
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 30 June 2022
Sec. Forest Management
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.937404
This article is part of the Research TopicVanishing old-growth forests: What are their roles and values for society under global change?View all 7 articles
In the last century, a synchronous beech expansion has been observed for many mixed mountain forests in southeastern Europe. This change is associated with the interaction of various disturbances. We analyzed structural changes in the Pecka old-growth forest in Slovenia during the last century, using several inventories of the tree layer, regeneration, and site factors. Throughout the observation period, the density of silver fir in the regeneration layer and in the overstory steadily decreased. In 1893, silver fir accounted for about 60% of the growing stock, whereas in 2013 it accounted for less than 13%. This is likely because of silver fir’s decline in the canopy layer due to air pollution, successive windthrows, and overbrowsing. However, climate change may also have played an important role, as silver fir also declined in southeastern European old-growth forests where air pollution was less pronounced and ungulate densities were low. A gradual decline of silver fir in the overstory resulted in a decrease of overall tree density to 231 trees ha–1, while growing stock remained relatively high at 712 m3 ha–1. Median diffuse light at 1.3 m was 3.7% and regeneration density was 19,954 ha–1. Beech was dominant (94%), followed by silver fir (4%), and sycamore maple (2%). No silver fir seedlings larger than 0.2 m were recorded. Silver fir, sycamore maple, and beech regeneration showed 87, 76, and 45% browsing damage, respectively. Regression models indicated some evidence of niche partitioning between silver fir and beech. However, many processes may be masked by the silver fir’s avoidance strategy. Given current red deer densities, climate change, and existing forest structure, the Pecka old-growth forest will likely reach an alternative stable state dominated by beech in a few decades. This calls for immediate reduction of ungulate populations. Despite the interaction of multiple disturbances, the Pecka old-growth forest has maintained a relatively high overall growing stock, a favorable microclimate, and succession pathway with shade-tolerant beech. This indicates the intrinsic resilience of natural forests. The mechanisms discussed here can be applied to the future governance of old-growth and managed montane mixed forests.
Angiosperms are generally more adaptable to environmental changes than gymnosperms, which occasionally gives them a competitive advantage. This is one of the reasons for the significant decline of gymnosperms since the Mid-Cretaceous period (Condamine et al., 2020) and for the exacerbation of this decline in the era of modern environmental change (McDowell and Allen, 2015). Among the conifers of the temperate zone of Europe, silver fir (Abies alba Mill.) is particularly sensitive to environmental change. It is susceptible to fluctuations in forest microclimate, especially to drought and to an abrupt increase in solar radiation, as well as to polluted air (Elling et al., 2009; Cater and Diaci, 2017). At the same time, silver fir is highly palatable to deer and very sensitive to browsing, as it grows slowly, preferably in the shade, and recovers poorly from browsing damage (Eiberle and Nigg, 1987; Gill, 1992; Motta, 1996). Beech (Fagus sylvatica L.), the competing species in mixed montane forests, is a less preferred browse species and can also survive heavy browsing for many years without significant mortality (Harmer, 2001; Rozenbergar and Diaci, 2014).
Numerous studies have reported a decline of silver fir due to the factors mentioned above in both managed (Ficko et al., 2011; Linares and Camarero, 2012; Keren et al., 2017) and old-growth forests (Firm et al., 2009; Vrska et al., 2009; Diaci et al., 2011). The decline of silver fir in southeastern Europe has been observed for several decades. It started before industrial air pollution was widespread, with the first more pronounced droughts in the 1920s and 1950s (Safar, 1951; Keren et al., 2014), and spread after the 1960s with increasing SO2 emissions and climate change (Mlinsek, 1964). After the reduction of industrial pollution (Amann et al., 2013), the health of silver fir improved (Dobrowolska, 1998; Büntgen et al., 2014); however, there have been a number of reports of growth declines during drought years, especially at the edge of its range (Linares and Camarero, 2012; Cailleret et al., 2014). In the era of recent climate change, silver fir is becoming increasingly important due to its resistance to drought and warming, special ecological niche and associated impacts on energy flow and matter cycling, as well as the fact that many organisms are dependent on it (Dobrowolska et al., 2017; Vitali et al., 2017; Vitasse et al., 2019). It is also a desirable species in mountain protection forests (Ott et al., 1997; Kupferschmid and Bugmann, 2013) and valuable in the wood supply chain as an alternative to the increasingly endangered spruce (Bouriaud and Popa, 2009; Vitali et al., 2017).
Dinaric fir-beech forests cover large areas in southeastern Europe (Boncina et al., 2014). Silver fir and beech have coexisted on these sites for millennia because they are fairly balanced in competition, but each occupies a slightly different ecological niche (Horvat et al., 1974; Matic, 1983). In addition to being sensitive to overbrowsing and air pollution, silver fir is also limited on microsites with abundant ground vegetation (Diaci, 2002; Albanesi et al., 2005; Dobrowolska et al., 2017). It can develop better on cooler moesic sites on siliceous parent material or on rockier microsites on calcareous parent material and sometimes on woody debris (Puncer, 1980; Orman and Szewczyk, 2015; Dobrowolska et al., 2017). Numerous studies have been conducted on the regeneration of Dinaric fir-beech old-growth forests, partially confirming the differences in regeneration niches (Mlinsek, 1967; Mayer and Neumann, 1981; Leibundgut, 1987; Nagel et al., 2014); however, these differences are often blurred due to the predominant influence of overbrowsing (Rozenbergar et al., 2007). In most cases, one-time surveys predominate, while repeated measurements of regeneration in old-growth forests are rare.
In an era of rapid environmental change, there is a high risk of cascading effects of interacting natural and anthropogenic disturbances that could lead to abrupt irreversible changes in forest structure and mixture and thus ecosystem functioning (Buma, 2015). The changes in vegetation may be so pronounced that intensive and costly restoration efforts will be required to return to the baseline condition. In this case, the ecosystem approaches an alternative stable state (Stromayer and Warren, 1997). Because of the longevity of trees and the need to exclude the confounding effects of management on forest structure, long-term studies of old-growth forests are needed to verify whether the ecosystem is approaching an alternative stable state. This type of research is rare because of the general lack of old-growth forests and dearth of long-term studies.
Thanks to a tradition of forest management planning that goes back more than a century, detailed long-term data on the stand structure and regeneration of the Pecka old-growth forest are available (Hufnagel, 1893). This makes it possible to gain a long-term insight into forest developmental dynamics and to predict future trends.
The objectives of the research were (1) to analyze the long-term dynamics of stand structure and regeneration and their relationships, (2) to examine the relationship between regeneration and ecological factors, especially light climate, and browsing, (3) to verify whether the Pecka old-growth forest is already approaching a beech-dominated alternative stable state, and (4) to propose guidelines for the governance of similar old-growth and managed montane forests in the Dinaric Mountains. Considering the numerous natural and anthropogenic disturbances that have occurred in the Pecka old-growth forest, we hypothesized that the growing stock would be significantly reduced, that light conditions on the forest floor would improve, and that pioneer and semi-light-demanding tree species would be represented in the regeneration. We also hypothesized that the risk of complete silver fir decline was reduced due to improved air quality and increased culling of ungulates.
The study was conducted in the Pecka Forest Reserve, which covers 60.2 ha and is one of the best-preserved and largest old-growth forest remnants in Slovenia. It is located within a large forest complex that is managed using a combination of selection and irregular shelterwood silvicultural systems (Boncina, 2011). The reserve is located on a karst plateau on the northeast edge of Kocevski Rog, at 800–910 m above sea level. Annual precipitation is about 1,500 mm and average annual temperature is between 6 and 7°C (Marincek and Marinsek, 2004). The predominant bedrock is limestone, on which well-drained rendzic leptosols and calcareous cambisols of varying depth have developed. The soil depth and macro- and micro-topography are highly variable, significantly altering site conditions over a very small area. In addition, karst phenomena such as sinkholes and rock outcrops at or near the surface are common. Forest sites in the reserve are classified as Omphalodo-Fagetum association according to Braun-Blanquet’s nomenclature (Marincek and Marinsek, 2004). Silver fir and beech dominate the upper tree layer of the reserve, accounting for 99.5% of the total tree cover. Other less common species include Norway spruce [Picea abies (L.) Karst.], sycamore maple (Acer pseudoplatanus L.), and mountain elm (Ulmus glabra Huds.). There has never been any major anthropogenic impact in the reserve, and it has been protected since 1893 (Hufnagel, 1893).
This study took into account nine tree inventories of the entire reserve. In 1893, trees were analyzed in an area twice the size of the present forest reserve, which was reduced compared to its original size; therefore, we present data only on the ratio of silver fir to beech (Hufnagel, 1893; Boncina et al., 2002, 2003). In the subsequent inventories of 1953, 1963, 1973, 1980, 1982, 1994, 2003, and 2013, all trees with a diameter at a breast height (DBH) greater than 10 cm in the entire old-growth forest were inventoried by tree species.
Systematic sampling of the regeneration started when a decline in silver fir regeneration was observed in the reserve (Mlinsek, 1967); thus, most of the years in which the regeneration was sampled differed from those of the stand structure inventories. The regeneration was sampled in 1963, 1980, 1988, 1992, 1995, 2007, and 2013. For the first inventory, three 2-m-wide transects were established and fully inventoried, twice along the maximum length of the old-growth forest and once along its maximum width (Mlinsek, 1967). All subsequent sampling was carried out on 2 × 2 m sample plots laid out on three transects along the maximum length of the old-growth forest. The distance between transects was 100 and 21 m between plot centers along transects (Turk et al., 1985; Debeljak, 1997). The regeneration of each tree species was categorized into different age/height classes and counted (Table 1).
The regeneration was assessed using a three-point scale for browsing damage (1—limited: < 10% damage and non-browsed terminal shoots, 2—moderate: 10–50%, 3—severe: > 50% damage). In 2007, tree regeneration coverage per species from the forest floor to a height of 0.2 m (small seedling coverage), from 0.2 to 1.3 m (larger seedling coverage), and from 1.3 to 2.5 m (small sapling coverage) were estimated on the regeneration plots (Table 1). In the same year, total, diffuse and direct light were assessed at a height of 0.2, 1.3, and 5.0 m using a digital fisheye camera (sensu Rozenbergar et al., 2011). Additionally, total ground vegetation, rock and coarse woody debris (CWD) coverage, and plot inclination were estimated to the nearest one percent. The basal area of the stand surrounding the regeneration plot was determined using the Bitterlich Relascope method. Developmental phases were mapped during three inventories using the methodology of Leibundgut (1987), throughout the old-growth stand in 1980, and by sampling the area surrounding the regeneration plots in 1995 and 2007. For a detailed description of the developmental phases and method, see Boncina (2000).
Data were analyzed in Microsoft Excel version 2019 and R version 3.6.1 (R Core Team, 2019). To compare the ecological niches of silver fir and beech, we developed statistical models for predicting regeneration success. Only the 2007 inventory data were used for model calculations because we measured most ecological factors in that year and because the low silver fir densities were not sufficient for modeling the 2013 inventory. Regeneration density is not a suitable indicator for comparing different developmental stages of regeneration due to a decrease in density with increasing height; therefore, we focused on regeneration coverage. For beech, we compared and modeled coverage at three regeneration height layers. The densities and coverage of silver fir were extremely low; therefore, we converted the coverage data to binomial form (presence/absence) and used logistic regression. Still, all inventoried silver fir regeneration fell within the category lower than 0.2 m and is thus comparable with the same category of beech. In the models, we used all assessed ecological factors described in section “Sampling Design and Recordings” as explanatory variables (light components; ground vegetation, rock and CWD coverage; plot inclination; basal area; developmental phase: optimal vs. other), including the coverage of the higher and lower regeneration layers than the layer analyzed. One-year-old seedlings were not included in the modeling analyses. The models were built using a series of generalized linear models (GLM) following standard protocols described by Zuur et al. (2009). When necessary, variables were transformed using the common logarithm function or the arcsine function to meet the assumptions of normality and linearity. Models were developed with the drop1 function. For model diagnostics, we analyzed the residuals using graphical summaries (Robinson and Hamann, 2011).
In the last 60 years, growing stock decreased from 940 to 710 m3 ha–1 (Figure 1.1). This was due to the continuous decline of silver fir in the growing stock, which was not fully offset by the increase of beech. The greatest decline of silver fir occurred in the 1980s. In the last 120 years, the proportion of beech in the growing stock increased and the proportion of silver fir decreased by 45 percentage points (Figure 1.2). From 1980 to 2007, the proportion of the optimal developmental phase decreased from 75 to 25% (Figure 1.3). In the first period (1980–1995) a transition from the optimal to the selection and terminal phase was recorded, while in the second period (1995–2007) the share of the initial phase increased at the expense of the other three phases. This is likely related to the second windthrow in 2004 and a further decline of silver fir that typically characterizes the selection phase.
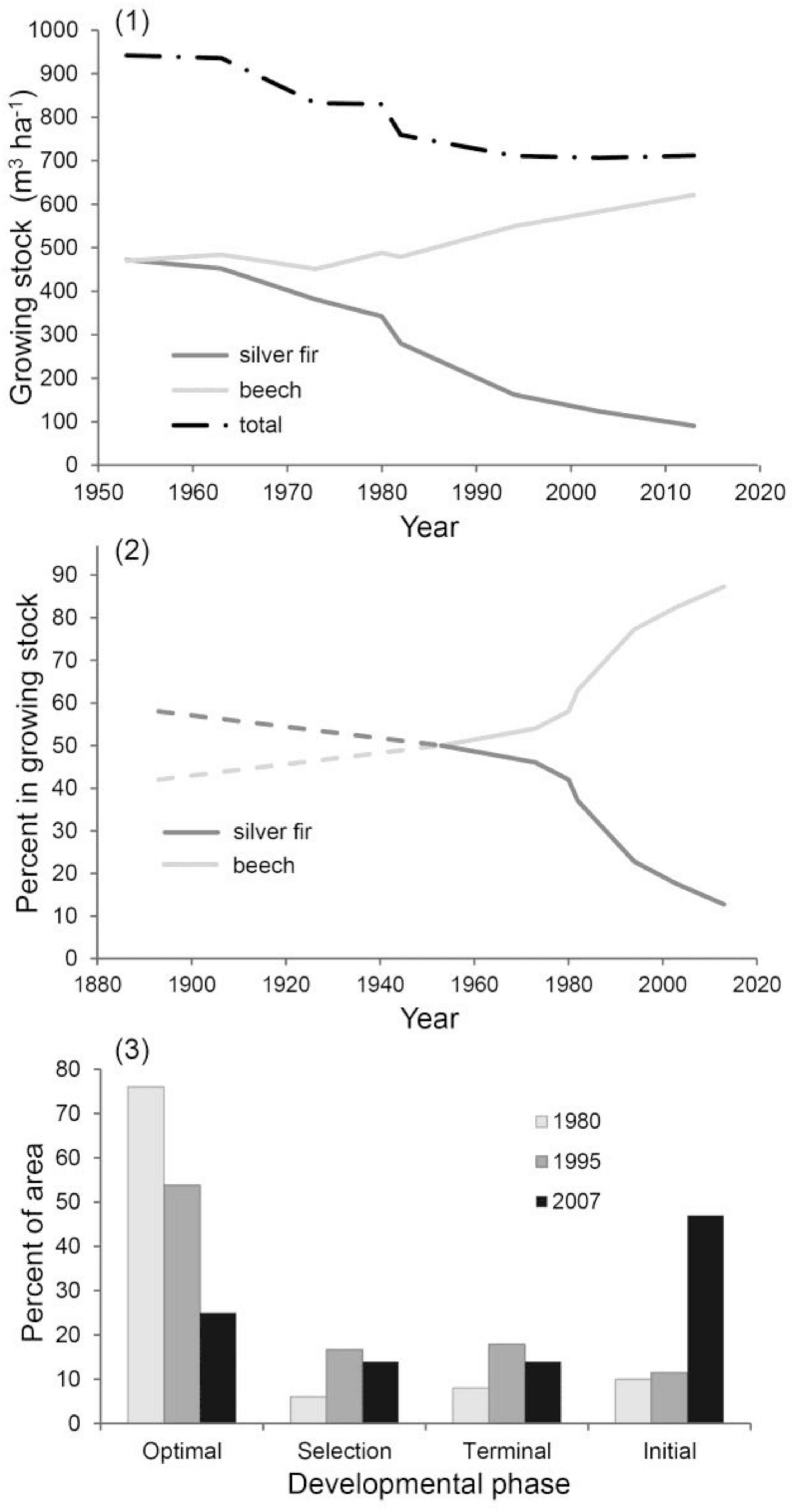
Figure 1. (1) Dynamics and structure of growing stock in the last half century, (2) decrease in the proportion of silver fir in the growing stock over a 120-year period and (3) percentage of forest area per developmental phase.
In the period 1953–2013, the cumulative distribution of tree densities by DBH class showed a decrease in the first DBH class by 1994, followed by an increase in subsequent years. In contrast, the density of medium diameter trees (up to 50 cm DBH) decreased. Tree density in the larger DBH classes remained relatively stable, while density of the largest trees decreased. In the last 40 years, the total tree density decreased from 294 to 231 trees per hectare, or 22% (Figure 2.1). Most of this was due to the decline in silver fir of 68 trees ha–1, which was only partially offset by the increase in beech of 3 trees ha–1. The recorded decline of silver fir began in the 1960s and was most pronounced in the small and medium diameter classes. During the period 1980–1994 trees > 75 cm DBH were strongly affected by the windthrow of 1983, while earlier (1960s) and latter (2003) windthrow events seemingly had a rather low impact on the large overstory trees (Figure 2.2). The most recent inventory showed a sharp decline in silver fir density within the first diameter class, most likely due to persistent overbrowsing. During the same period, beech distribution shifted toward larger diameter classes (Figure 2.3); however, the number of trees in the range of 20–50 cm in DBH decreased. The curves for cumulative and species-specific DBH distribution followed a rotated sigmoid shape when plotted on a semi-logarithmic scale (logN) (graphs not shown). In the old-growth forest of Pecka, the decrease in tree density following the disturbances has not yet recovered, while the growing stock remained relatively stable. This means that there are fewer trees with larger trunks after all the above-mentioned disturbances.
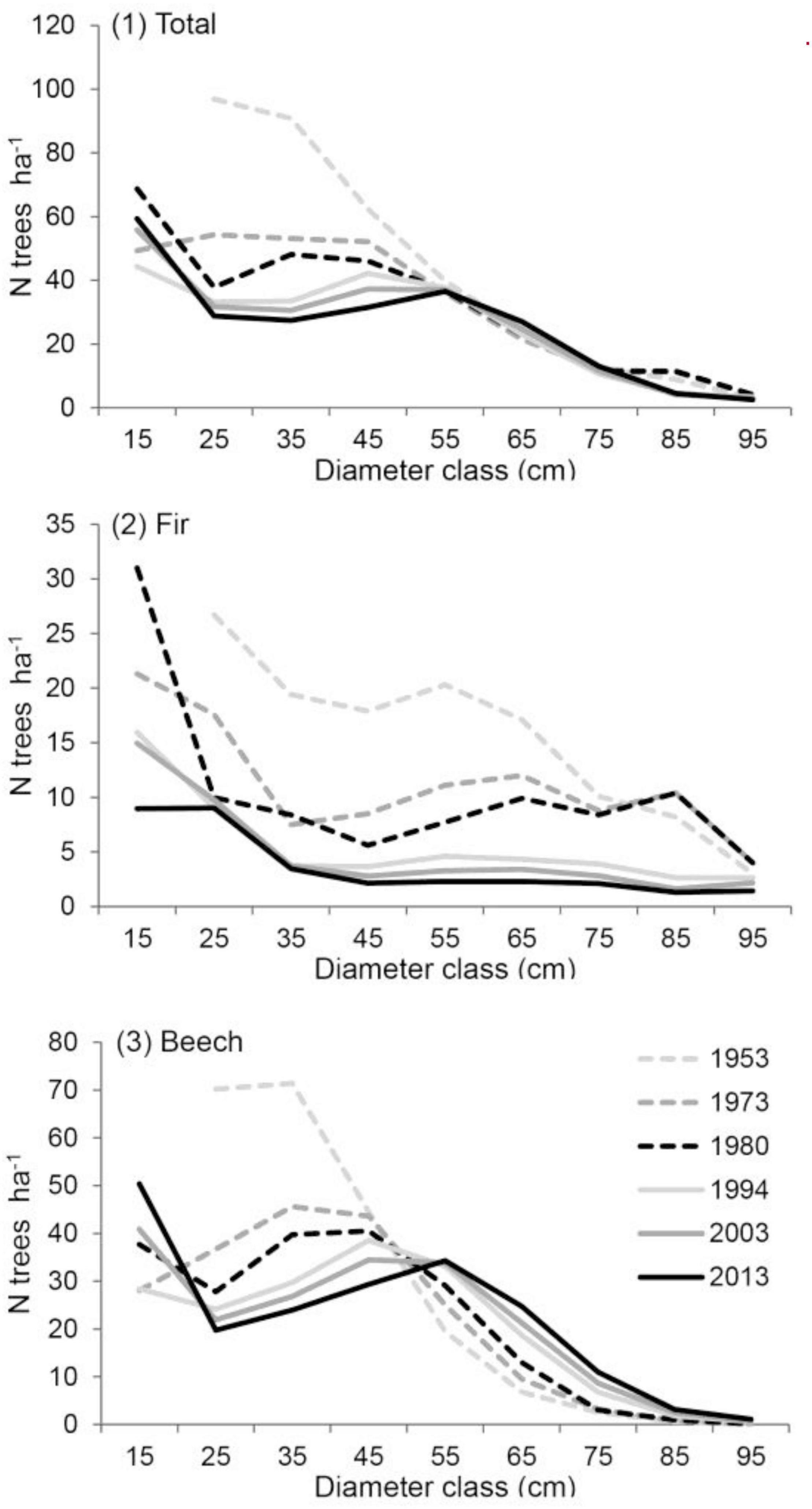
Figure 2. Development of the distribution of the number of trees by DBH class in the Pecka old-growth forest. (1) total, (2) silver fir, (3) beech.
Since the first measurement in 1963, the total density of regeneration, including 1-year-old seedlings, mostly ranged between 23,000 and 51,000 ha–1. The highest values were reached in 1980, while densities decreased after 1988 (Figure 3.1). Beech densities were significantly higher than those of silver fir in all measurements. For example, the total densities of beech and silver fir were 44,100 and 3,182 ha–1, respectively, in 1963 and 12,576 and 412, respectively, in 2013. From 1980 to 2013, the density of beech seedlings decreased due to recruitment to higher regeneration classes and self-thinning (Figure 3.2). The proportion of silver fir in the class up to 20 cm varied between 55 and 97% in the first three measurements and decreased to 7 and 43% after 1992. The proportion of silver fir taller than 21 cm was 22% in 1988 and has not exceeded 8% since, being particularly low after 1995 (Figure 3.3).
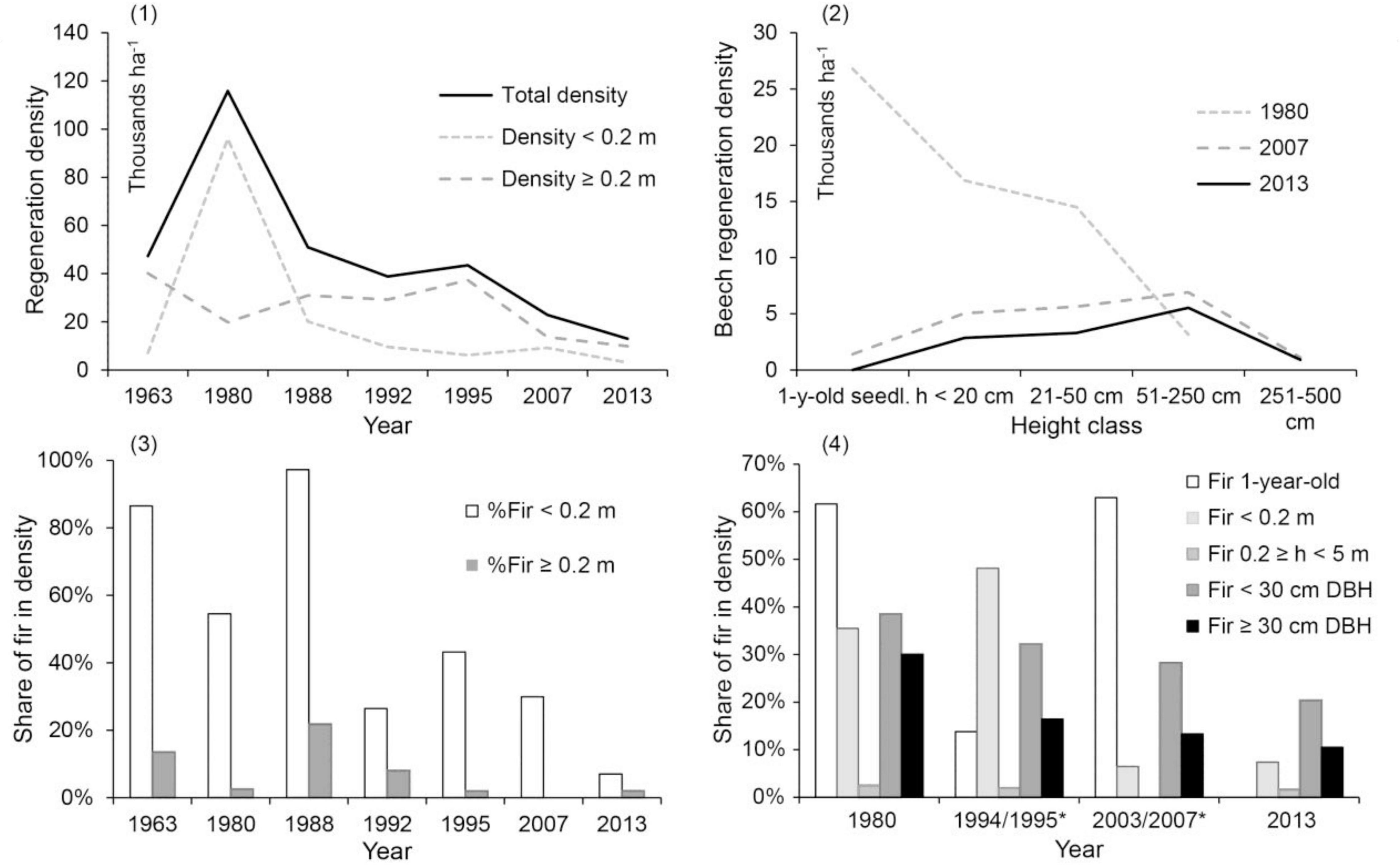
Figure 3. (1) Trends in total regeneration densities by year. (2) Changes in beech regeneration density per height class between years. (3) Trends in the proportion of silver fir per height class. (4) Comparison of the proportion of silver fir in the total tree density in three developmental stages of regeneration and in two extended DBH classes. *For the 1994 and 2003 columns, regeneration data originates from 1995 and 2007, respectively. Graphs (1) and (3) include 1-year-old seedlings within the small seedling category (h < 0.2 m).
To infer future trends of silver fir development, its proportion in successive regeneration height and DBH classes are shown (Figure 3.4). Declines were observed over time in all developmental stages of the silver fir population. This is most evident in the developmental stage between 0.2 and 5.0 m in height, where the chronic overbrowsing of recent decades is most directly reflected. With respect to other tree species, we also found sycamore maple and Norway spruce during the 2007 inventory. Their share in 1-year-old seedlings was 2%. Among smaller seedlings (h < 0.2 m), we recorded only sycamore maple with 9%, while among larger seedlings and saplings (0.2 ≤ h < 2.5 m), we did not find any species other than beech. In 2007, 87, 76, and 45% of silver fir, sycamore maple and beech regeneration (older than 1-year-old seedlings and h < 2.5 m), respectively, was damaged by browsing.
In 54 years and seven regeneration inventories, no silver fir taller than 0.5 m was found in the regeneration. Low overall silver fir density was noted as early as 1963 (Figure 4.1). The peak of silver fir seedling density occurred in the 1980s during the acute decline of silver fir. It was followed by a marked decline that continues to this day. In all inventories, at least 50% of silver fir seedlings were damaged by deer (Figure 4.2). Beech browsing damage was assessed only in the last two inventories. In 2007, the percentages of undamaged, moderately browsed and severely browsed beech seedlings were 56, 33, and 11%, respectively; in 2013, the percentages were 49, 35, and 16%, respectively.
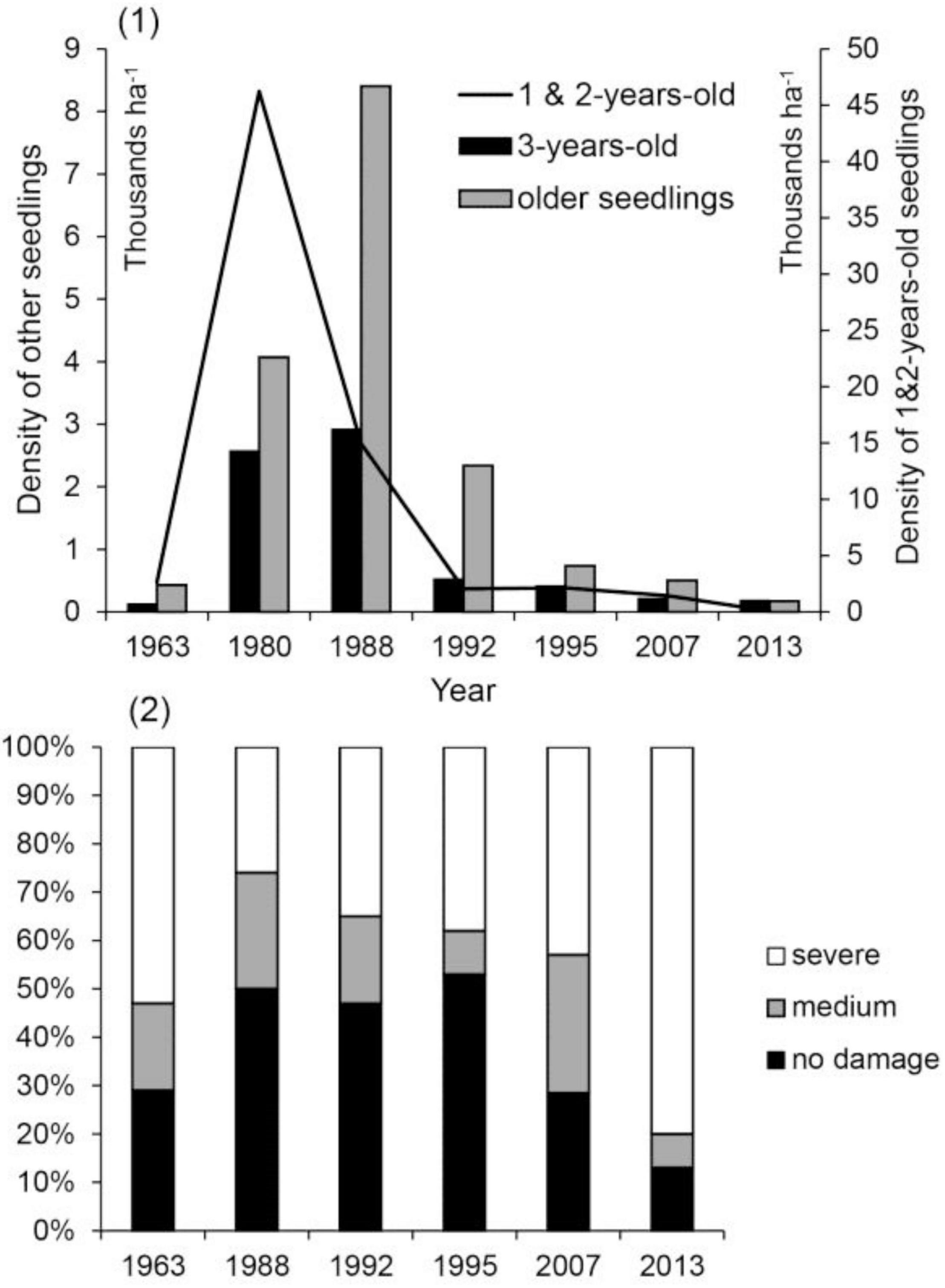
Figure 4. (1) Dynamics of silver fir regeneration density by age class and (2) browsing damage to small silver fir seedlings by year.
In 2007, the average density and cover of small silver fir seedlings (h < 0.2 m) were 351 ha–1 and 0.1%, respectively, while the average density and cover of small beech seedlings was 5,046 ha–1 and 3.8%, respectively. While there were no larger silver fir seedlings, the density and coverage of beech seedlings (0.2 m ≤ h < 1.3 m) were 10,351 ha–1 and 15.5%, respectively, while small beech sapling density (1.3 m ≤ h < 2.5 m) and coverage were 2,180 ha–1 and 16.1%, respectively. Median diffuse light values at 0.2, 1.3, and 5.0 m were 3.6, 3.7, and 3.8%, respectively.
A binary logistic regression model for the presence of small silver fir seedlings, excluding ephemeral 1-year-old seedlings, indicated a positive association with beech sapling coverage (likelihood-ratio-test—LRT = 14.1), followed by total light at 1.3 m (LRT = 12.8) and developmental phase (LRT = 6.1), with higher density in the optimal phase. The best predictors for small beech seedling coverage (h < 0.2 m) were rock coverage (scaled Deviance—sD = 25.0) followed by beech basal area (sD = 23.4) and diffuse light at 0.2 m (sD = 14.0) (Table 2). The most important predictors for larger beech seedling coverage were rock coverage (sD = 7.2) followed by developmental phase (sD = 4.1) and diffuse light at 5 m (sD = 4.1) (Table 2 and Figure 5). The best predictors for beech sapling coverage were larger beech seedling coverage (sD = 35.4) followed by total basal area (sD = 20.7).
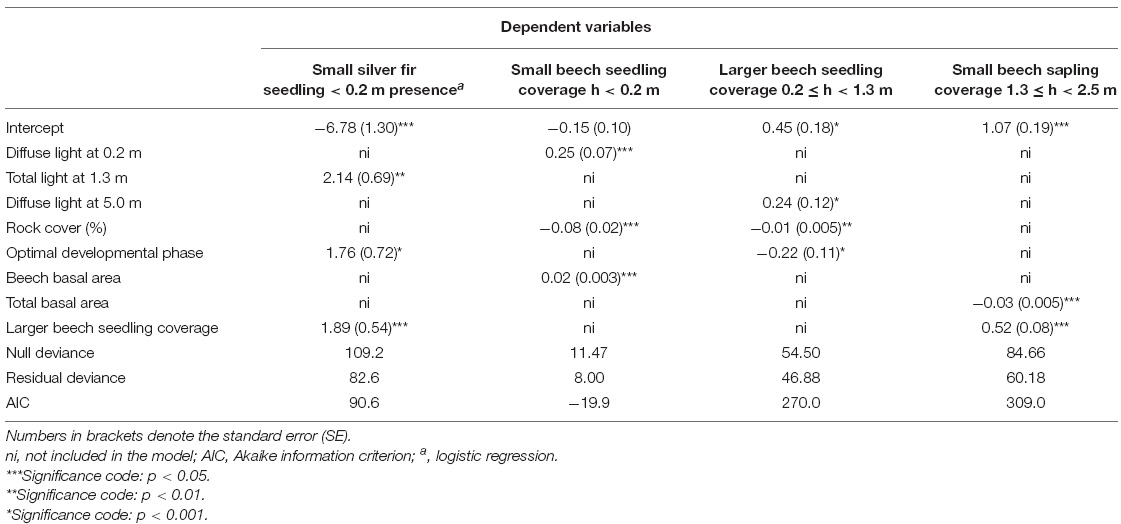
Table 2. Results of the GLM analysis for silver fir presence (h < 0.2 m) and beech seedling and sapling cover predicted by light components, rock cover, developmental phase, beech basal area, and beech seedling coverage for 164 regeneration plots.
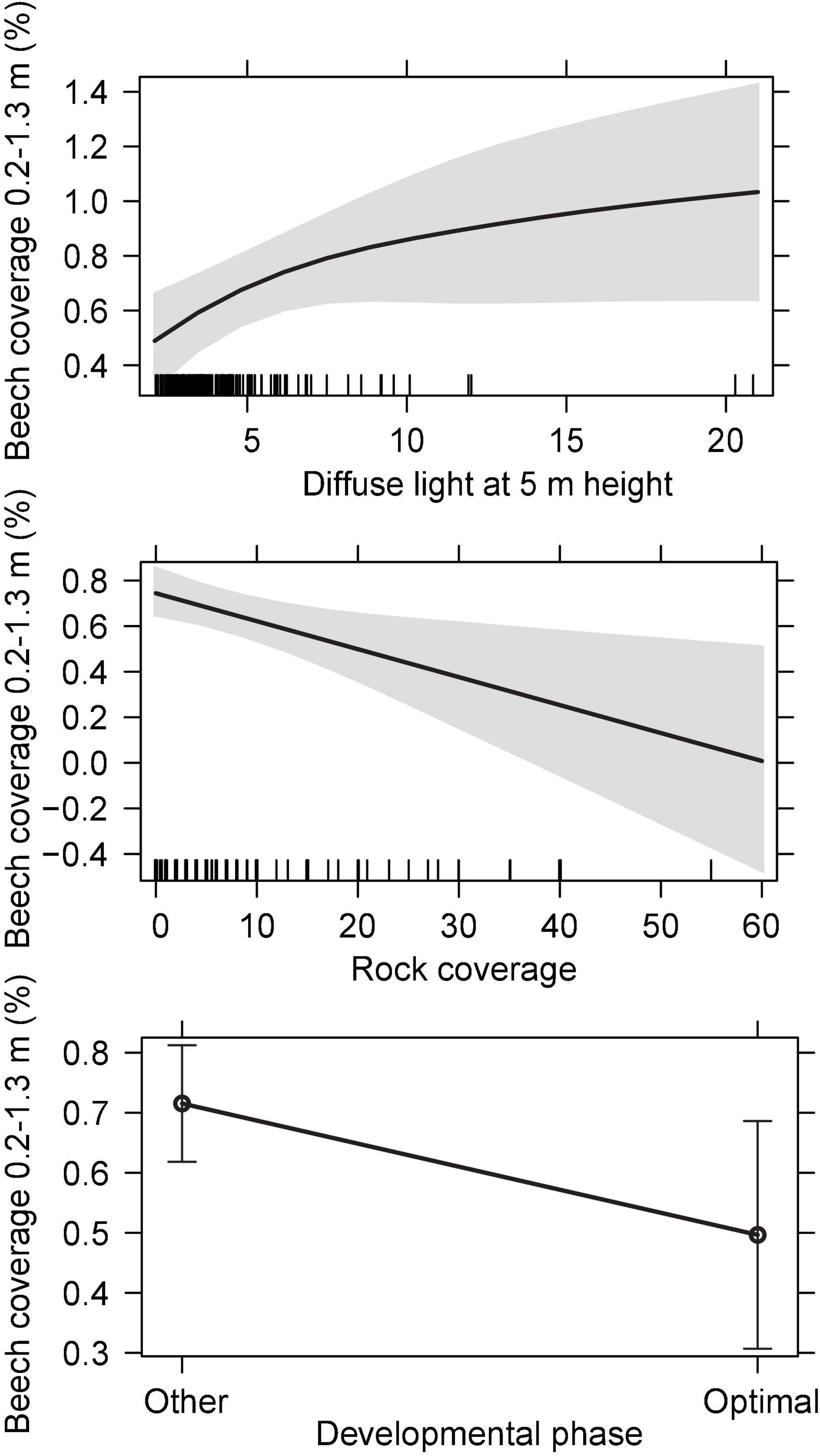
Figure 5. Effect display for significant factors in the regression model for the coverage of beech between 0.2 and 1.3 m height. The gray areas represent 95-percent confidence envelopes around the fitted effects.
Small silver fir seedling presence was associated with the optimal phase, while larger beech seedlings were associated with other developmental phases. Small beech seedling and sapling coverage were not associated with developmental phases. Small and larger beech seedling cover were negatively correlated with rockiness. Small silver fir seedlings were frequently present under beech regeneration. While the coverage of small beech seedlings indicated a positive relationship with the basal area of beech, the relationship between small beech sapling coverage and total basal area was negative, indicating a higher light requirement.
In the last 100 years, the tree species composition (DBH ≥ 10 cm) of the Pecka old-growth forest has changed drastically. The mixture has changed from the predominance of silver fir in the canopy layer to an almost complete dominance of beech. The decline of silver fir within the reserve has been documented over the last 60 years, and was particularly pronounced between the 1980s and mid-1990s. From the 1960s to the most recent inventory, growing stock and tree density declined, and the predominant optimal phase with a closed canopy changed to an initial phase with an open canopy. The main drivers of these changes have been natural and anthropogenic disturbances and their cascading effects. Three windthrows were identified in the archives and through dendroecological analyses between 1960 and 2004, primarily affecting the tallest firs (Leibundgut, 1987; Nagel et al., 2007). The 1983 and 2004 windthrows reduced total tree density by 9.3 and 4.8%, respectively (Nagel and Diaci, 2006). In addition to natural disturbances, the decline of silver fir during the last quarter of the twentieth century was significantly influenced by air pollution (Elling et al., 2009). The current total tree density of 231 tree ha–1 is only 68% of the average of several successive inventories of 15 silver fir-beech-Norway spruce old-growth forests in Slovenia, Croatia, Slovakia, and Bosnia and Herzegovina (Diaci et al., 2011).
The first reports of regional silver fir mortality in southeastern Europe date from the late 1920s (Safar, 1951). It occurred along the boundaries of fir’s natural range, where it was more pronounced on drier slopes. It was probably triggered by harsh winters and hot, dry summers. Silver fir decline has often been accompanied by bark beetle calamities. The intensification of silver fir dieback took place after the mid-1950s, when it also affected younger trees and spread to larger areas (Mlinsek, 1964). Several factors played a role (Krause et al., 1986); however, acute silver fir dieback in the 1970s and 1980s was primarily influenced by regional SO2 air pollution combined with climatic extremes (Elling et al., 2009). The Pecka old-growth forest is also close to the natural boundary of the silver fir-beech forest habitat toward the Subpannonian region, where silver fir is less competitive than beech due to lower precipitation and climatic extremes (Puncer, 1980).
Studies of similar mixed montane old-growth forests in Europe have also reported a decline in silver fir (Korpel, 1995; Vrska et al., 2009), although it has been somewhat less pronounced when compared to Pecka or other old-growth forests in Slovenia (Boncina et al., 2003; Nagel et al., 2015). The decline was significantly less pronounced elsewhere in southeastern Europe (Prpic and Seletkovic, 1996; Diaci et al., 2011; Boncina et al., 2014; Keren et al., 2014). This was probably due to the lower atmospheric load of SO2 and more frequent silicate soils that are more suitable for silver fir (Boncina et al., 2002; Dobrowolska et al., 2017). There, silver fir is probably more competitive with ground vegetation, and soil moisture conditions are better compared to those on limestone (Ott et al., 1997; Leuschner and Ellenberg, 2017). The silver fir population in Slovenia has also been affected by chronic overbrowsing (Diaci et al., 2010; Klopcic et al., 2010).
Despite the interplay of various disturbances, the Pecka old-growth forest has maintained a relatively high overall growing stock over the last century. In 2013, it was 712 m3 ha–1, which is slightly below 724 m3 ha–1, the average growing stock of repeated inventories of 15 silver fir-beech-Norway spruce old-growth forests (Diaci et al., 2011). The lowest recorded growing stock of 707 m3 ha–1 was measured in 2003. Trees remaining in the canopy layer after disturbance compensated for the reduction in competition by growing more intensively. Beech, in particular, is capable of rapid lateral canopy growth and the formation of large tree crowns. The high growing stock and rapid canopy closure indicated by the light measurements preserved the forest microclimate and thus the moesic old-growth forest (Morecroft et al., 1998; Aussenac, 2000). The understory light climate was comparable to measurements in similar old-growth forests in Slovenia (Rozenbergar et al., 2007; Diaci et al., 2012) and in temperate old-growth forests in general (Messier et al., 2009). Preserved canopy trees were also important as seed banks (Cremer et al., 2012). Other researchers have also reported continuously high levels of growing stock in mixed montane forests despite natural and anthropogenic disturbances (Leibundgut, 1987; Korpel, 1995).
The temporal dynamics of silver fir seedling density was probably associated with changes in the canopy layer and chronic overbrowsing. Low silver fir seedling densities at the first inventory in 1963 may have been due to competition from a well-developed layer of small- and medium-diameter trees in the sub-canopy layer at that time. The high densities during the 1980s may be associated with the decline in medium-diameter silver fir trees due to air pollution and increased light on the forest floor due to the 1983 windthrow (Nagel and Diaci, 2006; Diaci, 2011). The sharp decline afterward was probably due to the declining number of silver fir seed trees as a result of air pollution, the two windthrows and chronic overbrowsing (Nagel et al., 2015). A comparison of the regeneration density in 2007 with that of similar mixed montane old-growth forests shows that seedling (h < 0.5 m, excluding 1-year-old seedlings), sapling (0.5 ≤ h < 3–4 m) and small tree (8–10 ≤ DBH < 20 cm) densities at 10,686, 8,018, and 59 ha–1, respectively, amounted to 80, 225, and 39% of the averages for similar mixed montane forests (Diaci et al., 2011). The overabundance of the sapling development stage indicates a synchronous beech regeneration following silver fir canopy decline and two windthrows within the last 30 years. This is confirmed by previous dendroecological studies in the Pecka old-growth forest, which showed that regeneration in the sapling stage was between 8 and 30 years old and small diameter trees were between 50 and 140 years old (Nagel et al., 2006, 2007). According to anecdotal evidence, the extraordinary wave of beech regeneration was also the result of increased ungulate control, as their densities peaked in the early 1990s with almost 20 red deer km–1 (Nagel et al., 2015). A partially unexpected result was the increase in silver fir regeneration density in the 1980s. This may seem surprising since red deer densities during this period were relatively high (ca. 10 km–1). However, at that time, there was still a relatively high density of adult silver fir trees as potential seed trees in the canopy layer. In addition, the vast majority of seedlings were in the size class below 20 cm, where ground vegetation or snow cover protected the regeneration from overbrowsing. Therefore, there was a lag between ungulate density and regeneration density/damage. The subsequent decline in silver fir regeneration was probably due to the rapidly declining density of silver fir seed trees and the low vigor of many of them (Turk et al., 1985), as well as to browsing.
In addition to significant changes in the canopy structure and partially also in micro-sites (CWD, pit and mound topography) due to disturbances, we may hypothesize, based on similar research in the region, that it is very likely that the regeneration was significantly influenced by ungulates (Rozenbergar et al., 2007; Nagel et al., 2015). Browsing damage on beech and especially on silver fir regeneration was high. Lower browsing damage was reported for other central European old-growth forests (Leibundgut, 1987; Korpel, 1995), while browsing damage was significantly lower in the old-growth forests of southeastern Europe (Rozenbergar et al., 2007; Keren et al., 2014). Silver fir is extremely sensitive to browsing and is affected even by small injuries; therefore, the browsing index is not a reliable indicator of ungulate impact (Blossey et al., 2017). Deer densities are more important and indicate that overbrowsing has persisted in the region for at least 60 years (Nagel et al., 2015). Chronic overbrowsing was also indicated by the sharp decline of silver fir in the first diameter class (10 cm ≤ DBH < 20 cm) in Pecka during the most recent inventory. Silver firs with a DBH ≥ 10 cm are 30–100 years old (Ferlin, 2002), suggesting that they regenerated during a period of increased ungulate density that lasted more than half a century. Similar deviations from the shape of the rotated sigmoid for silver fir in the first DBH class were also observed in other old-growth forests in Slovenia with chronic overbrowsing, while such patterns were not detected in other old-growth forests in southeastern Europe (Diaci et al., 2011). However, accumulated with beech the shape of DBH distributions resembled rotated sigmoid. Population densities of red deer (Cervus elaphus L.) and roe deer (Capreolus L.) in the research area were extremely low immediately after World War II, increased rapidly in the 1960s, peaked in the early 1990s (about 20 red and 1 roe deer km–1) and declined to current levels of about 13 red and 1 roe deer km–1 due to intensified culling (Nagel et al., 2015).
Because of the reduction in canopy cover, we expected a higher proportion of light-demanding species. However, regeneration inventories did not confirm this; we observed only an increased proportion of 1-year-old seedlings of sycamore maple and Norway spruce and small sycamore maple seedlings (h < 20 cm). On the one hand, this was probably due to the strong response of advanced beech regeneration that rapidly occupied the available growing space from canopy opening and on the other hand, to selective overbrowsing by ungulates. Poor regeneration of light-demanding and intermediate species following disturbance has also been reported for similar beech-dominated and mixed montane old-growth forests (Marinsek and Diaci, 2004; Nagel et al., 2006, 2015).
Despite the high densities of deer populations, statistical models confirmed the expected complementarity of micro-site niches for silver fir and beech, e.g., the association of silver fir seedlings with the optimal phase and vice versa for beech and the negative association of small and larger beech seedlings with rock cover. Similar results have been reported by other researchers (Gessler et al., 2004; Vacek et al., 2015; Dobrowolska et al., 2017; Diaci et al., 2020). The association of silver fir seedlings with larger beech seedlings is somewhat unexpected. This is likely due to overbrowsing because silver fir seedlings are harder to notice among larger beech seedlings, i.e., a variant of the avoidance strategy (Danell et al., 2006). Ground vegetation was not a negative factor for regeneration as has often been reported (Rozenbergar et al., 2007; Dobrowolska et al., 2017). This is probably related to the low light levels in the understory and the low water holding capacity of soils on limestone. All developmental stages of regeneration were at least indirectly positively associated with higher light levels; for example, small beech sapling density was negatively related with total basal area. This suggests that light is a resource that is in short supply in this type of old-growth forest. We did not find large differences in the light environment at different heights in the understory. It also appears that microsite conditions (light climate, microrelief, CWD) were less important than the overall conditions in the middle and upper stand layers.
The results of this study indicate a rapid decline of silver fir in both the regeneration and canopy layers. Although several factors have contributed, it is likely that overbrowsing is significantly hindering fir’s recruitment, similar to other Slovenian mixed mountain forests (Diaci et al., 2010; Nagel et al., 2015). The question is how much time is left for interventions in deer populations to allow a return to the predicted vegetation community based on the prevailing ecological conditions, i.e., a montane mixed forest of silver fir and beech (Stromayer and Warren, 1997). One of the ways to answer this question is to analyze the remaining number of seed trees present and the trend of their decline. On average, there are still 33 silver firs ha–1 with DBH greater than 10 cm or 24 ha–1 trees with DBH greater than 20 cm that can produce seeds. The latter are on average more than 150 years old in the Pecka old-growth forest (Nagel et al., 2007). Based on observations, we estimate that most silver firs with DBH < 20 cm in montane mixed old-growth forests are under canopy and stressed and do not produce seeds.
The number of seed trees of silver fir should still be sufficient to support seeding and long-term recovery, assuming immediate reductions in wild ungulate densities. However, given the average yearly rate of silver fir decline of 1.7 trees ha–1 over the last 40 years, the complete loss of silver fir can be expected within 10–30 years, depending on natural disturbances. This means that the ecosystem is rapidly approaching an alternative stable state, with problems due to inbreeding occurring even sooner (Piovani et al., 2010). Without silver fir seed trees, the return to a mixed forest will be extremely difficult, especially since active management interventions such as planting silver fir are not allowed in old-growth forests for the sake of their integrity. A similar fate awaits managed montane mixed forests and many similar forests in central Europe unless ungulate populations are reduced immediately. Nevertheless, the situation in Slovenian and southeastern European managed mixed forests is better than that in old-growth forests, probably due to the fact that silver fir is favored by silvicultural prescriptions and tree marking (Boncina et al., 2014; Adamic et al., 2016; Keren et al., 2017).
The research results document how combinations of natural and anthropogenic disturbances can completely change the tree species mixture of old-growth forests in a relatively short period of time compared to their developmental cycle. In addition to several windthrows, periods of air pollution and overbrowsing, the Pecka old-growth forest is also characterized by its location at the border of mixed montane forests, which is why the silver fir decline has been particularly pronounced. As such, it can serve as a warning for other old-growth mixed forests in central and western Europe which are threatened with a similar fate.
The high growing stock and well-preserved forest microclimate, despite the interactions of disturbances, indicates the high resilience of mixed montane forests. Although the disturbances most likely deviated from the natural disturbance regime, the forest has recovered due to the advanced regeneration of shade-tolerant beech, while successional pathways with pioneer species have not yet been activated. From this point of view, it does not seem appropriate to overemphasize the importance of pioneer stages in managed forests for adaptation to a higher intensity of natural disturbances, especially because in managed forests, due to the lower growing stocks, the development of pioneer stages is more probable even at lower disturbance intensities. Compared to old-growth forests, managed forests are also in an advantageous position because of the possibility to support endangered species through silvicultural measures such as planting, protection from browsing and competition regulation. At the same time, extremely endangered old-growth forests such as Pecka serve as a warning that changes in the management of ungulate populations are long overdue.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JD, TA, GF, and DR developed the conception and the method of the study, performed the data analysis, and contributed to manuscript revision, read and approved the submitted version. TA, GF, and DR collected the most of the data. JD edited and organized the database, set up and performed the regression model statistics, and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the Slovenian Research Agency (ARRS) under grants P4-0059 and J4-1765 and by the Pahernik Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamic, M., Diaci, J., Rozman, A., and Hladnik, D. (2016). Long-term use of uneven-aged silviculture in mixed mountain Dinaric forests: a comparison of old-growth and managed stands. Forestry 90, 279–291. doi: 10.1093/forestry/cpw052
Albanesi, E., Gugliotta, O., Mercurio, I., and Mercurio, R. (2005). Effects of gap size and within-gap position on seedlings establishment in silver fir stands. Forest 2, 358–366.
Amann, M., Klimont, Z., and Wagner, F. (2013). Regional and global emissions of air pollutants: recent trends and future scenarios. Annu. Rev. Environ. Resour. 38, 31–55. doi: 10.1146/annurev-environ-052912-173303
Aussenac, G. (2000). Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 57, 287–301. doi: 10.1051/forest:2000119
Blossey, B., Dávalos, A., and Nuzzo, V. (2017). An indicator approach to capture impacts of white-tailed deer and other ungulates in the presence of multiple associated stressors. AoB PLANTS 9:lx034. doi: 10.1093/aobpla/plx034
Boncina, A. (2000). Comparison of structure and biodiversity in the Rajhenav virgin forest remnant and managed forest in the Dinaric region of Slovenia. Glob. Ecol. Biogeogr. 9, 201–211. doi: 10.1046/j.1365-2699.2000.00155.x
Boncina, A. (2011). History, current status and future prospects of uneven-aged forest management in the Dinaric region: an overview. Forestry 84, 467–478. doi: 10.1093/forestry/cpr023
Boncina, A., Cavlovic, J., Curovic, M., Govedar, Z., Klopcic, M., and Medarevic, M. (2014). A comparative analysis of recent changes in Dinaric uneven-aged forests of the NW Balkans. Forestry 87, 71–84. doi: 10.1093/forestry/cpt038
Boncina, A., Diaci, J., and Cencic, L. (2002). Comparison of the two main types of selection forests in Slovenia: distribution, site conditions, stand structure, regeneration and management. Forestry 75, 365–373. doi: 10.1093/forestry/75.4.365
Boncina, A., Gaspersic, F., and Diaci, J. (2003). Long-term changes in tree species composition in the Dinaric mountain forests of Slovenia. For. Chron. 79, 227–232. doi: 10.5558/tfc79227-2
Bouriaud, O., and Popa, I. (2009). Comparative dendroclimatic study of Scots pine, Norway spruce, and silver fir in the Vrancea Range, Eastern Carpathian Mountains. Trees 23, 95–106. doi: 10.1007/s00468-008-0258-z
Buma, B. (2015). Disturbance interactions: characterization, prediction, and the potential for cascading effects. Ecosphere 6, 1–15. doi: 10.1890/ES15-00058.1
Büntgen, U., Tegel, W., Kaplan, J. O., Schaub, M., Hagedorn, F., Bürgi, M., et al. (2014). Placing unprecedented recent fir growth in a European-wide and Holocene-long context. Front. Ecol. Environ. 12:100–106. doi: 10.1890/130089
Cailleret, M., Nourtier, M., Amm, A., Durand-Gillmann, M., and Davi, H. (2014). Drought-induced decline and mortality of silver fir differ among three sites in Southern France. Ann. For. Sci. 71, 643–657. doi: 10.1007/s13595-013-0265-0
Cater, M., and Diaci, J. (2017). Divergent response of European beech, silver fir and Norway spruce advance regeneration to increased light levels following natural disturbance. For. Ecol. Manag. 399, 206–212. doi: 10.1016/j.foreco.2017.05.042
Condamine, F. L., Silvestro, D., Koppelhus, E. B., and Antonelli, A. (2020). The rise of angiosperms pushed conifers to decline during global cooling. Proc. Natl. Acad. Sci. U.S.A. 117, 28867–28875. doi: 10.1073/pnas.2005571117
Cremer, E., Ziegenhagen, B., Schulerowitz, K., Mengel, C., Donges, K., Bialozyt, R., et al. (2012). Local seed dispersal in European silver fir (Abies alba Mill.): lessons learned from a seed trap experiment. Trees 26, 987–996. doi: 10.1007/s00468-012-0676-9
Danell, K., Bergstrom, R., Duncan, P., and Pastor, J. (2006). Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge, MA: Cambridge University Press. doi: 10.1017/CBO9780511617461
Debeljak, M. (1997). Jelka (Abies alba Mill.) v pomladku pragozda Pecka v zadnjih tridesetih letih. Zbornik Gozdarstva Lesarstva 53, 29–48.
Diaci, J. (2002). Regeneration dynamics in a Norway spruce plantation on a silver fir-beech forest site in the Slovenian Alps. For. Ecol. Manag. 161, 27–38. doi: 10.1016/S0378-1127(01)00492-3
Diaci, J. (2011). Silver Fir Decline in Mixed Old-Growth Forests in Slovenia: An Interaction of Air Pollution, Changing Forest Matrix and Climate. London: INTECH Open Access Publisher. doi: 10.5772/17962
Diaci, J., Adamic, T., and Rozman, A. (2012). Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: influences of light regime, herb competition and browsing. For. Ecol. Manag. 285, 20–28. doi: 10.1016/j.foreco.2012.08.010
Diaci, J., Rozenbergar, D., Anic, I., Mikac, S., Saniga, M., Kucbel, S., et al. (2011). Structural dynamics and synchronous silver fir decline in mixed old-growth mountain forests in Eastern and Southeastern Europe. Forestry 84, 479–491. doi: 10.1093/forestry/cpr030
Diaci, J., Rozenbergar, D., and Boncina, A. (2010). Stand dynamics of Dinaric old-growth forest in Slovenia: are indirect human influences relevant? Plant Biosyst. 144, 194–201. doi: 10.1080/11263500903560785
Diaci, J., Rozman, J., and Rozman, A. (2020). Regeneration gap and microsite niche partitioning in a high alpine forest: are Norway spruce seedlings more drought-tolerant than beech seedlings? For. Ecol. Manag. 455:117688. doi: 10.1016/j.foreco.2019.117688
Dobrowolska, D. (1998). Structure of silver fir (Abies alba Mill.) natural regeneration in the ‘Jata’ reserve in Poland. For. Ecol. Manag. 110, 237–247. doi: 10.1016/S0378-1127(98)00286-2
Dobrowolska, D., Boncina, A., and Klumpp, R. (2017). Ecology and silviculture of silver fir (Abies alba Mill.): a review. J. For. Res. 22, 326–335. doi: 10.1080/13416979.2017.1386021
Eiberle, K., and Nigg, H. (1987). Grundlagen zur Beurteilung des Wildverbisses im Gebirgswald. Schweiz Z Forstwes 138, 747–780.
Elling, W., Dittmar, C., Pfaffelmoser, K., and Rötzer, T. (2009). Dendroecological assessment of the complex causes of decline and recovery of the growth of silver fir (Abies alba Mill.) in Southern Germany. For. Ecol. Manag. 257, 1175–1187. doi: 10.1016/j.foreco.2008.10.014
Ferlin, F. (2002). The growth potential of understorey silver fir and Norway spruce for uneven-aged forest management in Slovenia. Forestry 75, 375–383. doi: 10.1093/forestry/75.4.375
Ficko, A., Poljanec, A., and Boncina, A. (2011). Do changes in spatial distribution, structure and abundance of silver fir (Abies alba Mill.) indicate its decline? For. Ecol. Manag. 261, 844–854. doi: 10.1016/j.foreco.2010.12.014
Firm, D., Nagel, T. A., and Diaci, J. (2009). Disturbance history and dynamics of an old-growth mixed species mountain forest in the Slovenian Alps. For. Ecol. Manag. 257, 1893–1901. doi: 10.1016/j.foreco.2008.09.034
Gessler, A., Keitel, C., Nahm, M., and Rennenberg, H. (2004). Water shortage affects the water and nitrogen balance in central European beech forests. Plant Biol. 6, 289–298. doi: 10.1055/s-2004-820878
Gill, R. M. A. (1992). A review of damage by mammals in north temperate forests: 3. Impact on trees and forests. Forestry 65, 363–388. doi: 10.1093/forestry/65.4.363-a
Harmer, R. (2001). The effect of plant competition and simulated summer browsing by deer on tree regeneration. J. Appl. Ecol. 38, 1094–1103. doi: 10.1046/j.1365-2664.2001.00664.x
Keren, S., Diaci, J., Motta, R., and Govedar, Z. (2017). Stand structural complexity of mixed old-growth and adjacent selection forests in the Dinaric Mountains of Bosnia and Herzegovina. For. Ecol. Manag. 400, 531–541. doi: 10.1016/j.foreco.2017.06.009
Keren, S., Motta, R., Govedar, Z., Lucic, R., Medarevic, M., and Diaci, J. (2014). Comparative structural dynamics of the janj mixed old-growth mountain forest in bosnia and herzegovina: are conifers in a long-term decline? Forests 5, 1243–1266. doi: 10.3390/f5061243
Klopcic, M., Jerina, K., and Boncina, A. (2010). Long-term changes of structure and tree species composition in Dinaric uneven-aged forests: are red deer an important factor? Eur. J. For. Res. 129, 277–288. doi: 10.1007/s10342-009-0325-z
Korpel, S. (1995). Die Urwälder der Westkarpaten. Stuttgart; Jena. New York, NY: Gustav Fischer Verlag.
Krause, G., Arndt, U., Brandt, C. J., Bucher, J., Kenk, G., and Matzner, E. (1986). Forest decline in Europe: development and possible causes. Water Air Soil Pollut. 31, 647–668. doi: 10.1007/BF00284218
Kupferschmid, A. D., and Bugmann, H. (2013). Timing, light availability and vigour determine the response of Abies alba saplings to leader shoot browsing. Eur. J. For. Res. 132, 47–60. doi: 10.1007/s10342-012-0653-2
Leuschner, C., and Ellenberg, H. (2017). Ecology of Central European Forests. Cham: Springer. doi: 10.1007/978-3-319-43042-3
Linares, J. C., and Camarero, J. J. (2012). Growth patterns and sensitivity to climate predict silver fir decline in the Spanish Pyrenees. Eur. J. For. Res. 131, 1001–1012. doi: 10.1007/s10342-011-0572-7
Marincek, L., and Marinsek, A. (2004). Vegetation of the Pecka virgin forest remnant. Hacquetia 3, 5–27.
Marinsek, A., and Diaci, J. (2004). Razvoj inicialne faze na vetrolomni povrsini v prajozdnem ostanku Ravna gora (Development of the initial phase after windthrow in the virgin forest remnant Ravna gora). Zb. Gozd. Lesar. 73, 31–50.
Matic, S. (1983). Utjecaj ekoloskih i strukturnih cinilaca na prirodno pomladivanje prebornih suma jele i bukve u Gorskom kotaru. Glasnik Za Sumske Pokuse 21, 223–400.
Mayer, H., and Neumann, M. (1981). Struktureller und entwicklungsdynamischer vergleich der fichten-tannen-buchen-urwälder rothwald-niederösterreich und corkova uvala-kroatien. Forstw. Cbl 100, 111–132. doi: 10.1007/BF02640624
McDowell, N. G., and Allen, C. D. (2015). Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Change 5, 669–672. doi: 10.1038/nclimate2641
Messier, C., Posada, J., Aubin, I., and Beaudet, M. (2009). “Functional relationships between old-growth forest canopies, understorey light and vegetation dynamics of old-growth forests,” in Old-Growth Forests, eds C. Wirth, G. Gleixner, and M. Heimann (Berlin: Springer), 115–139. doi: 10.1007/978-3-540-92706-8_6
Mlinsek, D. (1967). Pomlajevanje in nekatere razvojne znacilnosti bukovega in jelovega mladovja v pragozdu na Rogu. Zbornik Biotehniske Fakultete 15, 7–32.
Morecroft, M., Taylor, M. E., and Oliver, H. R. (1998). Air and soil microclimates of deciduous woodland compared to an open site. Agric. For. Meteorol. 90, 141–156. doi: 10.1016/S0168-1923(97)00070-1
Motta, R. (1996). Impact of wild ungulates on forest regeneration and tree composition of mountain forests in the Western Italian Alps. For. Ecol. Manag. 88, 93–98. doi: 10.1016/S0378-1127(96)03814-5
Nagel, T. A., and Diaci, J. (2006). Intermediate wind disturbance in an old-growth beech-fir forest in southeastern Slovenia. Can. J. For. Res. 36, 629–638. doi: 10.1139/x05-263
Nagel, T. A., Diaci, J., Jerina, K., Kobal, M., and Rozenbergar, D. (2015). Simultaneous influence of canopy decline and deer herbivory on regeneration in a conifer-broadleaf forest. Can. J. For. Res. 45, 265–274. doi: 10.1139/cjfr-2014-0249
Nagel, T. A., Levanic, T., and Diaci, J. (2007). A dendroecological reconstruction of disturbance in an old-growth Fagus-Abies forest in Slovenia. Ann. For. Sci. 64, 891–897. doi: 10.1051/forest:2007067
Nagel, T. A., Svoboda, M., and Diaci, J. (2006). Regeneration patterns after intermediate wind disturbance in an old-growth Fagus-Abies forest in southeastern Slovenia. For. Ecol. Manag. 226, 268–278. doi: 10.1016/j.foreco.2006.01.039
Nagel, T. A., Svoboda, M., and Kobal, M. (2014). Disturbance, life history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol. Applic. 24, 663–679. doi: 10.1890/13-0632.1
Orman, O., and Szewczyk, J. (2015). European beech, silver fir, and Norway spruce differ in establishment, height growth, and mortality rates on coarse woody debris and forest floor—a study from a mixed beech forest in the Western Carpathians. Ann. For. Sci. 72, 955–965. doi: 10.1007/s13595-015-0492-7
Ott, E., Frehner, M., Frey, H.-U., and Lüscher, P. (1997). Gebirgsnadelwälder: Praxisorientierter Leitfaden für eine standortgerechte Waldbehandlung. Bern: Verlag Paul Haupt.
Piovani, P., Leonardi, S., Piotti, A., and Menozzi, P. (2010). Conservation genetics of small relic populations of silver fir (Abies alba Mill.) in the northern Apennines. Plant Biosyst. Int. J. Deal. Aspects Plant Biol. 144, 683–691. doi: 10.1080/11263504.2010.496199
Prpic, B., and Seletkovic, Z. (1996). “The research in Croatian virgin forests and the application of results to natural forests,” in Skrb Za Hrvatske Sume Od 1946. do 1996.: Unapredenje Proizvodnje Biomase Sumskih Ekosustava, ed. B. Mayer (Jastrebarsko: Sumarski institut), 97–104.
Puncer, I. (1980). Dinarski Jelovo-Bukovi Gozdovi Na Kocevskem/Die Dinarischen Tannen-Buchenwälder im Gebiete von Kocevje. Ljubljana: Slovenian Academy of Science and Art.
R Core Team (2019). A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robinson, A. P., and Hamann, J. D. (2011). Forest Analytics with R: An Introduction. Berlin: Springer. doi: 10.1007/978-1-4419-7762-5
Rozenbergar, D., and Diaci, J. (2014). Architecture of Fagus sylvatica regeneration improves over time in mixed old-growth and managed forests. For. Ecol. Manag. 318, 334–340. doi: 10.1016/j.foreco.2014.01.037
Rozenbergar, D., Kolar, U., Cater, M., and Diaci, J. (2011). Comparison of four methods for estimating relative solar radiation in managed and old-growth silver fir-beech forest. Dendrobiology 65, 73–82.
Rozenbergar, D., Mikac, S., Anic, I., and Diaci, J. (2007). Gap regeneration patterns in relationship to light heterogeneity in two old-growth beech-fir forest reserves in South East Europe. Forestry 80, 431–443. doi: 10.1093/forestry/cpm037
Safar, J. (1951). Ugibanje i obnavljanje jele u prebornim sumama Gorskog Kotara. Sumarski List 75, 299–303.
Stromayer, K. A., and Warren, R. J. (1997). Are overabundant deer herds in the eastern United States creating alternate stable states in forest plant communities? Wildlife Soc. Bull. 25, 227–234.
Turk, V., Kastelic, A., and Hartman, T. (1985). Forest Reserves in Slovenia - Old-Growth Forest Pecka. Ljubljana: Univerza Edvarda Kardelja v Ljubljani, Biotehniska fakulteta.
Vacek, Z., Vacek, S., Podrázský, V., Bílek, L., Stefancík, I., Moser, W. K., et al. (2015). Effect of tree layer and microsite on the variability of natural regeneration in autochthonous beech forests. Polish J. Ecol. 63, 233–246. doi: 10.3161/15052249PJE2015.63.2.007
Vitali, V., Büntgen, U., and Bauhus, J. (2017). Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Change Biol. 23, 5108–5119. doi: 10.1111/gcb.13774
Vitasse, Y., Bottero, A., Rebetez, M., Conedera, M., Augustin, S., Brang, P., et al. (2019). What is the potential of silver fir to thrive under warmer and drier climate? Eur. J. For. Res. 138, 547–560. doi: 10.1007/s10342-019-01192-4
Vrska, T., Adam, D., Hort, L., Kolár, T., and Janík, D. (2009). European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) rotation in the Carpathians–A developmental cycle or a linear trend induced by man? For. Ecol. Manag. 258, 347–356. doi: 10.1016/j.foreco.2009.03.007
Keywords: Fagus sylvatica, stand structure, regeneration dynamics, overbrowsing, windthrow, air pollution, Abies alba
Citation: Diaci J, Adamic T, Fidej G and Rozenbergar D (2022) Toward a Beech-Dominated Alternative Stable State in Dinaric Mixed Montane Forests: A Long-Term Study of the Pecka Old-Growth Forest. Front. For. Glob. Change 5:937404. doi: 10.3389/ffgc.2022.937404
Received: 06 May 2022; Accepted: 13 June 2022;
Published: 30 June 2022.
Edited by:
Maxence Martin, Université du Québec à Chicoutimi, CanadaReviewed by:
Eike Feldmann, Northwest German Forest Research Institute, GermanyCopyright © 2022 Diaci, Adamic, Fidej and Rozenbergar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jurij Diaci, SnVyaWouRGlhY2lAYmYudW5pLWxqLnNp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.