- 1Ecology, Evolution and Marine Biology, University of California, Santa Barbara, Santa Barbara, CA, United States
- 2USDA Forest Service, Solvang, CA, United States
Introduction: Forest restoration is a powerful tool to combat the dual threats of drought and fire, both of which have been increasing in frequency and severity in recent years in the Western United States. The hard-hit region of Southern California is home to the endemic bigcone Douglas-fir, Pseudotsuga macrocarpa (Vasey), whose abundance and range have been impacted by multiple large fires within the last two decades.
Methods: To better understand the ecology of these trees, and thereby improve the potential for restoration in light of predicted future conditions, we outplanted 1,728 seedlings into burned areas with varying levels of pre-planting fire severity and proximity to water (near canyon bottom and upslope). Within each planting area, seedlings were planted into one of four microhabitats; under conspecifics, under the codominant oak species [Quercus chrysolepis (Liebm)], in the open (no woody canopy), or in the open within a microsite feature such as a log, rock or a small shaded hole. At each site and within each microhabitat, half the seedlings were treated with oak-soil amendments (soil from under the oak) and half with spring watering (4 months after planting). To better understand the influence of outplanting conditions, we tracked the survival of the seedlings over the next two years.
Results and discussion: Short-term (March to June) survivorship following planting was quite high and the most significant die-off of seedlings occurred during the first dry season (June to November) despite strong droughts in the second and third dry seasons. Overall, only 7.3% (127 of 1,728) of seedlings survived. Seedling success depended strongly on the microhabitat and summer watering, though not in necessarily intuitive ways. Seedlings that received supplemental water during the first summer did worse than unwatered seedlings. The most successful microhabitats for planting were open sites with microsite features and sites underneath canyon live oak, while sites under mature bigcone Douglas-firs had the lowest rates of survival. Position on the slope had no effect on outcomes and soil amendment had a weak negative effect on seedling survival.
1 Introduction
Globally, conifer forests are experiencing the detrimental impacts of climate change in the form of increased aridity and large high-intensity wildfires (Breshears et al., 2005; Allen et al., 2015; Halofsky et al., 2020; Stephens et al., 2020). Many forests are storing tones of carbon in the form of tree biomass and soil organic matter, the loss of which could be catastrophic for the balance of carbon budgets at regional to global scales (Hurteau et al., 2008). Additionally, forests are home to a diverse set of animals and plants reliant on an intact canopy, help to provide clean air and water, and protect lands from erosion and flooding. The management of forests and fires are thus critical for maintaining these ecosystem services. Ecological restoration of canopy trees can be an effective tool for combating the loss of stored carbon and other resources that decline when forests are decimated (Kashian et al., 2006).
While ecological restoration has been proposed as a tool to assist regions in reaching climate-related goals (Harris et al., 2006; Suding et al., 2015; Bustamante et al., 2019), the reality is that forest restoration goals are challenging to achieve particularly at scale. Restoration of forest species is often hindered by the harsh conditions present in degraded forests and by the reality that sites may burn again before canopy trees have become well established. Thus practitioners face site-specific hurdles and uneven success (Cruz-Alonso et al., 2019; Rodríguez-Uña et al., 2020; Nolan et al., 2021). Simultaneously, it is important to build broader frameworks that can save land managers and practitioners time and money in the planning and implementation of restoration projects. For these reasons, restoration studies should represent a balance between the testing and integration of ecological principles and the need for local but scalable management strategies.
Given the large footprints of recent wildfires (Juang et al., 2022), burned forests are often far from seed sources, necessitating active planting to recover the tree canopy. Fire-scorched areas are also frequently devoid of canopy cover, which can lead to increased soil drying, making it difficult for seedlings to establish and survive. Tools, such as irrigation, can help overcome barriers to seedling establishment, but they are impractical across large and in remote areas. Additional hurdles to seedling success include access to soil-derived inorganic nutrients, such as nitrogen and phosphorus, some of which influence the availability, and establishment of crucial symbiotic microbial communities (e.g., ectomycorrhizae).
Integration of mycorrhizal inoculations into restoration is becoming more common, but there is yet to be a general consensus on best practices (Teste et al., 2004; Grove et al., 2019). Ectomycorrhizal fungi associate with many temperate forest species, particularly conifers, and are estimated to exist on the roots of more than 60% of trees across the globe (Gfbi consortium et al., 2019). Thus, it may be beneficial to integrate symbiotic communities into restoration practices, particularly when seedling establishment is hampered by insufficient access to soil moisture and nutrients (Lu et al., 2016). Moreover, planted seedlings have been shown to benefit from established mycorrhizal communities in the soil, such as those associated with conspecifics or alternate hosts with overlapping fungal compatibility (Bingham and Simard, 2011).
In this study, we report results of a restoration experiment after the 2007 Zaca Fire, which burned through multiple vegetation types, including chaparral, mixed conifer, and hardwood (oak) forests (see Kibler et al., 2019) and left a large fire scar (972 km2) in southern California. The Zaca fire burned through a region that is home to an endemic conifer, Pseudotsuga macrocarpa, which we hereafter refer to as bigcone Douglas-fir or simply bigcone. This drought-tolerant sister species of the common western USA Douglas fir [Pseudotsuga menziesii (Mirb.)] is endemic to the southern California mountains, a region that has experienced multiple severe wildfires over the last two decades. These fires, alongside recent historic droughts and the restricted distribution of the species, have led to the listing of this species on the IUCN Red List as a near threatened species (Farjon, 2011). The challenges facing bigcone Douglas-fir are indicative of broader impacts that are predicted to accompany climate change, large scale fires, and future restoration efforts.
The primary goal of this study was to identify both environmental features and outplanting treatments that promote the greatest seedling survival of this conifer. To investigate the optimum combination of landscape-level factors and planting strategies, we designed a study testing the effects of pre-planting fire severity (high, moderate, low), slope position (low and high elevation), microhabitat type (e.g., developed tree canopy, open habitat), planting condition treatments (e.g., soil inoculation, watering), and their interactions on the restoration success of bigcone seedlings. We selected planting locations and treatments that are practical for implementation by restoration practitioners and land managers. We did not include irrigation since that is not realistic for these remote settings. It may be expected that sites with the greatest outplanting success would be those that experienced low mortality during fire and thus offer shade and soil microbial conditions (e.g., mycorrhizal networks) conducive to seedling growth rather than conditions in a large barren patch of ground. Yet planting into low-mortality burn sites does little toward the goal of restoring bigcone trees within their former range. Hence, it is critical to identify microsites and planting conditions that will lead to the greatest likelihood of survival within moderate- to high-mortality burn sites where substantial areas of forest canopy were removed by fire.
2 Materials and methods
2.1 Site selection
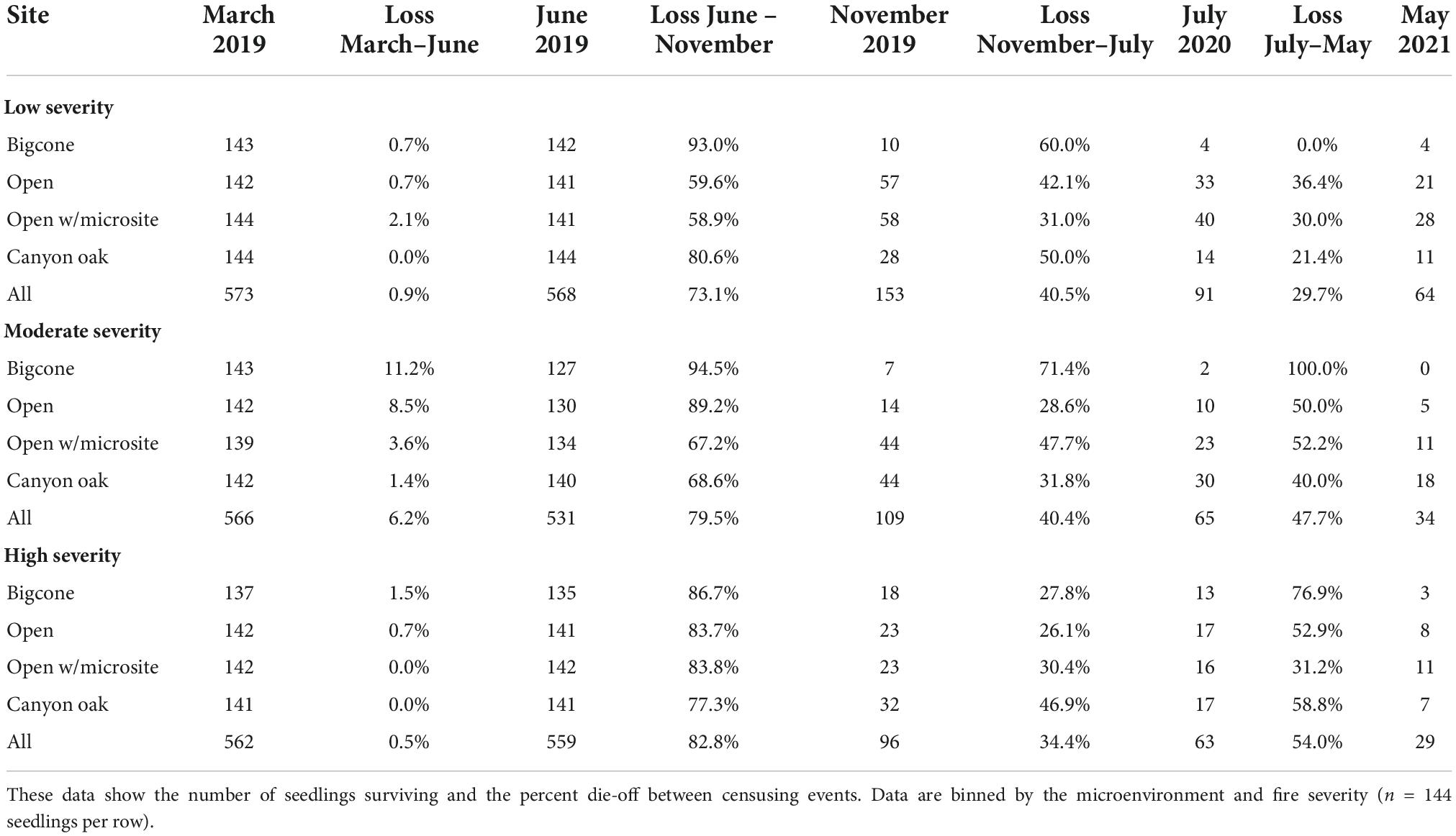
This study was initiated in response to the Zaca Fire that burned over 97,124 hectares in southern California from July to September 2007 including > 4,000 stands of bigcone Douglas-fir (Parkinson et al., 2022). Selection of study sites was confined to the Zaca Fire perimeter. Aerial imagery was used to evaluate the condition of bigcone stands before and after the fire. To better understand how fire influences the restoration potential of bigcone seedlings, we outlined three classes of fire severity based on the percent mortality of bigcone stands following the Zaca Fire. Areas were categorized as high-severity sites if greater than 75% of bigcone canopy died, moderate severity if between 75 and 25% bigcone canopy died, and low severity if less than 25% bigcone canopy died. We further refined the restoration areas by selecting planting sites outside of designated Wilderness Areas (due to permitting constraints), on north-facing slopes, accessible from trails or roads, and at least 0.12 hectares in size. From the list of criteria, two sites were selected from each of the three fire severity classes (high, moderate, and low; n = 6; Figure 1, see Supplementary material for shapefiles).
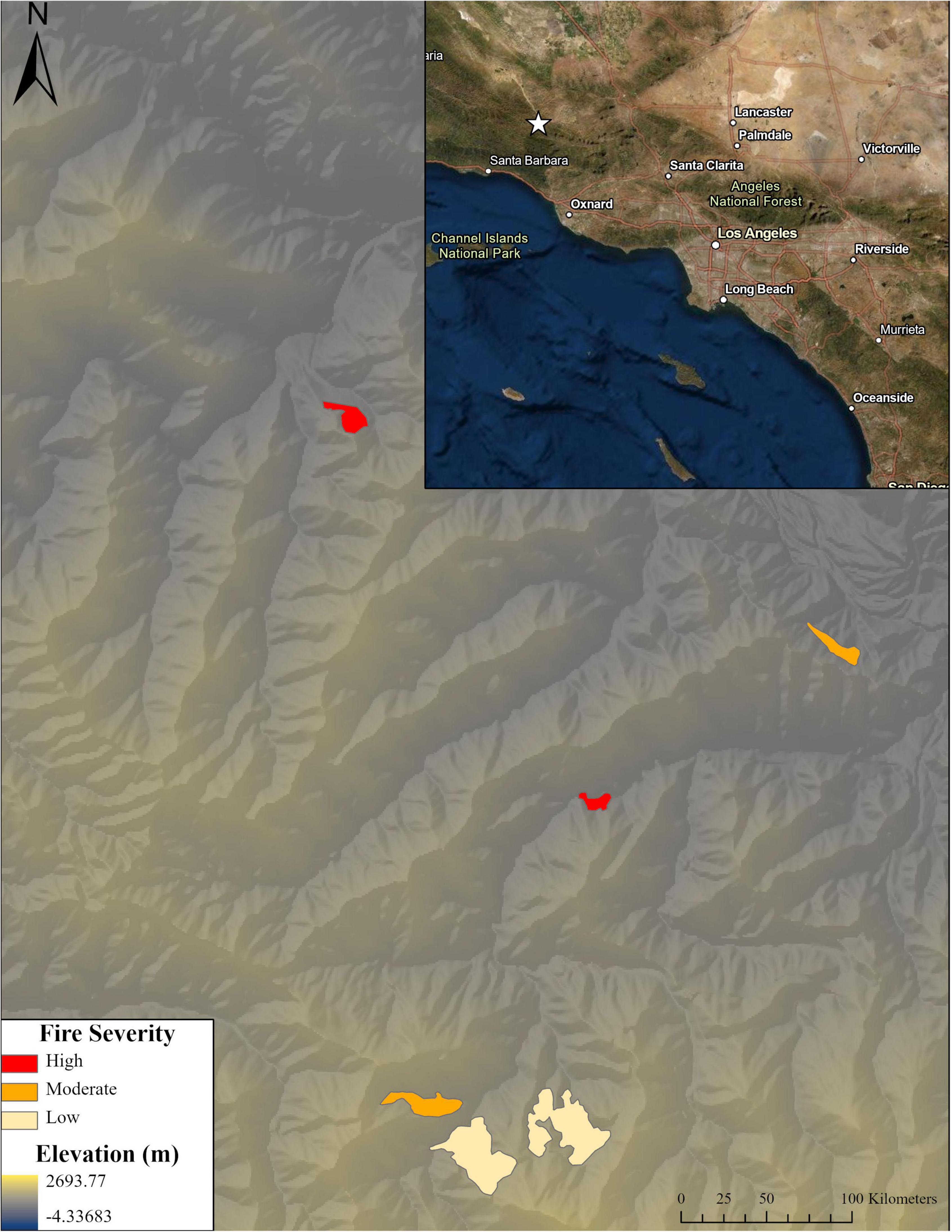
Figure 1. Map of study locations. Planting areas were chosen to be as close to one another as possible to constrain climate, elevation, and parent material. They follow CA HWY 33 on the north side of the Pine Mountain saddle (34.649917, –119.385418). Sites are colored by the severity of fire experienced in the area, calculated from percent die-off (see above). Shading represents elevation and is overlaid on a hillshade layer to display topography. Shapefiles are available in the Supplementary material. Inset map shows the location of sites (white star) within a broader geographic context.
Within each site, four microhabitat treatments were identified: near mature bigcone Douglas-fir (hereafter “bigcone”), near canyon live oak (“canyon oak”), areas without canopy or large topographic features (“open”), and areas without canopy but with topographic features (e.g., boulder, log) to provide shade to seedlings (“open with microsite”). Although many reforestation efforts incorporate site prep and even continue to remove vegetation after planting (i.e., “release”), the arid portions of the bigcone Douglas-fir range, where this study was conducted, have little competing vegetation (see Supplementary material for photos). The only changes made to sites were to increase the number of microsites for the “open with microsite” treatment by bringing in nearby logs or other “natural” shade structures. Canyon oaks were chosen as an alternate canopy site due to their abundance near bigcone stands. We also wanted to investigate a potential nurse plant relationship after anecdotal reports that naturally regenerating seedlings had been frequently observed near oaks. At the two high-severity burn sites, seedlings were planted below standing dead trees because there were no living bigcone trees remaining to plant under. These four microhabitat treatments were replicated at high (mid-slope to ridgeline) and low (toe slope closer to canyon) elevations on the slope to assess the effect of proximity to water source. These 48 locations—6 sites × 2 slopes × 4 microhabitats—constitute our “plots” for the study. This outplanting design, along with the information in the following section, is summarized in Figure 2.
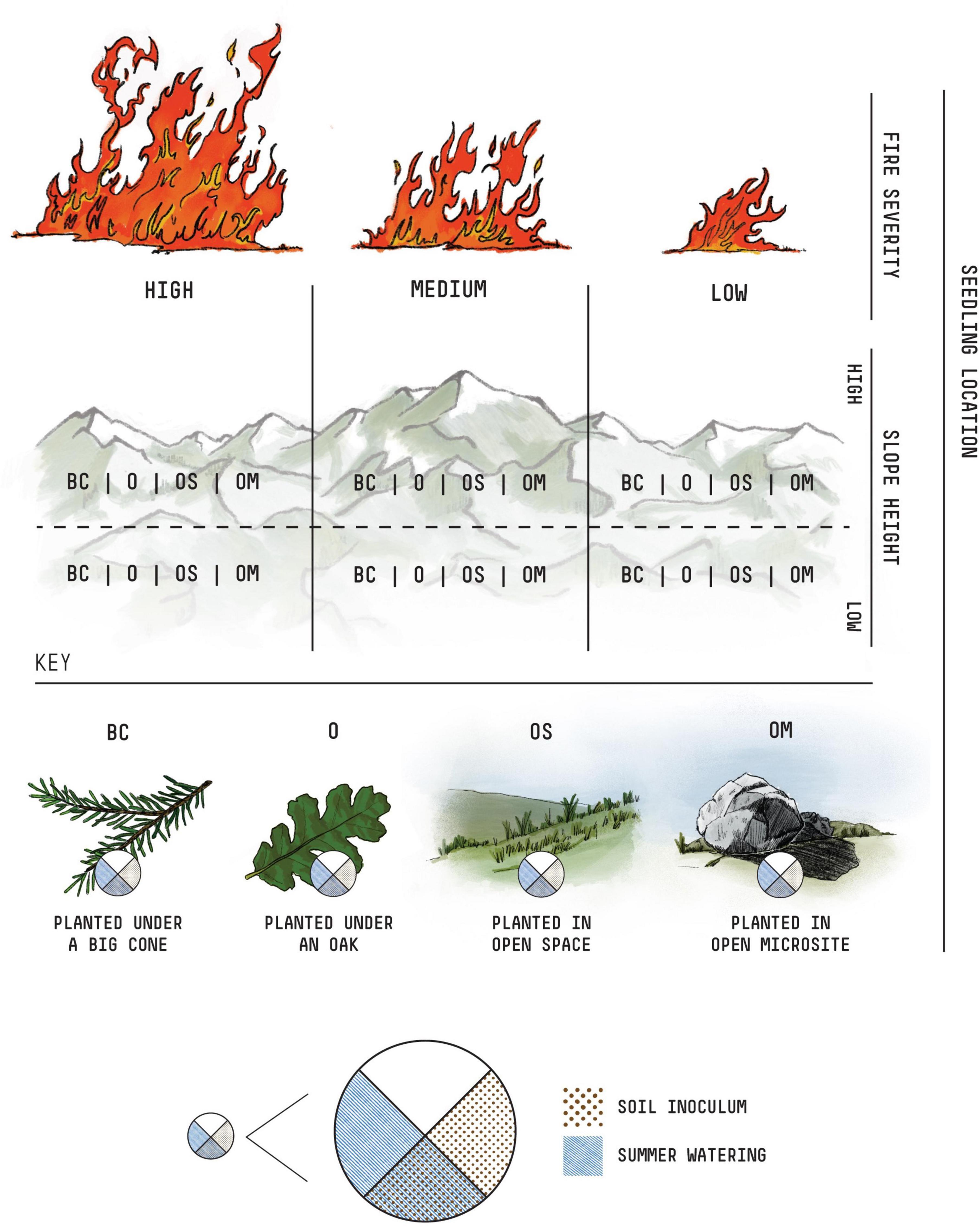
Figure 2. Outplanting design. Breakdown of seedling planting locations across the landscape. Three fire severity levels were replicated twice in planting areas shown in Figure 1. Within these areas, there were high and low elevation planting locations across which the microhabitat treatments were replicated (under bigcone Douglas-fir, under canyon live oak, in the open, and in the open with microsites). These sites (48 sites, 36 seedlings per site) were further broken down by inoculating half of the seedlings with live soil for mycorrhizal associates and watering half in June of the planting year such that nine seedlings had each of four combinations of soil inoculum or not and watering or not.
2.2 Planting and measurements
Seedlings were purchased from the united states department of agriculture (USDA) Placerville Forest Service nursery in Camino, California, approximately 4 months prior to the start of outplanting. The 2-year-old seedlings were received as bare-root plants with the soil, a synthetic potting mix in plastic bags (five seedlings to a bag). They were held in waxed cardboard boxes at 4°C in a walk-in cooler between the time of receiving and the time of planting. Seedlings were planted within 18 h after removal from the cooler.
Seedlings were planted plot by plot (n = 48 plots, 36 seedlings per plot) over the course of 1 month in March 2019. Southern California is characterized as having a Mediterranean climate with cool wet winters and hot dry summers. Planting in the late winter allowed for the avoidance of freezing conditions and the opportunity to capitalize on later winter-spring precipitation to aid in seedling establishment. The planting region received 8.48 cm of rain in the month of planting with an additional 4.29 cm coming in April and May combined. Only 2.31 cm of rain fell between June and November of that year (Supplementary Figure 1). Due to a history of low survival in bigcone restoration efforts, techniques were incorporated in this study that, while unorthodox, were believed to hold potential as ecologically justifiable manipulations that could be used in remote settings.
A total of 1,728 seedlings were planted across our mixed design. Seedling holes were dug with a hoedad (Forestry Suppliers #69088), after which soil samples were collected from the bottom of each hole for soil fungal community analyses (see below). Half of the holes at each plot received 200 ml of soil inoculum prior to the seedling being planted. This soil inoculum was collected from under a single canyon live oak (Quercus chrysolepis) from the same plot by first removing leaf litter and/or herbaceous vegetation. Homogenized soil from the first 15 cm was applied as inoculum to half of the seedlings. This low-cost, low-effort subsidy was intended as a potential restoration technique to introduce communities of soil symbiotic fungi to areas on the landscape that may have been devoid of such communities. Inoculum used at each plot originated from within the same plot and was not homogenized across plots.
On June 30th, prior to the full onset of the first summer, half of the seedlings across all treatments received 500 ml of water. Water was provided more than a month after meaningful levels of rainfall had ceased for the summer and soil was already dry by the time of watering. The goal of this strategy was to reach the seedlings before they had fully adapted to drought conditions, thereby extending the hospitable growing conditions of winter and spring. The volume of water was compromised due to the labor-intensive task of transporting large volumes of water up dangerous steep slopes where bigcones grow and was also intended to test a low-tech, scalable solution that could be realistically implemented by practitioners dealing with thousands of outplanted seedlings. This watering treatment resulted in nine replicates across each plot (36 seedlings per plot, crossed with soil inoculation and watering treatments).
The survival of seedlings was monitored five times over the next 26 months, including at the time of planting (March 2019), before (June 2019) and after the first summer (November 2019), and two summers (July 2020 and May 2021) following planting (Figure 3A and Table 1). Seedlings were marked as dead when needles were completely brown or completely missing. Because this species has the capacity to resprout even after losing all of their green needles, it is possible for seedlings that appeared dead in one census to be alive at a later point in time. This scenario was rare (n < 10), but when it did occur the seedling was retroactively given a living health score for any previous time step in which it was considered dead.
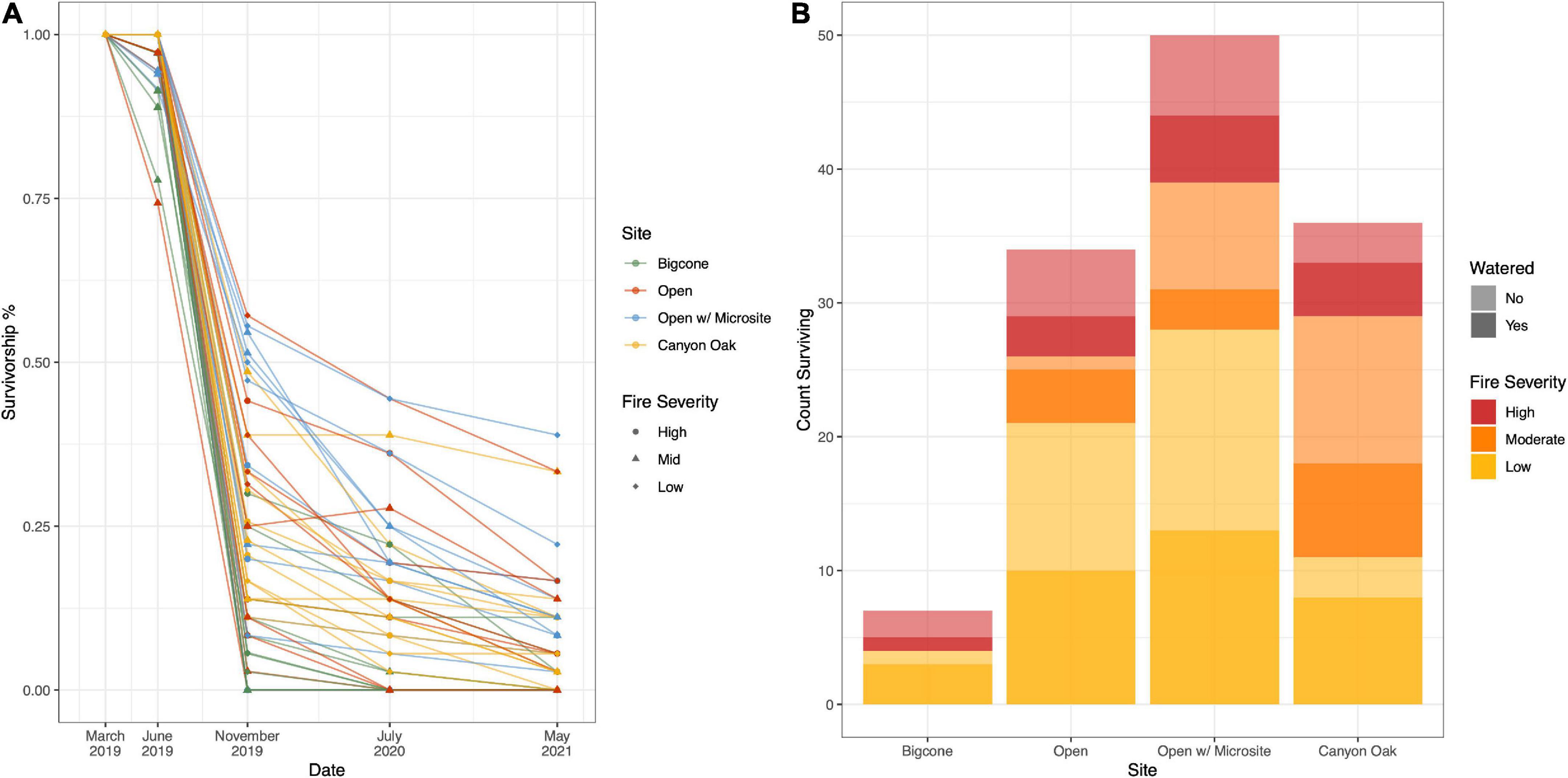
Figure 3. Seedling survival. Each of these panels depict the overall survival of seedlings throughout the study (March 2019–May 2021). (A) Shows the proportion of seedlings surviving within each planting area, with each microenvironment given a different color and each fire severity level given a different shape. (B) Shows the final count of seedlings surviving after 26 months. Here, seedlings are organized by microenvironment and stacked by fire severity. Within each fire severity bin (red, orange, yellow), seedling counts are shaded by whether or not they received a first summer watering (darker) or not.
2.3 Soil fungal analysis
To understand how soil fungal communities may influence the survivorship of outplanted seedlings, soil was collected from the bottom of every planting hole. Soil from each of the 36 seedlings was combined at the plot level and analyzed together. Following collection, soil was stored in a cooler (approx. 4–8°C), moved to a refrigerator (4°C) within 14 h, and a minimum of three homogenized subsamples were stored at −80°C within 5 days for molecular analysis.
Deoxyribonucleic acid (DNA) was extracted from 200 to 250 mg of soil using the Qiagen DNeasy Powersoil Pro Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Internal transcribed spacer 1 (ITS1) region was amplified using the GoTaq Green mastermix (Promega, USA) with the ITS1 primer pair ITS1F-KYO1 and ITS2-KYO1 (Toju et al., 2012) and the following thermal cycler steps: 95°C for 3 min, 35 rounds of 95°C for 30 s, 50°C for 30 s, 72°C for 30 s, then a final elongation step of 5 min at 72°C. Illumina indices were ligated with 3 min at 95°C, 10 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, then a final elongation step of 72°C for 5 min. Finally, samples were purified with AMPure XP beads and diluted to 10 ng/μL before multiplexing all samples for sequencing in two sequencing runs based on time of processing. Amplicons were sequenced on the Illumina MiSeq platform with 250-bp paired-end reads at the California NanoSystems Institute (UC Santa Barbara).
Amplicon sequences were processed using the DADA2 pipeline (Callahan et al., 2016). Adapter and primer sequences were removed using the cutadapt package and chimeras were removed before filtering (Martin, 2011). DADA2::filterandtrim settings removed any sequences with an “N” assignment, truncated reads at the first instance of a quality score at or below 2, and removed reads shorter than 50 bp. Amplicon sequence variants (ASVs) were given taxonomic assignments using the UNITE database for use in functional assignments with FUNGuild (Nguyen et al., 2016; Nilsson et al., 2019) [see Supplementary Figures 2, 3 for guild-specific principal coordinates analysis (PCoAs)].
Fungal community turnover (beta diversity) across plots was evaluated based on Bray-Curtis dissimilarity [vegdist() function in vegan] (Oksanen et al., 2008). Community structures were visualized using a PCoA. Finally, community dissimilarity was used as the response variable in a series of PERMANOVA tests [adonis() in vegan] to evaluate how fungal community composition shifted across the study plots as well as to assess the correlation between fungal community dissimilarity and seedling survival at the plot level (n = 36). Included in these tests were all factors relating to the plot as a whole: fire severity, microhabitat, position on slope, and seedling survival along with relevant interactions.
2.4 Seedling survival analyses
Due to the low rates of survival in the seedlings, we analyzed only binary survival data for all time points across the 2 years. Our Bayesian model was developed using the Rethinking package in R (McElreath, 2021). This package is built upon the package RStan (Stan Development Team, 2022) which allows the user to implement Hamiltonian Monte Carlo sampling to calculate the posterior probabilities of model parameters. Survival was predicted as a function of whether or not the individual seedling was alive at the prior time step, with the addition of relevant experimental parameters. We used random effects to account for the levels of nestedness of our data, selecting from our models the relevant model with the lowest tested Watanabe–Akaike information criterion (WAIC) score (Eqs 1, 2, see Data availability statement for full model and code). Models were iteratively built, incorporating all predictor variables (e.g., microhabitat, fire severity, water addition) and random effects (e.g., time, site, interactions) in a sequence corresponding to their level of expected impact, before WAIC-selection. If models were not improved by the addition of a predictor such that the model with additional predictors had a higher WAIC score, that predictor was removed prior to proceeding with further additions to the model. All models were run on four chains and checked for convergence by ensuring that all R-hat values were ≤ 1.1 (Gelman et al., 1995).
In the model (Eqs 1, 2), random effects are denoted with an α (alpha) scaling parameter and predictors are denoted with a β (beta) scaling parameter. This model design accounts for the noise associated with the mixed design to focus directly on the independent effects of microhabitat treatments, fire severity in the area, water addition in the summer, and added soil inoculum. The predictive power of each of the parameters in the final model were evaluated using posterior comparisons, wherein the relative effects of treatments are compared by taking the difference between posterior samples from each of the microhabitats.
3 Results
3.1 Seedling survival
In May of 2021, after more than 2 years of monitoring, 7.3% (127 of the 1,728) of the seedlings were still alive. The greatest seedling survival was in the “open microsite” microhabitat treatment (39% of surviving seedlings) and the sites with low fire severity (50% of surviving seedlings) (Figure 3B and Table 1). The best fit model describing seedling survivorship included the effects of microhabitat, fire severity, water, and soil inoculation. The effect of slope position was not included as a predictor in the model, though the interaction between slope and water was included.
Model posterior comparisons between tested microhabitats are shown in Figure 4A. The only statistically significant microhabitat treatment effect was the strong negative (predicting more die-off) effect of planting under mature or dead bigcone Douglas-firs. Among the remaining three treatments, open sites did the worst, with seedlings planted in open-microsites and those planted near canyon live oaks faring the best.
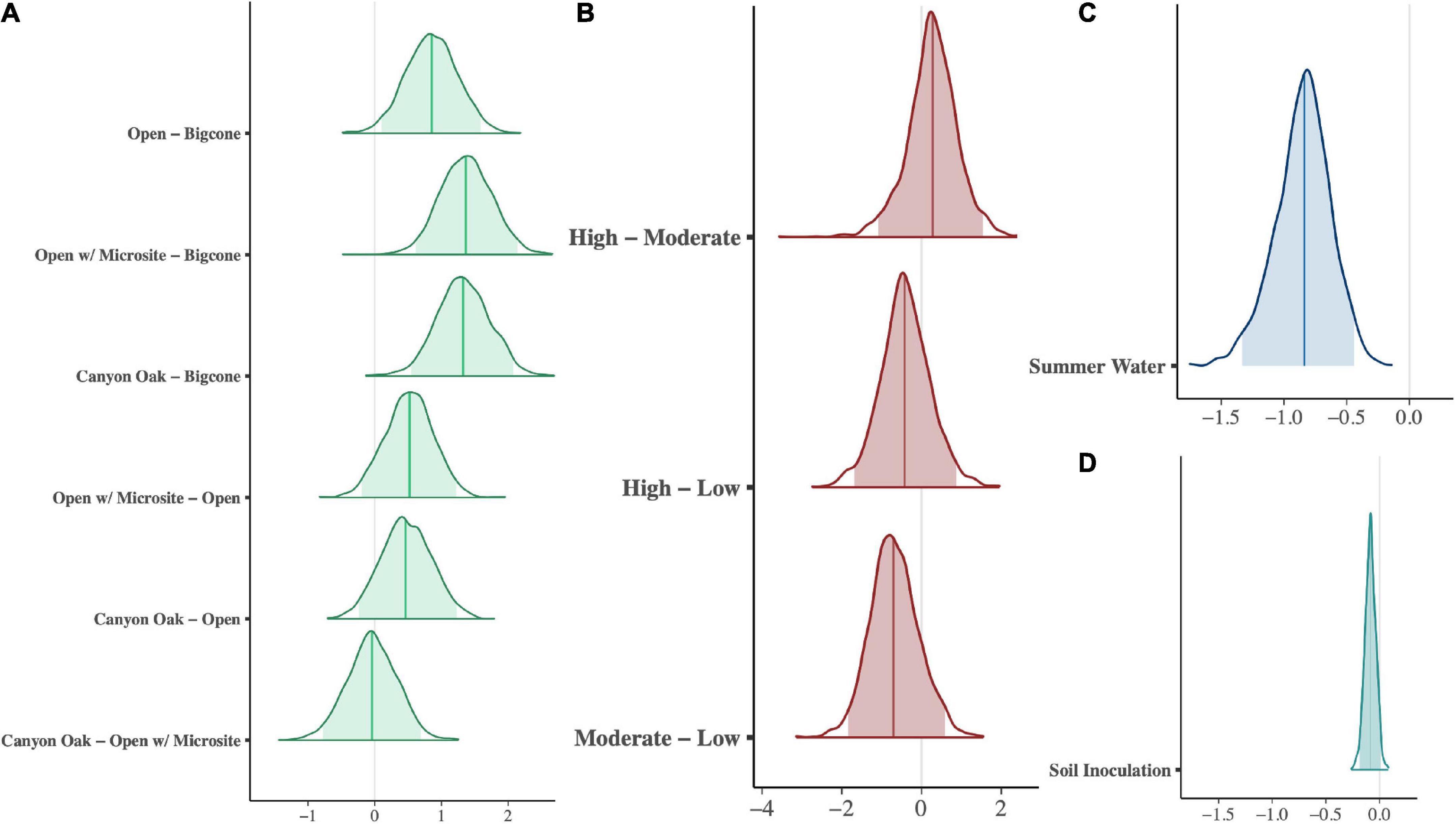
Figure 4. Posterior distributions of parameters in seedling-survival model. Shaded regions depict 95% probability in the distribution and the vertical line shows the median estimate. For categorical terms, microhabitat (A) and fire (B), the distributions show the contrasts between treatments. Binary terms, soil inoculation (C) and summer water (D), are centered at zero with +1 for treated seedlings and –1 for untreated seedlings. Positive x-axis values are related to increased survival of seedlings in our model (Eqs 1, 2).
Fire severity showed no distinguishable effect on seedling performance (Figure 4B). While seedlings in areas with low fire severity had the highest survival, the posterior comparisons between the three fire classes are centered around zero, showing no directional effect that was statistically significant.
The planting condition treatments (soil addition and summer watering) are also presented as posterior comparisons in Figures 4C, D. Soil addition had very little effect on the seedlings’ survival, though it tended to have a slightly negative effect. Summer watering, on the other hand, was strongly associated with negative seedling survival outcomes.
3.2 Soil fungi
Deoxyribonucleic acid (DNA) sequences were successfully extracted from 154 soil subsamples which were roughly evenly distributed across the six planting areas and four microhabitat treatments. We retained 4,890,524 reads with a mean (and standard deviation) read count of 31,757 (10,894) reads per sample. Fungal community turnover was best predicted by the spatial distribution of the samples. The planting site was the single strongest predictor of fungal dissimilarity, with an R2 value of 0.13 in the PERMANOVA. Because these sites are, by their nature, not replicated, and therefore have less relevance in connecting fungal community turnover to the ecology of regenerating forests, we chose to drop this factor and focus additional tests on experimental considerations, including fire severity, microhabitat, slope location, and survival. From these, we eliminated factors with R2 values below 0.02, leaving us with the strongest PERMANOVA that included fire severity, microhabitat treatment, and their interaction, which explained 17% of the total variation among samples. Fire severity, with the strongest predictive power apart from planting area, had an R2 of 0.07. Similarly, in the PCoA the most distinct pattern was a differentiation of communities based on fire severity (Figure 5).
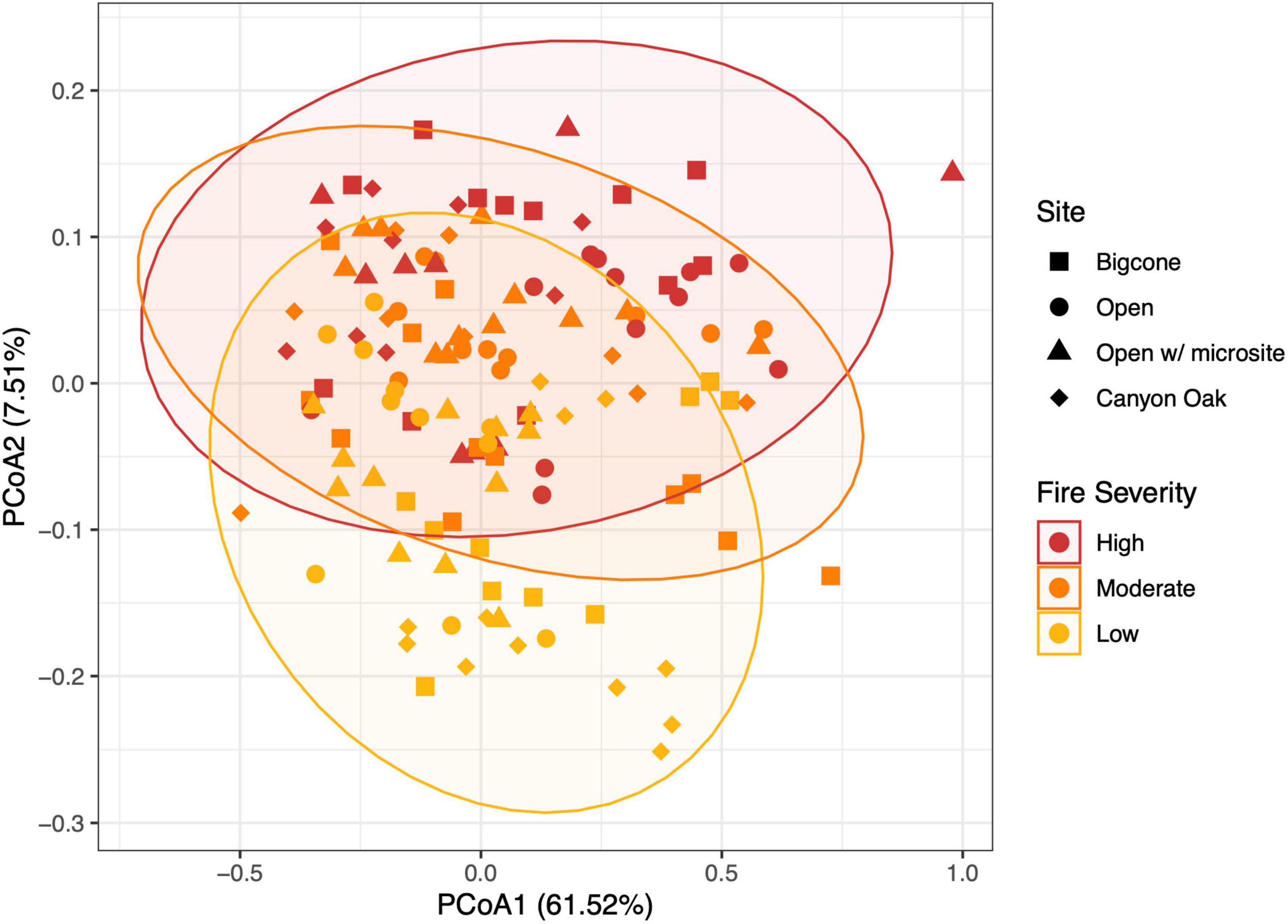
Figure 5. Principal coordinates analysis (PCoA) of soil fungal communities. Soil fungal community data from within each of the planting sites. Bray–Curtis dissimilarity was used to differentiate the community compositions. Shading and point shapes are used to distinguish fire severity and microenvironment, respectively. Ellipses represent the 95% confidence interval for each fire severity level.
4 Discussion
4.1 Putting ecology into practice
The aim of this study was to expand our understanding of seedling survival in post-fire environments and to better inform restoration of degraded landscapes, particularly those facing drought and fire. Many of the treatments with positive outcomes from our study can be put into practice, although uncertainties on specific recommendations still exist. Notably, this study was conducted during a period of prolonged drought in California and Southwestern North America more broadly (Williams et al., 2022). Planning for and studying these conditions is imperative for land managers, and that may mean planning for low rates of survival in some forest restoration projects. Importantly, the low survival in this study is higher than other known restoration efforts of the same species. Other studies have shown total die-off in censused seedlings (Guiterman et al., 2022) and personal communications with Forest Service employees reiterate these challenges. Literature describing dryland forest restoration is sparse, and we found low rates of survival (even below 10%) are not uncommon in studies with little to no seedling management post-planting (Höhl et al., 2020; Holl and Brancalion, 2020). Moreover, to provide greater nuance to our understanding of optimal seedling conditions, it was necessary to plant into a range of more and less suitable habitats leading, in part, to increased seedling mortality. Nonetheless, we argue that studies such as this are an important step in ground-truthing restoration theory and recentering academic pursuits on feasible restoration targets for practitioners interested in reforesting after disturbance. Within favorable sites, bigcone seedling survival was high.
4.2 Planting after fire
This study took place in what might be considered a model system for the predicted trajectory for forests across the temperate Western United States; a forest type defined by limited water and a history of fire (see Lombardo et al., 2009 for fire history of bigcone across the region prior to 2007). Arid regions are anticipated to get even drier over the coming decades (Putnam and Broecker, 2017) and large-scale high-severity fires will become more frequent (Moritz et al., 2012). We found that high fire severity alone is not a barrier to successful restoration. There is little measurable difference between sites that experienced varying fire severities, although the sites with the lowest severity had the greatest survivorship. Importantly, other factors, such as the microhabitat and post-planting treatment, were stronger predictors of the establishment success of this drought-adapted conifer.
Seedling microhabitat was a critical determinant of survivorship. Seedlings planted underneath mature bigcone Douglas-fir experienced the greatest mortality. This was counter to our expectation that open sites would be the most challenging to restore because of a lack of shade and litter. One possible explanation for this pattern is that the presence of host-specific pathogens outweighed the benefits of any host-specific mutualistic symbionts, such as mycorrhizae, as in the Janzen–Connell hypothesis (Janzen, 1970; Connell, 1971). We did not have the sampling resolution to assess the role of pathogen buildup in seedling die-off, but this is a common mechanism in observed negative plant-soil feedbacks (van der Putten et al., 2013). Microhabitats without a canopy or additional cover, referred to here as open sites, had greater seedling survival than under bigcone but not more than the open with microsites or the canyon live oak sites. The greater survivorship in open areas with microsites was likely associated with shading and, possibly, micro-topographical features with the potential to increase water availability to the seedlings. While we do not have soil moisture data, the intention of the experimental manipulation was to reduce water stress. Finally, the canyon live oak sites provided a successful habitat for bigcone seedlings to grow, which was statistically comparable to open with microsite sites. The benefits for a seedling under an oak could be some shading effect as in open with microsite sites, though this should have been true also of the bigcone sites. Similarly, the roots of the oaks may increase the pool of available soil water by hydraulic lift, effectively increasing the access of nearby plants to deep soil water (Caldwell et al., 1998). It is also possible that the seedlings under oaks were able to benefit from compatible symbiotic microbial communities (Runte et al., unpublished) without the negative effects of host-specific pathogens, which would have been present under mature bigcone trees. Previous greenhouse work by collaborators has also shown that bigcone seedlings in proximity to canyon live oaks are better able to withstand drought stress (Rodolf et al., 2019) and a previously unpublished survey found that natural seedlings in the field were almost always associated with canyon live oaks (Moritz et al., unpublished).
4.3 Elevation
Location on the slope was not a strong predictor of seedling survival or fungal community turnover. This effect was dropped in the process of model selection as any predictive power added to the model was outweighed by overfitting. We chose to still add slope into the soil fungal community PERMANOVA, but it was also removed due to a low R2 value. Nonetheless, it is remarkable that mature bigcone trees, particularly at low elevations, are frequently distributed in canyon, riparian settings as reported by the united states forest service (USFS) (Howard, 1992). Other studies have also found strong effects of hillslope position on seedling survivorship and forest composition, leading us to expect that our manipulation simply may not have captured the full strength of these differences between ridges and canyon bottoms (Frey et al., 2007; O’Brien and Escudero, 2022). Our downslope plots were lower but by no means equivalent to a riparian planting.
4.4 Summer watering
The increased mortality of watered seedlings was an unexpected outcome but one that may be highly relevant for restoration practitioners. There is great interest in irrigating seedlings in semi-arid environments with extreme interannual variation in precipitation, like southern California. Given the remote location and steep slopes of many restoration projects in the region, watering seedlings is labor intensive and a significant financial investment. In some cases, water addition has led to improved seedling survivorship in shrublands not far from the bigcone restoration sites (Dewees, unpublished). However, our work shows that early summer irrigation of bigcone was deleterious and ultimately resulted in substantial mortality. What remains to be understood is when and where additional water will be a benefit to seedlings and when it might be a hindrance.
Multiple explanations exist for the mechanism by which watering might negatively affect seedlings. A number of these mechanisms relate to plant hydraulics and rely on the assumption that bigcone Douglas-fir is an isohydric species, meaning it closes its stomata to conserve water as soil moisture decreases. Studies of P. menziesii, a close relative that is less drought tolerant, has confirmed this physiological approach (Kerhoulas et al., 2020). In our study, watering occurred after the end of the growing season, as soil moisture was likely dropping too low to allow for plant productivity. The addition of supplemental water to the surface soil may have induced a reactivation of the plants’ hydraulic system, leading to root embolisms lower in the soil. If this led to enough root die-off, the plant would have been more likely to die in the next dry season. Similarly, even if all roots were able to take up water, the water provided most likely evaporated, infiltrated, or was taken up quickly. If the plants were not able to respond quickly enough by re-closing their stomata, stems might have experienced severe cavitation, leading to irreparable damage. Another explanation that could have played a role in the demise of watered seedlings is an activation of dormant pathogens in the soil. Increased moisture may have made the environment temporarily more hospitable to dormant pathogens that could have devastated already weakened plants. The last notable factor related to watering would be the promotion of non-beneficial growth. Water in the topsoil could encourage seedlings to grow shallow roots over the deep roots that may be better equipped to seek out naturally available water. Watering could also promote greater leaf area, which would contribute to greater transpiration and vulnerability during droughts. However, in this and other unpublished work, we have observed that bigcone seedlings primarily grow in the late winter and spring, producing far less above-ground material after the onset of summer. Hence, it is unlikely that this mechanism is responsible for the increased die-off of the seedlings after watering.
4.5 Soil inoculation
While compatible fungal partners have been shown to increase the growth and success of seedlings in controlled environments (Lu et al., 2016; Moeller et al., 2016), the addition of soil inoculum collected from a co-occurring plant species did not benefit seedling survival in this field study. Previous field studies also found insignificant effects of inoculation on seedling survivorship (Teste et al., 2004; Grove et al., 2019). Many possible explanations exist for this phenomenon, including failure to form mycorrhizas or the negative effect of plant carbon loss in the subsidized development of new fungal networks that offsets any mycorrhizal benefits in the short term. Furthermore, seedlings in this field study may have all benefited equally from a mycorrhizal fungal network that was already in place and any effects of the inoculum may have been negligible. In greenhouse studies of the same species, we have found that we cannot discern mycorrhizas on root tips until 4–6 months from inoculation, suggesting that the symbiosis forms over longer periods of time even under well-watered conditions of a greenhouse. Seedlings would only gain significant benefits via mycorrhizae after the roots come into contact with compatible fungal spores, the spores germinate and grow hyphae out into the soil. In this study, we saw that the vast majority of seedling die-off came in the first 6 months and would likely, therefore, not have been impacted by any beneficial fungal partners. This, in addition to the relatively small sample size of surviving seedlings, makes it difficult to parse ectomycorrhizal-specific plant outcomes.
One potentially confounding factor in this treatment is the differences in soil inoculum between planting areas. This was both a practical and ethical decision. Firstly, it would have been a large hurdle to bring soil from all areas to all other areas in order to make a single uniform inoculum. Sites are miles apart and that volume of soil would have been extremely heavy. Secondly, from the point of view of careful land management, we did not want to unintentionally introduce possible pathogens across a broader range than the single hillslope on which they already existed. Nonetheless, while the replication is low, we did see clustering of fungal DNA-profiled communities from the bulk inoculum within the two high severity sites and the two low severity sites (Supplementary Figure 4) (moderate severity sites did not cluster with each other or either of the other severity levels). Moreover, fungal community profiling work in fire-burned areas shows changes in fungal composition in response to varying severity (Glassman et al., 2016; Pulido-Chavez et al., 2021).
A potential alternative for restoration practitioners would be to facilitate the formation of mycorrhizas with drought-adapted fungi in the greenhouse prior to outplanting. Previous work has shown that fungi differ strongly across water availability and other stress gradients in the field (Moeller et al., 2014; Bui et al., 2020). Stress-adapted fungi may better withstand harsh conditions of fire scars and other degraded areas, thereby providing substantial nutrient and water benefits to their host seedlings. Additionally, allowing seedlings to jumpstart fungal networks in the greenhouse, may bypass the initial carbon costs of establishing fungal partners in a field setting.
4.6 Limitations
The challenges to establishing bigcone Douglas-fir seedlings in burned sites are numerous. Among the hurdles to conducting this research were issues related to site selection, seedling propagation and preparation, and whether or how much to alter potential planting sites. The seedlings in this study were held in refrigeration for 4 months prior to outplanting. While this may be cause for concern for some, we expect that any mortality due to prolonged refrigeration would have been observable in the first few months after outplanting. Yet we saw almost no mortality prior to the first summer and therefore expect that any effect from refrigeration was small in comparison to field-experienced stresses.
4.7 Synthesis
Bigcone Douglas-fir is considered a drought adapted conifer (Lombardo et al., 2009). Across its range it occurs from within mixed conifer forests to mesic chaparral, and in some cases xeric open shrubland habitats. In more mesic chaparral sites, where chaparral is adjacent to or comprises the understory of bigcone stands, mature bigcones suffer very high mortality during fire (Parkinson et al., 2022). Hence restoration in those habitats makes little sense: conditions might appear to be more favorable initially due to somewhat more mesic conditions, but the ultimate fate of bigcone in chaparral vegetation is very poor due to the inevitability of stand-killing fires. By contrast, Parkinson et al. (2022) found that association with Q. chrysolepis and with more open understory increased the likelihood of at least partial stand survival during large scale fires. Stands that suffered some mortality during fire but are in these refugia (open understory, or associations with oaks) could be selected as targets for post-fire population enhancement through outplanting as done here. Because microsite manipulations are easy to conduct in the field and can dramatically improve survival, we recommend that the selection of outplanting sites be done to maximize both short term (adding protective microsites) and long term population survival (avoiding regions with greater potential for high fire intensity).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA859170.
Author contributions
CD’A, NM, and RO designed the experiment. GR led the planting, data collection, molecular analyses, and conducted all data processing and statistical analyses. SP and GR designed the statistical analyses. GR, RO, CD’A, and NM contributed to manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Fish and Wildlife Foundation (Grant ID:.19.064532).
Acknowledgments
We thank Ryan Fass, Ashley Grupenhoff, and Anne-Marie Parkinson for their efforts in planting these seedlings as well as Nicholas Saglimbeni for his assistance with field measurements and lab work on this project. We are also grateful to Aaron Ramirez, Max Moritz, and Holly Moeller for their insights in experimental design and methods. We thank to Hanna Yang at Aytch Wye Studio for designing and illustrating Figure 2 as well as to Shane Dewees for intellectual insights and his assistance on Figure 1. We also acknowledged the use of the Biological Nanostructures Laboratory within the California NanoSystems Institute, supported by the University of California, Santa Barbara and the University of California, Office of the President.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.995487/full#supplementary-material
References
Allen, C. D., Breshears, D. D., and McDowell, N. G. (2015). On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:art129. doi: 10.1890/ES15-00203.1
Bingham, M. A., and Simard, S. W. (2011). Do mycorrhizal network benefits to survival and growth of interior Douglas-fir seedlings increase with soil moisture stress? Ecol. Evol. 1, 306–316. doi: 10.1002/ece3.24
Breshears, D. D., Cobb, N. S., Rich, P. M., Price, K. P., Allen, C. D., Balice, R. G., et al. (2005). Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. U.S.A. 102, 15144–15148. doi: 10.1073/pnas.0505734102
Bui, A., Orr, D., Lepori-Bui, M., Konicek, K., Young, H. S., and Moeller, H. V. (2020). Soil fungal community composition and functional similarity shift across distinct climatic conditions. FEMS Microbiol. Ecol. 96:fiaa193. doi: 10.1093/femsec/fiaa193
Bustamante, M. M. C., Silva, J. S., Scariot, A., Sampaio, A. B., Mascia, D. L., Garcia, E., et al. (2019). Ecological restoration as a strategy for mitigating and adapting to climate change: lessons and challenges from Brazil. Mitig. Adapt. Strateg. Glob. Change 24, 1249–1270. doi: 10.1007/s11027-018-9837-5
Caldwell, M. M., Dawson, T. E., and Richards, J. H. (1998). Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113, 151–161. doi: 10.1007/s004420050363
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Connell, J. H. (1971). On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dyn. Popul. 298:312.
Cruz-Alonso, V., Ruiz-Benito, P., Villar-Salvador, P., and Rey-Benayas, J. M. (2019). Long-term recovery of multifunctionality in Mediterranean forests depends on restoration strategy and forest type. J. Appl. Ecol. 56, 745–757. doi: 10.1111/1365-2664.13340
Farjon, A. (2011). IUCN Red List of Threatened Species: Pseudotsuga macrocarpa. IUCN Red List Threat. Available online at: https://www.iucnredlist.org/en (accessed April 22, 2022).
Frey, B. R., Ashton, M. S., McKenna, J. J., Ellum, D., and Finkral, A. (2007). Topographic and temporal patterns in tree seedling establishment, growth, and survival among masting species of southern New England mixed-deciduous forests. For. Ecol. Manag. 245, 54–63. doi: 10.1016/j.foreco.2007.03.069
Gelman, A., Carlin, J. B., Stern, H. S., and Rubin, D. B. (1995). Bayesian Data Analysis, 1st Edn. London: Chapman and Hall.
Gfbi consortium, B. S., Crowther, T. W., Liang, J., Van Nuland, M. E., Werner, G. D. A., et al. (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569, 404–408. doi: 10.1038/s41586-019-1128-0
Glassman, S. I., Levine, C. R., DiRocco, A. M., Battles, J. J., and Bruns, T. D. (2016). Ectomycorrhizal fungal spore bank recovery after a severe forest fire: some like it hot. ISME J. 10, 1228–1239. doi: 10.1038/ismej.2015.182
Grove, S., Saarman, N. P., Gilbert, G. S., Faircloth, B., Haubensak, K. A., and Parker, I. M. (2019). Ectomycorrhizas and tree seedling establishment are strongly influenced by forest edge proximity but not soil inoculum. Ecol. Appl. 29, 1–12. doi: 10.1002/eap.1867
Guiterman, C. H., Gregg, R. M., Marshall, L. A. E., Beckmann, J. J., van Mantgem, P. J., Falk, D. A., et al. (2022). Vegetation type conversion in the US Southwest: frontline observations and management responses. Fire Ecol. 18:6. doi: 10.1186/s42408-022-00131-w
Halofsky, J. E., Peterson, D. L., and Harvey, B. J. (2020). Changing wildfire, changing forests: the effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol. 16, 4. doi: 10.1186/s42408-019-0062-8
Harris, J. A., Hobbs, R. J., Higgs, E., and Aronson, J. (2006). Ecological restoration and global climate change. Restor. Ecol. 14, 170–176. doi: 10.1111/j.1526-100X.2006.00136.x
Höhl, M., Ahimbisibwe, V., Stanturf, J. A., Elsasser, P., Kleine, M., and Bolte, A. (2020). Forest landscape restoration—what generates failure and success? Forests 11:938. doi: 10.3390/f11090938
Holl, K. D., and Brancalion, P. H. S. (2020). Tree planting is not a simple solution. Science 368, 580–581. doi: 10.1126/science.aba8232
Howard, J. L. (1992). Pseudotsuga Macrocarpa. Available online at: https://www.fs.usda.gov/database/feis/plants/tree/psemac/all.html (accessed July 13, 2022).
Hurteau, M. D., Koch, G. W., and Hungate, B. A. (2008). Carbon protection and fire risk reduction: toward a full accounting of forest carbon offsets. Front. Ecol. Environ. 6, 493–498. doi: 10.1890/070187
Janzen, D. H. (1970). Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528. doi: 10.1086/282687
Juang, C. S., Williams, A. P., Abatzoglou, J. T., Balch, J. K., Hurteau, M. D., and Moritz, M. A. (2022). Rapid growth of large forest fires drives the exponential response of annual forest-fire area to aridity in the Western United States. Geophys. Res. Lett. 49:e2021GL097131. doi: 10.1029/2021GL097131
Kashian, D. M., Romme, W. H., Tinker, D. B., Turner, M. G., and Ryan, M. G. (2006). Carbon storage on landscapes with stand-replacing fires. BioScience 56, 598–606.
Kerhoulas, L., Polda, W., Kerhoulas, N., and Berrill, J.-P. (2020). Physiology and growth of douglas-fir and redwood seedlings planted after partial harvesting. Front. For. Glob. Change 3:49. doi: 10.3389/ffgc.2020.00049
Kibler, C. L., Parkinson, A.-M. L., Peterson, S. H., Roberts, D. A., D’Antonio, C. M., Meerdink, S. K., et al. (2019). Monitoring post-fire recovery of chaparral and conifer species using field surveys and landsat time series. Remote Sens. 11:2963. doi: 10.3390/rs11242963
Lombardo, K. J., Swetnam, T. W., Baisan, C. H., and Borchert, M. I. (2009). Using bigcone douglas-fir fire scars and tree rings to reconstruct interior chaparral fire history. Fire Ecol. 5, 35–56. doi: 10.4996/fireecology.0503035
Lu, N., Yu, M., Cui, M., Luo, Z., Feng, Y., Cao, S., et al. (2016). Effects of different ectomycorrhizal fungal inoculates on the growth of pinus tabulaeformis seedlings under greenhouse conditions. Forests 7:316. doi: 10.3390/f7120316
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12. doi: 10.14806/ej.17.1.200
Moeller, H. V., Dickie, I. A., Peltzer, D. A., and Fukami, T. (2016). Hierarchical neighbor effects on mycorrhizal community structure and function. Ecol. Evol. 6, 5416–5430. doi: 10.1002/ece3.2299
Moeller, H. V., Peay, K. G., and Fukami, T. (2014). Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol. Ecol. 87, 797–806. doi: 10.1111/1574-6941.12265
Moritz, M. A., Parisien, M.-A., Batllori, E., Krawchuk, M. A., Van Dorn, J., Ganz, D. J., et al. (2012). Climate change and disruptions to global fire activity. Ecosphere 3:art49. doi: 10.1890/ES11-00345.1
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., et al. (2016). FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/j.funeco.2015.06.006
Nilsson, R. H., Larsson, K.-H., Taylor, A. F. S., Bengtsson-Palme, J., Jeppesen, T. S., Schigel, D., et al. (2019). The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. doi: 10.1093/nar/gky1022
Nolan, M., Dewees, S., and Ma Lucero, S. (2021). Identifying effective restoration approaches to maximize plant establishment in California grasslands through a meta-analysis. Restor. Ecol. 29:e13370.
O’Brien, M. J., and Escudero, A. (2022). Topography in tropical forests enhances growth and survival differences within and among species via water availability and biotic interactions. Funct. Ecol. 36, 686–698. doi: 10.1111/1365-2435.13977
Oksanen, J., Kindt, R., Legendre, P., O’Hara, B., Simpson, G. L., Solymos, P., et al. (2008). vegan: Community Ecology Package. Available online at: http://vegan.r-forge.r-project.org/ (accessed July 13, 2022).
Parkinson, A.-M. L., D’Antonio, C. M., and Moritz, M. A. (2022). Influence of topography, vegetation, weather, and climate on Big-cone Douglas-Fir fire refugia and high fire-induced mortality after two large mixed-severity wildfires. Front. For. Glob. Change 5:995537. doi: 10.3389/ffgc.2022.995537
Pulido-Chavez, M. F., Alvarado, E. C., DeLuca, T. H., Edmonds, R. L., and Glassman, S. I. (2021). High-severity wildfire reduces richness and alters composition of ectomycorrhizal fungi in low-severity adapted ponderosa pine forests. For. Ecol. Manag. 485:118923. doi: 10.1016/j.foreco.2021.118923
Putnam, A. E., and Broecker, W. S. (2017). Human-induced changes in the distribution of rainfall. Sci. Adv. 3:e1600871. doi: 10.1126/sciadv.1600871
Rodolf, P., DeGuzman, M., Post-Leone, P., and de Mello-Folson, J. (2019). Facilitation of Bigcone Douglas-Fir Seedling Recruitment by a Co-Occurring Species Canyon Live Oak. Paris: European Space Agency.
Rodríguez-Uña, A., Cruz-Alonso, V., Rohrer, Z., and Martínez-Baroja, L. (2020). Fresh perspectives for classic forest restoration challenges. Restor. Ecol. 28, 12–15. doi: 10.1111/rec.13093
Stan Development Team (2022). RStan: the R Interface to Stan. Available online at: https://mc-stan.org/ (accessed July 13, 2022).
Stephens, S. L., Westerling, A. L., Hurteau, M. D., Peery, M. Z., Schultz, C. A., and Thompson, S. (2020). Fire and climate change: conserving seasonally dry forests is still possible. Front. Ecol. Environ. 18, 354–360. doi: 10.1002/fee.2218
Suding, K., Higgs, E., Palmer, M., Callicott, J. B., Anderson, C. B., Baker, M., et al. (2015). Committing to ecological restoration. Science 348, 638–640. doi: 10.1126/science.aaa4216
Teste, F. P., Schmidt, M. G., Berch, S. M., Bulmer, C., and Egger, K. N. (2004). Effects of ectomycorrhizal inoculants on survival and growth of interior Douglas-fir seedlings on reforestation sites and partially rehabilitated landings. Can. J. For. Res. 34, 2074–2088. doi: 10.1139/x04-083
Toju, H., Tanabe, A. S., Yamamoto, S., and Sato, H. (2012). High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS One 7:e40863. doi: 10.1371/journal.pone.0040863
van der Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., et al. (2013). Plant-soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Keywords: restoration, fire, soil inoculation, ecological restoration, reforestation
Citation: Runte GC, Oono R, Molinari NA, Proulx SR and D’Antonio CM (2022) Restoring bigcone Douglas-fir post-fire in drought-stricken Southern California: Assessing the effects of site choice and outplanting strategies. Front. For. Glob. Change 5:995487. doi: 10.3389/ffgc.2022.995487
Received: 15 July 2022; Accepted: 30 November 2022;
Published: 15 December 2022.
Edited by:
Andres Holz, Portland State University, United StatesReviewed by:
Xiuwei Wang, Northeast Forestry University, ChinaFilip Jovanović, Institute of Forestry, Belgrade, Serbia
Copyright © 2022 Runte, Oono, Molinari, Proulx and D’Antonio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel C. Runte, Z2FiZS5ydW50ZUBsaWZlc2NpLnVjc2IuZWR1
 Gabriel C. Runte
Gabriel C. Runte Ryoko Oono1
Ryoko Oono1