- Institute of Environment & Sustainable Development, Banaras Hindu University, Varanasi, India
Home gardening is an indigenous practice of cultivation that has effectively adapted to local ecological conditions over generations. This study examined the effects of disturbance and garden size on biodiversity to develop a better understanding of vegetation cover and its role in livelihood and provision of forest management in the Vindhyan highlands. Data were collected from 60 gardens which were classified into large (> 650 m2), medium (400–650 m2), and small (< 400 m2), based on size and disturbance gradients viz., high, medium, and low. A total of 133 species from 50 families were recorded, in which trees (47.4%) were dominant followed by shrubs (18%) and herbs (16.5%). With respect to disturbance, the highest number of tree species (39) were found at low disturbance (LD) followed by 33 species in medium disturbance (MD) and 32 species in high disturbhance (HD). The total mean richness of species was greater at LD (20.3 ± 2.3) and lowest at HD (18.5 ± 2.2). Tree density was significantly (P ≤ 0.05) higher at LD (293.75 ± 16.1 individual ha–1) as compared to MD (221 ± 11.5 individual ha–1) and HD (210 ± 10.3 individual ha–1). However, the results for shrubs and herbs density were considerably different, where shrubs density was highest at HD (70 ± 6.9 individual per 1,000 m2) and lowest at LD (62.5 ± 5.8 individual per 1,000 m2), while the maximum density of herbs was recorded at MD (466.25 ± 29.8 individual per 100 m2) and minimum at LD (370 ± 21.4 individual per 100 m2). The summed dominance ratio indicated frequent use of garden plants in bio-fencing, vegetables, ornamental, and ethnomedicine. Diversity (P < 0.01) and species richness (P < 0.05) showed a significant positive correlation with garden size. The Principal Component Analysis (PCA) showed that the first component (PC1) accounted for 28.6% of variance, whereas the second explained 21.9% of variance in both disturbance and garden size with a cumulative variance of 50.5%. These components depicted the positive association with HD (14.34), SDiv (13.91), TCD (12.47), and HDiv (12.09). We concluded that the diversity of home gardens changed with disturbance, which crucially served as a refuge for native tree species in a degraded landscape. This pattern highlighted the importance of home gardens for plant biodiversity conservation and local livelihood, which must be a viable option for regeneration of deforested dry tropics, while also reducing the burden on dry tropical forest regions.
Introduction
Plants are the most important component of worldwide biodiversity, ensuring environmental sustainability and food security. Tropical forests contribute about 30% of global land covers and account for 50% of forest area (FAO, 2000, 2018). The prevailing vegetation of tropical forest is tropical dry forest (TDF) which occupies 42% of the forest cover (Galicia et al., 2008). The structure and distribution of tropical forests diversity are consequently changed by natural and human-induced factors at various spatial scales (Barlow et al., 2016; Betts et al., 2017). Anthropogenic disturbances have caused an extensive modification in the tropical forest which has consequently led to the loss of global biodiversity. Biodiversity loss is one of the most pressing threats to the TDF system which increasingly leads to the conversion of dense forest to scattered patches and savanna (Chaturvedi et al., 2011; Díaz et al., 2018). These threats have undergone extreme alteration in forms of habitat loss, forest fragmentation, and conversion of forest to agriculture (Laurance, 2007; Sharma et al., 2021). Along with these disturbances, tropical home gardens harbor rich floristic diversity which is equivalent to the natural vegetation of the surrounding ecosystem and potentially rejuvenate the degraded forest system (De Clerck and Negreros-Castillo, 2000; Shastri et al., 2002; Albuquerque et al., 2005). They are a diverse micros ecosystem, composed of various woody and non-woody vegetation with the association of wild and cultivated crops (Kumar and Nair, 2004; Kehlenbeck et al., 2007). In the current global climate change scenario, home gardens also act as carbon sinks and play an important ecological role in climate change mitigation (Saha et al., 2009).
Home gardening is a land-use practice involving deliberate conservation of multipurpose vegetation in intimate association with agricultural crops and livestock within the complex of individual houses. This system deliberately incorporates the safeguarding of forest species in association with agriculture crops and a large number of semi-domesticated plant species (Casas et al., 1997; Moreno-Calles et al., 2014). These practices create a mosaic of vegetation working as biological corridors at the landscape level which act as favorable habitat for a variety of related species (Perfecto and Vandermeer, 2008). These activities improve the availability of plant resources demanded by people as well as protecting and restoring the forest vegetation (Noble and Dirzo, 1997; Moreno-Calles et al., 2010). A large range of diversity is often emphasized as a characteristic feature of home gardens, especially in the tropical region (Peyre et al., 2006; Sunwar et al., 2006; Tiyakoat et al., 2010). Compared to the cultivated agroecosystem, gardening practices are a more diverse system that occasionally safeguards the diversity of surrounding natural forests (Sampanpanish and Jamroenprucksa, 1994; Gajaseni and Gajaseni, 1999).
The home gardening practice has received worldwide attention, and numerous studies have been reported from tropical as well as temperate regions of the world (Vlkova et al., 2011; Calvet-Mir et al., 2012; Clarke et al., 2014; Glavan et al., 2018; Boneta et al., 2019). A large number of studies are available from South Asia (Peyre et al., 2006; Mohri et al., 2013; Martin et al., 2019). Garden systems are essential to ensure required nutrition and food security, especially in the lower income countries of Africa and Asia (Maroyi, 2009; Galhena et al., 2013; Mellisse et al., 2018; Whitney et al., 2018a). The role of the home garden in the conservation and management of native species in TDF was reported by Bhagwat et al. (2008), Panyadee et al. (2018), Rendón-Sandoval et al. (2020). Home gardens in various countries contain about 50-80% of the plant diversity of their adjacent forests areas (Noble and Dirzo, 1997; Panyadee et al., 2016). Various studies also highlighted that the vegetation diversity of tropical home gardens was similar to the surrounding forest ecosystem (Shastri et al., 2002; Albuquerque et al., 2005). The role of these mosaics of forests for biological corridor formation at the landscape level characterized the importance of garden plants in habitat conservation and restoration of forest ecosystem (Perfecto and Vandermeer, 2008; Moreno-Calles et al., 2010).
In India, most of the marginal and tribal population of both rural and urban regions depend on home gardening for the subsidiary requirement of medicine, food, vegetables, and economic well-being, especially in the north-eastern and Himalayan regions (Sahoo and Rocky, 2015; Bargali, 2016), where several wild varieties of plant and crop species have been domesticated (Saikia et al., 2012; Padalia et al., 2015; Barbhuiya et al., 2016). Home gardens in Kerala have multipurpose implications of socio-economic and environmental sustainability (Peyre et al., 2006; Jeyaprabakaran and Rajendran, 2020). The conservation and management of key species in home gardens of the Pachmarhi biosphere reserve and the State of Jharkhand were reported by Kala (2010) and Shukla et al. (2017). However, no studies have been conducted on the vegetation diversity of home gardens with respect to environmental disturbance, especially in the Vindhyan highlands of India.
The Vindhyan region is one of the most densely forested regions of eastern Uttar Pradesh, India, characterized by a dry tropical monsoon climate with nine months of dry period (Singh et al., 2010). The majority of agriculture in the region is rainfed, which produces insufficient food for the people’s requirements. These circumstances result in lesser production, destruction of biodiversity, starvation, and malnutrition among the young population. About 11.5–49.7% of food requirements are fulfilled by the collection of wild food from the nearby forest and gardening (Singh and Singh, 1992). The expanding population in the region demands more farmlands for cultivation, thereby resulting in natural forest degradation, soil erosion, greenhouse gas (GHG) emission, and food insecurity (Deng et al., 2014; Tiwari et al., 2018). There are many studies from this region, which mainly focused on forest ecology (Sagar et al., 2003; Tiwari et al., 2019), biological invasion (Sharma and Raghubanshi, 2009), ethnomedicine (Singh et al., 2002, 2012), and agroecosystem (Singh and Singh, 1992). Nevertheless, there is still a gap in the research regarding the biodiversity of home gardens and their contribution to forest conservation and the livelihoods of locals to mitigating environmental disturbances in the dry tropical region of eastern Uttar Pradesh. This is the first report on species composition of indigenous gardens and their utilization in different use categories along with different disturbances. The main objective of this study was (i) to assess the effect of disturbance gradient and garden size on the distribution of plant diversity in home gardens. In addition, (ii) we aimed to advance the understanding of the value of garden plants in different use categories, forest management, and the livelihood of society. This study will provide opportunities to enhance the fundamental knowledge that how vegetation organization directly affects forest conservation and worldwide food security. In addition, this paper will highlight useful specific indicators for policy-making and planning to formulate management strategies of natural forest as well as food supplements in the highly disturbed dry tropical region of India.
Materials and Methods
Study Area
The study was conducted in three villages namely Hathawani, Majhouli, and Birar in the Duddhi Block of the Sonbhadra district, Uttar Pradesh (210 29′–250 11′ N and 780 15′–840 15′ E), situated in the Vindhyan Plateau, India (Figure 1). The average distance between these villages was 21.4 km. It is the most forested district of eastern Uttar Pradesh, where 36.79% of the geographical area is covered with forest. In total forest cover, 130 km2 is occupied by dense forest, 967 km2 moderate, and 1,443.29 km2 with open forest (Forest Survey of India [FSI], 2019). The district shares a border with four States, namely Madhya Pradesh in the west, Chhattisgarh in the south, Jharkhand in the south-east, and Bihar in the north-east. The climate is a tropical monsoon having three distinct seasons viz., summer, rainy, and winter. The annual rainfall fluctuates from 850 to 1,300 mm and most of the rain is received in the three months of June, July, and August. The remaining nine months are dry and suffer from moisture stress (Singh et al., 2010). The average monthly temperature of the region varies from 18.5°C to 43.7°C (Singh et al., 2010). Soils are ultisol, sandy loam, reddish to dark-gray in color, and highly nutrient-deficient (Singh et al., 1989). The study area is dominated by the tribal and marginalized community, which constitutes about 43.31% of the total population (Census of India, 2011). The deprived community of the region intensively depends on agriculture and the forest for food and nutrition. Physio-graphically, the region is characterized as a plateau and contains escarpments, hillocks, east-west trending gorse-lie valleys with flat basins as well as flat-topped ridges (Sagar et al., 2003).
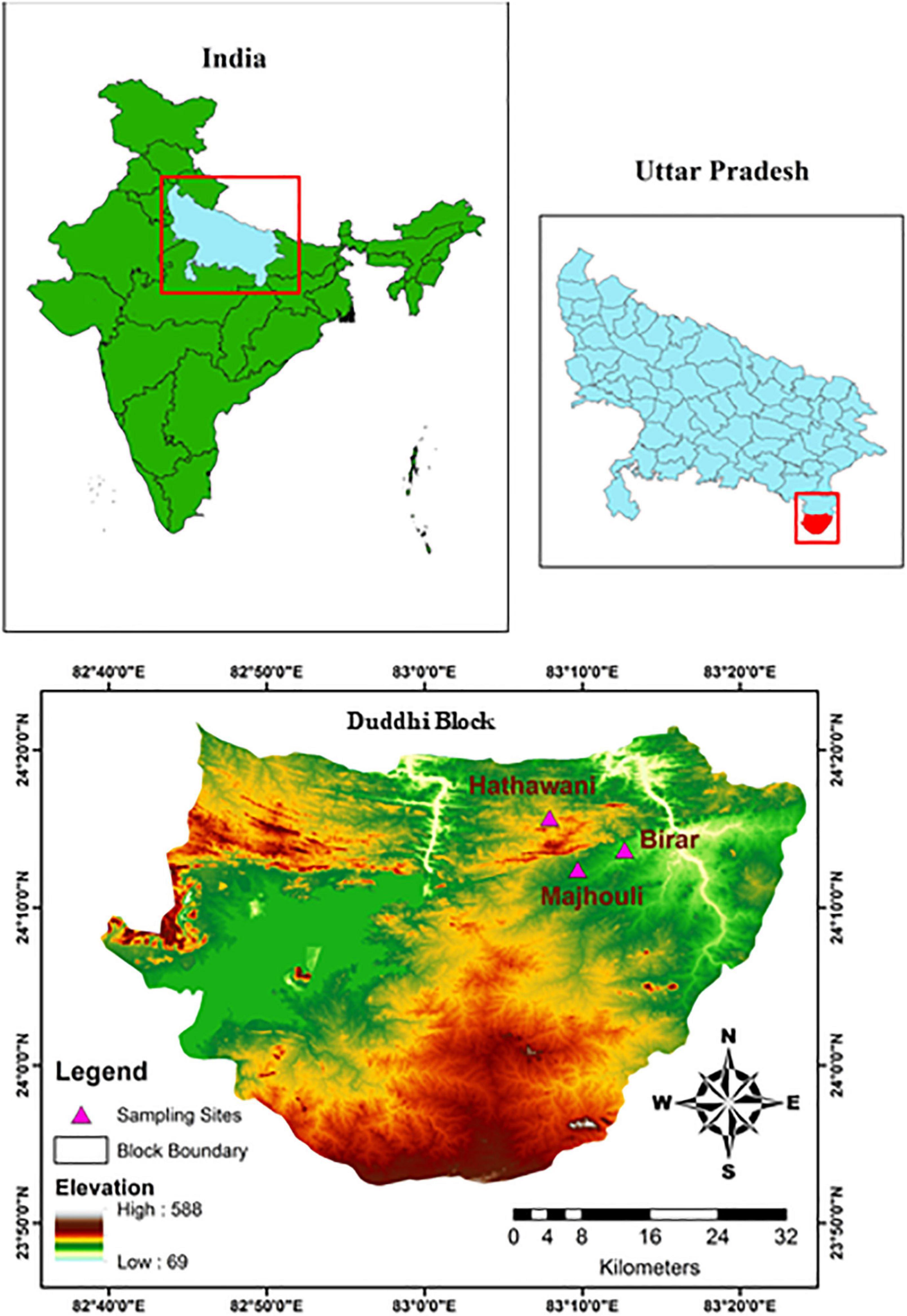
Figure 1. Geographical location showing three study villages (Hathawani, Majhouli, Birar) in a dry tropical region of Vindhyan highlands, India.
Data Collection
After reconnaissance of the study site, three villages namely Hathawani, Majhouli, and Birar were selected (based on distance from the center of Duddhi town) to represent the entire region of the study area. The study sites were ranked based on environmental disturbance representing both natural and human-made disturbance regimes. Anthropogenic disturbance included grazing, collection of non-timber products, cutting and lopping of herbs, shrubs, and trees for fodder and fuelwood, while natural disturbances included soil rockiness, soil erosion, forest fire, and drought. The disturbance regimes were evaluated for their relative impact on each of the three study sites (Table 1). Habitation close to markets and highways experienced more utilization pressure, and thus displayed higher disturbance. Therefore, a site with maximum distance from market and highway was assigned 1 impact factor. The impact for other sites was calculated as a ratio of the distance of this site to the distance of the other sites (Sagar et al., 2003). Tree stem cut cover (SCC) of a site was calculated following Malik et al. (2014), where, the percentage cut of SCC was calculated compared to the total basal cover of the tree. Whereas, the grazing impacts were analyzed following Chaturvedi et al. (2012) and Sahoo et al. (2020). The collection of non-timber products and attacks of wild animals from the nearby forest was determined by asking the individuals (N = 50) at each site. The agricultural impact was estimated by calculating the proportion of farmers in the total population of the village. On the other hand, a village with a high population was considered as highly impacted by population and vice-versa. In the above-mentioned disturbance, viz., cutting of SCC, grazing, collection of non-timber, and attack by wild animals had assigned 1 impact for low disturbance score. The succeeding higher impacts were assigned for greater disturbance score compared to the impact of the lower disturbance. For example, the percentage cut of SCC disturbance for Hathwani was minimum (9.34%) given 1 impact. The percentage cut of SCC for Majhouli (23.35%) and Birar (84.06%) was calculated as 23.35/9.34 equal to 2.5 and 84.06/9.34 to 9 impact for Majhouli and Birar, respectively. The overall disturbance index of a site was determined by the sum of all factors assigned to different factors (Table 1).
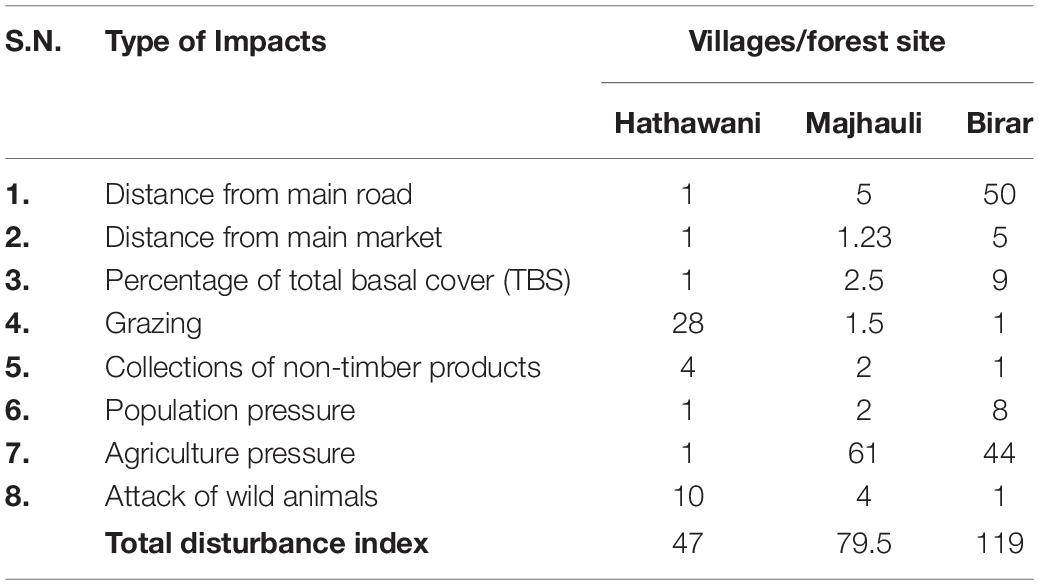
Table 1. Scoring of disturbance factors (score for related impact) at each of the three dry tropical village/forest sites as highly disturb (HD), moderately disturb (MD), and low disturb (LD).
A field survey of the study area was conducted from July 2017 to September 2019. We used purposive sampling to identify the variability in the type as well as the size of home gardens. Based on the disturbance index score, Hathawani was assigned as low disturbed (LD) with disturbance index (47), Majhouli had medium disturbed (MD) 79.5, and Birar as highly disturbed (HD) with disturbance index (119). From each disturbance, 20 home gardens (a total of 60 gardens) were selected for the detailed study. Large variability was found among the size of the garden, thus based on size, home gardens were classified into three size classes, large size home garden (LSG) > 650 m2, medium-size home garden (MSG) 400-650 m2, and small size home garden (SSG) < 400 m2 at each of the study villages (Subba et al., 2017; Vibhuti et al., 2018).
The investigation of the vegetation composition of each garden was performed by selecting a 500 m2 plot on each home garden to maintain uniformity in the area of gardens (Bernholt et al., 2009). The vegetation composition of 60 home gardens (20 from each village) with LSG 28, MSG 20, and SSG 12 were executed by the quadrats method. Trees were sampled in four quadrats of (10 × 10 m) size, within these quadrants, four (5 × 5 m) quadrats laid for shrubs and eight (1 × 1 m) quadrats for herbs (Barbhuiya et al., 2016). The identification of vegetation in the wild environment was done through local experts and officials of the forest department. The scientific identification was confirmed with the help of the International Plant Names Index1, Tropicos2, GRIN-Taxonomy3 and reference books (Khare, 2008; Joshi, 2019). Disturbance index and area of each home garden were measured, while sampling was organized in the peak growing season of plants (July-September and December-February). During sampling, the name of plants, habit, number, and their life-forms were noted down. Utilization of vegetation in different uses was asked and classified into different use categories namely fruits, vegetables, medicinal, ornamentals, fencing, and spices. Vegetation such as Madhuca indica, Ficus spp., Lagerstroemia parviflora, and Terminalia alata which are used as liquor, shade, timber, and fuelwood were grouped in the “other” use category.
Data Analysis
The number of species in each of the quadrats was counted and a significant difference between the mean numbers of species richness was estimated with the help of a t-test. Garden level density, frequency, and dominance were calculated, while important value index (IVI) was analyzed by adding, relative frequency, relative density, and relative dominance, following Cottom and Curtis (1956). Species diversity was calculated by Shannon and Weaver (1963) index of diversity (Equation1).
Where H’ = Shannon index of diversity; Pi is the proportion of the important value of the ith species (Pi = Ni / N), (Ni is the important value index of ith species and N is the important value index of all the species).
Simpson’s (1949) index of Dominance was determined by Equation 2.
Pielou (1969) species evenness index (E) by Equation 3.
Where H’ is the Shannon-Weaver diversity index and S is the total number of species.
Data obtained from various gardens on vegetation density, species richness, evenness, diversity indices, basal covers, and concentration of dominance for trees, shrubs, and herbs in two consecutive seasons (monsoon and winter) of a year were subjected to one-way-ANOVA and Post-Hoc (Tukey’s HSD) test to analyze the significant difference along with disturbance gradient and garden size. Heat map clustering was drawn to know the distribution of plant species in the different families at the different study sites. Pearson’s correlation analysis was performed to observe the interrelationships among the different diversity parameters of the garden. These statistical analyses were executed using SPSS software (ver. 23) package. To know the significant use of various plants in different use categories, summed dominance ratio (SDR) was calculated (McCune and Grace, 2002). In which, single SDR for each species was calculated by dividing IVI by 3, followed by adding up the single SDR values of all species within each of the use categories (Bernholt et al., 2009; Widayat et al., 2019). Multiple linear regression analysis was performed to know the factors determining the richness of species, diversity, and dominance in GraphPad Prism version 6. In these analyses, dependent variables were species richness (total number of species in each garden), diversity index, local species richness, and number of species’ used in different use categories. Independent variables included garden size, the distance of the garden from the local market, and disturbance index of each garden. We further applied Principal Component Analysis (PCA) using the “FactoMiner” package in R software (Lê et al., 2008), and “FactoExtra” package for visualization (Kassambara and Mundt, 2017). “FactoMiner” normalized the data before analysis, while “Multi-co-linearity” was reduced by removing parameters with a correlation coefficient higher than ± 0.7 (Verma et al., 2020).
Results
Species Composition, Richness, and Diversity
Sixty home gardens were investigated that harbored a diverse layer of useful plant species. A total of 133 species were recorded from 60 home gardens comprising three villages, in which trees were the dominating life form, comprising of 63 species (47.4%) followed by herbs with 24 species (18%) and 22 species of shrubs (16.5%) (Appendix 1). Climber vine species showed significant presence, contributing 22 species (16.5%) of which the majority were vegetables. The species found solely belonged to succulent and grass habit. In total, 81 species (60.9%) were wild, 31 species were cultivated (23.3%), and 21 species (15.8%) were both wild and cultivated. The area of the home gardens varied from 225 to 1,280 m2 with a mean area of 648 ± 249.3 m2 (Table 2). The total number of species in each garden ranges from 14 to 25, with a mean number of species 19.25 ± 2.45. Diversity in the home garden was a result of the intra-garden variations in composition. Based on the diversity and species richness analysis, the total species cover was found to come under 50 families. Fabaceae (20 species), Cucurbitaceae (14), and Moraceae (7) were the dominating families, followed by Apocynaceae (6), Combretaceae, and Euphorbiaceae (5 species each) across all sites. Out of the total, 27 families were mono-species (Figure 2). The heat map showed the domination of 14 families at Hathawani, in which Lamiaceae, Anacardiaceae, Mimosaceae, Cucurbitaceae, and Fabaceae were principal. While there were three dominant families at Majhouli, being Lythraceae, Myrtaceae, and Liliaceae, and seven for Birar including Moraceae, Rutaceae, and Apiaceae (Figure 2).
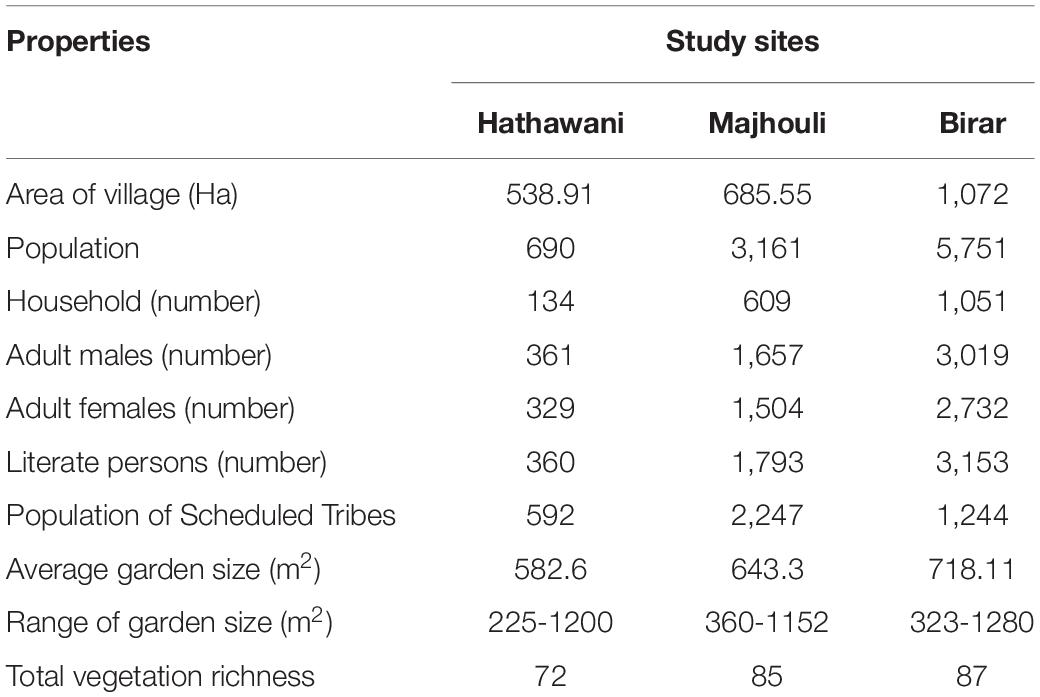
Table 2. Biophysical and demographic characteristics of three selected villages Hathawani (LD), Majhouli (MD), and Birar (HD).
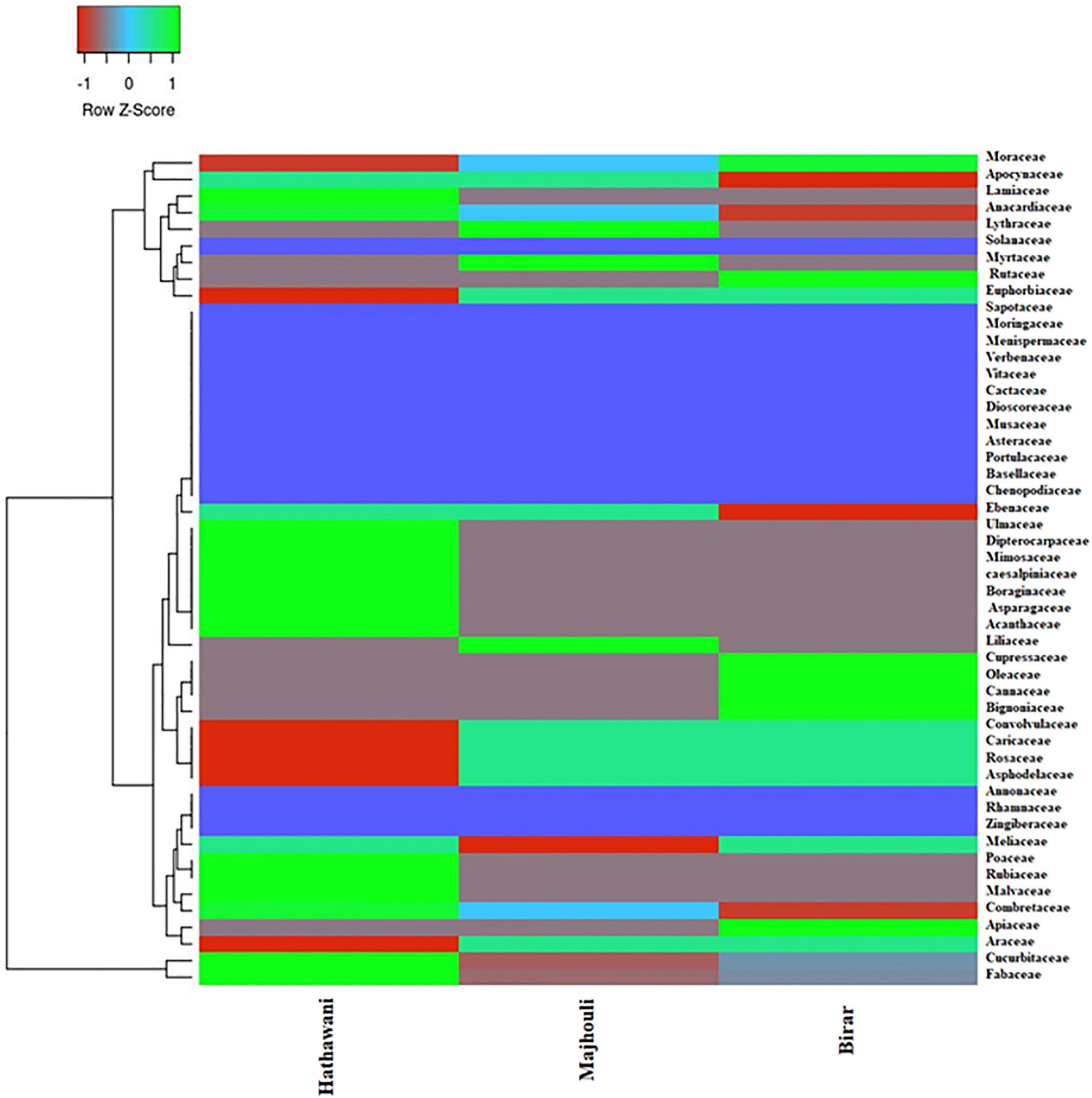
Figure 2. Heat map clustering showing the distribution of species individual under various families along different disturbances – low (Hathawani), Medium (Majhouli), and High (Birar) in a dry tropical region of the Vindhyan highlands, India. The extent of individual species is shown from green to red color on the basis of z-score.
Effect of Disturbance Gradient on the Plant Species Composition
Forest disturbances affect the distribution of vegetation in home gardens. In response to disturbance gradient, the highest number of tree species (39 species) were found in LD (Hathawani) followed by 33 species in MD (Majhouli) and 32 species in HD (Birar). In the case of herbs, 39 species were found in Hathawani and 33 species each from Majhouli and Birar, respectively. The result was different for shrubs, where maximum species were recorded from MD with 20 species (Appendix 1). The total mean species richness was greater for Hathawani (20.3 ± 2.3) followed by Majhouli (19.1 ± 2.6) and lower for Birar (18.5 ± 2.2). Shannon-Weaver diversity index was also in line with a similar trend along with different disturbance gradients, as Hathawani (2.7 ± 0.13), Majhouli (2.6 ± 0.21), and Birar (2.6 ± 0.13) respectively.
The highest mean tree density was recorded from LD (293.75 ± 16.1 individual ha–1) and the lowest from HD (210 ± 10.3 individual ha–1), but the results for shrubs and herbs density varied considerably. The maximum shrubs density was reported for HD (70 ± 6.9 individual per 1,000 m2) and minimum for LD (62.5 ± 5.8 individual per 1,000 m2). On the other hand, the MD site had reported the highest herbs density (466.25 ± 29.8 individuals per 100 m2) in comparison to HD (463.13 ± 37.9 individuals per 100 m2) and LD (370 ± 21.4 individuals per 100 m2) site (Figure 3). The total basal cover (TBC) of trees showed a mixed result similar to shrubs and herbs, where the highest TBC was at HD (170.38 ± 10.5 m2 ha–1) and the least at MD (117.72 ± 13.4 m2 ha–1). In terms of diversity index, Shannon-Weaver index for trees was greater at LD (1.88 ± 0.05) and lesser at MD (1.37 ± 0.04), while in shrubs, Shannon-Weaver index was high for HD (1.18 ± 0.05) followed by LD (1.01 ± 0.06) and MD (0.91 ± 0.06). In the case of herbs, the highest diversity index was recorded for HD (2.09 ± 0.05), while the lowest for MD (2.02 ± 0.06) and LD (1.93 ± 0.05). MD (0.19 ± 0.01) had the most concentration of dominance for trees, with the least differences in HD (0.17 ± 0.01) and LD (0.16 ± 0.01). Similar patterns were observed for shrubs, however, in the case of herbs, the trend was as follows LD > MD > HD (0.17 ± 0.01 > 0.16 ± 0.01 > 0.15 ± 0.01). The maximum species richness for trees was reported in LD (7.40 ± 0.26), and minimum in MD (6.20 ± 21), whereas the opposite result was observed for shrubs, where species richness was high in HD (3.65 ± 0.18) and low in MD (2.80 ± 0.17). In the case of trees, a greater species evenness was reported from HD (0.97 ± 0.01), MD (0.96 ± 0.01), and LD (0.96 ± 0.01), while shrubs and herbs displayed similar patterns for species evenness (Figure 3).
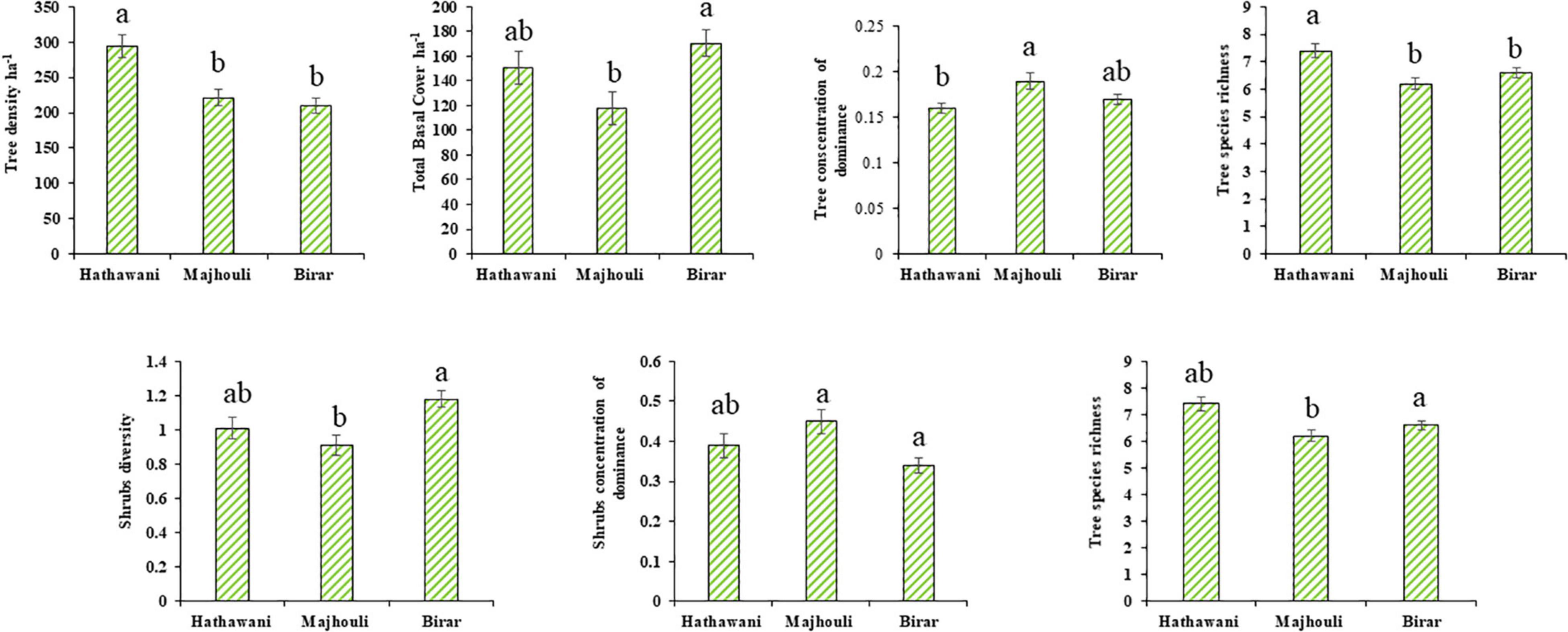
Figure 3. The distribution of diversity parameters in home gardens at high (Birar), medium (Majhouli), and low (Hathawani) disturbance. All the values are means ± S.E. The data were compared on the basis of p-values (p ≤ 0.05; 95% confidence level) by using one-way ANOVA. Different letters indicate significant differences in different disturbances as analyzed by Tukey’s HSD test.
The vegetation characteristics of home gardens were significantly affected by the disturbance gradient. Tree density of home garden at LD was higher than that at MD and HD (P ≤ 0.05). The total basal cover (m2 ha–1) of trees was significantly higher at HD than LD and MD. The Shannon-Weaver index of the shrubs was maximum for HD in comparison to LD as well as MD (P ≤ 0.05). Species richness of trees in LD was significantly higher than HD and MD, whereas species richness for shrubs was highest at HD in respect to LD as well as MD. Similarly, the concentration of dominance of trees was highest at MD than HD and LD, while, the shrubs concentration of dominance shows large dominance at MD compared to LD (Figure 3). Nevertheless, no significant (P ≤ 0.05) difference was found for shrubs and herbs density as well as Shannon-Weaver index for trees and herbs.
Effect of Garden Size on the Distribution of Vegetation Diversity
The highest tree density was observed in LSG (247.17 ± 15.18 individual ha–1) in comparison to MSG (241.25 ± 14.43 individual ha–1) and SSG (229.17 ± 11.02 individual ha–1) (Supplementary Figure 1). Varied results were obtained for shrubs and herbs density depending on the size of home gardens. MSG had maximum shrubs density (71 ± 7.34 individual per 1,000 m2) and minimum for LSG (62.50 ± 5.03), whereas herbs had just opposite result towards the trees where highest density was reported for SSG (455.21 ± 58.66 individual per 100 m2) which decreased from MSG (445.63 ± 31.70 individual per 100 m2) to LSG (414.73 ± 20.68 individual per 100 m2) respectively. TBC show related trends against trees density and follows patterns as LSG > MSG > SSG for basal cover. The Shannon-Weaver index for trees was found to be highest for LSG (1.85 ± 0.03) in comparison to SSG (1.77 ± 0.07) and MSG (1.76 ± 0.05), whereas shrubs and herbs follow trends as MSG > SSG > LSG (1.08 ± 0.06 > 1.03 ± 0.09 > 1.01 ± 0.05) and LSG > SSG > MSG (2.05 ± 0.04 > 2.01 ± 0.07 > 1.95 ± 0.05) respectively. The concentration of dominance in trees was highest in MSG (0.19 ± 0.01) and lowest in LSG (0.15 ± 0.01). A similar pattern was observed for herbs, but in shrubs, SSG (0.40 ± 0.04) had the highest concentration of dominance, followed by LSG (0.39 ± 0.02) and MSG (0.38 ± 0.02). Tree species richness followed the trend as LSG > SSG > MSG (6.93 ± 0.22 > 6.67 ± 0.78 > 6.5 ± 22) and vice-versa for the shrubs’ species richness. In the case of herbs LSG (9.61 ± 0.39) exhibited a higher species richness, and lower was found in SSG (9.33 ± 0.75) to MSG (8.75 ± 0.48). The tree species evenness was also followed similar patterns as in species richness. On the other hand, species evenness of shrubs was greater at MSG (0.92 ± 0.02) and lesser at SSG (0.89 ± 0.04). Whereas, in species evenness for herbs, only minor differences were detected in all three garden sizes (Supplementary Figure 1). The garden size was not significantly affected by the diversity of home gardens (P < 0.05).
Relationship Between Home Garden Diversity Parameters With Environmental Factor
Pearson correlation coefficients between diversity parameters across studied sites of the home garden showed a significant positive correlation. In which, tree density with tree species richness (TSRich) (0.650 P < 0.01) and tree diversity (TDiv) (0.411 P < 0.01). But tree density had a negative association with tree species evenness (TSevn) (−0.597 P < 0.01), tree concentration of dominance (TCD) (−0.289 P < 0.05), and herbs diversity (HDiv) (−0.255 P < 0.05). TDiv was shown to have a strong positive relationship with TSRich (0.854 P < 0.01) and a negative relationship with TCD (−0.833 P < 0.01). In the case of TCD, a strong negative association with TSRich (−0.800 P < 0.01) and TSevn (−0.465 P < 0.01). Similar to the tree density, shrubs density (SD) and herbs density (HD) had a positive relation with shrubs species richness (SSRich) (0.498 P < 0.01), shrubs diversity (SDiv) (0.415 P < 0.01), and herbs species richness (HSRich) (0.735 P < 0.01), and herbs diversity (HDiv) (0.586 P < 0.01). Whereas SDiv and HDiv were also strongly positively associated with SSRich (0.945 P < 0.01), SSevn (0.473 P < 0.01), and HDiv (0.586 P < 0.01), and HSRich (0.735 P < 0.01) as indicated in Table 3.
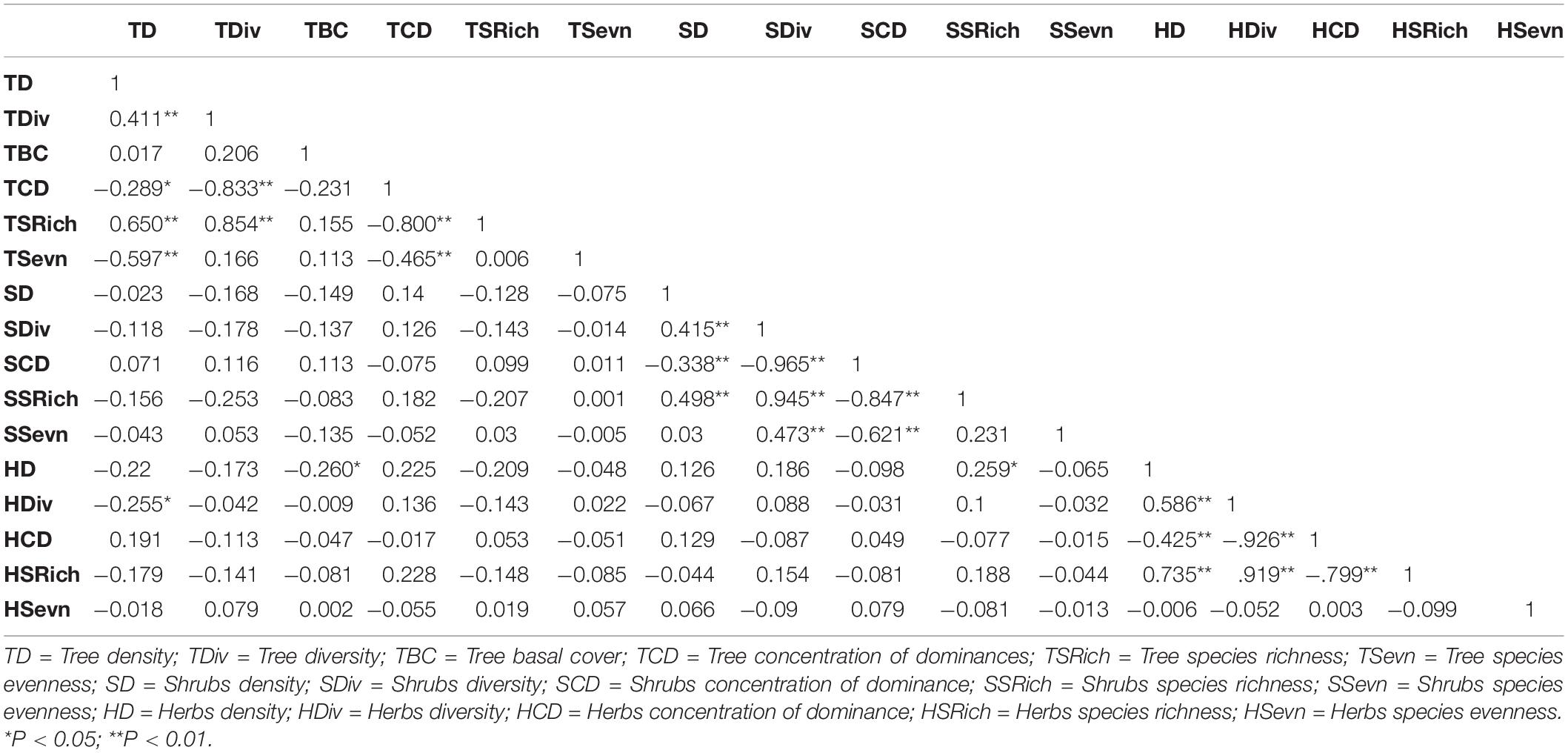
Table 3. Pearson correlation in between the different biodiversity parameters of home gardens (N = 60).
Total species richness, diversity, the richness of local species, and use of species in different use categories (N = 60) were also determined by environmental factors such as garden size, the distance of garden from the market, and disturbance index at the garden (Table 4). In multiple linear regression, species diversity (R2 = 0.109 P < 0.01) and species richness of local species (R2 = 0.107 P < 0.05) showed significant positive relations with garden size while non-significant positive relations with species richness (R2 = 0.036) and species used in different categories. Whereas similar positive relation of species richness, diversity index, and species use in different use categories was observed with the distance of gardens to market as shown in Table 4.
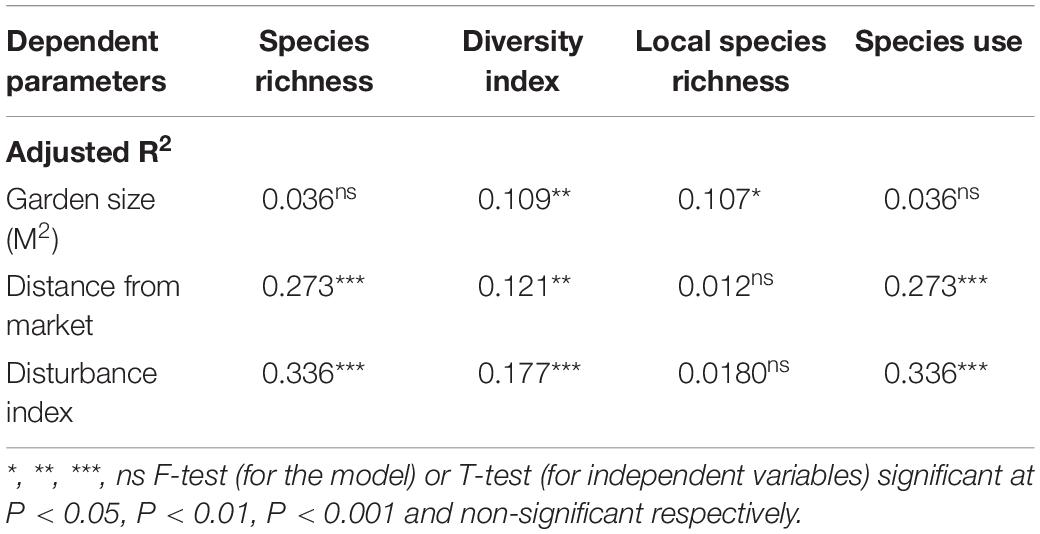
Table 4. Result of multiple regression in between garden size, distance of garden from main market and disturbance index with species richness, diversity index, local species richness and number of species in different categories use among different home garden in three selected villages.
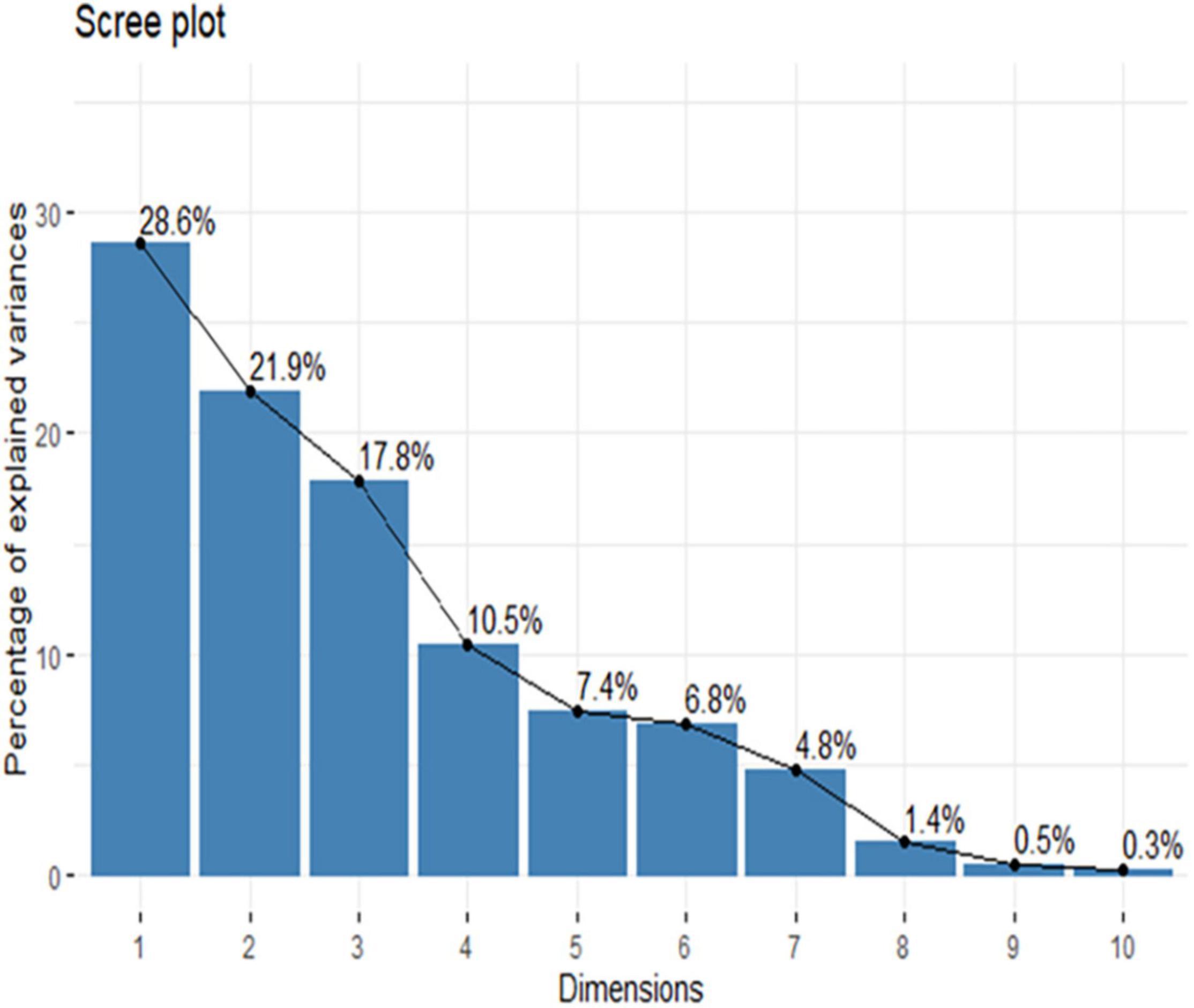
The PCA result of the diversity parameter of home gardens (N = 60) at disturbance and garden size has shown the domination of biological parameters on the major axis (Figure 4 and Supplementary Figure 1). The principal component 1 (pc1) accounted for 28.6% variance, whereas principle component 2 (pc2) had 21.9% variations in both disturbance and garden size with a cumulative score of 50.5%. In these results, the first four principal components had eigenvalues greater than 1. These first four principal components explained 78.1% of the variation in the data (Figure 5). The ordination biplot indicated that the spectra of biological parameter-based strategies of the home garden species at disturbance were similar to those of the species at the garden size. The primary axis (pc1) mainly represents HD (14.34), SDiv (13.91), TCD (12.47), and HDiv (12.09) with a positive association which significantly contributed to the plant diversity of home gardens. However, pc2 represented negative relationship with HCD (−0.772), SD (0.480) and SDiv (−0.473). The pc3 associated with a positive relationship with TDiv (0.661) and TCD (0.644) acted as an important factor for home gardens (Figure 6 and Supplementary Figure 1).
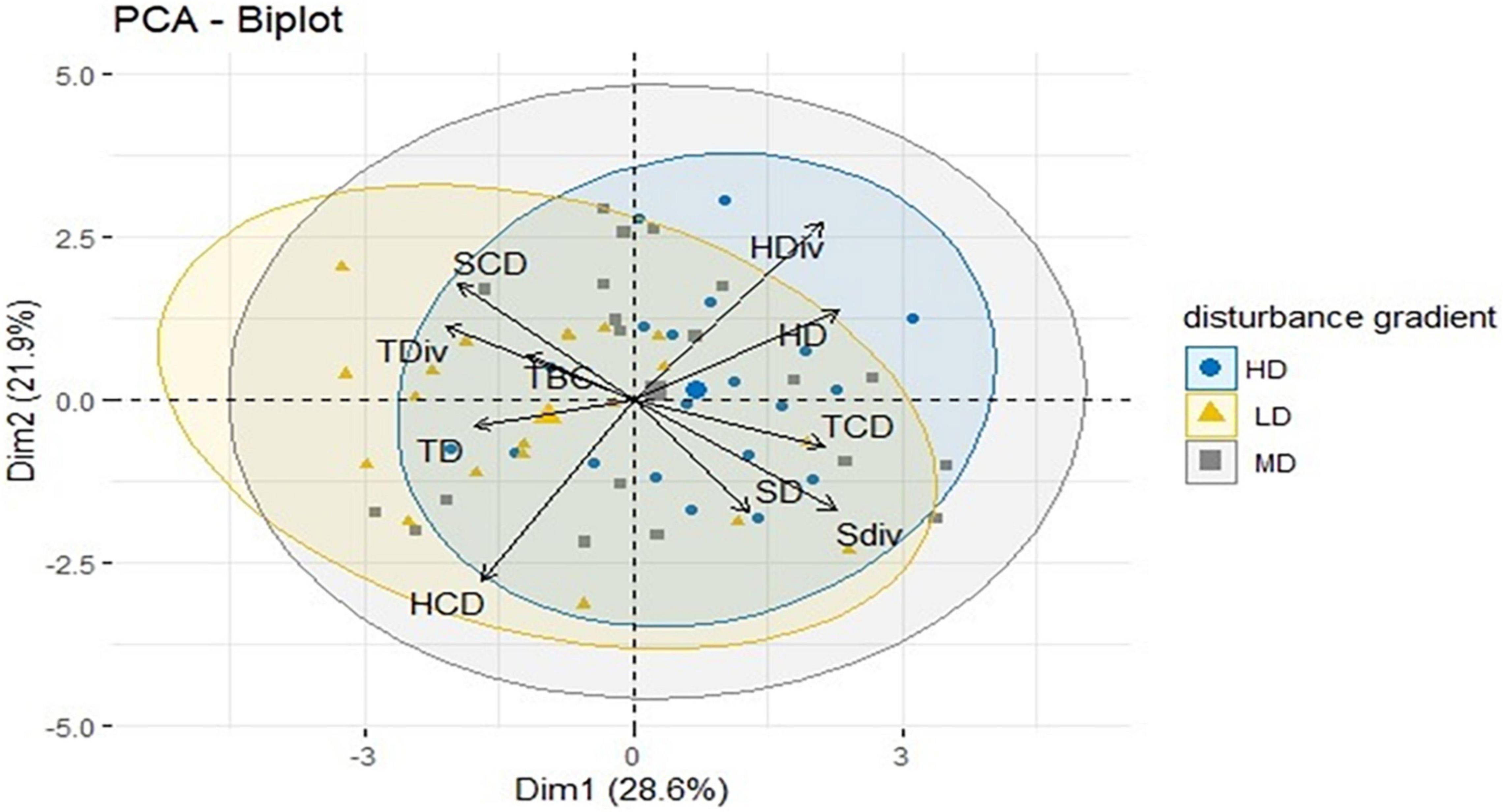
Figure 4. Principal Component Analysis (PCA) bipole plot of different diversity parameters at high, medium, and low disturb home garden. TD = Tree density; TDiv = Tree diversity; TBC = Tree basal cover; TCD = Tree concentration of dominances; SD = Shrubs density; SDiv = Shrubs diversity; SCD = Shrubs concentration of dominance; HD = Herbs density; HDiv = Herbs diversity; HCD = Herbs concentration of dominance.
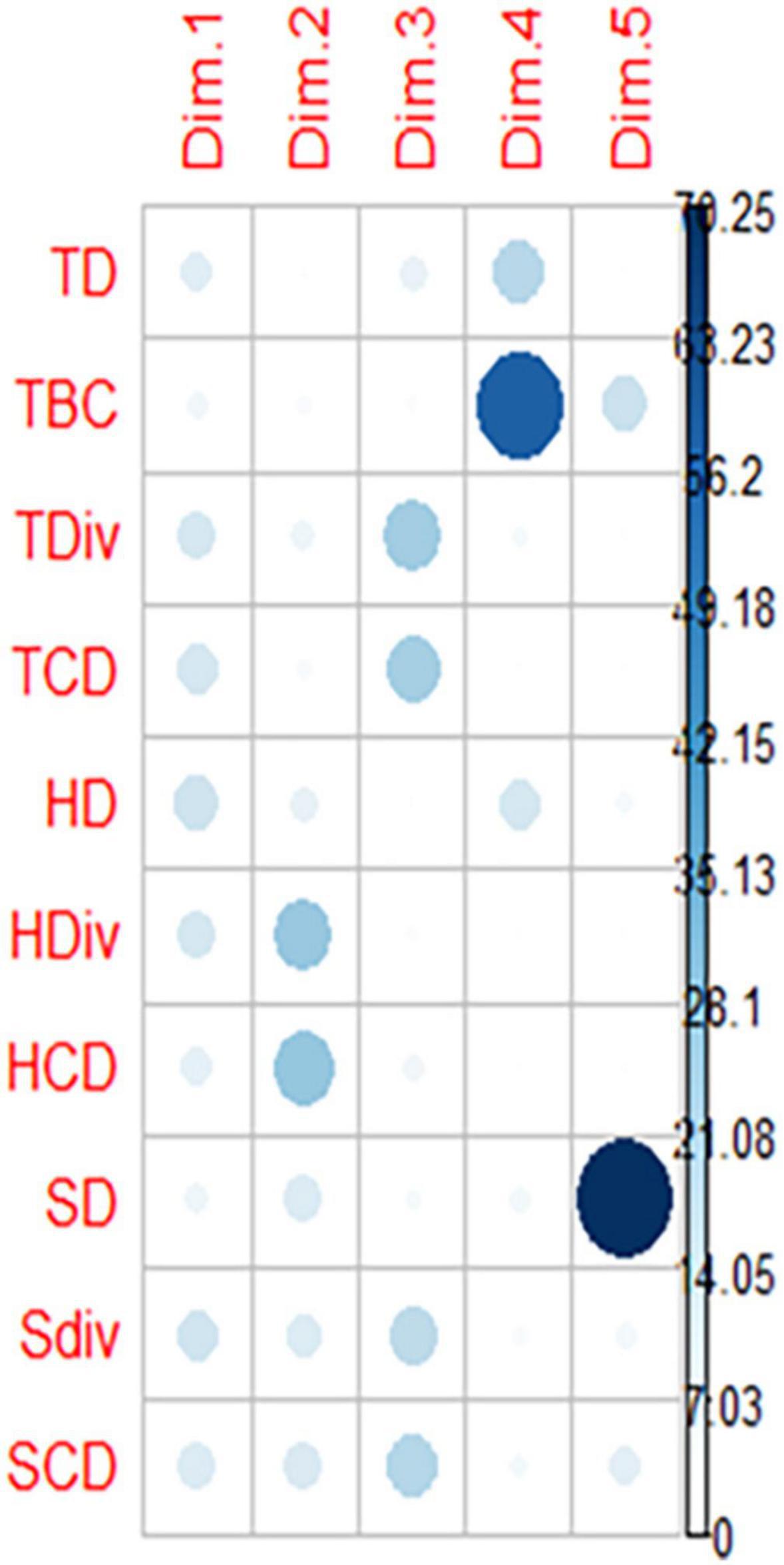
Figure 6. Loading contribution by different variables at various axis. TD, Tree density; TDiv, Tree diversity; TBC, Tree basal cover; TCD, Tree concentration of dominances; SD, Shrubs density; SDiv, Shrubs diversity; SCD, Shrubs concentration of dominance; HD, Herbs density; HDiv, Herbs diversity; HCD, Herbs concentration of dominance.
Distribution and Availability of Species in Different Use Categories
The use of vegetation from all home gardens (N = 60) was categorized into 11 use categories, including vegetables, bio-fencing, fruits, medicinal, ornamental, timber, religious, trades, spices, oil, and others (Figure 7). The SDR result indicates the proportionate contribution of different vegetation in various use categories at the three villages. Bio-fencing (LD = 17.1%, MD = 15.5%, and HD = 15.1%) was the most important use category at all study sites followed by vegetables. Fencing is effective to prevent the probable attack of the wild as well as domestic animals on crops, especially those located in forest fringe. Use of vegetables was dominated in LD with SDR (13.4%) and decreased in MD (11.6%) to HD (10.6). Plants in ethnomedicinal treatments were followed the analogous trends to bio-fencing and vegetables. On the other hand, fruit plants showed quite a high SDR at HD (15.1) followed by MD (12.9) and low at LD (10.0). Similar results were noticed for ornamental plants at all studied sites. Various use categories comprised of timber, religious practices, trades, spices, oil, and others are given in Figure 7.
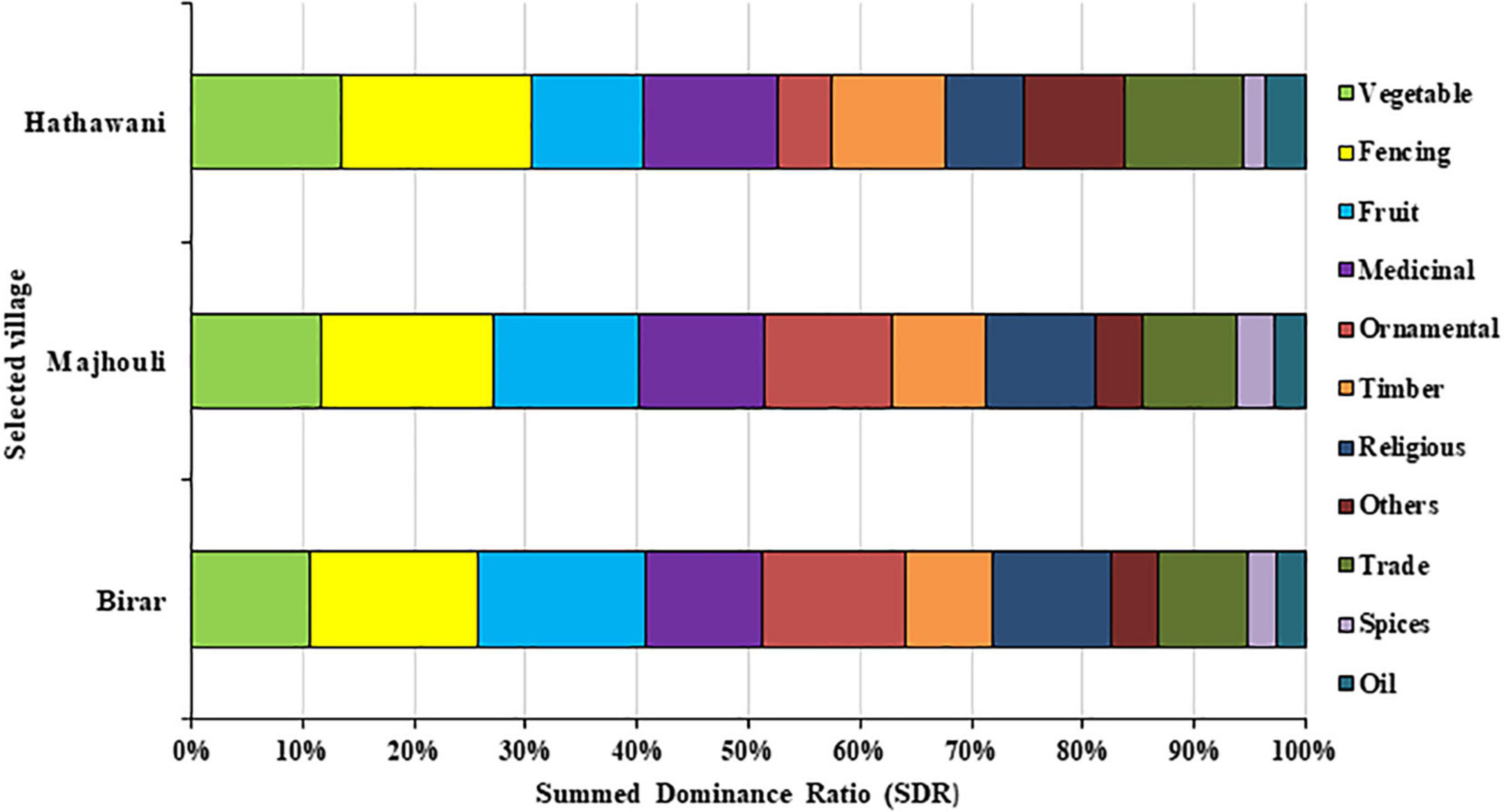
Figure 7. The proportion of plant use in different use categories was estimated with Summed Dominance Ratio (SDR) for 133 plant species of 20 home gardens in three selected villages.
All garden (N = 60) plants under different uses were categorized according to their seasonal availability across the three study sites. These uses indicate a diverse trend of garden plants to supplement vegetables, fruits, medicine, and scenic beauty. The products of this vegetation were available in all three seasons of the annual weather cycle. In respect to total plants (133) collected from home gardens, the maximum number of species (120) was used in the monsoon season. While the availability of species decreased in winter (113 species) and summer (102) (Table 5). In the monsoon season, decorative plants (33 species) prevailed, which is a common feature of residential gardens. Vegetable crops (24 species) came in second, followed by fencing and roofing (19) and medicinal plants (18). Like monsoon season, ornamental plants (31 species) dominated in winter with co-domination of timber and trade (22) and medicinal plants (20). On the other hand, the highest number of species were utilized in the category of timber and trade (31) followed by ornamental and medicinal uses (15 each) in the summer season (Table 5).
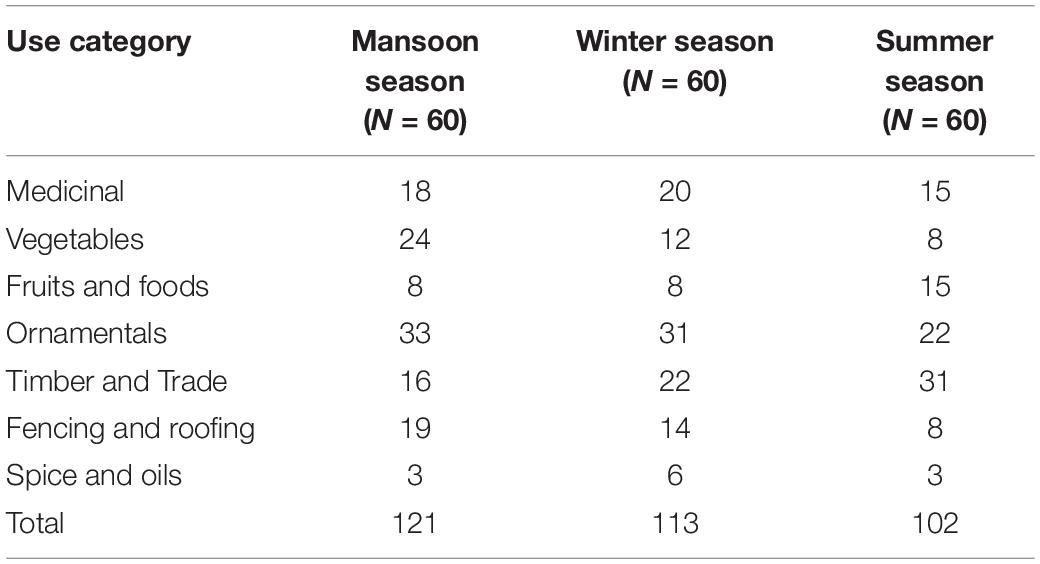
Table 5. Season-wise (monsoon, winter, and summer) distribution of total plant species in different use categories in the home gardens of the selected villages.
Discussion
The investigated home gardens comprised almost half of their vegetation as trees followed by shrubs and herbs. In the total collected vegetation, more than 60% of species were wild. The anthropogenic disturbance significantly affected the distribution of vegetation, tree species richness, mean garden species richness, and diversity indices. Home gardens were highly diverse in species composition, richness, and species diversity as reported in various studies elsewhere (Sujarwo and Caneva, 2015; Ferdous et al., 2016). In the present study, we reported 133 plant species (63 trees, 22 shrubs, 24 herbs, and 22 climbers) from 50 families. When considering the other dry tropical forests of the world, this result was similar to the 116 species from 50 families and 50 gardens in Niger (Bernholt et al., 2009), 148 species from 60 gardens of Vall Fosca (Calvet-Mir et al., 2011), while high compared to 79 species from 120 home gardens in the Republic of Sudan (Thompson, 2007). Similar studies in India reported 128 species from 58 families in 90 gardens of upper Asam (Barbhuiya et al., 2016), 127 species in 252 gardens of Kerala (Kumar et al., 1994), and 47 species in Central India (Kala, 2010). The mean species richness for home gardens was high, which made this system ecologically resilient and provide the ability of a better ecological function to conserve the degraded dry tropical forest (Olson et al., 2000; Vandermeer, 2002). The area of home gardens ranged from 0.0255–0.1280 ha which comparatively higher (0.003-0.007 ha) to central Himalaya and lower (0.04–0.52 ha) to Uganda but comparable with the average global range (0.10–0.50 ha) (Das and Das, 2005; Whitney et al., 2018a). This range also fell within the range of tropical home gardens reported by 0.01-0.02 ha in Ethiopia (Zemede, 2001), 0.09 ha from Cuba (Wezel and Bender, 2003), and 0.02-0.24 ha in Indonesia (Kehlenbeck and Maass, 2004).
Factors Influencing Plant Diversity in Home Gardens
The composition of vegetation in dry tropical regions is mainly altered by human-induced disturbance (Kumar and Ram, 2005; Zhu et al., 2007). The extent of the impact depends on the strength and categories of disturbance. In this study, the number of tree species was significantly affected by disturbance. In a dry tropical climate, the basic pattern of vegetation distribution is prone to disturbance, therefore trees dominate in undisturbed areas, shrubs in moderately disturbed areas, and herbs survive at high disturbed areas (Sahoo et al., 2020). This study found an equally high species of trees and herbs at LD, which decreased from MD to HD. This high number of tree species in home gardens validated the importance of this practice for in-situ conservation of trees. High tree diversity in home gardens compare to shrubs and herbs was also reported by Panyadee et al. (2018) in Thailand and Larios et al. (2013) in Mexico. The area of home gardens provide a microhabitat for trees and low anthropogenic disturbance promotes the large tree cover in the LD site (Khumbongmayum et al., 2005; Saikia and Khan, 2013). The forest inhabitants understand the crucial role of trees for non-timber forest products (NTFPs) to livelihood development and economic well-being. On the other hand, a large number of herbs was justified due to the availability of nutrients from leaf-litter and moisture as well as sunlight from open canopy during leaf-fall (Sagar et al., 2008). In the case of tree density, maximum density was reported by LD with subsequent decrease with MD and HD (Figure 3). The high tree density for a low disturbed zone has also been reported in the tropical region of Vindhyan highlands (Sagar et al., 2008) and Kalimantan, Indonesia (Marjokorpi and Ruokolainen, 2003). The shrubs and herbs showed the exact opposite results, where maximum density was found at HD and MD garden in respect to LD. The herbaceous plants invaded the garden area throughout the monsoon and post-monsoon seasons while open tree canopy and less grazing promoted the survival of shrubs and herbs vegetation in HD and MD. Due to the open tree canopy, more sunlight and adequate nutrient availability promoted the germination and establishment of herbaceous plants at the LD and MD regions. The high density and diversity of shrubs and herbs in MD to HD sites were in agreement with the findings of Birhane et al. (2020) in southern Ethiopia and Sagar and Singh (2005) in the Vindhyan region. Plants in home gardens generally depend on a priority of acclaim, disturbed urban and semi-urban people prefer cultivation of contemporary and attractive herbs and shrubs species in place of wild species (Regassa, 2016). However, in this study, two villages (Majhouli and Birar) situated nearer to Duddhi town had a large density of non-woody plants, whereas, Hathawani, far away from the town and situated into dense forest region, composed of high tree species density compared to the other two villages. Similar findings were made by Panyadee et al. (2018) and Lattirasuvan et al. (2010) in Thailand and Trinh et al. (2003) in northern Vietnam.
The diversity indices such as the Shannon-Weaver index, species richness, evenness, the concentration of dominance of trees, shrubs, and herbs represent the variability of vegetation composition and distribution in the garden at various disturbances. The tree Shannon-Weaver index varies from 1.73 to 1.88 across three disturbances. This result is comparable with the findings of other dry tropical home gardens of southern Ethiopia (1.87) (Birhane et al., 2020), Northern Bangladesh (1.64) (Jaman et al., 2016), and in central Vietnam (Vlkova et al., 2011). The mean Shannon-Weaver index for herbs (2.01), indicating a high distribution of herbs at all three disturbances, is justifiable with findings of (2.84) Southern Cameroon (Vidal, 2008) and (2.29) in Sri Lanka (Martin et al., 2019). The high range of total tree basal cover varies 117.72-170.78 m2 ha–1, shows a huge cover of tree in home-yard which significantly differ with disturbance gradient, and higher (17.4-32.6 m2 ha–1) to the result of Chiapas, Mexico (Valencia et al., 2014) and tropical lowlands of Tabasco, Mexico (11.7-16.1 m2 ha–1) (Alcudia-Aguilar et al., 2018). The basal cover was analogous to studies of Sahoo et al. (2020) (50-147 m2 ha–1) in dry tropical and Singh (2013) (18-100 m2 ha–1) in the Himalayan region. The mean species evenness for trees is 0.96, while shrubs and herbs have 0.91, which is high when compared to Ethiopia (0.48-0.61) (Bajigo and Tadesse, 2015) and India’s Eastern Ghats (0.60) (Gopalakrishna et al., 2015). However, these evenness values were found similar to home gardens (0.81) of Southern Ethiopia (Birhane et al., 2020) and (0.94-0.98) for Bali, Indonesia (Sujarwo and Caneva, 2015). Like wise, the value of TCD (0.18), SCD (0.39), and HCD (0.16) indicates the dominance of herbs followed by trees and shrubs resembles the study of the tropical forest of Nepal (0.39) by Mandal et al. (2016), the home garden study of Kerala (0.07-0.35) by Peyre et al. (2006), and south Andaman (Pandey et al., 2006).
Relationship of Disturbance and Biodiversity Parameters
In PCA analysis, most of the diversity parameters were found in pc1, which indicated the contribution of vegetation for home garden diversity. These layers of vegetation determined the existence of biodiversity in home-yard farming. Whereas, the negative association of pc2 with HCD and SCD specify the concentration of dominance reduces the density and diversity of garden plants. The domination of trees, shrubs, and herbs in density and diversity on the pc1 were also found in the home garden study of Nepal (Sunwar et al., 2006), Assam, and the central Himalaya of India (Saikia et al., 2012; Vibhuti et al., 2018). It was also observed that a diverse group of shrubs and herbs at MD and HD sites were maintained by the owner to meet their regular requirements and the ornamental desires of the house regardless of the disturbance gradient. Whereas the LD region has a higher tree richness because it experienced little disturbance and was located in a forest range where local people understand the importance of trees to their livelihood and survival (Pao and Upadhaya, 2017). This type of observation was also reported by the Panyadee et al. (2016, 2018) in the Karen home gardens of Thailand.
Environmental variables such as garden size, distance from market, and disturbance index of the garden significantly influenced species richness, diversity indices, the richness of local species, and usage of species in different use categories (Table 4). A large garden area provided more space for the germination of many types of vegetation while avoiding disturbance on natural vegetation, which promotes the richness of local varieties. Hence, the large garden size at Hathawani promoted the high richness of wild species. Several researchers have also described the positive relationship of wild plant species with the area of gardens (Albuquerque et al., 2005; Kehlenbeck et al., 2007; Bernholt et al., 2009). However, as we moved away from Duddhi town toward the forest region, wild species faced less utilization pressure and disturbance. The increase in the species richness (P > 0.001) and diversity index (P > 0.01) with the increase of distance from main markets were also consistent with the findings of other researchers (Abebe et al., 2006; Kabir and Webb, 2008; Barbhuiya et al., 2016). Diversity index (P > 0.001) and species richness (P > 0.001) were significantly related to disturbance because they promoted a mixture of vegetation systems in place of monoculture resulting in a positive association (Bhuyan et al., 2003; Fakhry and Aljedaani, 2020). It has been previously stated that Majhouli and Birar, which are located close to the Duddhi town and experience more disruptions, have encouraged a variety of decorative and cultivated herbs and shrubs species over wild and native species (Shrestha et al., 2002; Abdoellah et al., 2006; Jaganmohan et al., 2012). The number of species in different use categories was also positively associated with distance from the market (P > 0.001) and disturbance index (P > 0.001), indicating dependency of local people on garden products as constrained by elsewhere finding (Barbhuiya et al., 2016) and (Kala, 2010) in India. Whereas various other researchers such as Wiehle et al. (2014); Cruz-Garcia and Struik (2015), and Norfolk et al. (2015) reported the dependency of local people on timber and NTFPs products of gardens from other dry tropical regions of the world.
Conservation of Native Plant Species
Home gardens have an important role in the in-situ conservation of native plant species, which also conserve and store the rare ethnic wild species at different levels (Gautam et al., 2009b; Poot–Pool et al., 2015; Jhariya et al., 2021a,b, c). It is regarded as a living storehouse of a wide range of end products used in a variety of applications such as food, fiber, fodder, medicine, fuel, ornamental, and rituals (Galluzzi et al., 2010). The majority of studies revealed the dominance of exotic species (Bernholt et al., 2009), but in a few studies, the importance of home gardens for the conservation of local and wild species for various livelihood needs of the economically disadvantaged was also highlighted (Gautam et al., 2009a; Cruz-Garcia and Struik, 2015; Regassa, 2016). Most important native tree species which have multifarious application for society viz. Buchanania lanzan, Adina cordifolia, Ficus spp., Syzygium spp., and Madhuca indica were found in every site as wild/cultivated. In which, A. cordifolia and F. spp. were planted for shade and their leaf and wood were used in the summer season when most of the species shed their leaves. Whereas, B. lanzan, S. spp., and M. indica provided seasonal uses. Local people collect the fruits of S. spp. for food while the fruits of B. lanzan and M. indica were gathered for sale in the market (Sharma et al., 2021). Most of the tribal people used the flower of M. indica as dry food as well as the preparation of liquor by fermentation as also reported in dry tropics by Kala (2010) and Sharma et al. (2021). In herbs, species such as Dioscorea bulbifera, Cucumis Callosus, Trichosanthes cucumerina, Momordica dioica, Basella alba, and Amorphophallus paeoniifolius were wild and underutilized and their distribution is restricted only in home gardens. Such plants are one-of-a-kind for use as fruits and vegetables, and locals have been informed that they are solely adapted to climate change. The dominance of these native and wild plants in home gardens was explained by Whitney et al. (2018a,b) and Kala (2010). Our study found a variety of threatened species, including Abrus precatorious and A. paeoniifolius as endangered, B. lanzan and S. febrifuga as vulnerable, and Woodfordia fruticosa and Aegle marmelos are near threatened, which is consistent with the findings of Wagh and Jain (2015) in central India.
Home gardens are considered as the key center for ethnomedicinal diversity (Caballero-Serrano et al., 2019). The occurrences of medicinal plants such as Aloe barbadensis, Andrographis paniculata, Cissus quadrangularis, Tinospora cordifolia, and Ocimum tenuiflorum in this study recognized the home garden as an important reservoir of these plants. The existence of medicinal plant in home gardens reduces overexploitation of species from natural forests which minimizes forest degradation. High inter-specific diversity was found in the various species of the different families viz., Cucurbitaceae (three species of Cucumis and Luffa), Lamiaceae (two Ocimum species), and Fabaceae (six Lablab species). This could be attributed to the introduction of crops from wild sources and maintaining the wild plant concentration in the garden that worked as the center of domestication of wild species. The genetic exchange by natural crosses in the wild and domestic crops is reported as a mutual phenomenon (Hammer et al., 1999). The hybrid produced from the natural cross has high ability to overcome the environmental gradient than modern crops (Negri, 2005; Jackson et al., 2007). Many authors have also been reported the role of intraspecific diversity in enhancing an ecosystem’s ecological and biological abilities such as adaptation, survival, and breeding (Eyzaguirre and Linares, 2004; Feuillet et al., 2008; Calvet-Mir et al., 2012).
Contribution of Home Gardens to Food Supply and Income
Gardens have a variety of vegetation layers, including annual, biennial, and perennial plants that provide year-round services. Most annual crops and wild species were cultivated during the rainy season and a prominent number of nutritionally rich wild species such as B. alba, Benincasa hispida, Coccinia grandis, D. bulbifera, Lablab purpureus, Moringa oleifera, and T. cucumerina are regularly maintained throughout the year. The presence of these types of wild species was also reported by Hammer et al. (1999) in Italy, Norfolk et al. (2013) in South Sinai Egypt, and Whitney et al. (2018a) in Uganda. The perennial nature of the garden system supports a combination of fruit-based trees and shrubs with herbaceous vegetables highlighting a mixed and balanced production system that provides year-round fruits and vegetables (Barbhuiya et al., 2016). Gardens promote a nature-based climate change mitigation and management of scattered forest systems with future food security (Jhariya et al., 2019a,b). The % SDR for different plants varies by the utilization in different studies at disturbance gradient. Home gardens in LD dominated bio-fencing and vegetable plants (e.g., varieties of the vegetable family representing Cucurbitaceae and Fabaceae). These plants are available as vegetables for food and create bio-fencing on the outer boundaries of the home yard, which is effective in preventing wild animal attacks (Maroyi, 2009), as confirmed with banana planting at the border of the home yard (Whitney et al., 2018a) in Uganda and Jatropha curcas in Central India (Kala, 2010). Some other common shrubs species such as Euphorbia tithymaloides, Lantana camara, and W. fruticosa were mostly used as bio-fencing in this study as also reported from Uganda by Whitney et al. (2018b). The use category of “medicinal plant” (SDR 12%) was the third most dominant category at LD. The large use of species for medicinal reasons at Hathawani site was explained due to the location of the village far away from the larger town and the reliance of tribal communities on herbal medicine (Sharma et al., 2021). As we move from LD to HD, an increase in urban disturbance was found, which leads to an increase in the SDR of ornamental and fruit use categories. Species, namely Annona squamosa, Citrus limon, Morus alba, Murraya paniculata, and Platycladus orientalis are grown as ornamental plants. A similar study was also reported by Poot–Pool et al. (2015) in Mexico. SDR for the trade category was decreased with increasing disturbance, due to the dependency on trade-related activities of garden products.
Potential of Home Gardens to Achieve UN-SDGs and Forests Conservation
The home garden is a highly diverse system with a multi-layered vegetation structure coupled with a mixed balanced system of nutritional food, medicinal plants, and income sources. It has the potential to achieve United Nation-Sustainable Development Goals (UNSDGs) viz., no poverty (SDG-1), zero hunger (SDG-2), good health and well-being (SDG-3), gender equality (SDG-5), decent work and economic growth (SDG-8), responsible consumption and production (SDG-12), climate action (SDG-13) and life on land (SDG-15) (Figure 8). It effectively supports the target of the international year of fruits and vegetables-2021, as declared by the Food and Agriculture Organization of the United Nations (FAO). This indigenous practice has low input and is cost-effective, which is easily performed by marginal and poor farmers of almost every climatic region of the world. This system has a better adaptive capacity to cope with the adverse effect of climate change than the mono-cropping of modern agriculture. Such practices maintain a diverse system that may be a supplementary model for conservation, especially in the degraded dry tropical forests. It would be helpful to popularize the wild species for medicine, food, and economic upliftment and combating hunger in the tribal population through supplying quality diets.

Figure 8. Schematic representation of indigenous home garden contribution to achieving the target of Sustainable Development Goals (SDGs) providing food, fruit vegetable and livelihood for food security. These services contribute to achieving the targets of GDGs supporting SDGs 1 (no poverty), SDGs 2 (zero hunger), SDGs 3 (good health and well-being), SDGs 5 (gender equality), SDGs 8 (decent work and economic growth), SDGs 12 (responsible consumption and production), SDGs 13 (climate action) and SDGs 15 (life on land). Pictorial image show plant species and their contribution, (a) Momordica dioica, (b) Cucumis Callosus, (c) Sechium edule, (d) Dioscorea bulbifera, (e) Trichosanthes cucumerina (wild), (f) Carica papaya, (g) Punica granatum, (h) Annona squamosal, (i) Cucumis sativus, (j) Ziziphus xylopyrus, (k) Musa paradisiaca as ornamental plant, (l) Mangifera indica canopy for shed, (m) selling of garden vegetable in traditional market, (n) fencing by garden, (o) branches of Woodfordia fruticose as fodder, (p) Abelmoschus esculentus, (q) Cucurbita pepo, (r) Luffa operculata, (s) Lablab purpureus, (t) Trichosanthes cucumerina (cultivated).
Major Challenges to Recognize the Home Garden for Forests Conservation
The major challenges are increasing population, poverty, the need for firewood, squatter settlement growth, absence of legislation regulating tree clearance, and increased reliance on root crops such as Zingiber officinale, Allium cepa, A. paeoniifolius, and Colocasia esculenta, resulting in the rising elimination of trees (including A. cordifolia, Pterocarpus marsupium, and S. febrifuga) from nearby settlements of dry tropical forests. Secondly, the promotion of a wide variety of cash crops, such as Musa paradisiaca, B. hispida, Capsicum annuum, Z. officinale, and Curcurbita pepo in rural home gardens has led to the removal of diverse forested lands. Thirdly, the absence of appropriate germplasm, the risk of accidental fires, seedling survival in the dry season, and soil fertility are the key barriers to further improving home gardens or spreading them out to fields for increased productivity and revenue generation (Miller et al., 2006; Patel et al., 2020). Lastly, being a drought-prone area with poor soils such as rocky or stony lithosols, cost, and availability of land and water, insufficient labor, shortage of planting materials, and government support, result in the mortality of a large number of fruits, citrus species, and other trees and food plants that are only slightly suited to the moist environment.
Conclusion and Recommendations
The present study indicated that home gardens possess a rich repository of trees compared to shrubs and herbs among the total recorded vegetation. The presence of inter-specific varieties and the interdependence of diversity indices indicate the existence of multilayered vegetation that is mutually reliant. Home gardens serve as both the source and sink of tropical biodiversity because they provide shelter for native forest plants and supply subsistence to society for their survival and sustenance. The disturbance has an impact on the vegetation composition, which is a reflection of the unique biophysical environment and socio-cultural features of the garden. The most devastation was associated with agriculture expansion and pastoral commodities throughout the historical period of development in the dry tropical region. Home gardens preserve some elements of natural vegetation and thus, support ecosystem-based regeneration of forests in the deforested dry tropical areas. Local communities of this region utilized garden plants for a variety of purposes including bio-fencing, nourishment, medicine, and fuelwood. Further, home gardening advances the concept of supporting the most degraded regions of the world with a multifunctional food production system that is apparently superior to industrial agriculture in terms of biodiversity and forest protection. The ecological similarities of gardens with forest systems operate as an insurance against forest degradation because they buffer the utilization pressure on natural forest and rehabilitate the degraded ecosystem. This nature-based, low-cost, and climate-smart food production system would play a pivotal role in the management of forest ecosystems and in achieving several UNSDGs.
In the future, an extended methodology could be used for exploring both the broader and more detailed information of the climatic and socio-economic aspects of gardens. The amalgamation of traditional knowledge into scientific knowledge may enhance the production and conservation of the natural forest of the Vindhyan region. Future study can also be extended to the restoration of the dry tropical forest because home gardening has the potential to enhance the natural regeneration of wide tracts of deforested land with massive CO2 sequestration toward substantial costs of reforestation. Apart from these recommendations, the management and popularization of home gardens to sustain the production and conservation of forest system is given below.
(1) Enhancing the food production in changing climate crisis with the integration of home garden is a climate-smart approach that sustains traditional wild species with forest conservation and encourages a model for sustainable food production and forest management.
(2) Sensitization and awareness programs among the local population should be promoted to make garden cultivation for forest conservation a people’s movement.
(3) Collaboration and coordination among several stakeholders viz. native population, researchers, and policymakers, on an urgent basis to formulate effective strategies for promotion and conservation of home gardens for the nature-based solution of sustainable forest conservation and food security.
Data Availability Statement
The datasets presented in this article are not readily available because requests to access the datasets should be directed to GS, Z29wYWxzaW5naC5iaHVAZ21haWwuY29t.
Author Contributions
SP performed the field work, conceptualized methodology, and prepared the draft of the article as well as formal analysis and writing. AS helped in the field work, data analysis and prepared the early draft, and wrote the manuscript. AT prepared the location map and data analysis. RS reviewed and edited the English language and grammar. GS conceptualized and supervised the study and approved the final version of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to Dean and Director of Institute of Environment & Sustainable Development, BHU, Varanasi for Institutional support and home garden owners and Divisional Forest Officer, Renukoot, Sonbhadra, Uttar Pradesh, India for allowing them to work and conducting the survey in the vicinity of forest villages. We are also grateful to Pramit Verma for his critical inputs and suggestions in this work; SP thanks University Grants Commission, New Delhi for financial support in the form of fellowship.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.822320/full#supplementary-material
Footnotes
References
Abdoellah, O. S., Hadikusumah, H. Y., Takeuchi, K., Okubo, S., and Parikesit (2006). Commercialization of homegardens in an Indonesian village: vegetation composition and functional changes. Agrofor. Syst. 68, 1–13. doi: 10.1007/s10457-005-7475-x
Abebe, T., Wiersum, K. F., Bongers, F. J. J. M., and Sterck, F. (2006). “Diversity and dynamics in homegardens of southern Ethiopia,” in Tropical Homegardens: A Time-Tested Example of Sustainable Agroforestry, Advances in Agroforestry, eds B. M. Kumar and P. K. R. Nair (Dordrecht: Springer), 123–142. doi: 10.1007/978-1-4020-4948-4_8
Albuquerque, U. D., Andrade, L., and Caballero, J. (2005). Structure and floristics of homegardens in Northeastern Brazil. J. Arid Environ. 62, 491–506. doi: 10.1016/j.jaridenv.2005.01.003
Alcudia-Aguilar, A., van der Wal, H., Suárez-Sánchez, J., Martínez-Zurimendi, P., and Castillo-Uzcanga, M. M. (2018). Home garden agrobiodiversity in cultural landscapes in the tropical lowlands of Tabasco, México. Agrofor. Syst. 92, 1329–1339. doi: 10.1007/s10457-017-0078-5
Bajigo, A., and Tadesse, M. (2015). Woody species diversity of traditional agroforestry practices in Gununo watershed in Wolayitta zone, Ethiopia. For. Res. 4, 2168–9776. doi: 10.4172/2168-9776.1000155
Barbhuiya, A. R., Sahoo, U. K., and Upadhyaya, K. (2016). Plant diversity in the indigenous home gardens in the Eastern Himalayan Region of Mizoram, Northeast India. Econ. Bot. 70, 115–131. doi: 10.1007/s12231-016-9349-8
Bargali, K. (2016). Traditional homegardens as a sustainable ecosystem for maintenance of biodiversity: a case study from Kumaun Himalaya, India. J. Biodiver. 7, 88–100. doi: 10.1080/09766901.2016.11884761
Barlow, J. D., Gareth, F. J., Lennox, E., Berenguer, A. C., Lees, R., Mac Nally, R., et al. (2016). Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147. doi: 10.1038/nature18326
Bernholt, H., Kehlenbeck, K., Gebauer, J., and Buerkert, A. (2009). Plant species richness and diversity in urban and peri-urban gardens of Niamey, Niger. Agrofor. Syst. 77, 159–179. doi: 10.1007/s10457-009-9236-8
Betts, M. G., Wolf, C., Ripple, W. J., Phalan, B., Millers, K. A., Duarte, A., et al. (2017). Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 547, 441–444. doi: 10.1038/nature23285
Bhagwat, S. A., Willis, K. J., Birks, H. J. B., and Whittaker, R. J. (2008). Agroforestry: a refuge for tropical biodiversity? Trends Ecol. Evol. 23, 261–267. doi: 10.1016/j.tree.2008.01.005
Bhuyan, P., Khan, M. L., and Tripathi, R. S. (2003). Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodiver. Conser. 12, 1753–1773. doi: 10.1023/A:1023619017786
Birhane, E., Ahmed, S., Hailemariam, M., Negash, M., Rannestad, M. M., and Norgrove, L. (2020). Carbon stock and woody species diversity in homegarden agroforestry along an elevation gradient in southern Ethiopia. Agrofor. Syst. 94, 1099–1110. doi: 10.1007/s10457-019-00475-4
Boneta, A., Rufí-Salís, M., Ercilla-Montserrat, M., Gabarrell, X., and Rieradevall, J. (2019). Agronomic and environmental assessment of a polyculture rooftop soilless urban home garden in a Mediterranean city. Fron. Plant Sci. 10, 341. doi: 10.3389/fpls.2019.00341
Caballero-Serrano, V., McLaren, B., Carrasco, J. C., Alday, J. G., Fiallos, L., Amigo, J., et al. (2019). Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home gardens. Glob. Ecol. Conser. 17:e00524. doi: 10.1016/j.gecco.2019.e00524
Calvet-Mir, L., Calvet-mir, M., Vaqué-nuñez, L., and Reyes-garcí, V. (2011). Landraces in situ conservation: a case study in high-mountain home gardens in vall fosca, Catalan Pyrenees, Iberian Peninsula. Econ. Bot. 65, 146–157. doi: 10.1007/s12231-011-9156-1
Calvet-Mir, L., Gómez-Baggethun, E., and Reyes-García, V. (2012). Beyond food production: ecosystem services provided by home gardens. a case study in vall fosca, Catalan Pyrenees, Northeastern Spain. Ecol. Econ. 74, 153–160. doi: 10.1016/j.ecolecon.2011.12.011
Casas, A., Caballero, J., Mapes, C., and Zárate, S. (1997). Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bot. Sci. 61, 31–47.
Census of India (2011). Provisional Population Totals. New Delhi: Office of the Registrar General and Census Commissioner.
Chaturvedi, R. K., Raghubanshi, A. S., and Singh, J. S. (2011). Effect of small-scale variations in environmental factors on the distribution of woody species in tropical deciduous forests of Vindhyan Highlands, India. J. Bot. 2011:1537. doi: 10.17129/botsci.1537
Chaturvedi, R. K., Raghubanshi, A. S., and Singh, J. S. (2012). Effect of grazing and harvesting on diversity, recruitment and carbon accumulation of juvenile trees in tropical dry forests. For. Ecol. Manag. 284, 152–162. doi: 10.1016/j.foreco.2012.07.053
Clarke, L. W., Li, L., Jenerette, G. D., and Yu, Z. (2014). Drivers of plant biodiversity and ecosystem service production in home gardens across the Beijing Municipality of China. Urb. Ecosyst. 17, 741–760. doi: 10.1007/s11252-014-0351-6
Cottom, G., and Curtis, J. T. (1956). The use of distance measures in phytosociological sampling. Ecology. 35, 451–460. doi: 10.1371/journal.pone.0199980
Cruz-Garcia, G. S., and Struik, P. C. (2015). Spatial and Seasonal diversity of wild food plants in home gardens of Northeast Thailand 1. Econ. Bot. 69, 99–113. doi: 10.1007/s12231-015-9309-8
Das, T., and Das, A. K. (2005). Inventorying plant biodiversity in homegardens: a case study in Barak Valley, Assam, North East India. Curr. Sci. 5, 155–163. doi: 10.1023/A:1006322612362
De Clerck, F. A., and Negreros-Castillo, P. (2000). Plant species of traditional Mayan homegardens of Mexico as analogs for multistrata agroforests. Agrofor. Syst. 48, 303–317.
Deng, L., Liu, G. B., and Shangguan, Z. P. (2014). Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: a synthesis. Glob. Change Biol. 20, 3544–3556. doi: 10.1111/gcb.12508
Díaz, S., Pascual, U., Stenseke, M., Martín-López, B., Watson, R. T., Molnár, Z., et al. (2018). Assessing nature’s contributions to people. Science 359, 270–272. doi: 10.1126/science.aap8826
Eyzaguirre, P., and Linares, O. (2004). “Home gardens and agrobiodiversity,” in Home Gardens and Agrobiodiversity, eds P. Eyzaguirre and O. Linares (Washington, DC: Smithsonian Books D.C.), 1–28.
Fakhry, A. M., and Aljedaani, G. S. (2020). Impact of disturbance on species diversity and composition of Cyperus conglomeratus plant community in southern Jeddah, Saudi Arabia. J. King Saud Univ.-Sci. 32, 600–605. doi: 10.1016/j.jksus.2018.09.003
FAO (2000). Global Forest Resources Assessment. Main Report, FAO Forestry Paper, Vol. 140. Rome: Food and Agriculture Organization of the United Nations, 2001.
FAO (2018). Urban Agriculture. Available online at: http://www.fao.org/urban-agriculture/en/ (accessed January 20, 2018).
Ferdous, Z., Datta, A., Anal, A. K., Anwar, M., and Khan, A. M. R. (2016). Development of home garden model for year round production and consumption for improving resource-poor household food security in Bangladesh. NJAS Wagen. J. Life Sci. 78, 103–110. doi: 10.1016/j.njas.2016.05.006
Feuillet, C., Langridge, P., and Waugh, R. (2008). Cereal breeding takes a walk on the wild side. Trends Genet. 24, 24–32. doi: 10.1016/j.tig.2007.11.001
Forest Survey of India [FSI] (2019). State of Forest Report. Dehradun: Ministry of Environment Forest and Climate Change.
Gajaseni, J., and Gajaseni, N. (1999). Ecological rationalities of the traditional homegarden system in the Chao Phraya Basin, Thailand. Agrofor. Syst. 46, 3–23. doi: 10.1023/A:1006188504677
Galhena, D. H., Freed, R., and Maredia, K. M. (2013). Home gardens: a promising approach to enhance household food security and wellbeing. Agric. Food Secur. 2, 2–8. doi: 10.1186/2048-7010-2-8
Galicia, L., Zarco-Arista, A. E., Mendoza-Robles, K. I., Palacio-Prieto, J. L., and García-Romero, A. (2008). Land use/cover, landforms and fragmentation patterns in a tropical dry forest in the southern Pacific region of Mexico, Singapore. J. Trop. Geogr. 29, 137–154. doi: 10.1111/j.1467-9493.2008.00326.x
Galluzzi, G., Eyzaguirre, P., and Negri, V. (2010). Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers. Conser. 19, 3635–3654. doi: 10.1007/s10531-010-9919-5
Gautam, R., Sunwar, S., Subedi, A., Shrestha, P., and Sthapit, B. R. (2009b). Home gardens and their roles in domestication of wild and uncultivated plant genetic resources in Nepal. Acta Hortic. 806, 677–684. doi: 10.17660/ActaHortic.2009.806.84
Gautam, R., Sthapit, B., Subedi, A., Poudel, D., Shrestha, P., and Eyzaguirre, P. (2009a). Home gardens management of key species in Nepal: a way to maximize the use of useful diversity for the well-being of poor farmers. Plant Genet. Resour. Charact. Utiliz. 7:142. doi: 10.1017/S1479262108110930
Glavan, M., Schmutz, U., Williams, S., Corsi, S., Monaco, F., Kneafsey, M., et al. (2018). The economic performance of urban gardening in three European cities–examples from Ljubljana, Milan and London. Urb. For. Urb. Green. 36, 100–122. doi: 10.1016/j.ufug.2018.10.009
Gopalakrishna, S. P., Kaonga, M. L., Somashekar, R. K., Suresh, H. S., and Suresh, R. (2015). Tree diversity in the tropical dry forest of Bannerghatta National Park in Eastern Ghats, Southern India. Euro. J. Ecol. 1, 12–27. doi: 10.1515/eje-2015-0013
Hammer, K., Laghetti, G., and Perrino, P. (1999). A checklist of the cultivated plants of Ustica (Italy). Genet. Resour. Crop Evol. 46, 95–106. doi: 10.1023/A:1008601909574
Jackson, L. E., Pascual, U., Brussaard, L., de Ruiter, P., and Bawa, K. S. (2007). Biodiversity in agricultural landscapes: investing without losing interest. Agric. Ecosyst. Environ. 121, 193–195. doi: 10.1016/jagee.2006.12.011
Jaganmohan, M., Vailshery, L. S., Gopal, D., and Nagendra, H. (2012). Plant diversity and distribution in urban domestic gardens and apartments in Bangalore, India. Urb. Ecosyst. 15, 911–925. doi: 10.1007/s11252-012-0244-5
Jaman, M. S., Hossain, M. F., Islam, M. S., Helal, M. G. J., and Jamil, M. (2016). Quantification of carbon stock and tree diversity of homegardens in Rangpur District, Bangladesh. Int. J. Agric. For. 6, 169–180. doi: 10.5923/j.ijaf.20160605.01
Jeyaprabakaran, G., and Rajendran, K. (2020). Medicinal trees from home gardens of urban areas in Madurai District of Tamil Nadu, Southern India. Asian J. Ethnobiol. 3, 10–15. doi: 10.13057/asianjethnobiol/y030102
Jhariya, M. K., Banerjee, A., Meena, R. S., and Yadav, D. K. (2019a). Sustainable Agriculture, Forest and Environmental Management. Singapore: Springer Nature, 606. doi: 10.1007/978-981-13-6830-1_6
Jhariya, M. K., Yadav, D. K., and Banerjee, A. (2019b). Agroforestry and Climate Change: Issues and Challenges. Varthur: Tayler and Francis Group, 335. doi: 10.1201/9780429057274
Jhariya, M. K., Meena, R. S., and Banerjee, A. (2021a). Ecological Intensification of Natural Resources for Sustainable Agriculture. Singapore: Springer Nature, 655. doi: 10.1007/978-981-33-4203-3
Jhariya, M. K., Meena, R. S., Banerjee, A., and Meena, S. N. (2021b). Natural Resources Conservation and Advances for Sustainability. Amsterdam: Elsevier, 650.
Jhariya, M. K., Banerjee, A., Meena, R. S., Kumar, S., and Raj, A. (2021c). Sustainable Intensification for Agroecosystem Services and Management. Singapore: Springer Nature, 870. doi: 10.1007/978-981-16-3207-5
Kabir, M. E., and Webb, E. L. (2008). Can homegardens conserve biodiversity in Bangladesh? Biotropica 40, 95–103.
Kala, C. P. (2010). Home gardens and management of key species in the Panchmarhi Biosphere Reserve of India. J. Biodiver. 1, 111–117. doi: 10.1080/09766901.2010.11884722
Kassambara, A., and Mundt, F. (2017). Package ‘factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7, Vol. 76.
Kehlenbeck, K., and Maass, B. L. (2004). Crop diversity and classification of homegardens in Central Sulawesi. Indonesia. Agrofor. Syst. 63, 53–62. doi: 10.1023/B:AGFO.0000049433.95038.25
Kehlenbeck, K., Arifin, H. S., and Maass, B. (2007). “Plant diversity in homegardens in a socio-economic and agro-ecological context,” in Stability of Tropical Rainforest Margins, eds T. Tscharntke, C. Leuschner, M. Zeller, E. Guhardja, and A. Bidin (Berlin: Springer), 297–319.
Khare, C. P. (2008). Indian Medicinal Plant: An Illustrated Dictionary. Berlin: Springer Science and Business Media, LLC, 900.
Khumbongmayum, A. D., Khan, M. L., and Tripathi, R. S. (2005). Sacred groves of Manipur, northeast India: biodiversity value, status and strategies for their conservation. Biodiver. Conser. 14, 1541–1582. doi: 10.1007/s10531-004-0530-5
Kumar, A., and Ram, J. (2005). Anthropogenic disturbances and plant biodiversity in forests of Uttaranchal, central Himalaya. Biodiver. Conser. 14, 309–331. doi: 10.1007/s10531-004-5047-4
Kumar, B. M., and Nair, P. R. (2004). The enigma of tropical homegardens. Agrofor. Syst. 61, 135–152. doi: 10.1023/B:AGFO.0000028995.13227.ca
Kumar, B. M., George, S. J., and Chinnamani, S. (1994). Diversity, structure and standing stock of wood in the homegardens of Kerala in peninsular India. Agrofor. Syst. 25, 243–262. doi: 10.1007/BF00707463
Larios, C., Casas, A., Vallejo, M., Moreno-Calles, A. I., and Blancas, J. (2013). Plant management and biodiversity conservation in Náhuatl homegardens of the Tehuacán Valley, Mexico. J. Ethnobiol. Ethnomed. 9, 1–16. doi: 10.1186/1746-4269-9-74
Lattirasuvan, T., Tanaka, S., Nakamoto, K., Hattori, D., and Sakurai, K. (2010). Ecological characteristics of home gardens in northern Thailand. Tropics. 18, 171–184. doi: 10.3759/tropics.18.171
Laurance, W. F. (2007). Have we overstated the tropical biodiversity crisis? Trends Ecol. Evol. 22, 65–70. doi: 10.1016/j.tree.2006.09.014
Lê, S., Josse, J., and Husson, F. (2008). FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18. doi: 10.18637/jss.v025.i01
Malik, Z. A., Hussain, A., Iqbal, K., and Bhatt, A. B. (2014). Species richness and diversity along the disturbance gradient in Kedarnath Wildlife Sanctuary and its adjoining areas in Garhwal Himalaya, India. Int. J. Curr. Res. 6, 10918–10926.
Mandal, R. A., Jha, P. K., Dutta, I. C., Thapa, U., and Karmacharya, S. B. (2016). Carbon sequestration in tropical and subtropical plant species in collaborative and community forests of Nepal. Adv. Ecol. 529703, 1–7. doi: 10.1155/2016/1529703
Marjokorpi, A., and Ruokolainen, K. (2003). The role of traditional forest gardens in the conservation of tree species in West Kalimantan, Indonesia. Biodivers. Conserv. 12, 799–822. doi: 10.1023/A:1022487631270
Maroyi, A. (2009). Traditional homegardens and rural livelihoods in Nhema, Zimbabwe: a sustainable agroforestry system. Int. J. Sustain. Develop. World Ecol. 16, 1–8. doi: 10.1080/13504500902745895
Martin, M., Geiger, K., Singhakumara, B. M. P., and Ashton, M. S. (2019). Quantitatively characterizing the floristics and structure of a traditional homegarden in a village landscape, Sri Lanka. Agrofor. Syst. 93, 1439–1454. doi: 10.1007/s10457-018-0254-2
McCune, B., and Grace, J. B. (2002). Analysis of Ecological Communities MjM Software. Gleneden Beach, Oregon, USA. 28.
Mellisse, B. T., van de Ven, G. W., Giller, K. E., and Descheemaeker, K. (2018). Home garden system dynamics in Southern Ethiopia. Agrofor. Syst. 92, 1579–1595. doi: 10.1007/s10457-017-0106-5
Miller, R. P., Penn, J. W., and Van Leeuwen, J. (2006). “Amazonian homegardens: their ethnohistory and potential contribution to agroforestry development,” in Tropica Homegardens: A Time-Tested Example of Sustainable Agroforestry, eds B. M. Kumar and P. K. R. Nair (Dordrecht: Springer), 43–60. doi: 10.1007/978-1-4020-4948-4_4
Mohri, H., Lahoti, S., Saito, O., Mahalingam, A., Gunatilleke, N., Hitinayake, G., et al. (2013). Assessment of ecosystem services in homegarden systems in Indonesia, Sri Lanka, and Vietnam. Ecosyst. Serv. 5, 124–136. doi: 10.1016/j.ecoser.2013.07.006
Moreno-Calles, A. I., Galicia-Luna, V. J., Casas, A., Toledo, V. M., Vallejo-Ramos, M., Santos-Fita, D., et al. (2014). Etnoagroforestería: El estudio de los sistemas agroforestales tradicionales de México. Ethnobiología. 12, 1–16. doi: 10.15425/redepriv.52.2014.15
Moreno-Calles, A., Casas, A., Blancas, J., Torres, I., Masera, O., Caballero, J., et al. (2010). Agroforestry systems and biodiversity conservation in arid zones: the case of the Tehuacán Valley, Central México. Agrofor. Syst. 80, 315–331. doi: 10.1007/s10457-010-9349-0
Negri, V. (2005). Agro–biodiversity conservation in Europe: ethical issues. J. Agric. Environ. Ethics. 18, 1–25. doi: 10.1007/978-1-4020-6865-2_1
Noble, I. R., and Dirzo, R. (1997). Forests as human-dominated ecosystems. Science. 277, 522–525. doi: 10.1126/science.277.5325.522
Norfolk, O., Eichhorn, M. P., and Gilbert, F. (2013). Traditional agricultural gardens conserve wild plants and functional richness in arid South Sinai. Basic Appl. Ecol. 14, 659–669. doi: 10.1016/j.baae.2013.10.004
Norfolk, O., Power, A., Eichhorn, M. P., and Gilbert, F. (2015). Migratory bird species benefit from traditional agricultural gardens in arid South Sinai. J. Arid Environ. 114, 110–115. doi: 10.1016/j.jaridenv.2014.12.004
Olson, R. K., Schoeneberger, M. M., and Aschmann, S. G. (2000). “An ecological foundation for temperate agroforestry,” in North American Agroforestry: An Integrated Science and Practice, eds H. E. Garrett, W. J. Rietveld, and R. F. Fisher (Madison, WI: American Society of Agronomy), 31–61.
Padalia, K., Bargali, K., and Bargali, S. S. (2015). How does traditional home-gardens support ethnomedicinal values in Kumaun Himalayan Bhabhar belt, India? Afric. J. Tradit. Complemen. Alter. Med. 12, 100–112. doi: 10.4314/ajtcam.v12i6.10
Pandey, C. B., Lata, K., Venkatesh, A., and Medhi, R. P. (2006). Diversity and species structure of home gardens in South Andaman. Trop. Ecol. 47, 251–258.
Panyadee, P., Balslev, H., Wangpakapattanawong, P., and Inta, A. (2018). Karen Homegardens: Characteristics, functions, and species diversity. Econ. Bot. 72, 1–19. doi: 10.1007/s12231-018-9404-8
Panyadee, P., Balslev, H., Wangpakapattanawong, P., and Inta, A. (2016). Woody plant diversity in urban homegardens in Northern Thailand. Econ. Bot. 70, 285–302. doi: 10.1007/s12231-016-9348-9
Pao, N. T., and Upadhaya, K. (2017). Effect of fragmentation and anthropogenic disturbances on floristic composition and structure of subtropical broad leaved humid forest in Meghalaya, Northeast India. Appl. Ecol. Environ. Res. 15, 385–407. doi: 10.15666/aeer/1504_385407
Patel, S. K., Sharma, A., and Singh, G. S. (2020). Traditional agricultural practices in India: an approach for environmental sustainability and food security. Ener. Ecol. Environ. 5, 253–271. doi: 10.1007/s40974-020-00158-2
Perfecto, I., and Vandermeer, J. (2008). Biodiversity conservation in tropical agroecosystems. a new conservation paradigm. Ann. N. Y. Acad. Sci. 1134, 173–200. doi: 10.1196/annals.1439.011
Peyre, A., Guidal, A., Wiersum, K. F., and Bongers, F. J. J. M. (2006). Dynamics of homegarden structure and function in Kerala, India. Agrofor. Syst. 66, 101–115. doi: 10.1007/s10457-005-2919-x
Poot–Pool, W. S., van der Wal, H., Flores–Guido, S., Pat–Fernández, J. M., and Esparza–Olguín, L. (2015). Home garden agrobiodiversity differentiates along a rural peri–urban gradient in Campeche, México. Econ. Bot. 69, 203–217. doi: 10.1007/s12231-015-9313-z
Regassa, R. (2016). Useful plant species diversity in homegardens and its contribution to household food security in Hawassa city, Ethiopia. Afric. J. Plant Sci. 10, 211–233. doi: 10.5897/AJPS2016.1439
Rendón-Sandoval, F. J., Casas, A., Moreno-Calles, A. I., Torres-García, I., and García-Frapolli, E. (2020). Traditional agroforestry systems and conservation of native plant diversity of seasonally dry tropical forests. Sustainability 12:4600. doi: 10.3390/su12114600
Sagar, R., and Singh, J. S. (2005). Structure, diversity, and regeneration of tropical dry deciduous forest of northern India. Biodiver. Conser. 14, 935–959. doi: 10.1007/s10531-004-0671-6
Sagar, R., Raghubanshi, A. S., and Singh, J. S. (2003). Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For. Ecol. Manag. 186, 61–71. doi: 10.1016/S0378-1127(03)00235-4
Sagar, R., Raghubanshi, A. S., and Singh, J. S. (2008). Comparison of community composition and species diversity of understory and overstory tree species in a dry tropical forest of northern India. J. Environ. Manag. 88, 1037–1046. doi: 10.1016/j.jenvman.2007.05.013
Saha, S. K., Nair, P. K. R., Nair, V. D., and Kumar, B. M. (2009). Soil carbon stock in relation to plant diversity of homegardens in Kerala. India. Agrofor. Syst. 76, 53–65. doi: 10.1007/s10457-009-9228-8
Sahoo, T., Acharya, L., and Panda, P. C. (2020). Structure and composition of tree species in tropical moist deciduous forests of Eastern Ghats of Odisha, India, in response to human-induced disturbances. Environ. Sustain 3, 69–82. doi: 10.1007/s42398-020-00095-0
Sahoo, U. K., and Rocky, P. (2015). Species composition and plant diversity as influenced by altitude and size of homegardens in Mizoram, North-East India. Int. J. Ecol. Environ. Sci. 41, 195–215.
Saikia, P., and Khan, M. L. (2013). Population structure and regeneration status of Aquilaria malaccensis Lam. in homegardens of Upper Assam, northeast India. Trop. Ecol. 54, 1–13.
Saikia, P., Choudhury, B. I., and Khan, M. L. (2012). Floristic composition and plant utilization pattern in homegardens of Upper Assam. India. Trop. Ecol. 53, 105–118.
Sampanpanish, P., and Jamroenprucksa, M. (1994). Ecological characteristics of homegarden agroforestry system in Amphoe Muang, Changwat Nonthaburi. Thai J. For. 13, 114–124.
Shannon, C. E., and Weaver, W. (1963). The Mathematical Theory of Communities. Urbana, IL: University of Illinois Press.
Sharma, A., Patel, S. K., and Singh, G. S. (2021). Traditional knowledge of medicinal plants among three tribal communities of Vindhyan highlands, India: an approach for their conservation and sustainability. Environ. Sustain 4, 749–783. doi: 10.1007/s42398-021-00196-4
Sharma, G. P., and Raghubanshi, A. S. (2009). Lantana invasion alters soil nitrogen pools and processes in the tropical dry deciduous forest of India. Appl. Soil Ecol. 42, 134–140. doi: 10.1016/j.apsoil.2009.03.002
Shastri, C. M., Bhat, D. M., Nagaraja, B. C., Murali, K. S., and Ravindranath, N. H. (2002). Tree species diversity in a village ecosystem in Uttara Kannada district in Western Ghats, Karnataka. Curr. Sci. 82, 1080–1084.
Shrestha, P., Gautam, R., Rana, B. R., and Sthapit, B. (2002). “Home gardens in Nepal: status and scope for research and development,” in Home Gardens and in situ Conservation of Plant Genetic Resources in Farming Systems, eds J. W. Watson and P. B. Eyzaguirre (Witzenhausen: IPGRI), 105–118.
Shukla, G., Vineeta, A. K., and Chakravarty, S. (2017). Plant diversity, structure and uses of the plants in home garden of Jharkhand, India. Indian J. Trop. Biodiver. 25, 40–50.
Singh, A. K., Raghubanshi, A. S., and Singh, J. S. (2002). Medical ethnobotany of the tribals of Sonaghati of Sonbhadra district, Uttar Pradesh, India. J. Ethnopharmacol. 81, 31–41. doi: 10.1016/S0378-8741(02)00028-4
Singh, A., Singh, G. S., and Singh, P. K. (2012). Medico-ethnobotanical inventory of Renukoot forest division of district Sonbhadra, Uttar Pradesh, India. Indian J. Natur. Products Resour. 3, 448–457.
Singh, D. (2013). Forest Structure, Diversity, Growing Stock Variation and Regeneration Status of Different Forest Cover Types in Dudatoli Area of Garhwal Himalaya. Doctoral dissertation. Uttarakhand: HNB Garhwal University Srinagar Garhwal.
Singh, J. S., Pandey, V. C., Singh, D. P., and Singh, R. P. (2010). Influence of pyrite and farmyard manure on population dynamics of soil methanotroph and rice yield in saline rain-fed paddy field. Agric. Ecosyst. Environ. 139, 74–79. doi: 10.1016/j.agee.2010.07.003
Singh, J. S., Raghubanshi, A. S., Singh, R. S., and Srivastava, S. C. (1989). Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338, 499–500. doi: 10.1038/338499a0
Singh, V. P., and Singh, J. S. (1992). Energetics and environmental costs of agriculture in a dry tropical region of India. Environ. Manag. 16:495.
Subba, M., Pala, N. A., Shukla, G., and Chakravarty, S. (2017). Are size, distance and location responsible for species richness in home garden agroforestry systems. Indian fores. 143, 223–227.
Sujarwo, W., and Caneva, G. (2015). Ethnobotanical study of cultivated plants in home gardens of traditional villages in Bali (Indonesia). Hum. Ecol. 43, 769–778. doi: 10.1007/s10745-015-9775-8
Sunwar, S., Thornström, C. G., Subedi, A., and Bystrom, M. (2006). Home gardens in western Nepal: opportunities and challenges for on-farm management of agrobiodiversity. Biodiver. Conser. 15, 4211–4238. doi: 10.1007/s10531-005-3576-0
Thompson, J. L. (2007). The Gardens of Urban and Peri-Urban Khartoum, the Republic of the Sudan. MSc thesis. Germany: Faculty of Organic Agricultural Sciences, University of Kassel.
Tiwari, S., Singh, C., and Singh, J. S. (2018). Land use changes: a key ecological driver regulating methanotrophs abundance in upland soils. Ener. Ecol. Environ. 3, 355–371. doi: 10.1007/s40974-018-0103-1
Tiwari, S., Singh, C., Boudh, S., Rai, P. K., Gupta, V. K., and Singh, J. S. (2019). Land use change: a key ecological disturbance declines soil microbial biomass in dry tropical uplands. J. Environ. Manag. 242, 1–10. doi: 10.1016/j.jenvman.2019.04.052
Tiyakoat, W., Chllachakkawat, C., Dhompongsa, G., and Sarobol, S. (2010). “The migration of Tai Yai from Shan State-Myanmar, into Thailand,” in Procedings of the 1st International Conference on Culture, Toruism & Economy in Salween River Basin Reion, Mae Hong Son, Thailand, 10–11.
Trinh, L. N., Watson, J. W., Hue, N. N., De, N. N., Minh, N. V., Chu, P., et al. (2003). Agrobiodiversity conservation and development in Vietnamese home gardens. Agric. Ecosyst. Environ. 97, 317–344. doi: 10.1016/S0167-8809(02)00228-1
Valencia, V., García-Barrios, L., West, P., Sterling, E. J., and Naeem, S. (2014). The role of coffee agroforestry in the conservation of tree diversity and community composition of native forests in a Biosphere Reserve. Agric. Ecosyst. Environ. 189, 154–163. doi: 10.1016/j.agee.2014.03.024
Vandermeer, J. H. (2002). Tropical Agroecosystems. Boca Raton, FL: CRC Press. doi: 10.1201/9781420039887
Verma, P., Singh, R., Bryant, C., and Raghubanshi, A. S. (2020). Green space indicators in a social-ecological system: A case study of Varanasi, India, Sustain. Cities Societ. 60:102261. doi: 10.1016/j.scs.2020.102261
Vibhuti, B. K., Bargali, S. S., and Bargali, S. S. (2018). Effects of homegarden size on floristic composition and diversity along an altitudinal gradient in Central Himalaya, India. Curr. Sci. 114, 2494–2503. doi: 10.18520/cs/v114/i12/2494-2503
Vidal, S. (2008). Plant biodiversity and vegetation structure in traditional cocoa forest gardens in southern Cameroon under different management. Biodivers. Conser. 17, 1821–1835. doi: 10.1007/s10531-007-9276-1
Vlkova, M., Polesny, Z., Verner, V., Banout, J., Dvorak, M., Havlik, J., et al. (2011). Ethnobotanical knowledge and agrobiodiversity in subsistence farming: case study of home gardens in Phong My commune, central Vietnam. Genet. Resour. Crop Evol. 58, 629–644. doi: 10.1007/s10722-010-9603-3
Wagh, V. V., and Jain, A. K. (2015). Inventory of ethnobotanicals and other systematic procedures for regional conservation of medicinal and sacred plants. Environ. Syst. Decis. 35, 143–156. doi: 10.1007/s10669-015-9538-5
Wezel, A., and Bender, S. (2003). Plant species diversity of homegardens of Cuba and its significance for household food supply. Agrofor. Syst. 57, 39–49. doi: 10.1023/A:1022973912195
Whitney, C. W., Luedeling, E., Hensel, O., Tabuti, J. R., Krawinkel, M., Gebauer, J., et al. (2018a). The role of homegardens for food and nutrition security in Uganda. Hum. Ecol. 46, 497–514. doi: 10.1007/s10745-018-0008-9
Whitney, C. W., Luedeling, E., Tabuti, J. R., Nyamukuru, A., Hensel, O., Gebauer, J., et al. (2018b). Crop diversity in homegardens of southwest Uganda and its importance for rural livelihoods. Agric. Hum. Values. 35, 399–424. doi: 10.1007/s10460-017-9835-3
Widayat, D., Sumekar, Y., Wahyudin, A., Yuwariah, Y., and Farida, C. (2019). Effect of various dosage of ammonium glufosinate herbicide on suppressing weeds and growth and yield of corn. J. Res. Weed Sci. 2, 90–102. doi: 10.26655/JRWEEDSCI.2019.3.1
Wiehle, M., Prinz, K., Kehlenbeck, K., Goenster, S., Mohamed, S. A., Buerkert, A., et al. (2014). The role of homegardens and forest ecosystems for domestication and conservation of Ziziphus spina-christi (L.) Willd. in the Nuba Mountains, Sudan. Genet. Resour. Crop. Evol. 61, 1491–1506. doi: 10.1007/s10722-014-0124-3
Zemede, A. (2001). “The role of homegardens in the production and conservation of medicinal plants,” in Conservation and Sustainable use of Medicinal plants in Ethiopia, eds M. Zewdu and A. Demissie (Addis Ababa: Institute of Biodiversity Conservation and Research), 76–91. doi: 10.1186/s13002-018-0269-9
Keywords: indigenous species, disturbance gradient, Vindhyan region, summed dominance ratio (SDR), Principal Component Analysis (PCA)
Citation: Patel SK, Sharma A, Singh R, Tiwari AK and Singh GS (2022) Diversity and Distribution of Traditional Home Gardens Along Different Disturbances in a Dry Tropical Region, India. Front. For. Glob. Change 5:822320. doi: 10.3389/ffgc.2022.822320
Received: 25 November 2021; Accepted: 17 January 2022;
Published: 17 March 2022.
Edited by:
Rahul Bhadouria, University of Delhi, IndiaReviewed by:
Sachchidanand Tripathi, University of Delhi, IndiaG. N. Tanjina Hasnat, University of Chittagong, Bangladesh
Manoj Kumar Jhariya, Sant Gahira Guru Vishwavidyalaya, India
Copyright © 2022 Patel, Sharma, Singh, Tiwari and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gopal Shankar Singh, Z29wYWxzaW5naC5iaHVAZ21haWwuY29t
 Sanoj Kumar Patel
Sanoj Kumar Patel Anil Sharma
Anil Sharma Rinku Singh
Rinku Singh Amit Kumar Tiwari
Amit Kumar Tiwari Gopal Shankar Singh
Gopal Shankar Singh