
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 03 March 2020
Sec. Tropical Forests
Volume 3 - 2020 | https://doi.org/10.3389/ffgc.2020.00018
A correction has been applied to this article in:
Corrigendum: The Influence of Taxonomy and Environment on Leaf Trait Variation Along Tropical Abiotic Gradients
 Imma Oliveras1*
Imma Oliveras1* Lisa Bentley2
Lisa Bentley2 Nikolaos M. Fyllas3
Nikolaos M. Fyllas3 Agne Gvozdevaite1
Agne Gvozdevaite1 Alexander Frederick Shenkin1
Alexander Frederick Shenkin1 Theresa Peprah4
Theresa Peprah4 Paulo Morandi5
Paulo Morandi5 Karine Silva Peixoto5
Karine Silva Peixoto5 Mickey Boakye4,6
Mickey Boakye4,6 Stephen Adu-Bredu4
Stephen Adu-Bredu4 Beatriz Schwantes Marimon5
Beatriz Schwantes Marimon5 Ben Hur Marimon Junior5
Ben Hur Marimon Junior5 Norma Salinas7
Norma Salinas7 Roberta Martin8
Roberta Martin8 Gregory Asner8
Gregory Asner8 Sandra Díaz9
Sandra Díaz9 Brian J. Enquist10,11
Brian J. Enquist10,11 Yadvinder Malhi1
Yadvinder Malhi1Deconstructing functional trait variation and co-variation across a wide range of environmental conditions is necessary to increase the mechanistic understanding of community assembly processes and improve current parameterization of dynamic vegetation models. Here, we present a study that deconstructs leaf trait variation and co-variation into within-species, taxonomic-, and plot-environment components along three tropical environmental gradients in Peru, Brazil, and Ghana. To do so, we measured photosynthetic, chemical, and structural leaf traits using a standardized sampling protocol for more than 1,000 individuals belonging to 367 species. Variation associated with the taxonomic component (species + genus + family) for most traits was relatively consistent across environmental gradients, but within-species variation and plot-environment variation was strongly dependent on the environmental gradient. Trait-trait co-variation was strongly linked to the environmental gradient where traits were measured, although some traits had consistent co-variation components irrespective of gradient. Our results demonstrate that filtering along these tropical gradients is mostly expressed through trait taxonomic variation, but that trait co-variation is strongly dependent on the local environment, and thus global trait co-variation relationships might not always apply at smaller scales and may quickly change under future climate scenarios.
Over the past two decades, functional trait ecology has increasingly been used to understand and predict the structure and functioning of plant communities (Lavorel and Garnier, 2002; McGill et al., 2006). Plant traits and trait syndromes (consistent associations between traits, Kattge et al., 2011) reflect a combination of evolutionary and community assembly processes responding to biotic and abiotic constraints (Chapin et al., 1993; Grime, 1998; Valladares et al., 2007). As such, functional traits can be used to quantify these assembly processes across various ecological scales, such as within-species (Rozendaal et al., 2006; Valladares et al., 2007), across species (Rozendaal et al., 2006; Messier et al., 2017), and within and across populations and communities (Messier et al., 2010; Laughlin and Messier, 2015). In addition to ecological scales, assembly processes also vary across spatial scales and as such, studies have addressed trait variation at global (Wright et al., 2004, 2017; Díaz et al., 2016; Anderegg et al., 2018), regional (Fyllas et al., 2009; Asner and Martin, 2016) and local scales (Swenson and Enquist, 2007, 2009; Kraft et al., 2008; Messier et al., 2010). In paleoecology, fossil leaves and the generality of leaf-trait variation has also been of major interest to understand terrestrial paleoclimate evolution (Jacobs, 1999).
Functional traits are often measured across contrasting environments (Diaz et al., 1998; Ackerly, 2004; McGill et al., 2006; Anderson et al., 2011) to characterize how the mean community trait value varies within and across spatial scales. Therefore, they are often studied along vegetation continua derived from latitudinal (Shepherd, 1998; Swenson et al., 2012; Lamanna et al., 2014; Lawson and Weir, 2014), climatic (Hulshof et al., 2013), elevation (McCain and Grytnes, 2001; Körner, 2007; Bryant et al., 2008), temporal (Enquist et al., 2015), and vegetation gradients (Fernandes, 2000; Ackerly et al., 2002; Messier et al., 2017).
Importantly, it is not just the values of functional traits that are used to understand and predict the structure and functioning of plant communities, but the variation within and co-variation between and among traits (Violle et al., 2007, 2012; Albert et al., 2011; Taudiere and Violle, 2015; Neyret et al., 2016). The role of leaf functional trait variation and co-variation at different ecological scales has received renewed attention in the last decades, with efforts searching for general trait syndromes or spectra reflecting differences in ecological strategies, which are considered the result of natural selection (Grime, 1977; Coley et al., 1985; Westoby et al., 2002; Reich et al., 2003; Wright et al., 2004; Agrawal and Fishbein, 2006; Craine, 2009; Díaz et al., 2016). A major focus of these research efforts is to document the prevalence of the leaf economics spectrum (LES, Wright and Sutton-Grier, 2012; Osnas et al., 2013; Messier et al., 2016; Anderegg et al., 2018), with less attention paid to other leaf properties such as leaf chemistry [other than nitrogen which belongs to the LES, but see Fyllas et al. (2009), Asner and Martin (2016), hydraulic traits (Messier et al., 2017) or defense mechanisms (Agrawal and Fishbein, 2006)]. Given the importance of defining trait syndromes in plant ecology, a lack of knowledge related to functional trait variation across different scales limits our ability to identify and understand trait syndromes, which is essential to understand the ecology and evolution of diversity in plant form and function (Poorter et al., 2014; Díaz et al., 2016). This is particularly true in tropical areas, where relative remoteness, logistical challenges and high biodiversity continue to challenge our capacity to quantify and assess the generality of trait syndromes. There is also a need to hone trait-based approaches to develop more predictive mechanistic models of how ecological communities and ecosystems will respond to abiotic and biotic perturbations (Funk and Wolf, 2016).
Here we examine the variation among a set of key leaf functional traits with respect to plot-environment (i.e. abiotic conditions, such as climate and soil), taxonomic (species, genus, family) and within-species (i.e. specimens within species components across three contrasting tropical environmental gradients. Across all sites, traits were sampled using a standardized methodology, thereby providing a unique opportunity to test the consistency of trait variation and co-variation along environmental gradients without complicating factors of multiple collection and analysis methods. For clarity, we avoid the terms intraspecific and interspecific variation, as such terminology varies among studies (e.g. Fyllas et al., 2009; Asner and Martin, 2016; Messier et al., 2016; Rosas et al., 2019).
Using key leaf traits related to plant form and function, including photosynthetic capacity, leaf structure, and leaf chemistry, we ask the following questions:
(1) Does the partitioning of leaf trait variance across sources (within-species, taxonomic, plot-environment) reflect similar patterns across tropical environmental gradients of temperature, water, and land-use?
We hypothesize that the source of variance of photosynthetic and structural traits will be strongly taxonomy-driven, with a consistent degree of variation across gradients. However, we expect leaf nutrient traits to be strongly associated with the local abiotic conditions (i.e. to be dominated by the plot-environmental component of variation) reflecting the local soil which differ across the three gradients.
(2) To what extent do community-weighted mean trait values reflect the effect of within-species, taxonomic and plot-environment variation? Are these effects consistent across environmental gradients?
We hypothesize that community-weighted mean values of all key functional traits will be strongly site-specific, where environmental gradients driven by high species-turnover will exhibit higher taxonomic effects compared to gradients strongly driven by plot-environment variation.
(3) Are there differences in trait co-variation according to within-species, taxonomic and plot-environment variance components? Are these differences consistent across environmental gradients?
We hypothesize that trait co-variation will be consistent across environmental gradients. We expect the within-species component of trait variation to be important for photosynthetic traits reflecting acclimation and plasticity, the taxonomic component of trait variation to be important for structural traits reflecting the role of evolutionary processes, and the environmental components of trait variation to be important for chemical traits reflecting local abiotic filtering drivers.
To test these hypotheses, we sampled three distinct tropical environmental gradients. The Peru gradient spans more than 3,000 m altitude with concurrent differences in climate, the Ghana gradient spans precipitation regimes from wet to mesic and the Brazil gradient presents similar climatic conditions and soils but has a strong taxonomic turnover from shrubland savanna to semi deciduous forest. To our knowledge, this is the first study to deconstruct trait variation and co-variation across such broad biogeographic regions in the tropics using standardized methodology.
The study sites included in this work are part of the Global Ecosystems Monitoring Network (GEM1) and RAINFOR-ForestPlots network2. Sites were part of three gradients: (1) an altitudinal gradient spanning 330 km in Peru; (2) a rainfall gradient across > 200 km in Ghana; and (3) a forest-savanna vegetation gradient in Brazil across 25 km (Table 1). At all sites, we selected plots that were censored at 1–3 year intervals.
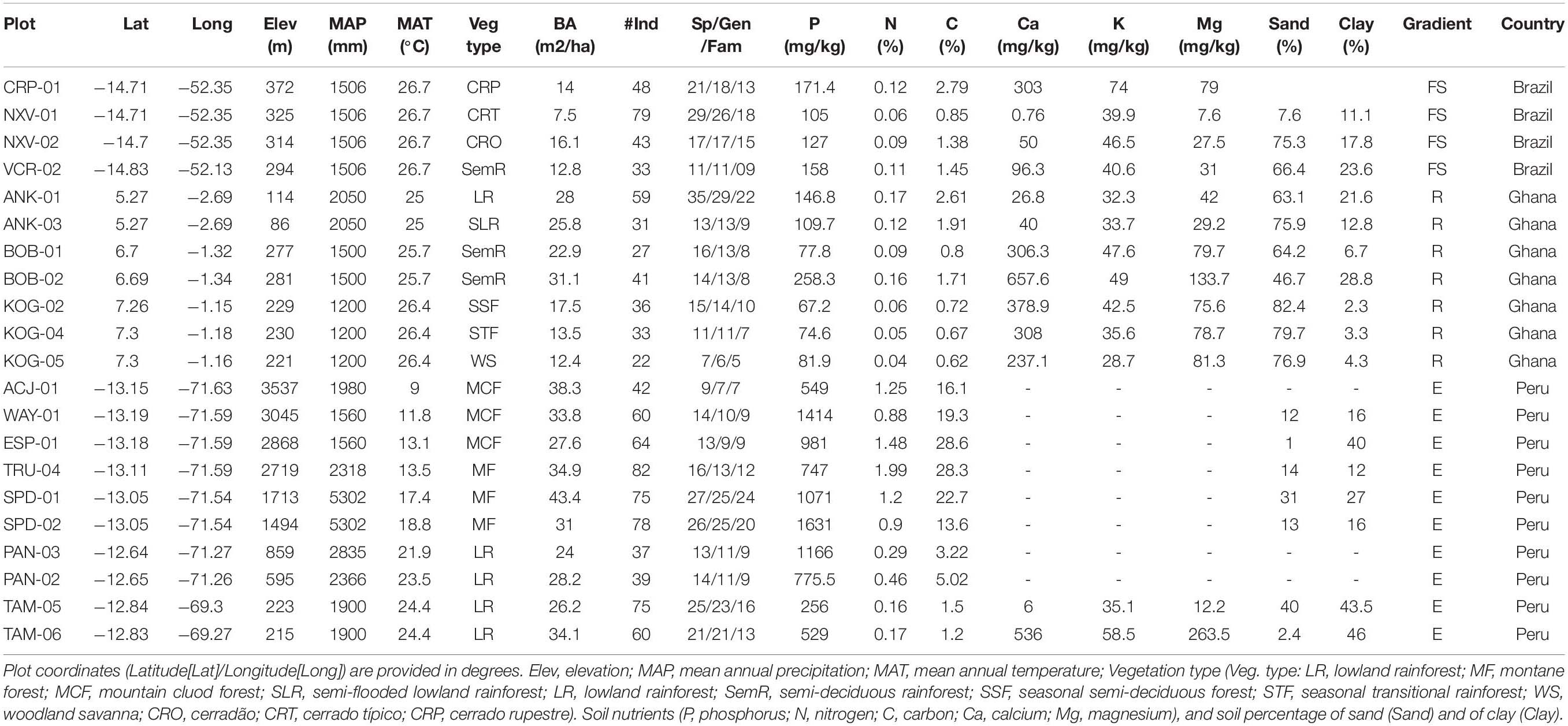
Table 1. Summary information of plot characteristics at each environmental gradient (E = elevation gradient, Peru; R = rainfall gradient, Ghana; V = forest-savanna gradient, Brazil).
Ten 1-ha permanent plots were sampled along an elevation gradient in the departments of Cusco and Madre de Dios in SE Peru (Table 1), ranging from 220 to 3500 m.a.s.l.). Six of the plots are montane plots in the Kosñipata Valley, spanning an elevation range 1,500–3,500 m (Malhi et al., 2010), two are submontane plots located in the Pantiacolla front range of the Andes (range 600–900 m.a.s.l.) and two plots are found in the Amazon lowlands in Tambopata National Park (elevation range 200–225 m.a.s.l.). All plots are operated by the Andes Biodiversity Ecosystems Research Group (ABERG3). The plots range in temperature from 25.2 to 9.0°C at the lowest to the highest elevation plot (Malhi et al., 2017). Plots are located in areas that have relatively homogeneous soil substrates and stand structure, and which have minimal evidence of human disturbance (Girardin et al., 2014). The montane plots were established between 2003 and 2013 and the two lowland plots were established in 1983. During plot establishment, all stems ≥ 10 cm diameter at breast height were tagged and identified to species-level, and in recent years plots have been measured at monthly intervals for carbon allocation and cycling following standard the GEM Network protocol (Marthews et al., 2014). As such, net primary productivity estimates (Girardin et al., 2010) and comprehensive descriptions of the carbon cycle exist for many of these plots (Girardin et al., 2014; Huasco et al., 2014; Malhi et al., 2014). From February 2013 to January 2014, mean annual air temperature varied from 9 to 24.4°C along the gradient and precipitation ranged from 1560 to 5302 mm y–1 across all sites along the gradient (Table 1). Precipitation peaks strongly at mid elevations (around 1500 m.a.s.l.).
Seven plots were sampled along a rainfall gradient spanning from 1200 to 2100 mm of mean annual precipitation (Moore et al., 2018, Table 1). On the driest end, three plots were located at the Kogyae Strict Nature Reserve), a 330 km2 protected area located in the north-eastern part of the Ashanti region. At Kogyae, three plots corresponded to three different vegetation types: savanna, savanna-woodland transition and dry semi-deciduous forest. The area experiences a bimodal annual rainfall distribution with a high precipitation period from March to July and another high precipitation period in September and October, and December and January are the driest months with less than 30 mm rain (Janssen et al., 2018). The geology of Kogyae belongs to the Voltarian system, and the rocks are reddish brown sandstone whenever exposed. The soil in the savanna and transition areas is Haplic Arenosols, showing thin, sandy loam topsoils and the soil of the forest sites Haplic Nitosols, more acidic than savanna soil (Domingues et al., 2010). Two semi-deciduous forest plots were sampled at Bobiri Reserve (Table 1). Soils at Bobiri are thought to be of similar origin as in Kogyae, and there may be local depositional features, or other possibility includes heavy cation deposition from Saharan dust during the Harmattan winds in January and February (Moore et al., 2018). The two plots at the wettest end were located in Ankasa National Park, south-western Ghana. This national park has an area of about 500 km2, with mean annual precipitation about 2,000 mm, mainly concentrated from March to mid-July and from September to November (Chiti et al., 2010). A dry period extends from December to February. Relative humidity is high through the years, ranging from over 90% at night to 75% in the early afternoon (Chiti et al., 2010). The soils are Oxisoils, deeply weathered, highly acidic (3.5–4.0 in pH). In Ankasa, one plot (moist evergreen forest) was situated on upland soils developed on coherent biotite-rich granites and another plot (swampy evergreen forest) was located on alluvial soils (Chiti et al., 2010).
This gradient included four plots (Table 1) along a forest-savanna transition in the state of Mato Grosso (Brazil). The region is characterized by two well-defined seasons: hot and wet from October to March, and cool and dry from April to September (Marimon et al., 2014). The area sits at the ecotone between Amazonia and Cerrado biomes, and has a rapid transition from an Amazonian semi-deciduous forest to various cerrado vegetation types. Local abrupt transitions in vegetation type are mediated by differences in soil physical and chemical properties (Marimon Junior and Haridasan, 2005). Three plots were located in Parque Municipal do Bacaba (CRP-01, NXV-01, NXV-02, Table 1) in Nova Xavantina, Mato Grosso and represented three distinct vegetation types of progressively decreasing woody biomass and stature [cerradão, cerrado tipico, cerrado rupestre, see Marimon Junior and Haridasan (2005), Maracahipes et al. (2011)]. The fourth site was located in a semi-deciduous forest, located 25 km away (SE) from Nova Xavantina in the reserve of Fazenda Vera Cruz (VCR-02, Table 1) (Marimon Junior and Haridasan, 2005; Marimon et al., 2014). All plots are located in areas that have relatively homogeneous soil substrates and stand structure (Table 1). Plots were established between in 2002, with all stems ≥ 5 cm basal diameter tagged and identified to species-level, except for the forest plot (VCR-02) where all stems ≥ 10 cm diameter at breast height tagged and identified at the species-level. Since its establishment, plots have been annually re-censured and since 2013 they are continuously being monitored for carbon allocation and cycling following the GEM protocol (Marthews et al., 2014). From Jan 2013 to June 2014, daily mean air temperature varied from 16.2 to 39.4°C and total precipitation was of 2,696 mm (BDMEP 2017) (Table 1).
Field campaigns to measure leaf traits were conducted using a standardized protocol between April 2013 and April 2015 in all plots (Supporting Information). Sampled leaves were chosen from individuals that corresponded to the most dominant species in each plot. To determine target species, we ranked and selected species contributing to up to 80% of the total basal area of the plot using data from the most recent plot census (2012–2013). For each selected species, we chose the largest 3–5 individuals to measure traits on 3–5 leaves. On each leaf we measured light-saturated rates of net photosynthesis at ambient CO2 (400 ppm) (Asat, μmol CO2 m2s–1), light-saturated maximum rates of net photosynthesis at saturated CO2 (2000 ppm) (Amax, μmol CO2 m2s–1), leaf thickness (LT, mm), leaf mass per area (LMA g m–2), leaf dry matter content (LDMC, mg g–1, calculated on saturated weight basis), leaf nitrogen content ([N],%), leaf phosphorus content ([P],%), leaf calcium content ([Ca],%), leaf magnesium content ([Mg],%) and leaf potassium content ([K],%). All leaf nutrient concentrations were calculated on a mass basis. Light-saturated photosynthetic rates are functional traits to provide valuable information of metabolic capacity, and together with LMA and [N] form the leaf economic spectrum (Wright et al., 2004). LDMC is a structural functional trait that correlates with toughness and physical hazards (e.g. herbivory, wind) and has also been shown to inversely correlate with growth, while LT plays a key role in determining the physical strength of leaves (Pérez-Harguindeguy et al., 2013). The selected nutrients play essential roles related to plant growth and performance. For example, [N], [P], [K], and [Mg] are key elements for photosynthesis (Taiz and Zeiger, 2006; Tränkner et al., 2018), [K] is also essential for plant osmotic processes and plays a critical role in long-distance water transport (Wang et al., 2013), and [Ca] is an important constituent of cell walls (Taiz and Zeiger, 2006).
We focused our statistical analysis on angiosperms only, thus data on palms and bamboos were excluded in order to reduce a level of complexity in the analysis of variance.
To investigate the partitioning of leaf trait variance across sources (within-species, taxonomic, plot-environment), traits were analyzed for their source of variance (Supplementary Figure S1) using a multilevel linear mixed effects analysis (Fyllas et al., 2009; Asner and Martin, 2016) on log10-transformed trait values:
Where μ is the fixed intercept that corresponds to the overall mean value of each trait Z, P is the plot-environment effect (f/g/s) represents the taxonomic structure of the data –species (s) includes each individual belonging to a species s, nested in genus (g), nested in family (f), and ε the within-species (i.e. individuals from the same species) variance plus measurement error, i.e. the residual (Anderegg et al., 2018). All effects were treated as random and parameters were estimated using the Residual Maximum Likelihood (REML) method with the lme4 package in R (Bates et al., 2018). Therefore, in each model, y is a leaf trait modeled as the sum of the mean value for the entire dataset (μ), the nested taxonomic effects (f/g/s), the site effect (P), and the within-species effect + residual error of measurement (ε). If in a given model, the term ε accounted for a high percentage of the total variance, we concluded that the site characteristics and taxonomy did not explain the data well (Asner and Martin, 2016). Asner and Martin (2016) highlighted that this method had the limitation only quantifying the entire pattern of phylogenetic grouping or lack thereof relative to site and residual effects, that is, it did not capture variation within taxa (as some taxa might have tightly clumped trait variation while others may vary widely). To maintain consistency among gradients and traits, we only included leaves collected in full sun (as opposed to leaves collected in the shade), and averaged values per tree in the analyses (i.e. within-tree variation was not examined). Some outlier values were removed from the analysis.
To investigate whether community-weighted mean trait values reflect the effect of within-species, taxonomic and plot-environment variation, and if these effects are consistent across environmental gradients, we extracted the random effects of the different components of variance (within-species, species, genus, family, plot-environment) for each trait. These terms were extracted as follows:
Where z = trait, μ is mean, ranef is the random effect of the linear effects model (Eq. 1), and e represents the components of variance (within-species, species, genus, family, plot-environment). The resulting value was back-transformed to obtain an actual value for each given trait when only that particular component of variance was considered (Fyllas et al., 2009).
In order to compare traits among plots and abiotic gradients, we derived community weighted means based on basal area at the plot level (CWM; Violle et al., 2007; Neyret et al., 2016) of each trait as well as the community weighted effect size (CWE).
We explored the significance CWE for each trait z and component of variance e by calculating their effect size (ESze) as:
Where σ2 corresponds to the community weighted variance.
To investigate the differences in trait co-variation according to the different variance sources we explored the bivariate relationships between community weighted leaf trait values for each component of variance (within-species, species, genus, family, plot-environment) using Kendall r- correlations and p-value corrections using Holm’s procedure for multiple comparisons (psych package in R, Legendre and Legendre, 1998).
We further explored trait co-variation among community weighted means of log transformed community weighted trait values by component of variance with standardized major axis (SMA) regressions (smatr package in R) (Legendre and Legendre, 1998). SMA regression lines represent the first axis of principal component analysis (of a correlation matrix) and are often used to evaluate trait interrelationships in plant allometry studies (Fyllas et al., 2009).
Finally, we tested the correlation between each trait community weighted plot-environment component and the following environmental variables for each gradient: elevation, mean annual temperature, mean annual temperature, percentage of sand in the soil, percentage of clay in the soil, and N, P, and C concentration in the soil (all soil variables calculated for the first 30 cm, Supplementary Table S1). We used Pearson correlation coefficients and their p-value with the cor.test() function in the stats package (R Core Team, 2018). Similarly, we also used Pearson correlation coefficients to test correlation between each trait community weighted taxonomic component (sum of species, genus, and families) and number of individuals, species and families per plot and environmental gradient.
The analysis of 10 different leaf functional traits from 1,064 trees belonging to 367 species, 326 genera, and 252 families across the three environmental gradients (Table 1) showed that variation associated with the within-species component across functional traits and gradients, contributing 40% on average of the total variance (median 42.8%, maximum 65% ([K] in the forest-savanna gradient) and minimum 14% ([Ca] in the elevation gradient). Taxonomic variation (i.e. species, genera, and families) among traits and across environmental gradientscontributed 42% of the trait variance on average (median 37.6%), with a maximum of 73.5% ([N] in the elevation gradient) and minimum of 22% ([P] in the rainfall gradient). Variance partitioning across different taxonomic levels was also not consistent across traits and environmental gradients (Figure 1 and Supplementary Table S1). The variation associated with the plot-environment component contributed 18% of the total trait variation on average (median 12.5%), with a maximum of 60% ([Ca] in the elevation gradient) and a minimum of 0.01% (LDMC in the forest-savanna gradient).
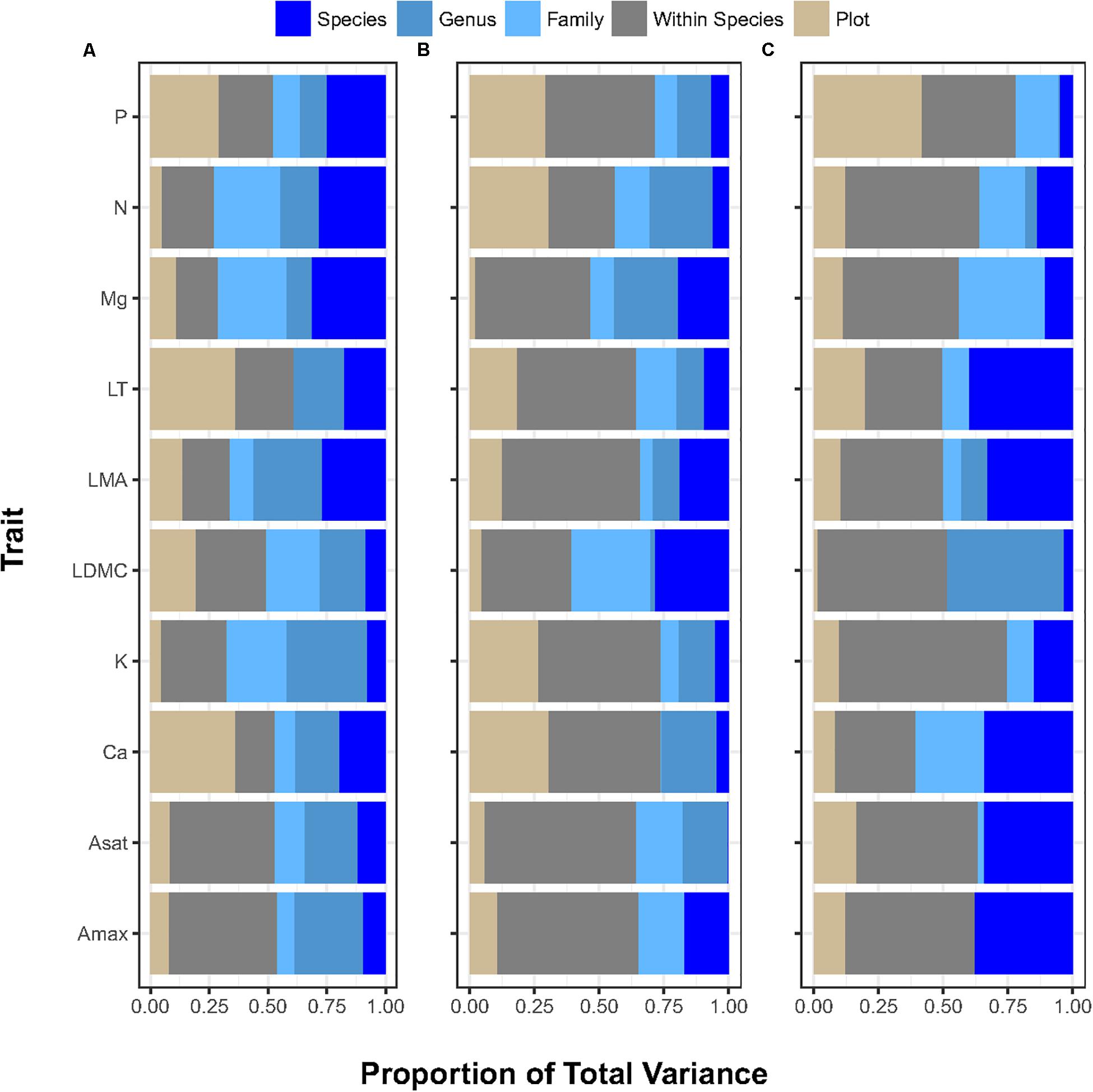
Figure 1. Proportion of variance (X-axis) explained by plot (environment) and by each ecological level (within-species, species, genus, family) contributing to the total variance for each trait (Y-Axis) in (A) elevation gradient, (B) rainfall gradient, (C) forest-savanna gradient. P: phosphous leaf concentration, N: nitrogen leaf concentration; Mg: magnessium leaf concentration; LT: leaf thickness; LMA: leaf mass per area; LDMC: leaf dry matter content; K: leaf potassium concentration; Ca: leaf calcium concentration; Asat: light-saturated rate of net CO2 assimilation at ambient (400 ppm) CO2; Amax: light-saturated rate of maximum CO2 at assimilation at 2000 ppm CO2.
Apart from these broad trends, some leaf nutrient traits ([N], [P], [Ca]) exhibited plot-environment effects, although these were not consistent across environmental gradients (Figure 1 and Supplementary Table S1). For example, leaf [N] presented little range of variation within plots across all gradients (2–4 fold), and [P] varied 3–7 fold (Table 2). However, the plot-environment variance component for leaf [N] ranged from 4.4% (elevation gradient) to 31% (forest-savanna gradient) whereas the plot-environment associated variance for leaf [P] represented 30–42% of the total variance (Figure 1 and Supplementary Table S1). Leaf [Ca] values showed a large range of variation in the elevation and rainfall gradients where they varied 6–18 fold (Table 2), with a variance associated with the plot-environmental component of 60 and 30% for elevation and rainfall gradients respectively (Figure 1 and Supplementary Table S1).
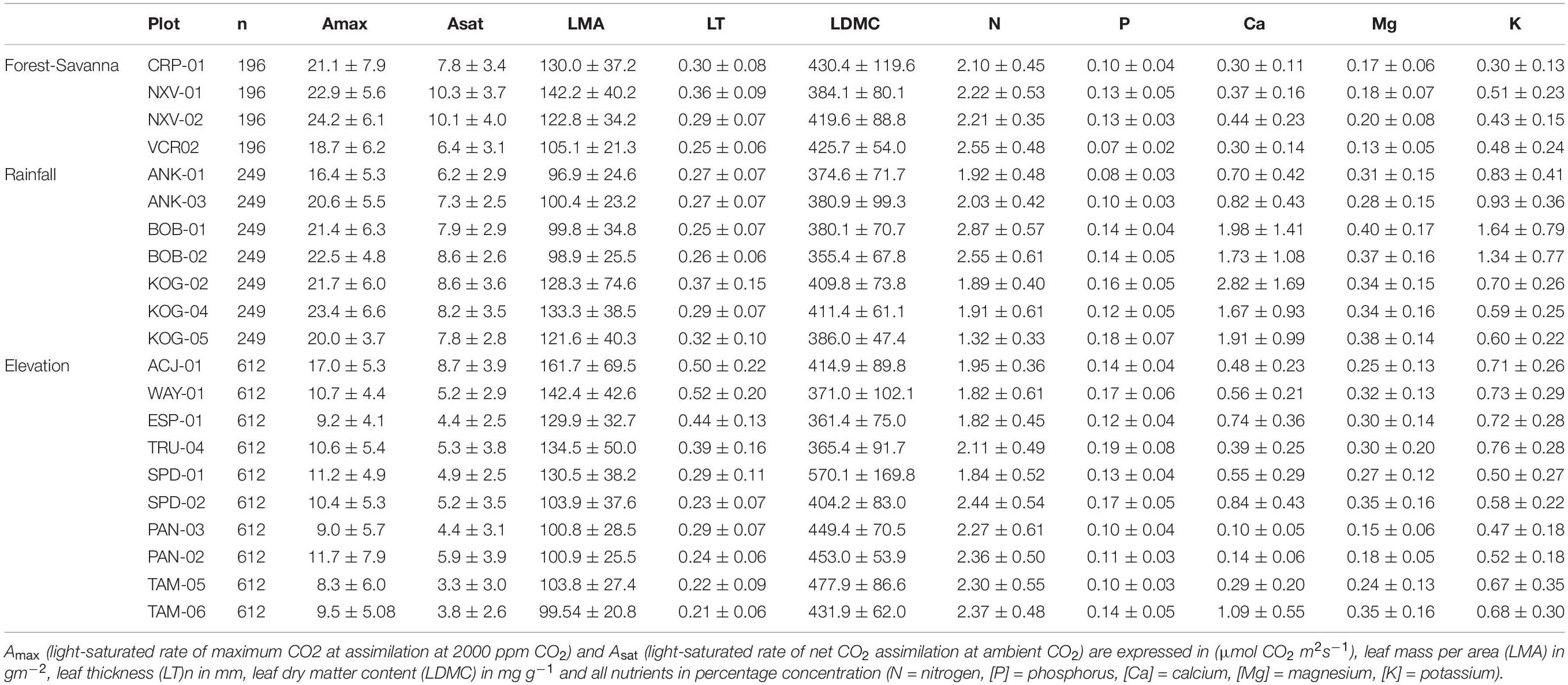
Table 2. Average ± standard deviation for the studied leaf traits in the forest-savanna, rainfall, and elevation gradients.
Photosynthetic traits showed the strongest variance associated with the within-species component (Figure 1 and Supplementary Table S1). Indeed, trait values associated with both Amax and Asat presented up to 140-fold variation along the elevation gradient, and 10 and 30-fold variation across the rainfall and forest-savanna gradients, respectively (Table 2).
The effect sizes of taxonomic and plot-environment components were consistently small for most traits and environmental gradients (Supplementary Figure S2), suggesting overall small differences between the trait values of these components and the CWM trait values. The effect sizes associated with the species and family effects had large errors bars, suggesting greater variability of these two taxonomic components within each environmental gradient. The rainfall gradient showed the greatest effect sizes, reflected in leaf thickness, LDMC, [N] and [P] by the plot-environment and species components.
There was little correlation between the community-weighted plot-environment component and the abiotic variables along the rainfall gradient and the forest-savanna gradient, whereas in the elevation gradient only LMA, leaf thickness and [P] presented significant correlations with elevation, precipitation, temperature, Nsoil, and Csoil (Supplementary Table S2). The plot-environment component of these three traits was positively related to temperature and negatively to elevation. The community weighted taxonomic component did not show significant correlations with number of trees, species, genera and families per plot for any environmental gradient or functional trait, with the exception of [P] in the rainfall gradient (Supplementary Table S3).
Trait correlations were strongly dependent on component of variance and on environmental gradient (Figure 2 and Supplementary Figures S3–S5). Unsurprisingly, Amax and Asat showed consistently significant correlations at all ecological levels in the three environmental gradients. There were only significant correlations at the environmental level for photosynthetic traits and leaf [Ca]–[Mg] in the forest-savanna gradient.
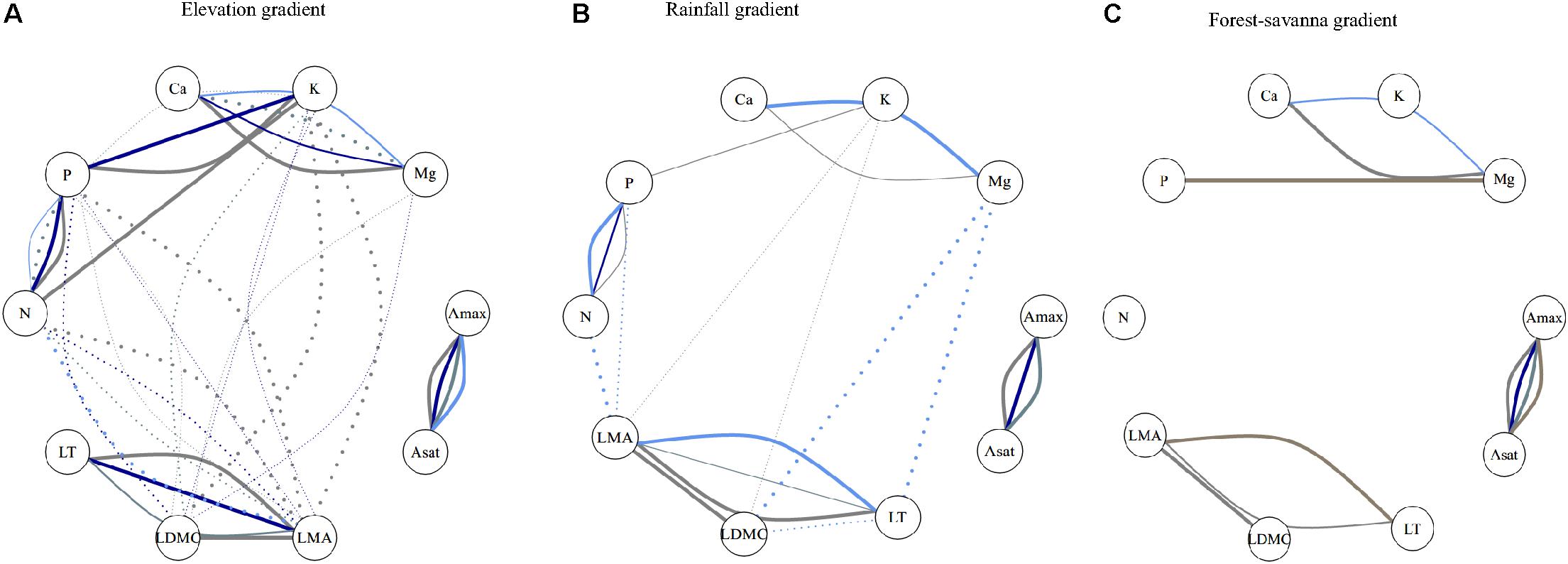
Figure 2. Kendall’r correlation network of leaf traits (inside the circles) at the different ecological levels for the three studied abiotic gradients. Line colors represent the different ecological levels: gray: within-species; darkblue: species; bluegray: genus; lightblue: family: brown: plot. Positive associations are depicted by solid lines, and negative associations are in dotted lines. Line width represents strength of statistical significance, with wide lines representing p < 0.001 and thinner p < 0.05 (after Holm’s correction). Full Kendall r association values are shown in Supplementary Figures S3–S5. (A) Elevation gradient, (B) rainfall gradient, and (C) forest-savanna gradient.
Leaf [P]–[Ca] showed a negative correlation for the plot-environment component in the elevation gradient (Figure 2A), and significant combined effects correlations in the rainfall and elevation gradients (Supplementary Figures S3, S4). Leaf thickness and [N] showed strong negative correlations in all three environmental gradients – none of these were, however, existent at the different components of variance (Figure 2 and Supplementary Figures S3–S5). There were significant within-species positive correlations between LMA- LDMC and LMA-LT in the three gradients, and also at the family level in the rainfall gradient. The elevation and rainfall gradient showed a significant negative trait bivariate correlation between leaf [N] and LMA, but not the forest-savanna gradient.
Along the elevation gradient, the within-species component presented the most significant trait bivariate correlations (Figure 2A and Supplementary Figure S3), while in the rainfall gradient the family component displayed most of the significant trait-trait correlations (Figure 2B and Supplementary Figure S4). The forest-savanna gradient presented only few within-species significant correlations and at the family level between leaf cation concentrations (Figure 2C and Supplementary Figure S5).
Of the 45 trait-trait relationships explored through the SMA regressions (Supplementary Tables S5–S7 and Supplementary Figures S6–S8), there were 25, 16, and 12 significant regressions (after the Holm’s correction for multiple tests) for the within-species component in the elevation, rainfall, and forest-savanna gradient, respectively. The number of significant trait-trait relationships for the species component in the elevation, rainfall and forest-savanna gradient were 15, 14, and 5, respectively; for the genus component 17, 8, and 8, and for the family 11, 4, and 4, respectively. The shared trait-trait significant regressions across the three gradients were, for the within-species component Asat- Amax, LT-LMA, LDMC-LMA, [N]-LMA, and [N]-LT (Figures 3–5 and Supplementary Tables S5–S7). The same trait-trait relationships were also significant across the three environmental gradients for the species and genus components, except LDMC-LMA. Asat -Amax, LT-LMA and [N]-LMA were also significant for the family component.
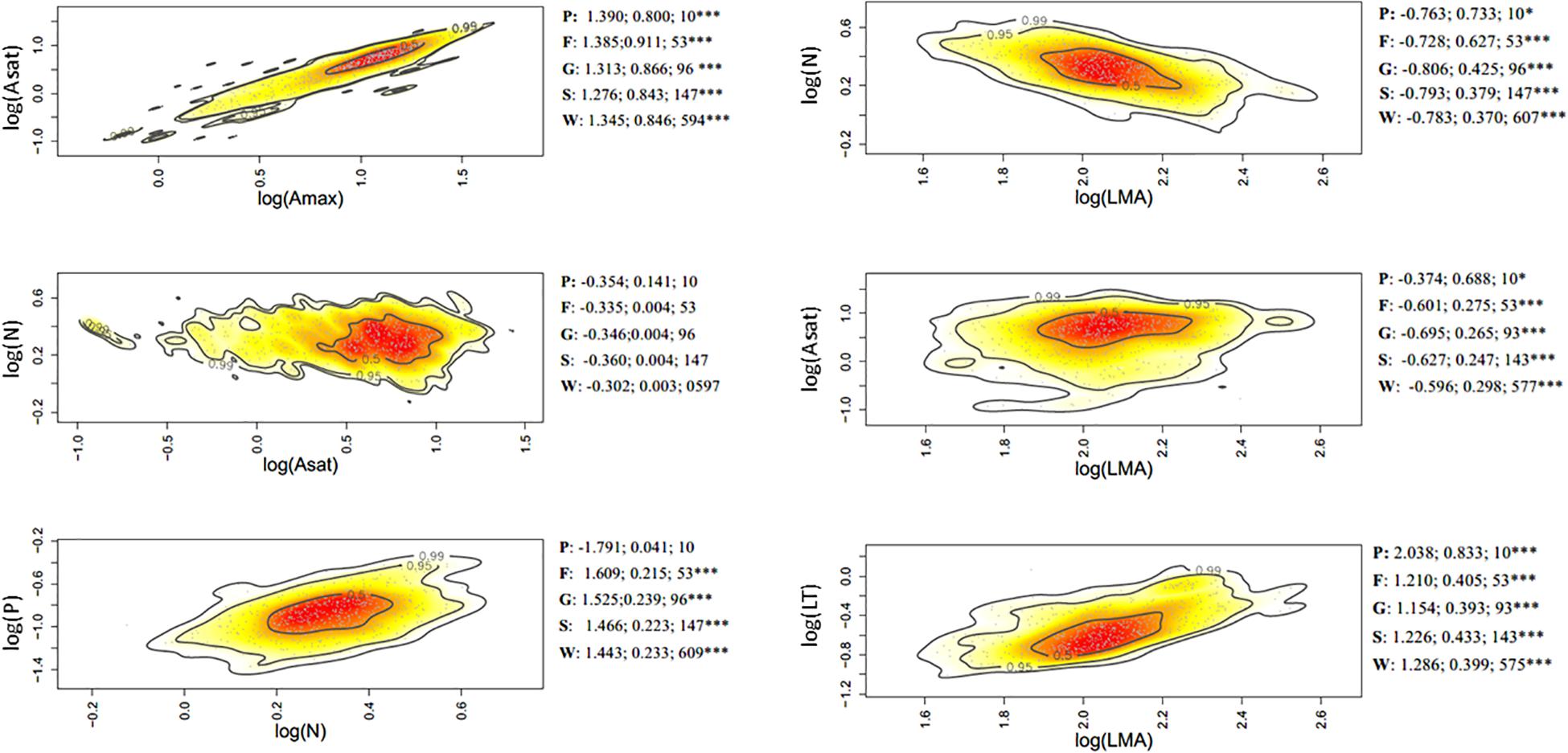
Figure 3. Bivariate trait relationships at the elevation gradient expressed through kernel density plots for selected trait bivariate relationships, and results of SMA regressions (slope, r2, degrees of freedom) for trait values community weighted at the tree level (W), species (S); genus (G); family (F), and plot (P). Stars *** represent significant after Holm correction. Full results of SMA regression analyses for the whole suite of traits are presented in Supplementary Table S4.
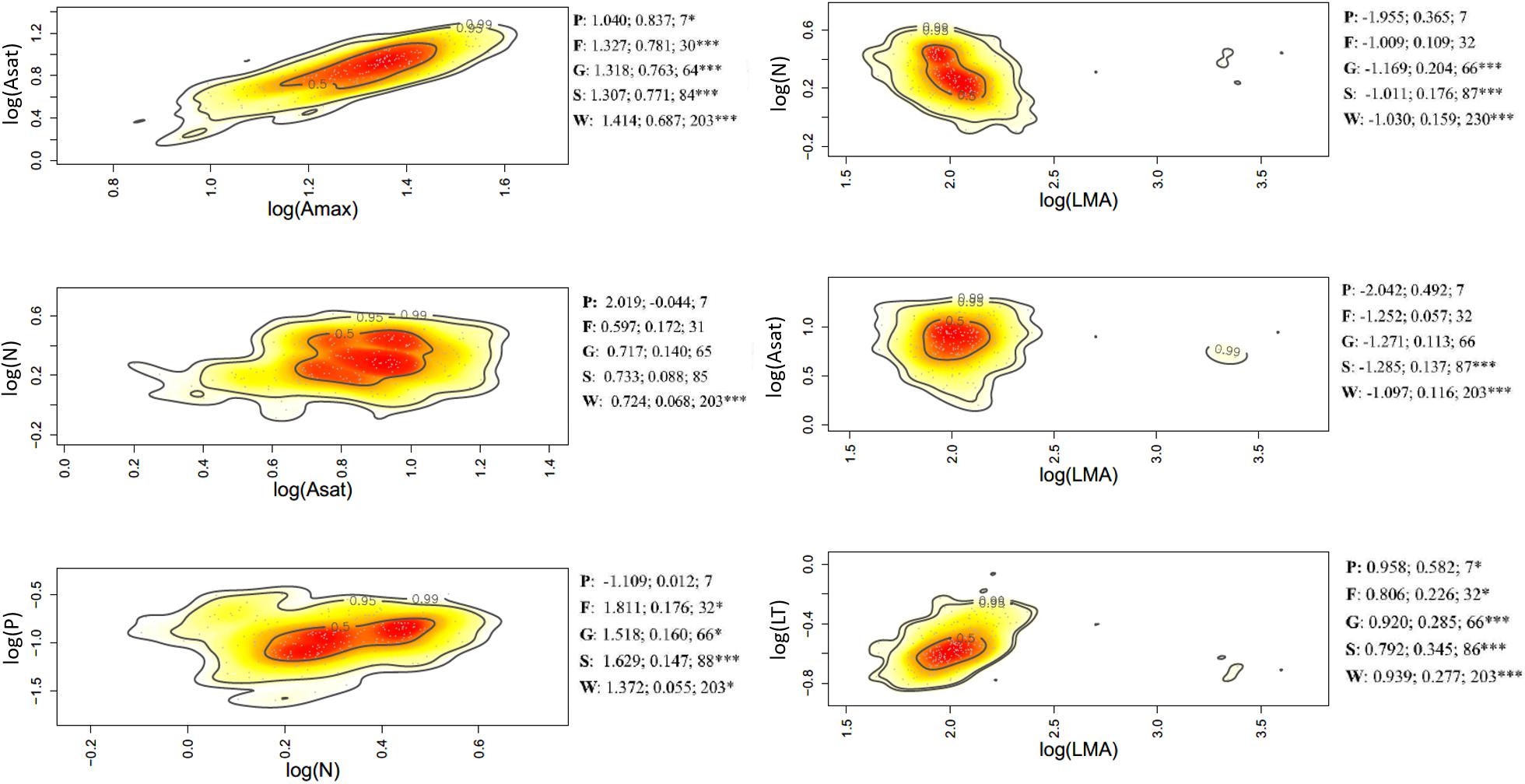
Figure 4. Bivariate trait relationships at the rainfall gradient expressed through kernel density plots for selected trait bivariate relationships, and results of SMA regressions (slope, r2, degrees of freedom) for trait values community weighted at the tree level (W), species (S); genus (G); family (F), and plot (P). Stars *** represent significant after Holm correction. Full results of SMA regression analyses for the whole suite of traits is presented in Supplementary Table S5.
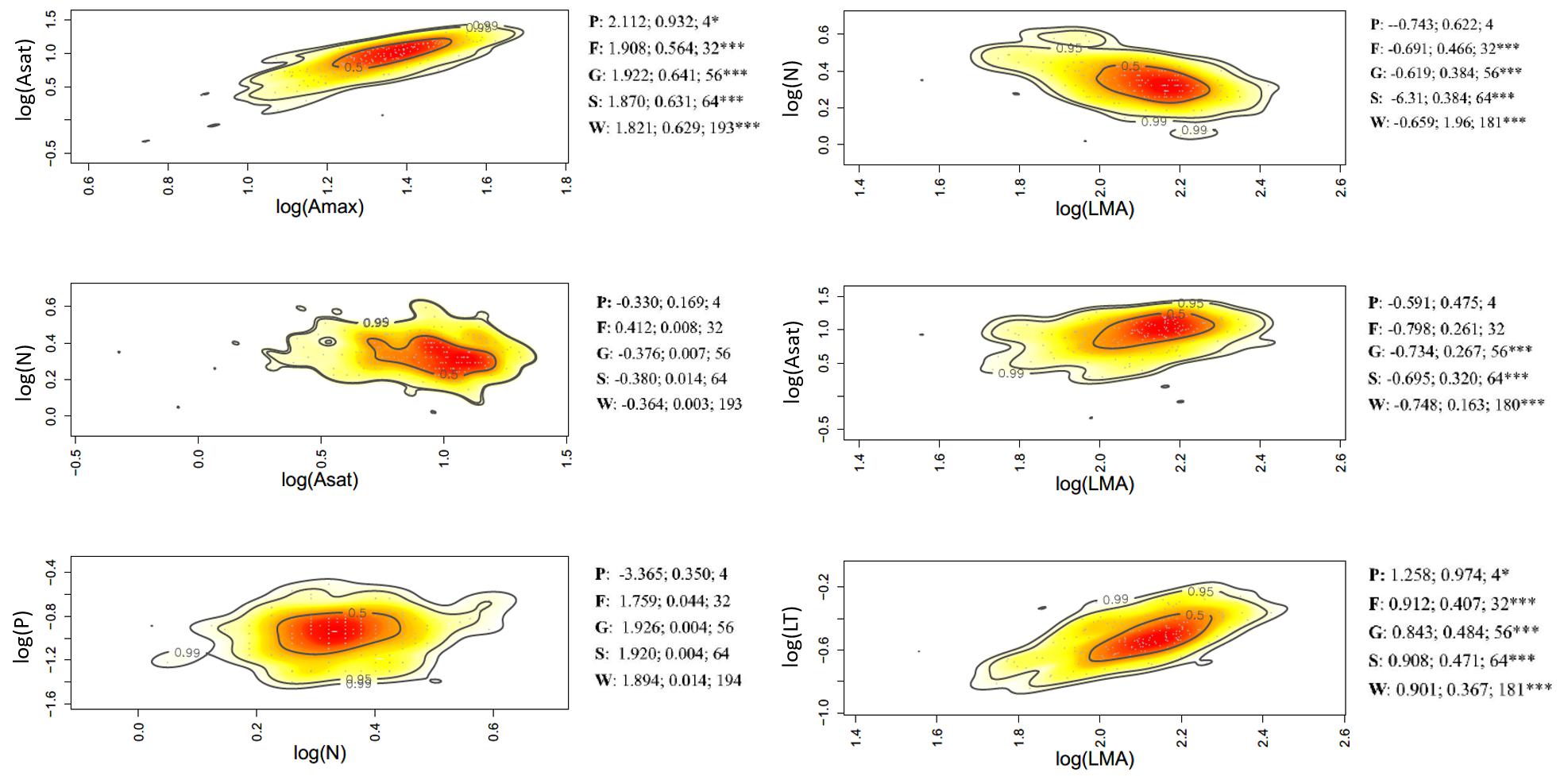
Figure 5. Bivariate trait relationships at the forest-savanna gradient expressed through kernel density plots for selected trait bivariate relationships, and results of SMA regressions (slope, r2, degrees of freedom) for trait values community weighted at the tree level (W), species (S); genus (G); family (F), and plot (P). Stars *** represent significant after Holm correction. Full results of SMA regression analyses for the whole suite of traits is presented in Supplementary Table S6.
Exploring leaf functional trait variation and co-variation across environmental gradients is essential given the importance of defining trait syndromes in plant ecology to develop a better understanding of ecosystem functioning and responses to global change. Importantly, this study presents the first examination of leaf trait variation across tropical environmental gradients, an understudied, but important biogeographic region, with a standardized sampling methodology (see also Asner and Martin, 2016). We show that: (1) the total amount of within-species, taxonomic and plot-environmental variation for a given trait is relatively consistent across environmental gradients; (2) community-weighted mean trait values are not consistently represented across environmental gradients, reflecting the varying strengths of local filtering in each gradient; (3) trait co-variation is strongly scale-dependent and site-dependent, although we found that traits associated to the leaf economic spectrum (Asat, Amax, LMA, N), and LT share a common axis of variation.
As we had hypothesized, the source of variation of photosynthetic and structural traits was strongly taxonomy driven. The total amount of within-species, taxonomic and plot-environmental variation for a given trait was relatively consistent across environmental gradients, suggesting that global sources of variation partition similarly across tropical abiotic gradients. In our study variation was more constrained by taxonomy than by environment, indicating that the influence of changing environmental conditions along these gradients is dominated by taxonomic turnover in response to environmental variation (Fyllas et al., 2009; Anderegg et al., 2018). Taxonomic variance ranged between 22 and 73%, with species level variance usually being the most important variance component along the forest-savanna gradient. Contrary to what we hypothesized, all leaf nutrient traits exhibited large taxonomic variance across gradients, and taxonomic variation explained most of the variation in nutrient traits along the elevation gradient. Along the elevation gradient, species turnover is high and species composition is highly selected for by abiotic conditions (e.g. soil chemistry). Indeed, Asner and Martin (2016) showed that species phylogeny played an important and significant role in explaining variation in leaf chemistry along this elevation gradient in Peru. Much of the taxonomically variation in [Ca] and [Mg] has been found to be mediated though taxonomy as well as through ontogeny, with differences in cation exchange capacities in the cell walls (White and Broadley, 2003; Fyllas et al., 2009), and specific Mg2 + transport genes (Gardner, 2003).
In general, traits associated with the LES had a large portion of their variation explained at the species level within a shared environment (Fyllas et al., 2009; Díaz et al., 2016; Anderegg et al., 2018). In particular, the variance partitioning of photosynthetic traits among environmental gradients was fairly consistent, with at least 50% of variance associated with individual tree variation. Studies have shown that changes in water availability have direct links to physiological responses, such as respiration and stomatal conductance, and therefore variation within an individual tree may be more important than variation between trees or species (Gvozdevaite et al., 2018). We found though, that the variance of photosynthetic traits was not strongly associated to taxonomy.
Plot-environmental variance was a minor source of variance in leaf structural traits (LMA, LDMC, LT) compared to taxonomic and within-species variance, with the exception of LT in the elevation gradient where the plot-environment component (reflecting strong altitudinal and temperature changes) was important. Some leaf nutrient traits showed a high proportion of plot variance, although they did not show a common pattern of response across gradients: leaf [Ca] exhibited large plot variance in the elevation and in the rainfall gradient, while leaf [P] showed large plot variance in the forest-savanna gradient. These results are consistent with Fyllas et al. (2009) and Asner and Martin (2016) who also found strong environmental components for [P] and [Ca]. However, the relative importance of the environment, and potentially other controls on leaf [Ca] uptake and supply are still poorly understood (Asner and Martin, 2016). Our study shows that leaf [P] and [Ca] have a significant response of soil fertility in the rainfall and elevation gradient, with stronger environmental associated variance in plots with higher P and Ca soil concentrations (Figure 2 and Supplementary Table S2).
The strong role of within-species and taxonomic components of trait variance is in accordance with other studies. For example, Messier et al. (2010) reported that, for LMA and LDMC, the total amount of within-species variation was roughly equivalent to the amount of taxonomic variation, and that plot-level variation was responsible for only a minute percentage of total variance. Interestingly, our study supported these results along the rainfall and forest-savanna gradients, but not in the altitudinal gradient. Fyllas et al. (2009) reported LMA to be highly constrained by taxonomic affiliation in the Amazon, whereas Anderegg et al. (2018) reported larger interspecific variation than intraspecific variation, with a particularly significant role of the family taxonomic scale in the traits associated to the leaf economic spectrum. Our results showed a stronger role of within-species variation than taxonomic of plot-environmental variation in the photosynthetic associated traits, suggesting that variance is not explained by these later components. A limitation of this study comes from the fact that the within-species component also includes potential variability due to error, and in the case of photosynthesis this could be associated to either a high plasticity on photosynthetic activity driven by micro-light conditions, or by the fact that despite all leaves were sun leaves they might have got different light intensity levels at the time of measurement that resulted in a wide variability at the individual level.
Community-weighted mean trait values were not consistent across environmental gradients, reflecting the varying strengths of local filtering and corroborating our second hypothesis that community-weighted mean trait values were strongly site-specific. Fyllas et al. (2009) and Asner and Martin (2016) found that soils strongly mediated leaf traits, especially chemical traits, in humid lowland and montane Amazonian and Andean forests. In our study, a plot-environment effect was associated with soil organic content and only for some traits and environmental gradients. There was an important effect of soil N and C for LMA, LT and leaf P in the elevation gradient. In the rainfall gradient, only soil C showed a significant effect on LMA, leaf P and leaf Ca. In the forest-savanna gradient, soil N and P were negatively correlated with Asat, N and C with LDMC and C with leaf K. Thus environmental filtering across these gradients was potentially partly driven by soil fertility.
Our third hypothesis referred to consistent patterns of trait co-variation across environmental gradients. However, we found that trait-trait correlations varied with the source of variation and with geographical context, and in general, there were not many universal patterns observed across gradients. Importantly, the strength of the trait-trait relationship varied with respect to scale (e.g., species, genus, family, plot-environmental). This might reflect relatively weak evolutionary or physiological trade-offs that can be reversed by plasticity, especially in the forest-savanna gradient, and corroborates evidence for little coordination between leaf traits in response to environmental gradients, with the combination of trait plasticity and species sorting driving the contrasting trait-trait and trait-environment patterns across taxonomic and ecological scales (Anderegg et al., 2018).
In order to better predict the impacts of current and projected anthropogenic global change on tropical forest ecosystems, a better understanding of the abiotic and biotic filters leading to community assembly is essential. By partitioning the sources of leaf trait variance across tropical environmental gradients of temperature, precipitation, and land-use, we determined that taxonomy and within-species variation play key roles in determining community assembly, often filtering abiotic associated variability. Consistent trait co-variation patterns were observed across gradients at the taxonomic level for traits associated with the leaf economic spectrum. While these results might imply a heavy importance of biotic filtering in community structuring, trait co-variation was strongly dependent on the environmental gradient considered, thus limiting the global applicability of trait co-variation models.
Currently, few vegetation models incorporate some degree of “trait filtering,” usually via defining plant functional types characterized by a series of community weighted mean traits values (e.g. Fyllas et al., 2014), and they do not take into account the trait variability associated with species and taxonomy. Our study highlights the need for vegetation models to improve representation of ecosystems and ecosystem function by representing inherent sources of leaf trait variance and co-variance due to phenotype and taxonomy. Across tropical environmental gradients, it is important to consider the role of within-species and taxonomic variation (i.e. controlling for phylogeny).
The datasets generated for this study are available on request to the corresponding author.
IO, LB, RM, NF, BE, and YM designed the idea. IO and YM received funding to collect the data. IO performed statistical analyses. IO, LB, NS, TP, RM, and AG led field campaigns, all authors participated in field data collection. IO led the manuscript writing with significant contributions from YM, LB, and BE. All authors commented on and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work is a product of the Global Ecosystems Monitoring (GEM) network (gem.tropicalforests.ox.ac.uk) the Andes Biodiversity and Ecosystems Research Group ABERG (andesresearch.org) research consortia. Fieldwork and analysis for all sites was supported through a European Research Council advanced investigator grant GEM-TRAITS (321131) to YM. In addition, fieldwork in Peru was further supported grants to YM from the UK Natural Environment Research Council (Grant NE/J023418/1), and in Brazil through a Marie Curie Fellowship to IO (FP7-2012-IEF-327990-TipTropTrans), and in Ghana through a Royal Society-Leverhulme Africa Capacity Building Award to YM and SA-B. GA, and RM were supported by a grant from the John D. and Catherine T. MacArthur Foundation and NSF grant 1457812. BM-J and BM were supported by the Brazilian National Council of Science and Technology [Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)] through CNPq/PPBio project (#457602), productivity grants (PQ-2), and CNPq/PELD (LTER) (#403725/2012-7).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00018/full#supplementary-material
Ackerly, D. (2004). Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 74, 25–44. doi: 10.1890/03-4022
Ackerly, D., Knight, C., Weiss, S., Barton, K., and Starmer, K. (2002). Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130, 449–457. doi: 10.1007/s004420100805
Albert, C. H., Grassein, F., Schurr, F. M., Vieilledent, G., and Violle, C. (2011). When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 13, 217–225. doi: 10.1007/s00442-016-3578-5
Anderegg, L. D. L., Berner, L. T., Badgley, G., Sethi, M. L., Law, B. E., and HilleRisLambers, J. (2018). Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 21, 734–744. doi: 10.1111/ele.12945
Anderson, M. J., Crist, T. O., Chase, J. M., Vellend, M., Inouye, B. D., Freestone, A. L., et al. (2011). Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28. doi: 10.1111/j.1461-0248.2010.01552.x
Asner, G. P., and Martin, R. E. (2016). Convergent elevation trends in canopy chemical traits of tropical forests. Glob. Chang. Biol. 22, 2216–2227. doi: 10.1111/gcb.13164
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., et al. (2018). Package lme4. R package version 1.0.
Bryant, J. A., Lamanna, C., Morlon, H., Kerkhoff, A. J., Enquist, B. J., and Green, J. L. (2008). Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci.U.S.A. 105, 11505–11511. doi: 10.1073/pnas.0801920105
Chapin, F. S. III, Autumn, K., and Pugntairet, F. (1993). Evolution of Suites of Traits in Response to Environmental Stress. Am. Nat. 142, S78–S92.
Chiti, T., Certini, G., Grieco, E., and Valentini, R. (2010). The role of soil in storing carbon in tropical rainforests: the case of Ankasa Park, Ghana. Plant Soil 331, 453–461. doi: 10.1007/s11104-009-0265-x
Coley, P. D., Bryant, J. P., and Chapin, F. S. (1985). Resource availability and plant antiherbivore defense. Science 230, 895–899. doi: 10.1126/science.230.4728.895
Diaz, S., Cabido, M., and Casanoves, F. (1998). Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 9, 113–122. doi: 10.2307/3237229
Díaz, S., Kattge, J., Cornelissen, J. H. C., Wright, I. J., Lavorel, S., Dray, S., et al. (2016). The global spectrum of plant form and function. Nature 529, 167–171. doi: 10.1038/nature16489
Domingues, T. F., Patrick Meir, P., Feldpausch, T. R., Saiz, G., Veenendaal, E. M., Schrodt, F., et al. (2010). Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 33, 959–980. doi: 10.1111/j.1365-3040.2010.02119.x
Enquist, B. J., Norberg, J., Bonser, S. P., Violle, C., Webb, C. T., Henderson, A., et al. (2015). Scaling from traits to ecosystems: developing a general trait driver theory via integrating trait-based and metabolic scaling theories. Adv. Ecol. Res. 52, 249–318. doi: 10.1016/bs.aecr.2015.02.001
Fernandes, G. W. (2000). Variations in leaf characteristics of two species of Miconia in the Brazilian cerrado under different light intensities. Trop. Ecol. 41, 47–60.
Funk, J. L., and Wolf, A. A. (2016). Testing the trait-based community framework: do functional traits predict competitive outcomes? Ecology 97, 2206–2211. doi: 10.1002/ecy.1484
Fyllas, N. M., Gloor, E., Mercado, L. M., Sitch, S., Quesada, C. A., Domingues, T. F., et al. (2014). Analyzing Amazonian forest productivity using a new individual and trait-based model (TFS v.1). Geosci. Model Dev. 7, 1251–1269. doi: 10.5194/gmd-7-1251-2014
Fyllas, N. M., Patiño, S., Baker, T. R., Nardoto, G. B., Martinelli, L. A., Quesada, C. A., et al. (2009). Basin-wide variations in foliar properties of Amazonian forest: phylogeny soils and climate. Biogeosciences 6, 2677–2708. doi: 10.5194/bg-6-2677-2009
Gardner, R. C. (2003). Genes for magnesium transport. Curr. Opini. Plant Biol. 6, 263–267. doi: 10.1016/s1369-5266(03)00032-3
Girardin, C. A. J., Espejo, J. E. S., Doughty, C. E., Huasco, W. H., Metcalfe, D. B., Durand-Baca, L., et al. (2014). Productivity and carbon allocation in a tropical montane cloud forest in the Peruvian Andes. Plant Ecol. Div. 7, 107–123.
Girardin, C. A. J., Malhi, Y., Aragão, L. E. O. C., Mamani, M., Huaraca Huasco, W., Durand, L., et al. (2010). Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 16, 3176–3192. doi: 10.1111/j.1365-2486.2010.02235.x
Grime, J. P. (1977). Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. doi: 10.1086/283244
Grime, J. P. (1998). Benefits of plant diversity to ecosystems: inmediate, filter and founder effects. J. Ecol. 86, 902–910. doi: 10.1046/j.1365-2745.1998.00306.x
Gvozdevaite, A., Oliveras, I., Domingues, T. F., Peprah, T., Boakye, M., Afriyie, L., et al. (2018). Leaf-level photosynthetic capacity dynamics in relation to soil and foliar nutrients along forest–savanna boundaries in Ghana and Brazil. Tree Physiol. 38, 1912–1925. doi: 10.1093/treephys/tpy117
Huasco, W. H., Girardin, C. A. J., Doughty, C. E., Metcalfe, D. B., Baca, L. D., Silva-Espejo, J. E., et al. (2014). Seasonal production, allocation and cycling of carbon in two mid-elevation tropical montane forest plots in the Peruvian Andes. Plant Ecol. Div. 7, 125–142. doi: 10.1080/17550874.2013.819042
Hulshof, C., Violle, C., Spasojevic, M. J., McGill, B., Damschen, E., Harrison, S., et al. (2013). Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. J. Veg. Sci. 24, 921–931. doi: 10.1111/jvs.12041
Jacobs, B. F. (1999). Estimation of rainfall variables from leaf characters in tropical Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 145, 231–250. doi: 10.1016/S0031-0182(98)00102-3
Janssen, T. A. J., Ametsitsi, G. K. D., Collins, M., Adu-Bredu, M., Oliveras, I., Mitchard, E. T. A., et al. (2018). Extending the baseline of tropical dry forest loss in Ghana (1984–2015) reveals drivers of major deforestation inside a protected area. Biol. Conserv. 218, 163–172. doi: 10.1016/j.biocon.2017.12.004
Kattge, J., Díaz, S., Lavorel, S., Prentice, I. C., Leadley, P., Bönisch, G., et al. (2011). TRY - a global database of plant traits. Glob. Chang. Biol. 17, 2905–2935.
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574. doi: 10.1016/j.tree.2007.09.006
Kraft, N. J. B., Valencia, R., and Ackerly, D. D. (2008). Functional traits and niche-based tree community assemble in an Amazonian forest. Science 322, 580–582. doi: 10.1126/science.1160662
Lamanna, C., Blonder, B., Violle, C., Kraft, N. J. B., Sandel, B., Šímová, I., et al. (2014). Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. U.S.A. 111, 13745–13750. doi: 10.1073/pnas.1317722111
Laughlin, D. C., and Messier, J. (2015). Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends Ecol. Evol. 30, 487–496. doi: 10.1016/j.tree.2015.06.003
Lavorel, S., and Garnier, E. (2002). Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16, 545–556. doi: 10.1046/j.1365-2435.2002.00664.x
Lawson, A. M., and Weir, J. T. (2014). Latitudinal gradients in climatic-niche evolution accelerate trait evolution at high latitudes. Ecol. Lett. 17, 1427–1436. doi: 10.1111/ele.12346
Malhi, Y., Gardner, T., Goldsmith, G. R., Silman, M. R., and Zelazowski, P. (2014). Tropical forests in the Anthropocene. Annu. Rev. Environ. Res. 39, 125–159.
Malhi, Y., Girardin, C. A. J., Goldsmith, G. R., Doughty, C. E., Salinas, N., Metcalfe, D. B., et al. (2017). The variation of productivity and its allocation along a tropical elevation gradient: a whole carbon budget perspective. New Phytol. 214, 1019–1032. doi: 10.1111/nph.14189
Malhi, Y., Silman, M., Salinas, N., Bush, M., Meir, P., and Saatchi, S. (2010). Elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Glob. Chang. Biol. 16, 3171–3175. doi: 10.1111/j.1365-2486.2010.02323.x
Maracahipes, L., Lenza, E., Marimon, B. S., Oliveira, E. A., de Pinto, J. R. R., and Junior, B. H. M. (2011). Estrutura e composição florística da vegetação lenhosa em cerrado rupestre na transição Cerrado-Floresta Amazônica Mato Grosso Brasil. Biota Neotrop. 11, 133–142.
Marimon, B. S., Marimon-Junior, B. H., Feldpausch, T. R., Oliveira-Santos, C., Mews, H. A., Lopez-Gonzalez, G., et al. (2014). Disequilibrium and hyperdynamic tree turnover at the forest–cerrado transition zone in southern Amazonia. Plant Ecol. Divers. 7, 281–292. doi: 10.1080/17550874.2013.818072
Marimon Junior, B. H., and Haridasan, M. (2005). Comparação da vegetação arbórea e características edáficas de um cerradão e um cerrado sensu stricto em áreas adjacentes sobre solo distrófico no leste de Mato Grosso, Brasil. Acta Bot. Bras. 19, 913–926. doi: 10.1590/s0102-33062005000400026
Marthews, T. R., Riutta, T., Oliveras-Menor, I., Urrutia, R., Moore, S., Metcalfe, D., et al. (2014). Measuring Tropical Forest Carbon Allocation and Cycling: A RAINFOR-GEM Field Manual for Intensive Census Plots (v3.0). Oxford: Global Ecosystems Monitoring Network.
McCain, C. M., and Grytnes, J. A. (2001). “Elevational gradients in species richness,” in Encyclopedia of Life Sciences (ELS), ed. R. Janson, (Chichester: John Wiley & Sons).
McGill, B. J., Enquist, B. J., Weiher, E., and Westoby, M. (2006). Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. doi: 10.1016/j.tree.2006.02.002
Messier, J., Lechowicz, M. J., McGill, B. J., Violle, C., and Enquist, B. J. (2017). Interspecific integration of trait dimensions at local scales: the plant phenotype as an integrated network. J. Ecol. 105, 1775–1790. doi: 10.1111/1365-2745.12755
Messier, J., McGill, B. J., Enquist, B. J., and Lechowicz, M. J. (2016). Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography 40, 685–697. doi: 10.1111/ecog.02006
Messier, J., McGill, B. J., and Lechowicz, M. J. (2010). How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 13, 838–848. doi: 10.1111/j.1461-0248.2010.01476.x
Moore, S., Adu-Bredu, S., Duah-Gyamfi, A., Addo-Danso, S. D., Ibrahim, F., Mbou, A. T., et al. (2018). Forest biomass productivity and carbon cycling along a rainfall gradient in West Africa. Glob. Chang. Biol. 24, e496–e510. doi: 10.1111/gcb.13907
Neyret, M., Bentley, L. P., Oliveras, I., Marimon, B. S., Marimon-Junior, B. H., Almeida de Oliveira, E., et al. (2016). Examining variation in the leaf mass per area of dominant species across two contrasting tropical gradients in light of community assembly. Ecol. Evol. 6, 5674–5689. doi: 10.1002/ece3.2281
Osnas, J. L. D., Lichstein, J. W., Reich, P. B., and Pacala, S. W. (2013). Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340, 741–744. doi: 10.1126/science.1231574
Pérez-Harguindeguy, N., Díaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234.
Poorter, H., Lambers, H., and Evans, J. R. (2014). Trait correlation networks: a whole-plant perspective on the recently criticized leaf economic spectrum. New Phytol. 201, 378–382. doi: 10.1111/nph.12547
R Core Team¡/snm¿, (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reich, A. P. B., Wright, I. J., Bares, J. C., Craine, J. M., Oleksyn, J., Westoby, M., et al. (2003). The evolution of plant functional variation : traits spectra and strategies. Int. J. Plant Sci. 164, S143–S164.
Rosas, T., Martínez-Vilalta, J., Mencuccini, M., Cochard, H., Barba, J., and Saura-Mas, S. (2019). Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phyt. 223, 632–646. doi: 10.1111/nph.15684
Rozendaal, D. M. A., Hurtado, V. H., and Poorter, L. (2006). Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 20, 207–216. doi: 10.1111/j.1365-2435.2006.01105.x
Shepherd, U. (1998). A comparison of species diversity and morphological diversity across the North American latitudinal gradient. J. Biogeogr. 25, 19–29. doi: 10.1046/j.1365-2699.1998.251172.x
Swenson, N. G., and Enquist, B. J. (2007). Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 94, 451–459. doi: 10.3732/ajb.94.3.451
Swenson, N. G., and Enquist, B. J. (2009). Opposing assembly mechanisms in a Neotropical dry forest: implications for phylogenetic and functional community ecology. Ecology 8, 2161–2170. doi: 10.1890/08-1025.1
Swenson, N. G., Enquist, B. J., Pither, J., Kerkhoff, A. J., Boyle, B., Weiser, M. D., et al. (2012). The biogeography and filtering of woody plant functional diversity in North and South America. Glob. Ecol. Biogeogr. 21, 798–808. doi: 10.1111/j.1466-8238.2011.00727.x
Taudiere, A., and Violle, C. (2015). cati: an R package using functional traits to detect and quantify multi-level community assembly processes. Ecography 39, 699–708. doi: 10.1111/ecog.01433
Tränkner, M., Tavakol, E., and Jákli, B. (2018). Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 163, 414–431. doi: 10.1111/ppl.12747
Valladares, F., Gianoli, E., and Gómez, J. M. (2007). Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763. doi: 10.1111/j.1469-8137.2007.02275.x
Violle, C., Enquist, B. J., McGill, B. J., Jiang, L., Albert, C. H., Hulshof, C., et al. (2012). The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. doi: 10.1016/j.tree.2011.11.014
Violle, C., Navas, M.-L. L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Wang, M., Zheng, Q., Shen, Q., and Guo, S. (2013). The critical role of potassium in plant stress response. Int. J. Mol. Sci. 14, 7370–7390. doi: 10.3390/ijms14047370
Westoby, M., Falster, D. S., Moles, A. T., Vesk, P. A., and Wright, I. J. (2002). Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 33, 125–159. doi: 10.1146/annurev.ecolsys.33.010802.150452
Wright, I. J., Dong, N., Maire, V., Prentice, I. C., Westoby, M., Díaz, S., et al. (2017). SI: global climatic drivers of leaf size. Science 357, 917–921. doi: 10.1126/science.aal4760
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827.
Keywords: environmental filtering, variance partitioning, trait covariation, interspecific, intraspecific
Citation: Oliveras I, Bentley L, Fyllas NM, Gvozdevaite A, Shenkin AF, Peprah T, Morandi P, Peixoto KS, Boakye M, Adu-Bredu S, Schwantes Marimon B, Marimon Junior BH, Salinas N, Martin R, Asner G, Díaz S, Enquist BJ and Malhi Y (2020) The Influence of Taxonomy and Environment on Leaf Trait Variation Along Tropical Abiotic Gradients. Front. For. Glob. Change 3:18. doi: 10.3389/ffgc.2020.00018
Received: 23 September 2019; Accepted: 04 February 2020;
Published: 03 March 2020.
Edited by:
Alice Catherine Hughes, Xishuangbanna Tropical Botanical Garden (CAS), ChinaReviewed by:
Tammo Reichgelt, University of Connecticut, United StatesCopyright © 2020 Oliveras, Bentley, Fyllas, Gvozdevaite, Shenkin, Peprah, Morandi, Peixoto, Boakye, Adu-Bredu, Schwantes Marimon, Marimon Junior, Salinas, Martin, Asner, Díaz, Enquist and Malhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imma Oliveras, aW1tYS5vbGl2ZXJhc0BvdWNlLm94LmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.