- 1Megafauna Ecology and Conservation Group, Southeast Asia Biodiversity Research Institute and Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan, China
- 2School of Environmental and Geographical Sciences, The University of Nottingham Malaysia, Semenyih, Selangor, Malaysia
- 3Faculty of Science, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei
- 4Institute for Biodiversity and Environmental Research, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei
- 5Department of Biology, Florida Museum of Natural History, University of Florida, Gainesville, FL, United States
- 6School of Science and Engineering, James Cook University, Cairns, QLD, Australia
- 7Kuala Gandah National Elephant Conservation Center (NECC), Lanchang, Malaysia
- 8Department of Wildlife and National Parks (DWNP), Kuala Lumpur, Malaysia
Megaherbivores exert strong top-down influence on the ecosystems they inhabit, yet little is known about the foraging impacts of Asian elephants (Elephas maximus) on the structure of Southeast Asia’s rainforests. Our goal was to document Asian elephants’ dietary composition, selectivity, and foraging impacts in a Sundaic rainforest and test whether these differed between habitats. We conducted controlled direct observations of five wild-born captive elephants feeding on six plant types (bamboo, grass, monocot herbs, palms, lianas, and trees) of different age classes in two habitats (mature vs. early successional forest) in Krau, Peninsular Malaysia. Palms, trees, and lianas formed the bulk of the elephants’ diet. In the mature forest, elephants showed a strong preference for monocots (preference ratio, PR = 5.1), particularly large palms (PR = 5.4), while trees were negatively selected (PR = 0.14). Conversely, in early successional habitats, large tree saplings were positively selected (PR = 1.6). Elephants uprooted (30%) and broke the main stem (30%) of the dicot trees, mainly large saplings, that they handled. Tree saplings broken by elephants had an average diameter of 1.7 ± 1.1 cm (up to 7 cm), with breaks happening at 1.1 ± 0.5 m of height. We estimated that, in a year, an elephant could damage (i.e., either uproot or break) around 39,000 tree saplings if it fed entirely in mature forest, and almost double the number (73,000) if it fed solely in early successional habitats. Assuming a density of 0.05–0.18 elephants/km2, elephant foraging could damage 0.2–0.6% of the tree sapling population per year. Slow growth rates of understory plants in mature forests could result in negative feedbacks, whereby elephants suppress palms, other monocots, and highly preferred tree species. Alternatively, elephants may initiate positive feedbacks by impeding succession along forest edges and in semi-open environments, thereby increasing the size of gaps and the availability of their preferred foodplants. Overall, our results show that Asian elephants act as ecological filters by suppressing the plants they prefer in Southeast Asia’s rainforests.
Introduction
As the largest terrestrial animals, elephants impact ecosystems by acting as important long-distance seed dispersers (Campos-Arceiz and Blake, 2011; Ong et al., 2022), mobilizing nutrients (Wolf et al., 2013), creating patch-scale heterogeneity and microhabitats (Pringle, 2008; Haynes, 2012), and modifying the structure and composition of vegetation through selective feeding (Dublin et al., 1990; Holdo, 2006; Berzaghi et al., 2023). Although the impact of elephant foraging in savannas has been extensively studied, much less is known about how elephant herbivory influences tropical forests (Hyvarinen et al., 2021).
Recent work has highlighted the importance of African forest elephants (Loxodonta cyclotis) in shaping Central African forests. Terborgh et al. (2016a) proposed the “megafaunal landscape” hypothesis to explain the relatively low hectare-scale diversity of trees in forests of equatorial Africa. They found in Gabon that low tree diversity was caused by a relatively low diversity of saplings and small trees, which they attributed to the filtering effect of browsing by megafauna, mostly elephants (Terborgh et al., 2016a). Moreover, the density and diversity of small trees in several forests in Gabon were inversely associated with elephant density (Terborgh et al., 2016b). Subsequent modeling work supported the megafaunal landscape hypothesis, showing that, by removing saplings, elephants facilitate the growth of large trees, which also increases above-ground carbon sequestration (Berzaghi et al., 2019). Selective browsing by forest elephants also stabilizes forest-savanna mosaics (Cardoso et al., 2020).
Less is known about the impacts of Asian elephant (Elephas maximus) foraging on ecosystems, particularly in equatorial wet forests of Southeast Asia. Asian elephants cause wide-spread crown distortion in small-stature trees in Sri Lanka’s dry evergreen forest (Mueller-Dombois, 1972), damage 1–3% of the trees (≥8 cm diameter at breast height, dbh) in recently recolonized forests of Nepal’s Bardia National Park (Pradhan et al., 2007), and contribute to the maintenance of forest gaps by damaging early successional Macaranga spp. trees in Sundaic rainforests of Sabah, Borneo (Matsubayashi et al., 2006). In Peninsular Malaysia, the density and diversity of tree saplings as well as the density of large palms and other monocots is higher in a forest where elephants have been absent for over 20 years (Krau) than in a forest where they are present and common (Belum; Terborgh et al., 2018). The frequency of stem break scars in Krau and Belum, however, does not differ and is much lower than in Gabonese forests (Terborgh et al., 2018), suggesting potential differences in the type of impacts caused by forest elephants in the two continents.
The influence of elephants on plant communities and forest structure is linked tightly to their foraging habits and dietary needs. Adult elephants can forage for 12–19 h and consume about 150 kg of fresh vegetation per day (Vancuylenberg, 1977; Sukumar, 2006). As mixed feeders, Asian elephants consume both grasses and browse (Sukumar and Ramesh, 1995; Sukumar, 2003), and their diet and foraging strategy can differ greatly with landscape and seasonal conditions (e.g., Sukumar, 1990; Chen et al., 2006; Joshi and Singh, 2008). With their prehensile trunk, body strength, and tusks (absent in Asian elephant females), elephants manipulate plants skillfully, being very versatile in processing the plants and plant parts they consume. Elephants handle plants by stripping out leaves from branches, debarking trees, breaking off trunks and branches, uprooting saplings, pulling down lianas, or shaking trees to harvest fruits (Ishwaran, 1983; Pradhan et al., 2007). Understanding elephants’ food preferences and the way they handle different food plants form the basis for determining their foraging impacts.
Peninsular Malaysia is home to approximately 1500 wild Asian elephants that roam through a mix of primary, selectively logged, and highly disturbed forests (Saaban et al., 2011; de la Torre et al., 2021). Once widespread throughout the Malay Peninsula (Olivier, 1978), elephant populations are now scattered in fragmented forest complexes (de la Torre et al., 2019). Under the current scenario of ongoing deforestation and defaunation (Miettinen et al., 2011; Tilker et al., 2019) it is important to close the knowledge gap on megafauna’s impacts on Southeast Asia’s rainforest ecosystems.
In our previous work (Terborgh et al., 2018), we used indirect methods (i.e., comparing vegetation structure, composition, and diversity in forests with and without elephants) to evaluate elephant foraging impacts in Southeast Asian rainforest, and we surmised that elephants act as ecological filters, suppressing the abundance of their preferred foodplants (Terborgh et al., 2018). Here we use direct methods—observations of captive elephants foraging unrestrained in forest and early succession habitats—to expand our understanding and test our previous conclusions. Particular objectives of this study were to document diet composition, selectivity among available plant materials, and impacts on forest structure; and to test whether these differ between two habitats. The elephants in our study (all females) had been captured as adults or subadults and hence had all experienced life in the wild. This work is fundamentally important to the understanding of megaherbivore and community ecology, as well as conservation and restoration efforts in Southeast Asia.
Materials and methods
Study area
We observed foraging female elephants in Krau Wildlife Reserve (hereafter Krau; 3°43′N, 102°10′E), located east of the Titiwangsa Range, in the State of Pahang, in Peninsular Malaysia (Supplementary Figure 1). Krau includes approximately 624 km2 of protected forest, ranging from lowland to hill dipterocarp, and montane forest (Nizam et al., 2006). There are no wild elephants in Krau, as the last individuals were translocated in 1993 to reduce conflicts with villages in and around the protected area.
The observed elephants came from the Kuala Gandah National Elephant Conservation Centre (NECC), a government-run facility adjacent to Krau that maintains captured elephants, including several resident elephants from the 1993 Krau translocation, and also elephants from other forests around Peninsular Malaysia, mostly areas with intense human-elephant conflicts. NECC is managed by the Elephant Capture and Translocation Unit for the protection and conservation of translocated wild elephants. The unit engages NECC elephants in wild elephant translocation operations; hence, NECC’s elephants often spend time in the forest (both in Krau and during translocation operations in other parts of the country). Unlike conventional paired bonds between a mahout and his elephant, all NECC’s mahouts share close bonds with several elephants.
Adequate food provision is an important component of NECC’s management. NECC provides elephants a diversity of food items throughout the day and evening, and NECC elephants are brought to forage in the surrounding forests nearly every day (Supplementary Table 1). Elephants receive balanced nutrition and physical and mental stimulation through forest enrichment activities, including scrubbing against trees, and bathing in rivers and mud pools. In this study, we conducted feeding observations in the morning, until noon, and elephants were fed as usual outside the observation times (refer to Supplementary Table 1), minimizing the disruption of their food provisioning during the study period.
Our study involved relatively close-distance (∼5 m) observations of elephant food choices and plant handling, which cannot be done with wild elephants. NECC’s captive elephants were thus adequate proxies to study Asian elephants’ food choices and plant handling behavior. During our observations, the elephants were generally comfortable with the presence of the observers, and this ensured the safety of the team as well as reducing errors that could arise from stress.
We conducted the study mostly in secondary lowland dipterocarp forests mixed with older stands, and observed the elephants in two different habitat types: (1) closed forest (ten patches) and (2) early successional habitat (seven patches). The patches were around half to one hectare in size, depending on how much the elephants moved, since they were free to roam during the observation periods (see below). The selected forest patches were largely undisturbed lowland dipterocarp forest. The early successional patches consisted of a matrix of forest edge and recovering agricultural plots. Some of the early successional sites were dominated by grasses and saplings of pioneer trees, whereas others contained shrubs growing amidst coppiced trees from shifting agriculture (Supplementary Figures 2, 3).
Research permits
Animal handling was carried out by the staff of Peninsular Malaysia’s Department of Wildlife and National Parks in accordance with research and ethics requirements by the Malaysian government [permit #JPHL%TN(IP): 80–4/2]. In addition, the first author received ethical approval for her Ph.D. research from the University of Nottingham Malaysia Science and Engineering Ethics committee (application identification number LO081016), with an emphasis on ethical concerns involving human participants.
Data collection
Feeding observations
We conducted direct feeding observations on five wild-born captive female elephants of different ages from NECC (Supplementary Table 2). Elephants were directed by the mahouts to the chosen patches where they were released to feed freely. The feeding patches were within 2 km from NECC (Supplementary Figure 1), and at least 200 m away from one another; most of which were previously unvisited by the elephants. Pairs of observers were assigned to each elephant, one who described the elephant’s foraging activities and another who recorded the first’s observations. Collectively, we were able to conduct observations of two or three elephants on a given day. Observations were made at distances of around 5 m from the elephants.
We recorded foraging in a total of 40 half-hour sessions—24 in mature forest and 16 in early successional sites for a week in May 2017. Specifically, we recorded the way elephants handled plants, the number of trunkfuls, and the type and size of plants consumed. We recognized six forms of plant handling: leaf stripping, branch breaking, stem breaking, debarking, uprooting, and pushing-over of trees (see Figure 1 and Supplementary Figure 4 for details). When elephants fed on grasses without uprooting them, we recorded the behavior as “stem breakage” (grass uprooting was recorded as such).
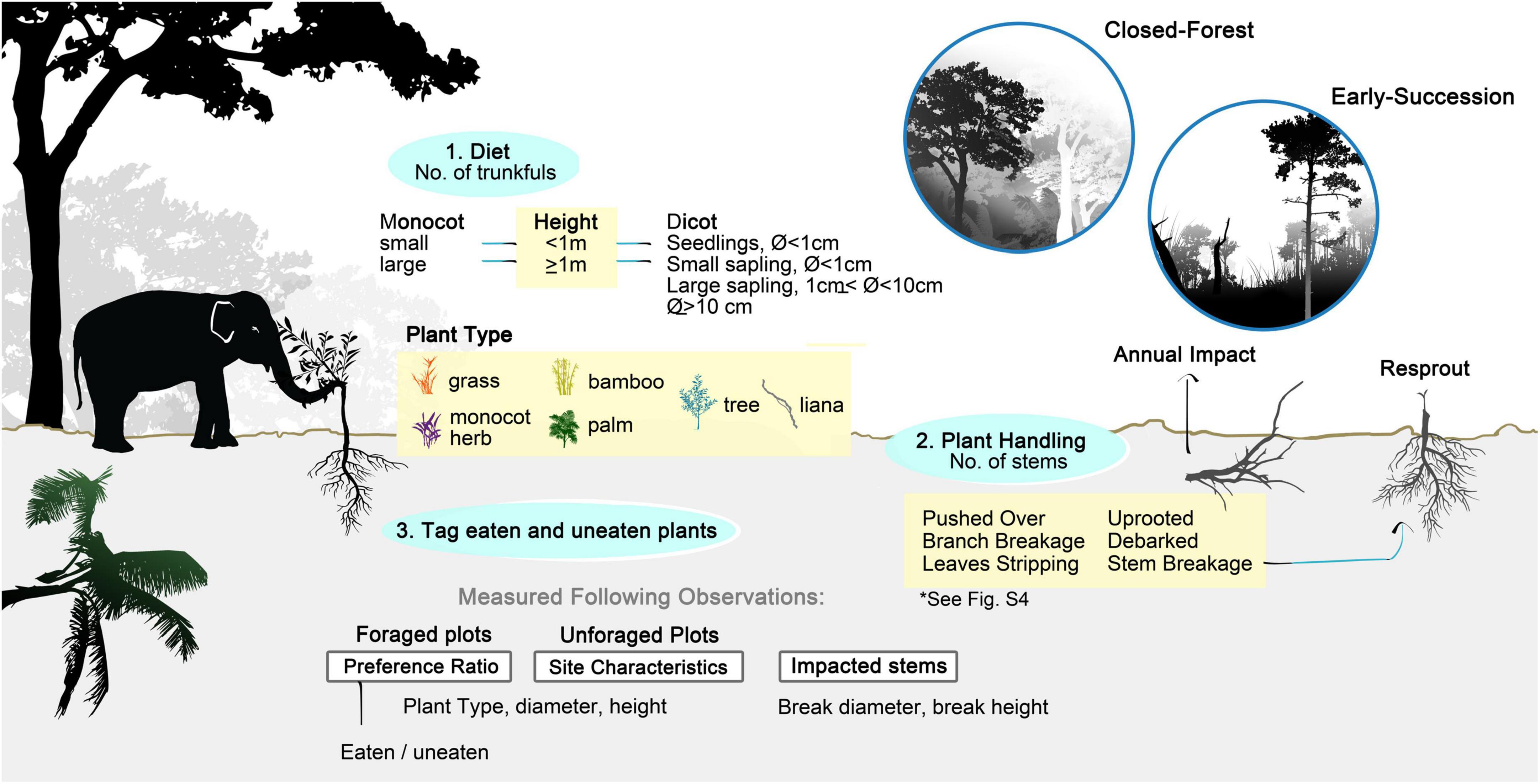
Figure 1. Sampling diet, food preference and impact of Asian elephant through direct observation and plot measurements. Plant tagging and measurements associated with diet and plant handling were recorded during direct observations, while information linked to preference ratio, site characteristics and impacted stems were collected following feeding.
We quantified feeding in trunkfuls of plant matter. We categorized monocots as bamboos, herbs (mostly gingers), grasses, and palms, distinguishing small (<1 m tall) and large (≥1 m tall) individual stems. Similarly, we classified dicots either as trees or lianas, also in size categories: seedlings (<1 m tall), small saplings (≥1 m tall and <1 cm dbh), large saplings (≥1 cm dbh and <10 cm), and trees (dbh ≥10 cm).
Tracing damaged stems
Elephants are destructive feeders and often break or uproot plants without eating them. During the feeding observations, we marked the plants broken by elephants with colored paper tags to distinguish between those “eaten” and those left “uneaten” (Figure 1). Following the observations, we recorded the plant type, category of plant handling, break height, and diameter of impacted stems from a total of 369 tagged stems. We scored them as damaged, debarked, broken at the main stem, or uprooted.
Food preference and impact plots
We analyzed vegetation plots to assess elephants’ preferences and herbivory impacts per area (Figure 1). At each feeding patch, we measured plant availability and utilization in three 2-m-radius circular plots (area = 12.6 m2), including two plots where elephants had been feeding and one control (i.e., un-foraged) plot. This plot size allowed us to capture fresh signs of elephant damage, concentrated mostly on saplings. We sampled a total of 49 plots including 20 foraged and ten un-foraged plots in the forest (from ten sites) and 13 foraged and six un-foraged plots in early successional habitat (from seven sites). In these plots, we measured only plants ≥1 m tall, as it was difficult to quantify plants below 1 m, many of which could have been uprooted by elephants leaving little trace of damage. Within each plot, we recorded plant types and stem diameter, breakage height and diameter, and whether the plants were eaten.
Data analysis
Principal vs. preferred foods
Following Petrides (1975), we considered principal foods as those consumed in the greatest quantities, and preferred foods as those consumed at a higher frequency than their availability. We determined the principal foods of both habitats by comparing the average proportion of plants consumed by the elephants per observation session. We estimated preference ratios (PR) modifying methods from previous studies in the region (Olivier, 1978; English et al., 2014), whereby we estimated preference ratios (PR) for all plant categories sampled as:
Plants that were broken but not eaten were not considered “used.” When a plant category was available but not used in a patch, we considered its PR as 0. A PR value of 1 indicates no selection (the plant is eaten in proportion to its availability), while values above 1 indicate preference and values below 1 indicate avoidance.
To compensate for small sample sizes, we simulated the final PR values from a range of 2-33 plots, depending on the availability of plant categories in the plots. We used Bayesian analysis (uninformative gamma prior) with Markov chain Monte Carlo (MCMC) methods to estimate a credible interval (CI) and range for each plant category in its respective habitat (package wiqid; Meredith, 2018); where negative CI numbers indicate possible unreliable estimations. These analyses were performed in R statistical environment 4.0.5 (R Core Team, 2021).
Elephant herbivory impact
We evaluated elephant foraging impacts by comparing the number of plants that were uprooted, or had their main stem broken, through direct observations. We traced damaged stems both within and outside plots (to increase the confidence of break estimates), to measure the diameter (N = 196) and height (N = 171) of tree stems broken by the elephants. We also measured the diameter of 13 broken lianas.
Taking into consideration that elephants forage around 12–19 h per day (Vancuylenberg, 1977; Sukumar, 2006), we estimated the potential annual herbivory impact (i.e., feeding for 5,475 h; 15 h × 365 days) caused by an elephant using probability density functions. While the potential annual impact of dicots was represented by stems that were uprooted and had their main stem broken, the potential annual impact of monocots was represented only by uprooted stems. With basally regenerating leaves and stems that are predominantly underground, we expected the impacts of stem breakage to be less severe for monocots. Since elephant movements and habitat use are highly variable (e.g., de la Torre et al., 2021), we considered three scenarios, estimating the foraging impacts of an elephant that spends (i) 100% of its time feeding in the forest, (ii) half in each habitat, and (iii) 100% in early successional habitats.
Models
We evaluated elephant diet and foraging impacts fitting negative binomial linear regressions that model over-dispersion with R’s glm.nb function in the “MASS” package (Venables and Ripley, 2002). We used likelihood-ratio tests to evaluate the effect of the tested variables. We determined the principal elephant food plants, and their combined effects on habitats using the number of trunkfuls of each plant category consumed per observation session by the elephants as the response variable. As explanatory variables, we used plant class (monocot vs. dicot), plant types (six categories), plant size (two categories for monocots; four categories for dicots), habitat (forest vs. early succession), and individual elephant (five subjects). The effects of elephant’s diet were determined using four models: (1) trunkfuls consumed ∼ plant class × habitat, (2) trunkfuls consumed ∼ plant class of different sizes × habitat, (3) trunkfuls consumed ∼ plant types × habitat, and (4) trunkfuls consumed ∼ plant types of different sizes × habitat. We determined the effects of elephants’ impacts (i.e., plant uprooting or main stem break) with two models: (1) number of stems impacted ∼ plant type × habitat, and (2) number of stems impacted ∼ plant type of different sizes × habitat.
Results
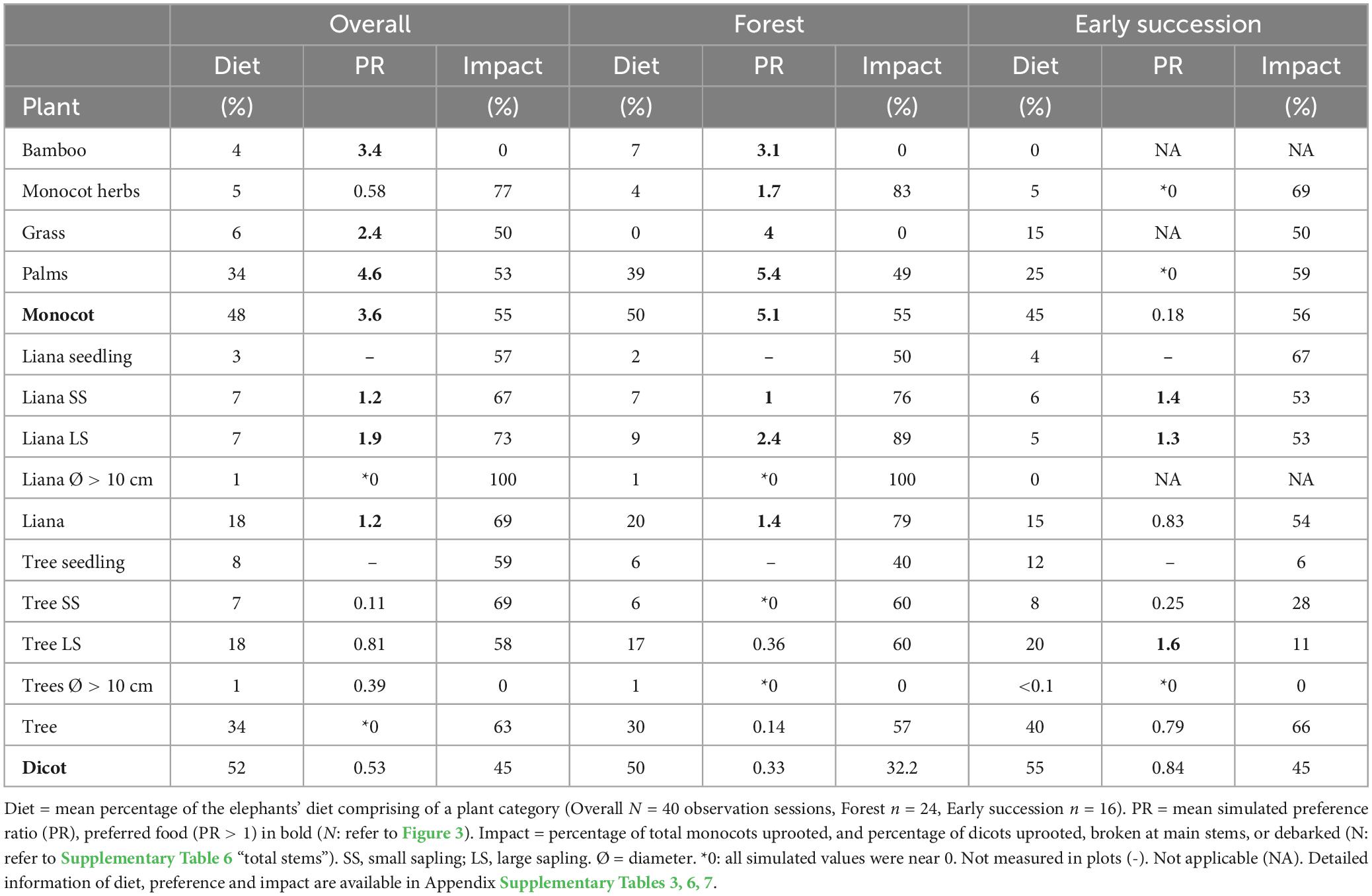
Diet composition
Elephants fed at a rate of 57.7 ± 20.3 trunkfuls per hour (N = 40 observation sessions, a total of 20 h). The principal diet of the elephants was palms and tree saplings, particularly palms above 1 m and large tree saplings (Figure 2, Table 1 and Supplementary Table 3). Their diet was explained by the differences between plant classes of different sizes (df = 5, X2 = 86.1, p < 0.001), plant types (df = 5, X2 = 33.6, p < 0.001), and plant types of different sizes (df = 15, X2 = 146.1, p < 0.001; Supplementary Figure 5 and Supplementary Tables 4, 5).
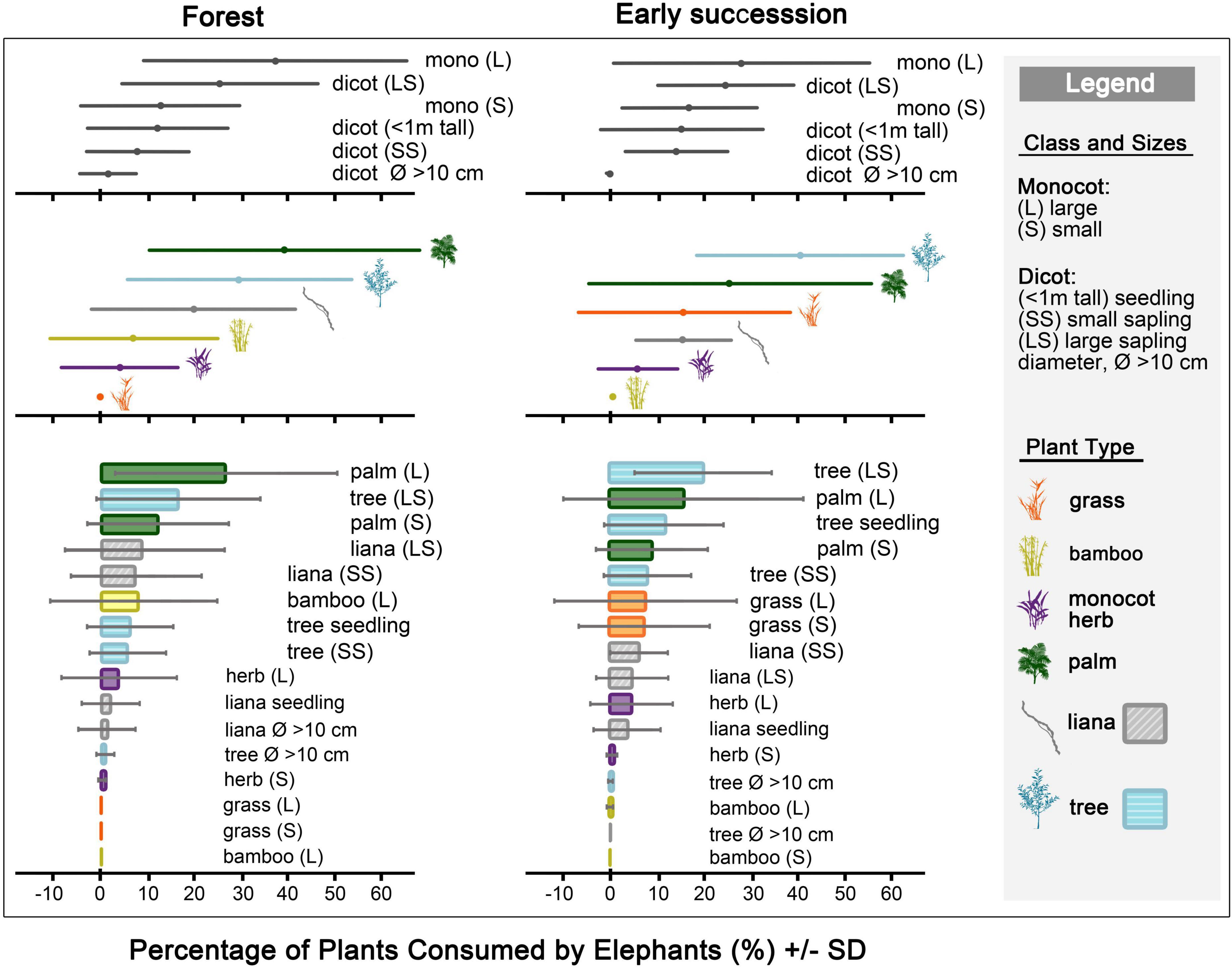
Figure 2. Relative percentage of plants (mean ± SD) consumed by elephants (N = 40 observation sessions) in the forest (n = 24 obs.), and in early successional habitats (n = 16 obs.).
In the forest (n = 24 obs.), palms, tree saplings, and lianas were the elephants’ main diet (Figure 2, Table 1 and Supplementary Table 3). Elephants consumed high quantities of monocots above 1 m tall, and large dicot saplings. Palms of all sizes, large tree saplings, bamboos above 1 m, and liana saplings were common elephant foodplants.
In early successional habitats (n = 16 obs.), trees formed the bulk of elephants’ diet, while palms were eaten in moderately high quantities (Figure 2, Table 1 and Supplementary Table 3). Large tree saplings and palms above 1 m tall were key elephant foodplants. Small tree seedlings and saplings, small palms (below 1 m tall), grasses of all sizes, and small liana saplings were moderately consumed.
Although we did not test for individual differences in elephant feeding habits, we found some noteworthy patterns. For example, the youngest elephant (Cherry, 8 years old), consumed more bamboo (25%, n = 3 obs.) than average (4%, N = 40 obs.); while the oldest elephant (Timur, 43 years old), consumed more trees (48%, n = 3 obs.) than average (34%, N = 40 obs.; Supplementary Tables 2, 3).
Food preferences
Elephant preferences varied by plant and habitat types (Figure 3, Table 1 and Supplementary Table 6). Overall, elephants showed a strong preference for palms (PR = 4.6), while they avoided trees (PR = 0.39). In the forest, elephants showed a strong preference for monocots (PR = 5.1) over dicots (PR = 0.33). Large palms (PR = 5.4) and large liana saplings (PR = 2.4) were the most preferred categories, whereas large tree saplings were harvested more selectively (PR = 0.36). In early successional habitats, elephants showed neither preference nor avoidance for dicots (PR = 0.84). Specifically, they showed a preference for large tree saplings (PR = 1.6), and avoided small tree saplings (PR = 0.25). As plants below 1 m were not sampled in the early successional habitats, the sample sizes of certain plant categories were too small to provide preference estimates or provided low-reliability estimates (as reflected by simulated negative credible intervals; Figure 3 and Supplementary Table 5).
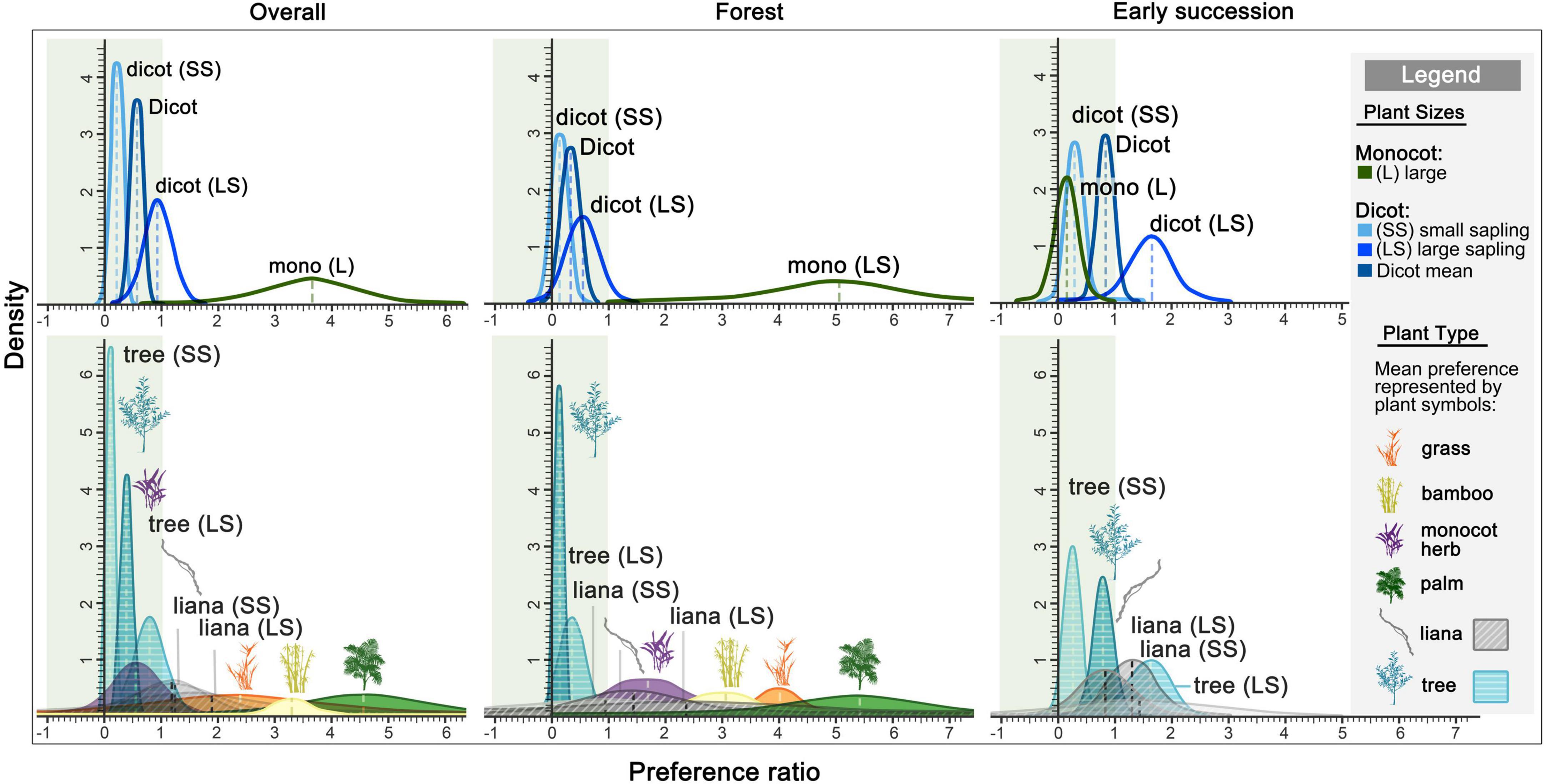
Figure 3. Density distribution of elephant preference for plants above 1 m tall simulated from sampled plots. Elephant preferred plants have a preference ratio (PR) of 1 or greater, while negative values imply less reliable simulations. The mean ratio of each plant type is represented by the plant symbol, while the mean ratio of plants in different sizes are labeled (legend). The breakdowns of PR and CI, and each sample size can be found in Supplementary Table 6.
Herbivory impacts
Plant handling
Elephants uprooted a high percentage (mean = 54.2%, range = 30–77%; Figure 4 and Supplementary Table 7) of the plants they handled (N = 873 stems), particularly herbs (77%, n = 74), and palms (52.5%, n = 204). They also broke many plants at their main stem (mean = 26%, range = 9.8–49.5%), notably trees (30.3%, n = 323), and lianas (28.7%, n = 129). Their impacts (uprooting or main stem breakage) were different between plant types (df = 18, X2 = 63.7, p < 0.001), plant types in different habitats (df = 20, X2 = 71.2, p < 0.001), plant types of different sizes (df = 45, X2 = 103, p < 0.001), and different-sized plant types in different habitats (df = 47, X2 = 112, p < 0.001; Supplementary Figure 6).
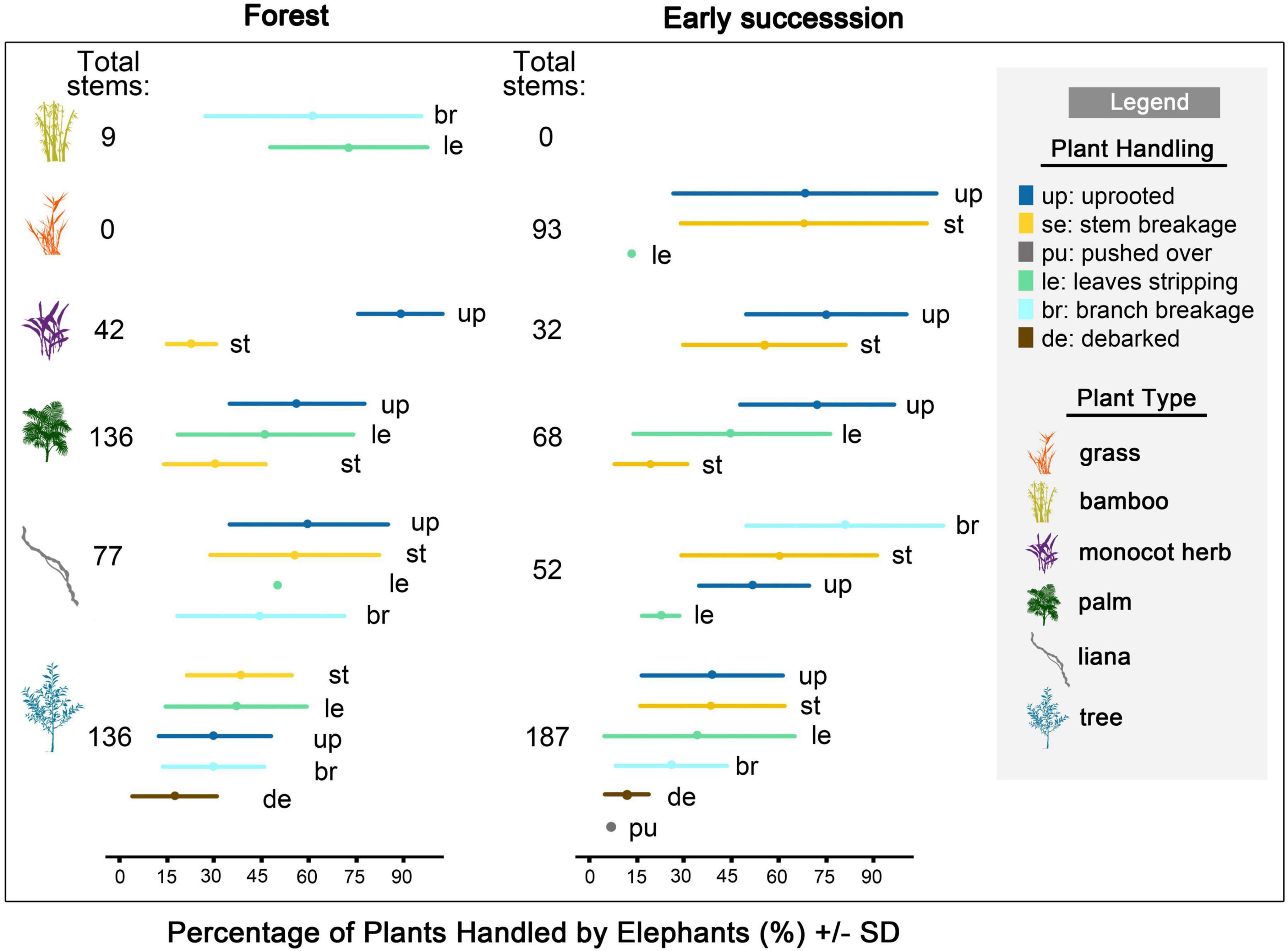
Figure 4. Total number of stems, and the relative herbivory impact of plants (%) handled by elephants in the forest and in early successional habitats (mean ± SD) (N = 40 observation sessions). Forest: bamboo (n = 4 observation sessions), monocot herb (n = 4), palms (n = 21), liana (n = 18), trees (n = 22); Early succession: grass (n = 8), monocot herbs (n = 9), palms (n = 10), liana (n = 15), tree (n = 16). Stems handled were either uprooted, broken at main stem, pushed-over, had their branches broken, leaves or bark stripped.
In the forest (N = 417 stems), many palms (49%, n = 136 stems) and a large proportion of herbs (83%, n = 42) handled were uprooted, while trees (29.4%, n = 136) were commonly broken. In early successional habitats (N = 456 stems), trees were frequently uprooted (33.2%, n = 187 stems) and broken (31%), while a large proportion of herbs (68.8%, n = 32) and palms (58.8%, n = 68) were uprooted (Figure 4 and Supplementary Table 7).
Out of 369 damaged stems tagged during the observations, 46% had been eaten and the remaining 54% had been damaged without being fed upon. These percentages exclude many uprooted small plants that had been consumed by the elephants entirely. The average diameter of trees uprooted and broken by elephants was 1.7 ± 1.1 cm (N = 196 stems; Supplementary Figure 7) and the average height of stem breaks was 1.1 ± 0.5 m tall (N = 171). Most broken tree stems were large saplings (≥1 cm dbh and <10 cm). The average diameter of uprooted and broken lianas was 1.2 ± 1.0 cm (N = 20; Supplementary Figure 7).
Site characteristics
Extrapolations from un-foraged plots showed that both habitats had similar overall stem densities: 153 ± 56 stems per 100 m2 (n = 10 plots) for forest patches and 153 ± 62 stems per 100 m2 (n = 6 plots) for early successional habitats. Nonetheless, finer comparison revealed higher tree density in the forest (111 ± 59 stems per 100 m2) than in early successional habitats (98 ± 29 stems per 100 m2). Large palms (12 vs. 5 per 100 m2), large tree saplings (65 vs. 44 per 100 m2), and large liana saplings (12 vs. 7 per 100 m2) were also more abundant in the forest than in early successional habitats, whereas monocot herbs and small liana saplings were more abundant in early successional habitats (Supplementary Table 8).
Annual foraging impacts
An elephant that spent a whole year feeding in a mature forest would damage ∼39,000 tree saplings (simulated based on data from N = 19 observation sessions), roughly uprooting (N = 12) and breaking (N = 18) the same number of trees. An elephant that spent a whole year feeding in an early successional habitat would damage ∼73,000 saplings (N = 15). An elephant that spent half of its time in each environment would damage ∼20,000 forest and ∼37,000 pioneer saplings (Figure 5).
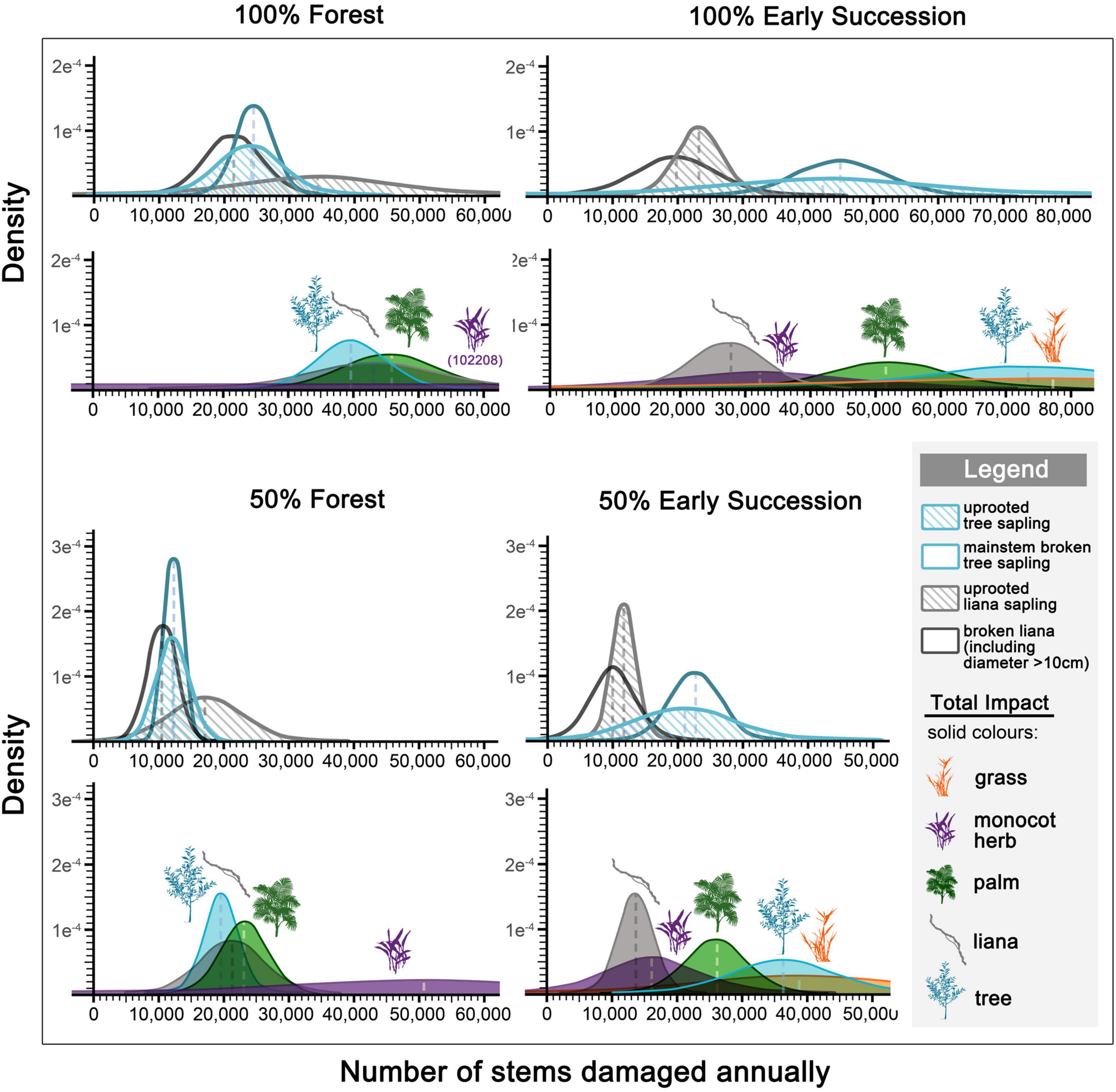
Figure 5. Density distribution of annual elephant herbivory impact (uprooted vs. broken mainstems) (outlined diagrams), and overall impact (i.e., uprooted monocots, and uprooted and broken dicots) on plants (colored fills). Estimations are based on the amount of time (either 100% or 50%) an elephant spent feeding in forest and early successional habitats, simulated from feeding observation sessions (N = 40; forest = 24, early successional habitats = 16).
Discussion
Free-ranging Asian elephants foraging in lowland dipterocarp forest and early successional habitats consumed monocots and dicots in similar proportions. They showed, however, strong selectivity toward monocots, particularly palms in the forest. In early successional habitat, elephants showed a preference for saplings of certain dicot trees, especially Macaranga spp. Foraging elephants uprooted and broke large numbers of palms and tree saplings. Size-selective browsing of small saplings supported the possibility of elephants reducing stem densities in forests over long-term feeding. They may initiate positive feedbacks and impede succession along forest edges, and negative feedbacks in mature forests by slowing the growth of the plants they prefer.
Habitat (mature forest vs. early succession) had a strong influence on elephant diet composition and food preferences. While palms and tree seedlings and saplings constituted the main components of the elephant diet, the proportions consumed differed between habitats (Figure 2). In early successional patches, elephants especially targeted large saplings of pioneer tree species (e.g., Macaranga and Mallotus spp., Euphorbiaceae) (PR = 1.6) which contrasted with a low preference for saplings in the forest (PR = 0.36). Although monocots, especially palms, were strongly preferred in the forest, we were not able to obtain reliable preference estimates for them in early successional plots.
Palms were the most preferred food and represented around one-third of what elephants ate in the forest, even though they were relatively rare (<10 % of stems in the forest). However, it is important to note that there have been no wild elephants in Krau for over 20 years and, in our previous work (Terborgh et al., 2018), we described how palms, particularly those >1 m tall, are four times more abundant in Krau than in Belum, another Malaysian forest where wild elephants are common. Given that many palms are slow growing (e.g., Lugo and Batlle, 1987) and elephants process them in highly destructive ways, it is likely that palm density in Krau would be much lower if wild elephants were still present. Where palms are rarer, we would expect a lower overall contribution to the diet and potentially even higher PR values. Olivier (1978) described Malaysian elephants as “palmivores,” a term that is likely to better describe elephants’ preference than their actual diet in areas where they have been continuously present. In such areas, dicot seedlings and saplings, followed by lianas, are likely to be the major components of the elephant diet.
We were surprised by how much time elephants spent pulling down lianas and even thin vines, a feeding behavior we would not have noticed if we had used any of the other methods to study elephant diet. We also observed situations in which elephants debarked saplings and uprooted saplings and other plants (e.g., gingers) to feed only on subterranean organs of the plant, roots, or tubers. The Orang Asli, the indigenous people of the Malay Peninsula have co-existed and adapted to living with elephants in the forest since their arrival around 55,000 years ago (Lim and Campos-Arceiz, 2022). They describe elephants as “forest cleaners” (T. Lim pers. comm.), hence, in the absence of elephants and the disturbance they produce, we can expect a higher presence of lianas and other understory plants.
Asian elephants can thrive along forest edges, since they show strong preferences for early successional vegetation; but they rarely venture far away from forest cover (e.g., Evans et al., 2018; Wadey et al., 2018; de la Torre et al., 2019, 2021). Their preference for edges is generally attributed to the higher availability of palatable plants in early successional habitats (Yamamoto-Ebina et al., 2016). Our results shed light on how feeding ecology underlies a preference for disturbed over mature forests, at least in Sundaic forests, and remind us that traditional shifting agriculture may improve elephant habitats (Olivier, 1978; Lim et al., 2019).
Combining information about stem density, elephant density, and damage rates, we can estimate Asian elephant foraging impacts in Sundaic forests. In our previous work (Terborgh et al., 2018), we described a mean density of 120.7 saplings per 100 m2 in Belum, an unlogged mature forest, where elephants are presumed to occur at near carrying capacity. While there is no reliable elephant density estimate for Belum, estimates exist for comparable environments: 0.05 elephants/km2 in Peninsular Malaysia’s Endau Rompin (Saaban et al., 2020); 0.07 elephants/km2 in northern Borneo (Cheah and Yoganand, 2022); and a strata-weighted density of 0.18 elephants/km2 in protected areas of southern Sumatra (Hedges et al., 2005). These values are close to the carrying capacity of 0.1 elephants/km2 predicted by Sukumar (2003, page 357) for Asia’s tropical rainforests. We can therefore assume a density of 0.05–0.18 elephants/km2 in Belum. And, in this study, we reported that an Asian elephant feeding in the forest could damage ∼39,000 saplings per year (half of them by means of uprooting and the other half by stem breaking). Altogether, these numbers (∼1,207,000 saplings and 0.05–0.18 elephants per km2, and ∼39,000 saplings damaged per elephant year–1) suggest that, in a forest like Belum, foraging by Asian elephants could be damaging 0.16–0.58% of the tree sapling population per year (Supplementary Table 9).
In our observations, 95% of the foraging damage happened to saplings ≤3.5 cm in diameter. Assuming a conservative annual diameter growth of 0.1 cm per year for tree saplings up to 3.5 cm in diameter (see Figure 4a in King et al., 2006 for sapling growth rates in Pasoh, Peninsular Malaysia), tree saplings (not considering the seedling stage) could spend 35 years in a “window of vulnerability” until they grow beyond the size where they are vulnerable to elephants. This simple back-of-the-envelope calculation shows the scale of Asian elephant foraging impacts in Southeast Asian forests. It is, however, important to note that not all damages will lead to mortality as roughly 90% of woody stems are able to resprout and continue growing following stem breakage (Ickes et al., 2003; Terborgh et al., in press). Moreover, saplings’ growth rates are highly variable between species and under different site-specific factors (Turner, 1990; Shono et al., 2007). Sapling densities in elephant-free Pasoh are probably higher than in comparable forests with elephants. Lower sapling densities in elephant-occupied forests could ease density-dependent growth (Berzaghi et al., 2019), thus reducing the period of vulnerability (Supplementary Table 9).
Given the slow growth rates of understory saplings, it would be possible for elephant foraging to result in negative feedbacks in the forest, i.e., depressing the abundance of their most preferred forage species, especially palms and other monocots. On the contrary, growth rates of plants in the accessible height range of elephants are high along edges and in semi-open environments. In such habitats, elephant feeding might trigger a positive feedback, whereby elephants contribute to decelerate succession, increasing the availability of their preferred food (e.g., Matsubayashi et al., 2006).
We observed captive elephants in an environment where wild elephants have been absent for over 20 years. Although this could bring some biases, e.g., due to the high abundance of palms, we believe the food preference of the wild-born elephants we observed are not likely to differ substantially from wild individuals. Plant consumption, food choices, and herbivory impacts could vary depending on a wide range of factors such as fluctuations in feeding intensity associated to specific elephant behaviors (e.g., resting vs. moving fast) or environmental conditions (e.g., increased browsing during the dry season; Wyatt and Eltringham, 1974), and natural (e.g., topography, habitat heterogeneity) and anthropogenic (e.g., barrier effects, human-elephant conflicts) factors that influence elephant movements and feeding strategies (Boettiger et al., 2011; Terborgh et al., 2016b; Neupane et al., 2019; Evans et al., 2020; Berzaghi et al., 2023). In Belum, for example, we might expect a higher relative impact on tree saplings due to a lower palm density as compared to Krau (Terborgh et al., 2018), where the estimate was derived from. Importantly, our studied elephants were all females, neglecting potential effects due to sex differences (e.g., Davies and Asner, 2019). With large tusks, male elephants have the ability to push over trees more often. This could be a contributing reason to why few trees were pushed-over in our study. We urge for more studies with larger sample sizes (across different ages and sexes) to be carried out in elephant landscapes with a similar approach to quantify these effects. Currently, there is no good method to study elephant diet due to sheer logistic problems and the difficulty of obtaining clear records amidst dense vegetation (e.g., differentiating consumed from unconsumed but damaged plants), and this approach of conducting direct observations at close range in the forest provided invaluable insights.
We demonstrated that Asian elephants play an important, yet poorly recognized, role as ecological filters in Southeast Asian forests, helping to serve as ecosystem engineers in mature forests through selective foraging. Despite their broad dietary breadth, elephants are selective feeders that cause severe damage to the plants they feed upon, and these impacts differ considerably between habitats (forest vs. early succession). Asian elephant foraging impacts translate into changes to forest structure, diversity, and composition (Terborgh et al., 2018). We have just begun to reveal the implications of Asian elephant herbivory on ecosystem function. A better understanding of elephant feeding selectivity and differential impacts on plants is important to understand the mechanisms of elephants’ filtering role in different ecosystems as well as its implications in broad-scale processes such as above-ground carbon sequestration (Berzaghi et al., 2019, 2023). As elephants continue to disappear from parts of their range (Williams et al., 2020), their loss will have cascading effects across ecosystems. These could include reduced above-ground carbon stocks due to the indirect effects of increasing fast-growing trees and liana loads (van der Heijden et al., 2015; Terborgh et al., 2016b; Berzaghi et al., 2023), seed dispersal limitation due to the loss of a dominant seed disperser (Campos-Arceiz and Blake, 2011; Ong et al., 2022), the co-extinctions of obligate plant species (Blake et al., 2009; Sekar et al., 2017), knock-on effects on animal communities due to changes in vegetation structure (e.g., Pringle, 2008), and loss of elephant-carved salt licks used by many species (Bowell et al., 1996). Conservation planning and practice should consider not only the population status of these charismatic animals, but also their important impacts on ecosystems. In highly disturbed environments, especially in mosaics of forest and early succession, elephants can occur at higher densities and utilize smaller home ranges. As elephant-inhabited forests continue to shrink and fragment, elephants concentrate on the edges, increasing the frequency of conflicts with humans (Neupane et al., 2019; de la Torre et al., 2021, 2022). It is important to preserve large reserves (Terborgh, 1974), and increase the connectivity of fragmented landscapes (de la Torre et al., 2019) to ensure that elephants continue to perform their ecological roles and to minimize their conflicts with local communities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study because Animal handling was carried out by the staff of Peninsular Malaysia’s Department of Wildlife and National Parks in accordance with research and Ethics requirements by the Malaysian government [permit #JPHL%TN(IP): 80–4/2]. In addition, the first author received ethical approval for her Ph.D. research from the University of Nottingham Malaysia Science and Engineering Ethics Committee (application identification number LO081016), with an emphasis on Ethical concerns involving human participants. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LO, AC-A, and JT conceived the idea. LO, AC-A, WT, LD, MKA, and JT designed the methodology and collected the data. MKA contributed critical resources. LO conducted data analysis and visualization. AC-A and KM supervised. LO wrote the first draft with support from AC-A, JT, and KM. All authors contributed critically to the drafts and gave final approval for publication.
Funding
We thank Yayasan Sime Darby (grant M0005.54.04) and the University of Nottingham Malaysia for funding LO scholarship and the field activities; and the Southeast Asia Biodiversity Research Institute (SEABRI: grant #Y4ZK111B01), Chinese Academy of Sciences for further development of the study.
Acknowledgments
We are very grateful to the Department of Wildlife and National Parks (DWNP), especially its Director General Dato’ Abdul Kadir bin Abu Hashim, for supporting this research. We are also very grateful to Nasharuddin bin Othman and the whole team from the Kuala Gandah National Elephant Conservation Center (NECC), especially its mahouts — Ali G., Fadhlul H.A.S, Khairul A., Muhammad I., Lukman M., Syed N.A., Sulaiman M., Rizuan M., and Mohamad S.A. for the arrangements and guidance of elephant feedings around Krau Wildlife Reserve. We appreciate the efforts of Param bin Pura, Husin Sudin A/L Din, Rizuan bin Angah, Vivienne P. W. Loke, Jeycern Loo, Ning Hii, and Alicia Solana-Mena for the time they devoted to the fieldwork; and Praveena Chackrapani for administrative assistance. Last but not least, we would like to dedicate this paper to the late Mike Meredith, who provided some feedback to an earlier version of this draft. Mike was previously Vice-Chair of the Biodiversity Conservation Society of Sarawak, and had been a dedicated and inspiring teacher of wildlife studies design and statistics to academics and conservationists in the region. Much of the statistics in this paper were adapted from his teachings.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1143633/full#supplementary-material
References
Berzaghi, F., Bretagnolle, F., Durand-Bessart, C., and Blake, S. (2023). Megaherbivores modify forest structure and increase carbon stocks through multiple pathways. Proc. Natl. Acad. Sci. U.S.A. 120:e2201832120. doi: 10.1073/pnas.2201832120
Berzaghi, F., Longo, M., Ciais, P., Blake, S., Bretagnolle, F., Vieira, S., et al. (2019). Carbon stocks in central African forests enhanced by elephant disturbance. Nat. Geosci. 12, 725–729. doi: 10.1038/s41561-019-0395-6
Blake, S., Deem, S. L., Mossimbo, E., Maisels, F., and Walsh, P. (2009). Forest elephants: Tree planters of the Congo. Biotropica 41, 459–468. doi: 10.1111/j.1744-7429.2009.00512.x
Boettiger, A. N., Wittemyer, G., Starfield, R., Volrath, F., Douglas-Hamilton, I., and Getz, W. M. (2011). Inferring ecological and behavioral drivers of African elephant movement using a linear filtering approach. Ecology 92, 1648–1657. doi: 10.1890/10-0106.1
Bowell, R. J., Warren, A., and Redmond, I. (1996). Formation of cave salts and utilization by elephants in the Mount Elgon region, Kenya. Geol. Soc. 113, 63–79. doi: 10.1144/GSL.SP.1996.113.01.06
Campos-Arceiz, A., and Blake, S. (2011). Megagardeners of the forest – the role of elephants in seed dispersal. Acta Oecol. 37, 542–553. doi: 10.1016/j.actao.2011.01.014
Cardoso, A. W., Malhi, Y., Oliveras, I., Lehmann, D., Ndong, J. E., Dimoto, E., et al. (2020). The role of forest elephants in shaping tropical forest–savanna coexistence. Ecosystems 23, 602–616. doi: 10.1007/s10021-019-00424-3
Cheah, C., and Yoganand, K. (2022). Recent estimate of Asian elephants in Borneo reveals a smaller population. Wildlife Biol. 2022:e01024. doi: 10.1002/wlb3.01024
Chen, J., Deng, X., Zhang, L., and Bai, Z. (2006). Diet composition and foraging ecology of Asian elephants in Shangyong, Xishuangbanna, China. Acta Ecol. Sin. 26, 309–316. doi: 10.1016/S1872-2032(06)60006-1
Davies, A. B., and Asner, G. P. (2019). Elephants limit aboveground carbon gains in african savannas. Glob. Chang. Biol. 25, 1368–1382. doi: 10.1111/gcb.14585
de la Torre, J. A., Cheah, C., Lechner, A. M., Wong, E. P., Tuuga, A., Saaban, S., et al. (2022). Sundaic elephants prefer habitats on the periphery of protected areas. J. Appl. Ecol. 59, 2947–2958.
de la Torre, J. A., Lechner, A. M., Wong, E. P., Magintan, D., Saaban, S., and Campos-Arceiz, A. (2019). Using elephant movements to assess landscape connectivity under Peninsular Malaysia’s central forest spine land use policy. Conserv. Sci. Pract. 1:e133. doi: 10.1111/csp2.133
de la Torre, J. A., Wong, E. P., Lechner, A. M., Zulaikha, N., Zawawi, A., Abdul Patah, P., et al. (2021). Towards tolerable human–elephant coexistence in tropical Asia. Anim. Conserv. 24, 740–742. doi: 10.1111/acv.12749
Dublin, H. T., Sinclair, A. R. E., and McGlade, J. (1990). Elephants and fire as causes of multiple stable states in the serengeti-mara woodlands. J. Anim. Ecol. 59, 1147–1164. doi: 10.2307/5037
English, M., Ancrenaz, M., Gillespie, G., Goossens, B., Nathan, S., and Linklater, W. (2014). Foraging site recursion by forest elephants Elephas maximus borneensis. Curr. Zool. 60, 551–559. doi: 10.1093/czoolo/60.4.551
Evans, L. J., Asner, G. P., and Goossens, B. (2018). Protected area management priorities crucial for the future of Bornean elephants. Biol. Conserv. 221, 365–373. doi: 10.1016/j.biocon.2018.03.015
Evans, L. J., Goossens, B., Davies, A. B., Reynolds, G., and Asner, G. P. (2020). Natural and anthropogenic drivers of Bornean elephant movement strategies. Glob. Ecol. Conserv. 22:e00906. doi: 10.1016/j.gecco.2020.e00906
Haynes, G. (2012). Elephants (and extinct relatives) as earth-movers and ecosystem engineers. Geomorphology 15, 99–107. doi: 10.1016/j.geomorph.2011.04.045
Hedges, S., Tyson, M. J., Sitompul, A. F., Kinnaird, M. F., Gunaryadi, D., and Aslan. (2005). Distribution, status, and conservation needs of Asian elephants (Elephas maximus) in Lampung Province, Sumatra, Indonesia. Biol. Conserv. 124, 35–48. doi: 10.1016/j.biocon.2005.01.004
Holdo, R. M. (2006). Elephant herbivory, frost damage and topkill in Kalahari sand woodland savanna trees. J. Veg. Sci. 17, 509–518. doi: 10.1111/j.1654-1103.2006.tb02472.x
Hyvarinen, O., Te Beest, M., Le Roux, E., Kerley, G., de Groot, E., Vinita, R., et al. (2021). Megaherbivore impacts on ecosystem and earth system functioning: The current state of the science. Ecography 44, 1579–1594. doi: 10.1111/ecog.05703
Ickes, K., Dewalt, S., and Thomas, S. C. (2003). Resprouting of woody saplings following stem snap by wild pigs in a Malaysian rain forest. J. Ecol. 91, 222–233. doi: 10.1046/j.1365-2745.2003.00767.x
Ishwaran, N. (1983). Elephant and woody-plant relationships in Gal Oya, Sri Lanka. Biol. Conserv. 26, 255–270. doi: 10.1016/0006-3207(83)90077-0
Joshi, R., and Singh, R. (2008). Asian elephant (Elephas maximus) and riparian wildlife corridors: A case study from lesser-Himalayan zone of Uttarakhand. J. Am. Sci. 4, 63–75.
King, D. A., Davies, S. J., and Noor, N. S. (2006). Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For. Ecol. Manag. 223, 152–158. doi: 10.1016/j.foreco.2005.10.066
Lim, T. W., Khan, M. K. M., and Campos-Arceiz, A. (2019). An environmental history of human-elephant relationships in Belum-Temengor, Malaysia. Malay. Nat. J. 2019, 1–26.
Lim, T., and Campos-Arceiz, A. (2022). A review of human-elephant ecological relations in the Malay Peninsula: Adaptations for coexistence. Diversity 14:1. doi: 10.3390/d14010036
Lugo, A. E., and Batlle, C. T. R. (1987). Leaf production, growth rate, and age of the palm Prestoea montana in the luquillo experimental forest, Puerto Rico. J. Trop. Ecol. 3, 151–161. doi: 10.1017/S0266467400001905
Matsubayashi, H., Lagan, P., and Abd Sukor, J. R. (2006). Utilization of Macaaranga trees by the Asian elephants (Elephas maximus) in Borneo. Mammal Study 31, 115–118.
Meredith, M. (2018). Wiqid: Quick and dirty estimates for wildlife populations. R package version 0.1.5. Available online at: https://CRAN.R-project.org/package=wiqid (accessed September 3, 2018).
Miettinen, J., Shi, C., and Liew, S. C. (2011). Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob. Change Biol. 17, 2261–2270.
Mueller-Dombois, D. (1972). Crown distortion and elephant distribution in the woody vegetations of Ruhuna National Park, Ceylon. Ecology 53, 208–226. doi: 10.2307/1934074
Neupane, D., Kwon, Y., Risch, T. S., Williams, A. C., and Johnson, R. L. (2019). Habitat use by Asian elephants: Context matters. Glob. Ecol. Conserv. 17:e00570. doi: 10.1016/j.gecco.2019.e00570
Nizam, M. S., Fakhrul-Hatta, M., and Latiff, A. (2006). Diversity and tree species community in the Krau Wildlife Reserve, Pahang, Malaysia. Malay. Appl. Biol. 35:81.
Olivier, R. C. D. (1978). On the ecology of the Asian elephant. PhD Thesis. Cambridge: University of Cambridge, 450.
Ong, L., McConkey, K. R., and Campos-Arceiz, A. (2022). The ability to disperse large seeds, rather than body mass alone, defines the importance of animals in a hyper-diverse seed dispersal network. J. Ecol. 110, 313–326. doi: 10.1111/1365-2745.13809
Petrides, G. A. (1975). Principal foods versus preferred foods and their relations to stocking rate and range condition. Biol. Conserv. 7, 161–169. doi: 10.1016/0006-3207(75)90012-9
Pradhan, N. M. B., Wegge, P., and Moe, S. R. (2007). How does a re-colonizing population of Asian elephants affect the forest habitat? J. Zool. 273, 183–191. doi: 10.1111/j.1469-7998.2007.00313.x
Pringle, R. M. (2008). Elephants as agents of habitat creation for small vertebrates at the patch scale. Ecology 89, 26–33. doi: 10.1890/07-0776.1
R Core Team (2021). R: A language and environment for statistical computing. R foundation for statistical computing. Vienna: R Core Team.
Saaban, S., Othman, N. B., Yasak, M. N. B., Burhanuddin, M. N., Zafir, A., and Campos-Arceiz, A. (2011). Current status of Asian elephants in Peninsular Malaysia. Gajah 35, 67–75.
Saaban, S., Yasak, M. N., Gumal, M., Oziar, A., Cheong, F., Shaari, Z., et al. (2020). Viability and management of the Asian elephant (Elephas maximus) population in the Endau Rompin landscape, Peninsular Malaysia. PeerJ 8:e8209. doi: 10.7717/peerj.8209
Sekar, N., Lee, C.-L., and Sukumar, R. (2017). Functional nonredundancy of elephants in a disturbed tropical forest. Conserv. Biol. 31, 1152–1162. doi: 10.1111/cobi.12907
Shono, K., Davies, S. J., and Chua, Y. K. (2007). Performance of 45 native tree species on degraded lands in Singapore. J. Trop. For. Sci. 19, 25–34.
Sukumar, R. (1990). Ecology of the Asian elephant in southern India. II. Feeding habits and crop raiding patterns. J. Trop. Ecol. 6, 33–53. doi: 10.1017/S0266467400004004
Sukumar, R. (2006). A brief review of the status, distribution and biology of wild Asian elephants Elephas maximus. Int. Zoo Yearbook 40, 1–8. doi: 10.1111/j.1748-1090.2006.00001.x
Sukumar, R. (ed.) (2003). “Moeritheres, mastodonts, and mammoths. elephant evolution in action,” in The living elephants: Evolutionary ecology, behavior and conservation, (Oxford: Oxford University Press), 3–54.
Sukumar, R., and Ramesh, R. (1995). “Elephant foraging: is browse or grass more important? A Week with Elephants,” in Proceedings of the International Seminar on the Conservation of Asian Elephants, (Oxford: Oxford University Press), 368–374.
Terborgh, J. (1974). Preservation of natural diversity: The problem of extinction prone species. Bioscience 24, 715–722. doi: 10.2307/1297090
Terborgh, J., Davenport, L. C., Niangadouma, R., Dimoto, E., Mouandza, J. C., Schultz, O., et al. (2016a). The African rainforest: Odd man out or Megafaunal landscape? African and Amazonian forests compared. Ecography 39, 187–193. doi: 10.1111/ecog.01643
Terborgh, J., Davenport, L. C., Niangadouma, R., Dimoto, E., Mouandza, J. C., Scholtz, O., et al. (2016b). Megafaunal influences on tree recruitment in African equatorial forests. Ecography 39, 180–186. doi: 10.1111/ecog.01641
Terborgh, J., Davenport, L. C., Ong, L., and Campos-Arceiz, A. (2018). Foraging impacts of Asian megafauna on tropical rain forest structure and biodiversity. Biotropica 50, 84–89. doi: 10.1111/btp.12488
Tilker, A., Abrams, J. F., Mohamed, A., Nguyen, A., Wong, S. T., Sollmann, R., et al. (2019). Habitat degradation and indiscriminate hunting differentially impact faunal communities in the Southeast Asian tropical biodiversity hotspot. Commun. Biol. 2:396.
Turner, I. M. (1990). Tree seedling growth and survival in a malaysian rain forest. Biotropica 22, 146–154. doi: 10.2307/2388407
Vancuylenberg, B. W. B. (1977). Feeding behaviour of the Asiatic elephant in South-East Sri Lanka in relation to conservation. Biol. Conserv. 12, 33–54. doi: 10.1016/0006-3207(77)90056-8
van der Heijden, G. M. F., Powers, J. S., and Schnitzer, S. A. (2015). Lianas reduce carbon accumulation and storage in tropical forests. PNAS 112, 13267–13271. doi: 10.1073/pnas.1504869112
Venables, W. N., and Ripley, B. D. (2002). Modern applied statistics with S. springer science & business media. New York, NY: Springer.
Wadey, J., Beyer, H. L., Saaban, S., Othman, N., Leimgruber, P., and Campos-Arceiz, A. (2018). Why did the elephant cross the road? The complex response of wild elephants to a major road in Peninsular Malaysia. Biol. Conserv. 218, 91–98. doi: 10.1016/j.biocon.2017.11.036
Williams, C., Tiwari, S. K., Goswami, V. R., de Silva, S., Kumar, A., Baskaran, N., et al. (2020). Elephas maximus. The IUCN red list of threatened species 2020:e.T7140A45818198. Gland: IUCN.
Wolf, A., Doughty, C. E., and Malhi, Y. (2013). Lateral diffusion of nutrients by mammalian herbivores in terrestrial ecosystems. PLoS One 8:e71352. doi: 10.1371/journal.pone.0071352
Wyatt, J. R., and Eltringham, S. K. (1974). The daily activity of the elephant in the Rwenzori National Park, Uganda. Afr. J. Ecol. 12, 273–289. doi: 10.1111/j.1365-2028.1974.tb01037.x
Keywords: Asian elephant, dipterocarp forests, ecological filtering, ecological function, food preference, foraging impacts, megafauna, megaherbivore
Citation: Ong L, Tan WH, Davenport LC, McConkey KR, Mat Amin MKAb, Campos-Arceiz A and Terborgh JW (2023) Asian elephants as ecological filters in Sundaic forests. Front. For. Glob. Change 6:1143633. doi: 10.3389/ffgc.2023.1143633
Received: 13 January 2023; Accepted: 16 May 2023;
Published: 27 June 2023.
Edited by:
Christopher Doughty, Northern Arizona University, United StatesReviewed by:
Jenna Keany, Northern Arizona University, United StatesAndrew Abraham, Aarhus University, Denmark
Copyright © 2023 Ong, Tan, Davenport, McConkey, Mat Amin, Campos-Arceiz and Terborgh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahimsa Campos-Arceiz, YWhpbXNhQHh0YmcuYWMuY24=
 Lisa Ong
Lisa Ong Wei Harn Tan
Wei Harn Tan Lisa C. Davenport
Lisa C. Davenport Kim R. McConkey
Kim R. McConkey Mohamad Khairul Adha bin Mat Amin7,8
Mohamad Khairul Adha bin Mat Amin7,8 Ahimsa Campos-Arceiz
Ahimsa Campos-Arceiz John W. Terborgh
John W. Terborgh