- 1Department Territorio e Sistemi Agro-Forestali, University of Padova, Legnaro, Italy
- 2Instituto de Biologia, Universidad Nacional Autonoma de Mexico, Mexico City, Mexico
Global climate change-induced droughts are provoking events of forest mortality worldwide, with loss of tree biomass and consequent ecosystem services. Ameliorating the effects of drought requires understanding the causes of forest mortality, with failure of the hydraulic system being an important contributor. Comparative anatomical data strongly suggest that, all else being equal, wider conduits are more vulnerable to drought-induced embolism than narrow ones. However, physiology experiments do not provide consistent support for such a link. If a vulnerability-diameter link exists, though, it would contribute not only to explaining and predicting forest mortality but also to interventions to render individual trees more drought resistant. Given that xylem conduits scale with plant height, taller plants have wider conduits. If there is a vulnerability-diameter link, then this would help explain why taller plants are often more vulnerable to climate change-induced drought. Links between conduit diameter, plant height, and vulnerability would also provide guidance for standardizing sampling of hydraulic variables across individuals and suggest that selecting for relatively narrow conduits at given height from the tree top could produce more drought resistant varieties. As a result, given current ambiguities, together with the potential importance of a link, it is important to maintain the vulnerability-diameter link as a research priority.
Introduction
A major effort is underway to understand the causes of death of millions of trees worldwide under climate change-induced drought (Breshears et al., 2013, 2018; Allen et al., 2015; Anderegg et al., 2015, 2016; Adams et al., 2017; Choat et al., 2018; Trugman et al., 2018, 2021; Brodribb et al., 2020). These studies reveal a multitude of factors involved in forest mortality, from the failure of water transport in the wood, to insect or fungal attack and fire. Identifying the ways mechanisms at the individual level result in the death of trees across the landscape requires integration factors across multiple scales. We wish to highlight that robust integration between experimental data on plant hydraulic function– xylem physiology– with comparative anatomical data– morphological variation across species– would provide better direction for research and more robust explanations than for comparative and experimental workers to continue research largely independently. The key point we focus on here is the possible link between vulnerability to drought-induced hydraulic failure and xylem water-transporting conduit diameter. If such a link exists, then it has a potentially very important role to play in explaining forest mortality events on large scales. Testing this possibility requires squaring comparative anatomical, across-species, data with the experimental results of xylem physiology studies.
Experimental Data Are Inconsistent With Regard to the Link
Physiology experiments have so far failed to find a consistent link between vulnerability to drought-induced embolism and conduit diameter, and likely as a result, recent reviews of the causes of forest mortality do not mention a possible vulnerability-conduit diameter link at all, suggesting that the link is not regarded as a significant potential player (Anderegg et al., 2015, 2016; Adams et al., 2017; Breshears et al., 2018; Stovall et al., 2019; Liang et al., 2020; Trugman et al., 2021). Some physiology experiments show a strong correlation, with the widest vessels in a given stem segment clearly being the first to embolize (Hargrave et al., 1994; Cai and Tyree, 2010). Others suggest that the link is sheer experimental error, known as the “open vessel artifact” (Martin-StPaul et al., 2014; Rockwell et al., 2014; Torres-Ruiz et al., 2015). Other studies examine situations in which the open vessel artifact would seem impossible and even so still fail to recover a vulnerability-diameter link (Cochard et al., 2015; Choat et al., 2016; Brodersen et al., 2018; Bouda et al., 2019; Jacobsen et al., 2019). These include minimally invasive techniques such as micro-computed x-ray tomography, which unlike traditional techniques do not require cutting stems into segments and deranging water columns under tension. Most such studies do not provide striking evidence that wider conduits are more vulnerable than narrow ones (Cochard et al., 2015; Choat et al., 2016; Brodersen et al., 2018; Bouda et al., 2019). At least one micro-ct study, though, suggests that vessels with larger volumes are indeed more susceptible to embolism (Jacobsen et al., 2019). Since conduits widen predictably from the tips of plant twigs to the trunk base and into the roots (Lechthaler et al., 2020; Olson et al., 2021), all else being equal (including xylem tension), roots should be more vulnerable than stems but this result is not always reported (Rodriguez-Dominguez et al., 2018; Wu et al., 2020). Thus, experimental evidence for the link is currently contradictory.
Moreover, there is not even a clear theoretical reason to expect a vulnerability-diameter link. This is in contrast to freezing-induced embolism, which has a consistent and well-understood relationship with conduit diameter (Cavender-Bares and Holbrook, 2001; Pittermann and Sperry, 2003, 2006; Cavender-Bares, 2005; Sevanto et al., 2012; Savage and Cavender-Bares, 2013). Failure of water transport under drought is certain to involve multiple factors, from wood density and pit membrane characteristics to internal conduit sculpture, the types of conductive cells present in the xylem and where gas exists in the xylem (Dalla-Salda et al., 2011; Sano et al., 2011; Li et al., 2016; Guan et al., 2021; Kaack et al., 2021). But all else being equal, wider conduits tend to be longer than narrow conduits, with a higher number of pits, which are often wider and with wider membrane pores (Martínez-Vilalta et al., 2002; Jacobsen et al., 2012, 2019). These traits should facilitate the spread of embolism in wider conduits. If wider conduits are also longer, then embolism propagation will occupy a greater volume in wider conduits (Comstock and Sperry, 2000; Jacobsen et al., 2012). If pit area is uniform across conduits, then conduits with larger volumes will have greater pit area. Greater pit area would offer more opportunity for air-seeding to adjacent conduits. So, even in the absence of a direct link between vulnerability and diameter, there are plausible reasons to suspect that natural selection could favor narrower conduits in situations of drought vulnerability. Yet studies fail to recover the predicted pit area-vulnerability relationships (Lens et al., 2011). Presumably it is on the basis of such inconsistent results regarding a link between vulnerability to drought-induced embolism and xylem conduit diameter that recent treatments of the causes of drought-induced mortality do not mention conduit diameter. And yet, if there were a link, it would contribute to explaining so much about forest mortality and plant adaptation in general.
What Is at Stake: What the Vulnerability-Diameter Link Would Explain If There Were One
Testing the link between drought-induced embolism vulnerability and conduit diameter remains a priority because of the explanatory reach it would have if such a link existed, and that it is consistent with comparative anatomical evidence often spanning hundreds or thousands of species (Olson, 2020). We now briefly turn to some examples relevant to studies of forest mortality and drought adaptation.
Why Taller Individuals Are More Vulnerable to Drought
Taller trees are often more vulnerable to drought than their smaller conspecifics (Lindenmayer and Laurance, 2016, 2017; Olson et al., 2018; Stovall et al., 2019; McGregor et al., 2020; Swemmer, 2020). Because death of large trees has disproportionate ecological consequences, explaining the higher vulnerability of large individuals is a focus of plant scientists (Bennett et al., 2015; Stovall et al., 2019; Bartholomew et al., 2020; McGregor et al., 2020). For example, within a community the tallest trees make up a disproportionate part of total aboveground biomass (Lutz et al., 2018; Enquist et al., 2019), making protecting old-growth forests key for forest carbon storage (Körner, 2017). Scientists have suggested that tall trees are more vulnerable because of the effects of gravity and resistance on their long hydraulic pathlengths, the higher vapor pressure deficit of the canopy, higher xylem demands for leaf-produced photosynthates, or that larger trees are simply more at risk of being pushed over by wind (Niklas, 1998; Koch et al., 2004; Givnish et al., 2014; McDowell and Allen, 2015; Trugman et al., 2018). The vulnerability-diameter link could be added to this list.
Taller plants have predictably wider conduits (Figure 1), and if wider conduits are more vulnerable to embolism (Figure 2A), then this pervasive scaling of conduit diameter with plant height would contribute to explaining the greater vulnerability of taller plants. In all terrestrial vascular plants studied to date, xylem conduits are very narrow in the terminal leaf veins, widening toward the petiole base (Sack et al., 2012; Gleason et al., 2018; Lechthaler et al., 2019, 2020). In the shoot-root system, conduits are narrowest at the twig tips, widening predictably toward the base (Anfodillo et al., 2006; Petit et al., 2009; Koçillari et al., 2021; Olson et al., 2021). Poiseuille’s Law shows that if conduits remain the same diameter but become longer, then flow to the leaves will decline in direct proportion to length, that is, the decrease in flow as a linear function of conductive path length.
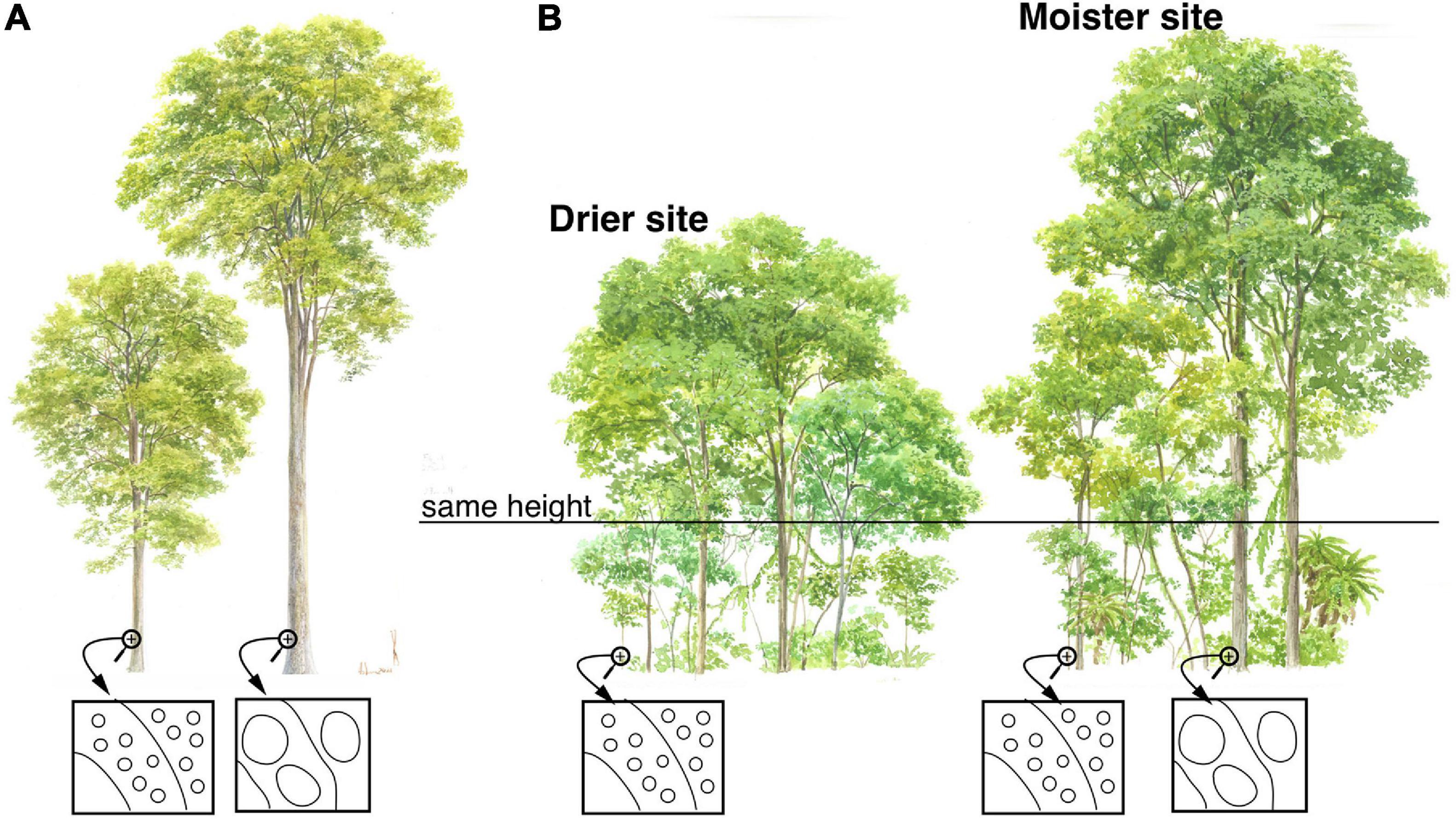
Figure 1. Conduit diameter scales predictably with plant height. The figure shows trees of differing heights, with schematic xylem cross-sections in boxes. The ellipses in the schematics represent vessels. The arcs represent successive layers of xylem produced concentrically by the vascular cambium. (A) Natural selection favors widening of conduits as trees grow taller, thus maintaining conductance to the leaves even though conductive pathlength becomes longer and therefore resistance potentially higher. This predictable widening means that when an individual is small, it has narrower conduits in the outermost wood than when it is tall. (B) Similar-sized plants have, on average, similar mean conduit diameters, regardless of climate. Similar-sized plants in dry sites have similar mean conduit diameters as similar-sized plants in moist areas. Because maximum and therefore mean height is taller in moist as compared to dry areas, community mean conduit diameter is wider in areas with higher moisture availability.
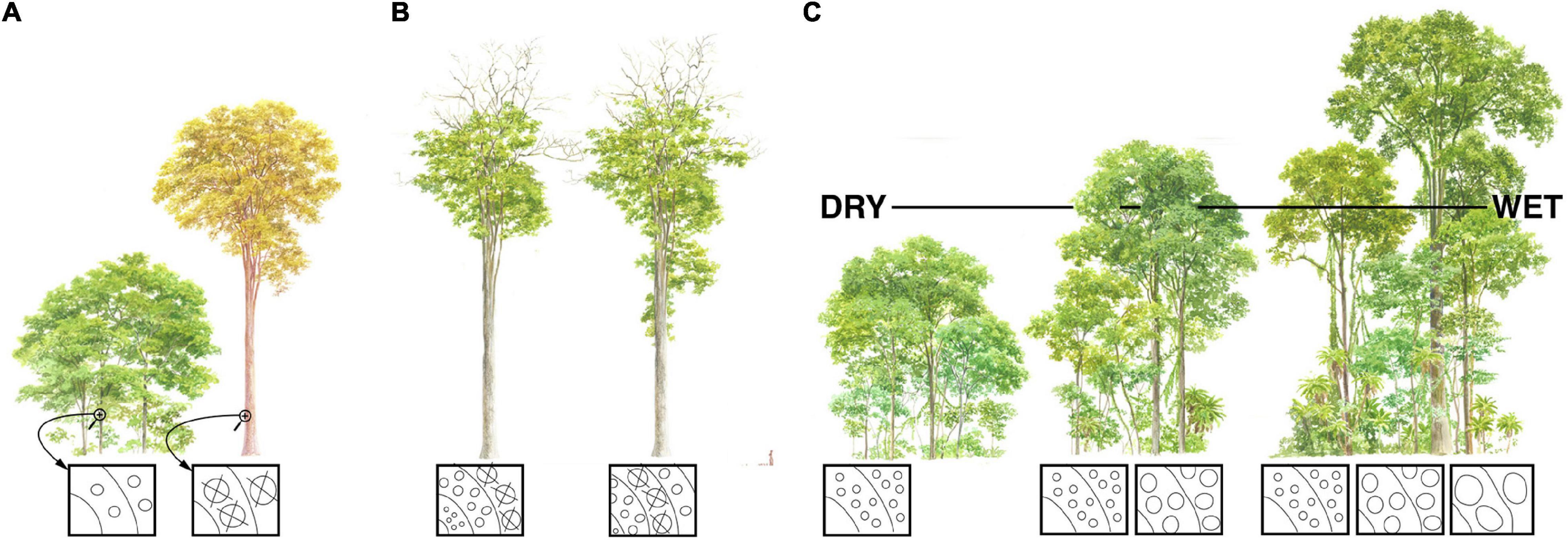
Figure 2. Conduit diameter scaling with plant height, the putative vulnerability-diameter link, and forest mortality. Symbology as for Figure 1; “X”s represent embolized vessels. (A) Large trees tend to be more vulnerable to drought induced mortality. Because taller trees have wider conduits, if wider conduits are more vulnerable to drought-induced embolism than narrow ones, then this would help explain why larger individuals are more susceptible to mortality than smaller conspecifics at the same locality. (B) If wider conduits are more vulnerable to drought induced embolism, it could help explain treetop dieback as an adaptation. If climates become drier, then trees find themselves at heights that are too tall, and therefore conduits too wide, for current drier conditions. Sacrificing terminal branches and resprouting at a lower height would lead to narrower, more embolism-resistant conduits better suited to current conditions. (C) Maximum plant height is taller in moister communities. If climate change-induced drought tends to kill the tallest individuals of a species because of their wide, embolism-vulnerable conduits, then mean forest height will become lower.
However, Poiseuille’s Law also shows that conductance depends on conduit diameter to the fourth power. This means that small increases in conduit diameter are sufficient to counteract the increase in resistance that increasing conductive path length creates. Given heritable within-species variation, all else being equal, individuals with conduits that widen slowly from the base and so are “too narrow” for a given stem length will have high resistance, low photosynthetic productivity, and low fitness. Those with conduits that widen quickly and so are “too wide” for a given stem length will, if wider conduits are more vulnerable to embolism, have a greater incidence of embolism, higher leaf dehydration and stomatal closure, and low fitness. The “just right” pattern is a hydraulic system made up of conduits that widen enough to overcome the resistance that arises as stems grow longer and conductive pathlength increases, but not so wide as to expose the individual to excessive embolism risk. Accordingly, across species, by far stem length is the variable that best predicts conduit diameter (r2≈0.6–0.9) (Anfodillo et al., 2006; Rosell and Olson, 2014; Olson et al., 2018, 2020b), as well as tip-to-base within individuals (r2≈0.8–0.9) (Anfodillo et al., 2006; Koçillari et al., 2021). As a result of this process of natural selection, taller trees [Figure 1; and longer lianas (Rosell and Olson, 2014)] have wider conduits. If wider conduits are more vulnerable to embolism, then this would help explain the greater vulnerability of taller individuals relative to others of the same species subject to the same conditions (Figure 2A) (Olson et al., 2018).
Why Some Individuals Die Whereas Others Do Not
Another aspect that a vulnerability-diameter link might help explain is the observation that one individual can die when apparently similar individuals in similar conditions survive (Trugman et al., 2021). A vulnerability-diameter link could help identify vulnerable individuals as those with relatively wide conduits for a given height. Even if the environmental conditions experienced by all individuals are identical, individuals with relatively wide conduits for a given plant height would be more vulnerable than individuals with narrower conduits. If this were the case, it would allow for relatively straightforward screening of populations to identify individuals with relatively wide conduits given height as the most vulnerable. It would allow for selection of more drought tolerant varieties by selecting those with relatively narrow conduits for a given height (Rosner et al., 2016). Additionally, it predicts that pruning in such a way that conductive path length is shortened and thus conduit diameter is narrower, would lower the drought vulnerability of a single individual.
Individual Tree Height
If narrower conduits are more drought resistant, and shorter plants have narrower conduits, then it seems likely that future forests will be shorter, with massive impacts on ecosystem services (Fajardo et al., 2019). If it were possible to select for individuals with slightly narrower conduits in the context of the same height, it might be possible to maintain tree biomass while still increasing drought resistance (cf. Rosner et al., 2016).
A vulnerability-diameter link would help explain the observed patterns of terminal branch dieback and resprouting that are observed in long-lived trees and in trees that survive drought (Figure 2B). Dead trunks, often hidden within taller foliage, at the tops of very old trees suggests that their terminal trunks have died and resprouted repeatedly over the centuries (Koch et al., 2004). Moist conditions impose less risk of embolism, permitting wider conduits. Wider conduits in turn permit greater conductive path lengths and thus height. When, over the decades or centuries, conditions become drier, trees can sacrifice their distalmost portions, shortening their total pathlenths. Given a constant rate of tip-to-base conduit widening, then shorter pathlengths lead to narrower conduits. If there is a link between vulnerability and conduit diameter, then these now-narrower conduits are better suited to the novel drier conditions that the trees are now subjected. The return of moister conditions again permits taller growth and the overtopping of the sacrificed trunk, accounting for dead snags in the tops of tall trees.
As with fluctuating heights in ancient trees, a vulnerability-diameter link would also contribute to explaining treetop dieback as an adaptive phenomenon (Rood et al., 2000; Olson et al., 2021). When drought sets in, trees find themselves at heights, and thus conduit diameters, that are excessive given current water availability. In such cases, natural selection favors shedding of terminal branches and re-sprouting at lower heights, allowing narrower, more embolism-resistant conduits better suited to current conditions (Figure 2B) (Rood et al., 2000; Olson et al., 2018). If a vulnerability-diameter relationship were to exist, no matter how indirect, it would help explain global patterns of vegetation height limitation, drought vulnerability, and dieback.
Why Maximum Plant Height Is Limited Across Sites of Differing Water Availability
If there were a link between drought-induced embolism vulnerability and conduit diameter, it would also help explain the global tendency for maximum vegetation height to be taller in areas of greater water availability. Minimum plant height tends to be the same across communities (Figure 2C). Where variation is most conspicuous is at the upper end of the height range. Drought-prone communities such as frost-free deserts have very short maximum plant heights (Olson et al., 2020a). In contrast, frost-free rainforests can support trees well above 60 m tall (Adams et al., 2017; Shenkin et al., 2019). If moister conditions permit wider, more embolism-vulnerable conduits, and wider conduits are associated with longer pathlengths, then it helps explain why taller ecological strategies are observed in moister areas (Figure 2C).
If there is a link between embolism vulnerability and conduit diameter, then given a constant rate of tip-to-base conduit widening with plant height, a clone planted on a dry site will require a narrow, embolism resistant mean conduit diameter. Because narrow conduits are associated with shorter pathlengths, then clones on a dry microsite will cease growth at a lower height, associated with narrower conduits, than those on moist sites. Clones on moist sites are exposed to lower embolism risk and can produce wider conduits. Wider conduits are associated with longer stems, so the clones planted on moist microsites should grow taller than those on dry ones. The ability to detect embolism risk and adjust conduit diameter and therefore height according to microsite conditions, should be part of the adaptive phenotypic plasticity of all woody plant species. The patterns of variation in plant height globally across communities, as well as within species across microsites, would at least partially seem explicable given a link between drought-induced vulnerability to embolism and conduit diameter (Olson et al., 2018).
Growth Rings in Drought-Prone Plants
The vulnerability-diameter link would also help explain why species with conduit diameters that vary within growth rings in drought-prone, frost-free areas always have wider conduits in earlywood and narrow ones in latewood (Carlquist, 2001; Silva et al., 2019, 2021). Wider conduits are produced early in the growing season when water is abundant and soil water potentials are not highly negative. As the rainy season wanes and drought begins to set it, more highly negative xylem tensions are required to draw water from the soil. These more negative tensions, in turn, require narrower, more embolism-resistant conduits, thus explaining why conduit diameters in dryland species with growth rings go from wide to narrow, and potentially even why tangential diameter might predict dieback better than radial (Rosner et al., 2016). The vulnerability-diameter link thus potentially participates in explaining not only currently puzzling patterns of global tree mortality, but also the action of natural selection in shaping plant hydraulic systems as a function of climate, habitat, microsite, and habit.
The Vital Interdependence Between Physiological and Comparative Anatomical Evidence of Xylem Function
For inferring xylem function, it is essential for xylem physiologists and comparative anatomists to work together. This is because “function” in biology implies adaptation (Garson, 2016; Olson, 2020). Because adaptation is an evolutionary process reaching into the distant and unobservable past, it requires adducing evidence from as many complementary sources as possible (Olson and Arroyo-Santos, 2015). Two very important sources are xylem physiology experiments and comparative anatomy studies. Both have their weaknesses, which are, happily, largely filled by the strengths of the other. Xylem physiology gains its relevance for inference of function via the assumption that the structure-function relations observed in an experiment are similar even in unobserved individuals, both contemporary and past. The comparative method tests this assumption (Olson et al., 2021). In xylem functional biology the most abundant comparative data are from comparative wood anatomy. For example, experiments show that narrow conifer tracheids are more resistant to freezing-induced embolism (Pittermann and Sperry, 2003, 2006; Sevanto et al., 2012), but these experiments have only covered small parts of a few species. Comparative data spanning hundreds of species are consistent with experimental observations, with conifers in very cold areas, as in plants at or above the treeline, having very narrow tracheids and short stature (e.g., Podocarpus nivalis, Phyllocladus trichomanoides var. alpinus, Microcachrys, etc.). In this way, experiments provide mechanistic detail but very limited generality; comparative wood anatomy provides maximal generality but very limited mechanistic detail. As a result, as in all of biology, there is an essential back-and-forth between experimental and comparative data (Mayr, 1982; Olson and Arroyo-Santos, 2015).
Conclusion: Pervasive Patterns Require Explanation
Optimal tests of the putative link between vulnerability to drought-induced embolism and conduit diameter, examining the vulnerability of a wide range of conduit diameters under similar tensions in species adapted to frost-free, drought-prone habitats, have never been performed. Most physiological data come from temperate zone plants, so adaptation to cold has shaped the conductive systems of these species. As a result, caution is warranted before rejecting outright the possibility of some link existing. The comparative evidence suggesting such a link, e.g., tip-to-base conduit widening, wide-to-narrow conduits in growth rings, wider maximum conduit diameters in moister areas, the wide variance in vessel diameters in lianas (Rosell and Olson, 2014), etc., include without a doubt among the most widespread and pervasive patterns in all of xylem structure. A vulnerability-diameter link would not only contribute to explaining these patterns but also in predicting and potentially mitigating drought-induced damage to forests. Given this explanatory reach, until a plausible alternative explanation is provided for these important comparative patterns, then the vulnerability-diameter link, however, indirect it might be, must remain a research priority for xylem hydraulic biology.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
TA and MO developed the idea and wrote the paper. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Consejo Nacional de Ciencia y Tecnología project A1-S-26934 and PAPIIT-DGAPA, UNAM, project IN210719 for funding. Illustrations by Aslam Narvaez-Parra.
References
Adams, H. D., Zeppel, M. J. B., Anderegg, W. R. L., Hartmann, H., Landhäusser, S. M., Tissue, D. T., et al. (2017). A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1, 1285–1291. doi: 10.1038/s41559-017-0248-x
Allen, C. D., Breshears, D. D., and McDowell, N. G. (2015). On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 1–55. doi: 10.1890/ES15-00203.1
Anderegg, W. R. L., Flint, A., Huang, C., Flint, L., Berry, J. A., Davis, F. W., et al. (2015). Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 8, 367–371. doi: 10.1038/ngeo2400
Anderegg, W. R. L., Klein, T., Bartlett, M., Sack, L., Pellegrini, A. F. A., Choat, B., et al. (2016). Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U.S.A. 113, 5024–5029. doi: 10.1073/pnas.1525678113
Anfodillo, T., Carraro, V., Carrer, M., Fior, C., and Rossi, S. (2006). Convergent tapering of xylem conduits in different woody species. New Phytol. 169, 279–290. doi: 10.1111/j.1469-8137.2005.01587.x
Bartholomew, D. C., Bittencourt, P. R. L., Costa, A. C. L., Banin, L. F., Britto Costa, P., Coughlin, S. I., et al. (2020). Small tropical forest trees have a greater capacity to adjust carbon metabolism to long-term drought than large canopy trees. Plant Cell Environ. 43, 2380–2393. doi: 10.1111/pce.13838
Bennett, A. C., McDowell, N. G., Allen, C. D., and Anderson-Teixeira, K. J. (2015). Larger trees suffer most during drought in forests worldwide. Nat. Plants 1:15139. doi: 10.1038/nplants.2015.139
Bouda, M., Windt, C. W., McElrone, A. J., and Brodersen, C. R. (2019). In vivo pressure gradient heterogeneity increases flow contribution of small diameter vessels in grapevine. Nat. Commun. 10:5645. doi: 10.1038/s41467-019-13673-6
Breshears, D. D., Adams, H. D., Eamus, D., McDowell, N. G., Law, D. J., Will, R. E., et al. (2013). The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant Sci. 4:266. doi: 10.3389/fpls.2013.00266
Breshears, D. D., Carroll, C. J. W., Redmond, M. D., Wion, A. P., Allen, C. D., Cobb, N. S., et al. (2018). A dirty dozen ways to die: metrics and modifiers of mortality driven by drought and warming for a tree species. Front. For. Glob. Change 1:4. doi: 10.3389/ffgc.2018.00004
Brodersen, C. R., Knipfer, T., and McElrone, A. J. (2018). In vivo visualization of the final stages of xylem vessel refilling in grapevine (Vitis vinifera) stems. New Phytol. 217, 117–126. doi: 10.1111/nph.14811
Brodribb, T. J., Powers, J., Cochard, H., and Choat, B. (2020). Hanging by a thread? Forests and drought. Science 368, 261–266. doi: 10.1126/science.aat7631
Cai, J., and Tyree, M. T. (2010). The impact of vessel size on vulnerability curves: data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant Cell Environ. 33, 1059–1069. doi: 10.1111/j.1365-3040.2010.02127.x
Cavender-Bares, J. (2005). “Impacts of freezing on long distance transport in woody plants,” in Vascular Transport in Plants, eds N. M. Holbrook and M. A. Zwieniecki (San Diego, CA: Academic Press), 401–424. doi: 10.1016/B978-012088457-5/50021-6
Cavender-Bares, J., and Holbrook, N. M. (2001). Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with contrasting habitats. Plant Cell Environ. 24, 1243–1256. doi: 10.1046/j.1365-3040.2001.00797.x
Choat, B., Badel, E., Burlett, R., Delzon, S., Cochard, H., and Jansen, S. (2016). Noninvasive measurement of vulnerability to drought-induced embolism by x-ray microtomography. Plant Physiol. 170, 273–282. doi: 10.1104/pp.15.00732
Choat, B., Brodribb, T. J., Brodersen, C. R., Duursma, R. A., López, R., and Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature 558, 531–539. doi: 10.1038/s41586-018-0240-x
Cochard, H., Delzon, S., and Badel, E. (2015). X-ray microtomography (micro-CT): a reference technology for high-resolution quantification of xylem embolism in trees: a reference method for xylem embolism. Plant Cell Environ. 38, 201–206. doi: 10.1111/pce.12391
Comstock, J. P., and Sperry, J. S. (2000). Theoretical considerations of optimal conduit length for water transport in vascular plants. New Phytol. 148, 195–218.
Dalla-Salda, G., Martinez-Meier, A., Cochard, H., and Rozenberg, P. (2011). Genetic variation of xylem hydraulic properties shows that wood density is involved in adaptation to drought in Douglas-fir (Pseudotsuga menziesii (Mirb.)). Ann. For. Sci. 68, 747–757. doi: 10.1007/s13595-011-0091-1
Enquist, B. J., Abraham, A. J., Harfoot, M. B. J., Malhi, Y., and Doughty, C. E. (2019). On the importance of the megabiota to the functioning of the biosphere. EcoEvoRxiv [Preprint]. doi: 10.32942/osf.io/hn9xs
Fajardo, A., McIntire, E. J. B., and Olson, M. E. (2019). When short stature is an asset in trees. Trends Ecol. Evol. 34, 193–199. doi: 10.1016/j.tree.2018.10.011
Givnish, T. J., Wong, S. C., Stuart-Williams, H., Holloway-Phillips, M., and Farquhar, G. D. (2014). Determinants of maximum tree height in Eucalyptus species along a rainfall gradient in Victoria, Australia. Ecology 95, 2991–3007. doi: 10.1890/14-0240.1
Gleason, S. M., Blackman, C. J., Gleason, S. T., McCulloh, K. A., Ocheltree, T. W., and Westoby, M. (2018). Vessel scaling in evergreen angiosperm leaves conforms with Murray’s law and area-filling assumptions: implications for plant size, leaf size and cold tolerance. New Phytol. 218, 1360–1370. doi: 10.1111/nph.15116
Guan, X., Pereira, L., McAdam, S. A. M., Cao, K., and Jansen, S. (2021). No gas source, no problem: Proximity to pre-existing embolism and segmentation affect embolism spreading in angiosperm xylem by gas diffusion. Plant Cell Environ. 44, 1329–1345. doi: 10.1111/pce.14016
Hargrave, K. R., Kolb, K. J., Ewers, F. W., and Davis, S. D. (1994). Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytol. 126, 695–705. doi: 10.1111/j.1469-8137.1994.tb02964.x
Jacobsen, A. L., Brandon Pratt, R., Venturas, M. D., Hacke, U. G., and Lens, F. (2019). Large volume vessels are vulnerable to water-stress-induced embolism in stems of poplar. IAWA J. 40, 4–22. doi: 10.1163/22941932-40190233
Jacobsen, A. L., Pratt, R. B., Tobin, M. F., Hacke, U. G., and Ewers, F. W. (2012). A global analysis of xylem vessel length in woody plants. Am. J. Bot. 99, 1583–1591. doi: 10.3732/ajb.1200140
Kaack, L., Weber, M., Isasa, E., Karimi, Z., Li, S., Pereira, L., et al. (2021). Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytol. 230, 1829–1843. doi: 10.1111/nph.17282
Koch, G. W., Sillett, S. C., Jennings, G. M., and Davis, S. D. (2004). The limits to tree height. Nature 428, 851–854. doi: 10.1038/nature02417
Koçillari, L., Olson, M. E., Suweis, S., Rocha, R. P., Lovison, A., Cardin, F., et al. (2021). The widenened pipe model of plant hydraulic evolution. Proc. Natl. Acad. Sci. U.S.A. 118:e2100314118.
Lechthaler, S., Colangeli, P., Gazzabin, M., and Anfodillo, T. (2019). Axial anatomy of the leaf midrib provides new insights into the hydraulic architecture and cavitation patterns of Acer pseudoplatanus leaves. J. Exp. Bot. 70, 6195–6201. doi: 10.1093/jxb/erz347
Lechthaler, S., Kiorapostolou, N., Pitacco, A., Anfodillo, T., and Petit, G. (2020). The total path length hydraulic resistance according to known anatomical patterns: what is the shape of the root-to-leaf tension gradient along the plant longitudinal axis? J. Theor. Biol. 502:110369. doi: 10.1016/j.jtbi.2020.110369
Lens, F., Sperry, J. S., Christman, M. A., Choat, B., Rabaey, D., and Jansen, S. (2011). Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. N. Phytol. 190, 709–723. doi: 10.1111/j.1469-8137.2010.03518.x
Li, S., Klepsch, M., Jansen, S., Schmitt, M., Lens, F., Karimi, Z., et al. (2016). Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 37, 152–171. doi: 10.1163/22941932-20160128
Liang, X., Ye, Q., Liu, H., and Brodribb, T. J. (2020). Wood density predicts mortality threshold for diverse trees. New Phytol. 229, 3053–3057. doi: 10.1111/nph.17117
Lindenmayer, D. B., and Laurance, W. F. (2016). The ecology, distribution, conservation and management of large old trees: ecology and management of large old trees. Biol. Rev. 92, 1434–1458. doi: 10.1111/brv.12290
Lindenmayer, D. B., and Laurance, W. F. (2017). The ecology, distribution, conservation and management of large old trees. Biol. Rev. 92, 1434–1458. doi: 10.1111/brv.12290
Lutz, J. A., Furniss, T. J., Johnson, D. J., Davies, S. J., Allen, D., Alonso, A., et al. (2018). Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 27, 849–864. doi: 10.1111/geb.12747
Martin-StPaul, N. K., Longepierre, D., Huc, R., Delzon, S., Burlett, R., Joffre, R., et al. (2014). How reliable are methods to assess xylem vulnerability to cavitation? The issue of ‘open vessel’ artifact in oaks. Tree Physiol. 34, 894–905. doi: 10.1093/treephys/tpu059
Martínez-Vilalta, J., Prat, E., Oliveras, I., and Piñol, J. (2002). Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia 133, 19–29.
Mayr, E. (1982). The Growth Of Biological Thought: Diversity, Evolution, and Inheritance. Cambridge, MA: Belknap Press.
McDowell, N. G., and Allen, C. D. (2015). Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 5, 669–672. doi: 10.1038/nclimate2641
McGregor, I. R., Helcoski, R., Kunert, N., Tepley, A. J., Gonzalez-Akre, E. B., Herrmann, V., et al. (2020). Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol. 231, 601–616. doi: 10.1111/nph.16996
Niklas, K. J. (1998). The influence of gravity and wind on land plant evolution. Revi. Palaeobot. Palynol. 102, 1–14. doi: 10.1016/S0034-6667(98)00011-6
Olson, M. E. (2020). From Carlquist’s ecological wood anatomy to Carlquist’s Law: why comparative anatomy is crucial for functional xylem biology. Am. J. Bot. 107, 1328–1341. doi: 10.1002/ajb2.1552
Olson, M. E., Anfodillo, T., Gleason, S. M., and McCulloh, K. A. (2021). Tip-to-base xylem conduit widening as an adaptation: causes, consequences, and empirical priorities. New Phytol. 229, 1877–1893. doi: 10.1111/nph.16961
Olson, M. E., Anfodillo, T., Rosell, J. A., and Martínez-Méndez, N. (2020a). Across climates and species, higher vapor pressure deficit is associated with wider vessels for plants of the same height. Plant Cell Environ. 43, 3068–3080. doi: 10.1111/pce.13884
Olson, M. E., and Arroyo-Santos, A. (2015). How to study adaptation (and why to do it that way). Q. Rev. Biol. 90, 167–191. doi: 10.1086/681438
Olson, M. E., Rosell, J. A., Martínez-Pérez, C., León-Gómez, C., Fajardo, A., Isnard, S., et al. (2020b). Xylem vessel diameter-shoot length scaling: ecological significance of porosity types and other traits. Ecological Monographs 90:e01410 doi: 10.1002/ecm.1410
Olson, M. E., Soriano, D., Rosell, J. A., Anfodillo, T., Donoghue, M. J., Edwards, E. J., et al. (2018). Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. U.S.A. 115, 7551–7556. doi: 10.1073/pnas.1721728115
Petit, G., Anfodillo, T., and De Zan, C. (2009). Degree of tapering of xylem conduits in stems and roots of small Pinus cembra and Larix decidua trees. Botany 87, 501–508. doi: 10.1139/B09-025
Pittermann, J., and Sperry, J. (2003). Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol. 23, 907–914.
Pittermann, J., and Sperry, J. S. (2006). Analysis of freeze-thaw embolism in conifers. The interaction between cavitation pressure and tracheid size. Plant Physiol. 140, 374–382. doi: 10.1104/pp.105.067900
Rockwell, F. E., Wheeler, J. K., and Holbrook, N. M. (2014). Cavitation and its discontents: opportunities for resolving current controversies. Plant Physiol. 164, 1649–1660. doi: 10.1104/pp.113.233817
Rodriguez-Dominguez, C. M., Carins Murphy, M. R., Lucani, C., and Brodribb, T. J. (2018). Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytol. 218, 1025–1035. doi: 10.1111/nph.15079
Rood, S. B., Patiño, S., Coombs, K., and Tyree, M. T. (2000). Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14, 248–257. doi: 10.1007/s004680050010
Rosell, J. A., and Olson, M. E. (2014). Do lianas really have wide vessels? Vessel diameter–stem length scaling in non-self-supporting plants. Perspect. Plant Ecol. Evol. Syst. 16, 288–295. doi: 10.1016/j.ppees.2014.08.001
Rosner, S., Světlík, J., Andreassen, K., Børja, I., Dalsgaard, L., Evans, R., et al. (2016). Novel hydraulic vulnerability proxies for a boreal conifer species reveal that opportunists may have lower survival prospects under extreme climatic events. Front. Plant Sci. 7:831. doi: 10.3389/fpls.2016.00831
Sack, L., Scoffoni, C., McKown, A. D., Rawls, M., Havran, J. C., Tran, H., et al. (2012). Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun. 3, 1–10. doi: 10.1038/ncomms1835
Sano, Y., Morris, H., Shimada, H., Ronse De Craene, L. P., and Jansen, S. (2011). Anatomical features associated with water transport in imperforate tracheary elements of vessel-bearing angiosperms. Ann. Bot. 107, 953–964. doi: 10.1093/aob/mcr042
Savage, J. A., and Cavender-Bares, J. (2013). Phenological cues drive an apparent trade-off between freezing tolerance and growth in the family Salicaceae. Ecology 94, 1708–1717. doi: 10.1890/12-1779.1
Sevanto, S., Holbrook, N. M., and Ball, M. C. (2012). Freeze/thaw-induced embolism: probability of critical bubble formation depends on speed of ice formation. Front. Plant Sci. 3:107. doi: 10.3389/fpls.2012.00107
Shenkin, A., Chandler, C. J., Boyd, D. S., Jackson, T., Disney, M., Majalap, N., et al. (2019). The world’s tallest tropical tree in three dimensions. Front. For. Glob. Change 2:32. doi: 10.3389/ffgc.2019.00032
Silva, M., dos, S., Funch, L. S., and da Silva, L. B. (2019). The growth ring concept: seeking a broader and unambiguous approach covering tropical species. Biol. Rev. 94, 1161–1178. doi: 10.1111/brv.12495
Silva, M. dos S., Funch, L. S., Silva, L. B., and Cardoso, D. (2021). A phylogenetic and functional perspective on the origin and evolutionary shifts of growth ring anatomical markers in seed plants. Biol. Rev. 96, 842–876. doi: 10.1111/brv.12681
Stovall, A. E. L., Shugart, H., and Yang, X. (2019). Tree height explains mortality risk during an intense drought. Nat. Commun. 10:4385. doi: 10.1038/s41467-019-12380-6
Swemmer, A. (2020). Locally high, but regionally low: the impact of the 2014–2016 drought on the trees of semi-arid savannas, South Africa. Afr. J. Range Forage Sci. 37, 31–42. doi: 10.2989/10220119.2020.1723696
Torres-Ruiz, J. M., Jansen, S., Choat, B., McElrone, A. J., Cochard, H., Brodribb, T. J., et al. (2015). Direct X-ray microtomography observation confirms the induction of embolism upon xylem cutting under tension. Plant Physiol. 167, 40–43. doi: 10.1104/pp.114.249706
Trugman, A. T., Anderegg, L. D. L., Anderegg, W. R. L., Das, A. J., and Stephenson, N. L. (2021). Why is tree drought mortality so hard to predict? Trends Ecol. Evol. 36, 520–532. doi: 10.1016/j.tree.2021.02.001
Trugman, A. T., Detto, M., Bartlett, M. K., Medvigy, D., Anderegg, W. R. L., Schwalm, C., et al. (2018). Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 21, 1552–1560. doi: 10.1111/ele.13136
Keywords: hydraulic architecture, tree height, tip-to-base xylem conduit widening, comparative anatomical data, climate change
Citation: Anfodillo T and Olson ME (2021) Tree Mortality: Testing the Link Between Drought, Embolism Vulnerability, and Xylem Conduit Diameter Remains a Priority. Front. For. Glob. Change 4:704670. doi: 10.3389/ffgc.2021.704670
Received: 03 May 2021; Accepted: 07 July 2021;
Published: 16 August 2021.
Edited by:
Giovanna Battipaglia, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Sabine Rosner, University of Natural Resources and Life Sciences Vienna, AustriaRobert Muscarella, Uppsala University, Sweden
Copyright © 2021 Anfodillo and Olson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark E. Olson, bW9sc29uQGliLnVuYW0ubXg=
 Tommaso Anfodillo
Tommaso Anfodillo Mark E. Olson
Mark E. Olson