
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 16 August 2019
Sec. Tropical Forests
Volume 2 - 2019 | https://doi.org/10.3389/ffgc.2019.00037
This article is part of the Research Topic Improving Environmental Sustainability in Oil Palm: Linking Science, Policy, & Practice across the Tropics View all 18 articles
Southeast Asian peatlands have undergone recent land use change with an increase in industrial agricultural plantations, including oil palm. Cultivating peatlands requires creating drainage ditches and other surface microforms (i.e., harvest paths, frond piles, cover plants, and next to the palm). However, it is currently unclear how these management actions affect rates of carbon losses from the peat. Here we report carbon fluxes from each of the different surface microforms measured monthly (soil CO2 [total soil respiration—Rtot] and stem CH4) and bimonthly (soil CH4, drain CO2 and drain CH4). We calculated annual carbon fluxes and partitioned heterotrophic (Rh) and root-rhizosphere respiration by sampling rhizosphere and root-free soil. Linear mixed effect models were used to determine which environmental factors best-predicted carbon fluxes, and to develop recommendations for management solutions that could reduce carbon losses. Carbon fluxes varied significantly between the different microforms; the greatest CO2 fluxes were measured next to the palm and the greatest CH4 fluxes were measured from the drainage ditches. Annual estimates of Rtot, Rh and drain CO2 were 22.08 ± 0.50, 17.75 ± 1.54, and 1.5 ± 0.10 Mg CO2-C ha−1 yr−1, respectively. Rh varied between the two plantations: Sebungan averaged 11.43 ± 1.37 Mg CO2-C ha−1 yr−1 and Sabaju averaged 24.08 ± 1.42 Mg CO2-C ha−1 yr−1. Net ecosystem CH4 fluxes averaged 61.02 ± 17.78 kg CH4-C ha−1 yr−1–similar to unmanaged swamp forests. The two plantations did not vary in overall CH4 flux, but did vary in transport pathway. CH4 fluxes from the soil, drains and stems followed a ratio of 50:50:0 from Sabaju (water table depth [WTD]: −0.49 ± 0.004 m) and 11:98:0 from Sebungan (WTD: −0.77 ± 0.007 m). Rh dominated the peat carbon losses. WTD controlled variation in Rh from Sebungan where the WTD was deeper. Air and soil temperature controlled variation in Sabaju, with greater fluxes from the harvest path, attributed to the absence of shade. These results suggest that shading the soil (e.g., through addition of frond piles) and raising the water table may be the most effective ways to reduce peat carbon loss from drained peat soils.
Palm oil currently makes a significant contribution to feeding the world, which is expected to continue with increased population growth, and helps with climate change mitigation, for example through high rates of photosynthesis taking CO2 from the atmosphere and it's use for bioenergy (Fowler et al., 2011; Fuss et al., 2016; IPCC, 2018; Meijaard et al., 2018). However, agriculture itself is not free from carbon emissions (Bajželj et al., 2014; Smith et al., 2014; Tubiello et al., 2015). Oil palm (Elaeis guineensis; the source of palm oil) plantations are grown on both industrial and small-holder scale throughout the tropics (FAOSTAT, 2019). Indonesia and Malaysia currently dominate both the production and export of palm oil (FAOSTAT, 2019; USDA, 2019). Since 1980, oil palm plantations have been established on peat soils in these countries, due to the decline in available suitable mineral soils, which are preferred (Silvius and Diemont, 2007; Corley and Tinker, 2008; Miettinen et al., 2016). However, oil palm plantations growing on peat soils are subject to debate concerning the magnitude of CO2 and CH4 losses from the peat (Evers et al., 2017; Wijedasa et al., 2017).
Southeast (SE) Asia has 24.7 Mha of peatlands, storing ~66 Gt C (Page et al., 2011; Miettinen et al., 2016). Industrial oil palm plantations are currently cultivated on 3.1 Mha of SE Asian peatlands (Miettinen et al., 2016). Undisturbed, these peatlands act as an overall long-term carbon sink, due to high water tables providing saturated conditions with low redox potentials (Dommain et al., 2015; Cobb et al., 2017; Hodgkins et al., 2018). Organic matter breaks down in these conditions through anaerobic degradation, releasing CH4 to the atmosphere (Sundh et al., 1994; Arai et al., 2014; Sjögersten et al., 2014).
Modifying peatlands for agricultural management can alter the biogeochemistry and trace gas fluxes from these systems due to the introduction of artificial drainage ditches that lower the water table and create an aerated zone for root growth, raising the soil redox potential (Hirano et al., 2012; Mishra et al., 2014; Tonks et al., 2017). In these more oxic conditions, methanogenesis is inhibited and aerobic degradation pathways predominate, namely peat oxidation from heterotrophic respiration (Rh; Hooijer et al., 2012; Miettinen et al., 2017; Warren et al., 2017). Additionally, significant CO2 and CH4 fluxes have been observed from the surface of drainage ditches in cultivated temperate and tropical peatlands (hereafter drain CO2, drain CH4 and stem CH4; Teh et al., 2011; Jauhiainen and Silvennoinen, 2012). Tree stems have also been shown to act as a pathway for CH4 transport from tropical peat swamp forests (Pangala et al., 2013, 2017).
Soil Rh and CH4 fluxes have been measured previously from oil palm plantations. Current estimates of Rh from oil palm plantations on peat suggest that these systems emit a mean flux of 12.2 Mg CO2-C ha−1 yr−1, with reported estimates ranging from 4.1 to 22.9 Mg CO2-C ha−1 yr−1 (Farmer, 2013; Melling et al., 2013; Dariah et al., 2014; Husnain et al., 2014; Comeau, 2016; Comeau et al., 2016; Hergoualc'h et al., 2017; Ishikura et al., 2018; Matysek et al., 2018). Only one study has quantified CH4 from oil palm plantations, finding annual emissions of −0.2 kg CH4-C ha−1 yr−1, suggesting that CH4 oxidation may predominate in these drained ecosystems (Melling et al., 2005a).
Furthermore, existing studies of Rh and soil CH4 have failed to fully account for spatial heterogeneity of the trace gas fluxes in oil palm systems, despite the fact that biogeochemical processes are known to be highly variable in space and time (i.e., “hot spots” and “hot moments,” sensu McClain et al., 2003). Oil palm plantations have different soil surface management microforms, namely highly compacted bare soil harvest paths, piles of decomposing fronds, rows of cover plants, and an extensive rhizosphere in the fertilizer circle around the palm (Manning, 2019; Manning et al., in preparation). Rh and soil CH4 measurements have predominantly been sampled from the harvest path only (Melling et al., 2005a, 2013; Farmer, 2013; Dariah et al., 2014; Hergoualc'h et al., 2017; Matysek et al., 2018). However, the frond piles and cover plants may give different results for Rh and CH4 due to potentially different environmental conditions.
Several CO2 and CH4 fluxes known to be important in temperate peatlands and tropical forest have not been measured in oil palm plantations on peat soils. In temperate peatlands, drainage ditches have been observed to act as hotspots for CH4 emissions, making a disproportionately large contribution to net ecosystem exchange (Minkkinen and Laine, 2006; Schrier-Uijl et al., 2010; Teh et al., 2011). Similarly the absence of stem CH4 may signify the oversight of a notable CH4 loss from oil palm plantations; it has been found that stem fluxes contribute between 62–87% (Brunei) and 42–53% (Amazon) of ecosystem CH4 fluxes from mature trees in swamp forests (Pangala et al., 2013, 2017), and may therefore represent an important but so far unexplored transport pathway for CH4 emission in oil palm systems.
Here we report CO2 (Rtot, Rh and drain) and CH4 (soil, drain and stem) fluxes from two mature oil palm plantations established on peat soils in Sarawak, Malaysian Borneo. Autotrophic fluxes (Ra and stem CO2) are noted due to being measured simultaneously. We include a wide spatial resolution, sampling CO2 and CH4 from all major surface microforms (i.e., next to palms, harvest paths, frond piles, cover plants, drainage ditches, and tree stems). Temporal trends in fluxes were determined by sampling at monthly (soil CO2 and stem fluxes) or bi-monthly (soil CH4 and drain fluxes) intervals. Lastly, we upscaled our surface fluxes and estimates of peat oxidation (i.e., Rh) to the plantation-level using proportional surface area-weighting and temporal integration. The overarching goal of this research was to determine rates of carbon losses that are broadly representative of production systems of this kind elsewhere in Southeast Asia, with a view to presenting effective solutions to reduce these carbon losses. During the course of this work we explored the following research questions:
1. Do CO2 and CH4 fluxes vary spatially and temporally in oil palm plantations?
2. What are the annual rates of Rh, drain CO2, soil CH4, drain CH4 and stem CH4 from oil palm plantations on peat soil—both individually and combined as total peat carbon losses?
3. What are the relationships between key environmental factors and trace gas fluxes?
4. Do opportunities exist to minimize or mitigate CO2 and CH4 fluxes, based on our understanding of the drivers of trace gas exchange in this system?
Data were collected from Sabaju (latitude 003° 12′ N, longitude 113° 30′ E) and Sebungan (latitude 003° 09′ N, longitude 113° 21′ E) oil palm plantations in Sarawak, Malaysia. Sabaju and Sebungan Estates have been established on peat soils broadly classified as histosols (FAO, 2006). The peat depth was measured to be 3.0 m at Sabaju and 4.0 m at Sebungan. The mean annual precipitation was ~3,200 mm (MET Malaysia, 2017). The northeast monsoon from October to January has the most rainfall, with a slightly drier southwest monsoon between May and August (MET Malaysia, 2017). The two principal dry seasons fall between February to April and in September. The mean annual temperature was 26.5°C (MET Malaysia, 2017).
Prior to planting, the land use was a mixed species swamp forest, which had been heavily logged. The land was converted to a plantation in 2006 and the palms were on their first crop rotation. The palms were 8 years old when measurements began. The plantations were laid out systematically with ~35 ha blocks and drainage ditches every 28 m leading to a larger ditch running down the center of the block. Palms were planted every 8 m in rows that were 8 m apart, with a planting density of 160 palms per ha. Within the palm blocks, four different surface management microforms were present and two different drain types (Figure 1):
• By palm or fertilizer circle—the ring of soil around the palm where the majority of oil palm roots grow and the fertilizer is applied.
• Harvest path—frequently weeded soil between the rows of palms and around the palms to allow access for workers.
• Frond pile—the location of the decomposing, harvested fronds.
• Cover plants—an area where weeds were left to grow freely.
• Field drains—small, 1.5 m wide drains dug every four rows of palms.
• Collection drains—larger, 3 m wide drains running down the center of the plantation blocks.
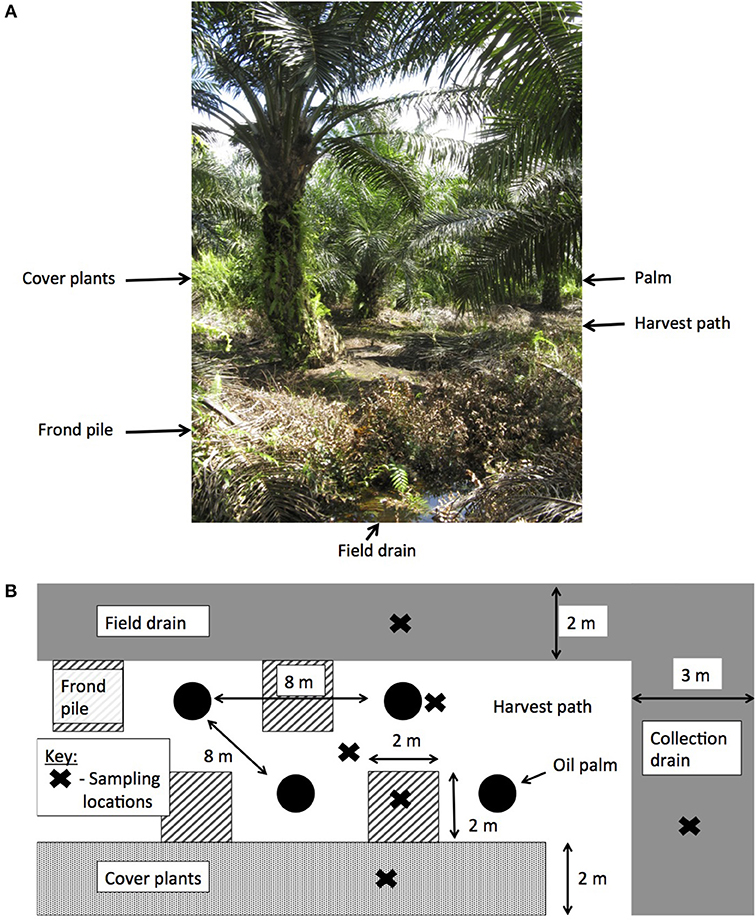
Figure 1. The layout of oil palm plantations on peat soil. (A) Sebungan oil palm plantation with the different surface microforms labeled, and (B) the experimental design in this study, showing the different sampling locations.
Six one hectare plots were established, three in Sabaju and three in Sebungan. Within each plot, three subplots were randomly placed. In each subplot, soil surface collars made from PVC plastic of dimensions 10 cm deep by 10.5 cm diameter were installed to 5 cm depth to measure from the harvest path, underneath a frond pile, in the cover plants and adjacent to three palms in order to take CO2 and CH4 measurements. Collars were installed 6 months prior to the commencement of sampling in order to avoid disturbance effects associated with chamber base installation (Varner et al., 2003).
A location was selected to sample the drain fluxes from both the field drain and the collection drain within each subplot. Soil surface collars of 10 cm depth and 10.5 cm diameter were installed into the base of each ditch to 5 cm, to prepare for sampling months when the drains were dry. Floating chambers were made for sampling when there was water in the drains (Figure 2A; Kent, 2018). Here two holes were drilled into plastic mixing bowls (30 cm rim diameter) and lengths of Tygon tubing (2.5 m long, 3 mm inner diameter) were attached to each hole using Swagelok fittings. Aluminum foil was taped over the bowls to prevent light from penetrating and foam cylinders were attached to the bowls to help them float.

Figure 2. Chambers adapted for this study: (A) a floating chamber to measure CO2 and CH4 fluxes from the drainage ditches, and (B) a novel stem chamber to measure stem CO2 and CH4.
Stem fluxes were only taken from one plot in Sabaju and one plot in Sebungan, due to time and resource limitations, adapting the chamber methodology used by Pangala et al. (2013) and Siegenthaler et al. (2016) to suit oil palms (Figure 2B; Figure S1; Manning, 2019). Here, five palms were selected from each plot, including three that had soil collars next to them. Permanent chamber bases were glued onto the palm surfaces—these were made out of 3 cm wide and 6 cm thick strips of neoprene, attached at 0.2 m and 0.7 m height. Expandable polyurethane (PU) foam was applied to the stem, filling any gaps between the neoprene strips and the palm surface that had been created by the uneven distribution of frond bases. Extra neoprene blocks were randomly placed inside the chamber base area to help maintain an even volume inside the chamber. The chamber itself consisted of a 2.5 m × 0.75 m × 0.003 m sheet of plastic, which was wrapped around the chamber base and fixed in place using two ratchet straps to attach the sheet to the neoprene strips, with a third ratchet strap around the middle of the chamber to keep the sheet closed. Prior to attachment, six fans (12 VDC 120 mm computer case fans; flow ~200 CFM) were distributed within the chamber base. After attaching the chamber, the chamber was wrapped first in plastic wrap to make it gas tight, and then in black plastic sheeting to block sunlight and prevent any photosynthesis. Furthermore, the palm stems were regularly cleaned of vegetation at least one week prior to flux measurement.
The soil chamber volume was determined by multiplying the height of the chamber (15 cm) with the surface area (radius 5.5 cm). Prior to each measurement, the depth of the collar was measured and included in the volume calculations. The floating drain chamber volume was 7 L. The volume inside the drain chamber did not change when the chamber was placed on the water.
The stem chamber volume needed to be calculated for each measurement due to the uneven surface of the palms and the chamber being flexible rather than rigid. Equation (1) was used to determine the stem chamber volume:
where V is the volume of the chamber, d is the mean depth of the chamber base (i.e., radially outwards from palm), h is the internal (vertical) height of each chamber, R is the radius of the outer chamber surface (i.e., that of the plastic sheet), and r is the radius of the inner chamber surface (i.e., the radius of the stem). Multiple measurements were made of d, h, R, and r for each palm and the mean values were used in this equation. More information can be found in Manning (2019).
Flux measurements were made using the static chamber approach (Livingston and Hutchinson, 1995). Soil and drain CO2 and CH4 fluxes were collected between June 2015 and May 2016 and stem fluxes were collected from May 2016 to May 2017. The fluxes were measured at different times due to limitations in resource availabilities. Soil, drain and stem fluxes were measured at the same time in May 2016 to ensure comparability. Furthermore, the different instruments were calibrated against each other to ensure compatibility (Manning, 2019).
Rtot measurements were made using an EGM-4 and SRC-1 chamber (PP Systems; Hansatech Instruments, Amesbury, USA). Two recordings were made in duplicate (one immediately after the other) and averaged during data processing because the EGM did not always save one of the recordings. For each replicate measurement, CO2 concentrations were measured over a 2 min enclosure period, with concentrations recorded at 3 s intervals, or until an increase of 100 ppm CO2 had been observed. Measurements were then made monthly for 12 months (per month n = 54 for by palm fluxes and n = 18 for each of the harvest path, frond pile and cover plants fluxes). Extra fluxes were measured next to the palm because of high variability in both Rtot, root biomass and Ra, and the desire to accurately capture plantation Rtot fluxes (Manning, 2019; Manning et al., in preparation).
Soil CH4, drain CO2, and drain CH4 measurements were made using syringe sampling (soil CO2 data were also obtained using this method but the EGM data are presented due to a larger dataset). Static chambers were placed on the soil collars and floating chambers were deployed on drains when there was water present, otherwise static chambers were used on the drain surface. These chambers were left in place for 40 min, and 30 ml samples were collected with syringes every 0, 10, 20, 30, and 40 min. These samples were transferred into 20 ml pre-evacuated vials, which had been sealed with chlorobutyl rubber septa and aluminum seals (Sigma-Aldrich, Dorset, UK), and the over-pressurized vials were shipped to the UK for analysis. Measurements were made in August 2016, September 2016, November 2016, January 2017, March 2017, and May 2017 providing per month n = 18 for each location. The air samples were sent to St. Andrews University for analysis where samples were manually injected into a Thermo TraceGC Ultra Gas Chromatograph, which was fitted with a FID and methanizer for determination of CO2 (Thermo Electro Corporation). August 2016 samples were analyzed at the University of Aberdeen on an Agilent 6890 Gas Chromatograph Analyser, fitted with an FID (Agilent). The two different analysers were cross-calibrated with three different BOC standards, which comprised of mixes of CO2 and CH4 in different concentrations (Manning, 2019).
Stem CO2 and CH4 fluxes were measured using a Los Gatos UGGA (Ultraportable Greenhouse Gas Analyzer; Los Gatos Research Inc, San Jose, California, USA). This UGGA was attached to the chamber by 2 m long tubing and data were continuously measured for 10 min at a frequency of 1 Hz. Data were stored on the field laptop and the dry air ppm were reported.
Ancillary measurements were made during and just after the completion of flux measurements. Ambient air temperature was measured at the same time as all flux measurements, using a thermometer (LCD Digital Thermometer, ATP Instrumentation, Leicestershire, UK; precision: ±1°C). Soil temperature and soil moisture measurements at 0–10 cm depth were taken following the completion of soil CO2 and CH4 sampling, adjacent to the soil collars, with soil moisture measured using an ML3-probe and HH2 moisture meter (Delta-T, Cambridge, UK; precision for soil moisture: ±1%). Soil moisture measurements at 0–10, 10–20, 20–30, and 30–40 cm intervals were made next to a subset of soil collars following each measurement, namely at one subplot in each plot, using a PR2-probe and HH2 moisture meter (Delta-T, Cambridge, UK; precision: ±4%). If soil collars were being used to measure drain fluxes, soil temperature and soil moisture at 0–10 cm were also sampled from the peat at the base of the drain following the completion of the flux measurement. When floating chambers were being used to measure drain fluxes, water temperature was measured following the completion of the flux measurement, using the same thermometer as used for air and soil temperature. Stem temperature was measured using a Fluke 62 Max Infrared Thermometer (MEA, Dubai, United Arab Emirates; precision: ±2°C). Throughout the study climate data were collected from two weather stations (Davis Vantage Pro2 Plus, Hayward, California, USA), one on each plantation. Measurements from these weather stations include precipitation, air temperature, UV index, and air humidity (precisions: ±4%, ±0.3°C, ±5 and ±2%, respectively).
Water table depth (WTD) and the depth of the water in the drains (drain water depth) were both measured after the flux measurements were completed. To prepare for WTD measurements from the soil, PVC pipes of 3 m length and 5 cm diameter had holes drilled into them at 10 cm intervals along the pipe, in all four directions. These pipes were then installed into the peat to depths of 2.5 m 1 month prior to the commencement of flux measurement. WTD was then measured by inserting a 2.5 cm diameter pipe with a measuring tape attached to it into the PVC pipe. Drain water depth was measured using a pipe with a measuring tape attached to it, which was lowered into the drain until it reached the bottom of the drain. If the drain was dry when CO2 and CH4 fluxes had been measured from the drain surface, the WTD was measured by digging a hole 0.5 m away from the flux collar from the drain surface to the water table and measuring this distance.
Fluxes were calculated using the HMR package in R version 2.15.1 (http://www.R-project.org; Pedersen et al., 2010). Here linear and non-linear regressions were applied to the fluxes in accordance with the HMR methodology, whereby both options were calculated and the fit with the lowest standard error was used. All of the data were included for the flux calculations when the data were collected by the EGM. The vial CO2 data were first plotted in conjunction with CH4 to determine whether any of the vials had leaked, before the HMR function was applied, with the leaked vials being excluded from the analysis. The units of the HMR outputs are μL m−2 s−1. These units were converted to Mg CO2-C ha−1 yr−1 or kg CH4-C ha−1 yr−1 using Equation (2):
where F is the C flux, ΔC is the change in CO2 or CH4 concentration over the measurements period (ppm), Δt is the duration of the measurement period (s), P is pressure (mb), T is temperature (K), V is volume (m3), A is surface area of the soil (m2), R is the Universal Gas Constant 8.31432 J mol−1 K−1, M is the relative molecular mass of CO2 or CH4 and Y is the conversion to upscale the flux to annual emissions.
Annual flux estimates were upscaled using spatial and temporal weighting. Fluxes from the soil and drain microforms were spatially weighted by multiplying the mean CO2 or CH4 flux by the proportional area of the microform (Tables S1, S2). Stem fluxes were first upscaled to the plot level fluxes (F′), expressed as Mg CO2-C ha−1 or kg CH4-C ha−1 soil—first the flux (F) was multiplied by the surface area (A) of each palm to calculate the mean stem flux (A−1) and then A−1 was then multiplied by the planting density (166 palm ha−1) to get the per hectare stem carbon flux. Second, each spatially weighted flux (F′) was linearly interpolated between months using Equation (3):
where t1 and t2 are the timings of the measurements in days. These temporally weighted fluxes F* were subsequently summed over one year to produce annual flux estimates (Ftot). The microform annual estimates were summed to provide component and plantation flux estimates.
The rate of peat oxidation (i.e., Rh) was calculated by sampling root-free soil 4 m away from the palms, based on prior work by Manning et al. (in preparation), where roots and Rtot were measured in transects at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, and 4 m distance from the palm, going into the harvest path, frond piles and cover plants, to 0.3 m depth. Root biomass was found to be negligible further than 1.0 m away from the palm and Rtot did not vary with increasing distance more than 0.75 m distance from the palm (Manning et al., in preparation). Therefore, at this site the contribution of Ra to Rtot was considered to be negligible more than 1 m from the palm and sites were chosen for Rh measurements exactly 4 m away from the palms (halfway between two palms).
A range of Rh values were proposed, assuming: (1) the harvest path only provided true Rh estimates, (2) the harvest path and frond pile gave Rh estimates, and (3) assuming that surface Rtot fluxes measured from the harvest path, frond pile and cover plants only contributed to Rh. We estimated Rh using this range of methods because peat oxidation occurs at all of these different microforms and potentially at different rates due to variations between the microclimates. These estimates of Rh were extrapolated to the plantation level by area-weighting each flux by the proportional surface area of its microform. For methods (1) and (2) the flux measured from the harvest path was used to represent Rh from the microforms that were not included in the specific Rh calculation, when spatially upscaling. In all three methods the harvest path flux was also assumed to represent the rate of Rh for the area next to the palm.
All statistical analyses were done in R (version 3.5.1). Kruskal-Wallis tests were used to test whether there were significant variations in the CO2 and CH4 fluxes between the two different plantations, the three plots within each plantation, the three subplots within each plot and the different surface microforms. Kruskal-Wallis tests were used to test for significant variation in the CO2 and CH4 fluxes between the months. Post-hoc multiple comparisons tests were performed using the R package pgirmess (Giraudoux et al., 2018).
CO2 and CH4 measurements were modeled as a function of environmental variables using Gaussian linear mixed effect models from the nlme package in R (Pinheiro et al., 2017). Log or square root transformations of CO2 and CH4 measurements were sometimes necessary to normalize model residuals. The random effect structure and correlation structure, the latter used to take into account seasonality, were determined by comparing model residual plots. Fixed effect variables were chosen by top down selection, with the non-significant variables removed one by one from models using the ANOVA function (see Data Sheets 1–5, for the selection processes for the fixed effects in each model). The following five models were used for this study, with * used to denote significant interactions between variables:
Precipitation, relative humidity and air temperature were measured from weather stations in Sabaju (November 2015–March 2017) and Sebungan (June 2015–March 2017). Annual means for climate variables can be found in Table 1. Annual precipitation was recorded at 2,015 mm in Sabaju, using an average of moving annual sums of the data available. Precipitation records from Sebungan were unreliable due to equipment failure. The dry season consisted of February, March and May—all of which recorded <100 mm rain in Sabaju. Collectively, the rainy season had significantly more rainfall than the dry season, averaging 216 ± 25 mm/month in the rainy season and 90 ± 46 mm/month in the dry season (Kruskal-Wallis: chi-squared = 4.5; d.f. = 1; p = 0.03). Relative humidity was significantly higher in Sabaju than in Sebungan in both measurement periods (2015–2016: Kruskal-Wallis: chi-squared = 6.2; d.f. = 1; p = 0.01; 2016–2017: Kruskal-Wallis: chi-squared = 12.8; d.f. = 1; p < 0.0001). Using averages taken between November 2015 and January 2017, where there were complete records of relative humidity from both plantations, relative humidity was significantly higher in the rainy season (88.0 ± 0.5%) than in the dry season (87.3 ± 1.1%; Kruskal-Wallis: chi-squared = 6.5; df = 1; p = 0.01). Annual mean air temperatures measured from the weather stations were the same for both plantations, in both measurement periods. Air temperature measured at the time and location of the soil flux sampling was significantly higher in Sabaju than in Sebungan (Sabaju: 30.7 ± 0.1°C; Sebungan: 29.2 ± 0.1°C; Kruskal-Wallis: chi-squared = 109.7; d.f. = 1; p < 0.0001). Air temperature measured at the same time as the stem fluxes was not significantly different between the two plantations. Air temperature did not vary significantly between the rainy and dry seasons.
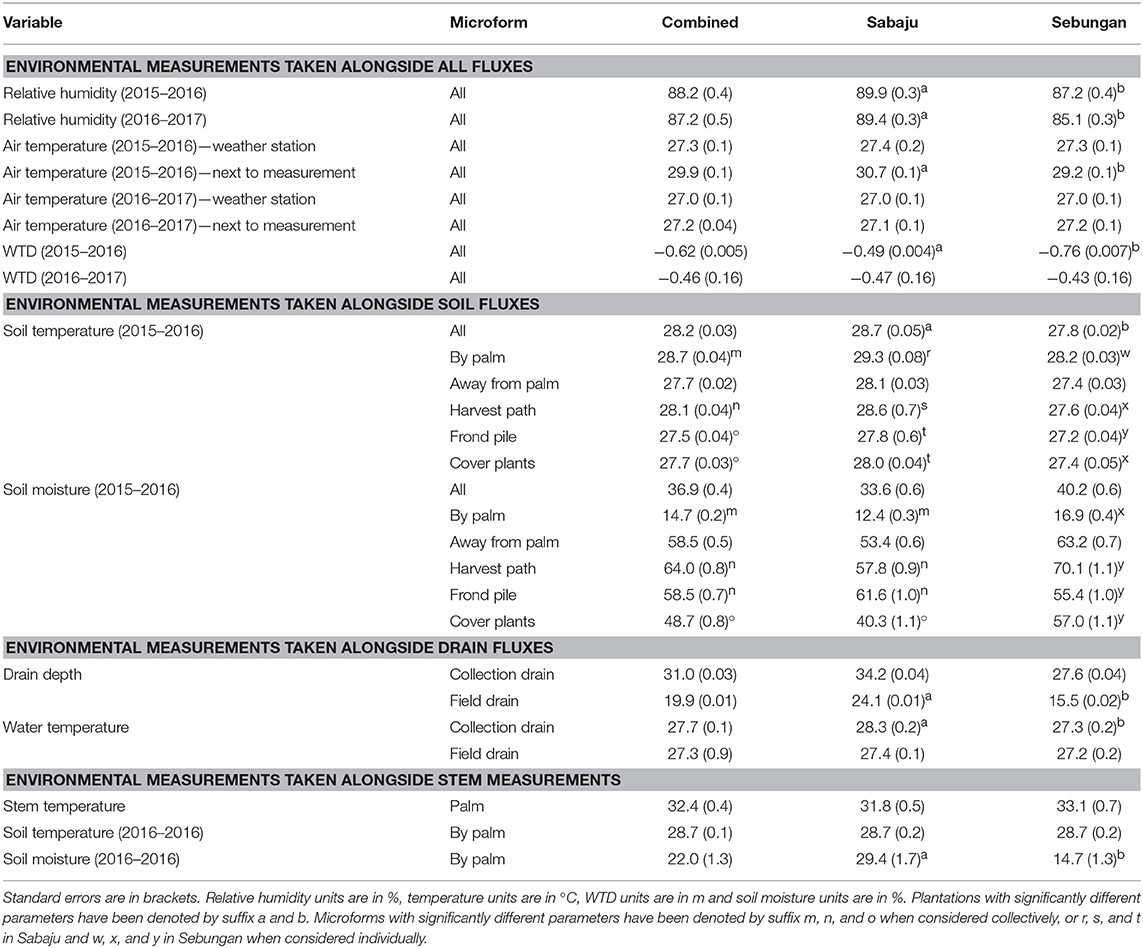
Table 1. Mean environmental variables measured from the two plantations, both combined and from Sabaju and Sebungan individually.
WTD varied between the two plantations during 2015–2016 but not during 2016–2017 (Table 1; Table S3). During 2015–2016, when the soil and drain measurements were made, mean WTD was −0.49 ± 0.0041 m in Sabaju and −0.76 ± 0.0072 m in Sebungan, which were significantly different (Kruskal-Wallis: chi-squared = 789.2; d.f. = 1; p < 0.0001). During 2016–2017, when the stem measurements were made, monthly mean WTD was −0.46 ± 0.16 m for Sabaju and −0.43 ± 0.16 m for Sebungan, which were not significantly different. Seasonal variation was observed in WTD; WTD was significantly higher in the dry season than in the rainy season in both measurement periods (2015–2016: dry season: −0.72 ± 0.00048 m; rainy season: −0.61 ± 0.00046 m; Kruskal-Wallis: chi-squared = 50.11; d.f. = 1; p < 0.001; 2016–2017: dry season: −0.49 ± 0.02 m; rainy season: −0.42 ± 0.02 m; Kruskal-Wallis: chi-squared = 5.68; d.f. = 1; p = 0.017).
In addition to climate and WTD measurements, different environmental measurements were made at the time of flux sampling, with annual averages reported in Table 1. Of particular interest are soil temperature and soil moisture, which varied significantly between the two plantations and between the different surface microforms. Soil temperature was significantly higher in Sabaju than in Sebungan (Kruskal-Wallis: chi-squared = 185.9; d.f. = 1; p < 0.0001) and varied significantly between the different surface microforms (Kruskal-Wallis: chi-squared = 253.7; d.f. = 3; p < 0.0001). Soil temperature was highest next to the palm and lowest beneath the frond piles, decreasing in order by palm > harvest path > cover plants > frond pile. Soil temperature was also significantly higher in the dry season (29.4 ± 0.05°C) than the rainy season (28.1 ± 0.05°C; Kruskal-Wallis: chi-squared = 29.2; d.f. = 1; p < 0.001). This seasonality was measured in both plantations (Sabaju Kruskal-Wallis: chi-squared = 10.60; d.f. = 1; p = 0.0011; Sebungan Kruskal-Wallis: chi-squared = 30.31; d.f. = 1; p < 0.001).
Soil moisture was significantly greater in Sebungan than in Sabaju (Kruskal-Wallis: chi-squared = 15.8; d.f. = 1; p < 0.0001). Soil moisture varied significantly between the different surface microforms (Kruskal-Wallis: chi-squared = 840.0; d.f. = 3; p < 0.0001). Soil moisture was consistently drier next to the palm than in the other microforms, decreasing in order harvest path > frond pile > cover plants > by palm, although soil moisture was highest in the frond pile in Sabaju and the harvest path in Sebungan. Soil moisture was significantly greater in the rainy season than in the dry season, with a mean of 39.4 ± 1.1% in the rainy season and 32.4 ± 1.0% in the dry season (Kruskal-Wallis: chi-squared = 23.8; d.f. = 1; p < 0.001). This relationship was seen in the different microforms, at the different plantations and in the different microforms at each plantation.
The CO2 fluxes are summarized in Table 2. Mean Rtot was recorded as 0.83 ± 0.048 g CO2-C m−2 hr−1. Drain CO2 averaged 0.14 ± 0.0012 g CO2-C m−2 hr−1. Mean stem respiration was 0.23 g ± 0.012 CO2-C m−2 hr−1. There was no significant difference in the overall mean CO2 flux between the plantations.
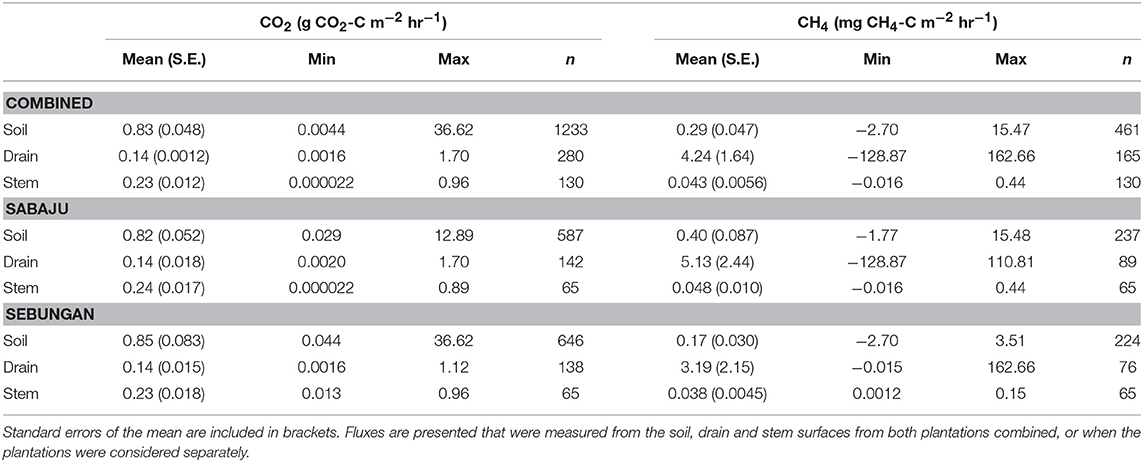
Table 2. Summaries of the mean, minimum, maximum and number of CO2 and CH4 fluxes measured in this study.
Significant differences were seen in the magnitude of the CO2 fluxes between the different surface microforms (Figure 3). Considering the plantations together, Rtot measured next to the palm gave the highest CO2 flux from the plantation (Kruskal-Wallis: chi-squared = 1,555.5; d.f. = 6; p < 0.0001). Within each plantation, we found differences in the magnitude of CO2 flux from each microform (Sabaju: Kruskal-Wallis: chi-squared = 572.6; d.f. = 6; p < 0.0001; Sebungan: Kruskal-Wallis: chi-squared = 1,078.6; d.f. = 6; p < 0.0001). For example, in Sabaju, the harvest path Rtot was significantly higher than Rtot measured from the frond piles and cover plants—although they themselves did not differ (multiple comparison test for Kruskal-Wallis: p ≤ 0.05). Drain fluxes were lower than soil fluxes but not significantly different to stem CO2 (multiple comparison test for Kruskal-Wallis: p ≤ 0.05). Stem CO2 fluxes were significantly lower than Rtot from next to the palm, the harvest path and beneath the frond piles, but not the cover plants (multiple comparison test for Kruskal-Wallis: p ≤ 0.05). In contrast, in Sebungan, frond pile Rtot was significantly higher than the field drain CO2 flux, but otherwise the soil, drain and stem fluxes did not differ significantly, apart from the high Rtot fluxes measured next to the palms (multiple comparison test for Kruskal-Wallis: p ≤ 0.05).
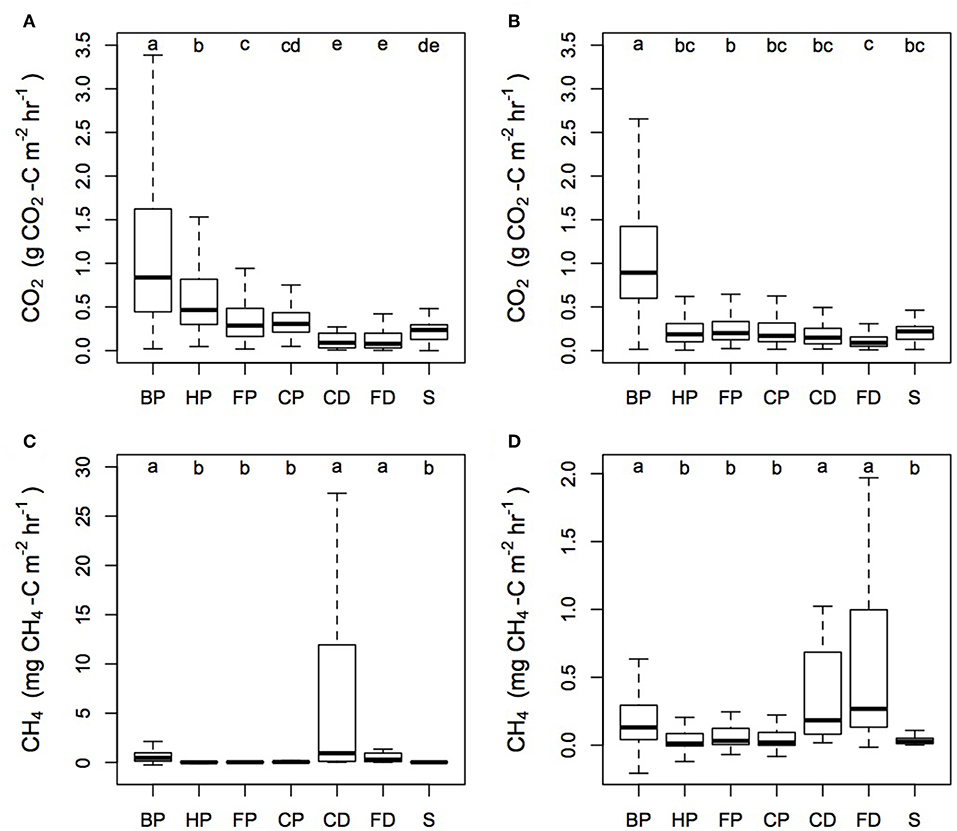
Figure 3. CO2 and CH4 fluxes from the different microforms in Sabaju and Sebungan. Microform acronyms are in the top left of each panel and are: BP - by palm; HP - harvest path; FP - frond pile; CP - cover plants; CD - collection drain; FD - field drain; and S - stem. (A) the median and interquartile range for CO2 fluxes from Sabaju and (B) Sebungan, and (C) the median and interquartile range for CH4 fluxes from Sabaju and (D) Sebungan. Outliers were excluded from the graph for visual clarity.
Summaries of the CH4 fluxes are presented in Table 2. The mean soil CH4 flux was 0.29 ± 0.047 mg CH4-C m−2 hr−1. The mean drain CH4 flux was 4.24 ± 1.64 mg CH4-C m−2 hr−1. The stem CH4 flux averaged 0.043 ± 0.0056 mg CH4-C m−2 hr−1. We did not observe a significant difference in the overall CH4 flux when comparing all the CH4 fluxes from the two plantations.
CH4 fluxes varied significantly among the different surface microforms (Figure 3; Kruskal-Wallis: chi-squared = 258.79; d.f. = 6; p < 0.0001). Multiple comparisons tests indicate that the CH4 fluxes from different microforms fell into one of two groups; the first (lower emission) group consisted of the harvest path, frond pile, cover plants and stem fluxes, while the second (higher emission) group consisted of the by palm, collection drain and field drain fluxes (multiple comparison test for Kruskal-Wallis: p ≤ 0.05). This trend was the same in both plantations.
Seasonal trends were observed in trace gas fluxes between the rainy and dry seasons. CO2 fluxes varied between the rainy and dry seasons, but this trend was not statistically significant. The rainy season flux averaged 0.74 ± 0.054 g CO2-C m−2 hr−1 and the dry season flux averaged 0.94 ± 0.081 g CO2-C m−2 hr−1.
Disaggregating the data by microform showed that each microform had different trends with respect to CO2 fluxes (Figure 4). Rtot measured next to the palm was significantly higher in the dry season than in the rainy season (Kruskal-Wallis: chi-squared = 25.6; d.f. = 11; p < 0.0001). Rtot measured from the harvest path, frond piles and cover plants did not vary significantly between the rainy and dry seasons. Significantly more CO2 was measured from the surfaces of the drainage ditches in the rainy season than in the dry season (Kruskal-Wallis: chi-squared = 11.54; d.f. = 1; p < 0.0001). Splitting the drains, the larger collection drains had significant seasonal variation in CO2 fluxes (Kruskal-Wallis: chi-squared = 8.1; d.f. = 1; p = 0.004), with higher CO2 fluxes in the rainy season than the dry season (multiple comparison test for Kruskal-Wallis: p ≤ 0.05) and the smaller field drains did not. Stem CO2 did not show significant variation between the rainy and dry seasons.
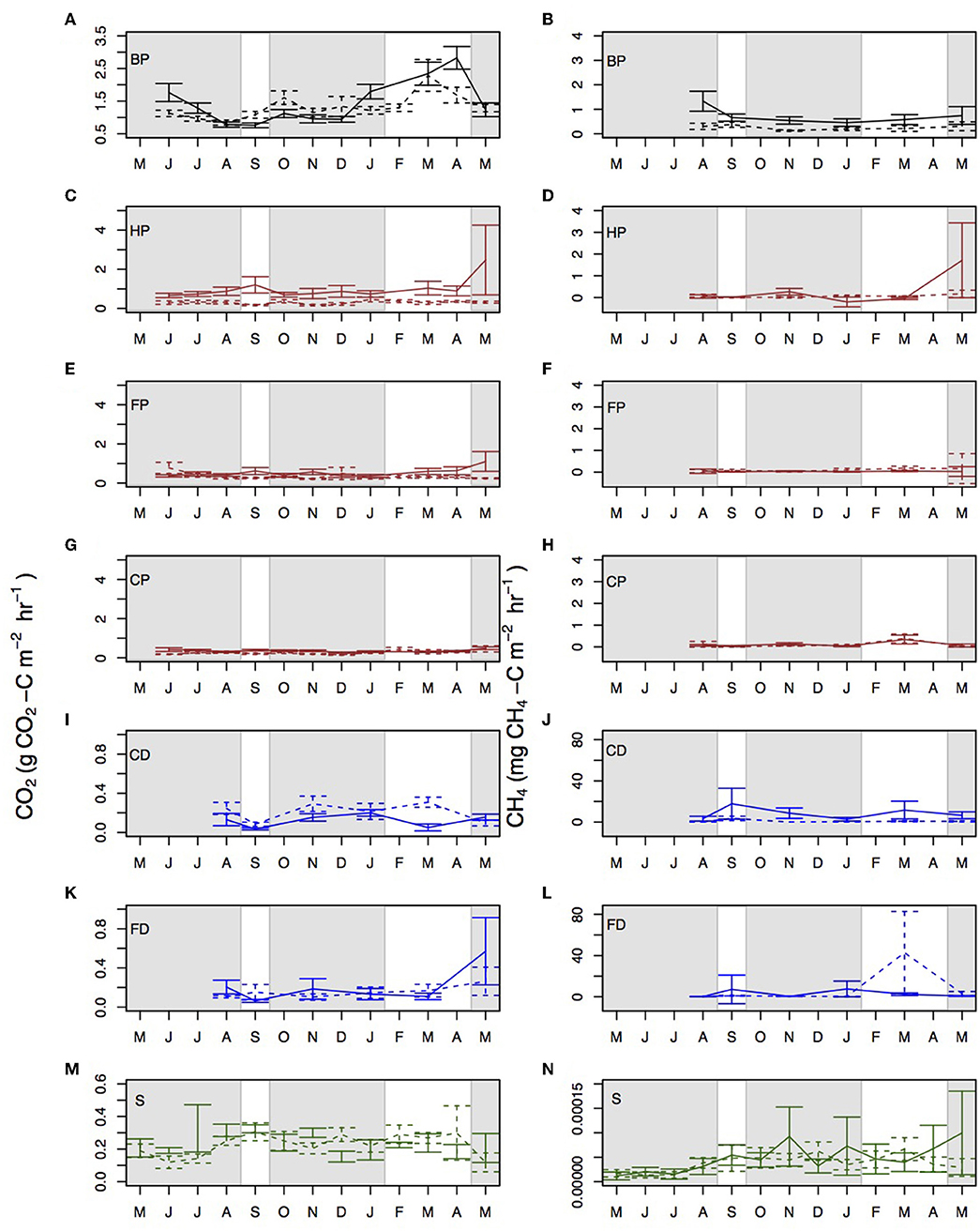
Figure 4. Monthly mean CO2 and CH4 measurements taken from the different surface microforms in Sabaju (continuous line) and Sebungan (dashed line) for (A) by palm CO2, (B) by palm CH4, (C) harvest path CO2, (D) harvest path CH4, (E) frond pile CO2, (F) frond pile CH4, (G) cover plants CO2, (H) cover plants CH4, (I) collection drain CO2, (J) collection drain CH4, (K) field drain CO2, (L) field drain CH4, (M) stem CO2, and (N) stem CH4. Microform acronyms are in the top left of each panel and are: BP - by palm; HP - harvest path; FP - frond pile; CP - cover plants; CD - collection drain; FD - field drain; and S - stem. Furthermore, the lines for by palm fluxes are plotted in black, the lines for harvest path, frond pile, and cover plants are plotted in brown, the drain fluxes are plotted in blue and the stem fluxes are plotted in green. The shaded gray areas signify the rainy seasons and the white areas signify the dry seasons. Standard errors are shown with bars. Data are presented for 2015–2016, apart from stem data which are presented for 2016–2017. Note the different y-axis scales.
Collectively, CH4 fluxes did not vary significantly between rainy and dry seasons, although the rainy season flux averaged 0.76 ± 0.17 mg CH4-C m−2 hr−1 and the dry season flux averaged 2.27 ± 1.14 mg CH4-C m−2 hr−1. Soil and stem CH4 fluxes did not show significant variation between the rainy and dry seasons at the different plantations. Drain CH4 fluxes were significantly higher in the rainy season than in the dry season (Figure 4; Kruskal-Wallis: chi-squared = 4.33; d.f. = 1; p = 0.04). Considering the plantations separately, there was no significant difference between the rainy and dry seasons in the different microforms or locations.
Annual estimates of CO2 fluxes were produced for the plantations both together and separately (Table 3). Combining Rtot and drain CO2 fluxes to give the total CO2 flux from the ground surfaces gave an average of 23.57 ± 0.50 Mg CO2-C ha−1 yr−1. Sabaju and Sebungan differed in their annual combined Rtot and drain CO2 flux. Sabaju was estimated to produce 29.54 ± 0.47 Mg CO2-C ha−1 yr−1 and Sebungan was estimated to produce 17.62 ± 0.53 Mg CO2-C ha−1 yr−1. The plantations differed in this CO2 flux because of the contribution from the harvest path at each plantation; Sebungan averaged 9.21 ± 1.40 Mg CO2-C ha−1 yr−1 from the harvest path, while Sabaju emitted 20.19 ± 1.33 Mg CO2-C ha−1 yr−1.
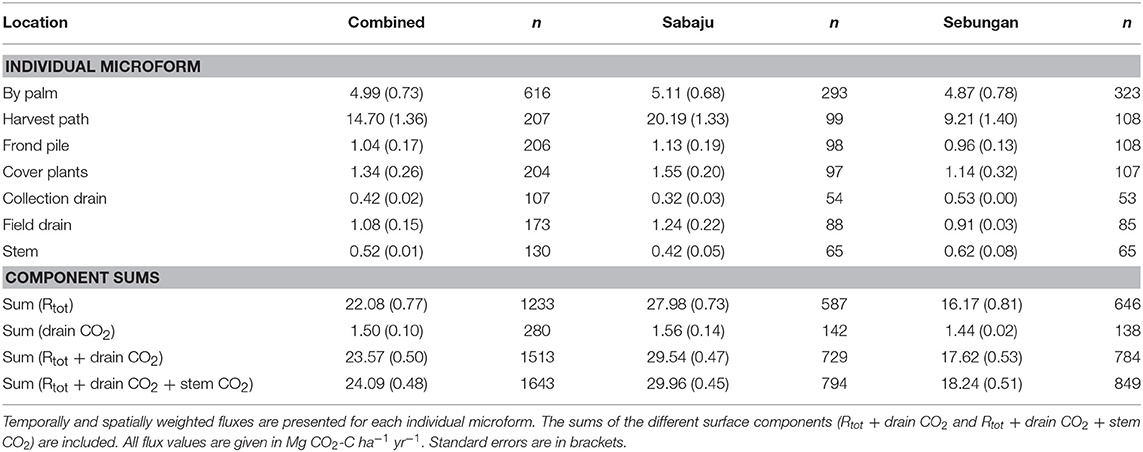
Table 3. Annual upscaled CO2 fluxes from the different surface microforms sampled at both plantations and at Sabaju and Sebungan individually.
Annual estimates of CH4 fluxes were calculated, collectively for both plantations and individually, with very little difference between the total CH4 flux (Table 4). Combined, the annual CH4 flux from oil palm plantations, including soil, drain and stem fluxes, was 61.02 ± 17.78 kg CH4-C ha−1 yr−1. In Sabaju, the annual CH4 flux was 60.84 ± 4.35 kg CH4-C ha−1 yr−1 and in Sebungan the annual CH4 flux was 61.19 ± 25.28 kg CH4-C ha−1 yr−1.
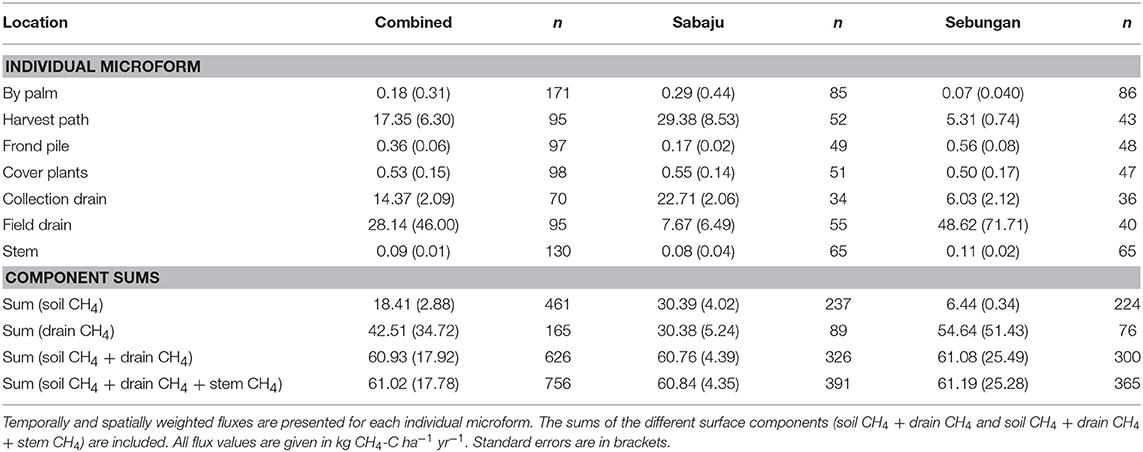
Table 4. Annual upscaled CH4 fluxes from the different surface microforms sampled at both plantations and at Sabaju and Sebungan individually.
Rtot was partitioned into Rh and Ra at both plantations (Table 5). Collectively, Rh ranged from 17.61 to 17.89 Mg CO2-C ha−1 yr−1 and Ra ranged from 4.19 to 4.47 Mg CO2-C ha−1 yr−1. Plantation Ra increased from 4.71 to 4.99 Mg CO2-C ha−1 yr−1 when stem respiration was included. Rh varied between the two plantations; Rh in Sabaju ranged from 23.89 to 24.39 Mg CO2-C ha−1 yr−1, while in Sebungan it ranged from 11.13 to 11.81 Mg CO2-C ha−1 yr−1. Considering the plantations separately, Ra ranged from 3.10 to 3.60 Mg CO2-C ha−1 yr−1 in Sabaju and from 4.36 to 5.04 Mg CO2-C ha−1 yr−1 in Sebungan. When stem respiration was combined with soil Ra, plantation Ra increased to range from 3.52 to 4.02 Mg CO2-C ha−1 yr−1 in Sabaju and from 4.98 to 5.66 Mg CO2-C ha−1 yr−1 in Sebungan.
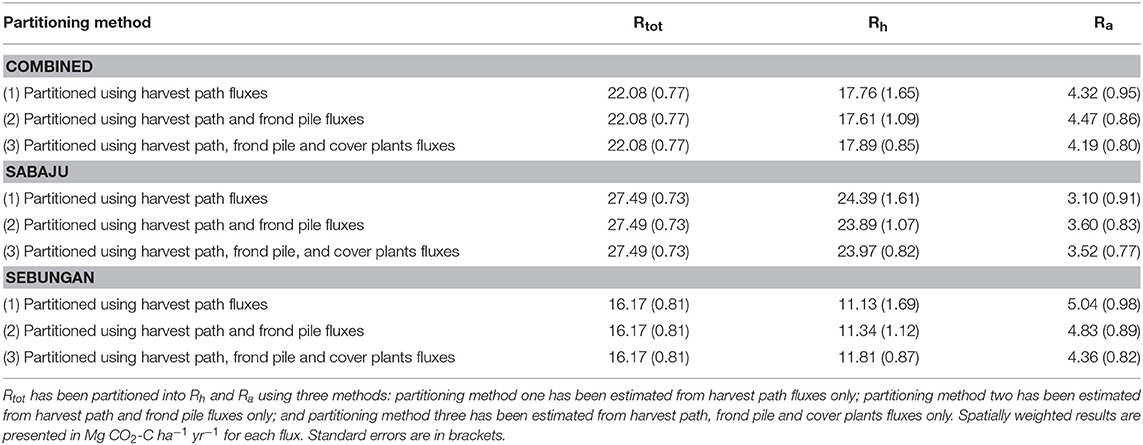
Table 5. Rtot, Rh and Ra estimates from both plantations combined, and Sabaju and Sebungan considered individually.
Plantation peat carbon losses were comprised of Rh, drain CO2, soil CH4, drain CH4, and stem CH4 components and were estimated in CO2−eq. Overall plantation carbon losses ranged from 72.35 ± 0.14 to 73.38 ± 0.16 Mg CO2−eq ha−1 yr−1. Plantation carbon losses varied between the two plantations. In Sabaju, the plantation carbon losses ranged from 95.58 ± 0.14 to 97.41 ± 0.17 Mg CO2−eq ha−1 yr−1. In Sebungan, the plantation carbon losses ranged from 48.37 ± 0.16 to 50.86 ± 0.19 Mg CO2−eq ha−1 yr−1.
Breaking the carbon losses down by component showed that 92% of the CO2 produced through Rh and drain CO2 pathways came from soils and 8% came from the drains (Table 6). This was similar for both plantations. In Sabaju, 94% of the CO2 produced through Rh or drain CO2 came from Rh and 6% came from drain CO2. In Sebungan, 89% of the CO2 produced through Rh or drain CO2 came from Rh and 11% came from drain CO2.
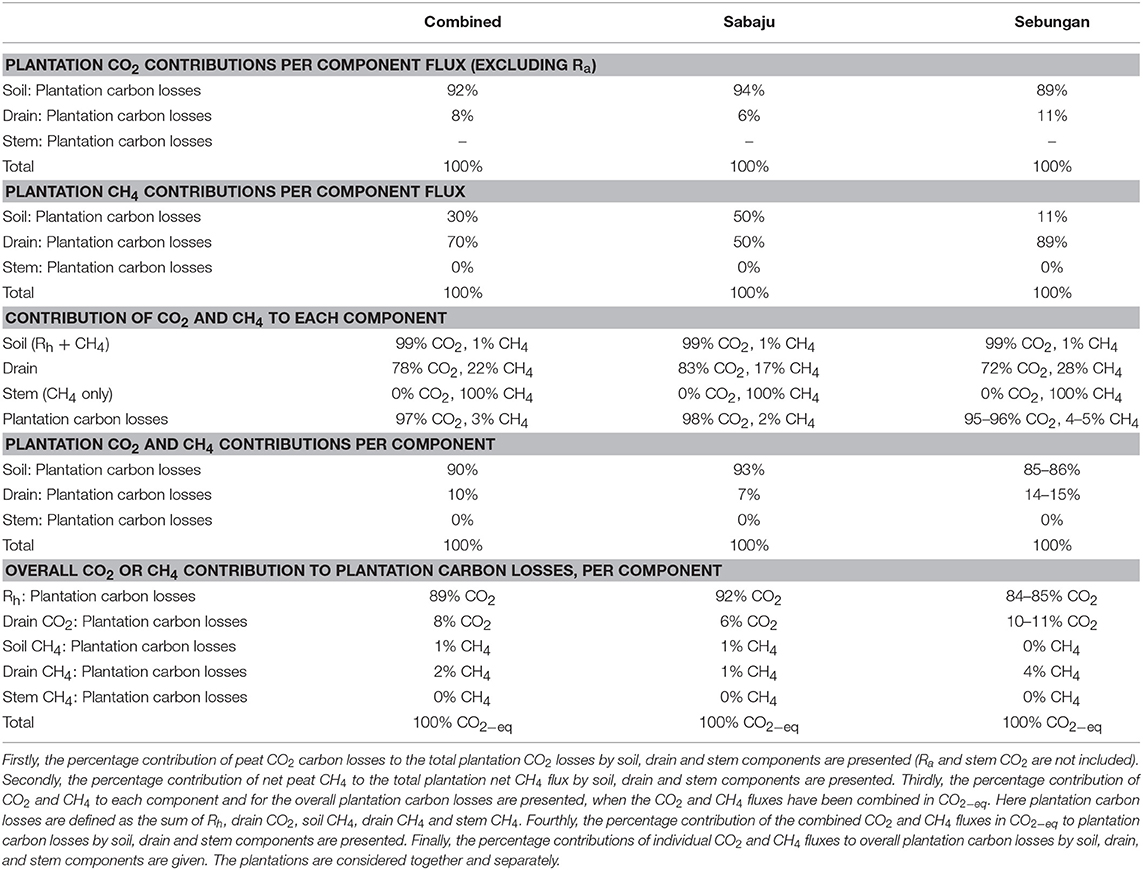
Table 6. Percentage contributions of the soil, drain, and stem components to plantation CO2, CH4 and CO2−eq fluxes.
The CH4 losses showed different component contributions to CO2 (Table 6). Overall, 30% of CH4 fluxes came from the soil and 70% of plantation CH4 fluxes came from the drainage ditches. Stem CH4 fluxes gave negligible contributions. The CH4 pathways varied between the two plantations. In Sabaju, 50% of the plantation CH4 flux was measured from the soil surface and 50% was recorded from the drainage ditches. In Sebungan, 11% of the plantation CH4 flux came from the soil and 89% came from the drainage ditches.
In order to isolate which pathways were dominating the plantation peat carbon losses, the fluxes were compared in CO2−eq (Table 6). Considering the soil component in isolation, 99% of soil peat carbon losses were attributable to Rh and 1% to CH4. Considering the drain carbon fluxes, 78% of the carbon losses came through drain CO2 and 22% of the carbon measured from the drain surfaces came from drain CH4.
Overall, 89% of plantation peat carbon losses came from Rh, 8% came from drain CO2, 1% from soil CH4, 2% from drain CH4, and 0% from stem CH4; 90% of plantation carbon losses came from the soil and 10% from drainage ditches (Table 6). In Sabaju, 92% of plantation peat carbon losses were attributable to Rh, 6% to drain CO2, 1% to soil CH4, 1% to drain CH4, and 0% to stem CH4; 93% of peat carbon losses came from the soil and 7% from the drainage ditches. In Sebungan, 84–85% of plantation carbon losses were from Rh, 10–11% were from drain CO2, 0% from soil CH4, 4% from drain CH4, and 0% from stem CH4; 85–86% came from the soil and 14–15% from the drainage ditches.
Rh, the most dominant component in the peat carbon losses, was controlled by variations in air temperature, WTD, soil temperature, and soil moisture (Table S4). Rh increased as air temperature increased (Figure 5). Whilst the interaction between air temperature and plantation was not significant, the rate of increase in Rh as air temperature increased was predicted to be almost three times greater in Sabaju than in Sebungan (Table S5). Unexpectedly, Rh increased as WTD rose, with a modeled increase of 0.75 Mg CO2-C ha−1 yr−1 for every 10 cm decrease in WTD. The interaction between WTD and plantation was significant, with opposite relationships seen in both plantations (Figure 5). In Sabaju, Rh increased by 0.85 Mg CO2-C ha−1 yr−1 for every 10 cm rise in WTD. Rh increased in Sebungan as the WTD was lowered, by a modeled rate of −0.29 Mg CO2-C ha−1 yr−1 for every 10 cm drawdown of WTD.
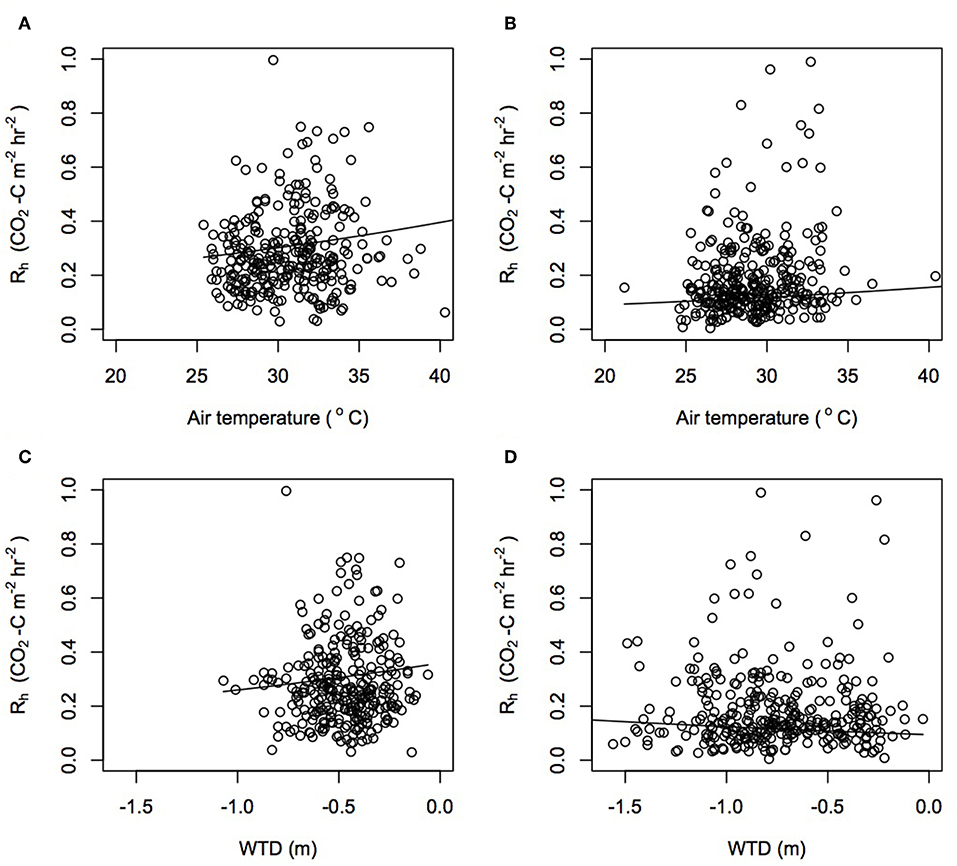
Figure 5. Rh estimates modeled from changes in (A) air temperature in Sabaju, (B) air temperature in Sebungan, (C) WTD in Sabaju, and (D) WTD in Sebungan, using the linear mixed effect model parameters. Actual data points from the harvest path, frond pile and cover plants are plotted.
Overall, Rh increased as soil temperature increased and Rh increased as soil moisture decreased (Tables S4, S5). Soil temperature and soil moisture had significant relationships with Rh when their individual interactions with plantation and microform were included in the model. Considering the plantations together and the microforms separately, the harvest path increased in Rh as soil temperature increased, and the frond pile and cover plants decreased in Rh as soil temperature increased. This pattern was seen in Sebungan but not in Sabaju when the plantations were considered separately. In Sabaju, Rh increased when soil temperature increased throughout the microforms. Increasing soil moisture decreased the rate of Rh, with the greatest effect beneath the frond pile, followed by the harvest path and then the cover plants. This trend was also observed when the microforms in Sebungan were considered in isolation. In Sabaju, rates of Rh decreased as soil moisture increased in the cover plants but not in the harvest path and frond piles.
Soil CH4 was controlled by soil moisture between 0 and 10 cm, soil moisture between 30 and 40 cm, WTD and soil temperature (Table S6). Soil CH4 increased as soil moisture between 0 and 10 cm increased (Table S5). The interaction between plantation, microform, WTD and soil moisture between 30 and 40 cm also significantly explained the variation in soil CH4. Here the expected relationship of soil CH4 increasing as WTD decreased was observed in the harvest path and next to the palm. This trend was seen when considering the plantations together or in Sabaju. In Sebungan, soil CH4 was modeled to increase as WTD decreased in the frond piles and cover plants. The opposite relationship was seen in the other microforms. Similarly, variation was seen in the relationship between soil CH4 and soil moisture between 30 and 40 cm. Soil CH4 increased as soil moisture between 30 and 40 cm increased in the harvest path, frond pile and next to the palm, with the opposite relationship in the cover plants. In Sabaju, soil CH4 increased as soil moisture between 30 and 40 cm increased in the harvest path and by the palm. In Sebungan, soil CH4 increased as soil moisture between 30 and 40 cm increased in the frond pile and cover plants. Soil CH4 increased as soil temperature decreased. The interaction between soil temperature and plantation was significant, with the rate of soil CH4 increasing twice as quickly in Sabaju than in Sebungan when soil temperature decreased.
Drain CO2 and drain CH4 had different environmental relationships (Tables S5, S7). Drain CO2 did not have a significant relationship with any of the environmental variables measured in this study, but did vary significantly depending on the size of the microform. Overall, the field drain had a greater drain CO2 flux. Drain CH4 increased significantly as air temperature increased. This was seen at both plantations.
Stem CH4 was explained by variations in monthly relative humidity, WTD, soil moisture, air temperature and soil temperature (Table S8). Stem CH4 decreased as monthly relative humidity increased (Table S5). This relationship was seen on both plantations and the interaction between the individual palms within each plantation with monthly relative humidity was significant. Stem CH4 increased as WTD increased. This was also seen at both plantations. For soil moisture, air temperature and soil temperature, opposite trends were seen between the two plantations. Stem CH4 increased as soil moisture increased, this trend was the same when the plantations were considered together and also in Sebungan when the plantations were considered separately, but not in Sabaju. Stem CH4 increased as air temperature and soil temperature increased, when both plantations were considered together. These trends were seen in Sebungan but not in Sabaju when the plantations were considered separately.
CO2 and CH4 fluxes varied significantly when measured from different surface microforms in two oil palm plantations on peat soil. The highest soil CO2 and CH4 fluxes were measured next to the palms. This pattern was attributed to the high density of roots in the palm rhizosphere; root biomass was greatest within the immediate 1 m radius around each palm, and decreased with increasing distance from the palm (Farmer, 2013; Dariah et al., 2014; Manning, 2019; Manning et al., in preparation). In these studies Rtot and soil respiration were measured at consistent intervals from the palm into the harvest path (Farmer, 2013; Dariah et al., 2014), frond pile and cover plants (Manning, 2019; Manning et al., in preparation) from mature oil palm plantations in Jambi, Indonesia (Farmer, 2013; Dariah et al., 2014) and this plantation (Manning, 2019; Manning et al., in preparation). Respiration from root biomass has been shown to drive high Rtot rates next to the palm, with a progressive decline in Rtot with increasing distance from each palm (Farmer, 2013; Dariah et al., 2014; Manning, 2019; Manning et al., in preparation). Likewise, high CH4 fluxes from the rhizosphere could be due to a number of causes, including accelerated organic matter turnover facilitated by the metabolism of root exudates (Girkin et al., 2018a,b), or transport of CH4 from deeper in the profile via porous tissues in the roots.
Outside the palm rhizosphere (i.e., >1 m distance from the palm), different soil surface management practices appeared to influence Rtot but not CH4. Rtot was significantly higher in the harvest path than from either the frond piles or beneath the cover plants. There was no significant difference in CH4 measured from these microforms. Considering the plantations separately gave different trends for Rtot, but did not change the pattern of CH4. For example, in Sebungan, there was no significant difference in Rtot between the non-rhizosphere fluxes. In contrast, in Sabaju the harvest path had significantly higher Rtot than the frond pile and cover plants. These differences in Rtot among microforms are noteworthy because few other studies consider the effects of surface management practices on Rtot, with the exception of work by Arifin et al. (2015), who also found that Rtot was significantly lower when measured from beneath cover plants than from the harvest path. These differences in Rtot among microforms are significant because they have direct bearing not only predicting and upscaling land-atmosphere fluxes, but also because they provide insight into how soil surface management practices are affecting soil CO2 and CH4 dynamics.
Drainage ditches made a substantial contribution to land-atmosphere CO2 and CH4 fluxes, particularly with respect to CH4. Drain CH4 was significantly higher than CH4 fluxes from all soil microforms, with the exception of the CH4 flux measured next to the palm. Comparing our drain data against other datasets, we found that our CH4 fluxes fell within the range observed in drained peatlands and drained Acacia sp. plantations in Kalimantan (Jauhiainen and Silvennoinen, 2012). These high drain CH4 fluxes could be attributable to the photochemical or microbial breakdown of DOC, or lateral transport of dissolved CH4 produced in the peat into surface waters (Billett and Moore, 2008; Teh et al., 2011; Cory et al., 2014; Logue et al., 2016). These data are significant because there are currently no published data on drain CO2 and CH4 fluxes from oil palm plantation drainage ditches, and they have implications for extrapolating both plot-level CO2 and CH4 fluxes to larger spatial scales. In particular, the estimates of CO2 and CH4 fluxes based on straight mean averaging or non-spatially explicit sampling may tend to overestimate the net release of CO2 to the atmosphere and underestimate the net release of CH4 from tropical peatlands, given that drainage ditches form a small but significant portion of the landscape.
Stem respiration fell within a similar range to drain CO2 and the Rtot fluxes when excluding the high fluxes next to the palm. Once again, disaggregation of the dataset by plantation revealed local differences in stem respiration relative to other CO2-emitting processes. Stem respiration was lower in Sabaju than in Sebungan. Furthermore, in Sabaju, stem respiration was significantly lower than Rtot measurements taken from next to the palm, the harvest path and the frond pile. In contrast, in Sebungan, stem respiration was similar to all other soil fluxes with the exception of Rtot measured next to the palm. We believe that these local differences in stem respiration may reflect differences in productivity between the two plantations. Generally, stem respiration correlates with photosynthetic rate (Yang et al., 2016); hence, the higher stem respiration in Sebungan may reflect that this plantation shows higher rates of CO2 uptake and growth than Sabaju, evidenced by higher rates of net primary productivity, larger fronds and formation of a denser, more closed canopy.
Stem CH4 fluxes were recorded from the palms in both plantations, with lower CH4 fluxes than those reported from the soil or drain surfaces, by factors of 22 and 200, respectively. Stem CH4 has been recorded from the stems of tropical trees growing in swamp forests in Brunei and the Amazon (Pangala et al., 2013, 2017). Stem fluxes from Brunei ranged between similar magnitudes to the stem fluxes measured at this site (here: −0.016–0.440 mg CH4-C m−2 hr−1; Brunei: 0.013–0.139 mg CH4-C m−2 hr−1) when the fluxes were considered per m−2 of stem surface area (Pangala et al., 2013). Stem fluxes in the Amazon were considerably higher than the stem fluxes in Borneo (0.248–435.75 mg CH4-C m−2 hr−1; Pangala et al., 2017). Stem CH4 has been shown to correlate with rates of evapotranspiration (Pangala et al., 2014). We propose that the similar rates of relative humidity between Sarawak and Brunei may contribute to the similarities in range of fluxes from the stems of plants at these sites. The CH4 oxidation reported from this site was measured four times and only when relative humidity exceeded 93% (Manning, 2019). We suggest that this may be due to methanotrophs on the palm surface, that are normally masked from stem CH4 fluxes, but observable when high relative humidity prevents transpiration (Raghoebarsing et al., 2005).
Both CO2 and CH4 fluxes were greater in the dry season than the rainy season, but these differences were not statistically significant. In this region, two rainy seasons occur, with the main rainy season between October and January, and a secondary rainy season between May and August. Dry season falls from February to April and in September.
Considering the surface microforms separately, significant seasonal variation was seen from Rtot measured next to the palm, with higher fluxes in the dry season than in the rainy season. Rtot next to the palm was predominately attributed to Ra (Farmer, 2013; Dariah et al., 2014; Manning et al., in preparation). Stem respiration gave similar overall trends to by palm Rtot, but differences were not statistically significant. Overall these fluxes, dominated by autotrophic processes, suggest strong seasonal growth from the palms, with more photosynthetic activity in the dry season than in the rainy season. Seasonality has been recorded from Rtot next to the palm in other plantations (Comeau, 2016; Hergoualc'h et al., 2017), as well as from Ra sampled from the edge of the canopy (Melling et al., 2013).
In contrast, Rtot measured from the harvest path, frond pile and cover plants did not show significant seasonal variation. Rtot fluxes measured from these locations were dominated by Rh. Rh was shown to have significant seasonality in a rubber plantation in Kalimantan, Indonesia growing on peat soil, where the seasonality in Rh was driven by seasonal changes in WTD (Wakhid et al., 2017). Similar to this study, those on other oil palm plantations have not found significant seasonality in Rh (Comeau, 2016; Hergoualc'h et al., 2017). The lack of apparent seasonality in Rh from oil palm plantations could be due to reduced variability in the environmental variables driving Rh. For example, Melling et al. (2013) found that Rh varied in the forest due to variations in WTD but not in the oil palm plantation where the WTD was more consistent.
Drain CO2 and CH4 fluxes showed seasonality; drain CO2 fluxes were significantly higher in the rainy season than the dry season and drain CH4 fluxes were significantly higher in the dry season than in the rainy season. Cook et al. (2018) found that drain discharge varied seasonally but total organic carbon (DOC plus particulate organic carbon) concentrations in the drains did not vary seasonally from Sabaju and Sebungan. Billett and Moore (2008) found that drain CO2 increased when flow rate increased, whilst DOC concentrations remained consistent regardless of flow rate.
Rh was estimated to range from 17.61 ± 1.65 to 17.89 ± 0.85 Mg CO2-C ha−1 yr−1 (mean: 17.75 ± 1.54 Mg CO2-C ha−1 yr−1). For these calculations, Rh was partitioned from Rtot using the distance from palm approach, as this was deemed sufficient in these plantations (Manning, 2019; Manning et al., in preparation). We present a range of estimates that include (1) only the harvest path—comprising of bare soil and often the only microform measured in Rh studies; (2) the harvest path and the frond pile—the latter being bare soil covered in dead fronds; (3) the harvest path, frond pile and cover plants—with the cover plants being included, despite the presence of cover plant roots, due to the increased Rh being potentially from priming, as opposed to Ra or decomposition from the cover plants (Manning et al., in preparation). In this study the lowest Rh estimate was obtained from when the harvest path and frond pile were both included in the calculations and the highest estimate came from when the cover plants were included as well. However, the estimates did not vary significantly regardless of which microforms were included.
Rh estimates from Sebungan were found to range from 11.13 ± 1.69 to 11.81 ± 0.87 Mg CO2-C ha−1 yr−1 (mean: 11.43 ± 1.37 Mg CO2-C ha−1 yr−1) and Rh from Sabaju was measured ranging between 23.89 ± 1.07 and 24.39 ± 1.61 Mg CO2-C ha−1 yr−1 (mean: 24.08 ± 1.42 Mg CO2-C ha−1 yr−1). In these estimates Rh was lowest from Sebungan when the harvest path only was measured, and highest when the cover plants were included. In Sabaju the lowest Rh estimate used the harvest path and frond pile data and the highest Rh estimate only used the harvest path results. The Sebungan Rh estimate fell within the reported range of Rh from oil palm plantations (4.1 to 22.9 Mg CO2-C ha−1 yr−1, but the Sabaju estimate was greater than this range (Farmer, 2013; Melling et al., 2013; Dariah et al., 2014; Husnain et al., 2014; Comeau, 2016; Comeau et al., 2016; Hergoualc'h et al., 2017; Ishikura et al., 2018; Matysek et al., 2018).
These data are relevant to policy because they suggest that the IPCC emissions factor for Rh is underestimated. For example, whilst Rh estimates from Sebungan have been shown to be similar to the IPCC (2014) emissions factor of 11 Mg CO2-C ha−1 yr−1, Rh from Sabaju is at least twice as large. This has implications when upscaling—estimating the Rh emissions from industrial oil palm plantations using the areal extent of plantations given in Miettinen et al. (2016) increases predicted Rh from 34.1 Tg CO2-C yr−1 if the IPCC emission factor is used to 55.8 Tg CO2-C yr−1 if the mean Rh from this study is used.
Another important policy-relevant finding is that area-weighted CH4 fluxes from these managed systems are similar to swamp forests in SE Asia, suggesting that drainage has not diminished the CH4 emissions potential of these systems. Annual estimates of CH4 were 61.02 ± 17.78 kg CH4-C ha−1 yr−1 including soil, drain and stem fluxes. Sabaju and Sebungan produced similar rates of CH4 of 60.84 ± 4.35 kg CH4-C ha−1 yr−1 and 61.19 ± 25.28 kg CH4-C ha−1 yr−1, respectively. Annual estimates from forest CH4 have been shown to range between 0.2 and 72 kg CH4-C ha−1 yr−1 from Malaysian, Indonesian and Brunei peatlands (Inubushi et al., 1998, 2003; Furukawa et al., 2005; Hadi et al., 2005; Jauhiainen et al., 2005; Melling et al., 2005b; Hirano et al., 2009; Pangala et al., 2013). The results from this study are potentially controversial because current conceptual ideas of peatland drainage predict a net reduction in CH4 emission linked to peatland drainage (Jauhiainen et al., 2005, 2008; Lai, 2009; Couwenberg et al., 2010). This is reflected in the IPCC (2014) emissions factors for oil palm plantations that are 0 kg CH4-C ha−1 yr−1 from the peat soil and 45.18 kg CH4-C ha−1 yr−1 from the drainage ditches. The results from this study show that draining peat does not reduce CH4 fluxes. The high CH4 fluxes from the drains suggest that either the drain sediments have increased rates of CH4 production, or that the CH4 transported in the water may be carried to the drains before being egressed, rather than diffusing through the soil and being oxidized by methanotrophs (Müller et al., 2015; Evans et al., 2016).
Combining rates of Rh, drain CO2, soil CH4, drain CH4, and stem CH4 in CO2−eq gave overall plantation peat carbon losses. Here, net peat carbon losses averaged across plantations ranged between 72.35 ± 0.14 and 73.38 ± 0.16 Mg CO2−eq ha−1 yr−1. Of this flux, 89% was attributed to Rh. Drain CO2 was the next largest driver, contributing 8% of the plantation carbon losses. CH4 contributed 2, 1, and 0% to plantation carbon losses for drain, soil and stem fluxes, respectively. We suggest this particularly low contribution from palm stems is due to two factors: (1) a strong gradient for CH4 transport to the atmosphere through the drainage ditches, and (2) the low density of palms growing on the soil. As previously mentioned, the measured stem CH4 fluxes were in the same range as the stem fluxes from trees in a swamp forest in Brunei (Pangala et al., 2013). Following upscaling the stem CH4 fluxes from Brunei were 10–23 times greater than the stem CH4 fluxes measured here (Pangala et al., 2013). However, the swamp forest trees were taller and more densely populated than oil palms, allowing for a greater overall stem surface area and therefore a greater total plot-scale stem CH4. Similarly, the total contribution of tree stem CH4 to plot-scale CH4 was 62–87% of the total ecosystem flux—but there were no drainage ditches in the forest plot, meaning that stem fluxes had a greater overall representation (Pangala et al., 2013). Excluding the drainage ditches from the estimates in this study increased the stem contribution to 0.5%.
Sabaju and Sebungan differed in their overall net peat carbon losses. Sabaju produced double the amount of peat carbon losses compared to Sebungan, with peat carbon losses ranging between 95.58 ± 0.14 and 97.41 ± 0.17 Mg CO2−eq ha−1 yr−1 in Sabaju. In Sebungan, net peat carbon losses fell within the range of 48.37 ± 0.16 to 50.86 ± 0.19 Mg CO2−eq ha−1 yr−1. Sabaju and Sebungan peat carbon losses were made up from slightly different proportions of CO2 and CH4. Rh dominated the proportional losses of peat carbon in Sabaju; 92% of the total flux was from Rh, 6% from drain CO2, 1% from soil CH4, 1% from drain CH4, and 0% from stem CH4. In Sebungan, Rh was still the predominant peat carbon loss pathway, but the drain CO2 and CH4 fluxes doubled in contribution compared to Sabaju; 84–85% of plantation carbon losses in Sebungan was made up from Rh, 10–11% was from drain CO2, 4% was from drain CH4, and 0% was from both soil and stem CH4.
Air temperature, soil temperature, WTD and soil moisture controlled rates of Rh. WTD, soil moisture at 0–10 cm, soil moisture at 30–40 cm and soil temperature controlled soil CH4 fluxes. Drain CO2 did not correlate with the environmental variables measured in this study. Drain CH4 was controlled by air temperature. Stem CH4 had significant relationships with relative humidity, WTD, air temperature, soil temperature and soil moisture.
Air and soil temperatures have been shown to control the rate of Rh, both on these plantations and in other studies (Jauhiainen et al., 2012; Farmer, 2013; Hergoualc'h et al., 2017). Temperature has been shown to increase the rate of Rtot and Rh due to increased activation energy for the chemical reactions (Lloyd and Taylor, 1994). Air temperature increased the rate of Rh in both plantations and the model predicted it was at a three times greater rate in Sabaju than in Sebungan. This steeper gradient in Sabaju might explain why Sabaju was only 1.5°C warmer than Sebungan but rates of Rh were twice as high. Rh also increased as soil temperature increased in both plantations—with differences seen in the relationships between the microforms. As soil temperature increased, Rh was predicted to increase in the harvest path and in the cover plants in Sabaju, which also did not always have a closed canopy, exposing the soil directly to the Sun. This relationship with temperature was not seen in the frond piles, potentially due to extra shading, a different microclimate and the interaction of other variables, such as moisture.
Hydrology also controlled rates of Rh, with soil moisture and WTD acting as proxies for redox potential. Increasing soil moisture lowered the rate of Rh, as supported by Farmer (2013), Comeau (2016) and Hergoualc'h et al. (2017). This was seen in every microform in Sebungan, particularly in the frond pile, and in the frond pile and cover plants in Sabaju. Rh increased as soil moisture increased in Sabaju, but the effect size was negligible. WTD had opposite relationships with Rh at both plantations. Sebungan gave the expected relationship with Rh increasing as the WTD lowered. This trend has also been seen by Hergoualc'h et al. (2017). However, Sabaju had the opposite relationship with WTD, with increasing WTD decreasing rates of Rh. The WTD measurements made in Sebungan were deeper and had a wider range than those made in Sabaju. Within the WTD range sampled, the more labile carbon may have already been oxidized in Sabaju, with fresher labile carbon exposed at the deeper WTDs in Sebungan, explaining the variation in relationship with WTD (Hooijer et al., 2012).
Soil CH4 was controlled by soil temperature, soil moisture between 0 and 10 cm, soil moisture between 30 and 40 cm and WTD. Melling et al. (2005b) also found that soil temperature, water filled pore space and WTD controlled soil CH4 fluxes. Here, soil CH4 increased as soil moisture between 0 and 10 cm increased. This result would be expected, as CH4 is more likely to be produced in anoxic conditions. The interaction between soil CH4, soil moisture between 30 and 40 cm, plantation and location was significant. In the different plantations and at different microforms, the expected relationship of soil CH4 increasing as WTD increased and soil moisture between 30 and 40 cm increased was seen, but not in every microform. It would be expected that soil CH4 increased as WTD decreased and soil moisture between 30 and 40 cm increased due to a larger volume of anoxic conditions for methanogens to break the peat down, and a lower volume of oxic conditions for methanotrophs to break down the CH4 (Iiyama et al., 2012; Carlson et al., 2015). Soil moisture at 0–10 cm did not have a significant interaction with soil moisture between 30 and 40 cm or WTD. Furthermore, Manning (2019) found that surface soil moisture correlated with climatic trends and soil moisture between 30 and 40 cm was determined by WTD. We propose that redox potential nearer the surface of the peat is more important than WTD for soil CH4 fluxes, with CH4 oxidized to CO2 regardless of WTD, if the surface of the peat does not inhibit methanotrophs. Finally, soil CH4 increased as soil temperature decreased. This may be due to seasonality and soil temperatures being cooler when the peat was wetter.
Drain CO2 and drain CH4 were controlled by different variables. Drain CO2, produced from DOC, did not have a significant relationship with the environmental variables but did have a significant relationship with drain type, being higher in the smaller field drains than the larger collection drains. DOC was greater in Sebungan than Sabaju during this measurement period and this was associated with the increased WTD at time of measurement (Cook et al., 2018). This same relationship was not observed in this study for drain CO2. The smaller field drains may therefore have a higher drain CO2 flux than the collection drains due to being the first drain that DOC reaches from the soil—after all the field drains feed into the collection drains. Drain CH4 was controlled by the rate of air temperature in both plantations. This was presumably due to the increase in rate of diffusion for the CH4 (Billett and Moore, 2008).
Stem CH4 was controlled by relative humidity, WTD, air temperature, soil temperature and soil moisture. Stem CH4 reduced as relative humidity increased. We hypothesize that this is due to reduced rates of evapotranspiration at higher relative humidities, from a reduced moisture gradient. Evapotraspiration has been shown to control the rates of stem CH4 (Pangala et al., 2014). Slowing the rate of water through the xylem would reduce the speed of transport for dissolved CH4 and thus reduce the rate of stem CH4. Increasing WTD increased the rate of stem CH4. This has been seen in a manipulation experiment by Pangala et al. (2014), who found that if there is soil volume between the WTD and plant roots, CH4 is oxidized before it reaches the roots. Air temperature, soil temperature and soil moisture gave opposite relationships with stem CH4 at each plantation, with stronger relationships in Sebungan where increasing air temperature, soil temperature and soil moisture all increased stem CH4. Increasing temperatures would be expected to increase the rate of methanogenesis, whilst increasing soil moisture would reduce the redox potential, also increasing methanogenesis or transport of stem CH4 to the roots (Pangala et al., 2014). These relationships were negative in Sabaju but had very small effects.
The most effective way to reduce carbon losses from oil palm plantations on peat soil is to reduce the rate of Rh. The strong influence of temperature and WTD on Rh suggest that means of controlling soil surface temperatures and WTD are the best means of mitigating carbon losses from the peat. For example, the impact of temperature could be reduced by providing better coverage of the soil surface—particularly when the plantation canopy has not closed. Here, in Sebungan the canopy was closed, soil and air temperatures were lower and the Rh flux was half the rate of Sabaju. In Sabaju, the canopy was open and air temperature increased the rate of Rh at three times the extent in Sebungan. Covering the soil in Sabaju with frond piles lowered the mean soil temperature (harvest path: 28.6 ± 0.7°C; frond pile: 27.8 ± 0.6°C) and increased mean soil moisture (harvest path: 57.8 ± 0.9%; frond pile 61.6 ± 1.0%); both changes in environmental conditions have been shown to reduce the Rh flux. Jauhiainen et al. (2014) found that Rh could be reduced by 30% if the tropical peat was shaded. Annual Rtot was 30% lower beneath the frond pile in Sabaju than from the harvest path—extending the shade could therefore reduce Sabaju Rh to range between 16.9 ± 1.61 and 17.4 ± 0.82 Mg CO2-C ha−1 yr−1, reducing the plantation net carbon losses to between 69.59 ± 0.62 and 71.75 ± 0.65 Mg CO2−eq ha−1 yr−1.
Raising the water table is also an effective way of suppressing Rh. This was particularly apparent in Sebungan, where the canopy was closed and temperatures were lower. Sebungan had a lower mean WTD than the RSPO recommendations at the time of measurement and thus fresh peat may have been exposed to heterotrophic bacteria, increasing the rate of Rh from Sebungan (Lim et al., 2012; Mishra et al., 2014; Carlson et al., 2015).
Furthermore, temperature control may be more important than WTD control. Sebungan had a lower WTD than Sabaju but Sabaju had higher rates of Rh. It would be expected that Rh decreased with WTD (Carlson et al., 2015). However, the result from Sabaju did not fit the trend. We propose this is due to the open canopy in Sabaju and thus there being no barrier for the Sun to heat up the peat and increase the rate of its decomposition.
CO2 and CH4 fluxes vary spatially and temporally in oil palm plantations on peat soil. Rtot and soil CH4 fluxes were both higher next to the palm than from the “away from palm” soil surface microforms (the bare soil harvest path, beneath frond piles and beneath cover plants). Drain CO2 did not differ significantly from the “away from palm” Rtot fluxes but drain CH4 was significantly greater than the “away from palm” soil CH4 fluxes. CH4 emitted through the palm stems, after being transported through the xylem from the soil, was measured at both plantations. Rtot next to the palm, drain CO2 and drain CH4 showed seasonality. Here Rtot and drain CO2 were higher in the dry season than in the rainy season. Drain CH4 was higher in the rainy season than in the dry season.
Annual CO2 fluxes varied between the two plantations but annual CH4 fluxes did not—and were within the range of CH4 reported from swamp forests in the literature—draining the peat did not reduce the CH4 flux (here 61.02 ± 17.78 kg CH4-C ha−1 yr−1). Rtot from Sabaju and Sebungan were 27.98 ± 0.73 Mg CO2-C ha−1 yr−1 and 16.17 ± 0.81 Mg CO2-C ha−1 yr−1, respectively. The larger flux in Sabaju was due to higher Rh measurements: Rh ranged from 23.89 ± 1.07 to 24.39 ± 1.61 Mg CO2-C ha−1 yr−1 in Sabaju and 11.13 ± 1.69 to 11.81 ± 0.87 Mg CO2-C ha−1 yr−1 in Sebungan.
Plantation carbon losses were dominated by Rh, with drain CO2, soil CH4, drain CH4 and stem CH4 also contributing, in order of proportion. In Sabaju the plantation carbon losses were between 95.58 ± 0.14 and 97.41 ± 0.17 Mg CO2−eq ha−1 yr−1, with 92% of the flux attributed to Rh. In Sebungan, the plantation carbon losses fell within the range of 48.37 ± 0.16 and 50.86 ± 0.19 Mg CO2−eq ha−1 yr−1, with 84–85% of the flux made up from Rh. Therefore, the optimal management strategies to reduce plantation carbon losses are to focus on reducing Rh.
We propose shading the peat and raising the WTD to reduce Rh. In Sabaju, where the canopy was open, air temperature dominated the drivers of Rh, with fluxes measured below the frond piles being 30% lower than fluxes measured from the bare soil harvest path. Temperatures were lower in Sebungan, attributed to a closed canopy, and rates of Rh were also lower. In Sebungan, WTD had a significant effect on rates of Rh. WTD was lower in Sebungan than in Sabaju, (Sabaju: −0.49 m; Sebungan: −0.77 m) providing a greater peat volume for Rh.
FM designed and conducted the study, performed the data analysis, and wrote the manuscript. TH and YT were integrally involved in the study design, data interpretation, and writing the manuscript. LK was involved in the study design, data collection, field support, and data interpretation. TC helped with the mixed model analysis.
This project was funded by the Natural Environmental Research Council, UK (grant code: 1368637) and the Malaysian Palm Oil Board (grant code: R010913000).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank the Director-General of the Malaysian Palm Oil Board for permission to publish this study. This study was part of a MPOB-University of Exeter-University of Aberdeen collaborative research program on tropical peat research. The authors would like to thank MPOB and Sarawak Oil Palm Berhad staff for all of their help and support for this project. We are grateful to Elizabeth Telford, Ham Jonathon, Steward Saging, Xytus Tan, Elisa Rampung, Cecylea Jimmy, Tiara Nales, Lilyen L. Ukat, Lukas Ellbiey, Ahmad Afiq Faris B. Mohd Razali, Mohamad Rizalasri B. Che Hashim, Muhammad Afiz B. Mazlan, Muhammad Syazwe B. Rusli, and Buit Tanyang for their help with the field work, particularly regarding installing WTD pipes and assisting with data collection. We would like to thank Frances Pusch for her help developing stem chamber designs. We would also like to thank Elizabeth Telford and Melanie Chocholek for helping with the GC analysis at the Universities of Aberdeen and St. Andrews, respectively. The authors are grateful to Laura Kruitbos for her logistical help. Finally, FM would like to thank Jodie Hartill for her discussions on methane fluxes from oil palm plantations and encourages the reader to look out for Jodie's publications.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2019.00037/full#supplementary-material
Arai, H., Hadi, A., Darung, U., Limin, S. H., Hatano, R., and Inubushi, K. (2014). A methanotrophic community in a tropical peatland is unaffected by drainage and forest fires in a tropical peat soil. Soil Sci. Plant Nutr. 60, 577–585. doi: 10.1080/00380768.2014.922034
Arifin, A., Atmojo, S. W., Setyanto, P., and Dewi, W. S. (2015). Temperature effect investigation toward peat surface CO2 emissions by planting leguminous cover crops in oil palm plantations in West Kalimantan. J. Agric. Sci. Technol. B 5, 170–183. doi: 10.17265/2161-6264/2015.03B.002
Bajželj, B., Richards, K. S., Allwood, J. M., Smith, P., Dennis, J. S., Curmi, E., et al. (2014). Importance of food-demand management for climate mitigation. Nat. Clim. Change 4:924. doi: 10.1038/nclimate2353
Billett, M. F., and Moore, T. R. (2008). Supersaturation and evasion of CO2 and CH4 in surface waters at Mer Bleue peatland, Canada. Hydrol. Process. 22, 2044–2054. doi: 10.1002/hyp.6805
Carlson, K. M., Goodman, L. K., and May-Tobin, C. C. (2015). Modeling relationships between water table depth and peat soil carbon loss in Southeast Asian plantations. Environ. Res. Lett. 10:074006. doi: 10.1088/1748-9326/10/7/074006
Cobb, A. R., Hoyt, A. M., Gandois, L., Eri, J., Dommain, R., Salim, K. A., et al. (2017). How temporal patterns in rainfall determine the geomorphology and carbon fluxes of tropical peatlands. Proc. Natl. Acad. Sci. U.S.A. 26:201701090. doi: 10.1073/pnas.1701090114
Comeau, L. P. (2016). Carbon dioxide fluxes and soil organic matter characteristics on an intact peat swamp forest, a drained and logged forest on peat, and a peatland oil palm plantation in Jambi, Sumatra, Indonesia (PhD Thesis), University of Aberdeen, United Kingdom.
Comeau, L. P., Hergoualc'h, K., Hartill, J., Smith, J., Verchot, L. V., Peak, D., et al. (2016). How do the heterotrophic and the total soil respiration of an oil palm plantation on peat respond to nitrogen fertilizer application? Geoderma 268, 41–51. doi: 10.1016/j.geoderma.2016.01.016
Cook, S., Whelan, M. J., Evans, C. D., Gauci, V., Peacock, M., Garnett, M. H., et al. (2018). Fluvial organic carbon fluxes from oil palm plantations on tropical peatland. Biogeosci. Discuss. 10, 7435–7450. doi: 10.5194/bg-15-7435-2018
Cory, R. M., Ward, C. P., Crump, B. C., and Kling, G. W. (2014). Sunlight controls water column processing of carbon in arctic fresh waters. Science 345, 925–928. doi: 10.1126/science.1253119
Couwenberg, J., Dommain, R., and Joosten, H. (2010). Greenhouse gas fluxes from tropical peatlands in south-east Asia. Glob. Change Biol. 16, 1715–1732. doi: 10.1111/j.1365-2486.2009.02016.x
Dariah, A., Setiari Marwanto, S., and Agus, F. (2014). Root- and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strat. Glob. Change 19, 831–843. doi: 10.1007/s11027-013-9515-6
Dommain, R., Cobb, A. R., Joosten, H., Glaser, P. H., Chua, A. F., Gandois, L., et al. (2015). Forest dynamics and tip-up pools drive pulses of high carbon accumulation rates in a tropical peat dome in Borneo (Southeast Asia). J. Geophys. Res. Biogeosci. 120, 617–640. doi: 10.1002/2014JG002796
Evans, C. D., Renou-Wilson, F., and Strack, M. (2016). The role of waterborne carbon in the greenhouse gas balance of drained and re-wetted peatlands. Aquat. Sci. 78, 573–590. doi: 10.1007/s00027-015-0447-y
Evers, S., Yule, C. M., Padfield, R., O'Reilly, P., and Varkkey, H. (2017). Keep wetlands wet: the myth of sustainable development of tropical peatlands–implications for policies and management. Glob. Change Biol. 23, 534–549. doi: 10.1111/gcb.13422
FAO (2006). World Reference Base for Soil Resources 2006: A Framework for International Classification, Correlation and Communication. Rome. Available online at: http://www.fao.org/ag/agl/agll/wrb/doc/wrb2006final.pdf
FAOSTAT (2019). FAOSTAT Database. Rome: FAO. Retrieved from: http://faostat3.fao.org/home/E (accessed May 15, 2019).
Farmer, J. (2013). Measuring and modeling soil carbon and carbon dioxide emissions from Indonesian peatlands under land use change (PhD Thesis), University of Aberdeen, United Kingdom.
Fowler, D., Nemitz, E., Misztal, P., Di Marco, C., Skiba, U., Ryder, J., et al. (2011). Effects of land use on surface–atmosphere exchanges of trace gases and energy in Borneo: comparing fluxes over oil palm plantations and a rainforest. Philos. Trans. R. Soc. B Biol. Sci. 366, 3196–3209. doi: 10.1098/rstb.2011.0055
Furukawa, Y., Inubushi, K., Ali, M., Itang, A. M., and Tsuruta, H. (2005). Effect of changing groundwater levels caused by land-use changes on greenhouse gas fluxes from tropical peat lands. Nutr. Cycl. Agroecosyst. 71, 81–91. doi: 10.1007/s10705-004-5286-5
Fuss, S., Jones, C. D., Kraxner, F., Peters, G. P., Smith, P., Tavoni, M., et al. (2016). Research priorities for negative emissions. Environ. Res. Lett. 11:115007. doi: 10.1088/1748-9326/11/11/115007
Giraudoux, P., Antonietti, J. P., Beale, C., Pleydell, D., and Treglia, M. (2018). pgirmess. R package version 1.6.9. Available online at: https://cran.r-project.org/web/packages/pgirmess/index.html
Girkin, N. T., Turner, B. L., Ostle, N., Craigon, J., and Sjögersten, S. (2018a). Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol. Biochem. 117, 48–55. doi: 10.1016/j.soilbio.2017.11.008
Girkin, N. T., Turner, B. L., Ostle, N., and Sjögersten, S. (2018b). Composition and concentration of root exudate analogues regulate greenhouse gas fluxes from tropical peat. Soil Biol. Biochem. 127, 280–285. doi: 10.1016/j.soilbio.2018.09.033
Hadi, A., Inubushi, K., Furukawa, Y., Purnomo, E., Rasmadi, M., and Tsuruta, H. (2005). Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutr. Cycl. Agroecosyst. 71, 73–80. doi: 10.1007/s10705-004-0380-2
Hergoualc'h, K., Hendry, D. T., Murdiyarso, D., and Verchot, L. V. (2017). Total and heterotrophic soil respiration in a swamp forest and oil palm plantations on peat in Central Kalimantan, Indonesia. Biogeochemistry 135, 203–220. doi: 10.1007/s10533-017-0363-4
Hirano, T., Jauhiainen, J., Inoue, T., and Takahashi, H. (2009). Controls on the carbon balance of tropical peatlands. Ecosystems 12, 873–887. doi: 10.1007/s10021-008-9209-1
Hirano, T., Segah, H., Kusin, K., Limin, S., Takahashi, H., and Osaki, M. (2012). Effects of disturbances on the carbon balance of tropical peat swamp forests. Glob. Change Biol. 18, 3410–3422. doi: 10.1111/j.1365-2486.2012.02793.x
Hodgkins, S. B., Richardson, C. J., Dommain, R., Wang, H., Glaser, P. H., Verbeke, B., et al. (2018). Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 9:3640. doi: 10.1038/s41467-018-06050-2
Hooijer, A., Page, S., Jauhiainen, J., Lee, W. A., Lu, X. X., Idris, A., et al. (2012). Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 9, 1053–1071. doi: 10.5194/bg-9-1053-2012
Husnain, H., Wigena, I. G. P., Dariah, A., Marwanto, S., Setyanto, P., and Agus, F. (2014). CO2 emissions from tropical drained peat in Sumatra, Indonesia. Mitig. Adapt. Strat. Glob. Change 19, 1–18. doi: 10.1007/s11027-014-9550-y
Iiyama, I., Osawa, K., and Nagai, T. (2012). A seasonal behavior of surface soil moisture condition in a reclaimed tropical peatland. Soil Sci. Plant Nutr. 58, 543–552. doi: 10.1080/00380768.2012.723222
Inubushi, K., Furukawa, Y., Hadi, A., Purnomo, E., and Tsuruta, H. (2003). Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 52, 603–608. doi: 10.1016/S.0045-6535(03)00242-X
Inubushi, K., Hadi, A., Okazaki, M., and Yonebayashi, K. (1998). Effect of converting wetland forest to sago palm plantations on methane gas flux and organic carbon dynamics in tropical peat soil. Hydrol. Process. 12, 2073–2080. doi: 10.1002/(SICI)1099-1085(19981030)12:13/14<2073::AID-HYP720>3.0.CO;2-K
IPCC (2014). Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands. Geneva: Intergovernmental Panel on Climate Change (IPCC).
IPCC (2018). Global Warming of 1.5°C: An IPCC Special Report on the Impacts of Global Warming of 1.5°C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Geneva: Intergovernmental Panel on Climate Change.
Ishikura, K., Hirano, T., Okimoto, Y., Hirata, R., Kiew, F., Melling, L., et al. (2018). Soil carbon dioxide emissions due to oxidative peat decomposition in an oil palm plantation on tropical peat. Agric. Ecosyst. Environ. 254, 202–212. doi: 10.1016/j.agee.2017.11.025
Jauhiainen, J., Hooijer, A., and Page, S. E. (2012). Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 9, 617–630. doi: 10.5194/bg-9-617-2012
Jauhiainen, J., Kerojoki, O., Silvennoinen, H., Limin, S., and Vasander, H. (2014). Heterotrophic respiration in drained tropical peat is greatly affected by temperature–a passive ecosystem cooling experiment. Environ. Res. Lett. 9:105013. doi: 10.1088/1748-9326/9/10/105013
Jauhiainen, J., Limin, S., Silvennoinen, H., and Vasander, H. (2008). Carbon dioxide and methane fluxes in drained tropical peat before and after hydrological restoration. Ecology 89, 3503–3514. doi: 10.1890/07-2038.1
Jauhiainen, J., and Silvennoinen, H. (2012). Diffusion GHG fluxes at tropical peatland drainage canal water surfaces. Suo 63, 93–105.
Jauhiainen, J., Takahashi, H., Heikkinen, J. E. P., Martikainen, P. J., and Vasander, H. (2005). Carbon fluxes from a tropical peat swamp forest floor. Glob. Change Biol. 11, 1788–1797. doi: 10.1111/j.1365-2486.2005.001031.x
Kent, M. S. (2018). Greenhouse gas emissions from channels draining intact and degraded tropical peat swamp forest (PhD thesis), Open University, Milton Keynes, United Kingdom.
Lai, D. Y. F. (2009). Methane dynamics in northern peatlands: a review. Pedosphere 19, 409–421. doi: 10.1016/S1002-0160(09)00003-4
Lim, K. H., Lim, S. S., Parish, F., and Suharto, R. (2012). RSPO Manual on Best Management Practices (BMPs) for Existing Oil Palm Cultivation on Peat. Kuala Lumpur: RSPO.
Livingston, G. P., and Hutchinson, G. L. (1995). “Enclosure-based measurement of trace gas exchange: applications and sources of error,” in Methods in Ecology. Biogenic Trace Gases: Measuring Emissions From Soil and Water, eds P. A. Matson, and R. C. Harriss (Malden, MA: Blackwell Science, 14–51.
Lloyd, J., and Taylor, J. A. (1994). On the temperature dependence of soil respiration. Funct. Ecol. 8, 315–323. doi: 10.2307/2389824
Logue, J. B., Stedmon, C. A., Kellerman, A. M., Nielsen, N. J., Andersson, A. F., Laudon, H., et al. (2016). Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 10:533. doi: 10.1038/ismej.2015.131
Manning, F. C. (2019). Carbon dynamics in oil palm agro-ecosystems (PhD Thesis), University of Aberdeen, United Kingdom.
Matysek, M., Evers, S., Samuel, M. K., and Sjogersten, S. (2018). High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetlands Ecol. Manage. 26, 415–424. doi: 10.1007/s11273-017-9583-6
McClain, M. E., Boyer, E. W., Dent, C. L., Gergel, S. E., Grimm, N. B., Groffman, P. M., et al. (2003). Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6, 301–312. doi: 10.1007/s10021-003-0161-9
Meijaard, E., Garcia-Ulloa, J., Sheil, D., Wich, S. A., Carlson, K. M., Juffe-Bignoli, D., et al. (2018). Oil Palmand Biodiversity: A Situation Analysis. Gland: IUCN Oil Palm Task Force.
Melling, L., Chaddy, A., Goh, K. J., and Hatano, R. (2013). Soil CO2 fluxes from different ages of oil palm in tropical peatland of Sarawak, Malaysia as influenced by environmental and soil properties. Acta Hortic. 982, 25–36. doi: 10.17660/ActaHortic.2013.982.2
Melling, L., Hatano, R., and Goh, K. J. (2005a). Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B 57, 1–11. doi: 10.3402/tellusb.v57i1.16772
Melling, L., Hatano, R., and Goh, K. J. (2005b). Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol. Biochem. 37, 1445–1453. doi: 10.1016/j.soilbio.2005.01.001
MET Malaysia (2017). Official Website of the Malaysian Meteorological Department. Available online at: http://www.met.gov.my/?lang=en
Miettinen, J., Hooijer, A., Vernimmen, R., Liew, S. C., and Page, S. E. (2017). From carbon sink to carbon source: extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 12:024014. doi: 10.1088/1748-9326/aa5b6f
Miettinen, J., Shi, C., and Liew, S. C. (2016). Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 6, 67–78. doi: 10.1016/j.gecco.2016.02.004
Minkkinen, K., and Laine, J. (2006). Vegetation heterogeneity and ditches create spatial variability in methane fluxes from peatlands drained for forestry. Plant Soil 285, 289–304. doi: 10.1007/s11104-006-9016-4
Mishra, S., Lee, W. A., Reuben, S., Sudiana, I. M., Idris, A., and Swarup, S. (2014). Microbial and metabolic profiling reveal strong influence of water table and land-use patterns on classification of degraded tropical peatlands. Biogeosciences 11, 1727–1741. doi: 10.5194/bg-11-1727-2014
Müller, D., Warneke, T., Rixen, T., Müller, M., Jamahari, S., Denis, N., et al. (2015). Lateral carbon fluxes and CO2 outgassing from a tropical peat-draining river. Biogeosciences 12, 5967–5979. doi: 10.5194/bg-12-5967-2015
Page, S. E., Rieley, J. O., and Banks, C. J. (2011). Global and regional importance of the tropical peatland carbon pool. Glob. Change Biol. 17, 798–818. doi: 10.1111/j.1365-2486.2010.02279.x
Pangala, S. R., Enrich-Prast, A., Basso, L. S., Peixoto, R. B., Bastviken, D., Hornibrook, E. R., et al. (2017). Large emissions from floodplain trees close the Amazon methane budget. Nature 552:230. doi: 10.1038/nature24639
Pangala, S. R., Gowing, D. J., Hornibrook, E. R. C., and Gauci, V. (2014). Controls on methane emissions from Alnus glutinosa saplings. New Phytol. 201, 887–896. doi: 10.1111/nph.12561
Pangala, S. R., Moore, S., Hornibrook, E. R. C., and Gauci, V. (2013). Trees are major conduits for methane egress from tropical forested wetlands. New Phytol. 197, 524–531. doi: 10.1111/nph.12031
Pedersen, A. R., Petersen, S. O., and Schelde, K. (2010). A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers. Eur. J. Soil Sci. 61, 888–902. doi: 10.1111/j.1365-2389.2010.01291.x
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and R Core Team (2017). nlme: Linear and Nonlinear Mixed Effect Models. R package version 2.1-131. Available online at: https://cran.r-project.org/web/packages/nlme/index.html
Raghoebarsing, A. A., Smolders, A. J., Schmid, M. C., Rijpstra, W. I., Wolters-Arts, M., Derksen, J., et al. (2005). Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153. doi: 10.1038/nature03802
Schrier-Uijl, A. P., Kroon, P. S., Leffelaar, P. A., van Huissteden, J. C., Berendse, F., and Veenendaal, E. M. (2010). Methane emissions in two drained peat agro-ecosystems with high and low agricultural intensity. Plant Soil 329, 509–520. doi: 10.1007/s11104-009-0180-1
Siegenthaler, A., Welch, B., Pangala, S. R., Peacock, M., and Gauci, V. (2016). Technical Note: Semi-rigid chambers for methane gas flux measurements on tree stems. Biogeosciences 13, 1197–1207. doi: 10.5194/bg-13-1197-2016
Silvius, M., and Diemont, H. (2007). Deforestation and degradation of peatlands. Peatlands Int. 2, 32–34.
Sjögersten, S., Black, C. R., Evers, S., Hoyos-Santillan, J., Wright, E. L., and Turner, B. L. (2014). Tropical wetlands: a missing link in the global carbon cycle? Glob. Biogeochem. Cycles 28, 1371–1386. doi: 10.1002/2014GB004844
Smith, P., Bustamante, M., Ahammad, H., Clark, H., Dong, H., Elsiddig, E. A., et al. (2014). “Agriculture, Forestry and Other Land Use (AFOLU),” in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds O. Edenhofer, R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlomer, C. von Stechow, T. Zwickel, and J. C. Minx (Cambridge; New York, NY: Cambridge University Press).
Sundh, I., Nilsson, M., Granberg, G., and Svensson, B. H. (1994). Depth distribution of microbial production and oxidation of methane in northern boreal peatlands. Microb. Ecol. 27, 253–265. doi: 10.1007/BF00182409
Teh, Y. A., Silver, W. L., Sonnentag, O., Detto, M., Kelly, M., and Baldocchi, D. D. (2011). Large greenhouse gas emissions from a temperate peatland pasture. Ecosystems 14, 311–325. doi: 10.1007/s10021-011-9411-4
Tonks, A. J., Aplin, P., Beriro, D. J., Cooper, H., Evers, S., Vane, C. H., et al. (2017). Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 289, 36–45. doi: 10.1016/j.geoderma.2016.11.018
Tubiello, F. N., Salvatore, M., Ferrara, A. F., House, J., Federici, S., Rossi, S., et al. (2015). The contribution of agriculture, forestry and other land use activities to global warming, 1990–2012. Glob. Change Biol. 21, 2655–2660. doi: 10.1111/gcb.12865
USDA (2019). Oilseeds: World Markets and Trade. United States Department of Agriculture Foreign Agriculture Service. Available online at: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed May 15, 2019).
Varner, R. K., Keller, M., Robertson, J. R., Dias, J. D., Silva, H., Crill, P. M., et al. (2003). Experimentally induced root mortality increased nitrous oxide emission from tropical forest soils. Geophys. Res. Lett. 30, 1–4. doi: 10.1029/2002GL016164
Wakhid, N., Hirano, T., Okimoto, Y., Nurzakiah, S., and Nursyamsi, D. (2017). Soil carbon dioxide emissions from a rubber plantation on tropical peat. Sci. Total Environ. 581, 857–865. doi: 10.1016/j.scitotenv.2017.01.035
Warren, M., Frolking, S., Dal, Z., and Kurnianto, S. (2017). Impacts of land use, restoration, and climate change on tropical peat carbon stocks in the twenty-first century: implications for climate mitigation. Mitig. Adapt. Strat. Glob. Change 22, 1041–1061. doi: 10.1007/s11027-016-9712-1
Wijedasa, L. S., Jauhiainen, J., Könönen, M., Lampela, M., Vasander, H., Leblanc, M. C., et al. (2017). Denial of long-term issues with agriculture on tropical peatlands will have devastating consequences. Glob. Change Biol. 23, 977–982. doi: 10.1111/gcb.13516
Keywords: oil palm, peat, peat oxidation, heterotrophic respiration, methane
Citation: Manning FC, Kho LK, Hill TC, Cornulier T and Teh YA (2019) Carbon Emissions From Oil Palm Plantations on Peat Soil. Front. For. Glob. Change 2:37. doi: 10.3389/ffgc.2019.00037
Received: 10 February 2019; Accepted: 24 June 2019;
Published: 16 August 2019.
Edited by:
Eleanor Slade, Nanyang Technological University, SingaporeReviewed by:
Ute Skiba, Centre for Ecology and Hydrology (CEH), United KingdomCopyright © 2019 Manning, Kho, Hill, Cornulier and Teh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frances Claire Manning, ZnJhbmNlcy5jbGFpcmUubWFubmluZ0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.