- 1College of Agricultural and Life Sciences, University of Idaho, Moscow, ID, United States
- 2Agriculture and Agri-Food Canada, Swift Current Research and Development Centre, Swift Current, SK, Canada
- 3Department of Soil Science, North Dakota State University, Fargo, ND, United States
In the Lake Tahoe Basin in California and Nevada (USA), managing nutrient export from watersheds into streams and the lake is a significant challenge that needs to be addressed to improve water quality. Leaching and runoff of phosphorus (P) from soils is a major nutrient source to the lake, and P loading potential from different watersheds varies as a function of landscape and ecosystem properties, and how the watershed is managed. In this research, P availability and speciation in forest and meadow soils in the Lake Tahoe Basin were measured at two watersheds with different parent material types. Soils developed on andesitic parent materials had approximately twice as much total P compared to those developed on granitic parent materials. Regardless of parent material, organic P was 79–92% of the total P in the meadow soils, and only 13–47% in the forest soils. Most of the soil organic P consisted of monoester P compounds, but a significant amount, especially in meadow soils, was diester P compounds (up to 30% of total extracted P). Water extractable P (WEP) concentrations were ~10 times greater in the granitic forest soils compared to the andesitic forest soils, which had more poorly crystalline aluminosilicates and iron oxides that retain P and thus restrict WEP export. In the meadow soils, microbial biomass P was approximately seven times greater than the forest soils, which may be an important sink for P leached from upland forests. Results show that ecosystem and parent material are important attributes that control P speciation and availability in the Lake Tahoe Basin, and that organic P compounds are a major component of the soil P and are available for leaching from the soils. These factors can be used to develop accurate predictions of P availability and more precise forest management practices to reduce P export into Lake Tahoe.
Introduction
Lake Tahoe, located in the Sierra Nevada Mountain range in California and Nevada, is the sixth largest lake by volume in the United States. It is classified as an ultra-oligotrophic lake, meaning that it has naturally low nutrient concentrations and low primary production, and it is renowned for the clarity of its water (Hatch et al., 2001; Goldberg et al., 2015). In recent years, however, water clarity in Lake Tahoe has declined, with Secchi depth readings decreasing from ~31 m in 1968 to 21.6 m in 2018 (Schladow, 2019). As a result of non-point-source nutrient loading, primary production in Lake Tahoe has increased by ~6% per year (Jassby et al., 1999; Roberts and Reuter, 2010).
Historically, algae growth in Lake Tahoe has been co-limited by nitrogen (N) and phosphorus (P) (Hatch et al., 1999). However, as has been observed for lakes worldwide (Elser et al., 2009), increased atmospheric N loading and N deposition have altered plankton species and N:P stoichiometry, shifting nutrient limitation in Lake Tahoe to P (Hatch et al., 1999; Goldberg et al., 2015). A recent lake-clarity model demonstrated that a return to the historical Secchi depth reading in Lake Tahoe would be possible within 20 years if P loading were reduced by at least 2.75% per year (Sahoo et al., 2010). However, to control P sources and subsequent loading into surface waters, a full understanding of P cycling and species in soils in the Lake Tahoe Basin is required.
The physical forms of P that can enter and cycle in lakes are defined as particulate P that is >0.45 μm, and solution P that can pass through a 0.45-μm filter that consists of dissolved and colloidal P (Bol et al., 2016). Colloidal P particles are 1 to 1,000 nm in diameter and the colloids less than 450 nm can pass through a 0.45 μm filter (Jiang et al., 2017), and can thus be mobilized through soils and remain suspended in surface waters. Dissolved, colloidal, or particulate P species can be organic [bound to a carbon (C) group] or inorganic (singular or multiple phosphate groups). Short-term changes in Lake Tahoe primary productivity are well-explained by dissolved inorganic and organic P loads from Lake Tahoe Basin streams, which contribute up to 1,000 kg of dissolved P annually (Hatch et al., 1999). Different forms of P, both physically and chemically, differ in their mobility, environmental reactivity, and bioavailability. Dissolved molybdate-reactive phosphate (MRP), also known as soluble reactive phosphate (SRP), is the most readily bioavailable form (Hatch et al., 1999; Sahoo et al., 2010). Thus, to manage Lake Tahoe Basin landscapes for P-load reduction requires knowledge of soil P species and pools, and their potential for release and transport into surface waters.
About 6% of the Lake Tahoe Basin is considered urban and has been developed for residential and commercial use. Phosphorus inputs within the urban portion of the Basin account for 18% of total P inputs into the lake (Sahoo et al., 2013). In the non-urban regions of the Basin, 50% are covered by forests with yellow pine associations (TRPA, 2015) containing Jeffrey pine (Pinus jeffreyi Balf.), white fir [Abies concolor (Gord. and Glend.) Lindl. Ex Hildebr.], incense cedar [Calocedrus decurrens (Torr.) Florin], and sugar pine (Pinus labertiana Douglas); and another 17% of the Tahoe Basin landscape are red fir associations containing red fir (Abies magnifica A. Murray bis), Jeffrey pine and Lodgepole pine (Pinus contorta Douglas ex Loudon). Wet and dry meadows comprise 2 and 1% of non-urban land, respectively, and contain grasses, sedges, and rushes (TRPA, 2015). Soils in the Lake Tahoe Basin are developed on andesitic, granitic, or mixed parent materials (Coats et al., 2016). Studies in the Eastern Sierra Nevada have shown that forest soils on granitic parent materials can have substantially higher extractable-P concentrations than those developed on andesitic parent materials (Johnson et al., 1997; Coats et al., 2016). However, it is unclear the mechanistic processes that are responsible for these differences.
In soils, inorganic and organic P molecular species have distinct potential for uptake by vegetation or mobilization out of the soil profile. Uselman et al. (2012) suggested that the amount of dissolved organic P in soil solution is largely dependent on the type and amount of above- and below-ground organic matter. Some forest-soil P is exported as particulate P (Prairie and Kalff, 1988). The amount of eroded particulate P that is exported from a site depends on three factors: (1) site geography (slope, climate, and geology); (2) site management (harvest, thinning, and development); and (3) wildfire history (Miller et al., 2006). Particulate P that enters streams and lakes is not directly available for uptake by aquatic organisms, although it can be released as dissolved P from the particles and then is bioavailable to support aquatic algae growth (Young et al., 1985; Reid et al., 2018).
A significant fraction of forest P exists in the plant litter and O horizons that can be illuviated into lower depths in the soil profile or be lost in runoff (Miller et al., 2010; Bol et al., 2016). Miller et al. (2005, 2006) observed that organic horizons on forest floors in Lake Tahoe Basin have high levels of water-soluble P that may be a source of P loading to streams via overland or subsurface flow, the latter of which moves through and reacts with soils. Phosphorus leached from O horizons can be transported into the soils through several mechanisms, depending on the soil physical properties that facilitate preferential vs. matrix flow (Julich et al., 2017; Luo et al., 2019). In alpine environments, spring snowmelt runoff is an important mechanism of P loading because it transports dissolved, colloidal, and particulate P from decomposed forest litter and soils, which can then emerge as subsurface P loading to streams and lakes (Backnäs et al., 2012).
Estimates suggest that groundwater sources make up 15% by mass of total P loading to Lake Tahoe (Roberts and Reuter, 2010). Furthermore, 61% (3,700 kg) of the annual total dissolved P that is found in Lake Tahoe Basin groundwater is believed to be derived from natural sources from unimpacted non-urban areas, predominantly from overlying forest litter P pools and P released from the adsorbed and mineral-bound soil P pool (U.S. Army Corp of Engineers, 2003). Sohrt et al. (2019) used an end-member mixing model that included soil water input to predict that up to 92% of the stream P in a mixed deciduous/evergreen forest in Europe was leached from the mineral soil horizons. Considering the hydrologic interfaces in the soil, it follows that in the Lake Tahoe Basin forests, soil P biogeochemistry is an important factor that controls P discharge into surface waters, and to reduce P loads released from forests it is imperative to understand how site and management factors impact P solubility and mobility.
Although meadows comprise a small area of the Lake Tahoe Basin watershed, they are important controllers of P entering streams because they are transitional zones connecting terrestrial and aquatic ecosystems and are commonly located adjacent to forests (Roby et al., 2015). Some meadows in the Lake Tahoe Basin are categorized as stream environment zones (SEZ), which is a designation used by the Lake Tahoe Basin Management Unit for an area of high value and management priority based on ecosystem services, including the filtering and storage of nutrients in runoff (Roby et al., 2015). Forest-derived P is commonly hydrologically transported through meadow ecosystems, which can act as either sinks that intercept P or sources that release P to streams and lakes. Several groups have studied the capacity of riparian systems to perform these functions. For example, Casey and Klaine (2001) studied P adsorption behavior in meadow soils, including Cumulic Humaquepts (similar taxa are found in some Lake Tahoe Basin meadows), and demonstrated the importance of sorption capacity as a mechanism of nutrient attenuation. They determined that soil P concentration was 100 times below concentrations that would cause soil solution P levels to exceed U.S. Environmental Protection Agency recommendations for lentic waters. In contrast, Hoffmann et al. (2006) found a net loss of P via leaching from soils in riparian meadows during two of three sampling years. In a later study, Hoffmann et al. (2009) concluded that, although sedimentation in riparian buffers is an important mechanism of P retention, these buffers may eventually become significant sources of dissolved reactive P release to surface or groundwater. Gergans et al. (2011) studied nutrient flow through a Lake Tahoe watershed that included a riparian meadow ecosystem and observed that the meadow soils were sources of phosphate into a nearby stream and that the release varied with season. The contrasting reports of nutrient retention and release from meadows highlight the complex nature of meadow biogeochemical processes that can make them either sources or sinks of P into surface waters.
Organic P species in forest soils have been shown to be a dominant loading factor to surface waters (Condron et al., 2005; Sohrt et al., 2017). Backnäs et al. (2012) observed higher soluble organic P (labile monoester and diester P species) in surface horizons of Podzol soils in a mixed-coniferous forest in Finland compared to deeper soils. Anderson and Magdoff (2005) observed higher levels of labile organic P than inorganic P in leachate from packed soil columns leached with DNA (diester P) and orthophosphate solutions. Missong et al. (2016) separated bulk soil extractions from forest soils into colloidal and electrolytic fractions and found most of the extractable P was organic bound P (diesters) on colloids. Brödlin et al. (2019b) studied P forms in soils from three different parent materials in deciduous forests and observed a tendency for organic P to dominate mobilized dissolved P. Bol et al. (2016) reviewed organic P in forested soils and concluded that, although it is a significant component of P cycling, the lack of knowledge of organic P species creates a “blind spot in ecosystem research.” Therefore, the dynamics and vulnerability of P leached from both forest and adjacent meadow soils needs to be investigated to understand the potential impact on water quality. This is especially true in watersheds like in the Lake Tahoe Basin, where nutrients leached through forest soils are major inputs into the lake.
In this paper, we investigated the influence of parent material and ecosystem type on soil P species and solubility in the Lake Tahoe Basin. We hypothesized that there would be distinct P biogeochemistry in forest and meadow ecosystems, and that granitic and andesitic parent materials would influence total and available soil P, as well as the amount and type of organic P. Speciation of P in the soils was determined by extraction and 31P nuclear magnetic resonance spectroscopy (P-NMR) to elucidate organic and inorganic P species. Water-extractable, exchangeable, and microbial-biomass bound soil P were measured to determine soil P fractions that are potentially soluble and labile. These extractions are good predictors of P immobilization and potential runoff from soils (Pote et al., 1996; Campo et al., 1998; Vadas et al., 2005; Wang et al., 2010; Pistocchi et al., 2018).
Methods
Study Sites and Sample Collection
Soils were sampled from two subalpine meadow systems (Paige Meadow and Meeks Meadow) and their adjacent forests. The research sites are located on the west shore of the Lake Tahoe Basin (Figure 1). Paige Meadow is an alluvial floodplain surrounded by forested hillslopes of terminal moraines at elevation ~2,115 m. Meeks Meadow is situated in an elongated glacial valley trough floodplain (elevation ~1,905 m), confined on both sides by steep forested hillslopes of lateral moraines. Separate lobes of the Sierran Ice Cap extended over the present-day locations of both meadows, carving out their current floodplain topographic environments (Ehlers and Gibbard, 2003). At Paige Meadow and its surrounding forest, soils developed on glacial deposits of eroded basaltic and andesitic rocks from Miocene- through Pleistocene-age volcanic activity (Kortemeier et al., 2018). The geologic substrate of the Meeks Meadow watershed is primarily granodiorite eroded from a glacial drift of till and outwash (Saucedo, 2005). Both meadows contain perennial grasses mixed with sedges, rushes, and forbs (Soil Survey Staff, 2007). The forest surrounding Paige Meadow is a red fir forest association, while the forest next to Meeks Meadow consists of a yellow pine association (Soil Survey Staff, 2007; TRPA, 2015). Climatic data from Tahoe City and Rubicon SNOTEL stations show approximate cumulative precipitation of 900 mm and a mean annual temperature of 7.5°C (USDA-NRCS, 2019). Soils at nearby SNOTEL stations fall under a xeric soil moisture and frigid soil temperature regimes. At each location, 3–5 soil profiles were viewed to 20–40 cm depth and characterized using either a shovel or corer. The descriptions of the soils were done following USDA NRCS soil description methods (Schoeneberger et al., 2012).
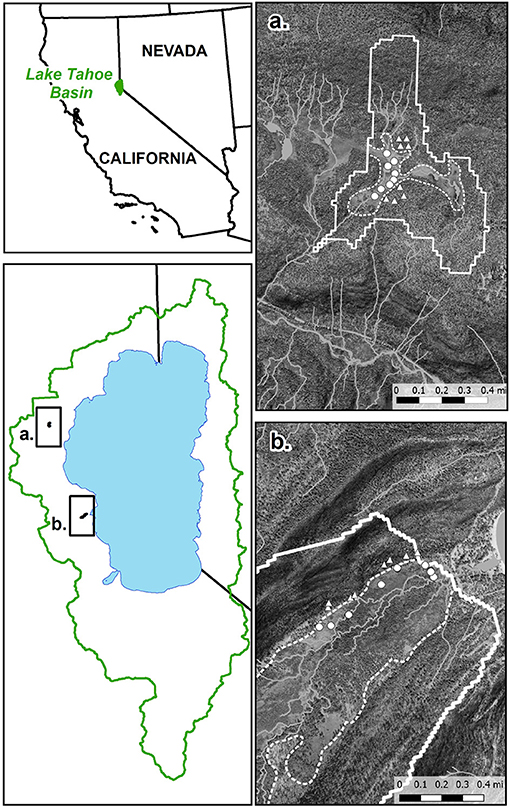
Figure 1. Andesitic watershed at Paige Meadow (a) and granitic watershed at Meeks Meadow (b). Solid lines are watershed boundaries. Dashed lines separate forest soils from meadow soils, based on map units from the SSURGO database. Circles are meadow sampling sites and triangles are forest sampling sites.
Eight locations from each ecosystem-parent material type were sampled in June, August, and October of 2018 (Figure 1). Samples were taken from the top 15 cm of the soil below the O horizon using a 10-cm diameter soil auger. At each of the eight replicate locations, a composite sample was collected by mixing three sub-samples from 1 m radius. After sampling, the soils were stored on ice while in transport to the lab. A portion of each sample was sieved (<2 mm) and oven-dried at 50°C, and the remainder was stored undried in re-sealable plastic bags at 4°C and sieved (<2 mm) immediately prior to analysis. At each site seven to fourteen 7-cm diameter by 15-cm depth intact cores were sampled for bulk density measurement. O-horizon samples were taken by compositing material from several locations at each forest site into a bag that was thoroughly mixed. For P-NMR analysis and P availability extractions, field-moist samples were used, and P concentrations were adjusted using the percent moisture content determined by the difference in mass of water between the field-moist and oven-dried samples. Other analyses used 50°C oven-dried soils.
Laboratory Analyses
Soil Characterization
Replicate samples from the June 2018 sampling were analyzed for pH, percent sand, and concentrations of total organic C (TOC), total N (TN), and oxalate-extractable iron (Fe), aluminum (Al), silicon (Si), and P. Soils pH was measured on soils at 1:1 soil to 18-megaohm deionized water mass ratio. Percent sand by mass was measured by sieving the <2 mm soil fraction through a 63 μm sieve. Bulk density was measured in cores dried at ~50°C and corrected for rocks using granite and andesite density of 2.65 and 2.60 g cm−3, respectively (Soil Survey Staff, 2014). Concentrations of TOC and TN were measured using a CNS dry combustion analyzer (Shimadzu Corporation, Oregon). Soils were extracted for poorly crystalline iron and aluminum oxides in a 1:50 solid-solution ratio of 0.2 M ammonium oxalate solution in darkness (Soil Survey Staff, 2014), shaken for 4 h, allowed to settle overnight, centrifuged (1,500 × g for 30 min), filtered (0.22 μm diameter PES membrane filter), and analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Thermo Scientific, Waltham, Massachusetts) that was calibrated using ISO traceable standards.
Soil Total P
A subset of samples from granitic meadow (n = 6), granitic forest (n = 6), andesitic meadow (n = 5), and andesitic forest (n = 5) soils were analyzed for total P (TP) concentration by an analytical laboratory (Bureau Veritas, Inc.; Vancouver, BC; ISO/IEC 17025 and ISO 9001) using a two-step multi-acid (HNO3-HClO4-HF, and HCl) heated-digestion and analysis by ICP-mass spectrometry.
Total Organic Soil P by Ignition
Total soil organic P concentrations of the same subset of samples used for P-NMR analysis were measured using the ignition method (Saunders and Williams, 1955; Cade-Menun and Lavkulich, 1997). Duplicate 0.5 g subsamples of oven-dried soil were weighed. One replicate was incinerated at 550°C over a 2 h ramp-up period and maintained at this temperature for an additional 1 h followed by a 2-hr cool down. Both samples were then extracted in 1:60 solid-solution ratio of 1 N H2SO4, shaken for ~16 h, centrifuged at 1,500 × g for 15 min, and the supernatant was decanted and analyzed colorimetrically (Murphy and Riley, 1962). Total organic P was calculated as the difference between incinerated and non-incinerated samples. The P concentration in the incinerated sample is an estimate of soil total P (TPinc).
Soil P Speciation by P-NMR Analysis
A subset of samples that included at least two replicates from each soil/ecosystem type were selected for P NMR analysis to identify concentrations and speciation of organic P in the soils. Following standard extraction procedures for P NMR (Cade-Menun and Preston, 1996; Cade-Menun and Liu, 2014), 2 g dry-mass equivalent undried soil subsamples were suspended in 25 ml of 0.5 M NaOH and 0.1 M Na2-EDTA solution, shaken for 4 h, centrifuged at 1,500 × g for 20 min, and the supernatant was decanted and freeze-dried. A 1 ml aliquot of extract was taken from each sample, diluted 1:10 with deionized water, and analyzed by ICP-AES for total P, Fe, and manganese (Mn) concentrations. The P-NMR spectroscopy was conducted at the University of Idaho's Department of Chemistry. Approximately 0.24 g of freeze-dried extract powder from each sample was dissolved in 0.9 ml of NaOH-EDTA solution and 0.1 ml and D2O and 0.5 ml of this solution was placed in a 5-mm NMR tube. The NMR spectra were obtained at 202.48 MHz on a 500 MHz Bruker Avance III spectrometer equipped with a 5-mm broadband probe. The 1D 31P spectra were acquired with 67.5° pulses, at 30°C, with proton decoupling, and a total recycle delay (pre-scan delay plus acquisition time) of 4 s, for 3,000–8,000 scans, determined by signal-to-noise ratios. This delay time will be sufficient for relaxation based on the ratio of P/Fe+Mn in these samples (McDowell et al., 2006; Cade-Menun and Liu, 2014). Spectra were plotted with 7 Hz line-broadening for the main spectra and 2 Hz line-broadening to assess finer details. Peak areas were computed by integration and visual inspection using NUTS software (Acorn NMR, Livermore CA, 2000 edition), with correction for the degradation of orthophosphate diesters (Cade-Menun and Liu, 2014; Schneider et al., 2016). Peak assignments were made from the literature and confirmed using phytate and β-glycerophosphate spikes (Cade-Menun, 2015).
Extractable Soil P
Concentrations of labile soil P were measured using water-extractable P (WEP), Bray-1 P (B1P), and microbial biomass P (MBP) methods. Field-moist soil samples were extracted for WEP in a 1:10 solid-solution ratio of 18 megaohm deionized water, shaken for 1 h, centrifuged (1,500 x g) for 10 min, and filtered through 0.45-μm diameter PES membrane filters (Kuo, 1996; Self-Davis et al., 2009). An aliquot was subsampled from the filtered extract for molybdate colorimetry (Murphy and Riley, 1962). Colorimetry measures phosphate that reacts with molybdate (MRP), which is used as an estimation of inorganic P in solution. However, some organic P compounds may hydrolyze during the colorimetric reaction and are included in the MRP measurement, while complex inorganic P compounds such as polyphosphates will not react with molybdate (Haygarth and Sharpley, 2000; Worsfold et al., 2016). Therefore, we hereafter refer to WEP MRP as WEPMR. The total P in the WEP was analyzed by ICP-AES. The difference between the total WEP and WEPMR concentrations is operationally defined as molybdate-unreactive (WEPMU), which primarily consists of P associated with organic, non-hydrolysable, and colloidal forms (Haygarth et al., 1997; Haygarth and Sharpley, 2000). In addition to soil extraction, five subsamples from composite O-horizon samples from each forest were ground, passed through a 2-mm sieve, and extracted at 1:50 solid solution ratio for WEP and WEPMR.
Field-moist soils were extracted for Bray-1 P (B1P) as described in Sims (2009). An aliquot of the Bray-1 extract was filtered through a 0.45-μm PES membrane filter and measured colorimetrically (B1PMR) and by ICP-AES (B1P). The difference between B1P and B1PMR is the B1P molybdate unreactive (B1PMU).
Microbial biomass P (MBP) was measured by treating a 1 g dry-mass equivalent sample of undried soil using 1 ml of chloroform, placing it under a vacuum with a beaker of ~30 ml of chloroform, allowing it to evaporate for 24 h, and then extracting with the Bray-1 P extractant (Voroney et al., 2008; Reddy et al., 2013). In acidic soils, Bray-1 is a better extract for microbial biomass P than Na-bicarbonate extract (Oberson et al., 1997; Wu et al., 2000). Microbial biomass P was calculated as the difference between chloroform-fumigated and unfumigated samples, without an efficiency correction factor.
Statistical Analyses
The three seasonal samples of WEP, B1P, and MBP were pooled in the statistical analysis using a mixed model to estimate the random and fixed effects. Extract concentrations that were below the method detection limit (MDL) of the ICP (0.05 mg kg−1 for WEP and B1P and 0.1 mg kg−1 for MBP) were assigned values ½ MDL. The extract data were analyzed with a generalized linear mixed model using a log-normal distribution. Landscape type, parent material, and their interaction were evaluated as fixed effects, and sample point was evaluated as a random effect. Repeated measurements on the concentrations from the sample points were modeled using a compound symmetry covariance structure. For all variables except total WEP and WEPMU, sample identification effects were estimated at each time point. Model fit was assessed by examining the log-likelihoods and inspecting residual plots. All analyses were performed in R version 3.6 (R Core Team, 2019) using the packages “nlme” (Pinheiro, 2019) for model building and ANOVA and “emmeans” (Lenth, 2019) for finding the estimated marginal means and conducting comparisons. Tukey honest significance difference (HSD) test (p < 0.05) was used to test significance for the following paired comparisons: andesitic-meadow vs. andesitic forest, granitic-meadow vs. granitic forest, andesitic forest vs. granitic forest, and andesitic meadow vs. granitic meadow.
All other soil data were tested for significance by fitting the data to the analysis of variance (ANOVA) linear models, and Tukey HSD test was used for assessing statistical differences (p < 0.05) between treatment means. Pearson's correlation coefficients were used to evaluate the strength of relationships between soil properties (Origin Lab, Northampton, MA).
Results
Soil Characterization
Upper soil profile descriptions for each watershed are listed in Supplementary Table 1. Both meadow soils are mapped as Inceptisols, and typically have aquic conditions in spring and early summer (Soil Survey Staff, 2007). Meadow soils had a darker chroma of 1 compared to chroma between 2 and 3 in the forest soils. The taxonomic descriptions of the meadow soils include subgroups Cumulic Humaquept at Paige Meadow and Cumulic Humaquept and Aquic/Oxyaquic Dystroxerept at Meeks Meadow. The difference between these subgroups is a higher seasonal water table and an epipedon thick enough to qualify as either mollic or umbric in Cumulic Humaquepts. The Aquic and Oxyaquic Dystroxerepts at Meeks Meadow have slightly deeper water tables and dark ochric epipedons (~ 15 cm) that verge on meeting the thickness requirement of a mollic or umbric epipedon. The forest soils surrounding Paige Meadow are mapped as Humic Vitrixerands. The forest soils at Meeks are mapped as Humic Dystroxerepts.
Organic horizons were ~6 cm thick at the granitic forest sites (Supplementary Table 1), and 1.5–2 cm thick at the andesitic sites. At the granitic forest sites, the decomposed litter could be separated into Oi and Oe horizons. In contrast, at sampling time (October 2018), only an Oe horizon was present in the litter at the andesitic forest site, suggesting a greater litter decomposition rate. Forest canopy coverage at the sites are similar: 49% at the andesitic site and 41% at the granitic site (Landfire, 2020). In the meadows, O horizons were 0.5–3 cm thick.
The pH of the meadow and forest soils ranged from pH 5.3 to 6.0, with meadows slightly lower than forests (Table 1). Average sand content was similar in the granitic meadow, granitic forest, and andesitic meadow (84, 87, and 88%, respectively), but it was significantly lower (75%) in the andesitic forest soils (Table 1). Bulk density of the forest and meadow soils ranged from 0.78 to 1.46 g cm−3. The andesitic soils had significantly lower bulk densities than the granitic soils (Table 1). The andesitic forest soils contain the most poorly crystalline iron and aluminum oxides (measured by oxalate extraction), which is consistent with Andisol classification by the USDA NRCS (Soil Survey Staff, 1999). Oxalate-extractable Fe and Al were not significantly different among the other three ecosystem-parent material types (Table 1). Oxalate-extractable Si concentrations followed the same patterns as Fe and Al concentrations. Oxalate-extractable P concentrations were significantly higher in andesitic forest soil than the other soils, and significantly lower in granitic meadow soils compared to granitic forest soils. Soil TOC concentrations were significantly different between the two forests (Table 1), but not the two meadows, and were not significantly different between meadows and forests within each watershed. Granitic forest soils contained the lowest average TOC concentration of all four ecosystem-parent material types, while andesitic forest soil had the highest average TOC concentration. The average TN concentration was approximately three times higher in the andesitic meadow soils compared to the andesitic forest soils (Table 1) and was higher in the andesitic forest soils than the granitic forest. Total N concentrations in the granitic forest and granitic meadow soils were not significantly different.
Soil Total P
The mean total soil P concentrations (TP) in the soils developed on andesitic parent materials were significantly higher than those for the soils developed on the granitic parent materials (Figure 2). Differences between forest and meadow soils within either watershed were not significant. The estimated total P concentrations via incineration and H2SO4 extraction for the soil samples analyzed by P-NMR were similar to the total P measured from three acid digestion (slope = 0.95, r2 = 0.95; Supplementary Table 2). The total P stocks for the 0–15 cm mineral soils calculated using the mean bulk densities were 0.69, 1.10, 1.22, and 1.81 Mg Ha−1 for the granitic meadow, granitic forest, andesitic meadow, and andesitic forest, respectively.
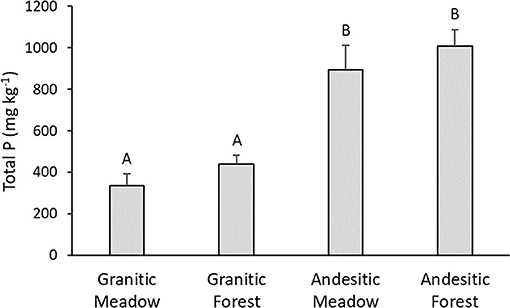
Figure 2. Mean total soil phosphorus. Error bars are standard errors. Values with the same letter are not significantly different (α =0.05), n = 6 for granitic parent material sites and n = 5 for andesitic parent material sites.
Speciation of Soil P
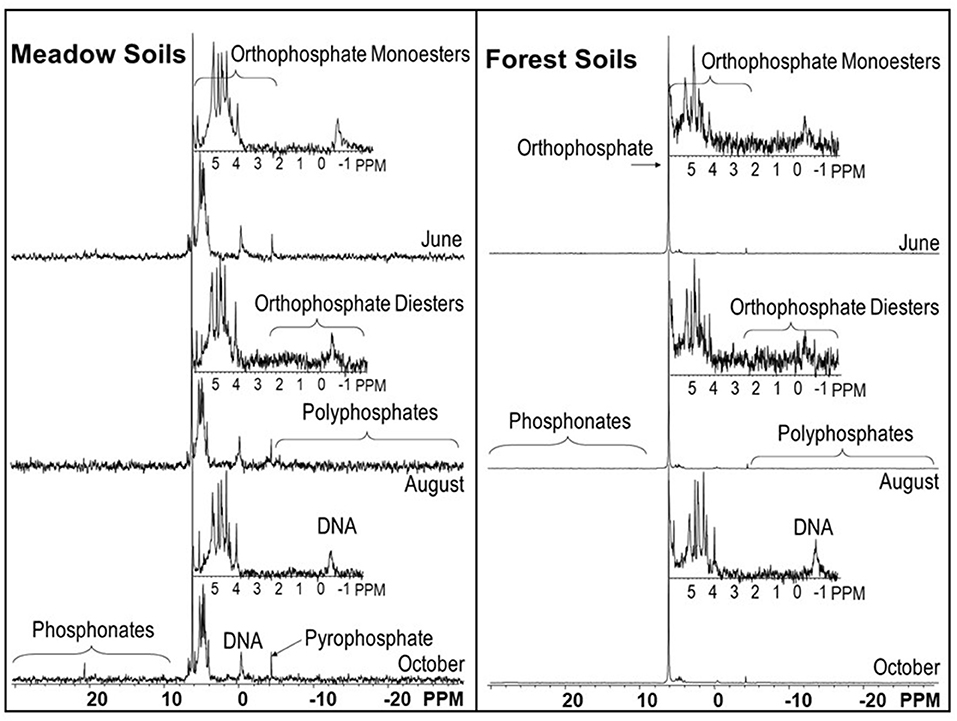
In the meadow soils, total organic P concentrations determined from incineration and H2SO4 extraction were 79–92% of the TPinc (Figure 3, Supplementary Table 2). Organic P concentrations in the forest soils were much lower (13–47% of the TPinc) than in the meadow soils.
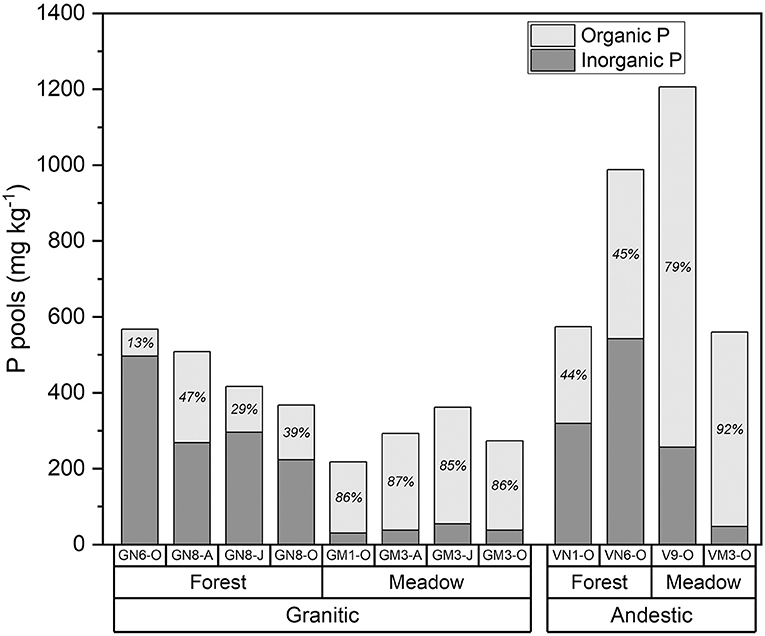
Figure 3. Organic and inorganic P in forest and meadow soils from andesitic and granitic parent materials determined by difference of H2SO4 extraction of non-incinerated and incinerated soils. Numbers within bars are percent organic P.
The NaOH-EDTA extraction efficiency ranged from 33 to 75% of total soil P (Supplementary Table 2). The P not extracted by NaOH-EDTA is considered to be predominantly mineral-bound inorganic P and not readily available to the soil solution or for biological cycling (Cade-Menun et al., 2015).
Example P-NMR spectra are shown in Figure 4, the concentrations (and percentage of extracted P) are shown in Supplementary Table 3, the grouping of these P species into pools (total organic and inorganic P) and compound classes (total polyphosphates, etc.) are shown in Supplementary Table 4, and the chemical shifts of the identified P compounds are shown in Supplementary Table 5. The concentrations of the main P compound classes within each ecosystem and parent material type are shown in Figure 5.
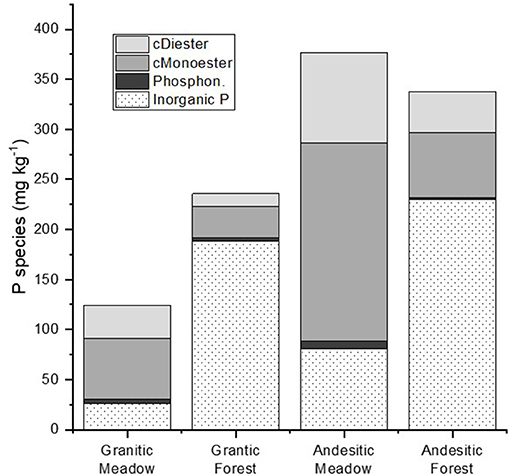
Figure 5. Mean P species in soils measured using 31-NMR analysis of NaOH-EDTA extracts of soils. The monoester and diester values were corrected for degradation (denoted with “c” prefix). Inorganic P is the sum of the inorganic orthophosphate and polyphosphate compounds.
Inorganic P compounds identified in the NaOH-EDTA extracts by NMR include orthophosphate, pyrophosphate, and polyphosphates. Pyrophosphate and polyphosphates were grouped together as total polyphosphates (Supplementary Table 4), and all three were summed together as inorganic P (Supplementary Table 4, Figure 5). For all soils, orthophosphate was the dominant inorganic P form, and for forest soils developed on both parent materials it comprised the majority of P in the NaOH-EDTA extracts (71.2–84.7% for granitic forests; 51.7–69.3% for andesitic forests). In contrast, the percentages and concentrations of all inorganic P compounds were much lower in meadow soils developed on both parent materials, averaging 21% of extracted P (Figure 5). There were no clear trends among the ecosystem and parent material soil types for pyrophosphate or polyphosphates, which were present in all samples, ranging from at 2.1–4.9% of NaOH-EDTA extracted P.
The percentage organic P determined by P-NMR on the soil extracts was directly correlated with the percentage determined using incineration and H2SO4 extraction (r2 = 0.95, Supplementary Figure 1). For all soil types, all the major organic P compound classes were identified: phosphonates, orthophosphate monoesters (hereafter called monoesters), and orthophosphate diesters (hereafter called diesters). The phosphonates included several different peaks (Supplementary Table 5), indicating that a number of different compounds were present, but these were not specifically identified. Concentrations of phosphonates ranged from 1.4 to 10.3 mg kg−1 (0.7–4.9% of extracted P) and were generally higher in meadows than forests.
Monoesters identified in the P-NMR spectra included four stereoisomers of inositol hexakisphosphate (IHP): myo-IHP (phytate), scyllo-IHP, neo-IHP, and D-chiro-IHP. Of these, myo-IHP was the predominant P form and was generally more abundant in meadows than forests. For most of the soil samples, myo-IHP exceeded the sum of the other three stereoisomers. Other specifically identified monoesters were glucose 6-phosphate (0.6–2.1% of extracted P), choline phosphate (0.3–1.3%), α-glycerophosphate (0.3–2.8%), β-glycerophosphate (0.7–5.7%), nucleotides (1.3–12.8%), and an unidentified peak at ~5 ppm, which was present in all samples at 0.7–9.2% of the extracted P. Although peaks for α-glycerophosphate, β-glycerophosphate, and nucleotides are present in the monoester region of spectra, they originate during NaOH-EDTA extraction and P-NMR analysis as a result of degradation of diesters in the original soil samples (Cade-Menun, 2015; Schneider et al., 2016). Thus, the peak areas from these compounds were subtracted from the monoester peak areas and included with the diesters.
Peaks representing diester compounds were separated into DNA (0.5–5.9%), Diester 1 (2.33 to −0.27 ppm, 0.6–8.7%), and Diester 2 (−0.9 to −3.72 ppm, 0.2–3.4%). The Diester 1 region included phospholipids and lipoteichoic acids, while the compounds in the Diester 2 region have not been specifically identified. The proportions and concentrations of P in these three diester regions were generally greater in meadows than forests for both parent materials. Total diesters (cDiesters), calculated by including the degradation compounds from the monoesters, confirmed that the percentages of cDiesters were greater in meadows than forests, and concentrations were greater in andesitic forests and meadows than the granitic forests and meadows, respectively (Supplementary Table 4, Figure 5).
The average of the replicate NMR results (Figure 5) shows that in the meadow soils the three major organic P compound classes were greater in concentration than in the forest soils. In meadow soils, cMonester was 50.3% (107 mg kg−1), cDiester was 25.8% (52.1 mg kg−1), and phosphonates were 3.0% (5.6 mg kg−1). In forest soils, cMonester was 15.7% (42.5 mg kg−1), cDiester was 8.5% (22.2 mg kg−1), and phosphonates were 1.3% (3.4 mg kg−1). The ratio of cMonesters to cDiesters was over 1 for all soils, indicating that monoesters were the dominant P compound class in both ecosystems. In meadow soils, total IHP concentrations comprised about one third of the cMonoesters 17.3% (33.5 mg kg−1) but were half of the cMonoesters in forest soils 7.7% (20.9 mg kg−1).
Effects of Parent Material and Ecosystem on Extractable P
In evaluating the extractable P concentrations, main effects were calculated as well as interactions (Supplementary Table 6). For all the WEP and B1P extractions, there were crossover interactions between parent material and ecosystem type (Supplementary Figure 3). Thus, the effect of ecosystem should be interpreted in context of parent material, and vice versa.
The mean total WEP (WEPTotal) concentrations from all the paired interactions (parent material within ecosystem type and ecosystem type within parent material type) were significantly different from each other (Supplementary Table 6). The granitic forest soils had the greatest mean WEPTotal concentration of the four ecosystem-parent material types (Figure 6). The andesitic forest soils had the lowest mean WEPTotal concentration. WEPMU comprised the largest fraction of WEP (Supplementary Table 6) in all ecosystem-parent material types. This suggests that most of the WEP exists as soluble organic P compounds or as inorganic P complexed to colloids instead of as dissolved phosphate.
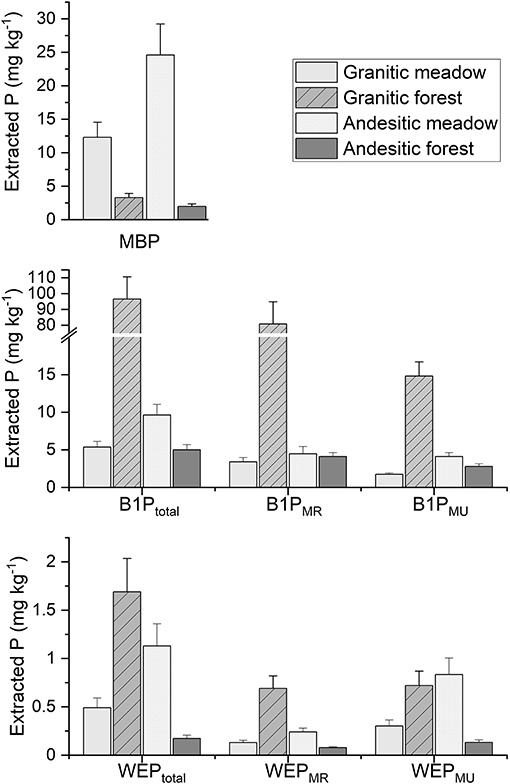
Figure 6. Estimated marginal means for water extractable P (WEP) (bottom), Bray 1 extractable P (B1P) (middle), and microbial biomass P (MBP) (top) from soil samples from the different ecosystem and parent material sites. WEP and B1P extract subscripts indicate total solution P, molybdate reactive P (MR), and molybdate unreactive P (MU). Error bars are the standard errors.
The mean total B1P concentration from the granitic forest soils was more than 10 times greater than the mean B1P concentration from the other soils (Figure 6). The B1P concentrations at the two forest soils were significantly different, as were the meadow to forest comparisons (Supplementary Table 6). However, the B1P from the andesitic meadow and granitic meadow soils were not significantly different. Most of the B1P was molybdate reactive P (Supplementary Table 6), suggesting B1P was predominantly inorganic P extracted from the soil.
The mean MBP concentrations for the meadow soils were approximately seven times more than the mean MBP concentrations from the forest soils (17.4 mg kg−1 compared to 2.55 mg kg−1, Supplementary Table 6). All paired comparisons for the interactions were significantly different. The andesitic meadow soils had the greatest MBP concentrations, followed by the granitic meadow soils (Figure 6).
Water Soluble P of Soil O Horizons
The WEP and WEPMR concentrations from composite O-horizon samples are shown in Table 2. The less decomposed Oi composite sample from the granitic site had greater WEP concentrations than the Oe sample.
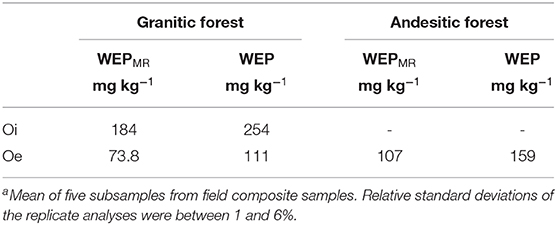
Table 2. Meana WEP and WEPMR extract concentrations from composite samples of the O horizons from the forested sites.
Discussion
Parent Material Effects on Soil P
Total P concentrations in unfertilized soils are typically linked to P content of the parent material, which is the source of P and is also an important control of soil mineralogy that affects P retention (Gardner, 1990; Porder and Ramachandran, 2013; Hahm et al., 2014). The granitic Meeks Meadow soils developed on granodiorite eroded from glacial till and outwash, while the andesitic soils at Paige Meadow developed on glacial deposits of eroded basaltic and andesitic volcanic rock. In a database of P concentrations of common rock types, mean total P concentrations of granodiorite and granite are 810 and 568 mg kg−1, respectively, while mean total P concentrations of basalt, andesite, and basaltic-andesite are 1,304, 1,150, and 1,551 mg kg−1 (Porder and Ramachandran, 2013). Total P concentrations in Tahoe soils developed in andesitic parent materials were over two times greater than in soils developed in the granitic parent materials (Figure 2), which is consistent with expected differences based on parent material.
In addition to P inputs from parent materials, other P inputs and losses from the soil also affect soil TP concentrations. Phosphorus released from parent material by weathering is taken up by plants and microbes and converted to other P forms and can also be leached out of the soil profile. It has been noted that total P concentrations in forest soils decrease over time (Yang and Post, 2011; Deiss et al., 2018; Nelson et al., 2020). There are no clear trends for WEP, B1P, or MBP concentrations between the granitic or andesitic watersheds (Figure 6, Supplementary Table 6). However, there were significant differences for WEP, B1P, and MBP when the interaction of parent material type and ecosystem were considered (Supplementary Figure 3). The granitic forest soils had much greater WEP and B1P concentrations than the andesitic forest soils, despite having lower TP concentrations. This suggests greater P availability, or more labile P, in the granitic forest soils than in the andesitic forest soils. The labile P is available to either efflux out of the soil or be taken up by plants, thus causing a decrease in TP concentration in the soil. Johnson et al. (1997) reported significantly higher concentrations for a B1P-type extraction from andesitic forest soils than granitic forest soils at other locations in the Lake Tahoe Basin, but did not report concentrations for other P pools such as TP. Brödlin et al. (2019a) observed that in sandy soils derived from glacial-till parent materials in a European deciduous forest, greater P leaching occurred than in soils derived from volcanic rock. Thus, for the granitic forest soils in Lake Tahoe, the decreased TP concentration is both due to the decreased parent material rock-P inputs and export of available P out of the soil. Since the meadow soils are depositional environments, the TP concentrations in these soils reflect the parent material trends as well: andesitic meadows have more TP than granitic meadows. But differences in labile P are not as great as the differences between the forest soils. Thus in the meadows, other ecosystem processes are more important in controlling the availability of the soil P.
In the soils of the current study, soil properties were not clearly delineated by parent material alone. Soil pH was more closely related to ecosystem type than parent materials (Table 1), and concentrations of oxalate-extractable Al, Fe, Si and P, and sand content of the andesitic forest were significantly different from the andesitic meadow and the granitic forest and meadow (Table 1). The andesitic forest soil properties are due to the andesitic-parent material contributions, while the andesitic meadow is in a depositional environment collecting eroded soils transported from surrounding landscapes. In addition, the hydraulic conditions and vegetative community dynamics of the meadow create pedogenic processes that can overshadow the andic soil properties. Thus, the meadow soil has less andesitic parent material influences, even though it is within a predominantly andesitic parent material landscape. The A horizon in the andesitic forest soils is only 6 cm thick, and thus some of the soil properties in the 15 cm cores sampled from this site were impacted by Bw horizon material (Supplementary Table 1). At nearly all of the other sites, except one andesitic meadow profile, A horizons are more than 15 cm thick, and thus the cores represent the pedogenic horizons. The inclusion of Bw material in the andesitic forest soil sample may have contributed to its distinct properties as compared to the other soil cores; however, the mineral properties of this sample as measured by the oxalate extractable Fe, Al, and Si (Table 1) are indicative of andic soils, which have high concentrations of poorly crystalline aluminum and iron oxides in both the surface and subsurface horizons. Thus, the andesitic soil samples, while composed of A and B horizon soils, have mineral properties that are indicative of the andesitic parent materials from which the soils developed and the soil P properties from this sample are indicative of the top 15 cm of the soil.
Soil P sorption capacity is related to clay minerals and iron and aluminum oxides (Gerard, 2016). The andesitic forest soils had the lowest sand content and the highest concentrations of amorphous iron hydroxides and aluminosilicates (Table 1), suggesting that P adsorption on mineral surfaces will be strong in these soils (Khare et al., 2005; Redel et al., 2008). Phosphorus complexed as Al or Fe-organic matter ternary complexes are also common in andesitic soils (Gerke and Hermann, 1992; Gerke, 2010). Oxalate-extractable P was positively correlated to oxalate extractable Fe, Al, and Si (r = 0.82, 0.85, 0.82, respectively; Supplementary Figure 2) and the highest concentrations of oxalate-extractable P were in the andesitic forest soils (Table 1), confirming the relation of the soil P to the andesitic soil minerals.
Sorption capacity will influence both inorganic and organic P forms. Total organic P in the Lake Tahoe soils does not appear to be related to the parent material, beyond the differences in TP already discussed, and is clearly more influenced by ecosystem (Figure 3; Supplementary Table 2). For specific organic P forms, correlations of concentrations of myo-IHP and other IHP stereoisomers with concentrations of oxalate-extractable Fe and Al have been widely reported for soils and are thought to demonstrate the sorption of these compounds to amorphous iron hydroxides and aluminosilicates (Jorgensen et al., 2015; Nelson et al., 2020). The average of the percentage of total extracted IHP (Supplementary Table 4) for the eight granitic soils was similar to that for the four andesitic soils (13.1 and 11.3% respectively), while the average concentrations of total IHP in andesitic soils were double those of the granitic soils (41.2 vs. 20.2 mg kg−1). The same trend was also observed for DNA and the general Diester 1 category (Supplementary Table 3). In acidic soils, adsorption of DNA occurs, and its NaOH-EDTA-extracted concentrations have been correlated with those of oxalate-extracted Fe and Al (Condron et al., 2005; Nelson et al., 2020). However, the Diester 1 category includes phospholipids and lipoteichoic acids, which do not sorb to soil minerals (Condron et al., 2005). The differences in concentrations of these compounds and compound classes are consistent with differences in total concentrations of P in the NaOH-EDTA extracts (Supplementary Table 2), which are consistent with total concentrations of soil P (Figure 2), so may simply reflect trends in total P rather than selective binding of P compounds.
Ecosystem Type and Soil P
Although TP concentration did not differ between ecosystem types at the two different parent material watersheds, total organic P measured by the incineration method was substantially greater in meadow soils than forest soils (85.5 vs. 36.2%, averaging data from Figure 3). The same trend was observed for total organic P determined by P-NMR, even though the recovery of total P was lower in NaOH-EDTA extracts from meadow soils (40.8%) than forest (61.9%, averaging data in Supplementary Table 2), which could underestimate inorganic P. Chiu et al. (2005) measured P-NMR spectra in NaOH-EDTA extracts from subalpine grassland and forest soils and observed a similar fractionation of inorganic and organic P forms between the two ecosystems. In addition to differences in total organic P, P-NMR revealed differences in P forms and compound classes between the two ecosystem types (Figure 3, Supplementary Table 4).
The forest and meadow ecosystems differ in vegetation, elevation, and slope position, all of which influence P cycling. The plant species in the meadows have above and below-ground vegetation that can readily decompose compared to forests (Margalef et al., 2017). In forests, litter is deposited onto the forest floor and gets incorporated into an Oi horizon that decomposes to an Oe horizon. An Oi horizon with identifiable pine needles was observed in the granitic soil, but not the andesitic forest. This may be due to andesitic soil properties that increase soil moisture retention, which facilitates greater microbial decomposition rates and thus quicker breakdown of forest litter (Sun et al., 2017). Different species of plants and even the same species of plants growing under different soil fertility conditions will contribute different P species (Noack et al., 2014) that can change with depth in the soil profile (Nelson et al., 2020).
The vegetation from these ecosystems was not analyzed by P-NMR, so we cannot say with certainty the P forms input from plants. However, myo-IHP is widely recognized as a plant P compound (Condron et al., 2005). Other compounds may originate from plants or microbes or can be produced by alteration of plant-P compounds (Condron et al., 2005). In the soils of this study, microbial P (MBP) concentrations were greater in meadow soils than they were in forest soils. However, in coniferous forests, the majority of microbial activity and P cycling occurs in the O horizon, associated with the hyphal mat of ectomycorrhizal fungi at the soil-organic matter interface (Plassard et al., 2011; Nelson et al., 2020). This may account for difference in P forms and MBP concentrations between these ecosystems and would be consistent with the substantially higher WEP concentrations in O horizons (Table 2) than mineral soils in the forest.
Availability of soil P controls P immobilization into microbial biomass (Olander and Vitousek, 2004; Yang and Post, 2011; Spohn and Widdig, 2017; Pistocchi et al., 2018). For example, Pistocchi et al. (2018) observed that during incubation of a deciduous forest soil with low available P, P cycling between soil and microbial biomass was conservative, while in soil with higher available P there was more exchange between microbial-bound P and inorganic soil P pools (i.e., mineral-bound P). Thus, in forest soils, when P availability exceeds biological demands, geochemical processes (adsorption and precipitation) predominate over immobilization by microbes (Olander and Vitousek, 2004); this implies that geochemical processes control P availability for leaching or root uptake in the mineral soil horizons. In the andesitic soils, the decreased labile P concentrations (Figure 6) inhibit microbial P fixation, causing low MBP. Aluminum toxicity is another cause of decreased MBP in the andic forest soils because high soluble Al concentrations inhibit microbial enzyme production, including phosphatase, thereby limiting P immobilization (Kunito et al., 2016).
Another possible cause of the MBP increase in the meadow soils compared to the forest soils is the increase in N availability in the meadow soils (Table 2). Microbes mineralize organic P for microbial uptake using phosphatase enzymes, which require N for production (Vitousek et al., 2010; Marklein and Houlton, 2012). Total N and MBP were significantly correlated (r = 0.81) (Supplementary Figure 3). Thus, the occurrence of sufficient N availability for phosphatase generation in the meadows facilitates degradation of organic P compounds, and subsequently, the biologically available P can be immobilized by microbes. Soil moisture may also play a factor, with greater moisture in the meadows increasing microbial activity.
Mycorrhizal association may also greatly influence P cycling. The ectomycorrhizal fungi found in forests will produce more phosphatases than endomycorrhizae associated with meadow plants (Plassard et al., 2011; Margalef et al., 2017). They will also produce organic acids such as oxalate (Plassard et al., 2011). These will desorb both inorganic and organic P, and both organic acids and phosphatases may need to be present simultaneously to mineralize organic P (Giles et al., 2018). This could also account for the reduced organic P concentrations in these forests compared to meadows.
In meadows, high seasonal water tables can have a significant impact on soil properties that influences P cycling. This, combined with high organic C concentrations, can mask the influence of mineralogy on labile P (Sah et al., 1989; Johnston et al., 1995). Sah et al. (1989) observed that C availability controlled P availability in wetland soils: when total organic C concentration exceeded 0.8%, it promoted the reduction of ferric (Fe3+) oxides, which decreased sorption capacity of the soil for P. Alternatively, during periods of flooding, the precipitation of ferrous iron-phosphate minerals such as vivianite may occur (Zhang et al., 2003; Heiberg et al., 2010; Rothe et al., 2016), which is less soluble upon drainage because the P remains occluded by oxidized iron-hydroxide minerals that form when the vivianite oxidizes (Sah and Mikkelsen, 1986b). The seasonal redox cycles that occur in wetland soils may decrease P leaching compared to unflooded soils, even after soils remained drained over 4 months (Sah and Mikkelsen, 1986a). In contrast, Gergans et al. (2011) proposed that excess sulfate in Tahoe Basin wetland soils facilitates production of iron sulfides during reducing conditions that make Fe unavailable when the soils re-oxidize to adsorb phosphate and organic P compounds, thus making soil P more available for continued leaching in these wetland soils. Based on the varying results of the studies discussed above, there are several factors that influence P speciation in Lake Tahoe Basin meadows, including seasonal flooding, high organic matter, and Fe biogeochemical transformations. These factors may be more important than parent materials for controlling P mobility. The presence of higher concentrations of phosphonates in meadow soils compared to forest soils is also consistent with higher moisture levels (Condron et al., 2005), and higher diester concentrations have also been reported for poorly drained soils compared to well-drained soils (Young et al., 2013).
Labile Soil P and Potential Loss to Lake Tahoe
Soil P buffering capacity is the degree to which soil can adsorb or release P from exchange sites to maintain dissolved P concentrations in the soil solution (Holford, 1997). Soils with larger total P reserves are considered to have greater buffering capacity to replenish P taken up by plants or leached out of the soil (Daly et al., 2015). An estimate of soil P buffering capacity is the P saturation index (PSI), which is calculated as concentrations of oxalated-extracted P divided by the sum of the oxalate-extracted Fe and Al (Schoumans, 2009). For the Lake Tahoe watershed soils, PSI was 0.029 and 0.072 for the granitic meadow and forest soils, and 0.047 and 0.029 for the andesitic meadow and forest soil (Table 1).
Of the extractants used in this study, WEP measures the most labile P, B1P extracts less labile P that is sorbed to the soil, and oxalate or NaOH-EDTA extract both labile P and P that is more tightly held by soil through either adsorption complexes, mineral-bound P, or larger organic P compounds. In all of the Lake Tahoe Basin soils, B1P concentrations were approximately an order of magnitude greater than WEP concentrations (Figure 6), suggesting a large amount of adsorbed P is released by the B1P extractant that is not released by water extraction. Although a stronger P buffering capacity is expected in the andesitic forest soils, where the highest TP was observed, the lower PSI in these soils suggests there is excess P sorption capacity on high-adsorption affinity iron and aluminum oxides, thus causing the lower concentrations of labile P (WEP and B1P) as compared to the granitic forest soils. The B1P extractant apparently did not access the P in the andesitic soils that was either strongly adsorbed, had formed Al or Fe-P mineral phases with low solubility (Negrín et al., 1996), or was complexed as Al or Fe-organic matter ternary complexes, which are common in andesitic soils (Gerke and Hermann, 1992; Gerke, 2010). The NaOH-EDTA extracts removed a much greater amount of the total P than the B1P extracts (Supplementary Table 6) but were similar in concentration to those of oxalate-extractable P (Table 1). Thus, there appears to be a large reserve of P associated with iron oxide and allophane minerals in the andesitic forest soils that is not readily available for release to the soil solution.
Analysis of the NaOH-EDTA soil extracts from the andesitic forests by P-NMR showed that 26–46% of the extracted P was organic P species (Supplementary Table 4). This organic P fraction in the andesitic soils may be slowly available to plants and microbes that release organic acids, which enhance P release through competitive exchange (Harrold and Tabatabai, 2006), and which may be synergistic with hydrolysis of organic P compounds by phosphatases (Giles et al., 2018).
Most of the WEP in forests and meadow soils from Lake Tahoe was not reactive with molybdate blue chemistry (WEPMU) (Figure 6, Supplementary Table 6). The source of water-extractable organic P compounds are inputs from plants and soil microbes. The predominance of WEPMU from both forest and meadow soils in the Lake Tahoe Basin is a potential source of mobile P that most likely consists of organic P compounds (Worsfold et al., 2016). The labile organic P compounds can be transported to Lake Tahoe by vertical and lateral transport processes, especially during high intensity events that cause preferential flow through macropores, which are common in coniferous forest soils (Luo et al., 2019). Organic P species have been reported in soil leachate, snowmelt runoff, and samples of river inlet and floodplain waters during flooding events (Toor et al., 2003; Cade-Menun et al., 2006; Wiens et al., 2019), and in water-extractable colloids from grasslands and forests (Missong et al., 2016; Jiang et al., 2017).
Water-soluble P from leaf litter is an important source of labile P return to the soil (Uselman et al., 2012; Sohrt et al., 2019). In a separate study of soluble P from O horizons from Lake Tahoe Basin (unpublished data), nine samples were collected from forest and meadow sites near the Paige Meadow and Meeks Bay watersheds. WEP concentrations in these samples ranged from 54 to 209 mg kg−1 (mean = 122 mg kg−1, standard deviation = 45 mg kg−1), indicating that WEP concentrations in the O-horizon samples are highly variable throughout the two watersheds. Based on the two Oe composite samples (Table 2), molybdate-reactive P (inorganic P) was the predominant phase of WEP (67% in both the granitic and andesitic forests). The concentration of P from the composite Oi horizon sample from the granitic site was more than twice that of the Oe horizon sample, suggesting P is lost from the litter as it decomposes (Table 2).
The Oe WEP concentration was ~58 times greater than soil A horizon WEP concentration in the granitic forest soils, and 690 times greater than in andesitic forest soils. Miller et al. (2005) measured soluble P from O horizons of Lake Tahoe Basin forest soils using laboratory simulated precipitation and snowmelt leaching experiments and observed 46 mg kg−1 of soluble P leached from the less decomposed Oi horizon and 28 mg kg−1 from the more decomposed Oe horizon. The O horizons in the Miller et al. (2005) study came from Jeffrey and Sugar Pine forests in a granitic watershed. Although the Miller et al. (2005) water extraction methods were different than those used in this study, both the Miller et al. (2005) leaching experiment and this study's granitic forest O-horizon samples (Table 2) have more water-soluble P in the minimally decomposed Oi horizons compared to the more decomposed Oe horizons.
Approximately one-third of the WEP from the O horizon samples was WEPMU, which could be a considerable source of organic P mobilized into the soil and possibly to the surface water, depending on the organic P species and reactivity. Both the granitic and andesitic sites have a similar vegetative density (41 vs. 49%) (Landfire, 2020), thus, vegetative P inputs to the soils should be similar in the two watersheds. However, based on the greater WEP and B1P concentrations in the granitic forest soils, P outputs to streams and groundwater are expected to be much greater from these systems; this is due to the lower sorption capacity of the soils that allows for a significant amount of P release in the extractions. Uhlig and von Blanckenburg (2019) estimated that P inventory of the forest litter in montane, temperate forest ecosystems can only sustain vegetative demand for a few decades, and that continuous release of P from parent rocks must occur to sustain forest growth. The different adsorption capacities of the andesitic and granitic soils in the Lake Tahoe forests can have a major influence on the timescales of P availability and its cycling between the forests, litter, and parent material.
There are several sinks for WEP from forest litter: it can be taken up by plants and microorganisms for internal cycling; leached into the soil where it may adsorb, be immobilized, or further leached into ground water; or be transported off site via surface runoff of dissolved P or eroded P-containing particles. Although concentrations of WEP in the O horizons are much greater than the soils, it is a much smaller total P pool in the ecosystem than soil P, which is large and stores much of the WEP leached into it from O horizons (Yang and Post, 2011). The high concentrations of soluble P in the Lake Tahoe Basin O horizon samples indicate that a large flux of available P can enter the soil. Much of this flux occurs during spring snowmelt. The fate of this O horizon-sourced P is a function of the characteristics of soil biological and physical properties and site hydrology.
Ohara et al. (2011) recorded that more than 90% of field-observed hillslope drainage in a Lake Tahoe watershed occurred as subsurface lateral flow through the soil. Thus, soil reactions are important processes controlling P transport to surface waters, which would be especially high during periods of continuous snowmelt. These processes are impacted by the species of soluble P in the soils, which both the soil extractions and soil P-NMR analyses suggest are both inorganic and organic P species.
When streams near our research sites experience peak discharge, molybdate-unreactive fractions make up 61–67% of filterable (<0.45 μm) P [Supplementary Figure 4 (USGS, 2016)]. Therefore, molybdate-reactive and unreactive fractions in these nearby streams during snowmelt more closely reflect the WEP fractionation of soils (50–74% WEPMU; Supplementary Table 6) than WEPMU from O horizons (28–36%; Table 2). A possible explanation of this is that during high-flow periods, the inorganic (molybdate-reactive) P species are attenuated by forest and meadow soils leading to net exports of organic forms. Thus, the flowing solution reflects the WEPMU fraction leaching from the soil. Bol et al. (2016) conducted an extensive review of P fluxes in forested ecosystems and concluded that P loss as colloidal-organic P that is exported from soil profiles through macropores during high-intensity rainfall events is likely a critical factor in P export. Colloidal organic P would be included in the WEPMU fraction in this study. Considering that organic P increases in the stream in the Lake Tahoe Basin during high-intensity events (Supplementary Figure 4), preferential-flow path loading is a likely scenario occurring in forest and meadow watersheds in the Lake Tahoe Basin. However, to explain the molybdate unreactive ratio of the stream water, there must be attenuation of the inorganic P as it moves through the preferential flow paths; otherwise the ratio of inorganic to organic P forms in the stream water would be more similar to the ratios in the O-horizon extracts. Alternatively, forest soils may be transporting P-laden water through preferential flow paths where P attenuation is minimal, but as the flow continues toward the streams, it is intercepted by riparian meadows that have fewer preferential flow paths, enhanced groundwater storage, and greater microbial activity that immobilizes orthophosphate, causing the soil water that exfiltrates into the streams to have a greater proportion of WEPMU than what is leached from the forest O horizons. A third mechanism of inorganic P attenuation that may enrich Lake Tahoe Basin stream waters with molybdate unreactive P is preferential adsorption of inorganic P within the stream on suspended particles eroded from soils. Since the highest total suspended solids occurs during high runoff events, adsorption may be significant enough to alter the dissolved inorganic and organic solution composition during these periods.
Because molybdate unreactive P (organic P) accounted for the majority of WEP from the Lake Tahoe Basin soils (Figure 6), it is likely the most vulnerable for transport as lateral flow during spring snowmelt or exfiltration from meadows, thereby increasing the P load in surface waters. The high concentrations of P released from litter suggests that forest management practices that remove timber and deposit deep layers of chopped fresh organic matter (mastication) to prevent erosion may be creating a potential source of P that can be leached into surface waters—at least in the short-term time it takes for the material to degrade. In watersheds with soils developed on granitic parent materials, this would be especially problematic. A beneficial focus of future research would be an examination of the speciation of P in the forest O horizons and comparison to P forms in both forest and meadow soils, as well as measurement of soil and stream water samples for P-species composition. Additionally, evaluation of the subsoil deeper than 15 cm should be done to account for how P reactions influence leaching through the deeper soil profile.
Conclusion
In soils in the Lake Tahoe Basin, P storage shifts from sorption on minerals in forests, to immobilization in microbial biomass in meadows. In forested hillslopes, adsorbed P may be gradually depleted if it is leached from the soil into ground and surface water. The degree of P depletion depends on the parent materials from which the soils developed (granitic vs. andesitic). In soils developed on andesitic parent materials, forest cycling of P is mediated by the high adsorption capacity of P on andic minerals, while in soils derived from granitic parent materials the increased resistance to weathering creates coarser-textured soils and fewer soil clays, causing a decreased P adsorption capacity. As a result, granitic soils have greater potential P mobilization into groundwater and lateral runoff into surface waters.
Organic P was a predominant water-extractable fraction from all soils. Total organic P concentration was greater in meadow soils than forest soils, and in all soils of this study, orthophosphate monoesters were the main organic P compound class, even after correcting for diester degradation during analysis. The organic P compounds in the soils can be leached into the surface waters. Once in the surface water, mineralization of the organic P compounds can make phosphate available to aquatic organisms, causing surface water quality degradation.
Results from this study provide insights into speciation of P in forest and meadow soils and show the importance of parent materials on P availability. This information can be used to better understand which ecosystems present the most risks for P loading into Lake Tahoe, which will allow for better forest management practices to prevent P export into feeder streams and groundwater that discharge into the lake. Current management strategies use controlled burns and erosion prevention strategies to prevent P loss from Lake Tahoe Basin soils into the lake. Resource managers need to consider the highly variable sources of P in the Basin to decide which watersheds are most vulnerable to P loss, such as the granitic forest watersheds, and match management strategy to site properties to optimize the site management for decreased P loss.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TH conducted the research and reported it as part of a MS thesis at the University of Idaho. DS is the corresponding author and led all aspects of the research and writing of this manuscript. MD, CD, EB, and CG designed the research and assisted with experiments and data interpretation. BC-M conducted NMR data analyses and interpretation as well as helped interpret all of the experimental data. JP did the mixed-model statistical analyses of the data and consulted in data interpretation. AC assisted with conducting experiments and collecting and analyzing data. All authors contributed to writing and editing of the article and approved the submitted version.
Funding
This work was supported by AFRI program (Grant No. 2016-67020-25320/project accession no. 1009827) from the USDA National Institute of Food and Agriculture.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate assistance with NMR experiments by Alex Blumenfeld in the University of Idaho Chemistry Department, and assistance with O horizon water extractable P measurements by Tiffany Perez.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.604200/full#supplementary-material
References
Anderson, B. H., and Magdoff, F. R. (2005). Relative movement and soil fixation of soluble organic and inorganic phosphorus. J. Env. Qual. 34, 2228–2233. doi: 10.2134/jeq2005.0025
Backnäs, S., Laine-Kaulio, H., and Klove, B. (2012). Phosphorus forms and related soil chemistry in preferential flowpaths and the soil matrix of a forested podzolic till soil profile. Geoderma 189, 50–64. doi: 10.1016/j.geoderma.2012.04.016
Bol, R., Julich, D., Brödlin, D., Siemens, J., Kaiser, K., Dippold, M. A., et al. (2016). Dissolved and colloidal phosphorus fluxes in forest ecosystems: an almost blind spot in ecosystem research. J. Plant Nutr. Soil Sci. 179, 425–438. doi: 10.1002/jpln.201600079
Brödlin, D., Kaiser, K., and Hagedorn, F. (2019a). Divergent patterns of carbon, nitrogen, and phosphorus mobilization in forest soils. Front. Glob. Chang. 2:66. doi: 10.3389/ffgc.2019.00066
Brödlin, D., Kaiser, K., Kessler, A., and Hagedom, F. (2019b). Drying and rewetting foster phosphorus depletion of forest soils. Soil Biol. Biochem. 128, 22–34. doi: 10.1016/j.soilbio.2018.10.001
Cade-Menun, B., and Liu, C. W. (2014). Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters. Soil Sci. Soc. Am. J. 78, 19–37. doi: 10.2136/sssaj2013.05.0187dgs
Cade-Menun, B. J. (2015). Improved peak identification in P-31-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 257, 102–114. doi: 10.1016/j.geoderma.2014.12.016
Cade-Menun, B. J., He, Z. Q., Zhang, H. L., Endale, D. M., Schomberg, H. H., and Liu, C. W. (2015). Stratification of phosphorus forms from long-term conservation tillage and poultry litter application. Soil Sci. Soc. Am. J. 79, 504–516. doi: 10.2136/sssaj2014.08.0310
Cade-Menun, B. J., and Lavkulich, L. M. (1997). A comparison of methods to determine total, organic, and available phosphorus in forest soils. Comm. Soil Sci. Plant Anal. 28, 651–663. doi: 10.1080/00103629709369818
Cade-Menun, B. J., Navaratnam, J. A., and Walbridge, M. R. (2006). Characterizing dissolved and particulate phosphorus in water with P-31 nuclear magnetic resonance spectroscopy. Env. Sci. Tech. 40, 7874–7880. doi: 10.1021/es061843e
Cade-Menun, B. J., and Preston, C. M. (1996). A comparison of soil extraction procedures for P-31 NMR spectroscopy. Soil Sci. 161, 770–785. doi: 10.1097/00010694-199611000-00006
Campo, J., Jaramillo, V. J., and Maass, J. M. (1998). Pulses of soil phosphorus availability in a Mexican tropical dry forest: effects of seasonality and level of wetting. Oecologia 115, 167–172. doi: 10.1007/s004420050504
Casey, R. E., and Klaine, S. J. (2001). Nutrient attenuation by a riparian wetland during natural and artificial runoff events. J. Env. Qual. 30, 1720–1731. doi: 10.2134/jeq2001.3051720x
Chiu, C. Y., Pai, C. W., and Yang, K. L. (2005). Characterization of phosphorus in sub-alpine forest and adjacent grassland soils by chemical extraction and phosphorus-31 nuclear magnetic resonance spectroscopy. Pedobiologia 49, 655–663. doi: 10.1016/j.pedobi.2005.06.007
Coats, R., Lewis, J., Alvarez, N. L., and Arneson, P. (2016). Temporal and spatial trends in nutrient and sediment loading to Lake Tahoe, California-Nevada, USA. J. Am. Water Resour. Assoc. 52, 1347–1365. doi: 10.1111/1752-1688.12461
Condron, L. M., Turner, B. L., and Cade-Menun, B. J. (2005). “Chemistry and Dynamics of Soil Organic Phosphorus,” in Phosphorus: Agriculture and the Environment, eds J. T. Sims and A. N. Sharpley (Madison, WI: American Society of Agronomy), 87–121. doi: 10.2134/agronmonogr46.c4
Daly, K., Styles, D., Lalor, S., and Wall, D. P. (2015). Phosphorus sorption, supply potential and availability in soils with contrasting parent material and soil chemical properties. Eur. J. Soil Sci. 66, 792–801. doi: 10.1111/ejss.12260
Deiss, L., de Moraes, A., and Maire, V. (2018). Environmental drivers of soil phosphorus composition in natural ecosystems. Biogeoscience 15, 4575–4592. doi: 10.5194/bg-15-4575-2018
Ehlers, J., and Gibbard, P. L. (2003). Extent and chronology of glaciations. Quaternary Sci. Rev. 22, 1561–1568. doi: 10.1016/S0277-3791(03)00130-6
Elser, J. J., Andersen, T., Baron, J. S., Bergstrom, A. K., Jansson, M., Kyle, M., et al. (2009). Shifts in Lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326, 835–837. doi: 10.1126/science.1176199
Gardner, L. R. (1990). The role of rock weathering in the phosphorus budget of terrestrial watersheds. Biogeochemistry 11, 97–110. doi: 10.1007/BF00002061
Gerard, F. (2016). Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils - a myth revisited. Geoderma 262, 213–226. doi: 10.1016/j.geoderma.2015.08.036
Gergans, N., Miller, W. W., Johnson, D. W., Sedinger, J. S., Walker, R. F., and Blank, R. R. (2011). Runoff water quality from a Sierran upland forest, transition ecotone, and riparian wet meadow. Soil Sci. Soc. Am. J. 75, 1946–1957. doi: 10.2136/sssaj2011.0001
Gerke, J. (2010). Humic (organic matter)-Al(Fe)-phosphate complexes: an underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 175, 417–425. doi: 10.1097/SS.0b013e3181f1b4dd
Gerke, J., and Hermann, R. (1992). Adsorption of orthophosphate to humic-Fe-Complexes and to amorphous Fe-oxide. Z. Pflanzen. Bodenk. 155, 233–236. doi: 10.1002/jpln.19921550313
Giles, C. D., Richardson, A. E., Cade-Menun, B. J., Mezeli, M. M., Brown, L. K., Menezes-Blackburn, D., et al. (2018). Phosphorus acquisition by citrate- and phytase-exuding Nicotiana tabacum plant mixtures depends on soil phosphorus availability and root intermingling. Physiol. Plantarum 163, 356–371. doi: 10.1111/ppl.12718
Goldberg, S. J., Ball, G. I., Allen, B. C., Schladow, S. G., Simpson, A. J., Masoom, H., et al. (2015). Refractory dissolved organic nitrogen accumulation in high-elevation lakes. Nat. Commun. 6:6347. doi: 10.1038/ncomms7347
Hahm, W. J., Riebe, C. S., Lukens, C. E., and Araki, S. (2014). Bedrock composition regulates mountain ecosystems and landscape evolution. Proc. Natl. Acad. Sci. U.S.A. 111, 3338–3343. doi: 10.1073/pnas.1315667111
Harrold, S. A., and Tabatabai, M. A. (2006). Release of inorganic phosphorus from soils by low-molecular-weight organic acids. Comm. Soil Sci. Plant Anal. 37, 1233–1245. doi: 10.1080/00103620600623558
Hatch, L. K., Reuter, J. E., and Goldman, C. R. (1999). Relative importance of stream-borne particulate and dissolved phosphorus fractions to Lake Tahoe phytoplankton. Can. J. Fish Aquatic Sci. 56, 2331–2339. doi: 10.1139/f99-166
Hatch, L. K., Reuter, J. E., and Goldman, C. R. (2001). Stream phosphorus transport in the Lake Tahoe basin, 1989-1996. Environ. Monit. Assess 69, 63–83. doi: 10.1023/A:1010752628576
Haygarth, P. M., and Sharpley, A. N. (2000). Terminology for phosphorus transfer. J. Env. Qual. 29, 10–15. doi: 10.2134/jeq2000.00472425002900010002x
Haygarth, P. M., Warwick, M. S., and House, W. A. (1997). Size distribution of colloidal molybdate reactive phosphorus in river waters and soil solution. Water Res. 31, 439–448. doi: 10.1016/S0043-1354(96)00270-9
Heiberg, L., Pedersen, T. V., Jensen, H. S., Kjaergaard, C., and Hansen, H. C. B. (2010). A comparative study of phosphate sorption in lowland soils under oxic and anoxic conditions. J. Env. Qual. 39, 734–743. doi: 10.2134/jeq2009.0222
Hoffmann, C. C., Berg, P., Dahl, M., Larsen, S. E., Andersen, H. E., and Andersen, B. (2006). Groundwater flow and transport of nutrients through a riparian meadow - field data and modelling. J. Hydrol. 331, 315–335. doi: 10.1016/j.jhydrol.2006.05.019
Hoffmann, C. C., Kjaergaard, C., Uusi-Kamppa, J., Hansen, H. C. B., and Kronvang, B. (2009). Phosphorus retention in riparian buffers: review of their efficiency. J. Env. Qual. 38, 1942–1955. doi: 10.2134/jeq2008.0087
Holford, I. C. R. (1997). Soil phosphorus: its measurement, and its uptake by plants. Aust. J. Soil Res. 35, 227–239. doi: 10.1071/S96047
Jassby, A. D., Goldman, C. R., Reuter, J. E., and Richards, R. C. (1999). Origins and scale dependence of temporal variability in the transparency of Lake Tahoe, California-Nevada. Limnol. Oceanogr. 44, 282–294. doi: 10.4319/lo.1999.44.2.0282
Jiang, X. Q., Bol, R., Cade-Menun, B. J., Nischwitz, V., Willbold, S., Bauke, S. L., et al. (2017). Colloid-bound and dissolved phosphorus species in topsoil water extracts along a grassland transect from Cambisol to Stagnosol. Biogeoscience 14, 1153–1164. doi: 10.5194/bg-14-1153-2017
Johnson, D. W., Susfalk, R. B., and Dahlgren, R. A. (1997). Nutrient fluxes in forests of the eastern Sierra Nevada mountains, United States of America. Global Biogeochem. Cy 11, 673–681. doi: 10.1029/97GB01750
Johnston, C. A., Pinay, G., Arens, C., and Naiman, R. J. (1995). Influence of soil properties on the biogeochemistry of a beaver meadow hydrosequence. Soil Sci. Soc. Am. J. 59, 1789–1799. doi: 10.2136/sssaj1995.03615995005900060041x
Jorgensen, C., Turner, B. L., and Reitzel, K. (2015). Identification of inositol hexakisphosphate binding sites in soils by selective extraction and solution P-31 NMR spectroscopy. Geoderma 257, 22–28. doi: 10.1016/j.geoderma.2015.03.021
Julich, D., Julich, S., and Feger, K.-H. (2017). Phosphorus in preferential flow pathways of forest soils in Germany. Forests 8:19. doi: 10.3390/f8010019
Khare, N., Hesterberg, D., and Martin, J. D. (2005). XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Env. Sci. Tech. 39, 2152–2160. doi: 10.1021/es049237b
Kortemeier, W., Calvert, A., Moore, J. G., and Schweickert, R. (2018). Pleistocene volcanism and shifting shorelines at Lake Tahoe, California. Geosphere 14, 812–834. doi: 10.1130/GES01551.1
Kunito, T., Isomura, I., Sumi, H., Park, H. D., Toda, H., Otsuka, S., et al. (2016). Aluminum and acidity suppress microbial activity and biomass in acidic forest soils. Soil Biol. Biochem. 97, 23–30. doi: 10.1016/j.soilbio.2016.02.019
Kuo, S. (1996). “Phosphorus,” in Methods of Soil Analysis, ed. D.L. Sparks. (Madison, WI: Soil Science Society of America), 869.
Landfire (2020). Forest Canopy Cover Layer, Landfire2.0.0. U.S. Department of the Interior, Geological Survey. Available online at: http://landfire.cr.usgs.gov/viewer/ [accessed March, 2020].
Lenth, R. (2019). “Emmeans: Estimated Marginal Means, aka Least-Squares Means,” in: Package for R. 1.4.2 ed.).
Luo, Z., Niu, J., Zhang, L., Chen, X., Zhang, W., Xie, B., et al. (2019). Roots-enhanced preferential flows in deciduous and coniferous forest soils revealed by dual-tracer experiments. J. Env. Qual. 48, 136–146. doi: 10.2134/jeq2018.03.0091
Margalef, O., Sardans, J., Fernandez-Martinez, M., Molowny-Horas, R., Janssens, I. A., Ciais, P., et al. (2017). Global patterns of phosphatase activity in natural soils. Sci. Rep. 7:1337. doi: 10.1038/s41598-017-01418-8
Marklein, A. R., and Houlton, B. Z. (2012). Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 193, 696–704. doi: 10.1111/j.1469-8137.2011.03967.x
McDowell, R. W., Stewart, I., and Cade-Menun, B. J. (2006). An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy. J. Env. Qual. 35, 293–302. doi: 10.2134/jeq2005.0285
Miller, W. W., Johnson, D. E., Loupe, T. M., Sedinger, J. S., Carroll, E. M., Murphy, J. H., et al. (2006). Nutrients flow from runoff at burned forest site in Lake Tahoe Basin. California Agriculture 60, 65–71. doi: 10.3733/ca.v060n02p65
Miller, W. W., Johnson, D. W., Denton, C., Verburg, P. S. J., Dana, G. L., and Walker, R. F. (2005). Inconspicuous nutrient laden surface runoff from mature forest Sierran watersheds. Water Air Soil Poll. 163, 3–17. doi: 10.1007/s11270-005-7473-7
Miller, W. W., Johnson, D. W., Karam, S. L., Walker, R. F., and Weisberg, P. J. (2010). A synthesis of Sierran forest biomass management studies and potential effects on water quality. Forests 1, 131–153. doi: 10.3390/f1030131
Missong, A., Bol, R., Willbold, S., Siemens, J., and Klumpp, E. (2016). Phosphorus forms in forest soil colloids as revealed by liquid-state 31P-NMR. J. Plant Nutr. Soil Sci. 179, 159–167. doi: 10.1002/jpln.201500119
Murphy, J., and Riley, J. P. (1962). A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 26, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Negrín, M. A., Espino-Mesa, M., and Hernández-Moreno, J. M. (1996). Effect of water: soil ratio on phosphate release: P, aluminium and fulvic acid associations in water extracts from Andisols and Andic soils. Eur. J. Soil Sci. 47, 385–393. doi: 10.1111/j.1365-2389.1996.tb01412.x
Nelson, L., Cade-Menun, B., Walker, I., and Sanborn, P. (2020). Soil phosphorus dynamics across a Holocene chronosequence of aeolian sand dunes on Calvert Island, BC, Canada. Front. For. Glob. Chang. 3:83. doi: 10.3389/ffgc.2020.00083
Noack, S. R., McLaughlin, M. J., Smernik, R. J., McBeath, T. M., and Armstrong, R. D. (2014). Phosphorus speciation in mature wheat and canola plants as affected by phosphorus supply. Plant Soil 378, 125–137. doi: 10.1007/s11104-013-2015-3
Oberson, A., Friesen, D. K., Morel, C., and Tiessen, H. (1997). Determination of phosphorus released by chloroform fumigation from microbial biomass in high P sorbing tropical soils. Soil Biol. Biochem. 29, 1579–1583. doi: 10.1016/S0038-0717(97)00049-7
Ohara, N., Kavvas, M. L., Easton, D., Dogrul, E. C., Yoon, J. Y., and Chen, Z. Q. (2011). Role of snow in runoff processes in a subalpine hillslope: field study in the Ward Creek Watershed, Lake Tahoe, California, during 2000 and 2001 water years. J. Hydrol. Eng. 16, 521–533. doi: 10.1061/(ASCE)HE.1943-5584.0000348
Olander, L. P., and Vitousek, P. M. (2004). Biological and geochemical sinks for phosphorus in soil from a wet tropical forest. Ecosystems 7, 404–419. doi: 10.1007/s10021-004-0264-y
Pistocchi, C., Meszaros, E., Tamburini, F., Frossard, E., and Bunemann, E. K. (2018). Biological processes dominate phosphorus dynamics under low phosphorus availability in organic horizons of temperate forest soils. Soil Biol. Biochem. 126, 64–75. doi: 10.1016/j.soilbio.2018.08.013
Plassard, C., Louche, J., Ali, M. A., Duchemin, M., Legname, E., and Cloutier-Hurteau, B. (2011). Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann. Forest Sci. 68, 33–43. doi: 10.1007/s13595-010-0005-7
Porder, S., and Ramachandran, S. (2013). The phosphorus concentration of common rocks-a potential driver of ecosystem P status. Plant Soil 367, 41–55. doi: 10.1007/s11104-012-1490-2
Pote, D. H., Daniel, T. C., Sharpley, A. N., Moore, P. A., Edwards, D. R., and Nichols, D. J. (1996). Relating extractable soil phosphorus to phosphorus losses in runoff. Soil Sci. Soc. Am. J. 60, 855–859. doi: 10.2136/sssaj1996.03615995006000030025x
Prairie, Y. T., and Kalff, J. (1988). Particulate phosphorus dynamics in headwater streams. Can. J. Fish Aquatic Sci. 45, 210–215. doi: 10.1139/f88-024
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reddy, K. R., Chua, T., and Richardson, C. J. (2013). “Organic phosphorus mineralization in wetland soils,” in Methods in Biogeochemistry of Wetlands, eds. K.R. Reddy and P.B. DeLaune. (Madison, WI: Soil Science Society of America), 683–700. doi: 10.2136/sssabookser10.c35
Redel, Y., Rubio, R., Godoy, R., and Borie, F. (2008). Phosphorus fractions and phosphatase activity in an Andisol under different forest ecosystems. Geoderma 145, 216–221. doi: 10.1016/j.geoderma.2008.03.007
Reid, K., Schneider, K., and McConkey, B. (2018). Components of phosphorus loss from agricultural landscapes, and how to incorporate them into risk assessment tools. Front. Earth Sci. 6:135. doi: 10.3389/feart.2018.00135
Roberts, D. M., and Reuter, J. E. (2010). Lake Tahoe Total Maximum Daily Load Technical Report-California and Nevada. Lahontan Water Board and Nevada Division of Environmental Protection.
Roby, K., O'Neil-Dunne, J., Romsos, S., Loftis, W., MacFaden, S., Saah, D., et al. (2015). “A review of stream environment zone definitions, field delineation criteria and indicators, classification systems, and mapping – collaborative recommendations for stream environment zone program updates”, (eds.) Spatial Informatics Group (SIG) University of Vermont and Spatial Analysis Laboratory (UVM-SAL) and the United States Department of Agriculture Natural Resource Conservation Service (NRCS).).
Rothe, M., Kleeberg, A., and Hupfer, M. (2016). The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth-Sci. Rev. 158, 51–64. doi: 10.1016/j.earscirev.2016.04.008
Sah, R. N., and Mikkelsen, D. S. (1986a). Sorption and bioavailability of phosphorus during the drainage period of flooded-drained soils. Plant Soil 92, 265–278. doi: 10.1007/BF02372640
Sah, R. N., and Mikkelsen, D. S. (1986b). Transformations of inorganic phosphorus during the flooding and draining cycles of soil. Soil Sci. Soc. Am. J. 50, 62–67. doi: 10.2136/sssaj1986.03615995005000010012x
Sah, R. N., Mikkelsen, D. S., and Hafez, A. A. (1989). Phosphorus behavior in flooded-drained soils. 3. Phosphorus desorption and availability. Soil Sci. Soc. Am. J. 53, 1729–1732. doi: 10.2136/sssaj1989.03615995005300060020x
Sahoo, G. B., Nover, D. M., Reuter, J. E., Heyvaert, A. C., Riverson, J., and Schladow, S. G. (2013). Nutrient and particle load estimates to Lake Tahoe (CA-NV, USA) for total maximum daily load establishment. Sci. Total Environ. 444, 579–590. doi: 10.1016/j.scitotenv.2012.12.019
Sahoo, G. B., Schladow, S. G., and Reuter, J. E. (2010). Effect of sediment and nutrient loading on Lake Tahoe optical conditions and restoration opportunities using a newly developed lake clarity model. Water Resour. Res. 46:W10505. doi: 10.1029/2009WR008447
Saucedo, G. J. (2005). Geologic Map of the Lake Tahoe Basin, California and Nevada, 1:100,000 Scale. Sacramento, CA: California Geological Survey.
Saunders, W. M. H., and Williams, E. G. (1955). Observations on the determination of total organic phosphorus in soils. J. Soil Sci. 6, 254–267. doi: 10.1111/j.1365-2389.1955.tb00849.x
Schneider, K. D., Cade-Menun, B. J., Lynch, D. H., and Voroney, R. P. (2016). Soil phosphorus forms from organic and conventional forage fields. Soil Sci. Soc. Am. J. 80, 328–340. doi: 10.2136/sssaj2015.09.0340
Schoeneberger, P. J., Wysocki, D. A., Benham, E. C., and Soil Survey Staff (2012). Field Book for Describing and Sampling Soils. Lincoln, NE: Natural Resources Conservation Service, National Soil Survey Center.
Schoumans, O. (2009). “Determination of the Degree of Phosphate Saturation in Noncalcareous Soils,” in Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, eds. J. Kovar and G. Pierzynski (Raleigh, NC: North Carolina State University; Southern Cooperative Series Bulletin).
Self-Davis, M., Moore, P., and Joern, B. (2009). “Water or Dilute Salt-Extractable Phosphorus in Soil,” in Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, eds. J. Kovar and G. Pierzynski. (Raleigh, NC: North Carolina State University: Southern Cooperative Series Bulletin), 22–24.
Sims, J. (2009). “Soil Test Phosphorus: Principles and Methods,” in Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, eds. J. Kovar and G. Pierzynski. (Raleigh, NC: North Carolina State University: Southern Cooperative Series Bulletin), 9–19.
Sohrt, J., Lang, F., and Weiler, M. (2017). Quantifying components of the phosphorus cycle in temperate forests. Wires Water 4, 1–36. doi: 10.1002/wat2.1243
Sohrt, J., Uhlig, D., Kaiser, K., von Blanckenburg, F., Siemens, J., Seeger, S., et al. (2019). Phosphorus fluxes in a temperate forested watershed: canopy leaching, runoff sources, and in-stream transformation. Front. Glob. Chang 2:85. doi: 10.3389/ffgc.2019.00085
Soil Survey Staff (1999). Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys. U.S. Department of Agriculture Handbook 436: Natural Resources Conservation Service.
Soil Survey Staff (2014). “Kellogg Soil Survey Laboratory Methods Manual”, in: Soil Survey Investigations Report No. 42 Version 5. (Lincoln, NE: Kellogg Soil Survey Laboratory).
Soil Survey Staff, N. R. C. S. (2007). Soil Survey of the Tahoe Basin Area, California and Nevada. Washington, DC: United States Department of Agriculture. Available online at: http://soils.usda.gov/survey/printed_surveys/ (accessed January 26, 2021).
Spohn, M., and Widdig, M. (2017). Turnover of carbon and phosphorus in the microbial biomass depending on phosphorus availability. Soil Biol. Biochem. 113, 53–59. doi: 10.1016/j.soilbio.2017.05.017
Sun, L., Teramoto, M., Naishin, L., Yazaki, T., and Hirano, T. (2017). Comparison of litter-bag and chamber methods for measuring CO2 emissions from leaf litter decomposition in a temperate forest. J. Ag Meteorol. 73, 59–67. doi: 10.2480/agrmet.D-16-00012
Toor, G. S., Condron, L. M., Di, H. J., Cameron, K. C., and Cade-Menun, B. J. (2003). Characterization of organic phosphorus in leachate from a grassland soil. Soil Biol. Biochem. 35, 1317–1323. doi: 10.1016/S0038-0717(03)00202-5
TRPA (2015). “2015 Threshold Evaluation Report”, (ed.) Tahoe Regional Planning Agency. Available online at: http://www.trpa.org/regional-plan/threshold-evaluation/ (accessed January 26, 2021).
U.S. Army Corp of Engineers (2003). Lake Tahoe Basin framework study ground water evaluation, Lake Tahoe Basin, California and Nevada (ed.). California: U.S. Army Corp of Engineers Sacramento District.
Uhlig, D., and von Blanckenburg, F. (2019). How slow rock weathering balances nutrient loss during fast forest floor turnover in montane, temperate forest ecosystems. Front. Earth Sci. 7:159. doi: 10.3389/feart.2019.00159
USDA-NRCS (2019). Snow Telemetry (SNOTEL) and Snow Course Data and Products. Available online at: https://www.wcc.nrcs.usda.gov/snow/: Natural Resource Conservation Service and National Water and Climate Center.
Uselman, S. M., Qualls, R. G., and Lilienfein, J. (2012). Quality of soluble organic C, N, and P produced by different types and species of litter: root litter versus leaf litter. Soil Biol. Biochem. 54, 57–67. doi: 10.1016/j.soilbio.2012.03.021
USGS (2016). USGS Water Data for the Nation. Available online at: https://waterdata.usgs.gov/nwis:USGS.
Vadas, P. A., Kleinman, P. J. A., Sharpley, A. N., and Turner, B. L. (2005). Relating soil phosphorus to dissolved phosphorus in runoff: a single extraction coefficient for water quality modeling. J. Env. Qual. 34, 572–580. doi: 10.2134/jeq2005.0572
Vitousek, P. M., Porder, S., Houlton, B. Z., and Chadwick, O. A. (2010). Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20, 5–15. doi: 10.1890/08-0127.1
Voroney, R. P., Brookes, P. C., and Beyaert, R. (2008). “Soil Microbial Biomass C, N, P and S,” in Soil Sampling and Methods of Analysis, eds. M.R. Carter and E.G. Gregorich. (Boca Raton, FL: CRC Press), 637–651.
Wang, Y. T., Zhang, T. Q., Hu, Q. C., Tan, C. S., O'Halloran, I. P., Drury, C. F., et al. (2010). Estimating dissolved reactive phosphorus concentration in surface runoff water from major Ontario soils. J. Env. Qual. 39, 1771–1781. doi: 10.2134/jeq2009.0504
Wiens, J. T., Cade-Menun, B. J., Weiseth, B., and Schoenau, J. J. (2019). Potential phosphorus export in snowmelt as influenced by fertilizer placement method in the Canadian prairies. J. Env. Qual. 48, 586–593. doi: 10.2134/jeq2018.07.0276
Worsfold, P., McKelvie, I., and Monbet, P. (2016). Determination of phosphorus in natural waters: a historical review. Anal. Chim. Acta 918, 8–20. doi: 10.1016/j.aca.2016.02.047
Wu, J., Li, J. B., Zhao, J., and Miller, R. (2000). Dynamic characterization of phospholipid/protein competitive adsorption at the aqueous solution/chloroform interface. Colloid Surface A 175, 113–120. doi: 10.1016/S0927-7757(00)00543-4
Yang, X., and Post, W. M. (2011). Phosphorus transformations as a function of pedogenesis: a synthesis of soil phosphorus data using Hedley fractionation method. Biogeoscience 8, 2907–2916. doi: 10.5194/bg-8-2907-2011
Young, E. O., Ross, D. S., Cade-Menun, B. J., and Liu, C. W. (2013). Phosphorus speciation in riparian soils: a phosphorus-31 nuclear magnetic resonance spectroscopy and enzyme hydrolysis study. Soil Sci. Soc. Am. J. 77, 1636–1647. doi: 10.2136/sssaj2012.0313
Young, T. C., DePinto, J. V., Martin, S. C., and Bonner, J. S. (1985). Algal-available particulate phosphorus in the Great Lakes Basin. J. Great Lakes Res. 11, 434–446. doi: 10.1016/S0380-1330(85)71788-1
Keywords: forest phosphorus, soil phosphorus, phosphorus NMR, phosphorus availability, phosphorus leaching, phosphorus speciation
Citation: Heron T, Strawn DG, Dobre M, Cade-Menun BJ, Deval C, Brooks ES, Piaskowski J, Gasch C and Crump A (2021) Soil Phosphorus Speciation and Availability in Meadows and Forests in Alpine Lake Watersheds With Different Parent Materials. Front. For. Glob. Change 3:604200. doi: 10.3389/ffgc.2020.604200
Received: 08 September 2020; Accepted: 23 December 2020;
Published: 23 February 2021.
Edited by:
Friederike Lang, University of Freiburg, GermanyReviewed by:
Joerg Schaller, Leibniz Center for Agricultural Landscape Research (ZALF), GermanyHui Wang, Chinese Academy of Forestry, China
Sara L. Bauke, University of Bonn, Germany
Copyright © 2021 Heron, Strawn, Dobre, Deval, Brooks, Piaskowski, Gasch, Crump and Her Majesty in Right of Canada as represented by the Minister of Agriculture and Agri-Food Canada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel G. Strawn, ZGdzdHJhd25AdWlkYWhvLmVkdQ==
 Thomas Heron1
Thomas Heron1 Daniel G. Strawn
Daniel G. Strawn Mariana Dobre
Mariana Dobre Barbara J. Cade-Menun
Barbara J. Cade-Menun Chinmay Deval
Chinmay Deval Erin S. Brooks
Erin S. Brooks Alex Crump
Alex Crump