
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 21 February 2022
Sec. Forest Management
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.844580
This article is part of the Research TopicCurrent Challenges in Forest Restoration and Sustainable Forest ManagementView all 5 articles
Worldwide, forestry must face several challenges during the UN Decade on Ecosystem Restoration. The decline of biodiversity and ecosystem services, ongoing deforestation, climate change, and biological invasions must be mitigated with forest restoration and by applying sustainable forest management. Experiences with the integration of non-native tree species into forest management in many parts of the world show benefits but also trade-offs regarding sustainability. In Central Europe, Douglas fir [Pseudotsuga menziesii (Mirbel) Franco], originating from Northern America, has been introduced by forestry and managed for more than one and a half centuries. Growth characteristics, drought tolerance, and timber quality are major reasons which make this tree species attractive for forestry and wood industry. Whether Douglas fir might be invasive with potential trade-offs regarding biodiversity, uncontrolled regeneration, and spread is not yet fully understood and controversially debated. We investigated the regeneration of Douglas fir in the Spessart mountains, a vast woodland in south-western Germany which has a considerable cover of anthropogenic coniferous afforestations. We sampled the regeneration of Douglas fir by differentiating height growth classes in various forest-stand types, taking the distance from mature mother trees, abiotic site conditions (e.g., water balance and soil properties), light supply, forest-stand characteristics, ground vegetation, and browsing pressure into account. Also integrating the individual regeneration of the accompanying tree species, we applied multivariate analyses. Most of our investigated variables did not show a significant correlation with Douglas fir regeneration. However, results point to a positive relationship of spontaneous Douglas fir regeneration at moist and light sites as well as in close distance to potential seed trees. The analysis of the current invasion potential did not reveal a major risk under the given site conditions in the study area.
Worldwide, forestry must face several challenges during the UN Decade on Ecosystem Restoration. The decline of biodiversity and ecosystem services, ongoing deforestation, climate change, biological invasions, natural hazards, and urbanization must be mitigated with forest restoration and by applying sustainable forest management (Mansourian et al., 2005; Aerts and Honnay, 2011; Zerbe, 2019). Large-scale forest fires in the tropics (e.g., Ferrante and Fearnside, 2020), a still high rate of deforestation in many countries due to land-use change (Bologna and Aquino, 2020), and the effects of climate change in combination with pests (Linnakoski et al., 2019) call for immediate action. In addition to the increasing role of timber as natural resource for many purposes (McEwan et al., 2020) forests provide a large variety of ecosystem services (Mengist and Soromessa, 2019).
One possible solution to overcome some of the problems mentioned above is to further develop ecosystems by slightly altering the combinations of tree species. This approach takes even forest types into account that do not exist in nature (Palmer et al., 2004). Such systems are not supposed to substitute natural systems, but may contribute in reducing the pressure on the few undisturbed ecosystems. Interestingly, such ecosystems consciously created to achieve multiple ecological, social, and economic goals already exist in forestry. Managed forests have already been composed by combining native with non-native tree species. Recent studies suggest that a solution for reconciling production-oriented goals with conservational interests may be found in mixtures of highly productive non-native and native tree species (Hildebrandt et al., 2010; Oxbrough et al., 2016).
Since more than one and a half centuries, forestry in Central Europe introduced non-native (synonymously, also “alien,” “exotic,” “non-indigenous” used) tree species, particularly from North America and Asia to enlarge the range of timber products and facilitate forest management. For some of these species, experiences of many decades concerning forest ecology, silviculture, and timber markets have been made. Economically most important species include Douglas fir [Pseudotsuga menziesii (Mirbel) Franco], Weymouth pine (Pinus strobus L.), Red oak (Quercus rubra L.), and Black cherry (Prunus serotina Ehrh.), all of them originating from North America (Brundu and Richardson, 2016; Pötzelsberger et al., 2020). Due to climate change, the introduction of other non-native species is suggested and already in an experimental phase (Messinger et al., 2015; Frischbier et al., 2019). Benefits and opportunities of these non-native species can be fast growth, high timber quality, easy-manageable regeneration, drought tolerance and thus better adaptation to climate change, and higher revenues on the timber markets (Pötzelsberger et al., 2020). However, if temperature increase due to climate change continues, a decline in productivity for Douglas fir is expected in already warm and dry regions (Eckhart et al., 2019).
However, trade-offs have been observed after the introduction and the integration of non-native tree species into forest management. The tree species can run out of control of silvicultural management and thus, might threaten native ecosystems during biological invasion (e.g., Terwei et al., 2013; Brundu and Richardson, 2016; Langmaier and Lapin, 2020). Biological invasion is hereby defined by Valéry et al. (2008, p. 1349) as consisting “of a species’ acquiring a competitive advantage following the disappearance of natural obstacles to its proliferation, which allows it to spread rapidly and to conquer novel areas within recipient ecosystems in which it becomes a dominant population.” One of the most prominent examples in Central and Western Europe is Black cherry which caused numerous activities on its eradication after having spread increasingly and uncontrolled in coniferous forests. The history of invasion as well as the change in the perception of this tree species by stakeholders has been comprehensively outlined by Starfinger et al. (2003). Biological invasions can cause considerable economic costs regarding their trade-offs (e.g., negative impact on land-use systems and human health) and their management (see Kowarik, 2010 for Central Europe). As a consequence of the risks and trade-offs of non-native tree species for land use, land management, and nature conservation, stakeholders implemented legal frameworks, international agreements, and certification schemes which limit an active introduction of non-native species (e.g., Kowarik, 2010; Bailey et al., 2011).
Douglas fir was planted in Germany since the 1820s (Booth, 1877; Knoerzer and Reif, 2002). Systematic silvicultural trials for provenances and ecological traits of Douglas fir started in the late 19th century, suggesting this tree species as a suitable candidate for forestry (Lavender and Hermann, 2014). Douglas fir originates from the Pacific Northwest of America and covers about 830,000 ha of forest land in Europe (Pötzelsberger et al., 2020). It is well investigated regarding biology, forest ecology, biodiversity, genetics, timber production, and timber quality as well as marketing (e.g., Podrázský et al., 2014; Schmid et al., 2014; Eckhart et al., 2017; Spiecker et al., 2019; Wohlgemuth et al., 2019; Eberhard et al., 2021). Various studies identified light supply on the forest floor, the composition and structure of understory vegetation, water availability, and the distance of diaspore sources as main factors for the natural regeneration of Douglas fir (Lavender et al., 1968; Broncano et al., 2005; Huth et al., 2011; Eggert, 2014; Eberhard and Hasenauer, 2018; Bindewald et al., 2021). Although Douglas fir seedlings have been reported up to 2 km away from a seed source, the majority of seeds fall within 100 m of seed parent trees (Barnhart et al., 1996; Broncano et al., 2005; Kennedy and Diaz, 2005). Besides its benefits and opportunities for forestry and timber production in Central Europe, however, concerns have been raised on its potential invasiveness and subsequent trade-offs for forest ecosystems such as, e.g., the change of the abiotic and biotic environment (Barnhart et al., 1996; Knoerzer, 1999; Goßner and Simon, 2002; Goßner and Utschik, 2002; Nehring et al., 2013; Wohlgemuth et al., 2021). Different approaches of invasiveness evaluations might lead to different assessments and implications for forestry (Spellmann et al., 2015).
As a contribution to the discussion of a potential invasiveness of Douglas fir in Central Europe, we investigate the natural regeneration of this tree species in the Bavarian Spessart mountains in south-west Germany as a case study. In this region, Douglas fir has a long silvicultural tradition, dating back to the 1880s (Mergner, 2018). We want to identify the main environmental factors influencing the regeneration of Douglas fir. Specifically, we hypothesize that the presence of Douglas fir increases with increasing proximity to seed trees and with reduced canopy closure of the mature stands. From these results, we assess and discuss the potential invasiveness of Douglas fir in the forests of the Spessart mountains and derive recommendations for the management of this non-native tree species.
The study area Spessart is a low mountain range with its highest peak being the Geierskopf (586 m a.s.l.). The Spessart is located in the very north-west of Bavaria in south-west Germany and stretches in the north into the adjacent federal state of Hesse (Figure 1). The bedrock is mainly red sandstone, locally influenced by loess (Matthes and Okrusch, 1965). Consequently, nutrient poor and acidic soils prevail throughout the mountain range. The climate is of sub-oceanic to oceanic character, with a mean annual precipitation ranging from 700 mm in the lower mountain altitudes (<300 m a.s.l.) to about 1,000 mm in the higher altitudes. The mean annual temperatures follow the same gradient varying between 8 and 9°C in the valley of the river Main and around 7°C in the upper Spessart (Zerbe, 1999).
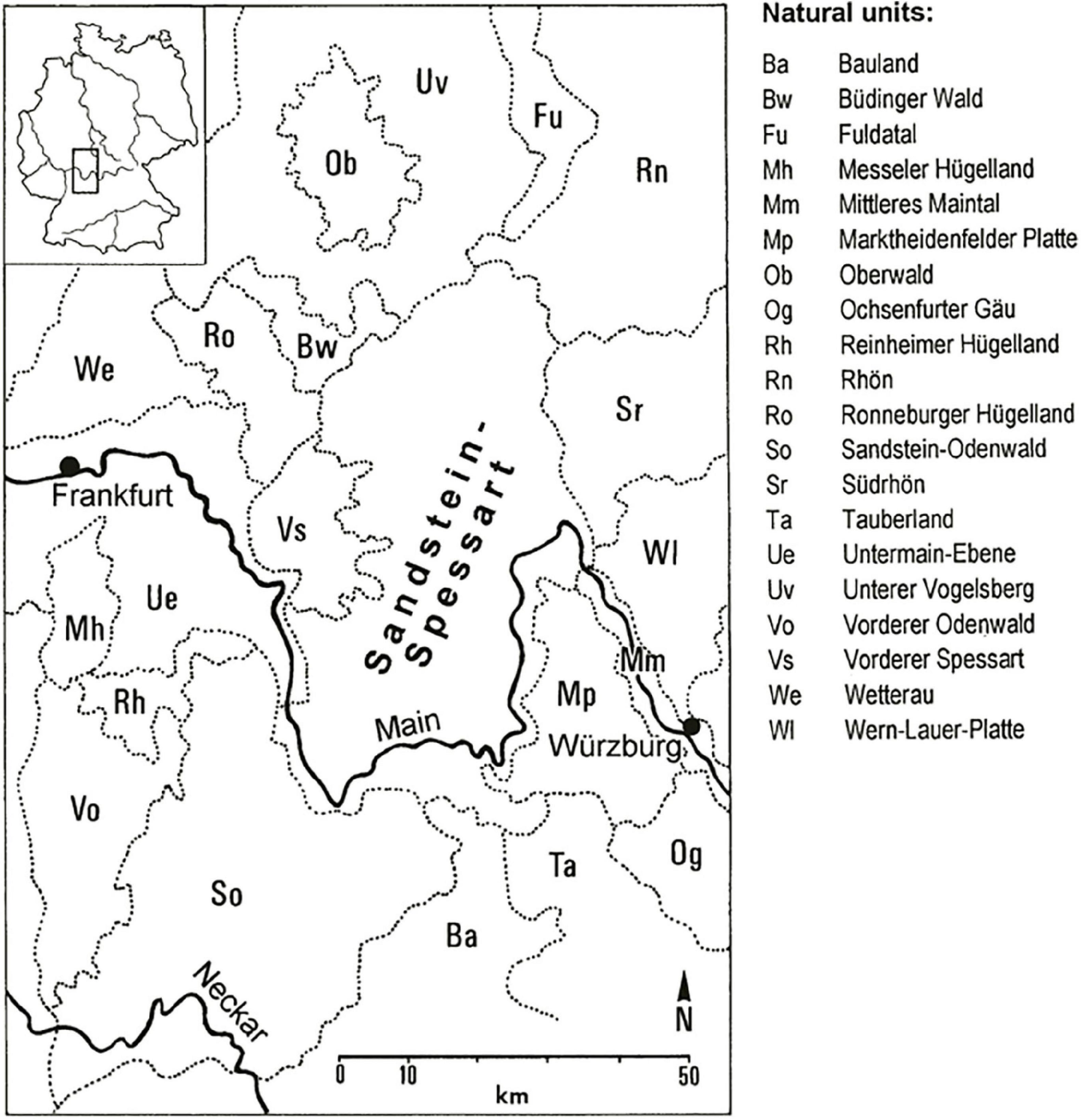
Figure 1. Study area “Sandstein-Spessart” with neighboring natural units [units and topographic names according to Meynen and Schmithüsen (1953) with an original scale of 1:1,100,000] and the river Main in SW Germany from Zerbe (2003).
Historically, forests in the Spessart underwent multifaceted phases of anthropogenic influences, with a spatially varying impact of forest glassworks, hunting, agriculture, and subsequent afforestation of degraded forest sites about 200 years ago. Particularly related to the history of hunting, this impact led to a distinguished separation of main forest types in the northern and southern upper Spessart (Zerbe, 1999). Thus, coniferous monocultures with mainly Norway spruce [Picea abies (L.) H. Karst.], Scots pine (Pinus sylvestris L.), and European larch (Larix decidua Mill.) are prevailing in the north, whereas in the south, near-natural broad-leaved forests with beech (Fagus sylvatica L.) and oak [mainly Quercus petraea (Matt.) Liebl.] are the main forest types (Zerbe, 2004). Additionally to the above-mentioned coniferous species, Douglas fir has been promoted in the northern upper Spessart as forest tree, dating back to the second half of the 19th century (Mergner, 2018). Consequently, we focus with our investigations on the northern upper Spessart. In the southern Spessart, however, selected habitats which are protected under Annex 1 of the EU Habitats Directive are included in the study area as these sites would be under threat of the invasion of a non-native species, thus losing its unique natural and near-natural character, respectively.
Our methodological approach encompasses three steps which are (1) the systematic selection of sample sites, (2) field sampling and analysis of the environmental factors affecting the regeneration of Douglas fir, and (3) the assessment of the potential invasiveness of Douglas fir in the Spessart mountains. We aim to investigate the environmental conditions on those sites where natural Douglas fir regeneration is expected to occur outside of already existing stands. These sites of interest are limited and selected through an approach which combines a systematic scan of potential sites based on forestry data and nature conservation maps as well as interviews with foresters. Stands with dominating Douglas fir were selected from geodata provided by the forest administration, whereby adjacent areas of these stands were considered being sites of potential regeneration. Following the results stated by Sankey (2008), Eggert (2014), and Tschopp et al. (2014), we searched within a maximum distance from the mother trees of 200 m. Protected areas are potentially susceptible to an invasion by Douglas fir (e.g., Knoerzer, 1999). Therefore, we included nature conservation areas which are protected by the Annex I of the EU Habitats Directive into our site selection. Consequently, Tilio-Acerion forests of slopes, screes, and ravines (EU Code: 9180), siliceous rocky slopes with chasmophytic vegetation (8220), and Nardus stricta grasslands on siliceous substrates in mountain areas (6230) were included. In order to integrate local forest-related knowledge (cp. Parrotta and Trosper, 2012) into our search for potential regeneration sites, we contacted those foresters in charge of our investigation area in the northern Spessart. The foresters were asked whether they know sites with natural regeneration of Douglas fir in their forest departments. Overall, a total area of 3,571.3 ha was determined as sites of interest for our study. A major part of this area was derived from forestry maps (3,467.0 ha), whereas sensitive habitats (23.8 ha) and interviews (80.5 ha) contributed only with small proportions.
At the sites of interest, random sample points were laid out with the random sample function of ArcGIS. The plot size was chosen according to the limitations of the GPS handheld’s accuracy (Trimble ® Juno SB) at a radius of 10 m around the plot center. Then, the presence or absence of Douglas fir regeneration on these plots was determined. Where natural regeneration was found occasionally on the way between random sample plots, additional plots were generated.
On 73 sample plots where natural regeneration of Douglas fir was found, a sub-sample of 5 m × 5 m was taken, by counting all tree saplings and seedlings. Height growth classes were differentiated in I: 20–50 cm, II: 51–130 cm, III: >130 cm and DBH <7 cm (Ohse et al., 2017). Data were then calculated for an area of 1 ha. The nomenclature of plant species follows Jäger et al. (2017). The environmental factors given in Table 1 which potentially influence the regeneration of Douglas fir were either extracted or calculated based on forest inventories, open-source data, or recorded in the field (see Table 1 for specific methods).
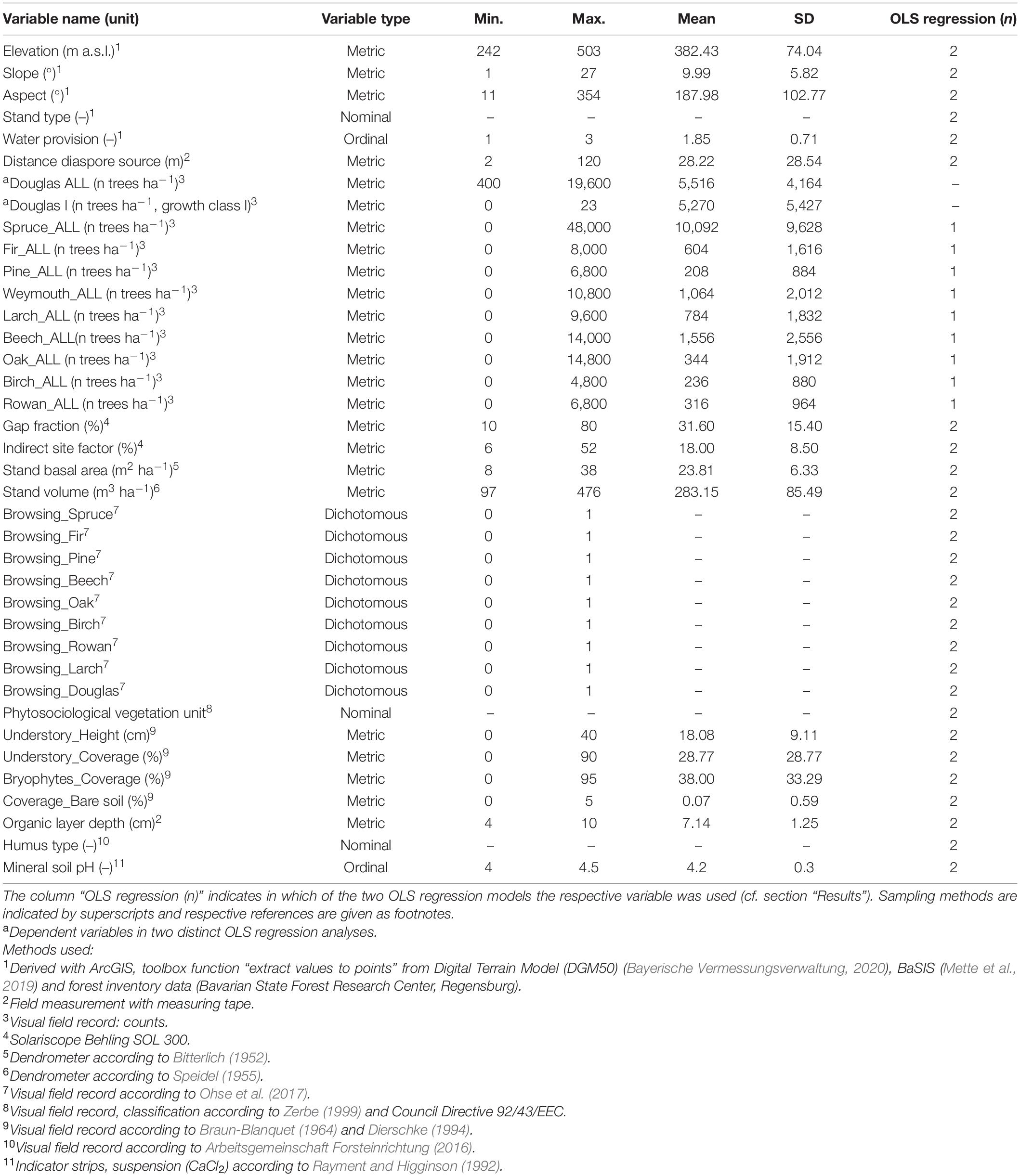
Table 1. Descriptive statistics of dependent and independent variables used to assess the decisive factors of Douglas fir regeneration and influencing site factors.
The assessment of the potential invasiveness of Douglas fir in the Spessart mountains was done by ordinary least-square regression (OLS) based on 73 observations (Table 1). First, all independent variables were tested regarding multi-collinearity which is indicated with a variance inflation factor (VIF) higher than 10 (according to Kutner et al., 2003). To check for non-linear dependencies, we secondly analyzed the scatter plots for each metric independent variable and the dependent variable. Finally, to check the assumption of homoscedasticity of the OLS regression, the scatterplot of residuals versus predicted values (residual analysis) was checked.
The invasiveness or potential invasiveness was assessed according to the model from Heger (2001; Figure 2) and the survey by Vor et al. (2015) on potentials and risks of non-native species introduced to Central Europe.
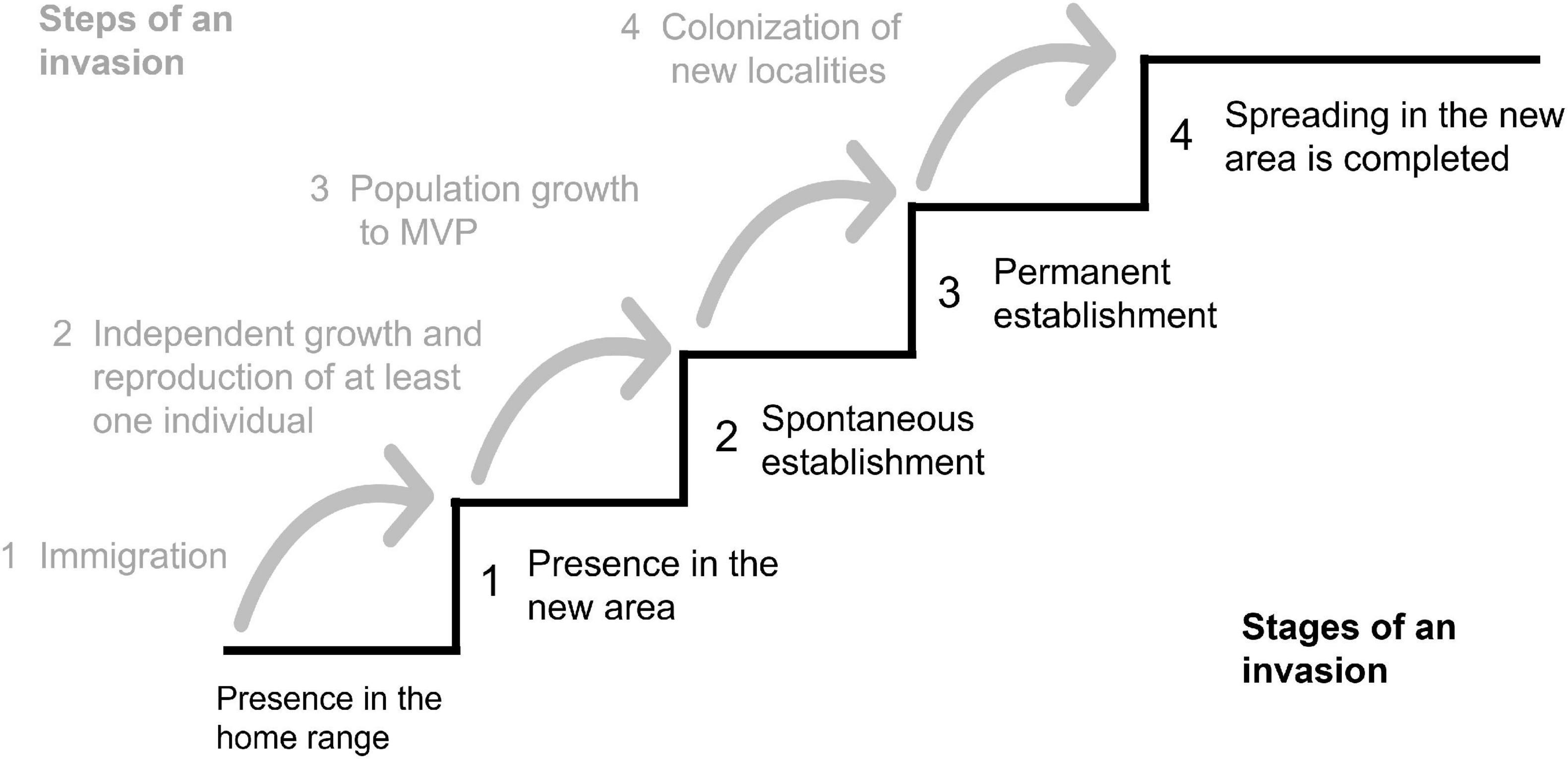
Figure 2. Chronological discrimination of an idealized invasion process into steps and stages. Different stages are reached by overcoming a sequence of steps during an invasion. The height of each step depends on the ability of the species to overcome environmental limitations; MVP, Minimum Viable Population (from Heger, 2001, modified).
On 52 out of 527 random sample plots, that were located within a radius of 200 m around already existing stands of Douglas fir, natural regeneration was observed which amounts for 9.9%. In the areas that were indicated within the foresters’ interviews, four out of 59 sample plots showed evidence of Douglas fir regeneration (6.8%). In the nature conservation areas according to the EU Habitats Directive (40 sample plots), no Douglas fir regeneration was recorded. Considering all visited random sample plots (n = 626), natural regeneration of Douglas fir was found in 8.9% of all cases. Additionally, occasional occurrences were recorded on 105 plots, but were excluded from the frequency analysis described above because they were not selected randomly. They contributed only to the analysis of remote data which is described in the following paragraph.
The analyses of 161 sample plots where natural regeneration of Douglas fir was found showed that 95% of all seedlings and saplings had established in a distance of less than 85 m to the next diaspore source (Figure 3). The largest distance measured was 120 m from an already existing Douglas fir stand. The data of the water regime showed that roughly 85% of the sample plots with Douglas fir regeneration belong to sites with fairly low water deficit during summer (transpiration difference between potential transpiration optimum and actual transpiration of forest stands, TDiff of only 0–10 mm according to Mette et al., 2019). The remaining 15% of the plots showed a higher water deficit (TDiff 10–20 mm).
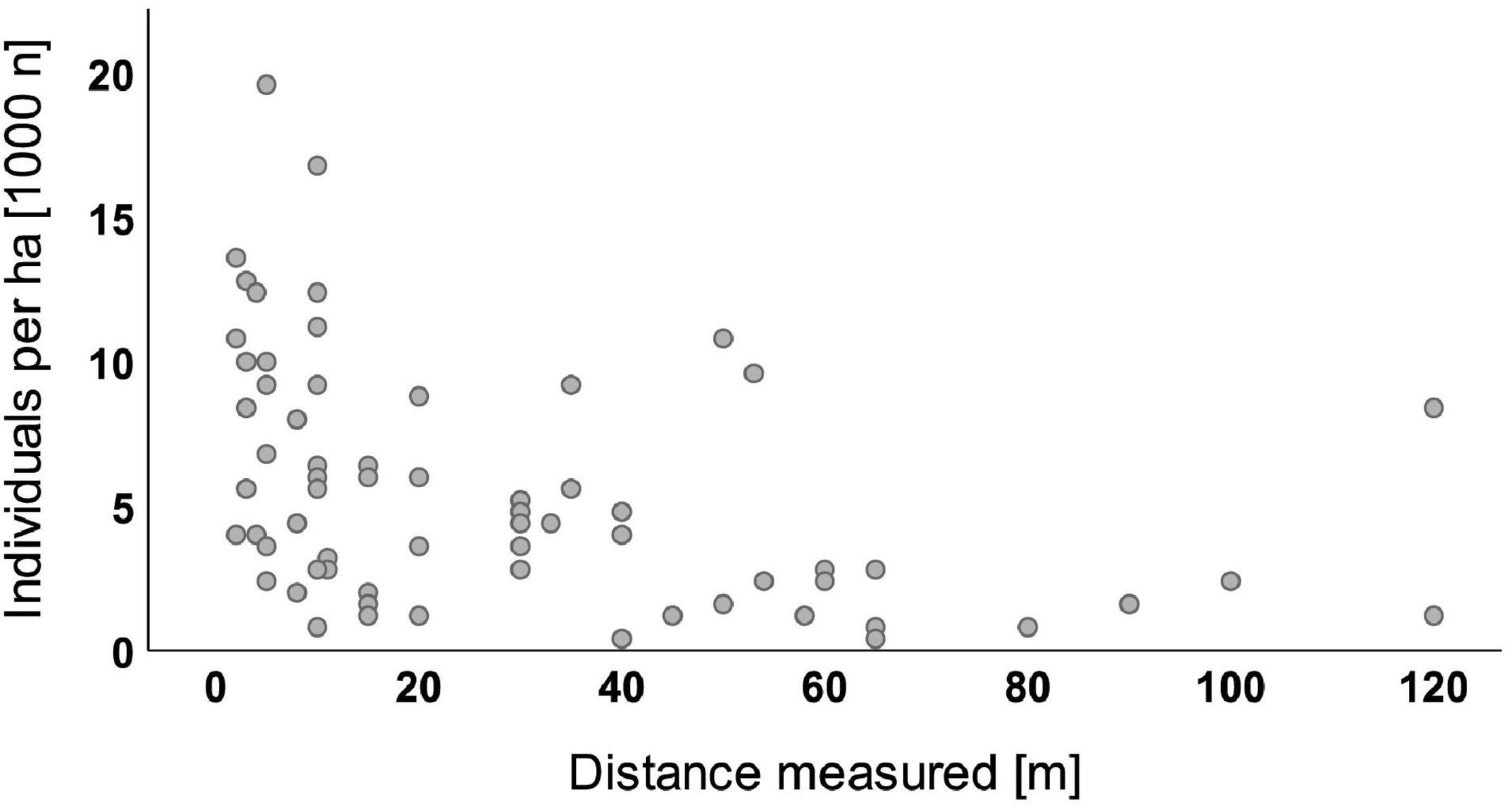
Figure 3. Douglas fir individuals per ha (1,000 n) by distance measured [bivariate regression with dependent variable “Douglas fir regeneration (all height growth classes),” n = 73 plots].
A total of 45% of the plots were located in stands dominated by Norway spruce (P. abies), followed by 25% beech (F. sylvatica) and 11% Scots pine (P. sylvestris) and Douglas fir, respectively. The predictor “stand type” turned out to be the only predictor with a significant influence on Douglas fir regeneration. Around 70% of the plots with natural Douglas fir regeneration were recorded in mixed forest stands and approximately 25% in pure coniferous stands, whereas only 1.2% of the plots were located in pure deciduous stands.
Throughout all recorded height growth classes, Douglas fir was the most abundant species in 24 out of 73 plots (33.8%). More than half of the plots (56%) were dominated by natural regeneration of Norway spruce. At all sample plots, regeneration of Douglas fir was coexisting with regeneration of other species thus not developing toward monospecific stands. Among the tree regeneration were the most abundant native, broad-leaved, and late-successional trees beech and oak, native pioneer species (e.g., rowan and Scots pine) as well as non-native coniferous tree species, which are cultivated in the forests such as, e.g., Weymouth pine, silver fir, and European larch (Figure 4). Those accompanying tree species which were mostly recorded in the height growth class III (>130 cm and DBH <7) were Norway spruce, beech, Weymouth pine, and European larch, thus partly tree species native to the Spessart mountains and partly non-native, however strongly promoted by silviculture during the past decades. In addition, correlation analysis showed that no other tree species significantly negatively correlated with Douglas fir regeneration, indicating that no species has been suppressed considerably at the sample plots (Table 2).
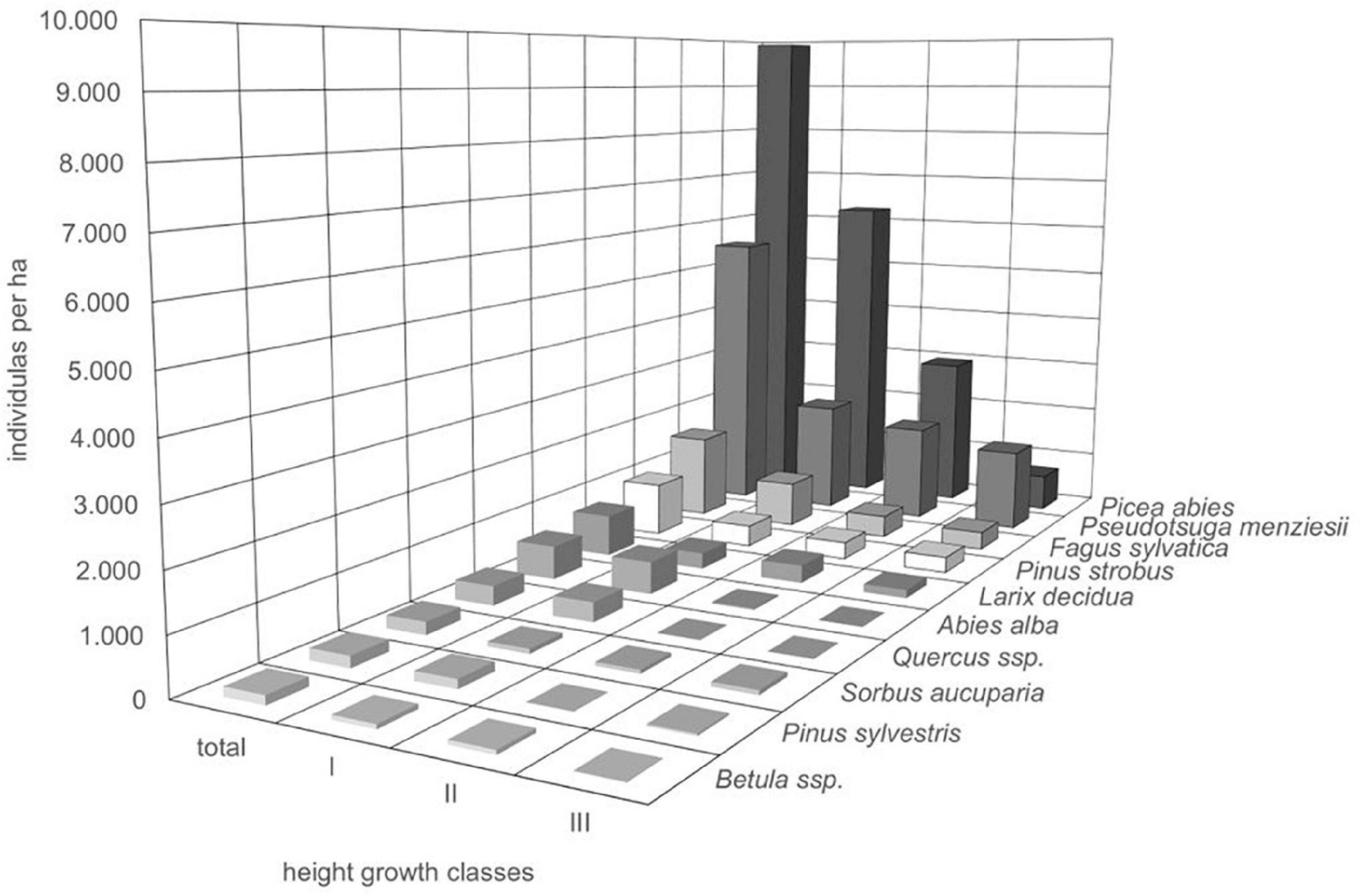
Figure 4. Mean individual numbers per ha of all tree species recorded in the regeneration layers (height growth classes I to III) on the sample plots (n = 73 plots).
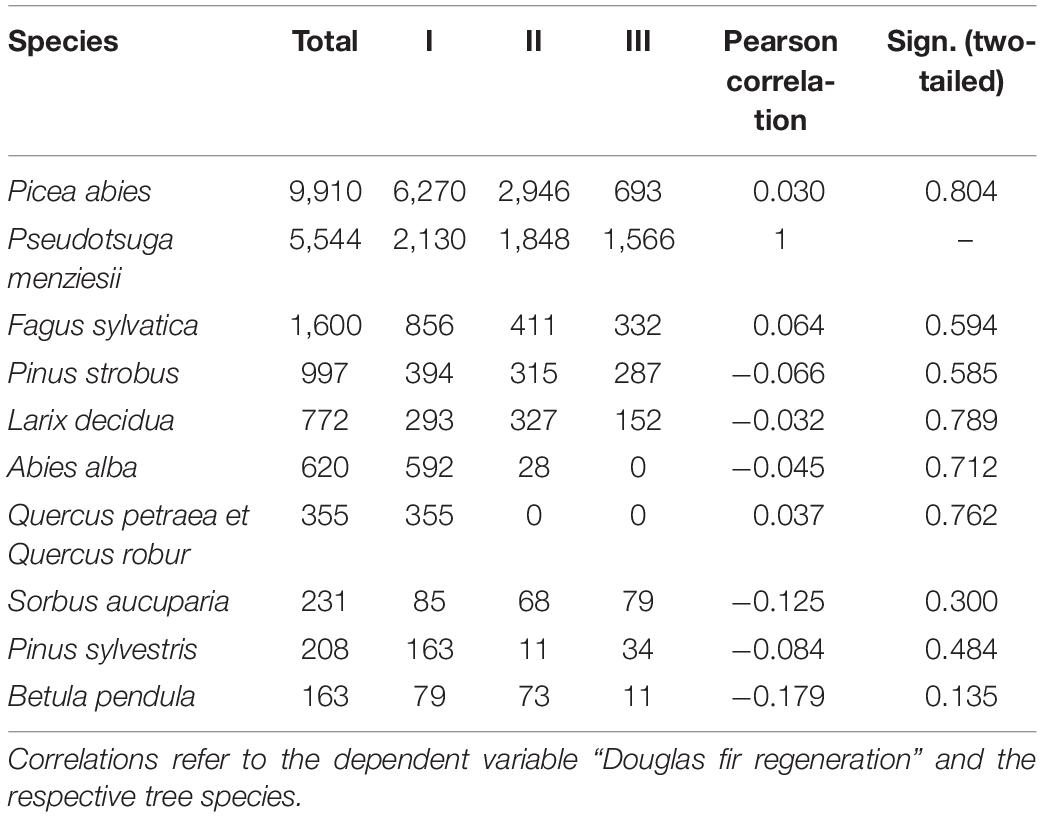
Table 2. Number of individuals per ha recorded by tree species (n = 73 plots), total and with height growth classes I: 20–50 cm, II: 51–130 cm, III: >130 cm and DBH < 7 cm.
Analyses of the scatter plots revealed no non-linear dependencies, the residual analyses revealed no noticeable patterns, and the Kolmogorov–Smirnov test proved the normal distribution of the dependent variables “Douglas fir regeneration (all height growth classes)” and “Douglas fir regeneration (height growth class I)” [asymp. sig. (two-tailed) > 0.05].
By analyzing correlations between Douglas fir regeneration and the regeneration of other tree species [OLS regression (1)], we found that the number of Douglas fir individuals on the plots (all height growth classes) is positively correlated with the occurrence of Norway spruce (height growth class I) and negatively correlated with the occurrence of Weymouth pine (height growth class III) (Table 3). By considering only the presence of Douglas fir regeneration in height growth class I as dependent variable, the number of trees is positively correlated with the occurrence of rowan (height growth class I), Weymouth pine (all height growth classes) and European Larch (height growth class I). However, the number of Douglas fir individuals is negatively correlated with the height growth class III of Weymouth pine (Table 4). Both OLS regressions revealed valid models (p < 0.05), the explanatory value (r2 = 0.321) when using the height growth class I of Douglas fir as dependent variable was slightly lower than for the OLS regression with all height growth classes of Douglas fir as dependent variable (r2 = 0.390).
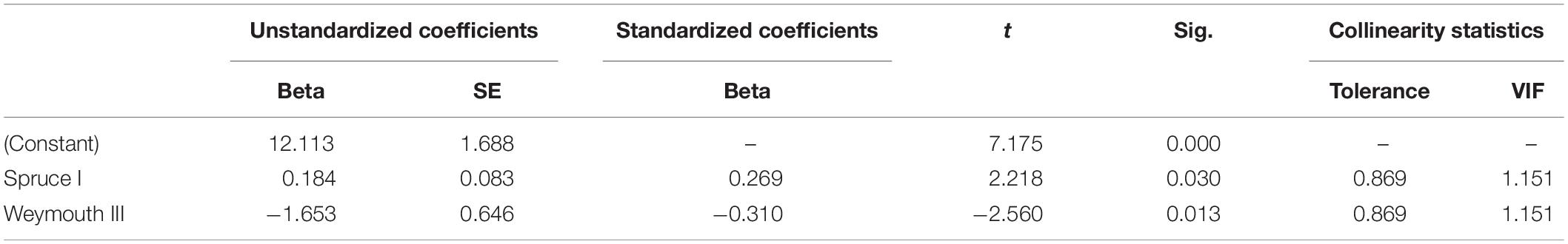
Table 3. Significant correlation of Douglas fir regeneration (all height growth classes) with the regeneration of Norway spruce (height growth class I) and Weymouth pine (height growth class III), with beta, standard error (SE), t value (t), significance (Sig.), tolerance, and variance inflation factor (VIF).
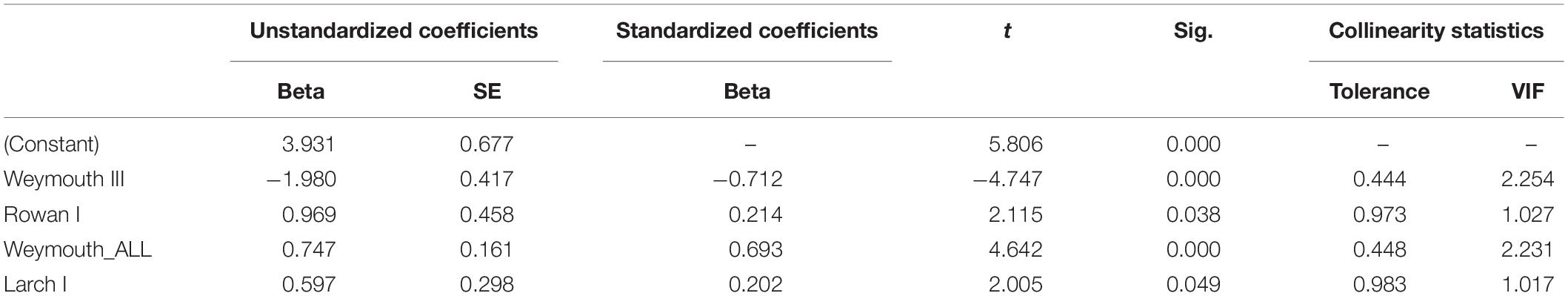
Table 4. Significant correlation of Douglas fir regeneration (height growth class I) with the regeneration of Rowan (height growth class I), Weymouth pine (all height growth classes and III), and European larch (height growth class I), with beta, standard error (SE), t value (t), significance (Sig.), tolerance, and variance inflation factor (VIF).
For the OLS regression (2) addressing the influence of environmental factors on the regeneration of Douglas fir, we considered as dependent variable the presence of Douglas fir of all height growth classes or only those in the lowest height growth class I. Thus, the presence of Douglas fir regeneration is positively correlated with browsing of Norway spruce and oak, but negatively correlated with the distance to the next diaspore source and with the diffuse light availability [indirect site factor (%); Figure 5]. However, the latter result may be due to the fact that our sample plots were not equally distributed along the light gradient (see section “Discussion”; Table 5).
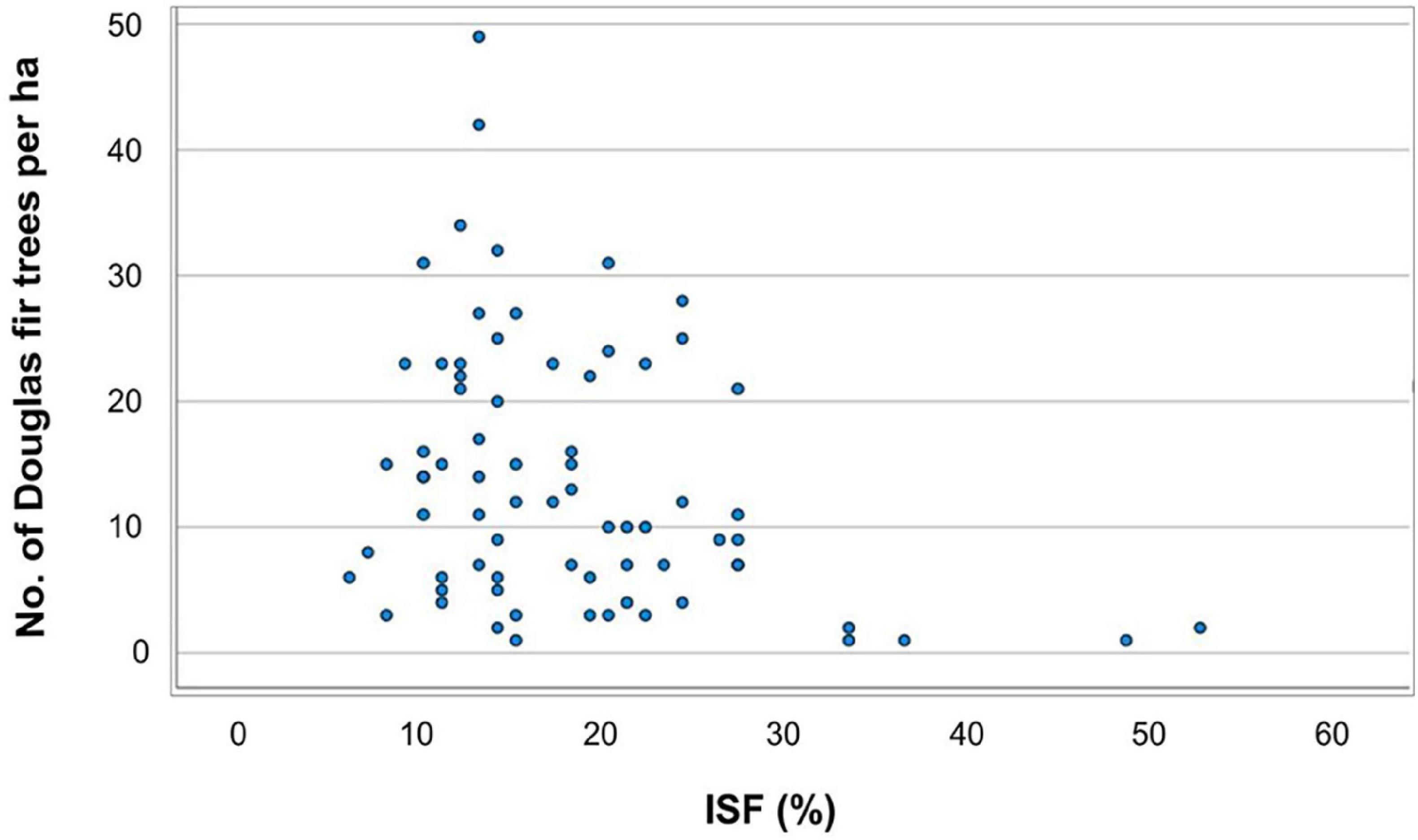
Figure 5. Dependent variable “Douglas fir regeneration (all height growth classes)” along the gradient of light supply (in %) as indirect site factor (ISF).
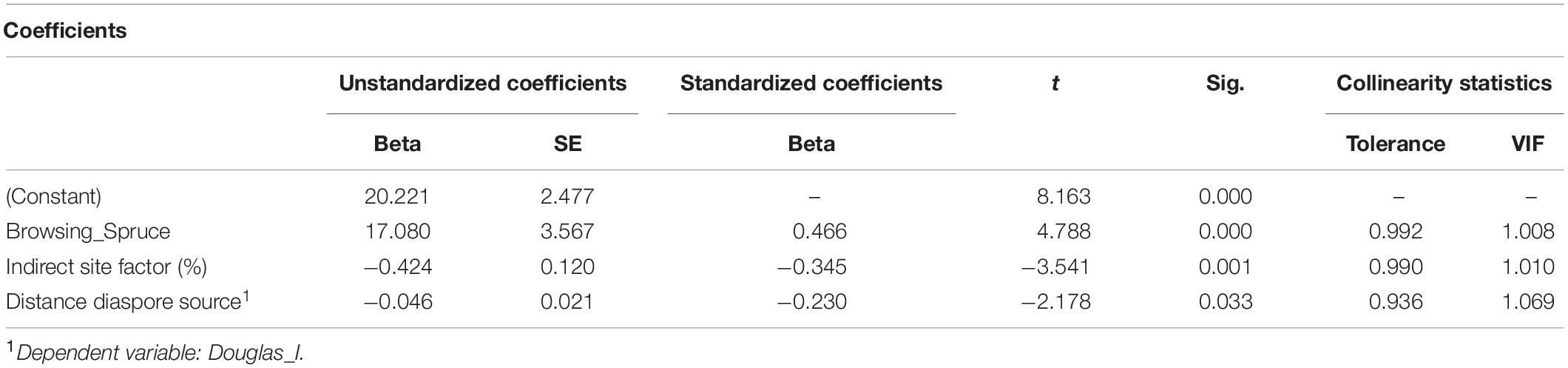
Table 5. Significant correlation of Douglas fir regeneration (all height growth classes and for distance to diaspore source only class I) with recorded environmental factors, with beta, standard error (SE), t value (t), significance (Sig.), tolerance, and variance inflation factor (VIF).
By applying the invasion model of Heger (2001), all four steps can be revealed for Douglas fir in the Spessart mountains which means that the non-native species is present (step 1), can establish spontaneously (step 2) as well as permanently (step 3), and a spread and colonization can be observed on many sites in the study area (step 4).
Our findings are based on data from 626 randomly and 105 occasionally located sample plots. With less than 10% of sample plots where Douglas fir regeneration occurred, we conclude an ability of Douglas fir to spread naturally, however not with high probability. As the analysis has shown, barely any spontaneous regeneration of Douglas fir was recorded in purely broad-leaved stands (e.g., beech and mixed oak-beech stands), whereas coniferous and mixed broad-leaved-coniferous forests, respectively, showed higher frequencies. The findings of Knoerzer et al. (1995) and Knoerzer and Reif (1996) confirm that deciduous stands, particularly if European beech is involved, are the least suitable for Douglas fir regeneration.
Our data reveal that higher soil moisture (TDiff < 20 mm), sufficient light (at least 10% of open field conditions) and close distance to seed sources are main environmental factors favoring Douglas fir regeneration in the Spessart mountains. The highest abundance of Douglas fir regeneration was found in stands with medium light levels and close distance to diaspore sources. Thus, our results confirm earlier findings that had identified the same environmental factors as important drivers of Douglas fir regeneration in Central Europe (Isaac, 1938; Lavender et al., 1968; Bindewald et al., 2021). However, this result needs careful interpretation. First, the number of plots were not equally distributed along the light gradient. Thus, we were lacking plots with established Douglas fir regeneration on plots with high light availability, but we do not know whether we could not find such plots because high light conditions restrict Douglas fir establishment or whether we just have sampled too few plots under such conditions. Second, we did not observe a clear pattern within the range of approximately 10–30% of full light. Instead, within this range the number of Douglas fir seedlings at a given light level varied strongly. We conclude that low to medium light conditions seem to promote the establishment of Douglas fir. This finding fits quite well with reports from the home range of Douglas fir in the Pacific Northwest (Hermann, 2003). However, with increasing size of the seedlings light demand is increasing as well (Hermann, 2003).
Despite many controversial contributions to the discussion on non-native species and their invasion potential, it is widely agreed on that time-lags and evolutionary effects represent uncertainties that make precise predictions of invasion risks very difficult (Kowarik, 1996; Simberloff, 2011; Heger, 2019). Careful observations of the current state of regeneration seems to be, however, a way to detect the potential of invasiveness and ongoing biological invasions (Simberloff, 2011). This supports effective management measures. In our study, we revealed the spontaneous regeneration and spread of Douglas fir in the Spessart. Thus, according to the model of Heger (2001), Douglas fir has already reached the final step of an invasion (cp. Figure 2). This assessment, however, is solely based on the presence of a non-native species in new localities. Based on the criteria of Vor et al. (2015), species are considered as “invasive” if they have negative impacts on site conditions, high reproductive potential, high spreading potential, suppressing capacity, and limited management options. The abundance of established Douglas fir regeneration can function as a proxy for the success rate of reproductive efforts (Vor et al., 2015). In our case study, the reproductive potential drastically decreased with increasing distance to mother trees. Therefore, both reproductive and spreading potential of Douglas fir in the Spessart are not considered as high, supporting recent assessments by Vor et al. (2015) and Bindewald et al. (2021). The correlation analyses further showed that no other tree species is heavily suppressed by Douglas fir at the sample plots. Accordingly, suppressing capacity, too, does not indicate invasiveness of Douglas fir at the studied plots. As discussed earlier, Douglas fir does not need much light for successful establishment of its natural regeneration. However, in later stages its light demand is strongly increasing (Hermann, 2003). As the light supply in managed forest stands is relatively easy to control, management options exist to prevent unwanted regeneration of Douglas fir. This assessment is also supported by the answers of local foresters who stated that Douglas fir is always outcompeted by beech and can easily be controlled by altering stand density. Interestingly, also Frei et al. (2022) found that Douglas fir is not able to compete with broad-leaved tree species in managed European beech forests.
Based on the data this study obtained and according to the above-mentioned criteria, we can conclude that Douglas fir is not invasive in the Spessart mountains. The fact that no occurrence of Douglas fir was found in sensitive habitats provides further support to this reasoning and underlines the conclusion of Schmid et al. (2014, p. 22) that “the existing studies suggest that forest ecosystems in Central Europe are able to deal with the introduction of Douglas fir comparably well. Until now, no severe ecological or economic consequences have been detected.” Furthermore, Kleinbauer (2010) found for a variety of climate change scenarios, that the area of suitable habitats for Douglas fir is not expected to increase. Therefore, future climate conditions might not increase the invasion risk of Douglas fir. However, uncertainties concerning possible time-lag effects remain. Lavender and Hermann (2014) state that seed production of mature Douglas fir reaches its maximum at an age of 200–300 years. According to the cultivation history in the Spessart (Albrecht, 2008; Mergner, 2018), the first trees might reach this climax in approximately 80 years. However, in most cases they will be harvested long before. Another uncertainty results from the likely increase of forest area where Douglas fir is cultivated which may increase the risk for invading sensitive habitats (Höltermann et al., 2008; Schmid et al., 2014). Additional monitoring and careful observations might be necessary in order to timely cope with potentially invasive developments due to time-lag effects and increasing cultivation area.
Our results show that the invasiveness of Douglas fir should be assessed in a differentiated approach. Our findings indicate that in the study area Spessart mountains where Douglas fir was strongly promoted in forestry during past decades does not tend to be generally invasive. On the contrary, across the relatively broad site conditions of the Spessart mountains, there were no indications that stands of other tree species worth of protection are being invaded by Douglas fir. This conclusion is in line with the large-scale assessment of Bindewald et al. (2021) who found no evidence for a high establishment and invasion potential of Douglas fir in Germany. As a precautionary measure, Douglas fir should, nevertheless, not be grown near dry and species-rich grassland, sparsely stocked rocky slopes or xerothermic open oak forests (Ammer et al., 2016). Outside these specific sites, however, Douglas fir does not seem to have any negative effects on plant diversity (Thomas et al., 2022). Especially, when mixed with the shade-tolerant European beech, the invasiveness potential of Douglas fir in Central Europe can therefore be considered very limited and controllable at any time.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CA, FL, GL, and SZ designed the study. FL conducted the data collection. FL and GL performed the data analysis. FL, CA, GL, AS, and SZ conceived, wrote, and edited the final manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Bavarian Ministry for Food, Agriculture and Forestry, Grant number F053. This manuscript was supported by the Open Access Publishing Fund of the Free University of Bozen-Bolzano.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful for the provision of forest inventory data by the Bavarian State Forest Research Center (Regensburg, Germany). We thank the local forest administration in the Spessart mountain for their fruitful cooperation.
Aerts, R., and Honnay, O. (2011). Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 11:29. doi: 10.1186/1472-6785-11-29
Ammer, C., Bolte, A., Herberg, A., Höltermann, A., Krüß, A., Krug, A., et al. (2016). Empfehlungen für den anbau eingeführter waldbaumarten. gemeinsames papier von forstwissenschaft und naturschutz. Natursch. Landschaftspl. 48, 170–171.
Arbeitsgemeinschaft Forsteinrichtung (2016). Forstliche Standortsaufnahme: Begriffe, Definitionen, Einteilungen, Kennzeichnungen, Erläuterungen. München: IHW-Verlag.
Bailey, S. A., Deneau, M. G., Jean, L., Wiley, C. J., Leung, B., and MacIsaac, H. J. (2011). Evaluating efficacy of an environmental policy to prevent biological invasions. Environ. Sci. Technol. 45, 2554–2561. doi: 10.1021/es102655j
Barnhart, S. J., McBride, J. R., and Warner, P. (1996). Invasion of northern oak woodlands by Pseudotsuga menziesii (Mirb.) Franco in the Sonoma Mountains of California. Madroño 43, 28–45.
Bayerische Vermessungsverwaltung (2020). DGM50. Available Online at: https://www.govdata.de/suchen/-/details/digitales-gelandemodell-50-m-gitterweite (accessed January 24, 2022).
Bindewald, A., Miocic, S., Wedler, A., and Bauhus, J. (2021). Forest inventory-based assessments of the invasion risk of Pseudotsuga menziesii (Mirb.) Franco and Quercus rubra L. in Germany. Eur. J. For. Res. 140, 883–899. doi: 10.1007/s10342-021-01373-0
Bologna, M., and Aquino, G. (2020). Deforestation and world population sustainability: a quantitative analysis. Sci. Rep. 10:7631. doi: 10.1038/s41598-020-63657-6
Booth, J. (1877). Die Douglas-Fichte und einige andere Nadelhölzer namentlich aus dem nordwestlichen Amerika in Bezug auf ihren forstlichen Anbau in Deutschland. Berlin: Springer.
Braun-Blanquet, J. (1964). Pflanzensoziologie. Grundzüge der Vegetationskunde. Wien: Springer, doi: 10.1007/978-3-7091-8110-2
Broncano, M. J., Vilà, M., and Boada, M. (2005). Evidence of Pseudotsuga menziesii naturalization in montane Mediterranean forests. For. Ecol. Manag. 211, 257–263. doi: 10.1016/j.foreco.2005.02.055
Brundu, G., and Richardson, D. M. (2016). Planted forests and invasive alien trees in Europe: a Code for managing existing and future plantings to mitigate the risk of negative impacts from invasions. NeoBiota 30, 5–47. doi: 10.3897/neobiota.30.7015
Dierschke, H. (1994). Pflanzensoziologie. Grundlagen und Methoden. Stuttgart: Ulmer, doi: 10.1002/fedr.19951060122
Eberhard, B., and Hasenauer, H. (2018). Modeling regeneration of Douglas fir forests in Central Europe. Austrian J. For. Sci. 135, 33–51. doi: 10.1007/s10530-019-02045-2
Eberhard, B. R., Eckhart, T., and Hasenauer, H. (2021). Evaluating strategies for the management of Douglas-Fir in Central Europe. Forests 12:1040. doi: 10.3390/f12081040
Eckhart, T., Pötzelsberger, E., Koeck, R., Thom, D., Lair, G. J., van Loo, M., et al. (2019). Forest stand productivity derived from site conditions: an assessment of old Douglas-fir stands (Pseudotsuga menziesii (Mirb.) Franco var. menziesii) in Central Europe. Ann. For. Sci. 76:19. doi: 10.1007/s13595-019-0805-3
Eckhart, T., Walcher, S., Hasenauer, H., and van Loo, M. (2017). Genetic diversity and adaptive traits of European versus American Douglas-fir seedlings. Eur. J. Forest Res. 136, 811–825. doi: 10.1007/s10342-017-1072-1
Eggert, M. (2014). Verjüngungspotenzial der Douglasie in Bayern. Naturschutz Landschaftspl. 46, 345–352.
Ferrante, L., and Fearnside, P. M. (2020). The Amazon’s road to deforestation. Science 369:634. doi: 10.1126/science.abd6977
Frei, E. R., Moser, B., and Wohlgemuth, T. (2022). Competitive ability of natural Douglas fir regeneration in Central European close-to-nature forests. For. Ecol. Manag. 503:119767. doi: 10.1016/j.foreco.2021.119767
Frischbier, N., Nikolova, P. S., Brang, P., Klumpp, R., Aas, G., and Binder, F. (2019). Climate change adaptation with non-native tree species in Central European forests: early tree survival in a multi-site field trial. Eur. J. For. Res. 138, 1015–1032. doi: 10.1007/s10342-019-01222-1
Goßner, M., and Simon, U. (2002). “Introduced Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) affects community structure of tree-crown dwelling beetles in a managed European forest,” in Biologische Invasionen – eine Herausforderung zum Handeln? Neobiota 1, eds I. Kowarik and U. Starfinger (Berlin: Springer), 167–179.
Goßner, M., and Utschik, H. (2002). “Douglas fir stands deprive overwintering bird species of food resource,” in Biological invasions - challenges for science. Neobiota 3, eds S. Klotz and I. Kühn (Berlin: springer), 105–112.
Heger, T. (2001). “A model for interpreting the process of invasion: crucial situations favouring special characteristics of invasive species,” in Plant Invasions. Species Ecology and Ecosystem Management, eds G. Brundu, J. H. Brock, I. Camarda, L. E. Child, and P. M. Wade (Leiden: Backhuys Publishers), 3–10.
Heger, T. (2019). “Can we predict whether a species will become invasive?” in Introduced Tree Species in European Forests: Opportunities and Challenges, eds F. Krumm and L. Vítková (Joensuu: European Forest Institute), 80–86.
Hermann, R. (2003). “Pseudotsuga menziesii (Mirb.) Franco, 1950,” in Enzyklopädie der Holzgewächse, eds P. Schütt, H. Weisgerber, H. J. Schuck, K. J. Lang, B. Stimm, and A. Roloff (Landsberg: Ecomed), 513–530.
Hildebrandt, P., Kirchlechner, P., Hahn, A., Knoke, T., and Mujica, R. (2010). Mixed species plantations in Southern Chile and the risk of timber price fluctuation. Eur. J. For. Res. 125, 935–946. doi: 10.1007/s10342-009-0284-4
Höltermann, A., Klingenstein, F., and Ssymank, A. (2008). Naturschutzfachliche Bewertung der Douglasie aus Sicht des Bundesamtes für Naturschutz (BfN). LWF Wissen 59, 74–81.
Huth, F., Körner, A., Lemke, C., Karge, A., Wollmerstädt, J., Wagner, S., et al. (2011). Untersuchungen zur Keimung und Keimlingsentwicklung der Douglasie (Pseudotsuga menziesii (Mirb.) Franco) in Abhängigkeit von Feuchte und Strahlung – ein Gewächshausversuch. Forstarchiv 82, 108–119. doi: 10.4432/0300-4112-82-10
Isaac, L. A. (1938). Factors Affecting the Establishment of Douglas fir Seedlings. USDA Circular No. 486. Washington: United States Department of Agriculture.
Jäger, E. J., Müller, F., Ritz, C. M., Welk, E., and Wesche, K. (eds) (2017). Rothmaler - Exkursionsflora von Deutschland. Gefäßpflanzen. Berlin: Springer.
Kennedy, P. G., and Diaz, J. M. (2005). The influence of seed dispersal and predation on forest encroachment into a California grassland. Madroño 52, 21–29. doi: 10.3120/0024-9637200552[21:TIOSDA]2.0.CO;2
Kleinbauer, I. (2010). Ausbreitungspotenzial ausgewählter neophytischer Gefäßpflanzen unter Klimawandel in Deutschland und Österreich. BfN Skripten 275:76.
Knoerzer, D. (1999). Zur Naturverjüngung der Douglasie im Schwarzwald. Inventur und Analyse von Umwelt- und Konkurrenzfaktoren sowie eine naturschutzfachliche Bewertung. Diss. Bot. 306, 1–283.
Knoerzer, D., Kühnel, U., Theodoropoulos, K., and Reif, A. (1995). “Zur Aus- und Verbreitung neophytischer Gehölze in Südwestdeutschland mit besonderer Berücksichtigung der Douglasie (Pseudotsuga menziesii),” in Gebietsfremde Pflanzenarten. Auswirkungen auf einheimische Arten, Lebensgemeinschaften und Biotope. Kontrollmöglichkeiten und Management, eds R. Böcker, H. Gebhardt, W. Konold, and S. Schmidt-Fischer (Landsberg: Ecomed), 67–81.
Knoerzer, D., and Reif, A. (1996). Die Naturverjüngung der Douglasie im Bereich des Stadtwaldes von Freiburg. AFZ Der Wald 20, 1117–1121.
Knoerzer, D., and Reif, A. (2002). “Fremdländische Baumarten in deutschen Wäldern,” in Biologische Invasionen - eine Herausforderung zum Handeln? Neobiota 1, eds I. Kowarik and U. Starfinger (Berlin: Springer), 27–35.
Kowarik, I. (1996). Auswirkungen von Neophyten auf Ökosysteme und deren Bewertung. Texte des Umweltbundesamtes 58, 119–155.
Kowarik, I. (2010). Biologische Invasionen. Neophyten und Neozoen in Mitteleuropa. Stuttgart: Ulmer.
Kutner, M. H., Nachtsheim, C. J., and Neter, J. (2003). Applied Linear Regression Models, 4th Edn. Illinois: Irwin Professional Pub.
Langmaier, M., and Lapin, K. (2020). A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests. Front. Plant Sci. 11:1349. doi: 10.3389/fpls.2020.524969
Lavender, D. P., Ching, K. K., and Hermann, R. K. (1968). Effect of environment on the development of dormancy and growth of Douglas-Fir seedlings. Bot. Gaz. 129, 70–83. doi: 10.1086/336415
Lavender, D. P., and Hermann, R. K. (2014). Douglas-fir - The Genus Pseudotsuga. Corvallis: Oregon State University Press.
Linnakoski, R., Kasanen, R., Dounavi, A., and Forbes, K. M. (2019). Forest health under climate change: effects on tree resilience, and pest and pathogen dynamics. Front. Plant Sci. 10:1157. doi: 10.3389/fpls.2019.01157
Mansourian, S., Vallauri, D., and Dudley, N. (eds) (2005). Forest Restoration in Landscapes. Beyond Planting Trees. New York: Springer.
Matthes, S., and Okrusch, M. (1965). Sammlung Geologischer Führer Band 44 Spessart. Berlin: Gebrüder Borntraeger.
McEwan, A., Marchi, E., Spinelli, R., and Brink, M. (2020). Past, present and future of industrial plantation forestry and implication on future timber harvesting technology. J. For. Res. 31, 339–351. doi: 10.1007/s11676-019-01019-3
Mengist, W., and Soromessa, T. (2019). Assessment of forest ecosystem service research trends and methodological approaches at global level: a meta-analysis. Environ. Syst. Res. 8:22. doi: 10.1186/s40068-019-0150-4
Mergner, W. (2018). 120 Jahre Douglasienanbau im ehemaligen Forstamt Heigenbrücken. Geschichtsbl. Heigenbrücken 2018, 24–27.
Messinger, J., Güney, A., Zimmermann, R., Ganser, B., Bachmann, M., Remmele, S., et al. (2015). Cedrus libani: a promising tree species for Central European forestry facing climate change? Eur. J. For. Res. 134, 1005–1017. doi: 10.1007/s10342-015-0905-z
Mette, T., Kolb, J., Schuster, O., Falk, W., and Klemmt, H. J. (2019). BaSIS-Wasserhaushalt wird bodensensitiver. Bayerisches Standortinformationssystem jetzt mit aktualisiertem Wasserhaushalt. LWF Aktuell 2, 50–52.
Meynen, E., and Schmithüsen, J. (1953). Handbuch der Naturräumlichen Gliederung Deutschlands. Remagen: Bundesanstalt für Landeskunde.
Nehring, S., Kowarik, I., Rabitsch, W., and Essl, F. (eds) (2013). Naturschutzfachliche Invasivitätsbewertungen für in Deutschland wild lebende gebietsfremde Gefäßpflanzen. BfN Skripten 352, 35–202.
Ohse, B., Seele, C., Holzwarth, F., and Wirth, C. (2017). Different facets of tree sapling diversity influence browsing intensity by deer dependent on spatial scale. Ecol. Evol. 7, 6779–6789. doi: 10.1002/ece3.3217
Oxbrough, A., García-Tejero, S., Spence, J., and O’Halloran, J. (2016). Can mixed stands of native and non-native tree species enhance diversity of epigaeic arthropods in plantation forests? For. Ecol. Manag. 367, 21–29. doi: 10.1016/j.foreco.2016.02.023
Palmer, M., Bernhardt, E., Chornesky, E., Collins, S., Dobson, A., Duke, C., et al. (2004). Ecology for a crowded planet. Science 304, 1251–1252. doi: 10.1126/science.1095780
Parrotta, J. A., and Trosper, R. L. (eds) (2012). Traditional Forest-Related Knowledge. Sustaining Communities, Ecosystems and Biocultural Diversity. New York: Springer.
Podrázský, V., Martiník, A., Matejka, K. J., and Viewegh, J. (2014). Effects of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) on understorey layer species diversity in managed forests. J. For. Sci. 60, 263–271.
Pötzelsberger, E., Spiecker, H., Neophytou, C., Mohren, F., Gazda, A., and Hasenauer, H. (2020). Growing non-native trees in European forests brings benefits and opportunities but also has its risks and limits. Curr. For. Rep. 6, 339–353. doi: 10.1007/s40725-020-00129-0
Rayment, G. E., and Higginson, F. R. (1992). Australian Laboratory Handbook of Soil and Water Chemical Methods. Melbourne: Inkata Press Pty Ltd.
Sankey, T. T. (2008). Spatial patterns of Douglas-fir and aspen forest expansion. New For. 35, 45–55. doi: 10.1007/s11056-007-9062-7
Schmid, M., Pautasso, M., and Holdenrieder, O. (2014). Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 133, 13–29. doi: 10.1007/s10342-013-0745-7
Speidel, G. (1955). Die Wertklasse als Gütemaßstab in der Forsteinrichtung. Forstarchiv 26, 217–224.
Spellmann, H., Weller, A., Brang, P., Michiels, H. G., and Bolte, A. (2015). “Douglasie (Pseudotsuga menziesii (Mirb.) Franco),” in Potenziale und Risiken Eingeführter Baumarten. Baumartenportraits mit Naturschutzfachlicher Bewertung, eds T. Vor, H. Spellmann, A. Bolte, and C. Ammer (Göttingen: Universitätsverlag Göttingen), 187–217. doi: 10.17875/gup2015-843
Spiecker, H., Lindner, M., and Schuler, J. K. (2019). Douglas-fir: An Option for Europe? Joensuu: European Forest Institute.
Starfinger, U., Kowarik, I., Rode, M., and Schepker, H. (2003). From desirable ornamental plant to pest to accepted addition to the flora? – the perception of an alien tree species through the centuries. Biol. Invasions 5, 323–335. doi: 10.1023/B:BINV.0000005573.14800.07
Terwei, A., Zerbe, S., Zeileis, A., Annighöfer, P., Kawaletz, H., Mölder, I., et al. (2013). Which are the factors controlling tree seedling establishment in North Italian floodplain forests invaded by non-native tree species? For. Ecol. Manag. 304, 192–203. doi: 10.1016/j.foreco.2013.05.003
Thomas, F. M., Rzepecki, A., and Werner, W. (2022). Non-native Douglas fir (Pseudotsuga menziesii) in Central Europe: ecology, performance and nature conservation. For. Ecol. Manage. 506:119956. doi: 10.1016/j.foreco.2021.119956
Tschopp, T., Holderegger, R., and Bollmann, K. (2014). Auswirkungen der Douglasie auf die Waldbiodiversität: eine Literaturübersicht. WSL Berichte 20, 1–55. doi: 10.7788/9783412514655.front
Valéry, L., Fritz, H., Lefeuvre, J. C., and Simberloff, D. (2008). In search of a real definition of the biological invasion phenomenon itself. Biol. Invasions 10, 1345–1351. doi: 10.1007/s10530-007-9209-7
Vor, T., Spellmann, H., Bolte, A., and Ammer, C. (eds) (2015). Potenziale und Risiken eingeführter Baumarten. Baumartenportraits mit Naturschutzfachlicher Bewertung. Göttingen: Universitätsverlag Göttingen.
Wohlgemuth, T., Hafner, J., Höltermann, A., Moser, B., Nehring, S., and Rigling, A. (2019). “Impact of Douglas-fir on forests and open land habitats,” in Douglas-fir – An Option for Europe?, eds H. Spiecker, M. Lindner, and J. Schuler (Joensuu: European Forest Institute), 57–62.
Wohlgemuth, T., Moser, B., Pötzelsberger, E., Rigling, A., and Gossner, M. M. (2021). Über die Invasivität der Douglasie und ihre Auswirkungen auf Boden und Biodiversität. Schweiz. Z. Forstwes. 172, 118–127. doi: 10.3188/szf.2021.0118
Zerbe, S. (1999). Die Wald- und Forstgesellschaften des Spessarts mit Vorschlägen zu deren zukünftigen Entwicklung. Mitt. Naturwiss. Mus. Aschaffenburg 19:354.
Zerbe, S. (2003). “The role of land use in the differentiation of cultural landscapes – a historical perspective,” in Multifunctional LandScapes: Continuity and Change. Advances in Ecological Sciences 16, Vol. III, eds Ü. Mander and M. Antrop (Southampton: WIT Press), 95–114.
Zerbe, S. (2004). Influence of historical land use on present-day forest patterns: a case study in south-western Germany. Scand. J. For. Res. 19, 261–273. doi: 10.1080/02827580410029291
Keywords: biological invasion, forest management, regeneration, Spessart mountains, risk assessment
Citation: Lange F, Ammer C, Leitinger G, Seliger A and Zerbe S (2022) Is Douglas Fir [Pseudotsuga menziesii (Mirbel) Franco] Invasive in Central Europe? A Case Study From South-West Germany. Front. For. Glob. Change 5:844580. doi: 10.3389/ffgc.2022.844580
Received: 28 December 2021; Accepted: 26 January 2022;
Published: 21 February 2022.
Edited by:
Piotr S. Mederski, Poznań University of Life Sciences, PolandReviewed by:
Panayiotis G. Dimitrakopoulos, University of the Aegean, GreeceCopyright © 2022 Lange, Ammer, Leitinger, Seliger and Zerbe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Zerbe, c3RlZmFuLnplcmJlQHVuaWJ6Lml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.