
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 26 October 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1267705
This article is part of the Research TopicInsect Conservation BehaviorView all 11 articles
 Hayat Mahdjoub1
Hayat Mahdjoub1 Rabah Zebsa2
Rabah Zebsa2 Hichem Amari3
Hichem Amari3 Soufyane Bensouilah4
Soufyane Bensouilah4 Abdelheq Zouaimia2
Abdelheq Zouaimia2 Abdeldjalil Youcefi5
Abdeldjalil Youcefi5 Rassim Khelifa1,6*
Rassim Khelifa1,6*Understanding habitat requirements of species of conservation concern is central for their conservation and management. Although much of the research attention has been focused on reproductive sites, the understanding of roosting behavior and microhabitat selection, and their potential fitness consequences is also crucial. Here, we assess the roosting behavior of an endangered endemic damselfly Calopteryx exul Selys in a lotic habitat of Northeast Algeria. Based on marked individuals, we specifically investigated their vertical and horizontal distribution at roosting sites, as well as the timing of roosting and its correlation with lifespan (as a measure of fitness). We found that individuals were philopatric to roosting sites and less so to vertical stratification. Roosting sites were used for both foraging and roosting. Individuals that occupied lower strata in roosting sites had longer lifespans and ceased roosting earlier. Average temperature of the day affected the timing of roosting such that on warm days roosting started later and ended earlier. Individuals with longer lifespans roosted earlier, suggesting potential scramble competition for roosting sites. Our results suggest that C. exul individuals show variability in the vertical and horizontal location as well as the timing of roosting, and these choices potentially have fitness consequences. This study highlights the importance of bank vegetation as roosting sites for lotic insects, and emphasizes the benefits of protecting these sites and including them as integral parts of the conservation plans of species.
Many threatened species of animals, including insects, are on the brink of extinction due to human activities (Maczulak, 2010; Wagner, 2020). Species of conservation concern need particular research attention because of their sensitivity to environmental disturbance (Maczulak, 2010; Samways et al., 2010; Samways, 2020). Conservationists have sounded the alarm about the rapid decline of insects in different regions worldwide due to various anthropogenic factors such as habitat destruction, pesticides, and climate change (Van Klink et al., 2020; Hallmann et al., 2021; Raven and Wagner, 2021; Uhler et al., 2021; Outhwaite et al., 2022). Thus, there is an increasing concern for threatened species because of their high sensitivity to habitat degradation and other anthropogenic stressors (New, 2009). Endemic species, in particular, are among the most threatened groups because their typical small range size limits their ability to cope with rapid changes in environmental conditions (Burlakova et al., 2011; Carmona et al., 2019). Because some anthropogenic factors are unavoidable in a human-dominated world, it is imperative to understand fundamental aspects of species’ ecological requirements to increase their resilience and promote their persistence in their natural habitat.
There is a need to develop a holistic understanding of the habitat requirements of threatened species to better protect them and manage their habitat (Deacon et al., 2020; Samways et al., 2020; Kietzka et al., 2021). This involves a better comprehension of the spatial and temporal dimensions that species occupy to perform their ecological functions and fulfill their biological needs (Samways et al., 2020). While conservationists have paid particular attention to foraging and reproductive sites as vital habitat components for the conservation of species (Foster and Soluk, 2006; Majewska and Altizer, 2020; Shipley et al., 2023), other aspects of habitat preferences such as roosting sites have been understudied despite their crucial role in the species’ life history (Grether and Switzer, 2000; Grether and Donaldson, 2007; Teng et al., 2012). Roosting sites may provide various services to individuals, including shelter, safety, and social information. Many species of animals, including mammals, birds, fish, and insects roost in communal aggregations of a dozen to millions of individuals (Krause and D., 2002). Compared to vertebrates (Laughlin et al., 2014; Deng et al., 2023), communal roosting behavior in insects has received far less research attention.
The evolution of communal aggregation behavior has been highly debated during the last centuries leading to various theories (Dwyer et al., 2018). Many of those theories such as thermal benefits (Vulinec, 1990), information center (Bijleveld et al., 2010), and aposematism (Turner, 1975) do not apply to a large number of insect groups (Grether and Switzer, 2000). The predator dilution theory as an antipredator defense mechanism; however, is one of the most applicable hypotheses to a wide range of taxa (Lack, 1968), including within insects (Vulinec, 1990). Studies have shown that species have specific preferences for the timing and location of communal roosting (Miller, 1989; Teng et al., 2012; Finkbeiner, 2014; Laughlin et al., 2014), often exhibiting high levels of philopatry in both vertebrates (Lewis, 1995; Beauchamp, 1999) and invertebrates (Miller, 1989; Grether and Donaldson, 2007; Finkbeiner, 2014). However, our understanding of the implication of microhabitat selection within roosting sites for the survival and fitness of individuals has not attracted comparable research attention.
Odonates are integral components of freshwater systems (Corbet, 1999; Martín and Maynou, 2016), occupying both terrestrial and aquatic habitats, and interacting with a wide range of taxa (Kaunisto et al., 2020). As adults, both dragonflies and damselflies spend the daytime foraging and reproducing near the water, however, at night, they occupy their roosting sites which are either near or far from the water (Corbet, 1999). Odonates are suitable organisms for the study of roosting behavior because they can be easily marked, recaptured, measured, and surveyed in the field throughout their entire lifespan (Cordero-Rivera and Stoks, 2008). Damselflies in particular perform mostly short-range movements and do not disperse frequently. There have been several records of communal roosting behavior in odonates (Neubauer and Rehfeldt, 1995; Grether and Switzer, 2000; Switzer and Grether, 2000; Rouquette and Thompson, 2007; Hykel et al., 2018), but only a few studies have explored microhabitat choice, site fidelity, and the potential fitness implications of roosting site selection.
Calopteryx exul is an endemic damselfly listed as Endangered on the IUCN Red list (Boudot, 2018). The species has a relatively patchy distribution with populations spanning from Morocco in the west to Tunisia in the East. In Algeria, the species had not been recorded for almost a century, from 1910 to 2007 (Khelifa et al., 2011). In later years, multiple subpopulations have been discovered in the Seybouse river, but several of them have perished following habitat degradation (Khelifa and Mellal, 2017). In more recent years, new sites have been recently discovered in the central North and east of Algeria (Chelli et al., 2019; Elafri, 2022), improving our understanding of its geographic distribution in the region. The historical sites of the country have encountered severe changes due to habitat destruction, exploitation (water pumping for irrigation), pollution (pesticides and fertilizers for agriculture), and climate change (severe drought and extreme heat) (Khelifa et al., 2021). Studies on the life history, reproductive behavior, habitat requirements, and geographic range dynamics have been carried out during the last decade (Khelifa, 2017; Mellal et al., 2018; Khelifa, 2019), furthering our understanding of the species ecology and behavior. However, there has not been any study on the roosting behavior in C. exul, restricting our holistic understanding of habitat preferences of the species. Filling in this gap of knowledge is crucial for the better management of natural habitats and maintenance of populations in their natural environment.
In this study, we investigate the roosting behavior of C. exul using capture-mark-recapture in the Seybouse river, Northeast Algeria. We marked individuals across a 100 m transect of the watercourse and surveyed their vertical stratification, horizontal distribution, timing of occupancy of roosting sites, and lifespan. We aim to test whether: 1) vertical and horizontal distribution differed between sexes, 2) vertical stratification and timing of roosting is correlated with fitness components (lifespan); and 3) temperature influences the timing of roosting. We hypothesize that: 1) there is sexual segregation in space and time due to behavioral differences between sexes; 2) vertical stratification and the timing of roosting are correlated with lifespan because better spatiotemporal choices have fitness consequences; and 3) timing of roosting depends on temperature such that individuals roost later on warm days.
Mature adults of C. exul spend their daytime mating in patches of vegetation floating on the water (territories). At the close of the day, the damselflies gather in specific areas along the watercourse, both males and females joining together in a communal roosting site. These areas are often nestled amidst the bank vegetation, providing foraging, resting, and roosting sites. Prior to roosting, damselflies perched on stems or leaves to forage using a sit-and-wait tactic and seize the passing insects that fly during the dawn. When it becomes dark, damselflies take their roosting position and posture (Figure 1), spreading their wings open and remaining in the same posture until sunrise. Thus, this behavioral display allows us to identify roosting individuals and investigate intraspecific variation in spatiotemporal distributions of roosting behavior.

Figure 1 A roosting mature male (left) and female (right) of Calopteryx exul adult early in the morning in the Seybouse river, Northeast Algeria. Wing spreading occurs only when individuals are roosting. The typical wing posture when individuals are perched is joint wings.
The research was conducted in the Seybouse river in the northeastern region of Algeria. The local climate is Mediterranean with hot and dry summers and cool and wet winters. In the Seybouse river, the mean annual rainfall varies between 350 mm upstream and 608 mm downstream (ABHCSM, 2009). The hydrology displays a wet season that encompasses the period from October to May, followed by a dry season that extends from June to September. The behavioral study was carried out upstream of the Seybouse River, approximately 5 km west of Guelma city (36°28023.16″N and 7°22032.73″E; 210 m elevation). The watercourse was a stream with a shallow depth and a 2-4 m width. The vegetation along the banks predominantly comprises Typha angustifolia L., Cyperus longus L., Juncus maritimus Lam., and Paspalum distichum L. In the same site, odonate assemblage is dominated by C. exul, C. haemorrhoidalis, P. subdilatata, and G. lucasii.
We took advantage of the daily capture-mark-recapture scheme that took place between 9:30 AM and 4:00 PM between late April and mid-July 2011 (total individuals marked 1417) as described in Khelifa et al. (2016). Six researchers carried out captures of adults with a hand net across a transect of 2 km. Adults were marked on the hindwing using permanent markers (Edding paint marker 780). Thus, a large proportion of individuals were marked throughout the flight season, which facilitated behavioral surveys of roosting behavior. Using repeated resightings, we were able to estimate the lifespan of individuals as the number of days between the first capture and the last resight.
To assess the roosting behavior of C. exul, we selected a transect of 100 m where the largest number of individuals occurred. A prior behavioral survey on the reproduction of the species took place in the same location (Khelifa, 2019). Two observers performed all behavioral surveys (each observer surveyed 50 m). To locate individuals within the studied area, we segmented the transect into 10 m sections using flags. The location within each 10 m-section was estimated visually to the nearest meter. The spatial and temporal distribution of roosting behavior was surveyed in the early morning between 5:00 am and 09:00 am and in the late afternoon between 05:30 pm and 09:00 pm for seven days (18-27 May 2023). We carried out scans every 10 minutes across the 100 m transect where we recorded the time of the day, individual ID, sex, location within the transect, and vertical stratification on the perching site. To estimate the vertical height of perching and roosting sites, we provided gridded sticks at different parts of the transect which allowed an estimation to the nearest 1-5 cm. The perching height of unmarked individuals was also estimated to determine the vertical stratification (involving a total number of observations of marked and unmarked individuals of 2357 and 589, respectively). Uniquely marked individuals included 122 adults, involving 70 females and 52 males.
We used R 4.2.2 to perform our statistical analyses (R Development Core Team, 2023). All mixed effects models (LME) were carried out using lme4 (Bates et al., 2015). To assess sexual differences in the vertical and horizontal distribution of individuals across the watercourse, we carried out a two-sample Kolmogorov-Smirnov test. To determine the temporal change in vertical stratification in the morning and the evening, we used a repeated measure correlation using the rmcorr package (Bakdash and Marusich, 2017). To assess philopatry to roosting sites and vertical stratification, we also analyzed the repeatability of location across days using the rpt function of the rptR package (Stoffel et al., 2017) with an LME including only the random effects of individual ID and sampling date. To determine whether there is a difference in the vertical stratification of individuals at communal roosting sites between sexes, marked and unmarked individuals, and time, we conducted an LME with sex, marking (marked or unmarked), and time of the day as explanatory variables, the height of roosting individuals as a response variable, and individual ID and sampling date as random effects. To test whether lifespan was correlated with vertical stratification, we used a generalized mixed-effects model (with negative binomial errors) that includes lifespan as a response variable, average roosting height (mean across days) and sex as explanatory variables, and individual ID as a random effect. To determine whether the temporal pattern of roosting differed between sexes and temperatures, we conducted a generalized LME (with binomial errors) for each period (morning and evening) with the presence/absence of roosting behavior as a response variable, time, sex, and average daily temperature as explanatory variables, and individual ID and sampling date as random effects. We used DHARMa package (Hartig, 2022) to perform residual diagnostics and check for the assumptions of the mixed-effects models (Supplementary material). Values shown in the text are mean ± SD.
During the sampling period, adults occupied an average height of 65.6 ± 40.6 cm (n= 2946; range= 1-200 cm) strata. On average, males occupied significantly higher strata (70.0 ± 42.4 cm) than females (62.5 ± 39.0 cm) (two-sample Kolmogorov-Smirnov test: D = 0.09, P<0.0001) (Figure 2). Vertical stratification showed a temporal decline in the morning (from dawn to early morning) (r = -0.20 [95% -0.24 − -0.15], P <0.0001) whereas a temporal increase in the evening (from late afternoon to dusk) (r = 0.45 [95% 0.40−0.50], P <0.0001).
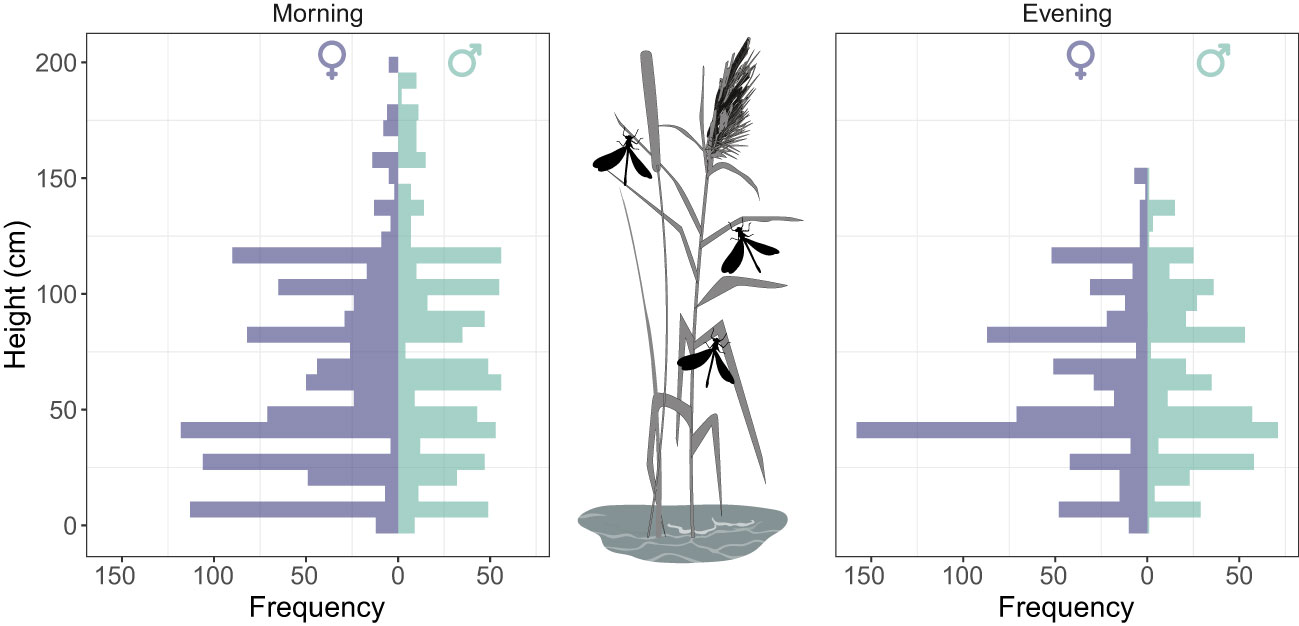
Figure 2 Vertical stratification of Calopteryx exul adult in roosting sites in early morning and evening in the Seybouse river, Northeast Algeria.
In roosting sites, when we consider only marked individuals, we found that there was no significant difference in vertical stratification between sexes (LME: χ² = 2.25, P=0.13) and across days (LME: χ² = 1.58, P=0.20). In fact, vertical stratification of roosting behavior showed a low but significant level of repeatability across days (R=0.13 [0-0.38], P = 0.008). Interestingly, there was a significant negative correlation between the average height of roosting and the lifespan of individuals (GLME: χ² = 4.54, P=0.03), revealing that individuals that had longer lifespans occupied lower strata (Figure 3).
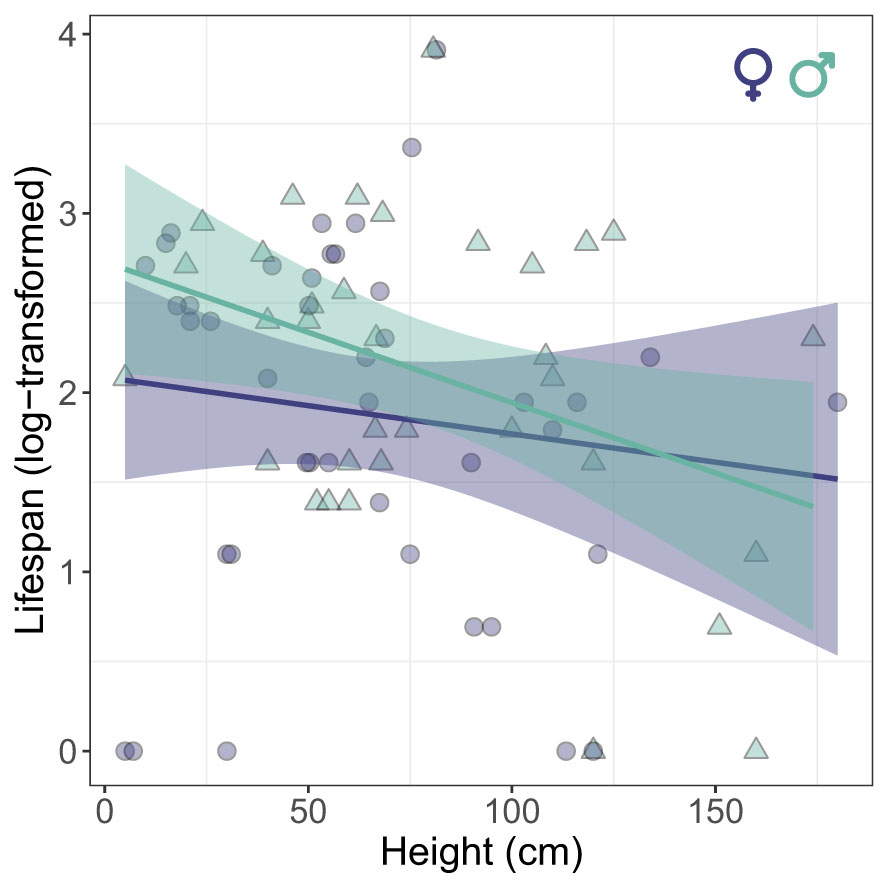
Figure 3 Relationship between the average height of vertical stratification at roosting sites and lifespan of Calopteryx exul adult in the Seybouse river, Northeast Algeria.
Roosting sites had a patchy horizontal distribution across the vegetated areas of the watercourse. Overall, males and females had a similar horizontal distribution of roosting sites in the evening (two-sample Kolmogorov-Smirnov test: D = 0.21, P=0.06) (Figure 4). Roosting site selection across the watercourse was significantly repeatable from one day to another (R=0.49 [0.03-0.77], P < 0.0001). Almost all individuals (94%) were recorded near the water with one individual (<1%) recorded roosting at 10 m away from the water.
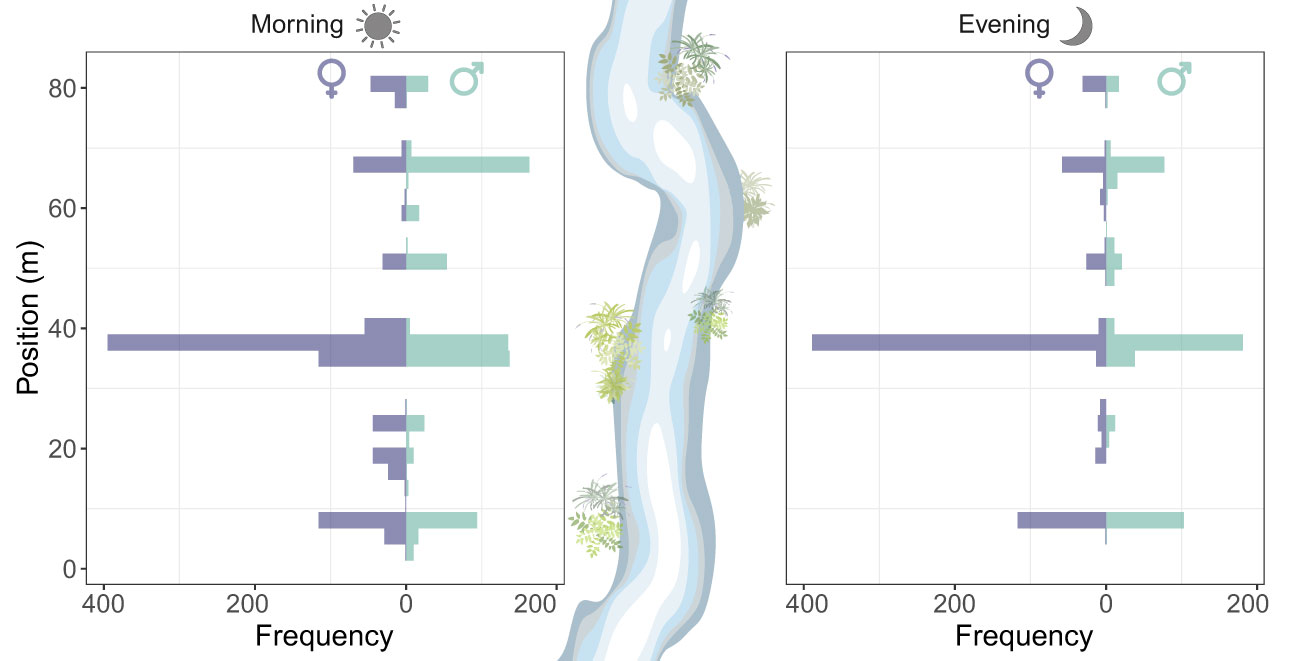
Figure 4 Horizontal distribution of the roosting sites of Calopteryx exul adult in early morning and evening in the studied stream in the Seybouse river, Northeast Algeria.
There was intraspecific variation in the timing of the beginning (in the evening) and ending (in the morning) of roosting. The proportion of roosting individuals increased gradually with time in the evening (LME: χ² = 58.8, P < 0.0001), and decreased in the morning (χ² = 88.4, P < 0.0001) (Figure 5), revealing a gradual chronological shift in the proportion of roosting individuals. The absence of time-by-sex interaction in the morning indicates that males and females had similar timing of roosting (χ² = 0.03, P=0.84), but in the evening the rate of roosting was slightly but not significantly faster in females than males (χ² = 0.71, P=0.39). Timing of roosting interacted significantly with the average temperature of the day in the morning (χ² = 58.1, P < 0.0001) and the evening (χ² = 32.6, P< 0.0001), revealing that individuals ceased the roosting posture earlier in the morning and started the roosting posture later in the evening in warmer days (Figure 5). There was a marginal negative correlation between the timing of roosting and lifespan in males but not in females, as revealed by the marginal interaction of lifespan and sex (χ² = 3.19, P = 0.08) (Figure 6).
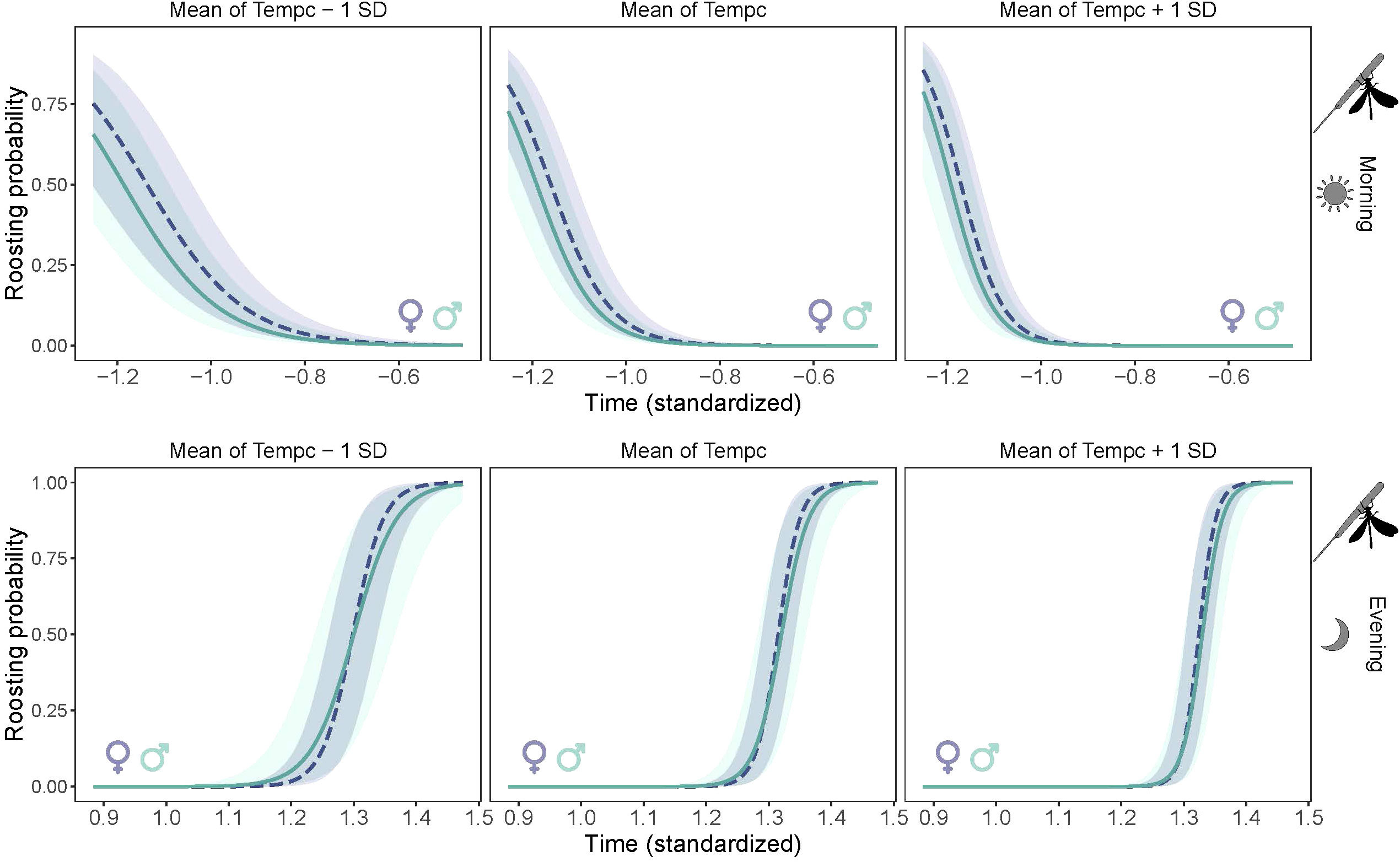
Figure 5 Temporal pattern of roosting (wing spreading posture) of Calopteryx exul adult at three levels of average daily temperature in early morning and evening in the Seybouse river, Northeast Algeria.
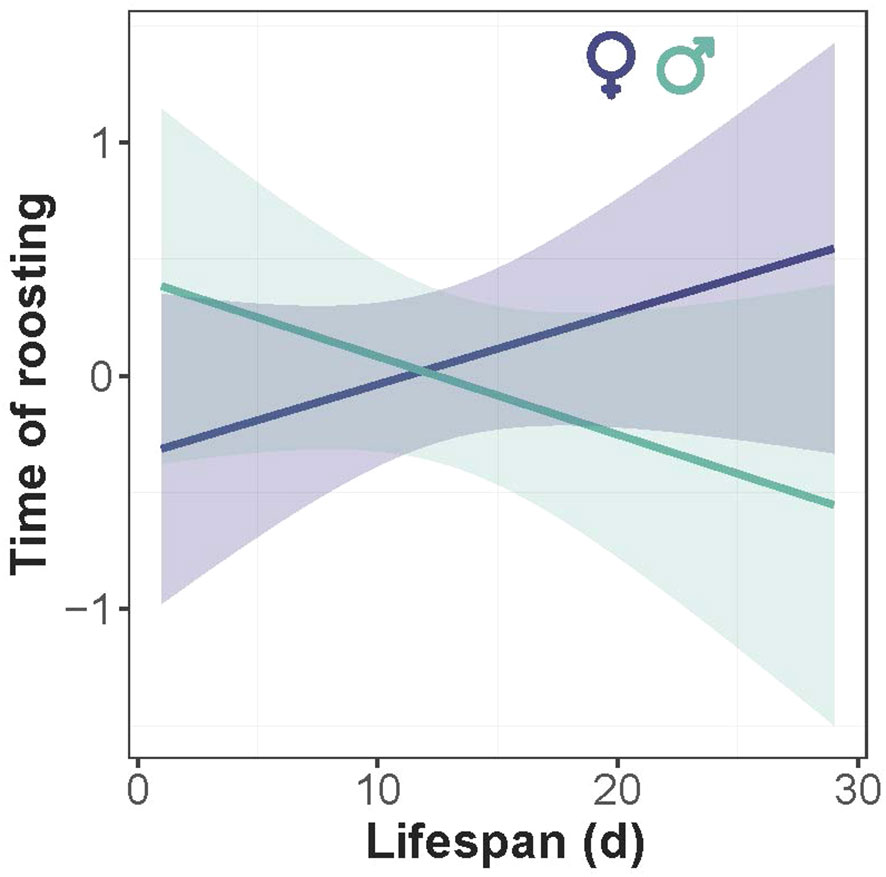
Figure 6 Relationship between the timing of roosting and lifespan of Calopteryx exul in the Seybouse river, Northeast Algeria. The fitted lines are predicted values from the linear mixed-effects model.
Although communal roosting behavior in odonates has been documented in both damselflies (Grether and Switzer, 2000; Rouquette and Thompson, 2007) and dragonflies (Miller, 1989), the study of its vertical stratification and timing and its potential fitness consequences has not attracted much research attention. In this study, we investigated the roosting behavior of C. exul, an endangered endemic damselfly that lives in lotic habitats of North Africa. Our behavioral survey showed that 1) roosting sites were restricted to small areas in the watercourse; 2) adults were philopatric to roosting sites but less so to vertical stratification; 3) vertical stratification and timing (marginal) of roosting were negatively correlated with lifespan; and 4) temperature of the day influenced the timing of roosting. This is the first study that investigates the beginning of roosting behavior was marginally correlated with individual lifespan.
In our study species, the non-random horizontal distribution of individuals suggests that the species exhibits site preferences for communal roosting. These roosting areas were relatively dense vegetated areas on the banks of the watercourse, which likely provided shelter against predators and resilience to withstand adverse nocturnal weather conditions (wind and rain) (Rouquette and Thompson, 2007; Hykel et al., 2018). Concordant with our hypothesis of site fidelity, we found that individuals were philopatric to roosting sites, which is similar to other species of Calopterygidae displaying comparable communal roosting behavior (Grether and Switzer, 2000). Grether and Switzer (2000) suggest that the location of the roosts is, at least partly, socially learned (i.e., traditional rather than habitat-related). In an experimental study on philopatry to roosting sites in a harvestman, Grether and Donaldson (2007) found that past communal usage was the best predictor of roosting site selection despite the occurrence of other similar sites (Grether and Donaldson, 2007). In many insects and other animal groups, philopatry is suggested to have evolved because of its fitness benefits (Stacey and Ligon, 1991; Hendry et al., 2004), which include familiarity with the environmental conditions including competition and predation risks, and minimization of the time and energy allocated to searching new locations (Switzer, 1993). Those benefits could also explain the occurrence of philopatry of odonate adults not only to roosting sites, but also to reproductive and emergence sites (Dolný et al., 2013).
In addition to selecting a particular location of roosting sites, adults of C. exul were also selective of vertical strata. The similarity in the vertical stratification between males and females (absence of sexual segregation) and the absence of mating attempts strengthens the hypothesis that vertical stratification is not directly related to mating (Grether and Switzer, 2000). There are many studies that show intraspecific and interspecific variation in the vertical distribution of adults during the active period of the day (Worthen and Jones, 2006; Worthen and Morrow, 2016), as well as larvae during emergence (Cordero, 1995; Hadjoudj et al., 2014). These studies suggest that the selection of heights has many fitness implications, including avoidance of predators, coping with harsh weather conditions, reducing competition, and exposure to sunlight (Switzer and Grether, 2000; Khelifa et al., 2013). Some of these benefits may apply to the vertical stratification of C. exul at roosting sites. In particular, roosting in specific strata may allow individuals to receive the first sunlight in the morning (Switzer and Grether, 2000), which could prolongate their hunting period. Importantly, our results support the hypothesis that lower strata likely provided better ecological conditions to roosting adults. In fact, we found a negative correlation between lifespan and vertical stratification of individuals at roosting sites, suggesting survival costs related to the selection of higher strata. It is likely that individuals that roosted in higher strata were more conspicuous to predators and thus more exposed to higher rates of mortality. This hypothesis goes in line with studies showing that more conspicuous individuals of Calopterygids are more often predated by birds (Svensson and Friberg, 2007). Further studies need to be conducted to assess whether the benefits of vertical stratification is condition-dependent, that is, lower strata could be less beneficial in some sites compared to others due to differences in predation or interspecific competition.
Unlike other species of Calopterygidae (Grether and Switzer, 2000), where foraging and roosting sites are distinct patches of vegetation, we found that roosting sites were often used as foraging sites for both males and females of C. exul. In the same roosting area, foraging was carried out prior to roosting in the evening and after becoming active in early morning, similar to other Calopterygidae (Switzer and Grether, 2000). However, the vertical strata used for both activities were quite different. As indicated by the temporal decline of vertical stratification in the evening and its gradual increase in the morning, the roosting strata were often lower than the foraging strata in C. exul. This might be a behavioral strategy to not only maximize foraging success at higher strata prior to or after roosting, but also reduce detectability and avoid predation at night during roosting. Interestingly, a high proportion of individuals concentrated near the water when roosting although there were vegetated areas farther from the water. Unlike other odonate species that roost away from the water (Rouquette and Thompson, 2007; Hykel et al., 2018), the nocturnal aggregation of individuals near the water renders the bank aquatic plants vital resources for feeding, resting, roosting, and emergence for C. exul.
Another finding in our study was that the average temperature of the day influenced the timing of roosting. On warmer days, both males and females started roosting later in the evening and became active earlier in the morning. As an ectotherm, damselfly activity is highly dependent on temperature (Angilletta, 2009), such that as body temperature increases earlier in the morning, individuals become active earlier (May, 1979). On warmer days, individuals also roosted later in the day probably because they spend more time mating and foraging. It is important to note that, since our survey was carried out in the spring, warmer days did not involve extreme heat. A similar influence of weather conditions on the timing of communal roosting behavior was recorded in a social bird (cattle egret) (Youcefi et al., 2019). This finding suggests that climate change could influence the diel pattern of activity and spatial distribution of the studied species (Yang et al., 2021).
Our results showed a marginal negative correlation between the timing of roosting and lifespan in males but not in females. It is likely that the temporal variation in roosting suggests competition for space at roosting sites. It is well known that high-quality males arrive earlier in breeding sites in birds and other taxa (Kokko, 1999; Morrison et al., 2019). If this hypothesis is true, we believe that such competition is more scramble than interference competition due to the rare intraspecific interactions between individuals at roosting sites. This is unlike the interspecific competition for space in perching sites usually recorded in different odonate assemblages where larger species tend to exclude smaller species from preferred habitats (Worthen and Jones, 2006; Worthen and Morrow, 2016). An alternative explanation is that, since foraging often precedes roosting, it is likely that individuals who are more fit (better at surviving longer) are more effective at foraging, thus roosting earlier than other individuals. Further studies are needed to shed light on the mechanisms underlying the negative relationship between the timing of roosting and fitness.
Our study presented some limitations due to the technical difficulty of performing field observations early in the morning and in the evening. Due to the rarity of the species and security issues, we could not perform the behavioral survey on multiple sites and throughout the entire season. We did not measure morphological traits such as body size and territoriality to determine their implication in vertical stratification and roosting behavior. Nevertheless, we believe that our fitness measure (lifespan) is a good proxy for quality because it captures the ability of individuals to survive and live longer. Further studies on roosting behavior need to explore potential behavioral variations of roosting across space and time.
Our results on the potential fitness consequences of roosting microhabitat selection highlight the importance of investigating roosting behavior and ecology. The dual function of roosting sites as both foraging and roosting areas makes them vital habitat elements and likely key criteria in habitat selection for C. exul, and other odonates. Studies have highlighted the pivotal role that bank vegetation and hydrophytes provide for the reproduction of damselflies (Oliveira-Junior et al., 2017), including C. exul (Khelifa, 2013; Mellal et al., 2018). Our results, together with the existing literature, highlight the importance of understanding the function of the bank vegetation of aquatic habitats (Guillermo-Ferreira and Del-Claro, 2011; Vilenica et al., 2022) and the ecological implications of its degradation on the aquatic community and ecosystem functioning (Da Silva Monteiro Júnior et al., 2013). The conservation plan for threatened species such as C. exul should not only focus on reproductive sites but also consider roosting sites as integral parts of the habitat requirements for the species (Rouquette and Thompson, 2007; Hykel et al., 2018). The conservation of these habitats probably has an umbrella effect on a diversity of terrestrial and aquatic taxa, thus promoting biodiversity and protecting the integrity of ecosystems. Future studies need to investigate the interplay between reproductive behavior, habitat preferences, foraging success, intrinsic traits, and lifetime mating success.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/s/b49f4f90592f2628def9.
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements.
HM: Data curation, Writing – original draft, Writing – review & editing, Formal Analysis, Visualization. RZ: Data curation, Writing – review & editing, Conceptualization, Investigation. HA: Data curation, Investigation, Writing – review & editing. SB: Data curation, Investigation, Writing – review & editing. AZ: Investigation, Writing – review & editing. AY: Data curation, Investigation, Writing – review & editing. RK: Writing – review & editing, Conceptualization, Data curation, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was conducted in the capacity of a Postdoctoral Research Fellow (project: P400PB_191139) awarded by the Swiss National Science Foundation (SNSF).
Thanks to the reviewers for their helpful comments and suggestions. We thank many students from the University of Guelma who helped us with the marking of damselflies. We are grateful to Nasr-Eddine Sakrane for providing transportation to and from the field site.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1267705/full#supplementary-material
ABHCSM (2009). Qualité des eaux superficielles dans les bassins du Kebir-Rhumel, de la Seybouse et de la Medjerda-Mellegue 2004–2007. Agence bassin hydrographique Constantinois-Seybouse-Mellegue. N° 12.
Angilletta M. J. (2009). Thermal adaptation: a theoretical and empirical synthesis (Oxford: Oxford University Press).
Bakdash J. Z., Marusich L. R. (2017). Repeated measures correlation. Front. Psychol. 8, 456. doi: 10.3389/fpsyg.2017.00456
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beauchamp G. (1999). The evolution of communal roosting in birds: origin and secondary losses. Behav. Ecol. 10, 675–687. doi: 10.1093/beheco/10.6.675
Bijleveld A. I., Egas M., Van Gils J. A., Piersma T. (2010). Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119, 277–285. doi: 10.1111/j.1600-0706.2009.17892.x
Boudot J.-P. (2018). Calopteryx exul. The IUCN red list of threatened species 2018, e.T60287A72725790. doi: 10.2305/IUCN.UK.2018-2301.RLTS.T60287A72725790.en
Burlakova L. E., Karatayev A. Y., Karatayev V. A., May M. E., Bennett D. L., Cook M. J. (2011). Endemic species: contribution to community uniqueness, effect of habitat alteration, and conservation priorities. Biol. Conserv. 144, 155–165. doi: 10.1016/j.biocon.2010.08.010
Carmona E. C., Musarella C. M., Ortiz A. C. (2019). Endemic Species (London, United Kingdom: BoD–Books on Demand).
Chelli A., Zebsa R., Khelifa R. (2019). Discovery of a new population of the endangered Calopteryx exul in central North Algeria (Odonata: Calopterygidae). Notulae Odonatol. 9, 150–154. doi: 10.5281/zenodo.3539748
Cordero A. (1995). Vertical stratification during emergence in odonates. Notulae Odonatol. 4, 103–105. Available at: https://natuurtijdschriften.nl/pub/593562/NOIOS1995004006004.pdf.
Cordero-Rivera A., Stoks R. (2008). “Mark-recapture studies and demography,” in Dragonflies and damselflies: Model organisms for ecological and evolutionary research. Ed. Córdoba-Aguilar A. (Oxford: Oxford University Press), 7–20.
Da Silva Monteiro Júnior C., Couceiro S. R. M., Hamada N., Juen L. (2013). Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int. J. Odonatol. 16, 135–144. doi: 10.1080/13887890.2013.764798
Deacon C., Samways M. J., Pryke J. S. (2020). Determining drivers of dragonfly diversity patterns and the implications for conservation in South Africa. Biol. Conserv. 245, 108548. doi: 10.1016/j.biocon.2020.108548
Deng Y., Belotti M. C. T., Zhao W., Cheng Z., Perez G., Tielens E., et al. (2023). Quantifying long-term phenological patterns of aerial insectivores roosting in the Great Lakes region using weather surveillance radar. Global Change Biol. 29, 1407–1419. doi: 10.1111/gcb.16509
Dolný A., Mižičová H., Harabiš F. (2013). Natal philopatry in four European species of dragonflies (Odonata: Sympetrinae) and possible implications for conservation management. J. Insect Conserv. 17, 821–829. doi: 10.1007/s10841-013-9564-x
Dwyer J. F., Fraser J. D., Morrison J. L. (2018). Evolution of communal roosting: A social refuge–territory prospecting hypothesis. J. Raptor Res. 52, 407–419. doi: 10.3356/JRR-17-101.1
Elafri A. (2022). New records of the endangered Calopteryx exul in a semi-arid territory of north-eastern Algeria (Odonata: Calopterygidae). Notulae Odonatol. 9, 451–454. doi: 10.60024/nodo.v9i9.a8
Finkbeiner S. D. (2014). Communal roosting in Heliconius butterflies (Nymphalidae): Roost recruitment, establishment, fidelity, and resource use trends based on age and sex. J. Lepidopterists’ Soc. 68, 10–16. doi: 10.18473/lepi.v68i1.a2
Foster S., Soluk D. (2006). Protecting more than the wetland: the importance of biased sex ratios and habitat segregation for conservation of the Hine’s emerald dragonfly, Somatochlora hineana Williamson. Biol. Conserv. 127, 158–166. doi: 10.1016/j.biocon.2005.08.006
Grether G. F., Donaldson Z. R. (2007). Communal roost site selection in a neotropical harvestman: habitat limitation vs. tradition. Ethology 113, 290–300. doi: 10.1111/j.1439-0310.2006.01328.x
Grether G. F., Switzer P. V. (2000). Mechanisms for the formation and maintenance of traditional night roost aggregations in a territorial damselfly. Anim. Behav. 60, 569–579. doi: 10.1006/anbe.2000.1511
Guillermo-Ferreira R., Del-Claro K. (2011). Oviposition site selection in Oxyagrion microstigma Selys 1876 (Odonata: Coenagrionidae) is related to aquatic vegetation structure. Int. J. Odonatol. 14, 275–279. doi: 10.1080/13887890.2011.621109
Hadjoudj S., Khelifa R., Guebailia A., Amari H., Hadjadji S., Zebsa R., et al. (2014). Emergence ecology of Orthetrum cancellatum: temporal pattern and microhabitat selection (Odonata: Libellulidae). Annales la Société Entomologique France (NS) 50, 343–349. doi: 10.1080/00379271.2014.938941
Hallmann C. A., Ssymank A., Sorg M., De Kroon H., Jongejans E. (2021). Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. 118, e2002554117. doi: 10.1073/pnas.2002554117
Hartig F. (2022). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models (R package version 0.4.6). Available at: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html.
Hendry A. P., Castric V., Kinnison M. T., Quinn T. P., Hendry A., Stearns S. (2004). “The evolution of philopatry and dispersal,” in Evolution illuminated: salmon and their relatives (New York: Oxford University Press), 52–91.
Hykel M., Harabiš F., Dolný A. (2018). Diel changes in habitat use by dragonflies: Nocturnal roosting site selection by the threatened dragonfly Sympetrum depressiusculum (Odonata: Libellulidae). Entomol. Sci. 21, 154–163. doi: 10.1111/ens.12292
Kaunisto K. M., Roslin T., Forbes M. R., Morrill A., Sääksjärvi I. E., Puisto A. I., et al. (2020). Threats from the air: Damselfly predation on diverse prey taxa. J. Anim. Ecol. 89, 1365–1374. doi: 10.1111/1365-2656.13184
Khelifa R. (2013). Flight period, apparent sex ratio and habitat preferences of the Maghribian endemic Calopteryx exul Selys 1853 (Odonata: Zygoptera). Rev. d’Ecologie (La Terre La Vie) 68, 37–45. doi: 10.3406/revec.2013.1674
Khelifa R. (2017). Partial bivoltinism and emergence patterns in the North African endemic damselfly Calopteryx exul: conservation implications. Afr. J. Ecol. 55, 145–151. doi: 10.1111/aje.12332
Khelifa R. (2019). Females A’ssist’ sneaker males to dupe dominant males in a rare endemic damselfly: sexual conflict at its finest. Ecology 100, e02811. doi: 10.1002/ecy.2811
Khelifa R., Mahdjoub H., Baaloudj A., Cannings R. A., Samways M. J. (2021). Effects of both climate change and human water demand on a highly threatened damselfly. Sci. Rep. 11, 7725. doi: 10.1038/s41598-021-86383-z
Khelifa R., Mellal M. K. (2017). Host-plant-based restoration as a potential tool to improve conservation status of odonate specialists. Insect Conserv. Diversity 10.2, 151–160. doi: 10.1111/icad.12212
Khelifa R., Zebsa R., Amari H., Houhamdi M., Khalil Mellal M., Mahdjoub H., Kahalerras A. (2016). A hotspot for threatened Mediterranean odonates in the Seybouse River (Northeast Algeria): are IUCN population sizes drastically underestimated? Int. J. Odonatol. 19, 1–11.
Khelifa R., Youcefi A., Kahlerras A., Al Farhan A., Al-Rasheid K. A., Samraoui B. (2011). L’odonatofaune (Insecta: Odonata) du bassin de la Seybouse en Algérie: intérêt pour la biodiversité du Maghreb. Rev. d’écologie 66, 55–66. Available at: https://hal.science/hal-03530643.
Khelifa R., Zebsa R., Amari H., Mellal M. K. (2013). Does wind affect emergence site selection in Odonata? Afr. Entomol. 21, 383–387. Available at: https://hdl.handle.net/10520/EJC141291
Kietzka G. J., Pryke J. S., Gaigher R., Samways M. J. (2021). Webs of well-designed conservation corridors maintain river ecosystem integrity and biodiversity in plantation mosaics. Biol. Conserv. 254, 108965. doi: 10.1016/j.biocon.2021.108965
Kokko H. (1999). Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. doi: 10.1046/j.1365-2656.1999.00343.x
Laughlin A. J., Sheldon D. R., Winkler D. W., Taylor C. M. (2014). Behavioral drivers of communal roosting in a songbird: a combined theoretical and empirical approach. Behav. Ecol. 25, 734–743. doi: 10.1093/beheco/aru044
Majewska A. A., Altizer S. (2020). Planting gardens to support insect pollinators. Conserv. Biol. 34, 15–25. doi: 10.1111/cobi.13271
Martín R., Maynou X. (2016). Dragonflies (Insecta: Odonata) as indicators of habitat quality in Mediterranean streams and rivers in the province of Barcelona (Catalonia, Iberian Peninsula). Int. J. Odonatol. 19, 107–124. doi: 10.1080/13887890.2016.1172991
May M. L. (1979). Insect thermoregulation. Annu. Rev. Entomol. 24, 313–349. doi: 10.1146/annurev.en.24.010179.001525
Mellal M. K., Bensouilah M., Houhamd M., Khelifa R. (2018). Reproductive habitat provisioning promotes survival and reproduction of the endangered endemic damselfly Calopteryx exul. J. Insect Conserv. 22, 563–570. doi: 10.1007/s10841-018-0085-5
Miller P. (1989). Communal roosting in Potamarcha congener (Rambur) and its possible functions (Anisoptera: Libellulidae). Odonatologica 18, 179–194.
Morrison C. A., Alves J. A., Gunnarsson T. G., Þórisson B., Gill J. A. (2019). Why do earlier-arriving migratory birds have better breeding success? Ecol. Evol. 9, 8856–8864. doi: 10.1002/ece3.5441
Neubauer K., Rehfeldt G. (1995). Roosting site selection in the damselfly species Calopteryx haemorrhoidalis (Odonata: Calopterygidae). Entomol. Generalis 19, 291–302. doi: 10.1127/entom.gen/19/1995/291
Oliveira-Junior J. M. B. D., De Marco P., Dias-Silva K., Leitão R. P., Leal C. G., Pompeu P. S., et al. (2017). Effects of human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon basin streams. Limnologica 66, 31–39. doi: 10.1016/j.limno.2017.04.007
Outhwaite C. L., Mccann P., Newbold T. (2022). Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 605, 97–102. doi: 10.1038/s41586-022-04644-x
Raven P. H., Wagner D. L. (2021). Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. 118. doi: 10.1073/pnas.2002548117
R Development Core Team (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing).
Rouquette J. R., Thompson D. J. (2007). Roosting site selection in the endangered damselfly, Coenagrion mercuriale, and implications for habitat design. J. Insect Conserv. 11, 187–193. doi: 10.1007/s10841-006-9030-0
Samways M. J., Barton P. S., Birkhofer K., Chichorro F., Deacon C., Fartmann T., et al. (2020). Solutions for humanity on how to conserve insects. Biol. Conserv. 242, 108427. doi: 10.1016/j.biocon.2020.108427
Samways M. J., Mcgeoch M. A., New T. R. (2010). Insect conservation: a handbook of approaches and methods (Oxford: Oxford University Press).
Shipley J. R., Gossner M. M., Rigling A., Krumm F. (2023). Conserving forest insect biodiversity requires the protection of key habitat features. Trends Ecol. Evol. 38, 788–791. doi: 10.1016/j.tree.2023.05.015
Stacey P. B., Ligon J. D. (1991). The benefits-of-philopatry hypothesis for the evolution of cooperative breeding: variation in territory quality and group size effects. Am. Nat. 137, 831–846. doi: 10.1086/285196
Stoffel M. A., Nakagawa S., Schielzeth H. (2017). rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Svensson E. I., Friberg M. (2007). Selective predation on wing morphology in sympatric damselflies. Am. Nat. 170, 101–112. doi: 10.1086/518181
Switzer P. V. (1993). Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 7, 533–555. doi: 10.1007/BF01237820
Switzer P. V., Grether G. F. (2000). Characteristics and possible functions of traditional night roosting aggregations in rubyspot damselflies. Behaviour 137, 401–416. doi: 10.1163/156853900502141
Teng B., Dao S., Donaldson Z. R., Grether G. F. (2012). New communal roosting tradition established through experimental translocation in a Neotropical harvestman. Anim. Behav. 84, 1183–1190. doi: 10.1016/j.anbehav.2012.08.022
Turner J. (1975). Communal roosting in relation to warning colour in two heliconiine butterflies (Nymphalidae). J. Lepidopterists Soc. 29, 221–226.
Uhler J., Redlich S., Zhang J., Hothorn T., Tobisch C., Ewald J., et al. (2021). Relationship of insect biomass and richness with land use along a climate gradient. Nat. Commun. 12, 1–9. doi: 10.1038/s41467-021-26181-3
Van Klink R., Bowler D. E., Gongalsky K. B., Swengel A. B., Gentile A., Chase J. M. (2020). Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420. doi: 10.1126/science.aax9931
Vilenica M., Rebrina F., Matoničkin Kepčija R., Šegota V., Rumišek M., Ružanović L., et al. (2022). Aquatic macrophyte vegetation promotes taxonomic and functional diversity of odonata assemblages in intermittent karst rivers in the Mediterranean. Diversity 14, 31. doi: 10.3390/d14010031
Vulinec K. (1990). “Collective security: aggregation by insects as a defense,” in Insect defenses (Albany, NY: State University of New York Press), 251–288.
Wagner D. L. (2020). Insect declines in the Anthropocene. Annu. Rev. Entomol. 65, 457–480. doi: 10.1146/annurev-ento-011019-025151
Worthen W. B., Jones C. M. (2006). Relationships between body size, wing morphology, and perch height selection in a guild of Libellulidae species (Odonata). Int. J. Odonatol. 9, 235–250. doi: 10.1080/13887890.2006.9748281
Worthen W. B., Morrow P. H. (2016). Perch selection by three Cooccurring species of Celithemis (Odonata: Libellulidae): testing for a competitive hierarchy among similar species. Psyche: A J. Entomol. 2016, 9028105. doi: 10.1155/2016/9028105
Yang L. H., Postema E. G., Hayes T. E., Lippey M. K., Macarthur-Waltz D. J. (2021). The complexity of global change and its effects on insects. Curr. Opin. Insect Sci. 47, 90–102. doi: 10.1016/j.cois.2021.05.001
Keywords: habitat preferences, resting, odonates, dragonflies, insects, Calopteryx exul
Citation: Mahdjoub H, Zebsa R, Amari H, Bensouilah S, Zouaimia A, Youcefi A and Khelifa R (2023) Potential fitness consequences of roosting spatiotemporal selection in an endangered endemic damselfly: conservation implications. Front. Ecol. Evol. 11:1267705. doi: 10.3389/fevo.2023.1267705
Received: 27 July 2023; Accepted: 09 October 2023;
Published: 26 October 2023.
Edited by:
Helen McCreery, Tufts University, United StatesReviewed by:
Adolfo Cordero-Rivera, University of Vigo, SpainCopyright © 2023 Mahdjoub, Zebsa, Amari, Bensouilah, Zouaimia, Youcefi and Khelifa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rassim Khelifa, cmFzc2ltLmtoZWxpZmFAY29uY29yZGlhLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.