- 1Forest Research Institute, Raszyn, Poland
- 2Laboratory of Dendrometry and Forest Productivity, Department of Forest Management Planning, Dendrometry and Forest Economics, Institute of Forest Sciences, Warsaw University of Life Sciences, Warsaw, Poland
- 3Department of Botany and Forest Habitats, Faculty of Forestry and Wood Technology, Poznań University of Life Sciences, Poznań, Poland
Scots pine (Pinus sylvestris L.) is the most widespread forest tree species in Central Europe. The range of Scots pine depends on the influence of forest management on stand species composition, as the potential for the natural regeneration of Scots pine monocultures is smaller than the current range of the species. To achieve regeneration, pine requires specific ecological conditions, including adequate soil preparation. The literature notes that the effective natural regeneration of pine requires fire or mixing the organic soil layer with the mineral layer. This hypothesis was critically evaluated carrying out work with the main objective of comparing the germination and growth dynamic of pine seedlings in two variants, simulating fire or soil scarification against natural conditions. The research focused on analyzing the growth of pine seedlings from germination to the final number of seedlings, which remained unchanged until the end of the experiment. The evaluation was carried out in soil monoliths from Kampinos National Park (KNP), in which seeds from a homogeneous mother stand were planted. The quantitative and qualitative characteristics of the seedlings were statistically evaluated, including analyses of their root system characteristics. The results confirmed the positive effect of mixing an organic and mineral layer at the germination stage and during the subsequent growth of the pine. The seedlings had a high survival rate (65.3%). However, the positive effect of fire on the regenerative capacity of pine could not be confirmed; the number of obtained seedlings (29.5%) was significantly lower than in the control variant. In addition, the “fire variant” was characterized by high seedling mortality immediately after germination. Root systems were important for the survival of the seedlings, the development of which was affected by the tested variant. The analyses performed may have implications for the development of research on the possible natural regeneration of Pines after natural disturbances. Additional topic that needs further research is the response of seedling root systems to changes in soil conditions.
Introduction
Scots pine (Pinus sylvestris L.) has a wide distribution range in Europe and Asia. Because of its natural plasticity, this species has colonized a wide range of habitats, from bogs to sandy inland and coastal dunes, with optimal climatic growing conditions in Baltic countries such as Estonia, Latvia, and Poland (see Interpretation Manual—EUR28) (European Commission, 2013). In Poland, pine is widely distributed: its share of the country’s forest area is 58.2% (State Forests of Poland, 2022) (lasy.gov.pl) 2020). The forest area occupied by pine exceeds the natural habitat conditions of its occurrence due to historical (Socha et al., 2021), natural (Nowakowska, 2007), and anthropogenic (Reich et al., 1996) factors. A trend currently observed in Poland is the decline of pine stands, especially in areas under various forms of nature protection (Przybylski et al., 2021). The reduced potential for pine stands is due to natural selection processes, including an increase in habitat availability (Socha et al., 2017) and the reduced impact of natural and anthropogenic disturbances that favor the natural regeneration of pine. One of the classic disturbances to plant communities is fire (Seidl et al., 2011). Although its role in shaping temperate forests is currently small due to the widespread use of fire suppression, fire has had a significant influence on the development of boreal habitats in the past (Niklasson et al., 2010). The recovery of fire-damaged vegetation begins almost immediately after a fire (Loster et al., 2011) and depends primarily on habitat conditions (Dobrowolska, 2008). The dynamic increase in the number of seedlings in burned areas compared to control areas is due, in part, to the absence of understory. Plant development is also positively influenced by residual chemical compounds from burned organic tissues. Burning the understory results in the germination of seeds that have been displaced deep into the soil profile, which is referred to as the soil seed bank. The germination process requires the cessation of physiological inhibitions associated with maintaining dormancy (Non-ogaki et al., 2010), beginning with rapid water uptake of the dry seed, and ending with embryo activation; this leads to the embryonic root piercing the seed coat (Job and Caboche, 2008; Non-ogaki et al., 2010). At the end of dormancy, biochemical changes occur in the seed to prepare the embryo for germination. A key factor in regulating the expression of genes responsible for dormancy-lifting is the carbon–nitrogen balance (Zhuo et al., 1999; Zheng, 2009), and the increased supply of these elements after fires accelerates this process (Van Staden et al., 2000). A study by Turner et al. (2007) and Boerner et al. (2009) documented higher concentrations of inorganic nitrogen in burned areas compared to controls. Nitrification improves growing conditions, especially in acidic soils, by increasing their alkalinity, stimulating microbial activity, and promoting plant germination (Pastor-López and Martin-Martin, 1995; Raison et al., 2009). These processes rapidly return nitrogen to the natural cycle (Gimeno-García et al., 2000). An increase in organic carbon is also observed after low-intensity fires, while humic acids may remain unchanged (Pardini et al., 2004; Badía-Villas et al., 2014). However, these changes do not last long (González-Vila et al., 2009), and excessive nitrogen can promote the growth of pathogenic fungi that can be toxic to developing embryos (Hilszczańska et al., 2008; Slama et al., 2021).
Soil scarification, which mainly includes mechanical site preparation (MSP) or natural boar bucking, is described by Kerr (2000) and Löf et al. (2012) as the best available method for preparing habitats for pine seedling germination and growth. The advantages of mixing the mineral and organic layers include reduced competition for light, water, and nutrients between seedlings and herbaceous plants (Nilsson and Örlander, 1999). This is especially important during the first and, usually, the second year of pine seedling life, as forest floor plants can hinder and delay germination, limiting seedling growth through allelopathic effects (Jäderlund et al., 1998). A negative effect of MSP can be the leaching of mineral nutrients, which results in soil depletion (Piirainen et al., 2009). MSP alters soil physical conditions such as water content, aeration, and temperature, together with chemical properties such as organic matter content, nutrient availability, and soil pH (Block and van Rees, 2002; Heiskanen et al., 2007). The assumptions of ecologically based sustainable forest management, which minimizes the impact of human activities on nature, limit the area covered by MSP in favor of the natural regeneration of pines resulting from disturbances such as fire and soil scarification. However, it is important to remember that the potential for natural pine regeneration is limited, and it is determined by climatic factors such as precipitation totals (and their distribution over the growing season) and mean air temperature (Puhlick et al., 2012). It also depends significantly on the sorption properties of the topsoil layers, especially the thickness of the litter and humus layers and their moisture content. The high organic matter content ensures that these layers dry out quickly and thus exhibit high variability in their moisture content (Hille and den Ouden, 2004). If the soil is not loosened, the thickness of the aforementioned layers often prevents or delays contact between the seedling root and the mineral soil (Ibáñez and Schupp, 2002). These factors make germination and seedling survival highly dependent on the volume and intensity of rainfall (Oleskog and Sahlén, 2000). The relationships between the factors that limit natural pine germination in stands can cause seeds to germinate in some places and make germination impossible in others. This phenomenon has been described as the “safe sites” hypothesis, originally proposed by Harper (1977). To test the “safe sites” hypothesis, the germination of seedlings was observed in experimental gaps in different environments and different species. The germination and emergence of seedlings in gaps is a response to a variety of favorable ecological factors. Gap formation is a prerequisite and occasionally a necessity for seedling development, while the possibility of seedling establishment is determined by, among other factors, the timing of gap formation, size, shape, soil microstructure, the presence or absence of litter, the influence of animals, and the temporal and spatial dispersal of seeds (Grubb, 1986).
Borkowska (2001) researched seedlings in gaps in mosaic vegetation depending on the strength of the associated disturbance. Severe disturbances simulated natural boar bucking, and weaker disturbances simulated foraging by herbivores. The control group consisted of undisturbed plots. In all variants, the gaps functioned as safe germination sites or regeneration niches (see Harper, 1977). In the context of the analyses cited by Borkowska (2001) and the results of a study conducted in Kampinos National Park (KNP) (Przybylski et al., 2021), it can be assumed that “safe sites” are the only possibility for the effective natural regeneration of pine. These observations led to the following research hypothesis: The occurrence of fire or soil scarification is a sufficient factor for the formation of “safe sites” in the stand and the initiation of effective natural regeneration of pine. The main objective of the study was to compare the dynamics and vigor of pine regeneration, initiated on the ground under controlled conditions: (a) following low-intensity fire and (b) in a habitat with soil scarification compared to natural (control) conditions. The study compared the germination dynamics up to the obtaining the final number of seedlings, which did not change by the end of the experiment. An additional qualitative evaluation of the obtained seedlings was performed by analyzing (a) seedling height and (b) the anatomical characteristics of the roots in all experimental variants. The results expand the scientific discourse on the possibilities of natural pine regeneration in contrast to the changing ecological conditions related to germination.
Materials and methods
Collection of plant material
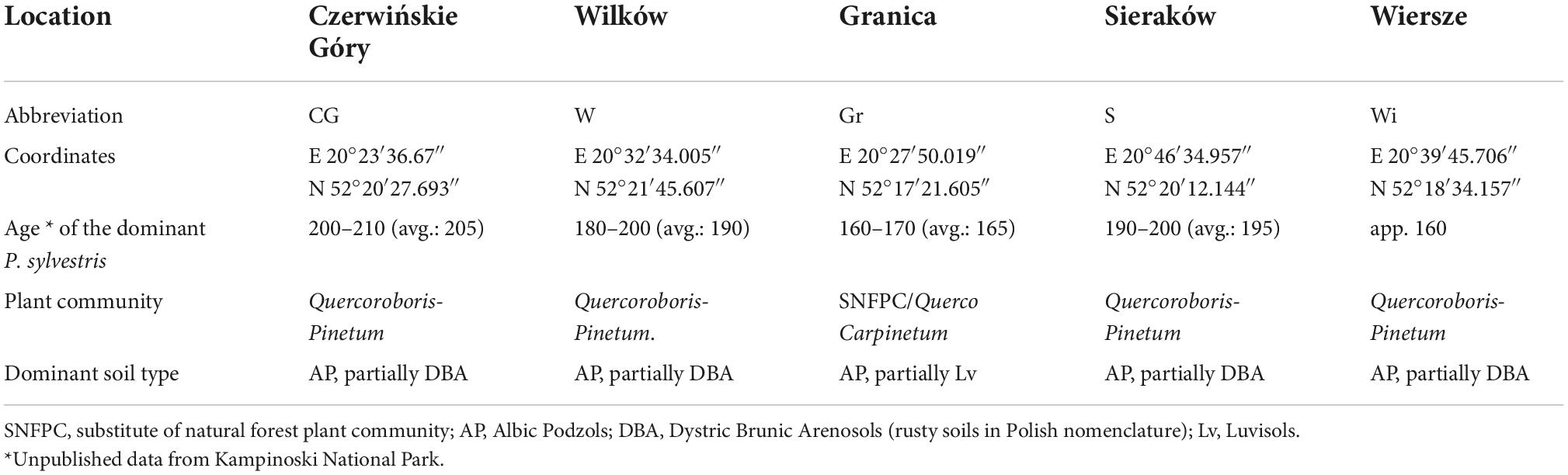
The study material was collected from standing trees growing at five sites (Table 1) in the strict protection area of KNP.
The study collected 50 cones per tree from 11 standing trees from each stand (Table 1 and Figure 1). In total, cones were collected from 55 trees for all stands; however, it should be noted that the collected seeds were separated for each population, so in further studies, to obtain the effect of adaptation to local growing conditions, no mixture of seeds was used. The evaluation was carried out for the selected population of seeds from 11 trees. The analysis of the quality characteristics of the collected seeds was previously described in detail in Przybylski et al. (2021), and the results showed very high average energy and germination (98.4–99.9%).
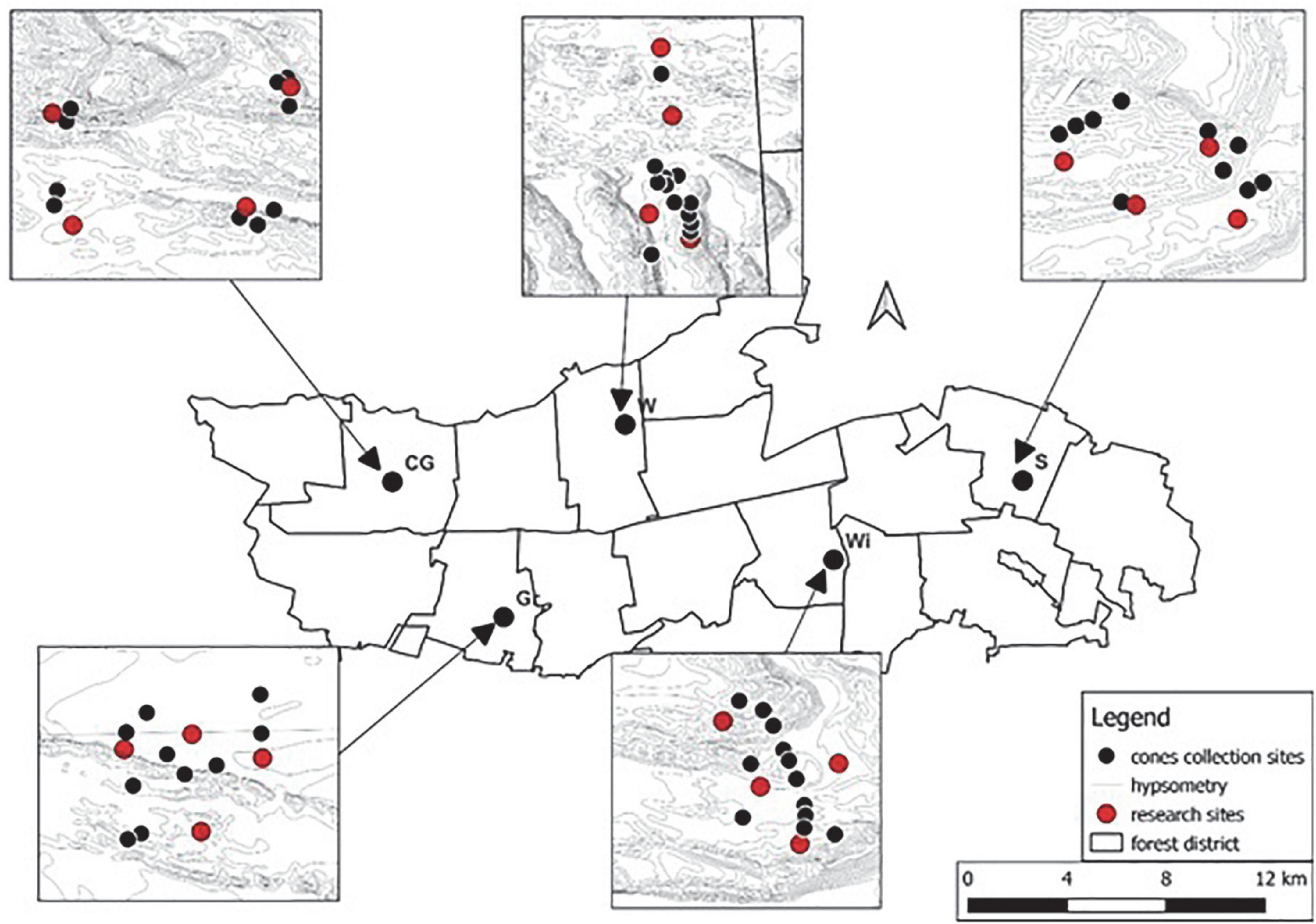
Figure 1. The main part of the figure shows the total area of Kampinos National Park together with the administrative subdivision into protected areas. The location of the stands selected for the study within the park is marked with black dots. For each stand there are detailed maps where black dots indicate the location of trees from which seeds were collected and red dots indicate the locations where soil monoliths were collected.
Collection of soil material and greenhouse experiments
Four stand fragments (research plot) were selected to conduct the experiment in the greenhouse mimicking natural habitat conditions (Supplementary Figures 1A,B), from which 3 soil monoliths measuring 40 × 50 × 30 cm were taken (Figure 1). A total of 12 soil monoliths were collected from each stand, 3 per research plot. The soil monoliths were immediately transported to the greenhouse of the Forest Research Institute (N 52°06′17.8″ E 20°52′53.1″). The collected soil monoliths were divided into the following experimental variants, noting that for each study plot, one soil monolith represents one replicate in the experimental variant (in total, each variant had 20 replicates with 5 replicates per stand):
(a) Natural, with unmodified habitat (N) growth conditions;
(b) Fire (artificial burning of the herbaceous vegetation layer), with simulated surface burning of herbaceous vegetation (F);
(c) Soil scarification (artificial soil mixing), with surface mixing of organic and mineral layers, simulating soil loosening by wild boars (SP).
For each experimental variant, 30 seeds from 11 trees from a homogeneous site were distributed in a fixed grid (to exclude random sowing and germination from the seed bank) on the study plot. Seeds were sown on April 30 year 2020 under controlled conditions, and then live seedlings were counted every 2 weeks (until October 6 year 2020). The observations were used to calculate the following values:
(a) Germination capacity of the field (GC), expressed as the maximum number of germinated seeds (%);
(b) Survival rate (S), expressed as the number of seedlings that survived to the end of the experiment (%);
(c) Mortality (M), expressed as the difference between GC and S.
At the end of the experiment, the height of the living seedlings was measured to the nearest 1 mm and analyses of the selected characteristics of their roots were performed.
Root characteristics analysis
Analysis of the selected root traits after seedling growth was performed after the removal of a root ball. Root characteristics were analyzed for all seedlings that survived to the end of the experiment. Root systems were then rinsed from the substrate by soaking them in water in several stages. The cleaned roots, after being wrapped in a paper towel, were placed in plastic bags, and the remaining water after rinsing was poured through sieves to extract any roots that may have become dislodged during rinsing. Root systems were scanned in a container filled with water on an EPSON Perfection V800/V850 scanner (software version 1.9 V3.93 3.9.3.4) adapted to Regent Instrument’s WinRHIZO software (version 2017). The scan was performed at a resolution of 600 dpi in 16-bit grayscale. This allowed the root system to be clearly separated from the background. The resulting image was saved in TIFF format. The measurement of root parameters was performed using WinRHIZO software (2017).
Statistical analysis
Survival rate variable
To compare the survival rates of pine regeneration in the three experimental variants (N, F, SP), a one-way analysis of variance was used. The significance of the model effects was tested by the Wald χ2-test for a type 3 analysis. Pairwise comparisons were made between least-square means with Tukey’s post-hoc test. Calculations were performed using the GENMOD procedure with the BINOMIAL option of SAS/STAT® v. 14.3 (SAS Institute Inc., 2017).
The MIXED procedure of the SAS program was used to model changes in survival rates through time. The REPEATED command with the TYPE = UN option was used, and, in this case, all effects were assigned intersubject degrees of freedom to provide better approximations of small samples to the corresponding sampling distributions.
The CALIS procedure of the SAS program was used to investigate the correlation between the studied traits. This procedure, dedicated to structural equations, allows for the determination of ML estimators with correction for the small sample size. Using the ROBUST option, modified statistics were obtained, which are asymptotically robust against violations of the assumption of normality of distributions and/or dedicated to small samples (Satorra and Bentler, 1988, 1994).
Other variables
In order to examine the effect of the experimental variants on the other traits of the aboveground parts of the seedlings (seedling height) and roots (length, surf. area, avg. diameter, root volume, and tips), a one-way analysis of variance and detailed comparisons of the means were performed using the Tukey test. Calculations were performed using the GENMOD procedure of the SAS program (without the BINOMIAL option).
Results
Based on the analysis of variance, significant differences were found between the survival rates of the experimental variants (Table 2).
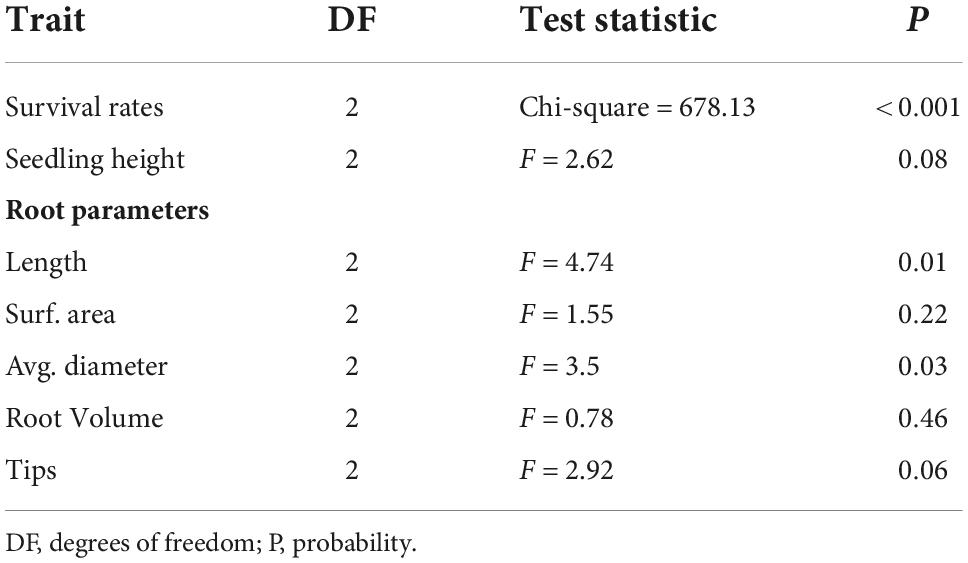
Table 2. The results of tests (Wald for survival rates (S) and F for other traits) testing differences between the experimental variants (N, F, SP) for tested traits.
Detailed analyses confirmed the presence of significant differences between the three variants (Table 3).
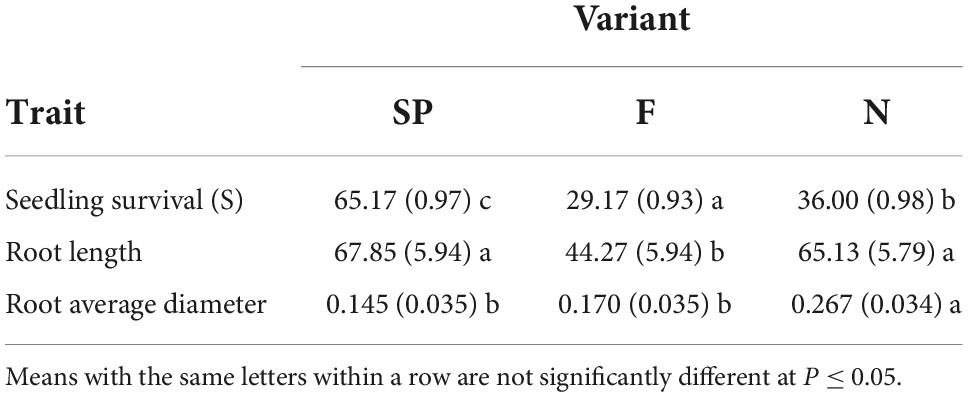
Table 3. Means and standard errors (in parentheses) of seedling tested traits; associated with the experimental variants (N, F, SP).
Germination began on April 30 and continued until June 10, when the maximum number of germinated seeds was reached. The highest number of seedlings was obtained for the SP variant (80% of seedlings) and the lowest for the F variant (48% of seedlings) (Figure 2). The seedlings in the initial stage germinated the fastest for variants SP and F, although it should be noted that the germination dynamics of variant F decreased significantly as the peak was approached. Variant N was characterized by lower germination dynamics throughout the measurement period, and the maximum number of seedlings (48% of seedlings) finally obtained did not differ significantly from the number of variant F seedlings due to the stability of germination through time (Figure 2). The death (Mor.) of germinated seedlings could be observed from the time of germination (June 10) to August 2 (Figure 2). Seedling mortality increased until July 12, and most seedlings died in variant F, in which the mortality process was the most intense and lasted the longest. The lowest Mor. rate was observed in the SP variant, while the process was the least dynamic in variant N (Figure 2). From August 2, there was no significant change in the number of seedlings that survived until the end of the experiment (stabilization phase). In the stabilization phase, the number of seedlings varied significantly among the variants until the end of the observation (Table 2 and Figure 2).
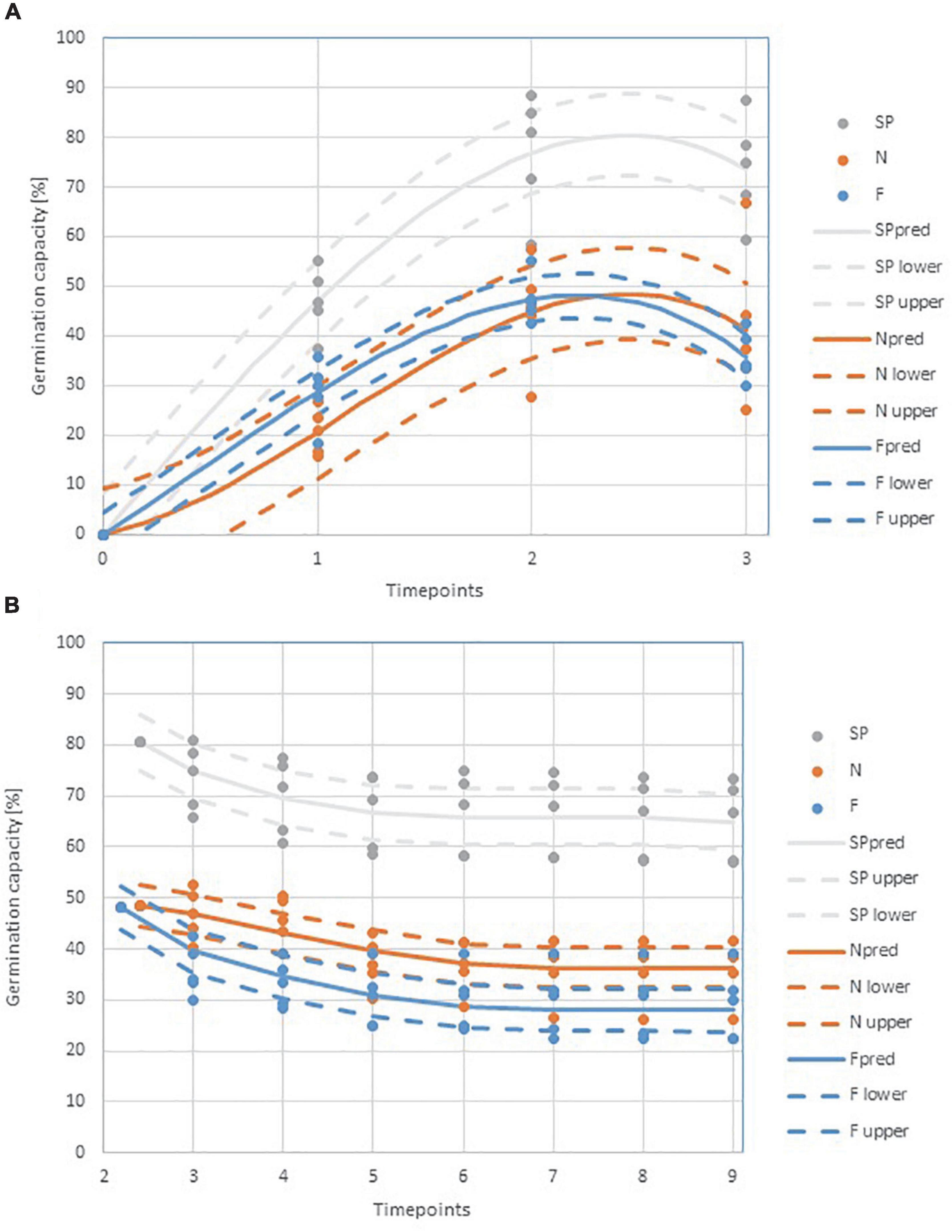
Figure 2. Germination dynamics of the tested pine seeds (A) from the beginning of the experiment to the peak of germination (B) and from the peak of germination to the end of the experiment in the studied variants (SP, F, N) at selected timepoints: 0 (planting) to 30 April; 1–24 May; 2–7 June; 3–18 June; 4–28 June; 5–12 July; 6–2 August; 7–22 August; 8–6 September; 9–end of experiment.
The SP variant had a significantly higher GC value compared to the other variants (Figure 2), with the difference between the values accounting for almost 30% of the possible seedlings. On the other hand, the variation in S for variants N and F is due to the Mor. of the obtained seedlings. It should be noted that, with almost identical GC values for N and F, the Mor. of the seedlings in F was about 6% higher.
The results also show that the germination capacity of seedlings in the field (GC) is demonstrably lower than the germination capacity of homogeneous seeds obtained under controlled conditions in seed evaluation stations (Forest Research Institute) (Przybylski et al., 2021). The results obtained were 22% lower for the SP variant and 52% lower for the N and F variants. At the end of the experiment, seedling height was measured for each variant (Table 4). At the end of the experiment, seedling height was measured for the variants. The highest seedlings were found in variant F (mean 8.59 cm) and the lowest in variant SP (mean 6.99 cm) (Supplementary Table 1). These results were subjected to a one-factor analysis of variance, which showed no significant differences between the variants (Table 2).
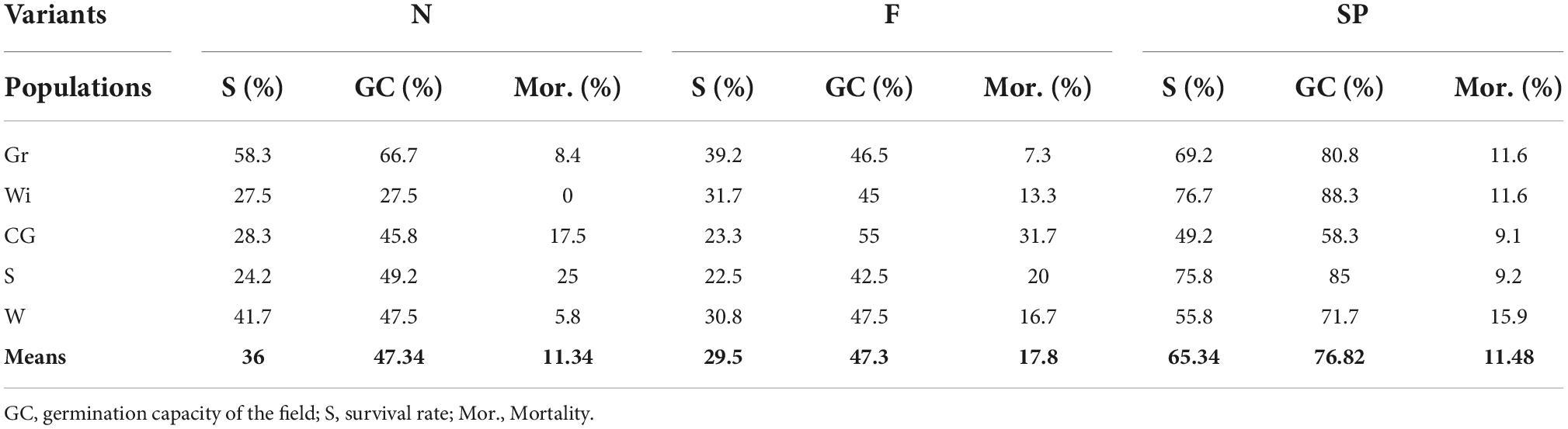
Table 4. Detailed variation of traits (GC, S, and Mor.) in the analyzed variants (N, F, SP) of the experiment.
After completing the experiment, the prepared root samples of a single plant were analyzed for selected characteristics, and the results were statistically evaluated. A preliminary analysis of the root thickness indicated differences between the tested variants. Variant F had the highest percentage of thin roots, while the thickest roots were found in variant N (Figure 3). The analysis of variance showed significant differences in root characteristics between variants (N, F, SP) for length and average diameter (Table 2). No significant differences were found for the surface area and root volume parameters. The tip parameter, despite its lack of statistical significance (P = 0.06), appears to be a biologically important discriminating factor. Therefore, it is included in the calculation of correlations between the traits that were studied.
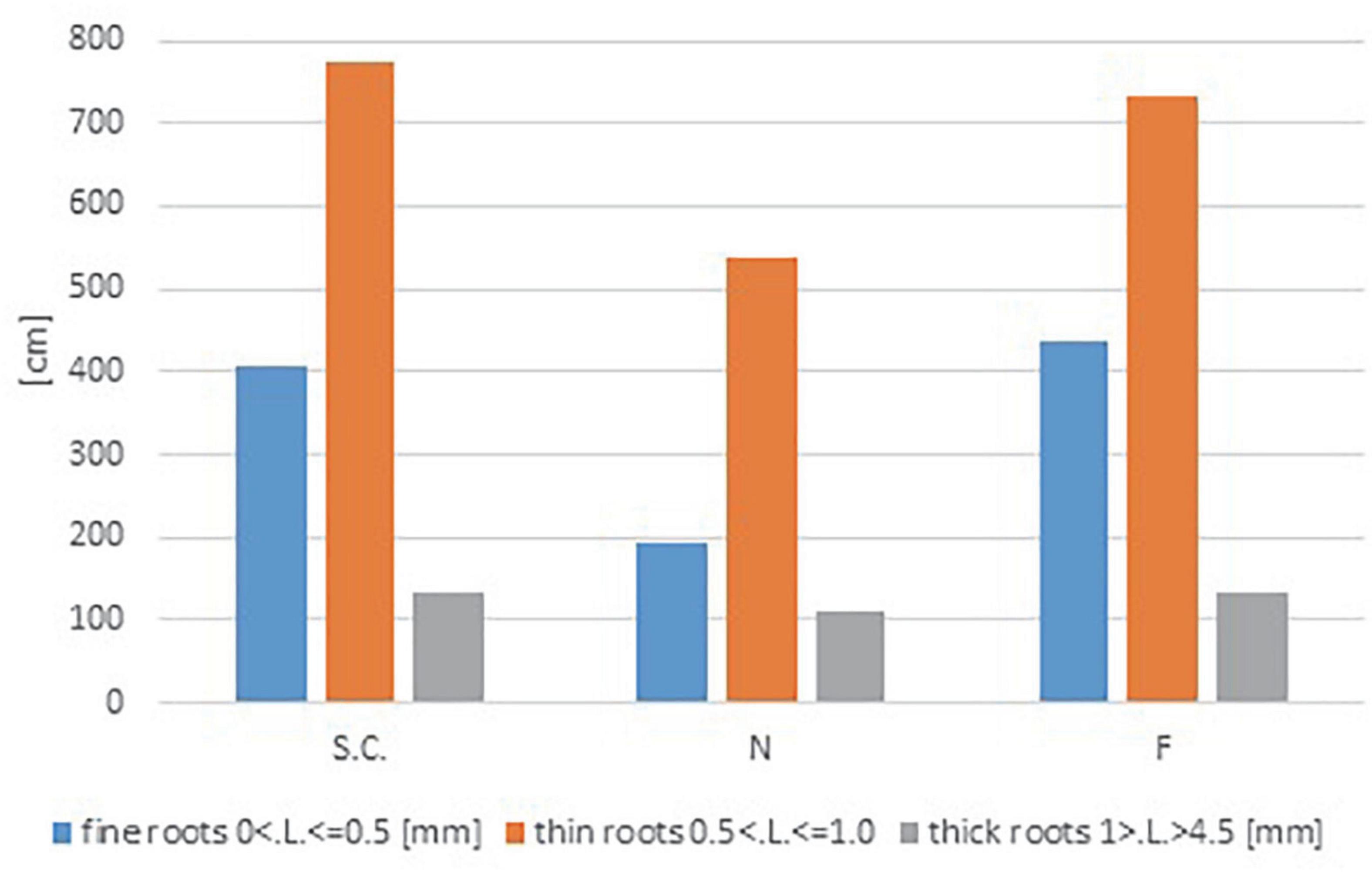
Figure 3. Quantitative breakdown (cm.) of total root length for all variants studied. Results shown were divided into root thickness classes (mm.): blue represents fine roots; orange represents thin roots; gray represents thick roots.
A statistical analysis of the root length characteristics between the tested variants revealed significant differences for SP and F and for F and N (Table 3). For SP and N, the difference was not significant.
Significant differences were found between the SP and N, F and N experimental variants in terms of the root average diameter trait (Table 3); For SP and F, the difference was not significant.
A correlation analysis was performed for the parameters that differed significantly from the experimental variants studied (Table 5). In variant N, a significant positive correlation between S and root parameter length (P = 0.02) and tips (P = 0.03) was demonstrated; the indicated dependencies of S in relation to the root parameters were confirmed in the evaluation of variant SP, in which a significant negative correlation with length (P = 0.04) and tips (P = 0.04) was demonstrated. In variant F, no correlation was found between S and the analyzed root characteristics.
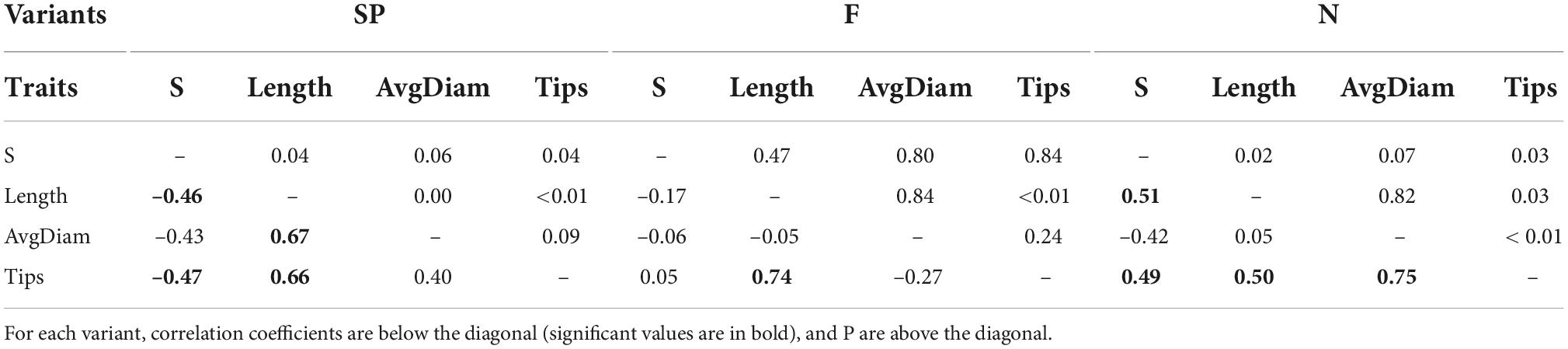
Table 5. Correlations between the survival rate and the significant root parameters for the experimental variants (N, F, SP): S, length (roots), average diameter (roots), and tips (roots).
Discussion
The research presented was conducted in KNP, which is an example of a legally protected forest ecosystem where pine dominates the species composition of the stand. In a national park, natural regeneration processes that spontaneously influence the species composition of the stand and its spatial structure can occur. In the context of pine, the possibility of its natural regeneration is limited, which is influenced, among other factors, by the features analyzed in the present study. According to Tomczyk (1990), one of the most important components limiting the natural regeneration of pine is the thickness of forest litter, where values of 6–7 cm practically prevent seedling survival. For this reason, a prerequisite for the survival of pine forests is the occurrence of large-scale natural disturbances, such as fires or local phenomena, which create safe germination sites (Borkowska, 2001). The above factors influence the removal of the vegetation cover from the forest floor, which significantly increases the probability of natural pine regeneration (Gmyz and Skrzyszewski, 2010). However, the present research only partially confirms this relationship. For the experimental variant in which a local habitat disturbance was simulated, i.e., mixing the mineral soil layer with the organic layer (SP), almost twice as many seedlings were obtained at the end of the measurement period (Table 4). On the other hand, the same effect could not be confirmed for the variant in which fire was simulated (F); moreover, the number of pine seedlings obtained in this variant was significantly lower (Table 3) than in the control variant of the experiment (N). In connection with these results, the study of Béland et al. (2000) was confirmed, according to which mineral soil is the optimal substrate for the germination of forest trees. Under these conditions, seeds have excellent moisture conditions due to the capillary transport of water to the soil surface. In contrast to seed germination in the mineral layer, seed germination in the organic layer is not favorable due to rapid drying and difficult wetting under low and steadily decreasing precipitation totals (Sewerniak et al., 2012). The results confirm the importance of natural or artificial habitat preparation for the success of pine regeneration processes. In Poland, forestry practices involve site preparation to initiate stand regeneration in the event of felling, which promotes natural regeneration, for example, by lateral seeding. Under natural conditions, mixing the organic and mineral layers occurs due, in part, to the activity of wild boar (Sus scrofa). There are differing views on the effects of wild boar on forest communities (Hone, 2002). In deciduous forest stands, such as in the Tilio–Carpinetum community, soil loosening caused by wild boars reduces seedling density (Piroznikow, 1998), but the effects on conifer stands may be the opposite, if only because of differences in forest litter thickness. In the context of the presented results, we should consider whether the natural regeneration of pine is possible in areas with strict protections. First of all, it is important to mention the limited size of the wild boar population, which is restricted by organized human hunting and reduced by predator pressure. In recent years, African swine fever (ASF) has been an additional factor limiting the wild boar population in Poland and Europe.
In the ongoing scientific debate, it is worth considering whether the present size and number of protected areas can sufficiently initiate the natural regeneration of Scots pine. The original natural forests of Europe were characterized by different dynamics; for deciduous forests, small-scale gaps and a mosaic of medium-scale development phases were typical. For coniferous forests, large-scale natural disturbances were typical, to which temperate forest ecosystems with pines were cyclically exposed. The most common large-scale disturbance is fires, which in modern Poland usually only reach small areas, the result of a comprehensive organizational and technical system meant to protect forest areas from fires. KNP is both the second largest national park and the largest forested park in Poland, with an area of 38,000 ha, and it is classified in the highest fire danger category (Tyburski et al., 2019). KNP experiences approximately 57% of the fires in all national parks in Poland each year (Szaga, 2015). In 2015, 61 fires were recorded in Kampinos Forest, including 52 ground-cover fires. The average size of a forest fire was only 0.36 ha (Szaga, 2015). The relatively small area of the average fire is the reason for the low extent of the observed natural pine regeneration in KNP (Przybylski et al., 2021). It should also be noted that, due to ongoing mandatory forest fire protection in Poland (which is also implemented in national parks), it is unlikely that the frequency of large fires will increase significantly. Therefore, it is worth considering whether small fires can have a significant impact on promoting natural pine regeneration. In the present study, the effect of forest litter fire on seed germination was investigated. During the first days, the germination process was dynamic and similar to variant a mixed organic and mineral layer (Figure 2A). In the later stages of the experiment, the seedlings of the fire variant started to die, so that the fire variant had the lowest survival rate at the end of the experiment (Figure 2B). The reasons for the high seedling mortality in the fire variant could be related to the ecological growing conditions. The restoration of a community after a disturbance occurring in a small area occurs due to seeds in the same ecosystem, possibly with a small contribution from light-seeded species (Faliński, 2001). Despite knowledge about the positive effect of the exposed mineral layer on pine seedling germination (Hille and den Ouden, 2004), it should be noted that litter removal favors an increase in understory species richness (Dzwonko and Gawroński, 2002). Moreover, plant growth in plots with a burned organic layer is more dynamic, and it depends on habitat moisture and fire intensity. In the process of secondary succession or fire regeneration, light-seeded species also appear, such as grasses, shrubs, and trees (Zaniewski and Otrȩba, 2017), which further increase competition with pine seedlings.
Increased plant growth results from a favorable ratio of carbon and nitrogen in the soil. Nitrogen is the nutrient most affected by fire (Mataix-Solera et al., 2011). In the first few years after a fire, inorganic nitrogen concentrations increase more in burned areas than in control areas (Turner et al., 2007; Boerner et al., 2009). Fire-induced changes in soil inorganic nitrogen can be attributed to the release of the compound from dead roots where it was previously stored (Rivas et al., 2012). According to Smithwick et al. (2005), nitrification has the most beneficial effect on plant growth, especially in acidic soils, because the reduced acidity increases microbial activity. It is possible to increase soil organic carbon in low-intensity fires by burning plant residues to compensate for the carbon lost during the fire (Knoepp et al., 2005). The results obtained in the present study for variant F demonstrate a larger number of germinated seeds compared with variant N during the first observation period, which could be due to an increased supply of carbon and nitrogen. However, it should be noted that, firstly, variant F had a lower number of germinated seeds compared with variant SP and, secondly, that variant F had the highest number of seedlings that died after germination of all the variants studied. The former observation confirms the thesis that mineral soil is the optimal environment for pine seed germination. In the context of seedling dieback, competition for resources, i.e., water and minerals, plays a dominant role, and the parameters of the root system are probably decisive. In the conducted study, the analysis of root thickness classes proved the differentiation of this trait among the experimental variants. Detailed analyses of root characteristics revealed significant differences among the variants for the root length and average diameter parameters. Tips are also an important parameter for pine seedlings; while it did not reveal any statistically significant differences between variants, its value (P = 0.06) proves the correctness of this trait choice in a biological context. The total length of the root system (length) revealed a statistically significant difference between the SP and N variants and the F and N variants, and the average root thickness (avg. diameter) between SP and F. Correlations between the seedling survival rate and the length and tips in the SP (negative correlation) and N (positive correlation) variants were found in the data obtained. These results confirm studies by other authors (Kottke, 2002; Leuschner et al., 2004; Ostonen et al., 2007) describing root plasticity as a response to local soil heterogeneity. The data obtained confirm the increased survival rate of pine seedlings characterized by longer roots, which is a priority under conditions of competition for water and minerals.
Synopsis
Our results allow us to form conclusions in relation to the objective of the study. The possibility of the natural regeneration of pines in boreal habitats is limited, and it requires the occurrence of natural or anthropogenic disturbances. The best results are obtained when the organic layer of the forest understory is mixed with or removed from the mineral layer. Under such conditions, pine seeds germinate intensively, and their survival rate is high. On the other hand, wildfire, especially at low intensity and on a relatively small area, does not provide conditions that trigger natural pine regeneration in the first year after fire. The results obtained in the present study indicate intensive seedling germination after fire, but followed by a high seedling mortality rate. Consequently, the number of seedlings at the end of the experiment was higher in the variant that simulated natural conditions without disturbance than in the variant that simulated a wildfire. The studied disturbances in the understory had no significant effect on the height of seedlings, but they significantly altered the root systems by changing their individual characteristics. In addition, the influence of fine root length on seedling survival was shown to be critical for pine regeneration in the context of competing for limited environmental resources. The results obtained raise research questions that can form the basis for further scientific projects. The role of fire as a factor ensuring the natural persistence of pine-dominated ecosystems does not emerge from the results. An interesting aspect related to a dynamically changing climate is the high plasticity of roots depending on current habitat conditions.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PP: conceptualization, methodology, writing—original draft preparation, writing—review and editing, visualization, and project administration. SJ: methodology and software. KU: formal analysis, validation, and data curation. ŁT: writing—review and editing and data curation. MK: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
Funding was received from Statutory Forest Research Institute (no. 900251).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.1023155/full#supplementary-material
References
Badía-Villas, D., González-Pérez, J. A., Aznar, J. M., Arjona-Gracia, B., and Martí-Dalmau, C. (2014). Changes in water repellency, aggregation and organic matter of a mollic horizon burned in laboratory: Soil depth affected by fire. Geoderma 213, 400–407. doi: 10.1016/j.geoderma.2013.08.038
Béland, M., Agestam, E., Ekö, P. M., Gemmel, P., and Nilsson, U. (2000). Scarification and seedfall affects natural regeneration of Scots pine under two shelterwood densities and clear-cut in southern Sweden. Scand. J. For. Res. 15, 247–255. doi: 10.1080/028275800750015064
Block, M. D., and van Rees, K. C. J. (2002). Mechanical site preparation impacts on soil properties and vegetation communities in the Northwest Territories. Can. J. For. Res. 32, 1381–1392. doi: 10.1139/x02-067
Boerner, R. E. J., Huang, J. J., and Hart, S. C. (2009). Impacts of fire and fire surrogate treatments on forest soil properties: A meta-analytical approach. Ecol. Appl. 19, 338–358. doi: 10.1890/07-1767.1
Borkowska, L. (2001). Zaburzenia Wywołane Eksperymentalnie w Zbiorowisku Niekoszonej ła̧ki Cirsietum Rivularis Ralski 1931, a Funkcjonowanie Niszy Regeneracyjnej, Ph.D thesis, Siedlce, PL: Siedlce University of Natural Sciences and Humanities.
Dobrowolska, D. (2008). Odnowienie naturalne na powierzchniach uszkodzonych przez pozar w nadlesnictwie rudy raciborskie. Les. Pr. Bad. 69, 255–264.
Dzwonko, Z., and Gawroński, S. (2002). Effect of litter removal on species richness and acidification of a mixed oak–pine woodland. Biol. Conserv. 106, 389–398. doi: 10.1016/S0006-3207(01)00266-X
European Commission. (2013). Interpretation Manual of European Union Habitats. https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (Accessed on Aug 2, 2021).
Faliński, J. B. (2001). Przewodnik do długoterminowych badań ekologicznych. Vademecum Geobotanicum. Warsaw: Wydawnictwo Naukowe PWN.
Gimeno-García, E., Andreu, V., and Rubio, J. L. (2000). Changes in organic matter, nitrogen, phosphorus and cations as a result of fire and water erosion in a Mediterranean landscape. Eur. J. of Soil Sci. 51, 201–210. doi: 10.1046/j.1365-2389.2000.00310.x
Gmyz, R., and Skrzyszewski, J. (2010). The influence of microsite diversity of mesic coniferous forest on number of Scots pine (Pinus sylvestris L.) natural regeneration. Sylwan 154, 173–181.
González-Vila, F. J., Almendros, G., González-Pérez, A., Knicker, H., González-Vázquez, R., Hernández, Z., et al. (2009). “Transformaciones de la materia orgánica del suelo por incendios naturales y calentamientos controlados en condiciones de laboratorio,” in Efectos de los incendios forestales sobre los suelos en España. El estado de la cuestión visto por los científicos españoles, eds A. Cerdà and J. Mataix-Solera (Valencia: Universitat de València), 219–267.
Grubb, P. J. (1986). “Problems posed by sparse and patchily distributed species in species-rich plant communities,” in Community Ecology, eds J. Diamond and T. T. Case (New York, NY: Harper and Row), 207–225.
Heiskanen, J., Mäkitalo, K., and Hyvönen, J. (2007). Long-term influence of site preparation on water-retention characteristics of forest soil in Finnish Lapland. For. Ecol. Manag. 241, 127–133. doi: 10.1016/j.foreco.2007.01.023
Hille, M., and den Ouden, J. (2004). Improved recruitment and early growth of Scots pine (Pinus sylvestris L.) seedlings after fire and soil scarification. Eur. J. For. Res. 123, 213–218. doi: 10.1007/s10342-004-0036-4
Hilszczańska, D., Malecka, M., and Sierota, Z. (2008). Changes in nitrogen level and mycorrhizal structure of Scots pine seedlings inoculated with Thelephora terrestris. Ann. For. Sci. 65:409. doi: 10.1051/forest:2008020
Hone, J. (2002). Feral pigs in Namadgi National Park, Australia: Dynamics, impacts and management. Biol. Conserv. 105, 231–242. doi: 10.1016/S0006-3207(01)00185-9
Ibáñez, I., and Schupp, E. W. (2002). Effects of litter, soil surface conditions, and microhabitat on Cerocarpus ledifolius Nutt. Seedling emergence and establishment. J. Arid Environ. 52, 209–221. doi: 10.1006/jare.2002.0988
Jäderlund, A., Norberg, G., Zackrisson, O., Dahlberg, A., Teketay, D., Dolling, A., et al. (1998). Control of bilberry vegetation by steam treatment—Effects on seeded Scots pine and associated mycorrhizal fungi. For. Ecol. Manag. 108, 275–285. doi: 10.1016/S0378-1127(98)00232-1
Job, D., and Caboche, M. (2008). Seed of life. Comp. Rend. Biol. 331, 711–714. doi: 10.1016/j.crvi.2008.08.001
Kerr, G. (2000). Natural regeneration of Corsican pine (Pinus nigra subsp. laricio) in Great Britain. Forestry 73, 479–488. doi: 10.1093/forestry/73.5.479
Knoepp, J. D., DeBano, L. F., and Neary, D. G. (2005). “Soil Chemistry,” in Wildland Fire in Ecosystems: Effects of Fire on Soils and Water, eds D. G. Neary, K. C. Ryan, and L. F. DeBano (Ogden, UT: Forest Service and Rocky Mountain Research Station), 53–71.
Kottke, I. (2002). “Mycorrhizae – rhizosphere determinants of plant communities,” in Plant Roots: The Hidden Halh, eds Y. Waisel, A. Eshel, and U. Kafkafi (New York, NY: Marcel Dekker), 919–932. doi: 10.1201/9780203909423.ch50
Leuschner, C., Hertel, D., Schmid, I., Koch, O., Muhs, A., and Holscher, D. (2004). Stand fine root biomass and fine root morphology in oldgrowth beech forests as a function of precipitation and soil fertility. Plant Soil 258, 43–56. doi: 10.1023/B:PLSO.0000016508.20173.80
Löf, M., Dey, D. C., Navarro, R. M., and Jacobs, D. F. (2012). Mechanical site preparation for forest restoration. New For. 43, 825–848. doi: 10.1007/s11056-012-9332-x
Loster, S., Dzwonko, Z., and Gawroński, S. (2011). “Early post–fire vegetation regeneration in a Scots pine forest site in southern Poland,” in Geobotanist and Taxonomist. A Volume Dedicated to Professor Adam Zaja̧c on the 70th Anniversary of His Birth, ed. B. Zemanek (Jagiellonian: Jagiellonian University), 117–130.
Mataix-Solera, J., Cerdà, A., Arcenegui, V., Jordán, A., and Zavala, L. M. (2011). Fire effects on soil aggregation: A review. Earth-Sci. Rev. 109, 44–60. doi: 10.1016/j.earscirev.2011.08.002
Niklasson, M., Zin, E., Zielonka, T., Feijen, M., Korczyk, A. F., Churski, M., et al. (2010). A 350–year tree–ring fire record from Białowieża Primeval Forest, Poland: Implications for Central European lowland fire history. J. Ecol. 98, 1319–1329. doi: 10.1111/j.1365-2745.2010.01710.x
Nilsson, U., and Örlander, G. (1999). Vegetation management on grass—dominated clearcuts planted with norway spruce in southern Sweden. Can. J. For. Res. 29, 1015–1026. doi: 10.1139/x99-071
Non-ogaki, H., Bassel, G. W., and Bewley, J. D. (2010). Germination – still a mystery. Plant Sci. 179, 574–581. doi: 10.1016/j.plantsci.2010.02.010
Nowakowska, J. A. (2007). Zmienność genetyczna polskich wybranych populacji sosny zwyczajnej (Pinus sylvestris L.) na podstawie analiz polimorfizmu DNA. Poland, Sȩkocin Stary: Forest Research Institute Prace IBL - dissertations and monographs. Sȩkocin Stary: Instytut Badawczy Leśnictwa
Oleskog, G., and Sahlén, K. (2000). Effect of seedbed substrate on moisture conditions and germination of Pinus sylvestris (L.) seeds in clear-cut. Scand. J. For. Res. 15, 225–236. doi: 10.1080/028275800750015046
Ostonen, I., Lohmus, K., Alama, S., Truu, J., Kaar, E., Vares, A., et al. (2007). Morphological adaptations of fine roots in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula Roth.) and black alder (Alnus glutinosa (L.) Gaertn.) stands in recultivated oil shale mining and semi-coke areas. Oil Shale 23, 187–202. doi: 10.3176/oil.2006.2.11
Pardini, G., Gispert, M., and Dunjó, G. (2004). Relative influence of wildfire on soil properties and erosion processes in different Mediterranean environments in NE Spain. Sci. Total Environ. 328, 237–246. doi: 10.1016/j.scitotenv.2004.01.026
Pastor-López, A., and Martin-Martin, J. (1995). “Potential nitrogen losses due to fire from Pinus halepensis stands in the Alicante Province (Southeastern Spain): Mineralomass variability,” in The biswell symposium: Fire issues and solutions in urban interface and wildland ecosystems, eds D. R. Weise and R. E. Martin (Albany, CA: Pacific Southwest Research Station), 199.
Piirainen, S., Finér, L., Mannerkoski, H., and Starr, M. (2009). Leaching of cations and sulphate after mechanical site preparation at a boreal forest clear-cut area. Geoderma 149, 386–392. doi: 10.1016/j.geoderma.2009.01.003
Piroznikow, E. (1998). The influence of natural and experimental disturbance on emergence and survival of seedilings in an oak-linden-hornbeam (Tilio-Carpinetum) forest. Pol. J. Ecol. 46, 137–156.
Przybylski, P., Konatkowska, M., Jastrzȩbowski, Sz, Tereba, A., Mohytych, V., Tyburski, Ł, et al. (2021). The possibility of regenerating a pine stand through natural regeneration. Forests 12:1055. doi: 10.3390/f12081055
Puhlick, J. J., Laughlin, D. C., and Moor, M. M. (2012). Factors influencing ponderosa pine regeneration in the southwestern USA. For. Ecol. Manag. 264, 10–19. doi: 10.1016/j.foreco.2011.10.002
Raison, R. J., Khanna, P. K., Jacobsen, K. L. S., Romanya, J., and Serrasolses, I. (2009). “Effect of fire on forest nutrient cycles,” in Fire Effects on Soils and Restoration Strategies, eds A. Cerdà and P. R. Robichaud (Singapore: Science Publishers), 225–256. doi: 10.1201/9781439843338-c8
Reich, P. B., Oleksyn, J., Modrzynski, J., and Tjoelker, M. G. (1996). Evidence that longer needle retention of spruce and pinepopulations at high elevations and high latitudes is largely aphenotypic response. Tree Physiol. 16, 643–647. doi: 10.1093/treephys/16.7.643
Rivas, Y., Huygens, D., Knicker, H., Godoy, R., Matus, F., and Boeckx, P. (2012). Soil nitrogen dynamics three years after a severe Araucaria-Nothofagus forest fire. Austral Ecol. 37, 153–163. doi: 10.1111/j.1442-9993.2011.02258.x
Satorra, A., and Bentler, P. M. (1988). “Scaling correction for chi-square ststistics in covariance structure analysis,” in Proceedings of the business and economic statistics section association, New Orleans, LA, 308–313.
Satorra, A., and Bentler, P. M. (1994). “Correction to test ststistics and standard errors in covariance structure analysis,” in Latent variables analysis: Applications for developmental research, eds A. von Eye and C. C. Clog (Thousand Oaks, CA: Sage), 399–419.
Seidl, R., Fernandes, P. M., Fonseca, T. F., Gillet, F., Jönsson, A. M., and Merganičová, K. (2011). Modelling natural disturbances in forest ecosystems: A review. Ecol. Model. 222, 903–924. doi: 10.1016/j.ecolmodel.2010.09.040
Sewerniak, P., Gonet, S. S., and Quaium, M. (2012). Impact of soil preparation with rotary tiller on growth of Scots pine plants on poor sites of the Bydgoszcz Forest. Sylwan 156, 871–880.
Slama, H. B., Bouket, A. Ch, Alenezi, F. N., Pourhassan, Z., Golińska, P., Oszako, T., et al. (2021). Potentials of endophytic fungi in the biosynthesis of versatile secondary metabolites and enzymes. Forests 12:1784. doi: 10.3390/f12121784
Smithwick, E. A. H., Turner, M. G., Mack, M. C., and Chapin, F. S. (2005). Postfire soil N cycling in northern conifer forests affected by severe, stand-replacing wildfires. Ecosystems 8, 163–181. doi: 10.1007/s10021-004-0097-8
Socha, J., Pierzchalski, M., Bałazy, R., and Ciesielski, M. (2017). Modelling top height growth and site index using repeated laser scanning data. For. Ecol. Manag. 406, 307–317. doi: 10.1016/j.foreco.2017.09.039
Socha, J., Solberg, S., Tymińska-Czabańska, L., Tompalski, P., and Vallet, P. (2021). Heightgrowth rate of Scots pine in Central Europe increased by 29% between 1900 and 2000 due to changes in site productivity. For. Ecol. Manage. 490:119102. doi: 10.1016/j.foreco.2021.119102
State Forests of Poland (2022). Forest resources. Available online at: https://www.lasy.gov.pl/en/our-work/forest-resources (accessed October 15. 2022).
Turner, M. G., Smithwick, E. A. H., Metzger, K. L., Tinker, D. B., and Romme, W. H. (2007). Inorganic nitrogen availability after severe stand-replacing fire in the greater yellowstone ecosystem. Proc. Natl. Acad. Sci.U.S.A. 104, 4782–4789. doi: 10.1073/pnas.0700180104
Tyburski, Ł, Zaniewski, P., Bolibok, L., Pia̧tkowski, M., and Szczepkowski, A. (2019). Scots pine Pinus sylvestris mortality after surface fire in oligotrophic pine forest Peucedano-Pinetum in Kampinos National Park. Folia For. Polonica 61, 51–57. doi: 10.2478/ffp-2019-0005
Van Staden, J., Brown, N. A. C., Jager, A. K., and Johnson, T. A. (2000). Smoke as a germination cue. Plant Species Biol. 15, 167–178. doi: 10.1046/j.1442-1984.2000.00037.x
Zaniewski, P., and Otrȩba, A. (2017). Response of vegetation to the surface fire in the pine forest Peucedano–Pinetum W. Mat. (1962) 1973 in the Kampinoski National Park. Sylwan 161, 991–1001.
Zheng, Z.-L. (2009). Carbon and nitrogen nutrient balance signalling in plants. Plant Signal. Behav. 4, 584–591. doi: 10.4161/psb.4.7.8540
Keywords: natural regeneration, Scots pine, seeds, seedlings, disturbances
Citation: Przybylski P, Jastrzȩbowski S, Ukalski K, Tyburski Ł and Konatowska M (2022) Quantitative and qualitative assessment of pine seedlings under controlled undergrowth disturbance: Fire and soil scarification. Front. For. Glob. Change 5:1023155. doi: 10.3389/ffgc.2022.1023155
Received: 22 August 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Thomas J. Dean, Louisiana State University, United StatesReviewed by:
Ajay Sharma, University of Florida, United StatesAsep Hidayat, Forest Research and Development Center, Indonesia
Copyright © 2022 Przybylski, Jastrzȩbowski, Ukalski, Tyburski and Konatowska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paweł Przybylski, cC5wcnp5Ynlsc2tpQGlibGVzLndhdy5wbA==
 Paweł Przybylski
Paweł Przybylski Szymon Jastrzȩbowski
Szymon Jastrzȩbowski Krzysztof Ukalski2
Krzysztof Ukalski2 Łukasz Tyburski
Łukasz Tyburski Monika Konatowska
Monika Konatowska