
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 25 January 2024
Sec. Conservation and Restoration Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fevo.2024.1301717
This article is part of the Research TopicBiodiversity Conservation and Sustainable Development of Protected AreasView all 12 articles
Due to the high level of disturbance in natural ecosystems and the progressive loss of habitats resulting from anthropic occupation, biodiversity conservation represents one of the greatest challenges today. Red lists of threatened species are essential tools for identifying species at risk of extinction and guiding conservation efforts. In this study, we assessed the vulnerability to extinction of 55 snake species that occur in the Atlantic Forest of northeastern Brazil in Paraíba state. We developed vulnerability indices based on 12 factors known to influence the survival of snake populations. To analyze the threat profiles and relative risk levels within the snake community, we employed principal component analysis (PCA) and cluster analysis. Additionally, we compared our findings with existing red lists of threatened species. Our results reveal that only 18% of the snake fauna in this region is free of any threat. The aquatic species Helicops angulatus and Oxyrhopus trigeminus were the snakes that presented the lowest risk of extinction, while Caaeteboia gaeli and Crotalus durissus presented the highest risk of extinction. Two groups of species were considered non-threatened and five groups were considered threatened. Our study provides the first overview on the conservation status of snake species in the northern portion of the Atlantic Forest and contributes to a better evaluation of conservation planning for this group in the region.
Red lists of threatened species are created based on various attributes of species biology, as well as the threats to which species are susceptible. These lists aim to identify species at risk of extinction and guide conservation actions and resource allocation (Collar, 1996). One of the most widely recognized red lists is produced by the International Union for Conservation of Nature (IUCN). The IUCN criteria primarily rely on population parameter estimates (criteria A, C, and D1), distribution data (criteria B and D2), and the probability of extinction (criterion E) (IUCN, 2018). These criteria, while designed to be applied on a global scale, have inspired several other lists, which apply the procedure at the regional level (Gärdenfors et al., 2001). Although the IUCN criteria effectively assess the extinction risk for many species (Rodrigues et al., 2006), certain groups, such as reptiles, pose challenges due to limited knowledge about their population parameters. As a result, many reptile species are evaluated solely based on inferred distribution or remain unassessed (Böhm et al., 2013). This scarcity of data hinders the comprehensive assessment of reptile populations using the IUCN criteria. Therefore, alternative approaches may be necessary to address the unique challenges faced by reptiles and ensure their proper conservation assessment and management.
Snakes, among reptiles, face significant challenges in accurately assessing their vulnerability to extinction. This is primarily due to the limited knowledge about the natural history of most snake species, which can be attributed to their prolonged periods of inactivity, elusive behavior, and low population densities (Seigel, 1993). Consequently, applying the IUCN criteria to evaluate the extinction risks of snake species becomes highly challenging. Recognizing this, several studies have explored alternative parameters to assess the vulnerability of reptiles, including snakes. These studies have investigated various hypotheses concerning intrinsic factors such as body size, litter size, and dietary specialization, as well as extrinsic factors like climate change and illegal trade in species, to evaluate the extinction risk of snake species (Filippi and Luiselli, 2000; França and Araújo, 2006; Luiselli, 2009; Tomović et al., 2015). These alternative criteria offer valuable insights for prioritizing conservation actions, particularly when accurate distribution and population data are lacking, which is often the case for many Brazilian snake species.
In a study on the preservation and conservation status of biodiversity worldwide, Myers et al. (2000) proposed 25 priority areas for conservation, one of which is the Atlantic Forest. Even though it has undergone extensive fragmentation over a prolonged time, the Atlantic Forest still harbors astonishing biodiversity, with more than 8000 endemic species of vascular plants, amphibians, reptiles, birds, and mammals (Myers et al., 2000). In Brazil, this biome stretches from Rio Grande do Norte and Paraíba in the north to Santa Catarina and Rio Grande do Sul in the south. The northeastern region of Brazil, particularly the portion of the Atlantic Forest located north of the São Francisco River known as the Pernambuco Endemism Center (Yi et al., 2017), has considerable species richness, harboring at least 143 species of reptiles, 91 of which are snakes (Pereira-Filho et al., 2023). This region has experienced rapid degradation over the years due to historical economic priorities, notably brazilwood and sugar cane (Coimbra-Filho and Câmara, 1996), and is considered an ideal “scenario” as described by Tabarelli et al. (2002), for local, regional, and even global species extinctions to occur.
Situated amidst this challenging scenario, the Atlantic Forest of Paraíba state has endured significant losses, with only 5% of its original area remaining (CEPED, 2012). Despite these constraints, the Paraíba Atlantic Forest stands out as a region of considerable snake species richness, with 55 species documented to date (Pereira-Filho et al., 2017), three of them recently described, i.e. the coral snake, Micrurus potyguara (Pires et al., 2014), the blind snake, Amerotyphlops arenensis (Graboski et al., 2015), and the ground snake Caaeteboia gaeli (Montingelli et al., 2020). Given the exceptional species diversity coupled with extensive biome degradation, it becomes imperative to assess the conservation status of these snake species, as a significant portion of this fauna may be facing the threat of extinction.
Therefore, our objective was to evaluate the vulnerability to extinction of snake species occurring in the Paraíba Atlantic Forest, northeast Brazil, to analyze the main factors that may threaten the viability of populations, to classify species in groups vulnerable to specific threats, and to compare our results with existing red lists of threatened species.
Paraíba state is situated in the northeastern region of Brazil, sharing borders with Rio Grande do Norte, Pernambuco, Ceará, and the Atlantic Ocean (Figure 1). Within this region lies the Paraíba Atlantic Forest (PAF), encompassing a total area of 5,994 km², which corresponds to approximately 11% of the state’s territory. The PAF spans across 63 municipalities, either fully or partially (SOS Mata Atlântica, 2018), and comprises diverse ecosystems, including forests, restinga (coastal woodland), and mangroves (Tabarelli et al., 2006).
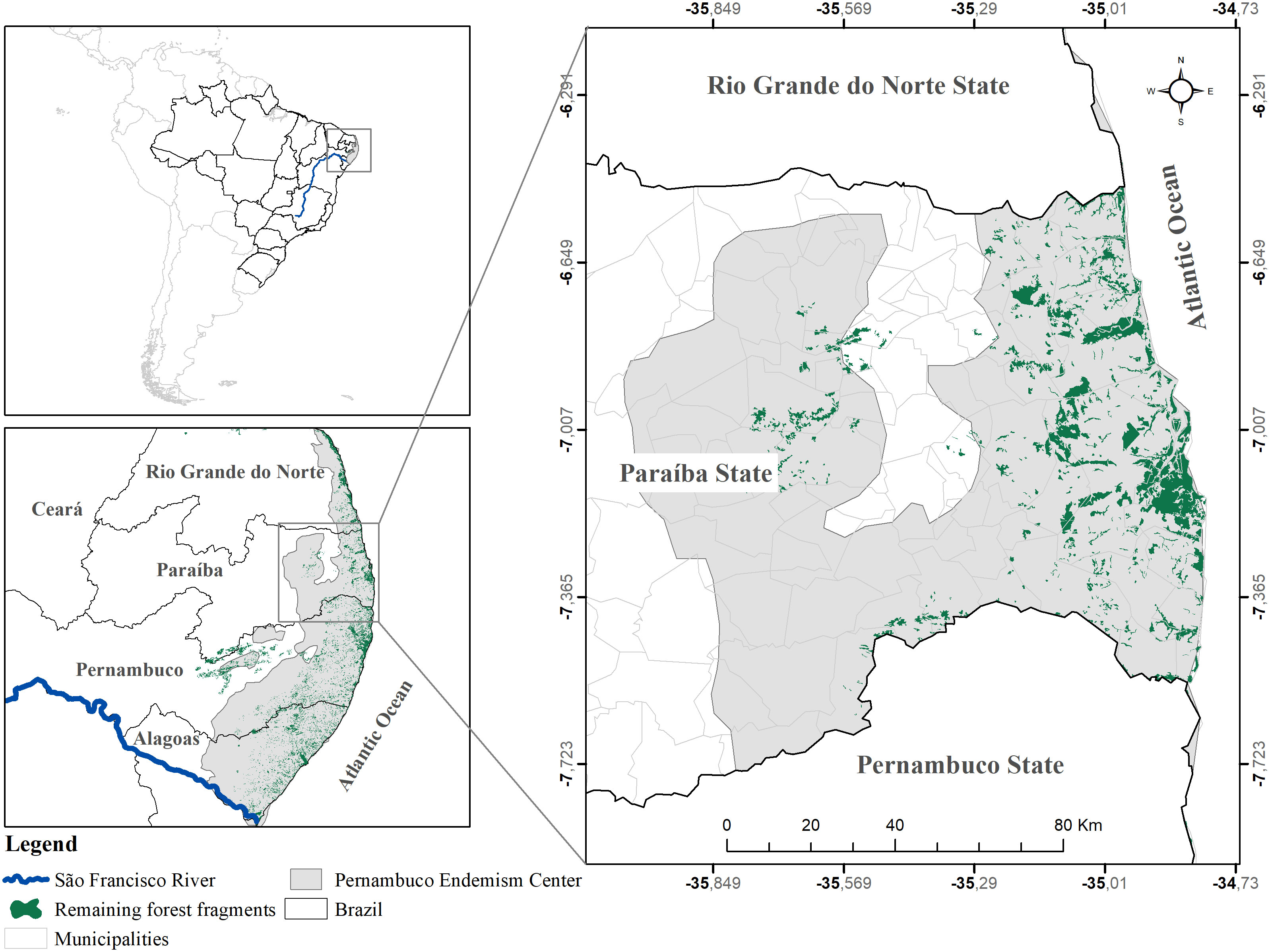
Figure 1 Map of the location of the Paraíba Atlantic Forest, with the original coverage of Atlantic Forest (gray), and the actual remnants (green).
In terms of climate classification, the region falls under the Köppen tropical wet and dry (As) climate category. It experiences rainfall predominantly during autumn and winter, with average temperatures of 26°C and an annual precipitation of approximately 1800 mm (CEPED, 2012). A total of 18 conservation units are in this region. Among these, the Área de Proteção Ambiental da Barra do Rio Mamanguape (14 640 ha) was established for sustainable use, and the Reserva Biológica Guaribas (4051 ha) for integral protection, stand out as the largest territories among these conservation units.
To assess the vulnerability of snake species within the Paraíba Atlantic Forest, we adopted a comprehensive approach inspired by previous studies conducted by Filippi and Luiselli (2000); França and Araújo (2006), and Tomović et al. (2015). These studies utilized ecological and geographic data to evaluate the conservation status of snakes in Italy, Brazil, and Serbia, respectively. Drawing from their methodologies, we employed a ranking method to generate vulnerability indices based on 12 factors known to influence the survival of snake populations.
To obtain the necessary data for classifying these threat factors, we conducted an extensive review of relevant literature, carried out fieldwork, and gathered valuable insights from the collection of the Universidade Federal da Paraíba. Each threat factor was assigned a score ranging from 1 (indicating a lower risk) to 2–4 (indicating a higher risk) based on careful consideration and evaluation.
1. Distribution breadth (DB): It is a crucial factor in assessing the vulnerability of species to extinction. The underlying principle is that species with smaller home ranges are more susceptible to extinction due to limited population densities, which can impact their ability to persist and survive during times of crisis (Purvis et al., 2000; Primack and Rodrigues, 2001). To determine the distribution breadth, we compiled data from species records within the municipalities encompassed by the Paraíba Atlantic Forest. The scoring system used for distribution breadth classification was as follows: 1 = wide distribution (present in > 80% of the territory); 2 = less broad (present in 50–80%); 3 = moderately restricted (present in 20–50%); 4 = restricted (present in <20%). In this work, we estimate the distribution area of the species through the minimum convex polygon (MCP) method. The MCP is the most used estimator to calculate the home range of a species and is designed to be the smallest possible polygon that covers all the points of record of the species (Laver and Kelly, 2008).
2. Habitat breadth (HB): The underlying principle is that species with specialized habitat requirements are more susceptible to the impacts of human activities, as they may have limited ability to adapt to disturbances (Purvis et al., 2000; Primack and Rodrigues, 2001). This threat factor was motivated by the occurrence of species in five habitat types of the Paraíba Atlantic Forest (see Pereira-Filho et al., 2017): brejos nordestinos, stational semidecidual forest (closed forest), stational semidecidual forest (tabuleiros or savannahs), mangrove, and restinga. 1 = generalist (found in at least four categories); 2 = less generalist (found in three categories); 3 = moderate specialist (found in two categories); 4 = specialist (found in only one category).
3. Endemicity (E): It is based on the principle that endemic species with a more restricted distribution may be especially vulnerable to extinction (Andreone and Luiselli, 2000; Işik, 2011; Tomović et al., 2015). The categories are: 1 = 0–10% of the species’ distribution occurs in the Paraíba Atlantic Forest; 2 = >10% of the species’ distribution occurs in the Paraíba Atlantic Forest.
4. Rarity in the Paraíba Atlantic Forest (RR): The underlying principle is that small and isolated populations are more susceptible to extinction due to factors such as accelerated inbreeding, increased stochastic effects, and genetic drift, which can lead to a loss of genetic variability (Primack and Rodrigues, 2001; Piratelli and Francisco, 2013). Our rarity categories were created based on the percentage of the quantity of registered specimens (2625 records) in the Paraíba Atlantic Forest. 1 = > 8% of total recorded specimens; 2 = 6-8%; 3 = 2-6%; 4 = < 2%.
5. Dietary breadth (DT): It is based on the principle that species with a more specialist diet are more vulnerable to extinction due to the possibility of loss of prey or destruction of their prey’s habitat caused by negative human interventions in their habitats (McKinney, 1997; Purvis et al., 2000; Boyles and Storm, 2007). The categories were created based on the level of taxonomic order of the prey and percentage of the main prey in the diet; 1 = generalist, main prey item < 30% of diet; 2 = low specialization, main prey 31–50% of diet; 3 = moderate specialization, main prey 51–70% of diet; 4 = highly specialized, main prey > 70% of diet.
6. Habitat use and activity period (HT): The underlying principle is that species with more secretive habits, such as fossorial (burrowing) and cryptozoic (cryptic or hidden) behavior, are generally less vulnerable to extinction. This is primarily because they are less likely to be detected by predators and less susceptible to direct harm from human activities (França and Araújo, 2006). 1 = fossorial species; 2 = species with nocturnal, cryptozoic, or aquatic activity; 3 = species with secretive diurnal activity; 4 = terrestrial species with diurnal activity.
7. Adaptability to altered environments (AH): The underlying principle is that species with greater adaptability to man-altered environments are generally less vulnerable to extinction, as they possess the ability to tolerate and persist in habitats that have been modified by human activities (Filippi and Luiselli, 2000). This category was based on the presence/absence of species in protected areas of the Paraíba Atlantic Forest: 1 = completely adapted (found even in urban environments); 2 = adapted (found in suburbs if there is natural environment nearby); 3 = less adapted (found in and near large natural environments); 4 = not adapted (found only within conservation units).
8. Direct anthropogenic effects on species conservation status (AE): The presence of direct anthropogenic effects can lead to a greater and faster reduction in the size of local populations, ultimately impacting their conservation status (Tomović et al., 2015). The categories were based on the presence of the following direct anthropogenic effects: roadkill (based on monitoring of road-killed snakes in the Paraíba Atlantic Forest (unpublished data; Pereira-Filho et al., 2017), consumption of snakes as human food, for medicinal, magic/religious, ornamental or decorative purposes, pets, target species of conflict (species that are commonly killed when in contact with humans) (Alves and Pereira-Filho, 2007; Pereira-Filho et al., 2017). The categories are: 1 = no effect; 2 = low effect (presence of one or two types of human impacts); 3 = medium effect (presence of three types of human impacts); 4 = high effect (presence of four or more types of human impacts) on the species.
9. Body size (BS): It is based on the principle that larger species tend to occur at lower densities, have larger home ranges, and reach sexual maturity later, making them more susceptible to negative human interventions in their habitats (McKinney, 1997; Purvis et al., 2000; Dulvy and Reynolds, 2002). The categories are: 1 = < 50 cm length; 2 = 51–100 cm length; 3 = 101–150 cm length; 4 = > 150 cm length.
10. Litter size (LS): Eggs or younglings; it is based on the principle that species with low fecundity are more vulnerable to extinction. This is because if such species experience a significant decrease in population size, it becomes more challenging for them to recover their original population levels (Purvis et al., 2000; Dulvy and Reynolds, 2002; Webb et al., 2002). The categories are: 1 = The maximum number of litters > 15; 2 = 11–15; 3 = 5–10; 4 = < 5.
11. Reproduction mode (RM): Viviparous species tend to produce fewer offspring than oviparous and are more prone to extinction risks (Andreone and Luiselli, 2000). The categories are: 1 = oviparity; 2 = viviparity.
12. Frequency of reproduction (FR): A taxon that can reproduce throughout the year can recuperate more easily when habitats are altered (Tomović et al., 2015; Vukov et al., 2015). The categories are: 1 = aseasonal reproduction; 2 = seasonal reproduction.
To determine the relative threat levels for each species of snake, the mean scores for the 12 threat factors mentioned above were calculated. Before computing the mean score, we standardized the scores for each variable, ranging them from zero to one. This standardization allows for a uniform comparison of the threat levels across different factors. Scores closer to 1 indicate higher risks of threat, while scores closer to zero indicate lower risks.
Next, we employed principal component analysis (PCA) and cluster analysis to assess how species are classified based on their similarity in terms of specific threats. The cluster analysis employed the UPGMA model, which generates an agglomerative hierarchical classification dendrogram. All analysis were conducted using the software R, version 3.2.0.
To evaluate the relative threat level for each snake species within the community, we followed the approach outlined by Tomović et al. (2015). We categorized the mean scores into five groups, based on the classification proposed by the IUCN. Specifically, 40% of the species with the lowest mean scores were considered Least Concern (LC, 0–40%). The remaining 60% of species were equally distributed among four categories of threatened species: near threatened (NT, 41–55%), vulnerable (VU, 56–70%), endangered (EN, 71–85%), and critically endangered (CR, 86–100%).
The results obtained in this study for the Paraíba Atlantic Forest snakes were compared with the results obtained by França and Araújo (2006) who evaluated the vulnerability to extinction of snakes in central Brazil using intrinsic and extrinsic factors. Additionally, we also compared our results with assessments conducted using the IUCN methodology and various regional red lists to examine if the same species exhibit similar degrees of vulnerability across different regions and methodologies. To assess the consistency of vulnerability levels, we referred to the red list of threatened species published by the IUCN (IUCN, 2022), the Brazilian red list of threatened species (MMA, 2022), and four regional lists: red list of threatened species of the Pernambuco state (SEMAS, 2017), red list of threatened species of the Bahia (SEMA, 2017), the Rio Grande do Sul state (Rio Grande do Sul, 2014), and of the Espírito Santo state (Vitória, 2022).
In Table 1 we present the scores for each species and each threat factor used to evaluate the vulnerability to extinction of the 55 snake species that were found in the Paraíba Atlantic Forest (more details see Supplementary Material, Table 1). The mean values of the scores for all species varied between 0.19 (lower risk) and 0.75 (higher risk). The categories, criteria, and amplitude of the scores for the five categories proposed by IUCN (LC, NT, VU, EN, CR) are presented in Table 2.
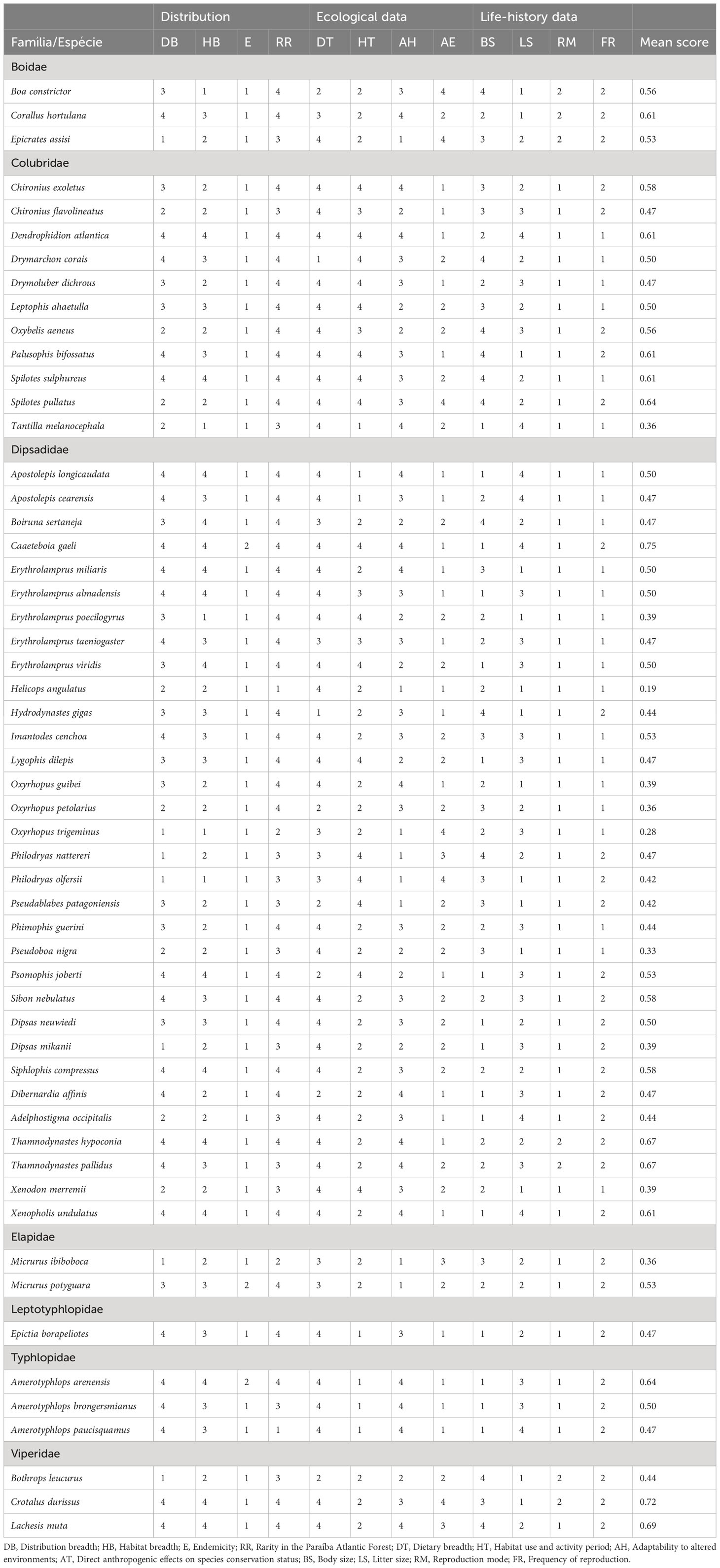
Table 1 Values for the 12 threat factors that may affect the survival of snakes in the Paraíba Atlantic Forest.
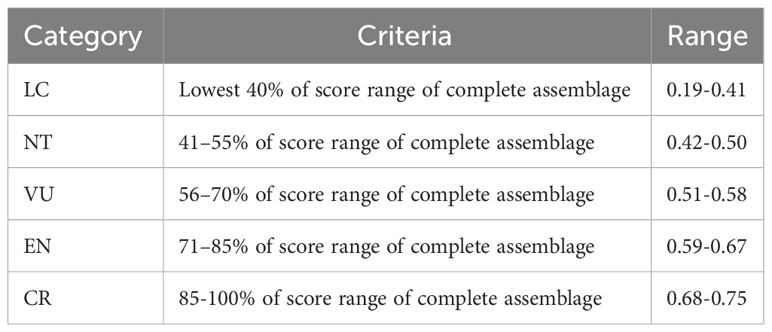
Table 2 Categories, criteria and score ranges for species of snakes of the Atlantic Forest of Paraíba.
Our results indicate that only 10 species (18%) of snakes present no risk of decline, 24 species (44%) are considered near threatened, 9 (16%) are considered vulnerable, 9 (16%) species are considered endangered, and 3 species (5%) are considered critically endangered. Helicops angulatus and Oxyrhopus trigeminus had the lowest mean scores of 0.19 and 0.28 respectively, while Caaeteboia gaeli and Crotalus durissus presented the highest mean scores, 0.75 and 0.72, respectively.
Threat factors related to species distribution contributed more to the mean scores of 21 species (Amerotyphlops arenensis, A. brongersmianus, Apostolepis cearensis, A. longicaudata, Boiruna sertaneja, Corallus hortulana, C. gaeli, Drymarchon corais, Erythrolamprus almadensis, E. miliaris, E. taeniogaster, Epictia borapeliotes, Hydrodynastes gigas, Imantodes cenchoa, Micrurus potyguara, Psomophis joberti, Siphlophis compressus, Sibon nebulatus, Dibernardia affinis, Thamnodynastes hypoconia, and Xenopholis undulatus). The factors related to ecology played a more prominent role in determining to the mean scores of 15 species (Chironius exoletus, Drymoluber dichrous, Dipsas mikanii, Erythrolamprus poecilogyrus, H. angulatus, Leptophis ahaetulla, Lygophis dilepis, Oxyrhopus guibei, O. trigeminus, Phimophis guerini, Pseudoboa nigra, Philodryas olfersii, Spilotes pullatus, Tantilla melanocephala, and Xenodon merremii), and the factors related to natural history contributed more to the mean scores of 7 species (Boa constrictor, Bothrops leucurus, Chironius flavolineatus, Epicrates assisi, Micrurus ibiboboca, Oxybelis aeneus, and Thamnodynastes pallidus). For 8 species (C. durissus, Dendrophidion atlantica, Dipsas neuwiedi, Erythrolamprus viridis, Lachesis muta, Palusophis bifossatus, Oxyrhopus petolarius, and Spilotes sulphureus), factors related to both distribution and ecology were the main contributors to the mean scores, while for three species (Amerotyphlops paucisquamus, Philodryas nattereri, and Adelphostigma occipitalis), factors related to both ecology and natural history were the main contributors, and for only one species (Pseudablabes patagoniensis), all factors contributed in the same way (Figure 2).
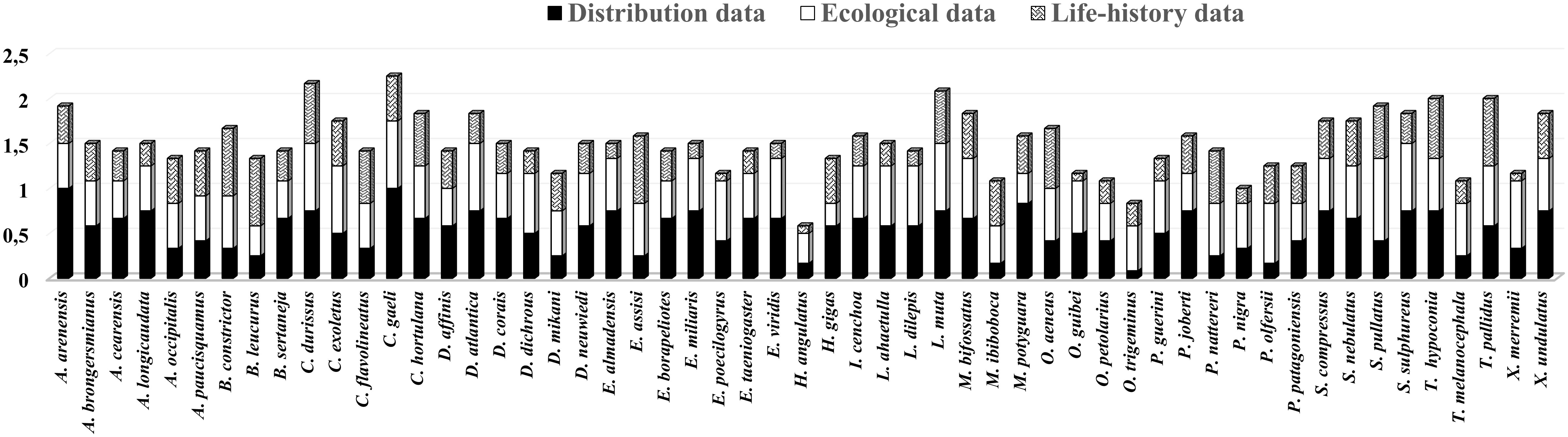
Figure 2 Mean scores for the distribution, ecology, and life-history data contributing to extinction risk of the Paraíba Atlantic Forest Snakes.
We used PCA to classify the snake species that occur in the Paraíba Atlantic Forest into groups of threat to specific factors; however, these groups are not easily visualized in the graph of the analysis (Figure 3), and so it was combined with the cluster analysis.
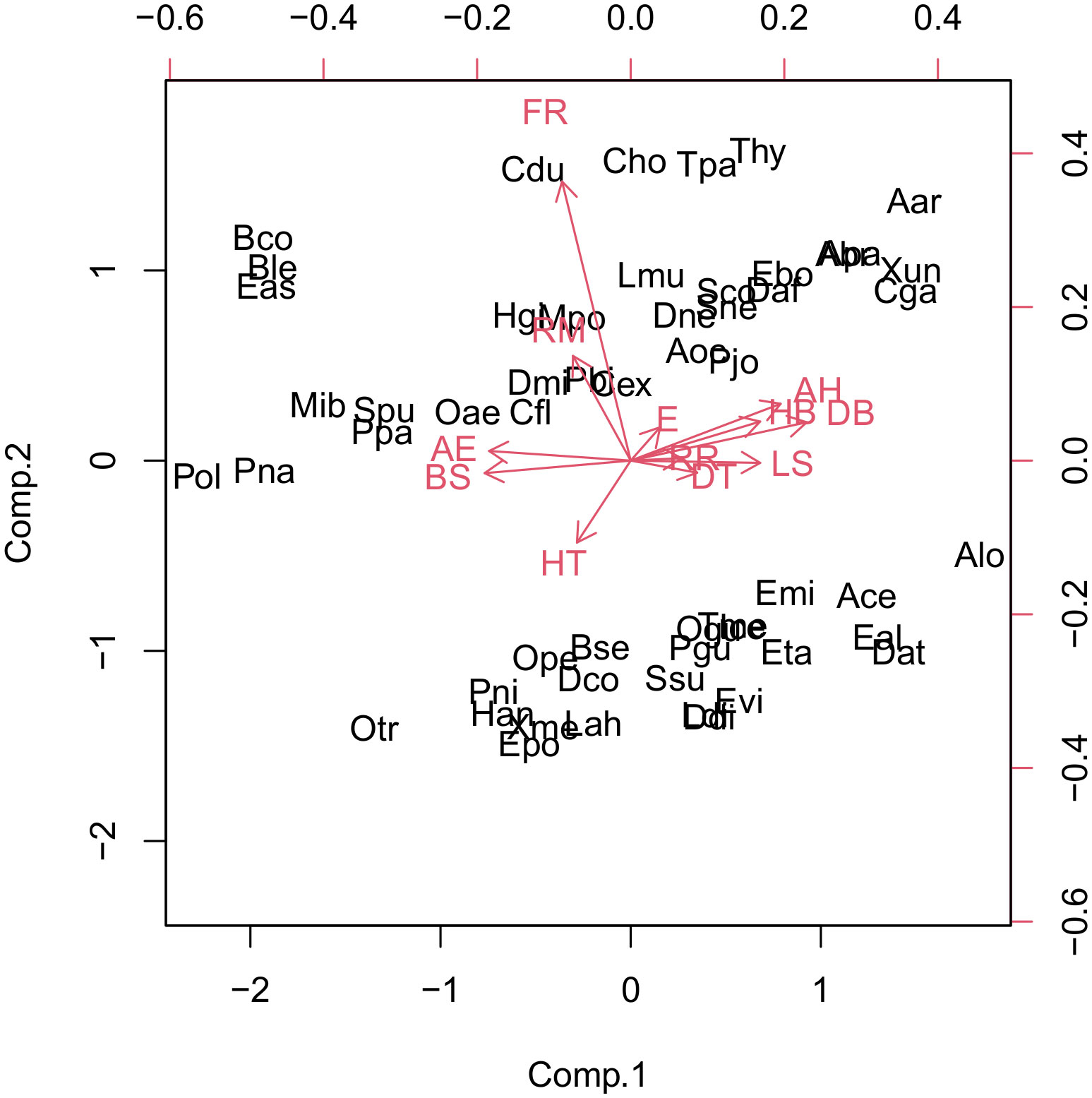
Figure 3 Scores of threat factors for the main components of the snake species of the Paraíba Atlantic Forest, showing some threatened groups. Aar, Amerotyphlops arenensis; Abr, Amerotyphlops brongersmianus; Ace, Apostolepis cearensis; Alo, Apostolepis longicaudata; Apa, Amerotyphlops paucisquamus; Bco, Boa constrictor; Ble, Bothrops leucurus; Bse, Boiruna sertaneja; Cdu, Crotalus durissus; Cex, Chironius exoletus; Cfl, Chironius flavolineatus; Cho, Corallus hortulana; Cga, Caaeteboia gaeli; Dat, Dendrophidion atlantica; Dco, Drymarchon corais; Ddi, Drymoluber dichrous; Dmi, Dipsas mikanii; Dne, Dipsas neuwiedi; Eal, Erythrolamprus almadensis; Eas, Epicrates assisi; Ebo, Epictia borapeliotes; Emi, Erythrolamprus miliaris; Epo, Erythrolamprus poecilogyrus; Eta, Erythrolamprus taeniogaster; Evi, Erythrolamprus viridis; Han, Helicops angulatus; Hgi, Hydrodynastes gigas; Ice, Imantodes cenchoa; Lah, Leptophis ahaetulla; Ldi, Lygophis dilepis; Lmu, Lachesis muta; Pbi, Palusophis bifossatus; Mib, Micrurus ibiboboca; Mpo, Micrurus potyguara; Oae, Oxybelis aeneus; Ogu, Oxyrhopus guibei; Ope, Oxyrhopus petolarius; Otr, Oxyrhopus trigeminus; Pgu, Phimophis guerini; Pjo, Psomophis joberti; Pna, Philodryas nattereri; Pni, Pseudoboa nigra; Pol, Philodryas olfersii; Ppa, Pseudablabes patagoniensis; Sco, Siphlophis compressus; Sne, Sibon nebulatus; Spu, Spilotes pullatus; Ssu, Spilotes sulphureus; Daf, Dibernardia affinis; Thy, Thamnodynastes hypoconia; Tme, Tantilla melanocephala; Aoc, Adelphostigma occipitalis; Tpa, Thamnodynastes pallidus; Xme, Xenodon merremii; Xun, Xenopholis undulates.
The values of the variables for the first three main components are presented in Table 3. The first two axes explained 49% of the variation in the data. The variables most significantly associated with the main component 1 were the direct anthropogenic effects on species conservation status, which was negatively related, and the adaptability to altered environments and distribution breadth, which were positively related. The variables most significantly associated with the main component 2 were frequency of reproduction and reproduction mode, both positively related.
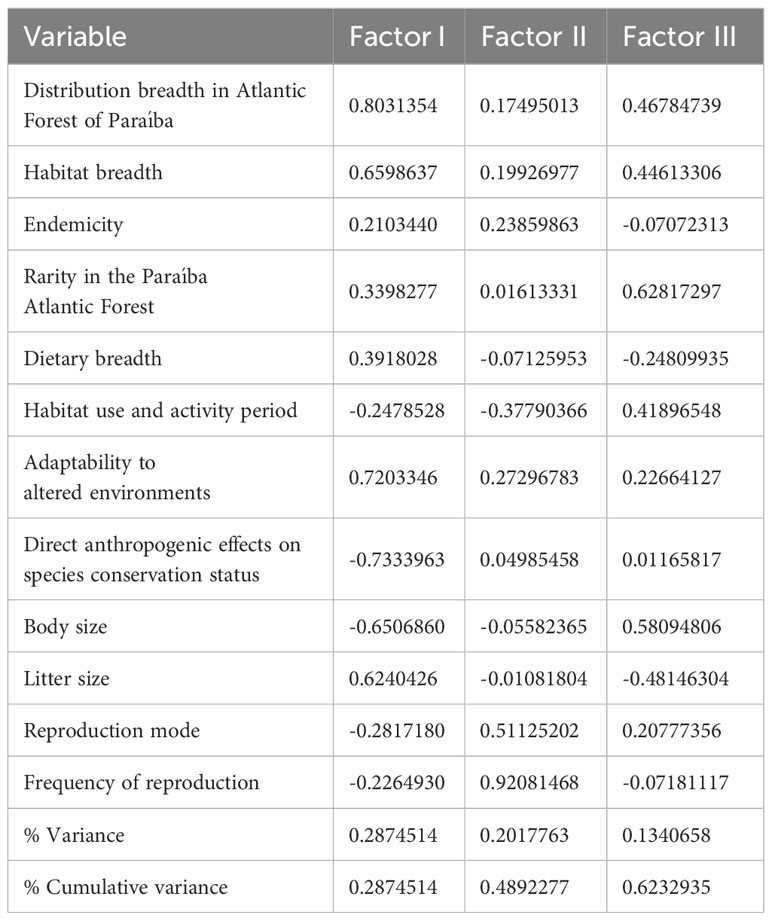
Table 3 Factor loadings of each variable on the first three principal components before VARIMAX rotation and proportion of the variance explained by each component.
We used PCA and cluster analysis to distinguish 10 groups of snake species in the Paraíba Atlantic Forest (Figure 4). Of these, two groups were considered non-threatened and five groups were considered threatened. All natural history information, distribution, and other threat factors refer to the data obtained for PAF snakes. The groups are described below.
Group 1= Non-endemic species, with large body size, produce large numbers of offspring, oviparous, and with seasonal reproduction (Non-threatened and Threatened): M. ibiboboca, P. nattereri, P. olfersii, P. patagoniensis, and S. pullatus.
Group 2= Non-endemic species, rare in PAF, with a large body size, produce large numbers of offspring, viviparous, and with seasonal reproduction (Threatened): B. constrictor, B. leucurus, and E. assisi.
Group 3= Species with wide distribution, non-endemic, adapted to altered environments, with aseasonal reproduction, and oviparous (Not Threatened): H. angulatus and O. trigeminus.
Group 4= Non-endemic species, adapted to altered environments, oviparous, and with seasonal reproduction (Non-threatened and threatened): A. brongersmianus, A. paucisquamus, C. flavolineatus, D. mikanii, D. neuwiedi, E. borapeliotes, O. aeneus, P. joberti, S. nebulatus, S. compressus, D. affinis, A. occipitalis and X. undulatus.
Group 5= Non-endemic species, rare in PAF, with a large body size, produce large numbers of offspring, oviparous, and with seasonal reproduction (Threatened): C. exoletus, H. gigas, L. muta and P. bifossatus.
Group 6= Species with restricted distribution, non-endemic, rare in PAF, with specialist diet, viviparous, and with seasonal reproduction (Threatened): C. hortulana, C. durissus, T. hypoconia and T. pallidus.
Group 7= Non-endemic species, rare in PAF, oviparous, and with aseasonal reproduction (Non-threatened and Threatened): A. cearensis, A. longicaudata, B. sertaneja, D. atlantica, D. corais, D. dichrous, E. almadensis, E. miliaris, E. poecilogyrus, E. taeniogaster, E. viridis, I. cenchoa, L. ahaetulla, L. dilepis, O. guibei, O. petolarius, P. guerini, P. nigra, S. sulphureus and X. merremii.
Group 8= Non-endemic species, generalists in habitat use, with a small body size, specialist diet, produce small numbers of offspring, oviparous, and with aseasonal reproduction (Not Threatened): T. melanocephala.
Group 9= Species with restricted distribution in PAF, endemic, rare in PAF, with specialist diet, and not adapted to altered environments (Threatened): A. arenensis and C. gaeli.
Group 10= Endemic species, rare in PAF, adapted to altered environments, oviparous, and with seasonal reproduction (Threatened): M. potyguara.
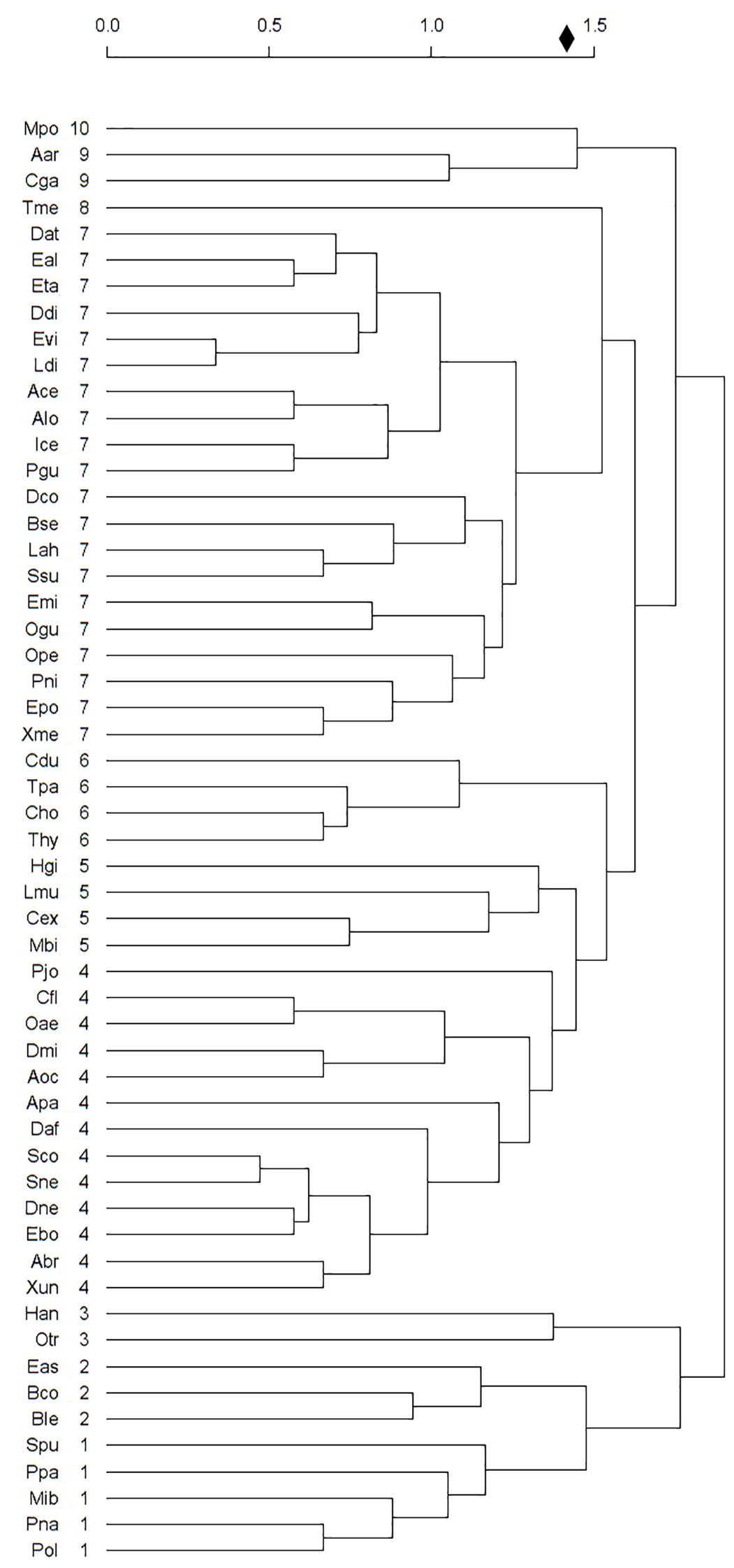
Figure 4 Cluster diagram showing the similarity groups for threatening factors among fifty-five snake species of Paraíba Atlantic Forest. Diamond indicates the point in cluster diagram where significance is achieved. Numbers to the right of species indicate the groups. The abbreviations are the same as those used in Figure 3.
Among the 55 snake species found in the PAF, 23 species also occur in Central Brazil, where the Cerrado Biome is present (Table 4). The species E. poecilogyrus and X. merremii were considered least concern in both localities, whereas B. constrictor was deemed vulnerable in both regions. The species C. exoletus and O. aeneus, considered as vulnerable in the PAF, were considered threatened for Central Brazil. C. durissus considered critically endangered in PAF, was considered vulnerable for Central Brazil. The species P. bifossatus, S. pullatus and T. hypoconia considered as endangered in PAF, were considered as vulnerable for Central Brazil and the species X. undulatus, considered endangered in PAF, was considered threatened for Central Brazil (Table 4).
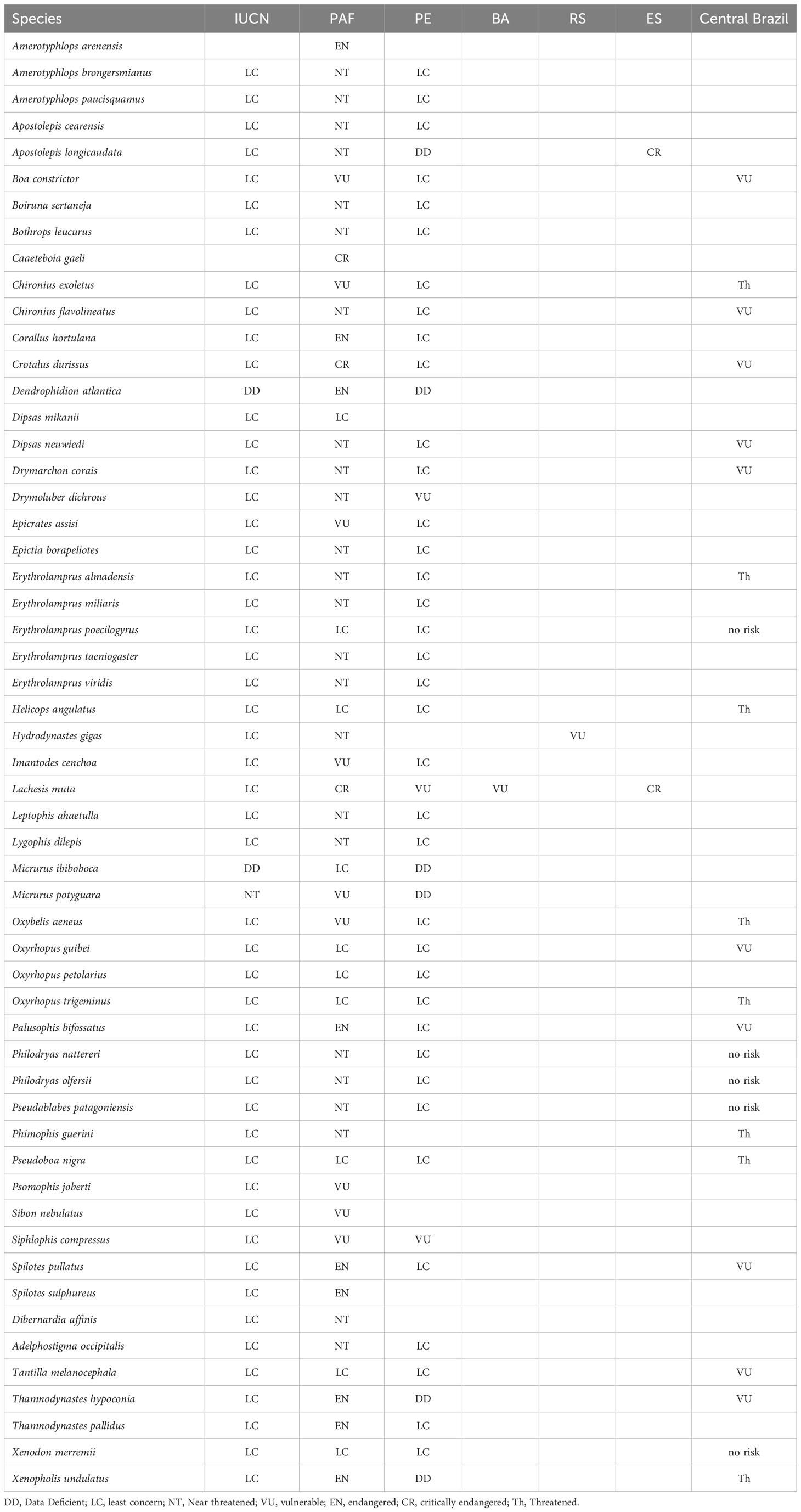
Table 4 Comparison between the degree of vulnerability to extinction of the snakes species of the Paraíba Atlantic Forest with preexisting assessments: International Union for Conservation of Nature (IUCN, 2022-2), Central Brazil (França and Araújo, 2006); Pernambuco state (PE) (SEMAS, 2017); Bahia (BA) (SEMA, 2017); Rio Grande do Sul state (RS) (Rio Grande do Sul, 2014) and Espı́rito Santo state (ES) (Vitória, 2022).
In reference to the IUCN list of threatened species, M. potyguara was listed as near threatened and D. atlantica as data deficient. Two species (A. arenensis and C. gaeli), have yet to be evaluated. The remaining species on the list were considered of least concern, implying a lower risk of extinction. It’s worth noting that none of the 55 snake species from the PAF are currently listed on the Brazilian threatened species list.
When comparing the regional red lists of threatened species from Pernambuco, Bahia, Rio Grande do Sul, and Espírito Santo states with the vulnerability assessments conducted in the PAF, interesting patterns emerge. The red list from Pernambuco state, which is a neighboring state to Paraíba, showed the highest similarity in terms of the degree of vulnerability of snake species. The species E. poecilogyrus, H. angulatus, O. guibei, O. petolarius, O. trigeminus, P. nigra, T. melanocephala, and X. merremii were considered as least concern both for the state of Pernambuco and for PAF, while S. compressus was considered vulnerable in both states. The species B. constrictor, C. exoletus, E. assisi, I. cenchoa, and O. aeneus, considered vulnerable, the species C. hortulana, T. pallidus, S. pullatus and P. bifossatus considered endangered, and C. durissus, considered critically endangered at the PAF, were considered of least concern to the Pernambuco state.
The species H. gigas was classified as vulnerable in the state of Rio Grande do Sul but considered near threatened in the PAF. While the species X. undulatus, considered threatened in Central Brazil and listed as data deficient (DD) for the state of Pernambuco, was considered endangered in the PAF. Furthermore, L. muta, which is vulnerable in the state of Pernambuco, and Bahia, was considered critically endangered in the state of Espírito Santo and in the present study. Although the faunas of Rio Grande do Sul, Bahia, and Espírito Santo states share few similar species with PAF, some species of the genus Corallus, Apostolepis, Philodryas, and Bothops that were considered threatened in the PAF were also considered threatened in these other lists.
Filippi and Luiselli (2000) proposed that factors associated with geographic distribution were the most important threats to the Italian snake fauna; however, factors related to the natural history of the species could also be influencing the viability of some species. For the Cerrado of Central Brazil França and Araújo (2006), found that both factors related to geographic distribution and natural history could affect the viability of snake populations. In contrast, the study conducted on Serbian snakes by Tomović et al. (2015) found that factors related to the natural history of the species contributed more to the conservation scores of the species. Regarding the PAF snakes and the study by Filippi and Luiselli (2000), the results indicate that factors related to species distribution (1–4) contribute more to the threat scores of snake species.
Our findings highlight several factors that contribute to the vulnerability of snake species in the Atlantic Forest, particularly in the Paraíba region. These factors include restricted distribution, rarity, and specialized diets, all of which increase the susceptibility of species to threats and population decline. Over the years, forest cover in the Paraíba region has been drastically reduced due mainly to the expansion of sugarcane cultivation and the development of activities related to shrimp farming in mangrove areas (Tabarelli et al., 2006). Currently, only small fragments remain on private property and some protected areas designated by the government (Barbosa et al., 2004). Unfortunately, these fragmented habitats are often insufficient to support the survival of many animal populations, including snakes. Thus, even species that present a very wide distribution, such as L. muta, which occurs throughout half of the Atlantic Forest and the Amazon, but only in large and well-preserved areas (Dixon and Soini, 1986; Marques et al., 2004), could become locally or regionally extinct in the PAF.
Numerous studies have demonstrated that snake species with specialized diets are more susceptible to extinction compared to generalists. This increased vulnerability stems from the potential loss of prey species or the destruction of their prey’s habitats, which directly impact the survival and reproductive success of these specialized snakes (Boyles and Storm, 2007). The significance of the diet factor in assessing the threat levels of snake species has been observed in various regions, including the PAF, the Cerrado of Central Brazil, and Italian snake populations. In these different contexts, studies such as França and Araújo (2006) and Filippi and Luiselli (2000) have consistently shown high vulnerability values associated with the diet factor, underscoring the importance of careful analysis and consideration of this factor in evaluating the conservation status of snake species.
When comparing the species groups formed through PCA in our present study with those of França and Araújo (2006), we identified both similarities and differences among certain groups. The species of the Boidae family are vulnerable or threatened in PAF and Cerrado, due mainly to their large size and by being significantly affected by direct anthropogenic effects, whereas contradictory results were observed among the Viperidae family. The viperids found in PAF were mainly threatened due to small population sizes and rarity, while in the Cerrado most species of this family exhibit wider distribution ranges and larger population sizes, reflecting low vulnerability levels.
Of the 23 species found in both the Atlantic Forest of Paraíba and the Cerrado (França and Araújo, 2006), the species E. poecilogyrus, X. merremii, and B. constrictor showed similar results in their degree of vulnerability. In addition, some other species (C. exoletus, C. durissus, P. bifossatus, S. pullatus, T. hypoconia, and X. undulatus) were identified as threatened in both the Atlantic Forest and the Cerrado, albeit at varying levels. This suggests that some species are subject to similar threats even in different biomes. For instance, C. exoletus and X. undulatus are rare species, with restricted distributions, and low adaptability to live in altered environments in both regions, while the conservation status of the species C. durissus is greatly affected by direct anthropogenic effects in both regions. The comparison of species conservation statuses in distinct habitats, using different methodologies that yield similar results, highlights the importance of assessing species vulnerability across different biomes. This comparative analysis underscores the need for careful evaluation and conservation considerations for species that exhibit consistent degrees of vulnerability in diverse habitats. Such species may be particularly susceptible to threats and require targeted conservation efforts to ensure their long-term survival.
Caaeteboia gaeli, a recently described species, exhibited the highest vulnerability index in the Paraíba Atlantic Forest, classifying it as critically endangered with a vulnerability index of 0.75. The species is known from only three specimens, with two individuals found in the Atlantic Forest of Paraíba and one in the state of Pernambuco (Montingelli et al., 2020). Prior to the description of C. gaeli, the only known species in the genus was C. amarali, which has a known distribution in the states of Bahia, Minas Gerais, São Paulo, Paraná, and Santa Catarina (Passos et al., 2012). C. amarali is sparsely represented in herpetological collections, with fewer than 15 specimens recorded until 2012 (Passos et al., 2012). In the red list of threatened species, this snake was considered endangered in the state of Bahia (SEMA, 2017), vulnerable in São Paulo (Marques et al., 2009), and data deficient in Paraná (Mikich and Bérnils, 2004). In our study, C. gaeli was considered threatened, mainly because of its rarity (only 3 known specimens), specialized in habitat and diet, and has not been found in altered environments. Consistent with Marques et al. (2009), the main threats to C. amarali in São Paulo state arise from habitat destruction and alteration caused by urbanization, housing development, and tourism along the coastal areas.
Our study findings reveal that the species C. durissus is critically endangered in the PAF (vulnerability index 0.72), mainly because it has a restricted distribution in PAF, is a specialist in habitat and diet, and is greatly affected by direct anthropogenic effects. Although the species has a wide distribution in Brazil (Boldrini-França et al., 2010), in PAF, this species was only found at a few localities (França et al., 2012; Mesquita et al., 2018). Besides the rarity, this species is threatened by anthropic use Notably, the skin and rattle of C. durissus have been associated with magical and religious rituals, particularly within Afro-Brazilian religions (Alves et al., 2012). Products such as rattlesnake rattles are frequently found in markets or specialty stores catering to mystical religious articles, primarily sought after by followers of Afro-Brazilian cults (Pereira-Filho et al., 2017). Furthermore, various parts of the snake’s body, including the skin, tail, cloaca, rattle, and fat, are used in traditional folk medicine for treating ailments such as asthma, thrombosis, rheumatism, skin diseases, tuberculosis, hanseniasis, and osteoporosis (Alves et al., 2009). Regrettably, the use of C. durissus for ornamental and decorative purposes has also been documented in the state of Paraíba. For instance, some hunters utilize rattlesnakes in the production of keyrings, and their skin is employed in the manufacturing of belts (Mendonça et al., 2014).
The species T. hypoconia also had a high vulnerability index in the PAF (0.67), being considered endangered in the region, mainly because it has a restricted distribution and is rare in the PAF, is a habitat and diet specialist, and has low adaptability to living in altered environments. In Brazil, this species is distributed in the Atlantic Forest (Marques et al., 2004; França et al., 2020), Cerrado (Marques et al., 2015), and Caatinga (Guedes et al., 2014) biomes. In Paraíba, this species is quite common in the Caatinga Biome, but is rare within the PAF, with only two recorded specimens found in a specific region called Brejo de Altitude Paraíba (Pereira-Filho et al., 2017; França et al., 2020). Furthermore, displays a specialized diet, primarily feeding on anurans (Bellini et al., 2013), which further increases its vulnerability to extinction.
Another species that presented a high vulnerability index (0.69) is L. muta. Historically, this species was classified into two subspecies: L. muta muta (Linnaeus, 1766) and L. muta rhombeata (Wied-Neuwied, 1824). The former was primarily found in the Amazon Forest, while the latter had a distribution range extending from northern Rio de Janeiro to Paraíba, with some isolated populations potentially present in moist enclaves of Ceará and Piauí (Cardoso et al., 2003). The subspecies L. muta rhombeata appeared in several state red lists, being considered vulnerable for the state of Espírito Santo, endangered for Rio de Janeiro, and critically endangered for Minas Gerais (Martins and Molina, 2008). In a review of the genus, Fernandes et al. (2004) considered the two names to be synonyms of L. muta, and as a result, excluded the species from the IUCN red list (IUCN, 2012). However, the populations identified in the Atlantic Forest should still be considered threatened due to the great deterioration of this Biome (Campbell and Lamar, 2004). The lack of comprehensive ecological data for L. muta, coupled with the challenge of encountering individuals in the field or scientific collections, underscores its rarity and the difficulties associated with studying this species (Lira-da-silva et al., 2009). However, recent studies utilizing telemetry on resident and translocated individuals in southern Bahia have provided new insights into the habitat preferences of L. muta. Contrary to previous assumptions, these findings suggest that L. muta may exhibit a greater level of tolerance towards agroforestry regions and areas undergoing early regeneration, rather than being solely reliant on well-preserved forests (Padrón et al., 2022).
The species X. undulatus, with only two specimens registered for PAF obtained a high vulnerability index (0.61). In the Cerrado (França and Araújo, 2006), this species also appeared as threatened, while in the list of species of the state of São Paulo (Marques et al., 2009), it appeared as being vulnerable. The main threat for this species indicated by Marques et al. (2009) is destruction of their habitat.
As with the rattlesnake, C. durissus (CR), other snakes, such as S. pullatus (EN), B. constrictor (VU), and E. assisi (VU), also had their vulnerability to extinction index greatly influenced by direct anthropogenic effects, such as roadkill, consumption as a human food, and use of the species for medicinal, magic/religious, ornamental, or decorative purposes. These factors need attention due to their uniqueness and growth in the last decades (Pereira-Filho et al., 2017).
The impact of roadkill on wild animal species has gained significant attention from researchers worldwide (Trombulak and Frissell, 2000). In Brazil, several studies have addressed this issue, revealing the common occurrence of road-killed snakes (Turci and Bernarde, 2009; Santos et al., 2012). In addition, studies show the existence of the practice of intentional roadkill, and explain that people generally try to kill snakes, especially for the belief that they are dangerous and pose a threat to human life (Secco et al., 2014). Even in species that present periods of nocturnal activities where car traffic would be less intense, such as E. assisi, O. trigeminus, and M. potyguara, the rate of road-killed animals is high on roads in the state of Paraíba. This threat factor, as well as others used here, are not included in the IUCN criteria for extinction risk assessments (IUCN, 2022-2) and their inclusion deserves to be assessed.
One of the primary objectives of conservation biology is to gain insight into the ecological mechanisms that contribute to the vulnerability of certain species and their decline (Caughley, 1994). By understanding these mechanisms, researchers can anticipate the potential for species extinction, thereby enhancing the chances of their survival. In general, the snakes of the Paraíba Atlantic Forest have restricted distribution, are rare and show diet specialization. Our results indicate that only 18% of snake species in the Paraíba Atlantic Forest have no risk of declining and revealed some patterns that can help to direct the conservation efforts for this fauna. We understand the importance of the formal IUCN system for assessing the risk of species extinction. Here, we are simply suggesting the parallel use of alternative parameters (e.g., data related to natural history and species ecology) to assess species’ vulnerability to extinction and define conservation priorities. This type of approach becomes necessary in situations where, for example, species population data is not available. It is worth noting that some species with high vulnerability indices in our study are not currently included in pre-existing red lists. This disparity highlights the importance of our research in identifying species that may have been overlooked or inadequately evaluated. Among these species are B. constrictor, C. exoletus, C. durissus, S. pullatus, and T. hypoconia, which appear as “least concern” or “data deficient” in the existing lists of threatened species. It is crucial to recognize that these species, despite their current classification, warrant further careful evaluation in future assessments.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RF: Writing – original draft, Writing – review & editing, Formal analysis, Conceptualization, Data curation, Project administration. MS: Writing – review & editing, Supervision, Conceptualization, Resources. FF: Writing – review & editing, Supervision, Conceptualization, Data curation, Resources.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
RCF thanks Paraíba State Research Foundation (FAPESQ) grant BLD-PDRP 09/2023. MS thanks CNPq for a research scholarship (304999/2015-6) and Alexander von Humboldt Foundation/CAPES for a grant (BEX 0585/16-5). FGRF thanks the financial support from CNPq (Universal grant 404671/2016-0) and Universidade Federal da Paraíba-UFPB (Edital PROPESQ/PRPG/UFPB No 03/2020 - PVP13459-2020).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1301717/full#supplementary-material
Alves R. R. N., Neto N. A. L., Santana G. G., Vieira W. L. S., Almeida W. O. (2009). Reptiles used for medicinal and magic religious purposes in Brazil. Appl. Herpetol. 6, 257–274. doi: 10.1163/157075409X432913
Alves R. R. N., Pereira-Filho G. A. (2007). Commercialization and use of snakes in North and Northeastern Brazil: implications for conservation and management. Biodivers. Conserv. 16, 969–985. doi: 10.1007/s10531-006-9036-7
Alves R. R. N., Rosa I. L., Neto N. A. L., Voeks R. (2012). Animals for the Gods: magical and religious faunal use and trade in Brazil. Hum. Ecol. 40, 751–780. doi: 10.1007/s10745-012-9516-1
Andreone F., Luiselli L. (2000). The Italian batrachofauna and its conservation status: a statistical assessment. Biol. Conserv. 96, 197–208. doi: 10.1016/S0006-3207(00)00070-7
Barbosa M. R. D. V., Agra M. D. F., Sampaio E. V. S. B., Cunha J. D., Andrade L. D. (2004). “Diversidade florística na Mata do Pau-Ferro, Areia, Paraíba,” in Brejos de altitude em Pernambuco e Paraíba: história natural, ecologia e conservação. Eds. Pôrto K. C., Cabral J. J. P., Tabarelli M.. Brasília, 111–121.
Bellini G., Arzamendia V., Giraudo A. R. (2013). Ecology of Thamnodynastes hypoconia in subtropical – temperate South America. Herpetologica 69, 67–79. doi: 10.2307/23352108
Böhm M., Collen B., Baillie J. E. M., Bowles P., Chanson J., Cox N., et al. (2013). The conservation status of the world’s reptiles. Biol. Conserv. 157, 372–385. doi: 10.1016/j.biocon.2012.07.015
Boldrini-França J., Corrêa-netto C., Silva M. M. S., Rodrigues R. S., de La Torre P., Pérez A., et al. (2010). Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteomics 73, 1758–1776. doi: 10.1016/j.jprot.2010.06.001
Boyles J. G., Storm J. J. (2007). The perils of picky eating: Dietary breadth is related to extinction risk in insectivorous bats. PLoS One 2, e672. doi: 10.1371/journal.pone.0000672
Campbell J. A., Lamar W. W. (2004). The Venomous Reptiles of the Western Hemisphere (Ithaca: Cornell University press). doi: 10.1016/j.trstmh.2004.12.002
Cardoso J. L. C., França F. O. S., Wen F. H., Málaque C. M. S., Haddad J. V. (2003). Venomous animals in Brazil: biology, clinic and therapeutics of envenomations. Rev. Inst. Med. Trop. Sao Paulo 45, 338–338. doi: 10.1590/S0036-46652003000600009
CEPED (2012). Atlas Brasileiro de Desastres Naturais 1991 a 2010: volume Brasil (Florianópolis: Centro Universitário de Estudos e Pesquisas sobre Desastres. CEPED).
Coimbra-Filho A. F., Câmara I. G. (1996). Os limites originais do bioma da Mata Atlântica na Região Nordeste do Brasil (Rio de Janeiro: FBCN).
Collar N. J. (1996). The reasons for red data books. Oryx 30, 121–130. doi: 10.1017/S0030605300021505
Dixon J. R., Soini P. (1986). The reptiles of the upper Amazon Basin, Iquitos Region, Peru (U.S.A: Milwaukee Public Museum). doi: 10.1111/j.1365-2028.1977.tb00406.x
Dulvy N. K., Reynolds J. D. (2002). Predicting extinction vulnerability in skates. Conserv. Biol. 16, 440–450. doi: 10.1046/j.1523-1739.2002.00416.x
Fernandes D. S., Franco F. L., Fernandes R. (2004). Systematic revision of the genus lachesis daudin 1803 (Serpentes, Viperidae). Herpetologica 60, 245–260. doi: 10.1655/02-85
Filippi E., Luiselli L. (2000). Status of the Italian snake fauna and assessment of conservation threats. Biol. Conserv. 93, 219–225. doi: 10.1016/S0006-3207(99)00138-X
França F. G. R., Araújo A. F. B. (2006). The conservation status of snakes in central Brazil. South Am. J. Herpetol. 1, 25–36. doi: 10.2994/1808-9798(2006)1[25:TCSOSI]2.0.CO;2
França R. C., Germano C. E. S., França F. G. R. (2012). Composition of a snake assemblage inhabiting an urbanized area in the Atlantic Forest of Paraíba State, Northeast Brazil. Biota Neotrop. 12, 183–195. doi: 10.1590/S1676-06032012000300019
França R. C., Morais M., França F. G., Rödder D., Solé M. (2020). Snakes of the Pernambuco Endemism Center, Brazil: diversity, natural history and conservation. ZooKeys 1002, 115–158. doi: 10.3897/zookeys.1002.50997
Gärdenfors U., Hilton-Taylor C., Mace G. M., Rodríguez J. P. (2001). The application of IUCN red list criteria at regional levels. Conserv. Biol. 15, 1206–1212. doi: 10.1046/j.1523-1739.2001.00112.x
Graboski R., Pereira Filho G. A., Silva A. A. A., Costa Prudente A. L., Zaher H. (2015). A new species of Amerotyphlops from Northeastern Brazil, with comments on distribution of related species. Zootaxa 3920, 443–452. doi: 10.11646/zootaxa.3920.3.3
Guedes T. B., Nogueira C., Marques O. A. V. (2014). Diversity, natural history, and geographic distribution of snakes in the Caatinga, Northeastern Brazil. Zootaxa 3863, 1–93. doi: 10.11646/zootaxa.3863.1.1
Işik K. (2011). Rare and endemic species: why are they prone to extinction? Turk J. Bot. 35, 411–417. doi: 10.3906/bot-1012-90
IUCN (2012). The IUCN Red List of Threatened Species. Version 2012-2. Available at: http://www.iucnredlist.org. (Accessed January 29, 2013).
IUCN (2018) The IUCN Red List of Threatened Species. Version 2018-2. Available at: http://www.iucnredlist.org (Accessed November 18, 2018).
IUCN (2022) The IUCN Red List of Threatened Species. Version 2022-2. Available at: http://www.iucnredlist.org (Accessed March 16, 2023).
Laver P. N., Kelly M. J. (2008). A critical review of home range studies. J. Wildl. Manage. 72, 290–298. doi: 10.2193/2005-589
Lira-da-silva R. M., Mise Y. F., Casais-e-silva L. L., Ulloa J., Hamdan B. (2009). Serpentes de importância médica do nordeste do Brasil. Gaz. Médica da Bahia 79, 7–20.
Luiselli L. (2009). A model assessing the conservation threats to freshwater turtles of Sub-Saharan Africa predicts urgent need for continental conservation planning. Biodivers. Conserv. 18, 1349–1360. doi: 10.1007/s10531-008-9486-1
Marques O. A. V., Eterovic A., Nogueira C., Sazima I. (2015). Serpentes do Cerrado: guia ilustrado (Ribeirão Preto: Holos).
Marques O. A. V., Eterovic A., Sazima I. (2004). Snakes of the Brazilian Atlantic Forest An Illustrated Field Guide for the Serra do Mar Range (Ribeirão Preto: Holos).
Marques O. A. V., Nogueira C. C., Sawaya R. J., Bérnils R. S., Martins M., Molina F. B., et al. (2009). “Répteis,” in Fauna Ameaçada de Extinção no Estado de São Paulo. Eds. Bressan P. M., Kierulff M. C. M. (São Paulo: Fundação Parque Zoológico de São Paulo, Secretária do Meio Ambiente de São Paulo), 286–327.
Martins M., Molina F. B. (2008). “Panorama geral dos répteis ameaçados do Brasil,” in Livro vermelho da fauna brasileira ameaçada de extinção. Eds. MaChado A. B., Drummond G., Paglia A. (Belo Horizonte: MMA, Brasília, Fundação Biodiversitas), 327–334.
McKinney M. L. (1997). Extinction vulnerability and selectivity:Combining Ecological and Paleontological Views. Annu. Rev. Ecol. Syst. 28, 495–516. doi: 10.1146/annurev.ecolsys.28.1.495
Mendonça L. E. T., Vieira W. L. S., Alves R. R. N. (2014). Caatinga Ethnoherpetology: Relationships between herpeto- fauna and people in a semiarid region of northeastern Brazil. Amphib. Reptil. Conserv. 8, 24–32.
Mesquita D. O., Alves B. C. F., Pedro C. K. B., Laranjeiras D. O., Caldas F. L. S., Pedrosa I. M. M. C., et al. (2018). Herpetofauna in two habitat types (tabuleiros and Stational Semidecidual Forest) in the Reserva Biológica Guaribas, northeastern Brazil. Herpetol. Notes 11, 455–474.
Mikich S. B., Bérnils R. S. (2004). Livro vermelho da fauna ameaçada no estado do Paraná (Curitiba: Instituto ambiental do Paraná).
MMA (Ministério do Meio Ambiente) (2022) Lista Nacional das Espécies Fauna Ameaçadas de Extinção (Portaria MMA N°. 148, de 07 de Junho de 2022). Available at: https://www.in.gov.br/en/web/dou/-/portaria-mma-n-148-de-7-de-junho-de-2022-406272733 (Accessed December 13, 2022).
Montingelli G. G., Barbo F. E., Alves G., Filho P., Santana G., Gustavo F., et al. (2020). A second new species for the rare dipsadid genus Caaeteboia Zaher et al. 2009 (Serpentes : Dipsadidae) from the atlantic forest of northeastern Brazil. Trabajo cuad. herpetol 34, 219–230. doi: 10.31017/CdH.2020.(2020-003)zoobank
Myers N., Mittermeier R., Mittermeier C., Fonseca G., Kent J. (2000). Biodiversity hotspots for conservation priorities. Conserv. Biol. 403, 853. doi: 10.1038/35002501
Padrón D. F., Mebert K., Pareja-Mejía D., Bauer A., Fernandes Vasconcelos L. D., Correia D., et al. (2022). Living in a mosaic of Brazilian Atlantic Forest and plantations: spatial ecology of five bushmaster Lachesis muta (Viperidae Crotalinae). Ethology Ecol. Evol. 35, 530–550. doi: 10.1080/03949370.2022.2123860
Passos P., Ramos L., Pereira D. N. (2012). Distribution, natural history, and morphology of the rare snake, Caaeteboia amarali (Serpentes: Dipsadidae). Salamandra 48, 51–57.
Pereira-Filho G. A., Guedes T. B., França R. C., Freitas M. A., Lourenço-de-Moraes R., Mesquita D. O., et al. (2023). “Composition, species richness, and conservation of the reptiles of the highly threatened northern Brazilian atlantic forest,” in Animal Biodiversity and Conservation in Brazil's Northern Atlantic Forest. Eds. Pereira Filho G. A., Franca F. G. R., Alves R. R. N., Vasconcellos A. (Cham: Springer International Publishing), 169–183.
Pereira-Filho G. A., Vieira W. L. S., Alves R. R. N., França F. G. R. (2017). Serpentes da Paraíba: Diversidade e Conservação (João Pessoa: G.A. Pereira Filho).
Piratelli A. J., Francisco M. R. (2013). Conservação da biodiversidade: dos conceitos às ações (Rio de Janeiro: Technical Books).
Pires M. G., Silva JR N. J., Feitosa D. T., Prudente A. L. C., Filho G. A. P., Zaher H. (2014). A new species of triadal coral snake of the genus Micrurus Wagler 1824 (Serpentes: Elapidae) from northeastern Brazil. Zootaxa 3811, 569–584. doi: 10.11646/zootaxa.3811.4.8
Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M. (2000). Predicting extinction risk in declining species. Proc. R. Soc B Biol. Sci. 267, 1947–1952. doi: 10.1098/rspb.2000.1234
Rio Grande do Sul (2014). Decreto Lei n° 51.797, de 8 de setembro de 2014. Publicado no DOE n.° 173, de 09 de setembro de 2014 (Declara as Espécies da Fauna Silvestre Ameaçadas de Extinção no Estado do Rio Grande do Sul). Available at: https://leisestaduais.com.br (Accessed November 5, 2022)
Rodrigues A. S. L., Pilgrim J. D., Lamoreux J. F., Hoffmann M., Brooks T. M. (2006). The value of the IUCN Red List for conservation. Trends Ecol. Evol. 21, 71–76. doi: 10.1016/j.tree.2005.10.010
Santos A. L. P. G., Rosa C. A., Bager A. (2012). Variação sazonal da fauna selvagem atropelada na rodovia MG 354, Sul de Minas Gerais – Brasil. Biotemas 25, 73–79. doi: 10.5007/2175-7925.2012v25n1p73
Secco H., Ratton P., Castro E., Lucas P. S., Bager A. (2014). Intentional snake road-kill: A case study using fake snakes on a Brazilian road. Trop. Conserv. Sci. 7, 561–571. doi: 10.1177/194008291400700313
Seigel R. A. (1993). “Summary: future research on snakes, or how to combat” lizard envy,” in Snakes: Ecology and Behavior. Eds. Seigel R. A., Collins J. T.(New York: McGraw-Hill), 395–402.
SEMA (2017). “Portaria SEMA n° 37 de 15 de agosto de 2017,” in Lista Oficial das Espécies da Fauna Ameaçadas de Extinção do Estado da Bahia (Bahia: Secretaria Estadual do Meio Ambiente).
SEMAS (2017). “Resolução SEMAS no 1 de 15 de maio de 2017,” in Lista Estadual Oficial de Espécies da Fauna Ameaçadas de Extinção – Répteis (Pernambuco: Secretaria de meio ambiente e sustentabilidade).
SOS Mata Atlântica (2018). Atlas dos Remanescentes Florestais da Mata Atlântica Período 2016–2017 (São Paulo: Relatório Técnico). Available at: http://mapas.sosma.org.br/dados/.
Tabarelli M., Marins J. F., Silva J. M. C. (2002). La biodiversidad brasileña, amenazada. Investig. Cienc. 308, 42–49.
Tabarelli M., Melo M. D., Lira O. C. (2006). “Nordeste; Piauí; Ceará; Rio Grande do Norte; Paraíba; Pernambuco e Alagoas: O Pacto Murici,” in Mata Atlântica: uma rede pela floresta. Eds. Campanili M., Prochnow M. (Brasília: RMA), 149–164.
Tomović L., Urošević A., Vukov T., Ajtić R., Ljubisavljević K., Krizmanić I., et al. (2015). Threatening levels and extinction risks based on distributional, ecological and life-history datasets (DELH) versus IUCN criteria: example of Serbian reptiles. Biodivers. Conserv. 24, 2913–2934. doi: 10.1007/s10531-015-0984-7
Trombulak S. C., Frissell C. A. (2000). Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30. doi: 10.1046/j.1523-1739.2000.99084.x
Turci L. C. B., Bernarde P. S. (2009). Vertebrados atropelados na Rodovia Estadual 383 em Rondônia, Brasil. Biotemas 22, 121–127. doi: 10.5007/2175-7925.2009v22n1p121
Vitória. (2022). Decreto n° 5237-R, de 25 de novembro de 2022. (Declara as Espécies de fauna Ameaçadas de extinção no Estado do EspÍrito Santo). Instituto de Meio Ambiente e Recursos Hídricos.
Vukov T. D., Tomović L., Krizmanić I., Labus N., Jović D., Džukić G., et al. (2015). Conservation issues of Serbian amphibians identified from distributional, life history and ecological data. Acta Zool. Bulg. 67, 105–116.
Webb J. K., Brook B. W., Shine R. (2002). What makes a species vulnerable to extinction? Comparative life-history traits of two sympatric snakes. Ecol. Res. 17, 59–67. doi: 10.1046/j.1440-1703.2002.00463.x
Keywords: Atlantic Forest, red listing, extinction risk, distribution, ecology
Citation: França RC, Solé M and França FGR (2024) Conservation status of Brazilian snakes inhabiting the Atlantic Forest of Northeastern Brazil. Front. Ecol. Evol. 12:1301717. doi: 10.3389/fevo.2024.1301717
Received: 25 September 2023; Accepted: 02 January 2024;
Published: 25 January 2024.
Edited by:
Wei Wang, Chinese Research Academy of Environmental Sciences, ChinaReviewed by:
Nikolay Natchev, Shumen University, BulgariaCopyright © 2024 França, Solé and França. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafaela C. França, cmFmYWVsYS5jYW5kaWRvLmZyYW5jYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.