
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 04 April 2023
Sec. Forest Management
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1135219
This article is part of the Research Topic Mountainous Forest Ecosystems: Challenges and Management Implications View all 16 articles
 Fozia Choudhary1
Fozia Choudhary1 Anil Bhardwaj1
Anil Bhardwaj1 Iqra Sayeed1
Iqra Sayeed1 Shabir Ahmad Rather2
Shabir Ahmad Rather2 Mohammad Abdul Hannan Khan2
Mohammad Abdul Hannan Khan2 Ali Asghar Shah1*
Ali Asghar Shah1*Nematodes are an integral part of soil biodiversity and play a vital role in soil nutrient cycling. The Himalayan mountainous ecosystems are amongst the extreme environments in the world. Still little is known about the diversity and distribution patterns of soil nematodes along the elevation gradient in the region, thus limiting our ability in understanding and comparing the structural patterns of nematode communities across different regions. To address this knowledge gap, we aim to disentangle the elevational patterns of soil nematode community structure and trophic diversity by studying the abundance, composition, diversity and functional indices, and metabolic footprint of soil nematodes at four elevation classes (Elev1, Elev2, Elev3, and Elev4 each being 400 m) along an altitudinal gradient (1,000–2,600 m) in the Trikuta mountain range of Pir-Panjal to Shivalik Himalaya. Overall, a total of 55 genera were found in the study region. The diversity and richness of soil nematodes varied significantly among the elevation classes, and exhibit a decreasing trend with elevation. Also, the nematode community composition varied significantly among the elevation classes along the elevational gradient. The bacterivores were the dominant trophic group at each elevation class. Further, the soil properties played a key role in explaining the changes in the nematode community composition across the elevation classes. Moreover, the total nematode, bacterivore and herbivore abundances showed an increasing trend, while as that of fungivores and predators exhibit a negative trend with elevation. In addition, a declining pattern in the overall maturity and metabolic footprint with elevation was observed, thus depicting the lower sustenance of k-strategic nematodes and their relatively lower contribution to belowground carbon cycling at higher elevations. The finding of our study could enhance our understanding of the overall community structure and diversity patterns of soil nematode along the elevation gradient, and the response of soil nematodes to ongoing climate change in the rapidly warming Himalayas.
Soil nematodes (popularly called as roundworms) are one of the major components of belowground faunal diversity (Kergunteuil et al., 2016; Kouser et al., 2021). With more than 27,000 known species, soil nematodes constitute approximately 80% of the total belowground soil metazoan taxonomic and functional diversity (Hodda et al., 2009; Hodda, 2011; Kouser et al., 2021). The nematodes generally occupy all the major trophic guilds of the soil food web, including herbivores, fungivores, bacterivores, predators, parasites, and omnivores (Yeates et al., 1993; Kergunteuil et al., 2016; Zhang et al., 2021). Also, functionally the nematodes can be assigned to a wide degree of ecological adaptations ranging from being acting as “colonizers” (i.e., r strategists) to “persistents” (i.e., K strategists), as well as all the stages occuring in between, along the colonizer-persistents (“cp”) continuum (Bongers, 1990; Kergunteuil et al., 2016; Kouser et al., 2021). Owing to vast trophic and functional diversity, nematodes play a key role in regulating several essential ecosystem processes including nutrient cycling, successional changes, and energy flow (Yeates, 2003; Bakonyi et al., 2007; Van Eekeren et al., 2010). As such, nematodes are considered to be the suitable indicators both for monitoring the ecosystem health and tracking environmental changes (Kitagami et al., 2017; Salamun et al., 2017; Zhang et al., 2021). Over decades, researchers have focused on studying the biogeographic distribution pattern of nematodes, however, such studied are mostly restricted to anthropogenic systems (Šalamún et al., 2014; Zhao et al., 2015; Kouser et al., 2021). Research focusing on the diversity and compositional patterns of soil nematodes in natural mountainous systems especially along elevational gradients is direly needed, as such studies can provide vital information on the casual relationship between belowground diversity, ecosystem functioning, soil properties, and local climate (van den Hoogen et al., 2019; Zhang et al., 2021).
Mountainous ecosystems provide an ideal and natural conditions for studying elevational variation in compositional structure of communities, and in unraveling the key environmental variables causing the variation, as both the climatic and edaphic variables change considerably with short distance along elevations (Körner, 2007; Kergunteuil et al., 2016; Wei et al., 2019; Yu et al., 2021; Wani et al., 2023). It has been reported that elevation has a strong filtering effect on soil nematode communities in terms of both climatic and edaphic factors (Thakur et al., 2017, 2019; Siebert et al., 2019; da Silva et al., 2020). For example, changes in the climatic variables (especially temperature and precipitation) directly affect the biotic environment and as such had a significant influence on the underlying community diversity and structure of soil nematodes (Nielsen et al., 2014; Song et al., 2017; Thakur et al., 2017; da Silva et al., 2020). Many studies have also reported the distinct elevational zonation of soil nematode communities due to varying climatic conditions along an elevational gradient (Kergunteuil et al., 2016; Dong et al., 2017). Also, the key role of changing soil physico-chemical properties including organic matter content, soil pH, and soil moisture, along elevational gradients in shaping the structure of soil nematode communities is well documented (Sun et al., 2013; Kitagami et al., 2017; Quist et al., 2019; Reid et al., 2019).
Recently, research efforts have been initiated to study the elevational patterns of nematode diversity and community structure, and draw diverse conclusions including a mid-elevation peak (Dong et al., 2017), increasing diversity with increase in elevation (Kergunteuil et al., 2016), decreasing diversity with elevation (Powers et al., 1998; Liu et al., 2019), irregular patterns to no change with elevation (Tong et al., 2010; Qing et al., 2015). Therefore, there is still a lack of consensus, which hampers our understanding of the overall biogeographical pattern of soil nematode across elevational gradients (Zhang et al., 2021). As such, additional studies in mountainous ecosystems need to be taken in order to arrive at general patterns about the effect of elevation on diversity and distributional patterns of soil nematodes (Dong et al., 2017; Afzal et al., 2021).
In the Himalayan biodiversity hotspot, few studies have been conducted to study the elevational patterns of soil nematodes (Devetter et al., 2017; Afzal et al., 2021). However, till date to the best of our knowledge, no study has attempted to study the elevational patterns of nematodes in conjugation with soil properties across the mountainous ecosystems of the Pir Panchal Himalaya. Toward bridging this knowledge gap, we aim to study the elevational pattern of nematodes along with the physico-chemical properties in soil system across the mountainous ecosystems. Specifically, our study aims to answer the following research questions: (1) whether the soil nematodes differ across elevational gradient? (2) Whether the different nematode trophic groups differ with elevation? (3) Whether the soil nematode diversity, abundance and derived functional indices (MI, PPI, CI, SI, and EI) change with increasing elevation? (4) Whether the soil nematode abundance and soil properties are correlated with each other (5) whether the soil properties change with elevation? Hopefully, the findings of our study can be immensely fruitful in assessing soil health and environmental change effects in the eco-fragile Himalayan mountainous ecosystem.
The present study was carried out along the Trikuta mountain range located in the Reasi district of North Western Himalaya. The average elevation of the region ranges from 1,000 to 4,500 m above sea level (a.s.l.). The region experiences mean annual temperature of 14°C and precipitation of 1,360 mm, respectively. The higher altitudes of the region receive a significant amount of precipitation in the form of snowfall. Distinct vegetation classes are found with changing elevation in the region. The vegetation at an elevation range of 1,000–1,800 masl comprise of mixed forest with Pinus roxburghii, Quercus leucotrichophora, Rhododendron arboreum, and Pyrus pashia as the dominant species. The elevational zone between 1,800 and 2 600 masl comprise of moist temperate deciduous forests with predominance of Pinus wallichiana, Abies pindrow, Quercus dilatata, and Picea smithiana. However, above the elevation of 3,000 masl, the vegetation mainly comprises of alpine tundra and meadows (Afzal et al., 2021).
We randomly carried soil sampling along a stratified altitudinal gradient in the month of September, 2020. In total, 48 soil samples were collected from four elevation classes (i.e., Elev1: 1,000–1,400 m; Elev2: 1,401–1,800 m; Elev3: 1,801–2,200 m; Elev4: 2,201–2,600 m) along elevation gradient. At each elevation class, four sites were selected which were separated from one another by an elevational distance of 100 m. From each of the selected site three samples and a total of 12 samples from each elevation class were collected (3 samples × 4 sites × 4 elevation classes). Each sample was taken by randomly laying 5 × 5 m quadrant and taking five sub-samples (four from the corners and one from the center) at a depth of 0–15 cm using a soil corer and mixing them together to form a composite sample of 500 g (Kouser et al., 2021; Kashyap et al., 2022) These soil samples were then packed in airtight polythene bags and carried to the laboratory for further analysis.
The soil physiochemical analysis was carried out at Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu. About 300 g of the bulk soil was used for measuring soil properties including pH, electrical conductivity, moisture, organic matter, total nitrogen, temperature, and soil texture). Soil pH and electrical conductivity were measured using a digital pH and conductivity meter fitted with glass electrodes, after shaking a soil water (1:2 w/v i.e., 50/100) suspension for 30 min on a rotator. The soil moisture was measured by gravimetric method (i.e., drying at 70°C till constant weight). The soil organic matter was estimated using loss on ignition method (Dar et al., 2015). The soil total nitrogen was estimated following the Kjeldahl method (Kjeldahl, 1883). The soil temperature was measured in the field using soil thermometer. Lastly, the soil texture was calculated as the relative proportion of sand and silt content expressed as percentage.
The nematodes were extracted from 100 g of soil samples following the sieving and decantation procedure as proposed by Baermann funnel method (Barker, 1985). According to this method, each soil sample was first taken into a 1 L beaker and mixed with tap water. Second, the water suspension was then stirred thoroughly and decanted into another 1 L beaker using a sieve of 2 mm mesh size to remove the stones and large debris. Third, the water suspension was then mixed further and decanted through 53 μm mesh sized sieve, and the material that was left on the sieve was collected in another 250 mL beaker and the extraction was repeated by following Baermann funnel method. The extracted nematodes were removed for 2 days, stored at 4°C, fixed in triethanolamine-glycerol fixative (TAF) and counted using an Olympus stereo -zoom microscope (model: SZX16), and simultaneously mounted on slides for identification to the genus level using various texts (Goodey, 1963; Jairajpuri and Ahmad, 1992; Andrássy, 2005). Also, the identified genera were assigned to different functional guilds based on their trophic groups and life history strategies (Yeates et al., 1993; Bongers and Bongers, 1998).
Using the NINJA online application (Sieriebriennikov et al., 2014),1 we calculated six nematode functional indices including maturity index (MI), sigma maturity index (ΣMI), plant parasitic index (PPI), enrichment index (EI), structure index (SI) and channel index (CI), based on c–p scale assigned to various genera of nematodes ranging from r-colonizers to k-persisters (cp1–cp5) (Sikora et al., 2018; Maina et al., 2022). The maturity index (MI), sigma maturity index (ΣMI), and plant parasitic index (PPI) were calculated following Bongers (1990), while as the enrichment index (EI) = (e/e + b) × 100, structure index (SI) = (s/s + b) × 100 and channel index (CI) = [0.8 Fu2/(3.2 Ba1 + 0.8 Fu2) × 100] which provide essential information on the soil food web dynamics and stability (Kashyap et al., 2022) were calculated following (Ferris et al., 2001). The structure index is an indicator of the maturity or stability of a system, with higher values reflecting structured and lower values representing a disturbed ecosystem (Afzal et al., 2021; Kashyap et al., 2022). Similarly, the channel index [CI = 0.8 Fu2/(3.2 Ba1 + 0.8 Fu2) × 100] provides information on the relative contribution of bacterial and fungal feeders in the decomposition pathway (Kashyap et al., 2022). In the above-mentioned enrichment and structure indices, the b component was calculated as Σkbnbwith kb indicating the weightage assigned to basal characteristic guilds of the soil food web [0.8 (Ba2 + Fu2)] and nb representing the nematode abundance of the corresponding guilds. Similarly, the enrichment and structure components were calculated based on the guilds representing enrichment [(3.2 Ba1) + (0.8 Fu2)] and structure (Ba3–Ba5, Fu3–Fu5, Ca2–Ca5), respectively, where Ba1-5 denote bacterivores of C–P group 1–5, Fu2-5 denotes fungivores of C–P group 2–5 and Ca2-5 denotes predators of C–P group 1–5 (Ferris et al., 2001). In addition, the metabolic footprints which acts as proxies to quantify the magnitude of ecosystem services and functions provided by nematode communities to soil food web (Afzal et al., 2021; Kashyap et al., 2022) were calculated following Andrassy (1956) [W = (D2 × L)/(1.6 × 106) and Ferris (2010)] [F = Σ(Nt (0.1 (Wt/mt) + 0.273 (Wt0.75)] using NINJA, where W represents the nematode biomass (μg), D and L denotes the maximum body diameter (μm) and body length (μm), respectively, Nt represents the number of nematodes in genus t, Wtrepresents the estimated body weight of genus t, and mt represents the c-p group of the genus t. We also calculated the Shannonfy the magnitude of ‘); Pielou’s evenness (J’); and generic richness (GR) indices for estimation of nematode diversity using the “vegan” package (Oksanen et al., 2020) in R statistical software (R Core Team, 2020).
To check whether our data meets the assumption of normality, we performed the Shapiro–Wilk test. The Shapiro–Wilk test showed that the data was non-normally distributed (p-value < 0.05). We therefore performed Kruskal–Wallis test to study whether the soil nematode abundance, diversity, functional and metabolic indices, and physicochemical properties differed significantly among the studied elevational classes. In cases where statistically significant differences were found, multiple post hoc comparisons among studied elevational classes were performed using the Dunn test. Also, owing to the non-normality of data, we performed Spearman’s multiple correlation among the studied soil physicochemical parameters and nematode trophic groups to study the relationship between them. The statistical significance of the resultant correlation was calculated at p < 0.05. Moreover, to investigate the relationship between soil physicochemical properties, nematode trophic groups and genera, we conducted the principal component analysis (PCA) using the “factoextra” package (Kassambara and Mundt, 2020) in R software.
Non-metric multidimensional scaling (NMDS) ordination based on Bray–Curtis distance was conducted using the “vegan” package (Oksanen et al., 2020) in R to investigate the differences in nematode communities along elevation gradient. Furthermore, the Permutational Multivariate Analysis of Variance (PERMANOVA) was used to quantify the compositional differences among the studied elevation classes, In PERMANOVA, we used both the abundance-based Bray-Curtis and incidence-based Jaccard indices to evaluate whether the observed compositional dissimilarity was due to species relative abundances or simply by the presence and/or absence of species (Shahabuddin et al., 2021). Furthermore, if significant differences were found, we also performed the pairwise multi-level comparisons using the vegan” package to assess differences between each pair of elevational classes. The associated statistical significance was assessed at p < 0.05 based on 999 permutations.
Along the elevational gradient, the studied soil physico-chemical parameters varied significantly between the elevational classes. More specifically, soil pH, electrical conductivity and temperature were significantly higher at the lower elevation class (i.e., Elev1) and lower at the highest elevation class (i.e., Elev4) (Figures 1A–C and Table 1). Contrary, the soil moisture, organic matter and texture were significantly lower at the lower elevation class (i.e., Elev1) and higher at the highest elevation class (i.e., Elev4) (Figures 1D–F and Table 1). Also, the soil pH at all the studied elevational classes was acidic in nature. Furthermore, the soil total nitrogen content was lower at the lowest elevation class and higher at the third elevation class (i.e., 1,801–2,200 m) (Figure 1G and Table 1).
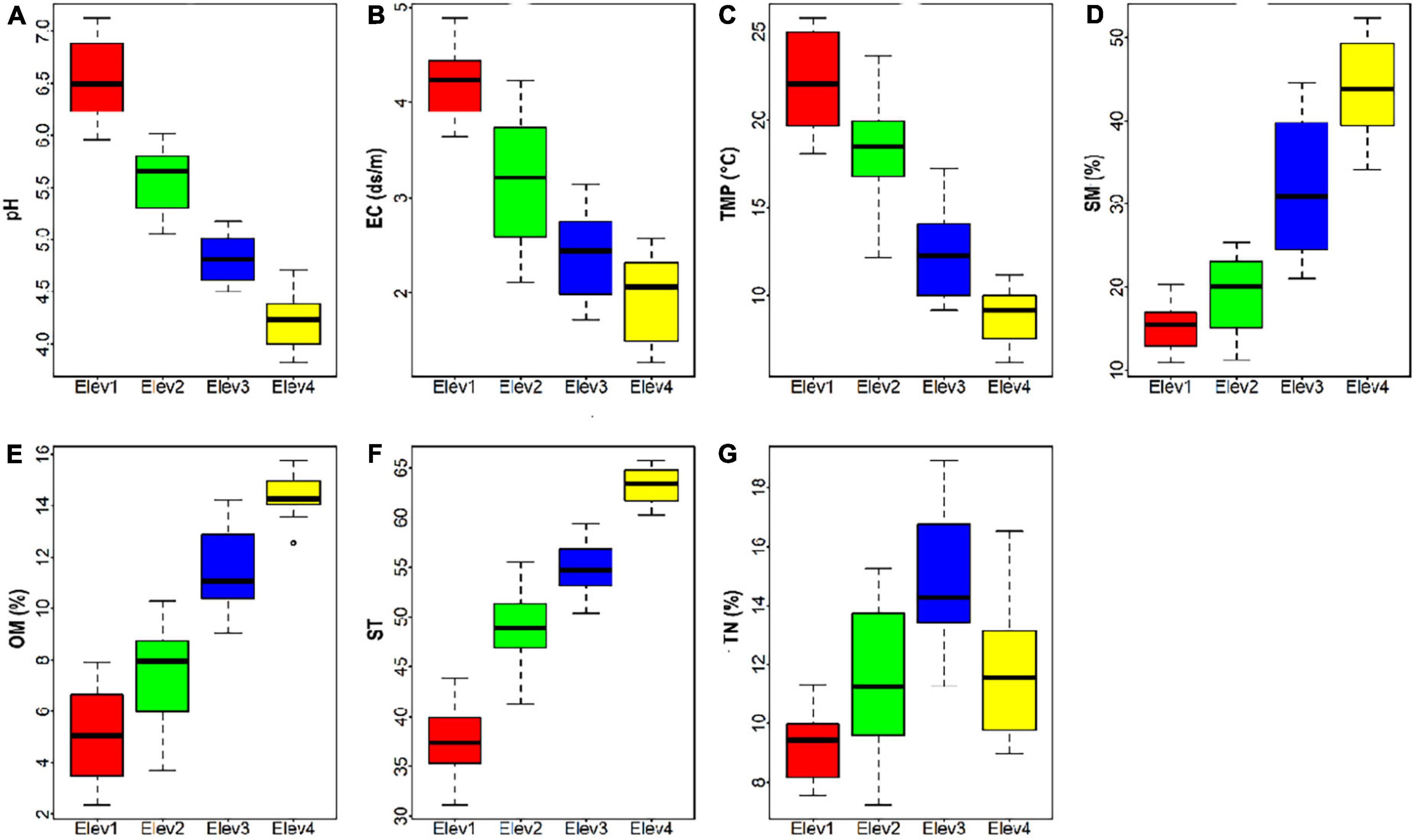
Figure 1. Soil physico-chemical properties across elevation classes (A) pH, (B) EC, electrical conductivity, (C) TMP, temperature, (D) SM, soil moisture, (E) OM, organic matter, (F) ST, soil texture, and (G) TN, total nitrogen. Elev, Elevation; where Elev1: 1,000–1,400 m, Elev2: 1,401–1,800 m, Elev3: 1,801–2,200 m, and Elev4: 2,201–2,600 m.
During the present study, we collected a total of 19,699 nematodes belonging to 55 genera and five trophic groups including bacterivores (21 genera), fungivores (8 genera), herbivores (10 genera), omnivores (6 genera), and predators (10 genera) (Table 2). The NMDS analysis using Bray-Curtis distance revealed that the soil nematode community composition differed considerably along the elevation gradient (Figure 2). Also, the PERMANOVA analysis showed that the nematode community composition differed significantly among the studied elevational classes both in terms of their relative abundances (Bray–Curtis: F = 2.13; p < 0.001) and number of species (Jaccard: F = 1.68; p < 0.001). Furthermore, the pairwise analysis showed a statistically significant difference in the nematode composition between all the elevation classes except between elevation 3 and elevation 4 (Table 3). Of the total 55 nematode genera identified, 14 genera differed significantly (p < 0.05) among the studied elevation classes (Table 2). More specifically, the nematode genera that differed significantly (p < 0.05) in their relative abundance among the studied elevation classes include bacterivores (Bunonema, Cuticularia, Diploscapter, Protorhabditis, Rhabditis), herbivores (Hemicriconemoides, Hoplolaimus, Psilenchus, Xiphinema), omnivores (Nygolamoides, Thornenema), and predators (Discolaimoides, Iotonchus, Mylonchulus) (Table 2). Moreover, 50, 44, 40, and 35 genera were reported from the first (1,000–1,400 m), second (1,401–1,800 m), third (1,801–2,200 m), and fourth (2,201–2,600 m) elevational class, respectively, (Table 2). Overall, the mean abundance of soil nematodes ranged from 203 to 886 (mean ± standard error: 410.40 ± 18.46) per 100 g of soil (Figure 3).
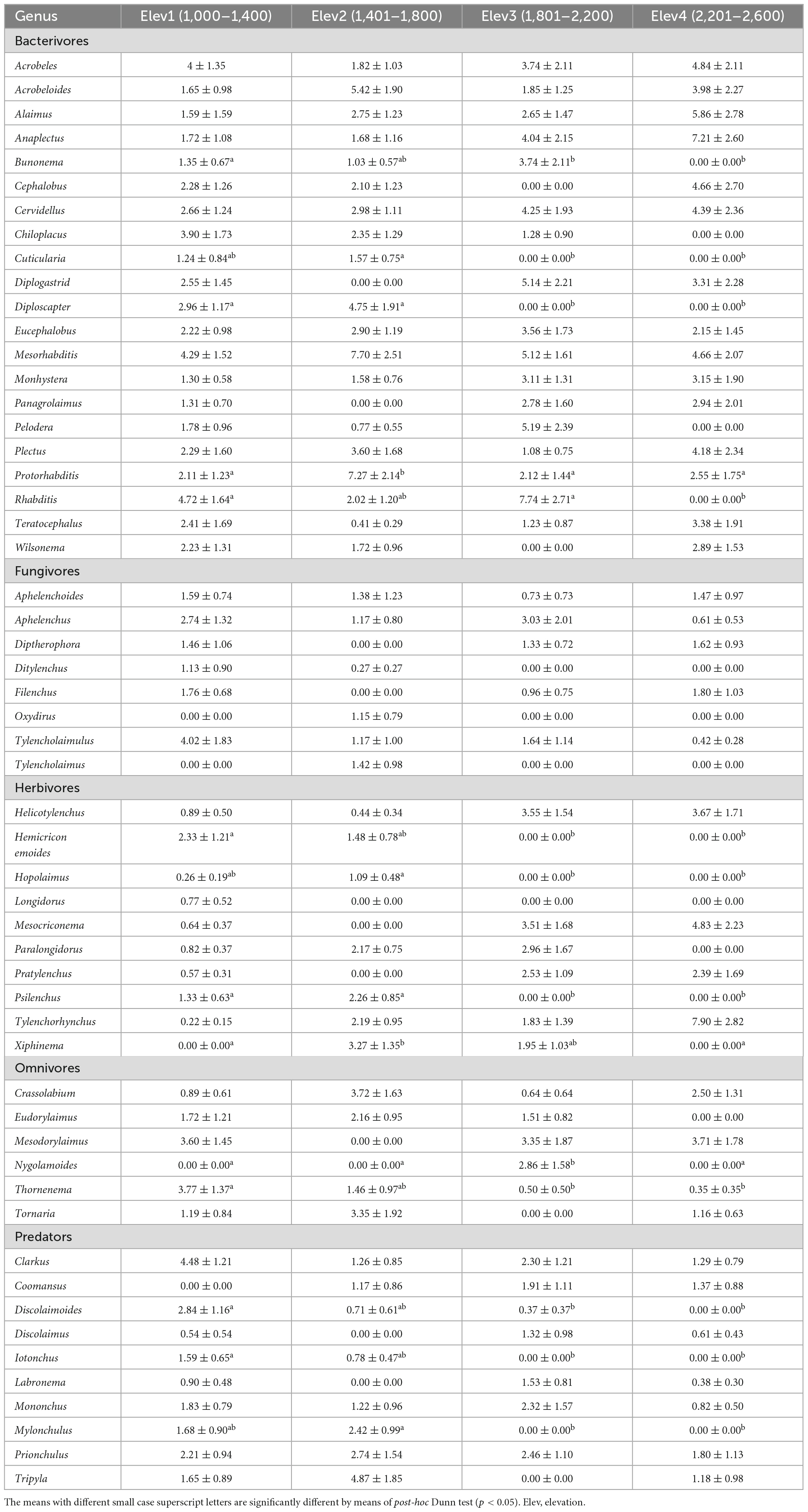
Table 2. Relative abundance of soil nematode genera at the studied elevation classes (mean ± standard error).
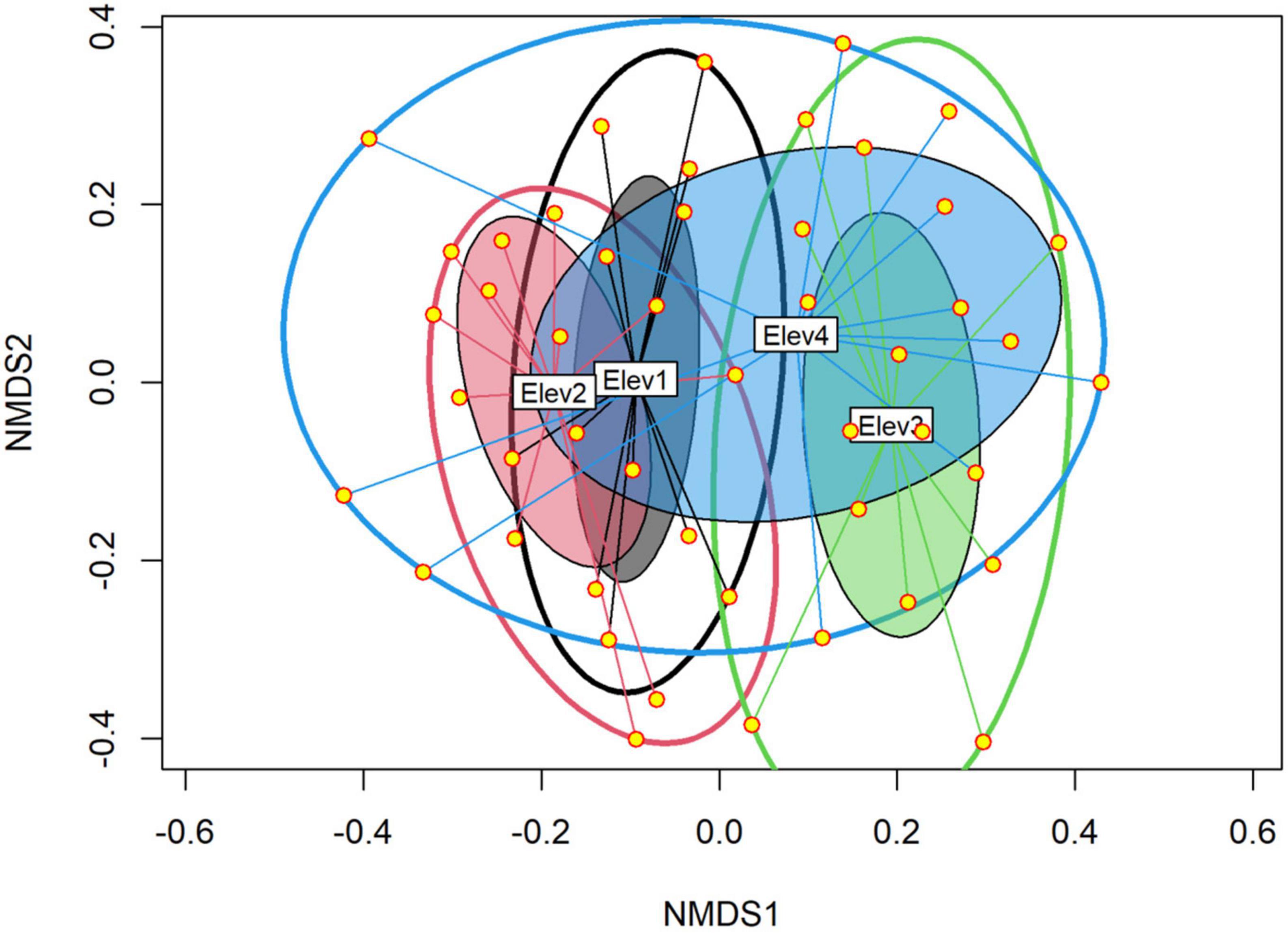
Figure 2. Non-metric multidimensional scaling (NMDS) plot of nematode communities based on Bray–Curtis dissimilarity metric. Each point represents the position of the nematode community in ordination space based on each individual sample. The size of each ellipse is proportional to within class dissimilarity, while as the degree of overlap between the two ellipses represents similarity in nematode composition between their respective elevational classes. Elev, Elevation; where Elev1: 1,000–1,400 m, Elev2: 1,401–1,800 m, Elev3: 1,801–2,200 m, and Elev4: 2,201–2,600 m.
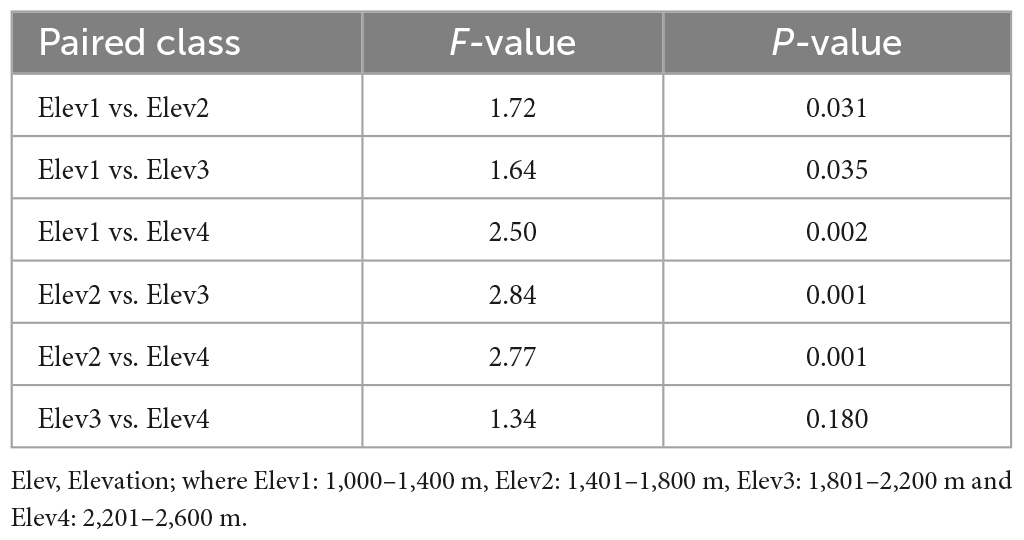
Table 3. Pairwise nematode compositional dissimilarity between the studied elevational classes using Bray–Curtis dissimilarity metric.
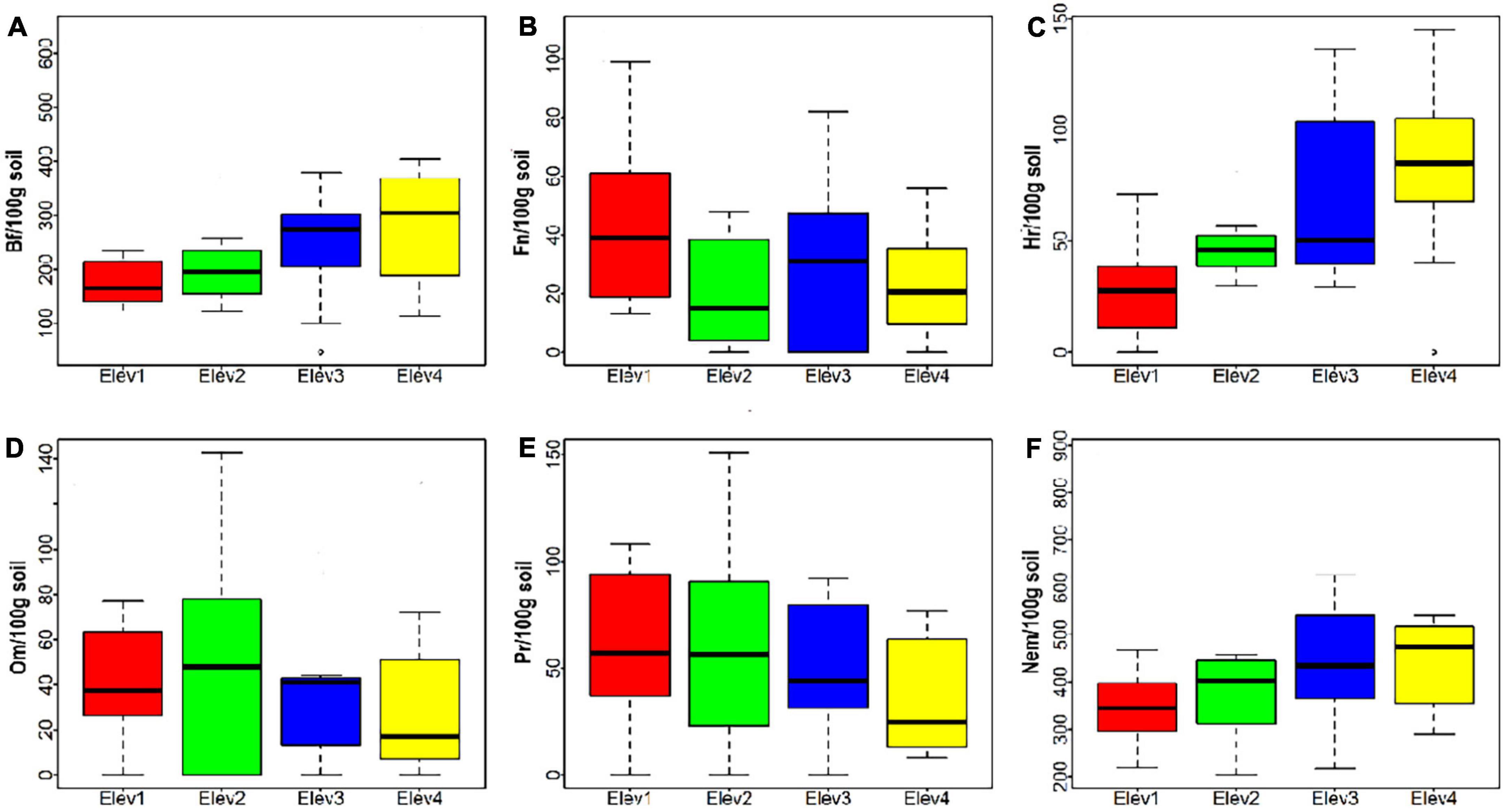
Figure 3. Nematode abundance of various trophic groups across studied elevational classes (A) Bf, bacterivores; (B) Fn, fungivores; (C) Hr, herbivores; (D) Om, omnivores; (E) Pr, predators; and (F) nem, total nematodes. Elev, elevation; where Elev1: 1,000–1,400 m, Elev2: 1,401–1,800 m, Elev3: 1,801–2,200 m, and Elev4: 2,201–2,600 m.
We found statistically significant differences in the bacterivores and herbivores only among the studied elevation classes (Figure 3 and Table 4). Bacterivores were found to be the most abundant trophic group across all the studied elevational classes, whereas, fungivores were least abundant nematode group except for lowest elevation class where in herbivores was the least abundant taxonomic group (Table 4). Also, the abundances of total nematodes, bacterivores and herbivores was found to increase with increasing elevation, while as that of fungivores and predators decreased with increasing elevation. However, the omnivore abundance was maximum at second elevational class (1,401–1,800 m) (Table 4).
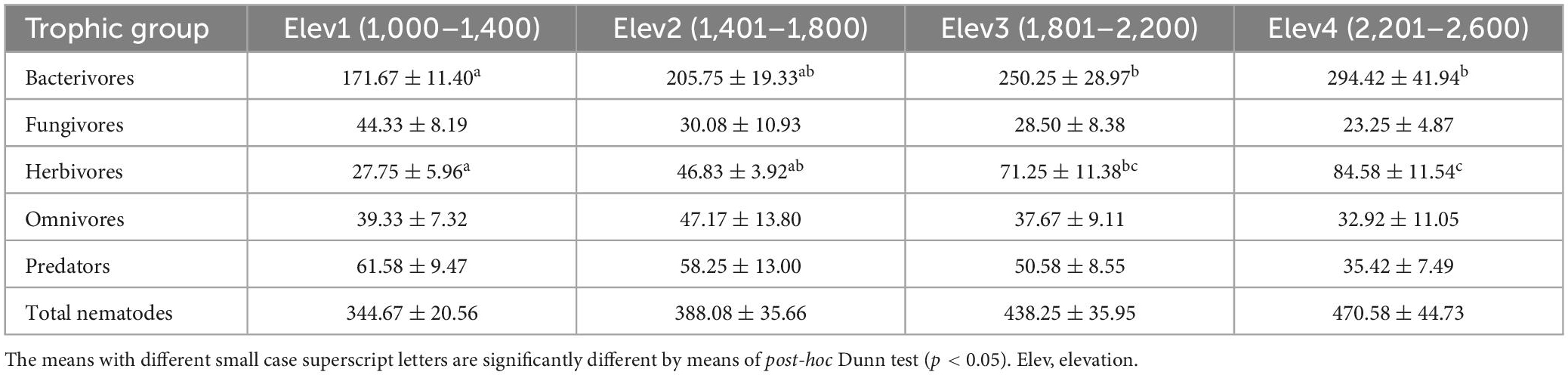
Table 4. Nematode abundance of various trophic groups across the studied elevation classes (mean ± standard error).
Our study showed that among the nematode maturity indices, only the MI25 differed significantly across studied elevational classes, with highest value found at lowest elevation class (Elev1) and lowest value at highest elevation class (Elev4) (Table 5). Also, the other maturity indices showed decreasing effect from lowest to the highest elevational class, however, the effect was not statistically significant (Table 5). Similarly, the PPI and EI showed decreasing effect from lowest to the highest elevational class, but the effect was once again non-significant (Table 5). In contrast, the SI different significantly across the elevational classes, with highest value observed at lowest elevational class (Elev1) and lowest at highest elevational class (Elev4) (Table 5). Likewise, the CI exhibited significant differences across the elevational classes, with highest value observed at highest elevational class (Elev4) (Table 5). Furthermore, the Shannon Weiner (H’), Pielou’s evenness (J’), and generic indices showed statistically significant difference among the elevational classes, with Shannon Weiner (H’), and Pielou’s evenness (J’) indices showing a decrease with increasing elevation (Table 5).
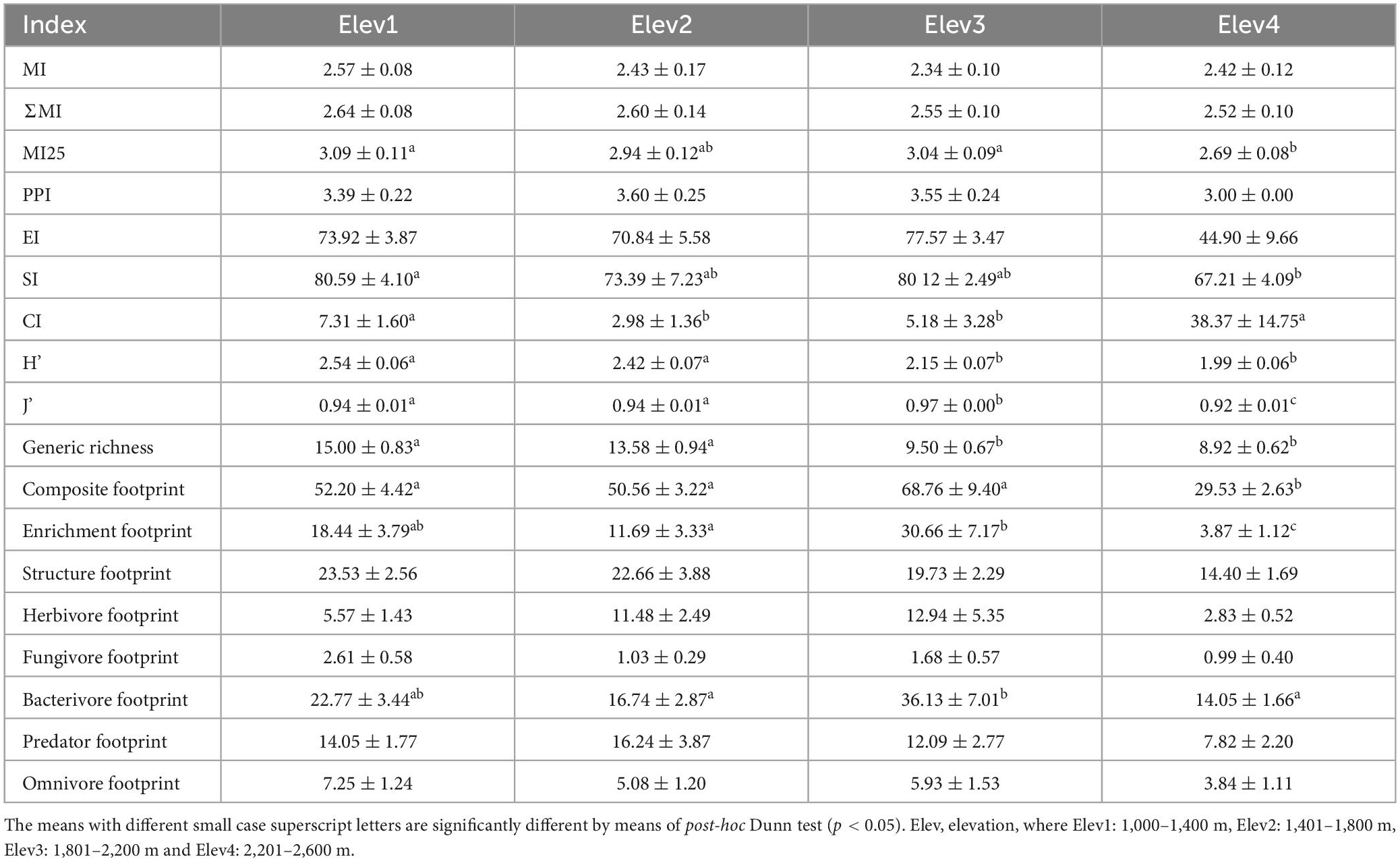
Table 5. Soil nematode functional diversity and metabolic indices across the studied elevation classes (mean ± standard error).
In addition, the soil nematode faunal profile using enrichment and structural indices (EI-SI) for all the studied elevational classes falls mainly under the quadrant B (indicating maturing, N-enriched, low C:, bacterial dominated and regulated system) except for some of the points from elevational classes third and four (Elev3 and Elev4) that fall under the quadrant C (indicating matured, fertile, moderate C: N, bacterial/fungal dominated and suppressive system) (Figure 4). Our study also showed that among the various nematode composite structure and trophic metabolic footprints, only the composite, enrichment and bacterivore footprints differed significantly among the elevational classes, with all of them showing higher values at third elevation (Elev3) (Table 5), while as the remaining footprints varied non-significantly among elevational classes (Table 5).
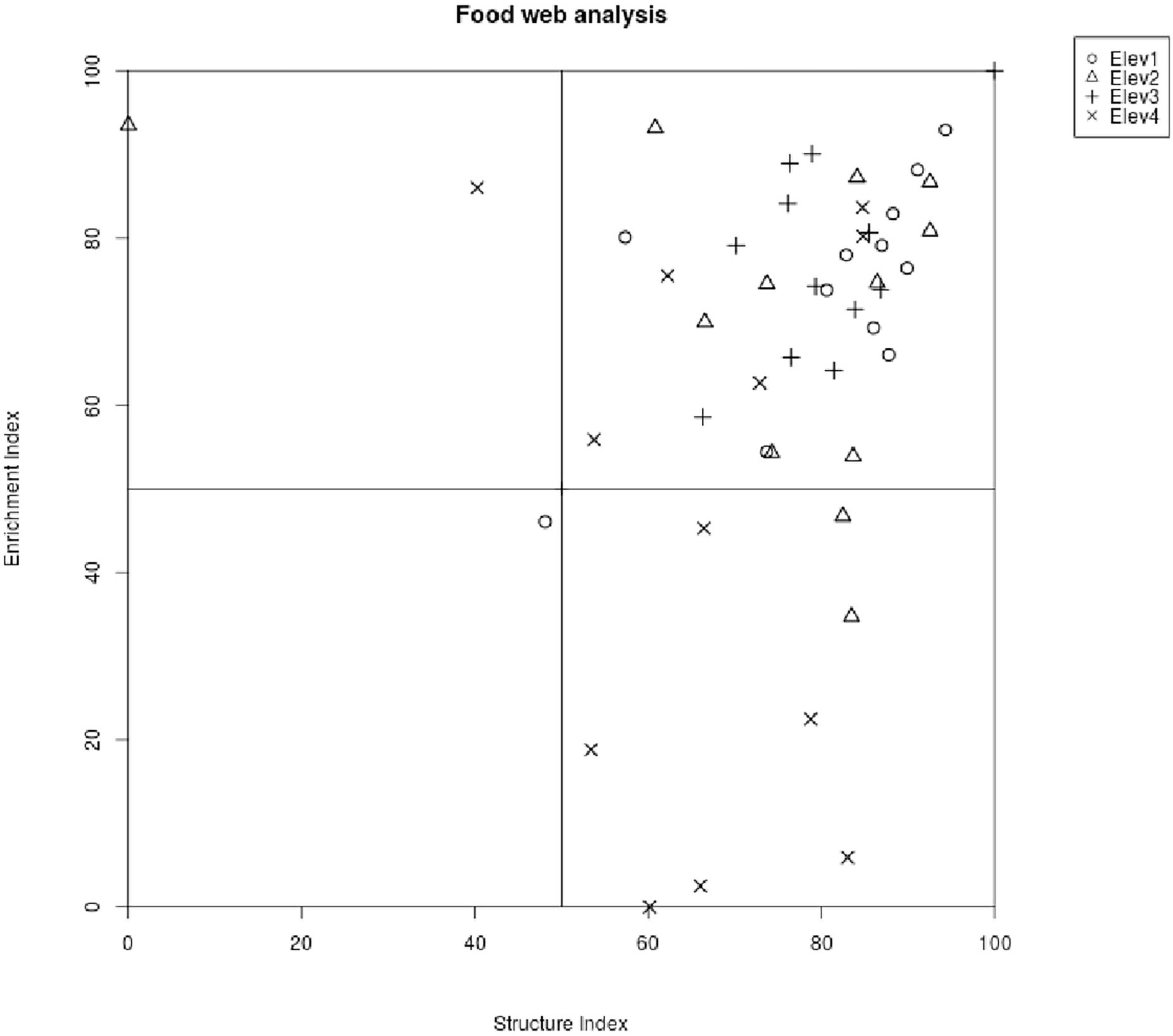
Figure 4. Food web diagnostic profile across the studied elevational classes. Elev, elevation; where Elev1: 1,000–1,400 m, Elev2: 1,401–1,800 m, Elev3: 1,801–2,200 m, and Elev4: 2,201–2,600 m.
The multiple correlation revealed that the soil properties and nematode communities were related to each other to a greater extent (Figure 5). More specifically, total nematode and bacterivore abundances showed a significant positive correlation with soil moisture, organic matter and texture and a significant negative correlation with soil electrical conductivity, pH and temperature (Figure 5). Similarly, herbivore abundance was significantly positively correlated with soil moisture, organic matter, total nitrogen and texture, but significantly negatively correlated with soil electrical conductivity, pH and temperature (Figure 5). In contrast, the fungivore, omnivore and predator abundances did not show statistically significant correlation with any of the soil variable (Figure 5). Moreover, the total nematode, bacterivore and herbivore abundances were significantly positively related with each other (Figure 5).
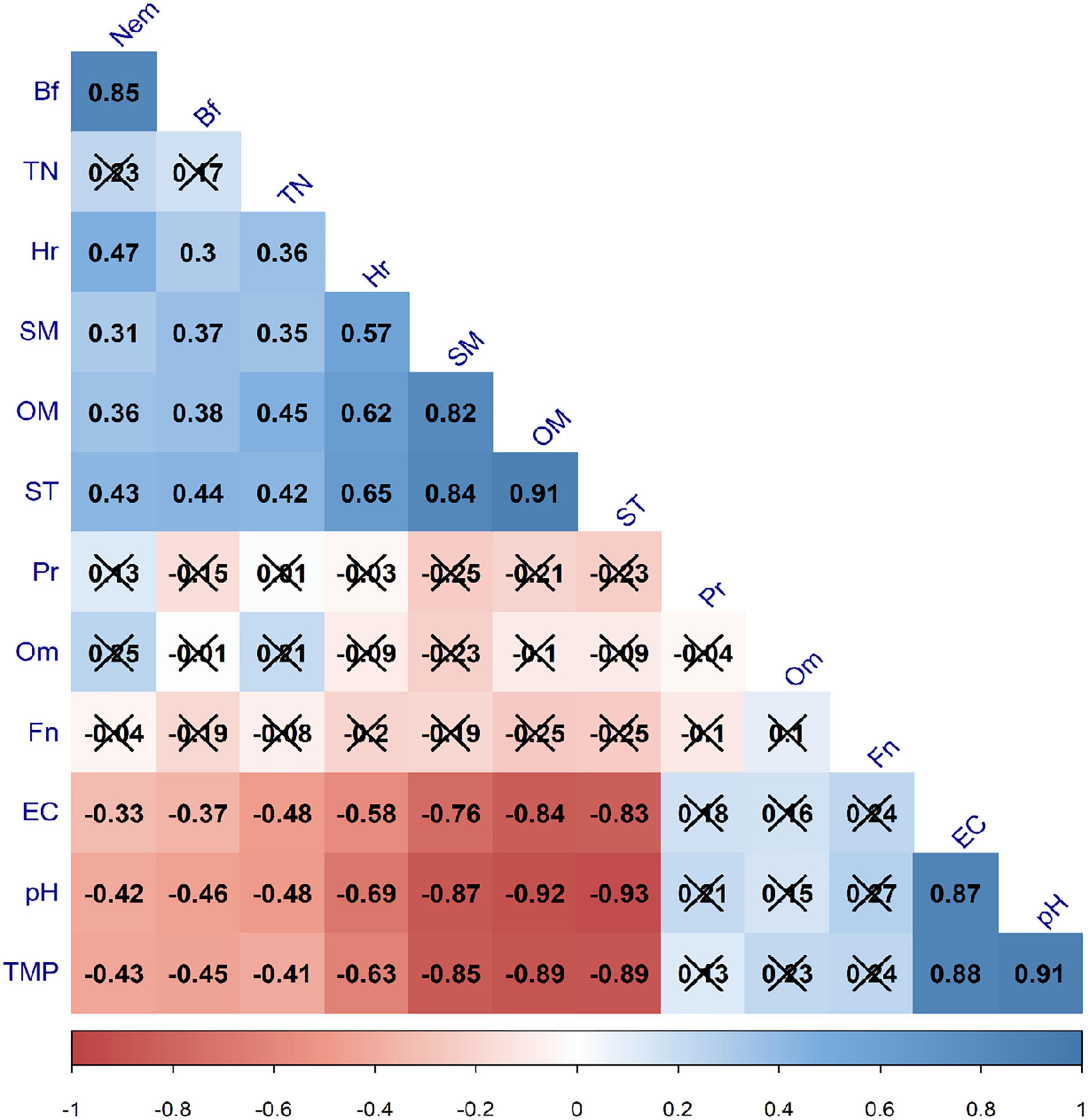
Figure 5. Correlation between soil nematode trophic groups and soil physico-chemical properties. The cross marked correlation coefficients denote non-significance at p = 0.05. BF, bacterivores; Fn, fungivores; Hr, herbivores; Om, omnivores; Pr, predators; nem, total nematode abundance; EC, electrical conductivity; OM, organic matter; SM, soil moisture; ST, soil texture; TMP, temperature; TN, total nitrogen.
The principal component analysis (PCA) explained 19.5% of the total variation in the data, with first component (Dim1) accounting for 13.6% and the second component (Dim2) explaining 5.9% of this variation (Figure 6). Once again, the PCA showed that the majority of genera belonging to bacterivores and herbivores were positively related with soil moisture, organic matter, total nitrogen and texture (i.e., most of the genera from bacterivores and herbivores are widely distributed across those sites having higher content soil moisture, organic matter and total nitrogen and richer soil texture), but showed negative relation with soil electrical conductivity, pH and temperature (Figure 6). In contrast, the genera from other trophic groups were distributed uniformly throughout regardless of the position of soil variables (Figure 6).
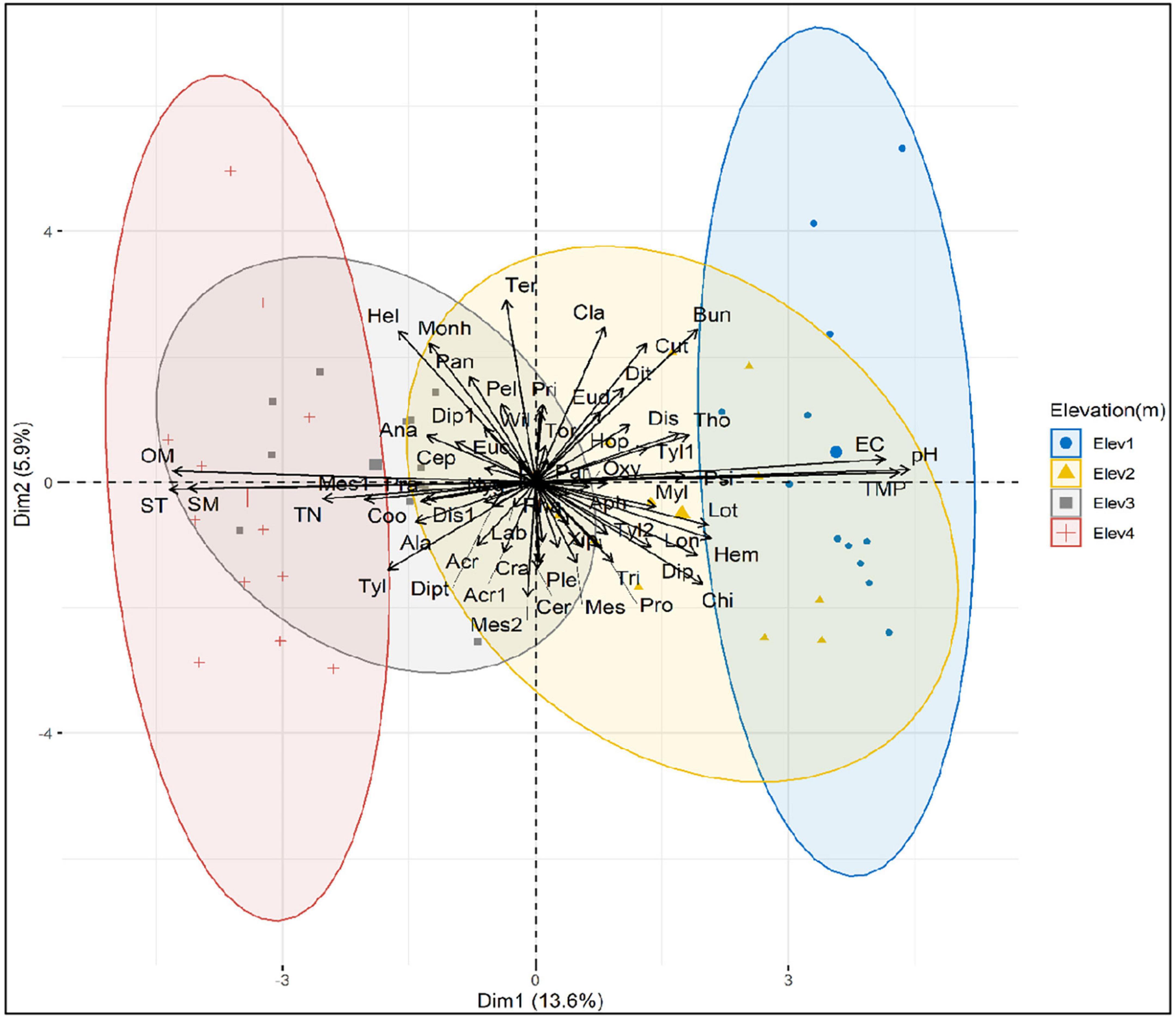
Figure 6. Principal component analysis (PCA) showing the relationship between soil nematode genera and soil properties across elevation classes.
In the mountainous ecosystems, elevation gradients along which environmental variables change considerably within shortest distances, remain one of the mainstays for contemporary research in the field of ecology and evolution, and thus play a key role in understanding the large-scale patterns in biodiversity, global change and conservation (Wei et al., 2019; Afzal et al., 2021).
The present study showed that both the soil nematode diversity and generic richness decreased significantly among the elevational classes with increasing elevation. Our result is in line with the findings of several published studies conducted in the forest ecosystems both in the Himalaya (Afzal et al., 2021) and elsewhere (Yeates, 2007; Liu et al., 2019; Yu et al., 2021; Zhang et al., 2021). This pattern of soil nematode richness and diversity can be attributed to a number of reasons: (1) the occurrence of adverse climatic conditions at the higher elevations in the region alter abiotic and biotic factors, which ultimately result in decreasing nematode diversity and richness at higher elevations (Afzal et al., 2021; Kashyap et al., 2022). (2) In our study region, the temperature remains below optimum during the winter months. The availability of lower temperatures at higher elevations decreases the soil microbial activity and associated decomposition process, leading to lower nutrient levels and negatively affecting soil nematode diversity and richness (Margesin et al., 2016; Albright et al., 2020; Afzal et al., 2021). (3) The mean annual precipitation also plays a key role in affecting the diversity and richness patterns of soil nematodes by changing the above ground vegetation composition and below ground microbial activities and soil nutrients (Bai et al., 2008; Kardol et al., 2011). Also, the higher amount of precipitation at higher elevation greatly reduces the diversity of soil nematodes by causing oxygen deprivation due to reduced oxygen levels (Afzal et al., 2021; Yu et al., 2021) and (4) The higher diversity and generic richness at lower elevations in the forest ecosystems as evidenced in our study can also be due to the more resilient, balanced and sustainable ecosystem occuring at lower elevations (Yeates, 2007; Kashyap et al., 2022).
The present study revealed that the soil nematode composition differed significantly among studied elevation classes along the elevation gradient. This result is in agreement with the findings of several other published studies especially from the mountainous ecosystems (Kergunteuil et al., 2016; Dong et al., 2017; Liu et al., 2019; Afzal et al., 2021; Yu et al., 2021). The plausible explanation for this can be that the vegetation composition, soil nutrients and bioclimatic variables vary considerably with elevation (Liu et al., 2019; Yu et al., 2021; Kashyap et al., 2022). All these abiotic and biotic factors shape soil nematode communities possibly due to net environmental filtering, varying environmental conditions and resource heterogeneity (Dong et al., 2017; Afzal et al., 2021). Another possible reason can be that the variation in the local floristic composition might cause changing nematode community structure along elevation gradients (Kergunteuil et al., 2016). For example, among the total nematode taxa characterizing the lowland communities, Tripylais mainly found associated with the tree species, while as Cervidellusis generally found to occur with the sub alpine cushions like Juniperus (Kergunteuil et al., 2016). More interestingly, our study showed one exception that soil nematode composition of the two highest elevation classes (Elev3 and Elev4) was comparatively similar. The result in in accordance with the study conducted by Kashyap et al. (2022) which reported similar nematode composition between the two higher elevation classes in Gangotri National Park. The possible reason for this effect can be that the micro-environmental condition between these two elevation classes may not vary to the extent that causes differences in the nematode composition.
Our study found that the response of nematode abundance to increasing elevation varied with trophic groups, with total nematode, bacterivore and herbivore abundances showing increasing effect with increasing elevation, while as fungivore and predator abundances exhibiting the opposite trend. The increasing effect of elevation on total nematode, bacterivore and herbivore abundances is contradictory with the studies from Afzal et al. (2021). However, our results are in agreement with several other studies that reported increasing bacterivore (Kergunteuil et al., 2016; Veen et al., 2017) and herbivore (Kouser et al., 2021) abundances and decreasing fungivore (Veen et al., 2017; Afzal et al., 2021) and predator (Afzal et al., 2021; Kashyap et al., 2022) abundances with elevation. Our findings are also in line with the study carried out by Veen et al. (2017) which reported that the soil nematode trophic groups can exhibit differential response to elevation under different vegetation types. Generally, the declining effect of elevation on nematode abundance is based on the notion that climate becomes adverse and harsher at high elevations (Afzal et al., 2021; Kashyap et al., 2022). In contrast, nematodes have been reported to withstand harshest environments in case of extreme polar regions (Yeates, 2010), thereby indicating that nematodes can survive more at higher elevations (Kouser et al., 2021). Therefore, the elevational patterns exhibited by different nematode trophic groups in different environmental settings is still debated and a matter of further investigation. One plausible explanation for the increasing herbivore nematode abundance with elevation can be the presence of well-developed dense root systems which provide shelter from various types of abiotic and biotic stress (Kergunteuil et al., 2016). Similarly, the declining effect of predatory nematode abundance with elevation can be possibly due to their key role in the soil food web, wherein they can flexibly switch on to a different prey species depending on the host availability (Afzal et al., 2021). Likewise, the declining effect fungivore nematode abundance with elevation can be possibly due to increasing pH along elevational gradient. Since it has been reported that fungi prefer acidic soils to develop mycelia, therefore the abundance of fungivore nematodes can decrease with increasing soil pH (Frostegård and Bååth, 1996; Kitagami et al., 2017). Our study also showed that among all trophic groups, bacterivores were the most abundant across the studied elevational classes, whereas fungivores were least abundant group. This result is in agreement with the finding of other studies (Dong et al., 2017; Kashyap et al., 2022). This in turn indicates that a relatively faster bacterial energy channel-based decomposition model predominates in the study region.
Consistent with several previous studies (Zhao et al., 2015; Song et al., 2016; Dong et al., 2017), we found that the soil physico-chemical properties were key environmental variables influencing nematode community structure. More specifically, our study showed that total nematode, bacterivore and herbivore abundances were associated with higher pH, soil organic matter, soil moisture and total nitrogen levels, which agrees with the findings of other studies as well (Savin et al., 2001; Postma-Blaauw et al., 2005; Wall et al., 2010; Song et al., 2016; Liu et al., 2019). As evidenced in the present study, the key role of soil pH in governing nematode community composition especially that of bacterivores can be best explained by the direct feeding relationships between bacterivore nematodes and bacteria, whose abundance are greatly influenced by soil pH (Shen et al., 2015, 2020; Wang et al., 2015; Nottingham et al., 2018). The possible explanation for significant influence of soil moisture on certain trophic groups as evidenced from our study can be due to its enhanced effects on decomposition rates (Dong et al., 2017). Similarly, the positive effect of total nitrogen on certain nematode group can be due to its key role in regulating the content of soluble nutrients in an ecosystem (Liang et al., 2007).
In recent times, a number of studies have advocated the usage of functional and metabolic indices, along with taxonomic indices for understanding the mechanisms of ecosystem functioning (Montoya et al., 2015; Kergunteuil et al., 2016; Kouser et al., 2021). The present study evaluated several key functional and community indices to better understand both the relative contribution of nematode trophic groups to soil food web structure and changes in soil ecosystem functioning along elevation gradients. Our study showed that the maturity indices decreased with elevation, however, the effect was significant for MI25 index only. This decrease in the maturity indices with elevation is in line with the findings of several the studies (Afzal et al., 2021; Kashyap et al., 2022). Since the maturity indices are indicative of degree of disturbance associated with the soil ecosystem with lower values representing more disturbances (Bongers and Ferris, 1999; Afzal et al., 2021), this in turn reflect a comparatively higher degree of disturbance among the highest elevation classes as compared to lowest elevation classes in our study region. Also, the evidence of decreasing maturity indices with elevation in our study suggests lesser tolerance of K-selected free living and plant parasitic nematodes toward stressful conditions typically encountered at higher elevations (Afzal et al., 2021). Our study also found a decrease in structure index (SI) and plant parasitic index (PPI) with elevation, although the effect was significant for SI only. These results of elevation wise decrease in SI and PPI are in accordance with the finding of Afzal et al. (2021) and highlight the existence of relatively simpler, loosely structured soil food webs having fewer connections at higher elevation classes as compared to those found in lower elevations. Moreover, our study also showed significant difference in the channel index (CI), among the elevation classes, with highest CI value found at the top elevation class (Elev4). As the CI index is simply the ratio of fungivore to bacterivore nematodes, our results indicate that in the study region the fungal decomposition pathways support greater nematode biomass than bacterial decomposition at higher elevation classes (Afzal et al., 2021). The possible reason for this effect can be that at higher elevations some of the plant traits including slow growing and longer leaf life span cause slow mineralization rates, and lesser fertile soils which generally enhance the fungal-based energy flows within ecosystems as compared to lowlands with more bacterial-dominated pathways (Zhao and Neher, 2014; Kergunteuil et al., 2016). Our study also revealed a significant difference both in the composite and enrichment footprints among the elevation classes, with lowest values observed at the highest elevation class (Elev4). Since, these metabolic footprints are indicators of the magnitude of carbon and energy flow in soil food webs (Ferris, 2010; Afzal et al., 2021), our results indicate that the degree of carbon and other resource assimilation in soil food web from autotrophic organisms is relatively lower at highest elevation class than the lower elevation classes in the study region, owing to slower decomposition rates in the former as compared to latter ones.
Our study also showed that soil physico-chemical parameters varied significantly between the elevation classes, with soil pH, electrical conductivity and temperature showing a decrease with elevation, the soil moisture, organic matter and texture showing an increase with elevation, while as the total nitrogen content showing maximum at middle elevation class (Elev3). Our finding of decreasing soil pH along elevation gradients is in line with other studies (Afzal et al., 2021; Kouser et al., 2021; Zhang et al., 2021). The possible explanation for this effect could be the accumulation of litter at higher altitudes due to slow rates of decomposition that negatively effects the soil biota (Kappes et al., 2007). Similarly, our result of decreasing soil EC along elevation gradient is in line with other studies conducted by Kouser et al. (2021) and Kashyap et al. (2022), and can possibly be a result of reduced mobility of ions due to leaching and washing out of the solutes at higher elevations. Likewise, our finding of increasing soil moisture with elevation is in accordance with several other studies (Kouser et al., 2021; Zhang et al., 2021), and can be attributed to an elevation wise increase in mean annual precipitation and a decrease in precipitation seasonality (Kouser et al., 2021). Lastly, our finding of mid elevational peak in total nitrogen content is in line with the study conducted by Dong et al. (2017) in Norikura mountains, and can be explained be the relatively slower decay of plant products at moderately cool temperatures of the middle elevations, thereby resulting in the accumulation of nitrogen in the upper layer of soil (Sarathchandra et al., 2001; Dong et al., 2017).
The present study provides a novel understanding of the diversity and compositional patterns of soil nematodes in the mountainous ecosystems of Pir-Panjal Himalaya. Overall, our study showed that soil nematodes exhibit distinct elevational patterns, which are consistent with the trends observed for other groups of organisms, thereby indicating how evident these patterns exit in nature. Taken together, we conclude that the variation in soil nematode diversity and community composition along the elevation gradient could be due to the shared effects of soil and climatic factors, thus highlighting the crucial role of interaction among multiple environmental factors in shaping soil biodiversity. Looking ahead, a better understating of relative importance of various ecological correlates in governing nematode diversity and distributional patterns can provide vital information on the response of soil nematode communities to climate and land-use change in the current era of anthropogenic global environmental change.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
FC: conceptualization, methodology, data collection, curation and analysis, investigation, and writing—original draft. AB, IS, and SR: writing—review. MK: supervision and writing—review. AS: conceptualization, supervision, validation, and writing—review. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afzal, S., Nesar, H., Imran, Z., and Ahmad, W. (2021). Altitudinal gradient affect abundance, diversity and metabolic footprint of soil nematodes in Banihal-Pass of Pir-Panjal mountain range. Sci. Rep. 11:16214. doi: 10.1038/s41598-021-95651-x
Albright, M. B., Johansen, R., Thompson, J., Lopez, D., Gallegos-Graves, L. V., Kroeger, M. E., et al. (2020). Soil bacterial and fungal richness forecast patterns of early pine litter decomposition. Front. Microbiol. 11:542220. doi: 10.3389/fmicb.2020.542220
Andrássy, I. (2005). “Free-living nematodes of Hungary (Nematoda errantia), I,” in Pedozoologica hungarica No. 3, eds C. Csuzdi and S. Mahunka (Budapest: Hungarian Natural History Museum).
Andrassy, I. T. (1956). Determination of volume and weight of nematodes. Acta Zool. Acad. Sci. Hungaricae 2, 1–15.
Bai, Y., Wu, J., Xing, Q., Pan, Q., Huang, J., Yang, D., et al. (2008). Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 89, 2140–2153. doi: 10.1890/07-0992.1
Bakonyi, G., Nagy, P., Kovacs-Lang, E., Kovacs, E., Barabás, S., Répási, V., et al. (2007). Soil Nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland. Appl. Soil Ecol. 37, 31–40. doi: 10.1016/j.apsoil.2007.03.008
Barker, K. R. (1985). “Nematode extraction and bioassays,” in An advanced treatise on meloidogyne, methodology, Vol. 2, eds K. R. Barker, C. C. Carter, and J. N. Sasser (Raleigh, NC: North Carolina State University Graphics), 19–35.
Bongers, T. (1990). The maturity index as an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83, 14–19. doi: 10.1007/BF00324627
Bongers, T., and Bongers, M. (1998). Functional diversity of nematodes. Appl. Soil Ecol. 10, 239–251. doi: 10.1016/S0929-1393(98)00123-1
Bongers, T., and Ferris, H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224–228. doi: 10.1016/S0169-5347(98)01583-3
da Silva, J. V. C. D. L., Hirschfeld, M. N. C., Cares, J. E., and Esteves, A. M. (2020). Land use, soil properties and climate variables influence the nematode communities in the Caatinga dry forest. Appl. Soil Ecol. 150:103474. doi: 10.1016/j.apsoil.2019.103474
Dar, T. A., Uddin, M., Khan, M. M. A., Hakeem, K. R., and Jaleel, H. (2015). Jasmonates counter plant stress: A review. Environ. Exp. Bot. 115, 49–57. doi: 10.1016/j.envexpbot.2015.02.010
Devetter, M., Hanel, L., Rehakova, K., and Anddolezal, J. (2017). Diversity and feeding strategies of soil microfauna along elevation gradients in Himalayan cold deserts. PLoS One 12:e0187646. doi: 10.1371/journal.pone.0187646
Dong, K., Moroenyane, I., Tripathi, B., Kerfahi, D., Takahashi, K., Yamamoto, N., et al. (2017). Soil nematodes show a mid-elevation diversity maximum and elevational zonation on Mt. Norikura, Japan. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-03655-3
Ferris, H. (2010). Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 46, 97–104. doi: 10.1016/j.ejsobi.2010.01.003
Ferris, H., Bongers, T., and De Goede, R. G. M. A. (2001). Framework for soil food web diagnostics extension of the nematode faunal analysis concept. Appl. Soil Ecol. 18, 13–29. doi: 10.1016/S0929-1393(01)00152-4
Frostegård, Å, and Bååth, E. (1996). The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertility Soils 22, 59–65. doi: 10.1007/BF00384433
Goodey, T. (1963). Soil and freshwater nematodes. Methuen, MA: Methuen and Cooperation Limited. doi: 10.1097/00010694-196404000-00018
Hodda, M. (2011). “Phylum Nematoda Cobb 1932,” in Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness, Vol. 3148, ed. Z.-Q. Zhang (Zootaxa), 63–95. doi: 10.11646/zootaxa.3148.1.11
Hodda, M. E., Peters, L., and Traunspurger, W. (2009). “Nematode diversity in terrestrial, freshwater aquatic and marine systems,” in Nematodes as environmental indicators, 1st Edn, eds M. Wilson and T. Kakouli-Duarte (Oxfordshire: CABI Publishing), 45–93. doi: 10.1079/9781845933852.0045
Jairajpuri, M. S., and Ahmad, W. (1992). Dorylaimida: Free-living, predaceous and plant-parasitic nematodes. Leiden: Brill.
Kappes, H., Lay, R., and Topp, W. (2007). Changes in different trophic levels of litter-dwelling macrofauna associated with giant knotweed invasion. Ecosystems 10, 734–744. doi: 10.1007/s10021-007-9052-9
Kardol, P., Reynolds, W. N., Norby, R. J., and Classen, A. T. (2011). Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 47, 37–44. doi: 10.1016/j.apsoil.2010.11.001
Kashyap, P., Afzal, S., Rizvi, A. N., Ahmad, W., Uniyal, V. P., and Banerjee, D. (2022). Nematode community structure along elevation gradient in high altitude vegetation cover of Gangotri National Park (Uttarakhand), India. Sci. Rep. 12, 1–13. doi: 10.1038/s41598-022-05472-9
Kassambara, A., and Mundt, F. (2020). Factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7. Available online at: https://CRAN.R-project.org/package=factoextra (accessed November 5, 2022).
Kergunteuil, A., Campos-Herrera, R., Sánchez-Moreno, S., Vittoz, P., and Rasmann, S. (2016). The abundance, diversity, and metabolic footprint of soil nematodes is highest in high elevation alpine grasslands. Front. Ecol. Evol. 4:84. doi: 10.3389/fevo.2016.00084
Kitagami, Y., Kanzaki, N., and Matsuda, Y. (2017). Distribution and community structure of soil nematodes in coastal Japanese pine forests were shaped by harsh environmental conditions. Appl. Soil Ecol. 119, 91–98. doi: 10.1016/j.apsoil.2017.05.030
Kjeldahl, J. (1883). New method for the determination of nitrogen. Chem. News 48, 101–102. doi: 10.1038/scientificamerican10061883-6470bsupp
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574. doi: 10.1016/j.tree.2007.09.006
Kouser, Y., Shah, A. A., and Rasmann, S. (2021). The functional role and diversity of soil nematodes are stronger at high elevation in the lesser Himalayan Mountain ranges. Ecol. Evol. 11, 13793–13804. doi: 10.1002/ece3.8061
Liang, W. J., Zhong, S., Hua, J. F., Cao, C. Y., and Jiang, Y. (2007). Nematode faunal response to grassland degradation in horqin sandy land. Pedosphere 17, 611–618. doi: 10.1016/S1002-0160(07)60072-1
Liu, J., Yang, Q., Siemann, E., Huang, W., and Ding, J. (2019). Latitudinal and altitudinal patterns of soil nematode communities under tallow tree (Triadicasebifera) in China. Plant Ecol. 220, 965–976. doi: 10.1007/s11258-019-00966-5
Maina, S., Karuri, H., and Mugweru, J. (2022). Nematode diversity and its association with soil properties in monocrop pigeon pea. J. Agric. Food Res. 9:100336. doi: 10.1016/j.jafr.2022.100336
Margesin, R., Minerbi, S., and Schinner, F. (2016). Litter decomposition at two forest sites in the Italian Alps: A field study. Arctic Antarctic Alpine Res. 48, 127–138. doi: 10.1657/AAAR0015-012
Montoya, D., Yallop, M., and Memmott, J. (2015). Functional group diversity increases with modularity in complex food webs. Nat. Commun. 6:7379. doi: 10.1038/ncomms8379
Nielsen, U. N., Ayres, E., Wall, D. H., Li, G., Bardgett, R. D., Wu, T., et al. (2014). Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 23, 968–978. doi: 10.1111/geb.12177
Nottingham, A. T., Fierer, N., Turner, B. L., Whitaker, J., Ostle, N. J., McNamara, N. P., et al. (2018). Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 99, 2455–2466. doi: 10.1002/ecy.2482
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). vegan: Community Ecology Package. R package version 2.5-7. Available online at: https://CRAN.R-project.org/package=vegan (accessed November 5, 2022).
Postma-Blaauw, M. B., de Vries, F. T., De Goede, R. G. M., Bloem, J., Faber, J. H., and Brussaard, L. (2005). Within-trophic group interactions of bacterivorous nematode species and their effects on the bacterial community and nitrogen mineralization. Oecologia 142, 428–439. doi: 10.1007/s00442-004-1741-x
Powers, L. E., Ho, M. C., Freckman, D. W., and Virginia, R. A. (1998). Distribution, community structure and microhabitats of soil invertebrates along an elevational gradient in Taylor Valley Antarctica. Arct. Alp. Res. 30, 133–141. doi: 10.2307/1552128
Qing, X., Bert, W., Steel, H., Quisado, J., and de Ley, I. T. (2015). Soil and litter nematode diversity of Mount Hamiguitan, the Philippines, with description of Bicirronemahamiguitanense n. sp (Rhabditida: Bicirronematidae). Nematology 17, 325–344. doi: 10.1163/15685411-00002870
Quist, C. W., Gort, G., Mooijman, P., Brus, D. J., van den Elsen, S., Kostenko, O., et al. (2019). Spatial distribution of soil nematodes relates to soil organic matter and life strategy. Soil Biol. Biochem. 136:107542. doi: 10.1016/j.soilbio.2019.107542
R Core Team (2020). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reid, M. L., Howes, A. S., and Emery, S. M. (2019). Dune soil communities primarily associated with climate factors, not exotic plant presence. Plant Soil 436, 503–515. doi: 10.1007/s11104-019-03944-y
Salamun, P., Hanzelová, V., Miklisová, D., Šestinová, O., Findoráková, L., and Kováčik, P. (2017). The effects of vegetation cover on soil Nematode communities in various biotopes disturbed by industrial emissions. Sci. Total Environ. 592, 106–114. doi: 10.1016/j.scitotenv.2017.02.238
Šalamún, P., Kucanová, E., Brázová, T., Miklisová, D., Renčo, M., and Hanzelová, V. (2014). Diversity and food web structure of nematode communities under high soil salinity and alkaline pH. Ecotoxicology 23, 1367–1376. doi: 10.1007/s10646-014-1278-7
Sarathchandra, S. U., Ghani, A., Yeates, G. W., Burch, G., and Cox, N. R. (2001). Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol. Biochem. 33, 953–964. doi: 10.1016/S0038-0717(00)00245-5
Savin, M. C., Gorres, J. H., Neher, D. A., and Amador, J. A. (2001). Biogeophysical factors influencing soil respiration and mineral nitrogen content in an old field soil. Soil Biol. Biochem. 33, 429–438. doi: 10.1016/S0038-0717(00)00182-6
Shahabuddin, G., Goswami, R., Krishnadas, M., and Menon, T. (2021). Decline in forest bird species and guilds due to land use change in the Western Himalaya. Glob. Ecol. Conserv. 25, e01447. doi: 10.1016/j.gecco.2020.e01447
Shen, C., Gunina, A., Luo, Y., Wang, J., He, J. Z., Kuzyakov, Y., et al. (2020). Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 22, 3287–3301. doi: 10.1111/1462-2920.15090
Shen, C., Ni, Y., Liang, W., Wang, J., and Chu, H. (2015). Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 6:582. doi: 10.3389/fmicb.2015.00582
Siebert, J., Sünnemann, M., Auge, H., Berger, S., Cesarz, S., Ciobanu, M., et al. (2019). The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-018-36777-3
Sieriebriennikov, B., Ferris, H., and de Goede, R. G. (2014). NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 61, 90–93. doi: 10.1016/j.ejsobi.2014.02.004
Sikora, R. A., Coyne, D., Hallmann, J., and Timper, P. (Eds.) (2018). Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford: CAB International, 62–83. doi: 10.1079/9781786391247.0000
Song, D., Pan, K., Tariq, A., Sun, F., Li, Z., Sun, X., et al. (2017). Large-scale patterns of distribution and diversity of terrestrial nematodes. Appl. Soil Ecol. 114, 161–169. doi: 10.1016/j.apsoil.2017.02.013
Song, M., Li, X., Jing, S., Lei, L., Wang, J., and Wan, S. (2016). Responses of soil nematodes to water and nitrogen additions in an old-field grassland. Appl. Soil Ecol. 102, 53–60. doi: 10.1016/j.apsoil.2016.02.011
Sun, X., Zhang, X., Zhang, S., Dai, G., Han, S., and Liang, W. (2013). Soil nematode responses to increases in nitrogen deposition and precipitation in a temperate forest. PLoS One 8:e82468. doi: 10.1371/journal.pone.0082468
Thakur, M. P., del Real, I. M., Cesarz, S., Steinauer, K., Reich, P. B., Hobbie, S., et al. (2019). Soil microbial, nematode, and enzymatic responses to elevated CO2, N fertilization, warming, and reduced precipitation. Soil Biol. Biochem. 135, 184–193. doi: 10.1016/j.soilbio.2019.04.020
Thakur, M. P., Tilman, D., Purschke, O., Ciobanu, M., Cowles, J., Isbell, F., et al. (2017). Climate warming promotes species diversity, but with greater taxonomic redundancy, in complex environments. Sci. Adv. 3:e1700866. doi: 10.1126/sciadv.1700866
Tong, F. C., Xiao, Y., and Wang, Q. L. (2010). Soil Nematode community structure on the northern slope of Changbai Mountain Northeast China. J. For. Res. 21, 93–98. doi: 10.1007/s11676-010-0016-0
van den Hoogen, J., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D. A., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198. doi: 10.1038/s41586-019-1418-6
Van Eekeren, N., De Boer, H., Hanegraaf, M., Bokhorst, J., Nierop, D., Bloem, J., et al. (2010). Ecosystem services in grassland associated with biotic and abiotic soil parameters. Soil Biol. Biochem. 42, 1491–1504. doi: 10.1016/j.soilbio.2010.05.016
Veen, G. F., De Long, J. R., Kardol, P., Sundqvist, M. K., Snoek, L. B., and Wardle, D. A. (2017). Coordinated responses of soil communities to elevation in three subarctic vegetation types. Oikos 126, 1586–1599. doi: 10.1111/oik.04158
Wall, D. H., Bardgett, R. D., and Kelly, E. (2010). Biodiversity in the dark. Nat. Geoence 3, 297–298. doi: 10.1038/ngeo860
Wang, J.-T., Cao, P., Hu, H.-W., Li, J., Han, L.-L., Zhang, L.-M., et al. (2015). Altitudinal distribution patterns of soil bacterial and archaeal communities along Mt. Shegyla on the Tibetan Plateau. Microb. Ecol. 69, 135–145. doi: 10.1007/s00248-014-0465-7
Wani, Z. A., Negi, V. S., Bhat, J. A., Satish, K. V., Kumar, A., Khan, S., et al. (2023). Elevation, aspect, and habitat heterogeneity determine plant diversity and compositional patterns in the Kashmir Himalaya. Front. For. Glob. Change 6:11. doi: 10.3389/ffgc.2023.1019277
Wei, D., Jia-Liang, W., Scott, M., Yi-Hao, F., Shuo-Ran, L., Xiao-Yan, Y., et al. (2019). Effects of sampling on the elevational distribution of nematode-trapping fungi. Res. Sq. BMC Microbiol. doi: 10.21203/rs.2.14154/v1
Yeates, G. W. (2003). Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertility Soils 37, 199–210. doi: 10.1007/s00374-003-0586-5
Yeates, G. W. (2007). Abundance diversity and resilience of Nematode assemblage in forest soils. Can. J. For. Res. 37, 216–225. doi: 10.1139/x06-172
Yeates, G. W. (2010). “Nematodes in ecological webs,” in Encyclopedia of life science, (Chichester: John Wiley & Sons, Ltd), 1–10. doi: 10.1002/9780470015902.a0021913
Yeates, G. W., Bongers, T., De Goede, R. G., Freckman, D. W., and Georgieva, S. S. (1993). Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 25, 315–331.
Yu, Z., Zou, S., Li, N., Kerfahi, D., Lee, C., Adams, J., et al. (2021). Elevation-related climatic factors dominate soil free-living nematode communities and their co-occurrence patterns on Mt. Halla, South Korea. Ecol. Evol. 11, 18540–18551. doi: 10.1002/ece3.8454
Zhang, Y., Ji, L., and Yang, L. (2021). Abundance and diversity of soil nematode community at different altitudes in cold-temperate montane forests in northeast China. Glob. Ecol.d Conserv. 29:e01717. doi: 10.1016/j.gecco.2021.e01717
Zhao, J., and Neher, D. A. (2014). Soil energy pathways of different ecosystems using nematode trophic group analysis: A meta-analysis. Nematology 16, 379–385. doi: 10.1163/15685411-00002771
Keywords: soil nematode, composition, diversity, elevation, trophic group, Himalaya
Citation: Choudhary F, Bhardwaj A, Sayeed I, Rather SA, Khan MAH and Shah AA (2023) Elevational patterns of soil nematode diversity, community structure and metabolic footprint in the Trikuta mountains of Northwestern Himalaya. Front. For. Glob. Change 6:1135219. doi: 10.3389/ffgc.2023.1135219
Received: 31 December 2022; Accepted: 20 March 2023;
Published: 04 April 2023.
Edited by:
Vikram S. Negi, Govind Ballabh Pant National Institute of Himalayan Environment and Sustainable Development, IndiaReviewed by:
Zeeshan Ahmad, Quaid-i-Azam University, PakistanCopyright © 2023 Choudhary, Bhardwaj, Sayeed, Rather, Khan and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Asghar Shah, YWFzaGFoQGJnc2J1LmFjLmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.