- 1Shanghai Botanical Garden, Shanghai Engineering Research Center of Sustainable Plant Innovation, Xuhui, Shanghai, China
- 2Ontario Ministry of Natural Resources and Forestry, Ontario Forest Research Institute, Sault Ste. Marie, ON, Canada
- 3Faculty of Natural Resources Management, Lakehead University, Thunder Bay, ON, Canada
Differential phenological responses
Plant phenology is shifting as a result of global warming (IPCC, 2019). General trends include advanced spring phenology (i.e., earlier budburst and leaf-out) and delayed leaf senescence, leading to an extended leaf-on period and possibly increased growth (Peñuelas et al., 2009; IPCC, 2019; Piao et al., 2019). However, responses vary among species, e.g., those with early spring phenology—referred to here as early season species—often show more pronounced advances in spring phenology (Abu-Asab et al., 2001; Beaubien and Hamann, 2011; Shen et al., 2014) than so called late season species. As global warming progresses, these among-species differences in leaf-on time or green-cover season may increase (Morin et al., 2009; Montgomery et al., 2020), leading to an expectation of possible changes in ecosystem structure and function (Polgar et al., 2014; Primack and Gallinat, 2016).
The annual development of plants in boreal and temperate regions is driven by the seasonal cycle of climatic conditions, although species-specific information about these changes is often lacking. Bud set, leaf senescence, and dormancy are induced by shorter daylength and lower temperatures in fall, while spring phenology is controlled primarily by temperatures, i.e., low chilling temperatures in fall and winter for dormancy release and high forcing temperatures for spring growth initiation (Chuine et al., 2016; Piao et al., 2019). As species chilling needs can be fulfilled long before spring arrives (see Figure 1), spring phenology is often not influenced by changes in cumulative winter chilling induced by global warming (Fu et al., 2015; Asse et al., 2018; Piao et al., 2019; Chu et al., 2021). Comparatively, early season species that need less accumulation of forcing temperatures (cumulative growing degree days or hours) to initiate spring growth are often more responsive or sensitive to rising temperatures (Abu-Asab et al., 2001; Beaubien and Hamann, 2011; Shen et al., 2014) than late season species. An early spring start is thought to help plants access resources and gain growth and competitive advantages (Polgar et al., 2014; Primack and Gallinat, 2016; Zettlemoyer et al., 2019; Montgomery et al., 2020). As early successional, exotic, and invasive species generally start growing early in spring, they are expected to benefit more from projected warming, resulting in proliferation of these species and therefore undesirable changes in ecosystems (Polgar et al., 2014; Zettlemoyer et al., 2019).
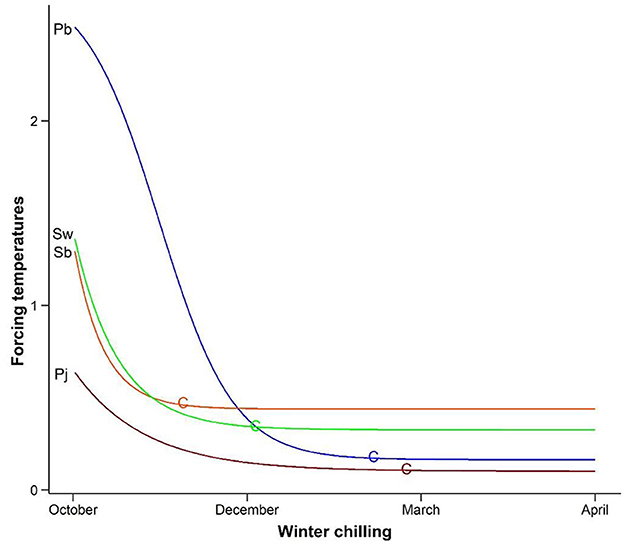
Figure 1. Diverse chilling-forcing relationships. Forcing temperatures (on a relative scale) needed for budburst decrease rapidly with an increase in winter chilling at low chilling and gradually stabilize at high chilling [modified from Man et al. (2017)]. Early season balsam poplar (Populus balsamifera-Pb) and jack pine (Pinus banksiana-Pj) need less accumulation of forcing temperatures for budburst than late season white spruce (Picea glauca-Sw) and black spruce (Picea mariana-Sb) after chilling requirement is met (indicated by letter C). The effects of chilling on phenological responses (i.e., changes in forcing temperatures needed for budburst per unit change in chilling) depend on chilling level and species chilling-forcing relationships but are not necessarily associated with species chilling/forcing requirements or functional groups.
However, a recent study by Chu et al. (2021) suggests that this theory is not supported by plant thermal balance in spring. Shifts in spring phenology driven by global warming are associated with changes in both timing of spring growth and forcing temperatures, with the latter more indicative of plant development and growth (Chuine et al., 2016; Man et al., 2017; Piao et al., 2019). Due to cumulative effects of spring temperatures, early season species gain fewer forcing temperatures from warming before growth initiation and more forcing temperatures after growth initiation than late season species. If warming-induced changes in forcing temperatures before and after growth initiation are both included, all plant species would equally benefit from warming in terms of total changes in forcing temperatures (Chu et al., 2021).
Factors influencing among-species differences
As shown by Chu et al. (2021), the more pronounced responses to global warming by early season species result largely from low temperatures in early and cold spring when these species leaf out, rather than their ability to track temperature changes. Although early season species resume growth early in spring and gain fewer forcing temperatures from their phenological shifts, at low temperatures they often need more time (or larger phenological advances) to accumulate forcing temperatures gained from warming. Therefore, leaf-out temperatures or spring phenology can strongly influence plant phenological responses to global warming. The effects of spring temperatures on phenological responses have occasionally been recognized (Shen et al., 2014), but never clearly elucidated. Depending on differences in the timing of spring growth and therefore leaf-out temperatures, early season species could also have similar or smaller phenological changes than late season species in response to global warming (Chu et al., 2021).
When winter chilling is insufficient, changes in cumulative winter chilling by global warming can accelerate or delay phenological responses from what would be expected solely from changes in forcing temperatures (Chu et al., 2021). Besides different chilling and forcing needs and chilling–forcing relationships (see Figure 1) (Chuine et al., 2016; Man et al., 2017; Piao et al., 2019), plants may also differ in temperature use efficiency through different threshold temperatures for chilling and forcing accumulations, leading to diverse phenological responses to global warming (Zhang et al., 2018). The lack of sufficient winter chilling can be offset by more spring forcing without affecting spring growth (Chuine et al., 2016; Man et al., 2017). Relative to spring forcing, the accumulation of winter chilling is more complex and experimentally studied on few species (Chuine et al., 2016; Man et al., 2017). Spring phenology can be also adversely affected by freezing temperatures (Man et al., 2021) or moisture stress that may progressively develop and differentially affect species with differing spring phenology (Zettlemoyer et al., 2019). Interest in the influence of photoperiod on spring phenology is increasing (Körner and Basler, 2010). However, critical photoperiods have not been found, other than weak and variable compensation of insufficient chilling effects by longer photoperiod (Körner and Basler, 2010; Way and Montgomery, 2015; Zohner et al., 2016). The photoperiod effects reported in the literature are often concluded from growth timing differences, with differences in forcing temperatures ignored (Chu et al., 2021). In photoperiod experiments, longer photoperiods are often associated with more hours of daytime high temperatures (Chu et al., 2021), in addition to extra heating effects from artificial light sources (Mellor et al., 1964).
The common explanation that early season species need less chilling for dormancy release and are therefore less restricted by chilling deficiency induced by global warming (Morin et al., 2009; Polgar et al., 2014; Primack and Gallinat, 2016) seems true in some cases (Beaubien and Hamann, 2011). It may not apply in cases where reduced or delayed phenological responses occur in species of all spring phenology (Abu-Asab et al., 2001; Fitter and Fitter, 2002). In boreal tree species, black spruce and white spruce (Picea mariana and Picea glauca) are late flushing/late successional conifers but need substantially less chilling than some early flushing/early successional conifer or broadleaf tree species (Man et al., 2017; see also Figure 1). Similarly, in temperate forests of eastern Canada, early season tree species generally have more chilling needs than late season species (Man et al., 2020). High chilling needs are more ecologically meaningful to early season species than to late season species in minimizing risks of frost damage (Morin et al., 2009; Tao et al., 2021).
Growing season length
Regardless the causes of differential phenological responses among species, the use of phenological responses as an indication of changes in growing season length or growth potential is misconceived and biased. First, the influence of daily temperatures on spring development increases during spring temperature recovery. Early season species could have longer leaf-on time from their phenological shifts but gain fewer forcing temperatures due to low leaf-out temperatures. The use of phenological responses to represent changes in growing season length (or growth potential) contradicts the convention that growing season is defined by temperatures for certain climatic conditions (Christiansen et al., 2011), not by leaf-on time for individual species. Second, in northern climate where spring development depends largely on temperatures, phenological models are generally based on the accumulation, rather than duration, of forcing temperatures (Chuine et al., 2016; Man et al., 2017; Piao et al., 2019). Forcing temperatures, a good predictor of plant development and growth, are used to estimate timing of spring growth, a poor predictor of plant development and growth; the latter is commonly used in discussing ecological implications of plant phenological changes, probably because it is comparable among different climatic conditions and connected to growing season length. Thus, changes in spring phenology do not necessarily represent changes in growing season length and alone would not be sufficient for understanding the ecological implications of plant phenological responses to global warming (Chu et al., 2021).
Conclusion
Temperatures at time of spring growth strongly influence plant phenological responses, with low temperatures associated with large responses and high temperatures with small responses (Prevéy et al., 2017; Chu et al., 2021; Wolkovich et al., 2021). Early season species start to grow at low spring temperatures and are often more advanced in spring phenology in response to warming. In the northern hemisphere, plant development and growth in spring is primarily restricted by low temperatures, rather than availability of light or soil moisture. Total changes in forcing temperatures from global warming do not differ among species with differing spring phenology (Chu et al., 2021). The use of phenological responses to represent changes in growing season length would include more early spring cold days and overestimate the potential benefits that may result from warming for early season species. Contrary to the suggestions by many (Fitter and Fitter, 2002; Polgar et al., 2014; Shen et al., 2014; Primack and Gallinat, 2016; Zettlemoyer et al., 2019; Montgomery et al., 2020), early start in spring is not necessarily beneficial, particularly if frost risk increases with the advance of spring phenology into early spring and late winter (Morin et al., 2009; Man et al., 2013; Ma et al., 2019; Tao et al., 2021). Ecologists have been interested in sensitivity of spring phenology to changes in temperatures and comparison of among-species differences in phenological responses to global warming. These differences, however, are not as ecologically meaningful as is often suggested in understanding global change effects on ecosystem structure and function.
Author contributions
RM and Q-LD generated the ideas. XC, RM, and Q-LD contributed to the writing of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Shanghai Landscaping & City Appearance Administrative Bureau (2022 science and technology project G220304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Asab, M. S., Peterson, P. M., Shetler, S. G., and Orli, S. S. (2001). Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodivers. Conserv. 10, 597–612. doi: 10.1023/A:1016667125469
Asse, D., Chuine, I., Vitasse, Y., Yoccoz, N. G., Delpierre, N., Badeau, V., et al. (2018). Warmer winters reduce the advance of tree spring phenology induced by warmer springs in the Alps. Agric. For. Meteorol. 252, 220–230. doi: 10.1016/j.agrformet.2018.01.030
Beaubien, E., and Hamann, A. (2011). Spring flowering response to climate change between 1936 and 2006 in Alberta, Canada. BioScience 61, 514–524. doi: 10.1525/bio.2011.61.7.6
Christiansen, D. E., Markstrom, S. L., and Hay, L. E. (2011). Impacts of climate change on the growing season in the United States. Earth Interact. 15, 1–17. doi: 10.1175/2011EI376.1
Chu, X., Man, R., Zhang, H., Yuan, W., Tao, J., Dang, Q-. L., et al. (2021). Does climate warming favour early season species? Front. Plant Sci. 12, 765351. doi: 10.3389/fpls.2021.765351
Chuine, I., Bonhomme, M., Legave, J. M., García de Cortázar-Atauri, I., Charrier, G., Lacointe, A., et al. (2016). Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Glob. Change Biol. 22, 3444–3460. doi: 10.1111/gcb.13383
Fitter, A. H., and Fitter, R. S. R. (2002). Rapid changes in flowering time in British plants. Science 296, 1689–1691. doi: 10.1126/science.1071617
Fu, Y. H., Zhao, H., Piao, S., Peaucelle, M., Peng, S., Zhou, G., et al. (2015). Declining global warming effects on the phenology of spring leaf unfolding. Nature 526, 104–107. doi: 10.1038/nature15402
IPCC (2019). “Summary for policymakers,” in Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems, eds P. R. Shukla, J. Skea, E. Calvo Buendia, V. Masson-Delmotte, H.-O. Pörtner, D. C. Roberts. doi: 10.1017/9781009157988.001
Körner, C., and Basler, D. (2010). Phenology under global warming. Science 327, 1461–1462. doi: 10.1126/science.1186473
Ma, Q., Huang, J-. G., Hänninen, H., and Berninger, F. (2019). Divergent trends in the risk of spring frost damage to trees in Europe with recent warming. Glob. Change Biol. 25, 351–360. doi: 10.1111/gcb.14479
Man, R., Colombo, S., Kayahara, G. J., Duckett, S., Velasquez, R., Dang, Q. L., et al. (2013). A case of extensive conifer needle browning in northwestern Ontario in 2012: winter drying or freezing damage? For. Chron. 89, 675–680. doi: 10.5558/tfc2013-120
Man, R., Lu, P., and Dang, Q. L. (2017). Insufficient chilling effects vary among boreal tree species and chilling duration. Front. Plant Sci. 8, 1354. doi: 10.3389/fpls.2017.01354
Man, R., Lu, P., and Dang, Q-. L. (2020). Effects of insufficient chilling on budburst and growth of six temperate forest tree species in Ontario. New For. 52, 303–315. doi: 10.1007/s11056-020-09795-1
Man, R., Lu, P., and Dang, Q-. L. (2021). Cold tolerance of black spruce, white spruce, jack pine, and lodgepole pine seedlings at different stages of spring dehardening. New For. 52, 317–328. doi: 10.1007/s11056-020-09796-0
Mellor, R. S., Salisbury, F. B., and Raschke, K. (1964). Leaf temperatures in controlled environments. Planta 61, 56–72. doi: 10.1007/BF01895390
Montgomery, R. A., Rice, K. E., Stefanski, A., Rich, R. L., and Reich, P. B. (2020). Phenological responses of temperate and boreal trees to warming depend on ambient spring temperatures, leaf habit, and geographic range. Proc. Natl. Acad. Sci. U.S.A. 117, 10397–10405. doi: 10.1073/pnas.1917508117
Morin, X., Lechowicz, M. J., Augspurger, C., O'Keefe, J., Viner, D., Chuine, I., et al. (2009). Leaf phenology in 22 American tree species during the 21st century. Glob. Change Biol. 15, 961–975. doi: 10.1111/j.1365-2486.2008.01735.x
Peñuelas, J., Rutishauser, T., and Filella, I. (2009). Phenology feedbacks on climate change. Science 324, 887–888. doi: 10.1126/science.1173004
Piao, S., Liu, Q., Chen, A., Janssens, I. A., Fu, Y., et al. (2019). Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 25, 1922–1940. doi: 10.1111/gcb.14619
Polgar, C., Gallinat, A., and Primack, R. B. (2014). Drivers of leaf-out phenology and their implications for species invasions: insights from Thoreau's concord. New Phytol. 202, 106–115. doi: 10.1111/nph.12647
Prevéy, J., Vellend, M., Rüger, N., Hollister, R. D., Bjorkman, A. D., Myers-Smith, I. H., et al. (2017). Greater temperature sensitivity of plant phenology at colder sites: implications for convergence across northern latitudes. Glob. Chang. Biol. 23, 2660–2671. doi: 10.1111/gcb.13619
Primack, R. B., and Gallinat, A. S. (2016). Spring budburst in a changing climate. Am. Sci. 104, 102–109. doi: 10.1511/2016.119.102
Shen, M., Tang, Y., Chen, J., Yang, X., Wang, C., Cui, X., et al. (2014). Earlier-season vegetation has greater temperature sensitivity of spring phenology in northern hemisphere. PLoS ONE 9, e88178. doi: 10.1371/journal.pone.0088178
Tao, J., Man, R., and Dang, Q. L. (2021). Earlier and more variable spring phenology projected for eastern Canadian boreal and temperate forests with climate warming. Trees For. People 6, 100127. doi: 10.1016/j.tfp.2021.100127
Way, D. A., and Montgomery, R. A. (2015). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 38, 1725–1736. doi: 10.1111/pce.12431
Wolkovich, E. M., Auerbach, J., Chamberlain, C. J., Buonaiuto, D. M., Ettinger, A. K., Morales-Castilla, I., et al. (2021). A simple explanation for declining temperature sensitivity with warming. Glob. Change Biol. 27, 4947–4949. doi: 10.1111/gcb.15746
Zettlemoyer, M. A., Schultheis, E. H., and Lau, J. A. (2019). Phenology in a warming world: differences between native and non-native plant species. Ecol. Lett. 22, 1253–1263. doi: 10.1111/ele.13290
Zhang, H., Liu, S., Regnier, P., and Yuan, W. (2018). New insights on plant phenological response to temperature revealed from long-term widespread observations in China. Glob. Chang. Biol. 24, 2066–2078. doi: 10.1111/gcb.14002
Keywords: spring phenology, phenological response, among-species difference, growing season length, global warming
Citation: Chu X, Man R and Dang Q-L (2023) Spring phenology, phenological response, and growing season length. Front. For. Glob. Change 6:1041369. doi: 10.3389/ffgc.2023.1041369
Received: 10 September 2022; Accepted: 29 March 2023;
Published: 18 April 2023.
Edited by:
James D. Blande, University of Eastern Finland, FinlandReviewed by:
Nan Cong, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (CAS), ChinaCopyright © 2023 Chu, Man and Dang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongzhou Man, rongzhou.man@ontario.ca
 Xiuli Chu
Xiuli Chu