- 1Department of Biology, University of Prince Edward Island, Charlottetown, PEI, Canada
- 2Department of Health Management, University of Prince Edward Island, Charlottetown, PEI, Canada
Bumble bees are essential pollinators that provide critical ecosystem services yet, studies are documenting global species declines while recognizing those declines may be understated due to insufficient baseline data. This study investigates bumble bee species richness and abundance across urban and natural sites in Prince Edward Island, Canada, focusing on Charlottetown (urban) and Prince Edward Island National Park (natural). We conducted fieldwork in August and September 2019 using a non-invasive photographic survey technique. We used published keys and sought feedback from citizen science platforms like iNaturalist and Bumble Bee Watch to verify species identification. Our results revealed nine bumble bee species, with Bombus impatiens being the most abundant and Bombus perplexus the rarest. Species richness was higher in natural sites, while urban sites demonstrated moderate levels of bumble bee diversity. Additionally, our findings suggest that sites containing a mix of natural and human-cultivated plant types, predominantly found in our urban study sites, may support higher diversity and evenness levels than those of homogenized plant types (natural or human-cultivated). This research illustrates the practicality of photographic surveys to document the species richness and diversity of bumble bees while avoiding disturbance to populations in urban and natural habitats of Prince Edward Island.
1 Introduction
Urbanization is recognized as a major cause of pollinator habitat loss, mainly promoted by the rapid growth and concentration of human populations in urban centers. From the ecological point of view, urban areas are a unique mosaic of residential, commercial, and industrial habitats ameliorated by green spaces (Breuste et al., 2008). Researchers frequently document urbanization’s negative impacts on species diversity and abundance of a broad range of taxa. Yet, with effective conservation measures, pollinators, including bumble bees, can successfully use urban habitats (Blackmore and Goulson, 2014) and may prefer urban spaces to agriculturally dominated spaces (Samuelson et al., 2018).
Bumble bees (genus Bombus) are a major group of bees comprising approximately 260 species globally (Fisher et al., 2022). They pollinate a wide range of flora, including species vital to agriculture (Milanoa et al., 2019; Samuelson et al., 2018), species of conservation concern (Baldock, 2020; Potts et al., 2016), and those that support global food security (Fauser-Misslin et al., 2013; Marshman et al., 2019). Bombus species possess several physiological (e.g., Heinrich, 1975; Heinrich and Kammer, 1973; Masson et al., 2017), morphological (e.g., variable tongue phenology; Arbulo et al., 2015; Grixti et al., 2009) and ecological (e.g., buzz pollination; Nunes-Silva et al., 2013) characteristics that contribute to their success as pollinators (Bond, 1994; Sheffield et al., 2003). Not only have bumble bees earned the label of keystone species within urban habitats (Goulson et al., 2011; Parrey et al. 2021), their nests can host parasitic and commensal species (Cameron et al., 2007), demonstrating their further value in providing ecosystem services (Winfree et al., 2007). The queens of parasitic species within the Bombus genus, also called cuckoo bumble bees (subgenus Psithyrus), locate established nests and kill or dominate the resident queen of a preferred Bombus host species (Lhomme and Hines, 2018). The dominant queen will then use the resident queen’s workers to rear her reproductive offspring (Lhomme and Hines, 2018); therefore, Psithyrus subgenus species lack worker castes.
Aside from collection reviews, scientists typically survey bumble bees using traps or bowls (Armistead, 2023; Bell et al., 2023), which are passive techniques involving lethal capture. Lethal capture techniques vastly reduce time and labor commitments (e.g., Brooks and Nocera, 2020; Montero-Castaño et al., 2022) but involve a certain level of disturbance in populations and ecological communities surveyed. Conducting surveys while limiting population disturbance is particularly important when studying rare or at-risk species (Montero-Castaño et al., 2022; Bell et al., 2023). A more labor-intensive yet still effective approach is netting to capture individuals for collections or subsequent analysis and release (Bell et al., 2023; Dominey, 2021). A non-lethal, non-invasive approach that may be used to survey pollinators, such as bumble bees, is the photographic survey. In this method, the researcher collects photos of individual animals and identifies them via their color patterns and physical characteristics (Williams et al., 2014). While requiring more labor than lethal capture, photographic surveys are very cost-effective. The studies that have used photographic surveys to document the presence or abundance of bumble bees have shown that this method of surveying can effectively quantify and distinguish bumble bee species provided the researchers possess expertise in bumble bee taxonomy (e.g., MacPhail et al., 2020). In addition, digital cameras are not required to take high-quality images of most bumble bee species. Most smartphones possess cameras that can provide excellent photos of bumble bees hovering around and on flowering plants. Citizen science platforms like iNaturalist and Bumble Bee Watch rely on photographic submissions and have become a popular approach to documenting bumble bee abundance and distribution (Falk et al. 2019; MacPhail et al., 2020; Suzuki-Ohno et al., 2017). Typically, experts take time to review submissions on citizen science platforms that improve accurate species identification. Yet, it is important to distinguish that some platforms allow any user to suggest identification. For example, Bumble Bee Watch submissions are verified by experts, whereas iNaturalist allows any user to suggest an identification that may falsely become considered “Research Grade.”
Within the urban context, green spaces may include human-made gardens, parks, playgrounds, trails, cemeteries, and enclaves of natural and semi-natural plant communities (Daniels et al., 2020; Wood et al. 2018). Some researchers studying bumble bees in urban areas have documented lower flower visitation rates, lower species richness, loss of rare species, and homogenization of species pools (Deguines et al., 2016; Harrison et al., 2019; Hernandez et al., 2009), while others are documenting that many large-bodied, social, and generalist pollinator species can thrive in urban environments (Liang et al., 2023). Some have found bumble bee diversity to be relatively higher in urban areas because of the assortment of flora that characterizes urban green spaces (Baldock et al. 2015; Theodorou et al., 2021), particularly individual and community gardens (Baldock, 2020). Studies have shown that bumble bees can colonize urban areas with a relatively small cover of green space (e.g., Hernandez et al., 2009; Matteson et al., 2008; Matteson and Langellotto, 2010; Tommasi et al., 2004), provided the green space offers favorable and beneficial characteristics (Nunes et al. 2024). Urban spaces may offset the negative impacts that surrounding agricultural (e.g., lower reproductive rates, smaller peak sizes) and rural areas (e.g., less variation of floral resources) may pose on bumble bee populations (Nunes et al. 2024; Samuelson et al., 2018). It is important to note that habitat requirements vary between bumble bee species, which also helps explain how studies focusing on specific bumble bee species can produce contrasting results (Liczner and Colla, 2020).
Prince Edward Island (PEI) is the smallest Canadian province with only 600,000 hectares of land (Kolinjivadi et al., 2020), situated in the Gulf of St. Lawrence on the eastern coast of Canada. Since European colonization, roughly three centuries ago, anthropogenic activities such as forestry, agriculture, and urbanization have altered the natural habitats of PEI. Some active research has been conducted on bumble bees in Eastern Canada (e.g., Brown, 2022; Dominey, 2021). However, only one study (Laverty and Harder, 1988) has supplied precise information about bumble bees in PEI. Laverty and Harder used museum and private collections and recorded nine bumble bee species in the natural areas of PEI: Bombus borealis, Bombus citrinus, Bombus fervidus, Bombus insularis, Bombus rufocinctus, Bombus sandersoni, Bombus ternarius, Bombus terricola, and Bombus vagans (1988). Unfortunately, they did not offer any details pertaining to bumble bee species in urban PEI.
Specifically, the objectives of this study were (1) to provide baseline data on species richness, diversity, and relative abundance of bumble bees occurring in urban and natural areas of PEI (2) to assess the usefulness of photographic surveys for monitoring bumble bee populations; and (3) to compare the diversity and composition of bumble bee communities between urban and natural areas of PEI. Based on studies conducted in other regions of North America, we predicted that the natural areas of Prince Edward Island would support a more diverse bumblebee community compared to urban areas. This is because of the availability of preferred native flora (Carvell et al. 2017) and less anthropogenic disturbance. We also expected higher bumble bee abundance levels in sites dominated by natural flora compared to human-cultivated flora (i.e., ornamental plants). Although Laverty and Harder did not document Bombus impatiens in PEI (1988), we expected to find this species in both urban and natural sites. This prediction was based on B. impatiens being a generalist species that is widely distributed in Eastern North America (e.g., Matteson and Langellotto, 2009) and their range has artificially expanded because of their usefulness in commercial crop pollination (Palmier and Sheffield, 2019; Velthuis and Doorn, 2005).
2 Materials and methods
2.1 Study sites
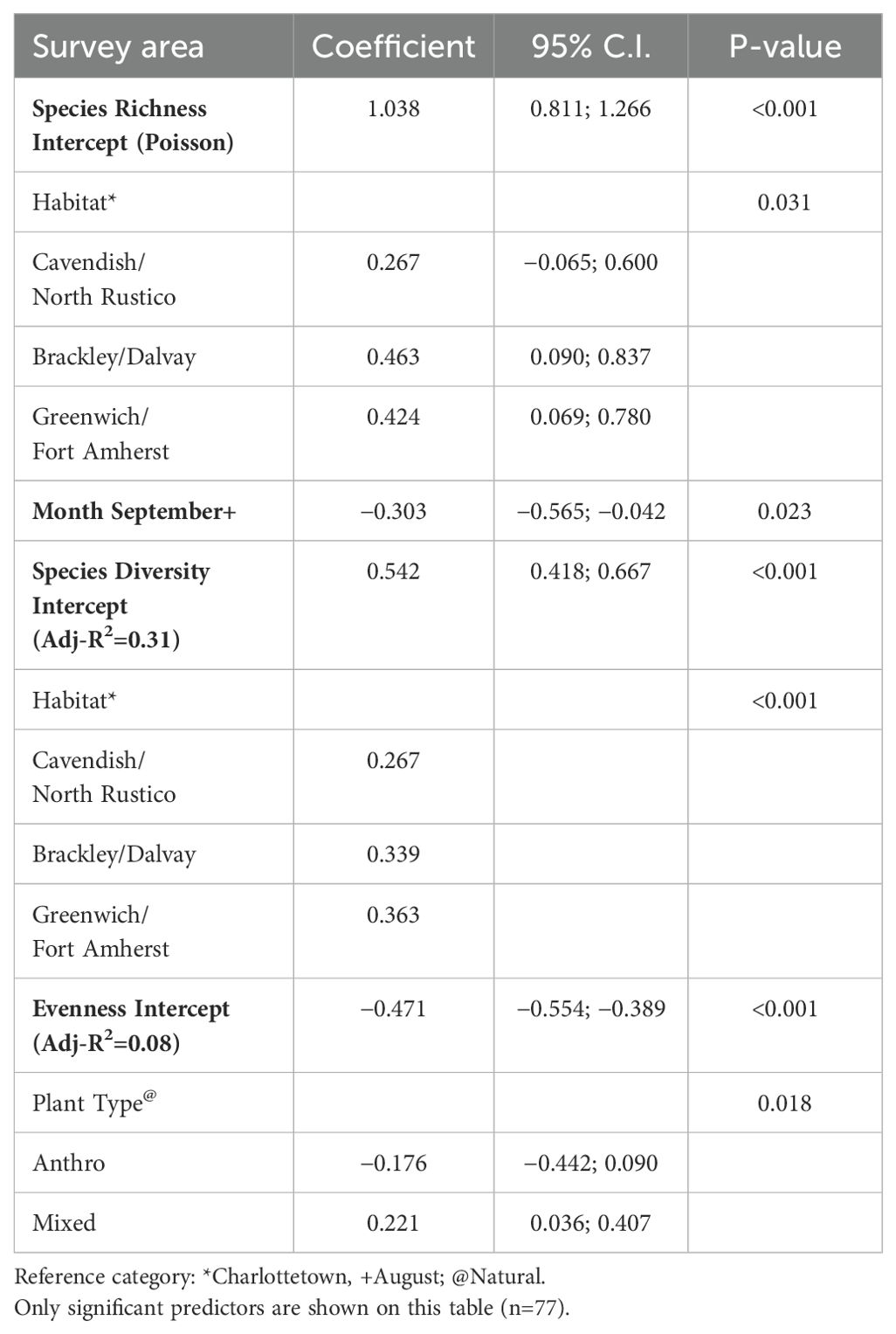
PEI is Canada’s smallest Atlantic province, located in the Gulf of St. Lawrence (Silva et al., 2005). The largest urban area of PEI is its capital, the city of Charlottetown (50 km2 area), which has a population of roughly 44,000 individuals (Statistics Canada, 2018) (Figure 1).
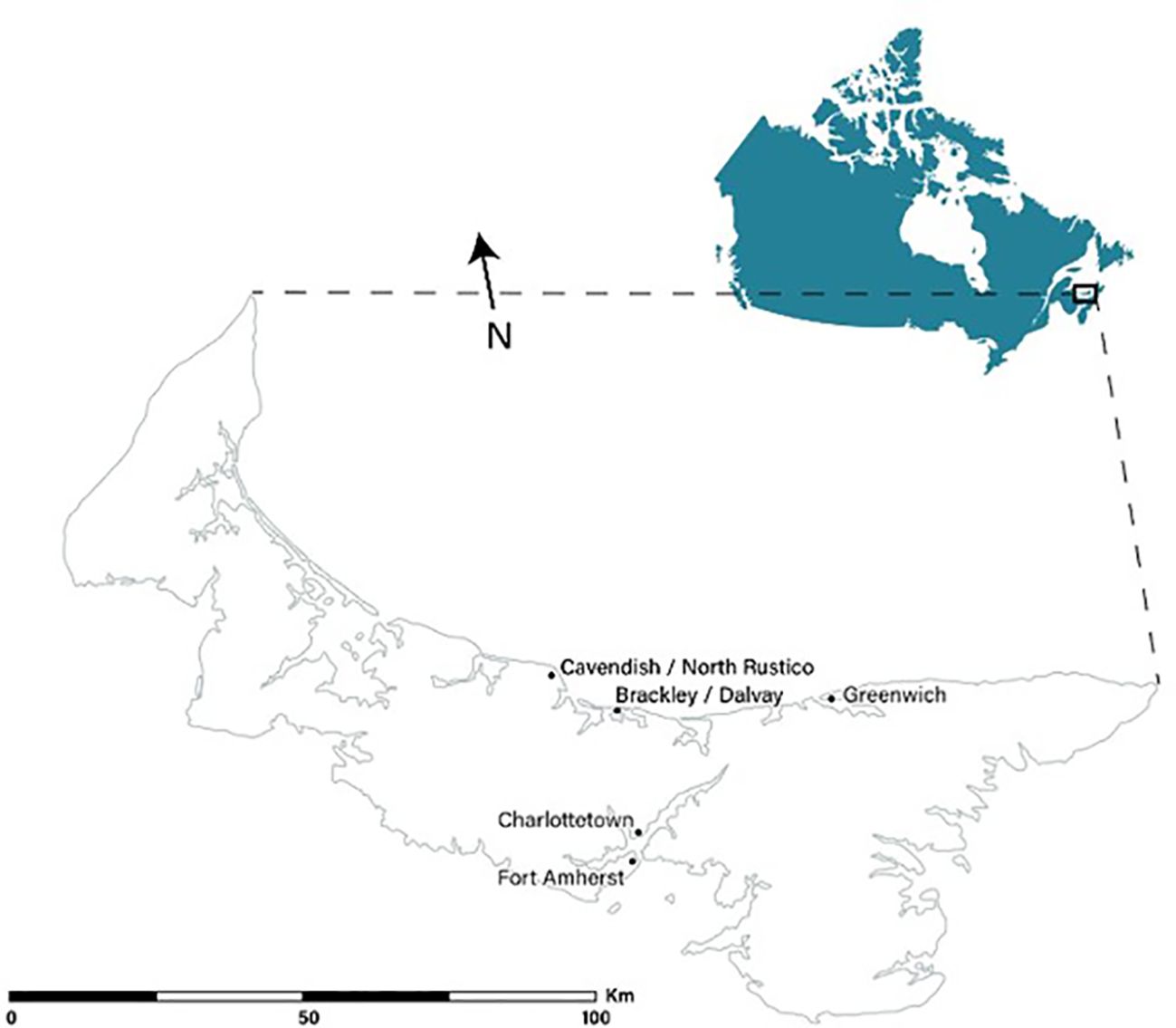
Figure 1. General location of bumble bee photographic surveys conducted in August and September 2019 between urban (Charlottetown, Prince Edward Island) and natural sites (Prince Edward Island National Park).
In order to compare the diversity and composition of bumble bee communities in urban and natural areas of PEI, a total of 20 sites were surveyed in PEINP and another 20 sites in Charlottetown (Figure 1). Charlottetown sites (20/20) included walking trails, parks, public gardens, and open green spaces, in locations that could represent the interior and boundaries of the city. Sites typically consisted of native and non-native naturally occurring species (e.g., aster spp., autumn hawkbit, clover spp., dandelion spp., goldenrod spp., and thyme spp.) and/or human cultivated species (e.g., begonia spp., chrysanthemum spp., cinquefoil spp., phacelia spp., and sedum spp.). PEINP sites consisted mainly of open green spaces with native and non-native wildflowers (aster spp., clover spp., common eyebright, goldenrod spp., knapweed spp., wild rose, and tufted vetch). PEINP contains 27 km2 of protected natural habitats but it is important to note that it also includes human infrastructures (e.g., campgrounds, visitor and interpretation centers) that are used predominantly during the summer. Given the substantial level of anthropogenic disturbance in Charlottetown, three major areas within PEINP were selected to encompass a broad spectrum, ranging from moderate anthropogenic disturbance to predominantly natural environments. The Cavendish/North Rustico sites (9/20) display moderate disturbance and the least natural characteristics. This area was selected for this study because of its similarity with Charlottetown, as it sustains a higher level of anthropogenic activities and human infrastructure while encompassing the least abundant amounts of green space compared to other PEINP locations. Disturbance is especially obvious during the summer when tourists visit PEINP and the town of Cavendish. In contrast, the Brackley/Dalvay sites (5/20) exhibit a moderate level of disturbance along with moderate instances of human infrastructure. Finally, the Greenwich/Fort Amherst sites (6/20), while geographically separated, demonstrate the least amount of infrastructure and rates of visitation, while displaying the highest influx of natural characteristics (forested areas, wetlands, and uncultivated fields), that could represent natural areas of PEI. In this study, we categorized the vegetation at all surveyed sites into three main groups: natural (including native and non-native plants), human-cultivated (such as ornamental plants), and mixed (a combination of natural and human-cultivated) based on the dominant plants present at each site. We used the provincial vegetation classification standards (e.g., Government of Prince Edward Island, 1977; Pollinator Partnership Canada, 2017) for this classification.
2.2 Photographic survey of bumble bees
For this study, we opted for a non-lethal, non-invasive photographic survey method at the request of Parks Canada. This decision was made to avoid capturing live animals within PEINP and to minimize disturbance to bumble bee populations. We chose photographic surveys because we believe photographs are a reliable survey instrument, especially when complimented via citizen science platforms utilized or managed by field experts that verify identification. We conducted a literature review to identify the specific characteristics of species expected to occur in PEI and used the identification key published by Williams et al. (2014). In addition, we also consulted local and regional experts to ensure that our list of characteristics was accurate and exhaustive. Experts from the citizen science platforms were consulted for species that exhibited similar physical characteristics or if we were unsure of an individual species identification.
2.3 Survey protocol
We surveyed each study site in August and September 2019, between 1000 and 1600, when the ambient temperature was at least 12°C, and no prolonged rain periods or strong winds during the survey time (sensu Pacific Northwest Bumble Bee Atlas, 2019). Even though bumble bees can fly in less suitable weather conditions relative to other bees, maintaining optimal weather conditions allows for high-quality photos of bumble bees, as strong winds can make selective focusing extremely challenging, and low light periods can greatly reduce photo quality. Two sites in Charlottetown and one in PEINP could not be re-surveyed in September for reasons out of our control. We opted not to use pre-determined transect lines to avoid the unintended exclusion of flora in bloom within the selected site (Nielsen et al. 2011). Therefore, transect lines varied slightly from August to September, as required, if the location of blooming floral resources had alternated. To reduce the possibility of double counting the same individual bumble bee, each survey was conducted for 10 minutes and involved continuously walking in a direction (e.g., meandering East to West) and only changing directions sharply if required (e.g., urban park site with flora positioned in a bordering L shape). Timers were paused if an individual bumble bee displayed uncharacteristic color patterns requiring significant photographs to further aid in accurate identification (e.g., individuals with lost hair, unusual pigmentation, and morphologically similar species).
Photographs were taken with a smartphone (Samsung S8 generation; 2268 x 4032 pixels) to provide an optimal view of the abdomen, face, and thorax of bumble bees (Figure 2). An initial attempt was made to distinguish each bumble bee at the species level during the photographing process in the field. Confirmation of the identification was made by examining the photographs on a laptop and noting distinct morphological features (e.g., abdomen/tail color, facial hair, and the number/pattern of bands) (sensu Laverty and Harder, 1988). We could confidently recognize most species in our study sites by assessing morphological traits. Field observations and photographs did not permit us to distinguish confidently between B. vagans and B. sandersoni (two species expected to occur in PEI), therefore we grouped all potential observations of individuals from these two species into one group for data analyses (B. sandersoni/B. vagans). The photographs depicting bumble bees with un-characteristic color patterns were posted on iNaturalist (iNaturalist, 2020) and/or Bumble Bee Watch (The Xerces Society et al., 2020) as well as individuals from each species with typical patterns to confirm surveyor validation.

Figure 2. Example of photographic procedure for each individual bumble bee so that multiple angles of the specimen are recorded to ensure accurate species identification (A–D). Photos taken by Janelle MacLeod of B. bimaculatus on August 17, 2019.
2.4 Data analysis
To assess community structure at each site in Charlottetown and PEINP, we computed species richness and Simpson’s index of diversity. We used evenness as a measure of relative abundance. Evenness values were calculated as the inverse of the Simpson’s index for each study site in both August and September (Krebs, 1989). Evenness reflects the relative abundance of species, indicating whether a community is dominated by a few species or if species are more evenly distributed. For example, if a community has high evenness, species are in similar abundance. Conversely, low evenness indicates that a few species are much more abundant than others. All community measurements, including species richness, species diversity, and evenness, were computed using Stata. Log transformation was applied to variables as needed to meet the assumptions of parametric statistical analyses. Multiple regression analyses were employed to investigate the influence of vegetation types and surveyed months on species diversity and evenness, while Poisson regression analysis was utilized to explore their effects on species richness.
3 Results
Overall, we observed 1,414 individual bumble bees in the 40 sites surveyed in this study (Table 1). Approximately the same number of bumble bees were photographed for Charlottetown (n = 705) and PEINP (n = 709). The most frequently observed species was B. impatiens representing ~79% of the observed bumble bees in Charlottetown sites and 58% in PEINP sites. B. perplexus and B. bimaculatus were only photographed in the Charlottetown sites (Figures 3–6). The average species richness in Charlottetown sites was 2.47 ± 1.33 (Table 2) and 3.56 ± 1.17 in PEINP (Table 3). Charlottetown displayed relatively lower species diversity, averaging 1.58 ± 0.67 compared to 2.13 ± 0.79 within PEINP. For evenness, the average in Charlottetown was 0.73 ± 0.23, while in PEINP, it was 0.62 ± 0.19.
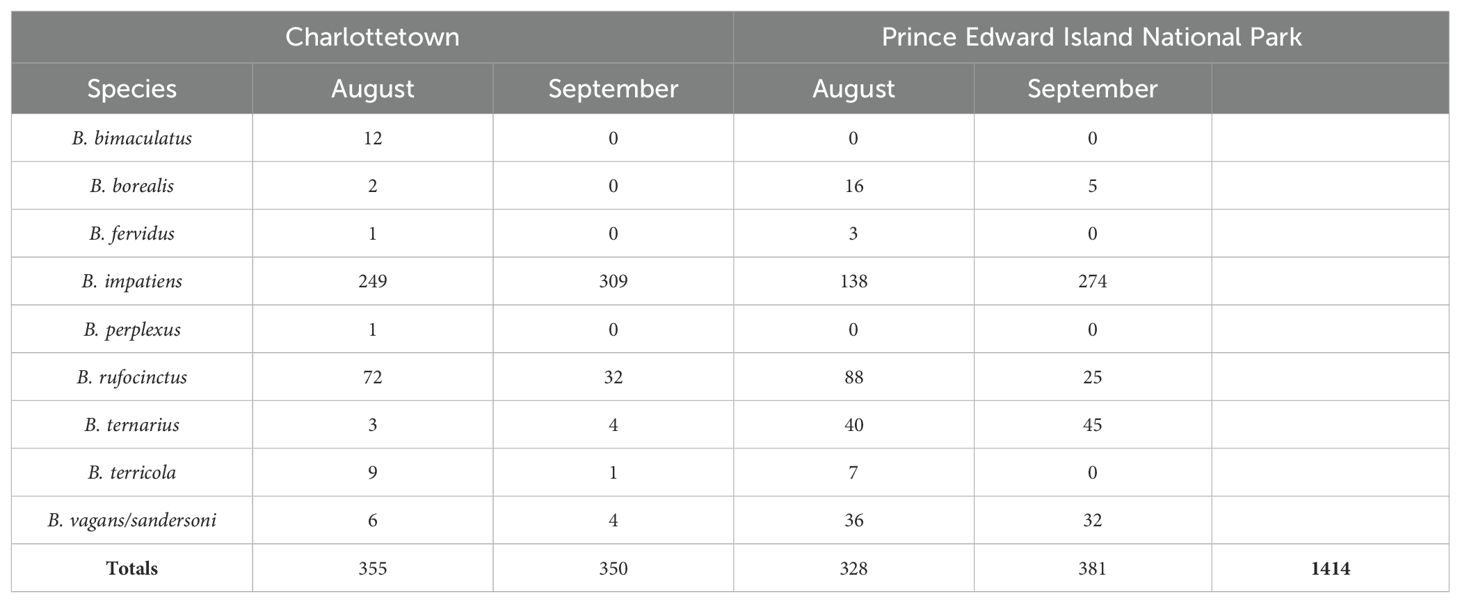
Table 1. Total bumble bee abundances based on photographic surveys conducted in August and September 2019 in Charlottetown, Prince Edward Island and Prince Edward Island National Park.
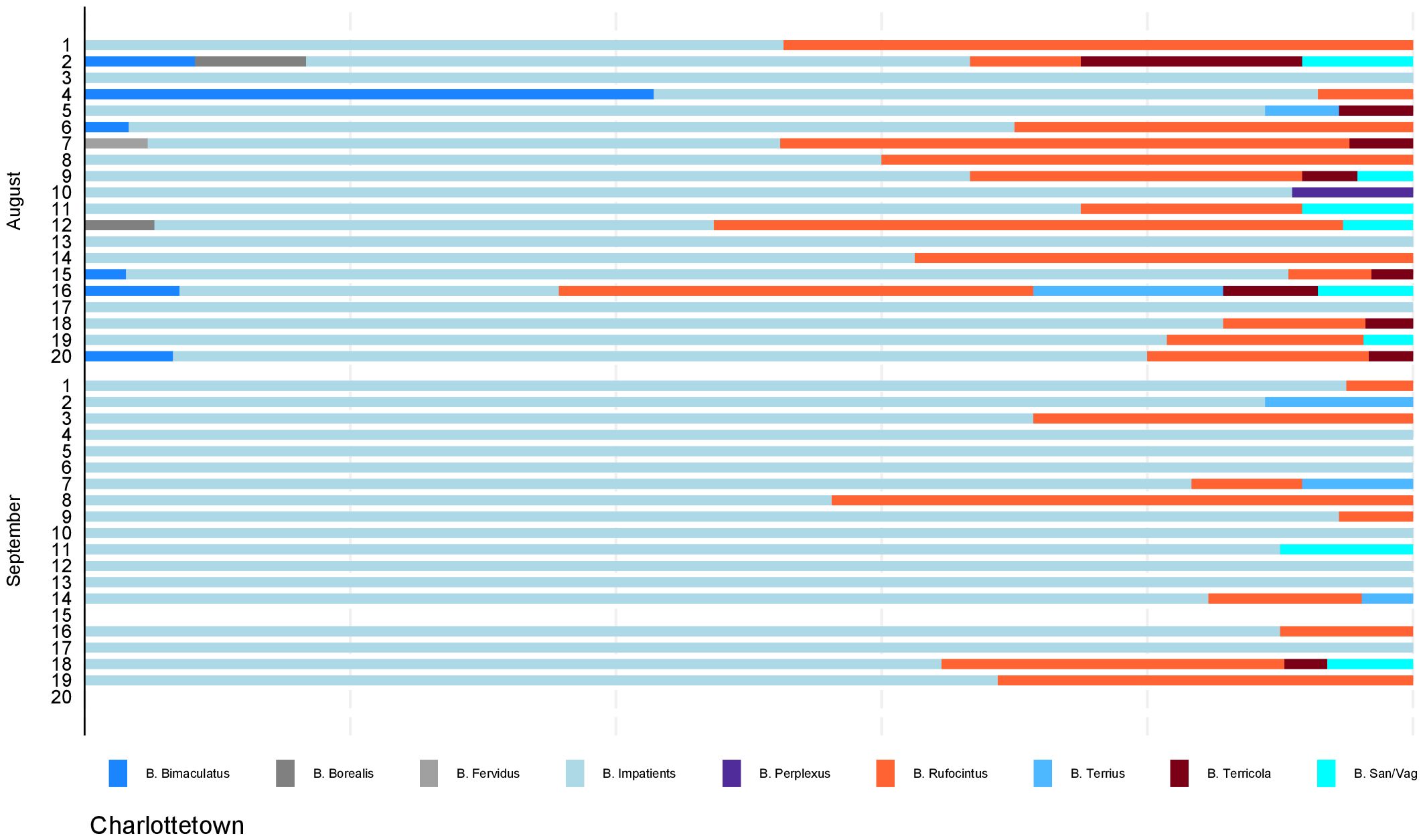
Figure 3. Distribution of bumble bees by site. Proportions of identified species by sampling month and location from photographic surveys conducted in Charlottetown, Prince Edward Island in August and September 2019. Site number is indicated on the y-axis which accounts for all sites within the study area. Sites were surveyed in August and re-surveyed in September; sites with no data in September indicate sites that could not be re-surveyed due to reasons out of our control.
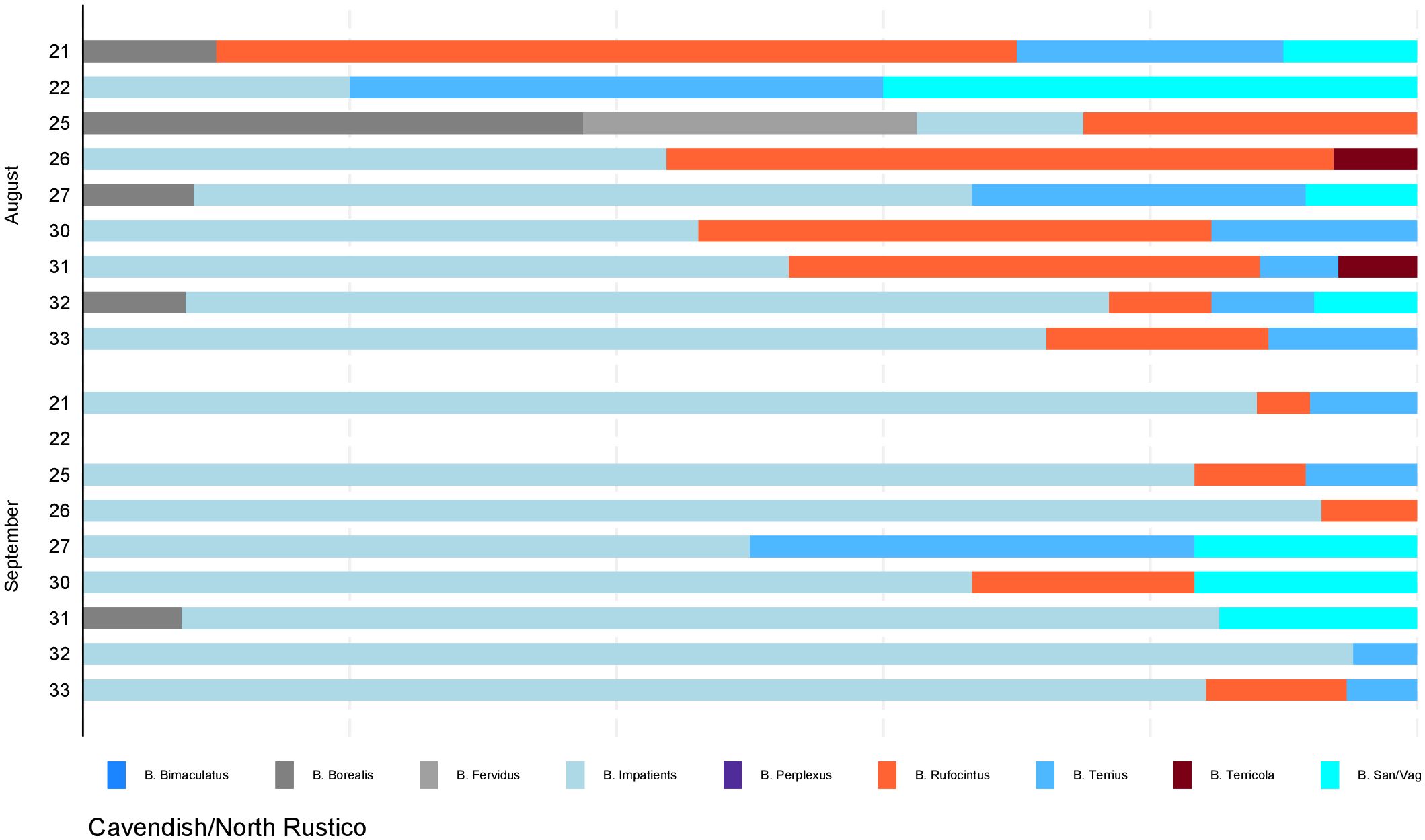
Figure 4. Distribution of bumble bees by site. Proportions of identified species by sampling month and location from photographic surveys conducted in the locations within Cavendish and North Rustico of Prince Edward Island National Park in August and September 2019. Site number is indicated on the y-axis which accounts for 9 of the 20 sites in the study area. Sites were surveyed in August and re-surveyed in September; sites with no data in September indicate sites that could not be re-surveyed due to reasons out of our control.
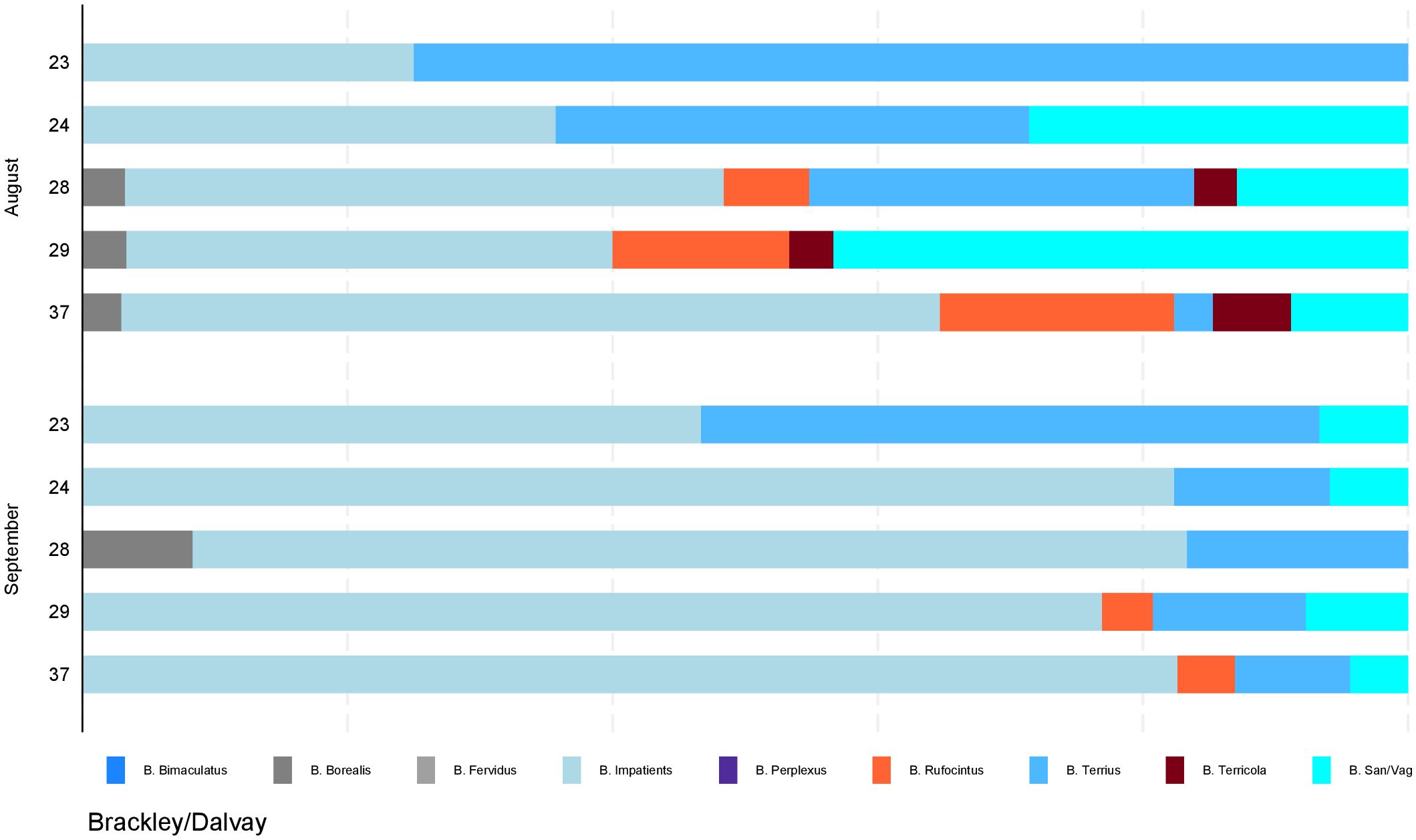
Figure 5. Distribution of bumble bees by site. Proportions of identified species by sampling month and location from photographic surveys conducted in the locations within Brackley and Dalvay of Prince Edward Island National Park in August and September 2019. Site number is indicated on the y-axis, accounting for 5 sites of the 20 sites in the study area. Sites were surveyed in August and re-surveyed in September.

Figure 6. Distribution of bumble bees by site. Proportions of identified species by sampling month and location from photographic surveys conducted in the locations within Greenwich and Fort Amherst of Prince Edward Island National Park in August and September 2019. Site number is indicated on the y-axis, accounting for 6 of the 20 sites in the study area. Sites were surveyed in August and re-surveyed in September.
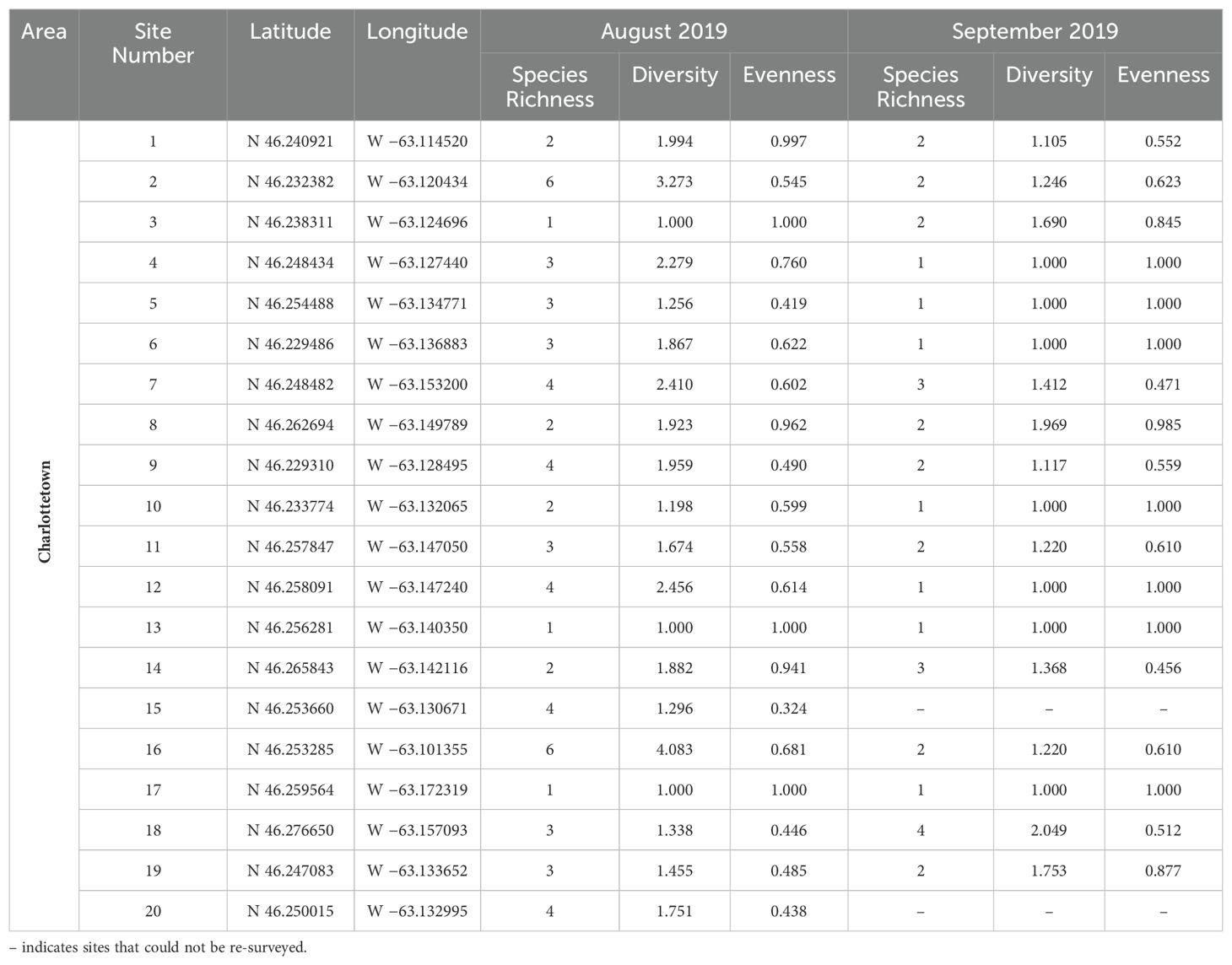
Table 2. Measurements of species richness, species diversity, and evenness for all photographic bumble bee survey locations in Charlottetown, Prince Edward Island, surveyed in August and September 2019.
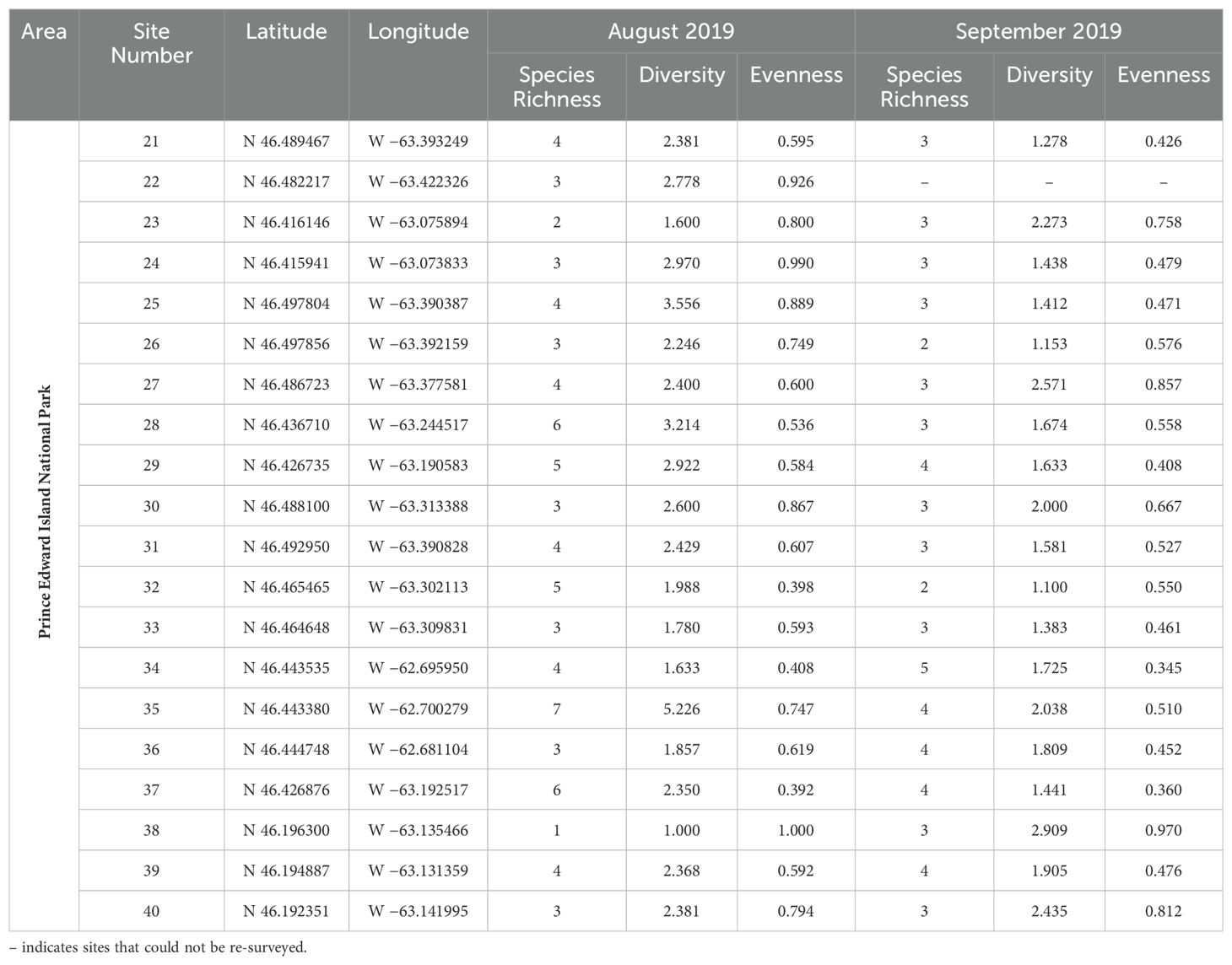
Table 3. Measurements of species richness, species diversity, and evenness for all photographic bumble bee survey locations in Prince Edward Island National Park, surveyed in August and September 2019.
Of the total individuals photographed in this study, 7.3% were submitted to either iNaturalist or Bumble Bee Watch platforms to attempt to ascertain the accuracy of our initial species identification. Identifications via iNaturalist were predominantly confirmed by expert John Ascher (curator for the platform and Assistant Professor at the National University of Singapore). Identifications via Bumble Bee Watch were confirmed by expert Victoria MacPhail (Environment and Climate Change Canada). Photographs of bumble bees identified as B.vagans/sandersoni were posted on iNaturalist and Bumble Bee Watch platforms but generally only confirmed to Pyrobombus. Based on all the responses from the experts on these platforms, only 3% of our preliminary identifications were inaccurately identified.
For the statistical analysis, month and site were significant for species richness (p-value = 0.0314). Species diversity was higher in all PEINP sites than Charlottetown in August and September (p-value = 0.0006). Cavendish/North Rustico sites displayed a 30% higher diversity index than Charlottetown. Plant type was the only significant predictor of evenness (p-value = 0.0184). Evenness index was lower in anthropogenic settings than natural, yet mixed settings displayed a higher index than natural by 24%. The results of all models are presented in Table 4.
4 Discussion
4.1 Baseline data for Charlottetown, PEI and PEINP
In our study sites, we observed nine bumble bee species, six of which were also recorded by Laverty and Harder in 1988. Two expected species, B. vagans and B. sandersoni, are difficult to differentiate without close inspection. Therefore, we only confirmed the subgenus Pyrobombus as representing one species, but both species may be present, which would account for seven of the nine species expected. In addition, we found two species, B. perplexus, and B. bimaculatus, only in Charlottetown sites, which had not previously been reported in PEI. This is an important finding suggesting that B. bimaculatus may be expanding its range into Atlantic Canada, confirming observations made by Dominey (2021) in the neighboring province of Nova Scotia. Furthermore, based on “Research Grade” iNaturalist records, B. bimaculatus is most abundant between July and early September. This may explain why all 12 individuals we observed from this species were photographed only in August.
We only observed one individual of B. perplexus, documented in urban site (Site 10) on the southern border of the survey area and on an ornamental plant (chrysanthemum sp.). While listed as secure for the research location (NatureServe, 2024), limited documentation is not completely surprising given with the restricted survey period. Yet, we observed relatively higher abundances of B. terricola; while B. terricola is considered a common species (NatureServe, 2024), they are listed as Species of Special Concern by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2015). Although the seasonal activity of B. perplexus typically reaches peak abundance slightly earlier than B. terricola, our observations showed overlap. The higher number of B. terricola (n=10) compared to B. perplexus (n=1) suggests that B. perplexus may be experiencing declines. However, additional studies are necessary to confirm this trend throughout the species’ active life cycle in PEI.
No specimens of B. citrinus or B. insularis were observed at any of our urban or natural sites. The most likely explanation for the absence of these two species is that they are parasitic species without worker castes, making them difficult to observe. According to both iNaturalist and Bumble Bee Watch, there are sufficient “Research Grade” sightings of individuals from both B. insularis and B. citrinus in early and mid-summer to confirm that these two species occur on PEI (iNaturalist, 2020; The Xerces Society et al., 2020). As our photographic surveys were conducted in August and September, it is evident that potential opportunities to capture images of these two species at our study sites may have been missed. To mitigate this issue, it is recommended that future studies incorporate surveys that encompass the entire seasonal activity of Bombus, preferably spanning from May to October. The acquisition of a more comprehensive dataset would enable scientists and conservationists to effectively gauge the focal areas for their endeavors and identify species warranting a conservation management program.
One species not previously recorded in PEI but expected to be present was B. impatiens, given its presence in nearby Canadian provinces and American states (Matteson and Langellotto, 2009). Our study revealed the species’ presence in urban (n=558) and natural sites (n=412). B. impatiens dominated most survey areas irrespective of month and site, indicating its adaptability to various conditions. These findings are consistent with those of Dominey (2021), indicating that while B. impatiens was not historically recorded in the study area, it is now firmly established within the region. Contrary to other species, B. impatiens populations typically reach peak abundance in September and continue their colony cycle into the fall (Colla and Dumesh, 2010), explaining its prevalence in our study sites during that period. The fact that Laverty and Harder (1988) did not document the presence of this species in PEI may be attributed to their study’s reliance on museum specimens rather than field surveys. Alternatively, the species may have expanded its distribution since their research, approximately 36 years ago. Future studies should explore other areas of PEI to assess the potential widespread distribution of this species throughout the province.
4.2 Photographic survey approach
The study used photographic surveys to examine bumble bee populations in urban and natural habitats and sought expert opinion via citizen science platforms to aid accurate identification. The effectiveness and cost-efficiency of this approach were successfully demonstrated, indicating its potential for application in areas where different species can be distinguished based on physical characteristics. However, our findings also revealed that photographic surveys of bumble bees had limitations when two or more species showed morphological resemblances, such as in the example of B. vagans and B. sandersoni. Individuals from these two species were indistinguishable based on photographs because of their similar morphological traits. Other photographic surveys have also found that with two or more morphologically similar species, only 94-98% of sightings could be identified to the species level (Armistead, 2023; Flaminio et al. 2021). To enhance taxonomic identification, we suggest researchers consider integrating netting to temporarily capture individuals, especially morphologically similar species. This would more effectively enable the differentiation between species that share similar morphological characteristics. Yet, in some cases, identification may be impossible without lethal capture (microscopic inspection and/or genetic analysis), for example, differentiating B. vagans and the subspecies B. vagans bolsteri. While more labor intensive than passive capture, this combination of surveys ensures minimal disturbance to populations. With the advancements posed by artificial intelligence, it is perceivable that future citizen science platforms may not require humans to dedicate time to confirm species identification (Montero-Castaño et al., 2022; Suzuki-Ohno et al., 2017).
Like any other citizen science approach, species identification based on photographs of bumble bees requires a certain level of taxonomic knowledge or expertise from the part of the surveyor. To verify surveyor expertise in accurately identifying bumble bee species, one should refer to published keys to test abilities before conducting field surveys (MacPhail et al., 2020; Montero-Castaño et al., 2022). However, the effectiveness of this approach also largely depends on what Bombus species are present. If the temporal region includes multiple species that are not easily distinguishable from field surveys, another approach may be deemed more appropriate. Limitations will still be present when conducting photographic surveys. Aside from morphologically similar species, some temporal regions support higher volumes of expected species, which can further complicate accurate identification in the field. When considering other pollinator species, such as solitary bee species, it can be impossible to differentiate without lethal capture and genetic analyses. Nonetheless, it is noteworthy that photographic surveys of bumble bees continue to offer high-quality data for preliminary assessments or to acquire informative diversity and richness indexes without necessitating permits or intrusive techniques. We suggest considering all possible limitations given the expected species for the region, and comparing the photographic survey approach to other capture methods (Armistead, 2023) to properly facilitate research objectives.
4.3 Bumble bee community comparison: urban vs. other sites
Our study found that PEINP sites exhibited the highest levels of species richness, relative abundance, and diversity compared to Charlottetown urban sites. The highest species richness observed in a single survey was within a Greenwich site (n=7). This site documented the presence of seven of the nine total species observed. Cavendish/North Rustico, while experiencing increased levels of anthropogenic activity, is predominantly surrounded by agriculture. This survey area would benefit from a comparative analysis to assess how exactly bumble bees use and adapt to each landscape use. A monthly analysis revealed a decrease in both species richness and abundance across all study sites in September. These findings are consistent with previous studies that have documented seasonal variations in the abundance of bumble bees, including some of the species found in PEI (e.g., Novotny et al., 2021). A plausible explanation for this decline is that most bumble bee species may have concluded their colony cycles by late August.
Interestingly, sites characterized by a mix of natural and human-cultivated (ornamental) vegetation—largely, sites within urban Charlottetown—showed greater bumble bee species diversity and abundance when compared to sites with solely natural vegetation or human-cultivated. Supporting this, other studies (Baldock et al. 2015; Kaluza et al. 2016; Marín et al. 2020; Nakamura and Kudo 2019; Sirohi et al., 2015) propose that urban green spaces with varied land use and moderate human activity promote species diversity by enhancing habitat and foraging diversity. For bumble bees, the mix of natural and human-cultivated vegetation offers a continuous and diverse supply of floral resources throughout the growing season, able to support a wider range of species with different foraging preferences and needs (Nakamura and Kudo 2019; Sikora et al., 2020). Furthermore, moderate disturbances like occasional mowing, planting, or construction in urban sites may prevent any single species from dominating the ecosystem. It is also possible that while ornamental plants may be visited, naturally occurring species (e.g., aster, clover, goldenrod) are the drivers for visitation within the area. To further this finding, studies should be conducted to encapsulate the entire active life cycle of bumble bees, as well as documenting floral preferences and visitation rates among each species.
5 Conclusion
Our study significantly contributes to the conservation of bumble bee species by demonstrating that photographic surveys offer a practical and cost-effective method for obtaining baseline data in urban sites and other areas where traditional or more invasive sampling methods may not be easily feasible. Citizen science platforms, when utilized by field experts, also create an opportunity to ensure the accuracy of surveyor identifications. Yet, they should not replace verification using taxonomic keys or expert consultation. Knowledge of the species in a given habitat is essential for practical conservation efforts. Considering the fragmentation caused by urbanization and agricultural activities on PEI, our findings suggest that urban green spaces, including, but not limited to, human-made gardens, parks, playgrounds, trails, cemeteries (Daniels et al., 2020; Wood et al. 2018), may serve as refuges for certain bumble bee species, aligning with the observations of Samuelson et al. (2018). Specifically, urban areas that incorporate a variety of native floral resources can play a crucial role in preserving bumble bee diversity, as observed in similar studies (Boone et al., 2022; Conflitti et al. 2022; Liang et al., 2023), especially when surrounded by habitats such as monoculture cropping (Deguines et al., 2016). Although human-introduced vegetation is sometimes undervalued and often removed from urban green spaces, we suggest further investigation into the role of these flora species in supporting urban fauna, including pollinators, before deciding on their removal.
In situations where several morphologically similar species are expected, we recommend adopting a survey methodology that integrates photography with temporary capture (netting) to facilitate the scrutiny of critical attributes and the accurate identification of these species (Armistead, 2023; Bell et al., 2023; Montero-Castaño et al., 2022). Accumulating additional data will inform and enhance conservation measures, especially for rare and specialized species; what benefits a generalist species may prove harmful to a rare species in peril (Liczner and Colla, 2020). Furthermore, it will help visualize which local species are stable and which are struggling beyond those officially listed by COSEWIC. Constructing a more comprehensive dataset combined with in-depth pollinator-plant interaction analyses will allow for additional advantages, such as informing local municipalities in targeted green space planning. It will also provide valuable insights to PEINP on enhancing their natural environments and constructing vital habitat corridors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because there was no handling of any animals in this study (photographic only).
Author contributions
JM: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. MS-O: Conceptualization, Formal Analysis, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. JS: Formal Analysis, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial support for this project was in-part provided by Parks Canada.
Acknowledgments
We are thankful to David McCorquodale, Cory Sheffield, Laurence Packer, and Vince Repaci for their help with the study design. We are grateful to Victoria MacPhail and John Ascher for their contribution to species identification. Our sincerest gratitude goes out to the PEINP students (Lindsey Burke, Lesley Macmillan, John Miller, Katie Sock, and Jenna Schellekens) who dedicated their time to aid the photographic surveys. Our gratitude also goes to the personnel of PEINP, especially Paul Ayles, as well as Kerry-Lynn Atkinson, and Hailey Paynter for their logistical support with surveys conducted at the park. Access to the provincial land covers digital information used for the maps was supplied by the Department of Agriculture and Forestry of Prince Edward Island. Finally, this research benefited from the hundreds of citizen scientists from Prince Edward Island who provided their bumble bee sightings to both iNaturalist and the Bumble Bee Project platforms.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arbulo N., Antúnez K., Salvarrey S., Santos E., Branchiccela B., Martín-Hernández R., et al (2015). High prevalence and infection levels of Nosema ceranae in bumblebees Bombus atratus and Bombus bellicosus from Uruguay. J. Invertebrate Pathol. 130, 165–168. doi: 10.1016/j.jip.2015.07.018
Armistead J. J. (2023). A comparison of blue vane trap, timed targeted netting, and timed photographic collection methods for evaluating Canadian bumble bee diversity (Brock University: Faculty of Mathematics and Science), 1009. Available at: http://hdl.handle.net/10464/17905.
Baldock K. C. (2020). Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 38, 63–71. doi: 10.1016/j.cois.2020.01.006
Baldock K. C. R., Goddard M. A., Hicks D. M., Kunin W. E., Mitschunas N., Osgathorpe L. M., et al (2015). Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 282, 20142849. doi: 10.1098/rspb.2014.2849
Bell C., Tronstad L., Hotaling S. (2023). Tailoring your bee sampling protocol: Comparing three methods reveals the best approaches to capturing bees. Agric. For. Entomol. 25, 477–488. doi: 10.1111/afe.12569
Blackmore L. M., Goulson D. (2014). Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect Conserv. Diversity 7, 480–484. doi: 10.1111/icad.12071
Bond W. J. (1994). “Keystone species,” in Biodiversity and Ecosystem Function. Eds. Schulze E. D., Mooney H. A. (Springer-Verlag, Berlin), 237–253. doi: 10.1007/978-3-642-58001-7_11
Boone M., Evans E., Wolf A., Minser H., Watson J., Smith T. (2022). Notes from rusty patched bumble bee (Bombus affinis Cresson) nest observations. Insect Conserv. Diversity 15, 380–384. doi: 10.1111/icad.12564
Breuste J., Niemelä J., Snep R. P. H. (2008). Applying landscape ecological principles in urban environments. Landscape Ecol. 23, . 1139–. 1142. doi: 10.1007/s10980-008-9273-0
Brooks D. R., Nocera J. J. (2020). Bumble bee (Bombus spp.) diversity differs between forested wetlands and clearcuts in the Acadian forest. Can. J. For. Res. 50, 1399–1404. doi: 10.1139/cjfr-2020-0094
Brown M. I. (2022). “Bumblebee response to glyphosate application within managed forests of New Brunswick, Canada,” (Moncton (NB): University of New Brunswick). Retrieved from https://unbscholar.lib.unb.ca/handle/1882/37313.
Cameron S. A., Hines H. M., Williams P. H. (2007). A comprehensive phylogeny of the bumble bees (Bombus). Biol. J. Linn. Soc. 91, 161–188. doi: 10.1111/j.1095-8312.2007.00784.x
Carvell C., Bourke A. F., Dreier S., Freeman S. N., Hulmes S., Jordan W. C., et al (2017). Bumblebee family lineage survival is enhanced in high-quality landscapes. Nature 543, 547–549. doi: 10.1038/nature21709
Colla S., Dumesh S. (2010). The bumble bees of southern Ontario: notes on natural history and distribution. J. Entomol. Soc. Ontario 141, 39–68.
Conflitti I. M., Imrit M. A., Morrison B., Sharma S., Colla S. R., Zayed A. (2022). Bees in the six: Determinants of bumblebee habitat quality in urban landscapes. Ecol. Evol. 12, e8667. doi: 10.1002/ece3.8667
COSEWIC (2015). COSEWICAssessment and Status Report on the Yellow-banded Bumble Bee Bombus Terricola in Canada (Ottawa: Committee on the Status of Endangered Wildlife in Canada).
Daniels B., Jedamski J., Ross-Nickoll R. O. M. (2020). A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PloS One 15, e0235492. doi: 10.1371/journal.pone.0235492
Deguines N., Julliard R., Flores M. D., Fontaine C. (2016). Functional homogenization of flower visitor communities with urbanization. Ecol. Evol. 6, 1967–1976. doi: 10.1002/ece3.2009
Dominey K. A. (2021). Are Yellow-banded Bumble Bee (YBBB) Bombus terricola and Gypsy Cuckoo Bumble Bee (GCBB) Bombus bohemicus at risk in Cape Breton. Sydney (NS): Cape Breton Univeristy.
Falk S., Foster G., Comont R., Conroy J., Bostock H., Salisbury A., et al. (2019). Evaluating the ability of citizen scientists to identify bumblebee (Bombus) species. PloS One 14, e0218614. doi: 10.1371/journal.pone.0218614
Fauser-Misslin A., Sadd B. M., Neumann P., Sandrock C. (2013). Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J. Appl. Ecol. 51, 450–459. doi: 10.1111/1365-2664.12188
Fisher K., Watrous K. M., Williams N. M., Richardson L. L., Woodard S. H. (2022). A contemporary survey of bumble bee diversity across the state of California. Ecol. Evol. 12, e8505. doi: 10.1002/ece3.8505
Flaminio S., Ranalli R., Zavatta L., Galloni M., Bortolotti L. (2021). Beewatching: A project for monitoring bees through photos. Insects 12, 841. doi: 10.3390/insects12090841
Goulson D., Rayner P., Dawson B., Darvill B. (2011). Translating research into action; bumblebee conservation as a case study. J. Appl. Ecol. 48, 3–8. doi: 10.1111/j.1365-2664.2010.01929.x
Government of Prince Edward Island (1977). Island woodland plants. Available online at: https://www.princeedwardisland.ca/sites/default/files/publications/pei_woodland_plants.pdf.
Grixti J. C., Wong L. T., Cameron S. A., Favret C. (2009). Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 142, 75–84. doi: 10.1016/j.biocon.2008.09.027
Harrison T., Gibbs J., Winfree R. (2019). Anthropogenic landscapes support fewer rare bee species. Landscape Ecol. 34, 967–978. doi: 10.1007/s10980-017-0592-x
Heinrich B. (1975). Thermoregulation in bumblebees. J. Compartive Physiol. 96, 155–166. doi: 10.1007/BF00706595
Heinrich B., Kammer A. E. (1973). Activation of the fibrillar muscles in the bumblebee during warm-up, stabilization of thoracic temperature and flight. J. Exp. Biol. 58, 677–688. doi: 10.1242/jeb.58.3.677
Hernandez J. L., Frankie G. W., Thorp R. W. (2009). Ecology of urban bees: A review of current knowledge and directions for future study. Cities Environ. 2. Available at: http://escholarship.bc.edu/cate/vol2/iss1/3.
iNaturalist (2020). Bumble bees (Genus bombus). Available online at: www.inaturalist.org.
Kaluza B. F., Wallace H., Heard T. A., Klein A.-M., Leonhardt S. D. (2016). Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 6, 1304–1316. doi: 10.1002/ece3.1941
Kolinjivadi V., Bissonnette J.-F., Mendez A. Z., Dupras J. (2020). Would you like some fries with your ecosystem services?: McDonaldization and conservation in Prince Edward Island, Canada. Geoforum 111, 73–82. doi: 10.1016/j.geoforum.2020.03.003
Laverty T. M., Harder L. D. (1988). The bumble bees of Eastern Canada. Can. Entomologist 120, 965–987. doi: 10.4039/Ent120965-11
Lhomme P., Hines H. M. (2018). Ecology and evolution of cuckoo bumble bees. Ann. Entomological Soc. America 112, 1–19. doi: 10.1093/aesa/say031
Liang H., He Y.-D., Theodorou P., Yang C.-F. (2023). The effects of urbanization on pollinators and pollination: A meta-analysis. Ecol. Lett. 26, 1629–1642. doi: 10.1111/ele.14277
Liczner A. R., Colla S. R. (2020). One-size does not fit all: at-risk bumble bee habitat management requires species-specific local and landscape considerations. Insect Conserv. Diversity 13, 558–570. doi: 10.1111/icad.12419
MacPhail V. J., Gibson S. D., Colla S. R. (2020). Community science participants gain environmental awareness and contribute high quality data but improvements are needed: insigfrom Bumble Bee Watch. PeerJ 8, e9141. doi: 10.7717/peerj.9141
Marín L., Martínez-Sánchez M. E., P. S., Navarrete D., Morales H. (2020). Floral visitors in urban gardens and natural areas: Diversity and interaction networks in a neotropical urban landscape. Basic Appl. Ecol. 43, 3–15. doi: 10.1016/j.baae.2019.10.003
Marshman J., Blay-Palmer A., Landman K. (2019). Anthropocene crisis: climate change, pollinators, and food security. Environments 6, 22. doi: 10.3390/environments6020022
Masson S. W., Hedges C. P., Devaux J. B., James C. S., Hickey A. J. (2017). Mitochondrial glycerol 3-phosphate facilitates bumblebee pre-flight thermogenesis. Sci. Rep. 7, 13107. doi: 10.1038/s41598-017-13454-5
Matteson K. C., Ascher J. S., Langellotto G. A. (2008). Bee richness and abundance in New York city urban gardens. Ann. Entomological Soc. America 101, 140–150. doi: 10.1603/0013-8746(2008)101[140:BRAAIN]2.0.CO;2
Matteson K. C., Langellotto G. A. (2009). Bumble bee abundance in New York city community gardens: implications for urban agriculture. Cities Environ. 2, 5. doi: 10.15365/cate.2152009
Matteson K. C., Langellotto G. A. (2010). Determinates of inner city butterfly and bee species richness. Urban Ecosyst. 13, 333–347. doi: 10.1007/s11252-010-0122-y
Montero-Castaño A., Koch J. B., Lindsay T.-T. T., Love B., Mola J. M., Newman K., et al. (2019). Comparative survival and fitness of bumble bee colonies in natural, suburban, and agricultural landscapes. Agriculture Ecosystems Environ. 284, 106594. doi: 10.1016/j.agee.2019.106594
Montero-Castaño A., Koch J. B., Lindsay T.-T. T., Love B., Mola J. M., Newman K., et al (2022). Pursuing best practices for minimizing wild bee captures to support biological research. Conserv. Sci. Pract. 4, e12734. doi: 10.1111/csp2.12734
Nakamura S., Kudo G. (2019). The influence of garden flowers on pollinator visits to forest flowers: comparison of bumblebee habitat use between urban and natural areas. Urban Ecosystems 22, 1097-1112. doi: 10.1007/s11252-019-00891-5
NatureServe (2024). Bombus perplexus. Available online at: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.116054/Bombus_perplexus.
Nielsen A., Steffan-Dewenter I., Westphal C., Messinger O., Potts S. G., Roberts S. P. (2011). Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 26, 969–983. doi: 10.1007/s11284-011-0852-1
Novotny J. L., Reeher P., Varvaro M., Lybbert A., Smith J., Mitchell R. J., et al. (2021). Bumble bee species distributions and habitat associations in the Midwestern USA, a region of declining diversity. Biodiversity Conserv. 30, . 865–. 887. doi: 10.1007/s10531-021-02121-x
Nunes L. A., Tai T., Zuckerberg B., Clare J. D., Jepsen S., Strange J., et al. (2024). Local floral abundance influences bumble bee occupancy more than urban-agricultural landscape context. Insect Conserv. Diversity 17, 215–228. doi: 10.1111/icad.12719
Nunes-Silva P., Hrncir M., Shipp L., Kevan P., Imperatriz-Fonseca V. L. (2013). The behaviour of Bombus impatiens (Apidae, Bombini) on tomato (Lycopersicon esculentum Mill., Solanaceae) flowers: pollination and reward perception. J. Pollination Ecol. 11, 33–40. doi: 10.26786/1920-7603(2013)3
Pacific Northwest Bumble Bee Atlas (2019). Participant handbook - 2019. Available online at: https://www.pnwbumblebeeatlas.org/uploads/1/1/6/9/116937560/participant_handbook_2019_web.pdf.
Palmier K. M., Sheffield C. S. (2019). First records of the Common Eastern Bumble Bee, Bombus impatiens Cresson (Hymenoptera: Apidae, Apinae, Bombini) from the Prairies Ecozone in Canada. Biodiversity Data J. 7, e30953. doi: 10.3897/BDJ.7.e30953
Parrey A. H., Raina R. H., Saddam B., Pathak P., Kumar S., Uniyal V., et al. (2021). Role of bumblebees (Hymenoptera: apidae) in pollination of high land ecosystems: A review. Agric. Rev. 43, 368–373. doi: 10.18805/ag.R-2159
Pollinator Partnership Canada (2017). Selecting plants for pollinators: A guide for gardeners, farmers, and land managers in the Prince Edward Island ecoregion. Available online at: https://pollinator.org/guides_Canada?c=c0a.
Potts S. G., Imperatriz-Fonseca V., Ngo H. T., Aizen M. A., Biesmeijer J. C., Breeze T. D., et al. (2016). Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. doi: 10.1038/nature20588
Samuelson A., Gill R., Brown M., Leadbeater E. (2018). Lower bumblebee colony reproductive success in agricultural compared with urban environments. R. Soc. Publishing 285, 20180807. doi: 10.1098/rspb.2018.0807
Sheffield C. S., Kevan P., Smith R. F., Rigby S. M., Rogers R. E., Westby S. (2003). Bee species of Nova Scotia, Canada, with new records and notes on bionomics and floral relations (Hymenoptera: apoidea). J. Kansas Entomological Soc. 76, 357–384.
Sikora A., Micholap P., Sikora M. (2020). What kind of flowering plants are attractive for bumblebees in urban green areas? Elsevier 48, 126546. doi: 10.1016/j.ufug.2019.126546
Silva M., Hartling L., Opps S. B. (2005). Small mammals in agricultural landscapes of Prince EdwardIsland (Canada): Effects of habitat characteristicsat three different spatial scales. Biol. Conserv. 126, 556–568. doi: 10.1016/j.biocon.2005.07.007
Sirohi M. H., Jackson J., Edwards M., Ollerton J. (2015). Diversity and abundance of solitary and primitively eusocial bees in an urban centre: a case study from Northampton (England). J. Insect Conserv. 19, 487–500. doi: 10.1007/s10841-015-9769-2
Statistics Canada (2018). Population and Dwelling Count Highlight Tables 2016 Census, s.l (Government of Canada).
Suzuki-Ohno Y., Yokoyama J., Nakashizuka T., Kawata M. (2017). Utilization of photographs taken by citizens for estimating bumblebee distributions. Sci. Rep. 7, 11215. doi: 10.1038/s41598-017-10581-x
Theodorou P., Baltz L. M., Paxton R. J., Soro A. (2021). Urbanization is associated with shifts in bumblebee body size, with cascading effects on pollination. Evolutionary Appl. , 14, 53–68. doi: 10.1111/eva.13087
The Xerces Society, Wildlife Preservation Canada, York University, University of Ottawa, The Montreal Insectarium, The London Natural History Museum, et al. (2020). Bumble Bee Sightings. Available online at: https://www.bumblebeewatch.org/sightings/bee/.
Tommasi D., Miro A., Higo H., Winston M. L. (2004). Bee diversity and abundance in an urban setting. Can. Entomologist 136, 851–869. doi: 10.4039/N04-010
Velthuis H. H., Doorn A. V. (2005). A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37, 421–451. doi: 10.1051/apido:2006019
Williams P. H., Thorp R. W., Richardson L. L., Colla S. R. (2014). Bumble Bees of North America: An Identification Guide (Princeton: Princeton University Press).
Winfree R., Williams N. M., Gaines H., Ascher J. S., Kremen C. (2007). Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 45, 793–802. doi: 10.1111/j.1365-2664.2007.01418.x
Keywords: bumble bees, urbanization, photographic surveys, pollinators, species richness, conservation, baseline data
Citation: MacLeod J, Silva-Opps M and Sanchez J (2024) Comparing species richness and abundance of bumble bees between urban and natural areas using a photographic survey approach. Front. Ecol. Evol. 12:1505827. doi: 10.3389/fevo.2024.1505827
Received: 03 October 2024; Accepted: 20 November 2024;
Published: 09 December 2024.
Edited by:
Kris Braman, University of Georgia, United StatesReviewed by:
Bodie Pennisi, University of Georgia, United StatesEmilee Poole, United States Department of Agriculture, United States
Copyright © 2024 MacLeod, Silva-Opps and Sanchez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janelle MacLeod, SmFuZWxsZW1hY2xlb2RAZ21haWwuY29t
 Janelle MacLeod
Janelle MacLeod Marina Silva-Opps
Marina Silva-Opps Javier Sanchez
Javier Sanchez