- 1CAS Key Laboratory of Forest Ecology and Silviculture, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, China
- 2Jilin Changbai Mountain West Slope National Research Station of Forest Ecosystem, Shenyang, China
- 3Yancheng Wetland and Natural World Heritage Conservation and Management Center, Yancheng, China
- 4School of Life Sciences, Anqing Normal University, Anqing, China
Introduction: Understory removal is a traditional practice in forest management to reduce fire risk and promote seedling regeneration. However, its effect on understory diversity, biomass and soil nutrients in temperate forest ecosystems is less known, which limits our assessment of the effectiveness of understory vegetation management.
Methods: We quantified the composition of the understory species, their diversity, and the biomass of the understory and factors driving changes in these parameters in primary mixed broad-leaved Pinus koraiensis forest (BKF), secondary Betula platyphylla forest (BF), and Larix gmelinii plantation (LF) in northeast China after a 5-year understory removal.
Results: After understory removal, the number of shrub and herb species in BKF and LF decreased, while the number of shrub species in BF increased significantly and that of herb species decreased; the species with strong light preference, Equisetum hyemale, Impatiens noli-tangere, and Filipendula Palmata, were dominant in the herb layer of the three forest types; Shannon–Wiener diversity, Pielou evenness, and Simpson diversity of the herb layer in LF increased significantly (P < 0.05), while those of the shrub and herb layers in BF and LF showed no significant changes (P > 0.05). The total understory biomass of understory of BKF and LF decreased by 0.94 t·hm−2 and 1.32 t·hm−2, respectively, while that of BF increased by 1.31 t·hm−2; soil -N and total phosphorus (TP) were the key factors regulating understory vegetation diversity and biomass, respectively.
Conclusion: These results suggest that understory removal is a beneficial management strategy for increasing shrub biomass and diversity in secondary forests, while it should be avoided in primary forests and plantations to prevent the reduction of understory plant diversity and soil nutrient loss.
1 Introduction
Understory vegetation accounts for a large proportion of the species diversity of forest ecosystems (Deng et al., 2023a) and is very crucial to maintaining community stability and regulating the structure and function of the forest ecosystem (Nilsson and Wardle, 2005; Bartels and Chen, 2013; Rodriguez-Rodriguez et al., 2023). The tree layer dominates the consumption of space, light (Bartels and Chen, 2010), water, and soil nutrients (Rybar et al., 2023) and has strong space heterogeneity (Yu et al., 2022), which strongly influences the composition and distribution of understory vegetation (Kumar et al., 2018). Nevertheless, understory vegetation has a detrimental impact on sharing environmental resources (De Lombaerde et al., 2020) or poses an interference competition against target overstory trees (Balandier et al., 2022) when it covers more forest land area. Moreover, overstory management, mainly thinning and pruning practices, can directly and indirectly affect the formation of understory vegetation (Wang et al., 2021). However, the process of forest management, such as understory removal, seldom attracts people's attention. Therefore, it is necessary to explore the ecological effects of understory removal, so as to implement high-quality forest ecosystem management based on improving forest complexity and versatility (Wang and Yu, 2023).
Understory removal is a sustainable forest management practice followed worldwide for plantations and natural forests (Povak et al., 2008) to decrease fire hazards (Jimenez et al., 2015) and promote the growth of target trees (Yildiz et al., 2011). Therefore, understory removal has stimulated much exploration about its effectiveness, producing some controversial results (Deng et al., 2023a). Understory removal can alter the characteristics of the understory environment, for example, soil water and nutrient availability (Zhao et al., 2011; Deng et al., 2023b), microbial community (Deng et al., 2023b) and light conditions (Kume et al., 2003), as well as limiting the build-up of litter (Navarro et al., 2010). Although previous studies have confirmed that, on the one hand, understory removal increases the understory diversity (Premer et al., 2016) and biomass by decreasing belowground competition and enhancing soil nutrients (Canteiro et al., 2011; Giuggiola et al., 2018), on the other hand, understory removal predominantly results in an increased seedling performance (De Lombaerde et al., 2021) and biomass. This contrasting finding is because it not only elicits significant increases in photosynthetic characteristics (Kume et al., 2003) but also reduces drought stress, resulting in an increased allocation of carbon into below-ground organs (Vandenberghe et al., 2006). Moreover, other researchers have argued that understory removal can prevent nutrient output and limit the build-up of litter and light distribution (Motsinger et al., 2010), thereby limiting plant recruitment (Premer et al., 2016) and ultimately leading to a decrease in understory biomass (Pires and Xavier, 2010). These contradictory reports on the effects of understory management on the understory characteristic may be attributed to differences in forest stand and types (Premer et al., 2016) and ages (Yildiz et al., 2011; Zhou et al., 2018b), the number (Dupuy and Chazdon, 2008) and times (De Lombaerde et al., 2021) of understory management, and vegetation types (Copeland et al., 2019). Therefore, it is imperative to understand how the understory community changes following understory removal, paying particular attention to the dynamic relationship between its characteristics (e.g., diversity and biomass) and soil properties (Ares et al., 2010; MacDonald et al., 2015), which will aid in a comprehensive assessment of the effectiveness of understory removal management.
The Changbai Mountains forest area is a typical temperate forest ecosystem and also is the largest forest area in Northeast China (Dai et al., 2011). Before the 1980s, the forest area was mainly used for timber production and therefore had a large area of logging. Following the complete implementation of the natural forest protection project in 2015, all commercial logging of natural forests in the forest area has ceased entirely (Qi et al., 2018). At present, the forest area is characterized by a small amount of undisturbed original broad-leaved Korean pine forest, a large number of Betula platyphylla secondary forests disturbed by logging, and some Larix gmelinii artificial forests replanted on clear-cutting land. Currently, the main forest management strategy is understory removal (Qi et al., 2018). Previous studies mainly focused on the effects of understory management on soil microorganisms (Deng et al., 2023b) and the effects of thinning on the characteristics of understory vegetation (Wang et al., 2021) in Changbai Mountains, which revealed the mechanism of the influence of vegetation on forest soil ecological process to a certain extent. However, it is unclear about the restoration characteristics of understory vegetation and their influencing factors in typical forests in Northeast China after understory removal.
In this study, our goal was to assess the effectiveness of understory removal management by determining how it affected understory plant community composition, diversity and biomass in a short-term (5 years) understory manipulation experiment in temperate forests (original broad-leaved Korean pine forest, B. platyphylla secondary forest, and L. gmelinii artificial forest). We examined the change in plant community and how it correlates with its driving soil contents and looked for indexes in plant community measures associated with management goals, such as understory plant abundance, height, and coverage. We particularly addressed the following questions: (1) What are the changes in understory vegetation composition in three typical stands before and after understory management? Considering that understory species have different demands for light and soil contents, we hypothesized that heliophile plants in understory removal treatments dominated the understory layer of the three stands; (2) After the short-term management, what are the characteristics of understory vegetation community in different stands and its driving factors? The removal of denser understory vegetation and under open understory conditions is expected to increase the biomass and diversity in the shrub layer faster than in the herb layer. Our findings will not only support forest managers' decision-making but also help to assess understory removal under which stand is the most justified and most conducive to understory regeneration.
2 Materials and methods
2.1 Study area
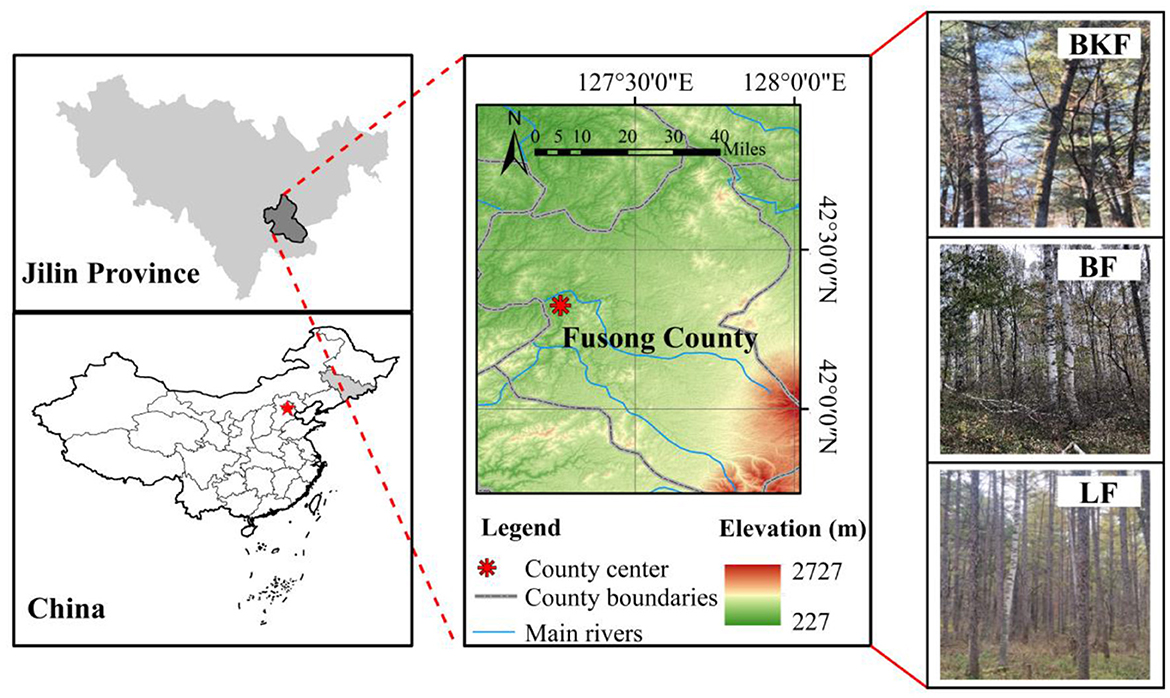
This study was conducted in Fusong County, Jilin Province in the northwest of Changbai Mountains (127°29′ to 128°02′E; 42°20′to 42°40′N; 600 to 800 m A. S. L., Figure 1). It is characterized by north temperate continental climate with prolonged and severe winters and hot and humid summer: long-term average annual precipitation recorded at the Baihe weather station ranged from 800 to 1,040 mm and the average annual temperature is 4.5–7.8°C. The forests are characterized by the original broad-leaved Korean pine forest and natural secondary forests regenerated after devastation with complex canopy, which is dominated by Pinus koraiensis, B. platyphylla, L. gmelinii, and Acer pictum. The soil is characterized as a mountain dark brown forest soil, and its average depth is about 50 cm (Deng et al., 2023b).
2.2 Experimental design
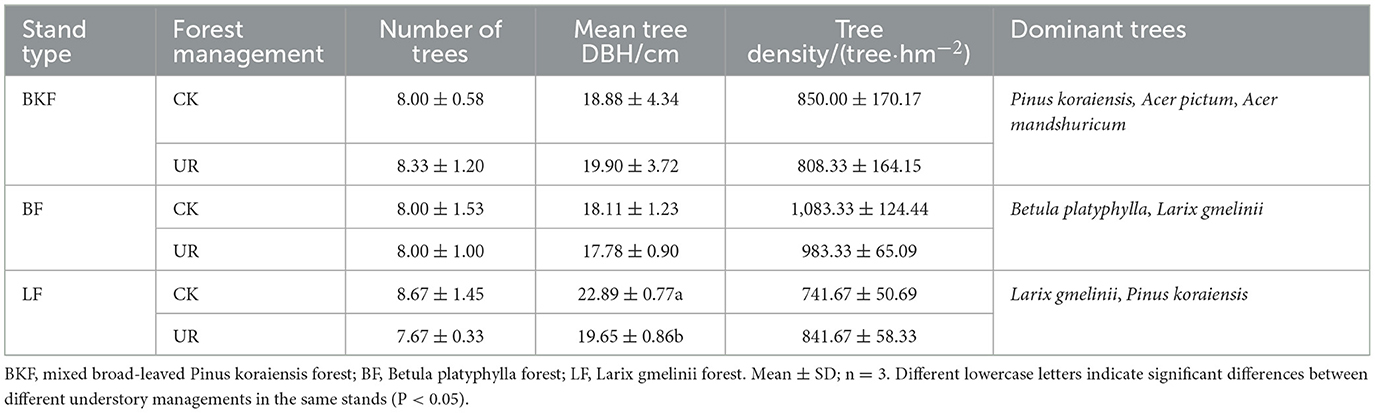
In the summer of 2015, two types, namely, understory removal plot (UR) and understory left intact plot (CK), of monitoring large plots with three repetitions (50 m × 50 m) were set up in the mixed broad-leaved P. koraiensis (BKF), B. platyphylla (BF), and L. gmelinii (LF) forests, respectively, and according to the forest inventory data from the local forestry bureau, the ages of the three stands were 195, 42, and 22 years, respectively. To minimize the edge effects, the large plots of each plot were replicated at least 20 m away from each other. The understory removal experiment in each stand was performed by a kind of machine that was used to cut the above-ground part of the understory shrubs, vines, and tall herbs that hinder the growth of seedlings, saplings, and trees. As a result, the plant residues were evenly stacked in the forests with some spacing. In July 2020, we randomly set up three subplots and their control plots with an area of 20 m × 20 m with similar abiotic conditions of topography, geology, soil, and climate in each large plot. The characteristics of each stand with two kinds of understory treatments are shown in Table 1.
2.3 Understory vegetation survey and measurement
2.3.1 Diversity survey
In July 2020, we set up four 1 m × 1 m subplots for herbaceous species at the four corners of each management plot. We collected basic information on all herbaceous species including their names, abundance, height, coverage, and total coverage, and then harvested the whole plant for all the herbs in a 50 cm × 50 cm random sample square in a 1 m × 1 m subplots. Meanwhile, four 5 m × 5 m subplots were set up for recording species names, abundance, height, canopy width and basal diameter of all shrub plants.
2.3.2 Obtaining biomass
We took the herbaceous samples back to the laboratory and dried them in an oven at 85°C for 48 h to constant weight and weighed their dry weight. The indicators (height, ground diameter, east-west and north-south, and crown width) of each shrub were placed into the corresponding allometric growth equations for obtaining biomass (Fan et al., 2011; He et al., 2011; Wang et al., 2016). The herb and shrub biomass per unit area were calculated based on coverage, the dry weight of the herbaceous plants, shrub biomass in each subplot, and the area of the entire subplot. The coverage of the plant refers to the ratio of the vertical projection area of above the ground part of the plant to the ground, and it was estimated in the field research by a visual method.
2.4 Soil sampling and analysis
Five soil cores (0–20 cm depth) of each subplot were collected randomly with an auger after removing the litter layer in July 2020 and were mixed with one composite sample. Soil samples of four plots from each treatment in each forest of each site were divided into two parts. One fresh part was dried and weighed to test its water content (SWC). The other part was air-dried for the determination of soil total carbon (TC), total nitrogen (TN), total phosphorus (TP), available phosphorus (AP), ammonium nitrogen (-N), and nitrate nitrogen (-N) content, as well as pH, resulting in a total of 24 soil samples.
Soil pH was determined using a pH meter (PHS-3C) in a 1:2.5 soil:water solution (weight/volume). Soil TC and TN contents were measured using a C/N analyzer (Elementar vario MACRO cube, German). Soil TP and AP contents were obtained by using a spectrophotometer (UV-9000S, China) with H2HClO4 digestion and 0.5 mol/L NaHCO3 extraction, respectively. Soil -N and -N contents were extracted by 1 mol/L potassium chloride solution and analyzed using dual-wavelength ultraviolet spectrophotometry.
2.5 Data analysis
2.5.1 Importance value of understory vegetation
To quantify the status and dominance of understory plants in the community, we calculated their importance value (IV, Equation 1), which was defined as the average of its relative abundance (Ar, Equation 2), relative coverage (Cr, Equation 3), and relative frequency (Fr, Equation 4) based on the obtained data. These parameters were calculated as follows (Arbainsyah et al., 2014):
where Ar is the relative abundance, Cr is the relative canopy, and Fr is the relative frequency.
2.5.2 Understory vegetation diversity
Margalef richness index (R, Equation 5), Shannon–Wiener diversity index (H, Equation 6), Simpson dominance index (D, Equation 7), and Pielou evenness index (J, Equation 8) were selected for calculating understory vegetation diversity (Hill and Pielou, 1970):
where S is the number of species in the sample plot, N is the total number of individuals of all species in the sample plot, and Pi is the ratio of the number of individuals of a species to the number of individuals of all species in the sample plot.
Importance values of understory species were calculated using Excel 2016. The vegetation diversity indices were calculated using the ‘vegan' package of RStudio software. Based on SPSS18.0 software, one-way analysis of variance (ANOVA) was performed to test understory removal effects on herbaceous diversity and biomass and test the difference between different forests (LSD, P = 0.05). Independent-sample t-test was performed to compare the difference between using different management strategies in the same forest. Redundancy analysis (RDA) was performed in Canoco 5.0 software to determine the extent to which the soil's physical and chemical properties can explain the quantitative characteristics of plant communities.
3 Results
3.1 Understory vegetation diversity
3.1.1 Species composition and importance values
Five years after understory removal, the number of herbaceous plants of all three stands decreased; the number of species, genera, and families of shrub plants of LF decreased significantly, BKF decreased little, whereas BF increased largely (Figure 2). In particular, there were no significant differences of the number of understory plants between different understory management in the same stand, the number of species, genera, and families of shrub plants of BKF was significantly higher than that of LF (P < 0.05).
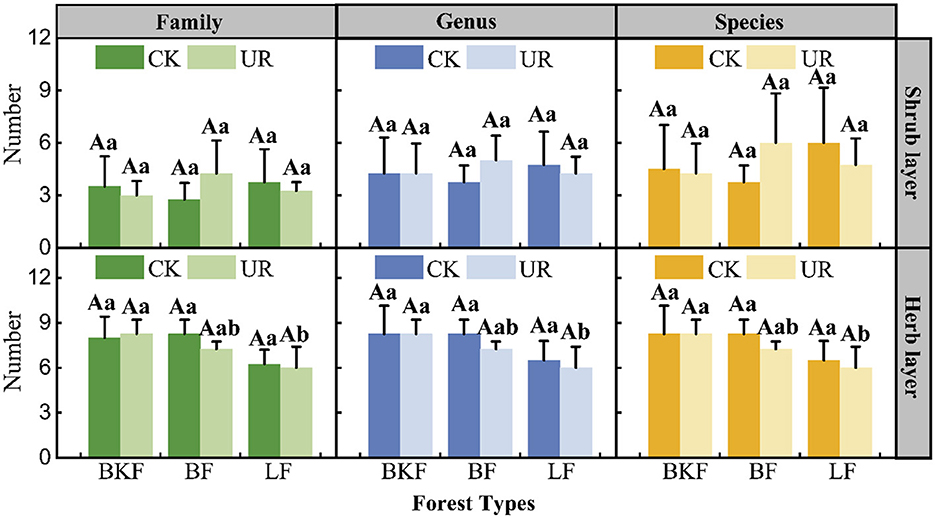
Figure 2. Changes in the number of families, genera, and species in understory left intact (CK) and removal (UR) stands. BKF, mixed broad-leaved Pinus koraiensis forest; BF, Betula platyphylla forest; LF, Larix gmelinii forest. Mean ± SD, n = 4. Different uppercase letters indicate significant differences between different understory managements in the same stands (P < 0.05), and different lowercase letters indicate significant differences among different forest types within a given understory management (P < 0.05).
The importance values of the main species in the three stands are shown in Table S1. Five years after understory removal, the light-demanding herbaceous plants, such as Filipendula palmata, Urtica angustifolia and Equisetum hyemale, which displayed a strong reproduction capacity and adaptability in the three stands, quickly occupied forest lands and their dominant position was enhanced, while the dominance of sciophiles, such as Ostericum grosseserratum, Meehania urticifolia, and Carex pilosa, decreased. The dominance of the shrub plants in the three stands did not vary congruously to understory removal, and the dominance of some heliophile shrubs, such as Acer ukurunduense (8.51 increased to 42.04) in BKF, Acer tegmentosum (1.81 increased to 10.13) in BF, and Ribes mandshuricum (3.57 increased to 13.55) in LF, increased. Whereas the dominance of some heliophile shrubs, such as Eleutherococcus senticosus and A. tegmentosum (importance value reduced to 0) in BKF, Acer pictum subsp. mono (importance value reduced to 0) in LF decreased after understory removal.
3.1.2 Plant diversity
Five years after understory removal, Shannon–Wiener diversity (H), Pielou evenness (J), Simpson diversity (D), and Margalef richness (R) indices showed no significant changes in the shrub layer of the three stands (P > 0.05), while Shannon–Wiener diversity (H), Pielou evenness (J), and Simpson diversity (D) indices of the herb layer of LF increased significantly (P < 0.05). However, there were no significant differences between the four diversity indices of the herb layer of BF and BKF (P > 0.05) (Figure 3).
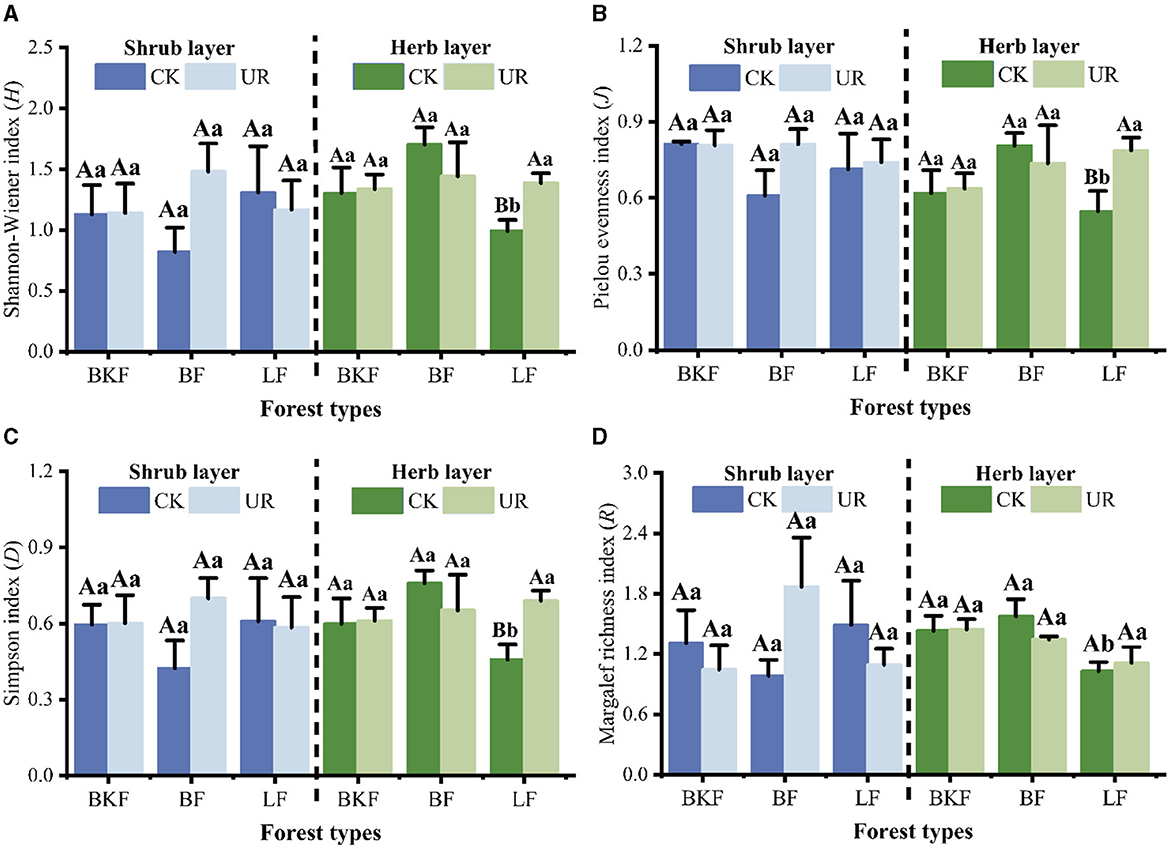
Figure 3. Changes in understory vegetation community diversity in understory left intact (CK) and removal (UR) stands. (A–D) represent Shannon-Wiener diversity index, Pielou evenness index, Simpson dominance index, and Margalef richness index of the understory, respectively.
3.2 Understory vegetation biomass
Understory removal significantly decreased the understory biomass of BKF and LF (P > 0.05) but can promote the increase of BF, and there were no significant differences among the three stands (Figure 4). Five years after understory removal, the total understory biomass of BKF and LF had decreased by 0.94 t·hm−2 and 1.32 t·hm−2, respectively, where there was a more than 25% decrease both in the biomass of the shrub and herb layers. In contrast, the total understory biomass of BF increased by 1.31 t·hm−2, and understory biomass increased by 82.63% and 21.77% in the shrub and herb layers, respectively, in understory removal stands as compared to that in the understory left stands.
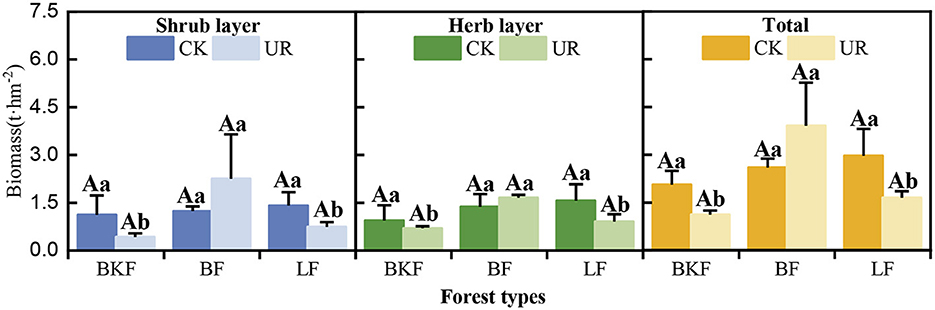
Figure 4. Changes in understory vegetation biomass in understory left intact (CK) and removal (UR) stands.
3.3 Soil properties
Understory removal had a greater impact on the soil's physical and chemical properties of BF and LF (Table 2). Five years after understory removal, there were no significant variations in soil properties in BKF (P > 0.05); the content of soil -N and available phosphorus (AP) of BF; while the water content (SWC), total nitrogen (TN), and total phosphorus (TP) content of LF increased significantly (P < 0.05). For the three stands, the total nutrient content (TN, TP, and total soil carbon (TC)) of BF in soil was the highest and significantly higher than BKF, followed by LF after understory removal; there were no significant differences in soil pH, SWC, and -N among the three stands.
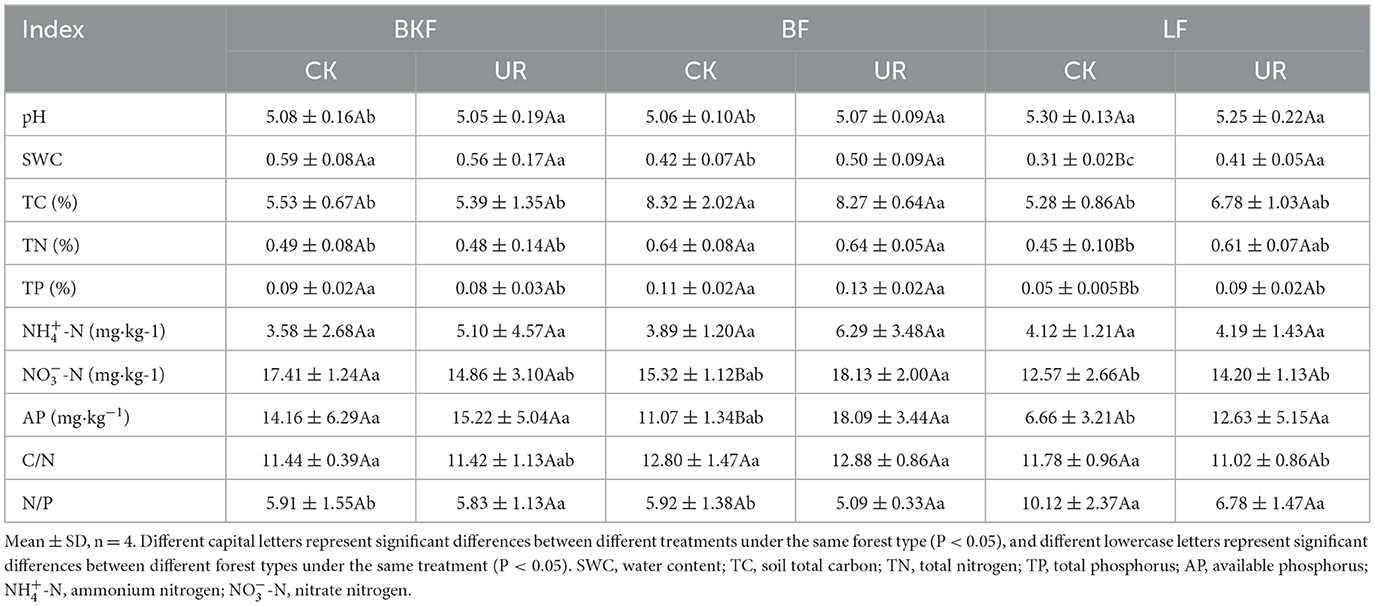
Table 2. Soil characteristics of the three stands with understory left intact (CK) and removal (UR).
3.4 Correlations of understory vegetation characteristics with soil properties
The biomass and diversity of understory vegetation were different due to different soil properties (Figure 5, Tables S2, S3). Redundancy analysis (RDA) was used to explore correlations of understory vegetation characteristics with soil properties (Table S2). Soil N/P was the primary factor impacting understory vegetation diversity and biomass prior to understory removal (Table S3), and as its value increased, shrub diversity and understory biomass increased, whereas herb diversity decreased (Figures 5A, C). Five years after understory removal, soil -N and TP were the key factors regulating understory vegetation diversity and biomass, respectively (Table S3), and as these values increased, shrub and herb diversity reduced while understory biomass increased (Figures 5B, D).
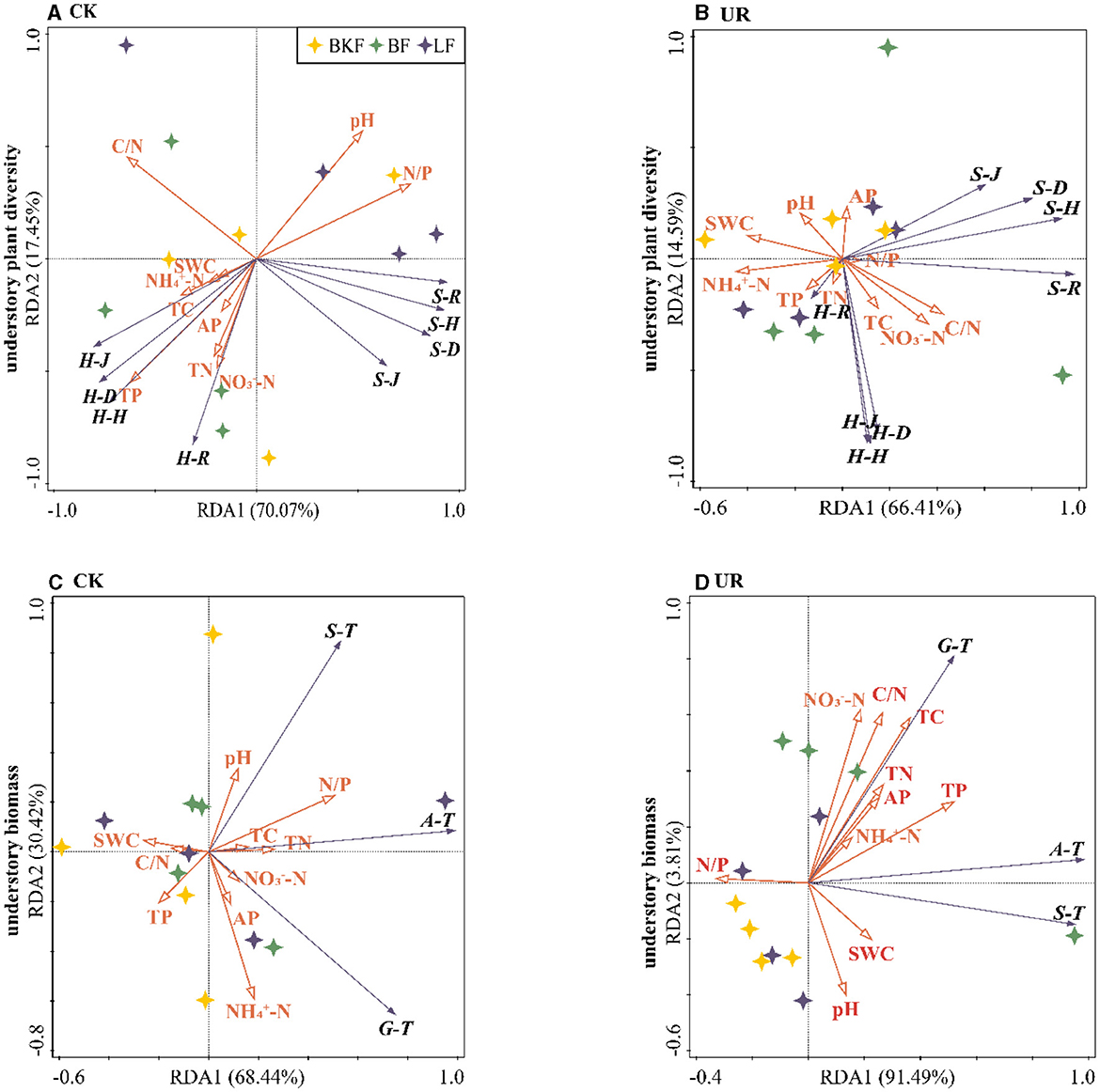
Figure 5. Redundancy analysis (RDA) for correlation between characteristics of understory vegetation and soil properties. (A, B) are redundant analyses of understory vegetation diversity and soil properties in understory left intact (CK) and removal (UR) stands, respectively; the first two axes explained 87.52% and 81% of understory plant diversity. (C, D) are the redundancy analysis of understory vegetation biomass and soil properties in understory removal and retaining stands, respectively, and the first two axes explained 98.96% and 95.3% of the understory biomass. S-R, S-H, S-D, and S-J and H-R, H-H, H-D, and H-J represent the Margalef richness index, the Shannon–Wiener diversity index, the Simpson dominance index and the Pielou evenness index of the shrub and herb layers, respectively. S-T, H-T, and A-T represent the shrub layer biomass, the herb layer biomass, and the total biomass of understory vegetation, respectively.
4 Discussion
4.1 Changes in understory diversity and biomass
Disturbance and the availability of resources, particularly light and soil conditions have been recognized as key factors influencing plant diversity in the understory of temperate forests (Hofmeister et al., 2009; Forster et al., 2017). After understory removal, the vertical light distribution changed dramatically, and 50% of the light absorbed by the higher canopy was released (Matsuo et al., 2021), resulting in an increase in photosynthetically active radiation reaching the forest land, as well as an increase in the availability of temperature, humidity, and nutrients in the forest (De Lombaerde et al., 2021). Furthermore, understory species or species groups that demand different environmental resources may respond in various ways to the canopy opening (Forster et al., 2017; Deng et al., 2023b). Although previous studies have confirmed that understory management decreased species richness in the semi-arid ecosystem (Jimenez et al., 2015), our study in temperate forests demonstrated that its impact varies by forest type and understory vegetation layers (Hart and Chen, 2008). In this study, the species diversity of understory increased rapidly in the three stands, especially for B. platyphylla forest (Figure 2). The response of herbaceous plants to interference is more sensitive than shrub plants (Wang et al., 2021), the dominance of strong adaptability and reproductive ability and heliophiles herbaceous species increased significantly after understory removal, such as Impatiens noli-tangere, F. palmata, and E. hyemale, which caused significant differences in three forest types. Because the standground was not fully expanded by the shrub plants, it directly reduced the dominance of sciophiles herbaceous species (Table S1). The growth cycle of shrubs is usually longer than that of herbaceous species, therefore shrub diversity was not affected by understory removal, and their recovery process still needs to be studied on a longer time scale (Copeland et al., 2019). Because broad-leaved forests (BF) have higher canopy light transmission (Messier et al., 1998), their soil water and light conditions are superior to coniferous forests (LF) (Hart and Chen, 2008), resulting in increasing vegetation diversity and resource availability, which was in line with other research studies, that is, increase of broad-leaved trees benefitted the understory plant richness via improved nutrient availability in the soil (Salemaa et al., 2023). Shrub plants of broad-leaved forests grow faster and have more plant species than coniferous forests. The increase in dominance of the shrub layer would lead to the loss of species diversity under shrubs (Pajunen et al., 2011). Therefore, after understory removal, BF has the highest shrub diversity and the lowest herb diversity, which is in contrast to LF (Figures 2, 3).
Biomass compensation for understory removals occurs significantly in BF, but not in BKF and LF after treatment. In an arid region forest, the researchers found the degree of biomass compensation was dependent on restoration term (Canteiro et al., 2011) and a fertilized environment (Pires and Xavier, 2010) after the removal, and they observed that there was a compensation for removals under high fertilization during the first 3 years but was reduced compensation after 9 years. Moreover, biomass compensation for plant removals can occur relatively rapidly in temperate ecosystems (Gonzalez et al., 2019). However, in contrast with our findings, biomass compensation after removals in the short term only appeared rapidly in the broad-leaved pure forest (that is BF) with a fast litter turnover and decomposition rate (Zhao et al., 2022b). After a 5-year removal, the litter's turnover mechanism (Qiao et al., 2014) and nutrient content (Peng et al., 2020) changed, the litter in broad-leaved forests increased and accumulated faster than in coniferous forests, and its biological return was greater (Zhou et al., 2018a), which promoted the rapid accumulation of soil nutrient content such as organic matter in soil in BF (Zhang et al., 2022). In addition, the canopy density of BF is higher than that of coniferous forests and coniferous and broad-leaved mixed forests, and the light environment of the lower layer of the forest has improved after understory removal (Wang et al., 2021), thus the species with light-demanding and strong reproductive ability (such as F. Palmata and A. ukurunduense) enter the forest first and occupy a large area under the forest. Finally, light-demanding and shade-tolerant shrubs exist at the same time, resulting in the recovery of the species in the short term (Table S1). However, soil nutrient contents in BKF and LF were lower than in BF (Table 2), and some shade-tolerant and hygrophilous plants grew slowly, which limited understory vegetation restoration in a short time. Overall, understory removal appeared to promote shrub diversity and biomass of broad-leaved forests more than that of conifer forests (Figure 4).
4.2 The relationship between understory and soil properties
In this study, we found that before the understory removal, soil N/P promoted the increase of understory vegetation biomass and shrub plant diversity (Figure 5). The effect of soil N/P on the diversity of the shrub layer and the herb layer was opposite, indicating that the shrub layer was more stable in response to changes in the soil's physical and chemical properties, which may be due to the understory canopy limiting the distribution of herbaceous plants. The shrub layer and the herb layer have a competitive relationship with environmental conditions such as light, water, and nutrients. Therefore, with the change in the soil's physical and chemical properties, the diversity of the shrub layer and the herb layer showed an opposite trend of change (Zhou et al., 2021).
After understory removal, the understory canopy closure decreased (Gurlevik et al., 2004; Matsushima and Chang, 2007), which increased light transmittance and, combined with the plant residues, increased the amount of rainwater in the coniferous forest (LF). This increase facilitated the availability of more nutrients to the soil with rainwater and significantly increased the soil water content of the forest land, which improved the utilization efficiency of nutrients in the ecosystem (Wang et al., 2023). Soil moisture in broad-leaved forests was comparable to those in coniferous and broad-leaved mixed forests, indicating that soil water content had little effect on the biomass and composition of the forest (Table 2). In addition, the plant residues from understory management could be used as biomass energy, which is conducive to the realization of carbon neutralization (Wang et al., 2023). The management process generates a significant number of branches, plant residues and other residues, which causes a rapid increase in organic matter on forest land, stimulates litter decomposition, and enhances soil nutrient input. Because of the strong demand for plant growth and its limited nutrients, increasing soil TP concentration promotes the development of plant roots, which is the primary determinant for the expansion of early biomass of understory plants (Zhao et al., 2022a). It promotes the growth of understory biomass in BF in particular, which shows the quickest microbial activity, decomposition rate, nutrient accumulation rate, and energy cycle (Li et al., 2016; Zhao et al., 2022b). Furthermore, some studies suggested that plant diversity decreased with the decrease in soil pH, that is, the increase of soil acidity (Zarfos et al., 2019), which is consistent with our findings on the shrub layer (Figure 4). The species richness and diversity of the herb layer increased, because the tolerance of the shrub layer to soil pH was higher than that of the herb layer, and the herbaceous plants were mostly shallow roots, and the competitiveness of soil nutrients was poor (Zhao et al., 2022a). -N is the basis of plant growth and development. This study found that with the increase of soil -N, the diversity of shrubs and herbs decreased and the biomass increased. It may be because the increase in plant biomass will increase the competition between species, thus reducing species diversity. In general, the coupling relationship between the diversity of the shrub layer and the herb layer and the physical and chemical properties of soil is complex and quite different. This may be because the structure and the growth pattern of shrubs and herbaceous plants are different (Keeley and Johnson, 1977), and the absorption of different elements in the soil is different (Salas-Luévano et al., 2017).
The diversity and biomass of understory vegetation is an important indicator to measure the effectiveness of forest management measures. Our study only analyzed the impact of soil properties on understory plant characteristics; however, for a full appreciation of the impact of understory removal on understory diversity and biomass, environmental parameters, such as light environment, should be taken into account to guide forest management and promote the restoration of understory vegetation in the future.
5 Conclusion
Understory management, which aims to reduce the competition between seedling and understory vegetation, affected the composition of understory species, their diversity, and biomass, and the primary soil properties driving these changes were different in three temperate typical stands. Five years after understory removal, the light conditions and soil temperature in the forest changed and the composition and diversity of herbaceous plants of BF decreased significantly, while its shrubs became established and grew faster than in BKF and LF. Thus, understory removal should be applied in the growth stage of birch seedlings to improve understory productivity and plant diversity, while understory removal should be avoided in BKF and LF to reduce understory plant diversity and soil nutrient loss. Considering the multiple effects of understory removal, forest managers should implement the current understory removal policy based on different forest types for better cultivating the forests. Further research should emphasize the effect of understory removal on the relationship between overstory and understory development in a long-term operation experiment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YZ: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. QY: Investigation, Methodology, Writing – original draft. JD: Writing – review & editing. LZ: Data curation, Project administration, Writing – review & editing. DY: Project administration, Writing – review & editing. WZ: Writing – review & editing. Q-WW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by NEYSF (Grant No. 32122059) and NSFC (Grant No. 41977423).
Acknowledgments
We thank Professor Jinshi Xu of Ludong University for his help in the revision of the article and the staff from Jilin Changbai Mountain West Slope National Research Station of Forest Ecosystem for their support with the fieldwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2024.1393772/full#supplementary-material
References
Arbainsyah de Iongh, H. H., Kustiawan, W., and de Snoo, G. R. (2014). Structure, composition and diversity of plant communities in FSC-certified, selectively logged forests of different ages compared to primary rain forest. Biodivers. Conserv. 23, 2445–2472. doi: 10.1007/s10531-014-0732-4
Ares, A., Neill, A. R., and Puettmann, K. J. (2010). Understory abundance, species diversity and functional attribute response to thinning in coniferous stands. For. Ecol. Manage. 260, 1104–1113. doi: 10.1016/j.foreco.2010.06.023
Balandier, P., Mårell, A., Prévosto, B., and Vincenot, L. (2022). Tamm review: forest understorey and overstorey interactions: so much more than just light interception by trees. For. Ecol. Manage. 526:120584. doi: 10.1016/j.foreco.2022.120584
Bartels, S. F., and Chen, H. Y. H. (2010). Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 91, 1931–1938. doi: 10.1890/09-1376.1
Bartels, S. F., and Chen, H. Y. H. (2013). Interactions between overstorey and understorey vegetation along an overstorey compositional gradient. J. Vegetat. Sci. 24, 543–552. doi: 10.1111/j.1654-1103.2012.01479.x
Canteiro, C., Pinto-Cruz, C., Simoes, M. P., and Gazarini, L. (2011). Conservation of Mediterranean oak woodlands: understorey dynamics under different shrub management. Agroforest. Syst. 82, 161–171. doi: 10.1007/s10457-011-9375-6
Copeland, S. M., Munson, S. M., Bradford, J. B., Butterfield, B. J., and Gunnell, K. L. (2019). Long-term plant community trajectories suggest divergent responses of native and non-native perennials and annuals to vegetation removal and seeding treatments. Restorat. Ecol. 27, 821–831. doi: 10.1111/rec.12928
Dai, L., Qi, L., Wang, Q., Su, D., Yu, D., Wang, Y., et al. (2011). Changes in forest structure and composition on Changbai Mountain in Northeast China. Ann. For. Sci. 68, 889–897. doi: 10.1007/s13595-011-0095-x
De Lombaerde, E., Baeten, L., Verheyen, K., Perring, M. P., Ma, S. Y., and Landuyt, D. (2021). Understorey removal effects on tree regeneration in temperate forests: a meta-analysis. J. Appl. Ecol. 58, 9–20. doi: 10.1111/1365-2664.13792
De Lombaerde, E., Blondeel, H., Baeten, L., Landuyt, D., Perring, M. P., Depauw, L., et al. (2020). Light, temperature and understorey cover predominantly affect early life stages of tree seedlings in a multifactorial mesocosm experiment. For. Ecol. Manage. 461:117907. doi: 10.1016/j.foreco.2020.117907
Deng, J., Fang, S., Fang, X., Jin, Y., Kuang, Y., Lin, F., et al. (2023a). Forest understory vegetation study: current status and future trends. Forest. Res. 3:6. doi: 10.48130/FR-2023-0006
Deng, J., Zhou, W., Dai, L., Yuan, Q., Zhou, L., Qi, L., et al. (2023b). The effects of shrub removal on soil microbial communities in primary forest, secondary forest and plantation forest on Changbai Mountain. Microb. Ecol. 85, 642–658. doi: 10.1007/s00248-021-01943-0
Dupuy, J. M., and Chazdon, R. L. (2008). Interacting effects of canopy gap, understory vegetation and leaf litter on tree seedling recruitment and composition in tropical secondary forests. For. Ecol. Manage. 255, 3716–3725. doi: 10.1016/j.foreco.2008.03.021
Fan, W., Li, M., and Yang, J. (2011). Forest biomass estimation models of remote sensing in Changbai Mountain forests. Scientia Silvae Sinicae 47, 16–20. doi: 10.11707/j.1001-7488.20111003
Forster, A., Becker, T., Gerlach, A., Meesenburg, H., and Leuschner, C. (2017). Long-term change in understorey plant communities of conventionally managed temperate deciduous forests: effects of nitrogen deposition and forest management. J. Vegetat. Sci. 28, 747–761. doi: 10.1111/jvs.12537
Giuggiola, A., Zweifel, R., Feichtinger, L. M., Vollenweider, P., Bugmann, H., Haeni, M., et al. (2018). Competition for water in a xeric forest ecosystem – Effects of understory removal on soil micro-climate, growth and physiology of dominant Scots pine trees. For. Ecol. Manage. 409, 241–249. doi: 10.1016/j.foreco.2017.11.002
Gonzalez, M. M., Crofts, A. L., and McLaren, J. R. (2019). Plant biomass, rather than species composition, determines ecosystem properties: results from a long-term graminoid removal experiment in a northern Canadian grassland. J.Ecol. 107, 2211–2225. doi: 10.1111/1365-2745.13169
Gurlevik, N., Kelting, D. L., and Allen, H. L. (2004). Nitrogen mineralization following vegetation control and fertilization in a 14-year-old loblolly pine plantation. Soil Sci. Soc. Am. J. 68, 272–281. doi: 10.2136/sssaj2004.2720
Hart, S. A., and Chen, H. Y. H. (2008). Fire, logging, and overstory affect understory abundance, diversity, and composition in boreal forest. Ecol. Monogr. 78, 123–140. doi: 10.1890/06-2140.1
He, L., Hang, X., Fan, X., Gao, Y., and Feng, Q. (2011). Estimation and analysis of understory shrub biomass in Changbai Mountains. J. Nanjing Forestry Univer. 35, 45–50. doi: 10.3969/j.jssn.1000-2006.2011.05.010
Hill, M. O., and Pielou, E. G. (1970). An introduction to mathematical ecology. J. Ecol. 58, 896. doi: 10.2307/2258549
Hofmeister, J., Hosek, J., Modry, M., and Rolecek, J. (2009). The influence of light and nutrient availability on herb layer species richness in oak-dominated forests in central Bohemia. Plant Ecology 205, 57–75. doi: 10.1007/s11258-009-9598-z
Jimenez, M. N., Spotswood, E. N., Canadas, E. M., and Navarro, F. B. (2015). Stand management to reduce fire risk promotes understorey plant diversity and biomass in a semi-arid Pinus halepensis plantation. Appl. Vegetat. Sci. 18, 467–480. doi: 10.1111/avsc.12151
Keeley, S. C., and Johnson, A. W. (1977). Comparison of pattern of herb and shrub growth in comparable sites in Chile and California. Am. Midland Natural. 97, 120–132. doi: 10.2307/2424690
Kumar, P., Chen, H. Y. H., Thomas, S. C., and Shahi, C. (2018). Linking resource availability and heterogeneity to understorey species diversity through succession in boreal forest of Canada. J.Ecol. 106, 1266–1276. doi: 10.1111/1365-2745.12861
Kume, A., Satomura, T., Tsuboi, N., Chiwa, M., Hanba, Y. T., Nakane, K., et al. (2003). Effects of understory vegetation on the ecophysiological characteristics of an overstory pine, Pinus densiflora. For. Ecol. Manage. 176, 195–203. doi: 10.1016/S0378-1127(02)00282-7
Li, H., Wang, B., Cao, Y., Liu, Q., and Li, D. (2016). Difference feature of planted vegetation biomass and litter biomass for three plantations and their relationship with soil nutrients in Lvliang Mountainous Region. Bullet. Botan. Res. 36, 573–580. doi: 10.7525/j.issn.1673-5102.2016.04.013
MacDonald, R. L., Chen, H. Y. H., Bartels, S. F., Palik, B. J., and Prepas, E. E. (2015). Compositional stability of boreal understorey vegetation after overstorey harvesting across a riparian ecotone. J. Vegetat. Sci. 26, 733–741. doi: 10.1111/jvs.12272
Matsuo, T., Martinez-Ramos, M., Bongers, F., van der Sande, M. T., and Poorter, L. (2021). Forest structure drives changes in light heterogeneity during tropical secondary forest succession. J.Ecol. 109, 2871–2884. doi: 10.1111/1365-2745.13680
Matsushima, M., and Chang, S. X. (2007). Effects of understory removal, N fertilization, and litter layer removal on soil N cycling in a 13-year-old white spruce plantation infested with Canada bluejoint grass. Plant Soil 292, 243–258. doi: 10.1007/s11104-007-9220-x
Messier, C., Parent, S., and Bergeron, Y. (1998). Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Vegetat. Sci. 9, 511–520. doi: 10.2307/3237266
Motsinger, J. R., Kabrick, J. M., Dey, D. C., Henderson, D. E., and Zenner, E. K. (2010). Effect of midstory and understory removal on the establishment and development of natural and artificial pin oak advance reproduction in bottomland forests. New Forests 39, 195–213. doi: 10.1007/s11056-009-9164-5
Navarro, F. B., Jiménez, M. N., Gallego, E., and Ripoll, M. A. (2010). Short-term effects of overstory reduction and slash mulching on ground vegetation in a Mediterranean Aleppo pine woodland. Eur. J. For. Res. 129, 689–696. doi: 10.1007/s10342-010-0374-3
Nilsson, M.-C., and Wardle, D. A. (2005). Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 3, 421–428. doi: 10.1890/1540-9295(2005)003(0421:UVAAFE)2.0.CO;2
Pajunen, A. M., Oksanen, J., and Virtanen, R. (2011). Impact of shrub canopies on understorey vegetation in western Eurasian tundra. J. Vegetat. Sci. 22, 837–846. doi: 10.1111/j.1654-1103.2011.01285.x
Peng, Y., Schmidt, I. K., Zheng, H., Heděnec, P., Bachega, L. R., Yue, K., et al. (2020). Tree species effects on topsoil carbon stock and concentration are mediated by tree species type, mycorrhizal association, and N-fixing ability at the global scale. For. Ecol. Manage. 478:118510. doi: 10.1016/j.foreco.2020.118510
Pires, A. L., and Xavier, R. (2010). Influence of vegetation management and fertilization on <i>Pinus pinaster </i> growth and on understory biomass and composition. Forest Syst. 19, 404–409. doi: 10.5424/fs/2010193-8927
Povak, N. A., Lorimer, C. G., and Guries, R. P. (2008). Altering successional trends in oak forests: 19 year experimental results of low- and moderate-intensity silvicultural treatments. Canad. J. Forest Res. 38, 2880–2895. doi: 10.1139/X08-118
Premer, M. I., Froese, R. E., Webster, C. R., and Nagel, L. M. (2016). Vegetation response to logging residue removals in Great Lakes aspen forests: Long-term trends under operational management. For. Ecol. Manage. 382, 257–268. doi: 10.1016/j.foreco.2016.09.048
Qi, L., Zhao, F., and Sun, J. (2018). An integrated multi-scale approach to restoring a degraded secondary forest ecosystem: a case study in the Changbai Mountains, northeastern China. Ecol. Eng. 125, 98–105. doi: 10.1016/j.ecoleng.2018.09.028
Qiao, Y. F., Miao, S. J., Silva, L. C. R., and Horwath, W. R. (2014). Understory species regulate litter decomposition and accumulation of C and N in forest soils: a long-term dual-isotope experiment. For. Ecol. Manage. 329, 318–327. doi: 10.1016/j.foreco.2014.04.025
Rodriguez-Rodriguez, J. C., Fenton, N. J., Kembel, S. W., Mestre, E., Jean, M., and Bergeron, Y. (2023). Drivers of contrasting boreal understory vegetation in coniferous and broadleaf deciduous alternative states. Ecol. Monogr. 93:1587. doi: 10.1002/ecm.1587
Rybar, J., Bosela, M., Marcis, P., Ujházyov,á, M., Polták, D., Hederov,á, L., et al. (2023). Effects of tree canopy on herbaceous understorey throughout the developmental cycle of a temperate mountain primary forest. For. Ecol. Manage. 546:121353. doi: 10.1016/j.foreco.2023.121353
Salas-Luévano, M. A., Mauricio-Castillo, J. A., González-Rivera, M. L., Vega-Carrillo, H. R., and Salas-Muñoz, S. (2017). Accumulation and phytostabilization of As, Pb and Cd in plants growing inside mine tailings reforested in Zacatecas, Mexico. Environm. Earth Sci. 76:7139. doi: 10.1007/s12665-017-7139-y
Salemaa, M., Hotanen, J.-P., Oksanen, J., Tonteri, T., and Meril,ä, P. (2023). Broadleaved trees enhance biodiversity of the understorey vegetation in boreal forests. For. Ecol. Manage. 546:121357. doi: 10.1016/j.foreco.2023.121357
Vandenberghe, C., Freléchoux, F., Gadallah, F., and Buttler, A. (2006). Competitive effects of herbaceous vegetation on tree seedling emergence, growth and survival: does gap size matter? J. Vegetat. Sci. 7, 481–488. doi: 10.1111/j.1654-1103.2006.tb02469.x
Wang, G. R., Sun, Y., Zhou, M., Guan, N. Q., Wang, Y. W., Jiang, R. H., et al. (2021). Effect of thinning intensity on understory herbaceous diversity and biomass in mixed coniferous and broad-leaved forests of Changbai Mountain. Forest Ecosyst. 8:331. doi: 10.1186/s40663-021-00331-x
Wang, J., Fu, J., Zhao, Z., Bing, L., Xi, F., Wang, F., et al. (2023). Benefit analysis of multi-approach biomass energy utilization toward carbon neutrality. The Innovation 4:100423. doi: 10.1016/j.xinn.2023.100423
Wang, L., Peng, Q., and Gen, S. (2016). Study on tree layer biomass and productivity in forest in Lushihe Forest Bureau of Changbai Mountains. Res. Soil Water Conservat. 23, 277–281+287.
Wang, Y., and Yu, G. (2023). Ecosystem quality-based management and the development of a new eco-friendly economy. The Innovation 4, 100491. doi: 10.1016/j.xinn.2023.100491
Yildiz, O., Cromack, K., Radosevich, S. R., Martinez-Ghersa, M. A., and Baham, J. E. (2011). Comparison of 5th- and 14th-year Douglas-fir and understory vegetation responses to selective vegetation removal. For. Ecol. Manage. 262, 586–597. doi: 10.1016/j.foreco.2011.04.015
Yu, J. T., Zhang, X. N., Xu, C. Y., Hao, M. H., Choe, C., and He, H. J. (2022). Thinning can increase shrub diversity and decrease herb diversity by regulating light and soil environments. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.948648
Zarfos, M. R., Dovciak, M., Lawrence, G. B., McDonnell, T. C., and Sullivan, T. J. (2019). Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States. Plant Soil 438, 461–477. doi: 10.1007/s11104-019-04031-y
Zhang, D., Man, X., Liu, S., and Xu, Z. (2022). Litter decomposition and nutrient release of typical forest communities in non-growing season in cold temperate zone. J. Beijing Forest. Univer. 44, 65–74.
Zhao, J., Wang, X., Shao, Y., Xu, G., and Fu, S. (2011). Effects of vegetation removal on soil properties and decomposer organisms. Soil Biol. Biochem. 43, 954–960. doi: 10.1016/j.soilbio.2011.01.010
Zhao, Y., Zhao, M. X., Qi, L. L., Zhao, C. S., Zhang, W. J., Zhang, Y. J., et al. (2022a). Coupled relationship between soil physicochemical properties and plant diversity in the process of vegetation restoration. Forests 13:648. doi: 10.3390/f13050648
Zhao, Y. Y., Li, Z. T., Xu, T., and Lou, A. R. (2022b). Leaf litter decomposition characteristics and controlling factors across two contrasting forest types. J. Plant Ecol. 15, 1285–1301. doi: 10.1093/jpe/rtac073
Zhou, J., Wang, Z. W., and Zhang, X. S. (2018a). Deposition and fate of mercury in litterfall, litter, and soil in coniferous and broad-leaved forests. J. Geophys. Res.-Biogeosci. 123, 2590–2603. doi: 10.1029/2018JG004415
Zhou, R., Tang, Y., Wang, M., Dong, H., Yu, F., Chen, C., et al. (2021). Species diversity and soil physicochemical properties at Eucalyptus robustaolantations of different ages in Weiyuan. Chinese J. Appl. Environm. Biol. 27, 742–748.
Zhou, X. G., Zhu, H. G., Wen, Y. G., Goodale, U. M., Li, X. Q., You, Y. M., et al. (2018b). Effects of understory management on trade-offs and synergies between biomass carbon stock, plant diversity and timber production in eucalyptus plantations. For. Ecol. Manage. 410, 164–173. doi: 10.1016/j.foreco.2017.11.015
Keywords: understory removal, understory vegetation restoration, biomass, diversity, soil properties, typical temperate forests
Citation: Zhang Y, Yuan Q, Deng J, Zhou L, Yu D, Zhou W and Wang Q-W (2024) Short-term effects of understory removal on understory diversity and biomass of temperate forests in northeast China. Front. For. Glob. Change 7:1393772. doi: 10.3389/ffgc.2024.1393772
Received: 29 February 2024; Accepted: 19 April 2024;
Published: 17 May 2024.
Edited by:
Ana Cristina Gonçalves, University of Evora, PortugalReviewed by:
Xueyong Pang, Chinese Academy of Sciences (CAS), ChinaSumit Chakravarty, Uttar Banga Krishi Viswavidyalaya, India
Zuoqiang Yuan, Northwestern Polytechnical University, China
Copyright © 2024 Zhang, Yuan, Deng, Zhou, Yu, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangming Zhou, emhvdXdhbmdtaW5nQDEyNi5jb20=; Qing-Wei Wang, d2FuZ3Fpbmd3ZWlAaWFlLmFjLmNu
 Yanyan Zhang
Yanyan Zhang Quan Yuan
Quan Yuan Jiaojiao Deng
Jiaojiao Deng Li Zhou1,2
Li Zhou1,2 Dapao Yu
Dapao Yu Wangming Zhou
Wangming Zhou Qing-Wei Wang
Qing-Wei Wang