
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 16 February 2023
Sec. Forest Ecophysiology
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.834669
This article is part of the Research Topic Living Far From the Ground: Strategies of Forest Epiphytes View all 10 articles
Vascular epiphytes are extraordinarily diverse in the tropical Andean region. Compared to trees and terrestrial herbs, epiphytes are more vulnerable to forest alteration due to their structural dependence on trees and environmental requirements. Based on experimental approaches for ecological purposes, monitoring air pollutants, and seeking propagation alternatives, the rescue and translocation of vascular epiphytes (mainly bromeliads and orchids) from a threatened forest to a safer forest has been recently conducted in Colombia. Preliminary assessments indicate that epiphytes benefit from such well-planned measures, and their mortality and survival might be associated with extrinsic and intrinsic factors, which remain to be understood. We evaluated the survival of 16 vascular epiphyte species after translocation into a secondary forest in Antioquia (Colombia) for 8 years. We assessed the role of intrinsic (foliar area, number of leaves, initial pseudobulbs, stems or rosettes, functional group, and epiphyte species) and extrinsic factors (host tree species, bark water-holding capacity, type of substrate, location on the host tree, nutrients, and hormone addition) and the effect of climatic variables on plant survival. The overall mortality rate in this study ranked 1–7% per year, and survival decreased annually, reaching 44% by the end of the 8th year. Host tree species and intrinsic factors such as the functional group and epiphyte species significantly affected the probability of survival. Bromeliads, in particular, exhibited high mortality, which their monocarpic growth form could explain. Another group of species showing high mortality were the miniature orchids, Masdevalia amanda and M. platyglossa, and are associated with short life cycles. Five host tree species appear to affect the survival of translocated epiphytes; however, the factors or characteristics involved remain unclear. A higher seasonality of precipitation was related to the percentage of overall mortality. This result indicates that extreme precipitation events or drought reduce epiphyte longevity. In conclusion, our study suggests that a wide range of epiphytes may be successfully translocated to secondary forests in the Colombian Andes and demonstrates that the effective introduction of epiphyte assemblages may be useful for ecological restoration efforts in Andean forests.
Vascular epiphytes are extraordinarily diverse in the tropical Andean region, reaching up to 50% of vascular plant species diversity at a local scale (Gentry and Dodson, 1987a,b). Compared to trees and terrestrial herbs, epiphytes are more vulnerable to forest alteration due to their environmental requirements such as higher humidity and substrate accumulation (Nadkarni, 2000; Köster et al., 2013; Barrancos et al., 2020). Seeds and spores of epiphytes arrive and colonize host trees, increasing species biomass and abundance in a well-established forest (Benavides et al., 2006). During the last few decades, land-use change has dramatically impacted tropical ecosystems, resulting in degraded landscapes with fragmented forests in asymmetric conservation states and submerged in a mosaic of agricultural and livestock patches. This condition affects the dispersion and natural regeneration processes of overall plant communities. It reduces dispersal opportunities for epiphytes, which require well-established and interconnected tree communities (Köster et al., 2009, 2013). However, fragmented forests can recover ecological attributes obtained by the epiphyte assemblage by introducing and maintaining populations of different species (Duarte and Gandolfi, 2013, 2017).
Andean forests in Colombia are home to one of the most extraordinary, highly endemic, and globally threatened diversities of epiphytes. Research on this enormous epiphytic species diversity has significantly progressed in Colombia. However, protecting this unique diversity is restricted to natural reserves and complemented by rescues and translocations of plants growing in forests, which will be destroyed to establish new infrastructure, agriculture, and livestock. Rescues and translocations of plants from forests threatened by extractive activities and land-use change are an alternative for saving epiphytes in Colombia, Brazil, and Peru (Ávila et al., 2017; Fernandez Barrancos et al., 2017). However, increasing pressure from the infrastructure sector requires a deep understanding of the factors associated with epiphyte survival after translocation.
Based on experimental approaches for ecological purposes, monitoring air pollutants, and seeking propagation alternatives, rescue, and translocation of vascular epiphytes (mainly bromeliads and orchids) from a threatened forest to a safer forest have been recently conducted (Malm, 1998; Callaway et al., 2002; Rapp and Silman, 2014). Surveys after 3 years of monitoring indicated that epiphyte survival was associated with both intrinsic and extrinsic factors, such as functional traits and spatial distribution (niche partition; Zotz, 2000; Petter et al., 2015; Duarte and Gandolfi, 2017; Izuddin et al., 2018; Agudelo et al., 2019; Domene, 2019; Faleiro et al., 2020). Studies have also indicated that an epiphyte spatial distribution on the host tree might respond to a niche partition (Wolf, 2005; Reyes-García et al., 2008; Petter et al., 2015; Agudelo et al., 2019). Deep knowledge of factors associated with the establishment and survival of epiphytes after translocation will contribute to maintaining epiphytes, which otherwise might move toward local or even global extinction scenarios.
This study provides the most comprehensive monitoring of translocated vascular epiphytes in Andean forests in Colombia. We evaluated the survival of 16 vascular epiphyte species after translocation into a secondary forest in Antioquia (Colombia) for 8 years. We assessed the role of intrinsic (foliar area, number of leaves, initial pseudobulbs, stems or rosettes, functional group, and epiphyte species) and extrinsic factors (host tree species, bark water-holding capacity, type of substrate, location on the host tree, nutrients, and hormone addition) and the effect of bioclimatic variables on plant survival.
Research activities were conducted in northwestern Colombia at 2,300 m in the municipality of Medellin (75° 30′8.04"W, 6°16′54.39"N). The area has an average temperature of 15°C, varying from 5 to 25°C, annual precipitation of 2,000 mm/year, and relative humidity of 89% (SIATA, 2011). The area corresponds to fragmented forests scattered and extensive coverage of cypress pine plantations (Cupressus lusitanica). These plantations offer conditions for well-established bryophyte mats on the ground and vascular epiphytes to grow profusely (Morales-Morales et al., 2015; Carmona Higuita et al., 2017).
We collected healthy epiphyte individuals of 16 species corresponding to aroids, bromeliads, and orchids from cypress plantation grounds in November 2013 (Table 1). We assigned functional groups to each species, according to Agudelo et al. (2018, Table 1). Orchids were selected rhizomatous plants with pseudobulbs or stems (corresponding to functional group 7) and ramicuals (FG 3). The bromeliads (FG 4) in this study exhibit sympodial growth; ramets mature by producing a terminal inflorescence, and after flowering, the rosette dies and produces one or two offshoots (rosettes). Aroids, nomadic vines, correspond to functional group 6. We sought initial size conditions to be homogenous among species (Table 1). For aroids, we obtained stem fragments of at least four internodes. Between 33 and 42 individuals per species were attached to the trunk of 70 selected host trees. Host trees were selected adjacent to the nearest tree with a diameter at breast height (DBH) >9 cm, with a straight trunk, and a height of the first branch >4 m. Epiphyte individuals were positioned every 0.5 m (starting at 0.5 m) until reaching 4 m along host trees. The designation of the position and host tree for each individual was randomly selected, except for nomadic vines (aroids), which we located at the base of host trees. Relocated individuals in 35 trees of the 70 trees were irrigated with water during the first 2 months (November and December 2013) and fertilized with phosphorus (Master® 13-40-13), and a synthetic plant hormone from the auxin group, which stimulates root production (Superthrive®), was added. During the same period, watering with sprinklers was conducted after more than 72 h without rain. Half of the individuals of each species were attached to host trees with a substrate made of fique (Sisal) fiber (Furcraea andina) or coconut mesocarps fiber (Cocos nucifera). We designed a pocket with the fique fiber and added ~40 g of peat. The second substrate consisted of coconut fibers and mesocarp fragments of an average granulometry between 2.5 and 10 mm. We fastened both substrates with strips of cotton and lycra. We registered each individual's initial number of leaves, pseudobulbs, rosettes, or stems. Annually, we registered the survival and the number of leaves until 2021.
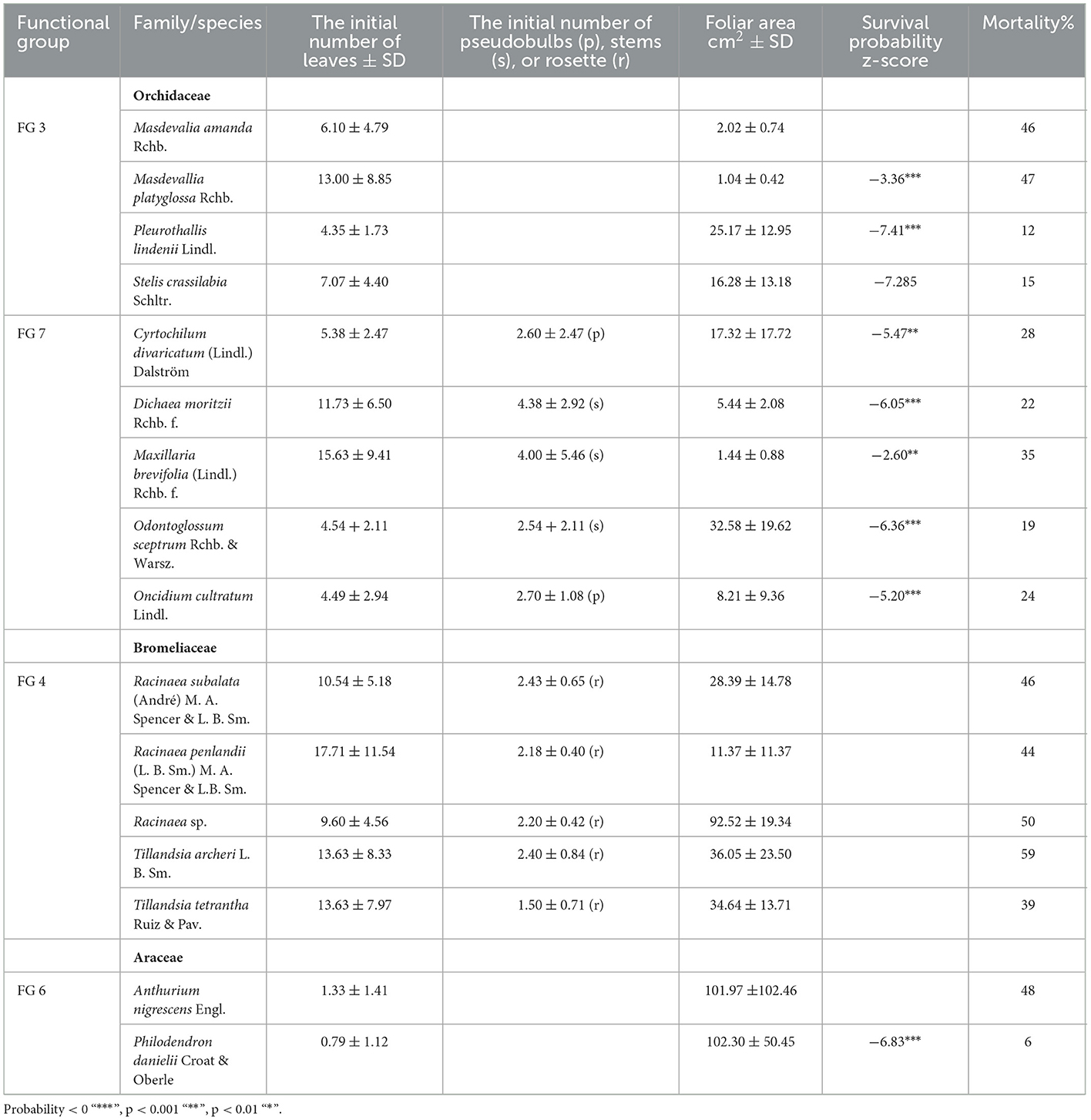
Table 1. Species functional group [sensu Agudelo et al., 2019], initial number of leaves or/and pseudobulbs (p), stems (s) or rosette (r), survival probability z-score, and percentage of mortality at the end of the 8th year*.
A total of five fully expanded and healthy leaves were photographed in situ or sampled from three individuals. When it was impossible to get five leaves per individual, we collected additional individuals until we obtained a minimum of 15 leaves per species. We calculated the area from the foliar area mean quantile and multiplied it by the number of leaves registered each year. We considered an individual alive when photosynthetic tissue (green) was evident, and meristems or lateral shoots were present. We reported the formation of flowers or fruits and dry peduncles as evidence of flower formation. Pseudobulbs were recorded at the beginning, but later measurements were not considered due to a high error in the observations because they were hidden by the substrate.
We identified the host tree species, and we determined their maximum water-holding capacity (WHC) at saturation per area. WHC was calculated based on three to five random samples (of ~2 cm2) that were chiseled from the bark at 1.3 m above ground (three tree species presented only one individual in the area). At the laboratory, each bark sample was cleaned; area and thickness were measured and oven-dried at 60°C for 48 h; their dry weight was determined. Maximum water-holding capacity was determined after soaking the samples in water for 24 h. Excess water was shaken off in a consistent manner, and the samples were weighed again (Einzmann et al., 2015).
Given that epiphyte species seem to be most affected by drought events, four bioclimatic variables related to precipitation dynamics were calculated: annual precipitation amount, precipitation amount of the driest month, precipitation seasonality, and mean monthly precipitation amount of the driest quarter (Karger et al., 2017). These variables were derived from diary precipitation data between 2014 and 2021, obtained from a meteorological station [Santa Helena (27010810)] located at Santa Elena rural township from Medellin (latitude: 6.1969, longitude: 75.5167, elevation: 2550 m asl). This meteorological station belongs to the meteorological monitoring network of the “Colombian Institute of Hydrology, Meteorology, and Environmental Studies” (Instituto de Hidrología, Meteorología y Estudios Ambientaes de Colombia, IDEAM). First, we evaluated variable collinearity among bioclimatic variables and plant survival annual probability and species functional group mortality using the Pearson test. This test allows us to check the dependence among variables, and then, correlated variables were removed. To assess the effect of precipitation bioclimatic variables on plant survival, we performed a multiple linear regression. These analyses were performed using the stats R package.
To estimate the probability of survival over time, we used the formula Surv (time, status) ~1 and the survfit() function to produce the Kaplan–Meier curve. We used the Cox proportional hazards (CoxPH) model to evaluate overall multifactor survival; we ran three models: (a) epiphyte functional groups, substrate, addition or not of fertilization, and height above the host tree; (b) tree species and media WHC; and (c) epiphyte species. The models were constructed using language R (R Development Core Team, 2021) and package survival version 3.2-13 (Therneau and Grambsch, 2000; Therneau, 2022).
The initial number of leaves varied between the species at the beginning of the experiment (Table 1). Aroids, Anthurium and Philodendron, presented 1.1 ± 1.3 leaves (functional group 6). Orchids, rhizomatous with ramicaul stems (FG 3), presented 7.58 ± 6.34 leaves. Rhizomatous plants with pseudobulbs or stems (FG 7) presented 8.28 ± 7.02 leaves, 2.6 ± 2 pseudobulbs, and 4.1 ± 4.5 stems. Bromeliads (FG 4) initially presented 2.3 ± 0.54 rosettes and 13.75 ± 8.46 leaves (Table 1). Masdevallia platiglossa showed the mean smallest leaf area (1.02 ± 0.42 cm2), in contrast to Philodendron danielii, the species with the highest mean leaf area (102.29 ± 50.45 cm2). However, there were no significant differences in the leaf area between species (F = 1.67, P = 0.058, Table 1). In general, the total leaf area was stable over the years for all species, with the exception of Philodendron danielii, which formed new leaves every year (Figure 1).
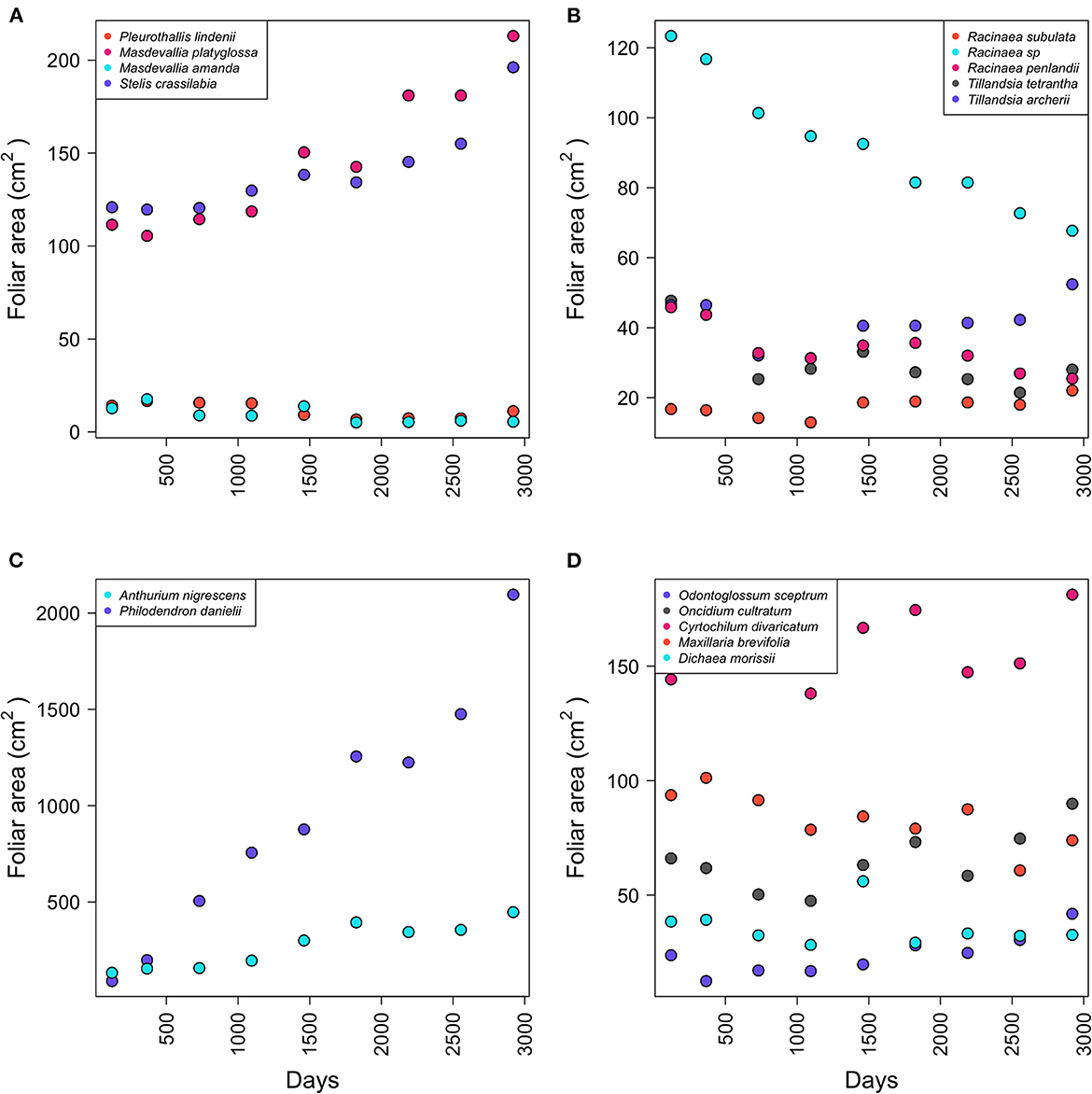
Figure 1. Total leaf area by species according to functional group. Epiphytic species correspond to the following functional groups (FG): (A) FG3, (B) FG4, (C) FG6, and (D) FG7.
The host tree's structure was relatively homogeneous (total host tree height 8.2 ± 1.7 m, first branch height 5.2 ± 1.09 m, and DBH 11.2 ± 2.3 cm), and the distance between host trees was, on average, 5.5 ± 4.2 m. Epiphytes were attached to 12 host tree species; Clusia ducu was the most abundant species, with 48% of host trees, followed by Sciodaphyllum trianae with 10%. Across host species, the maximum water-holding capacity (WHC) content ranged from 21 to 496 mg cm2, and the statistical result suggests that there is no significant difference between the study tree species bark WHC records (F = 1.714, p = 0.153, Table 2 supported by Figure 2); however, there was substantial variability in bark WHC within and between tree species (Table 2). The bark thickness ranged, on average, from 1.25 to 3.11 mm (Table 2). Although the measurement of bark WHC has been based on the area, samples chiseled from the bark presented a thickness, giving a volume to the water retention capacity. We checked whether thickness affects water retention capacity in the samples; however, it was not significant (p > 0.167; Supplementary material 2).
By the end of the 8th year, 44% of epiphytes survived. Bromeliads, functional group 4, presented the highest mortality, with ~ 47% of the plants dying at the end of the 8th year (Figure 3C, Table 1). Tillandsia archerii was the species with the highest mortality, with 59%. Orchids presented ~28% of mortality at the end of the 8th year with Masdevallia (FG 3), and miniature orchids with ramicuals presented the highest mortality (46–47%). Pleurothallis lindenii, with 11%, was the orchid with the lowest net mortality. Anthurium nigrescens (FG 6) presented high mortality (48%). By contrast, Philodendron danielii showed low mortality, with just two plants perishing during the study. Individuals that appeared dead during monitoring (dry and without evidence of photosynthetic tissue) exhibited evidence of survival as the formation of new leaves and living tissue during subsequent monitoring. This phenomenon was observed among 10–16 plants annually, being more frequent in orchids (FG 3, 30%), followed by aroids (24%), and uncommon in bromeliads (3%). All orchid and bromeliad species formed flowers and fruits during the study; 51% (327 individuals) and 31% (195) of the individuals were flowered and fructified, respectively. Philodendron danielii was observed initiating flowers in November 2021.
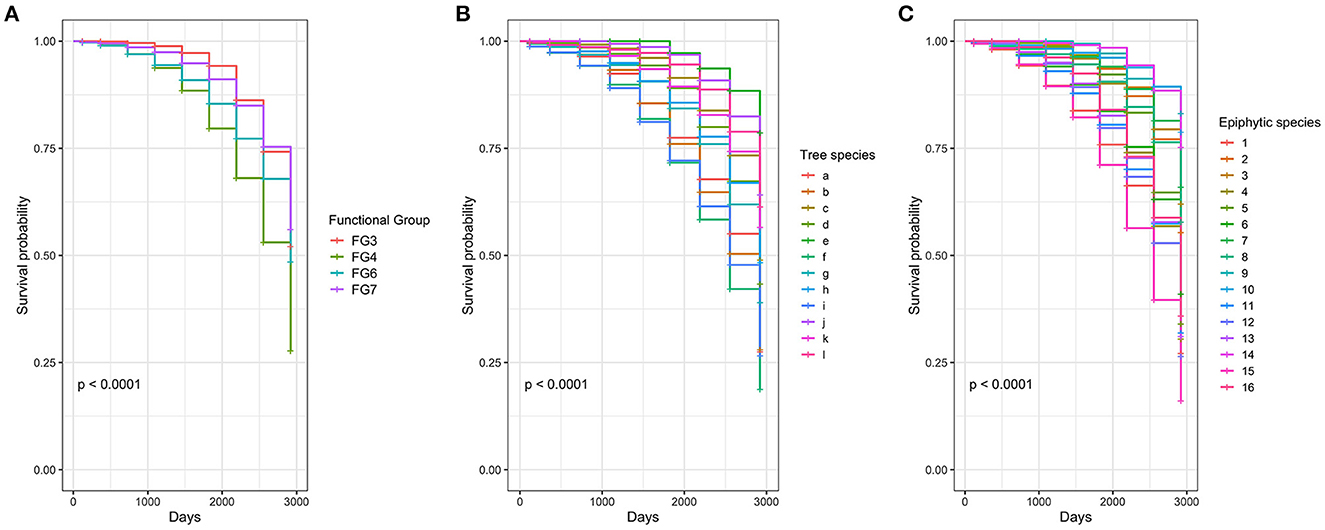
Figure 3. Survival probability according to (A) functional groups, (B) host tree species, and (C) epiphytic species. Letters in (B) are related to a, Alchornea acutifolia Müll. Arg.; b, Chrysochlamys myrcioides Planch. and Triana; c, Clethra fagifolia Kunth; d, Clusia ducu Benth.; e, Eschweilera antioquensis Dugand and Daniel; f, Ilex laurina Kunth; g, Ladenbergia macrocarpa (Vahl) Klotzsch; h, Myrcia cf. fallax (Rich.) DC.; i, Myrcia cf. popayanensis Hieron.; j, Persea chrysophylla L.E. Kopp; k, Sciodaphyllum trianae Planch. and Linden ex Marchal; l, Vismia guianensis (Aubl.) Choisy. Numbers in (C) correspond to 1, Anthurium nigrescens; 2, Cyrtochilum divaricatum; 3, Dichaea morissii; 4, Masdevallia amanda; 5, Masdevallia platyglossa; 6, Maxillaria brevifolia; 7, Odontoglossum sceptrum; 8, Oncidium cultratum; 9, Philodendron danielii; 10, Pleurothallis lindenii; 11, Racinaea penlandii; 12, Racinaea sp; 13, Racinaea subulata; 14, Stelis crassilabia; 15, Tillandsia archerii; and 16, Tillandsia tetrantha.
The mortality events, dead plants, fluctuated over the years. A total of 4 years presented mortalities ranging over 5% (37–44 dead plants; 2015, 2017, 2018, and 2019; Supplementary material 1). The annual precipitation amount in the last 8 years was 2,653.37 ± 498.51 mm year−1, where the least rainy years were 2014 and 2015; the precipitation amount of the driest month was 110.5 ± 51.36 mm year−1, the precipitation seasonality was 0.44 ± 0.06 mm year−1, and the mean monthly precipitation amount of the driest quarter was 3.91 ± 1.23 mm year−1 (Supplementary material 1). Regarding correlations between bioclimatic variables and survival dynamics, it was found that only precipitation seasonality showed a significant negative effect on the cumulative number of annual dead plants (r = 0.512; p < 0.05; Figure 4).
In CoxPH, (a) model, type of substrate, location on the host tree, nutrients, and hormone addition showed no significant effect on the probability of survival (p > 0.01). By contrast, FG 4, bromeliads, had a significant effect on the probability of survival (Figure 3A). In model (b), five host tree species, and in model (c), nine epiphyte species showed a significant effect on the probability of survival (Tables 1, 2, Figures 3B, C, respectively).
The overall mortality rate in this study ranked between 1 and 7% per year (Supplementary material 1), which is expected for annual epiphyte mortality (Matelson et al., 1993; Sarmento Cabral et al., 2015; Zuleta et al., 2016). In the Andes, non-mechanical factors, such as desiccation, accounted for a mortality rate of 1.9% per year, and mechanical factors, such as falling branches, accounted for a mortality rate of 5.6% per year (Zuleta et al., 2016). Our study had one of the highest first-year survival rates in the region, in particular for bromeliads and orchids (96 and 98%, respectively, Supplementary material 1). However, this high survival rate, which was within the expected range, did not prevent survival from declining in subsequent years. Enrichments and translocations of the same plant families conducted in forests in Brazil, Costa Rica, and Peru reported lower survivals of 60 and 80% for orchids and bromeliads, respectively (Duarte and Gandolfi, 2017). The particular conditions of the locality, such as high humidity and lower seasonality of precipitation, as well as irrigation during non-rainy periods during this first year, could have favored the survival of the species in this study.
In our study, species and functional groups responded differently over the 8 years of monitoring. Bromeliads (FG 4), in particular, exhibited high mortality, which could be explained by their monocarpic growth form; the plant dies after the fruit is developed. Although under natural conditions, new rosettes would form, and our observations indicated that a large number of the bromeliads detached or turned over, drying out on site, suggesting that no supporting roots were formed, which would limit the establishment of adult plants. Another group of species showing high mortality was the miniature orchids with ramicuals of the FG 3, Masdevalia amanda, and M. platyglossa. These are associated with short life cycles. However, the presence of seedbeds a few centimeters from the mother plant after 3 years was a remarkable finding for these species. The other species of orchids, FG 3, had longer life cycles and persisted with a stable proportion. The orchids of FG 7, rhizomatous plants with pseudobulbs or stems, presented homogeneous survival percentages below 35% as storage organs for water and nutrients; stems and pseudobulbs play an important role in the survival of orchids. Araceae presented a high contrast between the two species, showing different responses to vegetative reproduction, while Anthurium nigrescens presented one of the highest percentages of mortality, which was concentrated in the early years. Philodendron danielii showed favorable survival over the years. The ability to reiterate over extended periods of time was surprising; the resilience of many individuals who seemed to be dead for months and had the capacity to reiterate was observed in species of aroids and orchids, which evidences the capacity to reiterate and propagate clonally, as observed in other studies (Lasso et al., 2009; Benavides, 2010), and draws our attention to mortality studies over short periods or isolated observations.
Differences in survival between epiphyte species and functional groups indicate that attention should be paid to the differences required by each species, and therefore, epiphyte adaptations and lifespan must facilitate sexual and asexual reproductive processes that are effective in short periods of time (Zuleta et al., 2016). Although we did not focus on flowering and fruiting, numerous individuals flowered and fructified during this study, indicating the importance of the availability of pollinators in the translocated sites (Phillips et al., 2020). Therefore, it is more likely that epiphyte species producing seeds will increase populations within the forests (Duarte and Gandolfi, 2017). It is also recommendable to explore the viability of these seeds and their established processes to improve conservation outcomes. An unexpected result was the differential effect of survival according to host tree species; five species showed an effect on survival. However, it needs to be made clear which factors or characteristics of tree species affect survival; for example, there is no relationship between structural characteristics that were relatively homogeneous. Species-controlled experiments will be necessary to understand the mechanisms that might be favoring survival in certain species. Moreover, overall annual mortality was not related to other climatic variables associated with precipitation. However, a higher seasonality of precipitation was related to the percentage of overall mortality. This indicates that although epiphytic species are adapted to minimum levels of annual precipitation, they are more affected by extreme precipitation events or drought, indicating that the survival probability would be more favored by years with more homogeneous precipitation. This may be essential in order for epiphytes to continue surviving in a rapidly changing climate, as demonstrated by Nadkarni and Solano (2002), who found that increased climatic condition variability may reduce epiphyte assemblage longevity.
Epiphytes have shown a relationship with substrates that allow them to retain water (Dematte and Dematte, 1996; Ghosal et al., 1999). However, differences were not registered after using both substrates (one made of fique fiber and the other made of coconut mesocarp fibers) on individuals from the same species. Natural fibers used have effectively substituted substrates needed for the establishment, which under natural conditions might take years to accumulate (Nadkarni, 2000; Cobb et al., 2001). It is highly recommendable to evaluate the effectiveness of artificial substrates in further studies. Moreover, bark water-holding capacity varied widely among species, and we did not find a direct relationship with survival. In this study, we used plant-associated substrates that can minimize the direct effect of the bark. A similar effect may be occurring in cloud forests, like our site study, where it is common for soil and bryophytes to accumulate massively on top of branches and trunks, forming an interface between the bark and the plants. However, the effect of the bark water retention capacity could be more significant in other ecosystems, such as dry forests or lowland tropical forests with a low presence of fog. In addition, it is important to note that in this study, we only took a measure of the bark water-holding capacity at breast height (1.3 m), but the bark water-holding capacity can vary vertically, and it also depends on the age of the individual and the site conditions (Klamerus-Iwan et al., 2020).
Considering that microclimatic conditions in the first layers of a secondary forest are not expected to vary (Jucker et al., 2018), there was no effect on epiphyte survival within the first 4 m from the base of the host tree, where temperature and humidity facilitate their establishment. Selected host trees did not present branches or bifurcations below the first 4 m from their bases, and therefore, all individuals were positioned on the trunk. However, it is well-documented that branches of the host tree provide higher stability and an opportunity for natural accumulation and retention of substrate for epiphytes (Ingram and Nadkarni, 1993; Zuleta et al., 2016). In our experience, individuals weakly attached to the trunk are more likely to undergo death during the first 6 months after translocation. Therefore, we highly recommend firmly attaching (without movement) individuals to the trunk for at least 6 months, a time in which the majority of them will develop new roots. Moreover, establishing new plants from seedlings or juvenile stages directly in the host tree could guarantee a better long-term establishment of epiphytes, especially for bromeliads (FG 4), allowing the development of holdfast roots, characteristic of these species.
Overall survival of 44% of translocated individuals represents a medium survival percentage. This result indicates that translocation may be an effective conservation action for maintaining individual epiphytes of the selected species in secondary and fragmented forests. Translocation can be a cost-effective measure considering proximity and accessibility to the selected secondary forest and people involved (translocation of 629 epiphytic individuals on 70 host trees took four people and 10 working days). The average cost for the translocation in 2013 for each individual was estimated to be 2.5 dollars (COP 3700), not including technical or professional expenses. As implemented in sites prone to be deforested, this action is an ultimate measure of giving a second chance to these species. Achieving an effective ecological restoration of the epiphyte community requires a deep understanding of the biological aspects of the species as well as their responses to translocation protocols. Colombia's National Development Plan (2018–2022) aims to better leverage natural resources in service to the energy industry, which may increase pressures on the epiphytes assemblages that rely on these same natural areas. However, this context also represents an exciting and promising opportunity to engage local environmental authorities and communities toward biodiversity protection. As local environmental authorities and communities rely on their environments, it is more likely that community-based conservation approaches (engaging local communities in the protection of biodiversity actions) can contribute to protecting forests and their associated epiphyte assemblages. Based on our results, these forests can recover ecological attributes obtained by the epiphyte assemblages by introducing and effectively maintaining populations of different species.
According to the Ministry of Environment and Sustainable Development of Colombia, compensation processes for environmental damage caused to epiphytes (including lichens, bryophytes, orchids, and bromeliads) have mainly focused on strategies of translocation of a determined percentage of individuals to nearby forests and to host trees with similar structural conditions. Details of these processes, such as methods used for plant selection, nursery conditions (or step houses), types of ties used, and type of fertilization, remain to be described. Therefore, documentation and publication of these processes, including successes and failures, are urgently needed. Although compensation is not restricted to reintroduction processes, most compensation processes have focused on it in Colombia. Other measures may include research to generate better management, addressing fundamental questions on epiphyte ecology, adaptation to climate change, and mitigation of species loss. Evaluation of strategies that include propagation from seeds using in vitro protocols or nurseries must be included to enhance management and translocation success (Phillips et al., 2020).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AB designed the study, monitored each year, and wrote the manuscript. JC-C proved and analyzed the data. All authors jointly discussed and agreed to the final version.
The authors thank Maria Patricia Tobón, Diana Amaya, Martha Llano, and Beatriz Elena Araque, who have promoted the establishment and permanence of this study during the 8 years of monitoring. The Arví Park Corporation facilitated translocation and monitoring. Lorena Hernández coordinated the initial translocation. Gustavo Aguirre and Luis Pérez contributed key ideas for translocation. Roberto Parra and guides from Arví Park, Alex Nieto, and David Gallego collaborated during the translocation process. The translocation was carried out within the Pilot project for the conservation, restoration, and use of native ornamental species to benefit families in Santa Elena (Antioquia), financed by the sustainable forest management (SFM) program in the Andean region of Finland government. Numerous collaborators from Parque Arví and the Botanical Garden of Medellín have accompanied AB during the 8 years of monitoring.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.834669/full#supplementary-material
Agudelo, C. M., Benavides, A. M., Taylor, T., Feeley, K. J., and Duque, A. (2019). Functional composition of epiphyte communities in the Colombian Andes. Ecology 100, e02858. doi: 10.1002/ecy.2858
Ávila, J. O., Marín, A. V., and Pérez, J. F. B. (2017). Estimation of the transfer of vascular epiphytes, as a conservation strategy in the municipality of Aguazul, Casanare, Colombia. Rev. Invest. Agrar. Ambient 8, 27–37. doi: 10.22490/21456453.1830
Barrancos, E. P. F., Reid, J. L., and Hall, J. S. (2020). Lack of araceae in young forests highlights the importance of mature forest conservation. Trop. Conserv. Sci. 12, 1940082919849504. doi: 10.1177/1940082919849504
Benavides, A. M. (2010). Distribution and succession of vascular epiphytes in Colombian Amazonia. J. Trop. Ecol. 27, 299–314. doi: 10.1017/S0266467410000726
Benavides, A. M., Wolf, J. H., and Duivenvoorden, J. F. (2006). Recovery and succession of epiphytes in upper Amazonian fallows. J. Trop. Ecol. 22, 705–717. doi: 10.1017/S0266467406003580
Callaway, R., Reinhart, K., Moore, G., Moore, D., and Pennings, S. (2002). Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132, 221–230. doi: 10.1007/s00442-002-0943-3
Carmona Higuita, M. J., Agudelo Palacio, C. M., Zuleta, D., Monsalve Correa, S., Idárraga Piedrahíta, Á., Julio, B., et al. (2017). “Prioridades de conservación de la diversidad de orquídeas en el departamento de Antioquia (Colombia),” in Bosques Andinos, Estado Actual y Retos Para su Conservación en Antioquia, eds S. G.-C. E. Quintero, A. Benavides, and N. Moreno (Medellín: Jardín Botánico de Medellín), 155–178.
Cobb, A. R., Nadkarni, N. M., Ramsey, G. A., and Svoboda, A. J. (2001). Recolonization of bigleaf maple branches by epiphytic bryophytes following experimental disturbance. Can. J. Bot. Rev. Can. Bot. 79, 1–8. doi: 10.1139/cjb-79-1-1
Dematte, J. B. I., and Dematte, M. (1996). Water studies on substrates of vegetal origin for epiphytic orchid cultivation. Pesqui. Agropecu. Bras. 31, 803–813.
Domene, F. (2019). Reintroduction of Vascular Epiphytes in Forest Restoration Plantations. Universidade de São Paulo, São Paulo, Brazil. doi: 10.11606/T.11.2019.tde-04012019-091720
Duarte, M. M., and Gandolfi, S. (2013). Enriquecimento de florestas em processo de restauração: aspectos de epífitas e forófitos que podem ser considerados. Hoehnea 40, 507–514. doi: 10.1590/S2236-89062013000300010
Duarte, M. M., and Gandolfi, S. (2017). Diversifying growth forms in tropical forest restoration: enrichment with vascular epiphytes. For. Ecol. Manage. 401, 89–98. doi: 10.1016/j.foreco.2017.06.063
Einzmann, H. J., Beyschlag, J., Hofhansl, F., Wanek, W., and Zotz, G. (2015). Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. AoB Plants 7, plu073. doi: 10.1093/aobpla/plu073
Faleiro, R., Duarte, F., Calassa, C, Barros, J. C, and Barreira, S. (2020). Introdução e reavaliação de Aechmea bromeliifolia (Rudge) baker para fins de enriquecimento em area restaurada no bioma cerrado. Encicl. Biosfera 17, 125. doi: 10.18677/EnciBio_2020A11
Fernandez Barrancos, E. P., Reid, J. L., and Aronson, J. (2017). Tank bromeliad transplants as an enrichment strategy in southern Costa Rica. Restor. Ecol. 25, 569–576. doi: 10.1111/rec.12463
Gentry, A. H., and Dodson, C. H. (1987a). Contribution of nontrees to species richness of a tropical rain forest. Biotropica 19, 149–156. doi: 10.2307/2388737
Gentry, A. H., and Dodson, C. H. (1987b). Diversity and biogeography of neotropical vascular epiphytes. Ann. Mo. Bot. Gard. 74, 205–233. doi: 10.2307/2399395
Ghosal, S., Muruganandam, A. V., Chauhan, S, Kawanishi, K., Saiki, K., and Nadkarni, N. M. (1999). Crown humus: part I—the chemistry of the canopy organic matter of rain forests in Costa Rica. Indian J. Chem. Sect B Org. Chem. Incl. Med. Chem. 38, 67–75.
Ingram, S. W., and Nadkarni, N. M. (1993). Composition and distribution of epiphytic organic matter in a neotropical cloud forest, Costa Rica. Biotropica 25, 370–383. doi: 10.2307/2388861
Izuddin, M., Yam, T. W., and Webb, E. L. (2018). Specific niche requirements drive long-term survival and growth of translocated epiphytic orchids in an urbanised tropical landscape. Urban Ecosyst. 21, 531–540. doi: 10.1007/s11252-018-0733-2
Jucker, T., Hardwick, S. R., Both, S., Elias, D. M. O., Ewers, R. M., Milodowski, D. T., et al. (2018). Canopy structure and topography jointly constrain the microclimate of human-modified tropical landscapes. Glob. Change Biol. 24. 5243–5258. doi: 10.1111/gcb.14415
Karger, D. N., Conrad, O., Böhner, J., Kawohl, T., Kreft, H., Soria-Auza, R. W., et al. (2017). Climatologies at high resolution for the earth's land surface areas. Sci. Data 4, 170122. doi: 10.1038/sdata.2017.122
Klamerus-Iwan, A., Link, T. E., Keim, R. F., and Van Stan, J. T. II. (2020). “Storage and routing of precipitation through canopies,” in Precipitation Partitioning by Vegetation, eds J. van Stan, E. Gutmann, and J. Friesen (Cham: Springer), 17–34. doi: 10.1007/978-3-030-29702-2_2
Köster, N., Friedrich, K., Nieder, J., and Barthlott, W. (2009). Conservation of epiphyte diversity in an Andean landscape transformed by human land use. Conserv. Biol. 23, 911–919. doi: 10.1111/j.1523-1739.2008.01164.x
Köster, N., Kreft, H., Nieder, J., and Barthlott, W. (2013). Range size and climatic niche correlate with the vulnerability of epiphytes to human land use in the tropics. J. Biogeogr. 40, 963–976. doi: 10.1111/jbi.12050
Lasso, E., Engelbrecht, B. M, and Dalling, J. W. (2009). When sex is not enough: ecological correlates of resprouting capacity in congeneric tropical forest shrubs. Oecologia 161, 43–56. doi: 10.1007/s00442-009-1353-6
Malm, O. (1998). Use of epiphyte plants as biomonitors to map atmospheric. Sci. Total Environ. 213, 57–64. doi: 10.1016/S0048-9697(98)00074-6
Matelson, T. J., Nadkarni, N. M., and Longino, J. T. (1993). Longevity of fallen epiphytes in a neotropical montane forest. Ecology 74, 265–269. doi: 10.2307/1939523
Morales-Morales, P. A., Benavides, A. M., and Cardona, E. F. A. (2015). Guía de Campo del Parque Arví: Anturios, Bromelias y Orquídeas. Medellín: Alcaldía de Medellín, Corporación Parque Arví, Universidad de Antioquia, Corporación para Investigaciones Biológicas y Sociedad Colombiana de Orquideología.
Nadkarni, N. M. (2000). Colonization of stripped branch surfaces by epiphytes in a lower montane cloud forest, Monteverde, Costa Rica. Biotropica 32, 358–363. doi: 10.1111/j.1744-7429.2000.tb00479.x
Nadkarni, N. M., and Solano, R. (2002). Potential effects of climate change on canopy communities in a tropical cloud forest: an experimental approach. Oecologia 131, 580–586. doi: 10.1007/s00442-002-0899-3
Petter, G., Wagner, K., Wanek, W., Sánchez, E. J., Zotz, G, Cabral, J. S., et al. (2015). Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra and interspecific trait variability. and taxonomic signals. Funct. Ecol. 30, 188–198. doi: 10.1111/1365-2435.12490
Phillips, R. D., Reiter, N., and Peakall, R. (2020). Orchid conservation: from theory to practice. Ann. Bot. 126, 345–362. doi: 10.1093/aob/mcaa093
R Development Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rapp, J. M., and Silman, M. R. (2014). Epiphyte response to drought and experimental warming in an Andean cloud forest. F1000Research 3, 7. doi: 10.12688/f1000research.3-7.v2
Reyes-García, C., Griffiths, H., Rincón, E., and Huante, P. (2008). Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica 40, 168–175. doi: 10.1111/j.1744-7429.2007.00359.x
Sarmento Cabral, J., Petter, G., Mendieta-Leiva, G., Wagner, K., Zotz, G., and Kreft, H. (2015). Branchfall as a demographic filter for epiphyte communities: lessons from forest floor-based sampling. PLoS ONE 10. e0128019. doi: 10.1371/journal.pone.0128019
SIATA (2011). Sistema de Alerta Temprana del Área Metropolitana del Valle de Aburrá. Available online at: http://siata.gov.co/newpage/index.php (accessed October 31, 2021).
Therneau, T. (2022). A Package for Survival Analysis in R. R package version 3.4-0. Available online at: https://CRAN.R-project.org/package=survival (accessed October 31, 2022).
Therneau, T. M., and Grambsch, P. M. (2000). “The cox model,” in Modeling Survival Data: Extending the Cox Model (New York. NY: Springer), 39–77. doi: 10.1007/978-1-4757-3294-8_3
Wolf, J. H. D. (2005). The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. For. Ecol. Manage. 212, 376–393. doi: 10.1016/j.foreco.2005.03.027
Zotz, G. (2000). Size-related intraspecific variability in physiological traits of vascular epiphytes and its importance for plant physiological ecology. Perspect. Plant Ecol. Evol. Syst. 3, 19–28. doi: 10.1078/1433-8319-00002
Keywords: Andean forests, aroids, bromeliads, conservation actions, mortality rate, orchids, survival rate
Citation: Benavides AM, Calderón-Caro J and Canal D (2023) Surviving in a new host: Eight years of monitoring translocated aroids, bromeliads, and orchids in the Andean forests in Colombia. Front. For. Glob. Change 6:834669. doi: 10.3389/ffgc.2023.834669
Received: 13 December 2021; Accepted: 17 January 2023;
Published: 16 February 2023.
Edited by:
Paolo Giordani, University of Genoa, ItalyReviewed by:
John T. Van Stan, Cleveland State University, United StatesCopyright © 2023 Benavides, Calderón-Caro and Canal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana María Benavides,  YW5hbWFyaWEuYmVuYXZpZGVzQGpib3Rhbmljby5vcmc=
YW5hbWFyaWEuYmVuYXZpZGVzQGpib3Rhbmljby5vcmc=
†Present address: Duban Canal, Climate and Land Use Alliance, Bogotá, Colombia
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.