
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 03 January 2022
Sec. Tropical Forests
Volume 4 - 2021 | https://doi.org/10.3389/ffgc.2021.787533
This article is part of the Research Topic Drivers of Mangrove Forest Change and its Effects on Biodiversity and Ecosystem Services View all 13 articles
Mangroves are known for large carbon stocks and high sequestration rates in biomass and soils, making these intertidal wetlands a cost-effective strategy for some nations to compensate for a portion of their carbon dioxide (CO2) emissions. However, few countries have the national-level inventories required to support the inclusion of mangroves into national carbon credit markets. This is the case for Brazil, home of the second largest mangrove area in the world but lacking an integrated mangrove carbon inventory that captures the diversity of coastline types and climatic zones in which mangroves are present. Here we reviewed published datasets to derive the first integrated assessment of carbon stocks, carbon sequestration rates and potential CO2eq emissions across Brazilian mangroves. We found that Brazilian mangroves hold 8.5% of the global mangrove carbon stocks (biomass and soils combined). When compared to other Brazilian vegetated biomes, mangroves store up to 4.3 times more carbon in the top meter of soil and are second in biomass carbon stocks only to the Amazon forest. Moreover, organic carbon sequestration rates in Brazilian mangroves soils are 15–30% higher than recent global estimates; and integrated over the country’s area, they account for 13.5% of the carbon buried in world’s mangroves annually. Carbon sequestration in Brazilian mangroves woody biomass is 10% of carbon accumulation in mangrove woody biomass globally. Our study identifies Brazilian mangroves as a major global blue carbon hotspot and suggest that their loss could potentially release substantial amounts of CO2. This research provides a robust baseline for the consideration of mangroves into strategies to meet Brazil’s intended Nationally Determined Contributions.
Climate change velocity has outpaced models’ predictions spurring the implementation of natural climate solutions policies centered on ecosystems self-organizing properties to mitigate fossil fuels emissions and ensue adaptive capacity to future alterations in the climate system. Natural ecosystems have evolved mechanisms that allow them to shift among alternate states while remaining functional over geomorphic timescales (Holling, 1973). Such processes are evident in dynamic coastal sedimentary environments, which alternate between vegetated and unvegetated states (e.g., saltmarshes and mangroves versus mudflats and saltflats) in response to climate and millennial-scale changes in sea levels (Gabler et al., 2017; Saintilan et al., 2020). In particular, where sediment yield to coastal oceans has not been impaired and coastal floodplains still allows for inland expansion, rising sea levels can increase accommodation space along mangrove- and marsh-dominated environments sustaining continuous burial of terrigenous and marine organic sediments (Rogers et al., 2019).
Among tidal saline wetlands, mangroves are known for high rates of carbon sequestration in soils (mean = 222 gC m–2 yr–1; Jennerjahn, 2020; MacKenzie et al., 2020; Wang et al., 2020), that are 50 times higher than reported for terrestrial tropical and temperate forested biomes (mean = 4.5 gC m–2 yr–1; McLeod et al., 2011). Combined with comparable carbon sequestration rates in woody biomass (mean = 82.7 gC m–2 yr–1, range = 13–2,160 gC m–2 yr–1; Xiong et al., 2019), these intertidal wetlands can be a cost-effective strategy for some nations to compensate for part of their carbon dioxide (CO2) emissions (Taillardat et al., 2018). To date, however, few countries have the country-level inventories required to support the inclusion of coastal wetlands into national carbon credit markets (e.g., Holmquist et al., 2018 for the United States and Serrano et al., 2019 for Australia). Moreover, global estimates generally focus on carbon stocks within either soil or biomass (Hutchison et al., 2014; Jardine and Siikamäki, 2014; Atwood et al., 2017; Rovai et al., 2018, 2021b; Sanderman et al., 2018; Tang et al., 2018; Simard et al., 2019; Kauffman et al., 2020), which are important to determine potential CO2eq emissions from mangrove forest loss (see Adame et al., 2021), but do not provide comparable information in terms of mitigating current emission rates. Further, global estimates often do not accurately quantify within-country variability, relying, in many cases, on averaged reference values or model-based generalizations to extrapolate predictions to data-poor or data-absent nations when harnessing national datasets would be more appropriate to inform country-specific conservation targets (Worthington et al., 2020a).
Brazil is home to the second largest mangrove area in the world, with forests distributed across diverse coastal morphology and climate gradients (Hamilton and Casey, 2016; Worthington et al., 2020b). Despite accounting for over 9% of the world’s mangroves, Brazil still lacks an integrated inventory of carbon stocks and carbon sequestration rates that capture the diversity of coastline types and climatic zones in which mangroves are present. To fill this gap, we performed a comprehensive review of published global datasets to derive within-country estimates of carbon stocks and sequestration rates in mangrove soils and biomass that represent both geographic gradients and administrative divisions in Brazil. In addition to delivering state-level estimates, we provide a direct comparison between mangroves and Brazil’s other major vegetated biomes, identifying mangroves as a major carbon hotspot that can help meet intended Nationally Determined Contributions (NDC’s), in addition to their significance as global coastal carbon sinks.
Global mangrove aboveground biomass (AGB) and soil organic carbon stock (SOC) values were retrieved from various independent datasets that have explicitly mapped the spatial distribution parameters’ (Table 1). These global datasets were subsetted for Brazilian mangroves, and median statistics were computed from grided or vectorized datasets where available or directly from the original references. Where possible, uncertainties were assessed on the basis of bootstrapped 95% confidence intervals for medians using the bias corrected and accelerated (BCa) method (Carpenter and Bithell, 2000; Mangiafico, 2021).
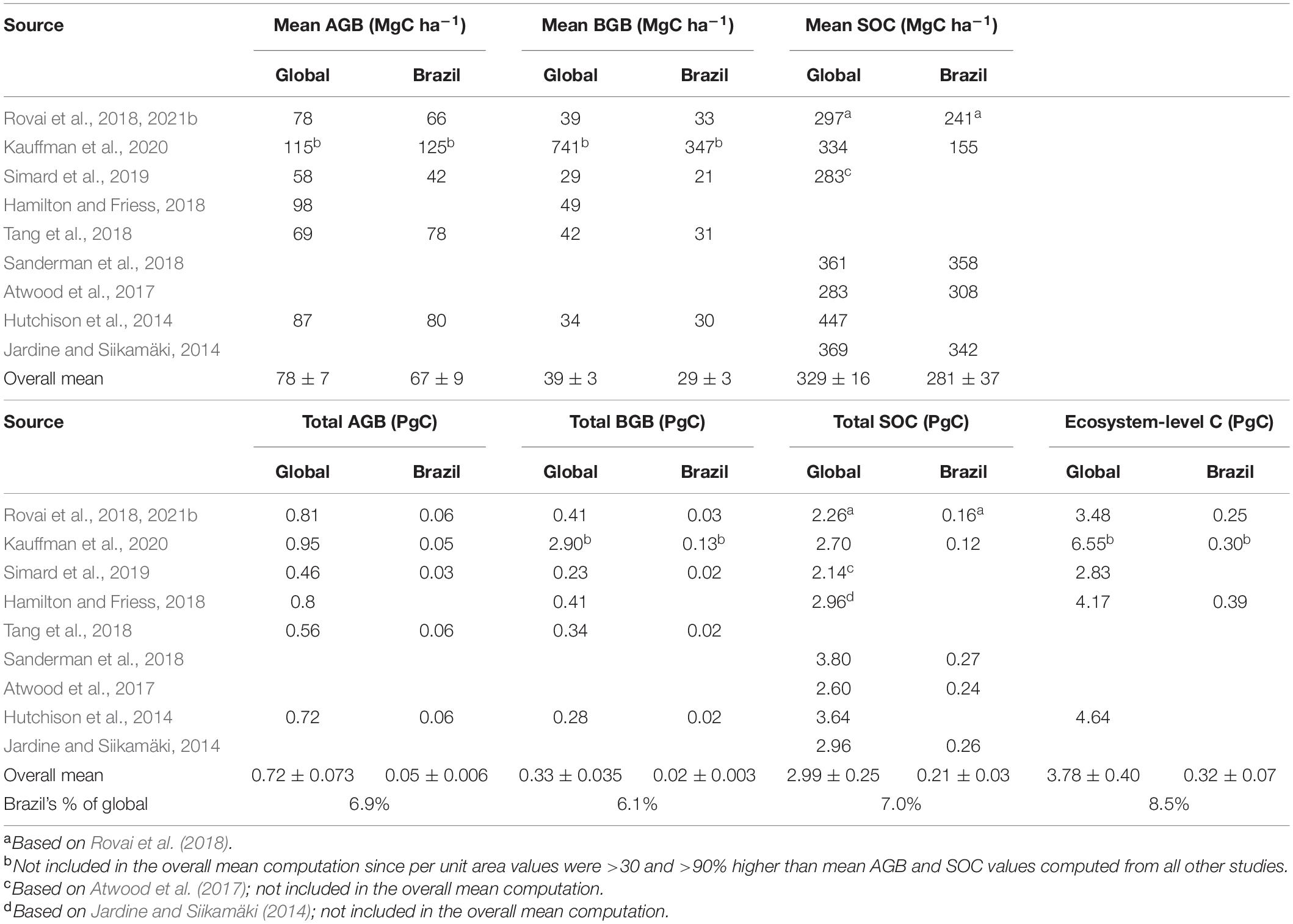
Table 1. Published above- and belowground biomass (AGB and BGB), and soil organic carbon (SOC) stock estimates for global and Brazilian mangroves.
As noted elsewhere (Bukoski et al., 2020), due to the scarcity of field observations there are no regional or global mangrove belowground biomass (BGB) maps. Thus, to be consistent with previous studies, we used a BGB:AGB ratio of 0.5 to estimate BGB across the world’s mangroves (IPCC, 2014; Hamilton and Friess, 2018; Rovai et al., 2021b). Further, biomass (both AGB and BGB) was converted to carbon units using a conversion factor of 0.475 (Hamilton and Friess, 2018).
To warrant direct comparison among independent sources, we standardized per-area (MgC ha–1) and total (TgC or PgC) carbon stock estimates across AGB and SOC datasets using a conservative mangrove extent of 82,849 and 7,675 km2 for the world’s and Brazilian mangroves, respectively (Table 1; after Hamilton and Casey, 2016 but see Hamilton et al., 2018; Worthington et al., 2020a for comprehensive discussions on existing mangrove extent databases).
Biomass (AGB and BGB) and SOC (top 1 meter) stock estimates for Brazilian mangroves used throughout this study were computed from Rovai et al. (2018, 2021b) respectively, given the comparatively larger number of observations (>900 forest plots for AGB and >65 sites for SOC stocks distributed only within Brazil’s mangroves; Supplementary Table 1) used in these studies. It is noteworthy that mean AGB and SOC estimates for global and Brazilian mangroves are consistent to mean values computed among previous studies (Table 1). Biomass (AGB and BGB) and SOC (top 1 meter) density in other Brazilian vegetated biomes (Amazon forest, Atlantic forest, Pampa grasslands, Cerrado savannas, Pantanal wetlands, and Caatinga forests) were extracted from harmonized biomass (Spawn et al., 2020) and soil (Hiederer and Köchy, 2011) databases. Due to some overlap between spatial datasets, cells containing mangroves were excluded when computing biomass and SOC density estimates for other Brazilian vegetated biomes. Global datasets were clipped to Brazil’s territory, split by state-level administrative divisions and classified into vegetated biomes according to the Brazilian Geography and Statistics Institute databases (IBGE, 2019).
Carbon sequestration in mangrove woody biomass and soils were estimated based on a comprehensive literature review performed online on Google Scholar, Science Direct, Web of Science, and the Brazilian SciELO databases. For carbon sequestration in woody biomass, we performed searches using the following expressions: “carbon sequestration,” “carbon accumulation,” “wood production,” “biomass production,” “stem growth,” “basal area increment,” and “DBH increment” always in combination with the terms “mangrove” and “Brazil.” Altogether the searches returned a total of 1,000 articles (Google Scholar = 815, Science Direct = 51, and Web of Science = 134). For carbon sequestration in mangrove soils, we used the expressions “carbon sequestration,” “carbon accumulation,” “carbon burial,” and “carbon accretion” again always in combination with the terms “mangrove” and “Brazil.” Initial searches returned a total of 3,725 articles (Google Scholar = 3,240, Science Direct = 404, and Web of Science = 81). Searches performed at the Brazilian SciELO database included generic Portuguese terms “carbono” (for carbon) and “mangue*” (for mangrove or mangal), which returned a total of 19 studies. Only studies conducted in Brazilian mangroves that presented data on carbon sequestration in either woody AGB (N = 2) or soils (N = 7) were included in our analyses. Carbon sequestration rates in mangrove woody biomass and soils were classified into one of four coastal geomorphic types along Brazil’s shoreline: deltas, estuaries, lagoons or open coasts (after Worthington et al., 2020b). Differences among those coastal typologies were assessed using analysis of variance for unbalanced designs (ANOVA function from R “car” package; Fox and Weisberg, 2019).
Carbon dioxide equivalents (CO2eq) for both carbon stock and carbon sequestration rate values were estimated using a CO2:C stoichiometric ratio of 3.67 (i.e., CO2/C = 44/12 = 3.67), which is used as a multiplying factor to convert carbon atoms to CO2 molecules. Potential CO2eq emissions were computed on a “stock-difference” basis (sensu Kauffman et al., 2017) using published mangrove biomass and soil carbon stock estimates (based on Rovai et al., 2018, 2021b as detailed above) and carbon sequestration rates (from the literature review). Further, we coupled degradation-specific carbon emission factors (after Sasmito et al., 2019: Erosion AGB = 1, SOC = 1; Clearing AGB = 0.7, SOC = 0.21; Settlement AGB = 1, SOC = 0.66; Extreme weather AGB = 0.31, SOC = 0.14; Agriculture and aquaculture AGB = 0.83, SOC = 0.52) with a high-resolution map of drivers of mangrove forest loss (covering the period 2000–2016; after Goldberg et al., 2020) to determine the dominant historical cause of mangrove degradation for each Brazilian state. While some mangrove loss drivers may change over time, dominant degradation causes, particularly those driven by climate (e.g., erosion caused by sea level rise and extreme weather events, which affects 85% of the country’s mangrove coverage; Goldberg et al., 2020), are likely to remain as a result of global climate change. Likewise, agriculture or aquaculture and clearing may be harder to reduce in Brazil in the years to come due to increasing relaxation of environmental regulations. Once determined, dominant state-level emission factors were multiplied by carbon stocks in AGB and in soils (top 1 meter) separately and then summed to compute ecosystem-level potential CO2eq emissions for each mangrove cell in the gridded dataset (that is, AGB and SOC density estimates combined from Rovai et al., 2018, 2021b).
All raster and vector manipulations and geospatial analyses were performed using R (R Core Team, 2020) packages ‘geobr’ (Pereira and Goncalves, 2021), ‘raster’ (Hijmans, 2020), and ‘rgdal’ (Bivand et al., 2020).
Based on recent global estimates (Table 1), Brazil holds on average 8.5% (or 0.32 PgC) of the world’s mangrove organic carbon stocks, partitioned among AGB (0.05 PgC or 6.9% of global stocks), BGB (0.02 PgC or 6.1% of global stocks) and soils (0.21 PgC or 7.0% of global stocks). On a per-area basis, Brazilian mangroves store on average 66, 33, and 241 MgC ha–1 in AGB, BGB and soils, respectively (from Rovai et al., 2018, 2021b for AGB and BGB, and SOC, respectively). Standardized to the same mangrove forest coverage, these values are comparable to and often more conservative than other studies’ estimates. However, our ecosystem-level carbon stock estimate for Brazilian mangroves is 36% lower than that reported in Hamilton and Friess (2018) due to overestimated SOC density estimates for Brazil (from Jardine and Siikamäki, 2014) used in that study.
Over 80% of all mangrove carbon stocks in Brazil are found in the states of Maranhão (91.3 TgC), Pará (61.2 TgC) and Amapá (47.3 TgC), reflecting extensive coverage which amounts to more than 80% of the country’s total mangrove area (Table 2).
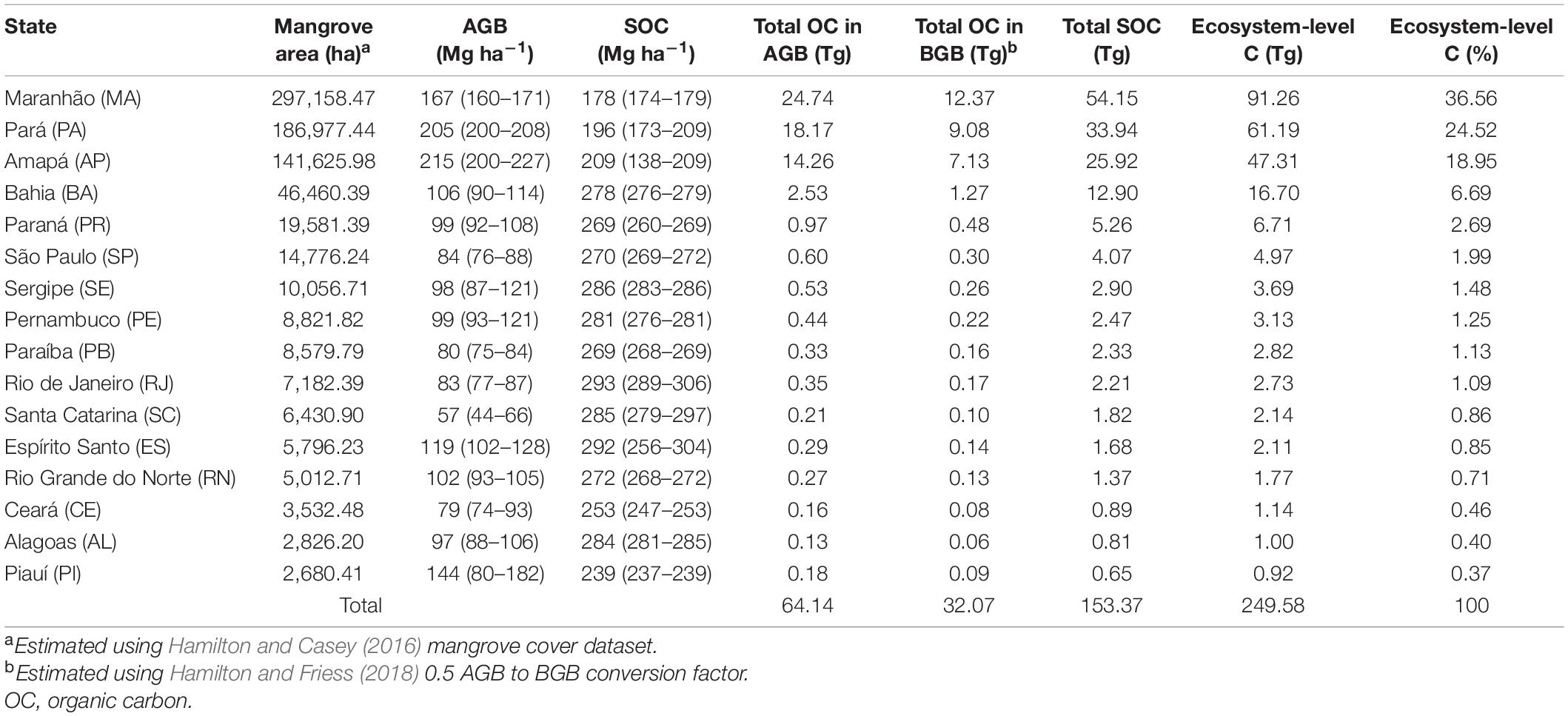
Table 2. Median (95% Confidence Intervals) and total values for above- and belowground biomass (AGB and BGB) and, soil organic carbon (SOC) stock estimates for Brazilian states.
Largest per-area AGB values are also found in these three states (215.5, 205.3, and 166.7 Mg ha–1 in Amapá, Pará and Maranhão, respectively) as well as in Piauí (143.4 Mg ha–1), where mangroves develop in nutrient-rich deltaic systems. In contrast, lowest per-area AGB was found in Santa Catarina (56.8 Mg ha–1), near the austral distribution limit for mangrove forests in the Southwestern Atlantic (Schaeffer-Novelli et al., 1990; Soares et al., 2012). AGB was also lower in São Paulo (84.2 Mg ha–1) and Rio de Janeiro (83.1 Mg ha–1), where extensive mangrove areas have been impacted by industrial activities and urban expansion (Soares, 1999; Ferreira and Lacerda, 2016; Moschetto et al., 2021). AGB values <100 Mg ha–1 were also found in Paraíba, Sergipe, Pernambuco, Ceará and Alagoas where shrimp farming has compromised the structural and functional integrity of Brazil’s drier-climate mangrove forests (Lacerda et al., 2021). AGB values >100 Mg ha–1 were found in Espírito Santo and Bahia mangroves where the multidecadal stability of more than 70% of the mangrove coverage suggests that the integrity of core areas have been maintained over time (Diniz et al., 2019). Predicted median AGB for Rio Grande do Norte mangroves was also >100 Mg ha–1 despite mangroves developing in a semi-arid climate and historical damage from shrimp farming (Lacerda et al., 2021). However, this result is likely due to the small number of observations used to constrain biomass predictions for that region (only two AGB values available for Rio Grande do Norte at the time Rovai et al., 2021b study was conducted; Supplementary Table 1). Regarding SOC stocks, deltaic mangroves in Piauí, Amapá, Pará and Maranhão states had lower soil carbon density due to higher inorganic-to-organic ratio per soil volume characteristic of coastal deltaic floodplains when compared to predominantly estuarine or lagoonal mangroves (Rovai et al., 2018; Sanderman et al., 2018; Jennerjahn, 2020; MacKenzie et al., 2020) found in other Brazilian states (Table 2). When summed, carbon stocks in biomass (AGB+BGB) and soils across Brazilian mangroves averaged 341 MgC ha–1 (range: 297–397 MgC ha–1), showing little variation among states (e.g., maximum difference of 23% or ∼80 MgC ha–1) (Table 2). This relatively small variability in per-unit area carbon stocks reflect mangrove plants’ resource partitioning strategies in response to broad geographical gradients (Rovai et al., 2021b), chiefly the role of coastal geomorphology in controlling the ratio between inorganic and organic matter in mangrove soils (Twilley et al., 2018; Jennerjahn, 2020).
Comparatively, on a per-area basis mangroves store between 2.2 and 4.3 times more carbon in the top meter of soil relative to other Brazilian vegetated biomes (Figure 1). Regarding mean carbon stocks in biomass (AGB and BGB combined), mangroves are second only to the Amazon forest, and 2.7–4.7 times higher than other Brazilian vegetation formations.
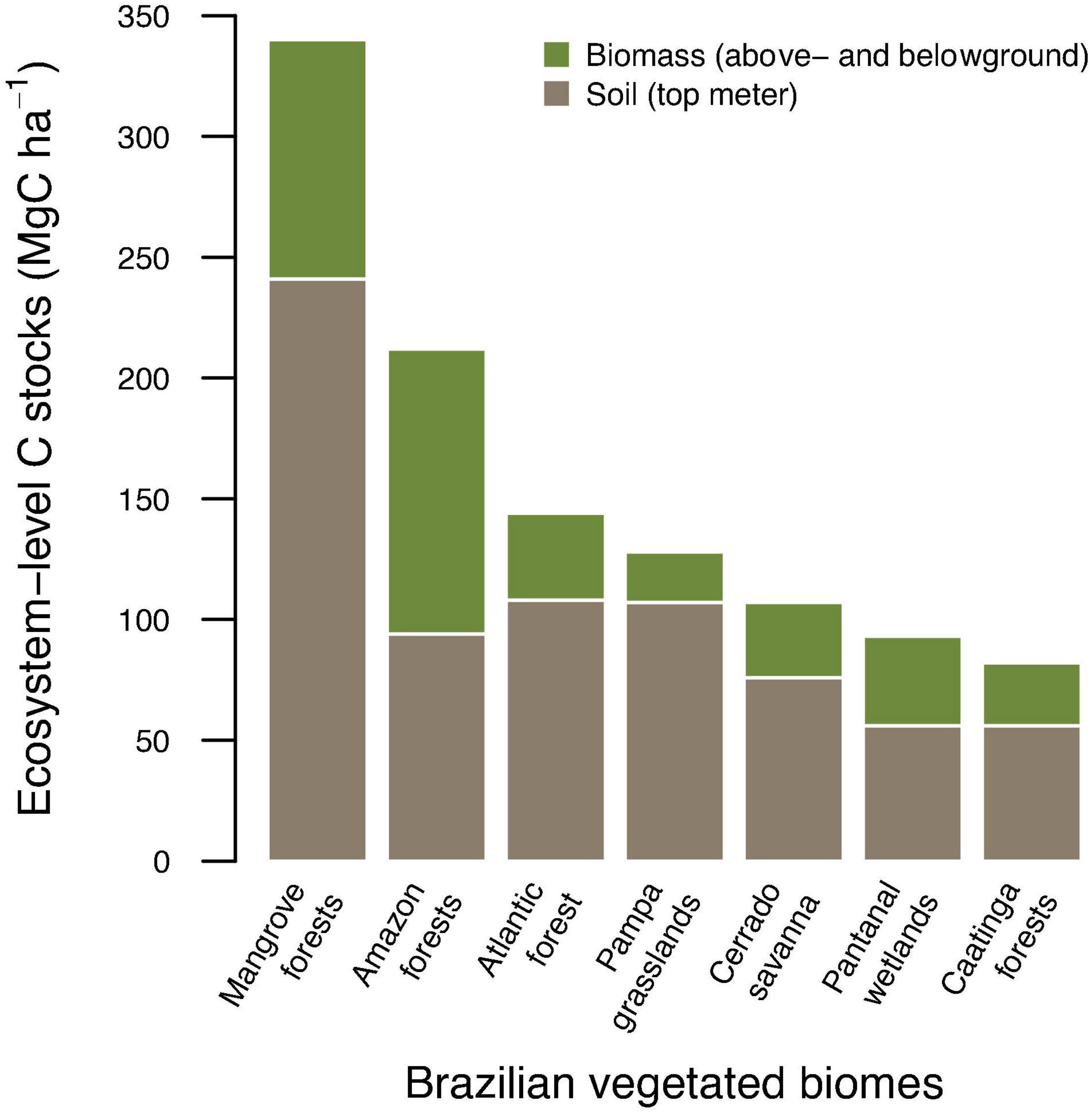
Figure 1. Comparison of ecosystem-level carbon stocks (above-, belowground biomass and soil organic carbon in the top 1 meter combined) among major Brazilian vegetated biomes.
Currently, only two studies in Brazil report on carbon sequestration in mangrove woody AGB (Table 3). From these studies, carbon sequestration in Brazilian mangroves’ woody AGB was estimated at 3.18 MgC ha–1 yr–1, consistent with values reported for a diversity of coastal typologies worldwide (Table 3). Thus, we used this reference value to produce a first order country-level estimate of annual carbon sequestration in Brazilian mangrove AGB, which totals 2.44 TgC yr–1, equivalent to 10% of all carbon sequestered in mangroves AGB globally.
Long-term carbon sequestration rates (mostly 210Pb-dated cores) in Brazilian mangrove soils was estimated at 2.81 MgC ha–1 yr–1 (Table 4). While there were no differences (P > 0.05, results not shown) across the coastal geomorphic types found along Brazil’s shoreline, this value is 15–30% higher than recent global estimates (e.g,. 1.94–2.39 MgC ha–1 yr–1; Jennerjahn, 2020; MacKenzie et al., 2020; Wang et al., 2020), likely due to the predominance of minerogenic coastlines (deltaic, which accounts for >80% of the country’s mangrove area, and meso- and macrotidal estuarine systems) where deposition of both autochthonous (mangrove detritus) and allochthonous (terrestrial and marine detritus) sediments are amplified (Adame et al., 2010; Kusumaningtyas et al., 2019; Cragg et al., 2020). Importantly, when this national median value is multiplied by the country’s mangrove area coverage, annual carbon sequestration in Brazilian mangroves soils was estimated at 2.14 TgC yr–1, corresponding to about 13.5% of the total amount of carbon buried annually in the world’s mangroves.
Highest potential CO2eq emissions (>900 MgCO2eq ha–1) resulting from loss of existing mangrove forests were estimated for Rio de Janeiro, Alagoas, Piauí, Pará, Amapá, and Maranhão states driven by the dominance of erosion (Figure 2 and Table 5) where eventually all carbon stored in soils (here based on top 1 meter) and in AGB is lost to the atmosphere. It should be noted, however, that while eroded SOC is rapidly mineralized in aerobic estuarine waters (Sapkota and White, 2021), carbon release back to the atmosphere from biomass loss is not immediate given slow decomposition rates of downed wood in mangrove forests (Romero et al., 2005). Notably, when considering only the top 1 meter of soil to compute such estimates, these values are amongst the highest CO2eq emissions reported in the literature for other mangrove sites worldwide (Kauffman et al., 2017; Alongi, 2020; Adame et al., 2021). Further agriculture/aquaculture- and settlement-based losses (emission factors of 0.83 and 1.00 for AGB and 0.52 and 0.66 for SOC, respectively) were also anticipated to cause high potential CO2eq emissions (>500 MgCO2eq ha–1) in Espírito Santo, Pernambuco, Rio Grande do Norte, São Paulo, and Santa Catarina states as these activities represent a considerable loss of both aboveground and soil compartments (Figure 2). Lowest potential CO2eq emissions were linked to episodic extreme weather events that have the potential to release smaller fractions on carbon stored in AGB (31%) and soils (14%) followed by clearing, which can remove substantial carbon stocks in aboveground (70%) and soil (21%) compartments. These estimates are conservative considering only carbon stored in AGB and soils (but not in BGB, since emission factors for this plant compartment have not been established yet) were used to compute potential CO2eq emissions resulting from mangrove forest loss.
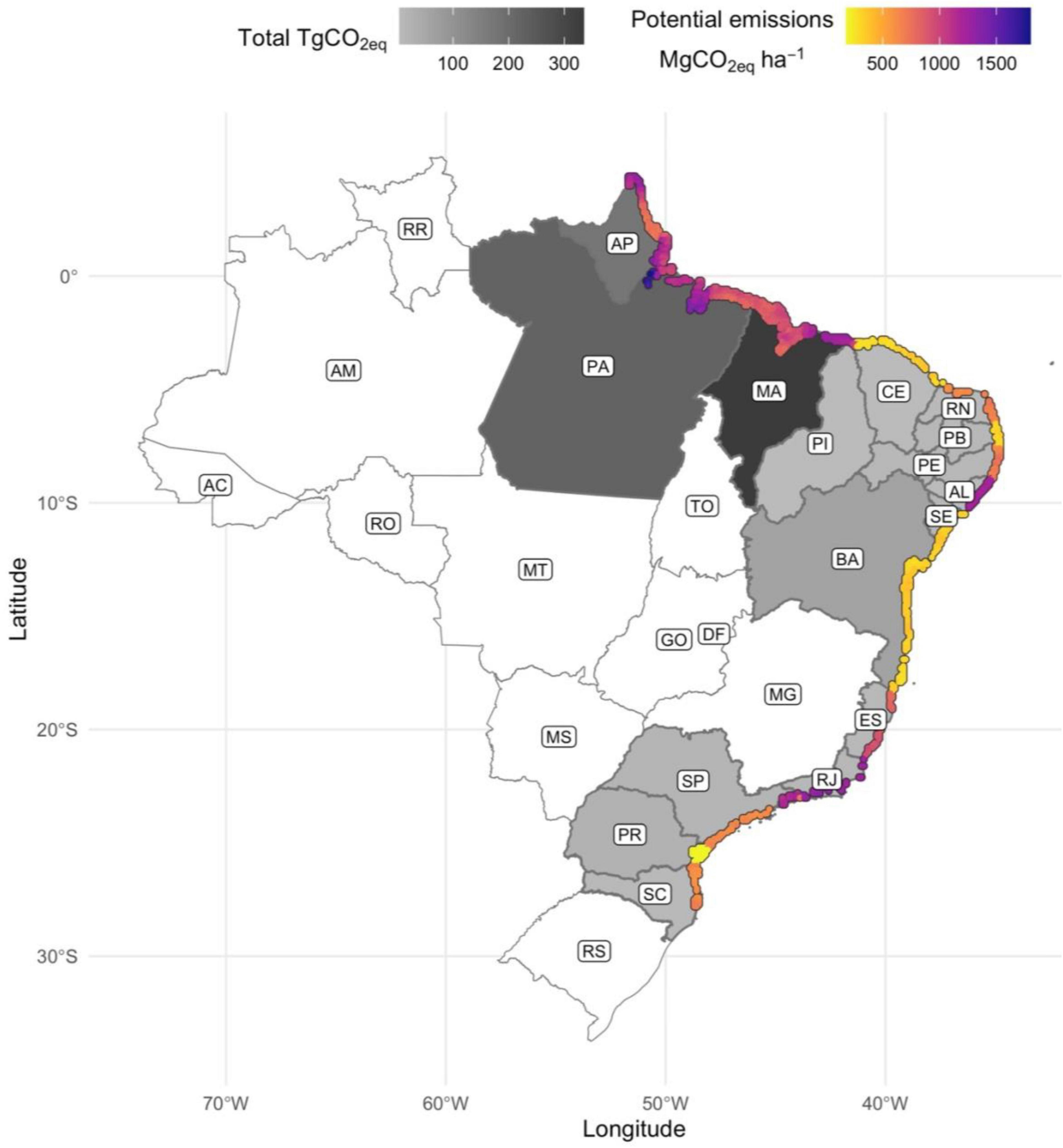
Figure 2. Total CO2eq stored in biomass and soils (grayscale top left legend) and variability in potential CO2eq emissions (colored scale top right legend) across Brazilian mangroves. Mangrove coverage exaggerated to improve visualization. Estimates per state are given on Table 5. Mangrove states: AP, Amapá; PA, Pará; MA, Maranhão; PI, Piauí; CE, Ceará; RN, Rio Grande do Norte; PB, Paraíba; PE, Pernambuco; AL, Alagoas; SE, Sergipe; BA, Bahia; ES, Espírito Santo; RJ, Rio de Janeiro; SP, São Paulo; PR, Paraná; and SC, Santa Catarina. Non-mangrove states: RR, Roraima; AM, Amazonas; AC, Acre; RO, Rondônia; MT, Mato Grosso; TO, Tocantins; GO, Goiás; DF, Distrito Federal; MS, Mato Grosso do Sul; MG, Minas Gerais; RS, Rio Grande do Sul.
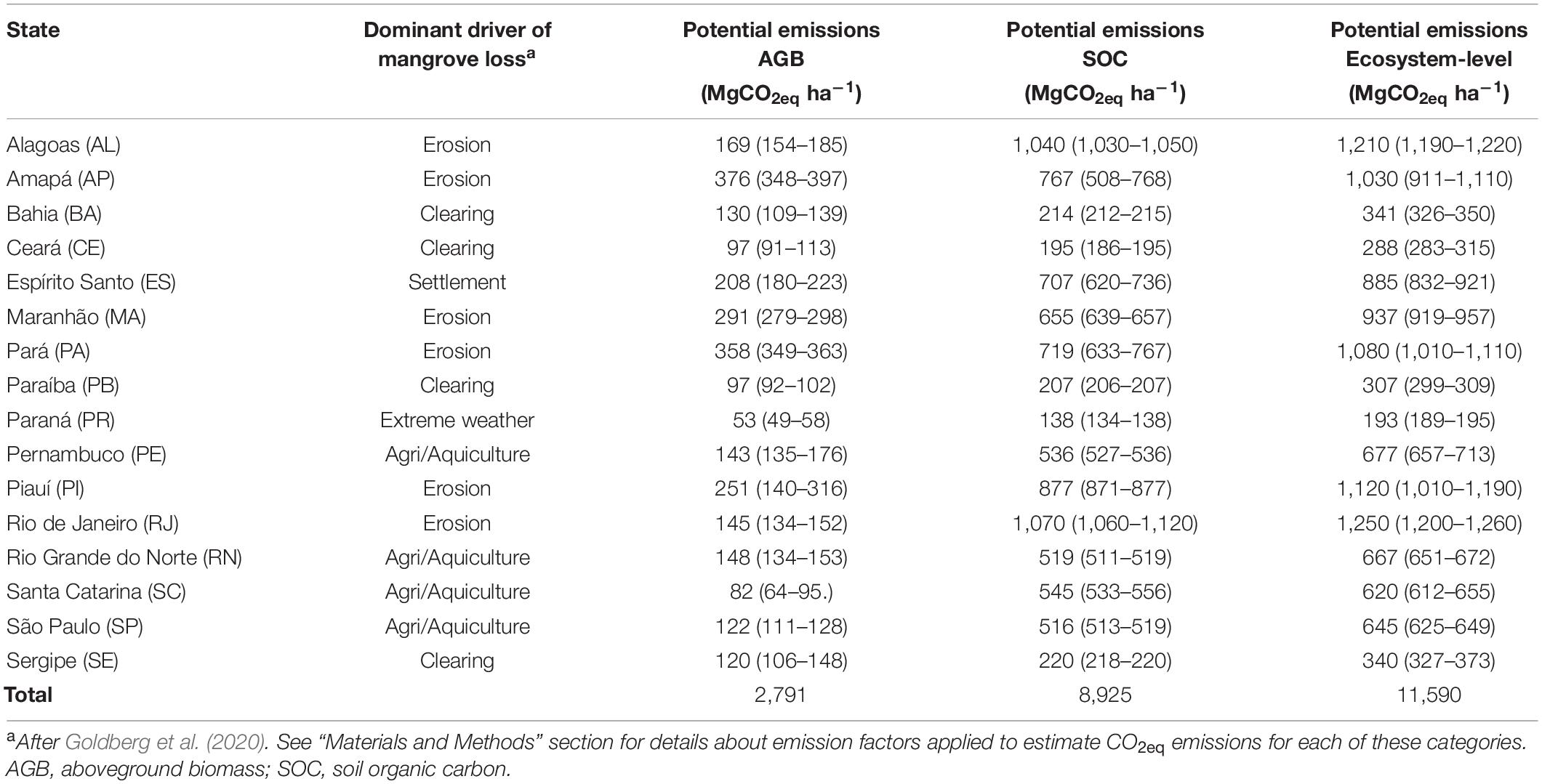
Table 5. Median values (95% Confidence Intervals) for potential CO2eq emissions resulting from mangrove forest loss across Brazil.
The loss of carbon sequestration potential after mangrove forests are degraded was assumed here to be 100% considering that soil and vegetation loss represent either acute stressors, which ceases mangrove production altogether, or chronic stressors that leave the system more susceptible to eventually collapse (Lugo et al., 1981; Lewis et al., 2016; Krauss et al., 2018). Further, there are currently no consistent degradation-specific emission factors available to estimate loss of carbon sequestration potential as there is for change in carbon stocks resulting from distinct mangrove deforestation causes (e.g., Sasmito et al., 2019).
Based on the current reference value of 2.81 MgC ha–1 yr–1 (Table 4), we estimated an annual loss of 10.31 MgCO2 ha–1 yr–1 that would otherwise be buried in mangrove soils. Combined with loss of carbon sequestration potential in woody biomass, based on the current reference value of 3.18 MgC ha–1 yr–1 (Table 3), foregone carbon sequestration in Brazilian mangroves annually could total 22 MgCO2 ha–1 yr–1, in line with estimates reported for other mangroves worldwide (23-254 MgCO2 ha–1 yr–1as reviewed in Alongi, 2014).
Here we deliver the first integrated assessment of mangrove carbon stocks, carbon sequestration rates and potential CO2eq emissions for each Brazilian state. While more data are needed (e.g., particularly on carbon sequestration and emission factors) to better quantify national level statistics, this study provides compelling information to both aid the inclusion of mangroves in national (or state-level) carbon credit markets and establish Brazilian mangroves as hotspots within the context of global blue carbon policies. Our estimates suggest that Brazilian mangroves can potentially release substantial amounts of carbon following mangrove forest loss, with CO2eq emissions nearing those estimated for other carbon-rich mangrove forests. In addition, loss of carbon sequestration potential in both woody biomass and soils following deforestation amplifies cumulative emissions annually, shortening the country’s capacity to mitigate its fossil fuel emissions and meet intended NDC’s.
In summary, we showed that Brazil is home of 9.3% of the world’s mangroves, commensurably holding 8.5% of the global mangrove carbon stocks (biomass and soils combined). When compared to other Brazilian vegetated biomes, on a per-area basis mangroves store between 2.2 and 4.3 times more carbon in the top meter of soil. While for carbon stocks in biomass, Brazilian mangroves are second only to the Amazon forest, and store between 2.7 and 4.7 times more carbon than other vegetated biomes. Moreover, on a per-area basis organic carbon sequestration rates in Brazilian mangroves are 15–30% higher than recent global estimates. Importantly, integrated over the country’s area, carbon sequestration in Brazilian mangroves soils account for 13.6% of the carbon buried in world’s mangroves annually. Likewise, carbon sequestration in Brazilian mangroves woody biomass is also higher than global estimates, accounting for nearly 10% of carbon accumulation in mangrove woody biomass globally.
This study also highlights important research gaps and uncertainties in Brazilian mangroves carbon inventories. For example, the greatest carbon sink capacity in mangroves lies in the soils since this ecosystem compartment continuously fixes and preserves layers of millennia-old atmospheric carbon beneath the surface. However, we still know very little about the carbon sequestration potential of Brazilian mangroves soils, particularly the contribution of the Amazon Macrotidal Mangrove Coast (AMMC) to global carbon budgets. To date, we have found only one study reporting on soil carbon sequestration rates in this region (Table 4). Overall, several of Brazil’s northern and northeastern states, where >80% of the country’s mangroves are present, lack data; seven and nine states out of the 16 mangrove states in Brazil still lack data on soil organic carbon stocks and sequestration rates, respectively (Supplementary Table 1). It should also be noted that, while this study focused on carbon stocks and long-term carbon sequestration rates in biomass and soils, real time air-sea CO2 fluxes, and DOC (dissolved organic carbon), DIC (dissolved inorganic carbon), and alkalinity (bicarbonate) export are important mechanisms of the carbon cycling in mangroves (e.g., Sippo et al., 2016; Carvalho et al., 2017; Cotovicz et al., 2019; Cabral et al., 2021) and should be taken into account to better constrain carbon budgets for Brazilian mangroves.
Mangrove AGB density has been consistently mapped across Brazilian mangroves, but disparities exist. For instance, no data was apparent for Alagoas’ mangroves and only a few plots have been implemented in Amapá (2 plots), Piauí (2 plots), Rio Grande do Norte (2 plots), and Paraíba (6 plots) states (Supplementary Table 1). While for carbon sequestration in woody biomass, currently only two states (Rio de Janeiro and São Paulo) are represented (Table 3). The situation is far more critical for BGB density and productivity estimates. In this study we used a 0.5 BGB:AGB ratio to estimate BGB across Brazilian mangroves; however, to our knowledge, there are only two studies that have comprehensively (using trenching vs. coring techniques; see Adame et al., 2017 for a comprehensive discussion) assessed actual BGB distribution in Brazilian mangroves (Santos et al., 2017 in Rio de Janeiro and Virgulino-Júnior et al., 2020 in Pará). Moreover, BGB productivity and root necromass, which are important contributors to refractory carbon stored in mangroves soils (Kihara et al., 2021), remain unknown for Brazilian mangroves.
It is imperative that future research efforts and funding opportunities focus on addressing these data coverage issues. This is particularly pertinent for the data-poor northern states, where the AMMC is located, as carbon fluxes are more intense due to the synergistic contribution or riverine and tidal forcings that dictate coastal and ecological processes (e.g., deposition, erosion, mineralization, export). We recommend future carbon inventories in Brazilian mangroves to look beyond carbon stocks in biomass and soils and prioritize carbon fluxes via biomass (e.g., woody biomass growth) and soils (long-term carbon sequestration) as well as export of other carbon forms (e.g., DOC, DIC, alkalinity), which provide a direct comparison to greenhouse gases emission rates. Overall, this study consolidates the scientific basis demonstrating the significance of Brazilian mangroves to achieve NDC’s both by enforcing environmental regulations to protect the country’s existing mangroves and by promoting mangrove restoration where feasible to increase carbon crediting potential.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AR conceived the study, collated and analyzed data, and wrote the draft. RT, TW, and PR analyzed data and wrote the draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SC declared a past collaboration with one of the authors TW to the handling editor.
The reviewer JB declared a past co-authorship with the authors to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AR is thankful to the Brazilian foundations CAPES and CNPq, and their Science Without Borders program for having funded the Ph.D. research (BEX1930/13-3) in which much of the data used in this study has originated. We are grateful to Jacob Bukoski for providing insightful comments that have much improved the quality of our original draft.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.787533/full#supplementary-material
Adame, M. F., Cherian, S., Reef, R., and Stewart-Koster, B. (2017). Mangrove root biomass and the uncertainty of belowground carbon estimations. For. Ecol. Manage. 403, 52–60. doi: 10.1016/j.foreco.2017.08.016
Adame, M. F., Connolly, R. M., Turschwell, M. P., Lovelock, C. E., Fatoyinbo, T., Lagomasino, D., et al. (2021). Future carbon emissions from global mangrove forest loss. Glob. Chang. Biol. 27, 2856–2866. doi: 10.1111/gcb.15571
Adame, M. F., Neil, D., Wright, S. F., and Lovelock, C. E. (2010). Sedimentation within and among mangrove forests along a gradient of geomorphological settings. Estuar. Coast. Shelf Sci. 86, 21–30. doi: 10.1016/j.ecss.2009.10.013
Alongi, D. M. (2014). Carbon cycling and storage in mangrove forests. Ann. Rev. Mar. Sci. 6, 195–219. doi: 10.1146/annurev-marine-010213-135020
Alongi, D. M. (2020). Global significance of mangrove blue carbon in climate change mitigation. Science 2:67. doi: 10.3390/sci2030067
Atwood, T. B., Connolly, R. M., Almahasheer, H., Carnell, P. E., Duarte, C. M., Ewers Lewis, C. J., et al. (2017). Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 7, 523–528. doi: 10.1038/nclimate3326
Bivand, R., Keitt, T., and Rowlingson, B. (2020). R Package Rgdal: bindings For The Geospatial Data Abstraction Library. 65. Available online at: https://cran.r-project.org/web/packages/rgdal/rgdal.pdf (accessed November 30, 2021).
Bukoski, J. J., Elwin, A., MacKenzie, R. A., Sharma, S., Purbopuspito, J., Kopania, B., et al. (2020). The role of predictive model data in designing mangrove forest carbon programs. Environ. Res. Lett. 15, 084019. doi: 10.1088/1748-9326/ab7e4e
Cabral, A., Dittmar, T., Call, M., Scholten, J., de Rezende, C. E., Asp, N., et al. (2021). Carbon and alkalinity outwelling across the groundwater-creek-shelf continuum off Amazonian mangroves. Limnol. Oceanogr. Lett. 6, 369–378. doi: 10.1002/lol2.10210
Carpenter, J., and Bithell, J. (2000). Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat. Med. 19, 1141–1164. doi: 10.1002/(SICI)1097-0258(20000515)19:9<1141::AID-SIM479>3.0.CO;2-F
Carvalho, A. C. O., Marins, R. V., Dias, F. J. S., Rezende, C. E., Lefèvre, N., Cavalcante, M. S., et al. (2017). Air-sea CO2 fluxes for the Brazilian northeast continental shelf in a climatic transition region. J. Mar. Syst. 173, 70–80. doi: 10.1016/j.jmarsys.2017.04.009
Cotovicz, L. C., Knoppers, B. A., Deirmendjian, L., and Abril, G. (2019). Sources and sinks of dissolved inorganic carbon in an urban tropical coastal bay revealed by δ 13 C-DIC signals. Estuar. Coast. Shelf Sci. 220, 185–195. doi: 10.1016/j.ecss.2019.02.048
Cragg, S. M., Friess, D. A., Gillis, L. G., Trevathan-Tackett, S. M., Terrett, O. M., Watts, J. E. M., et al. (2020). Vascular plants are globally significant contributors to marine carbon fluxes and sinks. Ann. Rev. Mar. Sci. 12, 16.1–16.29. doi: 10.1146/annurev-marine-010318-095333
Diniz, C., Cortinhas, L., Nerino, G., Rodrigues, J., Sadeck, L., Adami, M., et al. (2019). Brazilian mangrove status: Three decades of satellite data analysis. Remote Sens. 11:808. doi: 10.3390/rs11070808
Estrada, G. C. D., Soares, M. L. G., Fernadez, V., and de Almeida, P. M. M. (2015). The economic evaluation of carbon storage and sequestration as ecosystem services of mangroves: A case study from southeastern Brazil. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 11, 29–35. doi: 10.1080/21513732.2014.963676
Ferreira, A. C., and Lacerda, L. D. (2016). Degradation and conservation of Brazilian mangroves, status and perspectives. Ocean Coast. Manag. 125, 38–46. doi: 10.1016/j.ocecoaman.2016.03.011
Fox, J., and Weisberg, S. (2019). An {R} Companion to Applied Regression, 3rd Edn. Thousand Oaks, CA: Sage.
Gabler, C. A., Osland, M. J., Grace, J. B., Stagg, C. L., Day, R. H., Hartley, S. B., et al. (2017). Macroclimatic change expected to transform coastal wetland ecosystems this century. Nat. Clim. Chang 7, 142–147. doi: 10.1038/NCLIMATE3203
Goldberg, L., Lagomasino, D., Thomas, N., and Fatoyinbo, T. (2020). Global declines in human-driven mangrove loss. Glob. Chang. Biol. 26, 5844–5855. doi: 10.1111/gcb.15275
Hamilton, S. E., and Casey, D. (2016). Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 25, 729–738. doi: 10.1111/geb.12449
Hamilton, S. E., and Friess, D. A. (2018). Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat. Clim. Chang. 8, 240–244. doi: 10.1038/s41558-018-0090-4
Hamilton, S. E., Castellanos-Galindo, G. A., Millones-Mayer, M., and Chen, M. (2018). “Remote sensing of mangrove forests: Current techniques and existing databases,” in Threats to Mangrove Forests, eds C. Makowski and C. W. Finkl (Cham: Springer), 497–520. doi: 10.1007/978-3-319-73016-5_22
Hatje, V., Masqué, P., Patire, V. F., Dórea, A., and Barros, F. (2021). Blue carbon stocks, accumulation rates, and associated spatial variability in Brazilian mangroves. Limnol. Oceanogr. 66, 321–334. doi: 10.1002/lno.11607
Hiederer, R., and Köchy, M. (2011). Global soil organic carbon estimates and the Harmonized World 20 Soil Database, JRC Scientific and Technical Reports, 68528/EUR 25225 EN. Ispra: Joint ResearchCentre.
Hijmans, R. J. (2020). raster: Geographic Data Analysis and Modeling. R package. 249. Available online at: https://cran.r-project.org/package=raster (accessed November 30, 2021).
Holling, C. S. (1973). Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. doi: 10.1146/annurev.es.04.110173.000245
Holmquist, J. R., Windham-Myers, L., Bliss, N., Crooks, S., Morris, J. T., Megonigal, J. P., et al. (2018). Accuracy and precision of tidal wetland soil carbon mapping in the conterminous United States. Sci. Rep. 8:9478. doi: 10.1038/s41598-018-26948-7
Hutchison, J., Manica, A., Swetnam, R., Balmford, A., and Spalding, M. (2014). Predicting Global Patterns in Mangrove Forest Biomass. Conserv. Lett. 7, 233–240. doi: 10.1111/conl.12060
IBGE (2019). Biomas e Sistema Costeiro-Marinho do Brasil: Compatível com a escala 1:250.000 / IBGE, Coordenação de Recursos Naturais e Estudos Ambientais. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística IBGE.
IPCC (2014). 2013 Supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: Wetlands. Geneva: IPCC.
Jardine, S. L., and Siikamäki, J. V. (2014). A global predictive model of carbon in mangrove soils. Environ. Res. Lett. 9:104013. doi: 10.1088/1748-9326/9/10/104013
Jennerjahn, T. C. (2020). Relevance and magnitude of “Blue Carbon” storage in mangrove sediments: carbon accumulation rates vs. stocks, sources vs. sinks. Estuar. Coast. Shelf Sci. 247:107027. doi: 10.1016/j.ecss.2020.107027
Kauffman, B. J., Arifanti, V. B., Hernández Trejo, H., del Carmen, Jesús García, M., Norfolk, J., et al. (2017). The jumbo carbon footprint of a shrimp: carbon losses from mangrove deforestation. Front. Ecol. Environ. 15:183–188. doi: 10.1002/fee.1482
Kauffman, J. B., Adame, M. F., Arifanti, V. B., Schile-Beers, L. M., Bernardino, A. F., Bhomia, R. K., et al. (2020). Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 90:e01405. doi: 10.1002/ecm.1405
Kihara, Y., Dannoura, M., and Ohashi, M. (2021). Estimation of fine root production, mortality, and decomposition by using two core methods and litterbag experiments in a mangrove forest. Ecol. Res. doi: 10.1111/1440-1703.12275
Krauss, K. W., Demopoulos, A. W. J., Cormier, N., From, A. S., McClain-Counts, J. P., and Lewis, R. R. R. III (2018). Ghost forests of marco island: mangrove mortality driven by belowground soil structural shifts during tidal hydrologic alteration. Estuar. Coast. Shelf Sci. 212, 51–62. doi: 10.1016/j.ecss.2018.06.026
Kusumaningtyas, M. A., Hutahaean, A. A., Fischer, H. W., Pérez-Mayo, M., Ransby, D., and Jennerjahn, T. C. (2019). Variability in the organic carbon stocks, sources, and accumulation rates of Indonesian mangrove ecosystems. Estuar. Coast. Shelf Sci. 218, 310–323. doi: 10.1016/j.ecss.2018.12.007
Lacerda, L. D., Ward, R. D., Godoy, M. D. P., Meireles, A. J., de, A., Borges, R., et al. (2021). 20-Years cumulative impact from shrimp farming on mangroves of northeast brazil. Front. For. Glob. Change 4:653096. doi: 10.3389/ffgc.2021.653096
Lewis, R. R. R., Milbrandt, E. C. E. C., Brown, B., Krauss, K. W. K. W. K. W., Rovai, A. S. A. S., Beever, J. W. J. W., et al. (2016). Stress in mangrove forests: Early detection and preemptive rehabilitation are essential for future successful worldwide mangrove forest management. Mar. Pollut. Bull. 109, 764–771. doi: 10.1016/j.marpolbul.2016.03.006
Lugo, A. E., Cintrón-Molero, G., and Goenaga, C. (1981). “Mangrove ecosystems under stress,” in Stress Effects On Natural Systems, eds G. W. Barret and R. Rosenberg (Chichester: John Wiley and Sons), 129–153.
MacKenzie, R., Sharma, S., and Rovai, A. R. (2020). “Environmental drivers of blue carbon burial and soil carbon stocks in mangrove forests,” in Dynamic Eedimentary Environments Of Mangrove Coasts, eds F. Sidik and D. A. Friess (Amsterdam: Elsevier Inc), 275–294. doi: 10.1016/b978-0-12-816437-2.00006-9
Mangiafico, S. S. (2021). Rcompanion: Functions to Support Extension Education Program Evaluation. 2.4.1. New Brunswick, NJ: Rutgers Cooperative.
McLeod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9:552–560. doi: 10.1890/110004
Moschetto, F. A., Ribeiro, R. B., and De Freitas, D. M. (2021). Urban expansion, regeneration and socioenvironmental vulnerability in a mangrove ecosystem at the southeast coastal of São Paulo, Brazil. Ocean Coast. Manag. 200:105418.
Pereira, R. H. M., and Goncalves, C. N. (2021). geobr: Download Official Spatial Data Sets of Brazil, 38. Available online at: https://cran.r-project.org/web/packages/geobr/geobr.pdf (accessed November 30, 2021).
R Core Team (2020). R: A Language And Environment For Statistical Computing. Available online at: http://www.r-project.org/ (accessed November 30, 2021).
Rogers, K., Kelleway, J. J., Saintilan, N., Megonigal, J. P., Adams, J. B., Holmquist, J. R., et al. (2019). Wetland carbon storage controlled by millennial-scale variation in relative sea-level rise. Nature 567, 91–95. doi: 10.1038/s41586-019-0951-7
Romero, L. M., Smith, T. J., and Fourqurean, J. W. (2005). Changes in mass and nutrient content of wood during decomposition in a south Florida mangrove forest. J. Ecol. 93, 618–631. doi: 10.1111/j.1365-2745.2005.00970.x
Rovai, A. S., Twilley, R. R., Castañeda-Moya, E., Midway, S. R., Friess, D. A., Trettin, C. C., et al. (2021b). Macroecological patterns of forest structure and allometric scaling in mangrove forests. Glob. Ecol. Biogeogr. 30, 1000–1013. doi: 10.1111/geb.13268
Rovai, A. S., Coelho, C., de Almeida, R., Cunha-Lignon, M., Menghini, R. P., Twilley, R. R., et al. (2021a). Ecosystem-level carbon stocks and sequestration rates in mangroves in the Cananéia-Iguape lagoon estuarine system, southeastern Brazil. For. Ecol. Manage. 479:118553. doi: 10.1016/j.foreco.2020.118553
Rovai, A. S., Twilley, R. R., Castañeda-Moya, E., Riul, P., Cifuentes-Jara, M., Manrow-Villalobos, M., et al. (2018). Global controls on carbon storage in mangrove soils. Nat. Clim. Change 8, 534–538. doi: 10.1038/s41558-018-0162-5
Saintilan, N., Khan, N. S., Ashe, E., Kelleway, J. J., Rogers, K., Woodroffe, C. D., et al. (2020). Thresholds of mangrove survival under rapid sea level rise. Science 368, 1118–1121. doi: 10.1126/science.aba2656
Sanderman, J., Hengl, T., Fiske, G., Solvik, K., Adame, M. F., Benson, L., et al. (2018). A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 13:055002. doi: 10.1088/1748-9326/aabe1c
Sanders, C. J., Eyre, B. D., Santos, I. R., Machado, W., Luiz-silva, W., Smoak, J. M., et al. (2014). Elevated rates of organic carbon, nitrogen, and phosphorus accumulation in a highly impacted mangrove wetland. Geophys. Res. Lett. 41, 2475–2480. doi: 10.1002/2014gl059789
Sanders, C. J., Smoak, J. M., Naidu, A. S., and Patchineelam, S. R. (2008). Recent sediment accumulation in a mangrove forest and Its relevance to local sea-level rise (Ilha Grande, Brazil). J. Coast. Res. 24, 533–536. doi: 10.2112/07-0872.1
Sanders, C. J., Smoak, J. M., Naidu, A. S., Sanders, L. M., and Patchineelam, S. R. (2010b). Organic carbon burial in a mangrove forest, margin and intertidal mud flat. Estuar. Coast. Shelf Sci. 90, 168–172. doi: 10.1016/j.ecss.2010.08.013
Sanders, C. J., Smoak, J. M., Sanders, L. M., Sathy Naidu, A., and Patchineelam, S. R. (2010c). Organic carbon accumulation in Brazilian mangal sediments. J. South Am. Earth Sci. 30, 189–192. doi: 10.1016/j.jsames.2010.10.001
Sanders, C. J., Smoak, J. M., Naidu, A. S., Araripe, D. R., Sanders, L. M., and Patchineelam, S. R. (2010a). Mangrove forest sedimentation and its reference to sea level rise, Cananeia, Brazil. Environ. Earth Sci. 60, 1291–1301. doi: 10.1007/s12665-009-0269-0
Santos, D. M. C., Estrada, G. C. D., Fernandez, V., Estevam, M. R. M., Souza, B. T., and Soares, M. L. G. (2017). First assessment of carbon stock in the belowground biomass of brazilian mangroves. An. Acad. Bras. Cienc. 89, 1579–1589. doi: 10.1590/0001-3765201720160496
Sapkota, Y., and White, J. R. (2021). Long-term fate of rapidly eroding carbon stock soil profiles in coastal wetlands. Sci. Total Environ. 753:141913. doi: 10.1016/j.scitotenv.2020.141913
Sasmito, S. D., Taillardat, P., Clendenning, J. N., Cameron, C., Friess, D. A., Murdiyarso, D., et al. (2019). Effect of land-use and land-cover change on mangrove blue carbon: a systematic review. Glob. Chang. Biol. 25, 4291–4302. doi: 10.1111/gcb.14774
Schaeffer-Novelli, Y., Cintrón-Molero, G., Adaime, R. R., and Camargo, T. M. (1990). Variability of mangrove ecosystems along the Brazilian coast. Estuaries 13, 204–218. doi: 10.2307/1351590
Serrano, O., Lovelock, C. E., Atwood, T. B., Macreadie, P. I., Canto, R., Phinn, S., et al. (2019). Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 10:4313. doi: 10.1038/s41467-019-12176-8
Simard, M., Fatoyinbo, L., Smetanka, C., Rivera-Monroy, V. H., Castañeda-Moya, E., Thomas, N., et al. (2019). Mangrove canopy height globally related to precipitation, temperature and cyclone frequency. Nat. Geosci. 12, 40–45. doi: 10.1038/s41561-018-0279-1
Sippo, J. Z., Maher, D. T., Tait, D. R., Holloway, C., and Santos, I. R. (2016). Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export. Glob. Biogeochem. Cycles 30, 753–766. doi: 10.1111/1462-2920.13280
Soares, M. L. G. (1999). Estrutura vegetal e grau de perturbação dos manguezais da Lagoa da Tijuca, Rio de Janeiro, RJ, Brasil. Rev. Bras. Biol. 59, 503–515. doi: 10.1590/S0034-71081999000300016
Soares, M. L. G., Estrada, G. C. D., Fernandez, V., and Tognella, M. M. P. (2012). Southern limit of the Western South Atlantic mangroves: assessment of the potential effects of global warming from a biogeographical perspective. Estuar. Coast. Shelf Sci. 101, 44–53. doi: 10.1016/j.ecss.2012.02.018
Spawn, S. A., Sullivan, C. C., Lark, T. J., and Gibbs, H. K. (2020). Harmonized global maps of above and belowground biomass carbon density in the year 2010. Sci. Data 7:112. doi: 10.1038/s41597-020-0444-4
Taillardat, P., Friess, D. A., and Lupascu, M. (2018). Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 14:20180251. doi: 10.1098/rsbl.2018.0251
Tang, W., Zheng, M., Zhao, X., Shi, J., Yang, J., and Trettin, C. (2018). Big geospatial data analytics for global mangrove biomass and carbon estimation. Sustainability 10:472. doi: 10.3390/su10020472
Twilley, R. R., Rovai, A. S., and Riul, P. (2018). Coastal morphology explains global blue carbon distributions. Front. Ecol. Environ. 16:503–508. doi: 10.1002/fee.1937
Virgulino-Júnior, P. C. C., Carneiro, D. N., Nascimento, W. R., Cougo, M. F., and Fernandes, M. E. B. (2020). Biomass and carbon estimation for scrub mangrove forests and examination of their allometric associated uncertainties. PLoS One 15:e0230008. doi: 10.1371/journal.pone.0230008
Wang, F., Sanders, C. J., Santos, I. R., Tang, J., Schurech, M., Kirwan, M. L., et al. (2020). Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 8:nwaa296. doi: 10.1093/nsr/nwaa296
Worthington, T. A., Andradi-Brown, D. A., Bhargava, R., Buelow, C., Bunting, P., Duncan, C., et al. (2020a). Harnessing big data to support the conservation and rehabilitation of mangrove forests globally. One Earth 2, 429–443. doi: 10.1016/j.oneear.2020.04.018
Worthington, T. A., Ermgassen, P. S. E., Friess, D. A., Krauss, K. W., Lovelock, C. E., Thorley, J., et al. (2020b). A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 10:14652. doi: 10.1038/s41598-020-71194-5
Keywords: Brazil, mangrove forests, blue carbon, hotspot, CO2 equivalent emissions
Citation: Rovai AS, Twilley RR, Worthington TA and Riul P (2022) Brazilian Mangroves: Blue Carbon Hotspots of National and Global Relevance to Natural Climate Solutions. Front. For. Glob. Change 4:787533. doi: 10.3389/ffgc.2021.787533
Received: 30 September 2021; Accepted: 09 December 2021;
Published: 03 January 2022.
Edited by:
Dominic A. Andradi-Brown, World Wildlife Fund, United StatesReviewed by:
Steven Canty, Smithsonian Marine Station (SMS), United StatesCopyright © 2022 Rovai, Twilley, Worthington and Riul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andre S. Rovai, YXJvdmFpMUBsc3UuZWR1
†ORCID: Andre S. Rovai, orcid.org/0000-0003-4117-2055; Robert R. Twilley, orcid.org/0000-0002-6173-6033; Thomas A. Worthington, orcid.org/0000-0002-8138-9075; Pablo Riul, orcid.org/0000-0003-4035-1975
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.