
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 16 February 2023
Sec. Tropical Forests
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1041268
This article is part of the Research Topic Human-mediated effects on biodiversity conservation at Atlantic Forest: threats and solutions View all 6 articles
Landscape changes due to habitat loss and fragmentation can result in complex changes in biodiversity and functional diversity. On the other hand, the functional diversity changes also reflect the modifications in the ecosystem functions, patterns of resources use by the species, and species interactions. In the present work, we evaluated how habitat loss at a landscape scale influences the functional diversity of different bird communities (total community, frugivorous, and insectivorous birds) in landscapes of 5–60% of forest cover in the Bahia Atlantic Forest. In a sample design that aimed to minimize the effects of some landscape-scale possible bias, we randomly selected twelve 6 km × 6 km landscapes, and we surveyed eight plots randomly located in forested areas within each landscape. We focused on the species classified as forest-dependent. We calculated the total richness and each species’ relative abundance in each landscape. To evaluate functional diversity, 19 functional traits were chosen for the total community, 11 for the frugivore birds, and 12 for the insectivore birds. The choice of traits represents how species use their resources and the use of these in other studies of functional diversity. As biodiversity changes to habitat loss could be non-linear, we evaluated the response pattern of bird functional diversity to habitat loss using three different metrics (FRic, FEve, and FDiv) for all communities (total community, frugivorous and insectivorous birds). Model selection was used to evaluate the response models (null, linear, and logistical). Our results indicated that as forest amount decreases, we found a sharp decrease in FRic, significantly below 30% forest cover. That suggests a reduction in resource use by species in those landscapes. FEve also showed a sharp decline in landscapes below 15% of habitat, indicating a possible reduction in the structural complexity. Fdiv also decreases dramatically in landscapes below 15% of forest amount, which suggests a decrease in functional dissimilarity between species, probably due to environmental filtration, which can lead to taxonomic homogenization. Therefore, we assessed the importance of forests for providing the resources for the permanence of species and their functions, and as a population source. Our study provides quantitative indicators of the relationship between functional diversity and habitat loss, which can be crucial in implementing more robust conservation actions to preserve the Atlantic Forest and its ecosystem services.
Landscape changes due to habitat loss and fragmentation can result in complex changes in biodiversity, particularly in functional diversity (Zambrano et al., 2019). Functional diversity is a component of biodiversity that evaluate the diversity and distribution of functional traits in communities (Flynn et al., 2009; Meynard et al., 2011). These traits are related to the species functional roles (ecological functions) within a community (De Coster et al., 2015; Dehling et al., 2016). Thus, habitat loss, can drive an erosion or a turnover of functional traits within communities (De Coster et al., 2015; Farneda et al., 2015; Almeida-Gomes et al., 2019), causing changes in the functions provided by these communities. There is a need for research beyond taxonomic diversity (species richness), which can be achieved through functional approaches (Cadotte et al., 2011; Mouillot et al., 2013). Linking the consequences of habitat changes that shape communities to the ecosystem functioning can be essential to maintain a greater diversity of ecological functions (Cadotte et al., 2011; Mouillot et al., 2013; Chapman et al., 2017).
Functional diversity can be considered a measure of diversity that better assesses the functioning of the ecosystem than the species richness (Cadotte et al., 2011) because it does not consider species as equivalent, as is the case with species richness. Species with different characteristics play different roles in the ecosystem functioning (Carmona et al., 2017), and they can reveal effects of habitat changes other than species richness. For example, functional diversity can witness how the extinction of functionally different species can have a more significant impact on the functioning of the ecosystem than species with similar traits (Díaz et al., 2007; Cadotte et al., 2011; Mouillot et al., 2013). Therefore, understanding how community patterns influence changes in ecosystem functions through responses to functional diversity can be of great value (McKinney, 2008; Marzluff, 2017).
In addition, different from taxonomic diversity, there has yet to be a consensus on how functional diversity can persist in landscapes altered by human actions (Riemann et al., 2017). It is known that factors such as intraspecific variation, species substitution, and niche overlap can influence functional diversity to behave differently from species richness (Díaz and Cabido, 2001). Besides, the sensitivity of surviving species to changes in habitat structure will be influenced by their functional traits (Burivalova et al., 2015). These characteristics can affect the dispersion capacity of individuals or influence the establishment of new habitats (fragments) or the permanence of the existing ones (Tscharntke et al., 2012; Zambrano et al., 2019). So, functional diversity can remain constant or decline regardless of how species richness changes (Cadotte et al., 2011).
Additionally, communities of redundant species for the same function can be functionally nested in impacted landscapes. In this way, their functions may be prone to disappear faster than others as the impact increases (Almeida-Gomes et al., 2019). Thus, it is still being determined whether the remaining habitats derived from habitat loss can maintain functional diversity comparable to before of impact and consequently maintain functions (Riemann et al., 2017).
Some evidence has already found reduced functional diversity in some taxa after modifications and intensity of habitat use (Riemann et al., 2017). For example, De Coster et al. (2015) and Boesing et al. (2018) observed a reduction in functional integrity and functional diversity, respectively, with an increase in habitat loss in tropical forests. However, studies that tested how environmental impacts and landscape changes affect functional diversity are still scarce and limited to small spatial gradients (Barbaro and Van Halder, 2009; Lohbeck et al., 2012; Magioli et al., 2015). Consequently, our knowledge remains limited to changes in species composition; therefore, we lack knowledge about state of ecosystem functions (De Coster et al., 2015). Assessing the relationship between functional diversity and habitat loss on a landscape scale can be an excellent way to elucidate the mechanisms that drive changes in functional diversity and infer how these can influence ecosystem processes.
Soon, as birds have a wide variety of functional traits and are impacted by different aspects of environmental change (Alexander et al., 2019), they become a valuable model to evaluate changes in habitat structure or functioning of the ecosystem and for functional diversity studies (Bregman et al., 2016; Prescott et al., 2016). In addition, they are widely known for performing essential ecological functions such as seed dispersal, pollination, pest control, nutrient cycling, and soil formation (Sekercioglu, 2006). Therefore, this study evaluated how habitat loss at the landscape scale influences the functional diversity of birds for different groups (bird community, frugivores, and insectivorous birds) in a gradient of forest cover from 5 to 60% at the landscape scale in Bahia Atlantic Forest. This study is expected to elucidate aspects of the relationship between functional diversity and habitat loss, which can also help build more robust knowledge, especially for threatened biomes, such as the Brazilian Atlantic Forest.
This study was part of a larger multi-taxa project on Extinction thresholds due to habitat loss at the landscape scale, developed by a research team of the Federal University of Bahia. We aimed to investigate the effects of the habitat amount at the landscape scale over different groups (CNPq/FAPESB research founding PNX0016_2009). The conceptual basis for this study was built in simulated landscapes (Andrén, 1994; Fahrig, 2003), and predicted several landscapes and patch features essential to population dynamics, such as the mean distance between patches, edge density metrics, and mean patch size, to be dependent and correlated (linearly or non-linearly) to habitat amount at the landscape scale (Gustafson and Parker, 1992). Also predicted that biodiversity persistence in the landscape will depend on the habitat amount at the landscape scale. Theoretical models were built for populations but furthermore were tested with communities’ responses in real landscapes (e.g., Rigueira et al., 2013; Lima and Mariano-Neto, 2014; Morante-Filho et al., 2015). Pardini et al. (2010) also pointed out that the correlation between biodiversity metrics and local landscape metrics will also depend on the habitat amount: in landscapes with large amounts of habitat or very scarce habitat cover, biodiversity metrics will have a poor correlation with the patch size. In the first case, rescue effect from large fragments could maintain biodiversity even in small patches. And in the former, the scarcity of habitat increases the distance between patches and makes recolonization unfeasible after local extinctions, and all fragments will tend to suffer biodiversity erosion with time.
The study area includes areas of the Bahia Atlantic Forest, which is currently very fragmented (Ribeiro et al., 2009). This region is formed by several forest formations that extend throughout Brazil, such as ombrophilic forest, mountainous forest, seasonal semideciduous forest, sandbank, and mangrove (Tonhasca, 2005), with an annual average temperature of 25°C.
Atlantic Forest landscapes were sampled in Bahia, Brazil, in a wide region of 600 km × 150 km along the Atlantic coast, approximately 93.500 km2. Between latitudes 11° 80′ and 18° 49′ S and longitudes 21° 24′ and 40° 08. We used forest cover maps (SOS Mata Atlântica and Instituto Nacional de Pesquisas Espaciais, 2008) in a Geographic Information System (GIS), and in the entire region, we allocate 1,500 non-overlapping cells of 6 km × 6 km (36 km2). We consider these cells as landscapes wide enough to test the effects of the landscape at community levels. In this universe we calculate percentages of forest habitat coverage in each landscape, and we randomly choose 12 landscapes in a range of forest percental cover from 5 to 60% with a 5% step. We allowed a variation of ±2% in each desired percentual.
To reduce undesirable variability and ensure more homogeneous landscape contexts, the following criteria were also considered for landscapes validation: (1) matrix composition: 80% of the 6 km × 6 km landscape matrix must be composed of non-forest physiognomies, that prevented the matrix from acting as an alternative habitat for the species (Dixo and Martins, 2008); (2) external source areas: we aimed to reduce the likelihood of large forest remnants in the vicinity of the landscape acts as source areas. We considered a larger 18 km × 18 km landscape surrounding the 6 km × 6 km target landscapes (the eight neighbor landscapes), these larger landscapes could not have an LPI (LPI - Larger Patch Iindex, McGarigal and Marks, 1995) greater than the 6 km × 6 km target landscape LPI. LPI is a metric that assesses whether the adjacent forest remnants could act as areas of origin around the 6 km × 6 km landscape; and (3) the 18 km × 18 km landscape must have a native vegetation cover like the 6 km × 6 km landscape.
After checked these criteria, a landscape of each forest cover percentual was selected randomly from the universe of possible final landscapes: Ilhéus (5%, S 14° 44’ 32″ W 39° 06′ 20″), Itambé (10%, S 15° 10′ 58″ W 40° 20′ 28″), Presidente Tancredo Neves (15%, S 13° 23′ 28″ W 39° 19′ 06″), Itapetinga (20%, S 15° 14′ 46″ W 38° 56′ 25″), Valencia (25%, S 13° 20′ 32″ W 39° 11′ 43″), Ubaíra (30%, 13° 07′ 19″ W 39° 39′ 34″), Nilo Peçanha (35%,13° 38′ 58″ W 39° 12′ 37″), Wenceslau Guimarães (40%, 13° 33′ 14″ W 39° 42′ 07″), Camamu(45%, 14° 00′ 51″ W 39° 10′ 56″), Iguaí (50%, 14° 38′ 38″ W 40° 09′ 12″), Jaguaripe (55%, 13° 11′ 14″ W 39° 01′ 26″), and Itamaraju (60%, 16° 59′ 30″ W 39° 27′ 19″) (Figure 1). Each landscape comprised areas of the Atlantic Forest in intermediate, or advanced stages of regeneration and a predominantly non-forested and non-urban matrix (fields, pastures, agriculture). After selecting the landscapes, they were validated in the field to verify that all criteria were accomplished. The validation occurred before sampling through visits in landscapes verifying all spatial criteria.
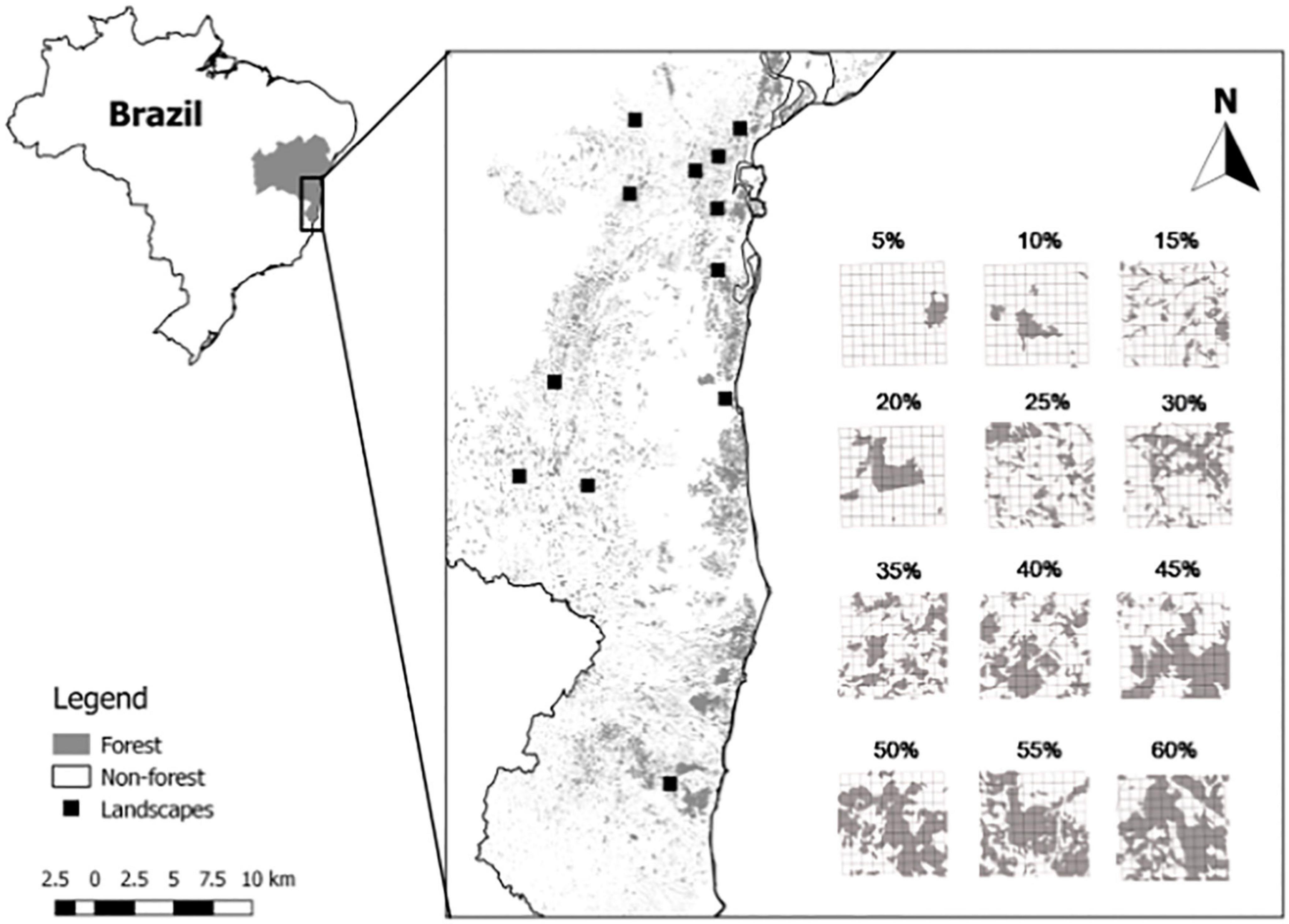
Figure 1. Map of sampled areas in the state of Bahia, Brazil. 5% (S 14° 44′ 32″ W 39° 06′ 20″), 10% (S 15° 10′ 58″ W 40° 20′ 28″), 15% (S 13° 23′ 28″ W 39° 19′ 06″), 20% (S 15° 14′ 46″ W 38° 56′ 25″), 25% (S 13° 20′ 32″ W 39° 11′ 43″), 30% (13° 07′ 19″ W 39° 39′ 34″), 35% (13° 38′ 58″ W 39° 12′ 37″), 40% (13° 33′ 14″ W 39° 42′ 07″), 45% (14° 00′ 51″ W 39° 10′ 56″), 50% (14° 38′ 38″ W 40° 09′ 12″), 55% (13° 11′ 14″ W 39° 01′ 26″), 60% (16° 59′ 30″ W 39° 27′ 19″).
In each landscape, the bird community was sampled in eight plots of 0.6 km × 0.6 km randomly distributed only in forests, as this is a landscape scale survey, plots could be in different forest patches. One plot was sampled each day. Our sampling strategy consisted of defining in each plot four sampling points 100 m away and at least 50 m from the forest edge (Bibby et al., 1992). We recorded all birds seen and heard at each sampling point for 20 min. Sampling was conducted from 5:30 am to 9:00 am, an interval that included the period of most significant activity of the birds (5:30 am–10:00 am). After identifying the species, the classification system proposed by Parker et al. (1996) lists species dependent on forest, classified as those that only had forest habitats. The species richness of each landscape was calculated by the sum of the species of each plot, and the relative abundance was calculated using the occurrence of each species in each plot divided by the total plots (eight plots).
Only species dependent on forest habitats (n = 210) were used to evaluate the functional diversity because they are more sensitive to habitat loss. To evaluate whether there would be differences in the responses of species communities to habitat loss we assessed the functional diversity of the total community, frugivores, and insectivorous birds. For this, 19 functional traits were chosen for the total community, 11 for the frugivore birds, and 12 for the insectivore birds (Table 1). The choice of traits in the evaluation of this study represents how species use their resources and the use of these in other studies of functional diversity. The functional traits used in this study were obtained from Parker et al. (1996), Dunning (2008), Sigrist (2013) and the websites WikiAves (2017)1 and Del Hoyo et al. (2014) but also from personal knowledge (foraging period).

Table 1. Traits used to assess species response to habitat loss over the entire community, frugivorous and insectivorous birds.
Due to the inherent difficulty in finding traces for all species, some standardization has been made. For species that did not have data from the “clutch size” category (n = 37) we used the value of “2 eggs.” Because according to Jetz et al. (2008) this is the general average of egg laying. In the category “nest location,” for the species that we did not find information (n = 11), we used the data from phylogenetically close species, following the classification by Clements et al. (2017).
Functional diversity metrics evaluate different aspects of species functionality in the community, such as uniformity and dispersion of functional traits, and none single metric can evaluate all these aspects simultaneously (Villéger et al., 2008). Therefore, three independent metrics were used to assess functional diversity (FRic, FEve, and FDiv). According to Villéger et al. (2008), these three metrics complement and constitute a suitable combination to assess functional diversity.
Fric is a metric that considers the total area occupied in the functional space (Villéger et al., 2008) and represents the amount of functional space filled by the community (niche) (Villéger et al., 2008). A low functional richness (FRic) could indicate that the available resources are not used (Mason et al., 2005). FEve measures whether the species’ traits are evenly distributed in the occupied space. In other words, low values of FEve may demonstrate the existence of overuse of resources by some species in the community (Schleuter et al., 2010), associated with less efficiency in the resources use. On the other hand, FDiv measures the relative abundance of species with unique traits, which can indicate a niche differentiation. Therefore, a low FDiv represents a low abundance of species with unique characteristics, with possibly increased competition between species (Schleuter et al., 2010).
Each metric (FRic, FEve, and FDiv) was calculated for each community in the sampled landscapes (three metrics × three communities × twelve landscapes). As we had continuous and categorical variables (traits), Gower’s distance was used to estimate functional diversity in all communities (Podani and Schmera, 2006; De Bello et al., 2010).
The estimated values of each element of the functional diversity were used to model the responses of the communities (total bird community, frugivorous, and insectivorous birds) to changes in forest cover at the landscape scale. To evaluate the response type, we used a model selection approach, with three possible responses of the bird functional diversity to forest cover, which was: No effect of forest cover on the metrics of functional diversity (null model), with linear effect on (f (x) = ax + b) (gradual and linear change of functional diversity) and, with effect non-linear and abrupt change of functional diversity, modeled with a four-parameter logistic function (f (x) = d + (a/(1 + exp ((bx)/c))).
We selected the best model using the Akaike Information Criterion corrected for small sample sizes (AICc), which gives the probability of a model being the best model and is calculated based on its likelihood. The model with the highest AICc weight (ranging from 0 to 1), which considers AICc values and parameter amount, was accepted as the most plausible. After the model selection, we also analyzed the best models’ residual distributions and the parameters’ confidence intervals. Models with AICc weights values with a difference of less than two decimals was considered equally probable. Further analysis of residuals (overdispersion and heterocedasticity) and parameters confidence intervals was used to decide which model to consider. In addition, a Pearson correlation analysis of the estimated values of functional diversity (FRic, FEve, and FDiv) with the species richness of each community was used to evaluate its influence (species richness) on functional diversity. All analysis was performed in R 2.15 (R Development Core Team, 2012) and the packages used for analysis were: FD, stats, and bbmle.
We registered a total of 273 bird species in the landscapes belonging to 48 families, 210 of which were classified as forest-dependent species. All 210 species were used in the community functional diversity analysis, 104 for insectivorous, 32 for frugivorous, 38 for omnivorous species, 20 for granivore species, 9 for nectarivore species, and 8 for carnivore species.
Pearson correlation analysis showed a positive and significant correlation of species richness and the FRic metric for the total community (r = 0.78; p = 0.002), for frugivorous (r = 0.79; p = 0.002), and for insectivorous (r = 0.74; p = 0.005). There was also a negative and significant correlation between the FEve metric and the total community (r = −0.72; p = 0.007), and with the insectivorous species (r = −0.59; p = 0.040). There was no correlation of species richness between the FEve metric and the frugivorous species (r = −0.46; p = 0.123), and between the FDiv metric and the total community (r = 0.21; p = 0.494), nor frugivorous species (r = −0.23; p = 0.456), and insectivores (r = 0.124; p = 0.699).
The linear model was selected as the best relation between forest amount at landscape scale and the total community FRic (Wi = 0.570). For FEve and FDiv the logistic model was selected (Wi = 0, 35; Wi = 0.691, respectively). For the frugivorous birds, the logistic model was selected (Wi = 0.526) for the FRic metric, and null models were selected for the FEve (Wi = 0.811) and FDiv (Wi = 0.770) metrics. To the insectivorous birds, null models was selected for the FRic metric (Wi = 0.728) and FDiv (Wi = 0.837), and the logistic model for the FEve metric (Wi = 0.040) (Figure 2 and Table 2).
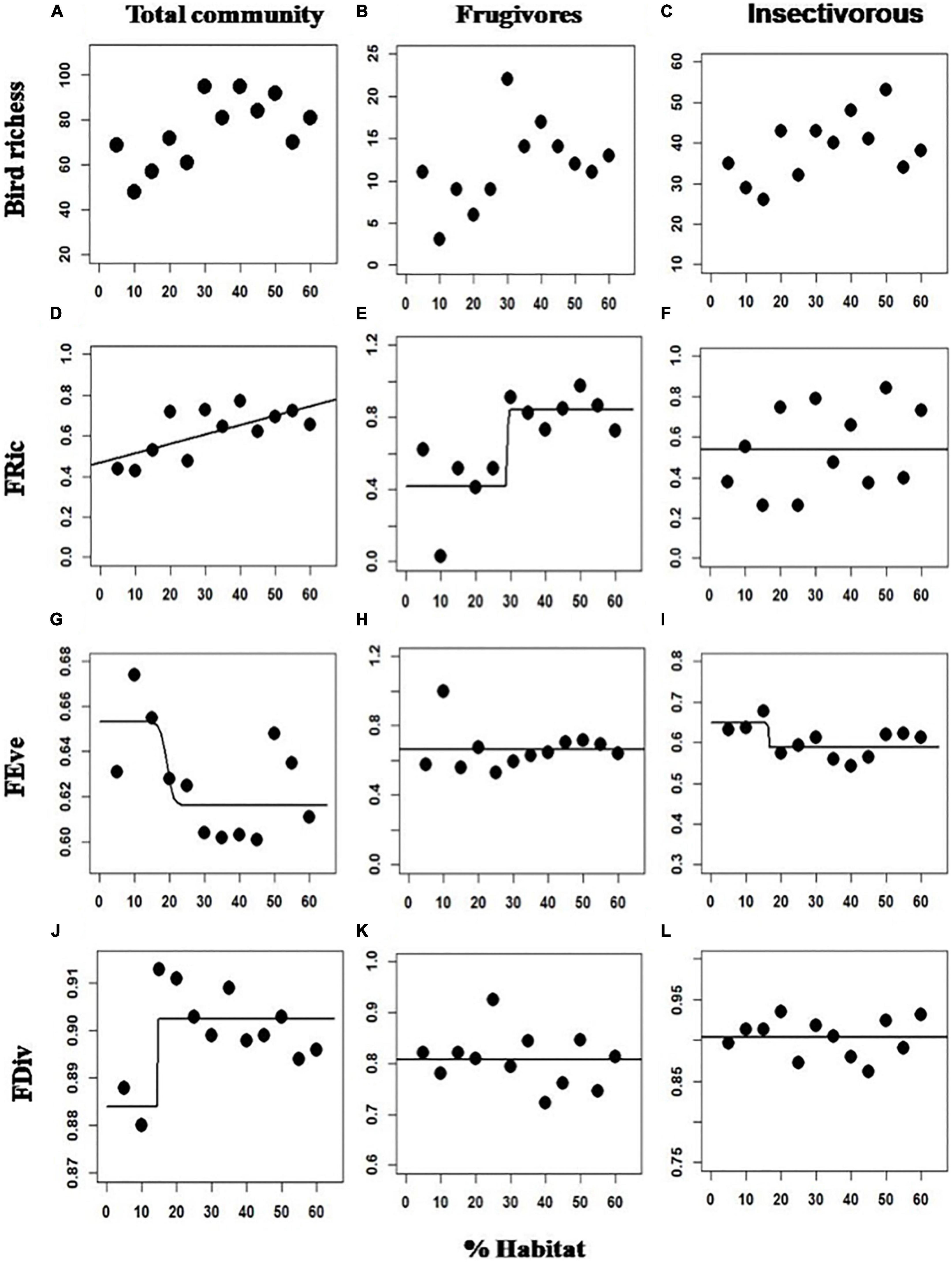
Figure 2. Responses of functional diversity variables and species richness to habitat loss, rows represent responses of the species richness FRic, FEve, and FDiv metrics from different bird communities (columns). The total community (A,D,G,J), Frugivores (B,E,H,K), and Insectivorous (C,F,I,L). Atlantic Forest of Bahia, Northeast Brazil.
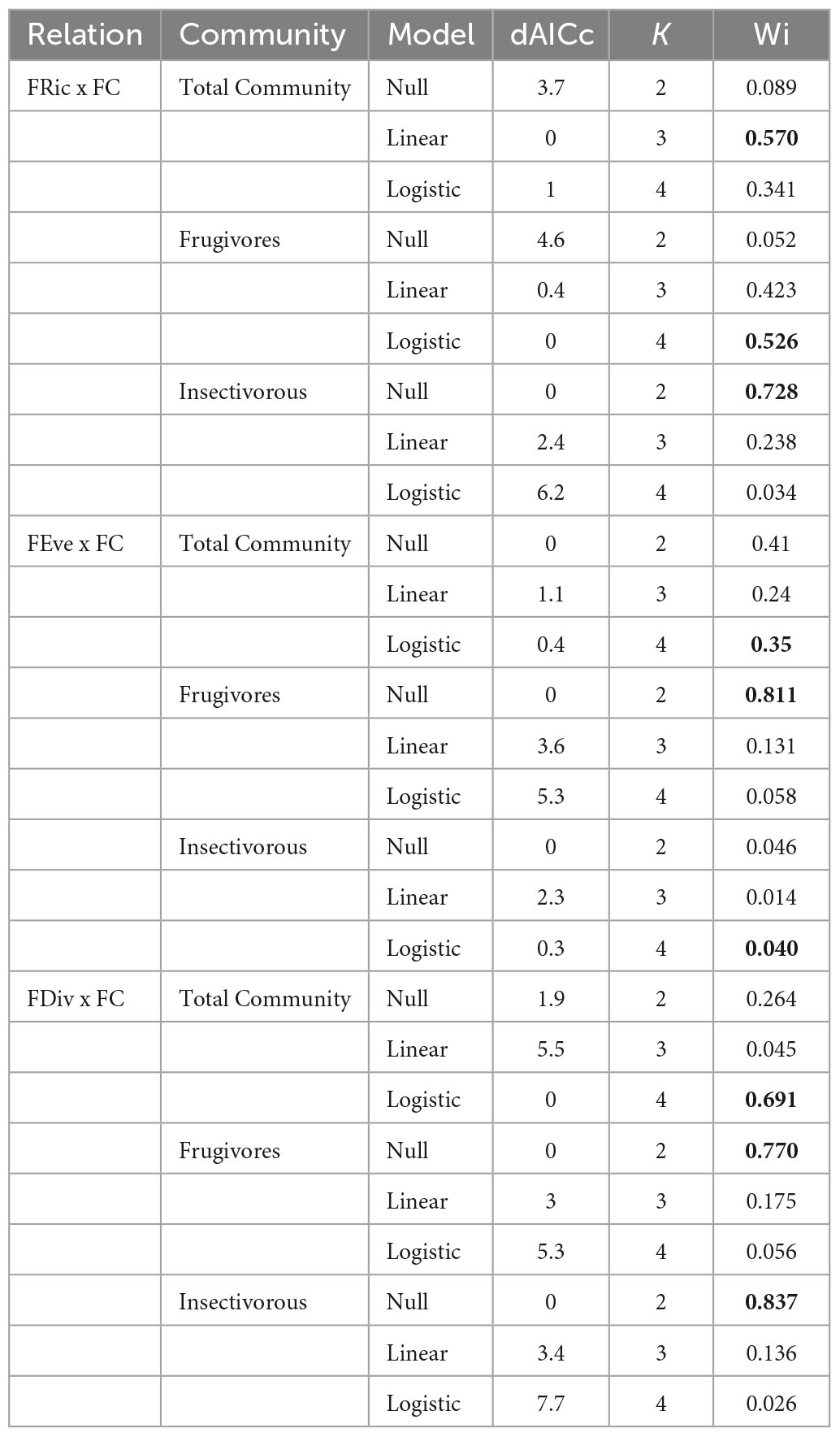
Table 2. Model selection of the relation between functional diversity (Fric, FEve, and FDiv) and the forest cover (FC), to different bird communities (total community, frugivores, and insectivorous). dAICc is the difference between the Akaike information criterion value, corrected to small samples (AICc) of the model and the best model; K is the number of the model parameters; Wi is Akaike weight. In bold are the selected models. N = 12 for all analyses. Atlantic Forest of Bahia, Northeast Brazil.
The three metrics used in this study measure different aspects of functional diversity in a community, and thus there is a complexity in conceptualizing changes in space with specific ecological issues by single metric (Boersma et al., 2016; Kuebbing et al., 2018). Our study found different results from the effect of habitat loss for each community depending on the metric of functional diversity that was used.
The Fric declined linearly with forest reduction for the total community and non-linearly for frugivorous species. It is a metric that assesses the volume of functional space occupied by species, and thus, communities with high FRic values may have a greater variety of functional traits, which potentially corresponds to greater use of resources by species (Cannon et al., 2019). It is known that FRic could be correlated with species richness (Cadotte et al., 2011), and we also found this in all communities. Which means that the larger and more diverse the community in landscapes with larger amounts of forests, the larger the volume of the functional space (Pakerman, 2011). As forest cover decreases, there is a consequent change in the landscape structure (e.g., distance and patches size), with a decrease in the number of species followed by a decrease in the diversity of functional traits, decreasing the volume of the functional space (FRic) for the total and frugivorous bird community.
Other studies have found evidence of a decrease in FRic with specific changes in landscape structure due to habitat loss. For example, Bovo et al. (2018) found a decrease in traces of frugivorous birds when there was a decrease in the patches size, Santillán et al. (2019) found a decrease in FRic in fragmented forests, and Cannon et al. (2019) found a decrease in FRic with an increase in the distance between continuous forests.
Additionally, these communities responded differently to forest amount decrease, with a linear decrease in FRic for the total community (Figure 2D), and non-linear for the frugivorous community, with a sharp decrease from 30% forest cover (Figure 2E). Possibly, for the total community, if conditions are favorable to species richness increase in larger amounts of habitat, the assemblies will be characterized by redundant species (Pakerman, 2011). Therefore, despite the non-linear decline in species richness in the total community, following forest reduction the decrease in functional traits occurred more slowly (Figures 2A, B).
However, for frugivorous birds, the decline in FRic may have been fast (non-linear) precisely because of the smaller number of redundant species in the community. According to Ibarra and Martin (2015), assemblies with few species are expected to show low functional richness due to the absence of functional redundancy. We find a marked richness decrease of frugivores from approximately 40% of the forest cover (Figure 2B), which may have reflected in a smaller variety of functional traits and a FRic decline in this community. So, as FRic is also associated with the amount of use of resources and the functions performed by species in the community, it is possible that ecosystem functions performed by frugivores birds may already be compromised in landscapes below 30% of habitat.
However, there was no effect of forest cover on the insectivore community FRic, although the species richness of this community decreased with the forest loss (Figure 2C). According to Murray et al. (2017), changes in species richness without effect on FRic would only occur if all species added or removed in communities were functionally redundant, and this may have occurred in our study for the insectivorous bird community. Insectivorous birds seem to have high levels of redundancy due to the high number of species, thus allowing a greater capacity to adapt to changes in the landscape (Luck et al., 2013). In addition, at least 50% of the birds are essentially insectivorous (in our study 49%), which may have been why this community had no effect of FRic with habitat loss.
We found a smooth non-linear decrease in FEve when forest cover increased from 15% for the total community and the insectivorous bird, with no effect for the frugivorous. High FEve values may indicate that communities use the resource efficiently, as abundances are evenly distributed (Cannon et al., 2019) while low FEve values represent that some parts of the functional space are empty while others are densely populated. Although a slight decrease in FEve, in landscapes with larger amounts of forest could have been an increased competition caused by higher species richness in those landscapes, and a greater competition for resources caused a decrease in FEve.
Likewise, our result corroborates the studies by Pakerman (2011) and Ding et al. (2013), who found an increase in FEve due to increased disturbance in habitats and fragmentation. Therefore, low levels of FEve were indicative of locals with little disturbance and, therefore, habitats that the competition has a lot of importance in the structure of the communities (areas with low disturbances). FEve can be high in more disturbed habitats where competition should be less important in structuring the community (Pakerman, 2011).
In addition, the FEve of the frugivorous bird community may not have felt the effect of habitat loss precisely because there are fewer redundant species and consequently low competition, and his community was able, in theory, to maintain efficient use of the resource in relation to the gradient of habitat loss. In addition, some empirical and simulated studies (Villéger et al., 2008; Mouchet et al., 2010; Ibarra and Martin, 2015) also did not find a relationship between FEve of bird communities when the richness increases, while other studies found a lower FEve in pasture areas when compared to remnants of forests (Prescott et al., 2016), or equivalents between remnants of forests and monocultures (oil palm) (Edwards et al., 2013). Thus, it is not yet clear what are the FEve patterns of bird communities to forest loss, and thus, as suggested by Sayer et al. (2017), further studies are needed to better understand this relationship.
For the FDiv metric, there was a smooth non-linear decrease below 15% of its coverage for the total community while for the frugivorous and insectivorous birds, there was no effect of forest cover on this metric. FDiv assesses levels of niche differentiation (Cannon et al., 2019), and the decline in FDiv can be associated with low dissimilarity between the most abundant species and other species, and with taxonomic homogenization (Ibarra and Martin, 2015). This relationship between FDiv and forest cover for the total community possibly happened because when the forest cover decreases, it also does the habitat area, and the number of niches and resources to be explored by the species (Tews et al., 2004). Also may have reflected in the decrease in functionally unique species when forest cover declined below 15%. This decline can indicate that the use of resources in these landscapes is less efficient, compromising the role of birds in the functioning of the ecosystem (Mason et al., 2005).
Additionally, habitat loss did not affect FDiv from the frugivorous and insectivorous birds. This probably occurred because in these communities the most abundant species did not have very distinct functional traits (same functional group) and are not grouped around the average values of the traits (Ding et al., 2013) when compared with the total community. That is, the frugivorous and insectivorous bird communities are more like each other, and probably these communities did not have functionally unique species, which probably influenced the FDiv.
According to our results, finding three patterns of habitat loss influence on functional diversity was possible. First, we observe a reduction in the amount of resource use by species when the habitat loss increased (FRic total community and frugivorous birds). According to Tilman et al. (1997), a greater diversity of traits increases the likelihood of less niche overlap (complementarily), a greater use of the resource, and an increase in the number of functions in the ecosystem. Thus, as species respond differently to the disturbance (Henle et al., 2004) when forest cover decreases below 30%, frugivorous birds dramatically decrease the amount of resource use. Bovo et al. (2018) found that the dispersion of seeds by large frugivorous birds in small fragments can be reduced, which is responsible for the dispersion of seeds. There is a predicted decrease in the size of fragments as habitat area decreased in the landscapes (Gustafson and Parker, 1992, and we also found this result in our real landscapes (Table 3). This means that functions performed by birds can likely be compromised below 30%.
Approximately 90% of woody species are dispersed by animals (Jordano, 2016), being birds an important group in the absence of other vertebrates (Holbrook et al., 2002). Considering the importance of the bird’s role in forest regeneration through seed dispersal (Silva and Tabarelli, 2000) our results are worrisome. Also, in the long run, the decline of seed dispersion could decrease the recruitment of plants (Galetti et al., 2013) important to birds, and increase the vulnerability of this group. It is possible that landscapes below this percentage of forest (< 30%) have a reduced capacity for regeneration and resilience to forest disturbances.
Second, the decrease of forest cover may have caused a decrease in the structural complexity of the habitat in landscapes below 15%, and influenced how species consume their resources. According to García-Morales et al. (2016) and Schleuter et al. (2010), high values of FEve (e.g., total community and insectivores in landscapes <15%) may suggest that the habitat is not structurally complex, besides this metric is associated with quite disturbed environments (Pakerman, 2011; Ding et al., 2013). Indeed, increased habitat loss can increases the edge habitats, causing changes in communities through abiotic changes, and changes in biotic interactions (Murcia, 1995). These can lead to a decrease in the viability of resources and how it is consumed by the species, especially in landscapes below 15% forest cover.
Third, if habitat loss indicates a decrease in habitat’s structural complexity, it is possible that increased habitat loss may be leading to environmental filtration. In other words the environment act as a filter, selecting only species with functional traits that can tolerate the habitat changes (Knapp and Kühn, 2012). Our results indicate that below 15%, there was a smooth decline in species dissimilarity, a possible taxonomic homogenization, as forests become habitable only for species with functional traits that can exploit resources in these environments.
Our results show that the increase in habitat loss can lead to less consumption and a decrease in the viability of resources by species (FRic and FEve), in addition, the competition can have increased between species as already highlighted by Schleuter et al. (2010) (Fdiv). All these factors together will act as environmental filters for the persistence of species in less forested landscapes, decreasing their resistance to disturbances, and its resilience.
Our results corroborate with other studies on the importance of forested landscapes as population sources and necessary resources for the species’ permanence (Gilroy and Edwards, 2017; Cannon et al., 2019). This way, conservation efforts that aim at maintaining and increasing forested habitats above extinction thresholds would be of great value because, above this percentage, natural landscapes can keep their ecosystem functions and provide important ecosystem services for humanity. It also indicates that landscapes around the extinction threshold (∼30%) may receive attention and be indicated as priorities to restoration efforts. Avoiding their functioning compromised, as Pardini et al. (2010) suggested.
On the other hand, in landscapes smaller than 15% of habitat, it is possible that there will be a bird taxonomic homogenization due to structural changes in the landscapes, and this lead to severe changes in the functions and services performed by this group. The situation becomes more worrying in Brazilian scenario because the Brazilian Atlantic Forest is extremely fragmented (more than 240,000 patches) and with only 16% of its original cover, 42% of which are fragments of less than 250 ha (Ribeiro et al., 2009). Birds functional decrease risk compromising ecosystem services such as restoring disturbed ecosystems, trees reproduction, insect control, rodent regulation, nutrient cycling, and economic and cultural uses such as birdwatching tourism (Sekercioglu et al., 2004; Barbaro et al., 2017).
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Both authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
RS was funded by CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—http://www.capes.gov.br with doctoral scholarship and EM-N was funded by CNPq for the productivity grant. CNPq/FAPESB research founding PNX0016_2009 founded fieldwork.
Thanks to the reviewers of the criticisms and suggestions. We also thank to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a doctoral scholarship to EM-N and to CNPq for the productivity grant from EM-N (310107/2018-0).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1041268/full#supplementary-material
Alexander, J., Smith, D. A. E., Smith, Y. C. E., and Downs, C. T. (2019). Drivers of fine-scale avian functional diversity with changing land use: An assessment of the effects of eco-estate housing development and management. Land. Ecol. 34, 537–549. doi: 10.1007/s10980-019-00786-y
Almeida-Gomes, M., Vieira, M. V., Rocha, C. F. D., and Melo, A. S. (2019). Habitat amount drives the functional diversity and nestedness of anuran communities in an Atlantic Forest fragmented landscape. Biotropica 51, 874–884. doi: 10.1111/btp.12687
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71, 355–366.
Barbaro, L., Rusch, A., Muiruri, E. W., Gravellier, B., Thiery, D., and Castagneyrol, B. (2017). Avian pest control in vineyards is driven by interactions between bird functional diversity and landscape heterogeneity. J. Appl. Ecol. 54, 500–508. doi: 10.1111/1365-2664.12740
Barbaro, L., and Van Halder, I. (2009). Linking bird, carabid beetle and butterfly life-history traits to habitat fragmentation in mosaic landscapes. Ecography 32, 321–333. doi: 10.1111/j.1600-0587.2008.05546.x
Bibby, C. J., Burgess, N. D., and Hill, D. A. (1992). Birds census techniques. San Diego, CA: Academic Press.
Boersma, K. S., Dee, L. E., Miller, S. J., Bogan, M. T., Lytle, D. A., and Gitelman, A. I. (2016). Linking multidimensional functional diversity to quantitative methods: A graphical hypothesis-evaluation framework. Ecology 97, 583–593. doi: 10.1890/15-0688
Boesing, A. L., Nichols, E., and Metzger, J. P. (2018). Biodiversity extinction thresholds are modulated by matrix type. Ecography 41, 1–14. doi: 10.1111/ecog.03365
Bovo, A. A. A., Ferraz, K. M. P. M. B., Magioli, M., Alexandrino, E. R., Hasui, E., Ribeiro, M. C., et al. (2018). Habitat fragmentation narrows the distribution of avian functional traits associated with seed dispersal in tropical forest. Perspect. Ecol. Conserv. 16, 90–96. doi: 10.1016/j.pecon.2018.03.004
Bregman, T. P., Lees, A. C., MacGregor, H. E. A., Darski, B., de Moura, N. G., Aleixo, A., et al. (2016). Using avian functional traits to quantify the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. Lond. B Biol. Sci. 283:20161289. doi: 10.1098/rspb.2016.1289
Burivalova, Z., Lee, T. M., Giam, X., Sekercioglu, C. H., Wilcove, D. S., and Koh, L. P. (2015). Avian responses to selective logging shaped by species traits and logging practices. Proc. R. Soc. Lond. B Biol. Sci. 282:20150164. doi: 10.1098/rspb.2015.0164
Cadotte, M. W., Carscadden, K., and Mirotchnick, N. (2011). Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087. doi: 10.1111/j.1365-2664.2011.02048.x
Cannon, P. G., Gilroy, J. J., Tobias, J. A., Anderson, A., Haugaasen, T., and Edwards, D. P. (2019). Land-sparing agriculture sustains higher levels of avian functional diversity than land sharing. Glob. Chang. Biol. 25, 1576–1590. doi: 10.1111/gcb.14601
Carmona, C. P., Guerrero, I., Morales, M. B., Oñate, J. J., and Peco, B. (2017). Assessing vulnerability of functional diversity to species loss: A case study in Mediterranean agricultural systems. Funct. Ecol. 31, 427–435. doi: 10.1111/1365-2435.12709
Chapman, P. M., Tobias, J. A., Edwards, D. P., and Davies, R. G. (2017). Contrasting impacts of land-use change on phylogenetic and functional diversity of tropical forest birds. J. Appl. Ecol. 55, 1604–1614. doi: 10.1111/1365-2664.13073
Clements, J. F., Schulenberg, T. S., Iliff, M. J., Roberson, D., Fredericks, T. A., Sullivan, B. L., et al. (2017). The eBird/Clements checklist of birds of the world: v2016. Available online at: http://www.birds.cornell.edu/clementschecklist/download/ (accessed April 29, 2017).
De Bello, F., Lavorel, S., Díaz, S., Harrington, R., Cornelissen, J. H. C., Bardgett, R. D., et al. (2010). Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 19, 2873–2893. doi: 10.1007/s10531-010-9850-9
De Coster, G., Banks-Leite, C., and Metzger, J. P. (2015). Atlantic forest bird communities provide different but not fewer functions after habitat loss. Proc. R. Soc. B 282:20142844. doi: 10.1098/rspb.2014.2844
Dehling, D. M., Jordano, P., Schaefer, H. M., Bohning-Gaese, K., and Schleuning, M. (2016). Morphology predicts species’ functional roles and their degree of specialization in plant –frugivore interactions. Proc. R. Soc. B 283:20152444. doi: 10.1098/rspb.2015.2444
Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A., and de Juana, E. (2014). Handbook of the birds of the world alive. Barcelona: Lynx Edicions.
Díaz, S., and Cabido, M. (2001). Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. doi: 10.1016/S0169-5347(01)02283-2
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., and Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. U.S.A. 104, 20684–20689. doi: 10.1073/pnas.0704716104
Ding, Z., Feeley, K. J., Wang, Y., Pakeman, R. J., and Ding, P. (2013). Patterns of bird functional diversity on land-bridge island fragments. J. Anim. Ecol. 82, 781–790. doi: 10.1111/1365-2656.12046
Dixo, M., and Martins, M. (2008). Are leaf-litter frogs and lizards affected by edge Effects due to forest fragmentation in Brazilian Atlantic Forest? J. Trop. Ecol. 24, 551–554. doi: 10.1017/S0266467408005282
Dunning, J. B. Jr. (2008). CRC Handbook of avian body masses. Boca Raton, Fl: CRC Press. doi: 10.1201/9781420064452
Edwards, F. A., Edwards, D. P., Hamer, K. C., and Davies, R. G. (2013). Impacts of logging and conversion of rainforest to oil palm on the functional diversity of birds in Sundaland. Ibis 155, 313–326. doi: 10.1111/ibi.12027
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Syst. 34, 487–515.
Farneda, F. Z., Rocha, R., Lopez-Baucells, A., Groenenberg, M., Silva, I., Palmeirim, J. M., et al. (2015). Trait-related responses to habitat fragmentation in Amazonian bats. J. Appl. Ecol. 52, 1381–1391. doi: 10.1111/1365-2664.12490
Flynn, D. F. B., Gogol-Prokurat, M., Nogeire, T., Molinari, N., Richers, B. T., Lin, B. B., et al. (2009). Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 12, 22–33. doi: 10.1111/j.1461-0248.2008.01255.x
Galetti, M., Guevara, R., Côrtes, M. C., Fadini, R., Von Matter, S., Leite, A. B., et al. (2013). Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090. doi: 10.1126/science.1233774
García-Morales, R., Moreno, C. E., Badano, E. I., Zuria, I., Galindo-González, J., Rojas- Martínez, A. E., et al. (2016). Deforestation impacts on bat functional diversity in tropical landscapes. PLoS One 11:e0166765. doi: 10.1371/journal.pone.0166765
Gilroy, J. J., and Edwards, D. P. (2017). Source-sink dynamics: A neglected problem for landscape-scale biodiversity conservation in the tropics. Curr. Land. Ecol. Rep. 2, 51–60. doi: 10.1007/s40823-017-0023-3
Gustafson, E. J., and Parker, G. R. (1992). Relationships between landcover proportion and indices of landscape spatial pattern. Land. Ecol. 7, 101–110. doi: 10.1007/BF02418941
Henle, K., Davies, K. F., Kleyer, M., Margules, C., and Settele, J. (2004). Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 13, 207–251. doi: 10.1023/B:BIOC.0000004319.91643.9e
Holbrook, K. M., Smith, T. B., and Hardesty, B. D. (2002). Implications of long-distance movements of frugivorous rain forest hornbills. Ecography 25, 745–749. doi: 10.1034/j.1600-0587.2002.250610.x
Ibarra, J. T., and Martin, K. (2015). Biotic homogenization: Loss of avian functional richness and habitat specialists in disturbed Andean temperate forests. Biol. Conserv. 192, 418–427. doi: 10.1016/j.biocon.2015.11.008
Jetz, W., Sekercioglu, C. H., and Bohning-Gaese, K. (2008). The Worldwide Variation in Avian Clutch Size across Species and Space. PLoS Biol. 6:e303. doi: 10.1371/journal.pbio.0060303
Jordano, P. (2016). Chasing ecological interactions. PLoS Biol. 14:e1002559. doi: 10.1371/journal.pbio.1002559
Knapp, S., and Kühn, I. (2012). Origin matters: Widely distributed native and non-native species benefit from different functional traits. Ecol. Lett. 15, 696–703. doi: 10.1111/j.1461-0248.2012.01787.x
Kuebbing, S. E., Maynard, D. S., and Bradford, M. A. (2018). Linking functional diversity and ecosystem processes: A framework for using functional diversity metrics to predict the ecosystem impact of functionally unique species. J. Ecol. 106, 687–698. doi: 10.1111/1365-2745.12835
Lima, M. M., and Mariano-Neto, E. (2014). Extinction thresholds for Sapotaceae due to forest cover in Atlantic Forest landscapes. Forest Ecol. Manag. 312, 260–270.
Lohbeck, M., Poorter, L., Paz, H., Pla, L., Van Breugel, M., Martínez-Ramos, M., et al. (2012). Functional diversity changes during tropical forest succession. Perspect. Plant Ecol. Evol. Systemat. 14, 89–96. doi: 10.1016/j.ppees.2011.10.002
Luck, G. W., Carter, A., and Smallbone, L. (2013). Changes in bird functional diversity across multiple land uses: Interpretations of functional redundancy depend on functional group identity. PLoS One 8:e63671. doi: 10.1371/journal.pone.0063671
Magioli, M., Ribeiro, M. C., Ferraz, K. M. P. M. B., and Rodrigues, M. G. (2015). Thresholds in the relationship between functional diversity and patch size for mammals in the Brazilian Atlantic Forest. Anim. Conserv. 18, 499–511. doi: 10.1111/acv.12201
Marzluff, J. M. (2017). A decadal review of urban ornithology and a prospectus for the future. Ibis 159, 1–13. doi: 10.1111/ibi.12430
Mason, N. W. H., Mouillot, D., Lee, W. G., and Wilson, J. B. (2005). Functional richness, evenness and divergence are the primary components of functional diversity. Oikos 111, 112–118. doi: 10.1111/j.0030-1299.2005.13886.x
McGarigal, K., and Marks, B. J. (1995). FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. USDA forest service general technical report PNW-351. Corvallis, OR: USDA.
McKinney, M. (2008). Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Meynard, C. N., Devictor, V., Mouillot, D., Thuiller, W., Jiguet, F., Mouquet, N., et al. (2011). Beyond taxonomic diversity patterns: How do α, β and γ components of bird functional and phylogenetic diversity respond to environmental gradients across France? Glob. Ecol. Biogeogr. 20, 893–903. doi: 10.1111/j.1466-8238.2010.00647.x
Morante-Filho, J. C., Faria, D., Mariano-Neto, E., and Rhodes, J. (2015). Birds in Anthropogenic Landscapes: The Responses of Ecological Groups to Forest Loss in the Brazilian Atlantic Forest. PLoS One 10:e0128923. doi: 10.1371/journal.pone.0128923
Mouchet, M. A., Villéger, S., Mason, N. W. H., and Mouillot, D. (2010). Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 24, 867–876. doi: 10.1111/j.1365-2435.2010.01695.x
Mouillot, D., Graham, N. A., Villéger, S., Mason, N. W., and Bellwood, D. R. (2013). A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. doi: 10.1016/j.tree.2012.10.004
Murcia, C. (1995). Edge effects in fragmented forests - implications for conservation. Trends Ecol. Evol. 10, 58–62. doi: 10.1016/S0169-5347(00)88977-6
Murray, B. D., Holland, J. D., Summerville, K. S., Dunning, J. B. Jr., Saunders, M. R., and Jenkins, M. A. (2017). Functional diversity response to hardwood forest management varies across taxa and spatial scales. Ecol. Appl. 27, 1064–1081. doi: 10.1002/eap.1532
Pakeman, R. J. (2011). Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J. Ecol. 99, 1143–1151. doi: 10.1111/j.1365-2745.2011.01853.x
Pardini, R., Bueno, A. A., Gardner, T. A., Prado, P. I., and Metzger, J. P. (2010). Beyond the Fragmentation Threshold Hypothesis: Regime Shifts in Biodiversity Across Fragmented Landscapes. PLoS One 5:e13666. doi: 10.1371/journal.pone.0013666
Parker, T. A. III, Stotz, D. F., and Fitzpatrick, J. W. (1996). “Ecological and distributional databases. Neotropical birds: Ecology and conservation,” in Neotropical birds: ecology and conservation, eds D. F. Stotz, J. W. Fitzpatrick, T. A. Parker III, and D. K. Moskovits (Chicago, IL: University of Chicago Press).
Podani, J., and Schmera, D. (2006). On dendrogram-based measures of functional diversity. Oikos 115, 179–185. doi: 10.1111/j.2006.0030-1299.15048.x
Prescott, G. W., Gilroy, J. J., Haugaasen, T., Uribe, C. A. M., Foster, W. A., and Edwards, D. P. (2016). Reducing the impacts of Neotropical oil palm development on functional diversity. Biol. Conserv. 197, 139–145. doi: 10.1016/j.biocon.2016.02.013
R Development Core Team (2012). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., and Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is there remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153. doi: 10.1016/j.biocon.2009.02.021
Riemann, J. C., Ndriantsoa, S. H., Rödel, M. O., and Glos, J. (2017). Functional diversity in a fragmented landscape — Habitat alterations affect functional trait composition of frog assemblages in Madagascar. Glob. Ecol. Conserv. 10, 173–183. doi: 10.1016/j.gecco.2017.03.005
Rigueira, D. M. G., Rocha, P. L. B., and Mariano-Neto, E. (2013). Forest cover, extinction thresholds and time lags in woody plants (Myrtaceae) in the Brazilian Atlantic Forest: Resources for conservation. Biodiv. Conserv. 22, 3141–3163. doi: 10.1007/s10531-013-0575-4
Santillán, V., Quitián, M., Tinoco, B. A., Zárate, E., Schleuning, M., Bohning-Gaese, K., et al. (2019). Different responses of taxonomic and functional bird diversity to forest fragmentation across an elevational gradient. Oecologia 189, 863–873. doi: 10.1007/s00442-018-4309-x
Sayer, C. A., Bullock, J. M., and Martin, P. A. (2017). Dynamics of avian species and functional diversity in secondary tropical forests. Biol. Conserv. 211, 1–9. doi: 10.1016/j.biocon.2017.05.004
Schleuter, D., Daufresne, M., Massol, F., and Argillier, C. (2010). A user’s guide to functional diversity indices. Ecol. Monogr. 80, 469–484. doi: 10.1890/08-2225.1
Sekercioglu, C. H. (2006). Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. doi: 10.1016/j.tree.2006.05.007
Sekercioglu, C. H., Daily, G. C., and Ehrlich, P. R. (2004). Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. U.S.A. 101, 18042–18047. doi: 10.1073/pnas.0408049101
Silva, J. M. C., and Tabarelli, M. (2000). Tree species impoverishment and the future flora of the Atlantic Forest of northeast Brazil. Nature 404, 72–74. doi: 10.1038/35003563
SOS Mata Atlântica and Instituto Nacional de Pesquisas Espaciais. (2008). Atlas dos remanescentes forestais da Mata Atlântica, period of 2000 at 2005. Available online at: http://www.sosmatatlantica.org.br/ (accessed August 8, 2010).
Tews, J., Brose, U., Grimm, V., Tielborger, K., Wichmann, M. C., Schwager, M., et al. (2004). Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 31, 79–92. doi: 10.1046/j.0305-0270.2003.00994.x
Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M., and Siemann, E. (1997). The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. doi: 10.1126/science.277.5330.1300
Tscharntke, T., Tylianakis, J. M., Rand, T. A., Didham, R. K., Fahrig, L., Batáry, P., et al. (2012). Landscape moderation of biodiversity patterns and processes-eight hypotheses. Biol. Rev. 87, 661–685. doi: 10.1111/j.1469-185X.2011.00216.x
Villéger, S., Mason, N. W., and Mouillot, D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
WikiAves (2017). A Enciclopédia das Aves do Brasil. Available online at: http://www.wikiaves.com.br (accessed August 3, 2017).
Keywords: ecological functions, ecosystem services, environmental filtration, functional diversity, habitat fragmentation
Citation: Mariano-Neto E and Santos RAS (2023) Changes in the functional diversity of birds due to habitat loss in the Brazil Atlantic Forest. Front. For. Glob. Change 6:1041268. doi: 10.3389/ffgc.2023.1041268
Received: 10 September 2022; Accepted: 26 January 2023;
Published: 16 February 2023.
Edited by:
Marcelo Lopes Rheingantz, Universidade Federal do Rio de Janeiro, BrazilReviewed by:
Paula Lira, Pontifical Catholic University of Rio de Janeiro, BrazilCopyright © 2023 Mariano-Neto and Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo Mariano-Neto,  bWFyaWFub25AZ21haWwuY29t
bWFyaWFub25AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.