- 1College of Agriculture and Forestry Technology, Hebei North University, Zhangjiakou, China
- 2Hebei Forestry and Grassland Survey and Planning Design Institute, Shijiazhuang, China
Introduction: The hybrid hazelnut is an important fruit tree species known for its high productivity and disease resistance. However, during the growth process of hybrid hazelnuts, issues such as branch withering and bud wrinkling have attracted significant attention. These phenomena severely impact the normal development and yield of the plants. This study aimed to investigate the relationship between the resistance to shoot emergence and physiological changes in six hazel tree varieties by comprehensively analyzing their resistance to shoot emergence.
Method: Six widely cultivated hazelnut hybrid varieties were selected for physiological measurements and field surveys using one-year-old branches and buds as test materials. The resistance to shoot emergence of different hazel tree varieties was evaluated through correlation analysis, principal component analysis, and membership function evaluation.
Results: The results showed that the length and width of hazel buds gradually increased while the thickness first increased and then decreased before and after bud burst. The carbohydrate levels of the ‘Dawei’, ‘Pingou 110’, and ‘84–237’ varieties were significantly higher than those of other varieties. The content of reducing sugars and starch in hazel buds generally increased and then decreased during the bud burst process, while α-amylase activity showed a decreasing trend followed by an increasing trend. The antioxidative enzyme activity of ‘Dawei’ was significantly higher than that of other varieties on different sampling dates. The soluble protein content, POD enzyme activity, and CAT enzyme activity of hazel buds for the six varieties gradually decreased. The moisture content of ‘Pingou 210’ shoots was significantly higher than that of other varieties during the peak emergence period. The correlation analysis results showed that the bud burst rate of hazel trees was positively correlated with the length, width, thickness, reducing sugar content, starch content, POD enzyme activity, CAT enzyme activity, and shoot moisture content, while it was negatively correlated with α-amylase activity and relative conductivity of shoots. Through a comprehensive evaluation using correlation analysis, principal component analysis, and membership function analysis, the resistance to shoot emergence of the six hazel tree varieties was ranked in the following order: ‘Dawei’, ‘Pingou 110’, ‘84–237’, ‘Pingou 210’, ‘Yuzhui’, and ‘Liaozhen 3’.
Discussion: This study fills a research gap regarding the physiological changes of hazelnuts before and after sprouting, and provides theoretical guidance for further research on the anti-withering ability of hazelnut trees.
1 Introduction
Ping’ou hybrid hazelnut (Corylus heterophylla Fisch × Corylus avellana L.) is a hazelnut variety obtained through interspecific hybridization, with the wild Ping’ou hazelnut as the maternal parent and European hazelnut as the paternal parent. As a widely cultivated hazelnut variety in China, hazelnut exhibits characteristics such as cold resistance, drought resistance, barrenness resistance, early fruiting, high yield, good profitability, and long lifespan, making it an important ecological and economic tree species (Zhang, 2015; Wang, 2018; Luo et al., 2023). However, during the growth process of hybrid hazelnuts, the phenomenon of branch withering and bud shriveling often attracts attention. Branch withering refers to the drying of branches, shrinkage of the epidermis, and whitening and drying of the wood in the growth process of hybrid hazelnut. Bud shriveling refers to the contraction and deformation of bud scales, limited bud growth, and hindered normal development of winter and spring buds. These phenomena severely impact the normal development and yield of the plants (Li, 2023), resulting in a significant decline in economic benefits (Xie, 2016; Ma, 2020).
Firstly, the level of carbohydrates is one of the important factors affecting hazelnut shoot growth. Plants convert carbon dioxide into carbohydrates through photosynthesis and store them as starch or reducing sugars, which play a crucial role in energy supply and physiological metabolism of plants. Studies have shown that sufficient carbohydrates play a vital role in the growth and development of fruit trees. For instance, Liu et al. (2014) conducted experiments using apple trees in northern regions and found that exogenous glucose treatment significantly promoted apple flower formation. Similarly, Cao et al. (2021) and Hu et al. (2021) determined through physiological and biochemical measurements in walnuts that high levels of reducing sugars and starch significantly promoted the physiological differentiation of female flower buds. Therefore, there is a close relationship between carbohydrate levels and the growth and development of fruit trees. Additionally, antioxidant enzyme activity plays a significant role in fruit trees when they experience environmental stress, such as drought or cold, leading to increased generation of reactive oxygen species within plant cells and exacerbating oxidative damage. However, most fruit trees possess high antioxidant enzyme activity, enabling them to better withstand oxidative stress. Antioxidant enzymes can eliminate reactive oxygen species within cells and alleviate the impact of oxidative damage on cell structure and function. For example, studies by Hu et al. (2021) found that increased activities of peroxidase (POD) and other antioxidant enzymes in walnuts, enhanced their ability to combat adversity and promote normal tree growth and development. Wang (2022) showed that the higher the antioxidant enzyme activity of hazelnut, the higher the content of soluble proteins, the stronger the hazelnut resistance to twitching ability; Furthermore, Li et al. (2023) demonstrated that in the adverse conditions of cold and drought, water content, soluble sugar content, and soluble protein content in pear trees were significantly positively correlated with germination rate. Additionally, environmental stress such as drought or low temperatures can cause a decrease in branch water content in hybrid hazelnut plants. In this situation, the plant’s transpiration rate increases while its water absorption capacity remains limited, resulting in cellular water deficiency and subsequently affecting normal plant growth and development. The decrease in branch water content may lead to a decrease in cell turgor pressure and damage to cell membrane integrity, leading to branch withering and bud shriveling. Research has found that the occurrence of shoot withering in hazelnuts is often caused by cold and drought stress during winter (Zhang, 2013; Xue, 2015; Changsheng, 2019), and recent studies indicate that larger xylem vessels and well-developed root systems can effectively absorb water to withstand cold and drought environments (Zhang, 2013; Xue, 2015). Wang (2022) demonstrated that higher antioxidant enzyme activity and soluble protein content are associated with stronger resistance to shoot withering in hazelnuts.
The cold resistance of fruit trees is closely related to physiological indicators such as carbohydrate content, antioxidant enzyme activity, and water content. However, there have been few reports on the physiological changes of hazelnuts before and after bud break. Therefore, this study aims to establish the relationship between the physiological changes before and after bud break and the resistance to shoot withering in different hazelnut varieties. By combining principal component analysis, correlation analysis, and membership function analysis, the study utilizes 12 physiological indicators before and after bud break as evaluation criteria for the resistance to shoot withering in six hazelnut varieties, ultimately assessing their resistance capabilities. This study not only fills the research gap regarding the physiological changes before and after bud break in hazelnuts but also effectively evaluates the resistance to shoot withering before and after bud break, providing theoretical guidance for the global selection of hazelnut varieties with stronger resistance to shoot withering.
2 Materials and design
2.1 Test material
The test material was widely cultivated Ping ou hybrid hazelnut varieties (lines) 1-year-old branches and shoots, including ‘Liaozhen 3’, ‘Yuzhui’, ‘84–237’, ‘Dawei’, ‘Ping’ou 110’, and ‘Ping’ou 210’, a total of six varieties. The experiment was conducted in the hazelnut nursery of Hebei North University South Campus, the nursery base of Hebei Zhangjiakou Academy of Agricultural Sciences, and the physiology and biochemistry laboratory of Hebei North University South Campus, located in Zhangjiakou Jingkai District, Hebei Province, China. The experimental site is located at 114°50′E longitude and 40°10′N latitude and has a temperate continental monsoon climate. The climate is characterized by four distinct seasons, with cold and long winters, dry and sandy springs, hot and short summers with concentrated precipitation, and sunny and moderately warm autumns (Kang, 2022).
2.2 Experimental design
This test in 2023 on March 5, March 20, March 25, April 1, April 13 sampling five times, each sampling time to select a clear morning around 10:00, each variety of randomly selected tree body healthy 5 trees, randomly intercepted the growth potential of the same, branching body of the stout 1 year-old branches of 3, each time the sampling of each variety of sampling 15 and with the label to indicate the variety number and the time of collection. Hazelnut buds of different varieties were removed in the laboratory with a razor blade, wrapped into tin foil, put in a labeled bag, marked, and quickly placed in a −20°C refrigerator to prevent inactivation of the relevant antioxidant enzyme activity.
Firstly, the relevant indexes of the branches were measured, including dry weight, fresh weight, water content, relative conductivity, and the methods are shown below; secondly, the relevant indexes of the shoots were measured, including morphological indexes, reducing sugar content, starch content, α-amylase activity, soluble protein content, catalase (CAT) activity, and peroxidase (POD) activity. The measurement of enzyme activity should be carried out at about 0°C to prevent inactivation of enzyme activity; finally, during the survey of field drawdown rate at the hazelnut nursery of the South Campus of Hebei North University and the nursery base of Hebei Zhangjiakou Academy of Agricultural Sciences on 25th March, five hazelnut trees of each variety were randomly selected with good growth, and the number of buds germinated on the first-year branches was investigated to determine the ability of different varieties of Ping’ou hybrid hazelnut to resist shoot shriveling.
2.2.1 Measurement of morphological indicators
Observe and record the morphological changes of hazelnut buds before and after hazelnut sprouting, take samples of 10 hazelnut buds randomly selected from each species using vernier calipers to measure their length, width and thickness, and finally take their average values.
2.2.2 Measurement of physiological indicators
2.2.2.1 Determination of reducing sugar and starch content and α-amylase activity
Reducing sugars were extracted and determined by 3,5-dinitrosalicylic acid method using the method of Gao (2006) as reference. The starch content was determined by iodine chromatography with reference to the method of Xu et al. (1998). Determination of α-amylase activity The method of Zhang et al. (2020) was used to determine the α-amylase activity by the 3,5-dinitrosalicylic acid method. All the above three determination methods were improved in due course.
2.2.2.2 Measurement of soluble protein content and peroxidase and catalase activities
The soluble protein content was determined with reference to the method of Wang (2006), and the method was improved in due course. Weigh 0.5 g of hazelnut buds, add a small amount of PVPP, add 2.5 mL of phosphate buffer (pH 7.8, 0.05 mol/L), grind in an ice bath, and then rinse the residue with 2 mL of phosphate buffer in three times, and then transfer to a centrifuge tube. 0–4°C, centrifugation at 12000 rpm for 30 min, and then keep it under cold storage after centrifugation, and then use enzyme solution for the determination of soluble protein, POD and CAT. The enzyme solution was used for soluble protein, POD and CAT determination. The soluble protein content was determined by using the method of Liang et al. (2023), and the method was improved in the course of the determination by using Thomas Brilliant Blue G-250. For the determination of peroxidase (POD) activity, the method of guaiacol method was referred to that of Wang et al. (2010), and the method was improved in the course of the determination. The reaction mixture was as follows: 100 mL of 0.1 mol/L phosphate buffer (pH 6.0) was mixed with 70 μL of guaiacol, heated and stirred until dissolved, cooled down, and then 50 μL of 30 %H O22 was added, mixed well, and stored in the refrigerator. 50 μL of the enzyme solution was taken and added to 3 mL of the reaction mixture, and 50 μL of phosphate buffer (pH 7.8) was substituted for the enzyme solution as a blank, and then the enzyme activity was measured by colourimetry at 470 nm and timed. The colorimetry was carried out at 470 nm and timed at 1 min intervals (OD values at 0, 1 and 2 min), and the change in OD470 per minute was used to indicate the size of the enzyme activity.
POD activity (U.min-l.g-lFW) = ΔA470 × Vt/0.01 × W × Vs × t.
Where, △A470: change in absorbance value during the reaction time; Vt: total volume of extracted enzyme solution (mL); W: fresh weight of the sample (g); Vs: volume of enzyme solution taken during the determination (mL); t: reaction time (min); 0.01: increase of 0.01 per minute as a unit of enzyme activity.
Catalase (CAT) activity was determined by the hydrogen peroxide reduction method of Kato and Shimizu (1987) and the method was improved in due course.
2.2.2.3 Determination of branch dry and fresh weights and relative water content and relative conductivity
Determination of dry and fresh weights and moisture content of branches Three sets of parallel samples were set up from cut 1-year old branches. Firstly, the fresh weight of the branches was weighed (M1). Secondly, the weighed branches were put into the oven and set to be killed at 105°C for 30 min and baked at 100°C for 6 h until constant weight, and the dry weight of the branches was weighed (M2). The moisture content was then calculated from the dry fresh weight.
The relative conductivity of the branches was determined by the conductivity meter method, each sampling of the treated branches, cut into 2–3 cm long small sections (as far as possible to maintain the same size of the surface area), avoiding the eyes of the shoots, weighing the sample 0.25 g in a triangular flask, placed in 10 mL of distilled water, after 12 h of resting, conductivity value S1 was measured. Then the boiling water bath was used to kill the tissues for 15 min, and then cooled down to room temperature immediately after the removal of the sample to measure the boiling conductivity S2. 3 groups of parallel samples were set up. Conductivity S2. 3 sets of parallel samples were set up.
2.2.3 Field survey of branch extraction rate
On 25 March 2023, six varieties (lines) of hazelnut sampled from the resource nursery were investigated for shoot shriveling, referring to the method of Li (2010): Grade 0, all annual branches sprouted; Grade 1, more than 3/4 of the annual branches sprouted; Grade 2, 1/2 to 3/4 (including 1/2) of the annual branches sprouted; Grade 3, 1/4 to 1/2 (including 1/4) of the annual branches sprouted; Grade 4, less than 1/4 of the annual branches sprouted. Five sets of data were randomly investigated for each variety to compare the resistance of different varieties of Ping’ou hybrid hazelnut to shoot shriveling based on the number of sprouts on one-year old branches.
2.2.4 Correlation analysis and comprehensive evaluation of different hazelnut varieties on their resistance to shoot shriveling
Principal component and correlation analyzes were performed using SPSS Statistics26.0 software. Based on the results of the principal component analysis, the fuzzy mathematical affiliation function method was used to comprehensively evaluate the physiological indicators before and after the emergence of different varieties of hazelnut trees. Firstly, we need to calculate the value of the affiliation function of a certain principal component index of each variety, and then calculate the average affiliation function value of each variety (line), and the larger the value, the stronger the resistance of hazelnut trees of this variety to shoot shriveling. The formula is as follows:
μ(Xi) = (Xi-Xmin)/(Xmax-Xmin).
Where: μ(Xi) denotes the value of the affiliation function of the ith index, Xi denotes the measured value of the ith index, Xmin denotes the minimum value of the ith index, and Xmax denotes the maximum value of the ith index. According to the mean value of each index affiliation function value for comprehensive evaluation, the larger the mean value, the stronger the ability to resist the drawbar.
2.3 Data processing and analysis
Microsoft Excel 2021 was used for the processing of the experimental data and the analysis of the affiliation function method, SPSS Statistics 26.0 software was used for ANOVA and Duncana, b significance of difference analysis (p < 0.05), and principal component and correlation analysis of the experimental data was carried out.
3 Results
3.1 Morphological comparison of buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting
As shown in Table 1, the length and width of hazelnut buds generally increased over time, while the thickness of the buds exhibited an initial increase followed by a decrease. The length of buds from ‘Liao Zhen 3’ and ‘Yuzhui’ reached a significant level on April 1 (p < 0.05), while the length of buds from ‘84–237’ and ‘Dawei’ reached a significant level on March 25 (p < 0.05). The length of buds from ‘Ping’ou 110’ reached a significant level on April 13 (p < 0.05), and the length of buds from ‘Ping’ou 210’ reached a significant level on March 20 (p < 0.05). The width of buds from ‘Liao Zhen 3’, ‘Dawei’, and ‘Ping’ou 210’ reached a significant level on March 25 (p < 0.05), while the width of buds from ‘Yuzhui’ and ‘84–237’ reached a significant level on March 20 (p < 0.05). The width of buds from ‘Ping’ou 110’ reached a significant level on April 1 (p < 0.05). The thickness of buds from all six varieties reached its maximum value on March 25 and then gradually decreased.
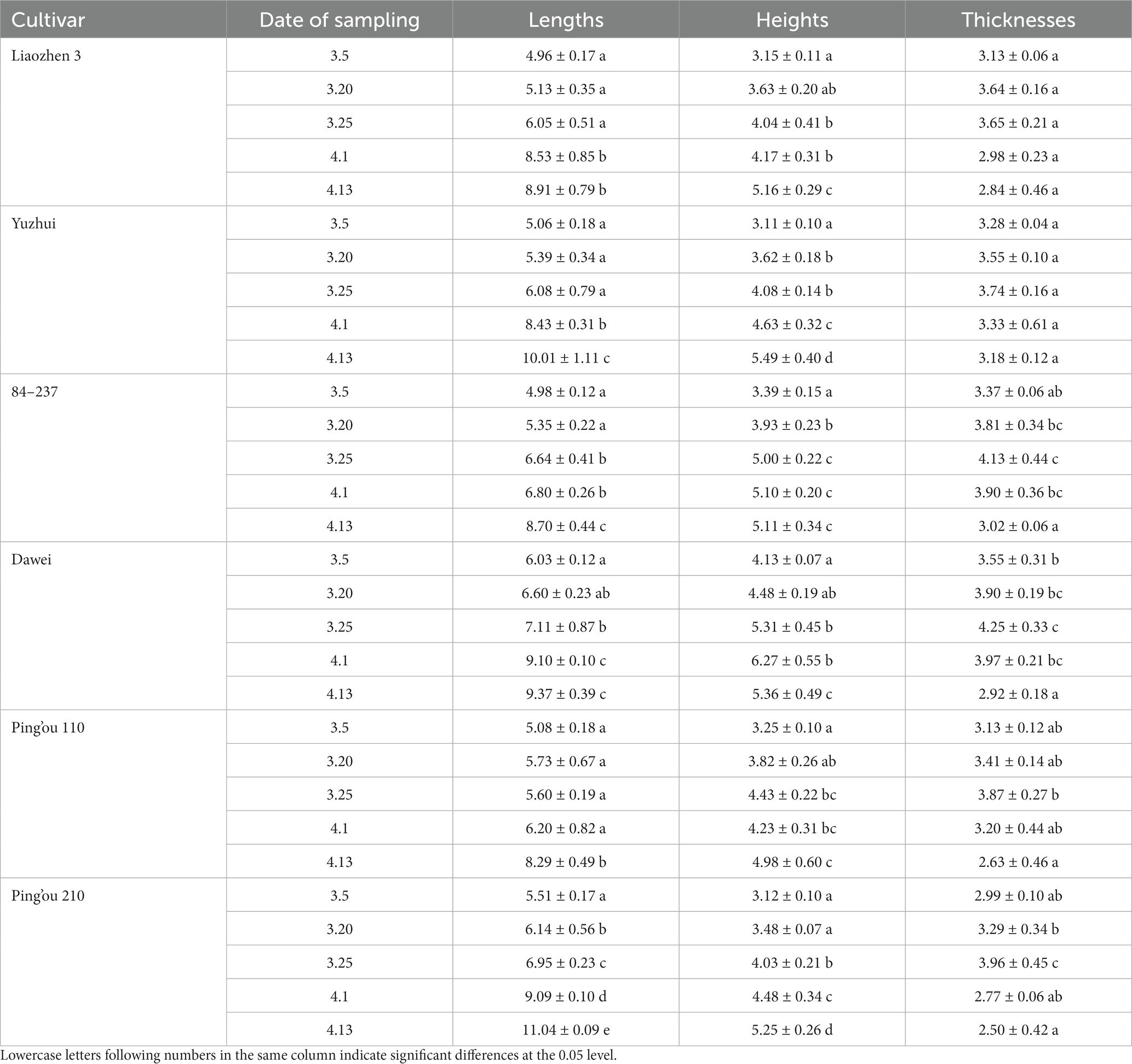
Table 1. Comparison of morphological development of different varieties of Ping’ou hybrid hazelnut before and after sprouting of hazelnut buds (unit: mm).
Based on the morphological comparison of the buds, it can be observed that the male floral clusters of the hazelnut buds were covered by numerous bracts arranged in a tile-like pattern. The female flowers of all six varieties were enclosed within the bracts from March 5 to March 20. By March 25, the female stigma gradually emerged with a dark red color, and the thickness of the buds reached its maximum during this period. On April 1, the number of stigmas protruding from the bracts increased, with nearly 70% of the stigmas exposed. The color of the stigmas also became brighter. By April 13, the stigmas of all six varieties gradually lost their luster and withered.
Based on the observation and measurement of the bud morphology, it can be inferred that the pre-sprouting period of hazelnut buds in the northwest region of China occurs around March 5 to March 20. During this time, the length, width, and thickness of the buds gradually increase, and the female stigmas begin to appear. The peak sprouting period of the buds is around March 25 to April 1, during which the length, width, and thickness of the buds significantly increase, and a large portion of the stigmas are exposed, showing a bright luster. The post-sprouting period of the buds is around April 13, during which the changes in length, width, and thickness are not significant, and the stigmas gradually wither.
3.2 Comparison of physiological changes in branches and buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting
3.2.1 Comparison of carbohydrate and amylase activity in hazelnut shoots of different varieties of Ping’ou hybrid hazelnut before and after germination
As shown in Figure 1A, the reducing sugar content of ‘Ping’ou 110’ and ‘Dawei’ was significantly higher than the other four varieties in all five sampling dates. On March 20, the reducing sugar content of ‘Dawei’ and ‘Ping’ou 110’ reached its maximum values: 28.68 μg.g − 1 and 29.06 μg.g − 1, significantly higher than the other varieties. The reducing sugar content of ‘Ping’ou 110’ was significantly higher than the other five varieties on March 5, March 25, April 1, and April 13. The reducing sugar content of the six varieties generally increased initially and then decreased, reaching its maximum value on March 20. Subsequently, as the hazelnut trees grew, the reducing sugar content gradually decreased. These findings suggest that a high level of reducing sugars may contribute to sufficient nutrient storage in hazelnut buds before and after sprouting, providing a solid material foundation for flowering and fruit setting as well as enhancing stress resistance.
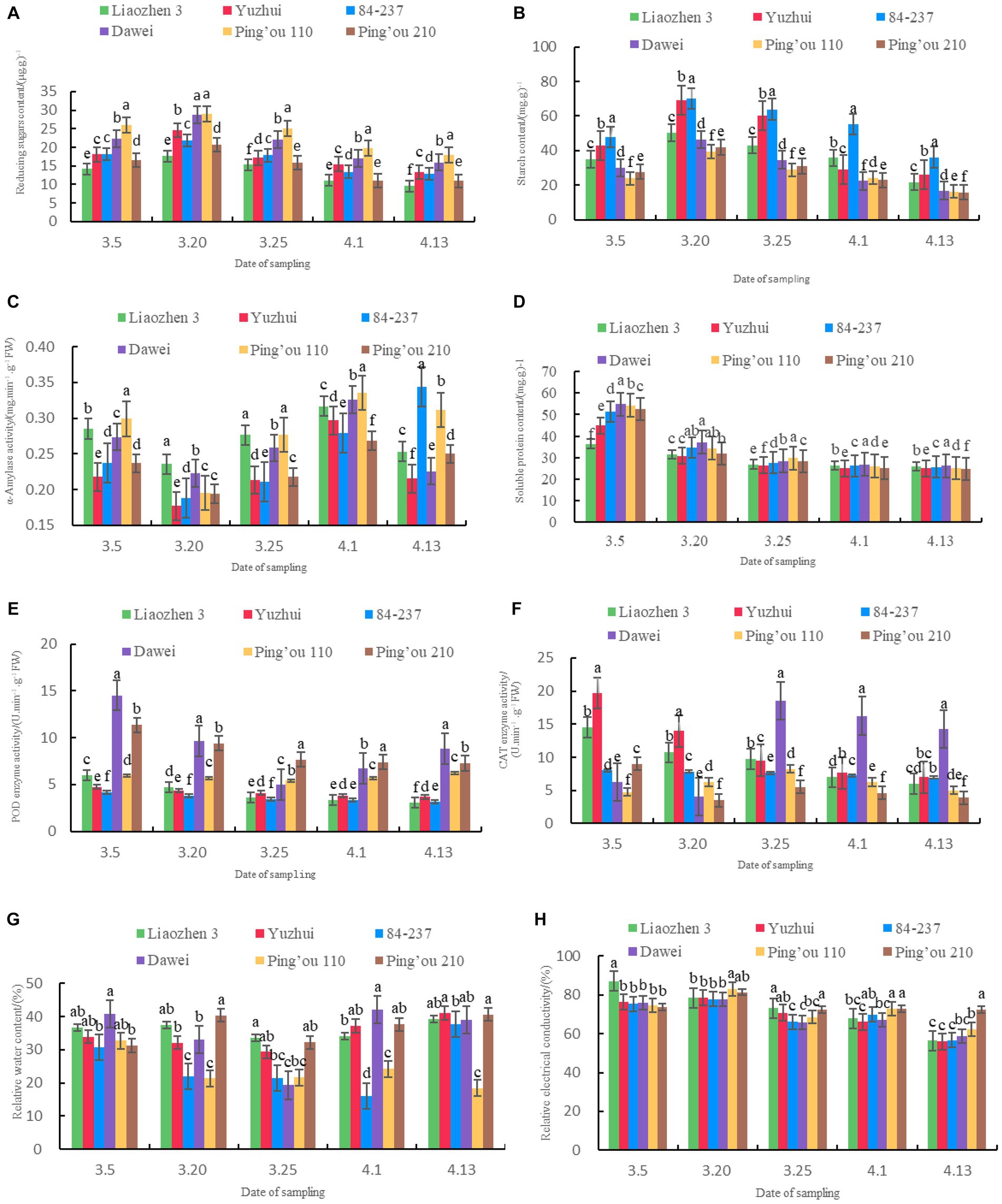
Figure 1. (A) Comparison of reducing sugar content in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting; (B) Comparison of starch content in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting; (C) Comparison of α-amylase activity in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting; (D) Comparison of soluble protein content in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting; (E) Comparison of POD enzyme activity in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting. (F) Comparison of CAT enzyme activity in shoots of different varieties of Ping’ou hybrid hazelnut before and after germination; (G) Comparison of water content in branches of different varieties of Ping’ou hybrid hazelnut before and after germination; (H) Comparison of relative electrical conductivity in branches of different varieties of Ping’ou hybrid hazelnut before and after germination. Lower case letters outside the data labels indicate that the data of different varieties on the same sampling date are significantly different at the 0.05 level.
As shown in Figure 1B, the starch content of ‘Yuzhui’ and ‘84–237’ was significantly higher than the other four varieties at different sampling dates. On March 20, the starch content reached 69.03 mg.g −1 and 70.27 mg.g −1 for the two varieties, significantly higher than the other four varieties. The starch content of all six varieties reached its maximum on March 20 and significantly increased by 43.54, 60.76, 46.85, 53.87, 64.79, and 50.92% compared to the sampling on March 5 (p < 0.05). The starch content of ‘84–237’ was significantly higher than the other varieties on all sampling dates, reaching 55.38 mg.g −1 on April 1, which was significantly higher than the other five varieties (p < 0.01). The starch content of the six varieties generally increased initially and then decreased.
As shown in Figure 1C, the α-amylase activity of ‘Yuzhui’ and ‘84–237’ was significantly lower than the other four varieties at different sampling dates. On March 20, the α-amylase activity of ‘Yuzhui’ and ‘84–237’ was 0.1768 mg.min-1.g−1FW and 0.1865 mg.min−1.g−1FW, significantly lower than the other varieties. On April 1, the α-amylase activity of ‘Yuzhui’ and ‘Ping’ou 110’ was significantly higher than the other four varieties, and ‘Ping’ou 110’ reached its maximum value: 0.3351 mg.min−1.g−1FW. On April 13, the α-amylase activity of ‘84–237’ and ‘Ping’ou 110’ was significantly higher than the other varieties, with ‘84–237’ reaching its maximum value. The α-amylase activity of the buds from all six varieties generally exhibited an initial decrease followed by an increase. The α-amylase activity of all six varieties reached its minimum on March 20, decreasing by 0.2356 mg.min−1.g−1FW, 0.1768 mg.min−1.g−1FW, 0.1875 mg.min−1.g−1FW, 0.2227 mg.min−1.g−1FW, 0.1949 mg.min−1.g−1FW, and 0.1938 mg.min−1.g−1FW compared to the sampling on March 5.
3.2.2 Comparison of soluble protein content and antioxidant enzyme activities in buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting
As shown in Figure 1D, the soluble protein content of ‘Dawei’ was significantly higher than the other varieties on March 5, March 20, April 1, and April 13. The soluble protein content of ‘Ping’ou 110’ was significantly higher than the other varieties on March 25. The soluble protein content of the six varieties generally exhibited a decreasing trend, with a significant decrease observed from March 5 to March 20. The soluble protein content of the six varieties decreased from 36.45 mg.g−1, 44.86 mg.g−1, 51.43 mg.g−1, 54.82 mg.g−1, 54.21 mg.g−1, and 52.57 mg.g−1 on March 5 to 31.37 mg.g−1, 30.72 mg.g−1, 34.66 mg.g−1, 37.10 mg.g−1, 34.31 mg.g−1, and 31.75 mg.g−1 on March 20. ‘Yuzhui’, ‘84–237’, ‘Dawei’, ‘Ping’ou 110’, and ‘Ping’ou 210’ exhibited a significant decrease in soluble protein content on March 20 (p < 0.05). ‘Liao Zhen 3’ showed a smaller decrease in soluble protein content before and after bud sprouting.
As shown in Figure 1E, the peroxidase (POD) enzyme activity of ‘Dawei’ and ‘Ping’ou 210’ was significantly higher than the other varieties at different sampling dates. On March 25 and April 1, the POD enzyme activity of ‘Ping’ou 210’ was significantly higher than the other five varieties. ‘Dawei’ exhibited significantly higher POD enzyme activity than the other varieties on March 5 and April 13, reaching its maximum value of 14.52 U.min−1.g−1FW on March 5. The POD enzyme activity of the buds from all six varieties generally showed a decreasing trend. ‘Dawei’ exhibited a larger decrease with a significant reduction of 33.47% from March 5 to March 20. The POD enzyme activity briefly increased to 8.84 U.min−1.g−1FW from March 25 to April 13 but showed an overall decreasing trend.
As shown in Figure 1F, the catalase (CAT) enzyme activity of ‘Yuzhui’ and ‘84–237’ was significantly higher than the other varieties at different sampling dates. On March 5 and March 20, ‘Yuzhui’ exhibited significantly higher CAT enzyme activity than the other varieties, reaching its maximum value of 19.68 U.min−1.g−1FW on March 5. ‘Dawei’ showed significantly higher CAT enzyme activity than the other varieties on March 25, April 1, and April 13, reaching its maximum value of 18.50 U.min−1.g−1FW on March 25. The CAT enzyme activity of ‘Liao Zhen 3’, ‘84–237’, ‘Ping’ou 210’, and ‘Dawei’ generally exhibited a decreasing trend, while ‘Yuzhui’ and ‘Ping’ou 110’ showed an overall increasing trend. ‘Yuzhui’ exhibited a significant decrease of 51.73% on March 25 compared to March 5. ‘84–237’ showed a smaller decrease in CAT enzyme activity. ‘Dawei’ showed a fluctuating pattern of decrease–increase–decrease, but with an overall increasing trend and reaching its maximum value of 18.50 U.min−1.g−1FW on March 25. ‘Ping’ou 110’ showed a pattern of increase–decrease with an overall increasing trend, reaching its maximum value of 8.25 U.min−1.g−1FW on March 25.
3.2.3 Comparison of branch water content and relative conductivity before and after germination of different varieties of Ping’ou hybrid hazelnut
As shown in Figure 1G, the branch water content of ‘Dawei’ was significantly higher than the other varieties on March 5 and April 1. The branch water content of ‘Ping’ou 210’ was significantly higher than the other varieties on March 20, March 25, and April 13. ‘Liao Zhen 3’, ‘Yuzhui’, ‘84–237’, and ‘Ping’ou 210’ showed an overall decreasing trend in branch water content. ‘Dawei’ and ‘Ping’ou 110’ showed an overall increasing trend. The water content of ‘Liao Zhen 3’, ‘Yuzhui’, ‘84–237’, and ‘Ping’ou 210’ decreased from 36.74, 33.97, 30.75, and 31.29% on March 5 to 39.26, 41.04, 37.75, and 40.64% on April 13. ‘Dawei’ showed a pattern of decrease–increase–decrease, but with an overall decreasing trend, reaching its maximum value of 42.04% on April 1. ‘Ping’ou 110’ showed a pattern of increase–decrease with an overall increasing trend, reaching its maximum value of 24.30% on April 1.
As shown in Figure 1H, the relative conductivity of the six hazelnut tree branches showed an overall decreasing trend. The relative conductivity of the six varieties decreased by 30.60, 20.44, 18.73, 17.57, 12.36, and 1.44% from March 5 to April 13. On March 20, ‘Yuzhui’, ‘84–237’, ‘Dawei’, ‘Ping’ou 110’, ‘Ping’ou 210’, and ‘Liao Zhen 3’ exhibited a brief increase in relative conductivity. ‘84–237’, ‘Dawei’, ‘Ping’ou 110’, and ‘Ping’ou 210’ showed a significant increase in relative conductivity on April 1. The overall trend of relative conductivity was decreasing, indicating improved tolerance to adversity.
3.3 Survey of field drawdown rates of different varieties of Ping’ou hybrid hazelnut before and after germination
According to field observations of bud sprouting rates, the average sprouting rates of the six hybrid hazelnut varieties from weakest to strongest were ‘Dawei’, ‘Yuzhui’, ‘Liao Zhen 3’, ‘84–237’, ‘Ping’ou 210’, and ‘Ping’ou 110’. Based on field observations, ‘Dawei’ had the lowest sprouting rate, with a robust and tall tree, as well as a strong and thick root system (Table 2).
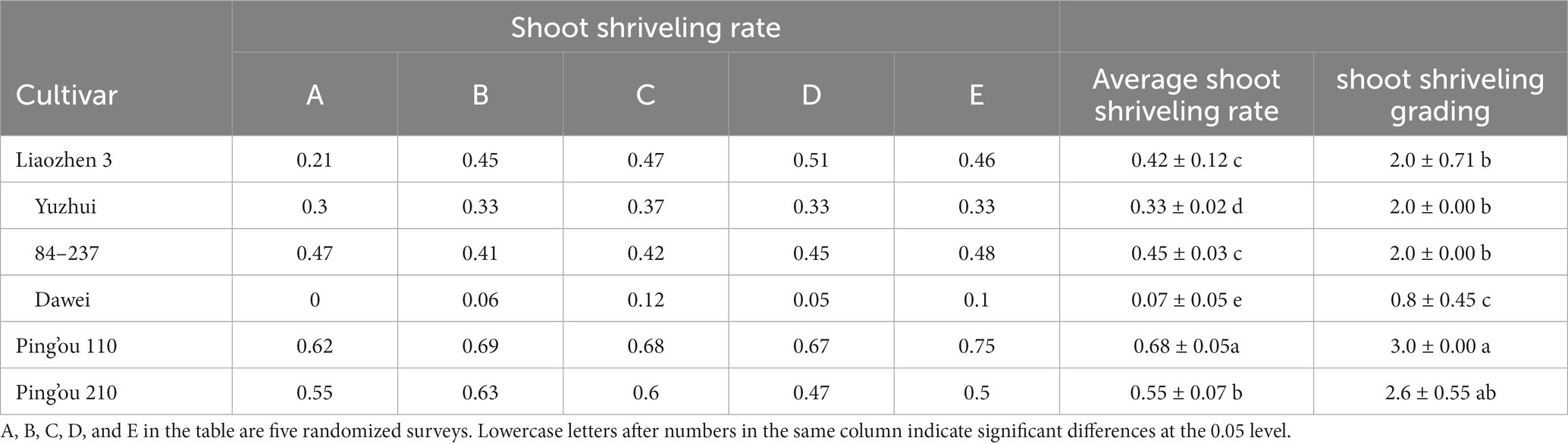
Table 2. Comparison of sampling rate of different varieties of Ping’ou hybrid hazelnut in field survey.
3.4 Correlation analysis and comprehensive comparison of physiological indicators of branches and buds before and after sprouting of different varieties of Ping’ou hybrid hazelnut
3.4.1 Correlation analysis
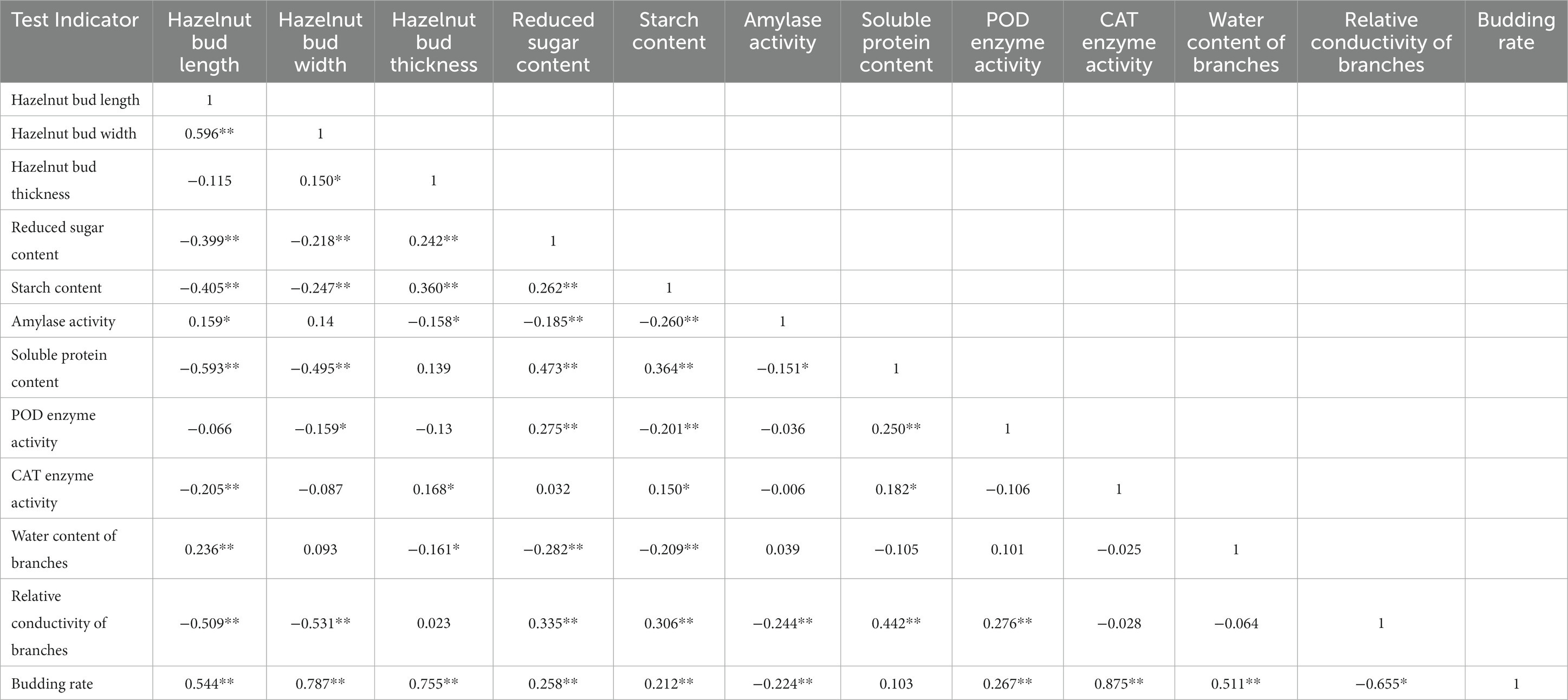
To further select the main evaluation indicators, a correlation analysis was conducted on these 12 indicators (see Table 3) in this study. The results showed that the germination rate of six hazelnut varieties was significantly positively correlated (p < 0.01) with the length, width, thickness, reducing sugar content, starch content, POD enzyme activity, CAT enzyme activity, and branch water content of the hazelnut buds. This indicates that the higher the length, width, thickness, and the higher the content of reducing sugar, starch, POD enzyme activity, CAT enzyme activity, and branch water, the higher the germination rate and the stronger the resistance to bud break of hazelnut trees. The germination rate of hazelnut trees showed a significant negative correlation with the amylase activity and relative conductivity of the branches, meaning that a lower amylase activity and relative conductivity of the branches are beneficial to hazelnut germination and stronger resistance to bud break. The germination rate of hazelnut trees was positively proportional to the soluble protein content, but the correlation was not significant. In conclusion, when hazelnut trees have higher water content, carbohydrate levels, and antioxidant enzyme activity, the germination rate and resistance to bud break are higher.
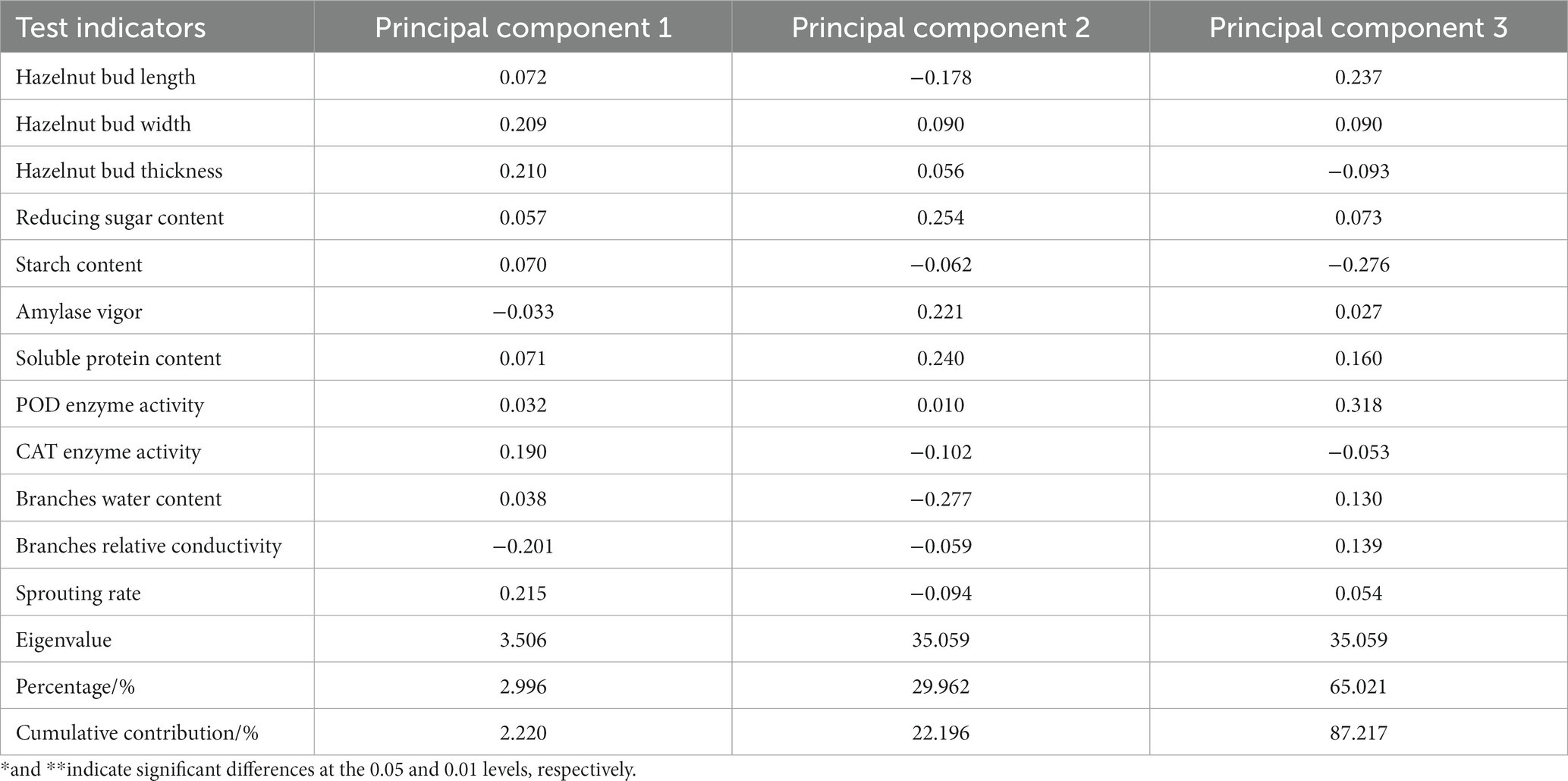
Table 3. Correlation analysis between indicators before and after sprouting of Ping’ou hybrid hazelnut.
3.4.2 Principal component and affiliation function analysis
Principal component analysis was performed on the 12 indicators (hazelnut bud length, width, thickness, reducing sugar content, starch content, amylase activity, soluble protein content, POD enzyme activity, CAT enzyme activity, branch water content, branch relative conductivity, and germination rate) during the germination period of the six hazelnut varieties. The loadings of the principal components were obtained (see Table 4). The variance contribution rate and cumulative contribution rate of the eigenvalues were calculated, and the number of principal components was determined based on a cumulative contribution rate ≥ 85%. Three principal components were obtained in this study. According to Table 4, hazelnut bud width, thickness, CAT enzyme activity, and germination rate had a positive influence on the first principal component, while branch relative conductivity had a negative influence. Reducing sugar content, amylase activity, and soluble protein content had a positive influence on the second principal component, while branch water content had a negative influence. The third principal component mainly represented hazelnut bud length, starch content, and POD enzyme activity, with starch content having a negative influence and hazelnut bud length and POD enzyme activity having a positive influence. The scores of the three principal components were denoted as X1, X2, and X3, and their corresponding membership function values μ1, μ2, and μ3 were calculated using the formula μ(Xi) = (Xi-Xmin)/(Xmax-Xmin). Finally, the comprehensive ranking was obtained by μcomprehensive = μ10.4086 + μ20.2985 + μ3*0.2928. After analyzing the changes in various indicators before and after germination in the six hazelnut varieties, it was found that a single indicator could not objectively evaluate the resistance to bud break of the tested hazelnut varieties. Therefore, based on the results of the principal component analysis and factor loading matrix, the fuzzy mathematical membership function method was used to analyze and evaluate the test data (see Table 5). The results showed that among the six hazelnut varieties tested, ‘Dawei’ had the strongest resistance to bud break, followed by ‘Pingou 110’, ‘84–237’, and ‘Pingou 210’. Based on the comprehensive analysis, the ability of shoot differentiation among the six hazelnut varieties from best to worst is as follows: ‘Da Wei’, ‘Ping Ou 110’, ‘84–237’, ‘Ping Ou 210’, ‘Yu Zhui’, and ‘Liao Zhen 3’.
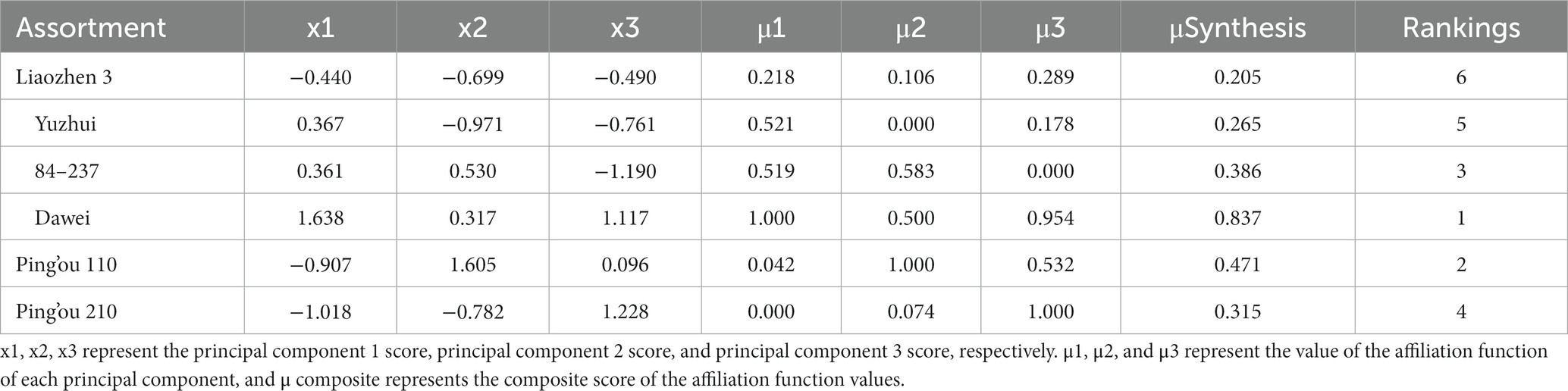
Table 5. Principal component scores and composite scores of affiliation functions for different varieties of hazelnut trees.
4 Discussion
4.1 Morphological comparison of buds of different varieties of Ping’ou hybrid hazelnut before and after sprouting
In this study, the length and width of hazelnut buds significantly increased with time before and after budburst, while the thickness of the buds showed a trend of initial increase followed by decrease. The changes in bud morphology may be related to the utilization of early nutrients. Zhao (2020) and Yuan (2021) observed the morphological changes during the bud differentiation period of apricot and walnut, and found that the length and width of apricot and walnut buds gradually increased during this period. Li (2023) discovered in a study on apple bud before and after budburst that there was vigorous nutrient activity during this period, such as synthesis of reducing sugars and starch. Combining previous studies, the increase in length and width of hazelnut buds in this stage is a characteristic of hazelnut development. At this stage, hazelnut begins bud differentiation, and metabolic activity is robust. Therefore, hazelnut needs to store sufficient nutrients, resulting in the increase in length and width of buds. However, the thickness of hazelnut buds showed a trend of initial increase followed by decrease before and after budburst, which may be related to the utilization of nutrients during this period. Zhao et al. (2015) and Sun et al. (2017) showed through phenological observations on apricot that bud differentiation is influenced by nutrient allocation within the tree. When the tree enters the late budburst stage and leaf expansion stage, nutrients are preferentially supplied for leaf development. Based on this, it can be inferred that the reason for the decrease in bud thickness after the peak budburst period is twofold: firstly, the nutrients before budburst are fully utilized and the contents decrease; secondly, during the late budburst stage, the tree enters the leaf expansion stage, and nutrients are preferentially supplied for leaf development, thus providing a good material basis for fruit development.
4.2 Comparison of carbohydrate content of buds of different varieties of Ping’ou hybrid hazelnut before and after germination
Among the six hybrid hazelnut varieties, ‘Dawei’ and ‘Ping Ou 110’ had significantly higher carbohydrate levels than other varieties. In this study, the reducing sugar and starch contents of hazelnut buds showed an initial increase followed by a decrease before and after budburst, while the α-amylase activity showed an overall trend of decrease followed by increase. On March 20th, the reducing sugar and starch contents of hazelnut buds in all six varieties increased significantly, while α-amylase activity decreased significantly. This is similar to previous research by Li (2023), Richardson et al. (2010), Wang et al. (2011), who studied the carbohydrate content of apple buds, kiwi buds, and peach buds before and after budburst, respectively, and found that the reducing sugar and starch contents showed an initial increase followed by a decrease. The temporary increase in reducing sugar and starch content is mainly due to the decomposition of sucrose and other non-reducing sugars in the buds at the early stage of budburst, part of which is converted into reducing sugars such as glucose, and part of which is synthesized into starch, thus laying the material foundation for the vigorous budburst period. At the same time, after entering the peak budburst period, the temperature increases and sap flow accelerates, leading to a decrease in reducing sugar and starch content. This is because the internal physiological metabolic activity of hazelnut buds rapidly increases at this time, requiring more abundant nutrients for growth and metabolism. Additionally, lower reducing sugar and starch contents may result in a higher osmotic potential within the bud, facilitating the continuous transport of nutrients to the developing buds. Therefore, the reducing sugar and starch contents show an initial increase followed by a decrease before and after budburst.
4.3 Comparison of antioxidant activity of buds of different varieties of Ping’ou hybrid hazelnut before and after germination and field investigation
The antioxidative enzyme activity of the ‘Dawei’ variety was significantly higher than that of other varieties on different sampling dates. The overall trend of soluble protein, POD enzyme activity, and CAT enzyme activity for the six varieties showed a decrease before and after hazel bud burst. This is consistent with previous studies by Zhao et al. (2018), Liu et al. (2022), and Guo et al. (2018), who found that under adverse conditions such as cold stress, the soluble protein content and antioxidative enzyme activity of apple, pear, and jujube increased, indicating that these three fruit trees enhance antioxidative enzyme activity and soluble protein content to resist stress. Therefore, when hazel trees are exposed to unfavorable environmental conditions such as low temperature and drought, the osmotic regulatory activity within the plant is disrupted, leading to physiological and metabolic disorders (Xiong et al., 2023). POD and CAT are important antioxidative enzymes in plants. When plants are subjected to abiotic stress, they increase the activity of their own antioxidative enzymes, enabling them to resist unfavorable growth environments and maintain normal physiological activities (Liu et al., 2019). Additionally, soluble proteins act as osmotic regulators, and when plants are in unfavorable growth environments, adjusting the content of soluble proteins helps them resist negative environmental interference. In conclusion, it can be inferred that the variations in soluble protein content, POD enzyme activity, and CAT enzyme activity of hazel buds of different varieties directly affect their cold resistance. On March 25th, the POD enzyme activity of the ‘Dawei’ variety decreased significantly by 65.56% compared to March 5th. Considering the morphological characteristics and carbohydrate comparison of hazel buds mentioned earlier, the reducing sugar content and starch content of ‘Dawei’ hazel buds reached their peak on March 20th, with a significant increase of 28.38% (p<0.05) and 64.76%, respectively, compared to the sampling on March 5th. The length, width, and thickness of ‘Dawei’ hazel buds were slightly larger than those of other varieties, suggesting that this variety has a larger storage capacity, allowing it to store more nutrients to promote growth and development. Through field surveys, the average shoot emergence rate of the six hazel varieties ranked from low to high as ‘Dawei’, ‘Yuzhui’, ‘Liao Hazelnut 3’, ‘84–237’, ‘Pingou 110’, ‘Pingou 210’. The ranking of shoot emergence rates in the field survey is consistent with the results obtained from physiological indicators, which indicate that ‘Dawei’ has strong resistance to shoot emergence.
4.4 Comprehensive analysis of physiological indicators of branches and buds before and after germination of different varieties of Ping’ou hybrid hazelnut
This study used 12 physiological indicators of hazel trees before and after bud burst to evaluate the resistance of the six hazel varieties to shoot emergence. Firstly, principal component analysis was used to transform multiple indicators into a few key indicators, and the load matrix of the principal components was used to express the influence of variables on the corresponding main components (Li et al., 2023; Wen et al., 2023). Then, the relative importance of each component was determined based on the contribution rate of each component, and finally, the resistance to shoot emergence of different hazel varieties was evaluated based on the values of their membership functions. The results of this study show that by using principal component analysis to simplify multiple measurement indicators, extracting the main component factors, and then using membership function analysis to comprehensively evaluate the resistance to shoot emergence of different hazel varieties, the evaluation method is scientifically reasonable, reduces the influence of subjective factors, and not only reflects the resistance to shoot emergence before and after hazel bud burst, but also provides theoretical guidance for the selection of hazel varieties with stronger resistance to shoot emergence internationally.
This experiment selected six widely cultivated hybrid hazelnut varieties to study the physiological changes of hazelnut buds before and after germination. The experiment first clarified the physiological changes of different hybrid hazelnut varieties before and after germination, and established the resistance to budding ability of hybrid hazelnuts through field investigations and comprehensive evaluations. Further research can be conducted on different sampling time points of hybrid hazelnuts for transcriptome sequencing. By combining phenotypic, physiological indicators, and gene expression, it is possible to discover the true cause of hazelnut resistance to bud germination.
5 Conclusion
This study aims to investigate the physiological changes in branches and buds of different hazelnut varieties before and after bud break and comprehensively evaluate the relationship between these changes and the resistance to shoot withering. The results indicate that the length and width of hazelnut buds gradually increase, while the thickness shows an increasing trend followed by a decrease after bud break. The carbohydrate levels of the ‘Dawei’, ‘Pingou 110’, and ‘84–237’ varieties are significantly higher than those of other varieties. The content of reducing sugars and starch in hazelnut buds of all six varieties initially increases and then decreases during bud break, while the activity of α-amylase shows a decreasing trend followed by an increasing trend. The antioxidant enzyme activity of the ‘Dawei’ variety is significantly higher than that of other varieties on different sampling dates. Additionally, the content of soluble protein, POD enzyme activity, and CAT enzyme activity gradually decrease in all six hazelnut varieties. The water content of the ‘Pingou 210’ branches during the peak bud break period is significantly higher than that of other varieties. The correlation analysis results show a highly significant positive correlation between bud germination rate and the length, width, thickness, content of reducing sugars, starch, POD enzyme activity, CAT enzyme activity, and branch water content. On the other hand, there is a significant negative correlation between bud germination rate and amylase activity and relative conductivity of branches. By integrating the results of correlation analysis, principal component analysis, and membership function analysis, the resistance to shoot withering among the six hazelnut varieties is ranked in the following order from high to low: ‘Dawei’, ‘Pingou 110’, ‘84–237’, ‘Pingou 210’, ‘Yuzhui’, and ‘Liao Zhen 3’. These findings lay a theoretical foundation for further studying the relationship between resistance to shoot withering and physiological changes in hazelnuts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. ZJ: Conceptualization, Resources, Visualization, Writing – review & editing. LZ: Investigation, Software, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
This research was funded by the Hebei Key Research and Development Program (18227502D), the Hebei Science and Technology Program (16236802D-8) and the Hebei North University School Research Project (2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1304271/full#supplementary-material
References
Cao, Y., Liang, S., Hou, D., Xi, H., Wang, W., Zhang, M., et al. (2021). Branch characteristics and cold resistance evaluation of six pinyon European hybrid hazelnut varieties during natural overwintering. North. Hortic. 24, 32–38.
Changsheng, Z. (2019). Analysis of the causes of the dryness of the branches of Ping’ou Corylus heterophylla and countermeasures. J. Jilin For. Sci. Technol. 2019:11.
Gao, J. F. (2006). Experimental guide to plant physiology. Beijing: Higher Education Press, pp. 210–218.
Guo, J., Feng, H., Shi, Y., and Yu, Y. (2018). Comprehensive evaluation of cold resistance in Ziziphus jujuba superior lines. Southwest China J. Agric. Sci. 10, 2060–2068. doi: 10.16213/j.cnki.scjas.2018.10.012
Hu, H., Meng, J., Yang, J., Liu, Y., Huang, J., Zhang, C., et al. (2021). Relationship between physiological changes in dormant buds and bud sprouting rate in different varieties of apple and pear. J. Tianjin Agric. Univ. 2, 39–42. doi: 10.19640/j.cnki.jtau.2021.02.008
Kang, F. Q. (2022). Current situation and protection of ancient trees in Zhangjiakou City. J. Hebei North Univ. 38, 56–57.
Kato, M., and Shimizu, S. (1987). Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent Peroxidative degradation. Can. J. Bot. 65, 729–735. doi: 10.1139/b87-097
Li, C. N. (2010). Study on the causes and control measures of shoot Shrivelling in hybrid hazelnut, Doctoral dissertation. China: Central South University of Forestry and Technology.
Li, Y. (2023). Changes in sugar and related enzyme activities in apple branch buds during the dormancy and Budbreak periods, Master’s thesis. Shaanxi: Northwest A&F University.
Li, C. N., Dong, F. X., Zhang, R. Q., Wang, G. X., and Liang, L. S. (2023, 2010). Research Progress in the study of shoot Shrivelling in fruit trees. Chin. Agric. Sci. Bull. 26, 138–141.
Li, K., Lin, Z., Liu, J., Liao, M., Yuan, H., Liang, Y., et al. (2023). Comprehensive evaluation of plum fruit quality based on principal component analysis and cluster analysis. Food Ind. Sci. Techn. 1–15. doi: 10.13386/j.issn1002-0306.2023060002
Liang, Y., Deng, C., Qin, S., Wei, G., Wei, K., Wei, F., et al. (2023). Expression and physiological response of StNCED1 in Acacia auriculiformis under drought and rehydration conditions. Molecu. Plant Breed. 1–15. Available at: http://kns.cnki.net/kcms/detail/46.1068.S.20230427.1331.004.html
Liu, C., Dong, L. A., Lin, J., and Liu, X. (2019). Research progress on reactive oxygen metabolism and regulation mechanism in plants under stress. Life Sci. Res. 3, 253–258. doi: 10.16605/j.cnki.1007-7847.2019.03.012
Liu, M., Wang, Q., Lu, M., Yan, X., Wu, C., Zhang, M., et al. (2022). Evaluation of cold resistance and study on leaf tissue anatomy structure of some pear varieties. Northeast. Agric. Sci. 5, 93–97. doi: 10.16423/j.cnki.1003-8701.2022.05.021
Liu, X., Yang, F., Li, Y., and Chen, Z. (2014). Research Progress on physiological differentiation of walnut flower buds. Hubei Agric. Sci. 21, 5065–5068. doi: 10.14088/j.cnki.issn0439-8114.2014.21.003
Luo, R., Wu, Z., Song, F., and Shi, Y. (2023). Characteristics of ion flux in roots of pinyon European hybrid hazelnut seedlings under salt stress and their response to ion transport inhibitors. Acta Physiol. Plant. 45, 889–898. doi: 10.13592/j.cnki.ppj.100456
Ma, W. C. (2020). The effects of exogenous glucose on apple flower bud differentiation and functional analysis of candidate gene MdSC35_2 (Master’s thesis, Northwest A&F University). Available at: https://link.cnki.net/doi/10.27409/d.cnki.gxbnu.2020.001295
Richardson, A. C., Walton, E. F., Meekings, J. S., and Boldingh, H. L. (2010). Carbohydrate changes in kiwifruit buds during the onset and release from dormancy. Sci. Hortic. 124, 463–468. doi: 10.1016/j.scienta.2010.02.010
Sun, J., Liao, K., Dong, S., Zhang, S., Qi, Y., Yang, W., et al. (2017). Flower bud morphological differentiation of ‘Lajiao apricot’. Econ. For. Res. 2, 188–193. doi: 10.14067/j.cnki.1003-8981.2017.02.032
Wang, X. K. (2006). Principles and techniques of plant physiology and biochemistry experiments. Beijing: Higher Education Press.
Wang, G. X. (2018). Research on breeding and utilization of Corylus species plants in China (part II) - morphological development, physiology, and molecular biology research. For. Sci. Res. 31, 113–121. doi: 10.13275/j.cnki.lykxyj.2018.01.014
Wang, F. (2022). Preliminary study on the correlation between meteorological factors and shoot Shrivelling index of hybrid hazelnut, Doctoral dissertation. China: Shanxi Agricultural University.
Wang, H., Li, L., Tan, Y., Li, D., Tan, Q., Chen, X., et al. (2011). Changes in carbohydrate metabolism and expression of related genes during dormancy of peach flower buds. Acta Physiol. Plant. 6, 595–600. doi: 10.13592/j.cnki.ppj.2011.06.004
Wang, W. L., Wang, Z., and Wang, J. Y. (2010). Optimization of plant peroxidase activity determination method. Lab. Res. Explor. 29, 21–23.
Wen, J., Cao, W., Wang, Y., He, Y., Sun, Y., Yuan, P, et al. (2023). Comprehensive evaluation of fruit quality of kiwifruit based on principal component analysis and cluster analysis. Food Industry Sci. Techn. 1–16. doi: 10.13386/j.issn1002-0306.2023020245
Xie, X. Y. (2016). Changes in nutrient content in walnut female bud during physiological differentiation. Hubei Agric. Sci. 55:4. doi: 10.14088/j.cnki.issn0439-8114.2016.19.037
Xiong, L., Feng, G., Liu, D., Liu, C., Yan, Z., Yang, X., et al. (2023). Effects of exogenous CaCl2 and Vc on seed germination and physiological activity of Phaseolus vulgaris under drought stress. J. Heilongjiang Univ. Eng. 1, 106–112. doi: 10.13524/j.issn2095-008x.2023.01.015
Xu, C., Chen, W., Chen, K., and Zhang, S. L. (1998). A simple method for determining starch content - the iodine staining method. Biotech. 2, 41–43. doi: 10.16519/j.cnki.1004-311x.1998.02.012
Xue, J. H. (2015). Physiological mechanism of resistance to shoot Shrivelling in Ping’ou Corylus heterophylla elite strains, Master’s thesis. China: Shanxi Agricultural University.
Yuan, P. (2021). Genetic characteristics of Tibetan walnut progeny populations and genome-wide association analysis of four traits, Doctoral dissertation. China: Huazhong Agricultural University.
Zhang, Q. (2013). Preliminary study on the mechanism of shoot Shrivelling in Ping’ou Corylus heterophylla, Master’s thesis. China: Shanxi Agricultural University.
Zhang, L. (2015). Physiological mechanisms of salt-alkali tolerance in pinyon European hybrid hazelnut and evaluation of salt tolerance. Doctoral dissertation, Beijing: Chinese Academy of Forestry.
Zhang, X., Tian, H., Liu, C. Y., and You, J. (2020). Determination of α-amylase activity by visible spectrophotometry. Chem. Biolog. Eng. 3, 65–68.
Zhao, T. (2020). Morphological observation of flower bud differentiation and transcriptomic analysis of pistil abortion in ‘Li Guang apricot’ from Dunhuang, Master’s thesis. China: Gansu Agricultural University.
Zhao, S., Liao, K., Liu, J., Peng, X., Dong, S., Du, R., et al. (2015). Study on winter phenological period and flower bud differentiation characteristics of late-maturing apricot varieties. J. Xinjiang Agric. Univ. 1, 31–35.
Keywords: Ping’ou hybrid hazelnut, germinating buds, shoot shriveling, physiological changes, comprehensive assessment
Citation: Gao H, Jia Z, Zhang L and Li Z (2024) Comprehensive evaluation of the resistance to shoot shriveling before and after sprouting in different varieties of Ping’ou hybrid hazelnut. Front. For. Glob. Change. 6:1304271. doi: 10.3389/ffgc.2023.1304271
Edited by:
Pedro Giovâni Da Silva, Federal University of Minas Gerais, BrazilReviewed by:
Shashikant Patil, Atlas SkillTech University, IndiaTiantian Zhao, Chinese Academy of Forestry (CAF), China
Copyright © 2024 Gao, Jia, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Li, bmt4end6bEAxNjMuY29t
 Haizhe Gao
Haizhe Gao Zhiguo Jia1
Zhiguo Jia1 Lirong Zhang
Lirong Zhang