- 1Institute of Psychology, Polish Academy of Sciences, Warsaw, Poland
- 2Department of Biological Sciences, Fordham University, Bronx, NY, United States
- 3Department of Ecology and Evolutionary Biology, University of California, Los Angeles, Los Angeles, CA, United States
- 4Institute for Pharmacology and Toxicology & Center for Behavioral Brain Sciences, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
- 5Laboratory of Veterinary Ethology, The University of Tokyo, Tokyo, Japan
Editorial on the Research Topic
Trends toward naturalistic, field assays with free-ranging animals as contemporary alternatives to laboratory models
“If the system is more than the sum of its parts, ultimate understanding requires observation of the intact, functional whole” (Eberhardt and Thomas, 1991).
Field and laboratory research provide two fundamentally different, but potentially complimentary, paradigms for addressing experimental hypotheses. As early as the 17th century, the backbone of science, medicine and pharmacology has been constructed from highly-controlled laboratory studies. During the early stages of animal research, laboratories were necessary for breeding, husbandry, and experimentation on animals that were genetically-uniform (Gluck et al., 2002). The advantages included the provision of standardized contexts and conditions that could be repeated by other researchers using many animals of the same strains. Field research on the other hand, became widespread by the 19th century (Brinitzer and Benson, 2022), often required travel from campus to the natural environment sometimes in hard-to-reach locations, and provided a foundation for our understanding of animal ecological studies, conservation, animal welfare and wildlife management. Field studies were criticized because they provided less control over experimental designs, and by necessity, had fewer animal replicates (Eberhardt and Thomas, 1991). Yet, field studies provide some of the important contexts such as weather, shelter and conspecifics that were intentionally-removed from standardized tests (Figure 1). Both paradigms have merits and neither is perfect on its own. Indeed, for over a century, researchers have attempted to bridge the gap by bringing wild animals into the laboratory, or by providing more realistic settings in the laboratory (Fendt et al., 2020; Stryjek et al., 2021).
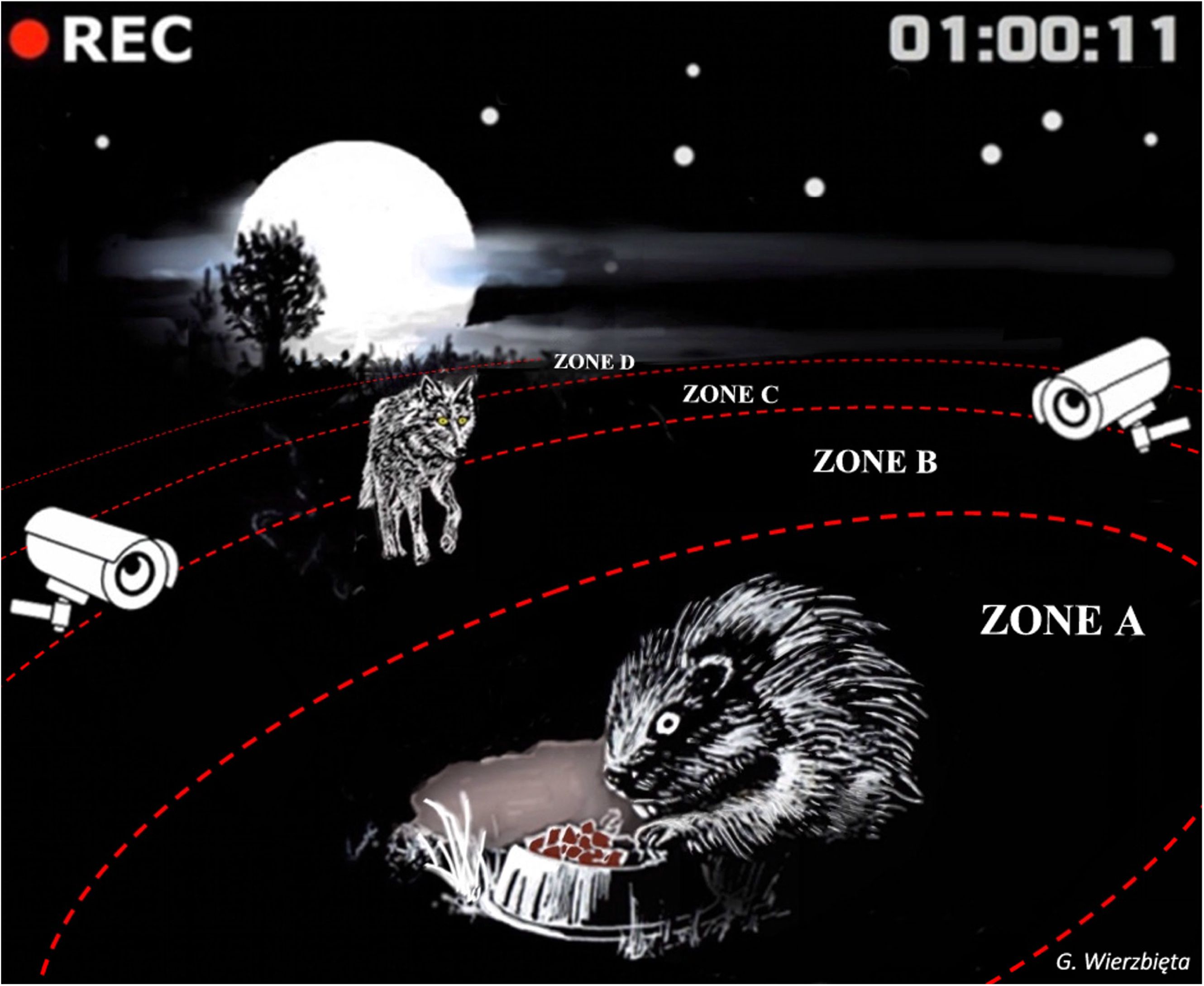
Figure 1 Representative image suggesting a palatable food it being offered to attract prey into a surveillance system where they are assessed for their behavior in response to natural risk cues in an active predator zone.
The majority of behavioral, medical and physiological research are conducted strictly in the laboratory. Therefore, we should not be surprised to learn that that, for over 50 years, researchers have been calling more naturalistic assays to support, compliment, or in some cases, replace laboratory assays (Wolff, 2003; Richter et al., 2009). The purpose of this special topic was to bring together researchers from a range of disciplines to present field assays that have replaced or supplemented tests that have been restricted to the laboratory. While such studies are not overly-common, we expected these willing researchers to discuss the strengths and weaknesses of their assays and emphasize how the natural context has strengthened confidence in their findings.
Therefore, in preparing this Research Topic, we brought together researchers from a range of countries including Poland, United States, Germany, Japan, Czechoslovakia, United Kingdom, Austria, and Indonesia, studying such diverse fauna as rhesus macaques (Macus mulatta), European catfish (Siuluris glanus), yellow necked mice (Apodemus flavicollis), striped field mice (Apodemus agrarius), Tanimbar corelllas (Cacatua goffiniana) and prairie voles (Microtus ochrogaster).
A number of critical themes emerge from this collection of diverse fauna, principally that researchers have gathered naturalistic data that would supplement similar findings in the laboratory. For instance, while studying the diel cycles of pigmented and albino European catfish (Siluris glanus), Valchářová et al. found that the availability of natural shelter influenced the rhythmicity of locomotor activity and thus, changes in aggression in both types, while light-induced stress was more apparent in albinos than pigmented catfish. Overall, this paper showed that laboratory experiments may have significant drawbacks when carried out on albino phenotypes, because these have exaggerated stress responses and altered reactions that are not seen in natural phenotypes. These findings underline the need for assessing pigmentation patterns occurring naturally in the wild to yield more accurate and ecologically-valid results, and the need to shift toward field assays with free-ranging animals to get as close as possible to the natural environment and behaviors of individuals.
Similarly, Cooper et al. tested urinary compounds as biomarkers of age-related changes in free-ranging rhesus macaques (Macaca mulatta). Typically, immunological assays have been restricted to the laboratory, yet again we have a case where a physiological test performed in the wild could provide highly complementary data to tests obtained withing laboratory conditions. In the wild, age-related changes in macaques were more pronounced under natural conditions than in laboratory bred animals.
Another critical and emergent theme was related by Parsons et al. who deployed laboratory-style chambers into the field to assay free-ranging rodents’ response to predator and control scents. Two species of wild mice (Apodemus flavivollis and Apodemus agrarius) were exposed to scents that often evoke fear or inspection. Unbeknownst to the researchers, the animals likely recognized the chamber was a shelter, and thus they were safe from predators. Instead of responding with fear, both species of wild mice, interacted with the scents—ostensibly to gauge the level of risk instead of demonstrating fear. Similarly, O’Hara et al. produced a naturalist assay to study wild Tanimbar corelllas (Cacatua goffiniana) by attracting them as ‘volunteers’ into their assay. They motivated them to participate by providing dry corn, which permitted the birds’ impressive cognitive abilities to be studied in nature. Lastly, Sailer et al. used semi-natural outdoor enclosures to explore complex behaviors in a more ecologically-valid setting, and hence further emphasized the utility of field-based studies to supplement traditional laboratory models. Specifically, this paper investigated how hippocampal damage influenced mating behaviors and spatial usage in prairie voles. The researchers discovered that, through making lesions in the dorsal CA1 region of the hippocampus, non-monogamous males—typically displaying more exploratory behavior and having more extensive home ranges—showed reduced spatial activity, as compared to monogamous males.
A final trend that was implied in several papers in our topic, but not explicitly stated within those papers, was that naturalistic assays such as these bring together wild organisms that ‘volunteer’ to be studied. Animals in the wild are hungry, and the provision of food and/or shelter is akin to paying subjects for their work. Human attitudes towards animal welfare continue to ‘evolve’, thus, creating ways to study unrestrained animals in nature may be valued more. Indeed, scales are already being developed to allow animals to indicate their own choices and degree of discomfort within natural and restricted assays (Pfefferle, 2024). Thus alternatives to laboratory studies may be appreciated by researchers and society alike (Parsons et al., 2023). Together, these studies in this Research Topic have revealed a few of the many benefits of using a mixed-approach paradigm involving the complementary use of field and laboratory studies.
Author contributions
RS: Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. DB: Writing – original draft, Writing – review & editing. MF: Writing – original draft, Writing – review & editing. YK: Writing – original draft, Writing – review & editing.
Conflict of interest
MP was employed by Centre for Urban Ecological Solutions, LLC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brinitzer C., Benson E. (2022). Introduction: what is a field? Transformations in fields, fieldwork, and field sciences since the mid-twentieth century. Isis 113, 108–113. doi: 10.1086/718147
Eberhardt L., Thomas J. (1991). Designing environmental field studies. Ecol. Monogr. 61, 53–73. doi: 10.2307/1942999
Fendt M., Parsons M. H., Apfelbach R., Carthey A., Dickman C. R., Endres T., et al. (2020). Context and trade-offs characterize real-world threat detection systems: A review and comprehensive framework to improve research practice and resolve the translational crisis. Neurosci. Biobehav. Rev. 115, 25–33
Gluck J. P., Dipasquale T., Orlans F. B. (2002). Applied ethics in animal research: philosophy, regulation, and laboratory applications (West Lafayette, IN, USA: Purdue University Press).
Parsons M. H., Stryjek R., Fendt M., Kiyokawa Y., Bebas P., Blumstein D. T. (2023). Making a case for the free exploratory paradigm: animal welfare-friendly assays that enhance heterozygosity and ecological validity. Front. Behav. Neurosci. 17, 1228478. doi: 10.3389/fnbeh.2023.1228478
Pfefferle D. (2024). Choice-based severity scale (CSS): assessing the relative severity of procedures from a laboratory animal’s perspective. PeerJ. 12, e17300.
Richter S. H., Garner J. P., Würbel H. (2009). Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat. Methods 6, 257. doi: 10.1038/nmeth.1312
Stryjek R., Parsons M. H., Fendt M., Święcicki J., Bębas P. (2021). Let’s get wild: A review of free-ranging rat assays as context-enriched supplements to traditional laboratory models. J. Neurosci. Methods 362, 109303. doi: 10.1016/j.jneumeth.2021.109303
Keywords: bench-to-bedside gap, domesticated laboratory animals, lab-to-field studies, real-world neuroscience, translational medicine
Citation: Stryjek R, Parsons MH, Blumstein DT, Fendt M and Kiyokawa Y (2024) Editorial: Trends toward naturalistic, field assays with free-ranging animals as contemporary alternatives to laboratory models. Front. Ecol. Evol. 12:1455394. doi: 10.3389/fevo.2024.1455394
Received: 26 June 2024; Accepted: 03 July 2024;
Published: 15 July 2024.
Edited and Reviewed by:
Jordi Figuerola, Spanish National Research Council (CSIC), SpainCopyright © 2024 Stryjek, Parsons, Blumstein, Fendt and Kiyokawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafal Stryjek, UnN0cnlqZWtAd3AucGw=; Michael H. Parsons, UGFyc29ucy5ITWljaGFlbEBnbWFpbC5jb20=
†Present address: Michael H. Parsons, Centre for Urban Ecological Solutions, LLC., Spring, TX, United States
 Rafal Stryjek
Rafal Stryjek Michael H. Parsons
Michael H. Parsons Daniel T. Blumstein
Daniel T. Blumstein Markus Fendt
Markus Fendt Yasushi Kiyokawa
Yasushi Kiyokawa