- 1Department of Geosciences, Boise State University, Boise, ID, United States
- 2Institute for Geosciences, Johannes Gutenberg University Mainz, Mainz, Germany
- 3Department of Biology, Zoology and Functional Morphology of Vertebrates, Kiel University, Kiel, Germany
- 4Vetsuisse Faculty, University of Zurich, Zurich, Switzerland
Hydrogen and oxygen isotope ratios in proteinaceous tissues have been used for some time in migratory, ecological, and archaeological studies. While the result of isotopic variation in drinking water and diet has been investigated with controlled feeding experiments and studies in the wild, there are few controlled feeding studies that manipulate the diet components and diet type, and this across different taxa. In this experiment, the diet fed to rats, guinea pigs, and quail varied from plant-based to insect-based and meat-based pelleted diets. We report the diet to tissue offsets for δ2H (denoted Δδ2H) and δ18O (Δδ18O) of tissue-bound organic matter in two tissue types: muscle and dentine collagen. The diet to tissue offset varies by diet type in muscle of all three species, by up to 16 ‰ (Δδ2H) and 2 ‰ (Δδ18O). In dentine collagen, a range of ~20 ‰ in Δδ2H and ~1.5 ‰ in Δδ18O are observed across diets, though in a smaller number of samples. Additionally, we note large variation in Δδ2H and Δδ18O by tissue type (δ2H = ~60 ‰, δ18O = ~3–4 ‰) and more moderate differences by species (up to δ2H = 7.4 ‰, δ18O = 1.5 ‰). The difference in consumer tissue Δδ2H and Δδ18O by diet type is important to consider as a source of isotopic variability for some studies such as migratory research or diet or drinking water reconstructions and (palaeo-)climate inferences drawn from them, particularly in species that may vary their dietary habits.
1 Introduction
Hydrogen isotopes in animal organic tissues have been exploited successfully to trace migration (a large literature; see the volume Hobson and Wassenaar, 2019) and are being investigated for ecological applications (reviewed by Vander Zanden et al., 2016) and palaeoenvironmental reconstruction (e.g., Gröcke et al., 2017; Reynard et al., 2020). Similarly, efforts are underway to include oxygen isotopes in organic matrices in these types of analyses (Kirsanow et al., 2008; Ehleringer et al., 2008; Koehler et al., 2019). The isotopic relationship between inputs (diet, water, and inspired O2; Longinelli, 1984; Kohn, 1996; Feng et al., 2022, 2024) and tissues is key to understanding tissue isotopic data and the limits of interpretation.
The literature on diet to tissue H and O isotope differences in organic tissues is modest, consisting of both controlled feeding experiments and observational studies. Most of the controlled studies vary the drinking water isotopic input to study its contribution to tissues; variations of the diet are fewer in number and manipulate the macronutrient proportions (Hobson et al., 1999) and/or the isotopic composition of diet components (Hobson et al., 1999; Wolf et al., 2012; Newsome et al., 2017; Topalov et al., 2019). It is not known how dietary habits (e.g., herbivory, omnivory) modulate the isotopic relationship from diet to tissue. Any isotopic variations between animals consuming different diet types are important to consider in interpretations based on underlying drinking water variation (e.g., migration studies) and to advance applications such as dietary reconstruction with tissue H and O isotopes.
In the controlled feeding study we report here, we hold the drinking water input isotopically constant and examine the H and O isotope relationship between diet and tissues on three different diet types: herbivorous, omnivorous, and insectivorous diets. Each of these diets was supplied to three different model animals (rats, guinea pigs, quail), and we analyzed muscle in all taxa and dentine collagen in rats and guinea pigs.
2 Materials and methods
The feeding experiments were performed at the Vetsuisse Faculty, University of Zurich and are the same as described thoroughly in Weber et al. (2020; Sr isotopes in tooth enamel) and Leichliter et al. (2021; nitrogen isotopes in tooth enamel). Tissues from three species were investigated: muscle from rats, guinea pigs, and quail; dentine collagen from incisors of rats and guinea pigs. We also used some quail feathers to supplement the other tissues as part of a complementary methodological study of δ2H measurements in proteinaceous tissues and animal diets (Supplementary Information).
The animals were adult female WISTAR (RjHan : WI) rats (Rattus norvegicus forma domestica), adult female Dunkin Hartley (HsdDhl : DH) guinea pigs (Cavia porcellus), and quail (Coturnix japonica). After an acclimatization period of five days with still available supplier food, the animals were held on one of three experimental diets for 54 days, after which they were euthanized and tissues collected. Starting and final body weights, respectively, were 198 ± 17 g and 245 ± 17 g for rats, 253 ± 23 g and 290 ± 24 g for quail, and 401 ± 16 g and 577 ± 104 g for guinea pigs (Supplementary Table 1). After enzymatic maceration of the skulls at 55°C, the rootward portion (i.e., last mineralized section) of rat and guinea pig lower incisors were sampled, resulting in segments ~5 mm long. Rat and guinea pig incisors are ever-growing teeth and should be expected to reflect the experimental diet and approximately 17–25 days’ growth (Law et al., 2003; Hillson, 2005; Müller et al., 2015). The Swiss Cantonal Animal Care and Use Committee, Zurich approved the experiment, licence N° ZH135/16.
The different pelleted diets were custom-made for the feeding experiments and comprise a) plant-based, with 56 wt% lucerne (hereafter plant); b) insect-based, with 26 wt% black soldier fly larvae insect protein meal (hereafter insect); c) meat-based, with 25 wt% lamb meat (hereafter meat). The balance of the diets was plant-based, including wheat- and oat-meal (Table 1). The diets were formulated to be isonitrogenous but not controlled for total hydrogen and oxygen content (Table 1; Leichliter et al., 2021); however, the experimental diets were nevertheless similar to each other in total hydrogen amount (5.9–6.2% H in whole diets, 5.5–5.6% H in non-lipid diets) and oxygen amount (37.7–38.4% O in whole diets, 36.9–40.2% O in non-lipid diets, Supplementary Table 3). Animals consumed food and water ad libitum. The diets from the animal suppliers were also analyzed (Supplementary Table 6). Zurich tap water was supplied to all animals (δ2H = -82 ‰, δ18O = -11.6 ‰) using nipple drinkers (reducing evaporation), with the exception of a subset of quail who were supplied 18O-enriched water (δ18O = 5.9 ± 3.5 ‰, quail tissue results in Supplementary Table 2).
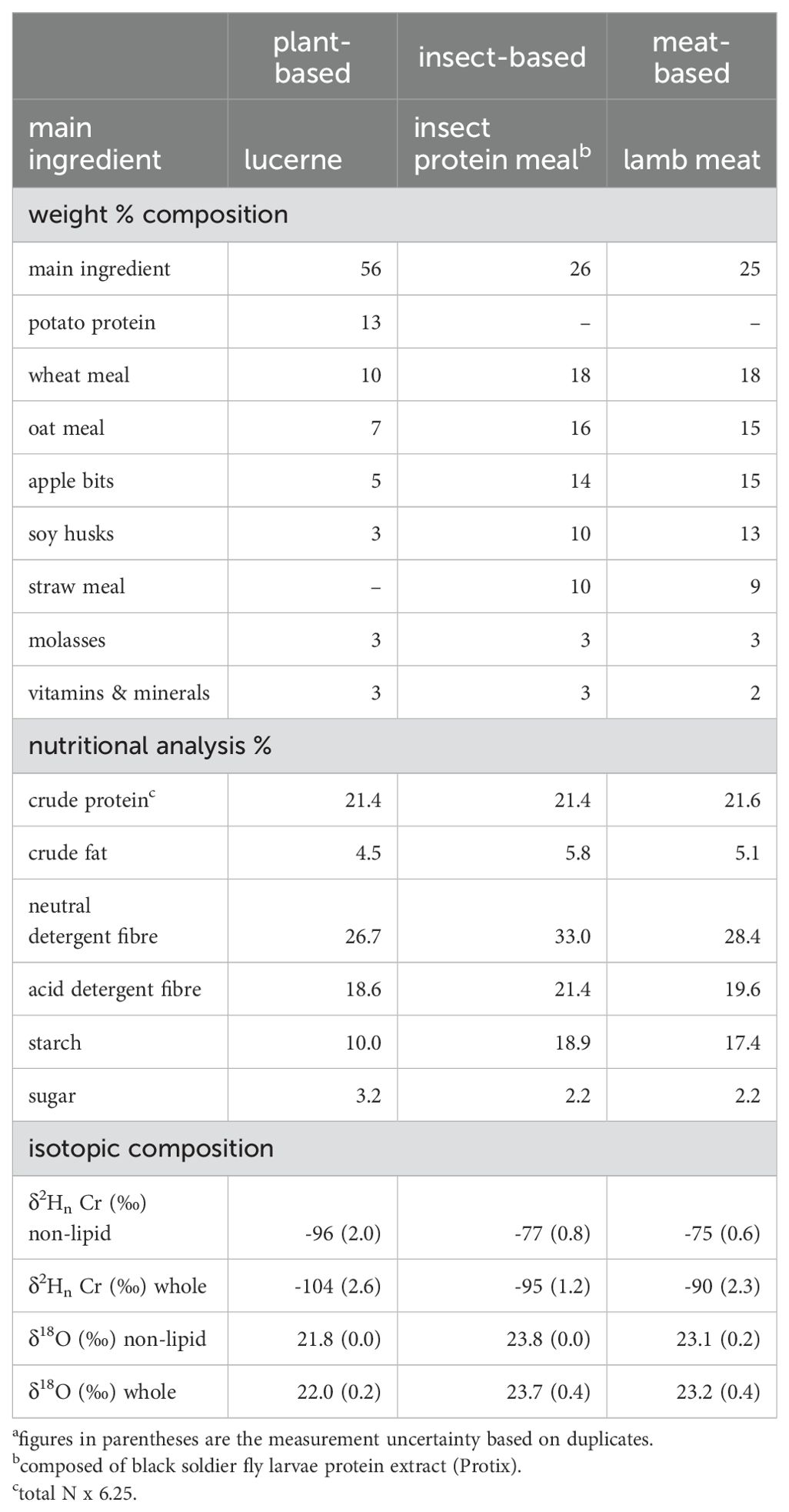
Table 1. Diet main ingredients, composition, and isotopic compositiona.
Tissue sample preparation and isotope ratio mass spectrometry were performed at Boise State University, in Boise, Idaho, USA. Muscle and diet samples were solvent-treated to remove lipids with 2:1 (v/v) chloroform:methanol for three sequential 24 hour treatments (Hobson et al., 1999; Soto et al., 2013; Newsome et al., 2017). After each soak the supernatant was pipetted off and refreshed. After the final 24 hour soak, the samples were given a brief final rinse in 2:1 chloroform:methanol and allowed to air dry. Tests showed minimal H and small and consistent O isotopic differences between petroleum ether (also used as a solvent for lipid removal, Wolf et al., 2012) and chloroform:methanol as a solvent on test rat and beef muscle samples (Supplementary Table 7). The diets were also analyzed without solvent-extraction (whole diet). Tooth segments (~ 5 mm long) were demineralized in 0.5 M EDTA over the course of several days, rinsed 8-10 times with deionized water in 2 mL tubes, and freeze dried, resulting in dentine collagen (Tuross, 2012). Feathers (for the methodological tests) were soaked overnight in 2:1 chloroform:methanol, rinsed in fresh 2:1 chloroform:methanol, and allowed to air dry.
Samples were prepared and analyzed by two methods: one, determination of δ2H of nonexchangeable hydrogen with thermal conversion using a chromium-packed reactor (Reynard and Tuross, 2016; Reynard et al., 2019); two, determination of δ18O with thermal conversion with a glassy carbon packed reactor. The latter technique also provided a second different δ2H determination (unexchanged with a glassy carbon packed reactor). The chromium-powder reactor was modified to include 7 cm of chromium powder, rather than 3 cm as before, and correspondingly fewer glassy carbon chips to maintain the same height of total reactor filling (Reynard et al., 2019).
For δ2H of nonexchangeable H (δ2Hn), following Meier-Augenstein et al. (2011), ~300 μg sample aliquots of muscle, dentine collagen, or diet were packed into silver capsules and folded loosely. Each tray of samples was placed in a glass desiccator with a ground glass joint sealed with vacuum grease, along with a beaker of 50 ml of water of known H isotope composition (δ2HwaterA = 155.2 ‰). Each sample was replicated in another tray and exchanged with a water of a second different known isotopic composition (δ2HwaterB = -224.7 ‰). After 4 days’ equilibration, the sample trays were quickly moved to a plastic vacuum desiccator and left under vacuum for 7 days, after which they were rapidly transferred to a zero-blank autosampler (Costech) and analyzed with the Cr-packed reactor configuration.
Samples were pyrolyzed at 1450°C (with glassy carbon reactor) or 1200°C (with Cr-packed reactor) in a Thermal Conversion Elemental Analyzer (TC/EA, Thermo Scientific). The resultant gases were separated with a 1.8 m long 5 Å molecular sieve gas chromatograph and then analyzed with a Delta V Plus mass spectrometer (Thermo Scientific). δ2H and δ18O values were normalized on the VSMOW-SLAP scale, using aliquots of VSMOW and SLAP in silver tubes (United States Geological Survey, Reston, VA) in each run. We estimate uncertainties of ±0.4 ‰ (1 SD) for δ18O and ±3 ‰ (1 SD) for δ2Hn (Cr-packed) based on long-term reproducibility data for the former and replicates of standard materials exchanged with water and analyzed in the same manner as the samples for the latter.
We computed δ2Hn as follows (Meier-Augenstein et al., 2011):
where δ2HA and δ2HB are δ2H values of sample exchanged with water A or B, respectively; δ2HwaterA and δ2HwaterB are the δ2H values of water A or B, respectively; and f is the fraction of H that is exchangeable under these experimental conditions.
We compute the diet-tissue isotopic offsets as Δδ2H = δ2Htissue – δ2Hdiet and Δδ18O = δ18Otissue – δ18Odiet.
We also computed a ‘Cr-equivalent’ δ2Hn value for four dentine collagen samples where only the glassy carbon reactor result was available, using the mean offset between δ2Hn-Cr and δ2H-glassyC of other dentine collagen samples in this data set, resulting in δ2Hn-Cr = δ2H-glassyC +10.4 ‰. This offset agrees with the result previously obtained from collagen δ2Hn-Cr = δ2H-glassyC +10.1 ‰ (Reynard et al., 2019). The δ2Hn-Cr and δ2H-glassyC relationship for all sample types analyzed here is given in the Supplementary Material.
Statistical analysis was performed with the program R and the ‘stats’ statistical package, using standard methods including analysis of variance (ANOVA) and Tukey’s Honest Significant Differences (Tukey’s HSD) for multiple comparisons (R Core Team, 2023). Tukey’s HSD test can be used with groups of different sample size (Quinn and Keogh, 2002).
3 Results
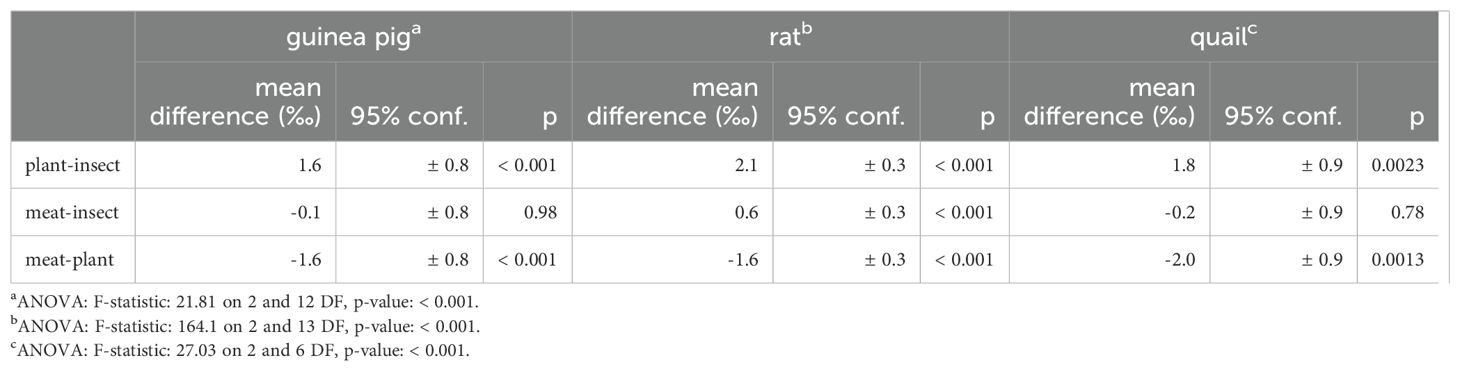
3.1 Isotopic differences by diet: muscle
Hydrogen and oxygen isotope values in muscle are grouped by diet treatment (Figures 1, 2). Diet-tissue offsets (Δδ2H and Δδ18O) in muscle vary by diet in a consistent manner in all three species (Figures 3, 4; Table 2). Δδ2H is smallest for plant, mid-sized for insect, and largest for the meat diets. The difference between the diet groups is in the range of Δδ2H = 4−16 ‰ (Table 3). Similarly, Δδ18O is smallest for the plant diet and a greater and overlapping difference for the insect and meat diets, for all three animal groups (excluding 18O-enriched treatment quail), with differences of ~1.2−2.0 ‰ (Figure 4, Table 4). The eight quail given 18O-enriched drinking water have higher δ18O in muscle than the quail consuming regular drinking water (12.9−13.4 ‰ vs. 8.1−9.7 ‰, respectively, Supplementary Table 2), consistent with the quail incorporating the drinking water and experimental new diets into the muscle tissue.

Figure 1. Tissue δ2Hn in muscle and dentine collagen of guinea pigs, rats and quails (the latter muscle only) fed different pelleted plant-based (green squares), insect-based (red circles), and meat-based (blue diamonds) diets (see Table 1 for details). The error bar shows the estimated measurement uncertainty of ± 3 ‰ (1 SD). For comparison the diet (lipid and non-lipid extracted) and water δ2H values are shown by the bars at the right.
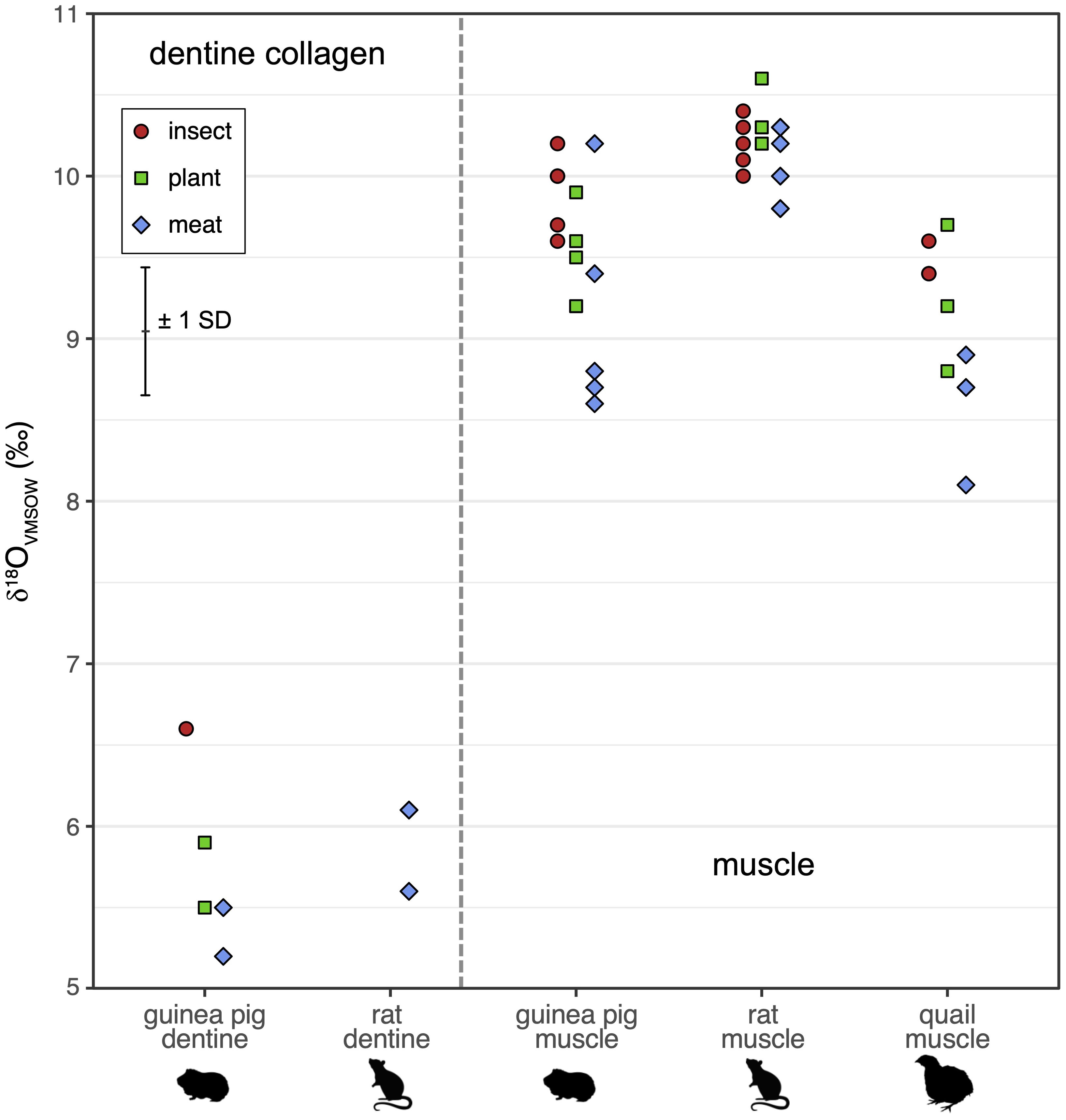
Figure 2. Tissue δ18O in muscle and dentine collagen of animals fed on plant-based (green squares), insect-based (red circles), and meat-based (blue diamonds) diets, in rats, guinea pigs, and quail (the latter muscle only). The error bar shows the estimated measurement uncertainty of ± 0.4 ‰ (1 SD). The diet and water δ18O values are outside the range of the figure (diets ~22−24 ‰, water -11.6 ‰, see Table 1).

Figure 3. Tissue Δδ2Hn (non-lipid diet) in muscle and dentine collagen of animals fed with plant-based (green squares), insect-based (red circles), and meat-based (blue diamonds) diets, in rats, guinea pigs, and quail (the latter muscle only).
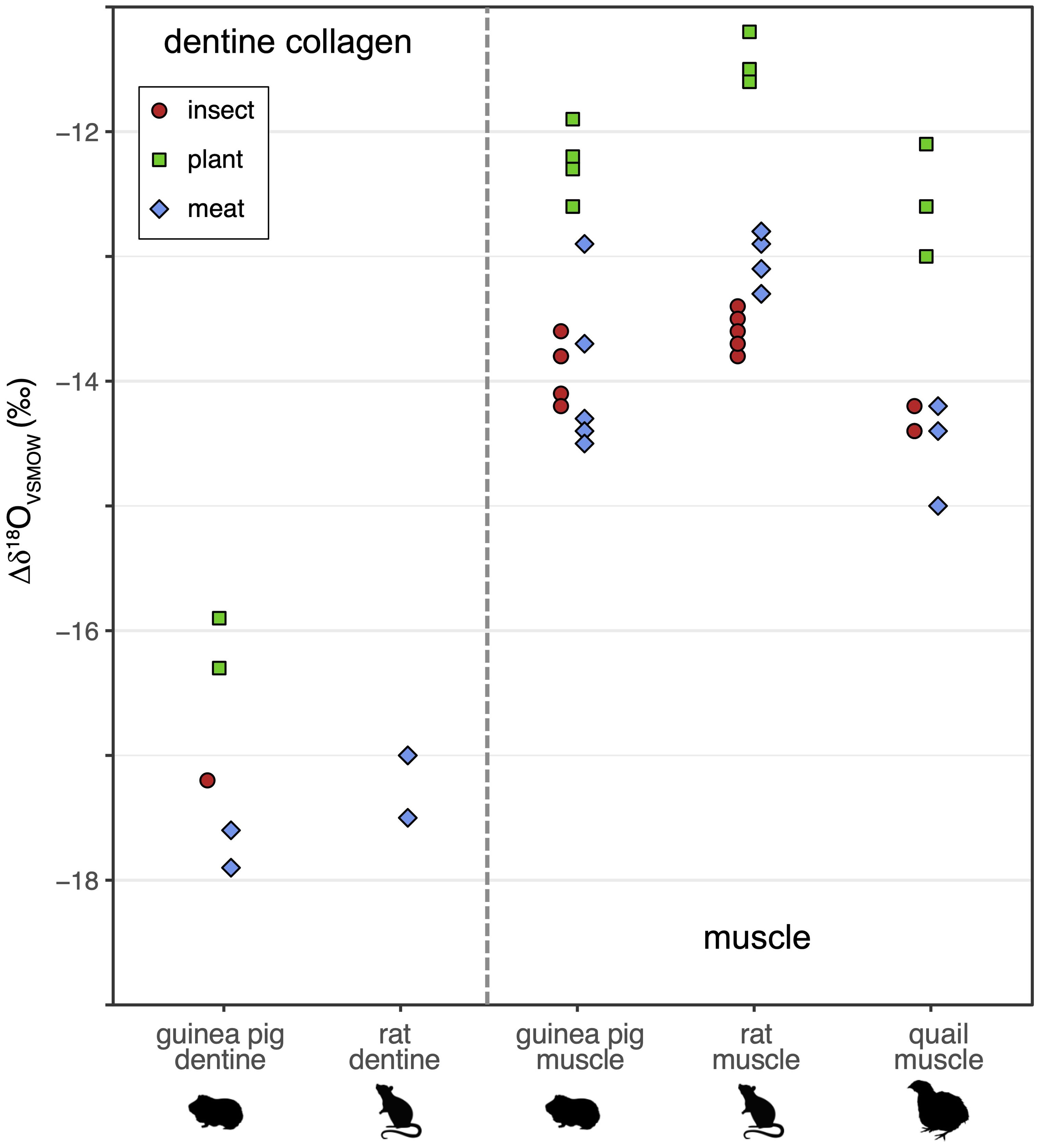
Figure 4. Tissue Δδ18O (non-lipid diet) in muscle and dentine collagen of animals fed with plant-based (green squares), insect-based (red circles), and meat-based (blue diamonds) diets, in rats, guinea pigs, and quail (the latter muscle only).

Table 2. Mean δ2H, δ18O, Δδ2H, and Δδ18O by diet groupa.
3.2 Isotopic differences by diet: dentine
In dentine collagen, there is a large variation in the Δδ2H and Δδ18O values, but some isotopic patterning by diet broadly similar to the muscle results. In dentine collagen, Δδ2H is smaller in the meat-containing diet than the plant- or insect-based diets for the guinea pigs by ~20 ‰ (Tukey HSD p ≤ 0.0031) and ~12 ‰ for rats (non-significant differences); there is a larger and equal Δδ2H for the insect and plant diets (Figure 3; Table 5). While the small number of samples precludes a strong comparison, Δδ18O is smallest on the plant diet and larger on the meat and insect diets for the guinea pig, in agreement with the pattern observed for Δδ18O in muscle (Figure 4).
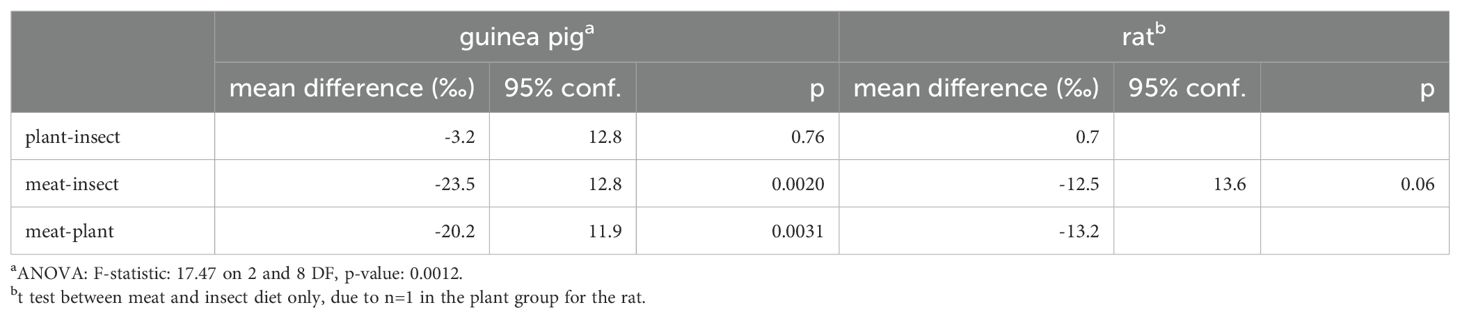
Table 5. ANOVA, Tukey’s honest significant differences, and t test results for dentine collagen Δδ2H by diet.
3.3 Isotopic differences by tissue
There are large and systematic isotopic differences by tissue between muscle and dentine collagen for both H and O in both rats and guinea pigs (Table 2): higher δ2H and δ18O values in dentine collagen than muscle (Figures 1, 2); a big positive diet-tissue Δδ2H in dentine collagen (~ +10−40 ‰) vs. ~ -20 ‰ (opposite direction) Δδ2H in muscle (Figure 3); and ~4 ‰ greater magnitude (negative) Δδ18O in dentine collagen than muscle (Figure 4).
3.4 Isotopic differences by species
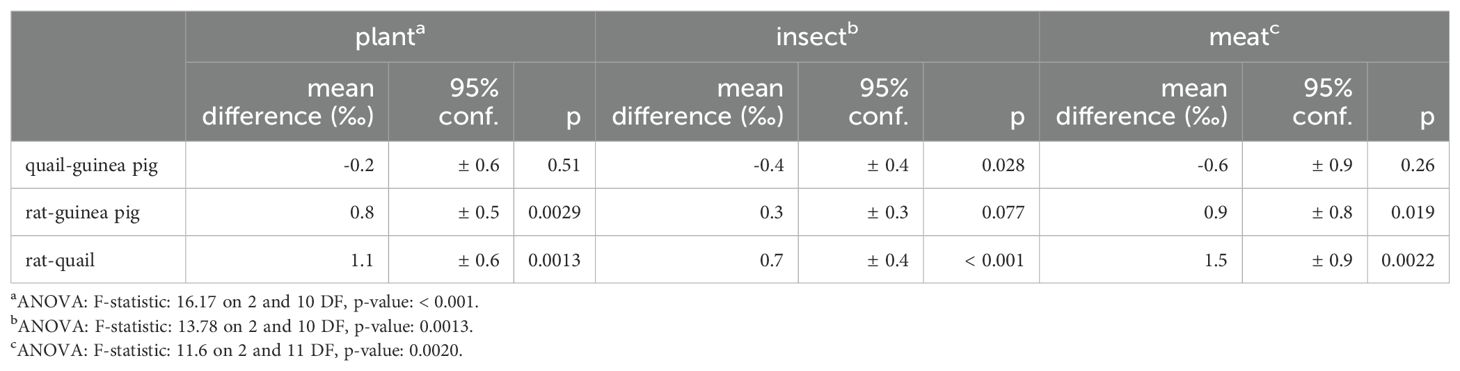
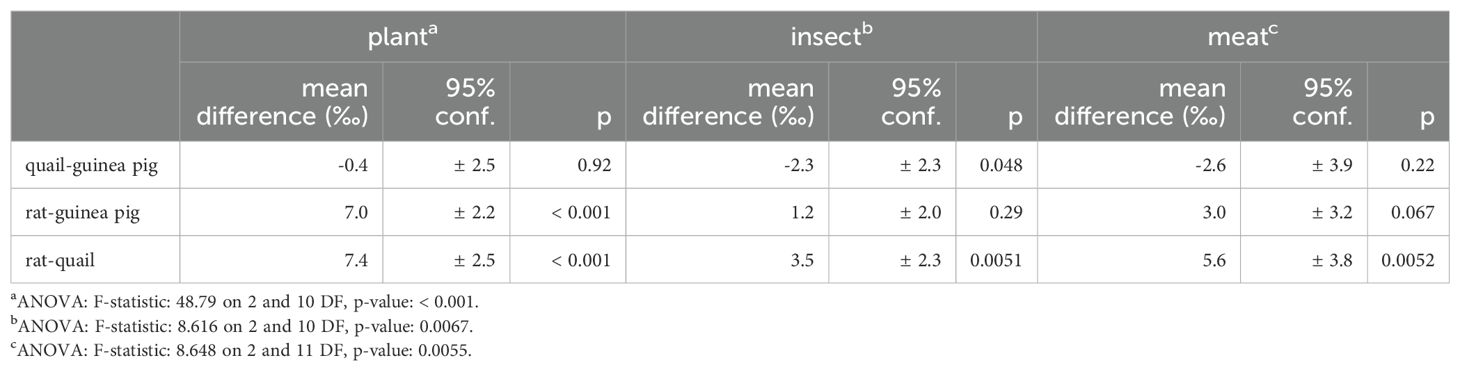
Inter-species isotopic differences are generally smaller than the differences between diets. However, there are δ2H differences between rat and guinea pig dentine collagen (by ~20 ‰ on average, though with a large range and SD of the mean of 7−11 ‰), and in muscle for plant-fed rats compared to plant-fed guinea pig and quail (7−8 ‰). Rat muscle δ18O is somewhat higher than in guinea pigs and quail on all three diets (by 0.7−1.1 ‰, Table 2).
4 Discussion
4.1 Isotopic differences by diet
The muscle δ2H and δ18O isotope values are tightly grouped by diet and species, and diet type affects the diet-tissue H and O isotope offset in muscle in all three species in the same way. In this and other controlled feeding experiments, tissue H and O isotope values are often tightly grouped within each diet or water treatment; the typical intra-group uncertainties are 2.4 ‰ for δ2H and 0.44 ‰ for δ18O (median values of 2 x the standard error of the mean, compiled in Figures 5, 6; Hobson et al., 1999; Tuross et al., 2008; Podlesak et al., 2008; Kirsanow and Tuross, 2011; Wolf et al., 2012; Wolf et al., 2013; Newsome et al., 2017; Rodriguez Curras et al., 2018; Topalov et al., 2019). Our isotopic differences by diet greatly exceed this intra-group variability. The magnitude of the difference for Δδ2H of ~ 4–16 ‰ and Δδ18O up to ~2 ‰ is relatively small but not negligible (Figure 4; Tables 3, 4); greater than typical intra-group uncertainties, but also smaller than the variance found in some studies (discussed further in sections 4.4 and 4.5, e.g. δ2H can range up to 20–80 ‰ in bird keratin, Hobson et al., 2014). In dentine collagen in the guinea pig Δδ2H varies by ~20 ‰ and Δδ18O by ~1.5 ‰ between the diets (diet group mean, Figures 3, 4).

Figure 5. Variances (2 x Standard Error of the mean) in δ2H by substrate and species for controlled feeding studies. Each bar represents one experimental group per study (same diet and water treatment). Results from the present work are denoted by asterisks (*). Data are from Hobson et al. (1999): quail keratin and muscle; Tuross et al. (2008): pig collagen and muscle; Podlesak et al. (2008): woodrat keratin; Kirsanow and Tuross (2011): rat collagen and keratin; Wolf et al. (2012): quail keratin and muscle; Wolf et al. (2013): quail keratin and muscle; Newsome et al. (2017): tilapia muscle; Rodriguez Curras et al. (2018): mouse muscle; Topalov et al. (2019): mouse collagen. Analytical uncertainties are ±1−4 ‰ (1 SD, or not specified) in these studies.
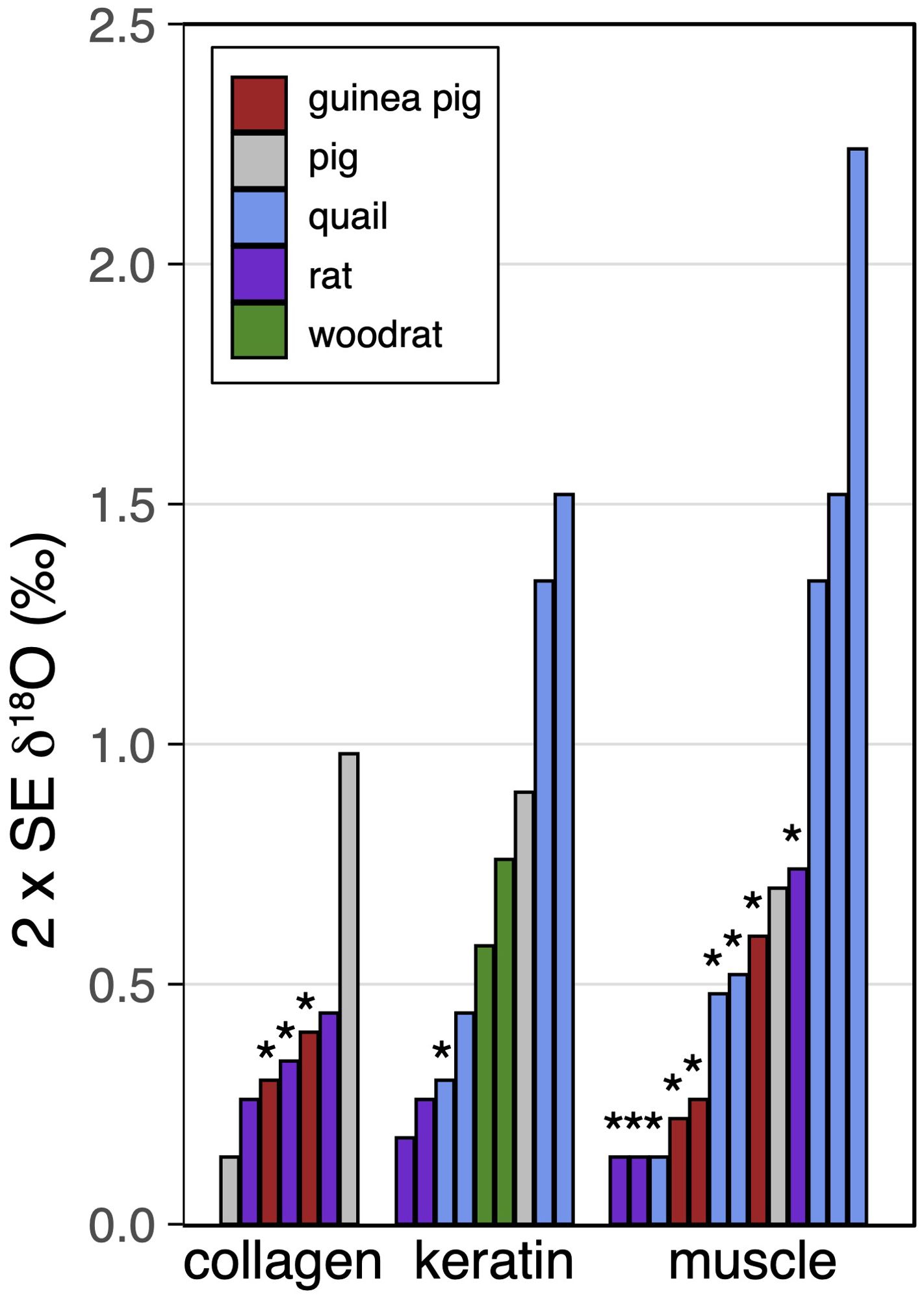
Figure 6. Variances (2 x Standard Error of the mean) in δ18O by substrate and species for controlled feeding studies. Each bar represents one experimental group per study (same diet and water treatment). Results from the present work are denoted by asterisks (*). Data are from Podlesak et al. (2008): woodrat keratin; Kirsanow and Tuross (2011): rat collagen and keratin; Wolf et al. (2013): quail keratin and muscle; Tuross et al. (2008): pig collagen and muscle. Analytical uncertainties are ±0.2−1 ‰ (1 SD, or not specified) in these studies.
In the same individual animal, dentine collagen and muscle Δδ2H and Δδ18O values are generally offset in parallel (Figure 7), indicating that both tissues are reflecting the same isotopic dietary input, and further, that conclusions drawn from the muscle results are probably generalizable to collagen (dentine and bone) and other proteinaceous tissues such as feathers.

Figure 7. (A) Δδ2H for guinea pig and rat dentine collagen and muscle, by diet; (B) Δδ18O for guinea pig dentine collagen and muscle, by diet. Tie lines join data points for individual animals.
This study does not directly elucidate the mechanisms responsible for these observed diet-tissue H and O isotope differences, and further work would be required to address this. Digestion, absorption, biochemical transformation, and differential incorporation of diet macronutrients and amino acids into tissues impart isotopic change (Macko et al., 1986; Bowen et al., 2009; Vander Zanden et al., 2016; Newsome et al., 2017; Magozzi et al., 2019; Newsome et al., 2020). The diet-tissue offset is plausibly affected by the protein composition and/or lipid and carbohydrate amounts (e.g. for nitrogen isotopes, protein quality influences diet-tissue offsets, e.g. Robbins et al., 2005).
The diets in this present study are isonitrogenous (Leichliter et al., 2021), but they differ in their major component of lucerne, lamb, or soldier fly larval insect meal (Table 1). We first consider the meat- and insect-based diets. These two diets are nearly the same in the non-meat/non-insect components (Table 1), so that the only difference is between the main component, the larval meal or lamb meat. The δ2H values of the lipid-removed diets are the same (non-lipid, -77 ‰ vs. -75 ‰, Table 1), which means that the main ingredient in these diets must have the same δ2H values as each other. There is no carbohydrate in lamb (U.S. Department of Agriculture and Agricultural Research Service, 2019), so the dried and defatted lamb meat is principally protein. The larval meal contains chitin, a glucosamine-based polymeric carbohydrate. In black soldier fly meal chitin levels can be in the range of 5−9% (dry matter basis, variable defatting; Finke, 2013; Schiavone et al., 2017; Caligiani et al., 2018). Recent work has shown that some mammals and birds express chitinases and can therefore hydrolyze chitin to the glucosamine monomer, but the expression and activity of Chia (one of the chitinases) varies between species (Tabata et al., 2018). Mice, chickens, and pigs have higher Chia expression than dogs or cattle; and guinea pigs lack the Chia gene (Tabata et al., 2018). One might predict rats and quail to have some ability to hydrolyze chitin. Glucosamines are absorbed by the gastrointestinal tract and can be further metabolized (Setnikar and Rovati, 2001; Anderson et al., 2005).
Despite the main component having the same δ2H value, Δδ2H in muscle varies significantly between the meat and insect diets (6–8 ‰, Tukey HSD p ≤0.002, Table 3). This suggests a few possibilities: first, if the protein δ2H value is the same in the meat- and insect-main components, then the protein quality or type affects the protein H utilization and diet-tissue fractionation (Δδ2H). Second, if the chitin has a different δ2H value than the insect-meal protein, then the inferred different protein δ2H between meat- and insect-diets could be the cause of the Δδ2H difference. Third, any incorporation of chitin H into tissue could also affect δ2H on the insect-based diet. Given the chitin component is likely small (<10% w/w of the main component), it is perhaps more likely that differences in protein quality and amino acid composition are important in the resulting Δδ2H offsets and the differences between the meat-based and insect-based diets.
In contrast, Δδ18O presents a more mixed picture in comparing the insect and meat diets. The insect and meat diet muscle δ18O values are fairly close at ~0.6 ‰ apart, and the resulting Δδ18O is significantly different between those diets only for the rat (0.6 ‰, Tukey HSD p < 0.001, Table 4).
The plant diet results in very different and smaller Δδ2H and Δδ18O in muscle than the other two diets (Figures 3, 4). The difference of the plant diet Δδ2H to that of other diets is significant (Δδ2H = 12−16 ‰ plant-meat, 4−10 ‰ plant-insect, Tukey HSD p ≤0.013, Table 3). Similarly, Δδ18O differences between plant and other diets are up to 2.1 ‰ (Table 4). The plant diet has a higher proportion of the main ingredient (lucerne), additional potato protein, and a lower proportion of the remainder of the diet components (to maintain an iso-nitrogenous condition, Table 1). The nutritional composition of the diets is similar, except the plant-based diet is somewhat lower than the other two diets in neutral detergent fibre and starch (Table 1). The variable dietary macronutrients (proportion of each and their isotope values) in the plant diet and/or the protein composition itself are possible reasons for the observed diet-tissue offset differences from the other two diets in both H and O.
The model elaborated by Magozzi et al. (2019) includes routing from dietary protein to tissue protein (keratin), meaning the protein component plays a strong role in setting the tissue isotope value (e.g., 60% of dietary protein H to keratin). Routing from the dietary protein with a different isotopic composition is plausibly why the plant diet in particular has different Δδ2H and Δδ18O than the insect and meat diets; a second effect may be variation in the fraction of dietary protein H and O routed to tissue between these diet types. In controlled feeding experiments where the water input is changed, the estimated total food contribution to tissues ranges from 70−85 % H in different proteinaceous tissues such as collagen/hair/feather/nail (Kirsanow and Tuross, 2011; Hobson et al., 1999; Topalov et al., 2019) and 30−80 % H in muscle (Hobson et al., 1999; Wolf et al., 2012); this variability may be a real reflection of different routing of atoms from diet component to tissue in different diet conditions as well as differences in how the experiments were conducted (see Vander Zanden et al., 2016 for more data and discussion).
4.2 Isotopic differences between tissues
The results here agree with previous studies showing that isotopic values vary by tissue (e.g., Tuross et al., 2008; Kirsanow and Tuross, 2011; Wolf et al., 2012, for H and O). Collagen H and O isotope values are reported in only a few other controlled feeding studies (rats: Kirsanow and Tuross, 2011; pigs: Tuross et al., 2008; mice: Topalov et al., 2019), and only one reports both muscle and collagen (Tuross et al., 2008). The isotopic pattern seen here agrees with bone collagen results found in pigs (Tuross et al., 2008): there are higher δ2H values and lower δ18O values in collagen (here, in dentine collagen) than in muscle. We find a large isotopic difference between dentine collagen and muscle of δ2H = ~55 ‰ (range 40−67 ‰) and δ18O = -3−4 ‰ with the same diet, species, and water combinations (Figures 1, 2). These inter-tissue differences are important to bear in mind in ecological or migration studies.
4.3 Isotopic differences between species
There is slight δ18O patterning in muscle by species for all three diets, with δ18O values following quail < guinea pigs < rats (0.2−1.5 ‰, Figure 2; Table 6). Hydrogen isotopes also vary between species on some diets in muscle, and more strongly in dentine collagen for all three diets (13−24 ‰, Figure 1; Table 7). Inter-species variation is not unexpected given the effect of metabolic rate and water flux (particularly from drinking water) on resultant tissue oxygen and hydrogen isotopes (O: Bryant and Froehlich, 1995; Kohn, 1996; H and O: Magozzi et al., 2019). Many of the relevant H and O fluxes scale with body mass with an allometric coefficient less than one (i.e., flux ∝ massx, where x < 1); some of these include the metabolic rate (and thus O2 and food consumption), total water flux, and water vapour loss. These fluxes have slightly different scaling relationships with body mass, so that the balance between them is important in determining the resultant body water and tissue isotopic compositions. Total water flux (and often thus the liquid water drinking rate) increases with body mass (Nagy and Peterson, 1988; Bryant and Froehlich, 1995; Kohn, 1996); albeit with different scaling relationships for captive and wild animals as well as between wild herbivorous mammals, wild omnivorous mammals, and wild birds (Nagy and Peterson, 1988).
In terms of isotopic effects, body water and tissue δ2H and δ18O values are predicted to decrease (body water in the direction of the drinking water isotopic value), with increasing liquid drinking water inputs and increasing total water flux. More isotopically fractionated water loss (as vapour), results in higher body water and thus higher tissue δ2H and δ18O values (Kohn, 1996). Given the complexity of the varying influences on H and O fluxes, of which only a few were mentioned above, it is difficult to predict simply the expected H and O isotope behaviour for these three species. In any case, these results highlight that it is important to bear this inter-specific isotopic variation in mind.
4.4 Implications for migration and ecology
Typically, isotope-based animal migration studies use an accessible and inert tissue, such as hair, feathers or claws. Controlled feeding experiments have shown that feathers or hair and muscle are isotopically related to each other; i.e. broadly speaking δ2H in tissues all shift systematically in concert with changes in water and/or dietary inputs (Hobson et al., 1999; Wolf et al., 2012; Newsome et al., 2017). As such the variations in muscle Δδ2H and Δδ18O by diet we observe here are likely relevant to migration and ecological studies using other tissues such as feathers.
Long-distance animal migration studies often have measured tissues with large variances in δ2H values between individuals in a given group. This present experiment’s Δδ2H differences with diet in muscle of ~4−16 ‰ and in dentine collagen of ~13−23 ‰ are small/moderate relative to the long-range geospatial variation in precipitation δ2H values and resultant tissue δ2H values; e.g., feathers can range up to ~80 ‰ in one species from across North America captured in one over-wintering location, and can typically be ~20−30 ‰ (Hobson et al., 2014). Idaho-resident and non-resident American kestrel claws differ in δ2H by up to ~40 ‰ (Ranck et al., 2023). The isotopic variation with diet type is small enough relative to the large geospatially-related variation in precipitation δ2H but might be important in cases with less of a δ2H gradient in environmental water and thus potentially blur the geospatial assignment of individuals.
Furthermore, an observational study also agrees with our experimental result that diet can affect tissue δ2H values. In non-migrant wild birds from the same locale (caught by mist nets, mostly smaller birds, van Wijk et al., 2021), feather δ2H values across species show differences between five dietary guilds (nectarivores, frugivores, granivores, omnivores, insectivores) of 2−30 ‰ – all much larger than the differences in muscle in this present controlled feeding experiment but somewhat closer to the differences we observe in dentine collagen. This variation in δ2H in the wild birds may result from a large variation in the δ2H values of the diet components, as well as any inter-species physiological differences.
The Δδ18O variations by diet in muscle of up to ~1.2−2.0 ‰ (Figure 4; Table 4) and ~1.5 ‰ in dentine collagen are relatively large (Figure 4; Tables 2, 4). While studies of oxygen isotopes in organic tissues are few thus far, feather δ18O reflects 60−80% of the variation in environmental water δ18O and thus is dampened in its response to changing water δ18O values, yielding a reduced range of tissue δ18O values (Hobson and Koehler, 2015; Magozzi et al., 2019). The greater δ18O variation in tissue (muscle, dentine collagen) relative to the reduced geospatially-related variation in δ18O (for feathers) means that in oxygen isotope studies for migration- and ecology-related questions, greater attention is needed to consider differences in diet type as a modulating factor.
However, this varied diet-tissue δ2H and δ18O offset by diet type is possibly mitigated by the fact that many species have rather consistent dietary habits. However, in the case of omnivores/generalists, humans, and species that may vary their diet type (e.g., seasonally or through ontogeny), the differences in diet-tissue isotopic offsets may add extra variability in tissue isotopic values.
4.5 Implications for palaeoecology and archaeology
The Δδ2H difference in dentine collagen with the meat-based diet has interesting implications for archaeological or palaeoecological samples where bone or dentine collagen is analyzed – that diet is an important factor to consider in resultant collagen δ2H values, particularly in omnivores such as humans. The effect of diet type is supported by studies on archaeological samples: in dentine collagen from human first molars δ2H varied significantly along incrementally sampled sections (up to 30 ‰ range), corresponding to changes over the first few years of life (Ryan et al., 2020). These variations were likely dietary in nature as juveniles changed their diets from mother’s milk to possibly plant-food gruel (cooked) and further towards an adult diet (as represented by adult bone isotopic values).
There are limited studies on δ18O in archaeological protein (collagen), but our results in dentine collagen suggest diet type will be an important consideration in interpreting δ18O values, among other parameters such as water flux, metabolism, and geographic locality. Data thus far are mixed: medieval bone collagen δ18O from the same site shows considerable differences between species (up to 3 ‰), including humans (Ryan et al., 2018); in contrast, little inter-species δ18O variation is seen in bone collagen in Bronze Age archaeological material but a large intra-species range is noted (Reynard et al., 2020). Magozzi et al. (2019) predict lowering of δ18O in tissue protein with trophic level in a strictly-modelled trophic system where the diet input is the protein from the previous trophic level.
There is no a priori expectation for the effect of protein type or amount on resultant tissue isotopic values. Generally the isotopic composition of macronutrients in a given plant rank from δ2H carbohydrate > δ2H protein > δ2H fat (Estep and Hoering, 1980; da Silveira Lobo Sternberg, 1989), δ18O carbohydrate > δ18O protein and δ18O carbohydrate > δ18O fat (Silva et al., 2015), and in animal muscle δ2H protein > δ2H fat (Supplementary Table 9) so that the result of any change in protein routing to tissue (and thus over- or under-representation) is not straightforward to predict.
Acknowledging the small number of dentine samples, our results suggest for both δ2H and δ18O in bone or dentine collagen that there are important inter-species differences to consider when interpreting archaeological material, as well as consideration of variation due to changes in diet. For example, inter-site or temporal comparisons should involve the same or very similar species (in diet and physiology). In species with varied diets, e.g. humans, care is needed in interpretations of small magnitude δ2H and δ18O variations, as diet may have played a role in the noted bone or tooth isotopic variability.
5 Conclusions
The three diets consumed (plant-, insect-, or meat-based) by the three different animals (guinea pigs, rats, and quail) result in different diet-tissue offsets; variation in hydrogen (Δδ2H) and oxygen (Δδ18O) isotope offsets are small but greater than intra-group variances, and thus important to consider in ecological and archaeological studies. Controlling for diet, there are also δ2H and δ18O variations noted between tissues and between species.
Consequently, the effect of diet type, tissue type, and species on diet-tissue isotopic offsets should be considered when using proteinaceous tissues for ecological, environmental, and diet reconstruction or migration studies. When the same species and tissue is under study, and if the diet type is relatively consistent, then the consequences of these isotopic variations may be mitigated. In addition, these results reinforce that caution is warranted in making inter-species H and O isotope comparisons using proteinaceous tissues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Swiss Cantonal Animal Care and Use Committee, Zurich. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LR: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JL: Project administration, Resources, Writing – review & editing. DW: Resources, Writing – review & editing. MC: Methodology, Resources, Writing – review & editing. TT: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for the analytical work was provided by a grant to LR from the Osher Institute for Lifelong Learning at Boise State University. This study was funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC CoG grant agreement no. 681450) to TT.
Acknowledgments
We thank Hubert Vonhof for measurement of the drinking water samples for H and O isotopes.We thank L. Martin, N. Schmid, K. Zbinden, D. Codron, A. De Cuyper, and S. Heldstab for taking care of the animals during the experiments at the Vetsuisse Faculty at the University of Zurich.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1516786/full#supplementary-material
References
Anderson J. W., Nicolosi R. J., Borzelleca J. F. (2005). Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem. Toxicol. 43, 187−201. doi: 10.1016/j.fct.2004.11.006
Bowen G. J., Ehleringer J. R., Chesson L. A., Thompson A. H., Podlesak D. W., Cerling T. E. (2009). Dietary and physiological controls on the hydrogen and oxygen isotope ratios of hair from Mid-20th century indigenous populations. Amer. J. Phys. Anthropol 139, 494−504. doi: 10.1002/ajpa.v139:4
Bryant J. D., Froehlich P. N. (1995). A model of oxygen isotope fractionation in body water of large animals. Geochim. Cosmochim. Acta 59, 4523−4537. doi: 10.1016/0016-7037(95)00250-4
Caligiani A., Marseglia A., Leni G., Baldassarre S., Maistrello L., Dossena A., et al. (2018). Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 105, 812−820. doi: 10.1016/j.foodres.2017.12.012
da Silveira Lobo Sternberg L. (1989). “Oxygen and hydrogen isotope ratios in plant cellulose: mechanisms and applications,” in Stable Isotopes in Ecological Research, vol. 68 . Eds. Ehleringer J. R., Nagy K. A., Rundel P. W. (New York: Springer-Verlag), 124−141.
Ehleringer J. R., Bowen G. J., Chesson L. A., West A. G., Podlesak D. W., Cerling T. E. (2008). Hydrogen and oxygen isotope ratios in human hair are related to geography. PNAS 105, 2788−2793. doi: 10.1073/pnas.0712228105
Estep M. F., Hoering T. C. (1980). Biogeochemistry of the stable hydrogen isotopes. Geochim. Cosmochim. Acta 44, 1197−1206. doi: 10.1016/0016-7037(80)90073-3
Feng D., Surma J., Tütken T., Löffler N., Heinemann G., Tröster G., et al. (2024). Triple oxygen isotopes of modern terrestrial mammalian tooth enamel – new implications for paleoenvironmental and physiological research. Geochim. Cosmochim. Acta 365, 21−34. doi: 10.1016/j.gca.2023.11.025
Feng D., Tütken T., Löffler N., Tröster G., Pack A. (2022). Isotopically anomalous metabolic oxygen in marine vertebrates as physiology and atmospheric proxy. Geochim. Cosmochim. Acta 328, 85−102. doi: 10.1016/j.gca.2022.05.008
Finke M. D. (2013). Complete nutrient content of four species of feeder insects. Zoo Biol. 32, 27−36. doi: 10.1002/zoo.21012
Gröcke D. R., Sauer P. E., Bridault A., Drucker D. G., Germonpré M., Bocherens H. (2017). Hydrogen isotopes in Quaternary mammal collagen from Europe. J. Archaeol. Sci.: Rep. 11, 12−16. doi: 10.1016/j.jasrep.2016.11.020
Hobson K. A., Atwell L., Wassenaar L. I. (1999). Influence of drinking water and diet on the stable-hydrogen isotope ratios of animal tissues. PNAS 96, 8003−8006. doi: 10.1073/pnas.96.14.8003
Hobson K. A., Koehler G. (2015). On the use of stable oxygen isotope (δ18O) measurements for tracking avian movements in North America. Ecol. Evol. 5, 799−806. doi: 10.1002/ece3.2015.5.issue-3
Hobson K. A., Van Wilgenburg S. L., Faaborg J., Toms J. D., Rengifo C., Sosa A. L., et al. (2014). Connecting breeding and wintering grounds of Neotropical migrant songbirds using stable hydrogen isotopes: a call for an isotopic atlas of migratory connectivity. J. Field Ornithol. 85, 237−257. doi: 10.1111/jofo.12065
Hobson K. A., Wassenaar L. I. (Eds.) (2019). Tracking animal migration with stable isotopes (London: Academic Press).
Kirsanow K., Makarewicz C., Tuross N. (2008). Stable oxygen (δ18O) and hydrogen (δD) isotopes in ovicaprid dentinal collagen record seasonal variation. J. Archaeol. Sci. 35, 3159−3167. doi: 10.1016/j.jas.2008.06.025
Kirsanow K., Tuross N. (2011). Oxygen and hydrogen isotopes in rodent tissues: Impact of diet, water and ontogeny. Palaeogeogr. Palaeoclimatol. Palaeoecol 310, 9−16. doi: 10.1016/j.palaeo.2011.03.022
Koehler G., Kardynal K. J., Hobson K. A. (2019). Geographical assignment of polar bears using multi-element isoscapes. Sci. Rep. 9, 9390. doi: 10.1038/s41598-019-45874-w
Kohn M. J. (1996). Predicting animal δ18O: Accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 60, 4811−4829. doi: 10.1016/S0016-7037(96)00240-2
Law K.-T., Lee C.-K., King N. M., Rabie A.-B. M. (2003). The relationship between eruption and length of mandibular incisors in young rats. Med. Sci. Monit 9, BR47−53.
Leichliter J. N., Lüdecke T., Foreman A. D., Duprey N. N., Winkler D. E., Kast E. R., et al. (2021). Nitrogen isotopes in tooth enamel record diet and trophic level enrichment: Results from a controlled feeding experiment. Chem. Geol. 563, 120047. doi: 10.1016/j.chemgeo.2020.120047
Longinelli A. (1984). Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 48, 385–390. doi: 10.1016/0016-7037(84)90259-X
Macko S. A., Estep M. L. F., Engel M. H., Hare P. E. (1986). Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim. Cosmochim. Acta 50, 2143−2146. doi: 10.1016/0016-7037(86)90068-2
Magozzi S., Vander Zanden H. B., Wunder M. B., Bowen G. J. (2019). Mechanistic model predicts tissue-environment relationships and trophic shifts in animal hydrogen and oxygen isotope ratios. Oecologia 191, 777−789. doi: 10.1007/s00442-019-04532-8
Meier-Augenstein W., Chartrand M. M. G., Kemp H. F., St-Jean G. (2011). An inter-laboratory comparative study into sample preparation for both reproducible and repeatable forensic 2H isotope analysis of human hair by continuous flow isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3331−3338. doi: 10.1002/rcm.5235
Müller J., Clauss M., Codron D., Schulz E., Hummel J., Kircher P., et al. (2015). Tooth length and incisal wear and growth in Guinea pigs (Cavia porcellus) fed diets of different abrasiveness. J. Anim. Physiol. Anim. Nutr. 99, 591−604. doi: 10.1111/jpn.12226
Nagy K. A., Peterson C. C. (1988). Scaling of Water Flux Rate in Animals (Berkeley and Los Angeles: University of California Press).
Newsome S. D., Nakamoto B. J., Curras M. R., Fogel M. L. (2020). Compound-specific δ2H analysis highlights the relationship between direct assimilation and de novo synthesis of amino acids from food and water in a terrestrial mammalian omnivore. Oecologia 193, 827−842. doi: 10.1007/s00442-020-04730-9
Newsome S. D., Wolf N., Bradley C. J., Fogel M. L. (2017). Assimilation and isotopic discrimination of hydrogen in tilapia: implications for studying animal diet with δ2H. Ecosphere. 8, e01616. doi: 10.1002/ecs2.2017.8.issue-1
Podlesak D. W., Torregrossa A.-M., Ehleringer J. R., Dearing M. D., Passey B. H., Cerling T. E. (2008). Turnover of oxygen and hydrogen isotopes in the body water, CO2, hair, and enamel of a small mammal. Geochim. Cosmochim. Acta 72, 19−35. doi: 10.1016/j.gca.2007.10.003
Quinn G. P., Keogh M. J. (2002). Experimental Design and Data Analysis for Biologists (Cambridge: Cambridge University Press).
Ranck S. C., Garsvo C. M., Schwartz D. M., Reynard L. M., Kohn M. J., Heath J. A. (2023). Sex, body size, and winter weather explain migration strategies in a partial migrant population of American Kestrels. Ornithology 140, ukad019. doi: 10.1093/ornithology/ukad019
R Core Team. (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Version 4.3.1. Available at: https://www.R-project.org/ (Accessed June 16, 2023).
Reynard L. M., Ryan S. E., Guirguis M., Contreras-Martínez M., Pompianu E., Ramis D., et al. (2020). Mediterranean precipitation isoscape preserved in bone collagen δ2H. Sci. Rep. 10, 8579. doi: 10.1038/s41598-020-65407-0
Reynard L. M., Ryan S. E., Tuross N. (2019). The interconversion of δ2H values of collagen between thermal conversion reactor configurations. Rapid Commun. Mass Spectrom. 33, 678−682. doi: 10.1002/rcm.v33.7
Reynard L. M., Tuross N. (2016). Hydrogen isotopic analysis with a chromium-packed reactor of organic compounds of relevance to ecological, archaeological, and forensic applications. Rapid Commun. Mass Spectrom. 30, 1857−1864. doi: 10.1002/rcm.7662
Robbins C. T., Felicetti L. A., Sponheimer M. (2005). The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 144, 534−540. doi: 10.1007/s00442-005-0021-8
Rodriguez Curras M., Fogel M. L., Newsome S. D. (2018). Assimilation and discrimination of hydrogen isotopes in a terrestrial mammal. Oecologia 188, 381−393. doi: 10.1007/s00442-018-4221-4
Ryan S. E., Reynard L. M., Crowley Q. G., Snoeck C., Tuross N. (2018). Early medieval reliance on the land and the local: An integrated multi-isotope study (87Sr/86Sr, δ18O, δ13C, δ15N) of diet and migration in Co. Meath, Ireland. J. Archaeol. Sci. 98, 59−71. doi: 10.1016/j.jas.2018.08.002
Ryan S. E., Reynard L. M., Pompianu E., van Dommelen P., Murgia C., Subirà M. E., et al. (2020). Growing up in Ancient Sardinia: Infant-toddler dietary changes revealed by the novel use of hydrogen isotopes (δ2H). PloS One 15, e0235080. doi: 10.1371/journal.pone.0235080
Schiavone A., De Marco M., Martínez S., Dabbou S., Renna M., Madrid J., et al. (2017). Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 8, 51. doi: 10.1186/s40104-017-0181-5
Setnikar I., Rovati L. C. (2001). Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review. Arzneimittelforschung 51, 699−725. doi: 10.1055/s-0031-1300105
Silva L. C. R., Pedroso G., Doane T. A., Mukome F. N. D., Horwath W. R. (2015). Beyond the cellulose: oxygen isotope composition of plant lipids as a proxy for terrestrial water balance. Geochem. Persp. Lett. 1, 33−42. doi: 10.7185/geochemlet.1504
Soto D. X., Wassenaar L. I., Hobson K. A. (2013). Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Funct. Ecol. 27, 535−543. doi: 10.1111/fec.2013.27.issue-2
Tabata E., Kashimura A., Kikuchi A., Masuda H., Miyahara R., Hiruma Y., et al. (2018). Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 8, 1461. doi: 10.1038/s41598-018-19940-8
Topalov K., Schimmelmann A., Polly P. D., Sauer P. E., Viswanathan S. (2019). Stable isotopes of H, C and N in mice bone collagen as a reflection of isotopically controlled food and water intake. Isotopes Environ. Health Stud. 55, 129−149. doi: 10.1080/10256016.2019.1580279
Tuross N. (2012). Comparative decalcification methods, radiocarbon dates, and stable isotopes of the VIRI bones. Radiocarbon 54, 837−844. doi: 10.1017/S0033822200047482
Tuross N., Warinner C., Kirsanow K., Kester C. (2008). Organic oxygen and hydrogen isotopes in a porcine controlled dietary study. Rapid Commun. Mass Spectrom 22, 1741−1745. doi: 10.1002/rcm.v22:11
U.S. Department of Agriculture, Agricultural Research Service (2019). (FoodData Central). Available online at: https://fdc.nal.usda.gov/fdc-app.html/food-details/174370/nutrients (Accessed February 3, 2024).
Vander Zanden H. B., Soto D. X., Bowen G. J., Hobson K. A. (2016). Expanding the isotopic toolbox: Applications of hydrogen and oxygen stable isotope ratios to food web studies. Front. Ecol. Evol. 4. doi: 10.3389/fevo.2016.00020
van Wijk R. E., Barshep Y., Hobson K. A. (2021). On the use of stable hydrogen isotope measurements (δ2H) to discern trophic level in avian terrestrial food webs. Diversity 13, 202. doi: 10.3390/d13050202
Weber M., Tacail T., Lugli F., Clauss M., Weber K., Leichliter J., et al. (2020). Strontium uptake and intra-population 87Sr/86Sr variability of bones and teeth-controlled feeding experiments with rodents (Rattus norvegicus, Cavia porcellus). Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.569940
Wolf N., Newsome S. D., Fogel M. L., del Rio C. M. (2012). An experimental exploration of the incorporation of hydrogen isotopes from dietary sources into avian tissues. J. Exp. Biol. 215, 1915−1922. doi: 10.1242/jeb.065219
Keywords: feeding experiments, guinea pigs, rats, quail, diet changes, dentine, muscle
Citation: Reynard LM, Leichliter JN, Winkler DE, Clauss M and Tütken T (2025) Hydrogen and oxygen isotopes in vertebrate tissues vary by diet type. Front. Ecol. Evol. 13:1516786. doi: 10.3389/fevo.2025.1516786
Received: 24 October 2024; Accepted: 24 January 2025;
Published: 18 February 2025.
Edited by:
Yuichi Naito, Central Research Institute of Electric Power Industry (CRIEPI), JapanReviewed by:
Ichiro Tayasu, Research Institute for Humanity and Nature, JapanThomas Larsen, Max Planck Institute for Geoanthropology, Germany
Copyright © 2025 Reynard, Leichliter, Winkler, Clauss and Tütken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda M. Reynard, bGluZGFyZXluYXJkQGJvaXNlc3RhdGUuZWR1; Thomas Tütken, dHVldGtlbkB1bmktbWFpbnouZGU=
†Present address: Jennifer N. Leichliter, Emmy Noether Group for Hominin Meat Consumption (HoMeCo), Max Planck Institute for Chemistry, Mainz, Germany
 Linda M. Reynard
Linda M. Reynard Jennifer N. Leichliter2†
Jennifer N. Leichliter2† Daniela E. Winkler
Daniela E. Winkler Marcus Clauss
Marcus Clauss Thomas Tütken
Thomas Tütken