- 1Department of Bioscience and Territory, University of Molise, Pesche, Italy
- 2Laboratory of Environmental and Applied Botany, Department of Biotechnology and Life Science, University of Insubria, Varese, Italy
The analysis of fine and coarse roots’ functional traits has the potential to reveal the performance of the root system, which is pivotal in tree growth, development, and failure in both natural and urban forest ecosystems. Furthermore, root traits may be a powerful indicator of tree resilience mechanisms. However, due to the inherent difficulties in measuring ‘the hidden half,’ and despite the recent advancements, the relationships among root functional traits and biotic and abiotic drivers still suffer from a lack of information. Thus, our study aimed to evidence knowledge milestones and gaps and to categorize, discuss, and suggest future directions for effective experimental designs in fine and coarse root studies. To this end, we conducted a systematic literature review supported by backward manual referencing based on 55 root functional traits and 136 plant species potentially suitable for afforestation and reforestation of natural and urban forest ecosystems. The majority of the 168 papers on fine and coarse root studies selected in our review focused predominantly on European natural contexts for a few plant species, such as Fagus sylvatica, Picea abies, Pinus sylvestris, and Pinus cembra, and root functional traits such as standing biomass, phenology production, turnover rate, and non-structural carbohydrates (NSC). Additionally, the analyzed studies frequently lack information and uniformity in experimental designs, measurements, and statistical analysis, highlighting the difficult integration and comparison of outcomes derived from different experiments and sites. Moreover, no information has been detected in selected literature about urban forest ecosystems, while most of the studies focus on natural forests. These biases observed during our literature analysis led us to give key indications for future experiment designs with fine and coarse roots involved, which may contribute to the building up of common protocols to boost the monitoring, managing, and planning of afforestation and reforestation projects.
1 Introduction
Both natural and urban forests deliver multiple vital functions and benefits deemed as ecosystem services that rely on physical, biological, and social modifications of the environment (Mori, 2017; Win, 2019). However, specific demands drive plant management by altering forests and tree attributes (Felipe-Lucia et al., 2018), which may affect their self-organization, adaptative capacity, and resilience (Nocentini et al., 2022). Short- and long-term innovation and optimization strategies are required for the sustainable provision of these ecosystem services (Mann et al., 2022), which, to be effective, should be based not only on increasing the tree cover (Przewoźna et al., 2022), but on an integrated perspective that includes the economic, environmental, and social dynamics of the rural-to-urban gradient (Feng et al., 2021). In this scenario, restoring, increasing, and preserving forest biodiversity, including a minimum of 10% of urban areas covered by tree canopy in all cities by 2050 (European Commission, Directorate-General for Environment, 2021, 2023), as well as integrating with their sustainable strategic planning also green space networks characterizing the peri-urban areas (Verdú-Vázquez et al., 2021), represent a crucial multiple task. Thus, in the next few years, European cities are expected to accomplish a large number of tree-based projects with a priority on the maintenance/management of already established urban and non-urban ecosystems. To make these projects effective, the development of guidelines and good practices is needed.
Due to global warming, central Europe is expected to experience more frequent and long summer droughts with a higher frequency and intensity of storms, windstorms, and other impacting climate events (Pauleit et al., 2005; Solomon, 2007; IPCC, 2014). These events are currently responsible in Europe for about 50% of tree decline, which occurs both in urban and non-urban forests because of the failure interplay between the above- and below-ground tissues (Taccoen et al., 2022). An inadequate rooting response to climate change events is one of the crucial factors in tree failure (Tamasi et al., 2005; Nicoll et al., 2008; Day et al., 2010; McCarthy et al., 2010; Correa et al., 2019), even if the relationship with the potential drivers is still an open question (van Haaften et al., 2021). Evidence points to the impacts of paving and soil compaction on tree root stability and nutrient uptake and their structural collapse caused by pathogen attacks or climate extremes on poorly managed trees (Manfra et al., 2022). Consequently, the root system is a key determinant of the functioning of the forest ecosystem, and the possibility to evaluate root health, decay, and injury is crucial not only to tree stability and management but also for the safety of forests and environments in both urban and non-urban contexts (Kabir et al., 2018; van Haaften et al., 2021).
Plant species vary widely in root characteristics and lifespan and thus create formidable challenges in defining the strategic and operational plans about “where” and “how” to choose tree species for the different areas or about performing interventions on already present plants in urban or non-urban areas. In this direction, the Italian strategic plan for urban forestry built up a list of different species suitable for the afforestation and reforestation of urban areas (NRRP; Protection and enhancement of urban and extra-urban green—M2C4 3.1). This list of species is expected to be the basis for the established planting of 6.6 million trees by 2024, underscoring the issue of the heterogeneity of the new planting species and recommending planting trees according to the biogeographical and ecological characteristics of the sites (Lenormand et al., 2018). In particular, this list includes the main species composing the flora of both Mediterranean and temperate forest ecosystems,1 being representative of the European and pan-European biogeographical regions. Thus, these species potentially play a pivotal role at the European scale in rethinking urban and non-urban designs through successful afforestation/reforestation interventions underpinned by a thorough knowledge of vegetation distribution and their capacity to deliver ecosystem services. In this direction, specific and standardized indexes/traits quantifying the value of plant ecosystem services in urban and non-urban contexts are needed.
As reviewed by Freschét et al. (2021b), root functional traits can be analyzed to obtain knowledge about resource acquisition, protection, and element cycling of plants. Additionally, the analysis of specific root functional traits can reveal plant resilience and adaptation mechanisms to disturbance (Montagnoli et al., 2023). Some of these traits are easily measurable and often vaguely related to a single or a few functions (i.e., “soft”), or difficult to measure and more often closely related to a precise function (i.e., “hard”) (Bakker et al., 2019; Freschét et al., 2021b). Root functional roles are strictly related to some root morphological traits, such as diameter, that permit categorization into fine and coarse roots. Fine roots, representing about 2–3% of the total biomass and 33–67% of the total annual net primary production in most terrestrial ecosystems, accomplish nutrient, oxygen, and water uptake by cooperating with associate mycorrhizae (Vogt et al., 1995; Finér et al., 2011; McCormack et al., 2015). Coarse roots are responsible for the storage of reserves, the distribution of nutrients/water to the above-ground part, and providing comprehensive physical support to the plant anchorage (Zhang and Wang, 2015; Montagnoli et al., 2020). Both fine and coarse roots contribute to plant adaptation to environmental conditions through the so-called “phenotypic plasticity” modulating the root dynamics (production and turnover rate) and morphology (length, diameter, and biomass), which are essential to soil exploration and anchorage (Iversen et al., 2017; Brunner et al., 2019; Dumroese et al., 2019; Iversen and McCormack, 2021). Root traits, such as length, branching characteristics, and diameter, have been quite often related to foraging strategies, with root length being assumed to be proportional to resource acquisition (benefit) and root mass being proportional to construction and maintenance (cost). Under drier soil conditions, plants modulate the production of longer and finer roots, which results in a relatively greater length per unit mass, thereby leading to an increase in specific root length (SRL) (Ostonen et al., 2007; Montagnoli et al., 2012). Moreover, in terms of nutrient acquisition, root production has been related to lateral root branching since a reduced density is beneficial for N capture by reducing competition among root axes (Lynch, 2019; Montagnoli et al., 2022). Zadworny et al. (2016, 2017) proposed two ways of phenotypic plasticity that plants use to react to water or nutrient deficiency: quick growth of fine roots for a fast enhancement of water/nutrient acquisition, or thick root production to go in the direction of long-term ability to store resources. More generally, Cudlin et al. (2007) explained that root growth stops and death (i.e., decrease in root length and biomass and increase in root turnover) might indicate that an overall reduction in root production becomes more functional when water shortage exceeds a certain limit in terms of content and time. In addition to their important role in the exploration and exploitation of water and nutrients, from a biomechanical point of view, preferential root production and growth occur in different directions to enhance anchorage along the axis of mechanical loading (Stokes et al., 2009). In slopy soils and/or under prevailing wind conditions, several authors have demonstrated that the tree anchorage is likely attributable to the forces of the roots pushing downward, hanging upward, or standing in the wind- or lee-ward direction (Danjon et al., 2005; Ghestem et al., 2011; Yang et al., 2014, 2017; Montagnoli et al., 2022). These findings together explain that plants continuously adjust their root growth in response to environmental conditions through the modulation of new root production, longitudinal or radial growth, and the turnover of standing roots (Amendola et al., 2017; Montagnoli et al., 2021) and toward the achievement of higher functional performance. However, the main drivers and production/decomposition patterns related to the phenotypic plasticity of fine and coarse roots still need to be studied to possibly link those to the overall tree performance, especially in a global climate change scenario (Mausolf et al., 2018; Brunner et al., 2019).
In general, the studies of the root systems are limited by their below-ground existence, making them hard to view and sample (Gyssels and Poesen, 2003), especially in urban contexts where the plants experience soils with environmental inputs that are strongly far and different from rural and natural forests. From this perspective, a high magnitude of efforts should be directed toward the appropriate development of urban forest ecosystems. Additionally, even with the same study aims or in similar conditions, the lack of standardized methods and the inhomogeneity of definitions in root studies affect the accuracy and the possibility of making comparisons among experiments and replicates (Reubens et al., 2007). For example, despite the identification of fine and coarse roots through their diameters, there is a lack of a unique definition. Reubens et al. (2007) define the fine roots as having a diameter lower than 3 mm, Zhang and Wang (2015) and Zhang et al. (2020) use the threshold of 2 mm, Dybzinski et al. (2019) of 1 mm, while other scientists still divide in classes the fine roots by their diameter minor than 2 mm or ranging between 2 and 5 mm (Steele et al., 1997; Xiao et al., 2003; Børja et al., 2008). This led to the impossibility of uniformly analyzing the studies to define the relationships among root traits and functions and developmental patterns, also from a species-specific perspective.
In this scenario, we performed a systematic review of the knowledge about the functional traits of fine and coarse roots of those species for afforestation and reforestation in urban and non-urban areas, considering those present in the “Urban and extra-urban afforestation plan” (Italian Ministry of Ecological Transition, 2021a,b), which extensively include different biological forms (e.g., trees, shrubs, herbs, and grasses) used in afforestation and reforestation projects across Europe (Dimitrova et al., 2022). Indeed, there is emerging evidence that planting a diverse mix of species with characteristics representative of a reference ecosystem can facilitate the succession of functional biodiverse ecosystems over time and successfully address climate change and biodiversity loss simultaneously (Andres et al., 2022). Based on this, we critically evaluated milestones and gaps of knowledge emerging from the analysis. Additionally, we performed a comprehensive analysis of the designs that drive the experiments on fine and coarse roots to highlight the peculiarities and general features. Such an overall assessment can lead to standardized procedures and methods applicable for experimental design in fine and coarse root research projects on tree species for afforestation and reforestation of urban and non-urban areas, boosting the development of applicative guidelines for the management of these peculiar ecosystems.
2 Methods
2.1 Searching strategy
This review is focused on the root functional traits of 136 plant species (including 79 trees and 57 shrubs) reported in the “Urban and extra-urban afforestation plan” (Italian Ministry of Ecological Transition, 2021a,b) as suitable for afforestation, reforestation, and tree planting in urban and non-urban areas. Indeed, these measures should take into consideration the high diversity of species, which also includes the different growth forms of plants. We performed a systematic literature search following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; Moher et al., 2009) using a query-based strategy and backward manual referencing of the literature. We first identified the root functional traits to be used as query terms for the automatic literature database interrogation by exploring the FRED 3.0 database that reports data related to root traits grouped in categories (Iversen and McCormack, 2021) and the paper of Freschét et al. (2021a). We considered those root traits related to architecture, chemistry, dynamics, morphology, physiology, and microbial associations together with the functional traits “root standing biomass” and “root standing necromass” crucial for the description of root dynamics in forest ecosystems. Lists derived after the exploration were crosschecked, and a comprehensive set of 55 root functional traits, categorized along with others and mapped in relation to one another, was adopted to perform the literature searches (Figure 1).
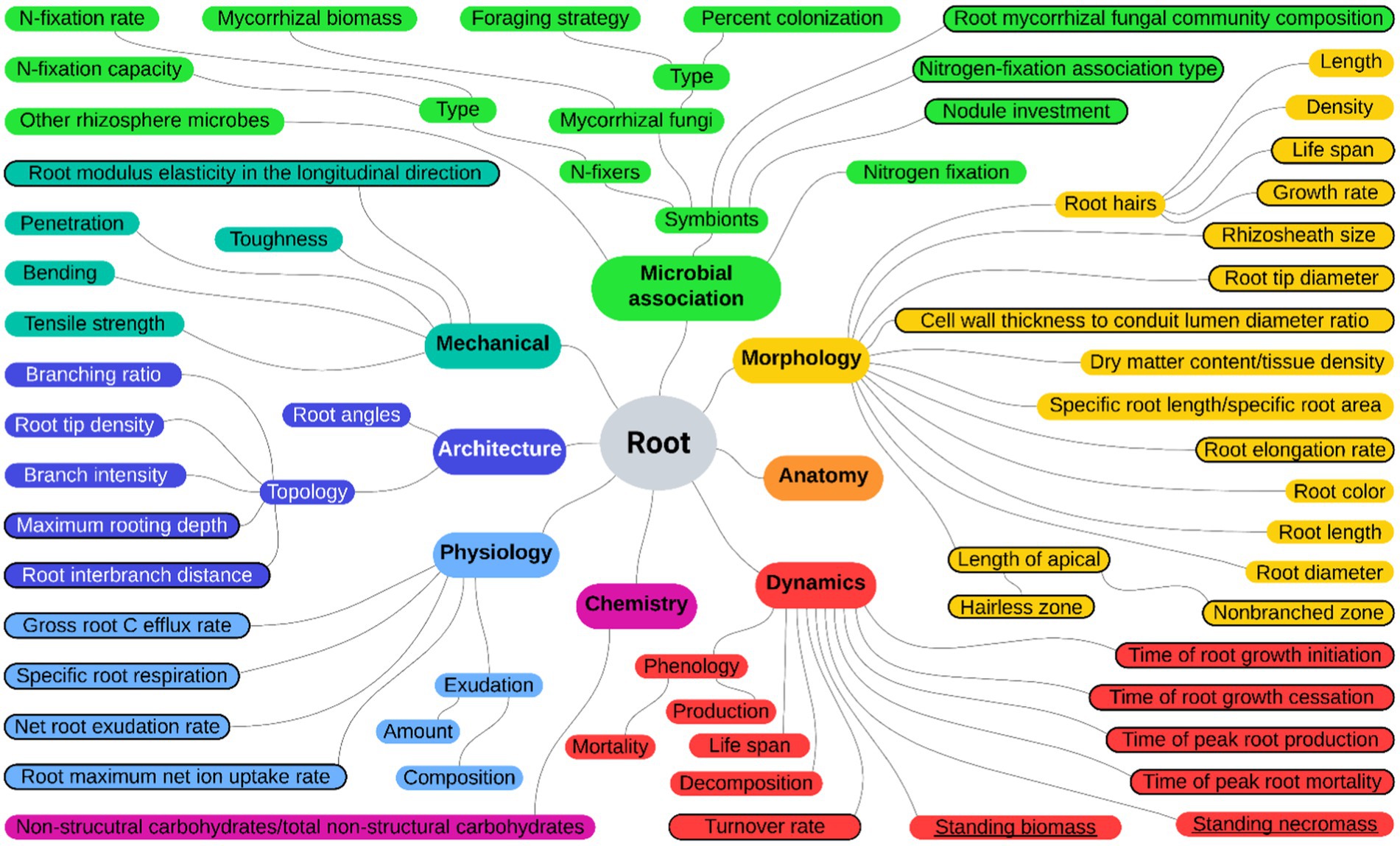
Figure 1. Root functional trait categorization and mapping. Map of the 55 root functional traits used as query terms from the FRED database and from Freschét et al. (2021a; black circled) together with “root standing biomass” and “root standing necromass” (underlined) categorized according to colors: yellow for morphology, orange for anatomy, red for dynamics, purple for chemistry, light blue for physiology, blue for architecture, sea green for mechanical traits, and green for microbial association.
The combination of the 55 root functional traits together with the names (Latin binomials and common) of the 136 tree species were used as query terms together with the words “root” and “roots.” The common species names were chosen according to those reported in the “Taxonomy” database of the National Center for Biotechnology Information (NCBI).2 In total, 7,480 queries (Supplementary File 1) were composed and used to perform searches through PubMed3 to identify articles with query terms matching their abstract, keywords, and title (last updated on 20 July 2023). The outputs were screened, edited, and tabulated by scripts in Perl (Wall et al., 2000). Additionally, the free accessible package pheatmap (v. 1.0.12; Kolde, 2019) in R (R Core Team, 2017) was used to draw a clustered heatmap based on the log scaled numbers of records identified for each species/trait combination. The clustering was done by complete hierarchical clustering based on Euclidean distances for both species and root functional traits, which were also categorized according to their recurrence in the literature search outputs. Subsequently, of all the identified records, only the scientific articles showing the terms “fine root(s)” and “coarse root(s)” in their abstract, keywords, and title and not related to the seedling stage and/or under controlled conditions were retained and considered relevant to our subsequent questions. Considering the wide range of related fields of the topic of our study, backward manual referencing based on snowballing (Wohlin et al., 2022) was also applied to identify relevant studies on the fine and coarse roots of the target species missed by query-based searches (Avenell et al., 2001; van Haaften et al., 2021). These papers, together with those identified through query-based searches, were included in the systematic literature review and underwent a full-text examination.
2.2 Data extraction and summary of selected studies
The information included in the selected original articles for the screening of their full text was categorized, considering three specific variables explained as follows: data were registered at the level of each selected original article.
The analysis of the first variable, “objective of the study,” was performed by categorizing the studies according to:
i. environmental constraints (e.g., drought, temperature, flooding, etc.),
ii. anthropogenic constraints (e.g., forest management, fertilization, irrigation, pollution, fire, etc.),
iii. other.
The analysis of the second variable, “root type,” was performed by categorizing the studies according to:
i. fine roots,
ii. coarse roots,
iii. other/not-specified types of roots as targets.
The analysis of the third variable, “experimental design,” was performed by categorizing the studies according to:
i. “population characteristics” (natural/afforestation/reforestation/agroforestry),
ii. “coordinates and localization,”
iii. “size of the population,”
iv. “plant age,”
v. “sampling method(s),”
vi. “sampling design (when, replicate, etc.),”
vii. “sampled organ(s),”
viii. “statistical method.”
Additionally, for each article, some features were reported, such as an ID (PubMed ID or EID from Scopus or DOI) and the number/type of species and root functional traits described in the article. This procedure was performed and cross-reviewed by the authors, allowing a detailed, summarized categorization of the knowledge present in each study.
2.3 Data visualization and statistics
The categories “species,” “traits,” “objective,” “type of root,” “maximum fine root diameter,” “minimum coarse root diameter,” “site type,” “country,” “number of plants,” “plant age,” “sampling method,” “experiment duration,” “sampling number,” “months of sampling,” “sampled organ,” and “statistical analysis” were considered. Plots and statistical analysis were carried out through R (R Core Team, 2017). Specifically, the R package “wordcloud” (Fellows, 2018) was used to generate a word cloud of the keywords associated with selected articles in the database, and the package ggplot2 (Wickham, 2016) was exploited for the graphical visualization of data. The package “rworldmap” (South, 2011) was used to plot the geographical distribution of the studies. For the Whittaker biome classification of the case studies, we plotted our database through the R package “BIOMEplots” (Stefan and Levin, 2018), acquiring climate data from WorldClim (Fick and Hijmans, 2017). Additionally, the publication-per-year trend of articles related to fine and coarse roots was plotted by the “ggplot2” RStudio package (Wickham, 2016).
3 Literature resource identification: milestones and knowledge gaps of plant species and root functional traits
The query-based search in PubMed identified a total number of records equal to 25.688. These records were considered together with those identified through backward manual referencing (42 papers) and, after duplicate removal, reduced to 2.424 (Supplementary File 2). Among all the identified records, only some were eligible for our criteria (281 papers). Records regarding experiments analyzing seedlings as plant material or plants growing under controlled conditions were also excluded (100 papers), as well as papers with no full text available (12 papers). Furthermore, a single record was excluded as not being related to an original research article. Finally, all articles with the full text available (168 papers) were included in the subsequent analysis (Figure 2). The full text of these papers was screened, and the included information was categorized according to specific variables and tabularly reported (Supplementary File 3).
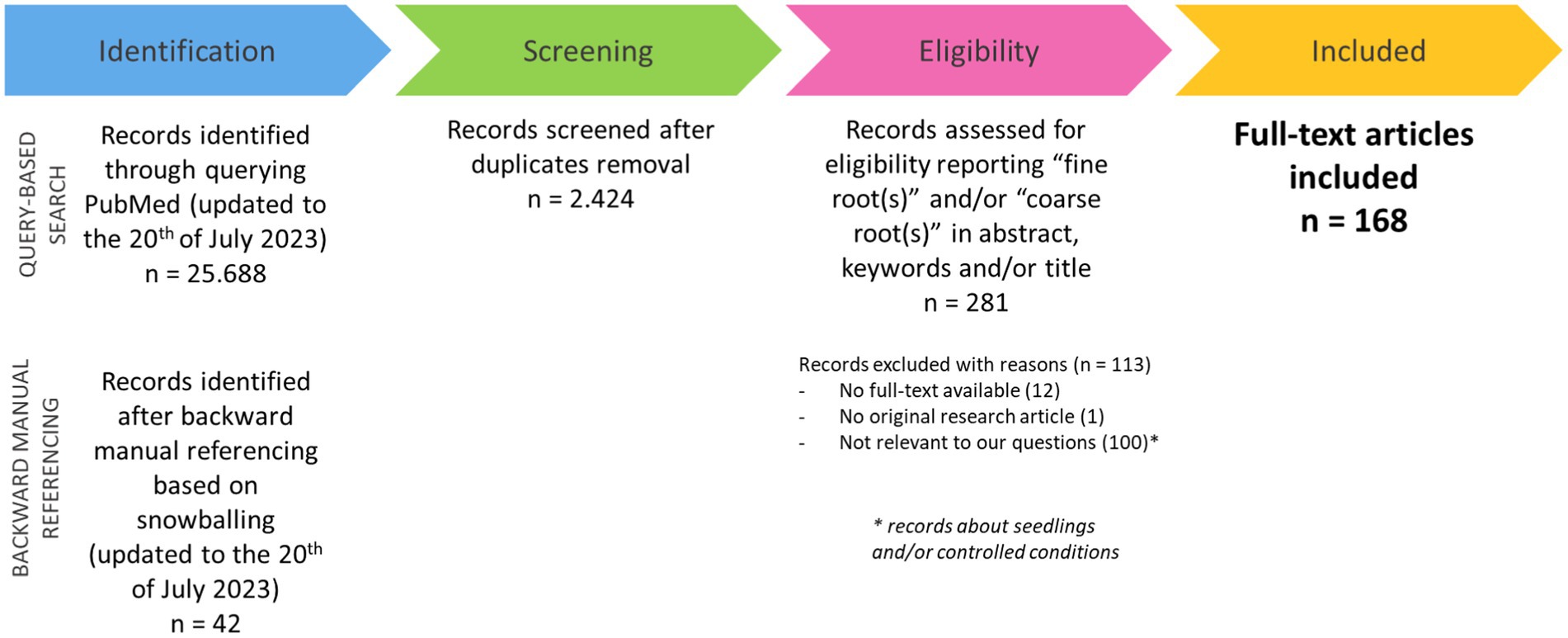
Figure 2. Flow chart of the process for selecting and including in the study the papers identified for the systematic review.
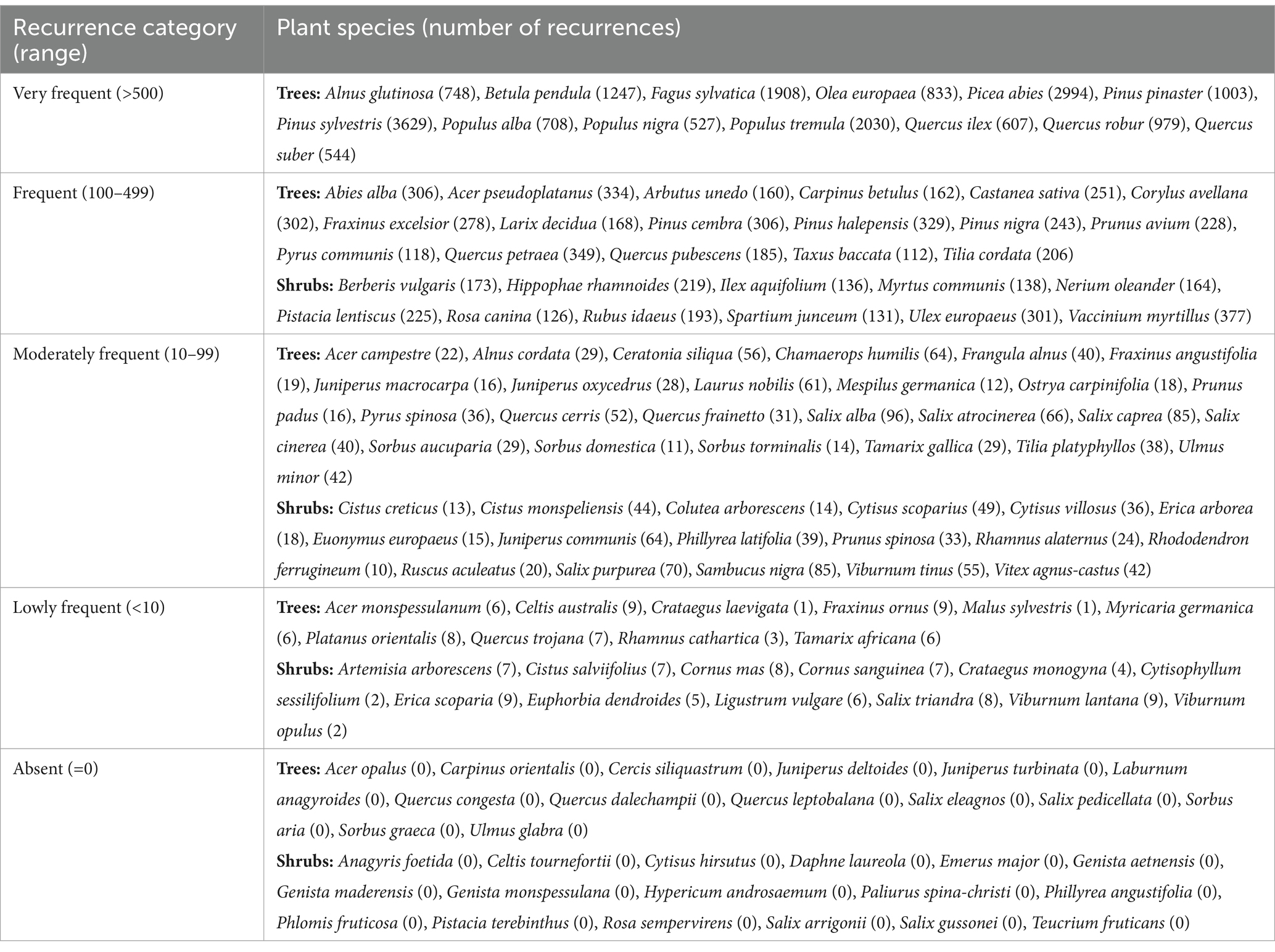
Plant species were categorized according to their recurrence in the literature as “very frequent” if identified with more than 500 recurrences (13 species), “frequent” if identified by a number of recurrences ranging between 100 and 499 (28 species), “moderately frequent” if identified by a number of recurrences ranging between 99 and 10 (42 species), “lowly frequent” if identified by a number of recurrences minor than 10 (22 species), and “absent” if not identified at all (31 species) (Table 1).
From a root functional traits perspective, we also performed a categorization considering their recurrence in the literature search outputs, subdividing the root functional traits into “very frequent” if identified with more than 500 recurrences (8 traits), “frequent” if identified by a number of recurrences ranging between 100 and 499 (41 traits), “moderately frequent” if identified by a number of recurrences ranging between 99 and 10 (6 traits), “lowly frequent” if identified by a number of recurrence minor than 10 (none), and “absent” if not identified at all (none) (Table 2).
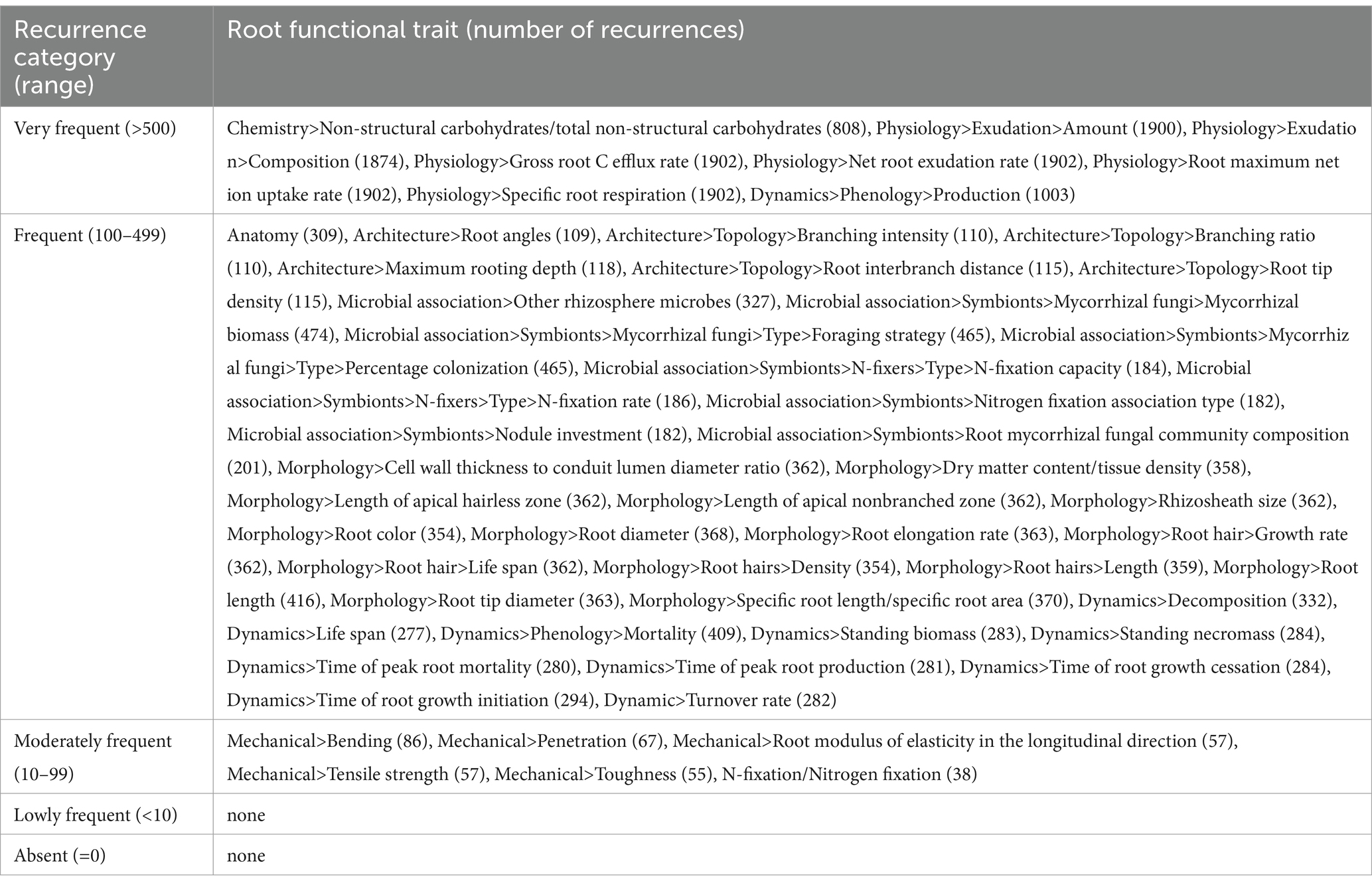
Table 2. Root functional trait categorization according to their recurrence in the literature search outputs.
The physiological-related root functional traits are the most investigated, both for all the species and for those categorized as “very frequent” (Figure 3). In addition, root dynamics- and chemistry-related functional traits such as “phenology production” and “non-structural carbohydrates/total non-structural carbohydrates,” respectively, fall within the category of “very frequent” both for all the species and for those categorized as “very frequent” (Figure 3).
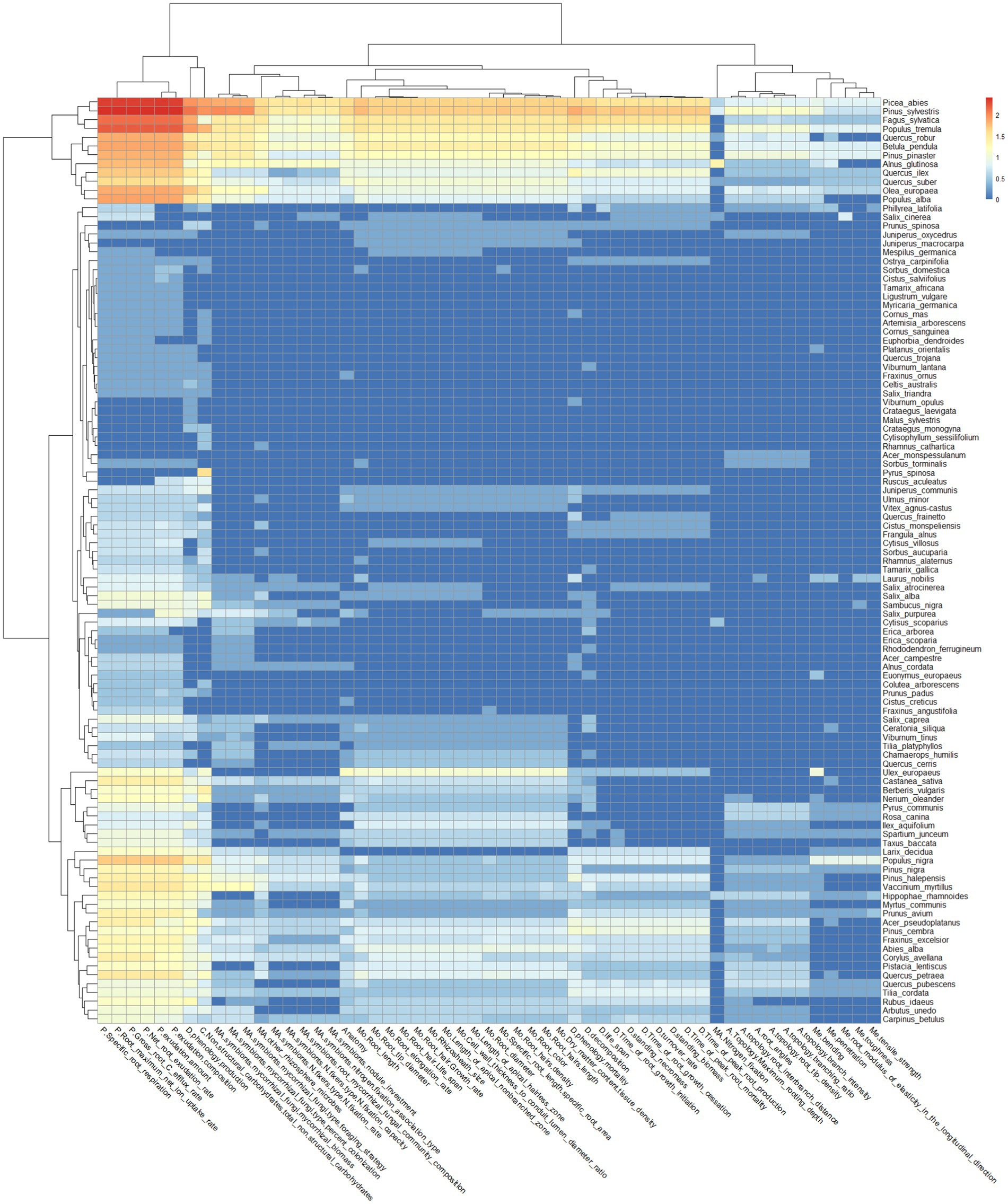
Figure 3. Heatmap of target species and root functional traits, which are represented in the PubMed search outputs. The x-axis shows the root functional traits, whose functional categories have been indicated as: A for architecture, C for chemistry, MA for Microbial Association, Me for mechanical, Mo for morphology, P for physiology, and D for dynamics. The y-axis shows the Latin names of target species present in the search output.
4 Information resulting from the screening of selected papers
A total of 168 full-text articles about fine and coarse roots were included in the database for the process of knowledge categorization based on the summary table (Supplementary File 3). The temporal analysis showed a general increase in the number of papers about fine and coarse roots published from 1992 to 2023, which is in consideration in our study (Figure 4). However, this literature production trend is characterized by alternate periods of high and low productivity. According to our results, the scientific name of the species Fagus sylvatica emerges as one of the most frequent keywords associated with selected papers, together with “fine root,” “Picea abies,” “Pinus sylvestris,” “non-structural carbohydrates,” and “minirhizotron” (Figure 5A). Indeed, among the 33 species present in the selected literature (Figure 5B), Fagus sylvatica is the most targeted species in fine and coarse root studies, as described in 55 out of 168 studies. This is followed by Picea abies in 50 studies, Pinus sylvestris in 39 studies, Pinus cembra in 13 studies, and Betula pendula in 10 studies. The rest of the species is targeted in a number of studies, including between 9 and 1 (Figure 5B).
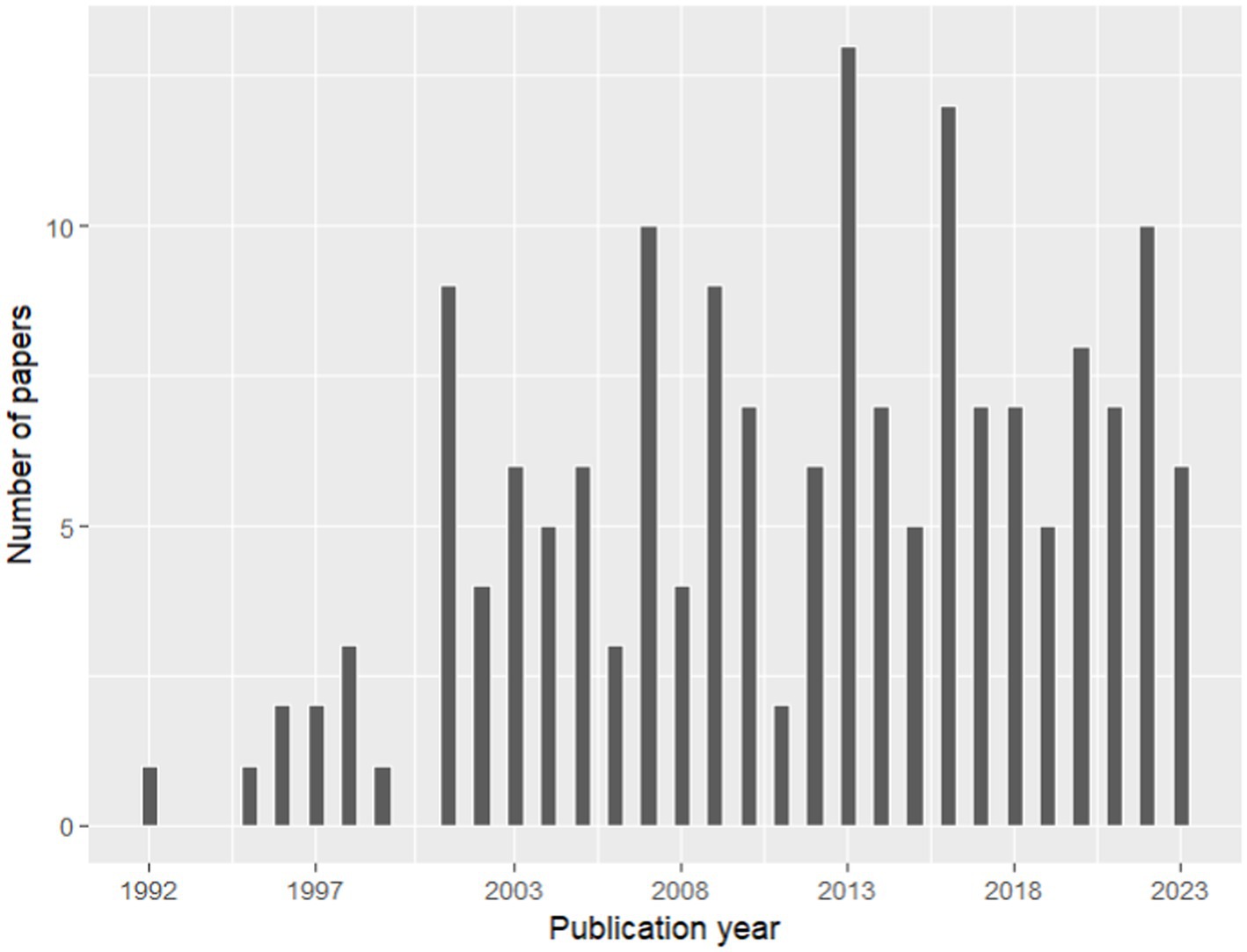
Figure 4. Temporal analysis per year of published papers present in our database (Source: PubMed, 1992–2023).
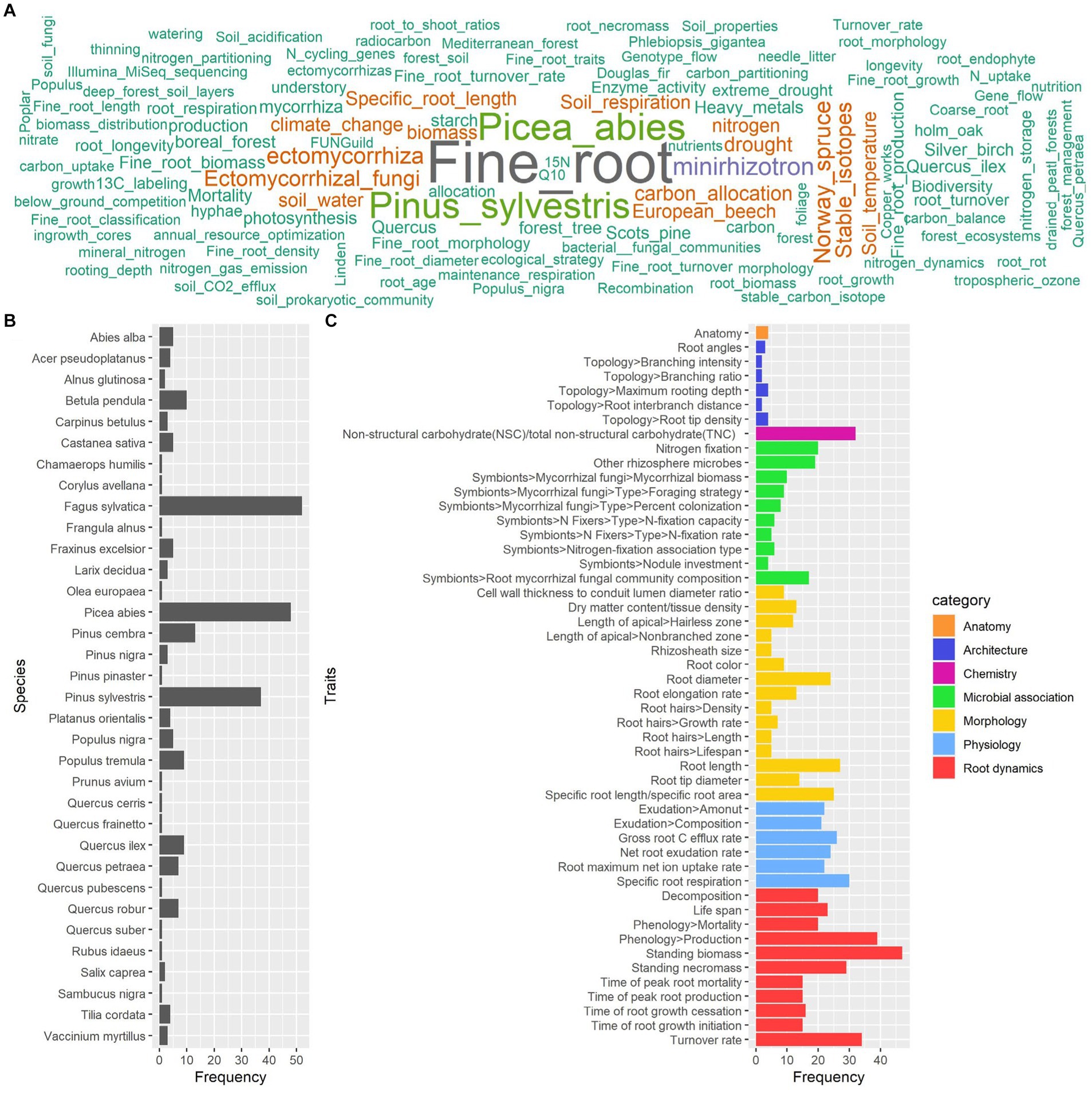
Figure 5. Word cloud of the keywords (A), occurrence of species (B), and of root functional traits (C) associated with screened papers. The colors in (A) trace the keyword occurrence. Colors in (C) trace the categories of the root functional traits.
The papers in our database regard 50 root functional traits, and the most investigated are related to root “dynamics” and, specifically, “standing biomass” in 48 out of 168 articles, “phenology production” in 41 articles, and “turnover rate” in 38 articles (Figure 5C). Also, other functional traits are widely represented, such as “non-structural carbohydrate (NSC)/total non-structural carbohydrate (TNC)” related to “chemistry” and described in 33 articles, or “specific root respiration” related to “physiology” and described in 31 articles (Figure 5C).
The geographical distribution of the carried-out studies showed that the most represented countries are Germany (32 publications), Switzerland (20 publications), Finland (16 publications), and Italy (15 publications), with other countries represented at a lower magnitude (Supplementary Figure 1).
Among global biomes, the most represented were temperate seasonal forests, woodland/shrubland, and boreal forests (Figure 6). Both gymnosperms and angiosperms are represented in these biomes, except for the boreal forest biome, which is more represented by angiosperms than gymnosperms. Tropical seasonal forest/savanna was represented in only one publication describing a gymnosperm. Thus, data extracted from the literature showed gaps related to some biomes, which are temperate and tropical rain forests, tundra and subtropical deserts, and temperate grassland/deserts, which represent the extremes in climate space. The data spanned a gradient of annual precipitation from 42.1 to 166.3 cm and of temperature from −0.7 to 24.66°C.

Figure 6. Whittaker biome plot of database locations (n = 169) showing mean annual temperature (°C) and mean annual precipitation (cm). Each point represents a plant species: gray for angiosperms and black for gymnosperms. Climate data are from WorldClim (Fick and Hijmans, 2017).
The greatest part of the studies analyzed had objectives other than investigating anthropogenic or environmental constraints (Figure 7A) and focused on the fine roots, a few on the coarse roots, a part targeted both fine and coarse roots, and the rest had no clear specification about this (Figure 7B). The studies were mostly carried out only in natural contexts, followed by afforestation sites, and only a few studies were carried out in agroforestry or reforestation sites or in combination of sites with diverse typologies (Figure 7C).
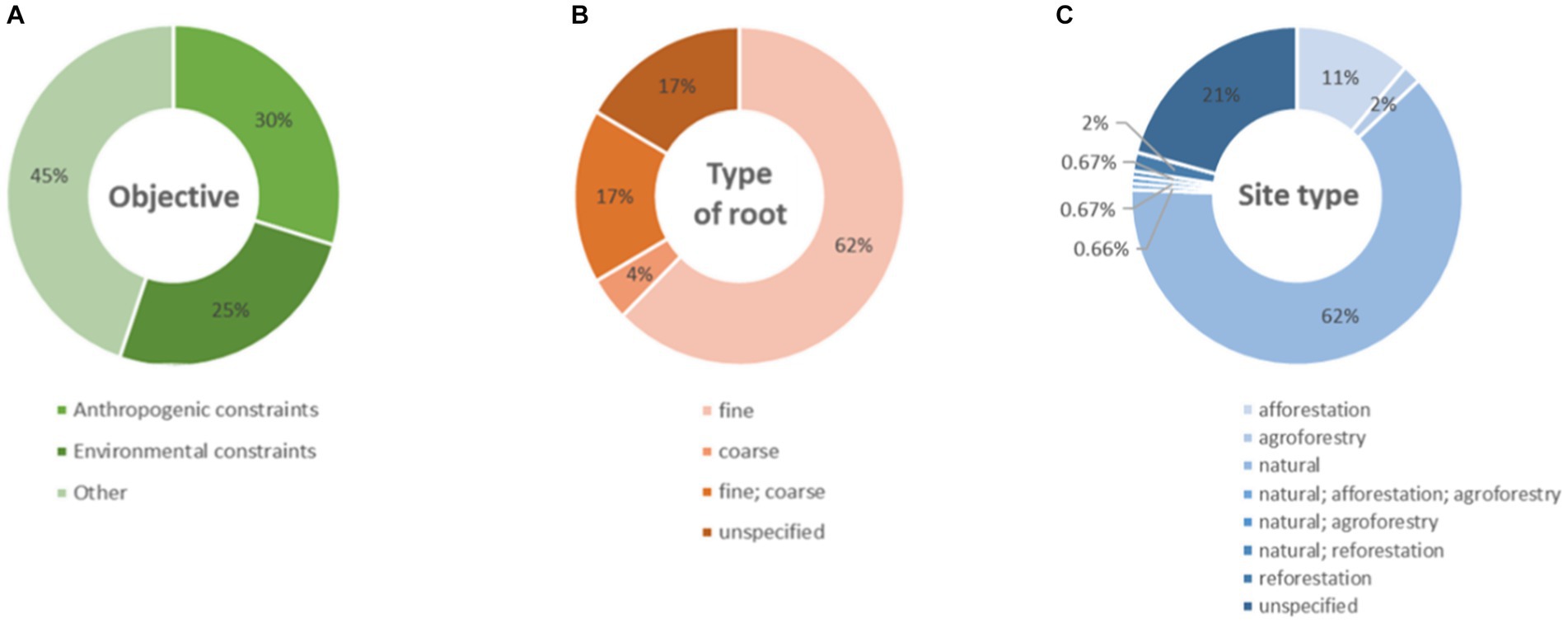
Figure 7. Donut charts represent (A) the percentages of objectives, (B) the type of targeted root(s), or (C) the site type(s) of the studies present in our database.
Notably, although the species used to search the literature are suitable for urban afforestation and reforestation, in fact, no research carried out in an urban environment emerges among the studies under analysis.
Plant age is another parameter of the experimental design considered in this analysis. A part of the selected literature (21 studies, equal to 12.5%) is based on experiments that indicated vague plant age, reporting it as a time range in years (Supplementary Figure 2A). On the contrary, about half of the studies (80 studies, equal to 47.62%) provided precise indications about this parameter (Supplementary Figure 2B). Nevertheless, most of the studies provided no information about plant age (67 studies, equal to 39.88%).
The months of sampling are clearly indicated in 71 studies (42.27%), mainly occurring in April (27 studies, 16.07%), July (28 studies, 16.67%), and October (26 studies, 15.48%), less those of the winter period (Supplementary Figure 2C). Some studies (16 studies, 9.52%) mentioned the sampling period by a season or with ambiguous indications like “before summer” and were considered as “vague.” Additionally, 81 studies (48.21%) made no description of the sampling period (Supplementary Figure 2C).
Almost half of the studies did not provide information regarding the number of plants included in the experiment (82 studies; 48.81%), and the rest described experiments including a number of plants ranging from 2 to 606 (Supplementary Figure 2D). There is a wide range of experiment duration spanning from 1 to 156 months (91 studies, equal to 54.17%; Supplementary Figure 2E).
However, most of the studies provided no indication about the experiment duration (56 studies equal to 33.34%) or were based on a single sampling (21 studies equal to 12.5%). Most experimental designs involved only root sampling (102 studies; 60.71%), while in other 26 studies (15.48%), the roots were sampled together with one or more additional organs, and in 40 studies (23.81%), the organs targeted for sampling were not specified (Figure 8A).
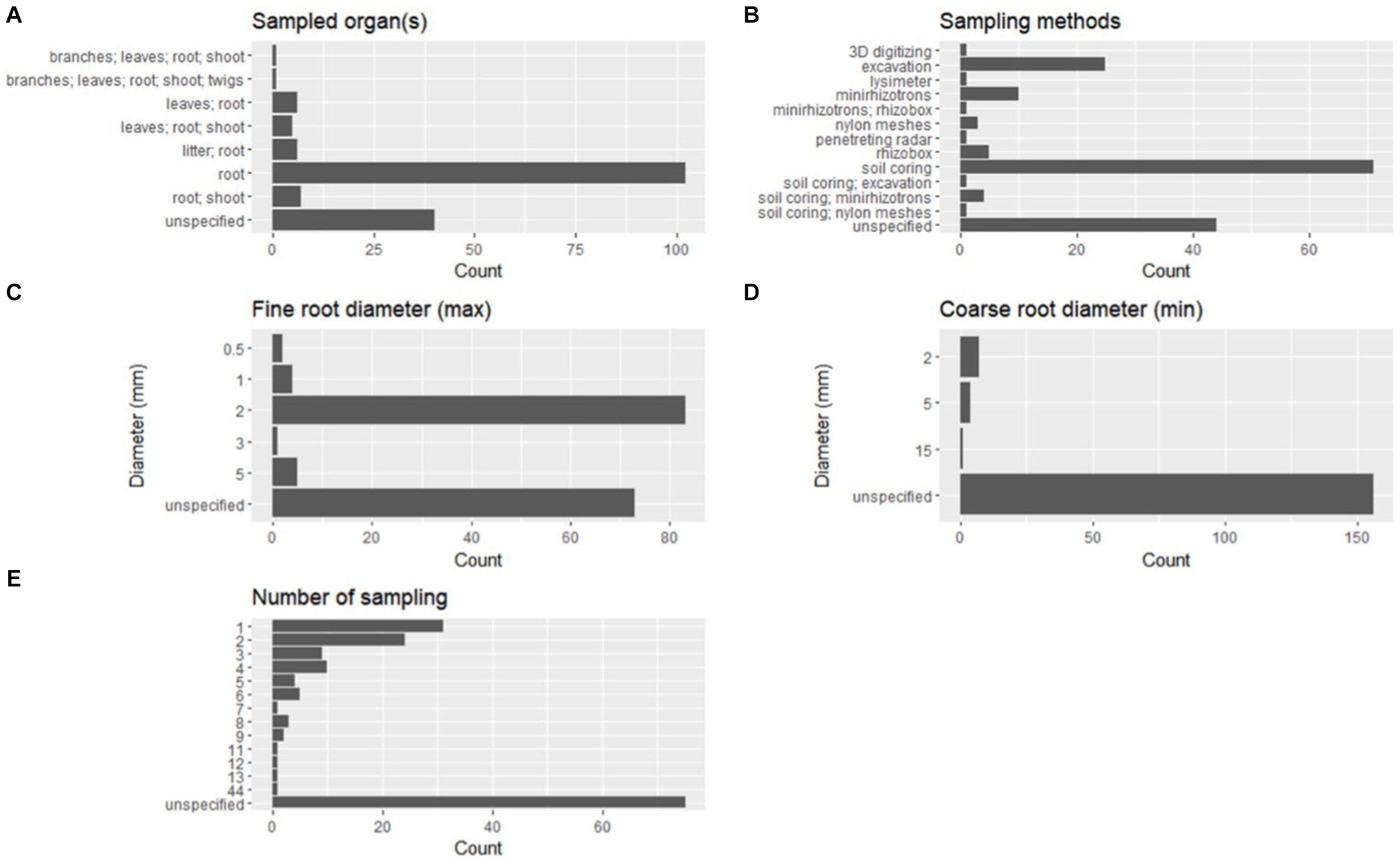
Figure 8. Features of the experimental designs in terms of sampled organs (A), sampling method (B), fine-root diameter (max, C), coarse root diameter (min, D), and number of samplings (E).
Among the sampling methods used alone, the most represented was soil coring (71 studies; 42.26%), followed by excavation, described in 25 studies (14.88%), and minirhizotrons (10 studies; 5.95%) (Figure 8B). Rhizoboxes were described in five studies (2.98%), nylon meshes in three studies (1.79%), and lysimeter, 3D digitizing, and penetrating radar were described only in one study (~0.59%), respectively (Figure 8B). The rest of the studies (seven, equal to 4.17%) describe a combination of two different methods. A number of studies equal to 44 (26.19%) have no specific description of the sampling method (Figure 8B).
The maximum diameter to define a fine root is frequently set at 2 mm (83 studies, 49.40%), while only some studies apply different thresholds equal to 0.5 mm (two studies, 1.19%), 1 mm (four studies, 2.38%), 3 mm (one study, 0.60%), or 5 mm (five studies, 2.98%; Figure 8C).
It is relevant to note that in 73 studies (43.45%), there are no indications about the diameter size adopted to identify fine roots.
Minimum diameters to identify coarse roots are defined only in eight studies (4.76%) and are set equal to 2 mm, 5 mm, or 15 mm (Figure 8D). However, the rest of the selected studies (160, corresponding to 95.24%) report no indications about this.
Concerning the number of samplings reported in the selected literature, 93 studies (55.36%) described a number of samplings ranging from 1 to 44 (Figure 8E), and among these, 31 studies are based on a single sampling (18.45%), while others are based on two samplings (24 studies, 14.29%), three samplings (nine studies, 5.36%), or four samplings (10 studies, 5.95%) (Figure 8E).
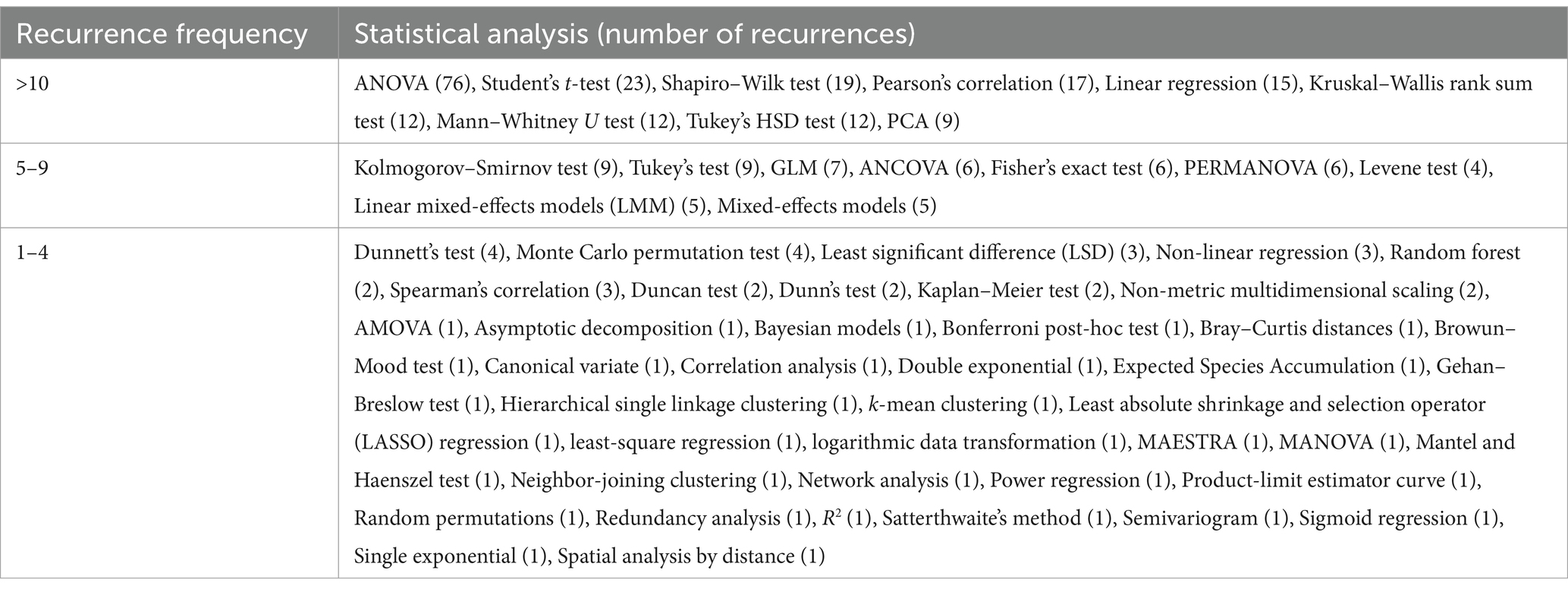
Of the others, 75 out of 168 studies (44.64%) did not report precise information (Figure 8E). In Table 3, results related to the occurrence of the statistical analysis are reported. Few statistical tests were frequently used, and among these, the most commonly used was ANOVA (45.24%), followed by Student’s t-test (13.69%), the Shapiro–Wilk test (11.31%), Pearson’s correlation (10.12%), linear regression model (8.93%), Kruskal–Wallis rank sum test (7.14%), Mann–Whitney U test (7.14%), Tukey’s HSD test (7.14%), and PCA (5.36%). Other statistical tests were applied in a number of studies ranging from 5 to 9, and the greatest part of the statistics were applied in less than four studies.
5 Discussion
We provide a general overview of the literature about root functional traits together with a revised and detailed dissection of the studies of fine and coarse roots of species targeted for afforestation and reforestation programs in urban and non-urban areas, evidencing common practices and criticisms of the applied experimental procedures.
5.1 Fine and coarse roots are mainly studied in European natural contexts for a few plant species and functional traits
Among the 136 target species, some of them, identified as “very frequent,” were well studied for their biological and economic relevance, such as Alnus glutinosa, Betula pendula, Fagus sylvatica, Olea europea, three Quercus genus, and Pinus pinaster (Majdi, 2001; Rastogi et al., 2015; Kostelenos and Kiritsakis, 2017; Mármol et al., 2019; Rey et al., 2019; Mader et al., 2020; Smeriglio et al., 2022), and/or are model species in plant biology investigations, such as Picea abies, Populus alba, and Populus tremula (Brunner et al., 2001, 2004; Vasiliauskas et al., 2007; Shorohova et al., 2011; Gauthier et al., 2015).
Among the traits analyzed, all those related to root physiology were classified as “very frequent” in the literature, and among these, root exudation was a key component since it played a crucial role in both plant–soil interactions, root carbon efflux, and microbe relationship. In particular, root exudates can modify soil characteristics and locally adapt plants to abiotic/biotic stressors, giving clear hints on plant morpho-physiological plasticity in response to biotic and abiotic stressors (Canarini et al., 2019). Therefore, despite the main drivers of root exudation still need to be identified, the study of root exudation remains a key to understanding plants as holobionts, especially in natural and urban forests (Lyu and Smith, 2022).
Despite the pivotal role that fine and coarse roots play in plant development and adaptation, and despite an increasing trend in scientific production, studies on these two root categories are still lacking. Furthermore, a large part of the root studies do not focus on environmental or anthropogenic constraints, and they are mainly performed in natural sites that do not experience any urban stressors. In detail, among the 136 candidate plant species, F. sylvatica is a common target species in fine and coarse roots studies (Montagnoli et al., 2014; Weigt et al., 2015; Montagnoli et al., 2018; Nikolova et al., 2020) and specifically in those analyzing fine roots biomass (Braun et al., 2005; Konôpka and Lukac, 2013; Jagodzinski et al., 2016; Montagnoli et al., 2016; Geilfus et al., 2017; Konôpka et al., 2020; Montagnoli et al., 2023). In root turnover studies, species other than F. sylvatica, such as Picea abies or Pinus sylvestris, are frequently involved (Godbold et al., 2003; Xiao et al., 2003; Vanninen and Mäkelä, 2005; Eriksson et al., 2012; Jacob et al., 2014; Mildner et al., 2014; Nacke et al., 2016; Yan et al., 2016; Zadworny et al., 2016; Grüning et al., 2017; Mariën et al., 2021). Root dynamics is frequently linked to environmental drivers such as drought, seasonality, or ozone concentration (Lopéz et al., 1998; Paoletti et al., 2007; Mainiero et al., 2009; Kuptz et al., 2011a,b; Montagnoli et al., 2014; Nickel et al., 2018; Montagnoli et al., 2019), together with traits associated with the chemistry of non-structural carbohydrate (Rosinger et al., 2020; Clausing et al., 2021). However, the greatest part of the other root functional traits is still at the beginning of the exploration of complex relationships with functions and environment, especially in the case of coarse roots (Freschét et al., 2021a). Our results also evidenced that the studies of the fine and coarse root functional traits are limited to countries that belong mostly to Central and North-Eastern Europe and North America and only to China and Mongolia for Asia, highlighting the need to expand the exploration to other regions and biomes.
5.2 Experimental designs lack crucial information
A large part of the studies was based on a single sampling or reported as having no or vague indications of the total duration of the experiment or the timing of the sampling, with no clear indications of dates and months. This approximation was also observed for information concerning both the number and age of the plants analyzed. In this perspective, our findings highlight the need for protocol uniformity about the tree species sampled and analyzed as individuals or populations as they might respond differently to environmental drivers (Rytter, 2013; Dawes et al., 2015; Mausolf et al., 2018; Lak et al., 2020; Freschét et al., 2021a). The relevance of the duration of the sampling period strongly affects the outcome of the research, especially in the root context where the phenotypic plasticity is high both in the short- and long-term periods (Brunner et al., 2013; Klavina et al., 2016). For example, in fine-root turnover studies, multiple sampling across different growing seasons is fundamental (Terzaghi et al., 2013; Kubisch et al., 2016, 2017) to avoid misestimation of production and mortality (Tingey et al., 2003).
Our findings underscored significant differences in various morphological parameters of the fine roots, except root length, when comparing different root sampling methods. Thus, increasing sampling effort (i.e., applying a combination of different sampling methods and/or considering samplings to avoid age-related bias) when designing a root experiment might lead to higher precision of the measured root characteristics (Tingey et al., 2003; Rytter, 2013; Klavina et al., 2016; Mausolf et al., 2018). In general, root studies based on samples collected from a few locations within the root zone have lower differentiating power than whole-plant sampling (Han et al., 2014), and increasing the sample size strongly impacts the outcome of the research as the root characteristics are affected by microsite variability (Rytter, 2013).
Our findings indicate that roots are usually sampled alone in most of the studies, although coupling the analysis of other organs might help to better understand ecosystem functionality. For example, tracing non-structural carbohydrates or labeled carbon or nitrogen in different organs improves the understanding of C dynamics (Maillard et al., 2001; Dyckmans and Flessa, 2002; Scartazza et al., 2015; Tang et al., 2022). Similarly, the analysis of the growth of both above- and below-ground organs has been linked to the presence of pollutants in the soil (Stobrawa and Lorenc-Plucińska, 2008; Dalle Fratte et al., 2022). Intriguingly, Kilpeläinen et al. (2022) identified resource competition among plant organs as a major driver of root plasticity, implicitly suggesting multiple organ studies as more complete. Therefore, we encourage root scientists to expand their collaboration, including plant scientists who might integrate analysis of the above-ground organ traits.
Another feature of the experimental design was related to the fine and coarse root definitions based on the diameter size. Fine roots are defined as short-lived, non-woody roots responsible for water and nutrient adsorption with a diameter lower than 2 mm (Rytter, 2013), which is the reference diameter we have found in the greatest part of the studies. However, from our results, diameter thresholds other than 2 mm are still used, and in the majority of studies, there is no precise indication provided about these thresholds. The coarse roots, which are woody roots crucial for soil exploration and plant stability, are frequently evaluated only as being not classified as fine roots (Rytter, 2013). Our results support this observation, as the greatest part of the authors give no threshold as a reference for the diameter of the coarse roots. Therefore, although detailed and precise information on root diameter is fundamental and can be linked to specific functional traits (Markkola et al., 1995; Menkis et al., 2004; Helmisaari et al., 2007; Børja et al., 2008; Gaul et al., 2009; Helmisaari et al., 2009; Meinen et al., 2009; Endrulat et al., 2010; Menkis et al., 2012; Terzaghi et al., 2013; Montagnoli et al., 2018; Kriiska et al., 2021; Mariën et al., 2021; Schwieger et al., 2021), our literature analysis reveals a diffuse failing in determining specific and functional diameter thresholds (Helmisaari et al., 2009; Nikolova et al., 2020).
5.3 The inadequate application of common a priori model-based statistics for interpreting complex dynamics of roots
Common issues related to root data collection are exacerbated by the application of complex and inappropriate statistical design (Ahrens et al., 2014; Thiese et al., 2015; Mausolf et al., 2018). Our findings are in line with this observation, which highlights the application of a large number of different statistical tests, which indicates a lack of clear and uniform statistical protocols in root studies. It is important to underline the need to build an optimal and univocal statistical approach to be applied for the study of a specific trait or set of traits that is tightly coupled with the sampling method and technique.
6 Future directions and perspectives
Our literature analysis evidenced the complete or limited root trait knowledge of the selected species for the afforestation and reforestation of urban and non-urban areas and, thus, the need to fulfill these knowledge gaps. The greatest part of the listed 136 plant species has already been reported to be widely used across Europe in afforestation and/or reforestation projects as well as in urban and non-urban areas (Dimitrova et al., 2022). For example, it has been seen that afforestation strategies in urban contexts, including A. glutinosa and B. pendula, induce a change in the soil properties, increasing acidity and nutrient contents after 45–60 years from introduction (Podwika et al., 2018). Furthermore, Quercus and Populus species have been shown to have great potential in urban afforestation and reforestation for their functionality in air quality improvement and climate regulation (Fusaro et al., 2015; Sun et al., 2017; He et al., 2018; Mariën et al., 2021). Thus, these species may serve as possible future targets to study root systems in urban contexts.
Our study evidenced the total absence of fine and coarse root studies in urban areas and limited coverage of world regions and biomes. Despite this, urbanization forecasts suggest world regions other than Central Europe and North America as being involved with higher urban extents, like Eastern and Western Europe, South America, some regions of the northern part of Africa, and Mid, Central, and Western Asia (Seto et al., 2012). In this perspective, studies on root systems must also concern species native to or adaptable to world regions that will experience high levels of urbanization within the next few years, and this task needs to consider that the urbanization process is usually a strong driver in modeling the changes of plant species and communities (Dimitrova et al., 2022; Ruas et al., 2022). Moreover, a large effort is needed for scientists from the most studied regions to apply their knowledge to less studied countries and ecosystems through specific funding opportunities.
With the aim of sharply providing take-home messages derived from our literature review, we followed a list of crucial points summarizing the main outcomes.
i. Population characteristics: Species other than common targets for their biological and economic relevance must be included in the root studies, and a wider analysis of functional traits should also be performed for the most common target species. Additionally, sites other than natural should be taken into account, considering the large efforts toward afforestation and agroforestry programs.
ii. Experimental design directions: plants of a known and uniform age and appropriate sampling (what, when, how many times, and how long): Plant age needs to be well-known and possibly uniform among the trees included in the experimental design. Sampling of multiple organs would give higher and more complete information about resource acquisition and utilization since organs often have complementary or competitive roles. Experiment duration must overlap and be extended beyond the period of root dynamics to fully appreciate parameter changes over time in accordance with the dynamics of the type of root category and the experiment aims. Thus, multiple samplings across spring, late summer, and/or early autumn are suggested for fine roots, while full autumn sampling with a yearly repetition is suggested for coarse roots.
iii. AI may enhance statistical models to fit with biological data complexity and dynamics: To capture the complex patterns and relationships in data derived from the intermingled and dynamic root system, complex algorithms based on machine learning, deep learning, and other artificial intelligence approaches should be considered, as they predict accurate relationships between variables without imposing any a priori model.
Taken together these observations, since they are coming from the analysis of the literature search outputs, may represent a solid base ground for the construction of indications for optimizing and standardizing the selection of plant species, root functional traits, and experimental design for natural and urban forest root studies.
Author contributions
DF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. AM: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. DT: Conceptualization, Project administration, Supervision, Writing – review & editing. PM: Data curation, Formal analysis, Writing – review & editing. GSc: Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Writing – review & editing. GA: Data curation, Formal analysis, Writing – original draft. DC: Supervision, Writing – review & editing. GSf: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for Tender No. 3138 of 16 December 2021, rectified by Decree No. 3175 of 18 December 2021 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP: H73C22000300001, Project title “National Biodiversity Future Center—NBFC.” The PhD scholarship of DF (XXXVIII cycle—Biology and Applied Science; DOT197K79Z) is supported by the “National Biodiversity Future Center” funded under the NRPP, Mission 4 Component 2 Investment 1.4—Project code CN_00000033, CUP: H73C22000300001.
Acknowledgments
AM and GA are grateful to the PhD course in Life Science and Biotechnology at the University of Insubria, which provides a PhD scholarship to GA (XXXIX cycle—Life Science and Biotechnology), funded under the NRPP (DM 118 Ricerca). AM and GS thank Enrique Andivia from the Universidad Complutense de Madrid for supporting the building of Whittaker biomes representation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2024.1322087/full#supplementary-material
Footnotes
1. ^https://www.mase.gov.it/sites/default/files/archivio/biblioteca/protezione_natura/ecoregioni_italia_eng.pdf
References
Ahrens, B., Hansson, K., Solly, E. F., and Schrumpf, M. (2014). Reconcilable differences: a joint calibration of fine-root turnover times with radiocarbon and minirhizotrons. New Phytol. 204, 932–942. doi: 10.1111/nph.12979
Amendola, C., Montagnoli, A., Terzaghi, M., Trupiano, D., Oliva, F., Baronti, S., et al. (2017). Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric Ecosyst. Environ. 239, 236–245. doi: 10.1016/j.agee.2017.01.025
Andres, S. E., Standish, R. J., Lieurance, P. E., Mills, C. H., Harper, R. J., Butler, D. W., et al. (2022). Defining biodiverse reforestation: Why it matters for climate change mitigation and biodiversity. Plants People Planet. doi: 10.1002/ppp3.10329
Avenell, A., Handoll, H. H., and Grant, A. M. (2001). Lessons for search strategies from a systematic review, in the Cochrane library, of nutritional supplementation trials in patients after hip fracture. Am. J. Clin. Nutr. 73, 505–510. doi: 10.1093/ajcn/73.3.505
Bakker, L. M., Mommer, L., and van Ruijven, J. (2019). Using root traits to understand temporal changes in biodiversity effects in grassland mixtures. Oikos 128, 208–220. doi: 10.1111/oik.05612
Børja, I., De Wit, H. A., Steffenrem, A., and Majdi, H. (2008). Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in Southeast Norway. Tree Physiol. 28, 773–784. doi: 10.1093/treephys/28.5.773
Braun, S., Cantaluppi, L., and Flückiger, W. (2005). Fine roots in stands of Fagus sylvatica and Picea abies along a gradient of soil acidification. Environ. Pollut. 137, 574–579. doi: 10.1016/j.envpol.2005.01.042
Brunner, I., Bakker, M. R., Björk, R. G., Hirano, Y., Lukac, M., Aranda, X., et al. (2013). Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362, 357–372. doi: 10.1007/s11104-012-1313-5
Brunner, I., Brodbeck, S., Büchler, U., and Sperisen, C. (2001). Molecular identification of fine roots of trees from the Alps: reliable and fast DNA extraction and PCR-RFLP analyses of plastid DNA. Mol. Ecol. 10, 2079–2087. doi: 10.1046/j.1365-294x.2001.01325.x
Brunner, A. M., Busov, V. B., and Strauss, S. H. (2004). Poplar genome sequence: functional genomics in an ecologically dominant plant species. Trends Plant Sci. 9, 49–56. doi: 10.1016/j.tplants.2003.11.006
Brunner, I., Herzog, C., Galiano, L., and Gessler, A. (2019). Plasticity of fine-root traits under long-term irrigation of a water-limited Scots pine Forest. Front. Plant Sci. 10:701. doi: 10.3389/fpls.2019.00701
Canarini, A., Kaiser, C., Merchant, A., Richter, A., and Wanek, W. (2019). Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 10:157. doi: 10.3389/fpls.2019.00157
Clausing, S., Pena, R., Song, B., Müller, K., Mayer-Gruner, P., Marhan, S., et al. (2021). Carbohydrate depletion in roots impedes phosphorus nutrition in young forest trees. New Phytol. 229, 2611–2624. doi: 10.1111/nph.17058
Correa, J., Postma, J. A., Watt, M., and Wojciechowski, T. (2019). Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 70, 6019–6034. doi: 10.1093/jxb/erz383
Cudlin, P., Kieliszewska-Rokicka, B., Rudawska, M., Grebenc, T., Al-Berton, O., Lehto, T., et al. (2007). Fine roots and ectomycorrhi- Zas as indicators of environmental change. Plant Biosyst. 141, 406–425. doi: 10.1080/11263500701626028
Dalle Fratte, M., Montagnoli, A., Anelli, S., Armiraglio, S., Beatrice, P., Ceriani, A., et al. (2022). Mulching in lowland hay meadows drives an adaptive convergence of above-and below-ground traits reducing plasticity and improving biomass: a possible tool for enhancing phytoremediation. Front. Plant Sci. 13:1062911. doi: 10.3389/fpls.2022.1062911
Danjon, F., Fourcaud, T., and Bert, D. (2005). Root architecture and wind-firmness of mature Pinus pinaster. New Phytol. 168, 387–400. doi: 10.1111/j.1469-8137.2005.01497.x
Dawes, M. A., Philipson, C. D., Fonti, P., Bebi, P., Hättenschwiler, S., Hagedorn, F., et al. (2015). Soil warming and CO2 enrichment induce biomass shifts in alpine tree line vegetation. Glob. Chang. Biol. 21, 2005–2021. doi: 10.1111/gcb.12819
Day, S. D., Wiseman, P. E., Dickinson, S., and Harris, J. R. (2010). Tree root ecology in the urban environment and implications for a sustainable rhizosphere. Arboricult. Urban For. 36, 193–205. doi: 10.48044/jauf.2010.026
Dimitrova, A., Csilléry, K., Klisz, M., Lévesque, M., Heinrichs, S., Cailleret, M., et al. (2022). Risks, benefits, and knowledge gaps of non-native tree species in Europe. Front. Ecol. Evol. 10:908464. doi: 10.3389/fevo.2022.908464
Dumroese, R. K., Terzaghi, M., Chiatante, D., Scippa, G. S., Lasserre, B., and Montagnoli, A. (2019). Functional traits of Pinus ponderosa coarse roots in response to slope conditions. Front. Plant Sci. 10:947. doi: 10.3389/fpls.2019.00947
Dybzinski, R., Kelvakis, A., McCabe, J., Panock, S., Anuchitlertchon, K., Vasarhelyi, M., et al. (2019). How are nitrogen availability, fine-root mass, and nitrogen uptake related empirically? Implications for models and theory. Glob Change Biol. 25, 885–899. doi: 10.1111/gcb.14541
Dyckmans, J., and Flessa, H. (2002). Influence of tree internal nitrogen reserves on the response of beech (Fagus sylvatica) trees to elevated atmospheric carbon dioxide concentration. Tree Physiol. 22, 41–49. doi: 10.1093/treephys/22.1.41
Endrulat, T., Saurer, M., Buchmann, N., and Brunner, I. (2010). Incorporation and remobilization of 13C within the fine-root systems of individual Abies alba trees in a temperate coniferous stand. Tree Physiol. 30, 1515–1527. doi: 10.1093/treephys/tpq090
Eriksson, D., Weiland, F., Hedman, H., Stenberg, M., Öhrman, O., Lestander, T. A., et al. (2012). Characterization of Scots pine stump-root biomass as feed-stock for gasification. Bioresour. Technol. 104, 729–736. doi: 10.1016/j.biortech.2011.10.102
European Commission, Directorate-General for Environment (2021). EU biodiversity strategy for 2030: bringing nature back into our lives. Publications Office of the European Union.
European Commission, Directorate-General for Environment (2023). Guidelines on biodiversity-friendly afforestation, reforestation and tree planting Publications Office of the European Union.
Felipe-Lucia, M. R., Soliveres, S., Penone, C., Manning, P., van der Plas, F., Boch, S., et al. (2018). Multiple forest attributes underpin the supply of multiple ecosystem services. Nat. Commun. 9:4839. doi: 10.1038/s41467-018-07082-4
Fellows, I. (2018). Wordcloud: word clouds. R package version 2.6. Available at: https://CRAN.R-project.org/package=wordcloud
Feng, H., Squires, V. R., and Wu, J. (2021). Ecosystem services provisioning, urban growth and the rural–urban interface: a case study from China. Land 10:337. doi: 10.3390/land10040337
Fick, S. E., and Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. doi: 10.1002/joc.5086
Finér, L., Ohashi, M., Noguchi, K., and Hirano, Y. (2011). Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manag. 262, 2008–2023. doi: 10.1016/j.foreco.2011.08.042
Freschét, G. T., Pagès, L., Iversen, C. M., Comas, L. H., Rewald, B., Roumet, C., et al. (2021a). A starting guide to root ecology: strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 232, 973–1122. doi: 10.1111/nph.17572
Freschét, G. T., Roumet, C., Comas, L. H., Weemstra, M., Bengough, A. G., Rewald, B., et al. (2021b). Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol. 232, 1123–1158. doi: 10.1111/nph.17072
Fusaro, L., Salvatori, E., Mereu, S., Marando, F., Scassellati, E., Abbate, G., et al. (2015). Urban and peri-urban forests in the metropolitan area of Rome: ecophysiological response of Quercus ilex L. in two green infrastructures in an ecosystem services perspective. Urban For. Urban Green. 14, 1147–1156. doi: 10.1016/j.ufug.2015.10.013
Gaul, D., Hertel, D., and Leuschner, C. (2009). Estimating fine root longevity in a temperate Norway spruce forest using three independent methods. Funct. Plant Biol. 36, 11–19. doi: 10.1071/FP08195
Gauthier, S., Bernier, P., Kuuluvainen, T., Shvidenko, A. Z., and Schepaschenko, D. G. (2015). Boreal forest health and global change. Science 349, 819–822. doi: 10.1126/science.aaa9092
Geilfus, C. M., Carpentier, S. C., Zavišić, A., and Polle, A. (2017). Changes in the fine root proteome of Fagus sylvatica L. trees associated with P-deficiency and amelioration of P-deficiency. J. Proteome 169, 33–40. doi: 10.1016/j.jprot.2017.06.012
Ghestem, M., Sidle, R. S., and Stokes, A. (2011). The influence of plant root systems on subsurface flow: implications for slope stability. Bio Sci. 61, 869–879. doi: 10.1525/bio.2011.61.11.6
Godbold, D. L., Fritz, H. W., Jentschke, G., Meesenburg, H., and Rademacher, P. (2003). Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol. 23, 915–921. doi: 10.1093/treephys/23.13.915
Grüning, M. M., Simon, J., Rennenberg, H., and L-M-Arnold, A. (2017). Defoliating insect mass outbreak affects soil N fluxes and tree N nutrition in scots pine forests. Front. Plant Sci. 8:954. doi: 10.3389/fpls.2017.00954
Gyssels, G., and Poesen, J. (2003). The importance of plant root characteristics in controlling concentrated flow erosion rates. Earth Surface Process. Landforms 28, 371–384. doi: 10.1002/esp.447
Han, S., Yoon, T. K., Han, S., Yun, S. J., Lee, S. J., Kim, S., et al. (2014). Fine Root Biomass in Pinus densiflora Stands using Soil Core Sampling and Minirhizotrons. J. Korean Forest. Soc. 103, 37–42. doi: 10.14578/jkfs.2014.103.1.37
He, D., Wan, X., Wang, B., Wan, X., and Lu, M. (2018). Poplars and willows, sustaining livelihoods in urban and periurban forests in China. Rome: FAO. 20 pp. Licence: CC BY-NC-SA 3.0 IGO.
Helmisaari, H. S., Derome, J., Nöjd, P., and Kukkola, M. (2007). Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 27, 1493–1504. doi: 10.1093/treephys/27.10.1493
Helmisaari, H. S., Ostonen, I., Lõhmus, K., Derome, J., Lindroos, A. J., Merilä, P., et al. (2009). Ectomycorrhizal root tips in relation to site and stand characteristics in Norway spruce and Scots pine stands in boreal forests. Tree Physiol. 29, 445–456. doi: 10.1093/treephys/tpn042
IPCC (2014). Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. 151 (Geneva, 2014).
Italian Ministry of Ecological Transition (2021a). Plan for the ecological transition (PTE). Available at: https://www.senato.it/service/PDF/PDFServer/BGT/1310524.pdf.
Italian Ministry of Ecological Transition (2021b). Urban and extra-urban forestry plan, 2021. Available at: https://www.mite.gov.it/sites/default/files/archivio/allegati/PNRR/PNRR_piano_forestazione.pdf
Iversen, C. M., and McCormack, M. L. (2021). Filling gaps in our understanding of belowground plant traits across the world: an introduction to a virtual issue. New Phytol. 231, 2097–2103. doi: 10.1111/nph.17326
Iversen, C. M., McCormack, M. L., Powell, A. S., Blackwood, C. B., Freschet, G. T., Kattge, J., et al. (2017). A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytol. 215, 15–26. doi: 10.1111/nph.14486
Jacob, A., Hertel, D., and Leuschner, C. (2014). Diversity and species identity effects on fine root productivity and turnover in a species-rich temperate broad-leaved forest. Funct. Plant Biol. 41, 678–689. doi: 10.1071/FP13195
Jagodzinski, A. M., Ziółkowski, J., Warnkowska, A., and Prais, H. (2016). Tree age effects on fine root biomass and morphology over Chronosequences of Fagus sylvatica, Quercus robur and Alnus glutinosa stands. PLoS One 11:e0148668. doi: 10.1371/journal.pone.0148668
Kabir, E., Guikema, S., and Kane, B. (2018). Statistical modeling of tree failures during storms. Reliabil. Eng. Syst. Saf. 177, 68–79. doi: 10.1016/j.ress.2018.04.026
Kilpeläinen, J., Domisch, T., Lehto, T., Piirainen, S., Silvennoinen, R., and Repo, T. (2022). Separating the effects of air and soil temperature on silver birch. Part I. Does soil temperature or resource competition determine the timing of root growth? Tree Physiol. 42, 2480–2501. doi: 10.1093/treephys/tpac092
Klavina, D., Pennanen, T., Gaitnieks, T., Velmala, S., Lazdins, A., Lazdina, D., et al. (2016). The ectomycorrhizal community of conifer stands on peat soils 12 years after fertilization with wood ash. Mycorrhiza 26, 153–160. doi: 10.1007/s00572-015-0655-2
Konôpka, B., Barna, M., Bosela, M., and Lukac, M. (2020). Biomass allocation to resource acquisition compartments is affected by tree density manipulation in European beech after three decades. Forests 11:940. doi: 10.3390/f11090940
Konôpka, B., and Lukac, M. (2013). Moderate drought alters biomass and depth distribution of fine roots in Norway spruce. For. Pathol. 43, 115–123. doi: 10.1111/efp.12005
Kostelenos, G., and Kiritsakis, A. (2017). “Olive tree history and evolution” in Olives and olive oil as functional foods. eds. F. Shahidi and A. Kiritsakis (New Dehli, India: Wiley)
Kriiska, K., Lõhmus, K., Frey, J., Asi, E., Kabral, N., Napa, Ü., et al. (2021). The dynamics of mass loss and nutrient release of decomposing fine roots, needle litter and standard substrates in hemiboreal coniferous forests. Front. For. Glob. Change 4:686468. doi: 10.3389/ffgc.2021.686468
Kubisch, P., Hertel, D., and Leuschner, C. (2016). Fine root productivity and turnover of ectomycorrhizal and arbuscular mycorrhizal tree species in a temperate broad-leaved mixed forest. Front. Plant Sci. 7:1233. doi: 10.3389/fpls.2016.01233
Kubisch, P., Leuschner, C., Coners, H., Gruber, A., and Hertel, D. (2017). Fine root abundance and dynamics of stone pine (Pinus cembra) at the alpine treeline is not impaired by self-shading. Front. Plant Sci. 8:602. doi: 10.3389/fpls.2017.00602
Kuptz, D., Fleischmann, F., Matyssek, R., and Grams, T. E. E. (2011a). Seasonal patterns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 191, 160–172. doi: 10.1111/j.1469-8137.2011.03676.x
Kuptz, D., Matyssek, R., and Grams, T. E. (2011b). Seasonal dynamics in the stable carbon isotope composition δ13C from non-leafy branch, trunk and coarse root CO₂ efflux of adult deciduous (Fagus sylvatica) and evergreen (Picea abies) trees. Plant Cell Environ. 34, 363–373. doi: 10.1111/j.1365-3040.2010.02246.x
Lak, Z. A., Sandén, H., Mayer, M., and Rewald, B. (2020). Specific root respiration of three plant species as influenced by storage time and conditions. Plant Soil 453, 615–626. doi: 10.1007/s11104-020-04619-9
Lenormand, M., Papuga, G., Argagnon, O., Soubeyrand, M., De Barros, G., Alleaume, S., et al. (2018). Biogeographical network analysis of plant species distribution in the Mediterranean region. Ecol. Evol. 9, 237–250. doi: 10.1002/ece3.4718
López, B., Sabaté, S., and Gracia, C. (1998). Fine roots dynamics in a Mediterranean forest: effects of drought and stem density. Tree Physiol 18, 601–606. doi: 10.1093/treephys/18.8-9.601
Lynch, J. P. (2019). Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 223, 548–564. doi: 10.1111/nph.15738
Lyu, D., and Smith, D. L. (2022). The root signals in rhizospheric inter-organismal communications. Front. Plant Sci. 13:1064058. doi: 10.3389/fpls.2022.1064058
Mader, M., Schroeder, H., Schott, T., Schöning-Stierand, K., Leite Montalvão, A. P., Liesebach, H., et al. (2020). Mitochondrial genome of Fagus sylvatica L. as a source for taxonomic marker development in the Fagales. Plants 9:1274. doi: 10.3390/plants9101274
Maillard, P., Guehl, J. M., Muller, J. F., and Gross, P. (2001). Interactive effects of elevated CO2 concentration and nitrogen supply on partitioning of newly fixed 13C and 15N between shoot and roots of pedunculate oak seedlings (Quercus robur). Tree Physiol. 21, 163–172. doi: 10.1093/treephys/21.2-3.163
Mainiero, R., Kazda, M., Häberle, K. H., Nikolova, P. S., and Matyssek, R. (2009). Fine root dynamics of mature European beech (Fagus sylvatica L.) as influenced by elevated ozone concentrations. Environ. Pollut. 157, 2638–2644. doi: 10.1016/j.envpol.2009.05.006
Majdi, H. (2001). Changes in fine root production and longevity in relation to water and nutrient availability in a Norway spruce stand in northern Sweden. Tree Physiol. 21, 1057–1061. doi: 10.1093/treephys/21.14.1057
Manfra, R., Massoca, M. S., Uras, P. M. C., Cavalari, A. A., and Locosselli, G. M. (2022). Avarage height of surrounding buildings and district age are the main predictors of tree failure on the streets of São Paulo/Brazil. Urban For. Urban Green. 74:127665. doi: 10.1016/j.ufug.2022.127665
Mann, C., Loft, L., Hernández-Morcillo, M., Primmer, E., Bussola, F., Falco, E., et al. (2022). Governance innovations for forest ecosystem service provision - insights from an EU-wide survey. Environ. Sci. Pol. 132, 282–295. doi: 10.1016/j.envsci.2022.02.032
Mariën, B., Ostonen, I., Penanhoat, A., Fang, C., Xuan Nguyen, H., Ghisi, T., et al. (2021). On the below- and aboveground phenology in deciduous trees: observing the fine-root lifespan, turnover rate, and phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula roth for two growing seasons. Forests 12:1680. doi: 10.3390/f12121680
Markkola, A. M., Ohtonen, R., Tarvainen, O., and Ahonen-Jonnarth, U. (1995). Estimates of fungal biomass in Scots pine stands on an urban pollution gradient. New Phytol. 131, 139–147. doi: 10.1111/j.1469-8137.1995.tb03063.x
Mármol, I., Quero, J., Jiménez-Moreno, N., Rodríguez-Yoldi, M. J., and Ancín-Azpilicueta, C. (2019). A systematic review of the potential uses of pine bark in food industry and health care. Trends Food Sci. Technol. 88, 558–566. doi: 10.1016/j.tifs.2018.07.007
Mausolf, K., Härdtle, W., Jansen, K., Delory, B. M., Hertel, D., Leuschner, C., et al. (2018). Legacy effects of land-use modulate tree growth responses to climate extremes. Oecologia 187, 825–837. doi: 10.1007/s00442-018-4156-9
McCarthy, J. K., Hood, I. A., Brockerhoff, E. G., Carlson, C. A., Pawson, S. M., Forward, M., et al. (2010). Predicting sapstain and degrade in fallen trees following storm damage in a Pinus radiata forest. For. Ecol. Manag. 260, 1456–1466. doi: 10.1016/j.foreco.2010.07.044
McCormack, M. L., Dickie, I. A., Eissenstat, D. M., Fahey, T. J., Fernandez, C. W., Guo, D., et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 207, 505–518. doi: 10.1111/nph.13363
Meinen, C., Hertel, D., and Leuschner, C. (2009). Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: is there evidence of below-ground overyielding? Oecologia 161, 99–111. doi: 10.1007/s00442-009-1352-7
Menkis, A., Allmer, J., Vasiliauskas, R., Lygis, V., Stenlid, J., and Finlay, R. (2004). Ecology and molecular characterization of dark septate fungi from roots, living stems, coarse and fine woody debris. Mycol. Res. 108, 965–973. doi: 10.1017/s0953756204000668
Menkis, A., Burokienė, D., Gaitnieks, T., Uotila, A., Johannesson, H., Rosling, A., et al. (2012). Occurrence and impact of the root-rot biocontrol agent Phlebiopsis gigantea on soil fungal communities in Picea abies forests of northern Europe. FEMS Microbiol. Ecol. 81, 438–445. doi: 10.1111/j.1574-6941.2012.01366.x
Mildner, M., Bader, M. K., Leuzinger, S., Siegwolf, R. T., and Körner, C. (2014). Long-term 13C labeling provides evidence for temporal and spatial carbon allocation patterns in mature Picea abies. Oecologia 175, 747–762. doi: 10.1007/s00442-014-2935-5
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Montagnoli, A., Baronti, S., Alberto, D., Chiatante, D., Scippa, G. S., Terzaghi, M., et al. (2021). Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: A rhizobox approach. Sci Total Environ. 751:141455. doi: 10.1016/j.scitotenv.2020.141455
Montagnoli, A., Di Iorio, A., Terzaghi, M., Trupiano, D., Scippa, G. S., and Chiatante, D. (2014). Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 133, 957–968. doi: 10.1007/s10342-014-0814-6
Montagnoli, A., Dumroese, R. K., Terzaghi, M., Onelli, E., Scippa, G. S., and Chiatante, D. (2019). Seasonality of fine root dynamics and activity of root and shoot vascular cambium in a Quercus ilex L. forest (Italy). For. Ecol. Manag. 431, 26–34. doi: 10.1016/j.foreco.2018.06.044
Montagnoli, A., Lasserre, B., Sferra, G., Chiatante, D., Scippa, G. S., Terzaghi, M., et al. (2020). Formation of annual ring eccentricity in coarse roots within the root cage of Pinus ponderosa growing on slopes. Plants 9:181. doi: 10.3390/plants9020181
Montagnoli, A., Lasserre, B., Terzaghi, M., Byambadorj, S. O., Nyam-Osor, B., Scippa, G. S., et al. (2022). Fertilization reduces root architecture plasticity in Ulmus pumila used for afforesting Mongolian semi-arid steppe. Front. Plant Sci. 13:878299. doi: 10.3389/fpls.2022.878299
Montagnoli, A., Terzaghi, M., Di Iorio, A., Scippa, G. S., and Chiatante, D. (2012). Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy Prealps: possible implications of coppice conversion to high forest. Plant Biosys. 146, 1012–1022. doi: 10.1080/11263504.2012.741626
Montagnoli, A., Terzaghi, M., Giussani, B., Scippa, G. S., and Chiatante, D. (2018). An integrated method for high-resolution definition of new diameter-based fine root sub-classes of Fagus sylvatica L. Ann. For. Sci. 75, 1–13. doi: 10.1007/s13595-018-0758-y
Montagnoli, A., Terzaghi, M., Magatti, G., Scippa, G. S., and Chiatante, D. (2016). Conversion from coppice to high stand increase soil erosion in steep forestland of European beech. Reforesta 1, 60–75. doi: 10.21750/REFOR.2.07.22
Montagnoli, A., Terzaghi, M., Miali, A., Chiatante, D., and Dumroese, R. K. (2023). Unusual late-fall wildfire in a pre-alpine Fagus sylvatica forest reduced fine roots in the shallower soil layer and shifted very fine-root growth to deeper soil depth. Sci. Rep. 13:6380. doi: 10.1038/s41598-023-33580-7
Mori, A. S. (2017). Biodiversity and ecosystem services in forests: management and restoration founded on ecological theory. J. Appl. Ecol. 54, 7–11. doi: 10.1111/1365-2664.12854
Nacke, H., Goldmann, K., Schöning, I., Pfeiffer, B., Kaiser, K., Castillo-Villamizar, G. A., et al. (2016). Fine spatial scale variation of soil microbial communities under European beech and Norway spruce. Front. Microbiol. 7:2067. doi: 10.3389/fmicb.2016.02067
Nickel, U. T., Weikl, F., Kerner, R., Schäfer, C., Kallenbach, C., Munch, J. C., et al. (2018). Quantitative losses vs. qualitative stability of ectomycorrhizal community responses to 3 years of experimental summer drought in a beech-spruce forest. Glob. Chang. Biol. 24, e560–e576. doi: 10.1111/gcb.13957
Nicoll, B. C., Gardiner, B. A., and Peace, A. J. (2008). Improvements in anchorage provided by the acclimation of forest trees to wind stress. Forestry 81, 389–398. doi: 10.1093/forestry/cpn021
Nikolova, P. S., Bauerle, T. L., Häberle, K. H., Blaschke, H., Brunner, I., and Matyssek, R. (2020). Fine-root traits reveal contrasting ecological strategies in European beech and Norway spruce during extreme drought. Front. Plant Sci. 11:1211. doi: 10.3389/fpls.2020.01211
Nocentini, S., Travaglini, D., and Muys, B. (2022). Managing Mediterranean forests for multiple ecosystem services: research progress and knowledge gaps. Curr. For. Rep. 8, 229–256. doi: 10.1007/s40725-022-00167-w
Ostonen, I., Puttsepp, U., Biel, C., Alberton, O., Bakker, M. R., Lohmus, K., et al. (2007). Specific root length as an indicator of environmental change. Plant Biosyst. 141, 426–442. doi: 10.1080/11263500701626069
Paoletti, E., Contran, N., Manning, W. J., and Tagliaferro, F. (2007). Ethylenediurea (EDU) affects the growth of ozone-sensitive and tolerant ash (Fraxinus excelsior) trees under ambient O3 conditions. ScientificWorldJournal 7, 128–133. doi: 10.1100/tsw.2007.21
Pauleit, S., Jones, N., Nyhuus, S., Pirnat, J., and Salbitano, F. (2005). “Urban Forest resources in European cities” in Urban forests and trees. eds. C. Konijnendijk, K. Nilsson, T. Randrup, and J. Schipperijn (Berlin, Heidelberg: Springer)
Podwika, M., Solek-Podwika, K., and Ciarkowska, K. (2018). Changes in the properties of grassland soils as a result of afforestation. iForest 11, 600–608. doi: 10.3832/ifor2556-011
Przewoźna, P., Mączka, K., Mielewczyk, M., Inglot, A., and Matczak, P. (2022). Ranking ecosystem services delivered by trees in urban and rural areas. Ambio 51, 2043–2057. doi: 10.1007/s13280-022-01722-2
R Core Team . (2017). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rastogi, S., Pandey, M. M., and Kumar Singh Rawat, A. (2015). Medicinal plants of the genus Betula–traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 159, 62–83. doi: 10.1016/j.jep.2014.11.010
Reubens, B., Poesen, J., Danjon, F., Geudens, G., and Muys, B. (2007). The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: a review. Trees 21, 385–402. doi: 10.1007/s00468-007-0132-4
Rey, M. D., Castillejo, M. Á., Sánchez-Lucas, R., Guerrero-Sanchez, V. M., López-Hidalgo, C., Romero-Rodríguez, C., et al. (2019). Proteomics, holm oak (Quercus ilex L.) and other recalcitrant and orphan Forest tree species: how do they see each other? Int. J. Mol. Sci. 20:692. doi: 10.3390/ijms20030692
Rosinger, C., Sandén, H., and Godbold, D. L. (2020). Non-structural carbohydrate concentrations of Fagus sylvatica and Pinus sylvestris fine roots are linked to ectomycorrhizal enzymatic activity during spring reactivation. Mycorrhiza 30, 197–210. doi: 10.1007/s00572-020-00939-x
Ruas, R. D. B., Costa, L. M. S., and Bered, F. (2022). Urbanization driving changes in plant species and communities – a global view. Glob. Ecol. Conserv. 38:e02243. doi: 10.1016/j.gecco.2022.e02243
Rytter, R. M. (2013). The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 33, 924–939. doi: 10.1093/treephys/tpt060
Scartazza, A., Moscatello, S., Matteucci, G., Battistelli, A., and Brugnoli, E. (2015). Combining stable isotope and carbohydrate analyses in phloem sap and fine roots to study seasonal changes of source-sink relationships in a Mediterranean beech forest. Tree Physiol. 35, 829–839. doi: 10.1093/treephys/tpv048
Schwieger, S., Blume-Werry, G., Ciesiolka, F., and Anadon-Rosell, A. (2021). Root biomass and root traits of Alnus glutinosa show size-dependent and opposite patterns in a drained and a rewetted forest peatland. Ann. Bot. 127, 337–346. doi: 10.1093/aob/mcaa195
Seto, K. C., Güneralp, B., and Hutyra, L. R. (2012). Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. U. S. A. 109, 16083–16088. doi: 10.1073/pnas.1211658109
Shorohova, E., Kneeshaw, D., Kuuluvainen, T., and Gauthier, S. (2011). Variability and dynamics of old-growth forests in the circumboreal zone: implications for conservation, restoration and management. Silva Fennica 45:72. doi: 10.14214/sf.72
Smeriglio, A., D'Angelo, V., Cacciola, A., Ingegneri, M., Raimondo, F. M., Trombetta, D., et al. (2022). New insights on phytochemical features and biological properties of Alnus glutinosa stem bark. Plants 11:2499. doi: 10.3390/plants11192499
Solomon, S. (2007). The physical science basis: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Intergovernmental panel on climate change. IPCC, 2014: Climate Change 2014: synthesis Report.
South, A. (2011). Rworldmap: a new R package for mapping global data. R J. 3, 35–43. doi: 10.32614/RJ-2011-006
Steele, S. J., Gower, S. T., Vogel, J. G., and Norman, J. M. (1997). Root mass, net primary production and turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiol. 17, 577–587. doi: 10.1093/treephys/17.8-9.577
Stefan, V., and Levin, S. (2018). Plotbiomes: R package for plotting Whittaker biomes with ggplot2 (v1.0.0). Zenodo. doi: 10.5281/zenodo.7145245
Stobrawa, K., and Lorenc-Plucińska, G. (2008). Thresholds of heavy-metal toxicity in cuttings of European black poplar (Populus nigra L.) determined according to antioxidant status of fine roots and morphometrical disorders. Sci. Total Environ. 390, 86–96. doi: 10.1016/j.scitotenv.2007.09.024
Taccoen, A., Piedallu, C., Seynave, I., Géout-Petit, A., and Géout, J. (2022). Climate change-induced background tree mortality is exacerbated towards the warm limits of the species ranges. Ann. For. Sci. 79:23. doi: 10.1186/s13595-022-01142-y
Sun, L., Ataka, M., Bengough, A. G., and Yoshimura, K. (2017). Relationship between fine-root exudation and respiration of two Quercus species in a Japanese temperate forest. Tree Physiol. 37, 1011–1020. doi: 10.1093/treephys/tpx026
Stokes, A., Atger, C., Seynave, I., Fourcaud, T., and Sidle, R. C. (2009). Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil. 324, 1–30. doi: 10.1007/s11104-009-0159-y
Tamasi, E., Stokes, A., Lasserre, B., Danjon, F., Berthier, S., Fourcaud, T., et al. (2005). Influence of wind loading on root system development and architecture in oak (Quercus robur L.) seedlings. Trees 19, 374–384. doi: 10.1007/s00468-004-0396-x
Tang, Y., Schiestl-Aalto, P., Saurer, M., Sahlstedt, E., Kulmala, L., Kolari, P., et al. (2022). Tree organ growth and carbon allocation dynamics impact the magnitude and δ13C signal of stem and soil CO2 fluxes. Tree Physiol. 42, 2404–2418. doi: 10.1093/treephys/tpac079
Terzaghi, M., Montagnoli, A., Di Iorio, A., Scippa, G. S., and Chiatante, D. (2013). Fine-root carbon and nitrogen concentration of European beech (Fagus sylvatica L.) in Italy Prealps: possible implications of coppice conversion to high forest. Front. Plant Sci. 4:192. doi: 10.3389/fpls.2013.00192
Thiese, M. S., Arnold, Z. C., and Walker, S. D. (2015). The misuse and abuse of statistics in biomedical research. Biochem. Med. 25, 5–11. doi: 10.11613/BM.2015.001
Tingey, D. T., Phillips, D. L., and Johnson, M. G. (2003). Optimizing minirhizotron sample frequency for an evergreen and deciduous tree species. New Phytol. 157, 155–161. doi: 10.1046/j.1469-8137.2003.00653.x
van Haaften, M., Liu, Y., Wang, Y., Zhang, Y., Gardebroek, C., Heijman, W., et al. (2021). Understanding tree failure—A systematic review and meta-analysis. PLoS One 16:e0246805. doi: 10.1371/journal.pone.0246805
Vanninen, P., and Mäkelä, A. (2005). Carbon budget for Scots pine trees: effects of size, competition and site fertility on growth allocation and production. Tree Physiol. 25, 17–30. doi: 10.1093/treephys/25.1.17
Vasiliauskas, R., Menkis, A., Finlay, R. D., and Stenlid, J. (2007). Wood-decay fungi in fine living roots of conifer seedlings. New Phytol. 174, 441–446. doi: 10.1111/j.1469-8137.2007.02014.x
Verdú-Vázquez, A., Fernández-Pablos, E., Lozano-Diez, R. V., and López-Zaldívar, Ó. (2021). Green space networks as natural infrastructures in PERI-URBAN areas. Urban Ecosyst. 24, 187–204. doi: 10.1007/s11252-020-01019-w
Vogt, K. A., Vogt, D. J., Palmiotto, P. A., Boon, P., O'Hara, J., and Asbjornsen, H. (1995). Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 187, 159–219. doi: 10.1007/BF00017088
Weigt, R. B., Häberle, K. H., Rötzer, T., and Matyssek, R. (2015). Whole-tree seasonal nitrogen uptake and partitioning in adult Fagus sylvatica L. and Picea abies L. [karst.] trees exposed to elevated ground-level ozone. Environ. Pollut. 196, 511–517. doi: 10.1016/j.envpol.2014.06.032
Win, T.L . (2019). World Economic Forum; 2019. The United Nations wants to grow urban forests in 30 countries. Available at: https://www.weforum.org/agenda/2019/09/un-plans-vast-urban-forests-fight-climate-change/
Wohlin, C., Kalinowski, M., Felizardo, K., and Mendes, E. (2022). Successful combination of database search and snowballing for identification of primary studies in systematic literature studies. Inf. Softw. Technol. 147:106908. doi: 10.1016/j.infsof.2022.106908
Xiao, C. W., Yuste, J. C., Janssens, I. A., Roskams, P., Nachtergale, L., Carrara, A., et al. (2003). Above-and belowground biomass and net primary production in a 73-year-old Scots pine forest. Tree Physiol. 23, 505–516. doi: 10.1093/treephys/23.8.505
Yan, C. F., Gessler, A., Rigling, A., Dobbertin, M., Han, X. G., and Li, M. H. (2016). Effects of mistletoe removal on growth, N and C reserves, and carbon and oxygen isotope composition in Scots pine hosts. Tree Physiol. 36, 562–575. doi: 10.1093/treephys/tpw024
Yang, M., Défossez, P., Danjon, F., Dupont, S., and Fourcaud, T. (2017). Which root architectural elements contribute the best to anchorage of Pinus species? Insights from in silico experiments. Plant Soil 411, 275–291. doi: 10.1007/s11104-016-2992-0
Yang, M., Défossez, P., Danjon, F., and Fourcaud, T. (2014). Tree stability under wind: simulating uprooting with root breakage using a finite element method. Ann. Bot. 114, 695–709. doi: 10.1093/aob/mcu122
Zadworny, M., McCormack, M. L., Mucha, J., Reich, P. B., and Oleksyn, J. (2016). Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol. 212, 389–399. doi: 10.1111/nph.14048
Zadworny, M., McCormack, M. L., Zytkowiak, R., Karolewski, P., Mucha, J., and Oleksyn, J. (2017). Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob. Chang. Biol. 23, 1218–1231. doi: 10.1111/gcb.13514
Zhang, C., Stratópoulos, L. M. F., Xu, C., Pretzsch, H., and Rötzer, T. (2020). Development of fine root biomass of two contrasting urban tree cultivars in response to drought stress. Forests 11:108. doi: 10.3390/f11010108
Keywords: functional traits, fine roots, coarse roots, literature metadata, experimental design, natural forest, urban forest
Citation: Fantozzi D, Montagnoli A, Trupiano D, Di Martino P, Scippa GS, Agosto G, Chiatante D and Sferra G (2024) A systematic review of studies on fine and coarse root traits measurement: towards the enhancement of urban forests monitoring and management. Front. For. Glob. Change. 7:1322087. doi: 10.3389/ffgc.2024.1322087
Edited by:
Ivika Ostonen, University of Tartu, EstoniaReviewed by:
Ziliang Zhang, University of Illinois at Urbana-Champaign, United StatesTimo Domisch, Natural Resources Institute Finland (Luke), Finland
Copyright © 2024 Fantozzi, Montagnoli, Trupiano, Di Martino, Scippa, Agosto, Chiatante and Sferra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriella Sferra, Z2FicmllbGxhLnNmZXJyYUB1bmltb2wuaXQ=
 Daniele Fantozzi
Daniele Fantozzi Antonio Montagnoli
Antonio Montagnoli Dalila Trupiano
Dalila Trupiano Paolo Di Martino1
Paolo Di Martino1 Gabriella Stefania Scippa
Gabriella Stefania Scippa Donato Chiatante
Donato Chiatante Gabriella Sferra
Gabriella Sferra