
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 February 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.935987
This article is part of the Research TopicCognition, Foraging, and Energetics in Extant and Extinct PrimatesView all 10 articles
 Jorin Veen1*‡
Jorin Veen1*‡ Haneul Jang2‡
Haneul Jang2‡ David Raubenheimer3‡
David Raubenheimer3‡ Bryndan O. C. M. van Pinxteren4‡
Bryndan O. C. M. van Pinxteren4‡ Vidrige Kandza2
Vidrige Kandza2 Patrick G. Meirmans1‡
Patrick G. Meirmans1‡ Nicole M. van Dam5,6,7†‡
Nicole M. van Dam5,6,7†‡ Susanne Dunker5,7,8‡
Susanne Dunker5,7,8‡ Petra Hoffmann5,7,8
Petra Hoffmann5,7,8 Anja Worrich5,6,9‡
Anja Worrich5,6,9‡ Karline R. L. Janmaat1,10,11,12‡
Karline R. L. Janmaat1,10,11,12‡The embodied capital theory states that the extended juvenile period has enabled human foragers to acquire the complex foraging skills and knowledge needed to obtain food. Yet we lack detailed data on how forager children develop these skills and knowledge. Here, we examine the seasonal diet composition, foraging behavior, and botanical knowledge of Mbendjele BaYaka forager children in the Republic of the Congo. Our data, acquired through long-term observations involving full-day focal follows, show a high level of seasonal fluctuation in diet and foraging activities of BaYaka children, in response to the seasonal availability of their food sources. BaYaka children foraged more than half of the time independent from adults, predominantly collecting and eating fruits, tubers, and seeds. For these most-consumed food types, we found an early onset of specialization of foraging skills in children, similar to the gendered division in foraging in adults. Specifically, children were more likely to eat fruit and seed species when there were more boys and men in the group, and girls were more likely than boys to collect tuber species. In a botanical knowledge test, children were more accurate at identifying plant food species with increasing age, and they used fruits and trunks for species identification, more so than using leaves and barks. These results show how the foraging activities of BaYaka children may facilitate the acquisition of foraging skills and botanical knowledge and provide insights into the development of embodied capital. Additionally, BaYaka children consumed agricultural foods more than forest foods, probably reflecting BaYaka’s transition into a horticultural lifestyle. This change in diet composition may have significant consequences for the cognitive development of BaYaka children.
The subsistence strategies and diet of human foragers are characterized by feeding on high-quality and difficult-to-acquire foods (Kaplan et al., 2000) such as meat (Milton, 1999), tubers (Laden and Wrangham, 2005), and honey (Crittenden, 2011). The embodied capital theory states that the extended juvenile period has enabled humans to acquire a wide variety of foraging skills to collect these foods and that this dietary shift has resulted in increased brain capacities (Kaplan et al., 2000). Referring to skills and knowledge as embodied capital, Kaplan et al. (2000, 2003) compared these processes of development with investments in stocks. They posit that the investment of learning extensive foraging strategies has coevolved with the large brain size, elongated childhood, and dietary shift in our species (Kaplan et al., 2003). In addition to hunted (e.g., animals) and extracted foods (e.g., tubers and honey), human foragers gather above-ground foods from wild plants, such as fruits and leaves. Even though fruits can be collected more easily regarding extraction costs, trees with ripe fruits are often sparsely distributed in space and time (Milton, 1993; Janmaat et al., 2016). The overall energetic costs of gathering have been found to exceed those of hunting and fishing (Gallois and Henry, 2021). Still, most studies on forager subsistence strategies have not considered fruit foraging and botanical knowledge, rather focusing on the importance of meat and tubers in the human diet (e.g., Jones and Marlowe, 2002; Walker et al., 2002; Tucker and Young, 2005; Gurven et al., 2006; Demps et al., 2012; Schniter et al., 2015). Moreover, previous studies on forager subsistence strategies have focused on adults, resulting in limited knowledge on the foraging contributions of children and how they acquire foraging skills and knowledge (Hewlett, 2017; but see Lew-Levy et al., 2017).
Here, we examined the seasonal diet composition, foraging behavior, and botanical knowledge of children in a contemporary forager society, the Mbendjele BaYaka (henceforth: BaYaka), in the Republic of the Congo. BaYaka children were observed to forage independently from a young age (Lew-Levy et al., 2020b), probably because foods of the BaYaka are distributed close to camp and the environment is considered relatively safe (Lewis, 2002), contrasting the ecology of other foraging societies (Blurton Jones et al., 1994; Draper, 2013). BaYaka start learning nut-cracking skills from early childhood, but their efficiency reaches maximum only later in adulthood (Boesch et al., 2019), suggesting that the children need a substantial learning period to acquire complex foraging skills (Lew-Levy et al., 2021). To understand children’s foraging and learning strategies for different types of food sources, it is important that foraging behaviors are observed throughout the year to account for seasonal changes in the types and abundance of available foods (Bahuchet, 1988; Kitanishi, 1995). In this study, we first investigated the diet composition and foraging activities of BaYaka children, while focusing on the seasonal variation and the ratio between forest and agricultural foods. Second, we examined what factors influence their collecting and eating probability of fruit/seed and tuber species, which are the most-consumed food types of the BaYaka children in our study community. Third, we examined the BaYaka children’s botanical knowledge of foraged plant species and which plant parts they used for species identification, by conducting tests with pictures of 12 different plant species.
In the Congo Basin the availability of different foods changes seasonally and annually (Bahuchet, 1988; Kitanishi, 1995), with a high availability of fruits and seeds in the wet season, a brief period of caterpillar abundance during the late wet season, and honey collecting and fishing during the dry season (Bahuchet, 1988; Hladik and Bahuchet, 1994; Kitanishi, 1995). Tubers are mainly collected during the dry season, even though their phenology is poorly known (Bahuchet, 1988). Meats are available in all seasons, although animal densities have likely declined over the years due to deforestation and high demands for bush meat. In addition to forest foods, the diet of the BaYaka include agricultural foods. They obtain these from a complex relationship with Bantu-speaking farmers on the edges of the forest (Bahuchet and Guillaume, 1982; Joiris, 2003; Takeuchi, 2005), in which agricultural foods are exchanged for labor and forest products (Kitanishi, 1994, 2003). In the studied community, the BaYaka also cultivate their own crops, including oil palm fruits (Elaeis guineensis), cassava (Manihot esculenta), papayas (Carica papaya), plantains (Musa × paradisiaca), taro (Colocasia esculenta), and sweet potatoes (Ipomoea batatas; Bombjaková, 2018).
Based on the seasonal variations in the availability of forest foods (Bahuchet, 1988; Kitanishi, 1995), we expected that BaYaka children’s diet composition and foraging activities will also fluctuate periodically. Seasonal foods such as caterpillars, honey, forest fruits, and seeds were expected to only be eaten for short periods, while less seasonal foods such as animals, fish, and leaves might be more consistently eaten throughout the year. When available, fruits were expected to be eaten frequently, as they are often sugar-rich and children in many societies prefer sweet foods (Desor and Beauchamp, 1987; Pellegrino et al., 2018). This preference for sweet foods, which is lower in adults, has been linked to physical growth during childhood (Coldwell et al., 2009). Additionally, fruits are generally less protected from consumption compared to other food types, such as leaves, mushrooms (e.g., chemical defenses), and seeds (e.g., hard shells that require access to iron or stone tools; see Panda oleosa in Boesch et al. (2019)). Besides fruits, we expected that children predominantly consume tubers, based on their importance in forager societies in the Congo Basin (Kitanishi, 1995; Sato et al., 2012). Finally, we expected meat to make up a substantial part of the diet as well, as was observed by Kitanishi (1995). Compared to Kitanishi (1995), who collected data in the same region as our study more than two decades ago (Supplementary Figure S1), we did expect to find higher percentages of agricultural foods in BaYaka children’s contemporary diet. The rapid deforestation over recent decades has likely caused a decrease in the availability of forest foods, potentially increasing their dependence on agricultural foods.
Based on the embodied capital theory (Kaplan et al., 2000) and previous research on gender-segregated children groups (Lew-Levy and Boyette, 2018; Jang et al., 2019b; Lew-Levy et al., 2020a) we expected children to learn and acquire foraging-related knowledge and skills from an early age and that this is related to a gendered division of foraging activities in adults. BaYaka men mainly hunt for animals and climb trees for fruits, seeds, and honey, whereas women spend more time collecting fish and plant foods such as tubers (Lewis, 2002). We propose that such a gendered division in foraging activities likely coincides with a division in specialized foraging skills, enabling the human species to have a much broader diet than our closest living relatives, the non-human great apes. Yet the question whether this gendered division in foraging activities is already present in childhood remains poorly understood. Hence, we also investigated whether boys and girls forage for different food items, and thus acquire distinctive foraging-related skills and knowledge. We specifically expected that the age and gender of the children and their foraging group composition will predict which foods will be collected. We expected that boys will primarily forage for fruits/seeds and girls for other plant foods such as tubers, based on the gendered division observed in adults (Lewis, 2002). However, as the BaYaka share the majority of food items within the community (Lewis, 2014), we expected that the age and gender would not specifically predict the food types and species consumed by children. We also expected that the BaYaka children are more likely to collect and consume fruits with higher sweetness levels as they have shown a strong preference for sweet foods (Kandza, 2018), like children in other societies (Desor and Beauchamp, 1987; Pellegrino et al., 2018).
Lastly, in the botanical knowledge test of foraging-related plant species, we expected boys to outperform girls as boys are expected to climb trees to collect fruits, seeds, and honey, and that older children are more accurate at identifying plant species than younger ones, as predicted by the embodied capital theory and age-structured knowledge (Kaplan et al., 2000; Lew-Levy et al., 2021). Furthermore, we expected that the children will identify the plant species mostly by using the fruit/seed part, as this is the part eaten and often brought back to camp to be shared. Children may use tree trunks as well for identification. Being able to identify a species by its trunk can enable foragers to detect potential trees from further distances and provides opportunities for inspection and fruit discovery (see Janmaat et al., 2013a). Children can also use leaves for species identification in their search, especially when inspecting the canopy or areas with fallen leaves.
We conducted our study with a total of 27 BaYaka children (Ngirls = 14, Nboys = 13), who volunteered to participate. The children lived with their community in a logging concession close to the Motaba river and the village Djoube, in the northern part of the Republic of the Congo (Supplementary Figure S1). Besides ‘BaYaka,’ the study community are often referred to as ‘Mbendjele,’ ‘Baaka,’ ‘Baka,’ or ‘Aka’ (Kitanishi, 1995; Köhler and Lewis, 2002; Bombjaková, 2018; Jang et al., 2019a). The children who participated in this study were estimated to be on average 9.7 years old (range: 4.5–17.1). Due to the absence of birth records kept by the BaYaka, the ages were estimated based on information about birth order provided by the family. Additionally, BaYaka or Bantu of whom the exact age was known were used as anchor points. Using these anchor points, the ages of other BaYaka were estimated based on an inter-birth span of 2.5 years, following Hill and Hurtado (2017).
During our data collection period, the BaYaka community resided in camp Mbaso from March to August 2016, camp Bongo from November 2019 to January 2020, and in camp Kuona from February to March 2020 (Supplementary Figure S1). The environment around the village Djoube has been described as Congolian Lowland Forests (Loubelo Madiela, 2018; Jang and Boyette, 2021), often with clear signs of logging activity. This logging activity causes rapid deforestation in those areas where the BaYaka are residing (Lewis and Nelson, 2006; Laporte et al., 2007). At each camp site, rainfall and temperature data were recorded daily over multiple fieldwork periods from 2015 to 2020. As expected from the seasonality described by Hewlett (1991), the proportion of rainy days per month was lower from November until February than from March until September, having a peak in August (Supplementary Figure S2). The temperature was relatively constant throughout the year (Supplementary Figure S2).
The focal children were accompanied on foraging trips during a rainy period of 6 months in 2016 (March – August; by V.K.) and during a dry period of 5 months in 2019–2020 (November – March; by J.V.), with most children being observed over multiple days (Supplementary Tables S1, S2). Observational data were collected using continuous focal sampling (Martin and Bateson, 2007), recorded by a combination of a GPS (Garmin 64S), that created location and time stamps, and a voice recorder (Jang et al., 2019a). The observation period for each child was split across two consecutive days to lower the potential effect of the long-term continuous presence of the researcher. In general, these 2 days were characterized as follows: the first day started with a picture test in a tent, after which observational data were collected from the moment the focal child left camp on a foraging trip until the end of the last meal of the day back in camp. On the second day, data were collected from sunrise until the first time the child would arrive back in camp after the start time of observations on the first day. This way, a full day of observation was ensured for each child. During observations, data were recorded on foraging (e.g., inspecting, digging), eating (e.g., food name, food type), and group composition (e.g., number of boys and girls). We defined inspection as moving one’s head in combination with a fixed gaze, either in the direction of the canopy or the ground (Janmaat et al., 2013b).
In total, the 27 children were observed for approximately 798 h, separated over 114 days covering every month of the year except for September and October. Since children were observed on two consecutive days, we analyzed these 114 days as 57 full-day observations (Supplementary Tables S1, S2). To visualize the variation in the diet composition and foraging activities throughout the year, the study period was divided into five periods (Supplementary Table S1). The focal child was asked to participate after a random selection depending on whether the child was present in the camp, in most cases alternating between girls and boys to account for seasonal variation in food availability. Informed consent was obtained from both the child and their parent(s)/caretaker(s) after the data collection method had been explained in their language.
Using the long-term observational data, we examined seasonal variation in diet composition and foraging activities of BaYaka children. Each food item was categorized into different food types including fruit, tuber, seed, leaf, honey, aquatic animal, caterpillar, and terrestrial animal (Supplementary Table S3). The food item was assigned as a fruit when the children ate the fleshy pulp that is often rich in sugars, whereas it was assigned as a seed when they ate the lipid-rich parts. Food items were furthermore distinguished between forest and agricultural foods (Supplementary Table S3). Similar to other dietary studies (see Lim et al., 2021), the time spent eating a certain food item was used as an indication of its percentage in the diet. This way, the behavior could be observed without interference by weighing each food item, which likely would have affected the children’s behavior such as eating and sharing. When a combination of multiple food items was eaten in a cooked dish (e.g., fish with Gnetum leaves), the total eating time was divided by the number of different food items eaten, assuming equal eating time on each food item. Foraging time was estimated based on the food items that were searched for and collected during foraging trips outside the camp, either in the forest or in gardens. Trips and behaviors unrelated to foraging were excluded from the calculation, including visiting other villages or camps, washing, swimming, collecting firewood, playing, dancing, cooking, and gardening. To estimate the total foraging time, it was assumed that a child would forage for a particular type of food (e.g., tubers) until they started looking for or collecting another food type (e.g., fruits). If it was not clear, the child was asked what they were looking for. Finally, travel time back to camp was not considered to be part of the foraging time when no food items were collected during travel.
Botanical identifications of food plants were performed at the Herbarium of the Institut de Recherche en Sciences Exactes et Naturelles (IRSEN) in Brazzaville, after which the dried samples were transported to the German Centre for Integrative Biodiversity Research (iDiv) to determine nutritional content. Samples were dried by storing them in small ziplock bags with silica. For the sugar extraction, 100 ± 5 mg of ground sample material was mixed with 1 mL of 70% methanol, vortexed and boiled for 5 min in a water bath (GFL, Burgwedel, Deutschland) at 90°C. Subsequently, samples were transferred to an ultrasonication bath (Thermo Fisher Scientific, Dreieich, Germany) for 15 min. Solid material was separated from the supernatant via centrifugation for 10 min at 14,000 × g in a benchtop centrifuge (Thermo Fisher Scientific, Dreieich, Germany) at room temperature. These supernatants were transferred to new reaction tubes (Eppendorf, Hamburg, Deutschland) and pellets were extracted a second time with 1 mL of 70% methanol and a 15 min ultrasonication step. After centrifugation, the supernatants were combined and the evaporation loss of methanol was compensated by bringing extracts to the predetermined average weight of Eppendorf tubes containing 2 mL of 70% methanol. Each sample material was extracted three times and samples were stored at −20°C until analysis. Extracted samples were measured with a high-performance anion-exchange chromatography system ICS-5000 with a pulse-amperometric detector (Thermo Fisher Scientific, Dreieich, Germany) for carbohydrate analysis. As mobile phase, eluents (12 mM and 150 mM NaOH) were manually prepared from 50% (w/w) NaOH (FisherChemical). A gradient program was used at a flow rate of 0.2 mL min−1 (Supplementary Table S4). As stationary phase a CarboPac-column (Dionex CarboPac, PA210-4 μm, 2 × 30 mm, Thermo Fisher Scientific, Dreieich, Germany) was used. The detection was performed via an electrochemical detector with a disposable Au-electrode and a quadrupole pulse- waveform at a sampling rate of 2 Hz. For carbohydrate quantification calibration curves were measured for D-(+)-glucose, sucrose, and D-(−)-fructose (10 Carbohydrate Kit, SigmaAldrich) in a range of 1–10 mg L−1. The samples were diluted accordingly to be detectable within the ranges of the calibration curves. The quantification was undertaken with the Software Chromeleon (Version 7.2.6). Standard and samples were injected with a volume of 2.5 μL. The sample compartment was cooled to 20°C and the column compartment heated to 30°C.
To determine sugar richness, the concentrations of glucose, fructose, and sucrose were summed (Supplementary Table S5). Subsequently, total sweetness was calculated based on the sweet perception in Aka foragers (Hladik et al., 1986) by multiplying these concentrations with 1.00, 6.55, and 7.76, respectively, and then taking the sum over the three sugar types (Supplementary Table S5).
After examining the seasonal variation in diet composition and foraging activities, we focused on the behavior while foraging for fruits, seeds, and tubers. As these were the most-consumed food types, we examined the factors influencing their collecting and eating probabilities. For some of the food items, availability was difficult to assess by independent ecological surveys. Hence, to determine the availability of each food species throughout the year, we supplemented our dataset with data collected similarly and simultaneously on foraging women (see Jang et al., 2019 for wet season). Based on all these data, a specific food species that was foraged and/or eaten on a specific date was assumed to be available the week before and after that date. This way, for each child a list of food species was created that could have potentially been collected and eaten. These available food species were the sample units of our models. Subsequently, the observational data of each child were used to determine for each food species whether it was collected and/or eaten or not. We analyzed the collecting and eating behavior as a binary response to circumvent potential issues with temporal autocorrelation (e.g., a child might be more likely to find more tubers after finding the first one of a particular species) and to minimize interobserver differences.
During the dry season of 2019–2020, we tested the botanical knowledge of the 18 children (Ngirls = 8, Nboys = 10) for whom behavioral data were collected (Supplementary Table S2). We selected 12 plant species that produce edible fruits or seeds known to be eaten by the BaYaka (Supplementary Table S6), then tested the ability of the children to correctly name these plant species. Providing the pictures of the plant parts had the advantages of testing the different parts separately and disentangling the possibility that children would have remembered individual tree species based on spatial knowledge from previous foraging experiences. For each of the 12 plant species, we located multiple individuals of which pictures were taken from the fruit/seed, leaf, trunk, and bark (Supplementary Figure S3). Most pictures of the fruits/seeds were taken previously during the wet season. The pictures of the trunk were taken at breast height, from a distance that made the base of the trunk visible (Supplementary Figure S3D). For the bark, the pictures were taken from a short distance including a rectangular incision in the tree ensuring that both the inner and outer bark were visible (Supplementary Figure S3E), similar to pictures provided in botanical books (Hawthorne and Jongkind, 2006). The children were all used to seeing 2D-pictures from previous research in 2013, and each year between 2015 and 2018. Nonetheless, to test for potential effects of 2D-pictures, we also included the actual leaves. These leaves were dried to simulate the leaves they would encounter in the leaf fall area surrounding the trees. Five different samples were used for each picture of the part of a certain plant species and its dried leaves. Subsequently, we randomly selected which sample was demonstrated on a laptop to a child and in which order. In total, all 60 combinations of plant parts and plant species were shown to each child: 12 species with for each species a picture of one of the four parts (i.e., fruit/seed, leaf, trunk, bark) and the actual leaf. One child with albinism was tested, but these data were excluded from the analysis since her eyesight limited her ability to interpret the pictures.
Since it was previously found that foraging-related plant knowledge is widely shared among camp members (Salali et al., 2016), we investigated whether there was an indication the children shared the answers of the botanical test with each other. To prevent this, the order and samples demonstrated were randomized and the children were not told which answers were correct. If they somehow would have figured out which 12 plant species were included in the test, we predicted that they would answer one of the names of these 12 plant species more frequently when they did not know the correct answer (i.e., guessing), which could have led to children tested later scoring higher on the test.
To examine the differences in foraging-related behaviors between boys and girls, we performed Mann–Whitney U tests. Based on the gendered division in adults we expected boys to spend more time foraging for fruits, seeds, caterpillars, and honey, and girls to spend more time foraging for fish and tubers. This might require different types of foraging behaviors since the boys are expected to forage for food items often located in the canopy while the girls are expected to forage on items found on the ground. Therefore, we expected boys to inspect the canopy more often and climb more trees. In contrast, girls were expected to inspect the ground more often and to collect larger quantities of tubers.
To examine the effects on the collecting and eating probabilities of fruit and seed species, we constructed two Generalized Linear Mixed Models (GLMMs; Baayen et al., 2008), using a binomial error structure with logit link function (models Fcol and Feat; Supplementary Table S7). The response variable consisted of the collecting (model Fcol) and eating (model Feat) probability (1 or 0) of all fruit and seed species that were expected to be available on the day of observation. As fixed effects, we included age, gender, weighted average number of boys and men in the group, sweetness, food habitat, and food type. The observation time per day varied (mean = 14.00 ± 2.05 h, range: 11.44–20.12), mostly depending on the moment the child would eat the last meal on the first day and would arrive back in camp on the second day. Therefore, observation time was log-transformed and included as an offset term. The random effects were observation day, child, and food species. For both models, we initially included all possible random slopes and their interactions to control type I error rate (Schielzeth and Forstmeier, 2009), creating a Maximal Model (Barr et al., 2013). These models included the random slopes of sweetness, habitat, and food type within observation day; of age, number of boys and men, sweetness, habitat, and food type within child; and of age, gender, and number of boys and men within food species. The models were subsequently simplified by the removal of the unidentifiable correlations between the random slopes of sweetness, habitat, and food type and their intercept observation day (Matuschek et al., 2017). For both models, we tested for multicollinearity by calculating the Variance Inflation Factors (VIFs) for the fixed effects in the models (Zuur et al., 2010). With a maximum VIF of 1.910, there was no indication of multicollinearity. Based on the stability analyses, the fixed effects of habitat and food type were slightly unstable for the Fcol model, as well as habitat in the Feat model. The direction of the effect of food type in Fcol and habitat in Feat was, however, stable. In total, 364 fruit/seed collecting and eating probabilities were included in each model, based on 57 observation days in which 27 children were observed with a total of 15 different species (species availability: mean = 6.39 ± 2.63, range: 1–10). We expected that older children are better at climbing trees and thus have a higher probability of collecting fruit and seed species compared to younger children. Especially boys were expected to focus more on this type of foraging. However, if the fruits and seeds are subsequently brought back to camp, we expected everyone to have an equal probability of eating the food by demand-sharing. In addition to the age and gender of the focal child, we also expected the number of boys and men in the group to have an effect if there is a gendered division in foraging activities. Groups with more boys and men were expected to spend more time on fruit/seed-related foraging trips and to have a higher probability of finding these food items. We expected children not yet able to climb trees to be dependent on older children to collect the fruits and seeds if they are high in the canopy. For this, the weighted average number of boys and men during trips outside of camp was calculated. To calculate this, we took into account the duration of each observed behavior and the according group composition. Finally, we expected the characteristics of the fruit/seed itself (i.e., sweetness, habitat, type) to influence the collecting and eating probability. Agricultural fruits and seeds were thought to have a less complex spatio-temporal distribution and to have some nutritional advantages over forest fruits, increasing the probability of collecting and eating them. As mentioned, we expected sweeter food items to be preferred. We therefore expected that the children prefer to collect and eat fruits over seeds.
For the collecting and eating probabilities of the tuber species, two GLMMs (Baayen et al., 2008) with binomial error structure and logit link function were constructed (models Tcol and Teat; Supplementary Table S7). In these models, the response variable consisted of all available tuber species for each child and whether they were collected/eaten or not (1 or 0). The fixed effects were age, gender, weighted average number of girls and women in the group, and habitat. Observation time (log-transformed) was again included as an offset term. The same random effects (i.e., observation day, child, food species) were included as in models Fcol and Feat. The created Maximal Models (Barr et al., 2013) had the random slope of habitat within observation day; of age, number of girls and women, and habitat within child; and of age, gender, and number of girls and women within food species. Subsequently, all the correlations between the random slopes and intercepts were removed to deal with convergence issues (Matuschek et al., 2017). The maximum VIF found for these models was 1.557, showing no indication of multicollinearity. Model stability analyses showed that the fixed effects were unstable for model Tcol, similar to habitat in model Teat. However, the direction of the effect of gender in Tcol and of habitat in Teat was found to be stable. The sample size in each model consisted of 250 collecting and eating probabilities of tuber species, using data from 57 observation days in which 27 children were observed with eight tuber species (species availability: mean: 4.39 ± 1.63, range: 2–8). We expected that older children are better at collecting tubers than the younger ones, but that the tubers will subsequently be shared and eaten by all children in the camp. Since tuber collecting is considered women’s work (Lewis, 2002), we expected that girls would have a higher probability to collect the tuber species than boys. In contrast to the fruit/seed models, we hypothesized that instead of the number of boys and men, here the weighted average number of girls and women in the group to have a positive effect on the collecting probability. As in the fruit/seed model, we also expected agricultural tubers to have a higher probability of being collected and eaten than forest ones because of their easier localization and higher nutrition, but also because of lower digging costs.
To examine the effect of age, gender, and plant part shown on the botanical identifications, we constructed a GLMM (Baayen et al., 2008), using a binomial error structure with logit link function (model Bknow; Supplementary Table S7). The probability of correctly identifying the plant species was used as a response variable, being either 1 or 0. The fixed effects of the model were age, gender, and the part of plants shown to the children, and as random effects, we included the sample, child, and plant species. After initially creating a Maximal Model following Barr et al. (2013), including the random slopes and their interactions with the intercepts (Schielzeth and Forstmeier, 2009), the model was simplified. In the Maximal Model we included random slopes of part shown within child; of age, gender, and part shown within plant species; and of age and gender within sample. The correlation between the random slope of gender and sample was unidentifiable and thus removed (Matuschek et al., 2017). All model assumptions were checked, including collinearity. With a maximum VIF value of 1.227 there was no indication of multicollinearity. Model stability analyses demonstrated that the fixed effects were stable. In total, 1,020 questions were asked to the 17 children consisting of four pictures (i.e., fruit, leaf, trunk, bark) and a dried leaf from 12 different foraging-related plant species. We expected that the children’s botanical knowledge linearly increases with age during childhood and that boys are better at identifying foraging-related plant species than girls are. The children are expected to be best at identifying the fruit/seed itself, followed by the trunk, leaf, and bark. If there is no effect of 2D-picture, equal scores are expected between the pictures of leaves and the actual leaves.
All statistical analyses were performed in R (version 1.1.419; Rstudio Team, 2016). For the GLMMs (Supplementary Table S7) we used the function glmer of the package lme4 (version 1.1-23; Bates et al., 2015). To increase the probability the model would converge, the optimizer bobyqa was used. The VIFs were calculated using the vif function in the car package (Fox and Weisberg, 2019). The functions glmm.model.stab, ranef.diagn.plot, and boot.glmm.pred were provided by dr. R. Mundry to assess model stability, to test the assumption that the Best Linear Unbiased Predictors (BLUPs; see Baayen et al., 2008) were normally distributed, and to calculate the bootstrapped 95% confidence intervals (using 1,000 bootstraps), respectively. Finally, effect sizes (Nakagawa et al., 2017) were calculated with the r.squaredGLMM function from the package MuMIn (version 1.43.17; Barton, 2020), with the marginal R2 reporting the effect size of the fixed effects and the conditional R2 reporting the effect size of fixed and random effects. All categorical fixed effects were manually dummy coded and centered, and all covariates were z-transformed to increase the likelihood that the models would converge and to make the estimates comparable. To test the overall effect of each model, likelihood ratio tests (LRTs) were used (Dobson, 2002), comparing the full model with the null model using the anova function. These null models consisted of the offset term and the random effect structure, lacking all fixed effects (Forstmeier and Schielzeth, 2011). For the individual effects, LRTs were used with the drop1 function, comparing all possible models lacking one of the terms (Barr et al., 2013; Harrison et al., 2018).
Out of the 798 h of observational data, 89 h were spent eating (11.2%) and 218 h were spent foraging (27.3%). More than half of the time the children foraged independently from the adults (52.4%), primarily in peer groups. Children spent on average most time eating fruits (39.6%), followed by tubers (29.1%), seeds (15.4%), leaves (4.3%), caterpillars (3.8%), aquatic animals (2.4%), honey (2.3%), and terrestrial animals (1.4%), with the remaining food items (e.g., mushrooms, insects other than caterpillars, or market foods only available in the village) making up 1.6% (Figure 1; Supplementary Table S3). Figure 1 shows that they spent most time foraging for tubers (40.5%), followed by fruits (16.2%), aquatic animals (15.8%), caterpillars (9.3%), honey (8.4%), seeds (5.1%), leaves (2.0%), terrestrial animals (1.8%), and others (1.1%).
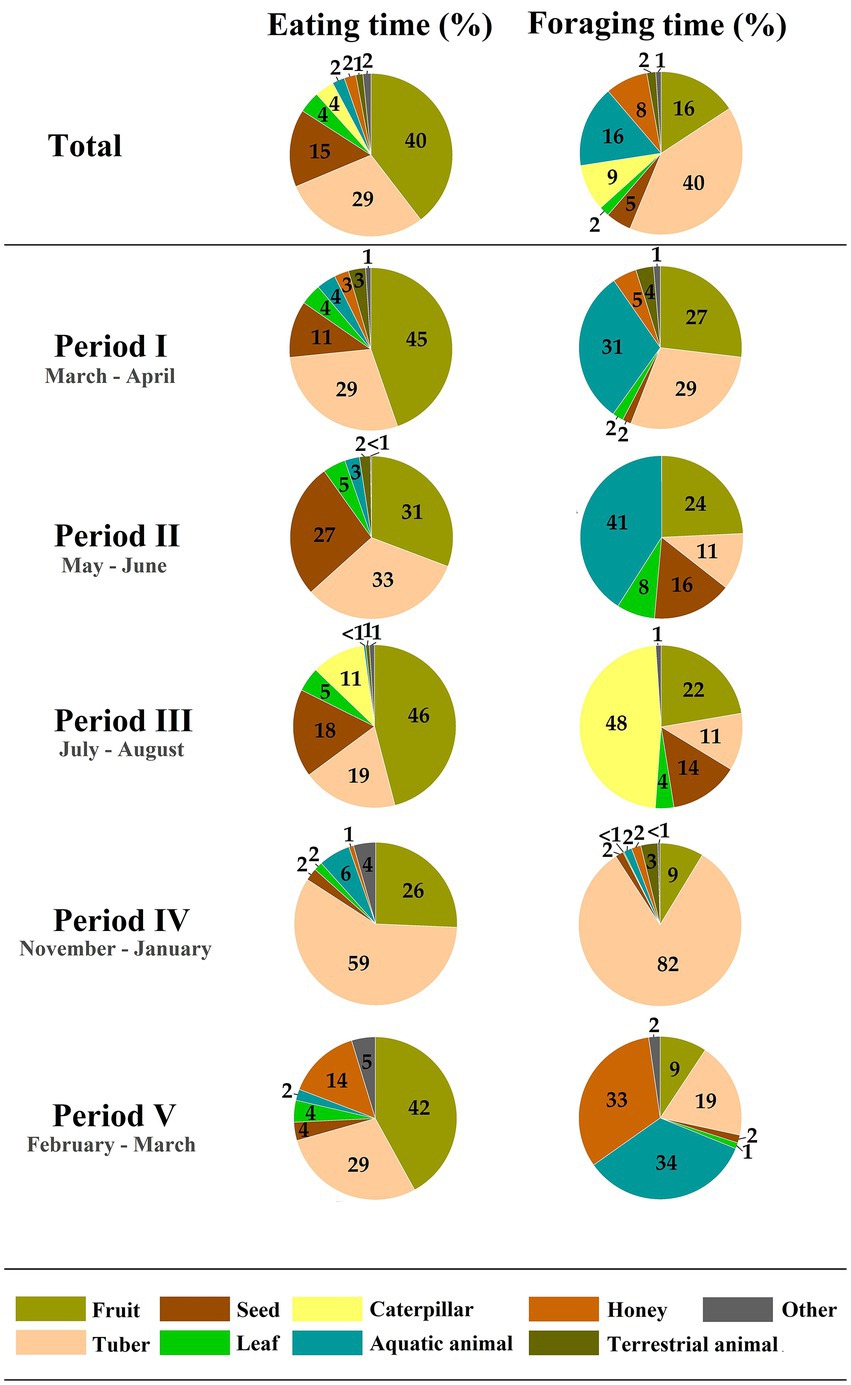
Figure 1. Diet composition and foraging activity. Shown are the percentages of time eating and foraging for different food types over the year. The periods visualize the fluctuation throughout the year. Period I to III corresponds with the wet season of 2016 and period IV and V with the dry season of 2019–2020.
The eating and foraging of these different food types varied across observation periods (Figure 1). With the exception of leaves and terrestrial animals, the food types show clear seasonal differences in eating and foraging time (Figure 1). Caterpillars, for example, were exclusively foraged and eaten during period III (Figure 1), which corresponds with the late wet season (Supplementary Figure S2). Foraging and feeding on honey had a clear peak during period V (Figure 1), the late dry season (Supplementary Figure S2). Furthermore, aquatic animals were also not consistently foraged throughout the year, being absent in periods III (late wet season) and IV (early dry season). Even though children were not observed to forage for aquatic animals during period IV, they did eat them. The foraging and feeding on seeds largely correspond with the wet season (period I to III; Figure 1). The seasonal variation in fruit foraging and eating becomes clear when focusing on the proportion of forest and agricultural fruits, with forest fruits exclusively being foraged and eaten during the wet season (period I to III; Supplementary Table S8).
Of the total eating time of the children, 42.8% concerned forest foods, compared to 55.5% agricultural foods and 1.7% food of other or unknown origin (Supplementary Table S3). The majority of the eating time on tubers consisted of agricultural species, with the exception of period IV, in which 83.2% of the eating time on tubers was on forest tubers (Supplementary Table S8). This was largely due to one species, Dioscoreophyllum cumminsii, which accounted for 96.9% of the time spent eating forest tubers (Supplementary Table S8). Even though forest tubers were eaten less than agricultural ones, more time was spent foraging for those forest tubers than for agricultural tubers (Supplementary Table S8). It furthermore took, on average, five times as long digging to find a forest tuber than an agricultural one during the dry season (302.03 ± 206.07 s and 57.79 ± 39.90 s, respectively).
The eating time and diet composition of boys and girls were highly similar, except for honey which was almost absent in the diet of girls (Figure 2). Boys spent a slightly lower proportion of the day foraging than girls (boys: 23.4%; girls: 31.6%), spending more time in camp (49.7 and 48.9%) and on trips unrelated to foraging (26.8 and 19.4%), such as washing or playing (see Methods; Figure 2). There were, however, larger differences between the foraging activities, with boys spending more time foraging for fruits (18.4%), honey (18.0%), and seeds (8.6%) than girls (13.7, 0.7, 2.2%, respectively; Figure 2). Girls, on the other hand, spent more time foraging for tubers (50.4%) and aquatic animals (20.4%) than did boys (28.1 and 10.9%, respectively, Figure 2).
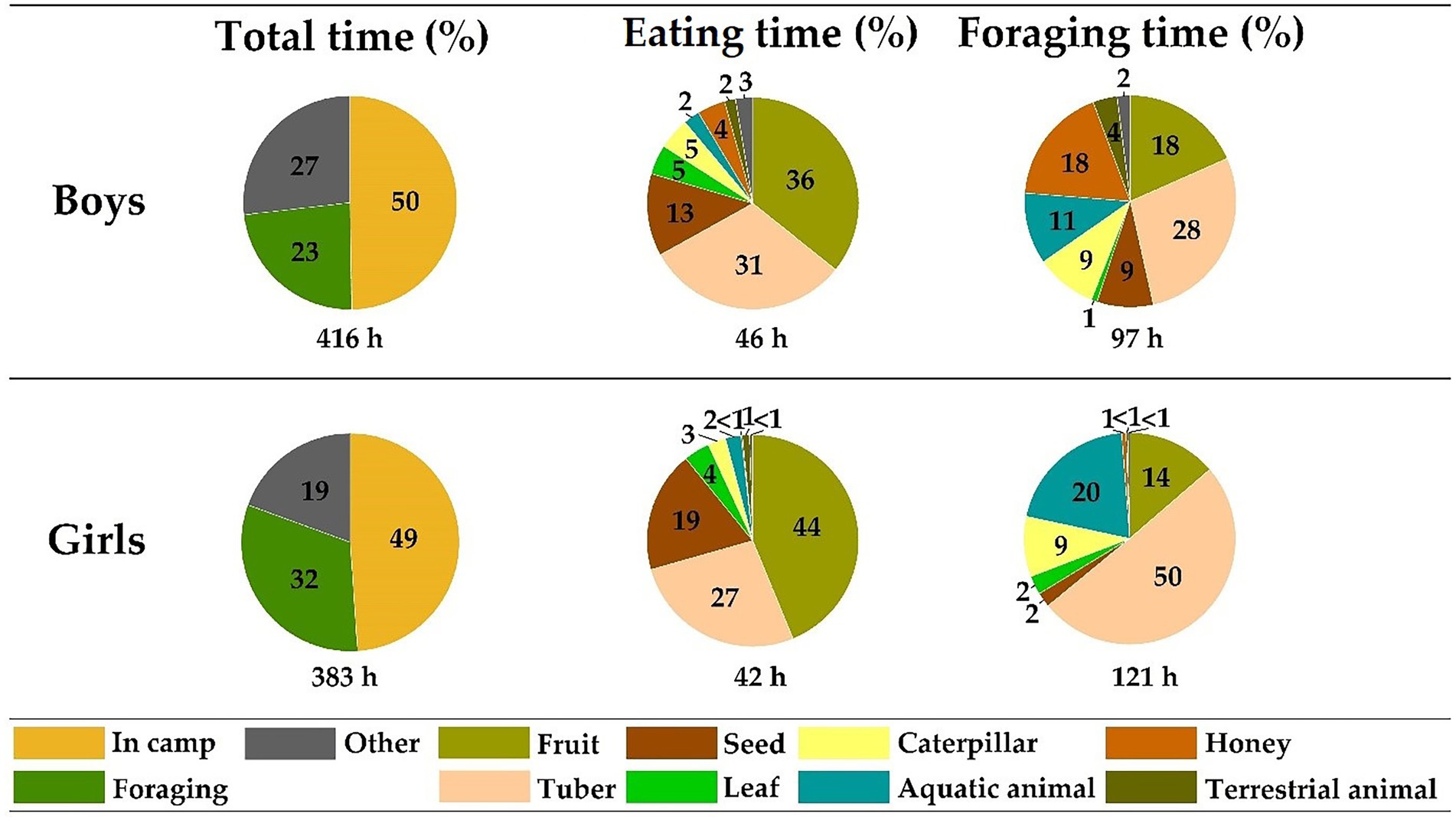
Figure 2. Differences in diet composition and foraging activities between boys and girls. Indicated is the difference in eating and foraging time on the food types between boys (N = 13, median age = 10.2, range: 4.5–16.5; observation time: 416 h, eating time: 46 h, foraging time: 97 h) and girls (N = 14, median age = 9.1, range: 5.5–17.1, observation time: 383 h, eating time: 42 h, foraging time: 121 h). The pie charts on the left indicate the percentage of time spent in camp, on foraging trips, and on trips unrelated to foraging. The middle and right pie charts indicate the percentage of time spent eating and foraging, respectively, on the different food types. For the definition and calculation of eating and foraging time, see Methods.
It was found that during foraging trips the canopy was scanned and inspected more often per hour by boys (Nboys = 13, medianboys = 2.542) than by girls (Ngirls = 14, mediangirls = 0.257, U = 14, p < 0.001). Additionally, boys spent a higher proportion of their foraging time climbing trees than did girls (medianboys = 0.030, mediangirls = 0.000, U = 42, p = 0.002). In contrast, girls tended to scan, inspect, and check – either with hand or machete – the ground more frequently per hour foraging (medianboys = 0.447, mediangirls = 1.206, U = 129.5, p = 0.065) and collected significantly more tubers per hour foraging (medianboys = 0.000, mediangirls = 0.204, U = 132.5, p = 0.041) than did boys. Finally, girls spent a higher proportion of their foraging time digging compared to boys (medianboys = 0.251, mediangirls = 9.932, U = 140, p = 0.018).
Contrary to our predictions, we did not find an effect of age, gender, number of boys and men or of any of the fruit/seed characteristics (i.e., sweetness, habitat, type) on the probability of fruit/seed species being collected (Fcol: Full-null model comparison: χ2 = 5.766, df = 6, p = 0.450; Table 1). However, for the eating probability, there was an overall effect of these factors (Feat: Full-null model comparison: χ2 = 14.791, df = 6, p = 0.022). It was found that fruit/seed species had a higher probability of being eaten when there were more boys and men in the group (χ2 = 4.049, df = 1, p = 0.044; Table 1). Furthermore, agricultural species were more likely to be eaten than forest species (χ2 = 7.991, df = 1, p = 0.005; Table 1).
For the tuber species collecting model, a significant overall effect was found of age, gender, number of girls and women in the group, and habitat on the probability of being collected (Tcol: Full-null model comparison: χ2 = 12.100, df = 4, p = 0.017). Specifically, girls were more likely to collect tuber species compared to boys (χ2 = 9.276, df = 1, p = 0.002; Table 2). For the eating probability, none of the factors were found to have an effect (Teat: Full-null model comparison: χ2 = 5.052, df = 4, p = 0.282).
Finally, we found an overall effect of age, gender, and plant part shown on the probability of correctly identifying the foraging-related plant species (Bknow: Full-null model comparison: χ2 = 28.284, df = 6, p < 0.001). There was a positive effect of age (χ2 = 12.712, df = 1, p < 0.001; Table 3; Figure 3) and an effect of plant part shown on the probability of correctly identifying the foraging-related plant species (χ2 = 9.885, df = 4, p = 0.042; Table 3; Figure 4). Specifically, the children were more likely to correctly identify the plant species based on a picture of the fruit/seed compared to a picture of the leaf (Estimate = 2.052, SE = 0.900, p = 0.023) or a picture of the bark (Estimate = 2.735, SE = 0.883, p = 0.002; Table 3; Figure 4). A picture of the trunk also had a higher probability of being correctly identified than a picture of the bark (Estimate = 1.556, SE = 0.573, p = 0.007; Table 3; Figure 4). In addition, children scored better on the leaf itself than on a picture of the bark (Estimate = 1.044, SE = 0.486, p = 0.032; Table 3; Figure 4). We found no statistical difference between the pictures of the leaves and the actual leaves themselves (Estimate = −0.358, SE = 0.368, p = 0.330; Figure 4), suggesting that the 2D-demonstration mode (i.e., the picture) did not have an effect. Spearman’s rank correlations indicated that the children had not learned the plant species names in the botanical picture test before they performed the test, as we found no significant correlation between the test number and the proportion of the 12 species incorrectly identified (ρ = −0.346, p = 0.160, N = 17). As expected from this, the children did not score higher with the end of the testing period approaching (ρ = −0.054, p = 0.832, N = 17).
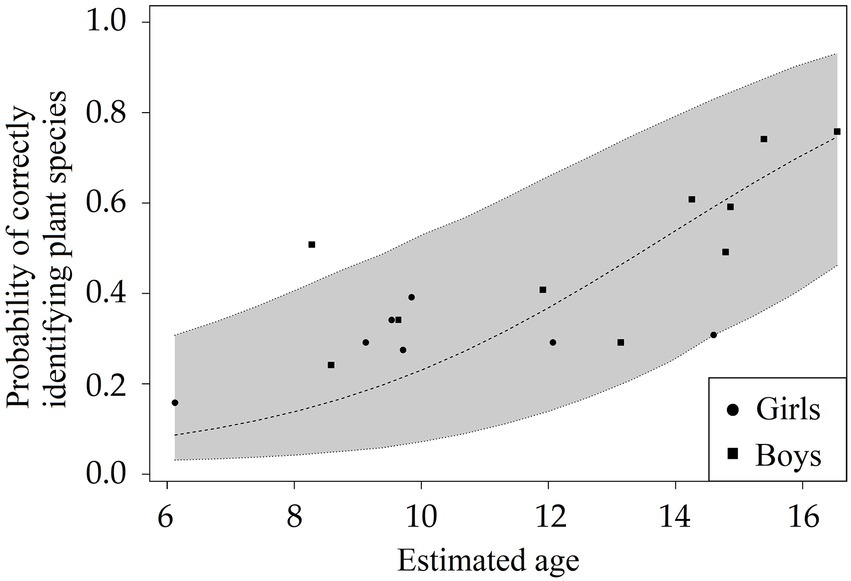
Figure 3. Botanical knowledge acquisition of foraging-related plant species during childhood. The relationship between age and the children’s (N = 17) probability to correctly answer 60 questions, in which they were asked to give the name of 12 foraging-related plant species based on pictures of different parts (i.e., fruit/seed, trunk, leaf, bark) and of the actual dried leaves. Data from girls are indicated with circles and data from boys with squares.
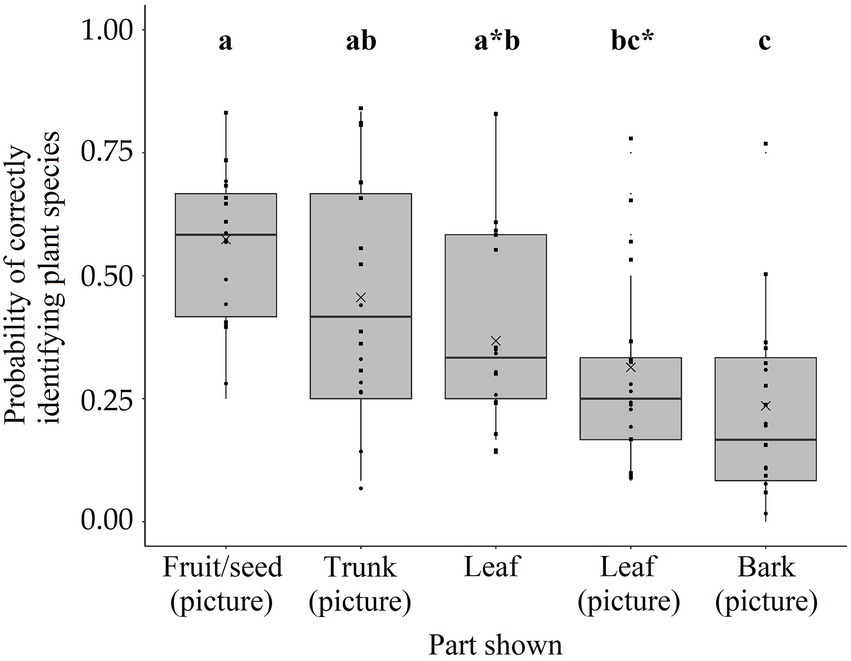
Figure 4. Botanical knowledge based on different plant parts. Effect of part shown on the probability of correctly identifying the plant species. Those parts with no corresponding letters indicate statistical significance (p < 0.05). There was also a trend for the difference between fruit/seed (picture) and leaf and between leaf (picture) and bark (picture) (p < 0.1), as indicated with an asterisk added to the letter. Data points are indicated with circles for girls and squares for boys. Finally, the mean probability per part shown is indicated with the cross.
Our results indicate the presence of a gender-specific early onset of the development of foraging skills and an age-related development of botanical knowledge in contemporary forager children, providing insight into the development of embodied capital. The BaYaka children spent most of their foraging time independent from adults, primarily eating and foraging on fruits, tubers, and seeds across seasons (Figure 1; Supplementary Table S3). Other food types, such as caterpillars and honey, were also widely eaten and foraged, but were more seasonally restricted (Figure 1; Supplementary Table S3). We also found a gendered division in the food types that were foraged (Figure 2). With more than half of their diet consisting of agricultural food, the children had a mixed-subsistence diet (Supplementary Table S3). Focusing on the fruit and seed species, children had a higher probability of eating the agricultural species (Table 1). A larger number of boys and men in the group increased the eating probability of fruit and seed species (Table 1). In addition, tuber species were more likely to be collected by girls (Table 2). Finally, the botanical knowledge of the children improved with increasing age (Table 3; Figure 3). The children were most likely to identify the foraging-related plant species based on its fruit/seed, followed by its trunk, leaf, and bark (Table 3; Figure 4).
The consistently high percentages of fruits and tubers in the diet of the BaYaka children is likely explained by a combination of preference and availability. In total, 17 different fruit species were observed being eaten, of which the agricultural oil palm fruit, papaya, and plantain were available during nearly the complete study period. For the tubers, eight species were eaten, of which both the agricultural species (i.e., cassava, taro, and sweet potato) and the forest ones, including D. cumminsii and Dioscorea spp., were seasonally widely available during the study period.
During period IV the agricultural tubers were rarely foraged and eaten, which is likely due to where the BaYaka resided during this period (i.e., camp Bongo). This camp is characterized by its fishing traps and numerous forest tuber patches, with no gardens nearby. Children were not observed to forage for aquatic animals during this period, even though they did eat fish. This is probably because the adults emptied the fish traps, compared to other fishing types such as dam-fishing in which children actively participate (Jang and Boyette, 2021). Hardly any fish were foraged and/or eaten during the peak of the wet season in period III, likely due to high levels of water making dam-fishing impossible. During this wet season, the children instead foraged for the highly seasonal caterpillars and seeds. Honey, another highly seasonal food item, was mainly foraged during the late dry season.
Our findings on diet composition are largely in line with previous research on the seasonal variation in diet and foraging activities of the Congo Basin foragers (e.g., Bahuchet, 1988; Kitanishi, 1995). As expected, forest fruits, seeds, caterpillars, and honey were highly seasonal, while terrestrial animals, fish, leaves, and tubers were more consistently collected and consumed throughout the year. These seasonal changes in dietary composition emphasize the importance of long-term data collection when focusing on subsistence strategies and the diet composition of human foragers. In addition, the high proportions of fruits and tubers were as expected based on previous research. Children prefer sweet foods (Desor and Beauchamp, 1987; Coldwell et al., 2009; Pellegrino et al., 2018), possibly explaining why fruits were eaten frequently by the children. This has also been observed in children of the Mbuti in the Democratic Republic of the Congo (Hart and Hart, 1986). The high proportion of tubers is consistent with previous studies claiming their importance for forager societies (Bahuchet et al., 1991; Dounias, 1993; Hladik and Dounias, 1993; Kitanishi, 1995; Sato, 2001; Marlowe and Berbesque, 2009; Sato et al., 2012).
When comparing diet composition across decades, it appears that meat was substantially less eaten in our study period than two decades ago (Kitanishi, 1995). This is, however, difficult to compare directly, as Kitanishi (1995) collected within-camp data focusing on adults rather than children. Compared to adults, with the men hunting at night, children might focus more on easily collected plant foods than on game (Crittenden et al., 2013). The apparent reduced meat consumption could, on the other hand, be explained by the decreased population sizes of animal species and the increased hunting pressure coinciding with the logging activities (Yasuoka, 2006; Laporte et al., 2007). This logging activity was especially visible around camp Bongo. The children spent 26.9% of their time outside camp Bongo on logging roads, primarily searching for tubers, which appear to be in higher densities in the open areas created by the logging roads (Yasuoka, 2013). Another indication that hunting might have decreased since Kitanishi (1995) is the seemingly increased time spent eating and foraging on fish. Kelly (2013) indicated that a decreased percentage of hunting often coincides with an increased dependence on fishing in forager societies.
Finally, this study reports considerably higher percentages of agricultural foods, with 55.5% compared to 22.2% by Kitanishi (1995). In agreement with Kitanishi (1995), these agricultural foods predominantly include oil palm with 46.4% in the present study compared to their reported 50%. This increase in agricultural foods likely indicates that contemporary BaYaka have a more horticultural lifestyle than two decades ago (Kitanishi, 1995; Thompson, 2018). Such a mixed-subsistence diet might have nutritional benefits, as was indicated by improved growth in Hadza children in Tanzania (Pollom et al., 2020). However, the effect of dietary change on the nutritional status might differ between forager societies, as it often coincides with increased nutritional stress (Crittenden and Schnorr, 2017), with negative effects in !Kung foragers in Namibia (Kirchengast, 1998) and potential future health disparities between settled and foraging BaAka in Central African Republic (Remis and Jost Robinson, 2014). The nutritional effects of changing from a diet dominated by forest foods to a mixed-subsistence diet in this community is thus an important focus for future research.
Children were more likely to eat fruit and seed species when there were more boys and men in the group, probably because boys and men often climb trees to collect these food types. In line with previous research on gender-segregated activities (Lew-Levy and Boyette, 2018; Lew-Levy et al., 2020a) as well as ranging patterns (Jang et al., 2019b) of BaYaka children, our results also provide evidence of the gendered division in children foraging activities. Specifically, we found that boys showed more canopy-related foraging behavior (i.e., scanning and inspecting the canopy, climbing trees) and spent more time foraging for canopy-related food items (i.e., fruits, seeds, caterpillars, honey) than did girls. Girls, in contrast, spent more time foraging for fish and digging for tubers. Especially the gendered division in honey collecting could be an interesting focus of future research, knowing its high value and potential importance later in life (e.g., as bride wealth; Lewis, 2002). However, we did not find gender differences on the probability of collecting and eating fruit and seed species. This could be explained by the way our data were collected as well as by the way these food items were shared. During fruit and seed foraging, but also with honey, often only one or a few of the boys that were strong and skilled enough climbed the trees, collected the food, and subsequently shared it with the rest of the foraging group, including the girls. Hence, the food was often not collected by the focal child and thus not considered in our data as collecting behavior, but as eating behavior by the focal child at the gathering spot. This potentially explains why the probability of eating the fruits and seeds was higher when more boys and men had been present in the foraging group.
By investigating the children’s collecting and eating models in more detail, the gendered division in foraging activities became clearer, with girls being more likely to collect tuber species. The eating probability of tuber species did not, however, differ between boys and girls, potentially because forest tubers were generally processed and consumed – and thus likely shared – in camp, with 78.8% of eating time being back in camp. This is a substantially larger percentage than forest fruits, which were eaten in camp only 15.8% of the time. This low percentage stresses the importance of data collection during foraging trips, which many previous studies have not done (see Thompson, 2018). Within-camp data collection would have largely underestimated the eating time on several food items, most notably caterpillars, forest fruits, and honey (for which 66.9, 84.2, and 100% of the eating time was outside the camp, respectively).
In contrast to the forest species, the eating time on agricultural fruit and tuber species in camp was similar (81.6 and 80.4%, respectively). This high percentage of agricultural fruits eaten in camp, compared to the forest species, likely increased the eating probability by frequent food sharing in camp. Agricultural fruits might furthermore have several advantages over forest fruits. Besides the nearly year-round availability, it is expected that they are spatially more clustered with predictable locations, having oil palm, papaya, and plantain trees in the camps and gardens. They might also have some nutritional benefits (e.g., sugar-rich with low levels of fibers) over forest species (McLennan and Ganzhorn, 2017; Pollom et al., 2020). These results provide another indication that the BaYaka are in transition into a more horticultural lifestyle (Thompson, 2018).
The absence of effects of the other variables included in the model could have multiple explanations. The heterogeneity of the food species caused the instability of some of the predictors. For example, while tubers were overall more often collected by girls, species such as Dioscorea semperflorens are often collected by boys. In addition, “availability” can be difficult to define since the phenology of fruiting tree species varies widely (Milton, 1993; Janmaat et al., 2016) and the phenology of tubers is largely unknown (Bahuchet, 1988). Importantly, when we assumed that food items were available only 3 days before or after being foraged or eaten (models F2col, F2eat, T2col, and T2eat; Supplementary Table S9) or during the entire observation period (models F3col, F3eat, T3col, and T3eat; Supplementary Table S10), the results reported proceed to be robust.
Another potential explanation for the lack of effect of age and gender on the eating probabilities is that in an egalitarian demand-sharing society, group performance might be of greater interest than individual performance. Having no hierarchy based on age, gender, or strength (Lewis, 2014), everyone is assumed to have roughly equal probabilities of eating the food items brought back to camp by sharing. This might also explain why, contrary to our expectations, we did not find a positive effect of sweetness on eating probability. Additionally, the fruit and seed models only compared the sweetness levels within these food types, which resulted in a relatively low variation in sweetness levels. Still, the high proportion of fruits in the diet, compared to other less sweet food types, supports our expectation that children have a higher probability to eat sweet foods.
We found an age-related development of the botanical knowledge of foraging-related plant species in forager children. The next generation of BaYaka children might lose this plant knowledge following the transition into a more horticultural lifestyle. Such a loss in knowledge has already been demonstrated in a study comparing forest-born with town-born BaYaka (Salali et al., 2020), indicating the urgency of studies on botanical knowledge. These results suggest that childhood might enable humans to acquire the botanical knowledge needed to forage for fruits and seeds. Contrary to our predictions based on the embodied capital theory (Kaplan et al., 2000), we did not find any difference between the knowledge acquisition of girls and boys. This could be due to the limited number of older girls present in camp at the time of testing, as the difference between boys and girls likely becomes more prominent later in childhood. For the plant parts, we did find differential effects, suggesting that fruits/seeds and trunks might be especially important identifiers of foraging-related tree species. Future research should investigate the relationship between individuals’ botanical knowledge and age and their foraging success rate on forest fruit and seed species.
Our study demonstrates a gendered division during childhood which provides insights into the development of embodied capital (Kaplan et al., 2000). Already from an early age, the gender-specific foraging activities offer the children an opportunity to learn the foraging skills required for the collection of a wide variety of food items such as fruits and tubers.
The energetic costs of gathering should not be underestimated (Gallois and Henry, 2021), and our observed behaviors indicate that food acquisition in a rainforest is a substantial challenge with regard to both its localization and collection. Children spent substantially more time foraging for the forest tubers than for the agricultural tubers. This may be because adults prevented them from entering the garden. Yet, a more likely explanation is that agricultural tubers were in known locations (i.e., gardens) whereas forest ones might be more patchily distributed and took longer to detect (Jang et al., 2019a). Once the tuber patches were found, the BaYaka children also took longer to dig up forest tubers compared to agricultural ones, which was consistent with earlier studies that indicated that foraging on forest tubers is particularly challenging (Dounias, 1993; Sato et al., 2012). Additionally, we found clear seasonal fluctuations in foraging activities, especially of forest fruits, caterpillars, and honey. This implies that food locations or species identification skills should be remembered over long time intervals, further challenging the forager’s cognition. Our results showed that their ability to identify foraging-related plant species by use of their fruit/seed and trunk of trees developed from a young age. This is exceptional knowing the tree species richness in this forest, with studies reporting as many as 72 tree species (DBH > 10 cm) for a single hectare in this research region (Loubelo Madiela, 2018).
Building on the theory of Kaplan et al. (2000), these findings clearly demonstrate the complexity involved with foraging. The activities of children include foraging for fruits and tubers, but also inspecting and climbing trees for honey or constructing dams to fish, each of which require specific skills that are developed during childhood. Investing in learning these wide variety of foraging strategies early in life possibly leads to higher productivity later in life, offering a likely explanation for our species’ extreme brain size and extended childhood (Kaplan et al., 2000, 2003). Future studies should focus on the stepwise acquisition of these skills, expecting a gradual increase in complexity and productivity as they age (Kaplan et al., 2000). The youngest children might focus more on easily targeted plant foods such as agricultural fruits while later in childhood both more and more diverse foods will be foraged (e.g., honey collection). This increase in complexity in foraging skills during childhood likely coincides with a division of foraging activities. It could be especially the early onset and development of a gender-based specialization, in combination with frequent sharing of foods, that enabled the human species to obtain a more energy-rich but mainly a more stable energy supply compared to that of our closest living relatives – a supply that ultimately enabled us to afford a substantially larger brain. Such speculations could be tested in the future by more detailed investigations on gender differences in specialized foraging skills in the BaYaka and other foraging communities, but especially between different primate species, taking a comparative phylogenetic approach (Nunn, 2011). Using a similar approach, Kraft et al. (2021) found that human foragers have, compared with great apes, increased energy acquisition rates, affording the energetic costs required for our extended childhood and enlarged brains.
Our findings raise interesting questions about the evolution of skill acquisition in human development, but also inform us on how to address these in comparative studies of other extant primates. For example, seeing the trunk triggers chimpanzees to stop and look up to the crown of trees to inspect for fruits, but only for species that are in season and not for those that are out of season (Janmaat et al., 2013a). This raises the question of whether this is achieved through remembering the location of suitable trees, or whether chimpanzees are able to distinguish tree species based on characteristics of the trunk alone. We were able to distinguish these possibilities for BaYaka children by factoring out tree location using pictures. Future studies on chimpanzee’s botanical knowledge could establish whether the capacity to recognize tree species using the trunk alone pre-dated humans, or evolved specifically in the context of the extended human life cycle.
BaYaka children predominantly ate a large proportion of agricultural species, which as noted above indicates that the BaYaka are in a transition into a more horticultural lifestyle. This transition does not stand on its own. Today, almost, if not all, remaining forager societies have a mixed-subsistence diet similar to the one reported here (Crittenden and Schnorr, 2017). The irreversible process of globalization and increased market integration will likely affect the children’s development of their embodied capital and corresponding foraging cognition, making them perhaps one of the last generations that can inform us about the development of foraging skills. Even though these contemporary foraging societies are not an analogy of our past, they can collectively provide knowledge of our evolutionary past (Kelly, 2013). By systematically documenting the last remaining forager diets, while considering aspects such as nutritional characteristics and seasonality, inferences can be made about the subsistence behavior of foraging societies in the past. Especially the focus on the foraging contributions of children can help us answer questions about the evolutionary function of the development of skills and knowledge, and our extended childhood and its role in brain size evolution.
Overall, these results stress the importance of the forest for the BaYaka and the potentially detrimental effects the current logging activity has on their diet composition, foraging skills, and botanical knowledge. The loss of this forest will coincide with the loss of an extensive foraging cognition developed from an early age that ranges from tuber foraging skills to the knowledge of a wide number of foraging-related tree species. The change in dietary composition may have inevitable consequences for the development of these and future BaYaka forager children. Even more important than documenting this rapid process is listening to the voices of the forager people concerned.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by relevant authorities of the Republic of the Congo (Research Approval Numbers for the research on BaYaka children: Nº070/MRSIT/IRSEN/DG/DS and Nº378/MRSIT/IRSEN/DG/DS from the Ministère de la Recherche Scientifique et de l’Innovation Technologique) and the Comité d’Ethique de la Recherche en Sciences de la Sante (CERSSA) (N˚095/MRSIT/IRSA/CERSSA). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JV, VK, and KJ: conceptualization. JV, HJ, BP, VK, and KJ: data collection. JV and KJ: formal analysis, investigation, and writing – original draft preparation. VK, ND, SD, PH, and AW: nutritional analyses. JV, HJ, DR, BP, PM, SD, and KJ: writing – review and editing. JV, ND, AW, and KJ: funding acquisition. PM and KJ: supervision. All authors contributed to the article and approved the submitted version.
JV thanks Alberta Mennega stichting, Amsterdams Universiteitsfonds (from Studiefonds Gouda de Vries), Treub-Maatschappij (the Society for the Advancement of Research in the Tropics), and Stichting het Kronendak. KJ thanks The Leakey Foundation and Templeton World Charity Foundation. AW and ND gratefully acknowledge the support of iDiv, in particular of the EcoMetEor platform, funded by the German Research Foundation (DFG–FZT 118, 202548816).
Firstly, we are thankful to L. Benoit, H. Cohen, M. Dzabatou, B. Loubello, and R. Ouamba for their contribution to the climate data, to R. Mundry for his statistical manuals and feedback on the models, and to C. Chittar and T. C. Vallina for additional feedback. For logistic support in Congo, we thank director C. Bouka-Biona at IRSEN and Marien Ngouabi University as well as M. J. Dzabatou and his daughter M. Dzabatou for transport and translations. We specifically thank J. M. Moutsambote for his help with the species identification at his herbarium at IRSEN. For introducing us to the family we thank D. Bombjaková, as well as for permission to adapt her figure of the research region. The University of Amsterdam and the Max Planck Institute of Animal Behavior are thanked for relevant permissions, insurance and their support during the repatriation of JV and BP following the COVID-19 pandemic. We are also grateful to the German Centre for Integrative Biodiversity Research (iDiv) for performing the nutritional analyses. We thank the editor and reviewers for their useful comments. Finally, eternal gratitude goes toward the BaYaka family, for welcoming us in their camp and letting us experience their culture and learn their language. Especially to the children we would like to say: Esengo ike!
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.935987/full#supplementary-material
Baayen, R. H., Davidson, D. J., and Bates, D. M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 59, 390–412. doi: 10.1016/j.jml.2007.12.005
Bahuchet, S. (1988). “Food supply uncertainty among the Aka Pygmies (Lobaye, C.A.R.)” in Coping with Uncertainty in Food Supply. eds. D. Garine and Harrison (Oxford: Oxford University Press), 118–149.
Bahuchet, S., and Guillaume, H. (1982). “Aka-farmer relations in the Northwest Congo basin” in Politics and History in Band Societies. eds. E. Leacock and R. Lee (Cambridge: Cambridge University Press), 189–211.
Bahuchet, S., McKey, D., and DeGarine, I. (1991). Wild yams revisited: is independence from agriculture possible for rain forest hunter-gatherers? Hum. Ecol. 19, 213–243. doi: 10.1007/BF00888746
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Barton, K. (2020). MuMIn: Multi-Model Inference. R package (version 1.43.17). Available at: https://cran.r-project.org/package=MuMIn
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Blurton Jones, N. G., Hawkes, K., and Draper, P. (1994). Foraging returns of !Kung adults and children: why Didn’t !Kung children forage? J. Anthropol. Res. 50, 217–248. doi: 10.1086/jar.50.3.3630178
Boesch, C., Bombjaková, D., Meier, A., and Mundry, R. (2019). Learning curves and teaching when acquiring nut-cracking in humans and chimpanzees. Sci. Rep. 9, 1515–1514. doi: 10.1038/s41598-018-38392-8
Bombjaková, D. (2018). The role of public speaking, ridicule, and play in cultural transmission among Mbendjele BaYaka forest hunter-gatherers. PQDT - UK & Ireland. Available at: https://search.proquest.com/docview/2116891091?accountid=13042%0Ahttp://oxfordsfx.hosted.exlibrisgroup.com/oxford?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&genre=dissertations+%26+theses&sid=ProQ:ProQuest+Dissertations+%26+Theses+G
Coldwell, S. E., Oswald, T. K., and Reed, D. R. (2009). A marker of growth differs between adolescents with high vs. low sugar preference. Physiol. Behav. 96, 574–580. doi: 10.1016/j.physbeh.2008.12.010
Crittenden, A. N. (2011). The importance of honey consumption in human evolution. Food Foodways 19, 257–273. doi: 10.1080/07409710.2011.630618
Crittenden, A. N., Conklin-Brittain, N. L., Zes, D. A., Schoeninger, M. J., and Marlowe, F. W. (2013). Juvenile foraging among the Hadza: implications for human life history. Evol. Hum. Behav. 34, 299–304. doi: 10.1016/j.evolhumbehav.2013.04.004
Crittenden, A. N., and Schnorr, S. L. (2017). Current views on hunter-gatherer nutrition and the evolution of the human diet. Am. J. Phys. Anthropol. 162, 84–109. doi: 10.1002/ajpa.23148
Demps, K., Zorondo-Rodríguez, F., García, C., and Reyes-García, V. (2012). Social learning across the life cycle: cultural knowledge acquisition for honey collection among the Jenu Kuruba, India. Evol. Hum. Behav. 33, 460–470. doi: 10.1016/j.evolhumbehav.2011.12.008
Desor, J. A., and Beauchamp, G. K. (1987). Longitudinal changes in sweet preferences in humans. Physiol. Behav. 39, 639–641. doi: 10.1016/0031-9384(87)90166-1
Dounias, E. (1993). “Perception and use of wild yams by the baka hunter- gatherers in South Cameroon” in Tropical Forests, People and Food: Biocultural Interactions and Applications to Development. eds. C. M. Hladik, A. Hladik, O. F. Linares, H. Pagezy, A. Semple, and M. Hadley, vol. 13 (Paris: UNESCO), 621–632.
Draper, P. (2013). “Social and economic constraints on child life among the !Kung” in Kalahari Hunter-Gatherers. eds. R. B. Lee and I. DeVore (Cambridge: Harvard University Press), 199–217.
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Fox, J., and Weisberg, S. (2019). CAR - An R Companion to Applied Regression. Thousand Oaks, CA: Sage.
Gallois, S., and Henry, A. G. (2021). The cost of gathering among the Baka forager-horticulturalists from southeastern Cameroon. Front. Ecol. Evol. 9:768003. doi: 10.3389/fevo.2021.768003
Gurven, M., Kaplan, H., and Gutierrez, M. (2006). How long does it take to become a proficient hunter? Implications for the evolution of extended development and long life span. J. Hum. Evol. 51, 454–470. doi: 10.1016/j.jhevol.2006.05.003
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794-32. doi: 10.7717/peerj.4794
Hart, T. B., and Hart, J. A. (1986). The ecological basis of hunter-gatherer subsistence in African rain forests: the Mbuti of Eastern Zaire. Hum. Ecol. 14, 29–55. doi: 10.1007/BF00889209
Hawthorne, W. D., and Jongkind, C. C. (2006). Woody Plants of Western African Forests, A Guide to the Forest Trees, Shrubs and Lianes From Senegal to Ghana. Kew: Royal Botanic Gardens.
Hewlett, B. S. (Ed.) (1991). “Aka pygmies of the western Congo basin” in Intimate Fathers. THE nature and Context of Aka Pygmy Paternal Infant Care (Ann Arbor, MI: The University of Michigan Press), 43–46.
Hewlett, B. S. (2017). Hunter-gatherer childhoods in the Congo basin. In B. S Hewlett. (Ed.) Hunter-Gatherers of the Congo Basin: Cultures, Histories and Biology of African Pygmies. New Brunswick: Transaction Publishers, 245–275.
Hill, K. R., and Hurtado, M. (2017). Ache Life History: The Ecology and Demography of a Foraging People. New York: Routledge.
Hladik, C. M., and Bahuchet, S. (1994). “Perception and utilization of rain forest fruits and honey by the Aka Pygmies (Central African Republic)” in Current Primatology. Vol. I, Ecology and Evolution. Selected Proceedings of the XIVth Congress of the International Primatological Society. eds. B. Thierry, J. R. Anderson, J. J. Roeder, and N. Herrenschmidt (Strasbourg: Université Louis Pasteur), 155–159.
Hladik, A., and Dounias, E. (1993). “Wild yams of the African forest as potential food resources” in Tropical Forests, People and Food: Biocultural Interactions and Applications to Development. eds. C. M. Hladik, A. Hladik, O. F. Linares, H. Pagezy, A. Semple, and M. Hadley (UNESCO-Parthenon Publishing Group), 163–176.
Hladik, C. M., Robbe, B., and Pagezy, H. (1986). Sensibilité gustative différentielle des populations Pygmées et non Pygmées de forêt dense, de Soudaniens et d’Eskimos, en rapport avec l’environnement biochimique. C. R. Acad. Sci. Série III, 303, 453–458.
Jang, H., Boesch, C., Mundry, R., Ban, S. D., and Janmaat, K. R. L. (2019a). Travel linearity and speed of human foragers and chimpanzees during their daily search for food in tropical rainforests. Sci. Rep. 9, 11066–11013. doi: 10.1038/s41598-019-47247-9
Jang, H., Boesch, C., Mundry, R., Kandza, V., and Janmaat, K. R. L. (2019b). Sun, age and test location affect spatial orientation in human foragers in rainforests. Proc. R. Soc. B Biol. Sci. 286:20190934. doi: 10.1098/rspb.2019.0934
Jang, H., and Boyette, A. H. (2021). Observations of cooperative pond fishing by the bayaka and bantu people in the flooded forest of the northern republic of Congo. Afr. Study Monogr. 41, 1–16. doi: 10.34548/asm.41.2.1
Janmaat, K. R. L., Ban, S. D., and Boesch, C. (2013a). Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Anim. Behav. 86, 1183–1205. doi: 10.1016/j.anbehav.2013.09.021
Janmaat, K. R. L., Ban, S. D., and Boesch, C. (2013b). Taï chimpanzees use botanical skills to discover fruit: what we can learn from their mistakes. Anim. Cogn. 16, 851–860. doi: 10.1007/s10071-013-0617-z
Janmaat, K. R. L., Boesch, C., Byrne, R., Chapman, C. A., Goné Bi, Z. B., Head, J. S., et al. (2016). Spatio-temporal complexity of chimpanzee food: how cognitive adaptations can counteract the ephemeral nature of ripe fruit. Am. J. Primatol. 78, 626–645. doi: 10.1002/ajp.22527
Joiris, D. V. (2003). The framework of Central African hunter-gatherers and neighbouring societies. Afr. Study Monogr. 28, 57–79. doi: 10.14989/68426
Jones, N. B., and Marlowe, F. W. (2002). Selection for delayed maturity: does it take 20 years to learn to hunt and gather? Hum. Nat. 13, 199–238. doi: 10.1007/s12110-002-1008-3
Kandza, V. (2018). What a growing brain wants and gets: food preference and consumption by Mbendjele foraging children in a tropical rainforest. Master’s Thesis. Brazzaville:Marien Ngouabi University.
Kaplan, H., Hill, K., Lancaster, J., and Hurtado, A. M. (2000). A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. doi: 10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7
Kaplan, H., Lancaster, J., and Robson, A. (2003). Embodied capital and the evolutionary economics of the human life span. Popul. Dev. Rev. 29, 152–182.
Kelly, R. L. (2013). The Lifeways of Hunter-Gatherers: The Foraging Spectrum. Cambridge:Cambridge University Press.
Kirchengast, S. (1998). Weight status of adult !Kung San and Kavango people from northern Namibia. Ann. Hum. Biol. 25, 541–551. doi: 10.1080/03014469800006782
Kitanishi, K. (1994). The exchange of forest products (Irvingia nuts) between the Aka hunter-gatherers and the cultivators in Northeastern Congo. Tropics 4, 79–92. doi: 10.3759/tropics.4.79
Kitanishi, K. (1995). Seasonal changes in the subsistence activities and food intake of the Aka hunter-gatherers in Northeastern Congo. Afr. Study Monogr. 16, 73–118.
Kitanishi, K. (2003). Cultivation by the Baka hunter-gatherers in the tropical rain forest of Central Africa. Afr. Study Monogr. 28, 143–157.
Köhler, A., and Lewis, J. (2002). “Putting hunter-gatherer and farmer relations in perspective. A commentary from Central Africa” in Ethnicity, Hunter-Gatherers, and the “Other”: Association or Assimilation in Africa. ed. S. Hunt (Washington: Smithsonian Institute), 276–305.
Kraft, T. S., Venkataraman, V. V., Wallace, I. J., Crittenden, A. N., Holowka, N. B., Stieglitz, J., et al. (2021). The energetics of uniquely human subsistence strategies. Science 374:eabf0130. doi: 10.1126/science.abf0130
Laden, G., and Wrangham, R. (2005). The rise of the hominids as an adaptive shift in fallback foods: plant underground storage organs (USOs) and australopith origins. J. Hum. Evol. 49, 482–498. doi: 10.1016/j.jhevol.2005.05.007
Laporte, N. T., Stabach, J. A., Grosch, R., Lin, T. S., and Goetz, S. J. (2007). Expansion of industrial logging in Central Africa. Science 316:1451. doi: 10.1126/science.1141057
Lewis, J. (2002). Forest Hunter-Gatherers and Their World: A Study of the Mbendjele Yaka Pygmies of Congo-Brazzaville and Their Secular and Religious Activities and Representations Dissertation. London: London School of Economics and Political Science.
Lewis, J. (2014). “Pygmy hunter-gatherer egalitarian social organization: the case of the Mbendjele BaYaka” in Congo Basin Hunter-Gatherers. ed. B. Hewlett, (New Brunswick, NJ: Transaction Publishers), 219–244.
Lewis, J., and Nelson, J. (2006). Logging in the Congo Basin. What hope for indigenous peoples’ resources, and their environments? Indigenous Affairs 4, 8–15.
Lew-Levy, S., and Boyette, A. H. (2018). Evidence for the adaptive learning function of work and work-themed play among aka forager and Ngandu farmer children from the Congo Basin. Hum. Nat. 29, 157–185. doi: 10.1007/s12110-018-9314-6
Lew-Levy, S., Boyette, A. H., Crittenden, A. N., Hewlett, B. S., and Lamb, M. E. (2020a). Gender-typed and gender-segregated play among Tanzanian Hadza and Congolese BaYaka hunter-gatherer children and adolescents. Child Dev. 91, 1284–1301. doi: 10.1111/cdev.13306
Lew-Levy, S., Kissler, S. M., Boyette, A. H., Crittenden, A. N., Mabulla, I. A., and Hewlett, B. S. (2020b). Who teaches children to forage? Exploring the primacy of child-to-child teaching among Hadza and BaYaka hunter-gatherers of Tanzania and Congo. Evol. Hum. Behav. 41, 12–22. doi: 10.1016/j.evolhumbehav.2019.07.003
Lew-Levy, S., Reckin, R., Lavi, N., Cristóbal-Azkarate, J., and Ellis-Davies, K. (2017). How do hunter-gatherer children learn subsistence skills?: a meta-ethnographic review. Hum. Nat. 28, 367–394. doi: 10.1007/s12110-017-9302-2
Lew-Levy, S., Ringen, E. J., Crittenden, A. N., Mabulla, I. A., Broesch, T., and Kline, M. A. (2021). The life history of learning subsistence skills among Hadza and BaYaka foragers from Tanzania and the Republic of Congo. Hum. Nat. 32, 16–47. doi: 10.1007/s12110-021-09386-9
Lim, J. Y., Wasserman, M. D., Veen, J., Després-Einspenner, M.-L., and Kissling, W. D. (2021). Ecological and evolutionary significance of primates’ most consumed plant families. Proc. Biol. Sci 288:20210737. doi: 10.1098/rspb.2021.0737
Loubelo Madiela, B. D. (2018). Cartographe du gradient de vegetation par teledetection pour l’amelioration des schemas de surfaces des modeles climatiques. Dissertation. Brazzaville: Marien Ngouabi University.
Marlowe, F. W., and Berbesque, J. M. (2009). Tubers as fallback foods and their impact on Hadza hunter-gatherers. Am. J. Phys. Anthropol. 140, 751–758. doi: 10.1002/ajpa.21040
Martin, P., and Bateson, P. (2007). Measuring Behaviour: An Introductory Guide. Cambridge:Cambridge University Press.
Matuschek, H., Kliegl, R., Vasishth, S., Baayen, H., and Bates, D. (2017). Balancing Type I error and power in linear mixed models. J. Mem. Lang. 94, 305–315. doi: 10.1016/j.jml.2017.01.001
McLennan, M. R., and Ganzhorn, J. U. (2017). Nutritional characteristics of wild and cultivated foods for chimpanzees (Pan troglodytes) in agricultural landscapes Int. J. Primatol. 38, 122–150. doi: 10.1007/s10764-016-9940-y
Milton, K. (1993). Diet and primate evolution. Sci. Am. 269, 86–93. doi: 10.1038/scientificamerican0893-86
Milton, K. (1999). A hypothesis to explain the role of meat-eating in human evolution. Evol. Anthropol. 8, 11–21. doi: 10.1002/(SICI)1520-6505(1999)8:1<11::AID-EVAN6>3.0.CO;2-M
Nakagawa, S., Johnson, P. C. D., and Schielzeth, H. (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14:20170213. doi: 10.1098/rsif.2017.0213
Nunn, C. L. (2011). The Comparative Approach in Evolutionary Anthropology and Biology. Chicago: University of Chicago Press.
Pellegrino, R., Sorokowska, A., Marczak, M., Niemczyk, A., Butovskaya, M., Huanca, T., et al. (2018). Mapping sweetness preference across the lifespan for culturally different societies. J. Environ. Psychol. 58, 72–76. doi: 10.1016/j.jenvp.2018.07.012
Pollom, T. R., Cross, C. L., Herlosky, K. N., Ford, E., and Crittenden, A. N. (2020). Effects of a mixed-subsistence diet on the growth of Hadza children. Am. J. Hum. Biol. 33:e23455-66. doi: 10.1002/ajhb.23455
Remis, M. J., and Jost Robinson, C. A. (2014). Examining short-term nutritional status among BaAka foragers in transitional economies. Am. J. Phys. Anthropol. 154, 365–375. doi: 10.1002/ajpa.22521
Rstudio Team (2016). RStudio: Integrated Development for R. RStudio (1.1.419). RStudio, Inc. Available at: http://www.rstudio.com/
Salali, G. D., Chaudhary, N., Thompson, J., Grace, O. M., van der Burgt, X. M., Dyble, M., et al. (2016). Knowledge-sharing networks in hunter-gatherers and the evolution of cumulative culture. Curr. Biol. 26, 2516–2521. doi: 10.1016/j.cub.2016.07.015
Salali, G. D., Dyble, M., Chaudhary, N., Sikka, G., Derkx, I., Keestra, S. M., et al. (2020). Global WEIRDing: transitions in wild plant knowledge and treatment preferences in Congo hunter-gatherers. Evol. Hum. Sci. 2, 1–14. doi: 10.1017/ehs.2020.26
Sato, H. (2001). The potential of edible wild yams and yam-like plants as a staple food resource in the African tropical rain forest. Afr. Study Monogr. 26, 123–134. doi: 10.14989/68404
Sato, H., Kawamura, K., Hayashi, K., Inai, H., and Yamaguchi, T. (2012). Addressing the wild yam question: how Baka hunter-gatherers acted and lived during two controlled foraging trips in the tropical rainforest of southeastern Cameroon. Anthropol. Sci. 120, 129–149. doi: 10.1537/ase.110913
Schielzeth, H., and Forstmeier, W. (2009). Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. doi: 10.1093/beheco/arn145
Schniter, E., Gurven, M., Kaplan, H. S., Wilcox, N. T., and Hooper, P. L. (2015). Skill ontogeny among Tsimane forager-horticulturalists. Am. J. Phys. Anthropol. 158, 3–18. doi: 10.1002/ajpa.22757
Takeuchi, K. (Ed.) (2005). “The Ambivalent symbiosis between the Aka hunter-gatherers and neighboring Farmers,” in Culture Conservation and Development in African Rain Forest (Research report of MEXT Grant-in-Aid for Scientific Research) (Toyama: Toyama University), 11–28.
Thompson, J. M. (2018). The Social Foraging Niche of the Mbendjele BaYaka Dissertation. London:University College London.
Tucker, B., and Young, A. G. (2005). Growing up Mikea: Children’s time allocation and tuber foraging in southwestern Madagascar. In Hunter-Gatherer Childhoods: Evolutionary, Developmental, and Cultural Perspectives, Eds. B. S. Hewlett and M. E Lamb. 129–147, New Brunswick: Transaction Publishers.
Walker, R., Hill, K., Kaplan, H., and McMillan, G. (2002). Age-dependency in hunting ability among the ache of eastern Paraguay. J. Hum. Evol. 42, 639–657. doi: 10.1006/jhev.2001.0541
Yasuoka, H. (2006). The sustainability of duiker (Cephalus spp.) hunting for the baka hunter-gatherers in southeastern Cameroon. Afr. Study Monogr. 33, 95–120. doi: 10.14989/68473
Yasuoka, H. (2013). Dense wild yam patches established by hunter-gatherer camps: beyond the wild yam question, toward the historical ecology of rainforests. Hum. Ecol. 41, 465–475. doi: 10.1007/s10745-013-9574-z
Keywords: botanical knowledge, cognitive development, embodied capital theory, forager diet, juvenile foraging, Mbendjele BaYaka subsistence, sedentarization, sugar content
Citation: Veen J, Jang H, Raubenheimer D, van Pinxteren BOCM, Kandza V, Meirmans PG, van Dam NM, Dunker S, Hoffmann P, Worrich A and Janmaat KRL (2023) Development of embodied capital: Diet composition, foraging skills, and botanical knowledge of forager children in the Congo Basin. Front. Ecol. Evol. 11:935987. doi: 10.3389/fevo.2023.935987
Received: 05 May 2022; Accepted: 03 January 2023;
Published: 08 February 2023.
Edited by:
David Andrew Gray, California State University, Northridge, United StatesReviewed by:
Alessandro Mondanaro, University of Florence, ItalyCopyright © 2023 Veen, Jang, Raubenheimer, van Pinxteren, Kandza, Meirmans, van Dam, Dunker, Hoffmann, Worrich and Janmaat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorin Veen, ✉ am9yaW52ZWVuQG91dGxvb2suY29t
†Present address: Nicole M. van Dam, Leibniz Institute of Vegetable and Ornamental Crops (IGZ), Großbeeren, Germany
‡ORCID: Jorin Veen https://orcid.org/0000-0003-1317-0343
Haneul Jang https://orcid.org/0000-0001-5994-6413
David Raubenheimer https://orcid.org/0000-0001-9050-1447
Bryndan O. C. M. van Pinxteren https://orcid.org/0000-0003-1920-7151
Patrick G. Meirmans https://orcid.org/0000-0002-6395-8107
Nicole M. van Dam https://orcid.org/0000-0003-2622-5446
Susanne Dunker https://orcid.org/0000-0001-7276-776X
Anja Worrich https://orcid.org/0000-0002-3116-2964
Karline R. L. Janmaat https://orcid.org/0000-0003-2533-6881
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.