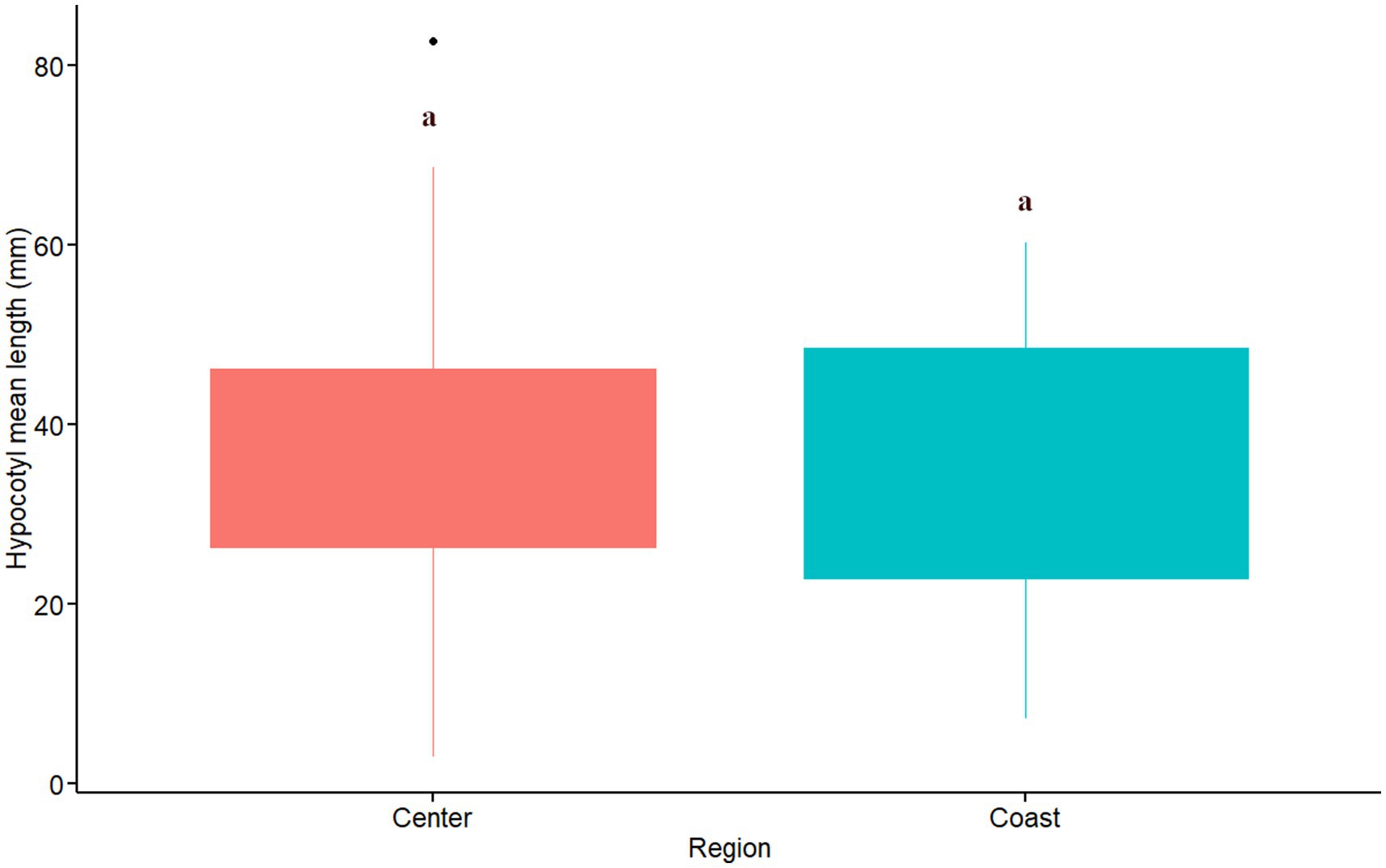
Figure 8. Hypocotyl length per region. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Equivalent letters indicate no statistically significant differences at p ≤ 0.05.
The sites also had a significant effect on the length of the radicle (F14,30 = 5.14, MSE = 356,6 p = 8.47×10-05) and the omega effect size was large (ω2 = 0.56). In contrast to the hypocotyl mean length, the effect of soil origin on radicle length is not clear. The main significant differences were observed among only two soil origins with the higher radicle length values and the six with the lower values of radicle length (Figure 9). The sites with radicle lengths ranging from 26.4 to 35.6 mm were not significantly different from the soil origins with lowest or highest radicle development. We did not find differences in the radicle mean length between regions (W = 193.5, p = 0.18; Figure 10).
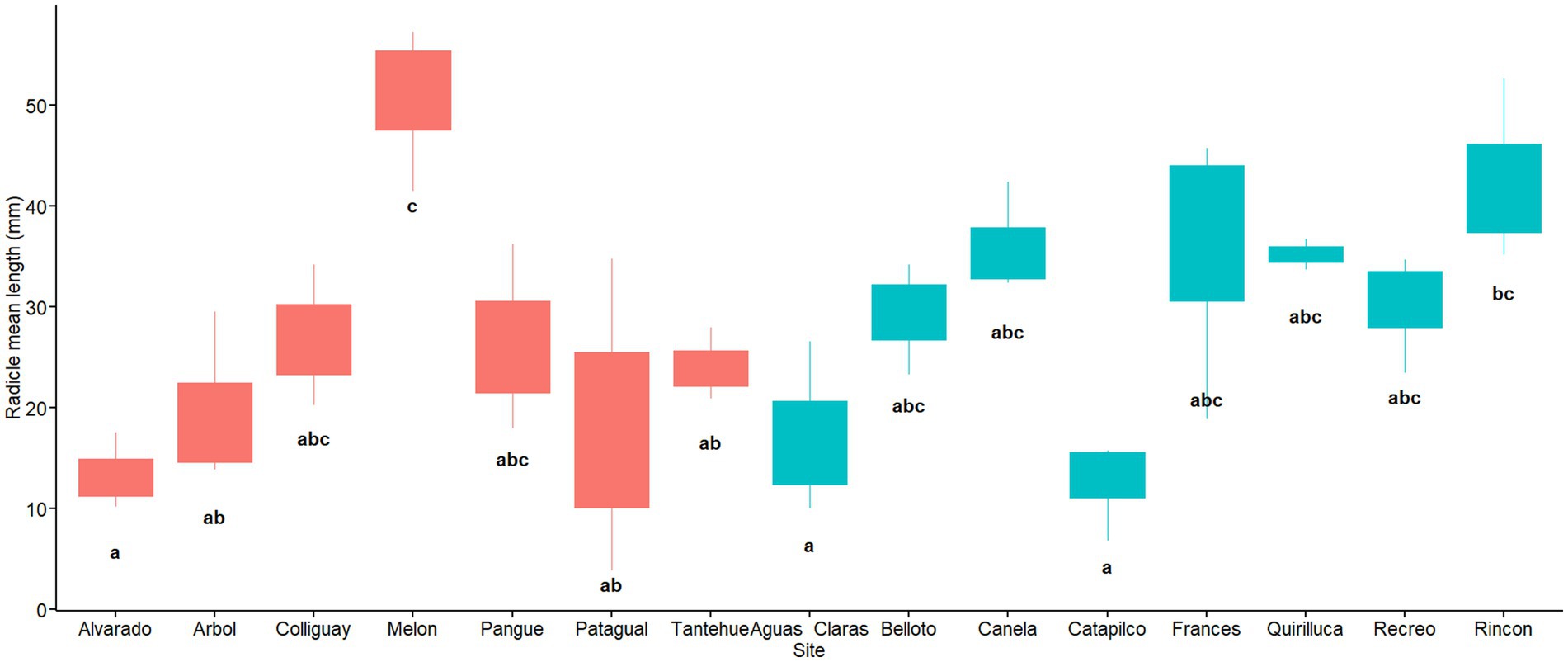
Figure 9. Radicle length per soil origin. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Different letters indicate statistically significant differences at p ≤ 0.05.
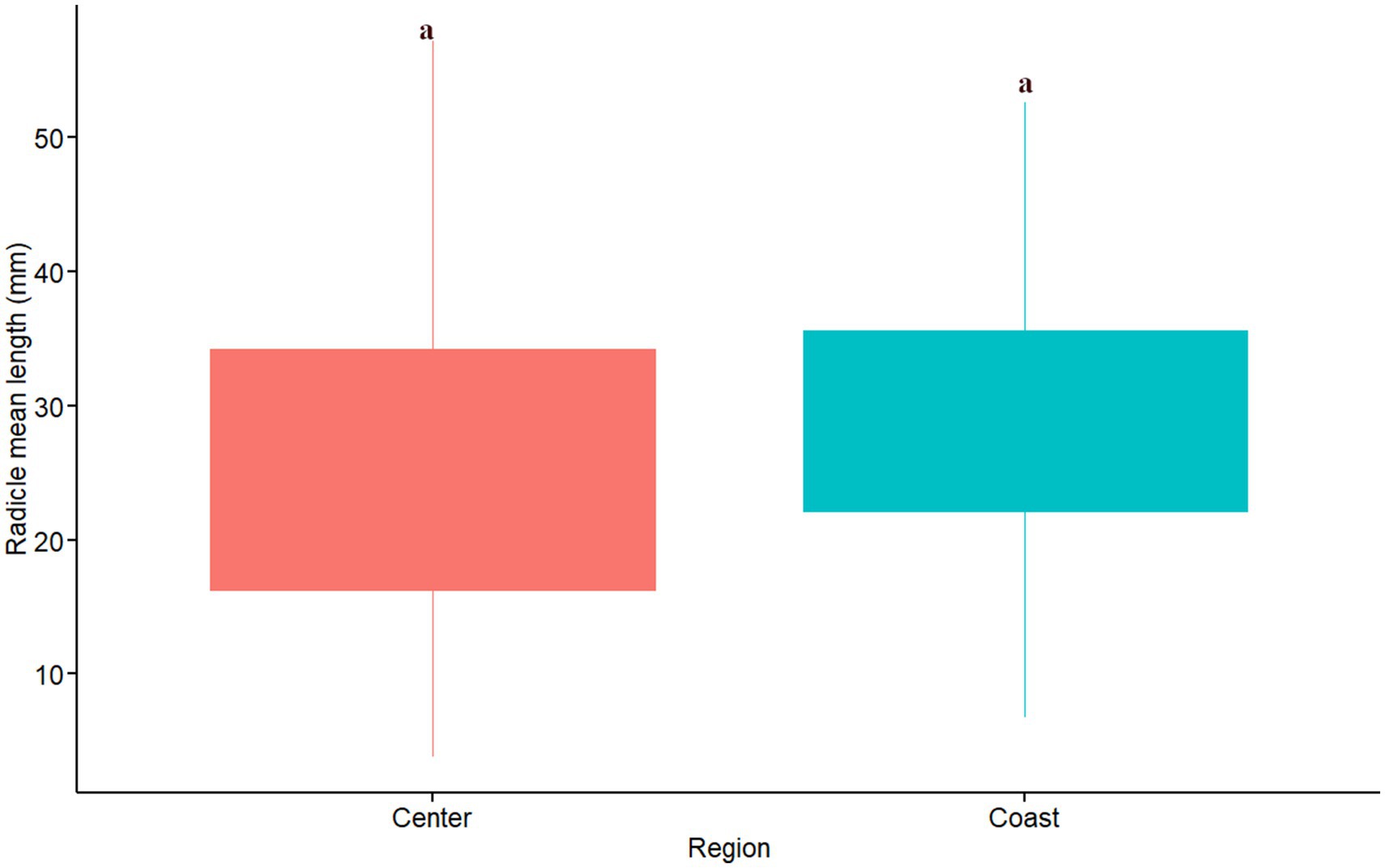
Figure 10. Radicle length per region. Red and blue colors indicate sites located in central and coastal areas as per Figure 1. Equivalent letters indicate no statistically significant differences at p ≤ 0.05.
The model showing the lowest qAIC for germination rate included organic matter, phosphorus, potassium, vegetational cover, difference in precipitation, and difference in minimum temperature as predictor variables. This model is able to predict differences in germination, but with low explanatory power (R2 = 0.32, χ28, 37 = 17.9, p = < 0.002). Organic matter (z = −0.34, df = 1, p = <0.001) and vegetational cover (z = 2.1, df = 1, p = 0.040) were the most significant variables explaining differences on germination rate between soil origins (Table 2).
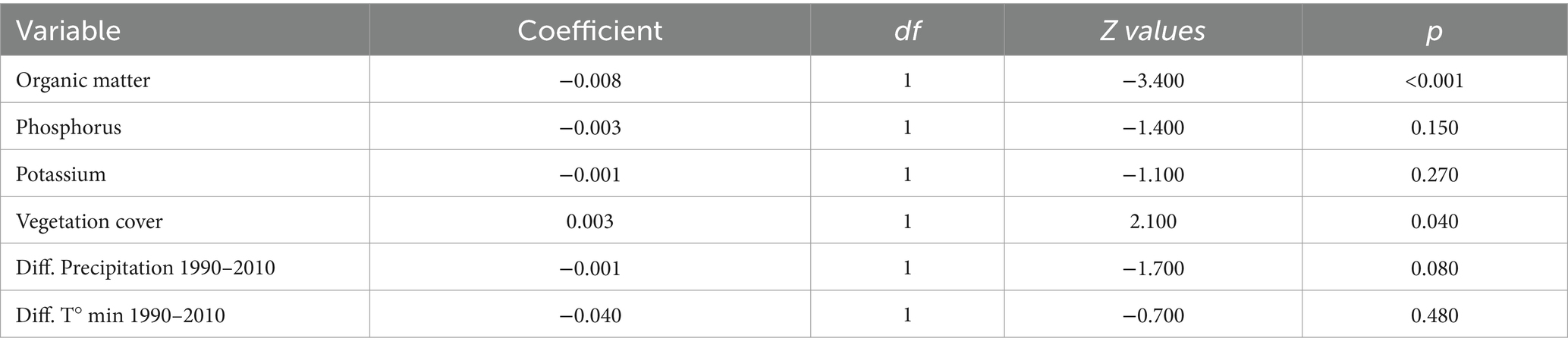
Table 2. Fixed effects table for the quassi-poisson regression fitted using the germination rate as the response variable.
In relation to the GSI, the final model included pH, electric conductivity, organic matter, potassium, vegetational cover, difference in precipitation and difference on minimum temperature. This model has a considerable explanatory power to predict differences in the germination speed index (R2 = 0.73, χ28, 37 = 58.6, p = <0.0001). All of the independent variables included in the model were significant (p ≤ 0.05; Table 3).
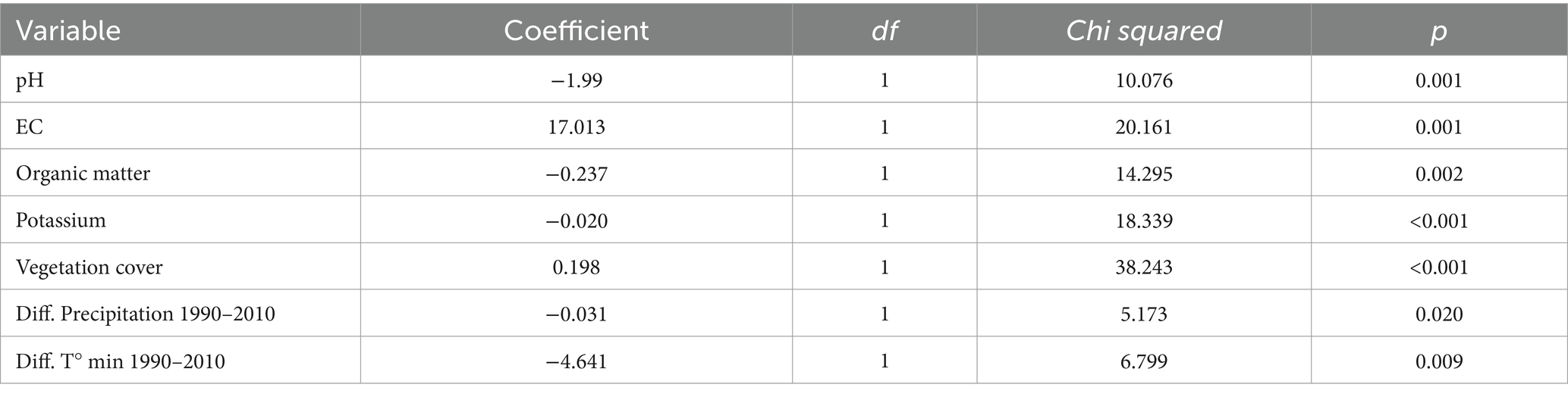
Table 3. Fixed effects table for the linear mixed model using the germination speed index as the response variable.
The best model to predict the hypocotyl length included pH, EC, organic matter, potassium, and vegetational cover. The model was able to predict changes in hypocotyl length with a moderate explanatory power (R2 = 0.45, χ25,40 = 27.6, p = < 0.0001). All the independent variables included in the model were statistically significant (Table 4).
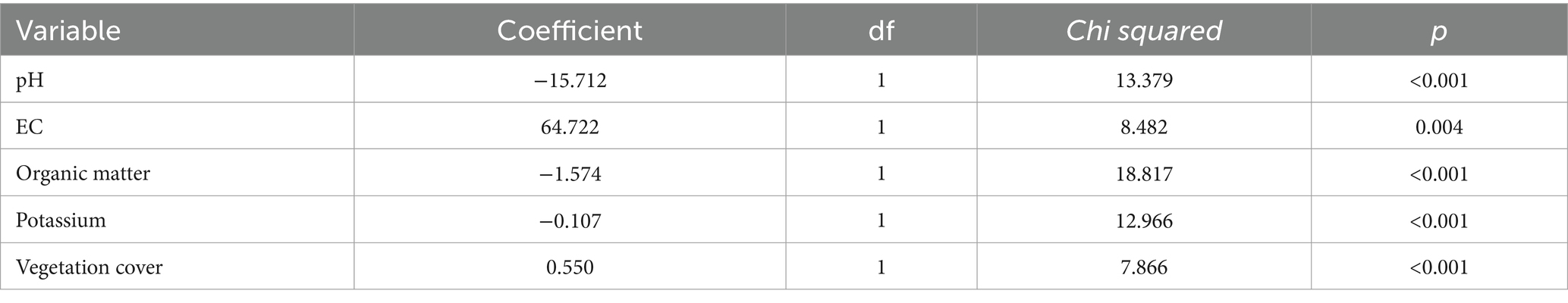
Table 4. Fixed effects table for the linear mixed model fitted using hypocotyl lengths as response variable.
For radicle length, the best model was achieved using only soil pH, EC, organic matter and potassium as explanatory variables, resulting in a significant model, but with low explanatory power (R2 = 0.25, χ24, 41 = 13.51, p = 0.009). Among the independent variables, only pH and organic matter were significant in explaining changes in radicle lengths (Table 5).
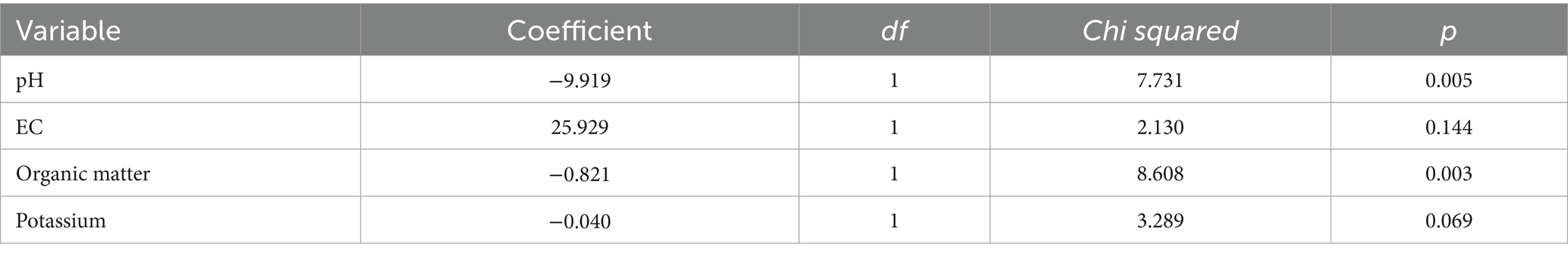
Table 5. Fixed effects table for the linear mixed model fitted using radicle lengths as response variable.
While we did find significant differences in germination and growth rates among soil origins, we did not find clear signs of germination limitations for any of the soils from the analyzed sites. Even though we were using a surrogate species, instead of seeds of B. miersii, our results suggest that all soil origins remain capable for recruitment, despite the observed variations in soils and sites conditions. These results were somewhat unexpected, given the lack of recruitment observed in the field at several sites, which was a pattern we were anticipating to also find in our laboratory experiment. An explanation for this may be related to the use a surrogate species (i.e., Lens culinaris) that requires different germination conditions that is also known to cope with limitation of nitrogen by fixing this nutrient from atmospheric sources. Nevertheless, we did not find nitrogen restrictions in any of the analyzed soils. Thus, other environmental limitations must be operating on sites, such as inadequate vegetation cover, harsh climatic conditions and increased herbivory, which were obviously not an issue at the laboratory setting.
We observed significant differences in the four analyzed variables (germination rate, germination speed, hypocotyl length, and radicle length) across different soil origins. Additionally, we identified specific site parameters associated with these variations, indicating that certain environmental factors at the site level play a crucial role in seedling recruitment. Yet, germination rates were relatively high, and mortality very low in all analyzed soils, indicating that the recruitment problems may be due to processes happening at seedling stages. Indeed, we were able to find some seedlings of B. miersii in 4 out of the 15 sites, ranging from 28 to 833 individuals per hectare, during our field work, but we did not see any evidence of seedling survival to sapling stage. Previous works have found that herbivory by exotic small-mammals [European rabbit (Oryctolagus cuniculus)] and dry summers may play a key role precluding the transition from seedlings to saplings in other mediterranean-type ecosystems (MacDougall et al., 2010; Granda et al., 2014), and the same effects has been also found for the Chilean Mediterranean ecosystem (Fleury et al., 2015). Thus, the same pressures could be precluding the recruitment of B. miersii on-site. Interestingly, we found that vegetation cover on-site was related to germination rates on the laboratory test, which may imply that vegetation cover could not only be relevant for decreasing water stress during the dry season but may also generate specific soil conditions that increases seed germination (Aponte et al., 2010).
The GSI tended to be higher for soil origins located in the coastal area, which could be attributed to variations on organic matter, canopy cover, species richness, nitrogen, and climatic conditions. However, among the aforementioned factors, only organic matter and nitrogen have been directly linked to germination speed changes according to the literature (Turk et al., 2004; Abazarian et al., 2011; De Souza Marinke et al., 2019; Cakir and Ceyhan, 2021). These two factors were relatively higher at the coastal sites, which may explain the differences on germination speed index (GSI). Nevertheless, our resulting explanatory model for GSI shows that organic matter is negatively related with GSI when all soil origins are considered, which may look counterintuitive. A deeper exploration of our data reveals that within the central sites there is a negative correlation (r = −0.57) between organic matter and nitrogen, which is not observed in coastal sites (r = −0.08). This difference may imply a differential effect of climatic change on these sites depending on their region. We argue that the decrease of precipitation and minimum temperature could cause the accumulation of organic matter and lower nutrients availability in soils due to reduced decomposition rates (e.g., Kirschbaum, 1995; Butenschoen et al., 2011). This process may be less pronounced in coastal areas, as there is a constant flow of humidity from the ocean that controls temperature and increases soil water content. Temperature is documented to have an influence on organic decomposition (e.g., Dwevedi et al., 2017), potentially increasing the decomposition rates in the hotter inland sites, but the dryer conditions of these areas may be limiting the decomposition processes. Despite the relationship observed between germination speed index and region, as well as the potential influence of certain independent variables, we acknowledge that our sample size is insufficient to draw definitive conclusions about these trends.
Both hypocotyl and radicle mean length showed significant differences among soil origins but not between regions. Patterns of hypocotyl and radicle growth were tightly related (r = 0.89), suggesting that these variables are responding to the same factors. Indeed, results from the mixed models show that both variables respond to the same factors (e.g., pH, EC, organic matter, potassium) but hypocotyl length also relates to site vegetation cover. Factors known to have an effect on hypocotyl growth include light intensity, temperature, water content, nutrient availability and organic matter (e.g., Xiong and Nilsson, 1999; Wang and Shang, 2020). However, in our study all these factors were controlled, except for soil nutrients and organic matter. Thus, the variability in nutrients such as nitrogen, phosphorous and potassium among sites may be a key factor explaining the differences in hypocotyl and radicle growth (Zhang and Wienhold, 2002; Abd El-hady et al., 2022). Nevertheless, contrary to what may be expected, potassium shows a negative effect on hypocotyl length. There is some evidence of a potential negative effect of an excess of potassium on plant growth which is mediated by modifications of the nutrient uptake processes (Xu et al., 2020, Papadakis et al., 2023). Our experimental setting had soils with relatively high concentrations of potassium; therefore, the excess of potassium could be a plausible explanation. However, this potential mechanism cannot be directly applied to B. miersii or other native species, as these have evolved to growth in naturally occurring high-potassium concentration soils.
Contrary to the prevailing notion that organic matter enhances plant growth, our study revealed a negative association between organic matter content and growth parameters (hypocotyl and radicle elongation). This seemingly paradoxical finding corroborates previous reports documenting the detrimental effects of organic matter on plant growth under specific conditions (e.g., Xiong and Nilsson, 1999). Nutrient depletion and the presence of allelopathic compounds, particularly polyphenols derived from evergreen litter, are potential mechanisms underpinning this negative relationship (Lopez-Iglesias et al., 2014). In addition, the potentially decreased availability of nutrients in soils due to slower decomposition rates can also influence growth (e.g., Kirschbaum, 1995, Butenschoen et al., 2011). While these factors could explain the negative relationship between organic matter and growth, this difference is more likely due to a positive relationship between organic matter and water retention (Lal, 2020). High moisture levels can hinder plant growth, for example, by reducing oxygen availability (Lacroix et al., 2021). In this study, the use of petri dishes without drainage, combined with high organic matter content in some soil samples, could have led to excessive moisture, thus causing the observed differences. In any case to have a conclusive answer field experiments will be needed.
Our results revealed a positive relationship between canopy cover and hypocotyl length. This finding is likely due to the close link between canopy cover and soil properties, particularly increased availability of nutrients like nitrogen and phosphorus (Gallardo, 2003). These nutrients have been shown to concentrate under tree canopies, with studies reporting contributions to the soil ranging from 49 to 90% (Prescott, 2002). Consequently, higher canopy cover could translate to potentially higher nutrient concentrations (N and P), which, due to their known positive impact on growth, can potentially influence hypocotyl length. This hypothesis appears to be supported by our data, as we observed a positive correlation between hypocotyl length and nitrogen levels (r = 0.57).
In terms of pH, results suggest a negative relationship between pH and hypocotyl and radicle length, meaning that there decrease in length when there is an increase in acidity of soil. Studies in other species such as red clover (Trifolium pratense L.) show that pH can have an impact on radicle length (Agić et al., 2009). However, it will depend on the tolerance range of the target species. Lens culinaris are tolerant to a wide range of soil pH, from moderately acid to slightly alkaline (Panuccio et al., 2022). The lentil cultivar we used in our study seems to present better growth responses in slightly acidic soils, but it did not show germination and growth problems in any of the assessed soils. We believe that the observed trends were perhaps explained by an interaction with other variables that were not studied.
The analyzed soil samples in this study do not show major restrictions for low recruitment of the surrogate species. Even the sites that have the worst performance still show reasonable germination and mortality rates, without major barriers to growth in the early stages of establishment. However, the main question of our study is whether the sites have the conditions to promote the recruitment of B. miersii. Most of these sites do not show evidence of successful recent regenerations as the youngest B. miersii individual we managed to find was dated to be 15 years old (Figure 1). From the 15 sites analyzed in this study, only four present seedlings, but no site had saplings. Interestingly, the age distribution of sites in the center was more homogeneous than in the coast and the four sites where seedlings were present were in coastal regions. These results are consistent with those of other species in the genus, such as Beilschmiedia tawa, where a lack of recruitment was also observed, and the youngest individual found was 45 years old (Morales, 2015a).
These results imply that other factors not analyzed in this study may explain the lack of B. miersii recruitment observed in the field, such herbivory, seed removal or specific microsite conditions. Seeds of B. miersii have short viability, and high humidity needs, especially after seeding in periods of drought. In addition, seeds require a leaf litter cover to secure germination and establishment (Becerra et al., 2004, Kremer et al., 2019). Studies have shown that B. miersii recruitment is highly dependent on regular water availability, with watering periods every two to 3 weeks to favor germination and promote the survival of seedlings. The amount of water in controlled studies have varied from 180 mm (dry year) to 419 mm (wetter year) (Becerra et al., 2004), and 502 mm (Kremer et al., 2019). The climatic data show that all the sites in our study have experienced a decrease in precipitation in the last 20 years (Table 1; Supplementary Figure S1). This reduction is significantly more intense during the first and second quarter of the year with an average decrease of 7 mm and 256 mm, respectively. A small decrease during the first quarter can be seen as marginal, however, is a critical time for the germination of B. miersii, as changes in humidity can have huge effects on the viability of recalcitrant seeds (Wen and Cai, 2014). Regarding litter, both studies highlight the importance of litter in successful germination, with a litter depth greater than 5 cm producing the best results (Kremer et al., 2019). However, it is important to note that in the present study, only 27% of the sites had a litter depth greater than 5 cm, which can also help to explain the lack of recruitment (Table 1).
We did not find any clear limitations, at least at the soil level, that could hinder germination and plant growth at the studied sites. B. miersii has recalcitrant seeds that have special requirements that are currently not met at all sites. The main current issues appear to be historical changes in climate variables (e.g., precipitation) and herbivory. Changes in other climatic variables, such as minimum and maximum temperature, can also cause disruptions in several processes and mechanisms that can exacerbate recruitment problems, and therefore are key for further analysis. Despite the limitation of using L. culinaris as a surrogate species, whose soil and water requirements may differ from B. miersii, it remains a valuable indicator of potential soil constraints for plant establishment. If this less tolerant species struggles to germinate and establish, it’s unlikely that native species like B. miersii would fare any better.
While this study employed a bioindicator species to assess soil limitations for B. miersii seed establishment, future studies in natural settings should prioritize native species, particularly B. miersii, to both minimize invasive risks and directly explore its recruitment strategies under shifting climatic conditions. In addition, coarse-scale climatic data, while valuable, lacks site-specific detail. To elucidate annual trends, sensor-driven research at site level on abiotic variables like temperature and soil moisture is essential to determine if the minimum conditions for recruitment are being met. Due to the complex interplay of different variables, future studies incorporating ecological modeling could help to evaluate the changes in these variables and their impacts on the viability of B. miersii dominated forests.
In conclusion, our study did not identify significant soil-related barriers to the germination and early growth of B. miersii, and the species’ recruitment appears to be influenced by other factors such as historical climate changes and herbivory. In Chile, efforts have been made to protect streams and gullies, which are the habitat of this species. However, changes in environmental factors, such as water availability, threaten their long-term viability. If this scenario continues, the species may face significant challenges. The use of a surrogate species highlighted potential soil constraints, but future research should focus on B. miersii itself to better understand its specific requirements and challenges. Detailed, site-specific climatic data and ecological modeling will be crucial in addressing the complex variables affecting B. miersii recruitment and ensuring the sustainability of its populations under changing environmental conditions.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
NM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. IF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. JR-A: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. EA: Investigation, Resources, Writing – review & editing, Validation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Chilean Native Forest Research Grant (Fondo de Investigación del Bosque Nativo), Project CONAF 009/2020: “Rol del cambio de uso de suelo, disturbios antrópicos y cambio climático en la degradación del hábitat y viabilidad de Belloto del Norte y Guayacán”. EA has received research support from ANID PIA/BASAL AFB240003.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can befound online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1394664/full#supplementary-material
Abazarian, R., Yazdani, M. R., Khosroyar, K., and Arvin, P. (2011). Effects of different levels of salinity on germination of four components of lentil cultivars. Afr. J. Agric. Res. 6, 2761–2766. doi: 10.5897/AJAR10.785
Abd El-hady, M. A., Abd-Elkrem, Y. M., Rady, M. O. A., Mansour, E., El-Tarabily, K. A., AbuQamar, S. F., et al. (2022). Impact on plant productivity under low-fertility sandy soil in arid environment by revitalization of lentil roots. Front. Plant Sci. 13:937073. doi: 10.3389/fpls.2022.937073
Agić, D., Gordana, B., Grljusic, S., and Beslo, D. (2009). Effect of pH on α-amylase activity and early seedling growth of red clover (Trifolium pratense L.). Notulae Bot. Horti Agrobot. 37, 77–80. doi: 10.15835/nbha3723197
Aponte, C., Marañón, T., and García, L. V. (2010). Microbial C, N and P in soils of Mediterranean oak forests: influence of season, canopy cover and soil depth. Biogeochemistry 101, 77–92. doi: 10.1007/s10533-010-9418-5
Araya-Osses, D., Casanueva, A., Román-Figueroa, C., Uribe, J. M., and Paneque, M. (2020). Climate change projections of temperature and precipitation in Chile based on statistical downscaling. Clim. Dyn. 54, 4309–4330. doi: 10.1007/s00382-020-05231-4
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, pp. 1–48. doi: 10.18637/jss.v067.i01
Becerra, P. I., Celis-Diez, J. L., and Bustamante, R. O. (2004). Effects of leaf litter and precipitation on germination and seedling survival of the endangered tree Beilschmiedia miersii. Appl. Veg. Sci. 7, 253–257. doi: 10.1111/j.1654-109X.2004.tb00617.x
Belasri, L., Hmimid, F., Cherki, M., and Ait Benichou, S. (2024). Seasonal comparison of the effect of landfill leachate from Mediouna (Casablanca, Morocco) on germination and α-amylase activity in Lens culinaris and Medicago sativa. Euro-Mediterr. J. Environ. Integr. 9, 1301–1309. doi: 10.1007/s41207-024-00511-5
Ben-Shachar, M. S., Lüdecke, D., and Makowski, D. (2020). Effectsize: estimation of effect size indices and standardized parameters. J. Open Source Softw. 5:2815. doi: 10.21105/joss.02815
Bogdziewicz, M. (2022). How will global change affect plant reproduction? A framework for mast seeding trends. New Phytol. 234, 14–20. doi: 10.1111/nph.17682
Bolker, B.R Development Core Team. (2022). Bbmle: Tools for general maximum likelihood estimation. Available at: https://CRAN.R-project.org/package=bbmle.
Boutin, C., Elmegaard, N., and Kjær, C. (2004). Toxicity Testing of Fifteen Non-Crop Plant Species with Six Herbicides in a Greenhouse Experiment: Implications for Risk Assessment, Ecotoxicol. 13, pp. 349–369. doi: 10.1023/B:ECTX.0000033092.82507.f3
Brevik, E. C. (2013). The potential impact of climate change on soil properties and processes and corresponding influence on food security. Agriculture 3, 398–417. doi: 10.3390/agriculture3030398
Brock, J. M. R., Craven, D., and Morales, N. S. (2023). Editorial: impacts of climate change on long term viability of tree species with recalcitrant seeds. Front. For. Glob. Change 6:354. doi: 10.3389/ffgc.2023.1264354
Brun, P., Zimmermann, N. E., Hari, C., Pellissier, L., and Karger, D. N. (2022). CHELSA-BIOCLIM+ a novel set of global climate-related predictors at kilometer-resolution. Envi Dat.
Butenschoen, O., Scheu, S., and Eisenhauer, N. (2011). Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biol. Biochem. 43, 1902–1907. doi: 10.1016/j.soilbio.2011.05.011
Cai, W., McPhaden, M. J., Grimm, A. M., Rodrigues, R. R., Taschetto, A. S., Garreaud, R. D., et al. (2020). Climate impacts of the el ñiño–southern oscillation on South America. Nat. Rev. Earth Environ. 1, 215–231. doi: 10.1038/s43017-020-0040-3
Cakir, C., and Ceyhan, E. (2021). Determination of salinity tolerances during germination period of some lentil (Lens culinaris medic.) cultivars. Selcuk J. Agric. Food Sci. 35, 173–177.
Caro, T. M., and O’Doherty, G. (1999). On the use of surrogate species in conservation biology. Conserv. Biol. 13, 805–814. doi: 10.1046/j.1523-1739.1999.98338.x
Chiapusio, G., Sánchez, A. M., Reigosa, M. J., González, L., and Pellissier, F. (1997). Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 23, 2445–2453. doi: 10.1023/b:joec.0000006658.27633.15
Cohen, J. (2013). Statistical Power Analysis for the Behavioral Sciences. 2nd edn. New York: Routledge.
De Souza Marinke, L., Catão, H. C. R. M., Martins, G. Z., and Castilho, I. M. (2019). Vigor of lentil seeds evaluated by the tests of accelerated aging and controlled deterioration/vigor de sementes de lentilha avaliadas pelos testes de envelhecimento acelerado e deterioração controlada. Braz. J. Dev. 5, 30846–30858. doi: 10.34117/bjdv5n12-194
De Silva, Y., Rajagopalan, U., Kadono, H., and Li, D. (2022). Effects of microplastics on lentil (Lens culinaris) seed germination and seedling growth. Chemosphere 303:135162:135162. doi: 10.1016/j.chemosphere.2022.135162
De Vitis, M., Hay, F., Dickie, J., Trivedi, C., Choi, J., and Fiegener, R. (2020). Seed storage: maintaining seed viability and vigor for restoration use. Restor. Ecol. 28, S249–S255. doi: 10.1111/rec.13174
Dupuy, J. M., and Chazdon, R. L. (2008). Interacting effects of canopy gap, understory vegetation and leaf litter on tree seedling recruitment and composition in tropical secondary forests. For. Ecol. Manag. 255, 3716–3725. doi: 10.1016/j.foreco.2008.03.021
Dwevedi, A., Kumar, P., Kumar, P., Kumar, Y., Sharma, Y. K., and Kayastha, A. M. (2017). “Soil sensors: detailed insight into research updates, significance, and future prospects” in New pesticides and soil sensors. ed. A. M. Grumezescu (Amsterdam, Netherlands: Elsevier), 561–594.
Ellis, E. C. (2015). Ecology in an anthropogenic biosphere. Ecol. Monogr. 85, 287–331. doi: 10.1890/14-2274.1
Fernández, I. C., Durán, L., Morales, N. S., Riquelme-Alarcón, J., Sharma, Y. K., and Koplow, T. (2023). Rol del cambio de uso de suelo, disturbios antrópicos y cambio climático en la degradación del hábitat y viabilidad de Belloto del Norte y Guayacán. Santiago, Chile: Fondo de Investigación del Bosque Nativo, 66.
Fernández, I. C., and Morales, N. S. (2016). A spatial multicriteria decision analysis for selecting priority sites for plant species restoration: a case study from the Chilean biodiversity hotspot. Restor. Ecol. 24, 599–608. doi: 10.1111/rec.12354
Figueroa, J. A., and Jaksic, F. M. (2004). Latencia y banco de semillas en plantas de la región mediterránea de Chile central. Rev. Chil. Hist. Nat. 77, 201–215. doi: 10.4067/S0716-078X2004000100016
Fleury, M., Marcelo, W., Vásquez, R. A., González, L. A., and Bustamante, R. O. (2015). Recruitment dynamics of the relict palm, Jubaea chilensis: intricate and pervasive effects of invasive herbivores and nurse shrubs in Central Chile. PLoS One 10:e0133559. doi: 10.1371/journal.pone.0133559
Fuentealba, D. A., Duran, M. L., and Morales, D. N. S. (2021). The impact of forest science in Chile: history, contribution, and challenges. Can. J. For. Res. 51, 753–765. doi: 10.1139/cjfr-2020-0471
Gallardo, A. (2003). Effect of tree canopy on the spatial distribution of soil nutrients in a Mediterranean Dehesa. Pedobiologia 47, 117–125. doi: 10.1078/0031-4056-00175
García-Valdés, R., Svenning, J. C., Zavala, M. A., Purves, D. W., and Araújo, M. B. (2015). Evaluating the combined effects of climate and land-use change on tree species distributions. J. Appl. Ecol. 52, 902–912. doi: 10.1111/1365-2664.12453
Granda, E., Escudero, A., and Valladares, F. (2014). More than just drought: complexity of recruitment patterns in Mediterranean forests. Oecologia 176, 997–1007. doi: 10.1007/s00442-014-3064-x
Henríquez, C. A., and Simonetti, J. A. (2001). The effect of introduced herbivores upon an endangered tree (Beilschmiedia miersii, Lauraceae), Biological Conservation, 98, pp. 69–76.
Henriquez, C. A., Sotes, G. J., and Bustamante, R. O. (2012). ‘Fenología reproductiva de Pouteria splendens (Sapotaceae)’, Gayana. Botánica 69, 251–255. doi: 10.4067/S0717-66432012000200004
Hijmans, R. J. (2023) Raster: geographic data analysis and modeling. Available at: https://CRAN.R-project.org/package=raster.
Jebara, S. H., Abdelkerim, S., Fatnassi, I. C., Chiboub, M., Saadani, O., and Jebara, M. (2015). Identification of effective Pb resistant bacteria isolated from Lens culinaris growing in lead contaminated soils, J. Basic Microbiol. 55, pp. 346–353. doi: 10.1002/jobm.201300874
Kalra, Y. P., and Maynard, D. G. (1991). Methods manual for forest soil and plant analysis. Edmonton, Alberta: Forestry Canada, Northwest Region, 116.
Karger, D. N., Conrad, O., Böhner, J., Kawohl, T., Kreft, H., Soria-Auza, R. W., et al. (2017). Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4:170122. doi: 10.1038/sdata.2017.122
Khazaei, H., Caron, C. T., Fedoruk, M., Diapari, M., Vandenberg, A., Coyne, C. J., et al. (2016). ‘Genetic diversity of cultivated lentil (Lens culinaris Medik.) and its relation to the world’s agro-ecological zones. Front. Plant Sci. 7:80
Kirschbaum, M. U. F. (1995). The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 27, 753–760. doi: 10.1016/0038-0717(94)00242-S
Kremer, K. N., Promis, Á. A., Mancilla, G., and Magni, C. R. (2019). Leaf litter and irrigation can increase seed germination and early seedling survival of the recalcitrant-seeded tree Beilschmiedia miersii. Austral Ecol. 44, 86–94. doi: 10.1111/aec.12655
Kuhn and Max (2008). Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26. doi: 10.18637/jss.v028.i05
Lacroix, E. M., Rossi, R. J., Bossio, D., and Fendorf, S. (2021). Effects of moisture and physical disturbance on pore-scale oxygen content and anaerobic metabolisms in upland soils. Sci. Total Environ. 780:146572. doi: 10.1016/j.scitotenv.2021.146572
Lal, R. (2020). Soil organic matter and water retention. Agron. J. 112, 3265–3277. doi: 10.1002/agj2.20282
Lenth, R. V. (2025). emmeans: Estimated Marginal Means, aka Least-Squares Means. Available at : https://rvlenth.github.io/emmeans/
Lloret, F., Peñuelas, J., and Estiarte, M. (2004). Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Glob. Chang. Biol. 10, 248–258. doi: 10.1111/j.1365-2486.2004.00725.x
Lopez-Iglesias, B., Olmo, M., Gallardo, A., and Villar, R. (2014). Short-term effects of litter from 21 woody species on plant growth and root development. Plant Soil 381, 177–191. doi: 10.1007/s11104-014-2109-6
Lynch, J. P., and St Clair, S. B. (2004). Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Field Crop Res. 90, 101–115. doi: 10.1016/j.fcr.2004.07.008
MacDougall, A. S., Duwyn, A., and Jones, N. T. (2010). Consumer-based limitations drive oak recruitment failure. Ecology 91, 2092–2099. doi: 10.1890/09-0204.1
Magni, C. R., Poch, P., Espinoza, S., Yáñez, M., Martínez, E., Promis, A., et al. (2023). Provenance influences seed germination and phenotypic responses to water restriction in the endemic Beilschmiedia miersii (gay) Kosterm. Front. For. Glob. Change 5:908. doi: 10.3389/ffgc.2022.1039908
Magni, C. R., Saavedra, N., Espinoza, S. E., Yáñez, M. A., Quiroz, I., Faúndez, Á., et al. (2022). The recruitment of the recalcitrant-seeded Cryptocarya alba (Mol.) looser, established via direct seeding is mainly affected by the seed source and forest cover. Plan. Theory 11:2918. doi: 10.3390/plants11212918
Mantyka-Pringle, C. S., Visconti, P., di Marco, M., Martin, T. G., Rondinini, C., and Rhodes, J. R. (2015). Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 187, 103–111. doi: 10.1016/j.biocon.2015.04.016
Marticorena, C., and Rodríguez, R. (2001) Flora de Chile, Vol. 2. Concepción,Chile: Universidad de Concepción.
Martinez-Harms, M. J., Wilson, K. A., Costa, M. D. P., Possingham, H. P., Gelcich, S., Chauvenet, A., et al. (2021). Conservation planning for people and nature in a Chilean biodiversity hotspot. People Nat. 3, 686–699. doi: 10.1002/pan3.10200
Montejano-Ramírez, V., and Valencia-Cantero, E. (2024). The importance of lentils: an overview. Agriculture 14:103. doi: 10.3390/agriculture14010103
Montgomery, R. A., Reich, P. B., and Palik, B. J. (2010). Untangling positive and negative biotic interactions: views from above and below ground in a forest ecosystem. Ecology 91, 3641–3655. doi: 10.1890/09-1663.1
Morales, N. S. (2015a). Factors affecting recruitment of Beilschmiedia tawa in northern New Zealand. N. Z. J. Bot. 53, 231–240. doi: 10.1080/0028825X.2015.1095212
Morales, N. S. (2015b). The role of post-dispersal regeneration processes in Beilschmiedia tawa forest fragments, Waikato, Northern New Zealand. Thesis. Auckland, New Zealand. Available at: https://researchspace.auckland.ac.nz/handle/2292/24535 (Accessed November 27, 2015).
Morales, N. S., Becerra, P. I., Arellano, E. C., and Gilabert, H. B. (2015). Effect of large and small herbivores on seed and seedling survival of Beilschmiedia miersii in Central Chile. Bosque 36, 127–132. doi: 10.4067/S0717-92002015000100014
Muñoz-Sáez, A., Choe, H., Boynton, R. M., Elsen, P. R., and Thorne, J. H. (2021). Climate exposure shows high risk and few climate refugia for Chilean native vegetation. Sci. Total Environ. 785:147399. doi: 10.1016/j.scitotenv.2021.147399
Newbold, T., Hudson, L. N., Hill, S. L. L., Contu, S., Lysenko, I., Senior, R. A., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. doi: 10.1038/nature14324
Nishida, S. (1999). Revision of Beilschmiedia (Lauraceae) in the Neotropics. Ann. Mo. Bot. Gard. 86, 657–701. doi: 10.2307/2666150
Northrup, J. M., Rivers, J. W., Yang, Z., and Betts, M. G. (2019). Synergistic effects of climate and land-use change influence broad-scale avian population declines. Glob. Chang. Biol. 25, 1561–1575. doi: 10.1111/gcb.14571
Novoa, P. (2004). Determinación del grado de amenaza del belloto del norte (Beilschmiedia miersii Kosterm, Lauraceae), mediante el uso de la metodología UICN 2001. Versión 3.1. Chloris Chilensis, no. 7.
OECD. (2006). Test no. 208: Terrestrial plant test: Seedling emergence and seedling growth test. Available at: https://www.oecd-ilibrary.org/content/publication/9789264070066-en.
Ostroumov, S. A. (2016). Toxicity testing of chemicals without use of animals. Russ. J. Gen. Chem. 86, 2933–2941. doi: 10.1134/S1070363216130028
Panuccio, M. R., Romeo, F., Marra, F., Mallamaci, C., Hussain, M. I., and Muscolo, A. (2022). Salinity tolerance of lentil is achieved by enhanced proline accumulation, lower level of sodium uptake and modulation of photosynthetic traits. J. Agron. Crop Sci. 208, 40–52. doi: 10.1111/jac.12560
Papadakis, I. E., Ladikou, E. V., Oikonomou, A., Chatzistathis, T., and Chatziperou, G. (2023). Exploring the impact of potassium on growth, photosynthetic performance, and nutritional status of lemon trees (cv. Adamopoulou) grafted onto sour orange and volkamer lemon rootstocks. Sustain. For. 15:15858. doi: 10.3390/su152215858
Pino-Palma, R., Baeza, C. M., and Stuessy, T. (2021). Caracterización citotaxonómica de cultivares y procedencias de Lens culinaris Medik. (Fabaceae) de Chile y Canada’, Gayana Bot. 78, pp. 86–94. doi: 10.4067/S0717-66432021000100086
Prescott, C. E. (2002). The influence of the forest canopy on nutrient cycling. Tree Physiol. 22, 1193–1200. doi: 10.1093/treephys/22.15-16.1193
Promis, A., and Allen, R. B. (2017). Tree seedlings respond to both light and soil nutrients in a Patagonian evergreen-deciduous forest. PLoS One 12:e0188686. doi: 10.1371/journal.pone.0188686
Quintana, J. M. (2012). Changes in the rainfall regime along the extratropical west coast of South America (Chile): 30-43o S. Atmósfera, 25, pp. 1–22.
R Core Team. (2023) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/.
Reyes Calvo, G., Gatica, G. Z., Torres, P., Escobar, G. G., Molina, P. C., and Hernández, B. Z.. (2015). Estudio agrológico Región Metropolitana: descripción de suelos materiales y símbolos, C578e 8114. CIREN. Available at: https://bibliotecadigital.ciren.cl/handle/20.500.13082/21330 (Accessed October 3, 2023).
Reynaert, S., de Boeck, H. J., Verbruggen, E., Verlinden, M., Flowers, N., and Nijs, I. (2021). Risk of short-term biodiversity loss under more persistent precipitation regimes. Glob. Chang. Biol. 27, 1614–1626. doi: 10.1111/gcb.15501
Román-Palacios, C., and Wiens, J. J. (2020). Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. 117, 4211–4217. doi: 10.1073/pnas.1913007117
Salazar, S. A., and Maldonado, H. A. (2019). Evaluation of cytotoxic potential of chlorpyrifos using Lens culinaris med as efficient bioindicator. Ecotoxicol. Environ. Saf. 183:109528. doi: 10.1016/j.ecoenv.2019.109528
Salazar, S. A., and Maldonado, H. A. (2020). Evaluation of the cytotoxic potential of sodium hypochlorite using meristematic root cells of Lens culinaris med. Sci. Total Environ. 701:134992. doi: 10.1016/j.scitotenv.2019.134992
Salazar, S. A., and Quintero, J. D. (2020). Determination of malathion's toxic effect on Lens culinaris Medik cell cycle. Heliyon 6:e04846. doi: 10.1016/j.heliyon.2020.e04846
Salazar, S. A., and Quintero, J. D. (2021). Use of Lens culinaris med test as environmental bioindicator to identify the cytogenotoxic effect of paraquat pesticide. Environ. Sci. Pollut. Res. 28, 51321–51328. doi: 10.1007/s11356-021-14352-0
Salazar, S. A., and Vega, D. G. (2023). Paracetamol ecotoxicological bioassay using the bioindicators Lens culinaris med. and Pisum sativum L. Environ. Sci. Pollut. Res. 30, 61965–61976. doi: 10.1007/s11356-023-26475-7
Schoenholtz, S. H., Van Miegroet, H., and Burger, J. A. (2000). A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. For. Ecol. Manag. 138, 335–356. doi: 10.1016/S0378-1127(00)00423-0
Schulz, J. J., Cayuela, L., Echeverria, C., Salas, J., and Rey Benayas, J. M. (2010). Monitoring land cover change of the dryland forest landscape of Central Chile (1975–2008). Appl. Geogr. 30, 436–447. doi: 10.1016/j.apgeog.2009.12.003
Segan, D. B., Murray, K. A., and Watson, J. E. M. (2016). A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 5, 12–21. doi: 10.1016/j.gecco.2015.11.002
Selwood, K. E., McGeoch, M. A., and Mac Nally, R. (2015). The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 90, 837–853. doi: 10.1111/brv.12136
Sinnett, D. E., Lawrence, V. K., Hutchings, T. R., and Hodson, M. E. (2011). Plants growing on contaminated and brownfield sites appropriate for use in organisation for economic co-operation and development terrestrial plant growth test. Environ. Toxicol. Chem. 30, 124–131. doi: 10.1002/etc.360
Sotes, G. J., Bustamante, R. O., and Henríquez, C. A. (2018). Leaf litter is essential for seed survival of the endemic endangered tree Pouteria splendens (Sapotaceae) from Central Chile. Web Ecol. 18, 1–5. doi: 10.5194/we-18-1-2018
Turk, M., Rahman, A., Tawaha, M., and Lee, K. D. (2004). Seed germination and seedling growth of three lentil cultivars under moisture stress. Asian J. Plant Sci. 3, 394–397. doi: 10.3923/ajps.2004.394.397
Urban, M. C. (2015). Accelerating extinction risk from climate change. Science 348, 571–573. doi: 10.1126/science.aaa4984
Venegas-González, A., Muñoz, A. A., Carpintero-Gibson, S., González-Reyes, A., Schneider, I., Gipolou-Zuñiga, T., et al. (2023). Sclerophyllous forest tree growth under the influence of a historic megadrought in the Mediterranean ecoregion of Chile. Ecosystems 26, 344–361. doi: 10.1007/s10021-022-00760-x
Vicuña, S., Vargas, X., Boisier, J. P., Mendoza, P. A., Gómez, T., Vásquez, N., et al. (2021). “Impacts of climate change on water resources in Chile” in Water resources of Chile. eds. B. Fernández and J. Gironás, vol. 8 (Cham: Springer International Publishing (World Water Resources)), 347–363.
Wach, M., Hellmich, R. L., Layton, R., Romeis, J., and Gadaleta, P. G. (2016). Dynamic role and importance of surrogate species for assessing potential adverse environmental impacts of genetically engineered insect-resistant plants on non-target organisms, Transgenic Res. 25, pp. 499–505. doi: 10.1007/s11248-016-9945-5
Walck, J., Hidayati, S., Dixon, K., Thompson, K., and Poschlod, P. (2011). Climate change and plant regeneration from seed. Glob. Chang. Biol. 17, 2145–2161. doi: 10.1111/j.1365-2486.2010.02368.x
Wang, H., and Shang, Q. (2020). The combined effects of light intensity, temperature, and water potential on wall deposition in regulating hypocotyl elongation of Brassica rapa. PeerJ 8:e9106. doi: 10.7717/peerj.9106
Wen, B., and Cai, Y. (2014). Seed viability as a function of moisture and temperature in the recalcitrant rainforest species Baccaurea ramiflora (Euphorbiaceae). Ann. For. Sci. 71, 853–861. doi: 10.1007/s13595-014-0388-y
Winkler, K., Fuchs, R., Rounsevell, M., and Herold, M. (2021). Global land use changes are four times greater than previously estimated. Nat. Commun. 12:2501. doi: 10.1038/s41467-021-22702-2
Xiong, S., and Nilsson, C. (1999). The effects of plant litter on vegetation: a meta-analysis. J. Ecol. 87, 984–994. doi: 10.1046/j.1365-2745.1999.00414.x
Xu, X., du, X., Wang, F., Sha, J., Chen, Q., Tian, G., et al. (2020). Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 11:904. doi: 10.3389/fpls.2020.00904
Yukich, O., Carpenter, J., Kelly, D., Timoti, P., Burns, B., Boswijk, G., et al. (2023). Global change explains reduced seeding in a widespread New Zealand tree: indigenous Tūhoe knowledge informs mechanistic analysis. Front. For. Glob. Change 6:326. doi: 10.3389/ffgc.2023.1172326
Zhang, R., and Wienhold, B. J. (2002). The effect of soil moisture on mineral nitrogen, soil electrical conductivity, and pH. Nutr. Cycl. Agroecosyst. 63, 251–254. doi: 10.1023/A:1021115227884
Keywords: biodiversity hotspot, Beilschmiedia miersii, climate change, Lens culinaris, long-term viability, mediterranean ecosystems, recruitment bottlenecks
Citation: Morales NS, Fernández IC, Riquelme-Alarcón J and Arellano EC (2025) Using a bioindicator species to evaluate soil limitations for the recruitment of a recalcitrant tree seed species of Central Chile threatened by climate change. Front. For. Glob. Change. 8:1394664. doi: 10.3389/ffgc.2025.1394664
Received: 01 March 2024; Accepted: 29 January 2025;
Published: 19 February 2025.
Edited by:
Barry Alan Gardiner, Institut Européen De La Forêt Cultivée, FranceReviewed by:
Panayiotis G. Dimitrakopoulos, University of the Aegean, GreeceCopyright © 2025 Morales, Fernández, Riquelme-Alarcón and Arellano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narkis S. Morales, bW9yYWxlc25AbGFuZGNhcmVyZXNlYXJjaC5jby5ueg==; Ignacio C. Fernández, aWduYWNpby5mZXJuYW5kZXouY0B1YWkuY2w=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.