
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol., 09 January 2024
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1347447
This article is part of the Research TopicOpportunities and Challenges for Wild Bee ConservationView all 13 articles
Protecting diverse solitary ground-nesting bees remains a pivotal conservation concern. Ground-nesting bees are negatively impacted by anthropogenic land use change that often removes suitable nesting habitat from the landscape. Despite their enormous ecological and agricultural contributions to pollination, solitary, ground-nesting bees are often neglected, partly due to the significant obstacle of discovering exactly where these bees establish their nests. To address this limitation, we have developed a ‘community science’ project to map aggregations of ground-nesting bees globally. In certain locations, their abundances reach astounding levels, sometimes in the millions, but are scarcely known. Utilizing the iNaturalist platform, which permits geo-referencing of site observations and bee identification, we are providing public education and seeking public engagement to document bee aggregations in order to understand the nesting requirements of diverse species and open new opportunities for their conservation. Conservation priorities may then unequivocally be directed to areas of high species richness, nest densities, and nesting sites of rare bees. Such community-led efforts are vital for successful long-term management of native bees and the biotic and abiotic landscape data from nest-site localities can allow modeling to predict nest-site suitability and to readily test such predictions on the ground. Here, we summarize the progress, current limitations, and opportunities of using a global mapping project (GNBee) to direct conservation efforts and research toward solitary ground-nesting bees.
Pollination services provided by bees are essential for sustaining the genetic variability in 85% of flowering plants and vital for securing yields of pollinator-dependent crops (Ollerton, 2017; Zattara and Aizen, 2021; Katumo et al., 2022). For 125 million years, bees have coevolved with and facilitated the vast radiation of flowering plants (300,000 angiosperm species), thus establishing terrestrial food webs (Vannette, 2020). To meet the extraordinary demand of pollinating diverse angiosperms, there are approximately 20,000 bee species, which differ greatly in morphology, life history, nesting habits, and the flower species with which they interact (Danforth et al., 2019). Despite the diversity of bee species, significant conservation concerns exist, and loss of bee diversity can negatively impact terrestrial ecosystems by reducing the genetic diversity of plants, which can lead to reduced ecosystem resilience (Potts et al., 2010).
Bees, like many organisms, face threats from human activities, primarily landscape changes, habitat loss, pesticide use, and invasive parasites (Willis Chan et al., 2019; Willis Chan and Raine, 2021; Zattara and Aizen, 2021). Studies, including those related to climate change, have consistently reported declines in bee populations, with shorter-term assessments at local, regional, or country levels (Biesmeijer et al., 2006; Goulson et al., 2008; Bartomeus et al., 2013; Ollerton, 2017; Powney et al., 2019; Simanonok et al., 2021; Janousek et al., 2023). Longer and broader assessments, biased toward the Northern Hemisphere, also confirm the decline in bee abundance and diversity (Sánchez-Bayo and Wyckhuys, 2019; Thomas et al., 2019). Zattara and Aizen (2021) conducted a global-scale study revealing a steady decline in the number of bee species observed since the 1990s, with 25% fewer species reported between 2006 and 2015 compared to before the 1990s. This collective evidence underscores the urgent need for swift actions to prevent further declines in bee populations.
Bees and their environmental struggles are currently experiencing increased attention in the media, and this is resonating with the public. However, this attention is largely centered around honey bees. The honey bee has been lauded as a conservation concern to the public, perhaps at the behest of commercial interests, and as a result, we are seeing an increase in backyard or rooftop honey bee husbandry. Unfortunately, honey bees, while great for inspiring public interest in insects, have overshadowed critical messaging about bee diversity and biologically sound conservation efforts. Managed honey bees, while beneficial in many agriculture settings, have been shown to outcompete native species (Iwasaki and Hogendoorn, 2022; Page and Williams, 2023) and can spread parasites and pathogens (Stout and Morales, 2009; Prendergast et al., 2022). Indeed, the honey bee is to bee diversity as the chicken is to bird diversity, and as a result, society is fixating on the wrong bees.
When people think of bees in the temperate zone, rather than only imagining a honey bee or bumble bee they should also envision solitary bees. Approximately 75% of described bee species are solitary, meaning each female constructs her own nest, provisions her own brood cells and lays her own eggs (i.e., there is no reproductive division of labor or cooperative brood care). If we combine brood parasitic bees, the solitary bees and their brood parasites account for ~90% of all bee species (Danforth et al., 2019).
Most of your average bee’s life occurs during development, from egg to larva to pupa, and these stages are often punctuated by diapause (a period of suspended development, either as adults or last instar larvae). Solitary bee flight activity, which may last only a few weeks in many species, begins with their emergence as newly formed adults. Males typically emerge first and mate with females that store sperm in their spermathecae (Danforth et al., 2019). Males then perish, and females are left to choose a nesting site and begin the process of provisioning for the next generation. While each individual species has a relatively short period of adult flight activity, the diversity of species in one area allows for continual emergence and activity that corresponds with the pollination needs of native flowering species in the region. Solitary female bees generally construct and provision brood cells one at a time. They are ‘single mothers hard at work’, and their work is typically out of sight and underground.
The solitary, below-ground-nesting strategy is believed to be ancestral in bees and is shared with their crabronid wasp ancestors (Debevec et al., 2012; Sann et al., 2018). Ground-nesting is observed in every bee family and all places where bees occur (Danforth et al., 2019). It is estimated that approximately 75% of all bee species are ground-nesting (Antoine and Forrest, 2020; Harmon-Threatt, 2020). A typical bee takes one (or sometimes more) year(s) to develop and receives no additional parental care after the egg is laid. Successful development can only be achieved when bees nest in soils suitable to their biology with preferred environmental conditions (Harmon-Threatt, 2020), and the nesting substrates chosen by females appear to be specific to each species (Cane, 1991; Antoine and Forrest, 2020).
Antoine and Forrest (2020) provide a comprehensive review of ground-nesting bee site preferences in their published paper. They summarize research on abiotic factors, including soil compaction, moisture, temperature, surface features, and slope, that influence suitable nesting habitats. Their review also covers biotic factors that may influence nesting, such as the abundance of natural enemies, the density of conspecifics, and the availability and quality of floral resources. It is therefore not necessary to re-synthesize these attributes here, however it is paramount to convey that there are still substantial gaps in our understanding of ground-nesting bee biology. In a survey of the literature on the approximately 3,000 bee species in America north of Mexico, Harmon-Threatt (2020) examined the literature on 527 randomly selected species and found that only 20% of those species had any information on nesting biology. Indeed, most of our knowledge regarding nesting biology (nest architecture, immature stages of bees, parasites etc.), come from field observations typically done at a single locality, making it difficult to confidently identify general characteristics of each species (Antoine and Forrest, 2020). Several studies of multiple nesting sites and bee species have begun to uncover and compare the nesting depths (Cane and Neff, 2011) and soil parameters of that characterize each species (Tsiolis et al., 2022; Ulyshen et al., 2023). However, these efforts are only scratching the surface of what is possible and what needs to be done. Therefore, we recognize substantial opportunities to improve our understanding of nesting behavior which can be used to improve bee conservation.
Bee conservation efforts for diverse wild bees principally focus on enhancing floral resources. As a result, ways to promote food resource availability are relatively well developed and include organized efforts, such as planting pollinator gardens, planting wildflower strips in public spaces, planting in unused agricultural lands or edge habitat, and community campaigns like No Mow May (Potts et al., 2003; Sheffield et al., 2008; Mader et al., 2011; Kirk and Howes, 2012; Rosa García and Miñarro, 2014; M’Gonigle et al., 2017). More recently, conservation efforts have expanded to include methods for enhancing nesting resources of above-ground cavity nesters, such as leaf-cutter bees and mason bees (MacIvor and Packer, 2015; Fortel et al., 2016). While the aforementioned strategies have had some positive and some mixed outcomes, they do not address the core limitations for most bee species (Gathmann and Tscharntke, 2002; Potts et al., 2005; Michener, 2007; Williams et al., 2011; Dicks, 2013). Rather, the vast majority of bee species are ground-nesting and limited by available nesting habitat, and with several notable exceptions discussed below and outlined in Table 1, few studies have tried to enhance nesting resources for ground-nesting bee species.
Particularly relevant to conservation of solitary ground-nesting bees, for most species, there is pronounced natal philopatry (i.e., females tend to nest in the same site as their mother), a condition unique, yet preset across diverse groups of animals (Byer and Reid, 2022). Nesting sites for many ground-nesting bee species can remain active for decades (Danforth et al., 2019) and we do not yet know the upper bounds of fidelity to a nesting location for ground-nesting bee species. This is a major component of ground-nesting bee biology that can build community engagement and facilitate research and conservation efforts. Clearly, nesting sites and nesting resources are not ubiquitous across the landscape and are not uniform in their ability to support bee communities (Potts et al., 2003; Grundel et al., 2010). Therefore, increased focus on the soil requirements and resources for ground-nesting species can improve conservation efforts.
To date, only a handful of studies have actively tried to promote the richness and abundance of ground-nesting bee species by constructing man-made or environmentally altered nesting habitat (Table 1). The most successful example of this work pertains to the sole species of managed ground-nesting bees, Nomia melanderi (Cane, 2008). Despite N. melanderi’s peculiar affinity to bare, smooth, damp, salty alkaline soils, this gregarious, generalist bee has become the best studied species of ground-nesting bee in the world (Cane, 2023). Its success as a managed pollinator in the US is largely driven by its ability to propagate within man-made bee beds constructed in the vicinity of alfalfa fields. Since it can tolerate colder temperatures, it emerges when many other bees remain inactive to pollinate alfalfa alongside another managed stem nesting bee, Megachile rotundata (Pitts-Singer and Cane, 2011). Together they produce seed valued at $22 billion annually. The pairing of ground-nesting bee biology with agricultural objectives can offer substantial opportunities and benefits in agricultural systems and similar outcomes may be possible for other agricultural crops and non-crop plant species. Thus, there is a natural alliance between farmers and native ground-nesting bees that should be nurtured.
Large-scale environmental science often requires a ‘community science’ approach (also called ‘citizen science’ or ‘participatory science’). In this research methodology, non-professionals contribute their time, energy or expertise to a research aim. Community science makes the activity of discovery and observation available to all, not just a privileged few, and is an effective method of upscaling research projects and adoption of innovations both temporally and spatially (Pocock and Evans, 2014). As a result, research that involves community science is becoming increasingly common and includes projects on climate change, invasive species, conservation biology, ecological restoration, and monitoring of all sorts (Silvertown, 2009; Dance, 2022). For example, the Christmas Bird Count, run by the National Audubon Society, has taken place every year since 1900, generating one of the most impressive biological datasets that we have (63 million observations). Indeed, in many countries, community scientists are the bedrock of biological recording and monitoring.
Community science has previously been applied to projects on bees; for example, identifying the diversity of bees found on flowers across an urban gradient in France (Deguines et al., 2016), and assessing the numbers of squash bees found on farmland in Michigan, USA (Appenfeller et al., 2020). In an encouraging study, Maher et al. (2019) used a community science approach to locate and investigate the nesting requirements of four species of gregarious ground-nesters (394 nesting sites across the UK and Ireland): Andrena cineraria and A. fulva (Andrenidae), Halictus rubicundus (Halictidae) and Colletes hederae (Colletidae). Even with the limited foraging ranges of most bees, locating nesting sites is a substantial challenge in studying and/or conserving ground-nesting bees (O’Connor et al., 2012; Antoine and Forrest, 2020). It is therefore significant that a community science project successfully overcame this obstacle, and Maher et al.’s (2019) study also suggests this approach could be used to discover nesting site locations at larger scales. However, to do so, a more robust and sustained effort must be employed.
Project GNBee (GNBee.org) champions a community science approach to research, conservation of ground-nesting bees. This project aims to connect amateur observers (nest site discoverers) to experts in real time, working together to identify and validate new ground-nesting bee records. To date, Project GNBee contains over 2,500 observations of over 240 bee species. Contributions have been made by over 1300 people worldwide, and real-time records can be found at iNaturalist (https://www.inaturalist.org/projects/ground-nesting-bees-3e6882c0-a112-4ddb-b043-1da25638ce96). All observations are geolocated and thus provide the basis for studies of nesting biology, behavior, and ecology of ground-nesting bee species at local, regional and national scales (Figure 1). Furthermore, sampling and gathering observational data at nesting sites can help develop species distribution models to predict where additional nest sites are located and also prioritize conservation efforts at local and regional scales.
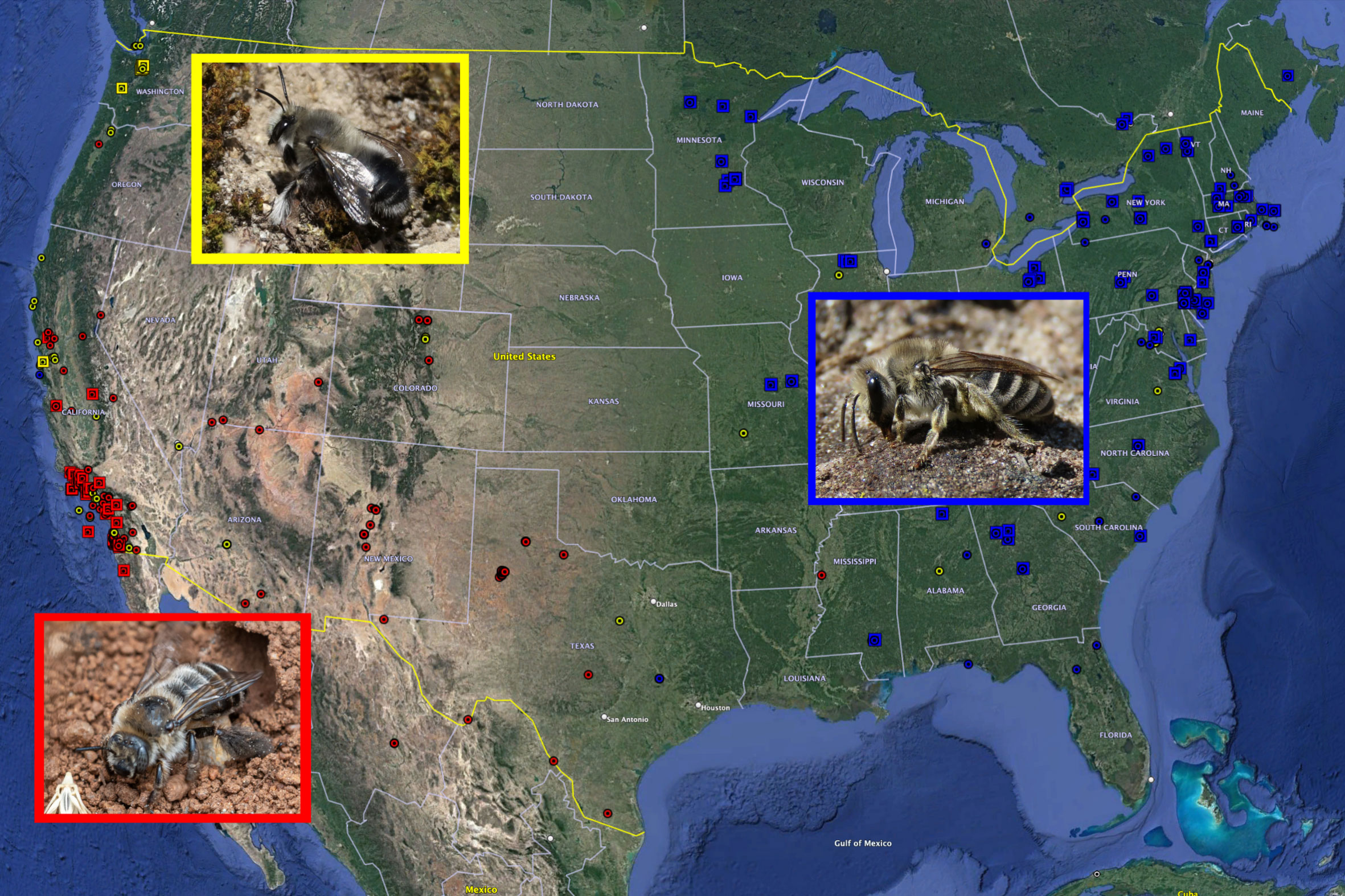
Figure 1 Potential to study bees at distinct scales and taxonomic levels: yellow = local and Anthophora, red = regional and Diadasia, and blue = national and Colletes. Yellow squares are records of Anthophora pacifica, red squares are records of Diadasia bituberculata, and blue squares are records of Colletes inaequalis.
The development of a robust global database that identifies ground-nesting bee sites has significant implications for understanding native bee ecology and offers new opportunities for native bee conservation. However, we must acknowledge several limitations. First, there is significant observation bias toward common bee species that make large and conspicuous aggregations. While such large aggregations are an intended focal target of Project GNBee, due to their sizable ecological contribution, many species nest at low densities with a few nests scattered over a large geographic area. Still others species nest under leaf litter or in dense vegetation. In these less visible cases, our community-driven approach to uncovering their nesting locations is far more difficult. Therefore, the detectability, which drives the species composition of our observations, will be biased. Second, the quality of our data is limited by the collective knowledge of our community. Thus, we seek experts and experienced amateurs to visit these sites and provide additional observations. Repeated observations from known sites, as well as observations in the literature, not currently available in Project GNBee, will help generate a consensus and improve the quality of the data by adding new sites and tracking bee seasonality and population dynamics through time.
Despite certain limitations and biases, Project GNBee can help fill current gaps in knowledge. The GNBee database has already incorporated rare bee nesting sites with high conservation priority, nest aggregations over 80 years in age, and numerous previously unknown high-density sites, several containing hundreds of thousands to well over a million individual solitary bees (Guilian et al., in prep; Hoge et al., in prep). Thus, we now can meaningfully prioritize discrete locations for research and conservation of ground-nesting bees.
Uniquely, aggregations can connect with people. A nesting aggregation is a place where bees live, much like a place in which humans live. One can return to nesting aggregations day after day to observe bees during their flight activity – a feature not possible in most animal community science projects. As such, these locations are part of a basic, local heritage. This can enhance efforts of property owners and land management agencies to prioritize the conservation of their resident bees. Signage (e.g., ‘Wild bee crossing’) that delivers educational information to the public should also be made available at these sites. Such on-site education and outreach could have profound impact on public sentiment and support. When possible, conservation agencies may seek to extend more robust protection to the most biologically significant nest sites, either through land acquisitions or through partnerships that establish guardians of these sites. We hope to make such recommendations in the future.
Beyond the conservation envelope, we are already able to study and compare the requirements of ground-nesting bees from locations in our own backyards to sites around the world. As such, we can move beyond single site descriptions of nesting biology and begin to understand the broader range of biotic and abiotic conditions that are required for a ground-nesting bee aggregation to persist. Furthermore, we can then attribute the degree of success (based on population size) of these local populations to their nesting conditions. This approach may help uncover meaningful predictors of nesting success within a species, across multiple species, and though space and time. While several attributes may be ‘reliably’ sourced using GIS, many attributes can be validated by the ‘community of scientists’ engaged with the project, who can send samples for further analysis. By using both remote sensing and community participation at scale, we plan to refine our models for predicting where individual bee species will be most likely to nest and how successful they are likely to become. Exploiting this framework, we may offer the building blocks needed to promote a more inclusive and robust community of pollinators that include the ground-nesting bees and lead to their successful management.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
JK: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. CD: Writing – review & editing. BD: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Cornell Atkinson Center for Sustainability – Academic Venture Fund and NSF grant 1398331.
We acknowledge and thank the entire iNaturalist community who collected, identified and publicly shared the ground-nesting bee observation data. We would like to thank John Asher, Zackary Portman, and several other unidentified individuals for their significant contributions to the platform.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1347447/full#supplementary-material
Antoine C. M., Forrest J. R. K. (2020). Nesting habitat of ground-nesting bees: a review. Ecol. Entomol. 46, 143–159. doi: 10.1111/een.12986
Appenfeller L. R., Lloyd S., Szendrei Z. (2020). Citizen science improves our understanding of the impact of soil management on wild pollinator abundance in agroecosystems. PloS One 15, e0230007. doi: 10.1371/journal.pone.0230007
Bartomeus I., Park M. G., Gibbs J., Danforth B. N., Lakso A. N., Winfree R. (2013). Biodiversity ensures plant–pollinator phenological synchrony against climate change. Ecol. Lett. 16, 1331–1338. doi: 10.1111/ele.12170
Biesmeijer J. C., Roberts S. P. M., Reemer M., Ohlemüller R., Edwards M., Peeters T., et al. (2006). Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. doi: 10.1126/science.1127863
Byer N. W., Reid B. N. (2022). The emergence of imperfect philopatry and fidelity in spatially and temporally heterogeneous environments. Ecol. Model. 468, 109968. doi: 10.1016/j.ecolmodel.2022.109968
Cane J. H. (1991). Soils of ground-nesting bees (Hymenoptera: apoidea): texture, moisture, cell depth and climate. J. Kansas Entomological Soc. 64, 406–413.
Cane J. H. (2008). A native ground-nesting bee (Nomia melanderi) sustainably managed to pollinate alfalfa across an intensively agricultural landscape. Apidologie 39, 315–323. doi: 10.1051/apido:2008013
Cane J. H. (2015). Landscaping pebbles attract nesting by the native ground-nesting bee Halictus rubicundus (Hymenoptera: Halictidae). Apidologie 46, 728–734.
Cane J. H. (2023). The extraordinary alkali bee, nomia melanderi (Halictidae), the world’s only intensively managed ground-nesting bee. Annu. Rev. Entomol. 69, null. doi: 10.1146/annurev-ento-020623-013716
Cane J. H., Neff J. L. (2011). Predicted fates of ground-nesting bees in soil heated by wildfire: Thermal tolerances of life stages and a survey of nesting depths. Biol. Conserv. 144, 2631–2636. doi: 10.1016/j.biocon.2011.07.019
Dance A. (2022). Community science draws on the power of the crowd. Nature 609, 641–643. doi: 10.1038/d41586-022-02921-3
Danforth B. N., Minckley R. L., Neff J. L. (2019). The Solitary Bees: Biology, Evolution, Conservation. (Princeton, NJ: Princeton University Press).
Debevec A. H., Cardinal S., Danforth B. N. (2012). Identifying the sister group to the bees: a molecular phylogeny of Aculeata with an emphasis on the superfamily Apoidea: Phylogeny of Aculeata. Zoologica Scripta 41, 527–535. doi: 10.1111/j.1463-6409.2012.00549.x
Deguines N., Julliard R., de Flores M., Fontaine C. (2016). Functional homogenization of flower visitor communities with urbanization. Ecol. Evol. 6, 1967–1976. doi: 10.1002/ece3.2009
Edwards M. (1996). Entomological survey and monitoring, Headley Heath, 1995–1996. Unpublished report, Commissioned by the National Trust.
Edwards M. (1998). Monitoring of bare ground for use by heathland insects. Unpublished report to the West Sussex Heathlands Project.
Fortel L., Henry M., Guilbaud L., Mouret H., Vaissière B. E. (2016). Use of human-made nesting structures by wild bees in an urban environment. J. Insect Conserv. 20, 239–253. doi: 10.1007/s10841-016-9857-y
Fortel L., Henry M., Guilbaud L., Mouret H., Vaissière B. E. (2016). Use of human-made nesting structures by wild bees in an urban environment. J. Insect Conserv. 20 (2), 239–253.
Fountain M. T., Tsiolis K., Silva C. X., Deakin G., Garratt M. P., O’Connor R., et al. (2023). Location and creation of nest sites for ground-nesting bees in apple orchards. Insects 14 (6), 490.
Gardein H., Fabian Y., Westphal C., Tscharntke T., Hass A. (2022). Ground-nesting bees prefer bare ground areas on calcareous grasslands. Glob. Ecol. Conserv. 39, e02289.
Gathmann A., Tscharntke T. (2002). Foraging ranges of solitary bees. J. Anim. Ecol. 71, 757–764. doi: 10.1046/j.1365-2656.2002.00641.x
Goulson D., Lye G. C., Darvill B. (2008). Decline and conservation of bumble bees. Annu. Rev. Entomol 53, 191–208. doi: 10.1146/annurev.ento.53.103106.093454
Gregory S., Wright I. (2005). Creation of patches of bare ground to enhance the habitat of ground-nesting bees and wasps at Shotover Hill, Oxfordshire, England. Conservation Evidence 2, 139–141.
Grundel R., Jean R. P., Frohnapple K. J., Glowacki G. A., Scott P. E., Pavlovic N. B. (2010). Floral and nesting resources, habitat structure, and fire influence bee distribution across an open-forest gradient. Ecol. Appl. 20, 1678–1692. doi: 10.1890/08-1792.1
Harmon-Threatt A. (2020). Influence of nesting characteristics on health of wild bee communities. Annu. Rev. Entomol. 65, 39–56. doi: 10.1146/annurev-ento-011019-024955
Iwasaki J. M., Hogendoorn K. (2022). Mounting evidence that managed and introduced bees have negative impacts on wild bees: an updated review. Curr. Res. Insect Sci. 2, 100043. doi: 10.1016/j.cris.2022.100043
Janousek W. M., Douglas M. R., Cannings S., Clément M. A., Delphia C. M., Everett J. G., et al. (2023). Recent and future declines of a historically widespread pollinator linked to climate, land cover, and pesticides. Proc. Natl. Acad. Sci. 120, e2211223120. doi: 10.1073/pnas.2211223120
Katumo D. M., Liang H., Ochola A. C., Lv M., Wang Q.-F., Yang C.-F. (2022). Pollinator diversity benefits natural and agricultural ecosystems, environmental health, and human welfare. Plant Divers. 44, 429–435. doi: 10.1016/j.pld.2022.01.005
Kirk W., Howes F. (2012). Plants for Bees: A Guide to the Plants That Benefit the Bees of the British Isles. (International Bee Research Association).
MacIvor J. S., Packer L. (2015). ‘Bee hotels’ as tools for native pollinator conservation: A premature verdict? PloS One 10, e0122126. doi: 10.1371/journal.pone.0122126
Mader E., Shepard M., Vaughan M., Hoffman Black S., LeBuhn G. (2011). Attracting native Pollinators: The Xerces Society Guide Protecting North America’s Bees and Butterflies. (The Xerces Society for Invertebrate Conservation).
Maher S., Manco F., Ings T. C. (2019). Using citizen science to examine the nesting ecology of ground-nesting bees. Ecosphere 10, e02911. doi: 10.1002/ecs2.2911
M’Gonigle L. K., Williams N. M., Lonsdorf E., Kremen C. (2017). A tool for selecting plants when restoring habitat for pollinators. Conserv. Lett. 10, 105–111. doi: 10.1111/conl.12261
Michener C. D. (2007). The Bees of the World. 2nd ed (Baltimore, MD: Johns Hopkins University Press).
Neumüller U., Burger H., Mayr A. V., Hopfenmüller S., Krausch S., Herwig N., et al. (2022). Artificial nesting hills promote wild bees in agricultural landscapes. Insects 13 (8), 726.
O’Connor S., Park K. J., Goulson D. (2012). Humans versus dogs; a comparison of methods for the detection of bumble bee nests. J. Apicultural Res. 51, 204–211. doi: 10.3896/IBRA.1.51.2.09
Ollerton J. (2017). Pollinator diversity: distribution, ecological function, and conservation. Annu. Rev. Ecology Evolution Systematics 48, 353–376. doi: 10.1146/annurev-ecolsys-110316-022919
Page M. L., Williams N. M. (2023). Evidence of exploitative competition between honey bees and native bees in two California landscapes. J. Anim. Ecol. 92, 1802–1814. doi: 10.1111/1365-2656.13973
Pitts-Singer T. L., Cane J. H. (2011). The alfalfa leafcutting bee, megaChile rotundata: the world’s most intensively managed solitary bee. Annu. Rev. Entomol. 56, 221–237. doi: 10.1146/annurev-ento-120709-144836
Pocock M. J. O., Evans D. M. (2014). The success of the horse-chestnut leaf-miner, cameraria ohridella, in the UK revealed with hypothesis-led citizen science. PloS One 9, e86226. doi: 10.1371/journal.pone.0086226
Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Potts S. G., Vulliamy B., Dafni A., Ne’eman G., Willmer P. (2003). Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84, 2628–2642. doi: 10.1890/02-0136
Potts S. G., Vulliamy B., Roberts S., O’Toole C., Dafni A., Ne’eman G., et al. (2005). Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 30, 78–85. doi: 10.1111/j.0307-6946.2005.00662.x
Powney G. D., Carvell C., Edwards M., Morris R. K. A., Roy H. E., Woodcock B. A., et al. (2019). Widespread losses of pollinating insects in Britain. Nat. Commun. 10, 1018. doi: 10.1038/s41467-019-08974-9
Prendergast K. S., Dixon K. W., Bateman P. W. (2022). A global review of determinants of native bee assemblages in urbanised landscapes. Insect Conserv. Diversity 15, 385–405. doi: 10.1111/icad.12569
Rosa García R., Miñarro M. (2014). Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agriculture Ecosyst. Environ. 183, 118–126. doi: 10.1016/j.agee.2013.10.017
Sánchez-Bayo F., Wyckhuys K. A. G. (2019). Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Sann M., Niehuis O., Peters R. S., Mayer C., Kozlov A., Podsiadlowski L., et al. (2018). Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evolutionary Biol. 18, 71. doi: 10.1186/s12862-018-1155-8
Severns P. M. (2004). Creating bare ground increases presence of native pollinators in Kincaid’s lupine seeding plots. Ecological Restoration 22, 234–235.
Sheffield C. S., Kevan P. G., Westby S. M., Smith R. F. (2008). Diversity of cavity-nesting bees (Hymenoptera: Apoidea) within apple orchards and wild habitats in the Annapolis Valley, Nova Scotia, Canada. Can. Entomologist 140, 235–249. doi: 10.4039/n07-058
Silvertown J. (2009). A new dawn for citizen science. Trends Ecol. Evol. 24, 467–471. doi: 10.1016/j.tree.2009.03.017
Simanonok M. P., Otto C. R. V., Cornman R. S., Iwanowicz D. D., Strange J. P., Smith T. A. (2021). A century of pollen foraging by the endangered rusty patched bumble bee (Bombus affinis): inferences from molecular sequencing of museum specimens. Biodivers Conserv. 30, 123–137. doi: 10.1007/s10531-020-02081-8
Stout J. C., Morales C. L. (2009). Ecological impacts of invasive alien species on bees. Apidologie 40, 388–409. doi: 10.1051/apido/2009023
Thomas C. D., Jones T. H., Hartley S. E. (2019). ‘Insectageddon’: A call for more robust data and rigorous analyses. Global Change Biol. 25, 1891–1892. doi: 10.1111/gcb.14608
Tsiolis K., Potts S., Garratt M., Tilston E., Burman J., Rintoul-Hynes N., et al. (2022). The importance of soil and vegetation characteristics for establishing ground-nesting bee aggregations. J. Pollination Ecol. 32, 186–200. doi: 10.26786/1920-7603(2022)682
Ulyshen M., Urban-Mead K. R., Dorey J. B., Rivers J. W. (2023). Forests are critically important to global pollinator diversity and enhance pollination in adjacent crops. Biol. Rev. 98, 1118–1141. doi: 10.1111/brv.12947
Vannette R. L. (2020). The floral microbiome: plant, pollinator, and microbial perspectives. Annu. Rev. Ecol. Evol. Syst. 51, 363–386. doi: 10.1146/annurev-ecolsys-011720-013401
Wesserling J., Tscharntke T. (1995). Homing distances of bees and wasps and the fragmentation of habitats. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 10, 323–326.
Williams N. M., Cariveau D., Winfree R., Kremen C. (2011). Bees in disturbed habitats use, but do not prefer, alien plants. Basic Appl. Ecol. 12, 332–341. doi: 10.1016/j.baae.2010.11.008
Willis Chan D. S., Prosser R. S., Rodríguez-Gil J. L., Raine N. E. (2019). Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 9, 11870. doi: 10.1038/s41598-019-47805-1
Willis Chan D. S., Raine N. E. (2021). Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Sci. Rep. 11, 4241. doi: 10.1038/s41598-021-83341-7
Keywords: ground-nesting bees, solitary bees, nesting aggregation, community science, citizen science (CS), iNaturalist, species occurrence data, conservation
Citation: Kueneman JG, Dobler CN and Danforth BN (2024) Harnessing community science to conserve and study ground-nesting bee aggregations. Front. Ecol. Evol. 11:1347447. doi: 10.3389/fevo.2023.1347447
Received: 30 November 2023; Accepted: 19 December 2023;
Published: 09 January 2024.
Edited by:
Kris Braman, University of Georgia, United StatesReviewed by:
Becky Griffin, University of Georgia, United StatesCopyright © 2024 Kueneman, Dobler and Danforth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jordan G. Kueneman, amsyODk5QGNvcm5lbGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.