- 1Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 2School of Biosciences, University of Nottingham, Loughborough, United Kingdom
- 3School of Science, Monash University Malaysia, Subang Jaya, Malaysia
- 4Sabah Forestry Department, Forest Research Centre, Sandakan, Malaysia
Despite the diverse ecosystem services that forested stream margins (“riparian buffer strips”) can provide in agricultural landscapes, understanding of their biodiversity impacts in the tropics is lacking. Stream invertebrates support many ecosystem functions and several groups are valuable bioindicators of environmental conditions. Semi-aquatic bugs (insects in Hemiptera that inhabit the water surface) are important within the aquatic food chain, acting as predators of other invertebrates and prey for larger animals. Since they inhabit the water surface, semi-aquatic bugs are potentially valuable indicators of within-stream health. Focusing on the impacts of conditions at the small-scale, we investigated how within-stream physical structure and the presence of riparian buffer strips affected the abundance, total biomass, richness, and community composition of semi-aquatic bugs in oil palm plantations in Sabah, Malaysia. We also assessed the effects on the proportion of juveniles and females of Ptilomera sp. (a common genus). Our focus on the small-scale make findings applicable for management both within smallholder and large-scale plantations. At the small-scale (10-m transect), oil palms streams with riparian buffers contained twice as many semi-aquatic bugs as those without (average richness in streams with buffers 3.55 (SE ± 0.42) compared to 1.40 (SE ± 0.22) in streams without). We found a total of 14 morphospecies in streams with buffers, compared to just seven in those without. There was no difference in total biomass or the proportion of female Ptilomera sp. in streams with or without buffers. There was a significantly higher abundance of semi-aquatic bugs in streams with wider wetted width, more isolated pools, shallower slopes, and lower percentage of deadwood. The proportion of juveniles was higher in streams with higher canopy openness, higher percentage of deadwood, lower percentage of pebbles, and narrower wetted widths. This study demonstrates that small-scale differences in stream conditions within oil palm can influence semi-aquatic bugs, opening up the possibility that oil palm management could be tailored to improve environmental conditions for stream communities. As our findings are based on only a few streams and at measurements collected at a single time-point, more studies are needed to validate what we have found.
Introduction
Globally, over 100,000 species (of plants as well as invertebrates and vertebrates) rely on freshwater systems for their habitat, but many of these systems have been degraded by land-use change, flow modification, pollution, invasive species, overharvesting, and management for hydropower (Carpenter et al., 2011; Cazzolla Gatti, 2016; Reid et al., 2019). The extinction or decline of freshwater species in modified systems is often driven by a change in physical and chemical environmental conditions within and around freshwater systems, as well as the loss or reduction of resources (Reid et al., 2019; Tanaka et al., 2021). In terms of land-use change, forest conversion causes erosion, sedimentation, and increased inputs of pollution into waterways (Dudgeon et al., 2006). These increased inputs also change the characteristics of waterways. For instance, an increased amount of sediment deposited at the bottom of streams can reduce the flow rate and alter aspects of the stream physical structure, such as reducing channel width and depth (Luke et al., 2017a). In addition, conversion often results in the loss of natural habitat margins surrounding freshwater systems, resulting in a reduction in canopy cover, which reduces litter inputs and increases water temperature, as well as increasing erosion and nutrient enrichment (Wantzen and Mol, 2013; Cole et al., 2020; Tanaka et al., 2021).
Whilst it is estimated that 83% of global freshwater biota populations have declined between 1970 and 2014, little information is available within the tropics, particularly about how species respond to each type of threat (WWF, 2018; Reid et al., 2019; Sundar et al., 2020). Environmental impacts in the tropics are expected to be more severe than in other regions (Dudgeon et al., 2006), since they house higher levels of biodiversity (Myers et al., 2000) and experience higher precipitation – hence more flash flood events and higher erosion (Tanaka et al., 2021). Therefore, more investigation is needed to improve evidence-based conservation policy and management for tropical freshwater ecosystems. Given the extensive scale of global tropical agricultural expansion, including in Southeast Asia (Halpern et al., 2022), studies addressing how land-use change affects tropical freshwater species and how conservation strategies can mitigate these impacts, are needed urgently, particularly at a scale at which land-managers can influence conditions.
Several management approaches have been proposed that could reduce impacts of habitat change on streams. These include conservation easement (leaving private land unexploited), retaining natural margins around streams (or “riparian buffer strips”), returning crop residue to the soil (reducing erosion by covering the soil surface with crop residue), reducing tillage in the surrounding catchment, and planting grass or vegetation strips around waterways and ditches. These strategies all aim to reduce erosion and sedimentation, or limit inputs of nutrients and pollutants into waterways (Blanco-Canqui et al., 2004; Cooper et al., 2004; Farmer et al., 2015; Luke et al., 2019; Du et al., 2022). Several studies have assessed the impacts of retaining riparian buffer strips on a range of freshwater species, including fishes and several groups of invertebrates, and have recorded benefits in terms of both abundance and diversity (e.g., Pusey and Arthington, 2003; Arnaiz et al., 2011; Ceneviva-Bastos and Casatti, 2014; Luke et al., 2017b).
Across tropical agricultural crops, oil palm is one of the main drivers of biodiversity loss (Sodhi et al., 2004; Foster et al., 2011), including within freshwater communities (e.g., Mercer et al., 2014; Giam et al., 2015; Luiza-Andrade et al., 2017; Luke et al., 2017b; Carvalho et al., 2018; Chellaiah and Yule, 2018). As a platform to improve the sustainability of palm oil production, the RSPO (the Roundtable on Sustainable Palm Oil) provides guidelines for less environmentally damaging production techniques, including for maintaining and managing riparian buffers strips. This includes recommendations on the width of buffer to be maintained and how to design riparian buffer strips within oil palm plantations, considering the type of soil, stream characteristics, and age of the plantation (Barclay et al., 2017). Retaining riparian buffer strips in oil palm in Malaysian Borneo has been shown to support higher species richness and abundance of adult dragonflies (Luke et al., 2017b), as well as altering the composition of benthic macroinvertebrate communities (Chellaiah and Yule, 2018). However, this strategy does not protect the diversity and abundance of larval dragonflies (Luke et al., 2017b) or other sensitive groups [such as larvae of aquatic insects, dung beetles, and large mammals (Deere et al., 2022)]. Furthermore, beyond these few studies, research on the effects of riparian buffer strips on freshwater communities in the tropics, including Southeast Asia (Luke et al., 2019), has been limited. Considering the large total area of oil palm in Southeast Asia (Pendrill et al., 2022), studies based in this region will be particularly useful for informing biodiversity conservation strategies, including platforms such as the RSPO, and assessing whether the benefits of maintaining buffer strips apply to a wide range of taxa in the region.
Invertebrates play critical functions in freshwater systems by acting as decomposers, herbivores, predators, or prey items (Bay, 1974; Malmqvist, 2002). Semi-aquatic bugs (Gerromorpha, Hemiptera) are predator-scavengers as well as being preyed upon by other animals (Foster and Treherne, 1981; Spence and Andersen, 1994). All stages within the lifecycle of semi-aquatic bugs occur on or in the water (Spence and Andersen, 1994), making their survival highly dependent on within-stream conditions. Within each population, there can be polymorphism in terms of the presence or absence of wings, and the form that they take. The proportion of winged and wingless adults can be related to the stability (Andersen, 2000) and quality of the habitat (Cunha et al., 2020), as well as whether it is the breeding season – although in the tropics, semi-aquatic bugs generally breed throughout the year (Andersen, 2000). For instance, a study by Ditrich et al. (2008) on semi-aquatic bug communities in spring areas in the Czech Republic found that, unlike in permanent pools and streams, temporary systems were dominated by winged individuals. As such, the quantification of winged forms of semi-aquatic bugs could potentially provide a valuable source of information about stream conditions in disturbed habitat sites. Consideration of ratio of juveniles to adults, and males to females within populations may also give valuable insights into stream and community conditions. A laboratory study found that the growth of mayflies (species investigated: Eurylophella prudentalis and Eurylophella macdunnough) was affected significantly by water temperature, with either an increase or decrease of five degrees Celsius causing higher mortality or a reduction in the hatch success of eggs (Sweeney, 1993). Consequently, this could reduce the number of juveniles and affect the proportion of juveniles to adults, if an increasing proportion of individuals cannot develop into the adult stage. Additionally, since female insects sometimes require more nutrition than males (Teder and Kaasik, 2023), a reduction in food resources, such as when the loss of riparian buffer strips causes a reduction in food inputs from the surrounding area, could reduce the proportion of females to males and the reproductive success of the population.
Studies have reported shifts in species composition of semi-aquatic bugs between streams with and without forested riparian margins in Brazilian savanna streams (Dias-Silva et al., 2020). Furthermore, richness of semi-aquatic bugs has been found to be lower in oil palm streams than in forest streams in Amazonia (Cunha et al., 2015), highlighting the impact of land-use change on semi-aquatic bugs. Another study found a higher number of winged individuals in forests than in oil palm in the Amazon (Cunha et al., 2020). However, no studies have yet assessed the impacts of maintaining riparian buffer strips on this group in Southeast Asia. In particular, studies seeking to understand how conservation strategies affect streams at a small scale within rivers could provide relevant information regarding the impacts of microhabitats on this group and could increase understanding of how microhabitat conditions affect demographic factors, such as reproduction and sex ratios, that influence population growth in semi-aquatic bugs. Work at the within-stream scale may be particularly relevant for conservation management and for informing sustainability guidelines, as this is the scale at which individual industrial or smallholder plantation managers can operate (Maddock, 1999).
In this study, we investigated the impacts of within-stream physical structure and maintaining riparian buffer strips on semi-aquatic bugs in oil palm streams in Sabah, Malaysia. We also assessed impacts on the demographic factors of the most widespread and easily sexed taxon in the study: Ptilomera sp. We asked the following questions:
1) What is the variability in within-stream physical structure across oil palm streams, and do conditions differ between streams with and without riparian buffer strips? We hypothesized that riparian areas with buffer strips would have different environmental conditions than those without, with streams without riparian buffer strips having a higher percentage of canopy openness, a lower percentage deadwood, as well as narrower wetted width, lower flow speed, and more homogenous flow regimes (due to a higher level of runoff and sediment deposition).
2) What is the impact of within-stream physical structure and the presence of riparian buffer strips on the richness, abundance, total biomass, and composition of semi-aquatic bugs in oil palm? Since semi-aquatic bugs live on the surface of the water and are therefore likely to be affected by the environmental conditions in and around streams, we hypothesized that oil palm streams without forest margins would have lower species richness, abundance, and total biomass of semi-aquatic bugs, compared to streams with forested river margins. As some species are likely to be more resilient to disturbed conditions, we also hypothesized that the absence of riparian buffers, and altered environmental conditions, would result in an altered community composition, with a higher abundance of disturbance-tolerant species.
3) What is the impact of within-stream physical structure and the presence of riparian buffer strips on the proportion of juvenile semi-aquatic bugs, proportion of winged and wingless adult individuals, as well as the proportion of female Ptilomera sp.? As different sexes, presence of wings in adults, and juvenile and adult semi-aquatic bugs may be affected by environmental conditions to different extents, we predict significantly altered proportions between stream types, with streams without riparian buffers having reduced numbers of females, higher numbers of wingless adults, and reduced numbers of juveniles.
Materials and methods
Stream sites
Streams within oil palm plantations were surveyed in July – September 2011 and in May – August 2012 in Sabah, Malaysia, where there is little seasonality throughout the year (tropical rainforest climate with average annual rainfall of 2,455 mm in the study sites) (Luke et al., 2017a). All the streams were natural channels present in established oil palm plantations (with drainage channels present in the surrounding area), either had or did not have forested riparian buffer strips, and were located within the SAFE (Stability of Altered Forest Ecosystems) Project study system, which was near the Kalabakan Forest Reserve, 116°570E to 117°420E, 4°380 N to 4°460 N (Ewers et al., 2011) (Figure 1). Oil palm sites with forested riparian buffers strips (OPB) were in Gaharu, Keruing, and Merbau estates (managed by Benta Wawasan). The width of the buffer strips differed between streams, with average widths of approximately 331 m (ranging from 75 m to 1,111 m), 68 m (ranging from 33 m to 173 m), and 26 m (ranging from to 2 to 57 m) in Gaharu, Keruing, and Merbau respectively, and buffers being continuous at all streams, but with plantation roads likely to cross them (Luke, 2016; Luke et al., 2017b; Supplementary Table 1). Exact buffer widths varied across the length of each stream (Luke, 2016; see Supplementary Table 1) and measurements were made on both sides of the stream at approximately 25 m intervals along a 500 m stretch to assess this (Luke, 2016). For the stream at Gaharu, measurements of buffer widths were conducted using a map of the plantation, while for the streams at Keruing and Merbau, measurements were conducted using GPS tracks that were walked in the field. Streams without forested riparian buffer strips (OP) were in Binuang and Selangan Batu estates, where oil palms were planted up to the margins of streams. Within oil palm sites without forested buffer strips, oil palms were managed and harvested as normal up to the stream edge.
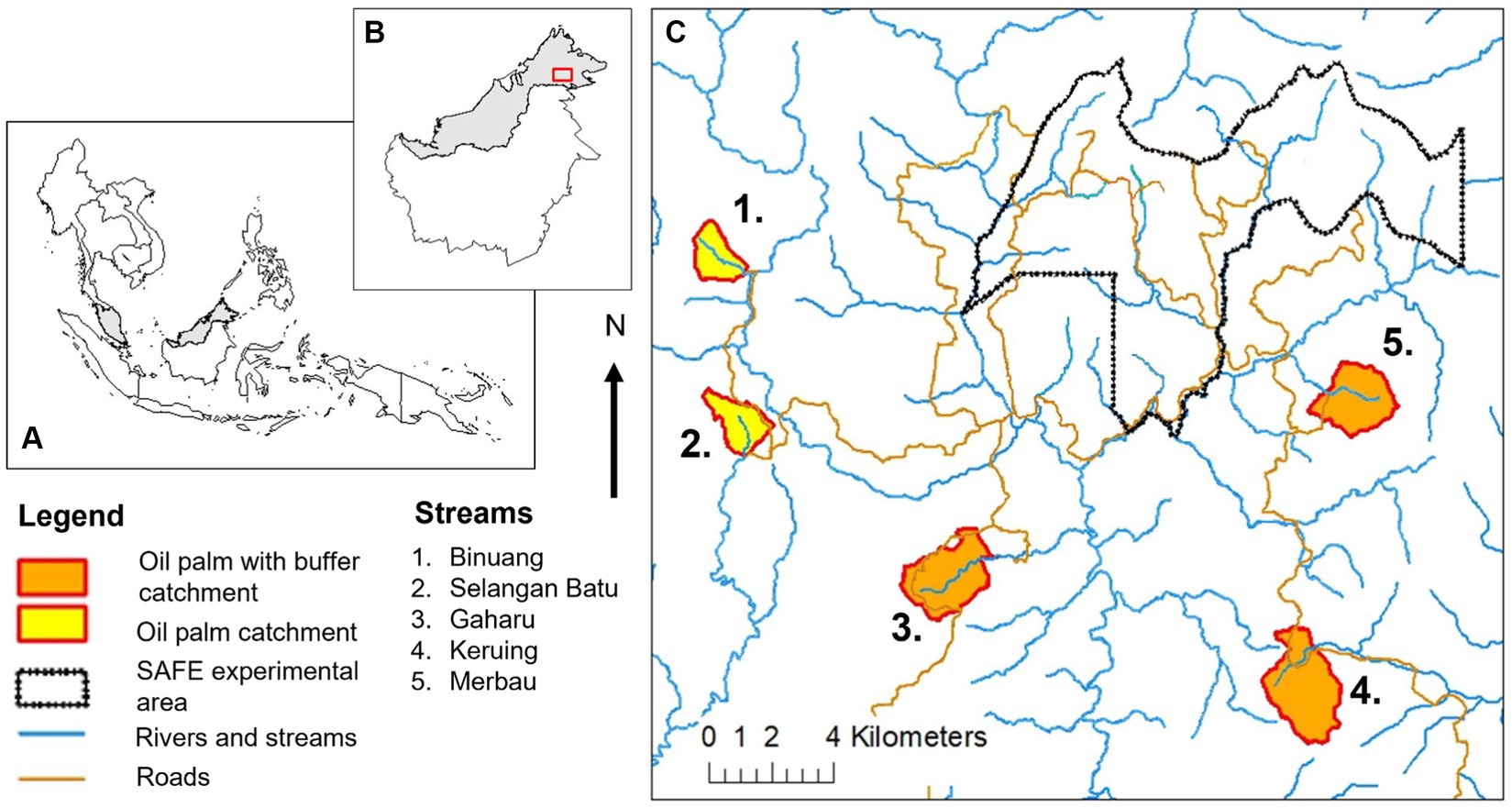
Figure 1. Map of streams in oil palm with and without riparian buffer strips in the SAFE (Stability of Altered Forest Ecosystems) Project sites in Sabah, Malaysian Borneo. The SAFE Project sites consist of streams within forest areas, an experimental area (“SAFE experimental area”) nearby the Kalabakan Forest Reserve, as well as oil palm streams with and without riparian buffer strips. See Luke et al., (2017a,b) for more details.
All streams originated within oil palm catchments (see Supplementary Table 1 for information on the sizes of catchments surrounding stream sites, average slopes, as well as channel and wetted widths of the streams), with surrounding oil palms being planted between 1999 and 2009 (see Supplementary Table 1 for the exact year of each plantation), and were therefore between two and thirteen years old at the time of this study (Luke et al., 2017b). At each stream, data collection was standardized to be conducted from approximately 2 km downstream from the stream source. This was to ensure catchment areas across streams were comparable, and this point is termed as the “0 m point” throughout this manuscript.
Data collection on within-stream physical structure
Stream environmental data at each site were collected once in May – August 2012 along a 200 m transect from the “0 m point” and going upstream. Data were collected during non-flood conditions. Stream physical structure was recorded at points at 10-m intervals along the transect and consisted of: flow speed (time needed for a tennis ball to travel along a 2 m string at the fastest flowing point along each transect (at rapids or riffles), repeated three times and then averaged), canopy openness (measured using a spherical densiometer (Lemmon, 1956) in the middle of each stream facing upstream, downstream, and to the left and right sides of the stream; average calculated), and wetted width of the stream, measured using a tape measure. Due to logistical constraints in the field, it was not possible to measure specific buffer widths at each sample point. However, as riparian width is likely to affect within-stream conditions downstream of the buffer location, rather than at the location itself, measurement at this scale is unlikely to reflect conditions at the sample locations themselves.
The physical structure of the whole river channel was also measured by recording percentage cover of rocks, pebbles, sand, dead-wood, rapids, riffles, connected pools, and isolated pools between successive pairs of 10-m points, and slope at each point using a clinometer. Deadwood were large chunks of wood from trees (tree trunks or portions of them, or very large branches), with coverage of >5% over the 10-m intervals. Pools, riffles, and rapids were assessed according to water speed (pools = still water, riffles = flowing water with a rippled surface, and rapids = fast-flowing white water). The average of each variable was calculated for subsequent analyses. We acknowledge that because we measured within-stream conditions at one time-period only, we are unlikely to have captured the full range of conditions experienced by bugs at each location, although our measurements do reflect conditions at the time of sampling. We also acknowledge that water chemistry and condition can be an important driver for semi-aquatic bugs, but we did not collect data on this at the scale of the 10-m transect, so we did not include this aspect of environmental conditions in our analyses. However, another study at a larger scale conducted at the same sites showed that, out of three parameters of water chemistry and condition that were recorded (water temperature, pH, and conductivity), streams with and without riparian buffer strips differed significantly only in terms of water temperature (Supplementary Table 2). Water temperature is associated with canopy openness (which we measured), so it is likely that this aspect of water condition can be implied from our canopy openness data.
Semi-aquatic bug collection and processing
Semi-aquatic bugs were collected once from five or ten 10-m sub-transects along the 200 m transects in July – September 2011 or June – August 2012, using hand-held nets (following Ditrich et al., 2008), with mesh size of 1 mm or less (hereafter, termed as “10-m transect”) (Supplementary Table 3). The bugs were collected from the same starting points as the measurements of stream environmental variables (the “0 m point”). After collection, all the bugs were preserved in 70% ethanol. Juveniles were identified to family level, while adults were identified to morphospecies level, using relevant identification guides and taxonomic papers (Andersen, 1982; Polhemus and Polhemus, 1988; Chen and Nieser, 1992, 1993a,b; Chen and Zettel, 1998). During the identification process, advice from taxonomic experts was also obtained (see Acknowledgements for details). Although morphospecies were split as accurately as possible, it is possible that morphospecies in this study do not align perfectly with true species identities.
In addition to identification, the body length of all individuals was measured to allow biomass calculations. Body length measurement was done to the nearest 1 mm using graph paper. Biomass was then calculated using power regression equations, following Harianja et al. (2023a) (derived from semi-aquatic bugs from the same study area). Family and body form of the bugs can influence the length-biomass relationship, thus we used three equations for the biomass calculations: y = 0.040x2.271 for Cylindrostethinae, Gerrinae, and Ptilomerinae; y = 0.072x2.218 for Halobatinae; and y = 0.041x2.320 for Veliidae, with y = the biomass and x = the body length of a bug (see Harianja et al., 2023a for further details). Total biomass was obtained by summing all individual biomass calculations of semi-aquatic bugs from each of the 10-m transects, and this total was used for subsequent analyses.
For assessment of the demographic structure of semi-aquatic bugs, we grouped adults and juveniles separately. The grouping was based on tarsal segments: juveniles have only one tarsal segment, while adults have at least two. For one morphospecies, Ptilomera sp., which has clear sexual dimorphism, we further grouped individuals into males and females: it was not feasible to distinguish sex reliably in other species. For each adult individual, we also recorded whether they were winged or wingless. However, because of the low number of winged adult individuals, we did not conduct further analysis on this aspect of the data.
Statistical analysis
We carried out analyses and visualization using R version 4.0.4 (R Core Team, 2021) and R Studio version 2022.07.1 + 554 (R Studio Team, 2022). For analysis, basic R syntax and package “dplyr” were used (Wickham et al., 2021). We used “plotrix” (Lemon, 2006) to calculate standard errors and “car” to run Levene’s Test for equality of variances (Fox and Weisberg, 2019). To check for association between the presence or absence of riparian buffer strips and each of the stream environmental variables, we used “ltm” package (Rizopoulos, 2006). To run Dunn tests to assess significant differences in species richness among streams with riparian buffer strips, we used the “FSA” package (Ogle et al., 2023).
To summarize and visualize stream environmental variables, we used a Principal Component Analysis (PCA), run through built-in codes in R and “factoextra” (Kassambara and Mundt, 2020), respectively. For analyses on impacts of stream physical structure and the presence or absence of riparian buffer strips on semi-aquatic bugs, we used “vegan” (Oksanen et al., 2020) to run Canonical Correspondence Analysis (CCA), and “lme4” (Bates et al., 2015) to run Linear Mixed-Effect Models (LMMs) and Generalised Linear Mixed-Effect Models (GLMMs). We used the following packages for all other visualizations in this study: “tidyverse” (Wickham et al., 2019), “cowplot” (Wilke, 2020), and “gridExtra” (Auguie, 2017).
Within-stream physical structure
To reduce dimensionality and summarize the environmental variables measured across all oil palm stream sites, we used PCA (Jolliffe, 1986), with data from each 10-m transect included as a separate data point. We did not transform any variables used in the analyses, and all variables were standardized first to account for differing units (using correlation matrix) (Jolliffe, 1986). In R, standardization was conducted using the following function: “scaling = TRUE.” Sand was excluded from the PCA because its value was implied from the total percentages of other measured percentage cover stream variables. Since the stream environmental variables could be correlated with the presence or absence of riparian buffer strips, we assessed whether buffer strip presence was associated with environmental variables. For this, we ran a biserial correlation test and found that there was a weak to moderate correlation, suggesting that additional information was included in the environmental data and presence/absence of buffer information (Supplementary Table 4). Therefore, for subsequent analyses, we included the presence of riparian buffer strips as a separate factor (hereafter “Riparian”). Finally, to assess if the stream physical structure differed between oil palm with and without riparian buffer strips, we ran separate Mann–Whitney U tests for the most influential stream Principal Component (PC) scores (i.e., PC scores 1–4 [“StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4”]), obtained from PCA between the two types of streams.
Impacts of within-stream physical structure and retaining riparian buffer strips on semi-aquatic bugs
To assess the impacts of measured stream environmental conditions as well as the presence or absence of riparian buffer strips on semi-aquatic bugs (abundance, total biomass, and richness), we ran LMMs or GLMMs, with each data point representing a 10-m transect site. For all analyses, we considered four PC scores on stream environmental variables (“StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4,” obtained from the PCA) as separate predictors, because PC scores explained similar levels of variation among stream environmental variables. In all models, stream identity was included as a random factor to take into account the non-independence of points within each stream (Zuur et al., 2009). We included stream as a random rather than a fixed effect, as we did not aim to specifically test the effect of stream identity on our dependent variables, but rather wanted to take into account the impact of non-independence of sampling points in our analyses. Rather than composite biodiversity indices, we chose to focus on simple measures of our community, as these allow assessments of specific impacts of environmental conditions (Barrantes and Sandoval, 2009).
For abundance, we ran negative binomial models due to overdispersion. For total biomass and richness, we ran LMM and GLMM with gaussian and Poisson distribution, respectively, according to the type of data (biomass and count). To assess the importance of each predictor (“StreamPC1”/ “StreamPC2”/ “StreamPC3”/ “StreamPC4”/ “Riparian” [“Riparian” indicates the presence/absence of riparian buffer strips]), we ran log-likelihood ratio tests. In particular, we used separate ANOVA tests to compare the full model (consisting of all predictors) with a model in which one of the predictors was dropped. To assess the effects on community composition, we ran CCA with 999 random permutation tests using stream environmental variables and buffer strip presence along with community data of adult bugs. To assess the impact of stream physical structure and retaining riparian buffer strips on the proportion of juveniles as well as the proportion of female Ptilomera sp., we ran GLMM with binomial family. Similarly, for assessing the importance of each predictor on the proportion of juveniles and female Ptilomera sp., we ran log-likelihood ratio tests. Since there were very few winged individuals found in this study, we carried out no formal analysis on these data, but present totals in the results.
Results
Variability and differences in within-stream physical structure between oil palm streams with and without riparian buffer strips
At the stream scale, PCA scores were spread evenly between axes 1, 2, 3, and 4 (“StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4,” respectively), with StreamPC1 and StreamPC2 explaining 24.5 and 18% of the variation in environmental variables measured in all stream sites, while “StreamPC3” and “StreamPC4” explained 15.3 and 11.7%, respectively, (Figure 2). In general, this means that multiple uncorrelated variables explained environmental differences between streams. “StreamPC1” scores were correlated with a higher percentage cover of rocks (0.387), rapids (0.366), and riffles (0.306), a steeper slope (0.374), and more rapid flow speed (−0.333, negative sign indicates that less time was needed for a tennis ball to travel the 2 m string), and with lower percentage cover of connected pools (−0.471) (Table 1). “StreamPC2” scores were associated with wider wetted width (0.464) and higher percentage of pebbles (0.417), and with lower percentage of canopy openness (−0.531) and deadwood (−0.332) (Table 1). “StreamPC3” scores were associated with a steeper slope (0.312), a higher percentage of rocks (0.475), pebbles (0.372) and isolated pools (0.359), and with lower percentage of riffles (−0.538) (Table 1). Finally, “StreamPC4” scores were correlated with a higher percentage of deadwood (0.427) and steeper slope (0.342), and with a lower percentage of isolated pools (−0.566) and narrower wetted width (−0.445) (Table 1). Streams with and without riparian buffer strips only differed significantly in “StreamPC4” scores (Table 2), with streams without riparian buffer strips having a higher percentage of deadwood but lower percentage of isolated pools, steeper slopes, and lower wetted widths. (Table 2).
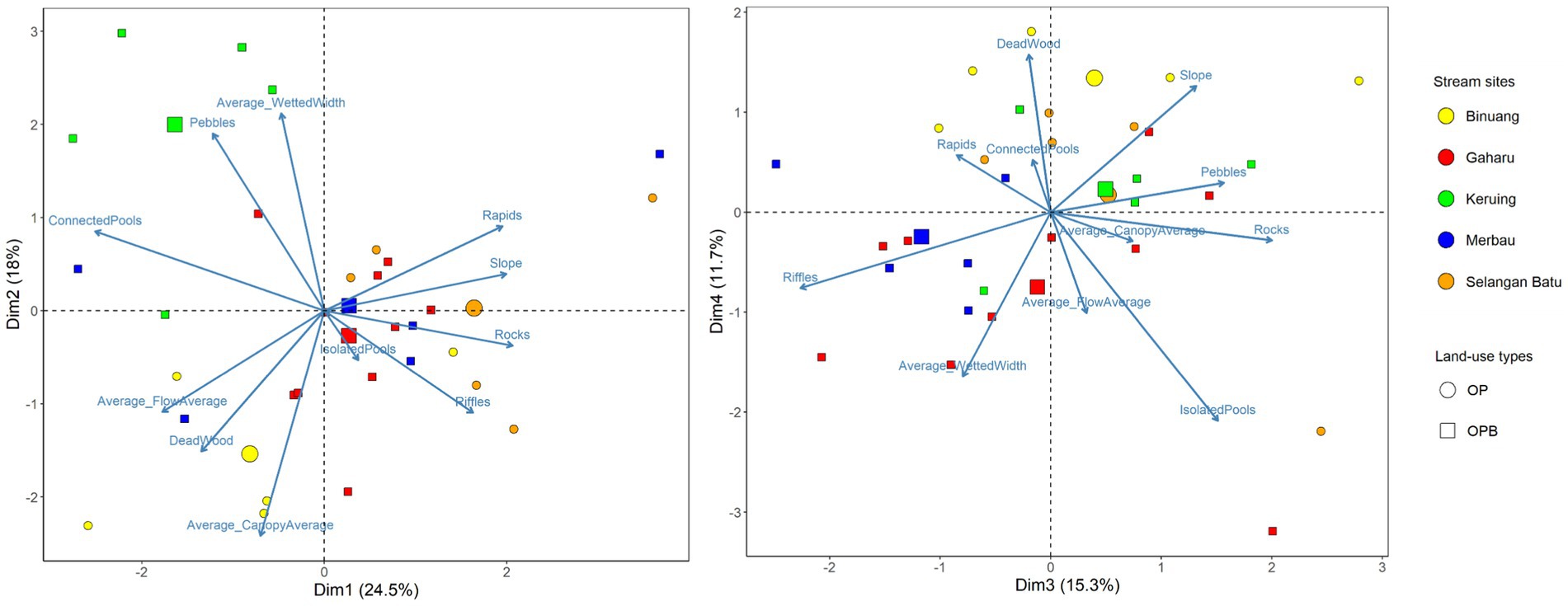
Figure 2. PCA (Principal Component Analysis) biplots showing PC1 and PC2 (“Dim1” and “Dim2” respectively, left panel) as well PC3 and PC4 (“Dim3” and “Dim4” respectively, right panel) site scores of streams in oil palm with (OPB) and without buffer strips (OP). Arrows represent environmental variables (representing within-stream physical structure), while circle and square points represent streams in oil palm with and without riparian buffer strips, respectively. Differing colors represent stream sites. Axes 1 and 2 explained 24.5 and 18% of the variation in environmental variables measured in all stream sites, while axes 3 and 4 explained 15.3 and 11.7%, respectively. Each smaller point represents a 10-m transect from each stream site, while larger points represent the average value for each stream site. N = 30 stream transects.
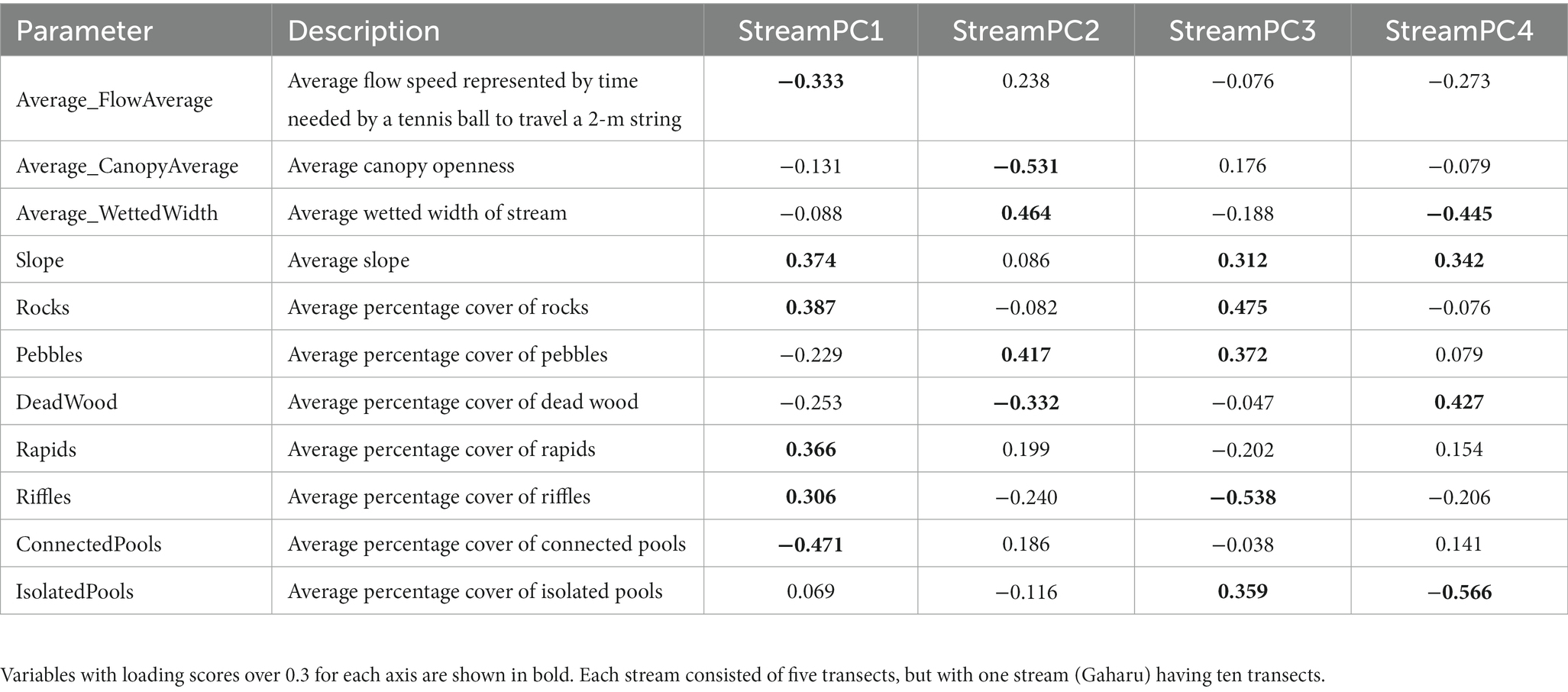
Table 1. Outputs of PCA (Principal Component Analysis) for 30 10-m transects representing streams with (OPB) and without riparian buffer strips (OP) (from three and two streams for OPB and OP, respectively), showing loading scores of each environmental variable within Principal Component (PC) axis 1 to 4 (“StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4,” respectively).

Table 2. Outputs of Mann–Whitney U-test used to assess the difference in stream physical structure between oil palm with (OPB, three streams) and without riparian buffer strips (OP, two streams), consisting of 20 and 10 10-m transects, respectively.
Impacts of within-stream physical structure and retaining riparian buffer strips on the abundance, total biomass, and richness of semi-aquatic bugs
We found 2,699 semi-aquatic bugs (including 1858 juveniles and 841 adults) across oil palm streams in this study. The bugs were from two families (Gerridae and Veliidae), eight genera, and 15 morphospecies (Supplementary Tables 5, 6). Higher “StreamPC4” scores were significantly associated with lower abundance of bugs (Figure 3, Supplementary Table 7), and the presence of riparian buffer strips (“Riparian”) was significantly associated with higher richness of bugs (Supplementary Table 7). Overall, the average abundance of semi-aquatic bugs in oil palm with and without riparian buffer strips were 104.35 (SE ± 11.81) and 61.2 (SE ± 17.41), respectively. Additionally, the average number of morphospecies of semi-aquatic bugs in oil palm streams with riparian buffer strips was 3.55 (SE ± 0.42), compared to 1.4 (SE ± 0.22) in oil palm streams without. There were no other significant effects of within-stream physical structure on the abundance, total biomass, or richness of semi-aquatic bugs (Figure 3, Supplementary Table 7). Nor was there an effect of the presence of riparian buffer strips on the abundance or total biomass of the bugs (Supplementary Table 7).
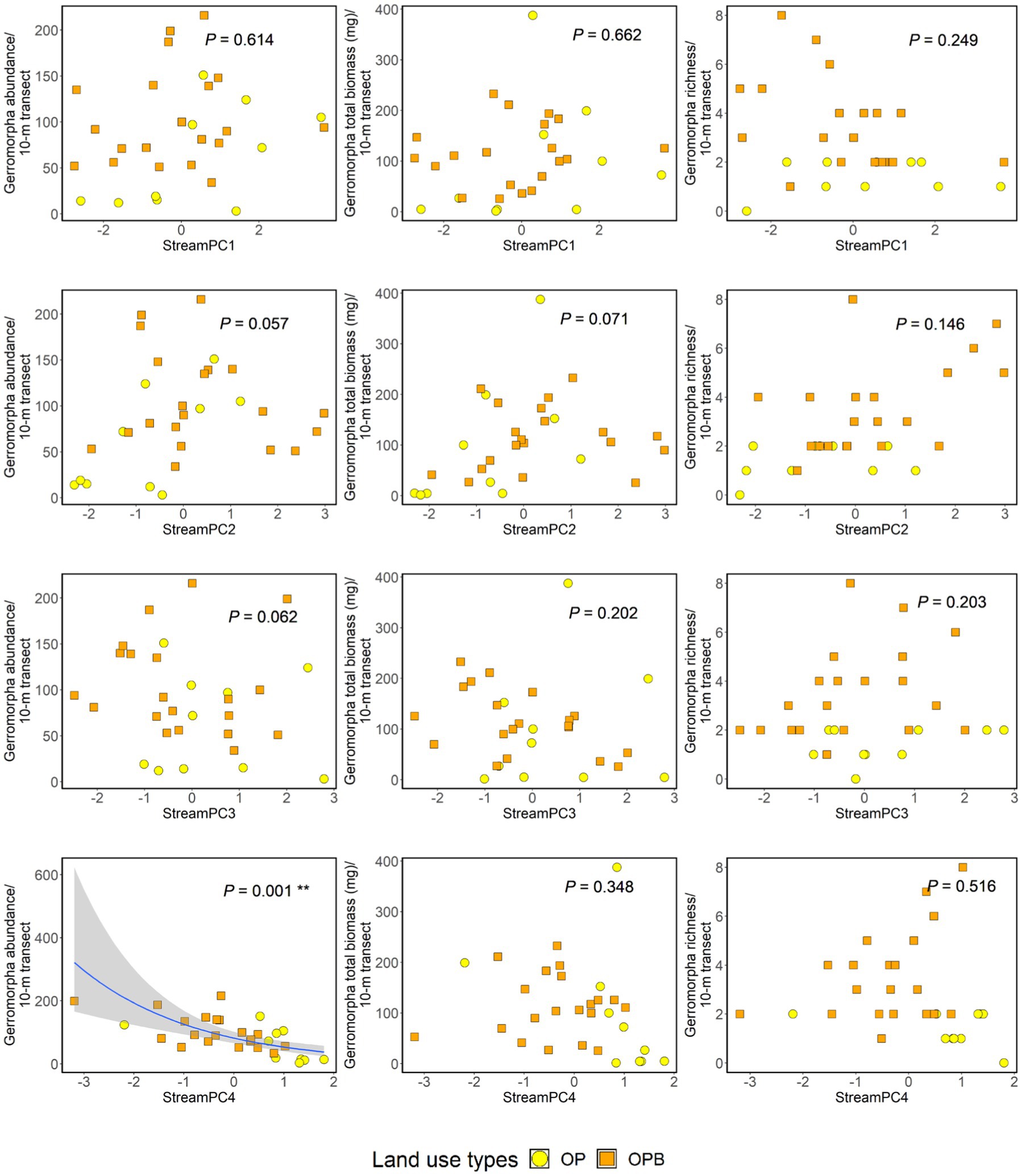
Figure 3. Impacts of within-stream physical structure (“StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4”) on the abundance (left-hand side panel), total biomass (middle panel), and richness (right-hand side panel) of semi-aquatic bugs. For analyses on abundance and total biomass, both juvenile and adult individuals were used. For richness analysis, only adult bugs were used. value of ps were obtained from ANOVA tests in the framework of log-likelihood ratio tests used to assess the importance of each predictor. Regression lines shown for significant associations fitted from “glm” with negative binomial family. Shaded areas show 95% confidence intervals. OP = Oil palm without buffer strips, OPB = Oil palm with buffer strips. Each point represents a 10-m transect from each stream site. N = 30 stream transects.
There was a differing community composition of semi-aquatic bugs associated with StreamPC1 and the presence of buffer strips, but not with other environmental variables representing stream physical structure (Table 3; Figure 4). In particular, the two commonest morphospecies, Ptilomera sp. and Rhagovelia sp. differed markedly in their distribution in relation to the presence of riparian buffer strips: Ptilomera sp. were found across sites, but Rhagovelia sp. were found almost exclusively in streams with buffers (Figure 4). Across the 15 morphospecies of adult bugs found across streams, only seven were found in oil palm without riparian buffer strips (while 14 morphospecies in total were found in oil palm streams with riparian buffer strips) and they were generally in low abundance (Supplementary Table 5). We found two genera that are known to be resilient to habitat change and pollution: Limnogonus and Rheumatogonus (Mohd Ishadi et al., 2014). On the other hand, a genus that is generally found in shaded streams only, Metrocoris, Polhemus (1990), was found at an extremely low abundance across streams in this study, with only one individual from streams without riparian buffer strips (Supplementary Table 6).
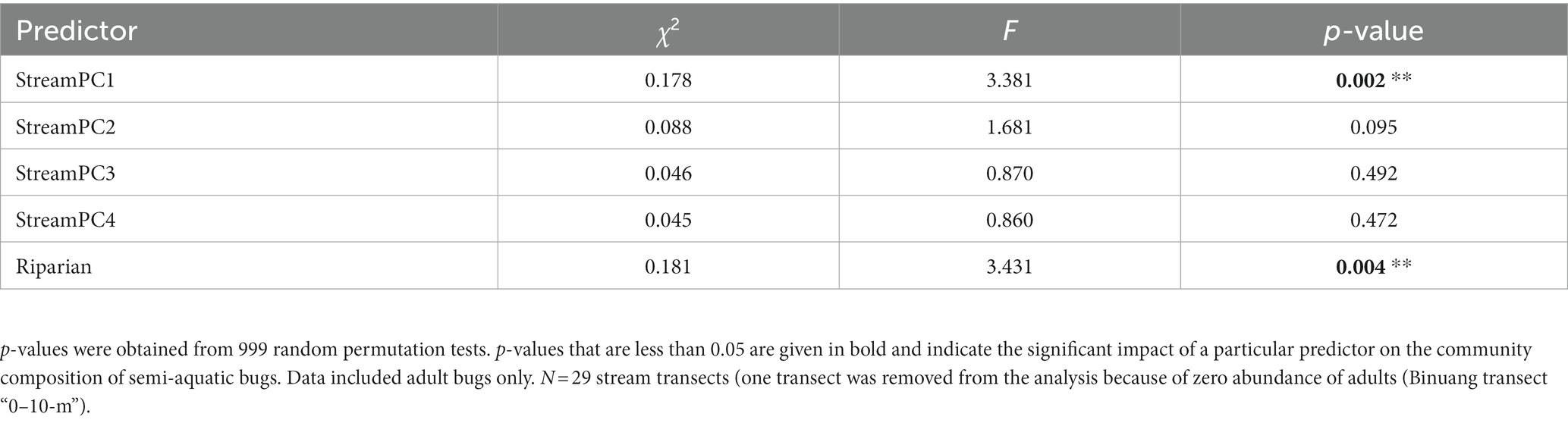
Table 3. Outputs of CCA (Canonical Correspondence Analysis) used to assess the impacts of within-stream physical structure (represented by “StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4”) and the presence of riparian buffer strips (“Riparian”) on the community composition of semi-aquatic bugs.
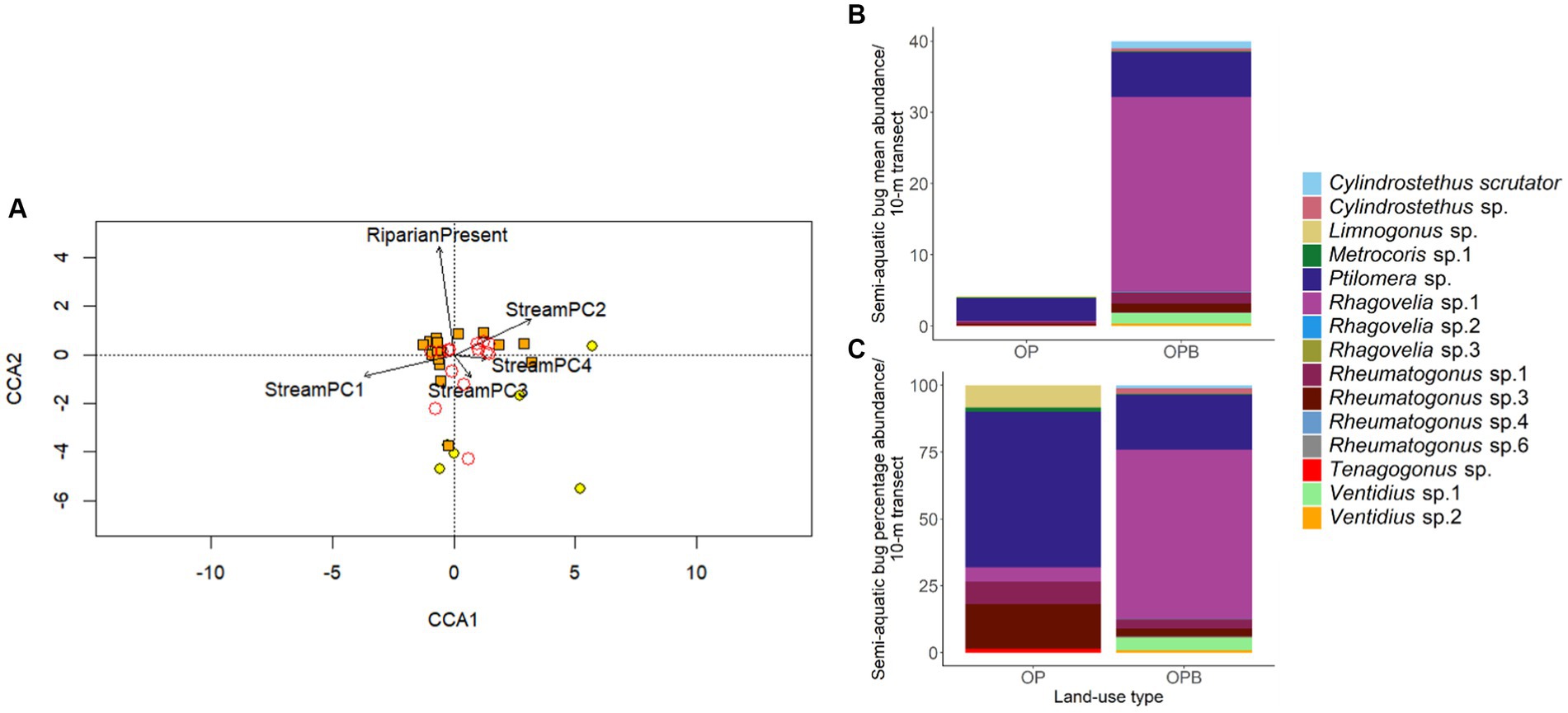
Figure 4. Community composition of semi-aquatic bugs in stream sites in oil palm with (OPB, orange points) and without buffer strips (OP, yellow points) in relation to environmental conditions (representing within-stream physical structure) measured at stream scales, visualized by a Canonical Correspondence Analysis (CCA) tri-plot (Panel A). The presence of riparian buffer strips is also shown. Environmental parameters are indicated as arrows. Community composition is shown by adult bug morphospecies, indicated by circles with no color but red borders. The position of a morphospecies represents the probability of finding that species in a stream. Mean and percentage abundance of each morphospecies in both stream types (OPB, OP) are also shown (Panel B and C, respectively). N = 30 stream transects (but for the CCA, one transect was removed from the analysis because of zero abundance of adults (Binuang transect “0–10-m”)).
Impacts of within-stream physical structure and retaining riparian buffer strips on the ratios of juveniles to adults and females to males in Ptilomera sp.
We found that StreamPC2 (wider wetted width and higher percentage of pebbles, and lower percentage of canopy openness and deadwood) significantly affected the proportion of juveniles across streams, with higher StreamPC2 scores being associated with a lower proportion of juveniles. We found no other effects of within-stream physical structure or retaining riparian buffer strips on the proportion of juveniles and females of Ptilomera sp. (Figure 5, Supplementary Table 8). We found a total of only 12 winged individuals: ten in streams with riparian buffers, two in streams without buffers.
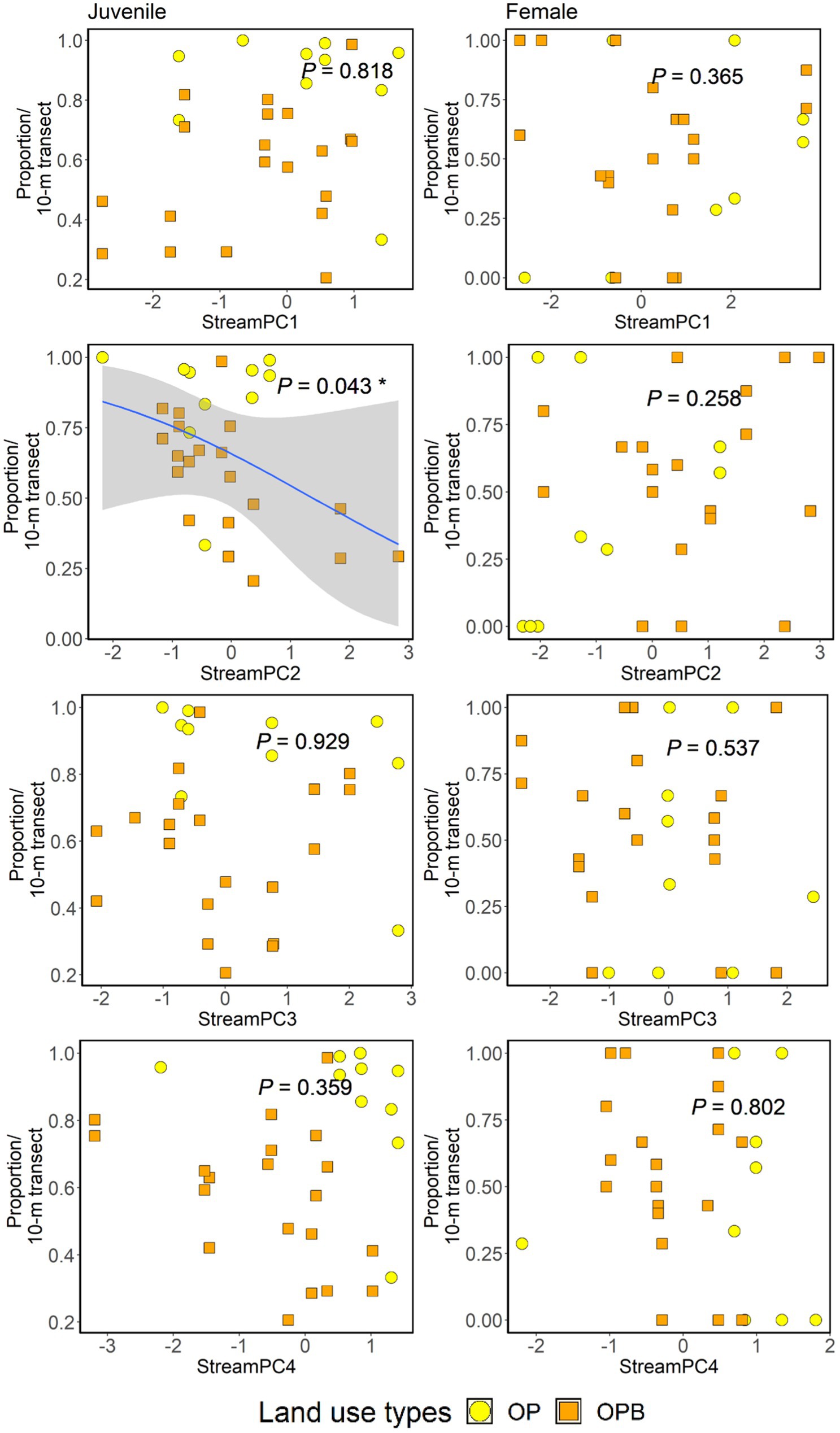
Figure 5. Impacts of stream physical structure (represented by “StreamPC1,” “StreamPC2,” “StreamPC3,” and “StreamPC4”) and the presence or absence of riparian buffer strips (Riparian) on the proportion of juveniles (left-hand side panel) and female Ptilomera sp. (right-hand side panel). P-values were obtained from ANOVA tests in the framework of log-likelihood ratio tests used to assess the importance of each predictor. Regression lines shown for significant associations fitted from “glm” with binomial family. Shaded areas show 95% confidence intervals. OP = Oil palm no buffer, OPB = Oil palm with buffer strips. Each point represents 10-m transect from each stream site. N = 30 stream transects.
Discussion
Small-scale conditions of streams with and without riparian buffer strips
At the small-scale (10-m transects), we found that streams without riparian buffer strips had a significantly higher percentage of deadwood, fewer isolated pools (although in general the presence of deadwood and isolated pools were low across all streams), steeper slopes, and lower wetted width (all were factors strongly associated with “StreamPC4” axis) than those with riparian buffers. The characteristics of streams without riparian buffer strips that we recorded, particularly steeper slopes and lower wetted widths, could be because riparian areas around smaller streams with narrower wetted widths are less likely to be protected during plantation establishment. In addition, smaller streams may be more likely to be found on steeper slopes (Burt et al., 2023, Supplementary Table 1). RSPO (Roundtable on Sustainable Palm Oil) (2018) advises that riparian buffer strips should be at least five meters wide for streams or rivers between one and five meters wide, and that wider strips should be maintained along larger waterways. As all oil palm streams in this study (both with and without riparian buffer strips) had average channel widths that were between five and 12 m (Supplementary Table 1), they should now all receive protection under RSPO guidelines. Those streams without buffer strips could have experienced more impacts of disturbance, such as higher surface runoff and erosion (Du et al., 2022).
It should be noted that buffers in our study areas were variable in size and condition (average widths of buffer strips were approximately 331 m, 68 m, and 26 m in Gaharu, Keruing, and Merbau, respectively, but with widths varying along the length of each stream; (Supplementary Table 1), and we only sampled two oil palm streams without buffers and three oil palm streams with buffers. Differences could therefore be heavily influenced by parameters associated with just one stream, and it is important to assess these findings in a larger-scale study. Additionally, we collected data on stream physical structure once at each site. Therefore, the conditions we recorded may not be representative of the overall conditions of streams or the conditions experienced by semi-aquatic bugs over longer time periods. However, some characteristics we recorded were less likely to be impacted by this than others (for example, percentage canopy openness, percentage cover of rocks, and steepness of slopes are less likely to vary over the year than wetted width or flow speeds).
In contrast, the lack of clear differences that we found in most factors (“StreamPC1” – “StreamPC3”) forming stream physical structure between streams with and without riparian buffer strips indicates that, in general, the presence of riparian buffer strips does not have significant impacts on within-stream physical structure. This could be because the influences of wider catchment properties, including management in the wider oil palm landscape, on stream conditions are more pervasive than maintaining buffer strips or not, necessitating Before-After Control-Impact (BACI) studies to assess the effects of entire catchments (Spray et al., 2022). Additionally, it could because the riparian buffers in this study were not able to protect streams from the influence of chemical runoff or erosion from the surrounding agricultural landscape (Xu et al., 2023; Harianja et al., 2023b).
Values of riparian buffer strips on semi-aquatic bugs
We found only a limited range of species in our study system, consisting of 15 morphospecies, from eight genera and two families. This is much lower than previous studies within forested areas in the region (e.g., up to 30 morphospecies from all forest sites in Harianja et al. (2023b). Other studies have also found a similar pattern, particularly that - regardless of the presence of riparian buffer strips - oil palm streams cannot support species that are sensitive to anthropogenic disturbance. For instance, some insect species [including some Odonata in Carvalho et al. (2018) and Luke et al. (2017b)], and aquatic insect larvae and dung beetles in Deere et al. (2022) are not found in oil palm streams.
We found that oil palm streams with riparian buffer strips had more species/morphospecies of semi-aquatic bugs and contained a distinct assemblage compared to streams without buffers. This is likely to be because oil palm with riparian buffer strips provides more food resources, such as leaf litter for invertebrates that are prey of semi-aquatic bugs or terrestrial invertebrates falling into the water (Maier, 1977; Mendes et al., 2019a; Popescu et al., 2021). Furthermore, the presence of the strips may buffer the microclimatic conditions within streams, so bug species with lower heat tolerance can be supported. Indeed, studies conducted on other aquatic invertebrates have found that oil palm streams house more species that are tolerant to the higher water temperatures found in these systems, caused by the loss of canopy cover, compared to forest streams (Mendes et al., 2019b).
It is notable that only seven of the 15 morphospecies in our study were found in streams without buffers: Limnogonus sp., Metrocoris sp., Ptilomera sp., Rhagovelia sp. 1, Rheumatogonus sp. 1 and sp. 3, and Tenagogonus sp.. Previous studies have found Limnogonus species in forest as well as oil palm streams, showing this genus to be resilient to habitat change (Al-Shami et al., 2011; Mohd Ishadi et al., 2014; Moy et al., 2022). In contrast, Metrocoris species are known to live in shaded streams (Polhemus, 1990), which may explain their low abundance in this study: only one individual was found in oil palm streams without riparian buffer strips and overall abundance of this genus across streams was generally extremely low (see Supplementary Table 6). Ptilomera and Rhagovelia species can tolerate fast-flowing water because of specialized structures for maintaining their position on the water surface in these habitats (Kim et al., 2022). This may explain their presence in oil palm streams, which can be highly variable in streamflow due to rapid surface runoff from surrounding agricultural land. Mohd Ishadi et al. (2014) also found Rhagovelia and Rheumatogonus bugs across stream sites exposed to various pollution sources (including from households, mining operations, and agriculture), demonstrating the resilience of these genera to changing stream conditions.
A notable difference in the community composition between streams with and without buffers was in the two commonest morphospecies of bugs, Ptilomera sp. and Rhagovelia sp.. Ptilomera sp. was found across stream types, but in contrast Rhagovelia sp. was largely restricted to streams with buffers. This could be owing to a marked size difference between the species: Rhagovelia spp. are relatively small and perhaps more vulnerable to variable climatic conditions, whereas Ptilomera sp. are much larger and therefore may be able to tolerate a wider range of microclimatic conditions (Kingsolver et al., 2011). We note that, as we only identified bugs to morphospecies level in this study, it is possible that some morphospecies may have contained more than one species. However, this is unlikely to significantly alter our key findings which are based on consistent differences observed at the genus and morphospecies level. More detailed taxonomic resolution of these communities could potentially reveal additional responses to changes in environmental parameters.
We also note that the three streams with buffer strips (Gaharu, Keruing, and Merbau) also differed in terms of average species richness. Keruing supported a markedly higher number of species than Gaharu and Merbau, but average abundance and total biomass (mg) were similar (Supplementary Tables 9, 10 and see Supplementary Table 6 for breakdowns of data for each stream). This difference did not seem to be related to buffer width; compared to Gaharu and Merbau, Keruing did not have markedly wider or narrower buffer strips. Hence this difference is also likely to have been driven by the catchment conditions. The three areas also differed in the age of oil palm surrounding them (Supplementary Table 1), which may also have influenced the trends observed. However, there are no obvious patterns, and this study was not designed to assess the effects of years explicitly. This area therefore merits further investigation.
Small-scale effects of the presence of riparian buffer strips and within-stream physical structure on semi-aquatic bugs
Along 10-m transects, we recorded a significantly higher abundance of bugs in streams with higher wetted width but lower percentage isolated pools, as well as shallower slopes and lower percentage cover of deadwood. It is likely to be related to habitat availability (although, again, the presence of deadwood and isolated pools were generally low across all streams). Streams with higher wetted width are likely to have a larger surface area, providing space for more individuals. We also found that at the small scale, streams with riparian buffer strips had more morphospecies of semi-aquatic bugs that streams without (average number of morphospecies in oil palm streams with riparian buffer strips was 3.55 (SE ± 0.42) and 1.4 (SE ± 0.22) in oil palm streams without). Additionally, we also found altered community composition. In particular, this was associated with slope, percentage cover of rocks, rapids, riffles, connected pools and flow speed. All of these factors, in addition to the presence of buffer strips, were likely to be related to varying habitat requirements needed by different species in this group (Andersen, 1982; Andersen and Weir, 1997), impacting the number of morphospecies and altering community composition.
In contrast to effects on abundance, richness, and composition, we did not identify any environmental factor or presence of buffer that affected total biomass of semi-aquatic bugs. However, other studies have found that the presence of riparian buffer strips is associated with a higher biomass of invertebrates (Burdon and Harding, 2008; Sargac et al., 2021). The contrasting trend observed in this study could have been because of species replacement/turnover across sites, stabilizing total biomass measurements. Although streams with buffer strips had a generally higher abundance of semi-aquatic bugs, this was from a combination of large- and small-bodied species across life stages, while streams without buffer strips were dominated by larger bodied bugs for both life stages. For instance, small species (up to 4 mm in body length) such as Rhagovelia spp. were common and abundant in streams with buffer strips, but the larger Ptilomera sp. adults (up to 19 mm in body length) and juveniles (up to 15 mm in body length) remained abundant in streams without buffers, maintaining a high total biomass owing to their large individual size (Slade et al., 2011).
Furthermore, at the small scale (10-m transects), we found that there were more juveniles in streams with higher canopy openness and deadwood, but lower wetted width and percentage cover of pebbles. This might show that juveniles can still survive and grow in streams with warmer water temperature caused by higher canopy openness, and indeed warmer conditions could result in higher growth rates for juvenile bugs, as it can (within an optimal range for growth) in other insects (Kingsolver et al., 2011). The higher incidence of deadwood could provide a substrate for egg-laying (Sweeney, 1993), potentially boosting numbers of juveniles in these stream sections. Finally, the lower wetted width could mean that these streams are less commonly used by adults, potentially allowing juveniles to escape competition, increasing their relative numbers (Spence and Carcamo, 1991).
We found an extremely low number of winged individuals at the small scale (10-m transects) across steams and found no relationship between any environmental factors or the presence of riparian buffer strips on the sex ratio of Ptilomera sp.. In terms of sex ratio, this may show that neither sex is disproportionately affected by habitat disturbance, at least in Ptilomera species. This could be owing to this species being robust to change or could be a finding that is true across species and merits further study. As a caveat to this, since Ptilomera semi-aquatic bugs in this study were identified to morphospecies level, this group might have included more than one species, so our results should be interpreted with caution.
Implications and conclusion
Our study found that certain stream environmental conditions and the presence of riparian buffer strips benefited semi-aquatic bugs within streams in oil palm landscapes, particularly their abundance and richness. Taxa that we found across all oil palm streams were generally tolerant of disturbance, although we found a significantly different community composition between streams with and without riparian buffer strips. More studies, particularly at a larger scale, are needed to confirm the findings from this study, particularly because we surveyed only five streams. In addition, over time there can be variation in semi-aquatic bug communities (Fernando, 1963) and stream physical parameters (including those that we did not measure in this study, such as percentage litter cover and erosion levels). It is therefore important to carry out studies over longer-time periods. Our study provides the first evidence of the effects of riparian buffer strips on semi-aquatic bugs, and extends the currently limited knowledge of the effects of riparian buffer strips on freshwater communities in Southeast Asia (Luke et al., 2019). Finally, whilst abundance, richness, and composition of semi-aquatic bugs were affected by stream physical structure and the presence of riparian buffer strips, this was not the case for total biomass, number of winged individuals, or for the sex ratio of Ptilomera sp.. This suggests that these factors may be fairly robust to the effects of environmental change, particularly for semi-aquatic bugs in permanent lotic habitats, as in this study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found at: https://zenodo.org/record/7813514#.ZDRlgXuSnb0 (Harianja et al., 2023c).
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SHL and ECT: conceptualization. SHL, HB, ECT, MFH, DCA, VKC, and WAF: developing methods and writing. MFH, SHL, HB, and ECT: conducting the research. MFH, ECT, and SHL: data analysis, data interpretation, and preparation figures and tables. All authors contributed to the article and approved the submitted version.
Funding
Jardine Foundation and the Cambridge Trust funded MFH, the Natural Environment Research Council (NERC) (studentship 1122589), Proforest, the Varley Gradwell Travelling Fellowship, the Tim Whitmore Fund, the Panton Trust, the Cambridge University Commonwealth Fund, and the Hanne and Torkel Weis-Fogh Fund funded SHL, and the ST Lee Fund provided funding for HB. DCA was supported by a Dawson Fellowship at St. Catharine’s College, Cambridge. The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We also thank the Sabah Biodiversity Council for permission for SHL and HB to conduct research in Sabah (access licence reference numbers, JKM/MBS.1000-2/2(03), JKM/MBS.1000-2/2(37), JKM/MBS.1000-2/2(68)), as well as to Southeast Asia Rainforest Research Partnership, Danum and Maliau Basin Management Committees, the SAFE Project, and Benta Wawasan for permission to work in the different areas and for logistical support in the field. We are grateful for the assistance from Min Sheng Khoo, Johnny Larenus, Alice Milton, and the SAFE Project research assistants during field data collection. We thank Jakob Damgaard, Lars Vilhelmsen, and Henrik Enghoff who helped SHL with the sample identification in Copenhagen; Mick Webb based in the London Natural History Museum who helped MFH to make connection with Herbert Zettel and obtained access to semi-aquatic bug collections in the Museum; and Herbert Zettel who provided guidance with semi-aquatic bug identification for MFH. We thank Konstans Wells and James Herbert-Read for their valuable feedback on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1203513/full#supplementary-material
References
Al-Shami, S. A., Md Rawi, C. S., Ahmad, A. H., Hamid, S. A., and Mohd Nor, S. A. (2011). Influence of agricultural, industrial, and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River basin, Penang, Malaysia. Ecotoxicol. Environ. Saf. 74, 1195–1202. doi: 10.1016/j.ecoenv.2011.02.022
Andersen, N.M. (1982). The semiaquatic bugs (Hemiptera, Gerromorpha): Phylogeny, adaptations, biogeography and classification Entomonograph. Klampenborg, Denmark: Scandinavian Science Press Ltd.
Andersen, N. M. (2000). The evolution of dispersal dimorphism and other life history traits in water striders (Hemiptera: Gerridae). Entomol. Sci. 3, 187–199.
Andersen, N. M., and Weir, T. A. (1997). The Gerrinae water striders of Australia (Hemiptera: Gerridae): taxonomy, distribution, and ecology. Invertebr. Taxon. 11, 203–299. doi: 10.1071/IT95047
Arnaiz, O. L., Wilson, A. L., Watts, R. J., and Stevens, M. M. (2011). Influence of riparian condition on aquatic macroinvertebrate communities in an agricultural catchment in South-Eastern Australia. Ecol. Res. 26, 123–131. doi: 10.1007/s11284-010-0767-2
Auguie, B. (2017). Grid extra: Miscellaneous functions for "grid" graphics. R package version 2.3. Available at: https://CRAN.R-project.org/package=gridExtra.
Barclay, H., Gray, C. L., Luke, S. H., Nainar, A., Snaddon, J. L., and Turner, E. C. (2017). RSPO manual on best management practices (BMPs) for the management and rehabilitation of riparian reserves. Guidance. Endorsed by the RSPO Biodiversity and High Conservation Values Working Group. doi: 10.13140/RG.2.2.17011.22561,
Barrantes, G., and Sandoval, L. (2009). Conceptual and statistical problems associated with the use of diversity indices in ecology. Rev. Biol. Trop. 57, 451–460.
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme 4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bay, E. C. (1974). Predator-prey relationships among aquatic insects. Annu. Rev. Entomol. 19, 441–453. doi: 10.1146/annurev.en.19.010174.002301
Blanco-Canqui, H., Gantzer, C. J., Anderson, S. H., Alberts, E. E., and Thompson, A. L. (2004). Grass barrier and vegetative filter strip effectiveness in reducing runoff, sediment, nitrogen, and phosphorus loss. Soil Sci. Soc. Am. J. 68, 1670–1678. doi: 10.2136/sssaj2004.1670
Burdon, F. J., and Harding, J. S. (2008). The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 53, 330–346. doi: 10.1111/j.1365-2427.2007.01897.x
Burt, E. I., Rimachi, D. H. C., Quispe, A. J. C., Atwood, A., and West, A. J. (2023). Isotope-derived young water fractions in streamflow across the tropical Andes mountains and Amazon floodplain. Hydrol. Earth Syst. Sci. 27, 2883–2898. doi: 10.5194/hess-27-2883-2023
Carpenter, S. R., Stanley, E. H., and van der Zanden, M. J. (2011). State of the world’s freshwater ecosystems: physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 36, 75–99. doi: 10.1146/annurev-environ-021810-094524
Carvalho, F. G., de Oliveira Roque, F., Barbosa, L., de Assis Montag, L. F., and Juen, L. (2018). Oil palm plantation is not a suitable environment for most forest specialist species of Odonata in Amazonia. Anim. Conserv. 21, 526–533. doi: 10.1111/acv.12427
Cazzolla Gatti, R. (2016). Freshwater biodiversity: a review of local and global threats. Int. J. Environ. Stud. 73, 887–904. doi: 10.1080/00207233.2016.1204133
Ceneviva-Bastos, M., and Casatti, L. (2014). Shading effects on community composition and food web structure of a deforested pasture stream: evidences from a field experiment in Brazil. Limnologica 46, 9–21. doi: 10.1016/j.limno.2013.11.005
Chellaiah, D., and Yule, C. M. (2018). Riparian buffers mitigate impacts of oil palm plantations on aquatic macroinvertebrate community structure in tropical streams of Borneo. Ecol. Indic. 95, 53–62. doi: 10.1016/j.ecolind.2018.07.025
Chen, P. P., and Nieser, N. (1992). Gerridae, mainly from Sulawesi and Pulau Buton (Indonesia). Notes on Malesian aquatic and semiaquatic bugs (Heteroptera) III. Tijdschrift Voor Entomologie 135, 145–162.
Chen, P. P., and Nieser, N. (1993a). A taxonomic revision of the oriental water strider genus Metrocoris Mayr (Hemiptera, Gerridae). Part I. Steenstrupia Zoological Museum, University of Copenhagen 19, 1–43.
Chen, P. P., and Nieser, N. (1993b). A taxonomic revision of the oriental water strider genus Metrocoris Mayr (Hemiptera, Gerridae). Part II. Steenstrupia Zoological Museum, University of Copenhagen 19, 45–82.
Chen, P. P., and Zettel, H. (1998). A taxonomic revision of the oriental water strider genus Ventidius distant (Hemiptera, Gerromorpha, Gerridae). Tijdschrift voor Entomologie 141, 137–208. doi: 10.1163/22119434-99900010
Cole, L. J., Stockan, J., and Helliwell, R. (2020). Managing riparian buffer strips to optimise ecosystem services: a review. Agric. Ecosyst. Environ. 296:106891. doi: 10.1016/j.agee.2020.106891
Cooper, C. M., Moore, M. T., Bennett, E. R., Smith, S., Farris, J. L., Milam, C. D., et al. (2004). Innovative uses of vegetated drainage ditches for reducing agricultural runoff. Water Sci. Technol. 49, 117–123. doi: 10.2166/wst.2004.0176
Cunha, E. J., de Assis Montag, L. F., and Juen, L. (2015). Oil palm crops effects on environmental integrity of Amazonian streams and heteropteran (Hemiptera) species diversity. Ecol. Indic. 52, 422–429. doi: 10.1016/j.ecolind.2014.12.024
Cunha, E. J., Guterres, A. P. M., Godoy, B. S., and Juen, L. (2020). Wing dimorphism in semiaquatic bugs (Hemiptera, Heteroptera, Gerromorpha) as a tool for monitoring streams altered by oil palm plantation in the Amazon. Ecol. Indic. 117:106707. doi: 10.1016/j.ecolind.2020.106707
Deere, N. J., Bicknell, J. E., Mitchell, S. L., Afendy, A., Baking, E. L., Bernard, H., et al. (2022). Riparian buffers can help mitigate biodiversity declines in oil palm agriculture. Front. Ecol. Environ. 20, 459–466. doi: 10.1002/fee.2473
Dias-Silva, K., Brasil, L. S., Juen, L., Cabette, H. S. R., Costa, C. C., Freitas, P. V., et al. (2020). Influence of local variables and landscape metrics on Gerromorpha (Insecta: Heteroptera) assemblages in savanna streams Brazil. Neotr. Entomol. 49, 191–202. doi: 10.1007/s13744-019-00748-8
Ditrich, T., Papáček, M., and Broum, T. (2008). Spatial distribution of semiaquatic bugs (Heteroptera: Gerromorpha) and their wing morphs in a small scale of the Pohořský Potok stream spring area (Novohradské Hory Mts.). Silva Gabreta 14, 173–178. Available at: https://www.researchgate.net/publication/267200310/Spatial_distribution_of_semiaquatic_bugs_Heteroptera_Gerromorpha_and_their_wing_morphs_in_a_small_scale_of_the_Pohorsky_Potok_stream_spring_area_Novohradske_Hory_Mts
Du, X., Jian, J., Du, C., and Stewart, R. D. (2022). Conservation management decreases surface runoff and soil erosion. Int. Soil Water Conserv. Res. 10, 188–196. doi: 10.1016/j.iswcr.2021.08.001
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 81, 163–182. doi: 10.1017/S1464793105006950
Ewers, R. M., Didham, R. K., Fahrig, L., Ferraz, G., Hector, A., Holt, R. D., et al. (2011). A large-scale forest fragmentation experiment: the stability of altered forest ecosystems project. Philos. Trans. R. Soc. Biol. Sci. 366, 3292–3302. doi: 10.1098/rstb.2011.0049
Farmer, J. R., Meretsky, V., Knapp, D., Chancellor, C., and Fischer, B. C. (2015). Why agree to a conservation easement? Understanding the decision of conservation easement granting. Landsc. Urban Plan. 138, 11–19. doi: 10.1016/j.landurbplan.2015.01.005
Fernando, C.H. (1963). Notes on aquatic insects colonizing an isolated pond in Mawai, Johore. Bull. Natl. Mus. (32).
Foster, W. A., Snaddon, J. L., Turner, E. C., Fayle, T. M., Cockerill, T. D., Farnon Ellwood, M. D., et al. (2011). Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philos. Trans. R. Soc. B Biol. Sci. 366, 3277–3291. doi: 10.1098/rstb.2011.0041
Foster, W. A., and Treherne, J. E. (1981). Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature 293, 466–467. doi: 10.1038/293466a0
Fox, J., and Weisberg, S. (2019). An {R} Companion to Applied Regression, 3rd. Thousand Oaks CA: Sage. Available at: https://www.john-fox.ca/Companion/index.html
Giam, X., Hadiaty, R. K., Tan, H. H., Parenti, L. R., Wowor, D., Sauri, S., et al. (2015). Mitigating the impact of oil-palm monoculture on freshwater fishes in Southeast Asia. Conserv. Biol. 29, 1357–1367. doi: 10.1111/cobi.12483
Halpern, B. S., Frazier, M., Verstaen, J., Rayner, P. E., Clawson, G., Blanchard, J. L., et al. (2022). The environmental footprint of global food production. Nat. Sustain. doi: 10.1038/s41893-022-00965-x
Harianja, M. F., Luke, S. H., Barclay, H., Chey, V. K., Aldridge, D. C., Foster, W. A., et al. (2023a). Length–biomass equations to allow rapid assessment of semi-aquatic bug biomass in tropical streams. Entomol. Exp. Appl. 171, 102–115. doi: 10.1111/eea.13247
Harianja, M.F., Turner, E.C., Barclay, H., Chey, V.K., Aldridge, D.C., Foster, W.A., et al. (2023b). The effects of land-use change on semi-aquatic bugs (Gerromorpha, Hemiptera) in rainforest streams in Sabah, Malaysia. Chapter 3 in “understanding the impacts of land-use change and management decisions within oil palm on insect assemblages in peninsular Malaysia and Borneo” by M.F. Harianja. PhD thesis. doi: 10.17863/CAM.102030
Harianja, M. F., Luke, S. H., Barclay, H., Chey, V. K., Aldridge, D. C., Foster, W. A., et al. (2023c). Data set and analytic codes supporting "the impacts of within-stream physical structure and riparian buffer strips on semi-aquatic bugs in southeast Asian oil palm" [data set]. Zenodo. doi: 10.5281/zenodo.7813514,
Kassambara, A., and Mundt, F. (2020). Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.7. Available at: https://CRAN.R-project.org/package=factoextra.
Kim, W., Pham, T. H., Nguyen, P. D., Tran, A. D., Ha, J., Jablonski, P. G., et al. (2022). Locomotion and flow speed preferences in natural habitats by large water striders, Ptilomera tigrina, with micro-morphological adaptations for rowing. J. Ethol. doi: 10.1007/s10164-022-00749-y
Kingsolver, J. G., Woods, H. A., Buckley, L. B., Potter, K. A., Mac Lean, H. J., and Higgins, J. K. (2011). Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 51, 719–732. doi: 10.1093/icb/icr015
Lemmon, P. E. (1956). A spherical densiometer for estimating forest overstory density. For. Sci. 2, 314–320.
Luiza-Andrade, A., Brasil, L. S., Benone, N. L., Shimano, Y., Farias, A. P. J., Montag, L. F., et al. (2017). Influence of oil palm monoculture on the taxonomic and functional composition of aquatic insect communities in eastern Brazilian Amazonia. Ecol. Indic. 82, 478–483. doi: 10.1016/j.ecolind.2017.07.006
Luke, S.H. (2016). The impacts of land-use on environmental conditions, invertebrate biodiversity, and ecosystem function of freshwater streams in Sabah, Malaysia. PhD thesis. University of Cambridge.
Luke, S. H., Barclay, H., Bidin, K., Chey, V. K., Ewers, R. M., Foster, W. A., et al. (2017a). The effects of catchment and riparian forest quality on stream environmental conditions across a tropical rainforest and oil palm landscape in Malaysian Borneo. Ecohydrology 10:e1827. doi: 10.1002/eco.1827
Luke, S. H., Dow, R. A., Butler, S., Chey, V. K., Aldridge, D. C., Foster, W. A., et al. (2017b). The impacts of habitat disturbance on adult and larval dragonflies (Odonata) in rainforest streams in Sabah Malaysian Borneo. Freshw. Biol. 62, 491–506. doi: 10.1111/fwb.12880
Luke, S. H., Slade, E. M., Gray, C. L., Annammala, K. V., Drewer, J., Williamson, J., et al. (2019). Riparian buffers in tropical agriculture: scientific support, effectiveness and directions for policy. J. Appl. Ecol. 56, 85–92. doi: 10.1111/1365-2664.13280
Maddock, I. (1999). The importance of physical habitat assessment for evaluating river health. Freshw. Biol. 41, 373–391. doi: 10.1046/j.1365-2427.1999.00437.x
Maier, C. T. (1977). The behavior of hydrometra championana (Hemiptera: Hydrometridae) and resource partitioning with Tenagogonus quadrilineatus (Hemiptera: Gerridae). J. Kansas Entomol. Soc. 50, 263–271.
Malmqvist, B. (2002). Aquatic invertebrates in riverine landscapes. Freshw. Biol. 47, 679–694. doi: 10.1046/j.1365-2427.2002.00895.x
Mendes, T. P., Amado, L. L., Ribeiro, R. A. B., and Juen, L. (2019a). Morphological diversity of Odonata larvae (Insecta) and abiotic variables in oil palm plantation areas in the eastern Amazon. Hydrobiologia 847, 161–175. doi: 10.1007/s10750-019-04079-y
Mendes, T. P., Benone, N. L., and Juen, L. (2019b). To what extent can oil palm plantations in the Amazon support assemblages of Odonata larvae? Insect Conserv. Divers. 12, 448–458. doi: 10.1111/icad.12357
Mercer, E. V., Mercer, T. G., and Sayok, A. K. (2014). Effects of forest conversions to oil palm plantations on freshwater macroinvertebrates: a case study from Sarawak Malaysia. J. Land Use Sci. 9, 260–277. doi: 10.1080/1747423X.2013.786149
Mohd Ishadi, N. A., Md Rawi, C. S., Ahmad, A. H., and Abdul, N. H. (2014). The influence of heavy metals and water parameters on the composition and abundance of water bugs (Insecta: Hemiptera) in the Kerian River basin, Perak Malaysia. Trop. Life Sci. Res. 25, 61–79. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4814147/
Moy, K. M., Brasil, L. S., Oliveira-Junior, J. M. B., Juen, L., Vieira, T. B., and Dias-Silva, K. (2022). Effects of environmental changes on Gerromorpha (Heteroptera: Hemiptera) communities from Amazonian streams. Hydrobiology 1, 111–121. doi: 10.3390/hydrobiology1010008
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Ogle, D.H., Doll, J.C., Powell Wheeler, A., and Dinno, A. (2023). FSA: simple fisheries stock assessment methods. R package version Available at: https://CRAN.R-project.org/package=FSA.
Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). Vegan: community ecology package. R package version 2.5-7. Available at: https://CRAN.R-project.org/package=vegan.
Pendrill, F., Gardner, T. A., Meyfroidt, P., Persson, U. M., Adams, J., Azevedo, T., et al. (2022). Disentangling the numbers behind agriculture-driven tropical deforestation. Science 377:eabm9267. doi: 10.1126/science.abm9267
Polhemus, D. A. (1990). A revision of the genus Metrocoris Mayr (Heteroptera: Gerridae) in the Malay archipelago and the Philippines. Ent. Scand. 21, 1–28. doi: 10.1163/187631290X00012
Polhemus, J. T., and Polhemus, D. A. (1988). Zoogeography, ecology, and systematics of the genus Rhagovelia Mayr (Heteroptera: Veliidae) in Borneo, Celebes, and the Moluccas. Insecta Mundi 2, 161–230.
Popescu, C., Oprina-Pavelescu, M., Dinu, V., Cazacu, C., Burdon, F. J., Forio, M. A. E., et al. (2021). Riparian vegetation structure influences terrestrial invertebrate communities in an agricultural landscape. Water 13:188. doi: 10.3390/w13020188
Pusey, B. J., and Arthington, A. H. (2003). Importance of the riparian zone to the conservation and management of freshwater fish: a review. Mar. Freshw. Res. 54, 1–16. doi: 10.1071/MF02041
R Core Team (2021). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reid, A. J., Carlson, A. K., Creed, I. F., Eliason, E. J., Gell, P. A., Johnson, P. T. J., et al. (2019). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873. doi: 10.1111/brv.12480
Rizopoulos, D. (2006). Ltm: an R package for latent variable modelling and item response theory analyses. J. Stat. Softw. 17, 1–25. doi: 10.18637/jss.v017.i05
RSPO (Roundtable on Sustainable Palm Oil). (2018). Principles and criteria for the production of sustainable palm oil. Available at: https://rspo.org/resources/.
Sargac, J., Johnson, R. K., Burdon, F. J., Truchy, A., Rîşnoveanu, G., Goethals, P., et al. (2021). Forested riparian buffers change the taxonomic and functional composition of stream invertebrate communities in agricultural catchments. Water 13:1028. doi: 10.3390/w13081028
Slade, E. M., Mann, D. J., and Lewis, O. T. (2011). Biodiversity and ecosystem function of tropical forest dung beetles under contrasting logging regimes. Biological Conservation, 144, 166–174. doi: 10.1016/j.biocon.2010.08.011
Sodhi, N. S., Koh, L. P., Brook, B. W., and Ng, P. K. L. (2004). Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660. doi: 10.1016/j.tree.2004.09.006
Spence, J. R., and Andersen, N. (1994). Biology of water striders: interactions between systematics and ecology. Annu. Rev. Entomol. 39, 101–128.
Spence, J. R., and Carcamo, H. A. (1991). Effects of cannibalism and intraguild predation on pondskaters (Gerridae). Oikos 62, 333–341.
Spray, C., Black, A., Bradley, D., Bromley, C., Caithness, F., Dodd, J., et al. (2022). Strategic design and delivery of integrated catchment restoration monitoring: emerging lessons from a 12-year study in the UK. Water 14, 1–12. doi: 10.3390/w14152305
Sundar, S., Heino, J., De Roque, F. O., Simaika, J. P., Melo, A. S., Tonkin, J. D., et al. (2020). Conservation of freshwater macroinvertebrate biodiversity in tropical regions. Aquat. Conserv. Mar. Freshwat. Ecosyst. 30, 1238–1250. doi: 10.1007/s10841-021-00298-8
Sweeney, B. W. (1993). Effects of streamside vegetation on macroinvertebrate communities of white Clay Creek in eastern North America. Proc. Acad. Natl. Sci. Phila. 144, 291–340.
Tanaka, Y., Minggat, E., and Roseli, W. (2021). The impact of tropical land-use change on downstream riverine and estuarine water properties and biogeochemical cycles: a review. Ecol. Process. 10, 40 doi: 10.1186/s13717-021-00315-3
Teder, T., and Kaasik, A. (2023). Early-life food stress hits females harder than males in insects: a meta-analysis of sex differences in environmental sensitivity. Ecol. Lett. 26, 1419–1431. doi: 10.1111/ele.14241
Wantzen, K. M., and Mol, J. H. (2013). Soil erosion from agriculture and mining: a threat to tropical stream ecosystems. Agriculture (Switzerland) 3, 660–683. doi: 10.3390/agriculture3040660
Wickham, H., François, R., Henry, L., and Müller, K. (2021). Dplyr: a grammar of data manipulation. R package version 1.0.4. Available at: https://cran.r-project.org/web/packages/dplyr/index.html
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., et al. (2019). Welcome to the tidyverse. J. Open Sour. Softw. 4:1686. doi: 10.21105/joss.01686
Wilke, C.O., (2020). Cowplot: streamlined plot theme and plot annotations for 'ggplot 2′. R package version 1.1.1. Available at: https://CRAN.R-project.org/package=cowplot.
WWF (2018) in Living planet report 2018: aiming higher. eds. M. Grooten and R. E. A. Almond (Gland, Switzerland: WWF International)
Xu, Q., Yan, T., Wang, C., Hua, L., and Zhai, L. (2023). Managing landscape patterns at the riparian zone and sub-basin scale is equally important for water quality protection. Water Res. 229:119280. doi: 10.1016/j.watres.2022.119280
Keywords: riparian buffer strips, oil palm, Gerromorpha (Hemiptera), semi-aquatic bugs, conservation management
Citation: Harianja MF, Luke SH, Barclay H, Chey VK, Aldridge DC, Foster WA and Turner EC (2024) The impacts of within-stream physical structure and riparian buffer strips on semi-aquatic bugs in Southeast Asian oil palm. Front. For. Glob. Change. 6:1203513. doi: 10.3389/ffgc.2023.1203513
Edited by:
Catherine M. Yule, University of the Sunshine Coast, AustraliaReviewed by:
Alan Feest, University of Bristol, United KingdomDarshanaa Chellaiah, Swedish University of Agricultural Sciences, Sweden
Copyright © 2024 Harianja, Luke, Barclay, Chey, Aldridge, Foster and Turner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina F. Harianja, bWZoNDZAY2FtLmFjLnVr; bWFydGluYS5mLmhhcmlhbmphQGdtYWlsLmNvbQ==
†ORCID: Martina F. Harianja orcid.org/0000-0002-9607-6151
Sarah H. Luke orcid.org/0000-0002-8335-5960
Holly Barclay orcid.org/0000-0002-0027-2570
Vun K. Chey orcid.org/0000-0003-3038-9494
David C. Aldridge orcid.org/0000-0001-9067-8592
William A. Foster orcid.org/0000-0002-2535-8012
Edgar C. Turner orcid.org/0000-0003-2715-2234
 Martina F. Harianja
Martina F. Harianja Sarah H. Luke1,2†
Sarah H. Luke1,2† Holly Barclay
Holly Barclay Vun K. Chey
Vun K. Chey William A. Foster
William A. Foster Edgar C. Turner
Edgar C. Turner