- 1Museo de Historia Natural Noel Kempff Mercado, Santa Cruz de la Sierra, Bolivia
- 2K. Lisa Yang Center for Conservation Bioacoustics, Cornell Lab of Ornithology, Cornell University, Ithaca, NY, United States
- 3Behavioral Ecology and Sociobiology Unit, German Primate Center – Leibniz-Institute for Primate Research, Göttingen, Germany
Editorial on the Research Topic
Duetting and turn-taking patterns of singing mammals: from genes to vocal plasticity, and beyond
One of the greatest challenges in evolutionary biology is tracing back the origins of human speech in the absence of fossilized vocal sounds. Since Darwin’s (1871) landmark treatise on the evolution of spoken language and music, the search for phylogenetic precursors of these two intimately connected fields has remained a major endeavor of scientific research (ten Cate and Honing, 2023).
As a prime signaling channel, acoustic communication is above all socially interactive and can take many forms in the animal kingdom, thus providing an evolutionary substrate for the emergence of human musicality and conversational speech (Snowdon et al., 2015; Levinson, 2016; Snowdon, 2017, 2021; Savage et al., 2020); it is also a useful system for understanding the evolutionary processes that shape phenotypic variation. In the wake of a Research Topic entitled “Turn-taking in Human Communicative Interaction” (Holler et al., 2015), the present collection of 13 articles brings together 47 authors who share ideas, data and methods on the theme of vocal duetting (i.e., a coordinated vocal exchange between two individuals who alternate and/or overlap their contributions) and turn-taking (i.e., a vocal exchange based on active overlap avoidance between individuals who take turns as callers and listeners) in singing mammals.
Approximately 6,400 living species of mammals populate Earth (Burgin et al., 2018). Of those that have been the subject of detailed bioacoustics analyses, only few have evolved the capacity for singing and fewer still have been reported to coordinate song (i.e., a string of modulated vocal sounds delivered in a predictable pattern), either as an intra- or intersexual display (Figure 1). Arguably, the champions are the “singing primates” (De Gregorio et al., 2022), an assemblage of ~70 arboreal species – roughly 14% of all extant primates – distributed in Southeast Asia (tarsiers, a Mentawai langur, gibbons and siamang), Madagascar (indri, Milne-Edward’s sportive lemur) and South America (titi monkeys).
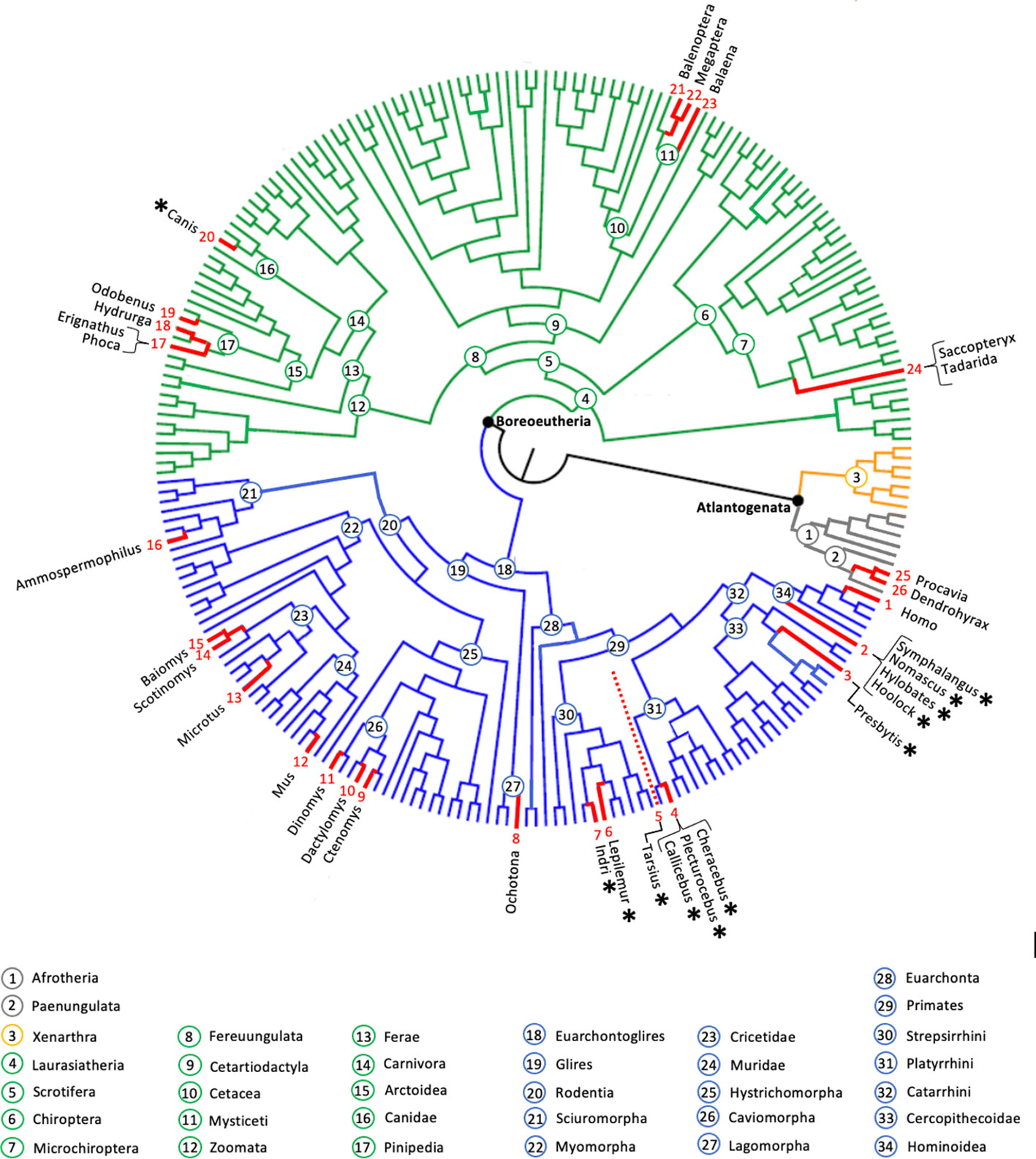
Figure 1 Ortho-phylogenetic tree of placental mammals (modified from Foley et al., 2023, with permission from the authors). Highlighted in red are mammalian genera endowed with species that have the ability to sing. Note that we consider howling in canids as a song-like vocalization performed as a duet by mated pairs or as a group chorus. In non-primate mammals, song appears to be a male prerogative (except in canids) leading some species to engage in intra-sexual counter-singing. Asterisks denote taxa in which mated pairs produce coordinated songs. The red dotted line corresponds to the Tarsiiformes, not included in the phylogenetic analysis of Foley et al. (2023). Where taxa differed from those originally reported in Foley et al. (2023), we elected the terminal branch which was most closely-related phylogenetically. Red numbers on the outer ring of the cladogram specify the relevant literature: 1- Mehr et al. (2019); 2- Geissmann (2002); 3- Tilson and Tenaza (1976); 4- Caselli et al. (2014); Adret et al. (2018); 5- Shekelle et al. (2019); 6- Méndez-Cárdenas and Zimmermann (2009); 7- Pollock (1986); 8- Somers (1973); 9- Amaya et al., (2016); 10- Emmons (1981); 11- Eisenberg (1974); 12- Holy and Guo (2005); 13- Rutovskaya (2020); 14- Banerjee et al. (2019); 15- Miller and Engstrom (2007); 16- Bolles (1988); 17-Ray et al. (1969); Fitch (2006); 18- Thomas and Golladay (1996); 19- Sjare et al. (2003); 20- Koler-Matznik et al. (2003); 21- Stafford et al. (2012); 22- Payne and McVay (1971); 23- Buchan et al. (2014); 24- Behr and von Helversen (2004); Bohn et al. (2009); 25- Demartsev et al. (2017); 26- Rosti et al. (2020).
While distinct phenotypes of coordinated acoustic signaling have been identified in a wide range of organisms (Pika et al., 2018; de Reus et al., 2021), a robust link between duetting, a long-lasting pair bond, and a non-migratory lifestyle marked by year-round territoriality is found primarily in homeothermic animals. To what extent these differences in communicative abilities are driven by genes, experience and the environment remains an active area of research. Notably, significant progress has been made by expanding the stage of experimentation to the emitter-receiver taken as the unit of investigation. Indeed, recent technological advances such as wireless dual recordings of vocalizations and concomitant brain activity can be considered as the gold standard to investigate social coordination in mammals (Rose et al., 2021), let alone the promising expectations from the field of Artificial Intelligence (Rutz et al., 2023).
Collectively, the contributions to this Frontiers Research Topic cover the four questions fundamental to behavioral research, namely causation, ontogeny, function and evolution. A final section is devoted to machine learning techniques with the goal of supporting primate conservation efforts.
1 Overview
Two articles introduce the Research Topic. Adret’s mini-review provides a concise synthesis of developmental plasticity in the coordinated songs of songbirds and singing primates. The broad relevance of linking duetting behavior and its neural underpinnings is made. Similarities and differences between the two fields of research are highlighted to help guide ongoing research. In a perspective article, Vanderhoff and Bernal Hoverud focus on the coordinated vocal exchanges of non-primate mammals, pointing out inconsistencies in term usage such as duetting, antiphony, counter-singing and turn-taking. Moving on, the authors present a case study from the elusive South-American bamboo rats and encourage researchers to search for more examples of mammals that communicate via coordinated vocalizations.
2 Causation
In a thought-provoking contribution, Ravignani et al. hypothesize on the role of the corpus callosum (CC) in facilitating turn-taking and duetting (TTD) behavior in mammals. Drawing on similarities from animal behavior, language and music, the authors argue that CC and TTD likely co-evolved to speed up interhemispheric communication during vocal exchanges in eutherian mammals. They propose to test this hypothesis by comparing CC size in duetting and non-duetting pairs of closely related mammalian species.
3 Ontogeny
Four articles compose this section. Abreu and Pika thoroughly review the development and acquisition of turn-taking skills in non-human mammals. Using a top-down approach, the authors highlight four building blocks of conversational speech and identify research biases and gaps after methodically sifting key-articles in this emerging system. The authors pinpoint fruitful research avenues to stir more interest in this field that will improve our understanding of turn-taking for language evolution. Following a decade of field recordings collected from eight family groups of indris (Indri indri) in Madagascar, De Gregorio et al. track the social dynamics underlying this unique lemur song display, which combines elements of solo singing, duetting and chorusing. The authors report a clear stochastic process of vocal turns resulting from non-random dyads between group members. Interestingly, the study provides evidence that each parent alters its singing while interacting with an offspring. In a singular paper, Yi et al. highlight the occurrence of co-singing episodes between offspring and mothers in a “non-duetting” gibbon, Hylobates moloch. Twelve consecutive years of field observations revealed that these joint vocalizations are transiently expressed from two to seven years of age, with striking sex differences, after which mature individuals produce only sex-specific solo songs. Working in captive settings, Hradec et al. undertake an analysis of adult male songs in the Southern yellow-cheeked gibbon, Nomascus gabriellae. The authors highlight structural differences between unpaired and paired males although further studies are needed to disentangle the respective effects of age and social status on song structure.
4 Function
Two articles make up this section. Dolotovskaya and Heymann investigate the adaptive value of duetting with an observational study of six groups of coppery titi monkeys, Plecturocebus cupreus, from Peruvian Amazon. A systematic mapping of duet records during periods of female receptivity, gestation, and lactation allows the researchers to combine their data with relevant ecological variables. The ensuing multifactorial analyses support a resource defense mechanism as opposed to a mate guarding strategy. Experimenting at the National Primate Research Center in Davis (California, USA), Lau et al. provide preliminary data on duet song perception in female coppery titi monkeys, Plecturocebus cupreus. Audio playback tests conducted both before and after pairing reveal noticeable behavioral and hormonal changes linked to the reproductive cycle. The work adds an important component to the broad picture of primate duetting, especially from the listener perspective.
5 Evolution
Two articles cover this section. The evolution of signal design is central to the Comella et al. article focusing on the duets of Gursky’s spectral tarsiers, Tarsius spectrumgurskyae, a nocturnal basal haplorrhine from Indonesia. Using unsupervised clustering methods, the authors show that individual pairs possess highly graded, sex-specific note repertoires, subject to morpho-physiological constraints between the rate of syllable repetition and note bandwidth. Such acoustic tradeoffs might represent one example of “species-universals” in vocal communication. Transcending the mechanistic view of duetting, Kaplan takes a multi-disciplinary and multimodal approach in formulating a “prosocial theory” for the evolution of human language. The author argues that the switch from self- to other-oriented behavior required expanding both cognitive and affective skills to foster intentional cooperation after social bonding has already been established. Within this framework, both gestural and vocal coordination were paramount to the emergence of human language.
6 Techniques
Passive acoustic monitoring (PAM) utilizes autonomous sensors for population surveys on broad spatial-temporal scales. On this basis, Clink et al. develop and test a machine learning approach for the automated detection and classification of female great calls in the Northern-grey gibbon, Hylobates funereus, on Malaysian Borneo. While performance of the open-source code for call detection was found satisfactory, the unsupervised clustering algorithm performed sub-optimally, thus impacting the ability to reliably discriminate individual females in the local population. Nonetheless, the proposed workflow constitutes a valuable effort on which further studies can build on. In a companion article using PAM, van Kuijk et al. investigate source level and detection range of duet songs in the cryptic red titi monkey, Plecturocebus discolor, of the Ecuadorian Amazon. To extract the target signal from audio recordings, the researchers apply a supervised template-based detection algorithm, which, compared with manual detection, significantly sped up data processing and will serve to implement future PAM studies of titi monkeys.
In conclusion, time will tell whether this Research Topic successfully achieved its goal to serve as a springboard for more empirical work in our quest to unravel pressing issues.
Author contributions
PA: Conceptualization, Writing – original draft, Writing – review & editing. DC: Writing – review & editing. SD: Writing – review & editing.
Acknowledgments
We wish to thank the Authors, Reviewers and invited Editors for their valuable contribution. PA is most grateful to Dr. Helen Kimbell and her team at Frontiers in Ecology and Evolution for inviting him to develop the theme of this article collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adret P., Dingess K. A., Caselli C. B., Vermeer J., Martínez J., Luna Amancio J. C., et al. (2018). Duetting patterns of titi monkeys (Primates: Callicebinae) and relationships with phylogeny. Animals 8, 178–211. doi: 10.3390/ani8100178
Amaya J. P., Areta J. I., Valentinuzzi V. S., Zuffiaurre E. (2016). Form and function of long-range vocalizations in a neotropical fossorial rodent: the anillaco tuco-tuco (Ctenomys sp.). PeerJ 4, e2559. doi: 10.7717/peerj.2559
Behr O., von Helversen O. (2004). Bat serenades–complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav. Ecol. Sociobiol. 56, 106–115.
Bohn K. M., Schmidt-French B., Schwartz C., Smotherman M., Pollak G. D. (2009). Versatility and stereotypy of free-tailed bat songs. PloS One 4 (8), e6746. doi: 10.1371/journal.pone.0006746
Bolles K. (1988). Evolution and variation of antipredator vocalisations of antelope squirrels, Ammospermophilus (Rodentia: Sciuridae). Z. Säugetierkunde 53, 129–114.
Buchan S. J., Hucke-Gaete R., Rendell L., Stafford K. M. (2014). A new song recorded from blue whales in the corcovado gulf, southern chile, and an acoustic link to the eastern tropical pacific. Endang. Species Res. 23, 241–252. doi: 10.3354/esr00566
Burgin C. J., Colella J. P., Kahn P. L., Upham N. S. (2018). How many species of mammals are there? J. Mammal. 99, 1–14. doi: 10.1093/jmammal/gyx147
Caselli C. B., Mennill D. J., Bicca-Marques J. C., Setz E. Z. F. (2014). Vocal behavior of black-fronted titi monkeys (Callicebus nigrifrons): Acoustic properties and behavioral contexts of loud calls. Am. J. Primatol. 76, 788–800. doi: 10.1002/ajp.22270
Darwin C. (1871). The descent of man and selection in relation to sex (London: John Murray, Albermarle Street).
De Gregorio C., Carugati F., Valente D., Raimondi T., Torti V., Miaretsoa L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 34, 205–219. doi: 10.1080/03949370.2021.2015451
Demartsev V., Ilany A., Kershenbaum A., Geva Y., Margalit O., Schnitzer I., et al. (2017). The progression pattern of male hyrax songs and the role of climactic ending. Sci. Rep. 7, 2794. doi: 10.1038/s41598-017-03035-x
de Reus K., Soma M., Anichini M., Gamba M., de Heer Kloots M., Lense M., et al. (2021). Rhythm in dyadic interactions. Phil. Trans. R. Soc B 376, 20200337. doi: 10.1098/rstb.2020.0337
Eisenberg J. F. (1974). “The function and motivational basis of hystricomorph vocalizations,” in The biology of hystricomorph rodents. Eds. Rowlands I. W., Weir B. J. (London: Symposia of the Zoological Society), 211–247. 34:1-482.
Emmons L. (1981). Morphological, ecological, and behavioral adaptations for arboreal browsing in Dactylomys dactylinus (Rodentia. Echimyidae). J. Mammal. 62, 183–189. doi: 10.2307/1380493
Fitch W. T. (2006). The biology and evolution of music: a comparative perspective. Cognition 100, 173–215. doi: 10.1016/j.cognition.2005.11.009
Foley N. M., Mason V. C., Harris A. J., Bredemeyer K. R., Damas J., Lewin H. A., et al. (2023). A genomic timescale for placental mammal evolution. Science 380, 6643. doi: 10.1126/science.abl8189
Geissmann T. (2002). Duet-splitting and the evolution of gibbon songs. Biol. Rev. 77, 57–76. doi: 10.1017/s1464793101005826
Holler J., Kendrick K. H., Casillas M., Levinson S. C. (2015). Editorial: Turn-taking in human communicative interaction. Front. Psychol. 6. doi: 10.3389/fpsyg.2015.01919
Holy T. E., Guo Z. (2005). Ultrasonic songs of male mice. PloS Biol. 3, e386. doi: 10.1371/journal.pbio.0030386
Koler-Matznick J., Brisbin I., Feinstein M., Bulmer S. (2003). An updated description of the new guinea singing dog (Canis hallstromi, troughton 1957). J. Zool. 261, 109–118. doi: 10.1017/S0952836903004060
Levinson S. C. (2016). Turn-taking in human communication-origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Méndez-Cárdenas M. G., Zimmermann E. (2009). Duetting - a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. doi: 10.1002/ajpa.21017
Mehr S. A., Singh M., Knox D., Ketter D. M., Pickens-Jones D., Atwood S., et al. (2019). Universality and diversity in human song. Science 366 (6468), eaax0868. doi: 10.1126/science.aax0868
Miller J. R., Engstrom M. D. (2007). Vocal stereotypy and singing behavior in baiomyine mice. J. Mammal. 88, 1447–1465. doi: 10.1644/06-MAMM-A-386R.1
Payne R. S., McVay S. (1971). Songs of humpback whales: Humpbacks emit sounds in long, predictable patterns ranging over frequencies audible to humans. Science 173, 585–597. doi: 10.1126/science.173.3997.585
Pika S., Wilkinson R., Kendrick K. H., Vernes S. C. (2018). Taking turns: bridging the gap between human and animal communication. Proc. R. Soc. B 285:20180598. doi: 10.1098/rspb.2018.0598
Pollock J. I. (1986). The song of the indris (Indri indri; primates; lemuroidea): Natural history, form, and function. Int. J. Primatol. 7, 225–264. doi: 10.1007/BF02736391
Ray C., Watkins W. A., Burns J. (1969). The underwater song of erignathus (bearded seal). Zoologica 54, 79–83. doi: 10.5962/p.203251
Rose M. C., Styr B., Schmid T. A., Elie J. E., Yartsev M. M. (2021). Cortical representation of group social communication in bats. Science 374, eaba9584. doi: 10.1126/science.aba9584
Rosti H., Pihlström H., Bearder S., Pellikka P., Rikkinen J. (2020). Vocalization analyses of nocturnal arboreal mammals of the taita hills kenya. Diversity 12, 473. doi: 10.3390/d12120473
Rutovskaya M. V. (2020). Acoustic communication in four species of subgenus alexandromys. Russian J. Theriol. 19, 21–36. doi: 10.15298/rusjtheriol.19.1.02
Rutz C., Bronstein M., Raskin A., Vernes S. C., Zacarian K., Blasi D. E. (2023). Using machine learning to decode animal communication. Science 381 (6654), 152–155. doi: 10.1126/science.adg7314
Savage P. E., Loui P., Tarr B., Schachner A., Glowacki L., Mithen S., et al. (2020). Music as a coevolved system for social bonding. Behav. Brain Sci. 44, e59. doi: 10.31234/osf.io/qp3st
Shekelle M., Groves C. P., Maryanto I., Mittermeier R. A., Salim A., Springer M. S. (2019). A new tarsier species from the togean islands of central sulawesi, indonesia, with references to wallacea and conservation on sulawesi. Primate Conserv. 33, 65–73.
Sjare B. L., Stirling I., Spencer C. A. (2003). Structural variation in the songs of atlantic walruses breeding in the canadian high arctic. Aquat. Mammals 29, 297–318.
Snowdon C. T. (2021). Animal signals, music and emotional well-being. Animals 11, 2670. doi: 10.3390/ani11092670
Snowdon C. T., Zimmermann E., Altenmüller E. (2015). Music evolution and neuroscience. Prog. Brain Res. 217, 17–34. doi: 10.1016/bs.pbr.2014.11.019
Somers P. (1973). Dialects in southern rocky mountain pikas, ochotona princeps (Lagomorpha). Anim. Behav. 21, 124–137. doi: 10.1016/S0003-3472(73)80050-8
Stafford K. M., Moore S. E., Berchok C. L., Wiig ØVerifyt. a. t., Lydersen C., Hansen E., et al. (2012). Spitsbergen’s endangered bowhead whales sing through the polar night. Endangered Species Res. 18 (2), 95–103. doi: 10.3354/esr00444
ten Cate C., Honing H. (2023). “Precursors of music and language in animals. PsyArXiv,” in Oxford handbook of language and music. Ed. Sammler D. (Oxford: Oxford University Press). doi: 10.31234/osf.io/4zxtr
Thomas J. A., Golladay C. L. (1996). “Geographic variation in leopard seal (Hydrurga leptonyx) underwater vocalizations. In Eds,” in Sensory systems of aquatic mammals. Eds. Kastelein R., Thomas J. A., Nachtigall P. E. (Woerden, The Netherlands: DeSpil Publishers), 201–221.
Keywords: primates, coordinated singing, turn-taking, social cooperation, evolutionary history, pairbonding, machine learning, language evolution
Citation: Adret P, Clink DJ and Dolotovskaya S (2023) Editorial: Duetting and turn-taking patterns of singing mammals: from genes to vocal plasticity, and beyond. Front. Ecol. Evol. 11:1279635. doi: 10.3389/fevo.2023.1279635
Received: 18 August 2023; Accepted: 30 August 2023;
Published: 14 September 2023.
Edited and Reviewed by:
Jordi Figuerola, Spanish National Research Council (CSIC), SpainCopyright © 2023 Adret, Clink and Dolotovskaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrice Adret, cGF0cmljZS5hZHJldEBnbWFpbC5jb20=
 Patrice Adret
Patrice Adret Dena J. Clink
Dena J. Clink Sofya Dolotovskaya
Sofya Dolotovskaya