
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 19 May 2022
Sec. Forest Ecophysiology
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.733636
This article is part of the Research Topic Functional Ecology and Conservation of Palms View all 10 articles
Environmental gradients influence plant establishment, survival, and functional traits. Along the Panama Canal Isthmus there is a strong rainfall gradient with an underlying mosaic of soil types ranging in soil nutrient availability. In this region, tree species distribution patterns are correlated with soil phosphorus availability and rainfall patterns, but how understory plant species such as palms relate to these factors is less clear. We hypothesized that due to greater resource use efficiency and optimal biomass allocation, specialist species will have greater seedling performance growing in home soil and sites compared to species not occurring there. To test this hypothesis, we used two specialist species (Chamaedorea tepejilote and Geonoma congesta) and two generalist species (Geonoma cuneata var. cuneata and Chamaedorea pinnatifrons), and for these four species we measured traits on seedlings and assessed their performance in shade house and field transplant experiments using five soils. Soils were sourced from five sites which varied in nutrient availability and rainfall, and were distributed along lowland tropical forests of the Panama Canal Isthmus. In the shadehouse experiment, leaf functional traits were determined by species rather than soil nutrient availability. However, in the shadehouse experiment, seedling biomass allocation, and relative growth rate were determined by interactions between species and soil, with weak support for home-site advantage for one of the species. In the field transplant experiment, seedling survival was strongly related to dry season water availability. However, species tended to have high survival at home sites and other sites with higher dry season rainfall. Together, results from these experiments suggest that understory palm species seedling performance are determined by species-specific responses to the combination of soil nutrient and water availability. This indicates that while soil nutrients influence seedling biomass allocation, dry season water availability determines both specialist and generalist seedling survival and therefore distributions along the soil nutrient and moisture gradient.
Abiotic environmental factors, including climate, soil nutrients, and light are drivers of seedling performance, including their survival, growth, and functional traits, and consequently influence species distribution patterns (Grubb, 1977; Grime, 2006; Engelbrecht et al., 2007). These abiotic factors do not operate independently of each other. For example, in lowland tropical forests, high mean annual precipitation (MAP) and temperatures leads to high rates of leaching of labile cations and phosphorous from the parent material during soil formation (Vitousek and Sanford, 1986; Sollins, 1998), and therefore result in nutrient limitation of rock-derived nutrients necessary for successful seedling regeneration (Alvarez-Clare et al., 2013). In addition to nutrient limitation, dry season soil moisture availability can also impose a filter on seedling regeneration and species composition (Baltzer et al., 2005; Engelbrecht et al., 2007; Condit et al., 2013). However, disentangling the effects of different abiotic drivers such as soil nutrient availability and dry season water deficit on seedling performance may reveal different mechanisms that drive habitat associations and ultimately species distribution patterns along environmental gradients (Fortunel et al., 2016).
Plants show trade-offs between “acquisitive” strategies aimed at rapid resource uptake driving fast growth rates, and “conservative” strategies aimed at the retention of resources and longer life spans (Diaz et al., 2004; Wright et al., 2004; Díaz et al., 2016). The traits underpinning these strategies are responsive to environmental gradients, including soil nutrient (Richardson et al., 2004; Peltzer et al., 2010), and moisture availability (Maharjan et al., 2011; Pollock et al., 2012; McLean et al., 2014). For example, plants growing in fertile soils allocate biomass aboveground and are associated with high relative growth rates (RGR), leaf-area ratios (LAR), specific leaf area (SLA; Aerts and Chapin, 2000; Palmiotto et al., 2004; Dent and Burslem, 2009), and height (Yavitt and Wright, 2008; Ali and Yan, 2017), whereas those growing in low-nutrient soils allocate more biomass belowground and are associated low RGR and SLA (Tilman and Wedin, 1991; Aerts and Chapin, 2000). Together, plant functional traits govern how species both influence and respond to their environment (Diaz et al., 2004) and can drive community assembly (Laughlin and Laughlin, 2013; Kumordzi et al., 2015). Among plant groups, monocots and particularly, palms, produce leaves with high leaf toughness (Dominy et al., 2008) and lignin (Santiago, 2007), slow decomposition rates, and low SLA and nutrient content compared to dicots, suggesting palms may have conservative plant economic strategies. However, despite the high species diversity and abundance of palm species in lowland tropical forests, we know little about how functional traits vary among palm species or the ecological significance of this variation compared to the breadth of information for their woody counterparts.
Understanding drivers of palm species distributions is central to understanding whole forest community ecology in lowland tropical forests because palms act as filters that affect tree seedling growth and survival (Farris-Lopez et al., 2004; Wang and Augspurger, 2004). Palms reduce seedling establishment by modifying microsite conditions through increasing leaf litter depth and decreasing light availability (Farris-Lopez et al., 2004). Therefore, it is important to better understand drivers of palm species distribution patterns. Palm species distribution patterns, phylogenetic structure, and ecological interactions are all influenced by soil conditions, such as nutrient and moisture availability (Clark et al., 1995; Emilio et al., 2013; Cámara-Leret et al., 2017; Muscarella et al., 2019). For example, palm species distribution patterns are strongly correlated with soil phosphorus and base cations across landscape scales in lowland Amazonia (Cámara-Leret et al., 2017) and soil nitrogen availability in lower montane forests in western Panama (Andersen et al., 2010a,b). Although there have been many studies examining the relationship between soil parameters and palm species distributions, fewer studies have experimentally tested the mechanisms responsible for driving these patterns along soil gradients or examined plant functional traits.
The Panamanian Isthmus presents an ideal study system for exploring species habitat specialization because of its mosaic of environmental conditions. The complex environmental variation in this region is due to a strong rainfall gradient which ranges from 1,500 to 3,000 mm MAP from the Pacific to Caribbean sides of the 60 km Isthmus (Paton, 2020a,b), as well as a diverse geology resulting in a range of soil properties (Turner and Engelbrecht, 2011; Cusack et al., 2018). Soil nutrients and moisture are the main abiotic factors that drive a near complete floristic turnover of woody species in the lowland tropical forests across the 60 km Panama Isthmus (Pyke et al., 2001; Engelbrecht et al., 2007; Condit et al., 2013). In addition to shifts in species composition, there are large changes in fine root biomass driven by variation in soil base cation availability (Cusack et al., 2018), and changes in community-level leaf trait values, such as SLA and leaf thickness, driven by variation in both soil moisture and phosphorus (Umaña et al., 2021). This demonstrates strong species selection and trade-offs along multiple resource gradients for trees, but it is unclear whether understory plant species follow the same patterns.
One of the most extensively studied understory palm communities occurs in the Fortuna Forest Reserve (Fortuna) and surrounding lower montane forests of western Panama, a hot spot for palm species diversity and endemism (Andersen et al., 2010a; Prada et al., 2017). In Fortuna, understory palm species community composition and functional traits are related to shifts in soil nitrogen, cation, and aluminum availability (Andersen et al., 2010a,2012), whereas tree (woody and palm) species composition is related to soil phosphorus availability and rainfall (Prada et al., 2017). Furthermore, seedling transplant experiments of understory palm species have revealed that whole-plant functional strategies of low nutrient specialist species give them a performance advantage at low soil nutrient sites (Andersen et al., 2010b,2014; Andersen and Turner, 2013). These findings suggest that functional traits and seedling performance drive understory palm species distribution patterns across the soil nutrient gradient at Fortuna, where species show “home-site advantages” when growing in soils they are associated with compared to species absent from the site. Here, we expand on these studies by using seedling experiments to test the effects of soil nutrient and moisture availability on seedling performance of four contrasting understory palm species along soil nutrient availability and rainfall gradient in lowland tropical forests of the Panama Canal Watershed.
To specifically examine the effects of soil nutrient availability on understory palm seedling performance and functional traits, we conducted a shadehouse experiment growing seedlings of four species in soil collected from five sites varying in soil nutrient availability along the Panama Canal (Table 1; Turner and Engelbrecht, 2011; Cusack et al., 2018). Nutrient availability varied from “low nutrient” soil that is depleted in phosphorus and base cations to “high nutrient” soil that is rich in phosphorus. We included two site specialist species (Chamaedorea tepejilote), from site Pipeline Road Plot 15 (an intermediate nutrient site), and Geonoma congesta, from site Fort Sherman (a low nutrient site). We also included two site generalist species (Geonoma cuneata var. cuneata and Chamaedorea pinnatifrons) from the lower montane Fortuna sites which have soils that range in nutrient availability (Table 2). In this study, a species was defined as a specialist if it is found at one site, i.e., that is has a single soil nutrient ranking. A species was classified as a generalist if it is found at multiple areas which vary in soil nutrient availability. While the ratio of generalist to specialist palm species across these sites is poorly understood, in western Amazonian lowland rainforests generalist species have wide tolerances to differences in soil fertility and outnumber specialist species (Ruokolainen and Vormisto, 2000). To further understand how abiotic factors (including both soil nutrients and moisture availability) influence seedling survival of the four palm species, we also transplanted seedlings of all four species into the five field sites. We expected that, due to higher resource use efficiency and optimal biomass allocation, the specialist species (C. tepejilote and G. congesta) would show greater seedling performance in the soil and at the site that they are associated with compared to species not occurring at those sites. On that basis we tested the following four hypotheses:
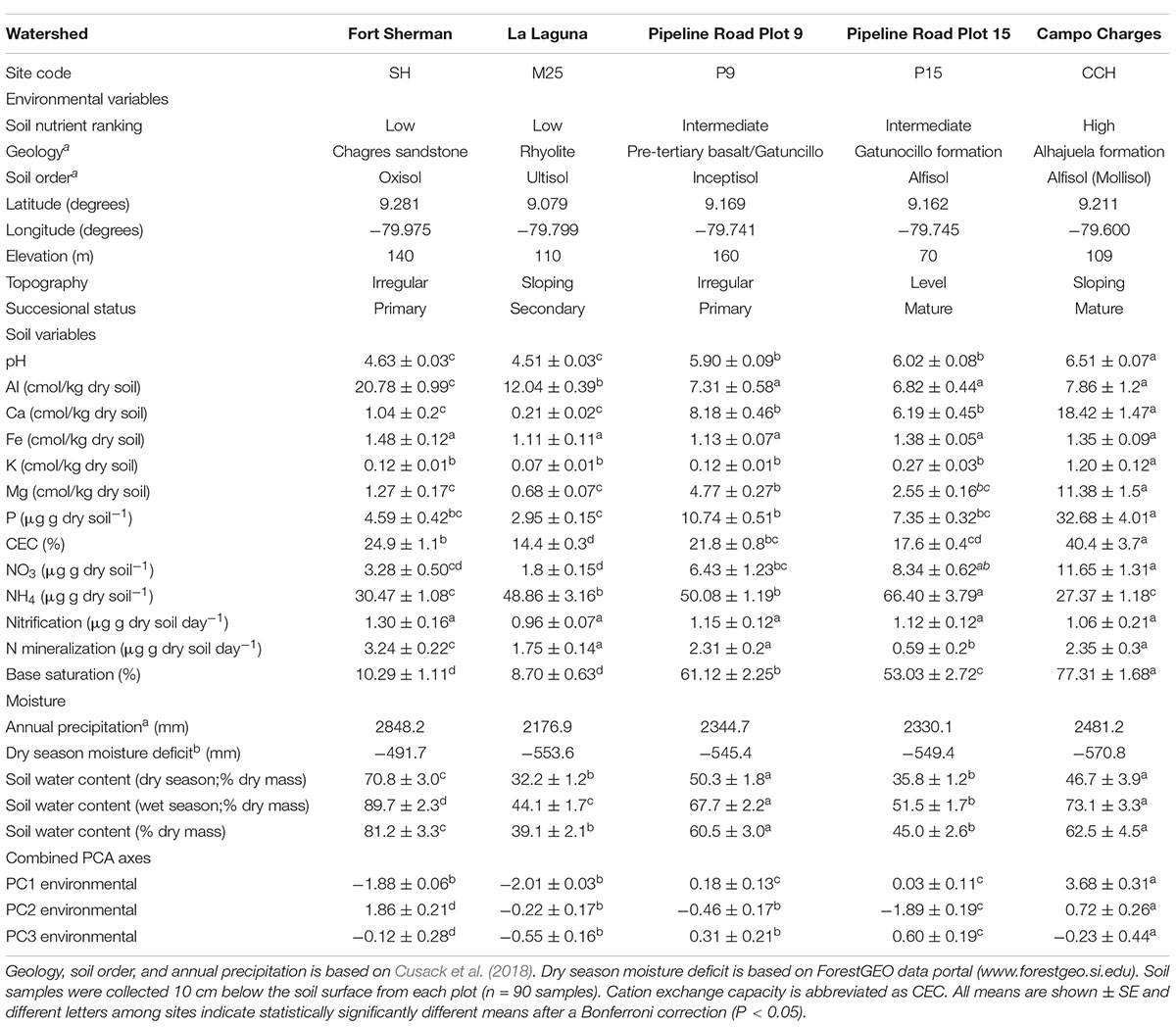
Table 1. Environmental conditions and plot locations of five sites along a soil nutrient gradient in lowland tropical forests of the Panama Canal Watershed.
(H1) To maximize returns of investment in leaf resources, leaf traits such as SLA, LAR, photosynthesis rates (Asat), and dark respiration rates (Rdark) will decline as soil fertility declines. Furthermore, we expected the species associated with low nutrient, phosphorus and base cation depleted soils (G. congesta) to have higher leaf resource-use efficiency and the species associated with intermediate nutrient soil (C. tepejilote) to have leaf traits to maximize carbon gains when grown in their home soil compared to species not absent from those soils.
(H2) Root mass ratio (RMR) should increase as soil fertility declines to meet nutrient demands at the expense of leaf mass ratio (LMR), stem mass ratio (SMR), and growth potential. The specialist species are expected to maximize allocation to leaf biomass to increase growth potential in their home soils compared to other species absent from those soils.
(H3) Due to increased resource-use efficiency (H1) and optimal biomass allocation patterns (H2), specialists growing in the soil they are associated with will achieve higher RGR compared to species not occurring in those soils.
(H4) Since specialist species have higher resource-use efficiency (H1), higher nutrient/water uptake efficiency biomass allocation patterns (H2), and higher RGR (H3), they will have higher seedling survival rates when growing at the sites where they naturally occur than will species not occurring at those sites.
By simultaneously examining the above hypotheses, we aim to advance our understanding of the mechanisms that influence establishment, survival, and functional traits of seedlings of understory palm species, and thus their distribution patterns along soil nutrient and moisture gradients in lowland tropical forests.
The present study was conducted at five one-hectare sites in lowland tropical forest along the Panama Canal (Pyke et al., 2001; Engelbrecht et al., 2007; Condit et al., 2013) ForestGEO data portal1. These sites following nomenclature from Pyke et al. (2001) and Cusack et al. (2018), and ForestGEO data portal (see text footnote 1) include the Fort Sherman/San Lorenzo crane site (SH), La Laguna/Rio Paja (M25), Pipeline Road plot 9 (P9), Pipeline Road plot 15 (P15), and Campo Charges (CCH; Table 1). MAP of these sites range from 2,177 mm at site M25 to 2,848 mm at site SH, with dry seasonal precipitation deficit ranging from 570 mm at site CCH to 492 mm at site SH (Condit et al., 2013); ForestGEO data portal (see text footnote 1). In general, soil nutrient availability and dry season water deficit increase as MAP decreases among sites (Condit et al., 2013; Cusack et al., 2018; Umaña et al., 2021). The M25 site represents a low nutrient site with low MAP, however, the fine texture soil allows it to maintain high soil available water capacity for a given gravimetric soil moisture content (Kursar et al., 2005).
To quantify the soil nutrient gradient, soil samples were collected in May 2004. At each site, three subsamples were collected from the top 10 cm of the soil, representing the main rooting zone (Cavelier, 1992), and bulked at eighteen locations in each of the 1-ha plots. Each of the eighteen composite samples per site were divided into four subsamples to measure soil moisture content, nitrate and ammonium, pH, and cation (Al, Ca, Fe, K, and Mg) and phosphorous concentrations. In addition, nitrogen mineralization and nitrification rates were estimated using in situ PVC incubation tubes at each of the soil sampling locations. After 30 days, soil from the tubes was collected and processed for ammonium and nitrate concentrations. For nitrate and ammonium analyses, 2.0 g of fresh soil were extracted with 2M KCl and analyzed colormetrically on a Latchat QuikChem flow injection analyzer (Hach Company, Loveland, CO, United States). Soil pH was measured in a 1:3 fresh soil solution with distilled water. Soil cation and phosphorus analyses were conducted by extracting 2.5 g fresh soil with Mehlich III extractant (Mehlich, 1984) and the filtrate analyzed by inductively coupled plasma optical emission spectroscopy on a Perkin-Elmer Optima 2000 (Perkin Elmer Inc., Shelton, CT, United States). Soil water content was measured by weighing fresh soil and soil samples dried at 100°C for 72 h to enable the calculation of the amount of dry soil used in all measurements. We used principal components analysis (PCA, see statistical analyses for details) to rank the sites by soil nutrient availability. The sites with the lowest nutrient availability were SH and M25, with sites P9 and P15 being intermediate, and site CCH having high soil fertility, with the main soil nutrient gradient driven by soil P and cation concentration and soil pH (Figure 1A).
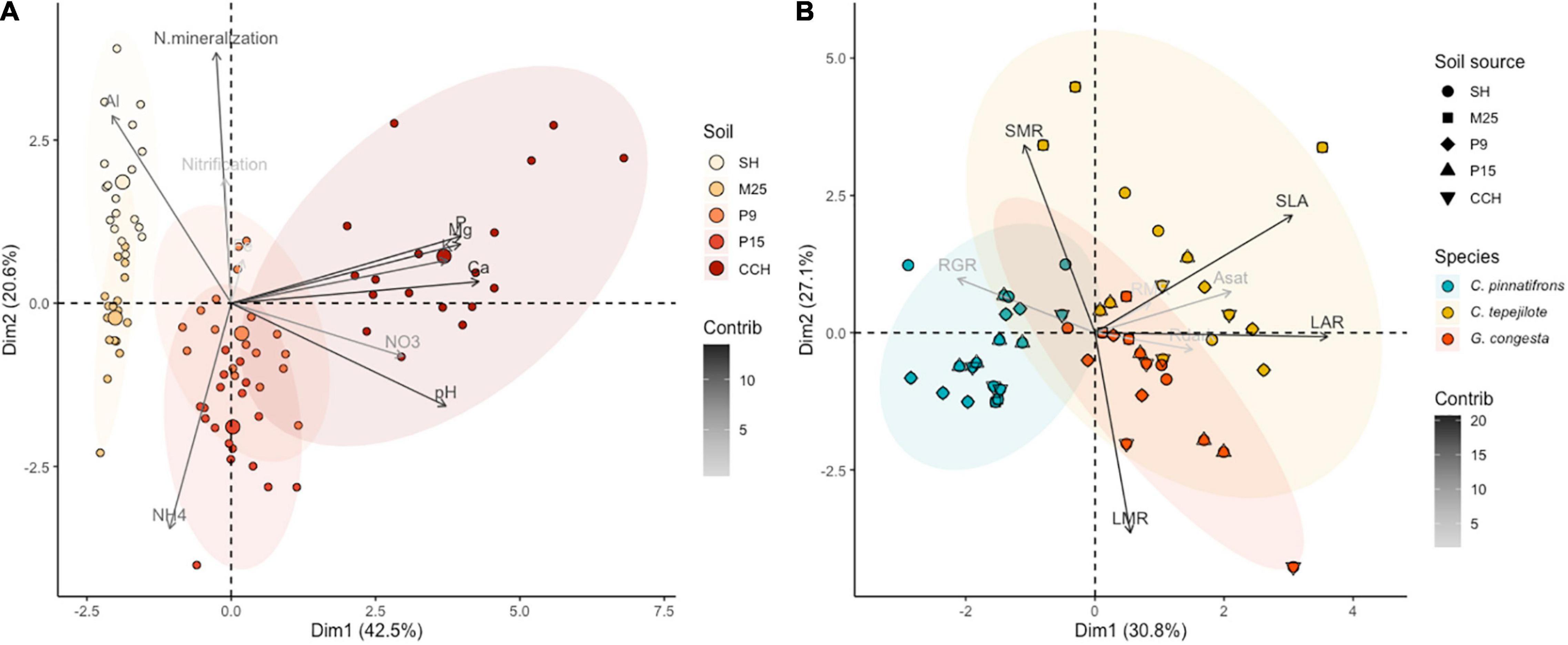
Figure 1. (A) Principal component analysis of soil data for each of the five sites, SH, M25, P15, P9, and CCH. Each point represents a separate sample (n = 90) and shading represents increasing soil nutrient availability from SH to the CCH site. Contribution of soil properties, AL, Ca, Fe, K, Mg, P, NH4, NO3, N mineralization, Nitrification, and pH, are indicated by arrow shading. Concentration ellipses are displayed for each of the five soils. (B) Principal component analysis of seedling trait data for three species at each of the five sites, with each point representing a separate sample (n = 59). Contribution of seedling traits are indicated by arrow shading. Concentration ellipses are displayed for each of the three species. The primary seedling trait ordination axis (PC 1) represents a shift from high relative growth rate (RGR) to investment in high leaf quality, specific leaf area (SLA), leaf area ratio (LAR), root mass ratio (RMR), maximum photosynthetic rate (Asat), and dark respiration rates (Rdark). The secondary seedling trait ordination axis (PC 2) represents a shift from high leaf mass ratio (LMR) to high stem mass ratio (SMR).
We focused on four common understory palms species within the subfamily, Arecoideae (Table 2): C. pinnatifrons (Jacq.) Oerst, C. tepejilote Liebm., G. congesta H. Wendl. ex Spruce, and G. cuneata subsp. cuneata (H. Wendl. ex Spruce; Baker et al., 2011; Henderson, 2011). All species are Neotropical long-lived perennials that differ in general morphology, life history, habitat association, and spatial distribution (Henderson et al., 2019). We define soil-based habitat associations on occurrence within the Panama Canal Watershed region. Preliminary surveys suggest that two of the four species are site specialists: C. tepejilote occurs at intermediate soil nutrient sites and G. congesta occurs at a low soil nutrient sites in the lowland tropical forests of the Panama Canal Watershed (Table 2). The other two species are generalist species in the lower montane forests of Fortuna Forest Reserve in western Panama, including the most abundant species in the lower montane forests of Fortuna, G. cuneata, as well as C. pinnatifrons (Andersen et al., 2010a). G. congesta, ranges from Honduras to Panama and occurs locally at the SH site where the seeds for the experiments were collected from. C. tepejilote, ranges from Mexico to Colombia and occurs at the P15 site and seeds of this species were collected along the Pipeline Road forests. G. cuneata, ranges from Nicaragua to Ecuador and contains a complex of subspecies (Henderson, 2011) including several within Panama. G. cuneata subsp. indivisa Henderson, occurs locally at SH, P9, and P15. However, the seeds used in the experiments were collected from G. cuneata subsp. cuneata “fortuna morphotype” (Henderson, 2011), which occurs across the soil nutrient gradient at the Fortuna Forest Reserve (Andersen et al., 2010a,2014). C. pinnatifrons, spans from Mexico to northern South America (Henderson et al., 2019). However, this species did not occur at any of the five sites used in our study and seeds were collected from the Fortuna Forest Reserve where it occurs across the soil nutrient gradient (Andersen et al., 2010a,2014). We collected seeds for all four species from the field between July and September 2005.
All seeds for the experiments were germinated at the Santa Cruz Experimental Site, Panama. Soils were collected from the five sites and mixed with washed sand in a 30:70 sand: soil ratio. Seeds were sowed into germination trays containing the soil type that the seedlings would be transplanted to for the shadehouse and field experiments.
To determine the effect of soil nutrient availability on seedling performance and functional traits, we conducted a completely randomized, factorial design shadehouse experiment by growing seedlings of the four species in soil from the five field sites. Once the seedlings had germinated with one fully expanded leaf, one seedling was transplanted per pot which contained freshly collected soils from the field sites that had been mixed with sand in a 30:70 sand:soil ratio. Ten seedlings per species * soil combination were planted in pots, resulting in a total of 200 plants. Pot size ranged from 0.5L for the smaller Geonoma species to 2L for the larger Chamaedorea species. Seedlings were watered during the dry season (January–April) and as needed to supplement natural rainwater during the wet season. Temperature was maintained at ambient conditions while light levels were maintained at 2% light conditions to simulate natural understory conditions within the shadehouse. Seedlings were moved around continuously during the experiment to avoid any bias in growing conditions.
Seedling survival was monitored for the experiment over 416 days, as at this time all the seedlings had produced multiple leaves and had started to reach the size limits of their pots. Light saturated photosynthetic capacity (Asat) and dark respiration (Rdark) were measured on the youngest fully mature leaf of all individuals surviving to the end of the experiment using a Li-Cor 6400 portable photosynthesis system (LI-COR Inc., Lincoln, NE, United States). Measurements were made with the following chamber conditions: <75% relative humidity, temperature of 25–28°C, air flow rate of 500 μmol⋅s–1 and CO2 of 400 ppm. Light saturation curves were performed to determine saturating light conditions for a subset of individuals of each species (data not shown), after which light levels were set to 400 μmol⋅m–2 s–1 for the Asat measurements (Chazdon, 1986). For Rdark, leaves were left for 15 min to stabilize with the light source turned off. The mean values of five-point measurements over 60 s were recorded for gas exchange measurements. After the gas exchange measurements were complete, all surviving seedlings were destructively harvested to measure leaf area and biomass allocation patterns. Seedlings were separated into leaf, stem, and root components and dried for 72 h at 65°C. Prior to drying, fresh leaves were digitally scanned using a Cannon CanoScan LiDE 100 flatbed scanner (Cannon, Melville, NY, United States) and leaf area was estimated using ImageJ software2. Harvest data were used to calculate SLA (leaf area per leaf mass; mm2⋅mg–1), LAR (leaf area per whole plant mass; mm2⋅mg–1), LMR (leaf mass divided by whole plant mass; LMR), SMR (stem mass divided by whole plant mass; SMR), and RMR (root mass divided by whole plant mass; RMR), and RGR (biomass at harvest subtracted by initial biomass divided by number of days since planting; RGR; mg⋅g–1⋅day–1). These parameters are indicative of resource use and acquisition, carbon allocation, and overall seedling performance. All seedlings of G. cuneata died before traits were measured at the end of the experiment.
To examine seedling survival under field conditions, we conducted a split-plot transplant experiment with ten gardens of paired exclosure and open (control) subplots randomly located at each field site. Transplant locations were chosen to represent closed canopy forest locations for all field sites. A regional study suggests that light transmittance to the understory of these forests are generally around 1–2% annually throughout the Panamanian Isthmus (Brenes-Arguedas et al., 2011). In November 2005, twenty seedlings of each of the four species were transplanted at each of the five sites, resulting in 400 seedlings in total. Within each site we set up ten randomly located gardens and established paired 50 × 50 cm subplots, i.e., a control subplot and a treatment subplot. One seedling per species was transplanted into each paired subplot (i.e., two seedlings per garden). Paired subplots were <1 m from each other and gardens were at least 20 m from each other. Exclosure subplots were covered by 1 mm transparent mesh to exclude herbivores, while control subplots were completely open to herbivores. Soil cores were taken adjacent to the experimental subplots at every census to monitor gravimetric soil moisture. Seedlings were monitored on average every 63 days on eight occasions, or 505 days in total. Seedling performance did not differ between the paired control and exclosure subplots and data was pooled prior to analysis by calculating the mean survival rate (%) per species per garden (n = 10 per site).
To examine how soil nutrients affect seedling leaf functional traits, biomass allocation and RGRs in the shadehouse experiment, we conducted two-way ANOVAs to test for differences in mean values among species, soil, and their interaction. We assessed whether model assumptions were met using graphical diagnostics. We used a backward elimination model simplification approach based on Akaike Information Criterion values and reported the statistics from the final (best fit) models. Tukey’s post hoc tests were then used to identify significant differences between means at P = 0.05, using the R package “emmeans” (Lenth, 2021).
To examine seedling survival in the field transplant experiment, we used generalized linear mixed effect models using the glmer function in the R package “lme4” (Bates et al., 2015). Mean seedling survival rates per garden were fitted using Poisson distribution and log link families with species, site, and their interactions as fixed effects and garden as a random effect. To examine species specific survival rates at the end of the field transplant experiment, generalized linear models (glm) were performed for each species separately followed by Tukey’s post hoc tests at P = 0.05 to examine differences in the mean survival among sites.
We conducted two separate PCA. The first analysis was performed on the soil nutrient data, to enable us to rank the five sites by overall soil nutrient availability. The second analysis was performed on the seedling trait and RGR data from the shadehouse experiment to examine whole-plant trade-offs among species and soil type. The PCAs were conducted using the R package FactoMineR (Lê et al., 2008). The data was centered and standardized to one unit of variance, but not transformed prior to analysis.
In the shadehouse experiment, the mean and standard error for SLA across all the seedlings was 306 ± 9.5 mm2 g–1. The best fit model for SLA showed that only species differed significantly (F2,86 = 70.91, p < 0.0001). Across all soil sources, C. tepejilote a significantly higher mean SLA (362 ± 7.9 mm2 g–1) than the other species, and G. congesta had a significantly higher SLA (276 ± 12.5 mm2 g–1) than C. pinnatifrons (187 ± 12.8 mm2 g–1; Figure 2A).
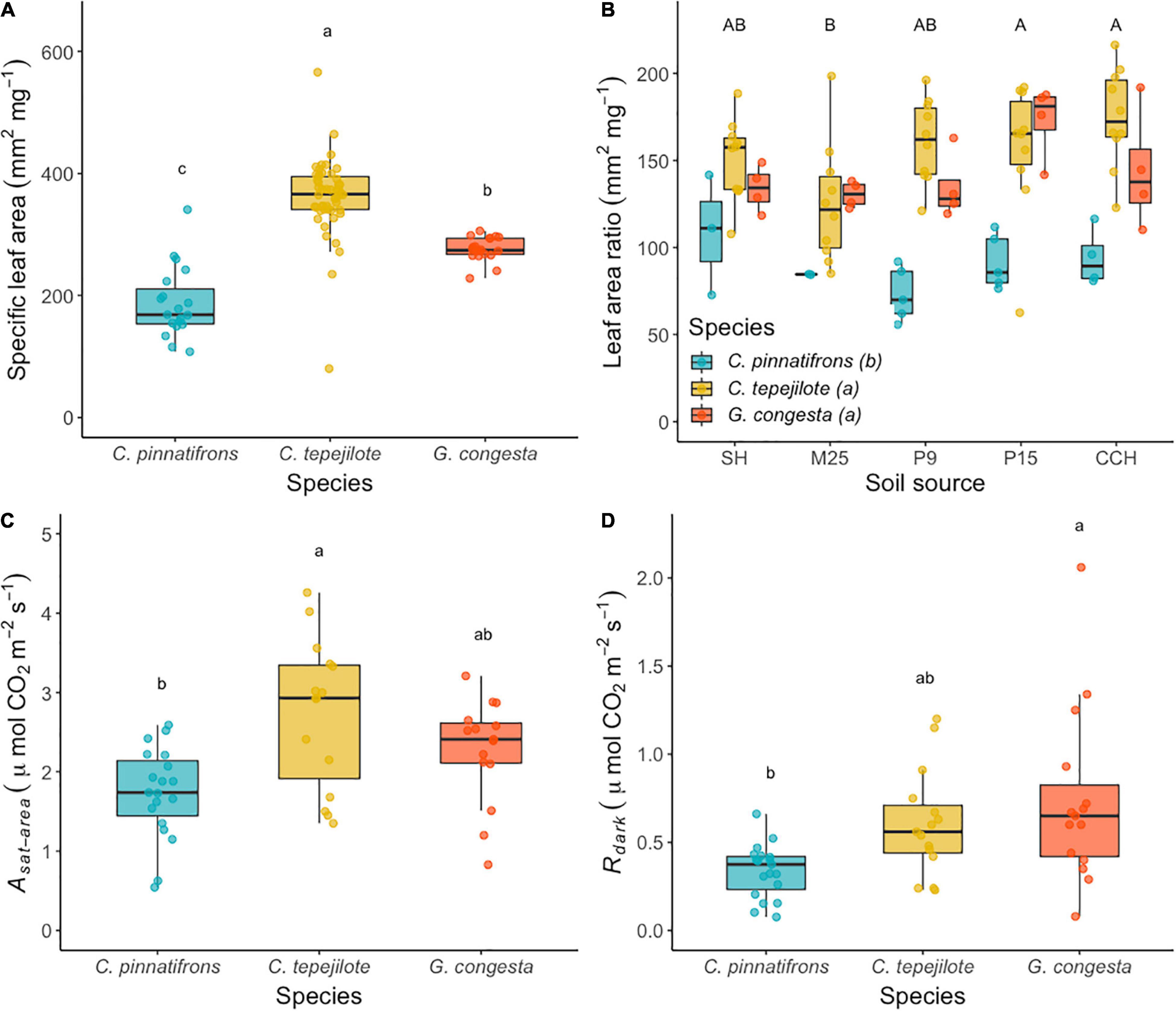
Figure 2. (A) Specific leaf area (SLA), (B) leaf area ratio (LAR), (C) photosynthetic rates (Asat), and (D) dark respiration rates (Rdark), in a greenhouse transplant experiment. Boxplots represent median shown by the solid black horizontal line in the middle of each box and bounds represent 25th and 75th percentiles and individual values. Soil is sourced from five sites across a soil nutrient gradient and sites are ranked and listed in order of increasing overall soil nutrient availability. Different letters above the bars and boxplots indicate statistically significant different means according to Tukey’s test (P < 0.05), with lower case letters representing species differences and capital letters representing soil differences.
The mean LAR across all the seedlings was 138 ± 4.1 mm2 g–1. The best fit model for LAR retained both species (F2,82 = 38.5, p < 0.0001) and soil (F4,82 = 3.99, p < 0.01), but not their interaction. C. pinnatifrons had a significantly lower mean LAR (86.6 ± 6.35 mm2 g–1) than either C. tepelijote (154.0 ± 3.88 mm2 g–1) or G. congesta (143.0 ± 6.13 mm2 g–1; Figure 2B). In addition, LAR was higher across all species growing in soil from P15 (143 ± 10 mm2 g–1) and CCH (150 ± 9.97 mm2 g–1) than M25 (122 ± 7.62 mm2 g–1), with intermediate values for SH and P9.
The mean light-saturated photosynthesis (Asat) across all seedlings was 2.29 ± 0.115 μmol⋅m–2⋅s–1. Photosynthetic rates significantly differed among species (F2,46 = 8.03, p < 0.001) but were unaffected by soil or its interaction with species. Photosynthetic rate was significantly higher for C. tepejilote (2.90 ± 0.131 μmol⋅m–2⋅s–1) than C. pinnatifrons (1.73 ± 0.131 μmol⋅m–2⋅s–1), with intermediate rates for G. congesta (2.21 ± 0.157 μmol⋅m–2⋅s–1; Figure 2C).
The mean Rdark for all seedlings was 0.54 ± 0.04 μmol⋅m–2⋅s–1. The best fit model for Rdark showed a significant difference between species means (F2,42 = 7.19, p < 0.01) with a non-significant soil effect retained in the model (F4,42 = 2.29, p = 0.08). Leaf respiration was significantly greater for G. congesta (0.74 ± 0.11 μmol⋅m–2⋅s–1) than for C. pinnatifrons (0.34 ± 0.035 μmol⋅m–2⋅s–1), with intermediate Rdark rates for C. tepejilote (0.61 ± 0.042 μmol⋅m–2⋅s–1; Figure 2D).
In the shadehouse experiment, the mean LMR across all seedlings was 0.463 ± 0.009. LMR was significantly influenced by the interaction between species and soil (F8,74 = 2.38, p < 0.05) and by the main effects of species (F2,74 = 15.17, p < 0.0001) and soil (F4,44 = 12.03, p < 0.0001; Figure 3A). Among species and soil combinations, LMR was highest for G. congesta in soil from P15 (0.58 ± 0.03) and lowest for C. tepejilote in soil from M25 (0.33 ± 0.02). We found that LMR for C. tepejilote was lower than C. pinnatifrons when growing in the M25 site soil (t = 5.89, p < 0.001). Furthermore, C. tepejilote LMR differed among soils, with lower LMR when growing in low nutrient site soils compared to high nutrient soils and had an intermediate LMR in the P9 soil, although LMR in P9 soil was significantly higher than in M25 soil (t = 5.26, p < 0.001).
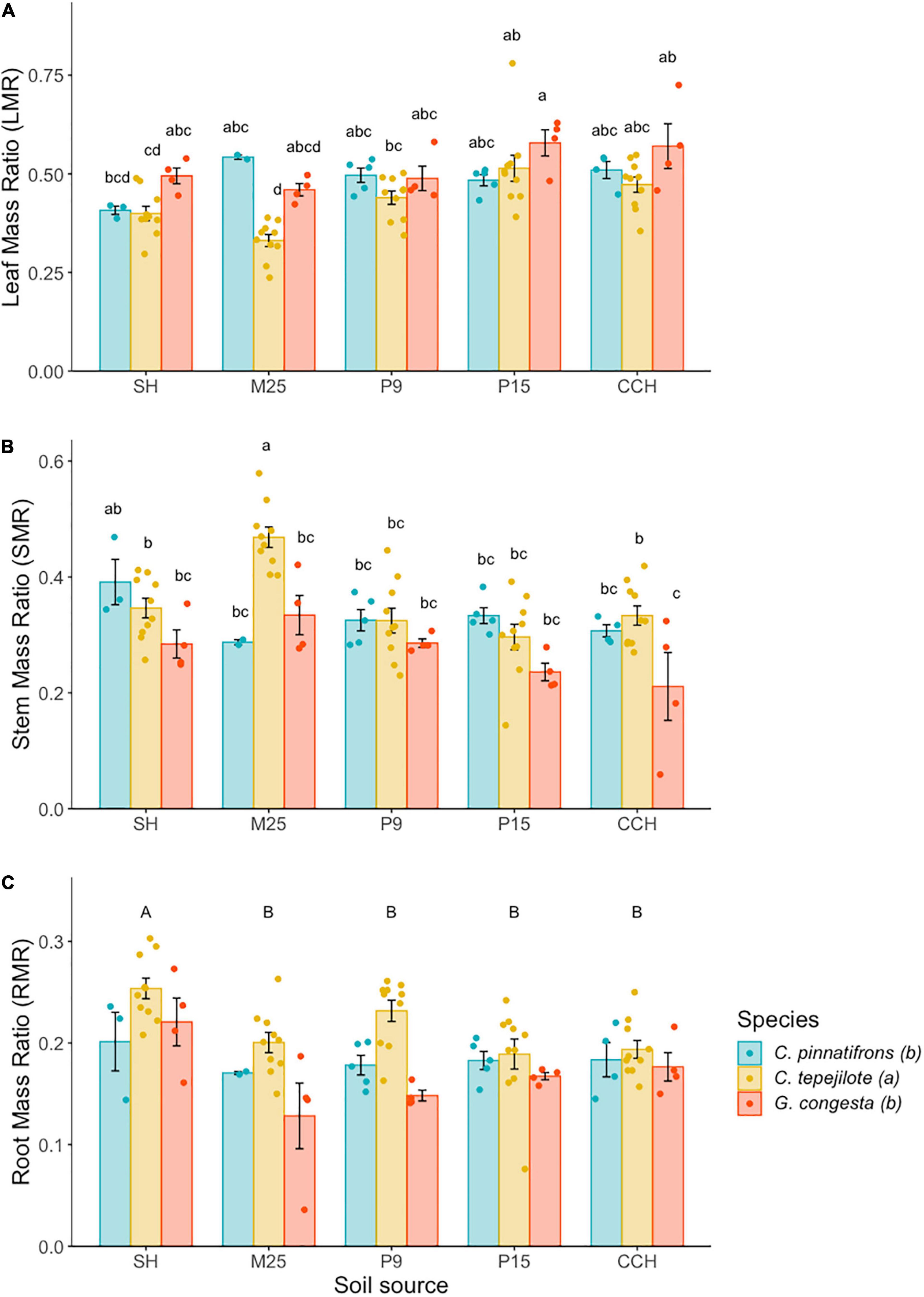
Figure 3. Biomass allocation (mean ± SE) of seedlings growing in soil from five sites along a soil nutrient gradient. (A) Leaf mass ratio (LMR), (B) Stem mass ratio (SMR), and (C) Root mass ratio (RMR). Soil is sourced from five sites across a soil nutrient gradient and sites are ranked and listed in order of increasing overall soil nutrition. Different letters above the bars indicate statistically significant different means according to Tukey’s test (P < 0.05), with lower case letters representing differences among species *soil source combinations and capital letters representing differences among soil sources. The legend represents species fill color for all panels and the letters represent species differences for RMR only. *Represents the interaction between species and soil.
The mean SMR across all seedlings was 0.330 ± 0.009. SMR was significantly influenced by the interaction between species and soil (F8,74 = 2.78, p < 0.01), and by the main effects of species (F2,74 = 14.82, p < 0.0001), and soil (F4,74 = 11.60, p < 0.0001; Figure 3B). For all soil and species combinations, C. tepejilote in soil from M25 had the highest SMR (0.47 ± 0.02) while G. congesta in soil from CCH had the lowest SMR (0.21 ± 0.03). C. tepejilote had higher SMR compared to the two other species when growing in the M25 site soil. Furthermore, for C. tepejilote, SMR was higher when growing in the M25 site soil compared to all other soil sources.
The mean RMR across all seedlings was 0.197 ± 0.005. There was no significant interactive effect between species and soil, but root mass was significantly influenced by the main effects of species (F2,74 = 13.58, p < 0.001) and soil (F4,74 = 7.45, p < 0.0001). RMR of C. tepejilote (0.21 ± 0.01) was higher than that of the other two species (Figure 3C). Across all species, RMR of seedlings growing in soils from SH (0.23 ± 0.01) was higher than for the other four soils (Figure 3C).
In the shadehouse experiment, the mean RGR across all species and soils was 11.5 ± 0.25 mg⋅g–1⋅day–1. There was a significant interaction between soil and species (F8,74 = 3.01, p < 0.01), and significant differences among the species (F2,74 = 9.76, p < 0.0001) and soil (F4,74 = 7.16, p < 0.0001) on RGR. For all soil and species combinations, C. tepejilote in soil from P15 had the highest RGR (14.5 ± 0.58 mg⋅g–1 day–1) while G. congesta in soil from CCH had the lowest RGR (9.41 ± 0.92 mg⋅g–1 day–1; Figure 4). When growing in the P15 soil, C. tepelijote had significantly higher RGR than G. congesta (t = 3.77, p = 0.05). Furthermore, C. tepejilote had significantly higher RGR growing in P15 soil, where it naturally occurs, than in lower nutrient soils, whereas RGR in the highest nutrient soil (CCH) was similar to all soils (Figure 4).
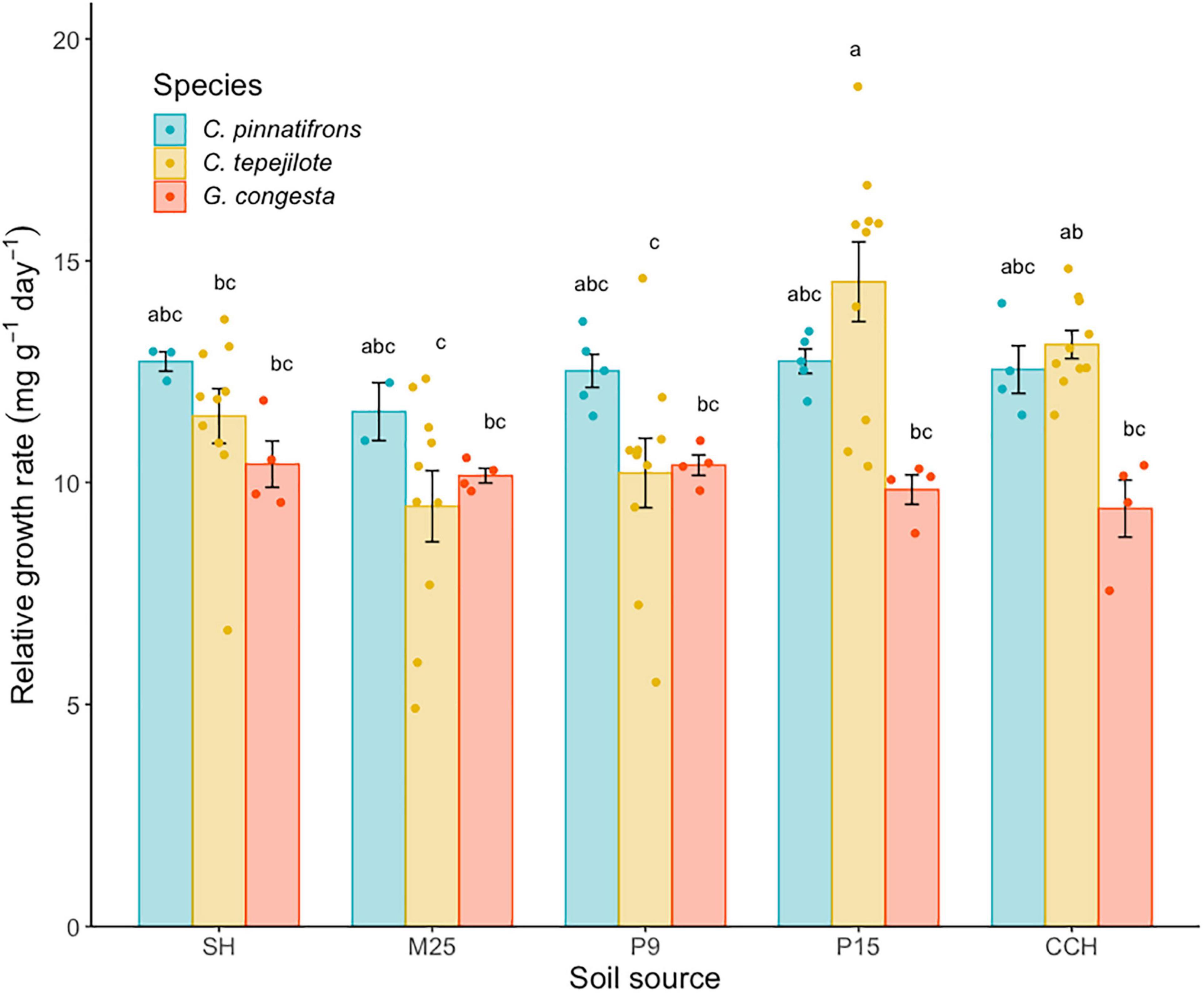
Figure 4. Relative growth rate (RGR; mean ± SE) of seedlings growing in soil from five sites along a soil nutrient gradient. Soil sources are ranked and listed in order of increasing overall soil fertility. Bars are shaded by species. Different letters above the bars indicate statistically significant differences among means according to Tukey’s test (P < 0.05).
For the PCA of the seedling trait data for the shadehouse experiment, the primary ordination axis (PC1) explained 30.8% of the total variation and represented increasing values of LAR and SLA, and more acquisitive leaf traits (Figure 1B). Species aligned along PC1. The second axis (PC2) explained 27.1% of the total variation and represented a shift from high leaf investment (LMR) to high stem investment (SMR). Soils aligned along PC2. As soil fertility increased (from soils from sites SH and M25 to soils from sites P9, P15, and CCH), there was an increase in seedling LMR and a decrease in SMR.
Seedling survival by the end of the field experiment was significantly influenced by the interaction between site and species (χ2 = 214, df = 12, p < 0.0001), and by the main effects of site (χ2 = 540, df = 5, p < 0.0001) and species (χ2 = 986, df = 4, p < 0.0001). After 505 days, overall survival across all species except C. tepejilote was highest at SH (low-nutrient, high-rainfall site; mean ± SE = 57.5 ± 5.23%), and lowest at CCH (high-nutrient, low-rainfall site; 0.0% ± 0.0) where all seedlings of all species died after 120 days during the first dry season (Figure 5). Among species, survival rates across all sites were highest for C. tepejilote (40.2 ± 5.69%) and lowest for C. pinnatifrons (26.3 ± 3.71%). For the species that naturally occurs at the P15 site (C. tepejilote), survival rates were 80.0 ± 2.83% at that site and did not differ from survival at the P9 or SH sites, but survival at these three sites were higher than that for M25 (45 ± 2.12%; z-ratio = 9.89, p < 0.0001) and for CCH (with zero seedlings surviving). For the species that naturally occurs at the SH site (G. congesta), survival was higher at its “home-site” (65 ± 2.5%) than at the other four sites, i.e., P15 (z-ratio = 2.89, p < 0.05), P9 (z-ratio = 11.36, p < 0.0001), M25 (z-ratio = 17.68, p < 0.0001), and CCH (z-ratio = 25.5, p < 0.0001; Figure 5C).
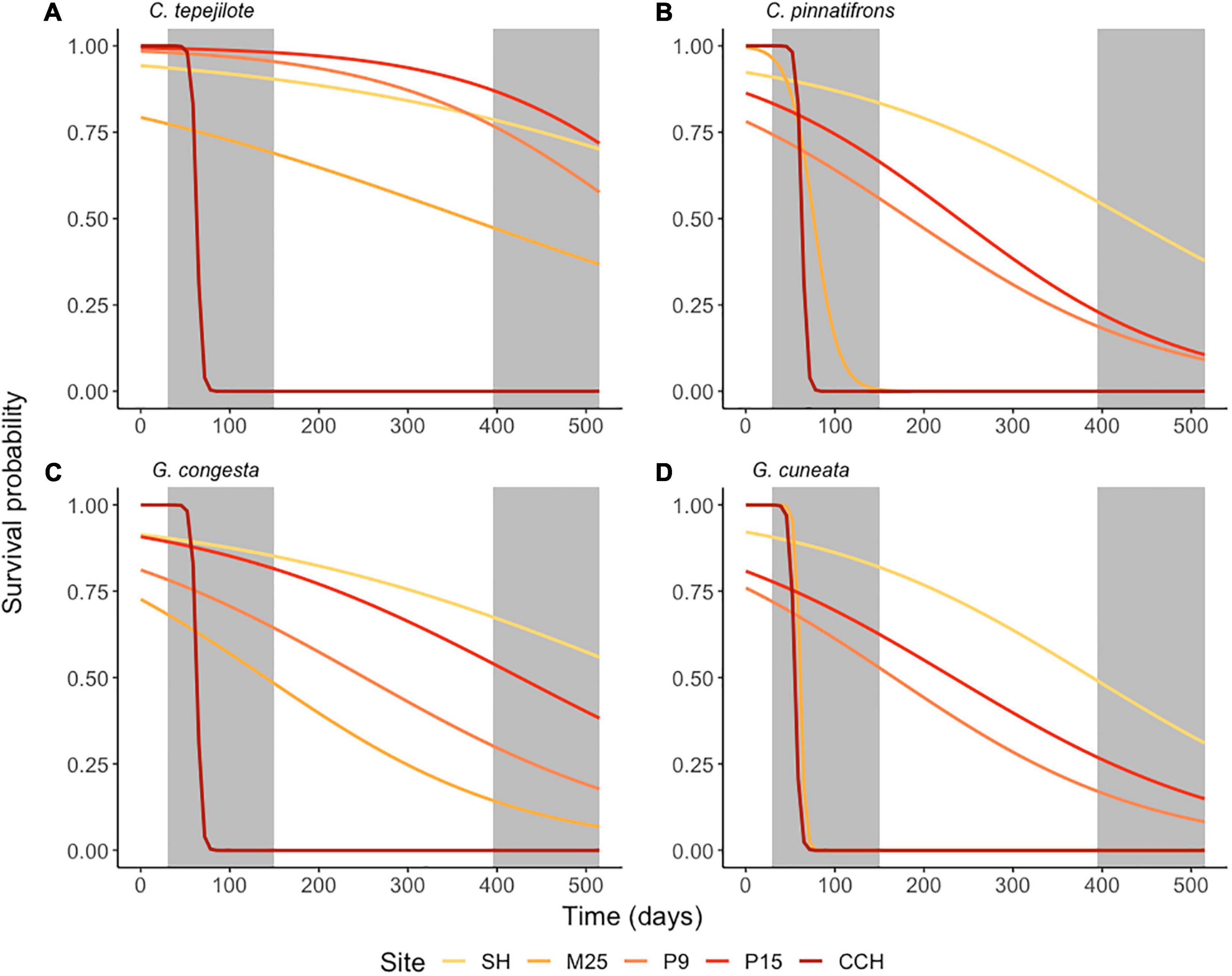
Figure 5. Field transplant experiment of seedling survival of four understory palm species, (A) Chamaedorea tepejilote, (B) C. pinnatifrons, (C) Geonoma congesta, and (D) G. cuneata. Seedlings of each species were grown at each of five sites that differed in soil fertility. Percent survival is based on the species mean per garden per site. Curves for each species are the outputs from generalized linear models (Survival rates ∼ Site). Shading of the curve increases intensity with soil nutrient availability from SH to CCH. Gray shaded boxes represent dry seasons in year 1 and 2 of the field experiment.
We used seedling experiments to better understand how four understory palm species respond to gradients of soil nutrients and rainfall in lowland tropical forests of Panama. In the shadehouse experiment, leaf functional traits were determined by species, whereas RMR was highest at the lowest nutrient site and with generally biomass allocation and RGR shifting with species-specific responses to soil source. By contrast, in the field transplant experiment seedling mortality was largely determined by dry season water availability rather than soil nutrient availability among the sites. Together, these results suggest that seedling performance is responsive to soil fertility when there is sufficient soil moisture. Thus, by pairing controlled and field experiments, we determined that seedling survival, and hence potentially species distribution, are strongly constrained by dry season water availability, whereas soil nutrient availability shapes subsequent seedling biomass allocation and RGRs.
In contrast to the predictions of our first hypothesis, we did not find that leaf morphological traits such as SLA and LAR, or physiological traits, such as Asat and Rdark, responded to soil fertility, and these traits were instead mainly determined by species identity. The only trait that differed among soils was LAR which was lower for seedlings growing in one of the low nutrient soils compared to high nutrient soils. In addition, we did not find any differences between generalist and specialist species leaf trait responses to soil fertility. Among the three species, the species associated with the intermediate fertility P15 site (C. tepejilote) had the most acquisitive leaf trait strategy whereas the generalist species found throughout the lower montane Fortuna sites in western Panama (C. pinnatifrons) had the most conservative leaf trait strategy. Our findings contrast experiments where leaf traits of seedlings grown in contrasting soil types follow the leaf economic strategy with higher SLA and LAR when growing in high resource environments (Baltzer et al., 2005; Dent and Burslem, 2009). Our findings also contrast with a parallel shadehouse experiment of five Chamaedorea species across the Fortuna soil gradient, where seedlings of C. pinnatifrons had higher photosynthetic rates than C. tepejilote and all species showed strong soil-driven shifts in photosynthetic rates (Andersen, 2021). One possible explanation for the discrepancy between these experiments is that soil nitrogen, a key nutrient for photosynthesis, is generally high across the lowland soil gradient (Figure 1A, PC2), whereas soil nitrogen partly drives the lower montane soil gradient (Andersen et al., 2010b,2014). This suggests that soil phosphorus and cations, the main drivers of the lowland soil nutrient gradient, do not strongly influence palm seedling leaf traits. However, evidence from tree species along the lowland Panama Isthmus shows changes in community-level leaf trait measures such as SLA and thickness are driven by variation in both soil phosphorus and moisture (Umaña et al., 2021). Thus, it is possible that understory palm leaf traits are more responsive to variation in soil nitrogen than phosphorus availability, while tree species are more responsive to variation to soil phosphorus among sites.
Our second hypothesis predicted that RMR for each species would increase as soil fertility declines to meet nutrient demands at the expense of LMR, SMR and overall growth potential. While palms use roots to access water and nutrients, in this shadehouse experiment, nutrient availability varied by soil source while water availability remained constant for all soil and species combinations. This suggests that root mass seems to increase in response to nutrient availability. In line with this we found that RMR was greatest at the least fertile SH site, suggesting that seedlings increased investment in roots to acquire limiting nutrients when grown in the lowest nutrient soil at the expense of allocation to leaf biomass. Both LMR and SMR had interactions between species and soil source. However, LMR generally increased with increasing soil nutrient availability, whereas SMR tended to decrease. This is in line with optimal allocation theory which predicts that allocation to belowground and support tissue increases with decreasing soil nutrient availability (Chapin, 1980; Poorter et al., 2012). In shadehouse experiments in French Guiana and Malaysia, seedlings of tree species showed similar trade-offs between above and belowground allocation with soil nutrient availability (Baraloto et al., 2005; Dent and Burslem, 2009). In palm seedling field experiments in Fortuna, RMR did not respond to nitrogen addition (Andersen et al., 2010a) but did increase as soil nutrient availability decreased (Andersen et al., 2014), suggesting that RMR and biomass allocation trade-offs for palm seedlings are driven by rock-derived nutrients such as soil P and cations. Furthermore, in the Fortuna transplant experiment, seedlings of species associated with the lowest nutrient site showed biomass allocation patterns that would promote growth compared to species not occurring at that site. However, here we found that despite the influence of soil source on biomass allocation, there was no indication that species were able to optimize their biomass allocation to promote growth in the soils they were associated with compared to species not occurring in that soil type.
Our third hypothesis predicted that specialist species growing in the soil they are associated with should have a higher RGR compared to species not occurring in those soils. We found that C. tepejilote had higher RGR when growing in the soil for which it is associated with compared to the low nutrient specialist species (G. congesta) but not the Fortuna generalist species C. pinnatifrons. Furthermore, C. tepejilote had higher RGR at higher compared to lower nutrient soils, suggesting that C. tepejilote may have adaptations that enhances growth and performance in higher fertility soils. Similarly, in a seedling transplant experiment in Fortuna, C. tepejilote was one of the species associated with the intermediate soil nutrient site Palo Seco and also had a growth advantage at home (Andersen et al., 2014), further supporting the hypothesis that this species is able to maximize its growth in soils where it occurs naturally. However, the mechanism for the enhanced growth performance of C. tepejilote in intermediate nutrient soils in lowland shadehouse experiment remains unclear as we did not observe greater resource use efficiency or optimal biomass allocation of this species compared to others. Our findings corroborate an Amazonian transplant study, where growth rates of habitat specialists were often but not always highest when growing in the soil they are associated with (Fortunel et al., 2016).
In contrast to C. tepejilote, the low-nutrient specialist, G. congesta, did not differ in RGR compared to other species when growing in the soil it is associated with. This contrasts with the general trends from the Fortuna transplant experiment, where species associated with low nutrient soils had strong growth advantages when grown at home (Andersen et al., 2014). Furthermore, G. congesta did not show significant differences in RGRs across the soil nutrient gradient, suggesting that G. congesta has a highly constrained and low RGR that may help it survive in low nutrient soils but may be outcompeted at higher nutrient sites as it had significantly lower RGR than C. tepejilote in the P15 soil. Alternatively, RGR of G. congesta may also be driven by other factors such as light and soil moisture, which may vary among sites in the field, but which were kept constant in the shadehouse experiment.
Our fourth hypothesis predicted that specialist species will have higher seedling survival rates at sites where they occur naturally compared to other species and compared to their survival at sites where they do not occur. In line with this, we found that the low nutrient specialist, G. congesta, had higher seedling survival rates growing at its home site compared to the other sites. However, C. tepejilote tended to have higher survival compared to other species regardless of lowland site. Nonetheless, for the site specialist C. tepejilote, survival rates were highest at its home site P15, and at sites P9 and SH, all of which have a lower dry season moisture deficit compared to the other sites. We also found that seedling survival for all species was high at the low nutrient site SH which has the highest annual rainfall (2,848 mm) and the highest soil water content during the wet season (89.7 ± 2.3 mm) and dry season (70.8 ± 3.0). Across all species, seedling survival rates were lowest at lowland site CCH which has the highest nutrient soil and dry season water deficit (571 mm). In the Fortuna seedling transplant experiment in lower montane forests, species showed strong home-site advantages in survival at low and high nutrient sites, but not intermediate nutrient sites (Andersen et al., 2014). However, the mechanisms driving home site survival advantages in the Fortuna lower montane experiment and this lowland experiment are expected to differ due to differences in MAP and seasonality. The lower montane soil gradient has a rainfall gradient of 4–10 m MAP with no substantial dry season compared to the 1.5–3 m MAP gradient and strong 4-month dry season at sites in this lowland experiment (Andersen et al., 2014). Furthermore, the lower montane experiment found that survival was mediated by herbivores, whereas protection from herbivores had no effect on survival in the lowland experiment. Similarly, our findings corroborate a transplant study where home-site survival advantages across lowland tropical forests in Amazonia were related to moisture and soil gradients, but not herbivory for seedlings of habitat specialist tree species (Fortunel et al., 2016).
Although the focus of this study was on belowground environmental variation, light availability can also influence seedling survival (Cintra and Horna, 1997; Record et al., 2016). Field transplant locations were chosen to represent closed canopy forest locations across all field sites. We do not have field light data, but a regional study suggests that light transmittance at the understory layer in these forests is generally around 1–2% annually throughout the Panamanian Isthmus (Brenes-Arguedas et al., 2011), and this is in line with previous measurements at two of our study sites, i.e., P9 (Gaviria et al., 2017) and SH (Brenes-Arguedas et al., 2011). Together, our results suggest that the main driver of seedling survival is dry season water availability, thus drought tolerance or avoidance may be important mechanisms, especially with more frequent droughts associated with extreme El Niño events, that determine species distributions along the Panama Canal Isthmus (Engelbrecht et al., 2007; Browne et al., 2021).
In this study, we found that although soil nutrient availability was an important driver of seedling biomass allocation and RGR in a controlled shadehouse setting, dry season water availability determines seedling survival in the field. This work demonstrates the necessity of transplant experiments to disentangle resource-based and climatic factors in understanding species pre-adaptions and response to environmental change. If lowland tropical forests in Panama are driven by soil nutrient and rainfall gradients, then changes in soil fertility due to nitrogen deposition (Hietz et al., 2011) and soil moisture availability caused by climate change have the potential to alter species distributions and forest dynamics (Engelbrecht et al., 2007). Conservation and restoration efforts of forests across the isthmus of Panama will need to consider the responses of individual species to such changes. Here, we observed the effects of limiting resources on four palm species. These understory species act as filters that affect seedling growth and survival in forest (Farris-Lopez et al., 2004; Wang and Augspurger, 2004). Therefore, factors that affect the growth and distribution of these palm species will indirectly impact tree seedling recruitment and forest regeneration. Further research on additional species is needed to obtain a more comprehensive view of forest dynamics in relation to spatial variation in the landscape of soil nutrient availability and rainfall patterns.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CC helped in statistical analysis and drafted the manuscript. DW helped to draft the manuscript. KA designed and carried out the study, helped in statistical analysis, and drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Smithsonian Tropical Research Institute for logistical support. This research was supported by funding from an NSF Dissertation Completion Grant, a Smithsonian Institution Predoctoral Fellowship, and funding from the Program in Ecology and Evolutionary Biology of the University of Illinois-Champaign/Urbana (KMA). Permits were provided by the Smithsonian Tropical Research Institute and the Panamanian Autoridad Nacional del Ambiente (ANAM).
Aerts, R., and Chapin, F. S. (2000). The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv. Ecol. Res. 30, 1–67. doi: 10.1016/S0065-2504(08)60016-1
Ali, A., and Yan, E. R. (2017). Functional identity of overstorey tree height and understorey conservative traits drive aboveground biomass in a subtropical forest. Ecolog. Ind. 83, 158–168. doi: 10.1016/j.ecolind.2017.07.054
Alvarez-Clare, S., Mack, M. C., and Brooks, M. (2013). A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 94, 1540–1551. doi: 10.1890/12-2128.1
Andersen, K. M. (2021). “Palm flora and drivers of distribution in Fortuna Forest Reserve,” in Fortuna Forest Reserve, Panama: Interacting Effects of Climate and Soils on the Biota of a Wet Premontane Tropical Forest, eds J. W. Dalling and B. L. Turner (Washington, D.C: The Smithsonian Institution, USA), 271–287. doi: 10.5479/si.14315990.v1
Andersen, K. M., and Turner, B. L. (2013). Preferences or plasticity in nitrogen acquisition by understorey palms in a tropical montane forest. J. Ecol. 101, 819–825.
Andersen, K. M., Turner, B. L., and Dalling, J. W. (2010a). Soil-based habitat partitioning in understory palms in lower montane tropical forests. J. Biogeogr. 37, 278–292. doi: 10.1111/j.1365-2699.2009.02192.x
Andersen, K. M., Corre, M. D., Turner, B. L., and Dalling, J. W. (2010b). Plant–soil associations in a lower montane tropical forest: physiological acclimation and herbivore-mediated responses to nitrogen addition. Funct. Ecol. 24, 1171–1180. doi: 10.1111/j.1365-2435.2010.01731.x
Andersen, K. M., Endara, M.-J., Turner, B. L., and Dalling, J. W. (2012). Trait-based community assembly of understory palms along a soil nutrient gradient in a lower montane tropical forest. Oecologia 168:519531. doi: 10.1007/s00442-011-2112-z
Andersen, K. M., Turner, B. L., and Dalling, J. W. (2014). Seedling performance trade-offs influencing habitat filtering along a soil nutrient gradient in a tropical forest. Ecology 95, 3399–3413. doi: 10.1890/13-1688.1
Baker, W. J., Norup, M. V., Clarkson, J. J., Couvreur, T. L., Dowe, J. L., Lewis, C. E., et al. (2011). Phylogenetic relationships among arecoid palms (Arecaceae: Arecoideae). Ann. Bot. 108, 1417–1432. doi: 10.1093/aob/mcr020
Baltzer, J. L., Thomas, S. C., Nilus, R., and Burslem, D. F. R. (2005). Edaphic specialization in tropical trees: physiological correlates and responses to reciprocal transplantation. Ecology 86, 3063–3077. doi: 10.1890/04-0598
Baraloto, C., Goldberg, D. E., and Bonal, D. (2005). Performance trade-offs among tropical tree seedlings in contrasting microhabitats. Ecology 86, 2461–2472. doi: 10.1890/04-1956
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Brenes-Arguedas, T., Roddy, A. B., Coley, P. D., and Kursar, T. A. (2011). Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia 166, 443–456. doi: 10.1007/s00442-010-1832-9
Browne, L., Markesteijn, L., Engelbrecht, B. M., Jones, F. A., Lewis, O. T., Manzané-Pinzon, E., et al. (2021). Increased mortality of tropical tree seedlings during the extreme 2015–16 El Niño. Glob. Chang. Biol. 27, 5043–5053. doi: 10.1111/gcb.15809
Cámara-Leret, R., Tuomisto, H., Ruokolainen, K., Balslev, H., and Munch Kristiansen, S. (2017). Modelling responses of western Amazonian palms to soil nutrients. J. Ecol. 105, 367–381. doi: 10.1111/1365-2745.12708
Cavelier, J. (1992). Fine-root biomass and soil properties in a semideciduous and a lower montane rain forest in Panama. Plant Soil 142, 187–201. doi: 10.1007/BF00010965
Chapin, F. S. (1980). The mineral-nutrition of wild plants. Annu. Rev. Ecol. Syst. 11, 233–260. doi: 10.1146/annurev.es.11.110180.001313
Chazdon, R. L. (1986). Light variation and carbon gain in rain forest understorey palms. J. Ecol. 1986, 995–1012. doi: 10.2307/2260229
Cintra, R., and Horna, V. (1997). Seed and seedling survival of the palm Astrocaryum murumuru and the legume tree Dipteryx micrantha in gaps in Amazonian forest. J. Trop. Ecol. 13, 257–277. doi: 10.1017/S0266467400010440
Clark, D. A., Clark, D. B., Sandoval, R. M., and Castro, M. V. C. (1995). Edaphic and human effects on landscape-scale distributions of tropical rain forest palms. Ecology 76, 2581–2594. doi: 10.2307/2265829
Condit, R., Engelbrecht, B. M., Pino, D., Pérez, R., and Turner, B. L. (2013). Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl. Acad. Sci. 110, 5064–5068. doi: 10.1073/pnas.1218042110
Cusack, D. F., Markesteijn, L., Condit, R., Lewis, O. T., and Turner, B. L. (2018). Soil carbon stocks across tropical forests of Panama regulated by base cation effects on fine roots. Biogeochemistry 137, 253–266. doi: 10.1007/s10533-017-0416-8
Dent, D. H., and Burslem, D. F. (2009). Performance trade-offs driven by morphological plasticity contribute to habitat specialization of Bornean tree species. Biotropica 41, 424–434. doi: 10.1111/j.1744-7429.2009.00505.x
Diaz, S., Hodgson, J. G., Thompson, K., Cabido, M., Cornelissen, J. H., Jalili, A., et al. (2004). The plant traits that drive ecosystems: evidence from three continents. J. Veg. Sci. 15, 295–304. doi: 10.1111/j.1654-1103.2004.tb02266.x
Díaz, S., Kattge, J., Cornelissen, J. H., Wright, I. J., Lavorel, S., Dray, S., et al. (2016). The global spectrum of plant form and function. Nature 529, 167–171. doi: 10.1038/nature16489
Dominy, N. J., Grubb, P. J., Jackson, R. V., Lucas, P. W., Metcalfe, D. J., Svenning, J. C., et al. (2008). In tropical lowland rain forests monocots have tougher leaves than dicots, and include a new kind of tough leaf. Ann. Bot. 101, 1363–1377. doi: 10.1093/aob/mcn046
Emilio, T., Quesada, C. A., Costa, F. R., Magnusson, W. E., Schietti, J., Feldpausch, T. R., et al. (2013). Soil physical conditions limit palm and tree basal area in Amazonian forests. Plant Ecol. Div. 7, 215–229. doi: 10.1080/17550874.2013.772257
Engelbrecht, B. M., Comita, L. S., Condit, R., Kursar, T. A., Tyree, M. T., Turner, B. L., et al. (2007). Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82. doi: 10.1038/nature05747
Farris-Lopez, K., Denslow, J. S., Moser, B., and Passmore, H. (2004). Influence of a common palm, Oenocarpus mapora, on seedling establishment in a tropical moist forest in Panama. J. Trop. Ecol. 2004, 429–438. doi: 10.1017/S0266467404001531
Fortunel, C., Paine, C. E. T., Fine, P. V. A., Mesones, I., Goret, J.-Y., Burban, B., et al. (2016). There’s no place like home: seedling mortality contributes to the habitat specialisation of tree species across Amazonia. Ecol. Lett. 19:12561266. doi: 10.1111/ele.12661
Gaviria, J., Turner, B. L., and Engelbrecht, B. M. (2017). Drivers of tree species distribution across a tropical rainfall gradient. Ecosphere 8:e01712.
Grime, J. P. (2006). Plant strategies, vegetation processes, and ecosystem properties. Hoboken, NJ: John Wiley and Sons.
Grubb, P. J. (1977). The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol. Rev. 52, 107–145. doi: 10.1111/j.1469-185X.1977.tb01347.x
Henderson, A. (2011). A revision of Geonoma (Arecaceae). Phytotaxa 17, 1–271. doi: 10.11646/phytotaxa.17.1.1
Henderson, A., Galeano, G., and Bernal, R. (2019). Field guide to the palms of the Americas. Princeton, NJ: Princeton University Press. doi: 10.1515/9780691197708
Hietz, P., Turner, B. L., Wanek, W., Richter, A., Nock, C. A., and Wright, S. J. (2011). Long-term change in the nitrogen cycle of tropical forests. Science 334, 664–666. doi: 10.1126/science.1211979
Kumordzi, B. B., de Bello, F., Freschet, G. T., Le Bagousse-Pinguet, Y., Leps, J., and Wardle, D. A. (2015). Linkage of plant trait space to successional age and species richness in boreal forest understorey vegetation. J. Ecol. 103, 1610–1620. doi: 10.1111/1365-2745.12458
Kursar, T. A., Engelbrecht, B. M. J., and Tyree, M. T. (2005). A comparison of methods for determining soil water availability in two sites in Panama with similar rainfall but distinct tree communities. J. Trop. Ecol. 21, 297–305. doi: 10.1017/s0266467405002324
Laughlin, D. C., and Laughlin, D. E. (2013). Advances in modeling trait-based plant community assembly. Trends Plant Sci. 18, 584–593. doi: 10.1016/j.tplants.2013.04.012
Lê, S., Josse, J., and Husson, F. (2008). FactoMineR: an R Package for Multivariate Analysis. J. Stat. Softw. 25, 1–18. doi: 10.18637/jss.v025.i01
Lenth, R. V. (2021). emmeans: Estimated MarginalMeans, aka Least-Squares Means. Rpackage version 1.5.5-1.
Maharjan, S. K., Poorter, L., Holmgren, M., Bongers, F., Wieringa, J. J., and Hawthorne, W. D. (2011). Plant functional traits and the distribution of West African rain forest trees along the rainfall gradient. Biotropica 43, 552–561. doi: 10.1111/j.1744-7429.2010.00747.x
McLean, E. H., Prober, S. M., Stock, W. D., Steane, D. A., Potts, B. M., Vaillancourt, R. E., et al. (2014). Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa. Plant Cell Env. 37, 1440–1451. doi: 10.1111/pce.12251
Mehlich, A. (1984). Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Comm. Soil Sci. Plant Anal. 15, 1409–1416. doi: 10.1080/00103628409367568
Muscarella, R., Bacon, C. D., Faurby, S., Antonelli, A., Kristiansen, S. M., Svenning, J. C., et al. (2019). Soil fertility and flood regime are correlated with phylogenetic structure of Amazonian palm communities. Ann. Bot. 123, 641–655. doi: 10.1093/aob/mcy196
Palmiotto, P. A., Davies, S. J., Vogt, K. A., Ashton, M. S., Vogt, D. J., and Ashton, P. S. (2004). Soil-related habitat specialization in dipterocarp rain forest tree species in Borneo. J. Ecol. 92, 609–623. doi: 10.1111/j.0022-0477.2004.00894.x
Paton, S. (2020a). Yearly Reports_Parque Natural Metropolitano Crane. The Smithsonian Institution. Dataset 2020:11799348. doi: 10.25573/data.11799348.v3
Paton, S. (2020b). Yearly Reports_San Lorenzo Crane. The Smithsonian Institution. Dataset 2020:11799309. doi: 10.25573/data.11799309.v2
Peltzer, D. A., Wardle, D. A., Allison, V. J., Baisden, W. T., Bardgett, R. D., Chadwick, O. A., et al. (2010). Understanding ecosystem retrogression. Ecolog. Monogr. 80, 509–529. doi: 10.1890/09-1552.1
Pollock, L. J., Morris, W. K., and Vesk, P. A. (2012). The role of functional traits in species distributions revealed through a hierarchical model. Ecography 35, 716–725. doi: 10.1111/j.1600-0587.2011.07085.x
Poorter, H., Niklas, K. J., Reich, P. B., Oleksyn, J., Poot, P., and Mommer, L. (2012). Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50. doi: 10.1111/j.1469-8137.2011.03952.x
Prada, C. M., Morris, A., Andersen, K. M., Turner, B. L., Caballero, P., and Dalling, J. W. (2017). Soils and rainfall drive landscape-scale changes in the diversity and functional composition of tree communities in premontane tropical forest. J. Veget. Sci. 28, 859–870. doi: 10.1111/jvs.12540
Pyke, C. R., Condit, R., Aguilar, S., and Lao, S. (2001). Floristic composition across a climatic gradient in a neotropical lowland forest. J. Veg. Sci. 12, 553–566. doi: 10.2307/3237007
Record, S., Kobe, R. K., Vriesendorp, C. F., and Finley, A. O. (2016). Seedling survival responses to conspecific density, soil nutrients, and irradiance vary with age in a tropical forest. Ecology 97, 2406–2415. doi: 10.1002/ecy.1458
Richardson, S. J., Peltzer, D. A., Allen, R. B., McGlone, M. S., and Parfitt, R. L. (2004). Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139, 267–276. doi: 10.1007/s00442-004-1501-y
Ruokolainen, K., and Vormisto, J. (2000). The most widespread Amazonian palms tend to be tall and habitat generalists. Basic Appl. Ecol. 1, 97–108.
Santiago, L. S. (2007). Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88, 1126–1131. doi: 10.1890/06-1841
Sollins, P. (1998). Factors influencing species composition in tropical lowland rain forest: does soil matter? Ecology 79, 23–30. doi: 10.1890/0012-9658(1998)079[0023:FISCIT]2.0.CO;2
Tilman, D., and Wedin, D. (1991). Plant traits and resource reduction for five grasses growing on a nitrogen gradient. Ecology 72, 685–700. doi: 10.2307/2937208
Turner, B. L., and Engelbrecht, B. M. J. (2011). Soil organic phosphorus in lowland tropical rainforests. Biogeochemistry 103, 297–231. doi: 10.1007/s10533-010-9466-x
Umaña, M. N., Condit, R., Pérez, R., Turner, B. L., Wright, S. J., and Comita, L. S. (2021). Shifts in taxonomic and functional composition of trees along rainfall and phosphorus gradients in central Panama. J. Ecol. 109, 51–61. doi: 10.1111/1365-2745.13442
Vitousek, P. M., and Sanford, R. L. Jr. (1986). Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Syst. 17, 137–167. doi: 10.1146/annurev.es.17.110186.001033
Wang, Y. H., and Augspurger, C. (2004). Dwarf palms and cyclanths strongly reduce Neotropical seedling recruitment. Oikos 107, 619–633. doi: 10.1111/j.0030-1299.2004.13328.x
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Keywords: biomass allocation, functional traits, home-site advantage, lowland tropical forest, seedling performance, understory palms
Citation: Collins C, Wardle DA and Andersen KM (2022) Palm Species Traits Determine Soil Nutrient Effects on Seedling Performance. Front. For. Glob. Change 5:733636. doi: 10.3389/ffgc.2022.733636
Received: 30 June 2021; Accepted: 25 April 2022;
Published: 19 May 2022.
Edited by:
Sanna Sevanto, Los Alamos National Laboratory (DOE), United StatesReviewed by:
Kelsey R. Carter, Los Alamos National Laboratory (DOE), United StatesCopyright © 2022 Collins, Wardle and Andersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colton Collins, Y29sdG9ubGEwMDFAZS5udHUuZWR1LnNn; Kelly M. Andersen, a2VsbHkuYW5kZXJzZW5AbnR1LmVkdS5zZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.