
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 28 September 2021
Sec. Forest Ecophysiology
Volume 4 - 2021 | https://doi.org/10.3389/ffgc.2021.711414
This article is part of the Research Topic Functional Ecology and Conservation of Palms View all 10 articles
Under the old taxon Principes, palms were once the Princes of the Kingdom Plantae. First on Engler’s list, they occupy a cherished place to botanists, and remain treasured centerpieces of many gardens. In turn, botanic gardens have put forward a decades-long effort to conserve these widely admired plants, keeping a number of palm species from extinction. Living palm collections also have critical value for comparative ecological studies. In this paper we highlight successful ex situ conservation programs for palms, review how the promising new field of collections genetics can guide ex situ conservation of palms, conduct a family-wide gap analysis for living collections in the Arecaceae, and provide an in-depth case study of ex situ conservation of the genus Sabal. These analyses highlight ways in which gardens can advance palm conservation following four recommendations: collect, cultivate, communicate, and collaborate.
In one of the earliest global florae, Engler (1889) designated the palms as the only family in the Order Principes, meaning “the first.” Through his coronation of Palmae (Arecaceae) as the princes of Kingdom Plantae, Engler reveals his own love for these cherished plants. Engler also brought together a fantastic palm collection at Botanic Garden and Botanical Museum Berlin-Dahlem, where he directed construction of their great Palm House. But Engler was certainly not alone in this regard—the Palm House was a potent status symbol in Victorian Britain, requiring not only expensive craftsmanship but also constant heat to protect these tropical gems throughout the year. Dedicated cultivation of these plants goes back much further, though (Figure 1). Representations of date palms (Phoenix dactylifera L.) and doum palms (Hyphaene thebaica Mart.)—ornamentally grown, not depicted as crops—date to 1450 BC in Thebes (Janick, 2002). The appreciation continues to our own era, with palms widely grown at civic landscapes and private gardens. At botanic gardens, palms remain a celebrated landscape feature that define spaces, frame vistas and evoke a tropical feel (Carricarte, 2021; Figure 2).
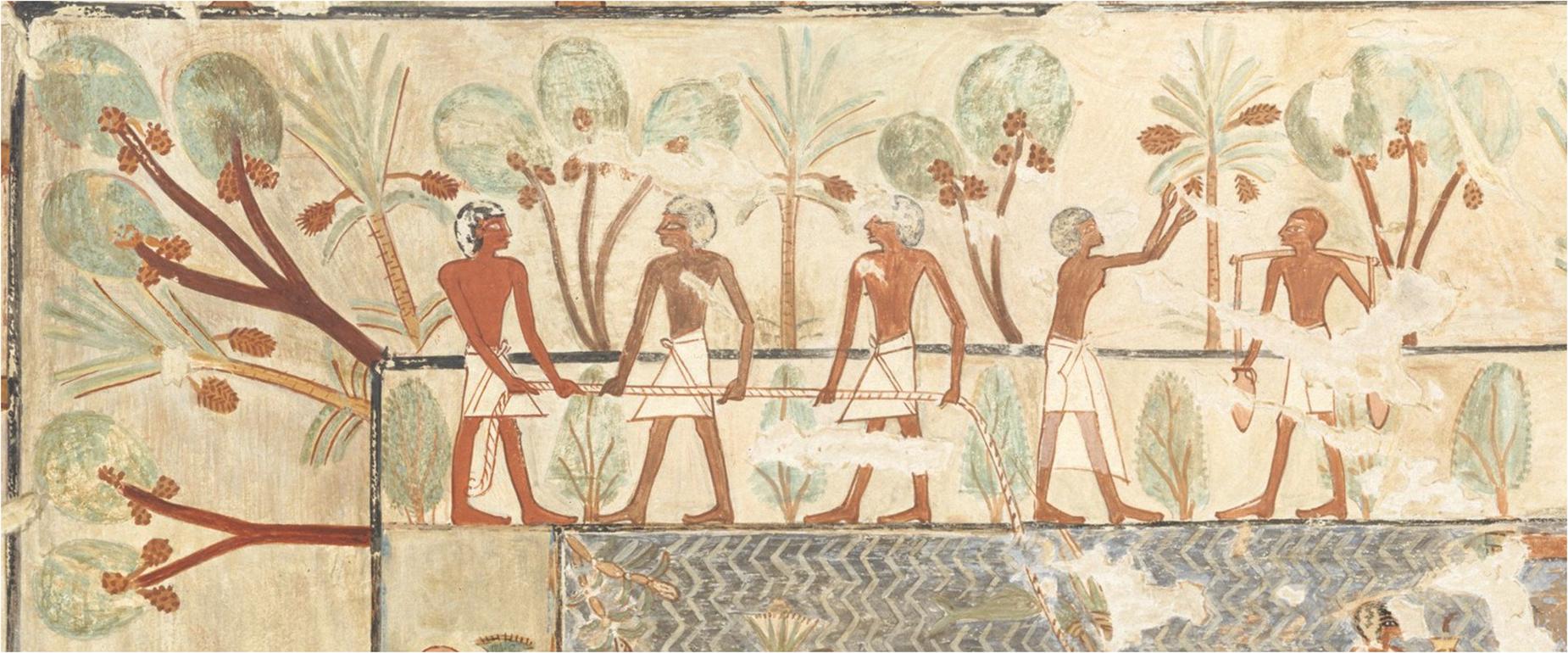
Figure 1. Ornamental palms recorded in ca. 1450 BC. Detail from Deceased Being Towed in a Boat, Tomb of Rekhmire; facsimile by Nina de Garis Davies. This scene shows Phoenix dactylifera and Hyphaene thebaica planted in an ornamental garden, perhaps the earliest known depiction of horticultural cultivation of palms.

Figure 2. A modern palm collection: Lowland Palmetum at the Montgomery Botanical Center, Florida, United States. While arranged and appreciated for aesthetics, these documented living collections also represent a rich resource for scientific study: between 2016 and 2020, 171 known publications made use of the plant resources at Montgomery.
Beyond the unrivaled aesthetic value of palms in these gardens, the utility of these living collections for scientific study is also vital. Many studies of comparative ecology have been facilitated by botanic garden palm collections (Tomlinson, 1979), as these collections bring a diversity of species into parallel cultivation. The robust and diverse holdings of botanic gardens make them well suited for studies of functional ecology (Perez et al., 2019). For example, abiotic natural selection for cyclone tolerance has been studied via differential morphology and mortality among palm collections (Griffith et al., 2008, 2013), relying on detailed records of provenance and taxonomy. Indeed, one of the best aspects of living palm collections is reliable taxonomy, as such collections are often used for systematics research and in some cases can even be associated with type specimens (Crane et al., 2009). A potential concern with using ex situ palm collections may be limited sampling (Pérez-Harguindeguy et al., 2013). This is increasingly a less relevant concern as gardens move from synoptic collections toward collections designed for genetic breadth (Oldfield, 2009). In many cases botanic gardens can provide more geolocated and taxonomically verified species than are available in field plots (Perez et al., 2019), making such collections especially useful for functional ecology studies. Realizing and activating the research value of the more passive palm collections in gardens would provide further resources for such studies.
Ex situ collections are well-established as an essential aspect of integrated plant conservation planning to help conserve plant species (Convention on Biological Diversity, 2020). Palms provide an apt model to illustrate the vital importance of living collections. For example, fewer than 25 Attalea crassispatha Burret survive in southern Haiti, but ex situ collections augment these numbers (Johnson, 1998). Seeds of these palms are eaten, limiting seedling recruitment. Copernicia fallaensis León is limited to 84 extant mature palms near Falla, Cuba, where it is overexploited for thatching (Verdecia, 2015). Establishment of a nearby ex situ collection of 50 plants helps to ensure its survival (Verdecia, 2015; Hodel et al., 2016). An extreme example is Pritchardia aylmer-robinsonii H. St. John, limited to 2 in situ plants on Niihau, which have not been observed to set seed for many years (Chapin et al., 2004). Thankfully, specimens growing in over 30 gardens have set seed (Chapin, 2005).
In addition, a number of palm species are fully extirpated from the wild, and survive only in cultivation. Corypha taliera Roxb., once native to Bangladesh and India, saw its last wild individual felled by mistake in 1979 (Johnson, 1998), but is known from at least 6 botanic gardens (Botanic Gardens Conservation International (BGCI), 2020). Hyophorbe amaricaulis Mart. survives as a single individual palm at Curepipe Botanical Gardens, Mauritius (Ludwig et al., 2010). Sabal miamiensis Zona was once known from Southeastern Florida, and now only survives in 5 ex situ collections (see below).
The examples above are also augmented by successes in restoration plantings derived from ex situ collections. Pseudophoenix sargentii H. Wendl. collections produced seedlings that have successfully augmented wild populations (Fotinos et al., 2015). Nypa fruticans Wurmb., a species widely used for thatching, has been used as restoration plantings in reclaimed agricultural areas (Bamroongrugsa et al., 2008). Research on seed collections of Pritchardia remota Becc. has provided guidance on breaking dormancy to better establish outplants for restoration on Nihoa (Pérez et al., 2008). All of these examples illustrate the great potential of ex situ collections to help secure survival of wild palm species.
While some palms can be propagated via in vitro tissue culture (Wang et al., 2003) or conventional vegetative propagation (Devanand and Chao, 2003), many threatened palms are in fact “exceptional species” sensu Pence (2013), and thereby require seed-grown living garden collections as ex situ safeguards, as the seeds do not survive conventional seedbanking storage (e.g., Porto et al., 2018). Advancements to in vitro propagation of threatened palms would help immensely, especially in the case of Hyophorbe amaricaulis mentioned above. Beyond such extreme cases, general research into the reproduction of palms in collections (e.g., Valdes et al., 2021; Tucker Lima et al., 2021) can inform how to functionally increase low census numbers within threatened in situ populations also.
Genetic analysis of in situ palm populations is a well-established tool for informing conservation actions. Many population genetic questions have been explored in Arecaceae, providing a robust background for conservation strategies. For example, Shapcott (1998) showed low genetic variation in the rare Ptychosperma bleeseri Burret, highlighting the threat of genetic swamping via ornamental palm production. In another example Bacon and Bailey (2006) demonstrated how accurate taxonomic circumscription advances palm conservation, using population genetics of Chamaedorea alternans H. Wendl.
Recent attention to the genetics of ex situ collections of palms has offered unique insight into how best to manage these resources for conservation benefit. An early pan-African survey of ex situ collections of Elaeis guineensis Jacq. (African Oil Palm; Hayati et al., 2004) examined polymorphism in this important genetic resource. Examination of the genetic variation held in a collection of Leucothrinax morrisii (H. Wendl.) C. Lewis and Zona (Namoff et al., 2010) demonstrated the value of maintaining multiple individuals from each palm population in cultivation. This work was followed by studies of how well garden palm collections represent variation in the wild, or how well these collections can help with restoration efforts. Asmussen-Lange et al. (2011) showed that collections of Hyophorbe lagenicaulis (L. H. Bailey) H. E. Moore can help to augment the eroded genetic diversity of the small, relict in situ population; as this very popular ornamental species is kept in at least 84 collections worldwide (Botanic Gardens Conservation International (BGCI), 2020), the potential for securing its survival is great. Ceroxylon quindiuense (H. Karst.) H. Wendl. is another spectacular, highly charismatic plant holding the record as the world’s tallest palm species. Comparing C. quindiuense populations with neighboring ex situ collections showed that diversity in collections was not as great as in the wild, and that careful selection among ex situ collections is critical for restoration efforts (Chacón-Vargas et al., 2020).
Another cherished charismatic megafloral palm, Pseudophoenix ekmanii Burret, provided a model to examine how combined holdings from multiple sites (i.e., “metacollections” sensu Griffith et al., 2019a) represented in situ diversity. It was found that pooled collections from more than one garden captured more genetic diversity than a single-garden collection, and captured it more efficiently (Griffith et al., 2020). Study of its sister species (P. sargentii) showed that tailored collection protocols for small and large populations should be considered, and that emphasis on maximizing maternal lines in a collection captures genetic diversity most efficiently (Griffith et al., 2021). Furthermore, comparing P. sargentii and P. ekmanii among non-palm species showed that taxonomic closeness does not predict genetic capture in ex situ collections (Hoban et al., 2020). This area of work shows great promise and potential for palm conservation collections. Further studies are underway at several labs and will help illustrate best practices for developing the most representative collections.
With all of the above reasons to cultivate and protect palms in networked botanic garden metacollections, it is important to grasp the broader, overall scope of what currently exists in ex situ living collections. Botanic gardens are museums of living collections, and initial intellectual control (i.e., cataloging) is an essential part of museum and garden management. Knowledge of holdings facilitates access and use of these living treasures (Perez et al., 2019) for research, conservation, education and aesthetic purposes. Thus, we seek to define and measure how thoroughly botanic garden collections represent the world’s extant palm diversity.
Records on ex situ palm holdings were obtained from BGCI PlantSearch (Botanic Gardens Conservation International (BGCI), 2020) on 25 September 2020. This dataset encompassed all palms recorded as present in living collections globally, and included taxonomy, site, and institution type (e.g., botanic garden, zoo, seed bank, gene bank, etc.) The raw data included 16,313 records of palm taxa held at a total of 523 sites, all self-reported to BGCI from participating botanic gardens and similar organizations.
First, these data were limited in scope to ex situ living sporophyte collections, i.e., excluding seed banks, tissue banks, networks which potentially duplicate records from other gardens, and observance data from the ornamental horticulture trade (e.g., Imada et al., 1989). This parsing left a total of 15,723 records. Then, garden hybrids (5 records) were removed, however, naturally occurring hybrids (e.g., Syagrus × costae Glassman) were retained.
Finally, these 15,718 records were reconciled against the World Checklist of Arecaceae (Govaerts et al., 2020). This continuously updated online resource supercedes the World Checklist of Palms (Govaerts and Dransfield, 2005), both of which have long provided vital, stable consensus taxonomy for this family with so many active researchers worldwide. This reconciliation removed unplaced names from older literature (e.g., Sabal ghiesbrechtii R. Pfister), corrected orthographic variants (e.g., “Sabal japa” = Sabal yapa C. Wright ex Becc.) and synonymized all records in accordance with Govaerts et al. (2020). Taxonomic reconciliation with the world checklist and removal of any subsequent duplicate records at each garden left a total of 14,779 records.
We also selected the genus Sabal for a more focused case study which also considered in situ threat level. We chose Sabal based on the experience and expertise of the authors. Methods for this case study were similar to the above, with exceptions noted below. BGCI PlantSearch contained 730 records of Sabal as of 25 September 2020. After excluding seed banks, checklists, and networks (as above), 699 records remained. We reconciled these records with Govaerts et al. (2020) as above, but with two exceptions: we chose to recognize Sabal guatemalensis Becc. and S. miamiensis as accepted species. Sabal guatemalensis is known from Guatemala, Southern Mexico, and Belize, and has sometimes been synonymized with S. mexicana Mart., a much more widespread species that (sensu lato) occurs from Texas to Central America. It is important to note that even though S. guatemalensis and S. mexicana look similar, they are not resolved as sister species in recent phylogenetic analysis (Heyduk et al., 2016). Sabal miamiensis was previously known from Broward County and Miami-Dade County, Florida, United States, but is sometimes synonymized with S. etonia Swingle, a species more widely distributed in Florida. Based on the phylogenetic analyses of Heyduk et al. (2016) and Cano et al. (2018), and the gene conflict analysis of Grinage et al. (2021), there is extensive gene conflict at the node leading to S. miamiensis and S. etonia, and we believe that further work with the advent of genomic data is necessary to more conclusively resolve the status of S. miamiensis. For these reasons and our familiarity with these species in the field and in collections, we are of the opinion that S. guatemalensis and S. miamiensis are each distinct taxa, and they are treated as such in multiple collections.
Four records in the dataset were unplaced names (published in 1853 and 1892), and 12 records were not validly published and appeared to be horticultural appellations (e.g., S. macrophylla). All of these 16 records were at older, European gardens, suggesting perhaps collection and “naming” prior to the wide establishment of modern taxonomic convention. Ninety of the remaining records were either synonyms or orthographic variants. For example one site self-reported 4 species all assigned to Sabal bermudana L. H. Bailey. After resolving synonymy and spelling, 634 records of Sabal remained.
IUCN Red List category was obtained from official sources (IUCN, 2020) for those palms with formal assessments published. For a number of Sabal spp. that are not formally listed we either reviewed literature for provisional assessments (e.g., Zona et al., 2007), reviewed literature for conservation information to provide our own provisional assessment (e.g., Paiz and Stuardo, 1999 for S. guatemalensis), and provided our own primary information from current ongoing fieldwork (e.g., Grinage pers. obs., Noblick pers. obs. for S. miamiensis).
Botanic gardens hold 1,380 of the world’s recognized wild palm taxa (out of 2,566 per Govaerts et al., 2020), in 178 of the 184 recognized genera (Figure 3), kept at 477 unique sites around the world (Table 1 and Figure 4; Botanic Gardens Conservation International (BGCI), 2020). Many sites (83) hold a single palm species in cultivation, and the largest number of species held at a single site is 863 (Nong Nooch Tropical Botanic Garden, Thailand; NNTBG). The median number of palm species at gardens with palms is 8, while the mean is 31, showing a large skew in distribution toward a few large gardens in the tropics, e.g., NNTBG, Cairns Botanical Garden (CBG), Singapore Botanic Garden (SBG), and Bogor Botanic Gardens (BBG). The 10 botanic gardens with the highest number of palm species collectively hold 1,207 taxa.
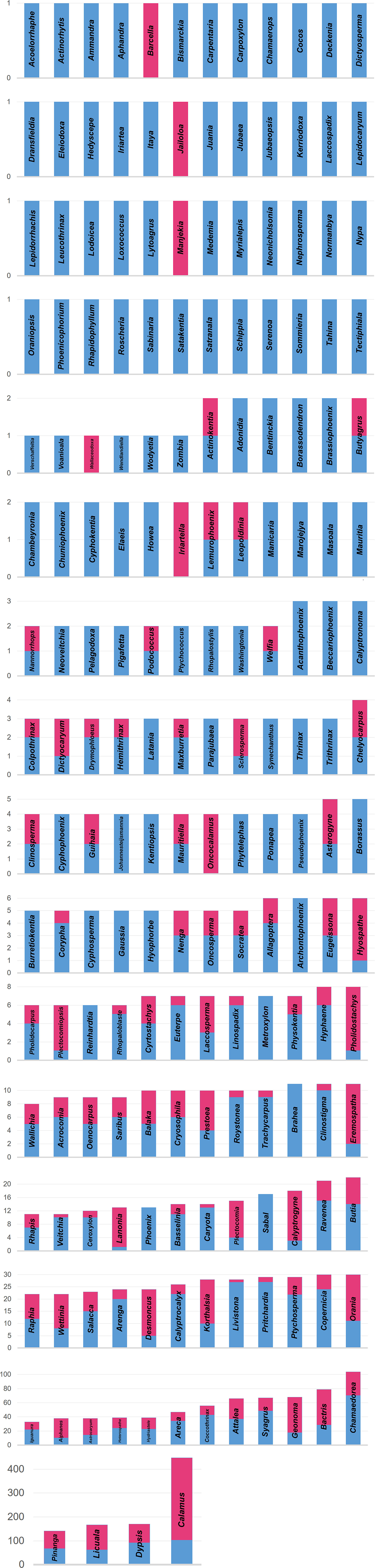
Figure 3. Conservation gap analysis of the palms by genus: Comparative number of species in Arecaceae genera, and presence of palm species in the global metacollection. The 184 accepted genera (Govaerts et al., 2020), are arranged by number of species (total spp. = 2,566). Proportion of species in ex situ collections shown in blue, and proportion of species not maintained in collections shown in magenta. Note changes in scale of Y-axis. Slightly over half of the world’s palm species (n = 1,380; 54%) are in protective cultivation.
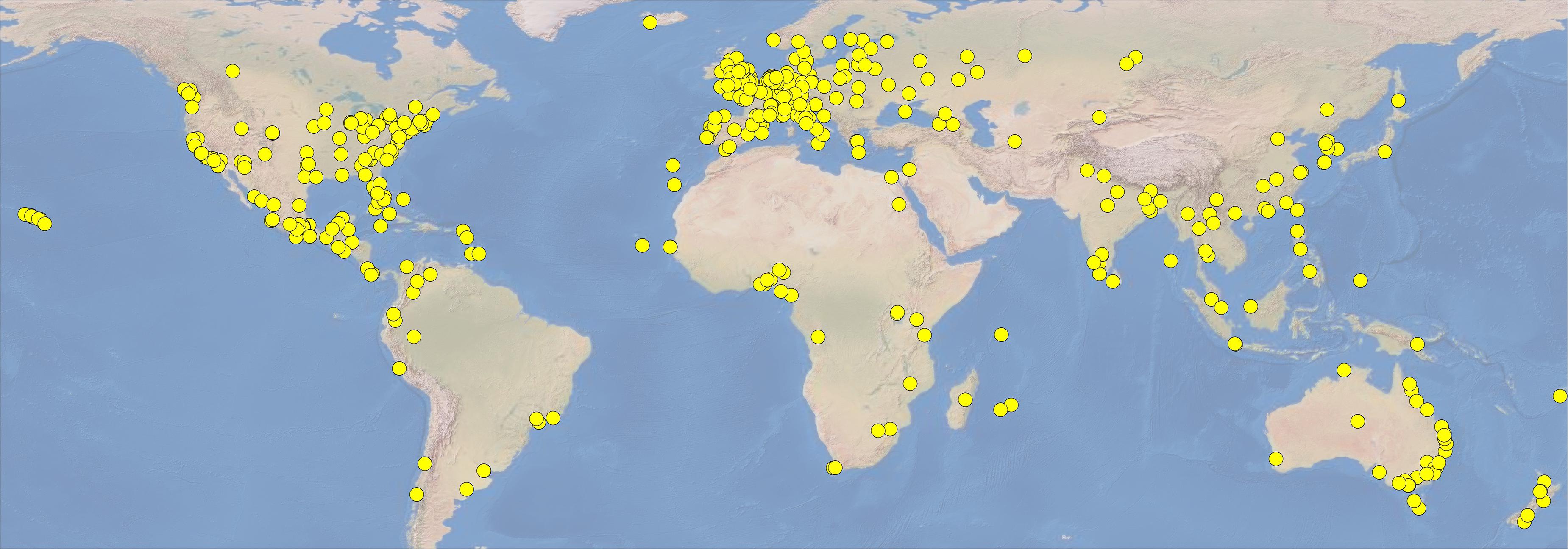
Figure 4. Location of the global palm metacollection. These 477 sites cultivate 1,380 spp. of palms collectively (Botanic Gardens Conservation International (BGCI), 2020). While palm species diversity is highest in the tropics and subtropics, the majority of palm-hosting sites are located in temperate regions. While this distribution of sites suggests a latitudinal bias toward the north (Pautasso and Parmentier, 2007; Mounce et al., 2017), the largest collections by number of palm species are in the tropics (see text).
Within this global metacollection, 347 palm species (including 58 Calamus spp.) are reported by only one garden. At the other end of the scale, Phoenix canariensis H. Wildpret is grown at 155 gardens, Trachycarpus fortunei (Hook.) H. Wendl. is grown at 183 gardens, and Chamaerops humilis L. is kept at 186 sites worldwide.
All 19 species of Sabal we recognize are currently kept in living botanic garden collections (Table 2 and Figure 5). The breadth of representation of these species varies in ways that suggest a correlation with threat level (Figure 5; R2 = 0.47 when ordered by threat level); for example, S. miamiensis, considered to be extinct in the wild (see below), is known in only 5 collections, while S. minor (Jacq.) Pers. is stable and listed as Least Concern (IUCN, 2020) and is recorded in 146 collections.
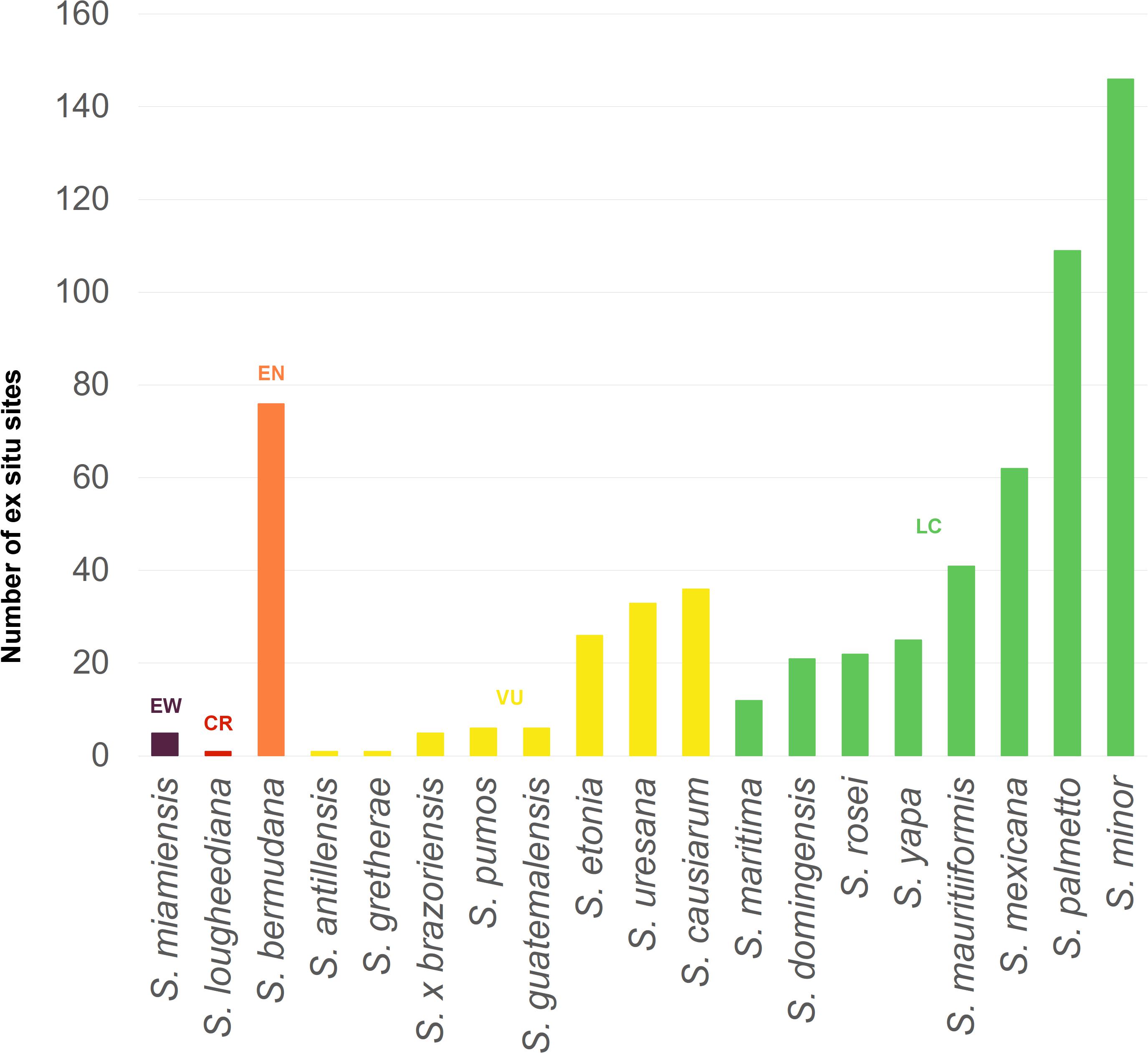
Figure 5. The global Sabal metacollection: Number of ex situ sites holding living palms of each species, arranged by IUCN Red List Category, and then arranged by number of sites (Botanic Gardens Conservation International (BGCI), 2020). This visualization highlights relative security for each taxon. For example, (1) Sabal miamiensis requires urgent propagation, distribution, and restoration efforts to ensure against extinction, as the plant is no longer found in the wild and only occurs in 5 gardens so far as known. (2) S. lougheediana must also be propagated and distributed in order to provide a more secure complement to in situ conservation efforts. (3) While efforts must be made to protect the remaining wild stands of S. bermudana, a robust group of gardens can provide germplasm for restoration efforts. (4) S. antillensis and S. gretherae are also high priorities for propagation and distribution. (5) At the other end of the security scale, S. palmetto and S. minor are of least concern for threats in its widespread natural range, and both are also widely grown at over 100 botanic gardens each. This breadth of plants in protective cultivation could prove vital if Lethal Bronzing greatly reduces the wild stands of these palms (Oates et al., 2020).
Slightly over half (54%) of the world’s palm species are in protective cultivation at botanic gardens and similar sites. Thus, this analysis highlights the great potential for developing further ex situ diversity in palm collections, as nearly 1,200 recognized palm species are not yet recorded in cultivation. Primary desiderata among these uncultivated taxa include the 6 genera not currently recorded at gardens (Figure 3): Barcella (1 sp.), Jailoloa (1 sp.), Manjekia (1 sp.), Wallaceodoxa (1 sp.), Iriartella (2 spp.), and Oncocalamus (4 spp.), given that these genera represent phylogenetically diverse lineages (Larkin et al., 2016).
Three of these genera are monotypic and only recently described from modern explorations on islands near northwestern New Guinea (Jailoloa, Manjekia, and Wallaceodoxa; Heatubun et al., 2014a). Notably, Manjekia was known to be in cultivation in one garden from at least 2012 (Heatubun et al., 2014b), but records from that garden in 2020 (current dataset) no longer report this palm species. This highlights the great importance of distributing collections among gardens—plant collections are not static and can change greatly over short time scales (Griffith et al., 2017a). This highlights the importance of regular reporting of collection data to such networked databases—BGCI PlantSearch encourages gardens to update their data annually.
Beyond these uncultivated genera, many other palm species are less obvious goals but still very important for collection and cultivation. One example is Coccothrinax jimenezii M. M. Mejía and R. G. García. This critically endangered species is limited to 61 individuals in the Dominican Republic and Haiti and is overexploited for broom making (Jestrow et al., 2016). Ex situ collections are recommended to safeguard this very imperiled species (Peguero et al., 2015; Harvey-Brown, 2018). As of September 2020, there is no record of this species in living collections. This is one example of many threatened palms that would benefit from protective cultivation. An Arecaceae-wide comparison of threat status with collections holdings would identify all such critical priorities and allow for informed conservation planning.
As noted above, the 10 largest palm collections together keep 1,207 species. This shows the important value of all gardens in stewarding palm diversity, as even the largest collections cannot keep all species, let alone all infraspecific (population) diversity. For example, Pinanga manii Becc. is currently only recorded at the Experimental Garden of the Botanical Survey of India (Kolkata), a site holding 2 palm species. This highlights the value that all ex situ sites of any size or type bring to a metacollection (Griffith et al., 2019a), and the importance of networking such collections in service of conservation goals. As noted above for Manjekia, facilitating distribution of such monosite collections should be an important safeguarding procedure for the palm metacollection. Natural disasters are but one example of why single-site ex situ collections should be avoided.
Also suggested here is a reversal of the “positive latitudinal bias” (Pautasso and Parmentier, 2007) shown in the overall species richness of botanic garden collections (Mounce et al., 2017) when palms alone are considered. While the global north has a large number of established botanic garden collections (as shown on Figure 4), many of the most diverse palm collections are found in the tropical latitudes and southern hemisphere (e.g., NNTBG, SBG, CBG, and BBG). Furthermore, the three most widely grown palms (Phoenix canariensis, Trachycarpus fortunei, and Chamaerops humilis) are considered the three most temperate species in the family. Thus, the species richness patterns of palm collections follow the species richness patterns of in situ palm diversity, with more diverse collections in tropical latitudes.
A caveat about this analysis is based in the breadth of coverage of this dataset. While BGCI PlantSearch is the widest-reaching and most complete global botanic garden collections database, coverage is estimated at only 34% of botanic gardens worldwide (Mounce et al., 2017). Community encouragement of all ex situ sites to freely upload collections data to PlantSearch would increase discoverability of other vital palm collections which likely exist (Vovides et al., 2018). For example, Attalea crassispatha is recorded at 3 gardens in PlantSearch, but at least 3 other ex situ sites also maintain living collections (Griffith, in prep.)—all of which are vital to conservation of this critically endangered species.
Another caveat of this analysis is based on the applicability of a single consensus taxonomy for palms. Certainly, for such a large and diverse family with voluminous active taxonomic research, consensus taxonomy is a major challenge, and we applaud the efforts of Govaerts et al. (2020) in assembling this important resource, as a common language for palm diversity allows clear communication and facilitates prioritization of coordinated conservation actions. However, reconciling the self-reported occurrence data with the up-to-date synonymy highlighted great variation in how individual gardens handle palm taxonomy: like systematists generally, some gardens appear to be “lumpers” and others are “splitters.” In the most extreme case, resolving synonyms reduced the number of species at a single garden by 72. The number of synonyms resolved in each collection was strongly correlated with overall collection diversity (n = spp. in collection vs. n = synonyms resolved: R2 = 0.77); more diverse collections seem to use more controversial labeling! At one of the authors’ gardens (Montgomery Botanical Center, Florida, United States) 6.5% of records were synonyms, reducing an initial 370 self-reported palm spp. to 346. But we are in good company: even Royal Botanic Gardens Kew—where this consensus taxonomy is produced—lost 7.5% of its self-reported palm diversity when the consensus was applied.
With our study of Sabal, we find that threat level correlates with the breadth of presence in collections (Figure 5 and Table 2), as is also seen in many other groups, including Australian plants (Botanic Gardens Conservation International (BGCI), 2013), conifers (Shaw and Hird, 2014), maples (Acer L.; Crowley et al., 2020), oaks (Quercus L.; Beckman et al., 2019; Carrero et al., 2020), and US plants (Botanic Gardens Conservation International (BGCI), 2014). This again illustrates the need for garden curators to actively propagate and distribute palm collections as a potential hedge against natural disasters (Griffith et al., 2008). Of particular concern in this way are the species Sabal lougheediana M. P. Griff. and Coolen and (to a lesser extent) S. antillensis M. P. Griff. Sabal lougheediana is Critically Endangered and currently limited to only 25 mature specimens in an area of less than 1 km2 due to overgrazing by feral ungulates (De Freitas et al., 2019; Griffith et al., 2019b). While S. antillensis is more secure in the wild, it remains vulnerable due to limited range and potential threats from invasive pests (Griffith et al., 2017b). Alarmingly, both taxa were currently known from only a single garden as of September 2020. Since that time, efforts to distribute both species to other gardens have been made. This work is especially important for the palm research community because the phylogenetic relationship with both S. lougheediana and S. antillensis are unknown in relation to the rest of the species of Sabal. Because both S. lougheediana and S. antillensis are distributed in the Leeward Antilles, these species represent a potentially important link to the biogeographic history of Sabal as only one other species [S. mauritiiformis (H. Karst.) Griseb. and H. Wendl.] is known from South America (Grinage et al., 2021).
Our study of Sabal also illustrates some of the challenges with stewarding a broad consensus taxonomy. In describing the already very-restricted species S. miamiensis, Zona (1985) highlighted that its habitat “is fast disappearing because of extensive urban development in the Miami area,” and “is in danger of extinction unless it can be brought into cultivation or its habitat can be preserved.” Collections at the Montgomery Botanical Center include plants of Sabal miamiensis which were obtained in habitat as seed or transplants (rescued from the path of development) shortly after the species was described. Revisits to these same sites in recent years, as well as sites of specimens cited in the protolog (Zona, 1985) yielded no further observations of these palms in the wild. The morphological distinctiveness of these collections compared to its supposed prior synonym (S. etonia) prompted our decision to retain the name S. miamiensis for these plants. If the consensus taxonomy was followed, these collections of extirpated palm diversity would be subsumed under S. etonia; while that synonymy is not problematic on its own, it does render this unique morphological, ecological and conservation phenomenon less discoverable and communicable. Planned upgrades to BGCI PlantSearch to store and retrieve accession-level data may help address such “lost information” wrought by such synonymy.
Synonymy is important to consider in a group such as Sabal for which there is a long botanical history (over 250 years). Similar to the taxonomy of S. miamiensis and S. etonia, S. minor s.l. was once divided into S. minor s.s. and S. louisiana (Darby) Bomhard (Small, 1926; Bailey, 1934; Bomhard, 1935; Bomhard, 1943). Sabal louisiana unlike S. minor is restricted in range to annual floodplain forests along the Mississippi Delta, United States. Furthermore, S. louisiana develops an above-ground trunk while S. minor does not. Aside from the presence of a trunk, the morphology of S. minor s.s. and S. louisiana is not easily distinguishable. This lack of distinguishing characteristics is one of the reasons it was synonymized by L. H. Bailey nearly 80 years ago (Bailey, 1944). Since then, new scientific tools (e.g., genetics and genomics) provide the ability to revisit these synonymies with modern methodologies. Luckily for S. louisiana, there are still enough wild populations available for study and ex situ collections development. This is a case where the synonymy obscures morphological diversity within a species considered “Least Concern” for extinction (IUCN SSC GTSG, 2020). We argue here that even though the current taxonomy combines these morphologies into one species, botanical gardens should strive to include all forms of diversity—not just taxonomic, but also morphologic, genetic and geographic. As technology continues to advance and destruction of wild habitats expands, it is important to maintain representatives of these original growth forms so that we can eventually solve these taxonomic and ecological puzzles.
This review and analysis illuminate a clear path forward for palm collections. In order to better serve the ecological field, the scientific community generally, the conservation field, students worldwide, and the broader global public, palm collections should collect, cultivate, communicate, and collaborate.
Gap analysis of the global palm metacollection highlights significant taxonomic gaps in worldwide collections holdings. Botanic gardens should prioritize bringing these taxa into collections that serve their communities through education, display, and research. Bring these palms into the gardens!
Active horticultural management of these living treasures is essential. Examples of palms brought into cultivation and then lost highlights the need for broader propagation and sharing of such rare material among gardens and others. Grow more palms!
Even the world’s broadest and most extensive networked database of palm collections still sees significant gaps in coverage. This is especially noted in cases of very rare and imperiled species. We encourage all gardens to upload their data to BGCI PlantSearch, and we especially implore the larger and more established botanic gardens to share resources and expertise to facilitate this process. Share your data!
As shown here, the global palm collection is greater than the sum of its parts. More deliberate networking of palm collections also demonstrably improves conservation outcomes. We encourage gardens to directly partner to advance conservation goals. Work together!
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MPG designed the article. MPG, AM, and AG performed analysis and wrote the article. All authors contributed to the article and approved the submitted version.
This work was funded by the Institute of Museum and Library Services (Grants MG-60-19-0064-19 and MG-245575-OMS-20) and funds from the authors’ organizations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Claudia Calonje, Michael Calonje, Eliza Gonzalez, Vickie Murphy, Larry Noblick, Joanna Tucker Lima, Paul Smith, and Andrew Street for discussion, input, and collections management. We also thank the Metropolitan Museum of Art for making the image for Figure 1 available, and all of the botanic gardens worldwide for uploading their plant records data to BGCI PlantSearch.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.711414/full#supplementary-material
Asmussen-Lange, C. B., Maunder, M., and Fay, M. F. (2011). Conservation genetics of the critically endangered Round Island bottle palm, Hyophorbe lagenicaulis (Arecaceae): can cultivated stocks supplement a residual wild population? Bot. J. Linn. Soc. 167, 301–310. doi: 10.1111/j.1095-8339.2011.01175.x
Bacon, C. D., and Bailey, C. D. (2006). Taxonomy and Conservation: a Case Study from Chamaedorea alternans. Ann. Bot. 98, 755–763. doi: 10.1093/aob/mcl158
Bamroongrugsa, N., Buachum, S., and Purintavaragu, C. (2008). Nipa Palm (Nypa fruticans Wurmb.) cultivation in salt affected paddy fields. J. Trop. Plants Res. 1, 93–102.
Bárrios, S., and Hamilton, M. A. (2018). Sabal causiarum. The IUCN Red List of Threatened Species 2018: e.T57356844A125646226. Gland: IUCN.
Beckman, E., Meyer, A., Denvir, A., Gill, D., Man, G., Pivorunas, D., et al. (2019). Conservation Gap Analysis of Native U.S. Oaks. Lisle: The Morton Arboretum.
Botanic Gardens Conservation International (BGCI) (2013). Botanic Gardens of Australia and New Zealand (BGANZ) ex situ collections survey. Chicago: BGCI-US.
Botanic Gardens Conservation International (BGCI) (2014). Progress report on Target 8 of the Global Strategy for Plant Conservation in the United States. Chicago: BGCI-US.
Botanic Gardens Conservation International (BGCI) (2020). PlantSearch online database. Botanic Gardens Conservation International. Richmond, U.K. Available online at: https://tools.bgci.org/plant_search.php (Accessed September 25, 2020).
Bomhard, M. L. (1935). Sabal louisiana, the correct name for the polymorphic palmetto of Louisiana. J. Wash. Acad. Sci. 25, 35–44.
Bomhard, M. L. (1943). Distribution and character of Sabal louisiana. J. Wash. Acad. Sci. 33, 170–182.
Cano, Á, Bacon, C. D., Stauffer, F. W., Antonelli, A., Serrano-Serrano, M. L., and Perret, M. (2018). The roles of dispersal and mass extinction in shaping palm diversity across the Caribbean. J. Biogeogr. 45, 1432–1443. doi: 10.1111/jbi.13225
Carrero, C., Jerome, D., Beckman, E., Byrne, A., Coombes, A. J., Deng, M., et al. (2020). The Red List of Oaks 2020. Lisle: The Morton Arboretum.
Chacón-Vargas, K., García-Merchán, V. H., and Sanín, M. J. (2020). From keystone species to conservation: conservation genetics of wax palm Ceroxylon quindiuense in the largest wild populations of Colombia and selected neighboring ex situ plant collections. Biodivers. Conserv. 29, 283–302. doi: 10.1007/s10531-019-01882-w
Chapin, M. (2005). Pritchardia aylmer-robinsonii St. John. Available online at: https://www.iucn.org/sites/dev/files/import/downloads/psg_pritchardia_aylmer_robinsonii.pdf (Accessed August 17, 2020).
Chapin, M. H., Wood, K. R., Perlman, S. P., and Maunder, M. (2004). A review of the conservation status of the endemic Pritchardia palms of Hawaii. Oryx 38, 273–281. doi: 10.1017/S003060530400050X
Convention on Biological Diversity (2020). Targets. Available online at: https://www.cbd.int/gspc/targets.shtml (Accessed August 19, 2020).
Copeland, A., and Roberts, A. (2016). Sabal bermudana. The IUCN Red List of Threatened Species 2016: e.T38691A101378743. Switzerland: IUCN.
Crane, P. R., Hopper, S. D., Raven, P. H., and Stevenson, D. W. (2009). Plant science research in botanic gardens. Trends Plant Sci. 14, 575–577. doi: 10.1016/j.tplants.2009.09.007
Crowley, D., Barstow, M., Rivers, M., and Harvey-Brown, Y. (2020). The Red List of Acer Revised and Extended. Richmond: Botanic Gardens Conservation International.
De Freitas, J., Camilleri, J., van Eijk, S., Posno, V., Valdes, I., Coolen, Q., et al. (2019). Sabalpalm (Sabal antillensis) recovery over 40 years: lessons for successful palm conservation. Palms 63, 57–68.
Devanand, P. S., and Chao, C. T. (2003). Genetic variation within ‘Medjool’and ‘Deglet Noor’date (Phoenix dactylifera L.) cultivars in California detected by fluorescent-AFLP markers. J. Hortic. Sci. Biotechnol. 78, 405–409. doi: 10.1080/14620316.2003.11511639
Engler, A. (1889). Die Natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten, insbesondere den Nutzpflanzen, unter Mitwirkung zahlreicher hervorragender Fachgelehrten begründet. Teil II, Abt. 3. Leipzig: W. Engelmann.
Fotinos, T. D., Namoff, S., Lewis, C., Griffith, M. P., Maschinski, J., and von Wettberg, E. J. B. (2015). Genetic evaluation of a reintroduction of Sargent’s Cherry Palm, Pseudophoenix sargentii. J. Torrey Bot. Soc. 142, 51–62. doi: 10.3159/torrey-d-14-00004.1
Goldman, D. H., Klooster, M. R., Griffith, M. P., Fay, M. F., and Chase, M. W. (2011). A preliminary evaluation of the ancestry of a putative Sabal hybrid (Arecaceae: Coryphoideae), and the description of a new nothospecies, Sabal × brazoriensis. Phytotaxa 27, 8–25. doi: 10.11646/phytotaxa.27.1.2
Govaerts, R., Dransfield, J., Zona, S., Hodel, D. R., and Henderson, A. (2020). World Checklist of Arecaceae. Richmond: Royal Botanic Gardens, Kew.
Griffith, M. P., Barber, G., Tucker Lima, J., Barros, M., Calonje, C., Noblick, L. R., et al. (2017a). Plant Collection “Half-life:” Can Botanic Gardens Weather the Climate? Curator 60, 395–410. doi: 10.1111/cura.12229
Griffith, M. P., De Freitas, J., Barros, M., and Noblick, L. R. (2017b). Sabal antillensis (Arecaceae): a new palmetto species from the Leeward Antilles. Phytotaxa 303, 56–64. doi: 10.11646/phytotaxa.303.1.4
Griffith, M. P., Beckman, E., Callicrate, T., Clark, J., Clase, T., Deans, S., et al. (2019a). TOWARD THE METACOLLECTION: Safeguarding plant diversity and coordinating conservation collections. San Marino: Botanic Gardens Conservation International US.
Griffith, M. P., Coolen, Q., Barros, M., and Noblick, L. R. (2019b). Sabal lougheediana (Arecaceae), a critically endangered, endemic palm species from Bonaire. Phytotaxa 420, 95–101. doi: 10.11646/phytotaxa.420.2.1
Griffith, M. P., Cartwright, F., Dosmann, M., Fant, J., Freid, E., Havens, K., et al. (2021). Ex Situ Conservation of Large and Small Plant Populations Illustrates Limitations of Common Conservation Metrics. Int. J. Plant Sci. 182, 263–276.
Griffith, M. P., Clase, T., Toribio, P., Piñeyro, Y. E., Jiménez, F., Gratacos, X., et al. (2020). Can a botanic garden metacollection better conserve wild plant diversity? A case study comparing pooled collections with an ideal sampling model. Int. J. Plant Sci. 181, 485–496. doi: 10.1086/707729
Griffith, M. P., Noblick, L. R., Dowe, J. L., Husby, C. E., and Calonje, M. A. (2008). Cyclone tolerance in New World Arecaceae: biogeographic variation and abiotic natural selection. Ann. Bot. 102, 591–598. doi: 10.1093/aob/mcn132
Griffith, M. P., Witcher, E., Noblick, L., and Husby, C. (2013). Palm stem shape correlates with hurricane tolerance, in a manner consistent with natural selection. Palms 57, 115–122.
Grinage, A., Landis, J., Majure, L. C., Valderrama, E., Gandolfo, M., and Specht, C. (2021). Deciphering the tales of sabal from the crypt: insights from past collections. Botany.
Harvey-Brown, Y. (2018). Coccothrinax jimenezii. The IUCN Red List of Threatened Species 2018: e.T129768110A129768126. Switzerland: IUCN.
Hayati, A., Wickneswari, R., Maizura, I., and Rajanaidu, N. (2004). Genetic diversity of oil palm (Elaeis guineensis Jacq.) germplasm collections from Africa: implications for improvement and conservation of genetic resources. Theor. Appl. Genet. 108, 1274–1284. doi: 10.1007/s00122-003-1545-0
Heatubun, C. D., Zona, S., and Baker, W. J. (2014a). Three new genera of arecoid palm (Arecaceae) from eastern Malesia. Kew Bull. 69:9525.
Heatubun, C. D., Zona, S., and Baker, W. J. (2014b). Three new palm genera from Indonesia. Palms 58, 197–202.
Heyduk, K., Trapnell, D. W., Barrett, C. F., and Leebens-Mack, J. (2016). Phylogenomic analyses of species relationships in the genus Sabal (Arecaceae) using targeted sequence capture. Biol. J. Linn. Soc. 117, 106–120. doi: 10.1111/bij.12551
Hoban, S., Callicrate, T., Clark, J., Deans, S., Dosmann, M., Fant, J., et al. (2020). Taxonomic similarity does not predict necessary sample size for ex situ conservation: a comparison among five genera. Proc. R. Soc. B 287:20200102. doi: 10.1098/rspb.2020.0102
Hodel, D. R., Verdecia Pérez, R., Suárez Oropesa, D., Rodríguez Lima, M., and Mera, L. A. (2016). Copernicia fallaensis The Greatest Fan Palm of Them All. PalmArbor 2016-4, 1–11.
Imada, C. T., Wagner, W. L., and Herbst, D. R. (1989). Checklist of native and naturalized flowering plants of Hawaii. Bishop Mus. Occas. Pap 29, 31–87.
IUCN (2020). The IUCN Red List of Threatened Species. Version 2020-1. Available online at: https://www.iucnredlist.org
IUCN SSC GTSG. (2020). Sabal minor. The IUCN Red List of Threatened Species 2020: e.T79521201A79521207. Gland: IUCN.
Janick, J. (2002). Ancient Egyptian agriculture and orgins of horticulture. Acta Hortic. 582, 23–39. doi: 10.17660/actahortic.2002.582.1
Jestrow, B., Peguero, B., Jiménez, F., Cinea, W., Hass, M., Reeve, A., et al. (2016). Genetic diversity and differentiation of the Critically Endangered Hispaniolan palm Coccothrinax jimenezii M.M. Mejía & García based on novel SSR markers. Biochem. Syst. Ecol. 66, 216–223. doi: 10.1016/j.bse.2016.04.013
Johnson, D. (1998). Corypha taliera. In: IUCN (2014). IUCN Red List of Threatened Species. Version 2014.1. Gland: IUCN.
Larkin, D. J., Jacobi, S. K., Hipp, A. L., and Kramer, A. T. (2016). Keeping all the PIECES: phylogenetically informed ex situ conservation of endangered species. PLoS One 11:e0156973. doi: 10.1371/journal.pone.0156973
Ludwig, N., Lavergne, C., and Sevathian, J. C. (2010). Notes on the Conservation Status of Mauritian Palms. Palms 54, 77–93.
Mounce, R., Smith, P., and Brockington, S. (2017). Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 3:795. doi: 10.1038/s41477-017-0019-3
Namoff, S., Husby, C. E., Francisco-Ortega, J., Noblick, L. R., Lewis, C. E., and Griffith, M. P. (2010). How well does a botanical garden collection of a rare palm capture the genetic variation in a wild population? Biol. Conserv. 143, 1110–1117. doi: 10.1016/j.biocon.2010.02.004
Oates, M. J., Abu-Khalaf, N., Molina-Cabrera, C., Ruiz-Canales, A., Ramos, J., and Bahder, B. W. (2020). Detection of Lethal Bronzing Disease in Cabbage Palms (Sabal palmetto) Using a Low-Cost Electronic Nose. Biosensors 10:188. doi: 10.3390/bios10110188
Oldfield, S. F. (2009). Botanic gardens and the conservation of tree species. Trends Plant Sci. 14, 581–583. doi: 10.1016/j.tplants.2009.08.013
Paiz, O., and Stuardo, H. (1999). Distribución y usos de la palma de escoba (Sabal guatemalensis Beccari) en el municipio de Guastatoya, El Progreso. Ph.D. thesis, Universidad de San Carlos de Guatemala, Guatemala.
Pautasso, M., and Parmentier, I. (2007). Are the living collections of the world’s botanical gardens following species-richness patterns observed in natural ecosystems? Bot. Helv. 117, 15–28. doi: 10.1007/s00035-007-0786-y
Peguero, B., Jiménez, F., Joseph, P. A., Cinea, W., Griffith, M. P., Francisco-Ortega, J., et al. (2015). Coccothrinax jimenezii – A Critically Endangered Palm from Hispaniola. Palms 59, 145–153.
Pence, V. C. (2013). In vitro methods and the challenge of exceptional species for Target 8 of the Global Strategy for Plant Conservation. Ann. Mo. Bot. Gard. 99, 214–220. doi: 10.3417/2011112
Perez, T. M., Valverde-Barrante, S. O., Bravo, C., Taylor, T. C., Fadrique, B., Hogan, J. A., et al. (2019). Botanic gardens are an untapped resource for studying the functional ecology of tropical plants. Phil. Trans. R. Soc. B Biol. Sci. 374:20170390. doi: 10.1098/rstb.2017.0390
Pérez-Harguindeguy, N., Diaz, S., Gamier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., et al. (2013). New handbook for standardized measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234.
Pérez, H. E., Criley, R. A., and Baskin, C. C. (2008). Promoting germination in dormant seeds of Pritchardia remota (Kuntze) Beck., an endangered palm endemic to Hawaii. Nat. Area. J. 28, 251–260. doi: 10.3375/0885-8608(2008)28[251:pgidso]2.0.co;2
Porto, K. C. N., Nunes, Y. R. F., and Ribeiro, L. M. (2018). The dynamic of recalcitrant seed banks of Mauritia flexuosa (Arecaceae) reveal adaptations to marsh microenvironments. Plant Ecol. 219, 199–207. doi: 10.1007/s11258-017-0788-9
Quero, H. J. (1998a). Sabal gretheriae. The IUCN Red List of Threatened Species 1998: e.T38692A10139282. Gland: IUCN.
Quero, H. J. (1998b). Sabal pumos. The IUCN Red List of Threatened Species 1998: e.T38693A10139394. Gland: IUCN.
Quero, H. J. (1998c). Sabal uresana. The IUCN Red List of Threatened Species 1998: e.T38694A10139483. Gland: IUCN.
Shapcott, A. (1998). The genetics of Ptychosperma bleeseri, a rare palm from the Northern Territory, Australia. Biol. Conserv. 85, 203–209. doi: 10.1016/s0006-3207(97)00147-x
Tomlinson, P. (1979). Systematics and Ecology of the Palmae. Annu. Rev. Ecol. Syst. 10, 85–107. doi: 10.1146/annurev.es.10.110179.000505
Tucker Lima, J. M., Reyes, J., and Salman, N. (2021). Flower Color Variation in Attalea phalerata (Arecaceae) Revisited. Palms 65, 27–33. doi: 10.15553/c2016v711a6
Valdes, I., Tucker Lima, J. M., and Noblick, L. R. (2021). Pollination of Nypa fruticans (Wurmb.) in a South Florida botanic garden. J. Pollinat. Ecol. 27, 57–64.
Verdecia, R. (2015). Copernicia fallaensis León. Available online at: https://www.iucn.org/sites/dev/files/import/downloads/psg_copernicia_fallaensis.pdf (Accessed August 10, 2020).
Vovides, A. P., Griffith, M. P., Stevenson, D. W., Li, N., Li, Y., Fang, S., et al. (2018). Botanic gardens cycad collections: 4th GBGC symposium report. Mem. N. Y. Bot. Gard. 117, 69–85.
Walter, K. S., and Gillett, H. J. (eds) (1998). 1997 IUCN red list of threatened plants. Switzerland: IUCN.
Wang, H. C., Chen, J. T., Wu, S. P., Lin, M. C., and Chang, W. C. (2003). Plant regeneration through somatic embryogenesis from zygotic embryo-derived callus of Areca catechu L.(Arecaceae). In Vitro Cell. Dev. Biol. Plant 39, 34–36. doi: 10.1079/ivp2002373
Zona, S. (1985). A new species of Sabal (Palmae) from Florida. Brittonia 37, 366–368. doi: 10.2307/2806549
Zona, S. (1990). A monograph of Sabal (Arecaceae: Coryphoideae). Aliso 12, 583–666. doi: 10.5642/aliso.19901204.02
Keywords: Arecaceae, curation, gap analysis, living collection, metacollection
Citation: Griffith MP, Meyer A and Grinage A (2021) Global ex situ Conservation of Palms: Living Treasures for Research and Education. Front. For. Glob. Change 4:711414. doi: 10.3389/ffgc.2021.711414
Received: 18 May 2021; Accepted: 13 August 2021;
Published: 28 September 2021.
Edited by:
Silvia Alvarez-Clare, Morton Arboretum, United StatesReviewed by:
Gerardo Avalos, University of Costa Rica, Costa RicaCopyright © 2021 Griffith, Meyer and Grinage. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Patrick Griffith, cGF0cmlja0Btb250Z29tZXJ5Ym90YW5pY2FsLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.