
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 11 August 2020
Sec. Pests, Pathogens and Invasions
Volume 3 - 2020 | https://doi.org/10.3389/ffgc.2020.00091
This article is part of the Research TopicTropical Plant-Pathogen Interactions in a Changing WorldView all 4 articles
 Patricia Chapple Wright1,2*
Patricia Chapple Wright1,2* Beatriz Otero Jimenez1,2
Beatriz Otero Jimenez1,2 Paul Rakotonirina2
Paul Rakotonirina2 Dina H. Andriananoely2
Dina H. Andriananoely2 Alexandra Shea3
Alexandra Shea3 Baovola Ratalata4
Baovola Ratalata4 Jean Claude Razafimahaimodison2
Jean Claude Razafimahaimodison2Pathogens are threatening crops worldwide, but little attention has been given to the threat to tree species in undisturbed rainforest. This communication reports the first case of a tree die off caused by a “wilt” in Madagascar. In 2016 while monitoring monthly tree phenology of Ranomafana National Park (RNP), the Centre ValBio research station observed that many Calophyllum adult trees had brown wilted leaves. There are three species of Calophyllum in this rainforest, C. paniculatum, C. drouhardii, and C. milvum, and all three have contracted this pathogen. Our goal was to document the spead of this suspected wilt in Calophyllum trees and determine if site, elevation and DBH had an influence on tree mortality. In 2019 we conducted an inventory of all Calophyllum trees in RNP and 42% of the observed trees were dead. The species with the highest mortality was C. paniculatum, with 53% of trees dead, followed by C. milvum with 18%, and C. drouhardii with only 2% of surveyed trees dead. Bark beetle traces were observed in all dead Calophyllum trees. Tree death caused by this suspected fungal pathogen has spread across a major river in the area and has been found at mid and high elevations. Our results show that C. paniculatum trees with a larger DBH have a higher mortality risk. Our report highlights the importance of fighting invasive pathogens that threaten protected ecosystems.
Madagascar holds the highest number of endemic families and genera of plants in the world (Myers et al., 2000; Kremen et al., 2008), and based on regional (Ganzhorn et al., 2001) and global estimates (Beech et al., 2017) of tree biodiversity, we estimate that Madagascar holds over 5% of the world’s tree diversity (Dunham et al., 2018). High species diversity, high endemism, and severe threats make Madagascar’s rainforests a focus of intense scientific and conservation interest. While we have an increasing understanding of the role of habitat loss and hunting on Madagascar’s tropical flora and fauna (Brown and Gurevitch, 2004; Mittermeier et al., 2006; Allnutt et al., 2008; Dunham et al., 2008; Irwin et al., 2010; Herrera et al., 2011; Schwitzer et al., 2014), we know little about how invasive disease affects the rainforests of Madagascar. Invasive vertebrates (including the Asian cane toad Marshall et al., 2018; Reardon et al., 2018) and invertebrates (including the marbled crayfish Jones et al., 2009; Gutekunst et al., 2018) have been introduced in the island of Madagascar within the last decade and had a devastating impact on native fauna. There is also evidence that pathogens are transferring from domestic animals (dogs) and invasive rodents (rats) to endemic forest species (lemurs) (Rasambainarivo et al., 2013; Pomerantz et al., 2016; Zohdy et al., 2019).
As with most other tropical plant species, little is known about the ecology and population status of Malagasy forest tree species. For this reason, the emergence of plant pathogens in Madagascar’s rainforest is of particular significance and can lead to undetected disease-induced population declines or extinctions (Anderson et al., 2004; Fisher et al., 2012). The majority of trees in tropical forests are dispersed by vertebrate frugivores (Terborgh et al., 2002) and provide critical resources for supporting vertebrate. In the southeastern rainforest of Madagascar, up to 85% of tree species support the region’s endemic vertebrate frugivores, including birds, bats, and the region’s diverse endemic primates, the lemurs. Loss or declines in tree species population could lead to greater ecosystem effects.
Although the impacts of selective logging precious hardwoods have been documented in Malagasy forests (Irwin et al., 2010; Herrera et al., 2011; Gerber et al., 2012) plant pathogens have not been describes as an important driver of fruit tree decline on the island. Scientific research on pathogens affecting wild plant populations has increased in recent years. This communication seeks to highlight the importance of continuing research in this topic, especially on the effect of invasive pathogens on tropical tree species. Here we present the incidence of the pathological condition on a rainforest population of trees in the family Calophyllaceae. We describe the condition and spread of the disease to Calophyllum species within Ranomafana National Park. More specifically, the aim of this study is to examine the effect of elevation, tree size, and location on mortality of Calophyllum paniculatum due to the wilt pathogen.
Vascular wilt diseases are caused by pathogenic fungi, bacteria or nematodes that enter the water-conducting xylem vessels of a plant, then proliferate within the vessels, causing water blockage (Tattar, 2012). Symptoms, including the Calophyllum wilt described here, include wilting and death of the leaves, followed often by death or serious impairment of the whole plant (Ploetz et al., 2011). As a group, the vascular wilts are among the most devastating plant diseases (Ploetz et al., 2011). Insects are one of the prominent invasive species groups worldwide (Biedermann et al., 2019). Bark beetles are one of the main dispersers of vascular wilt causing agents such as fungi and nematodes, and have devastated forests in North America, and Europe (Kudela et al., 1976; Wainhouse et al., 1998; Webber et al., 1999). However, most attention is given to plants that are used by humans as food crops (Mayfield et al., 2008) such as the avocado and mango, and not forest tree species (Ploetz et al., 2011; Rodgers et al., 2014; da Silva Galdino et al., 2016).
A vascular wild disease was detected in Calophyllum inophyllum trees on the island of Mauritius in 1939 just 1132 km from Madagascar (Webber et al., 1999). In 1994 on the island of Mahe on the Seychelles, 1834 km from Madagascar, the presence of the wilt was described affecting Calophyllum trees, and it was hypothesized that it was being spread by the bark beetle Cryphalus trypanus (Wainhouse et al., 1998; Webber et al., 1999). Additionally, this disease has been reported in other tropical regions such as, El Salvador and Cuba (Kudela et al., 1976). However, this is the first time that a wilt pathogen has been reported in Madagascar. Based on these studies and our field observations we suspect that the cause of the wilt disease in Calophyllum trees in RNP is the fungal pathogen dispersed by the bark beetles. However, our investigation of the cause of the wilt is ongoing.
Calophyllum trees can grow up to 30 meters in height and 90 DBH. Calophyllum wood is used to build boats, for construction, carpentry, flooring, furniture, cabinet work, and musical instruments (Damon, 2016). This tree is known for its chemistry with a variety of secondary metabolites isolated such as coumarins, xanthones, flavonoids and triterpenes which have cytotoxic, anti-HIV and antimicrobial properties (Vandana, 2017). Used as a medicinal plant by local healers, Calophyllum can treat peptic ulcers, tumors, infections, pain, and inflammation (Ratalata, 2007). In addition, Calophyllum trees have been reported as an important source of food for frugivorous birds (Pratt, 1982; Galetti, 1993), bats (Mello et al., 2005), and lemurs (Birkinshaw, 2001; Martinez and Razafindratsima, 2014).
Our study was conducted in Ranomafana National Park located in the southeastern rainforest belt of Madagascar (47°18′- 47°37′E, 21°02′- 21°25′S) and home to Centre ValBio research station (Wright et al., 2012; Figure 1). The park is comprised of 41,600 ha of evergreen montane rainforest, ranging in elevation from 600 to 1480 m (Wright and Andriamihaja, 2002). The park hosts over 330 tree species (Razafindratsima and Dunham, 2015), 85% of which are dispersed by frugivores (Razafindratsima and Dunham, 2016). The Centre ValBio tree phenology team has been recording fruiting phenology data since 1987 for 71 common tree species (representing 24 families and 46 genera). Trees are monitored for fruiting and flowering once a month (Wright et al., 2012). During the May 2016 monthly monitoring, the Centre ValBio Botanical team reported that C. paniculatum was dying. This discovery spearheaded an active search for Calophyllum trees in Ranomafana National Park.
In 2016 the first diseased C. paniculatum tree was detected by observation of dried and dead leaves during monthly phenological monitoring. In 2019 three Centre ValBio botanists systematically searched on trail for the Calophyllum trees in 5 sites within Ranomafana National Park. We surveyed each site 8 h every day for 6 days covering an area of approximately 3 km2 at each site. Our efforts resulted in the identification of more than 1,000 Calophyllum trees of all three species. For each Calophyllum tree the DBH, elevation, GPS coordinates, and tree condition (i.e., dead by disease, dead, or alive) were recorded. Trees were considered dead by disease if the presence of beetle tracks in the trunk and wilted leaves was observed. We estimated the effect of elevation, site, and DBH on tree survival through a binomial generalized linear model in R.
The disease progresses from a few leaves with welts, which turn brown, and die a few branches at a time (Figures 2A–C). More branches become progressively brown and the trunk is covered with vertical tracks (Figure 2D) and larvae (Figure 2E) that appear to be evidence of the bark beetle. The dead trees within the forest landscape show the dispersion of the Calophyllum trees (Figure 2A). In 2019 the disease was widespread in Calophyllum species and death by disease was detected in all 5 sites surveyed (Figure 3). All observed dead trees had evidence of contracting the pathogen and represented 42% (n = 702) of all the trees surveyed. The species with the highest mortality was C. paniculatum, with 53% of trees dead, followed by C. milvum with 18%, and C. drouhardii with only 2% of surveyed trees dead. Above 1000 m elevation, there were few C. paniculatum trees, and they are replaced by other species in this genus, C. drouhardii and C. milvum (Figure 3). Tree mortality varied by location and species (Figure 4). However, statistical analysis were only conducted for the most widespread species, C. paniculatum. Results from the generalized linear model (GLM) show that C. paniculatum trees of larger DBH are more likely to contract the disease and die (Table 1 and Figure 5). Elevation was not a significant variable in predicting tree survival (Table 1). Additionally, based on the GLM C. paniculatum trees present in the Talatakely site had a higher probability of dying from the disease than those in other sites (Table 1). During the time of the study bark beetles were never observed, but the vertical tracks implying bark beetle infestation were obvious on trunks of dead and dying trees, and larvae were observed (Figures 2D,E).
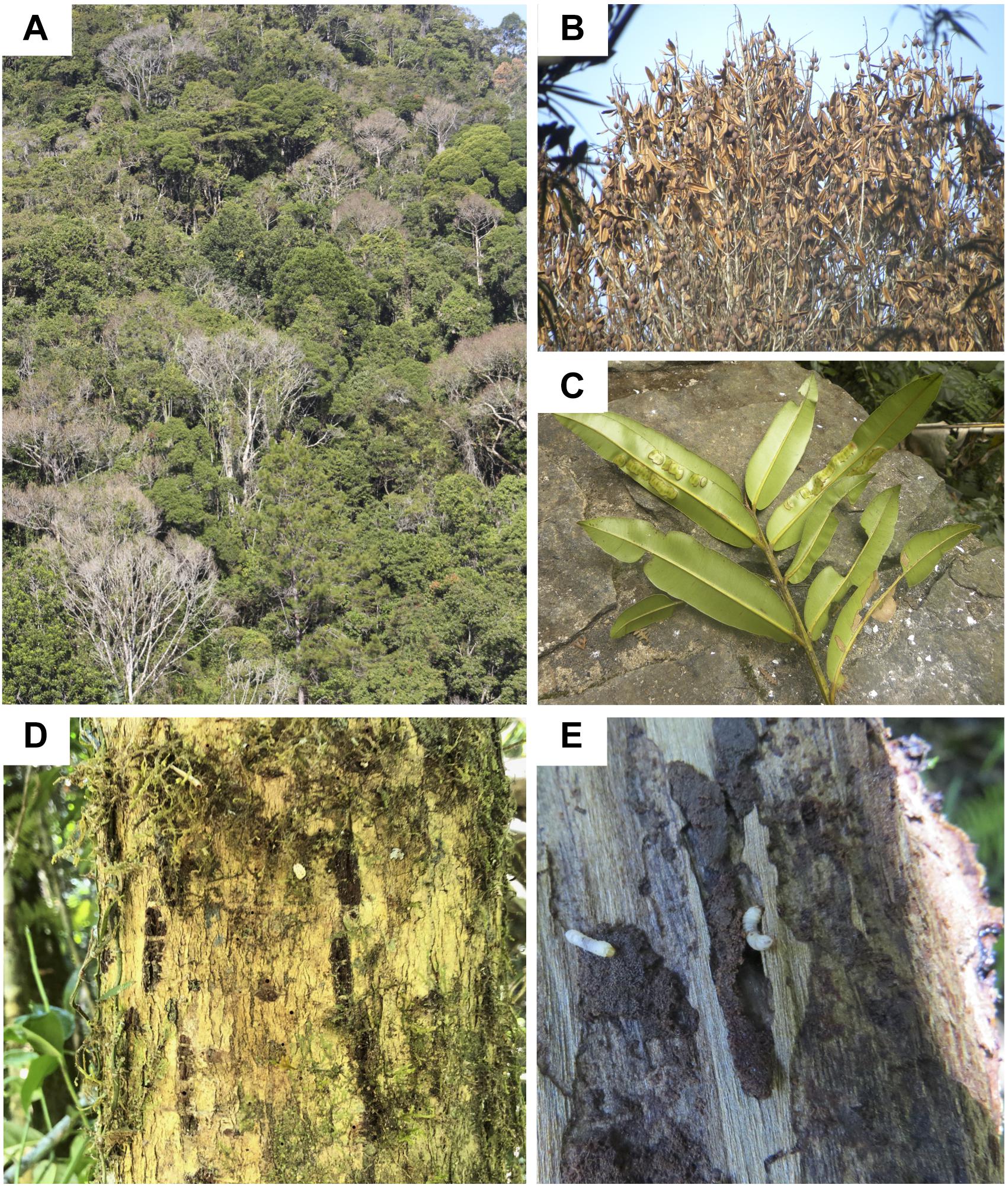
Figure 2. Photos of Calophyllum trees in Ranomafana National Park, (A) landscape of forest with dead trees within the park, (B) closeup of tree with dead leaves, (C) close up of the diseased leaves, (D) the bark of a dead C. paniculatum tree with grooves perhaps caused by bark beetles, and (E) suspected bark beetle larvae in Calophyllum tree trunk.
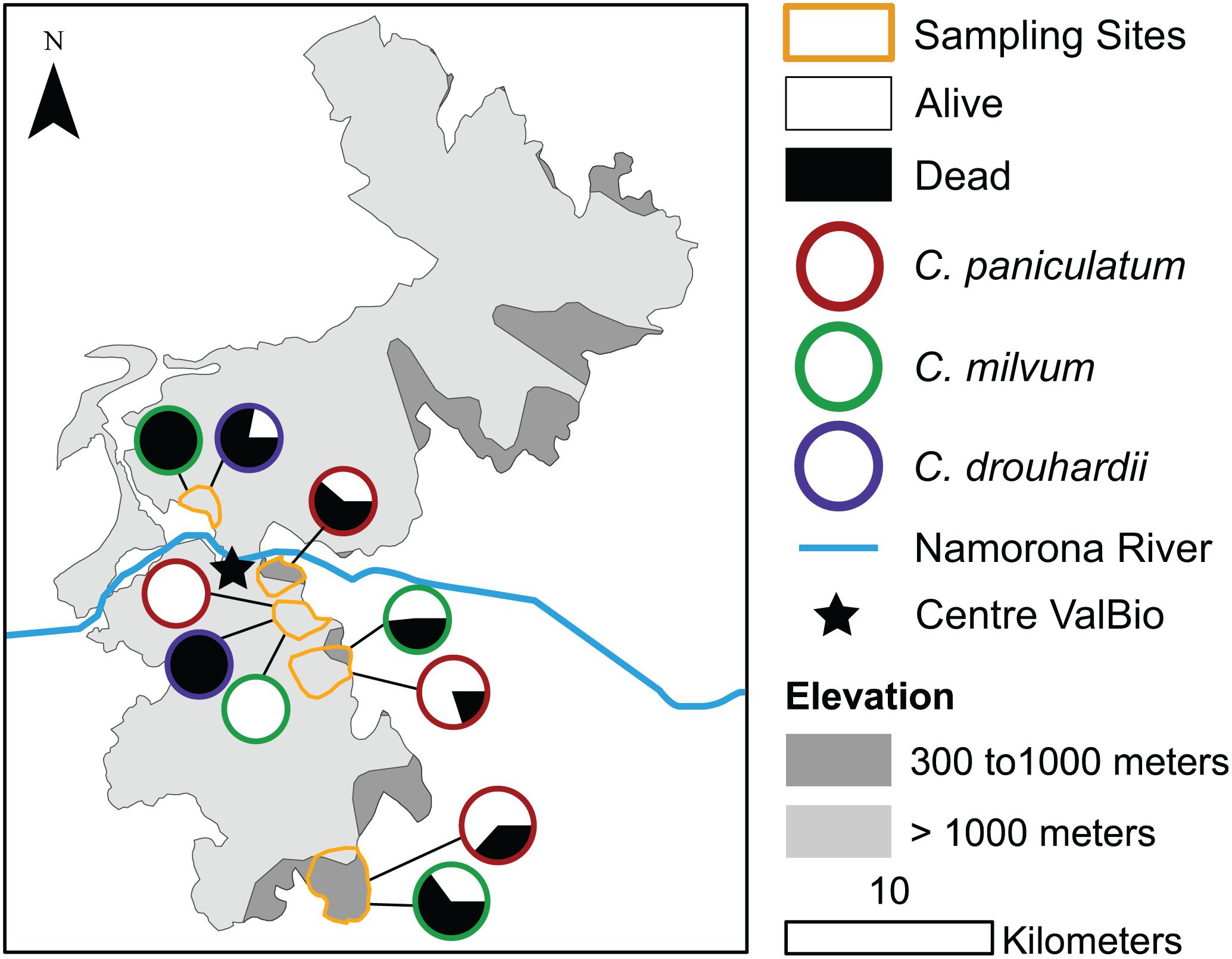
Figure 3. Map of surveys of Calophyllum spp. within five sites in Ranomafana National Park, Madagascar with elevation and proportion of dead and living trees by species.
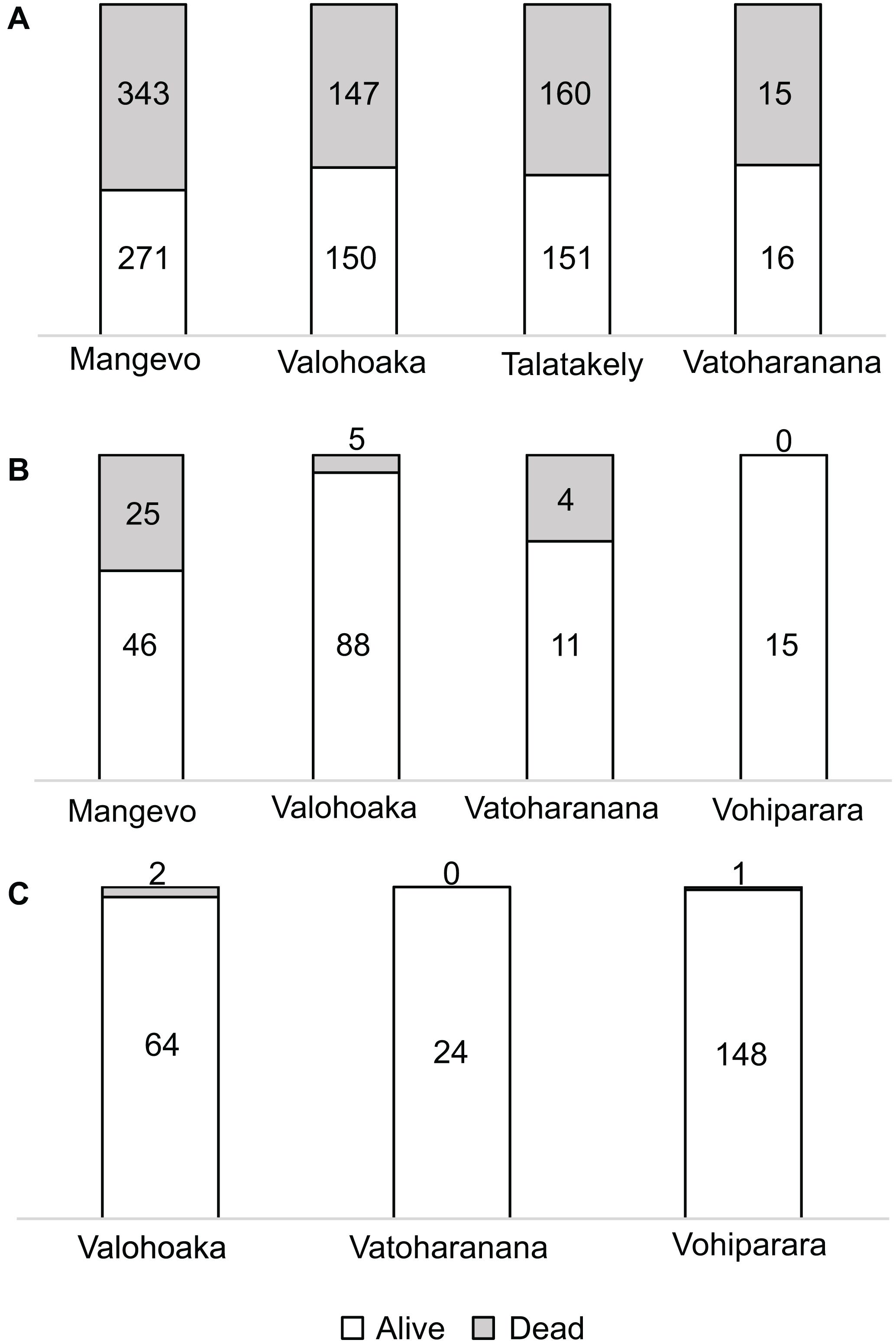
Figure 4. Proportion of dead (gray) and alive (white) trees per species of Calophyllum per site surveyed: (A) Calophyllum paniculatum, (B) Calophyllum milvum, and (C) Calophyllum drouhardii. Numbers within column represent the number of trees in each category.
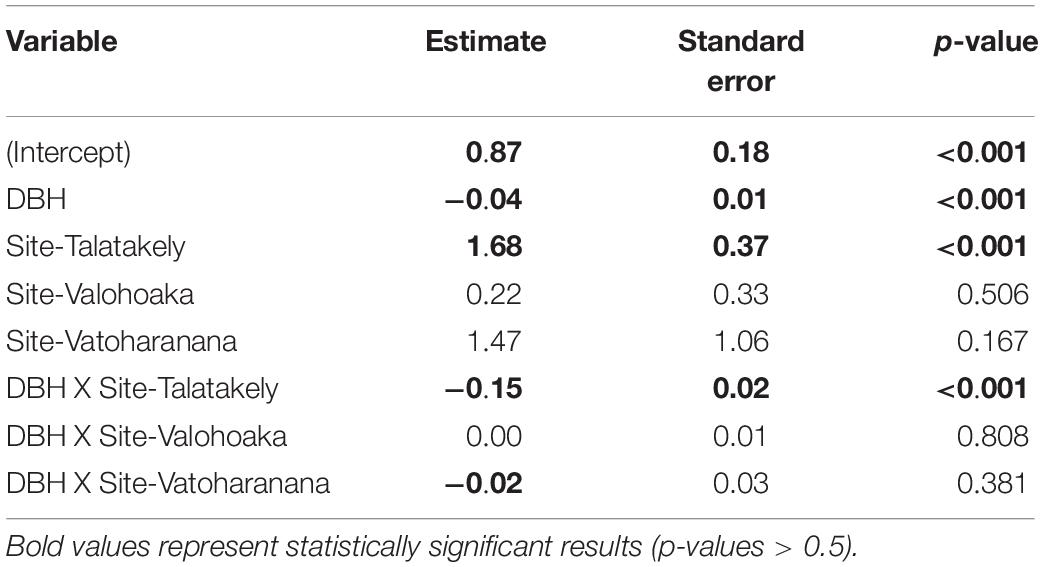
Table 1. Parameter estimates of general linear model for Calophyllum paniculatum survival including interaction term between sampling sites and DBH.
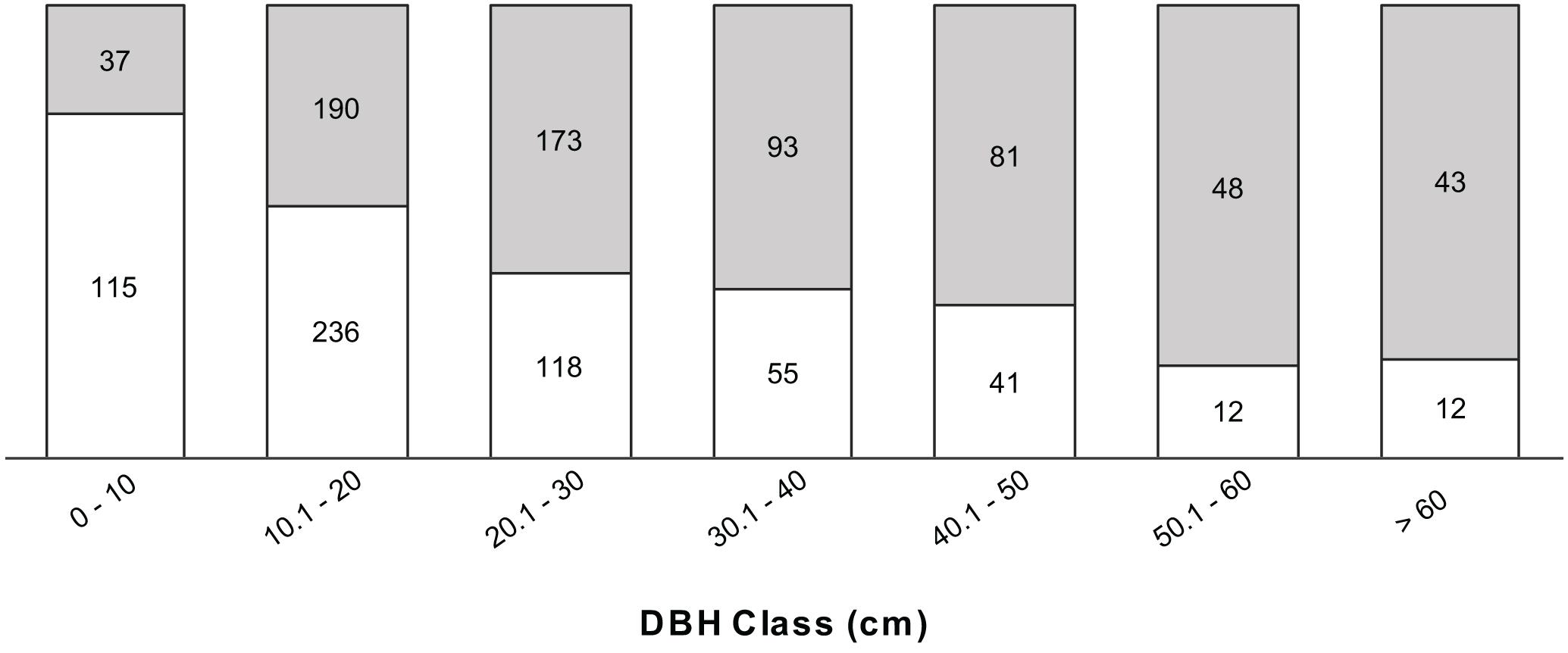
Figure 5. Proportion of dead (gray) vs. alive (white) trees per DBH class of Calophyllum paniculatum in Ranomafana National Park. Numbers within columns indicate the number of individual trees in each category (dead or alive).
Understanding how rapidly and how far disease epidemics can spread through species-rich tropical forests should be a priority for conservation planning (Gilbert and Hubbell, 1996; Anderson et al., 2004). Yet basic data on invasive tree pathogens in tropical forests is lacking. In this short communication we describe the first incidence of the pathological condition of wilt in Madagascar on a rainforest population of Calophyllum trees in the Ranomafana National Park.
In 2016 for the first time in the eastern rainforests of Madagascar a pathogen was detected to attack a primary rainforest tree. In this Malagasy case, the susceptibility of the trees may be caused by drought stress, and climate change might be implicated as dry seasons have become protracted in the last decade (Parmesan and Yohe, 2003; Ingram and Dawson, 2005; Wright, 2006; Hannah et al., 2008; Moritz and Agudo, 2013; Brown et al., 2015).
Our results highlighted the influence of tree DBH on tree survival to the wilt disease. We found that C. paniculatum with larger DBH were more likely to contract and die from the wilt disease. These results support patterns found on pine wilt disease in South Korea pine forests where researchers found higher risk rate of contracting the disease in pines with a DBH > 10 cm (Park et al., 2013). This pattern could be because trees with higher DBH have a larger canopy area and will be easier for beetles, the potential wilt fungi dispersers, to find these trees. We also found that C. paniculatum trees located in Talatekely site had a higher probability of contracting and dying due to the disease. The Talatekely site is closer to the entrance of the national park and has a higher number of visitors than other sites, increasing the potential of plant pathogen dispersal from outside the park.
We will need a census of pathogens involved tree symptoms in Madagascar and further work is needed to demonstrate Koch postulate.
Conservation biology is perhaps one of the most interdisciplinary in the biological sciences, but plant pathology has played only a peripheral role (Gilbert and Hubbell, 1996). The probability that introduced diseases will invade, spread and kill in protected tropical ecosystems is increasing with climate change and fighting these new pathogens should be a priority in conservation biology (Gilbert and Hubbell, 1996; Roy et al., 2017).
The datasets generated for this study are available on request to the corresponding author.
PW conceived the idea, spearheaded the writing and secured the financial contribution. BO contributed to the writing, the data analysis and created the figures. PR and DA collected field data, and created figures. AS edited and did literature review and references, BR identified the wilt in lab, and JR helped edit the manuscript. All authors contributed to the article and approved the submitted version.
Funding for the long-term monitoring of phenology was provided from the David and Lucile Packard Foundation, John D. and Catherine T. MacArthur Foundation, National Science Foundation (NSF BSC 0721233 and NSF DBI 1227143), Conservation International, and Stony Brook University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Madagascar’s Department of Water and Forests, the Ministry of Ecology, Environment, and Forests, Madagascar National Parks, and the CAFF/CORE committee for authorization to do this research. Thanks to Centre ValBio Research Station and MICET for logistical support. We acknowledge Centre ValBio’s botany field technicians: Pela Auguste, Razafitsiafajato Armand, Razafindraibe Dominique, and Razafindrakoto Georges for monitoring and survey data on Calophyllum.
Allnutt, T. F., Ferrier, S., Manion, G., Powell, G. V. N., Ricketts, T. H., Fisher, B. L., et al. (2008). A method for quantifying biodiversity loss and its application to a 50-year record of deforestation across Madagascar. Conserv. Lett. 1, 173–181. doi: 10.1111/j.1755-263x.2008.00027.x
Anderson, P. K., Cunningham, A. A., Patel, N. G., Morales, F. J., Epstein, P. R., and Daszak, P. (2004). Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. doi: 10.1016/j.tree.2004.07.021
Beech, E., Rivers, M., Oldfield, S., and Smith, P. P. (2017). GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. Forest. 36, 454–489. doi: 10.1080/10549811.2017.1310049
Biedermann, P. H. W., Müller, J., Grégoire, J. C., Gruppe, A., Hagge, J., Hammerbacher, A., et al. (2019). Bark beetle population dynamics in the Anthropocene: challenges and solutions. Trends Ecol. Evol. 34, 914–924. doi: 10.1080/10549811.2017.1310049
Birkinshaw, C. (2001). Fruit characteristics of species dispersed by the black lemur (Eulemur macaco) in the Lokobe forest, Madagascar. Biotropica 33, 478–486. doi: 10.1111/j.1744-7429.2001.tb00201.x
Brown, K. A., and Gurevitch, J. (2004). Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 101, 6045–6049. doi: 10.1073/pnas.0401456101
Brown, K. A., Parks, K. E., Bethell, C. A., Johnson, S. E., and Mulligan, M. (2015). Predicting plant diversity patterns in Madagascar: understanding the effects of climate and land cover change in a biodiversity hotspot. PLoS One 10:e0122721. doi: 10.1371/journal.pone.0122721
da Silva Galdino, T. V., Kumar, S., Oliveira, L. S., Alfenas, A. C., Neven, L. G., Al-Sadi, A. M., et al. (2016). Mapping global potential risk of mango sudden decline disease caused by Ceratocystis fimbriata. PLoS One 11:e0159450. doi: 10.1371/journal.pone.0159450
Damon, F. H. (2016). “A story of Calophyllum: from ecological to social facts,” in Trees, Knots, and Outriggers: Environmental Knowledge in the Northeast Kula Ring, ed. F. H. Damon (New York, NY: Berghahn Books), 180–246.
Dunham, A. E., Erhart, E. M., Overdorff, D. J., and Wright, P. C. (2008). Evaluating effects of deforestation, hunting, and El Niño events on a threatened lemur. Biol. Conserv. 141, 287–297. doi: 10.1016/j.biocon.2007.10.006
Dunham, A. E., Razafindratsima, O. H., Rakotonirina, P., and Wright, P. C. (2018). Fruiting phenology is linked to rainfall variability in a tropical rain forest. Biotropica 50, 396–404. doi: 10.1111/btp.12564
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. doi: 10.1038/nature10947
Galetti, M. (1993). Diet of the scaly-headed parrot (Pionus maximiliani) in a semideciduous forest in southeastern Brazil. Biotropica 25, 419–425.
Ganzhorn, J. U., Lowry, P. P., Schatz, G. E., and Sommer, S. (2001). The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. ORYX 35, 346–348. doi: 10.1046/j.1365-3008.2001.00201.x
Gerber, B. D., Arrigo-Nelson, S., Karpanty, S. M., Kotschwar, M., and Wright, P. C. (2012). Spatial ecology of the endangered Milne-Edwards’ Sifaka (Propithecus edwardsi): do logging and season affect home range and daily ranging patterns? Int. J. Primatol. 33, 305–321. doi: 10.1007/s10764-011-9576-x
Gilbert, G. S., and Hubbell, S. P. (1996). Plant diseases and the conservation of tropical forests. BioScience 46, 98–106. doi: 10.2307/1312812
Gutekunst, J., Andriantsoa, R., Falckenhayn, C., Hanna, K., Stein, W., Rasamy, J., et al. (2018). Clonal genome evolution and rapid invasive spread of the marbled crayfish. Nat. Ecol. Evol. 2, 567–573. doi: 10.1038/s41559-018-0467-9
Hannah, L., Dave, R., Lowry, P. P., Andelman, S., Andrianarisata, M., Andriamaro, L., et al. (2008). Climate change adaptation for conservation in Madagascar. Biol. Lett. 4, 590–594. doi: 10.1098/rsbl.2008.0270
Herrera, J. P., Wright, P. C., Lauterbur, E., Ratovonjanahary, L., and Taylor, L. L. (2011). The effects of habitat disturbance on lemurs at ranomafana national park, Madagascar. Int. J. Primatol. 32, 1091–1108. doi: 10.1007/s10764-011-9525-8
Ingram, J. C., and Dawson, T. P. (2005). Climate change impacts and vegetation response on the island of madagascar. Philos. Trans. A Math. Phys. Eng. Sci. 363, 55–59. doi: 10.1098/rsta.2004.1476
Irwin, M. T., Wright, P. C., Birkinshaw, C., Fisher, B. L., Gardner, C. J., Glos, J., et al. (2010). Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol. Conserv. 143, 2351–2362. doi: 10.1016/j.biocon.2010.01.023
Jones, J. P. G., Rasamy, J. R., Harvey, A., Toon, A., Oidtmann, B., Randrianarison, M. H., et al. (2009). The perfect invader: a parthenogenic crayfish poses a new threat to Madagascar’s freshwater biodiversity. Biol. Invasions 11, 1475–1482. doi: 10.1007/s10530-008-9334-y
Kremen, C., Cameron, A., Moilanen, A., Phillips, S. J., Thomas, C. D., Beentje, H., et al. (2008). Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320, 222–226. doi: 10.1126/science.1155193
Kudela, M., Hochmut, R., and Leontovyc, R. (1976). Forest protection in Cuba. Silvaecult. Trop. Subtr. 4, 97–112.
Marshall, M., Casewell, N., Vences, M., Glaw, F., Rakotoarison, A., Zancolli, G., et al. (2018). Widespread vulnerability of Malagasy predators to the toxins of an introduced toad. Curr. Biol. 28, R654–R655. doi: 10.1016/j.cub.2018.04.024
Martinez, B. T., and Razafindratsima, O. H. (2014). Frugivory and seed dispersal patterns of the red-ruffed lemur, Varecia rubra, at a forest restoration site in Masoala National Park, Madagascar. Folia Primatol. 85, 228–243. doi: 10.1159/000363408
Mayfield, A. E., Smith, J. A., Hughes, M., and Dreaden, T. J. (2008). First report of Laurel wilt disease caused by a Raffaelea sp. on avocado in Florida. Plant Dis. 92:976. doi: 10.1094/PDIS-92-6-0976A
Mello, M. A. R., Leiner, N. O., Guimarães, P. R., and Jordano, P. (2005). Size-based fruit selection of Calophyllum brasiliense (Clusiaceae) by bats of the genus Artibeus (Phyllostomidae) in a Restinga area, southeastern Brazil. Acta Chiropterol. 7, 179–182. doi: 10.3161/1733-5329(2005)7[179:sfsocb]2.0.co;2
Mittermeier, R. A., Valladares-Pádua, C., Rylands, A. B., Eudey, A. A., Butynski, T. M., Ganzhorn, J. U., et al. (2006). Primates in peril: the world’s 25 most endangered primates, 2004–2006. Primate Conserv. 20, 1–28.
Moritz, C., and Agudo, R. (2013). The future of species under climate change: resilience or decline? Science 341, 504–508. doi: 10.1126/science.1237190
Myers, N., Mittermeler, R. A., Mittermeler, C. G., Da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Park, Y.-S., Chung, Y.-J., and Moon, Y.-S. (2013). Hazard ratings of pine forests to a pine wilt disease at two spatial scales (individual trees and stands) using self-organizing map and random forest. Ecol. Informatic. 13, 40–46. doi: 10.1016/j.ecoinf.2012.10.008
Parmesan, C., and Yohe, G. (2003). A globally coherent fingerprint of climate change. Nature 421, 37–42. doi: 10.1038/nature01286
Ploetz, R. C., Peña, J. E., Smith, J. A., Dreaden, T. J., Crane, J. H., Schubert, T., et al. (2011). Laurel wilt, caused by Raffaelea lauricola, is confirmed in miami-dade county, center of florida’s commercial avocado production. Plant Dis. 95, 1589. doi: 10.1094/pdis-08-11-0633
Pomerantz, J., Rasambainarivo, F. T., Dollar, L., Rahajanirina, L. P., Andrianaivoarivelo, R., Parker, P., et al. (2016). Prevalence of antibodies to selected viruses and parasites in introduced and endemic carnivores in western Madagascar. J. Wildl. Dis. 52, 544–552. doi: 10.7589/2015-03-063
Pratt, T. K. (1982). Diet of the dwarf cassowary Casuarius bennetti picticollis at Wau, Papua New Guinea. EMU 82, 283–285. doi: 10.1071/mu9820283s
Rasambainarivo, F. T., Gillespie, T. R., Wright, P. C., Arsenault, J., Villeneuve, A., and Lair, S. (2013). Survey of Giardia and Cryptosporidium in lemurs from the Ranomafana National Park, Madagascar. J. Wildl. Dis. 49, 741–743. doi: 10.7589/2012-10-264
Ratalata, B. (2007). “Diplôme d’Etude Approfondie” Organic Chemistry Applied in Natural Products. Ph. D. Thesis, Antananarivo University, Antananarivo.
Razafindratsima, O. H., and Dunham, A. E. (2015). Assessing the impacts of nonrandom seed dispersal by multiple frugivore partners on plant recruitment. Ecology 96, 24–30. doi: 10.1890/14-0684.1
Razafindratsima, O. H., and Dunham, A. E. (2016). Frugivores bias seed-adult tree associations through nonrandom seed dispersal: a phylogenetic approach. Ecology 97, 2094–2102. doi: 10.1002/ecy.1434
Reardon, J. T., Kraus, F., Moore, M., Rabenantenaina, L., Rabinivo, A., Rakotoarisoa, N. H., et al. (2018). Testing tools for eradicating the invasive toad Duttaphrynus melanostictus in Madagascar. Conserv. Evid. 15, 12–19.
Rodgers, L., Derksen, A., and Pernas, T. (2014). Expansion and impact of laurel wilt in the Florida Everglades. Florida Entomol. 97, 1247–1250. doi: 10.1653/024.097.0335
Roy, H. E., Hesketh, H., Purse, B. V., Eilenberg, J., Santini, A., Scalera, R., et al. (2017). Alien pathogens on the horizon: opportunities for predicting their threat to wildlife. Conserv. Lett. 10, 477–484. doi: 10.1111/conl.12297
Schwitzer, C., Mittermeier, R. A., Johnson, S. E., Donati, G., Irwin, M., Peacock, H., et al. (2014). Averting lemur extinctions amid Madagascar’s political crisis. Science 343, 842–843. doi: 10.1126/science.1245783
Terborgh, J., Pitman, N., Silman, M., Schichter, H., and Núñez, P. (2002). “Maintenance of tree diversity in tropical forests,” in Seed Dispersal and Frugivory: Ecology, Evolution and Conservation, eds D. J. Levey, W. R. Silva, and M. Galetti (Wallingford: CAB International), 1–17.
Vandana, V. (2017). Synthesis of Calophyllum inophyllum esters as biofuel feed stock. Juniper Online J. Mater. Sci. 2:555583. doi: 10.19080/jojms.2017.02.555583
Wainhouse, D., Murphy, S., Greig, B., Webber, J., and Vielle, M. (1998). The role of the bark beetle Cryphalus trypanus in the transmission of the vascular wilt pathogen of takamaka (Calophyllum inophyllum) in the Seychelles. Forest Ecol. Manag. 108, 193–199. doi: 10.1016/S0378-1127(98)00234-5
Webber, J. F., Jacobs, K., and Wingfield, M. J. (1999). A re-examination of the vascular wilt pathogen of takamaka (Calophyllum inophyllum). Mycol. Res. 103, 1588–1592. doi: 10.1017/S0953756299001021
Wright, P. C. (2006). “Considering climate change effects in lemur ecology and conservation,” in Lemurs: Ecology and Conservation, eds L. Gould and M. L. Sauther (Boston, MA: Springer), doi: 10.1007/978-0-387-34586-4_18
Wright, P. C., and Andriamihaja, B. (2002). “Making a rain forest national park work in Madagascar: Ranomafana National Park and its long-term research commitment,” in. Making Parks Work: Strategies for Preserving Tropical Nature, eds J. Terborgh, C. von Schaik, L. Davenport, and R. Madhu (Washington, DC: Island Press), 112–136.
Wright, P. C., Erhart, E. M., Tecot, S., Baden, A. L., Arrigo-Nelson, S. J., Herrera, J., et al. (2012). “Long-term lemur research at Centre Valbio, Ranomafana National Park, Madagascar,” in Long-Term Field Studies of Primates, eds P. Kappeler and D. Watts (Berlin: Springer), 67–100. doi: 10.1007/978-3-642-
Keywords: Calophyllum, Madagascar, Vascular wilt disease, rainforest, Ranomafana National Park, invasive pathogen, tropical conservation
Citation: Wright PC, Otero Jimenez B, Rakotonirina P, Andriananoely DH, Shea A, Ratalata B and Razafimahaimodison JC (2020) The Progressive Spread of the Vascular Wilt Like Pathogen of Calophyllum Detected in Ranomafana National Park, Madagascar. Front. For. Glob. Change 3:91. doi: 10.3389/ffgc.2020.00091
Received: 29 November 2019; Accepted: 03 July 2020;
Published: 11 August 2020.
Edited by:
Julieta Benitez-Malvido, National Autonomous University of Mexico, MexicoReviewed by:
Luis Daniel Avila, National Autonomous University of Mexico, MexicoCopyright © 2020 Wright, Otero Jimenez, Rakotonirina, Andriananoely, Shea, Ratalata and Razafimahaimodison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Chapple Wright, cGF0Y2hhcHBsZXdyaWdodEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.