- 1Department of Microbiology, Oregon State University, Corvallis, OR, United States
- 2Fisheries Department, Hoopa Valley Tribe, Hoopa, CA, United States
- 3Yurok Tribe, Klamath, CA, United States
- 4Fish Health Services, Oregon Department of Fish and Wildlife, Corvallis, OR, United States
- 5California-Nevada Fish Health Center, United States Fish and Wildlife Service, Anderson, CA, United States
- 6Natural Resources Department, The Klamath Tribes, Chiloquin, OR, United States
- 7Oregon Department of Fish and Wildlife, Klamath Falls, OR, United States
- 8Fisheries Department, Yurok Tribe, Klamath, CA, United States
- 9Arcata Fish and Wildlife Office, Fish and Aquatic Conservation, United States Fish and Wildlife Service, Arcata, CA, United States
- 10Department of Fisheries Biology, California Polytechnic University, Humboldt, Arcata, CA, United States
- 11Department of Natural Resources, Karuk Tribe, Orleans, CA, United States
- 12National Oceanic and Atmospheric Administration (NOAA) Fisheries, U.S. Department of Commerce, Santa Cruz, CA, United States
- 13Oregon Department of Fish and Wildlife, Bend, OR, United States
The health of fish populations and the river systems they inhabit have broad ecological, cultural, recreational, and economic relevance. This is exemplified by the iconic anadromous salmonid fishes native to the West Coast of North America. Salmon populations have been constrained since the mid nineteenth century by dam construction and water reallocation. In the Klamath River (Oregon and California, USA), a series of dams built in the early-mid 20th century cut the basin in two and blocked anadromous fish access to more than 600 river kilometers. This dramatic loss of habitat, coupled with infectious diseases and resulting epizootics, have impacted the wellbeing of these salmonid populations. In 2023-2024, the Klamath River will undergo the largest river restoration project in US history. Removal of the four lowermost dams will cause profound physical changes to the river, including flow, water temperature, and channel geomorphology. The dam removals will reconnect the lower and upper portions of the basin, and provide fish passage after a century of segregation. Reestablishment of upstream and downstream fish movements will also alter the occupancy and abundance of the salmonid hosts and their pathogens. The increased habitat availability and longer migration routes will increase duration of pathogen exposure and potential impacts on juvenile survival and adult pre-spawn mortality. However, restoration of more natural flow and sediment regimes will decrease overall fish disease risk by disrupting complex parasite life cycles. To better understand these multifarious, competing factors, we review the salmonid species in the Klamath River, and provide an overview of their historical pathogen challenges and associated diseases and use this as a framework to predict the effects of dam removals on disease dynamics. Our review and predictions are a synthesis of expertise from tribal biologists, fish health specialists and fish biologists, many of whom have lived and worked on the Klamath River for decades. We conclude with recommendations for expansion of current pathogen monitoring and research efforts to measure changes in host-pathogen dynamics basin-wide.
1 Introduction
We are on the cusp of the largest dam removal project in history - four large dams on the Klamath River, USA, will be removed in 2023-2024, with key goals being ecosystem restoration and the recovery of salmon populations. In addition to meeting indigenous cultural and subsistence needs, salmon restoration will have far-reaching ecological, recreational, and economic benefits.
The Klamath River dams blocked access to more than 600 river kilometers of habitat for anadromous fishes and severed the basin in two. Consequences included the extirpation of anadromous salmon populations upstream of the dams and a shift in the dominant life history in salmonid populations both upstream and downstream of the barriers. The removal of these dams and the coincident closure of a major mitigation hatchery below the dams will change the distribution, species composition and life-history diversity of salmon populations (Quiñones et al., 2015). The opening of new habitat in the tributaries and mainstem river upstream of the dams will restore many historical processes, habitat features and populations of anadromous fishes, which include ecologically and economically important Chinook Salmon (Oncorhynchus tshawytscha), Coho Salmon (O. kisutch) and Steelhead (anadromous O. mykiss). After dam removals, the basin will become a more varied and dynamic environment that will allow for reexpression of diverse salmonid life-history types, leading to more resilient, viable and self-sustaining populations with varied migration timings and growth patterns, as anadromous species disperse into the Hydroelectric Reach and upstream (Williams et al., 2006).
Physical changes in the environment will alter fish-pathogen interactions and disease risk. Dam removals will result in changes to river sediment transport and geomorphology, flow and temperature regimes and water quality which are expected to benefit ecosystem function and salmon health (e.g., Bellmore et al., 2019). While many of these changes are likely to be positive in the long term, there will also be some challenges. Here, we use these interactions as a framework for our analysis of how salmon disease risk will respond to dam removals on the Klamath River. There are few published studies examining how dam removal impacts fish disease risk. In a much smaller system, Manatawny Creek Dam (PA, USA), a decreased incidence of fish parasites in former impoundments was observed (Hart et al., 2002). Prior to the removal of two large dams on the Elwha River (WA, USA; in 2011 and 2014), a targeted pathogen survey assessed the risk of anadromous fish introducing pathogens to isolated upriver populations, and detected only Renibacterium salmoninarum in non-anadromous fishes (Brenkman et al., 2008). The Elwha system has not had any detections of routinely monitored viral pathogens, either endemic or exotic, prior to and since the dams have been removed (M. House, NW Indian Fisheries Commission, pers com.). While these scenarios provide some insights into potential outcomes following dam removals, these systems differ markedly from the Klamath River, both in terms of scale and pathogen concerns.
Klamath River salmon encounter and host a variety of freshwater pathogens, first as juveniles migrating downstream to the ocean and then as adults returning to spawning grounds. The myxozoan parasite Ceratonova shasta is a primary factor affecting salmon recovery in the Klamath River (Fujiwara et al., 2011; Ray et al., 2014) because of its impacts on juvenile salmon, by direct mortality or predation associated with disease morbidity. Long-term monitoring and research on this parasite have informed current fisheries management, and resulted in models that can inform predictions on the effects of dam removal on this disease (Ray et al., 2015). Thus, our predictions in this paper will largely focus on C. shasta. However, other pathogens present in the Basin also have the potential to cause serious disease. Although large epizootics in salmonids are rarely documented, in summer 2002 thousands of adult salmon died as a result of combined infections of the ciliate parasite Ichthyophthirius multifiliis and the bacterial pathogen Flavobacterium columnare (Belchik et al., 2004). We use available data on presence and characteristics of these, and other pathogens of concern in the Klamath River, to predict how host-pathogen interactions and associated risks may change following removal of the four hydroelectric dams and reconnection of the upper and lower basins.
Herein, we present an overview of the Klamath River and the changes that are predicted to occur following dam removals, and of the salmonid species of concern and their historical pathogen challenges. We consider the effects of dam removals on infection dynamics and disease occurrence of important fish pathogens in salmonids in the Klamath River, then offer informed predictions of these effects on fish health. We draw on a combination of Western Science and Traditional Ecological Knowledge to inform these predictions. Our process involved hybrid workgroup meetings and discussions in the context of available data, and relied on expert opinions to develop conceptual models for perceived risks under the context of dam removals. This assessment provides a foundation to guide monitoring programs and management plans, and to understand the associated benefits and risks to salmonid populations in the Klamath River basin following dam removals.
2 Klamath River and hydroelectric project before dam removals
The Klamath River flows more than 400 km through southern Oregon and northern California, on the west coast of the United States (Figure 1). As of 2023, greater than 80% of the upper basin, previously freshwater marshland, has been converted to land that supports farming, ranching, and the city of Klamath Falls. Upper Klamath Lake (UKL), the source of the Klamath River, is fed by the Williamson and Sprague rivers, which originate in the high desert of southern Oregon. Downstream from Klamath Lake the river flows through a series of six dams that provide water storage for irrigation and hydroelectric power, before entering the canyon that flows through the Klamath Mountains to the Pacific Ocean. In the lower basin the river is fed by major tributaries that include the Shasta, Scott, Salmon and Trinity rivers. Given the complex and contentious nature of surface water allocation in the Basin, Federal Regulators issued directions to attempt to balance water budgets for irrigation while conserving fish. These Biological Opinions specified flow release requirements (for dilution, surface and deep flushing flows) to reduce disease incidence for Coho Salmon downstream of Iron Gate Dam (NMFS and USFWS (National Marine Fisheries Service and US Fish and Wildlife Service), 2013; NMFS (National Marine Fisheries Service), 2019).
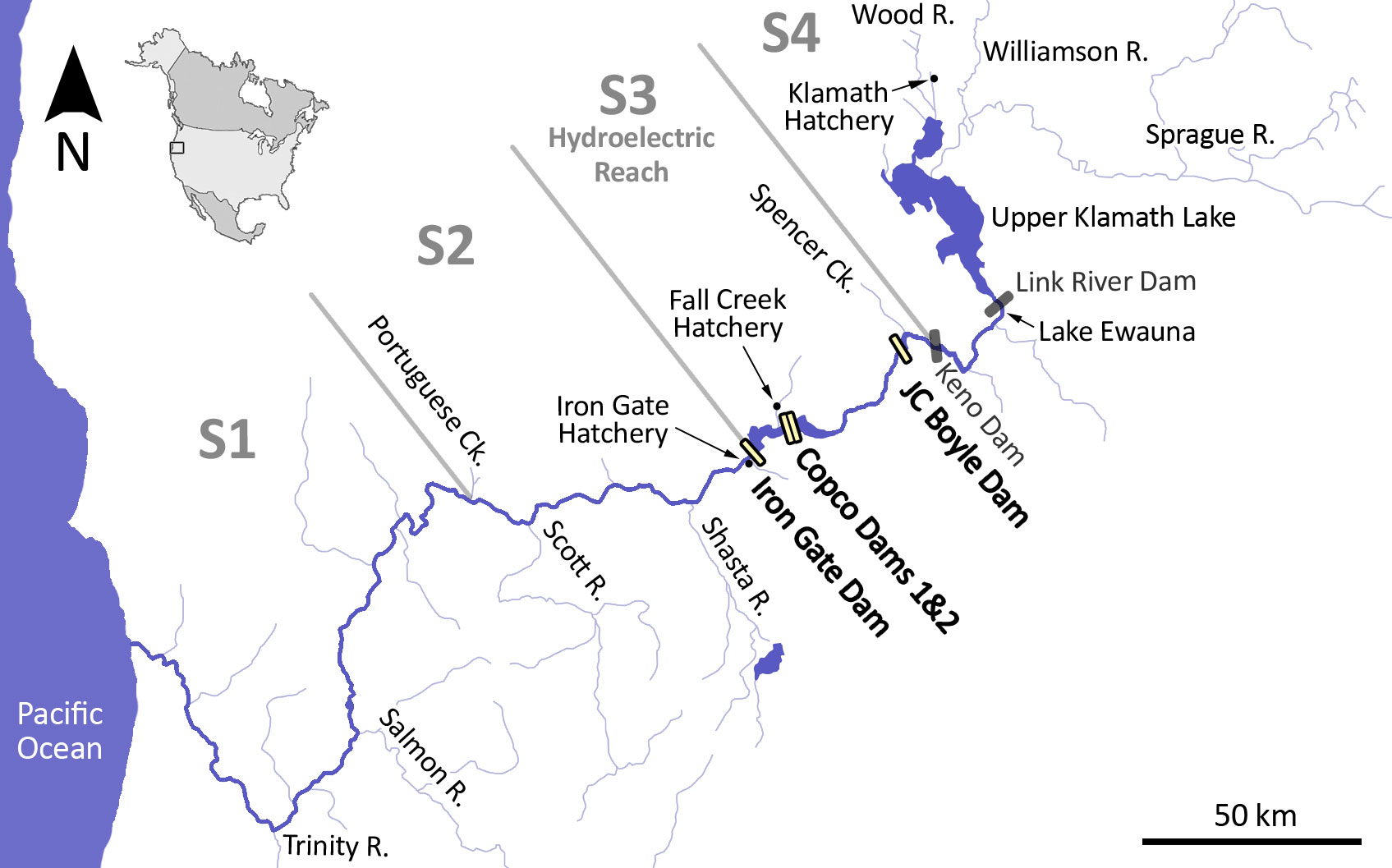
Figure 1 Map of the Klamath River Basin, showing major tributaries, features and sections mentioned in the text. Dams are shown as boxes; those with bold labels and outlines represent the four dams that are being removed. Fish ladders at Keno Dam and Link River Dam will allow upstream fish migration past those remaining barriers. Iron Gate Hatchery will be decommissioned with the removal of Iron Gate Dam; Fall-run Chinook Salmon and Coho Salmon production will be continued at Fall Creek Hatchery for up to eight years following dam removal. Klamath Fish Hatchery will be used for active reintroduction of Spring-run Chinook Salmon in the Upper Klamath Basin.
The Klamath River basin is home to multiple groups of indigenous peoples, including the Yurok, Karuk and Hoopa Valley tribes in the canyons of the lower basin, and the Klamath, Modoc and Yahooskin tribes in the upper basin. For thousands of years, people have harvested returning adult salmon and other aquatic resources that are central to their identity and existence. Western colonial expansion in the 19th and 20th centuries brought increased harvests of fish and timber, and hydrological alterations first for hydraulic mining for gold, then for agriculture and power generation. The Klamath Project, beginning in 1906, transformed the basin by diverting stream flows and converted marsh to agricultural use in the upper Klamath Basin.
Beginning in 1917, a series of dams was constructed between river kilometers (rkm) 305 and 409. These comprise the four lowermost mainstem dams that are being removed, Iron Gate, Copco I and II and JC Boyle, and two upstream dams, Keno and Link River, which will remain in place. The Link River Dam, at the mouth of Klamath Lake, stores most of the water used for irrigation of the Klamath Project and historically provided hydropower. Keno Dam impounds water to form Lake Ewauna, which supplies a limited amount of water for irrigation of adjacent land. In addition to these Project diversions, ongoing water withdrawals occur in all the major tributaries to the Klamath River.
In response to the impacts of the mainstem dams on anadromous salmon, in particular loss of access upstream of Copco I and II dams, a hatchery and rearing facility was established on Fall Creek (Figure 1). This facility ran from 1919-1948 as a hatchery, then up to early 2000s as rearing ponds. Completion of the furthest downstream and largest dam, Iron Gate, in 1961-1964, blocked all upstream movement of salmonids, including access to Fall Creek. Hence, Iron Gate Hatchery (1961-2023) was established to mitigate impacts of dam construction on Chinook Salmon and Coho Salmon. The hatchery, situated immediately below the dam at the congregation point for returning adults, was used to spawn these fishes, and then rear and release juveniles (yearlings and smolts) each spring and fall. Additionally, Trinity River Hatchery was constructed lower in the basin on the Trinity River to compensate for salmonid spawning habitat lost following the completion of Lewiston Dam in 1963.
3 Physical changes to the Klamath River with dam removal
The 50-year federal license for four of the Klamath River dams expired in 2006. The owner of these dams, PacifiCorp, entered into a Settlement Agreement that in 2016 resulted in transfer of ownership of the dams and related facilities to an independent nonprofit entity, the Klamath River Renewal Corporation. As a result of this agreement, the Corporation developed the schedule for dam removals in the 2018 Definite Plan Report, based on the assumptions and schedule outlined in the USBR analysis (U.S. Bureau of Reclamation [USBR], 2011; CSWRCB (California State Water Resources Control Board), 2018). Removal of the lowermost four Klamath River dams began in 2023 with deconstruction of Copco 2. Drawdown of the three reservoirs was scheduled for January-March 2024, which the Renewal Corporation identified as the period of least impact to aquatic species in the Klamath River downstream of Iron Gate Dam. By the end of 2024, all four dams will be removed, concurrent with instigation of restoration work, which will continue well beyond 2024.
Salmon disease risk is driven by a multitude of abiotic and biotic factors. The following sections detail the short- and long-term physical changes that dam removal will have on these factors. Abiotic effects that will alter salmon disease dynamics include changes to flow and temperature regimes, sediment transport, water quality, and re-establishment of connectivity. These changes will produce short- and long-term biotic responses.
3.1 Short-term changes
Short-term impacts of reservoir and dam removals will commence with reservoir draining (“drawdown”) starting January 2024 and continuing through approximately December 2024. The impacts we consider for salmon disease risk include immediate effects on river discharge, hereafter termed “flow,” temperature, release of trapped sediments, and water quality. We cover connectivity under long-term changes as fish movements in the short term will be disrupted.
3.1.1 Flow dynamics and thermal regime
Short-term alterations to flow and temperature regimes will result from the releases of water stored in the reservoirs during drawdown (planned for approximately January-March 2024), and following removal of physical structures (CSWRCB (California State Water Resources Control Board), 2018). During this period, water release patterns will be stable and low, with planned maximum rates corresponding to 3 - 15% of a peak flow magnitude that recurs every two years (or less than half of typical winter flows). Water temperatures are expected to be marginally warmer during drawdown due to the release of water from the reservoirs, if drawdown occurs on schedule (January-March 2024).
3.1.2 Sediment transport
Dam removals will result in short-term increases in suspended sediments and turbidity from the release of sediments trapped within the reservoirs. The estimated 12 million cubic meters of sediment accumulated in the reservoirs is comprised of inorganic particles including clay/silt (~85%), with sand (~12%), gravel and larger material (~3%) making up the remainder (Stillwater Sciences, 2010; U.S. Bureau of Reclamation [USBR] and California Department of Fish and Game [CDFG] (2012), CSWRCB (California State Water Resources Control Board), 2018). Mobilization and flushing of the majority of trapped sediments is expected to occur rapidly in the 4 to 8 months following reservoir drawdowns because of the dominance of fine particles. During the first year, sediment releases will bury mainstem salmonid spawning areas (e.g., Greig et al., 2005; Levasseur et al., 2006) and habitat for invertebrate hosts of parasites with complex life cycles (e.g., Doeg and Koehn, 1994). These effects will primarily occur in the 15 rkm downstream of Iron Gate Dam (Stillwater Sciences, 2008).
3.1.3 Water quality
The downstream transport of trapped sediment will also affect water quality over the short term. Released reservoir sediments will depress dissolved oxygen levels due to high concentration of organics (primarily derived from dead algae) in the reservoir sediments. The pulses of sediment that will co-occur with storm events until organic sediments are metabolized or flushed, will likely continue to drive periodic short-term dissolved oxygen sags (Stillwater Sciences, 2010).
3.2 Long-term changes
The long-term impacts of reservoir and dam removals include restored fish access (connectivity) to historical habitat upstream of Iron Gate Dam, and hydrograph, temperature, sediment transport and nutrient cycling dynamics that more closely resemble historical conditions.
3.2.1 Connectivity
Removal of the four lower Klamath River dams will reconnect the lower and upper basins, which have been divided for over a century. This provides the context for anadromous fish species’ long-term recovery and persistence (Ebersole et al., 1997; Williams and Reeves, 2003), and allows anadromous salmonids to use habitat that has been inaccessible for >100 years. These tributary and mainstem areas above the dams represent approximately 500 rkm, 125 rkm, and 700 rkm of historical habitat for Chinook Salmon, Coho Salmon, and Steelhead, respectively (Hamilton et al., 2005; Hamilton et al., 2011; Ramos and Ward, 2023). These habitats include Fall Creek, Shovel Creek, Spencer Creek, Big Springs, and their cool-water refugia (Hamilton et al., 2011).
3.2.2 Flow dynamics
In the Klamath River, dams altered the flow regime and decreased the magnitude, frequency, and duration of flooding events, and reduced flow variability. Dam removals will result in a flow regime more characteristic of historical pre-dam conditions, with greater intra- and inter-annual variability (Hardy et al., 2006). The greatest long-term changes in hydrology will occur in the reach comprising the four dams and associated reservoirs to be removed, and immediately downstream. The restoration of a more natural flow regime within sections currently subject to alteration (e.g., bypassing, hydropeaking and otherwise managed) will drive shifts in fish host distribution and abundance, and those of their pathogens.
Iron Gate Dam, completed in 1962 to re-regulate hydropeaking from the upriver Copco facilities, altered the timing, magnitude, and duration of downstream flows (peak and baseflow). Although Iron Gate Dam operations created a more natural hydrograph than one that hydropeaks daily, the resultant flow regime deviated from historical conditions. The outcome was higher discharge in fall, but significantly decreased discharge in spring and summer. The operations altered the timing of peak runoff and shifted the onset of baseflow at least two months earlier (in some years, baseflow began in March). These changes had important consequences for salmon disease. Dam removals will largely restore seasonality to the flow regime through tributary inflows and spring flow accretions (e.g., Big Springs, ~7 m3/s in the JC Boyle Bypass Reach).
3.2.3 Thermal regime
Temperature stratification occurs in large, deep reservoirs when the upper layer (epilimnion) warms and decreases in density, while cooler water remains on the bottom (hypolimnion). The release of water from the hypolimnion, which is common practice, decreases downstream temperature, particularly at lower flows (e.g., Petts, 1986). Two of the three reservoirs that will be restored to riverine habitats are large enough to have an effect on downstream water temperatures (Iron Gate and Copco). These reservoirs alter the timing of seasonal warming and cooling of river water temperatures (CSWRCB (California State Water Resources Control Board), 2018). For example, the largest of the reservoirs (Iron Gate), draws water from the hypolimnion, which delays warming in late winter and early spring. Thermal buffering from the reservoir also delays river water temperature cooling in fall, downstream.
Reservoir and dam removal are predicted to result in a 1 - 2.5°C increase in water temperatures during spring months and a 2 - 10°C decrease in water temperatures during the fall months (PacifiCorp, 2004; Dunsmoor and Huntington, 2006; North Coast Regional Board (North Coast Regional Water Quality Control Board), 2010; PacifiCorp, 2018). Elimination of the thermal lag caused by reservoirs will result in river temperatures consistent with those that historically co-occurred with salmon migration and spawning. The warmer spring temperatures will drive earlier fry emergence (Sykes et al., 2009), with potential consequences for disease risk. Fall-run Chinook Salmon spawning will gradually shift earlier and co-occur with cooler fall water temperatures, potentially reducing risks of pre-spawn mortality (Benda et al., 2015). In addition, the shift in discharge from thermally altered reservoirs to groundwater inputs (e.g., springs in the JC Boyle Bypass Reach are anticipated to account for 30 - 40% of the total summer discharge) will provide temperature relief for non-anadromous salmonids year-round, and in spring will benefit adult Spring-run Chinook Salmon during their migration. In addition to restoring a more natural thermal regime, dam removals will result in overall increases and diel variability in dissolved oxygen, and lower microbial oxygen demand due to decreased organic load.
3.2.4 Nutrient cycling and water quality
The upper Klamath River was naturally highly eutrophic, but damming and reservoir operations increased nutrient and organic loading and altered the temporal dynamics of nutrient cycling downstream (Asarian et al., 2009). Elevated nutrient levels stimulate the growth of periphyton (benthic algae), which serves as habitat for annelid hosts of salmon parasites. Similarly, species composition and densities of suspended algae and diatoms, which provide a food source for invertebrate hosts (e.g., annelid hosts of myxozoan parasites and snail hosts of trematodes), were elevated as a result of reservoirs.
The restoration of reservoir reaches to riverine habitat will decrease water residence time from several weeks to less than a day, resulting in reduction of primary productivity (including nuisance algae that produce toxic microcystin), and reduced settling of suspended particles. Reservoir and dam removals will also result in overall increases in dissolved oxygen due to aeration provided by a more dynamic river channel and shallower, more agradded bed and lower microbial oxygen demand, due to decreased organic load. The temporal dynamics of nutrient cycling will be more comparable to historical conditions.
3.2.5 Restored sediment transport and debris loading
Dam and reservoir removals should restore sediment dynamics to historical conditions. The greatest changes to sediment and debris transport will occur within and downstream of the Hydroelectric Reach (Figure 1). The Hydroelectric Reach lies within volcanic terrain, in a region characterized by being more bedrock-controlled and having lower rainfall and less sediment than below Iron Gate Dam (PacifiCorp, 2004; National Research Council, 2008). Stream gradients in this reach are higher than below the dams, but riverbed scour may be less frequent because of groundwater and springs in this river section relative to downstream river sections (PacifiCorp, 2004; PacifiCorp, 2018). Pool and riffle habitats, which are suitable for Fall-run Chinook Salmon, are abundant in the riverine sections of the Hydroelectric Reach (PacifiCorp, 2005; PacifiCorp, 2018). Following dam removals, the relative proportions of pool-riffle habitats are expected to remain similar, however the expansion of habitat to include areas previously flooded by reservoirs, will provide increased habitat not only for salmon but also for invertebrate hosts (e.g., Manayunkia occidentalis). In contrast, downstream of the location of Iron Gate Dam, the restored sediment input and transport following dam removals will create new gravel bars, a more heterogeneous and dynamic streambed, more suitable spawning habitat and reduced invertebrate hosts habitat.
3.3 Fish production and stocking changes
For over 50 years, Iron Gate Hatchery has reared and released up to 6 million salmonids annually (primarily Fall-run Chinook Salmon during late May-early June) to mitigate for Iron Gate Dam. Operations of this hatchery require Iron Gate Reservoir for adequate water supply. With removal of the dam, Iron Gate Hatchery will no longer be able to operate. To support fish production during the removal and transition to the cessation of hatchery operations, Fall Creek Hatchery will be updated to provide supplementation of Chinook Salmon and Coho Salmon for no more than eight years following dam removal. Preliminary numbers of Chinook Salmon smolts released from this facility will be lower than previous output from Iron Gate Hatchery, and release will be volitional, with one release occurring prior to March 31, and a second beginning May 1. Also, fewer yearling Coho Salmon and Chinook Salmon will be released mid-March to May and mid-October to mid-November, respectively (Klamath River Renewal Corporation and PacifiCorp, 2021).
The two remaining dams, Keno and Link River, have fish ladders to permit upstream migration, thus fish biologists expect volitional return of Coho Salmon upstream as far as Spencer Creek (inclusive), and Fall-run Chinook Salmon, Steelhead and Pacific Lamprey (Entosphenus tridentatus) upstream and into UKL and its headwater tributaries. Oregon plans to implement an active reintroduction program for Spring-run Chinook Salmon upstream of Link River Dam (Hereford et al., 2021), with the goal of re-establishing viable, self-sustaining populations that do not require hatchery supplementation. No changes are anticipated for Trinity River Hatchery production.
4 Scope and definitions used in this assessment
4.1 Salmonid species
Once the third most abundant salmon producing river in the US (excluding Alaska), Chinook Salmon (Spring- and Fall-run ecotypes) and Steelhead/Redband Trout (anadromous/non-anadromous O. mykiss) occurred throughout the Basin, including the tributaries of UKL1. Coho Salmon likely migrated as far upstream as Spencer Creek (rkm 366; and into Spencer Creek itself) (Hodge et al., 2016). Populations of Coastal Cutthroat Trout (O. clarkii clarkii) existed downstream of the location of Iron Gate Dam (Hamilton et al., 2005). Chum Salmon (O. keta) and Pink Salmon (O. gorbuscha) were observed in the lower portions of the Basin, but persistent populations were unlikely. Spring-run Chinook Salmon, believed to have once been the dominant Chinook Salmon life history in the Basin, have decreased in number by about 98% (Higgins et al., 1992). Coho Salmon are now ESA-listed as Threatened, and populations are at historically low levels (Olson, 1996; Federal Register, 1997). Fall-run Chinook Salmon runs decreased to an extent that prompted closure of the ocean commercial and sport fishing in 2008, 2009 and 2023 (CDFW- California Department of Fish and Wildlife- News, 2023). Herein, we focus on Chinook Salmon, Coho Salmon, and Steelhead/Redband Trout (Table 1; Figure 2); we do not discuss the other species because of their historically low numbers or limited distributions.
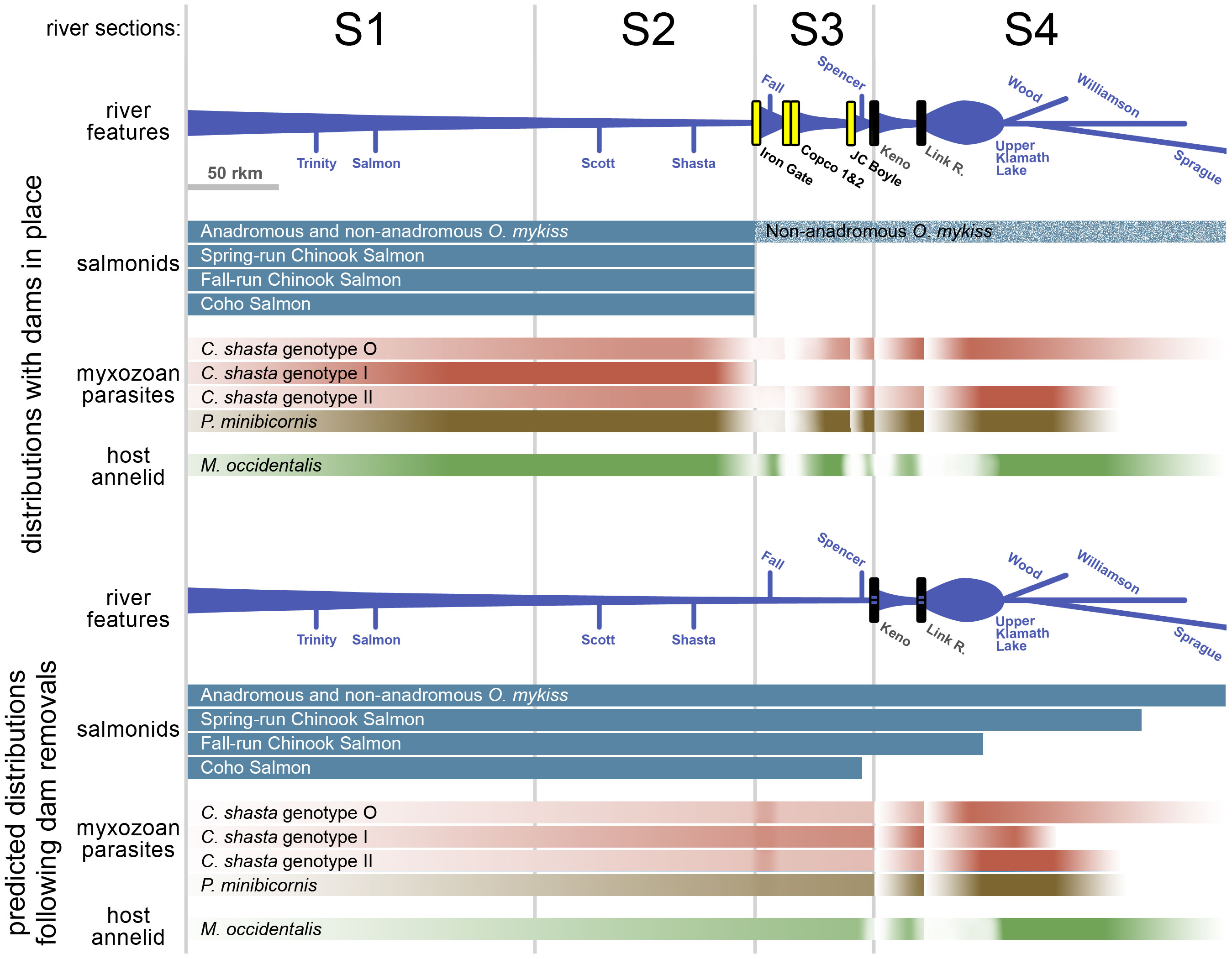
Figure 2 Schematic diagrams showing distributions and relative abundances of Klamath River salmonid fishes and the myxozoan pathogens Ceratonova shasta and Parvicapsula minibicornis, and their host annelid, Manayunkia occidentalis, during juvenile out-migration (spring). Major features of the river (dams, tributaries, lakes) are indicated. The top diagram shows fish distributions with dams in place, and the bottom diagram shows predicted distributions following removal of the lowermost four dams. Predicted fish ranges are based on historical data and represent habitat that will be accessible after dam removals; predicted pathogen and annelid distributions are based on changes to the river basin (refer to main text).
4.2 Pathogen species
We considered pathogens that were present historically (in the previous 25+ years) in the Klamath River and for which there has been documented evidence of salmonid disease. Disease in wild salmon populations is difficult to detect, as sick and moribund juvenile fish are removed rapidly by predators. Thus, what we know about disease impacts is often a result of large epizootics in adult fish, which may be less frequent but more visible. The occurrence of an adult salmon epizootic in the Klamath River in 2002 led to enactment of a long-term monitoring program (Bartholomew et al., 2022), which provided some of the most comprehensive data on fish pathogens in a large ecosystem (Lehman et al., 2020). This monitoring identified C. shasta as the dominant pathogen of juvenile Klamath River salmon and a primary factor limiting recovery of salmon populations in the Klamath system (Fujiwara et al., 2011). Thus this pathogen provides the primary scaffolding for structuring our predictions for salmon disease risk in a post dam ecosystem.
The other pathogens that we consider here represent a range of bacterial, protozoan and metazoan species, with life cycles that are direct (fish-to-fish, or fish-to-fish via an off-host development phase) or indirect (requiring other host/s) (Figure 3). They exhibit different host specificity, but all are endemic to and distributed throughout the Klamath River Basin (Supplemental Table 1). Pathogens present in salmonid populations elsewhere, for example Myxobolus cerebralis (causative agent of whirling disease), and a range of viruses, are not discussed here because they have not been detected in Klamath Basin populations of naturally produced and hatchery salmon in the previous 25+ years by the state and federal agencies that conduct fish health monitoring.
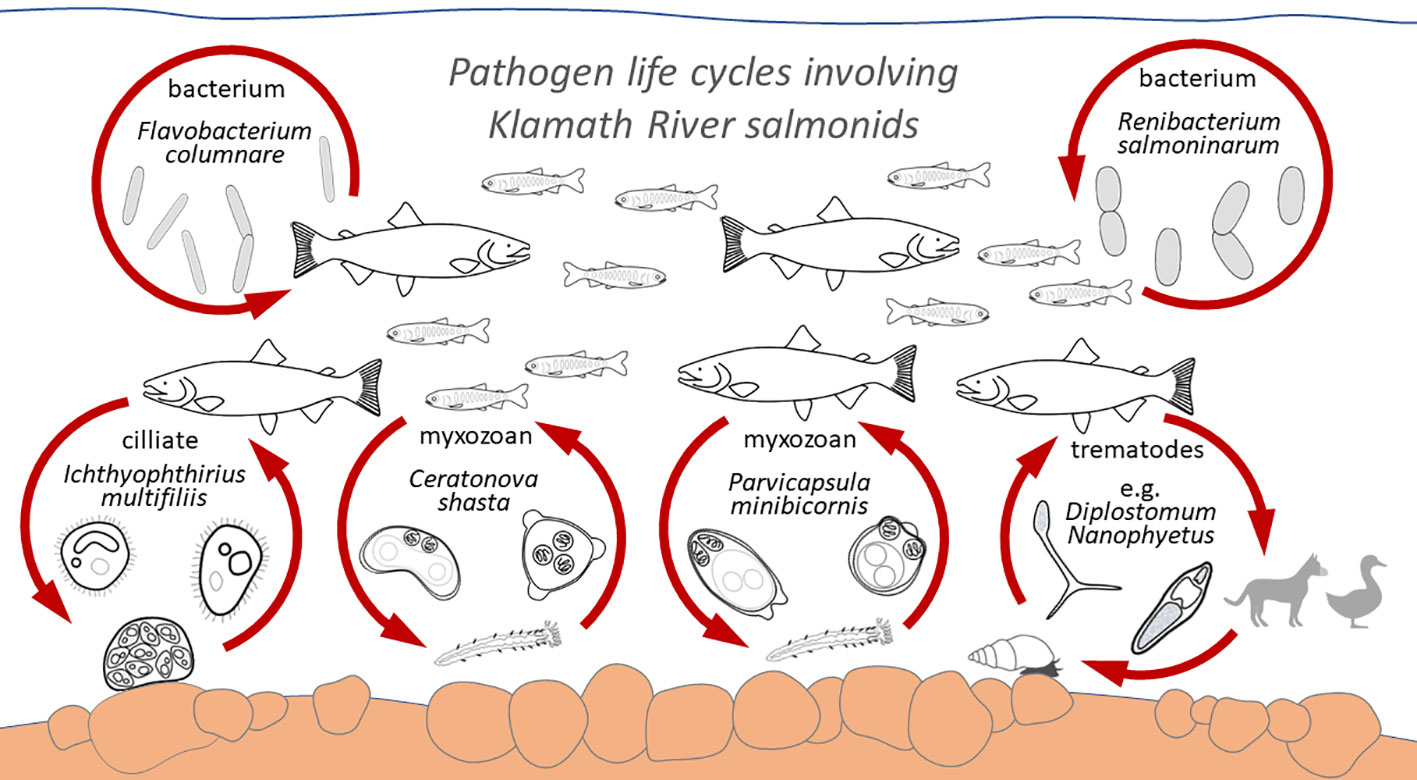
Figure 3 Life cycle relationships for the pathogens of concern to Klamath River salmonid fishes, showing life stages and alternate hosts. The hosts and pathogens are not shown to scale. Refer to main text for references.
4.2.1 Multi-host parasites (indirect life cycles): Myxozoan and trematode parasites
4.2.1.1 Ceratonova shasta
This myxozoan parasite (formerly Ceratomyxa shasta) only infects salmonid fishes in the Pacific Northwest of the US and Canada. In rivers where the parasite is endemic, like the Klamath River, native fish have developed a degree of resistance to severe disease. Nevertheless, C. shasta can be a major contributor to mortality in juvenile salmonids, depending on the environmental conditions. For example, an epizootic occurred in juvenile Chinook Salmon in the warm, dry spring of 2021. Weekly monitoring during out-migration that year documented a peak C. shasta infection prevalence of 98% and more than half of the fish sampled were determined to have a fatal infection (Voss et al., 2022). C. shasta is not considered a contributor to adult pre-spawn mortality in the Klamath River, as most adult salmon enter the river in the fall when parasite abundance is lower, and their freshwater residency time prior to spawning is short. However, the parasite has been implicated in pre-spawn mortality in salmon that have longer freshwater residencies (Chapman, 1986) and suffer from loss of immune functions (Dolan et al., 2016). Long-term monitoring (out-migrant sampling, sentinel fish exposures, annelid sampling and water sampling) has been critical to understanding the factors that result in disease and population level impacts in juvenile salmon (reviewed by Bartholomew et al., 2022); however, there are fewer data on infection prevalence and disease severity in adults.
The life cycle of C. shasta is indirect, involving an aquatic annelid host, Manayunkia occidentalis, and two waterborne spore stages (Figure 3; Bartholomew et al., 1997; Atkinson et al., 2020). The actinospore released from the annelid infects the fish through the gill. The parasite then travels through the bloodstream to reach the intestine, where it can cause severe inflammation and tissue necrosis. Here it matures into a myxospore, which is released into the water column. Adult salmon acquire C. shasta infections when re-entering fresh water in the lower river [there is no evidence for infection in ocean fish (Slezak, 2009)] and transport the parasite upstream as they migrate. Actinospores that infect adult salmon in the lower river come from annelids infected by myxospores released from out-migrating juveniles, whereas actinospores that infect juvenile salmonids further upstream come from annelids infected by myxospores from the previous season’s adult fish. In fatally-infected juvenile Chinook Salmon and Coho Salmon, myxospore release can occur during out-migration, 3 - 4 weeks after infection (Ray et al., 2012; Benson, 2014). In adults, myxospores are released after fish die post-spawn (Slezak, 2009; Kent et al., 2014; Foott et al., 2016b). In Steelhead/Redband juveniles and adults, myxospores may be released from apparently healthy infected fish (Bartholomew et al., 2022). The filter-feeding annelid host then ingests these myxospores to complete the cycle.
Effects of C. shasta differ among salmonid species and strains because C. shasta genotypes have differences in host specificity and virulence (Figure 2; Atkinson and Bartholomew, 2010a; Atkinson and Bartholomew, 2010b; Bartholomew et al., 2022). Infection in Chinook Salmon and Coho Salmon by genotypes I and II, respectively, can result in high mortality when conditions favor the parasite. In contrast, infections in Steelhead/Redband Trout with genotype 0 rarely result in overt disease and these fish may carry and disperse myxospores throughout their life. The severity of infection is a function of parasite dose and water temperature, which together affect disease progression in the fish, with 70,000 actinospores and 15°C considered a threshold for severe disease (mortality) in juvenile Chinook Salmon (Ray et al., 2012; Ray and Bartholomew, 2013); the threshold for mortality of Coho Salmon is lower (Hallett et al., 2012). Because C. shasta is not transmitted between fish, fish density is not directly a risk factor for infection. However, high densities of post-spawn adult salmon releasing myxospores in proximity to annelid hosts drive disease risk indirectly.
Before dam removals, C. shasta was well documented from the Williamson River in the upper basin, throughout the Klamath River mainstem to the estuary; it was not established in tributaries (Hendrickson et al., 1989; Hallett and Bartholomew, 2006; Stocking et al., 2006). Genotypes 0, I and II were found downstream from the dams to the estuary (Atkinson and Bartholomew, 2010a; Atkinson and Bartholomew, 2010b). An “infectious zone” of high waterborne actinospore abundance of genotypes I and II occurred episodically between the confluences of the Shasta and Salmon rivers, when water temperature rise in the spring, during juvenile salmon out-migration (Hallett et al., 2012; Voss et al., 2023). The myxospore input that drove this infectious zone likely came from the high densities of adult salmon that spawned directly downstream from Iron Gate Dam and the adjacent hatchery: a single adult salmon carcass can contribute millions of myxospores (Foott et al., 2016b). Upstream of the dams, genotype I was absent because migration of its Chinook Salmon host was blocked. Despite the absence also of Coho Salmon upstream of Iron Gate Dam (UKL, Williamson River), genotype II was present at high densities in water samples and annelids (Figure 2), suggesting that non-native trout or landlocked salmon serve as an alternate host. Genotype 0 persisted in the upper basin in non-anadromous O. mykiss (Redband Trout).
The obligate annelid host limits parasite distribution because transmission only occurs where there is spatial and temporal overlap between salmonids and infected annelids. Distribution and density of infected M. occidentalis are predictors of salmon disease risk and consequently, changes to either variable will have knock on effects. The annelids are patchily distributed throughout the mainstem Klamath River and in the Williamson River (Figure 2; Stocking and Bartholomew, 2007; Alexander et al., 2014; Alexander et al., 2016). While these annelids tolerate a broad range of environmental conditions (e.g., water temperature and dissolved oxygen extremes), their distribution is primarily driven by disturbance (flow events) and substrate. High M. occidentalis densities are generally restricted to stable substrates (boulder/bedrock) that co-occur with moderate depths and low velocities, but annelids are highly plastic in their habitat use and can use a range of substrates when disturbance is low (Stocking and Bartholomew, 2007; Jordan, 2012; Alexander et al., 2014; Alexander et al., 2016). In the reservoirs, M. occidentalis is restricted to the inflows and edges (Stocking and Bartholomew, 2007). It has not been observed in tributaries, which is likely related to the more dynamic flow regimes in those environments.
4.2.1.2 Parvicapsula minibicornis
Another myxozoan parasite, shares the same life cycle as C. shasta (Bartholomew et al., 2006), with the same annelid and salmonid hosts. Although its distribution mirrors that of C. shasta, it is generally detected earlier in the year and at higher densities (Bartholomew et al., 2007; Voss et al., 2023). P. minibicornis has intra-specific genetic differences that may map to specific salmon host species, but this is not as well established as for C. shasta (Atkinson et al., 2011). The parasite infects the gills and kidney glomeruli (Bradford et al., 2010) and spores are shed from the fish in urine, a dispersal mechanism that doesn’t require the death of the fish host. This difference in transmission strategy for migrating adult salmon (continuous shedding from live fish), likely results in greater dispersal of P. minibicornis myxospores than C. shasta.
While P. minibicornis is not considered a primary contributor to mortality in the Klamath River, out-migrating juvenile salmon have a high prevalence of infection with clinical disease signs (kidney swelling, multifocal glomerulonephritis and interstitial hyperplasia); however, fish collected in the estuary showed signs of recovery (Bartholomew et al., 2007; Voss et al., 2023). Juvenile fish are often co-infected with both C. shasta and P. minibicornis (Stone et al., 2008), and the parasite likely has sublethal effects in juvenile salmon as a stressor, particularly in co-infections, and its effects on saltwater survival are unknown.
In Klamath River adult salmon, high infection prevalence, with development to mature myxospores, has been observed in Chinook Salmon, Coho Salmon, and Steelhead, and disease (glomerulonephritis) was reported in Chinook Salmon (Bartholomew et al., 2007), but not identified as a cause of pre-spawn mortality. In other rivers, P. minibicornis impedes performance (Wagner et al., 2005) and contributes to morbidity and mortality of adult salmon (St-Hilaire et al., 2002; Jones et al., 2003; Bradford et al., 2010), particularly when there is a long freshwater residency.
4.2.1.3 Trematodes (flukes)
Several trematode species have been reported from salmonids in the Klamath Basin, including the eye fluke Diplostomum pseudospathaceum, the blood fluke Sanguinicola klamanthensis, Nanophyetus salmincola which infects muscle and kidney, and a gill fluke, possibly Apophallus sp. (Walker and Foott, 1993; USFWS unpublished survey data). These parasites have complex life cycles involving freshwater snails and often a second vertebrate host (e.g., bird, canid). Trematode snail hosts have a broad habitat preference, and have been reported from clean, running water and solid substrates, muddy-sand bottoms of small and medium lakes and from slow flowing streams (Clarke, 1981; Min et al., 2022). We lack comprehensive information on the distribution and abundance of snail hosts in the Klamath River.
There have been no reported health effects of trematode infections on salmon in the Klamath River. Generally, trematode-associated morbidity is linked to parasite density and fish size (thus younger fish are more likely to be affected), therefore, there is potential to have a localized effect if shedding snail populations are large and salmonid fry rear in the same edge habitat. In other rivers, adverse health effects and lower early marine survival were correlated with high N. salmincola loads in juvenile Steelhead (Chen et al., 2018) and Coho Salmon, with a lesser effect on Chinook Salmon (Jacobson et al., 2008). Reduced fitness (Ferguson et al., 2011; Ferguson et al., 2012) and increased sensitivity to other infections and predation (Jacobson et al., 2003; Roon et al., 2015; Puget Sound Steelhead Marine Survival Workgroup, 2018) have also been reported. For Diplostomum (eye fluke), prevalence and severity of infection is high in multiple fish species in UKL (Burdick et al., 2017).
As few data exist on the impacts of diseases caused by trematode infections and on the distribution of snail hosts in the Klamath River basin, we have little basis for specific predictions of disease risk following dam removals. Given that this group contains known pathogens of concern, these should be included in monitoring efforts following dam removals.
4.2.2 Single host parasites (direct life cycle with off-host development)
4.2.2.1 Ichthyophthirius multifiliis
“Ich”, a ciliated protozoan parasite that causes white spot disease, is named for the distinct trophont (feeding) stages that encyst in the skin and gills of its fish host. When present in high numbers, the parasites can disrupt osmoregulation and respiration, and the feeding wounds make the fish vulnerable to secondary bacterial and fungal infections. The life cycle of I. multifiliis is direct (no intermediate host, but has off-host development) and temperature dependent: the complete life cycle takes 3 - 4 d at 21 - 24°C, 10 - 14 d at 15°C and > 5 wks at 10°C (Warren, 1991). Because the parasite has a direct life cycle, it transmits rapidly when fish congregate at high densities, and disease impacts have been reported in spawning Sockeye Salmon in constrained spawning channels (Traxler et al., 1998), and in aquaculture production where disease is more easily observed. The parasite has low host specificity and can be transmitted between salmonid and non-salmonid fishes, and is present throughout the Klamath River. This parasite contributed to the 2002 epizootic in adult Chinook Salmon, which occurred during a low flow event that crowded the returning salmon in the lower Klamath River (Belchik et al., 2004). Following that mortality event, an adult salmon monitoring program was established (Belchik, 2015). High density of resident fish (e.g., speckled dace (Rhinichthys osculus)) harboring I. multifiliis, even at low prevalence or intensity, may provide a reservoir for the parasite in the lower river (Foott et al., 2016a). Additional outbreaks of I. multifiliis in returning adult salmon occurred in 2014 and 2016, but lethal events are thought to have been prevented by increasing river flow through the managed release of reservoir water (Bodensteiner et al., 2000; Belchik, 2015).
4.2.3 Single host pathogens (direct life cycle with no off-host development)
4.2.3.1 Flavobacterium columnare
Is a bacterium that infects the gills and skin of its host fish and causes columnaris disease. Disease signs include necrotic gills and skin, and frayed fins; fish also become lethargic, making them vulnerable to predation. This bacterium has a broad host range, and other fish present in the system can serve as reservoirs. It is present commonly in lakes and rivers and is transmitted rapidly at high fish densities and at warmer temperatures. F. columnare is a species complex composed of four distinct groups; however, the Klamath Basin has the “typic” F. columnare (LaFrentz et al., 2022).
This bacterium, along with I. multifiliis, contributed to the 2002 epizootic in adult salmon in the lower Klamath River. Disease epizootics in the Klamath River basin as a result of columnaris have been reported in other fish species; the earliest report of an epizootic in suckers and Tui Chub (Siphateles bicolor) in UKL in 1898 (Gilbert, 1898) was likely a result of F. columnare. Epizootics have been reported periodically since then (Perkins et al., 2000), including an outbreak in UKL in August 1971 where ~14 million fish died (Rohovec and Fryer, 1979). Fish affected by these epizootics include adult Shortnose Suckers (Chasmistes brevirostris), Lost River Suckers (Deltistes luxatus), Tui Chub, Blue Chub (Gila coerulea), Large Scale Suckers (Catostomus macrocheilus), Marbled Sculpin (Cottus Klamathensis) and rarely Rainbow Trout. In some reports, the dying fish were co-infected by various parasites such as Lernaea, leeches, Ichthyobodo, Trichodina and trematodes. Severe environmental conditions in UKL play a significant role in the predisposition and development of F. columnare infections. Hypoxia, caused by the collapse of blue green algae Aphanizomenon flos-aquae blooms, was identified as the primary mechanism that triggered the 1995 - 1997 fish kills (Perkins et al., 2000). Susceptibility of the fish to hypoxia was probably enhanced by chronic exposure to high water pH and ammonia levels, and low dissolved oxygen during the summer months; these water quality stressors increased susceptibility of fish to pathogens such as F. columnare. In August 2022, F. columnare caused the death of Chinook Salmon in the lower Klamath Basin, in a mortality event associated with higher water temperatures, the congregation of fish in cooler-water refugia, and co-infection with I. multifiliis.
F. columnare infections in juvenile Steelhead, Chinook Salmon, and Coho Salmon begin to occur when water temperatures reach 15°C and become progressively more severe as temperatures increase to 20 - 24°C (Holt et al., 1975). Most of the severe fish epizootics in UKL have occurred in August (summer) when water temperatures were 20 - 25°C; however, there are reports of high temperatures and fish loss or clinical disease as early as May (spring): e.g., mortality of Fathead Minnows (Pimephales promelas) and chubs in the Link River and head of Lake Ewauna in May 1987; detection of F. columnare gill lesions in apparently healthy adult suckers at the mouth of the Williamson River in May 1997 (Thorsteinson et al., 2011). Thus, infections of F. columnare can occur in the UKL watershed beginning in May and extending to September if water temperatures are elevated.
4.2.3.2 Renibacterium salmoninarum
Is the causative agent of bacterial kidney disease, a chronic granulomatous inflammatory infection of the kidney in salmonids (Delghandi et al., 2020). Although disease progresses most rapidly at higher temperatures (15 - 20°C), mortality is often highest at cooler temperatures (7 - 12°C) due to the chronic nature of the infection (Sanders et al., 1978). The bacterium can be transmitted both vertically (from adult to progeny through the egg) and horizontally (between fish), and its chronic nature makes detection of infected fish difficult as they may not display disease signs. Non-salmonid species are not infected by the bacterium and thus do not present a risk as reservoirs of infection.
The bacterium has been detected historically in Chinook Salmon, Coho Salmon, and Steelhead in the lower Klamath River (Walker and Foott, 1993), and from Rainbow Trout, Brown Trout (Salmo trutta), Brook Trout (Salvelinus fontinalis) and Kokanee Salmon (Oncorhynchus nerka) in the upper Klamath River basin (Oregon Department of Fish and Wildlife - ODFW - data). Chinook Salmon are particularly susceptible to severe disease (Elliott, 2017), and studies prior to dam removals show that exposure of naive Spring-run Chinook Salmon in Upper Klamath Lake for one week results in R. salmoninarum infection (ODFW-Oregon State University preliminary data).
4.2.4 Other pathogens
Other infectious agents have been detected in the Klamath River, but are not specifically considered in this assessment for one or more of the following reasons: they are rarely detected (e.g., IHNV), they have low pathogenicity (e.g., Trichodina sp., Ichthyobodo sp., Chilodonella sp., Gyrodactylus sp.), or we lack information on how basin changes could alter the pathogen’s effects (e.g., Lernaea salmonea, which has been detected episodically at high densities on Redband Trout in the UKL).
4.2.5 Other stressors
Physical and biological stressors can cause mortality on their own, or alter the host’s response to pathogens. Dam removals will alter the magnitude and timing of many physical stressors, with somewhat unknown consequences for fish communities (Brenkman et al., 2008). Acute mortality can be caused by physical stressors including: abrasions from passage through artificial structures (e.g., the remaining Keno Dam spillway and gates), turbidity associated with reservoir drawdowns, gas supersaturation (Weitkamp and Katz, 1980), predation, high water temperature, low dissolved oxygen, and high pH. Chronic stressors can cause sublethal effects that may increase susceptibility to pathogens and/or exacerbate infections that might otherwise be tolerated.
Additional stressors are associated with the effects of climate change. Southern Oregon and northern California have become warmer and drier, and experienced a multi-year drought with increased frequency of wildfires. In summer 2022, the McKinney Fire adjacent to the Klamath River created a burn area that together with unseasonably-heavy rains caused a flash flood and debris flow in the river. The resultant combination of low oxygen levels and high turbidity caused a local fish die-off and subsequent higher disease-related mortality that season, due to the additional stressors of persistent high turbidity and high river water temperatures (26°C).
Biological stressors include nutritional factors (e.g., thiamine), algal blooms and co-infections by other pathogens. Co-infection is common (e.g. C. shasta and P. minibicornis; I. multifiliis and F. columnare), yet effects on hosts are complicated and unpredictable. For example, prior infection with N. salmincola increased mortality when Chinook Salmon were exposed to certain bacterial pathogens, including Vibrio anguillarum (Jacobson et al., 2003) and F. columnare, but not in co-infection with Aeromonas salmonicida (Roon et al., 2015). In contrast, some pathogens can actually increase fish performance (McElroy et al., 2015; Lauringson et al., 2023), demonstrating that infection does not always result in a disease state.
4.3 River sections used in this assessment
Based on fish and pathogen distributions, river conditions, and barriers to fish movement, prior to dam removals, we stratified the river into four sections: numbered from the estuary to the headwaters (Figure 1). Other studies have used different schemes (e.g., geomorphology) for delineating river reaches, but for the purposes of this assessment we defined the sections to best characterize anticipated changes in disease risk in response to dam removals.
• S1: Estuary to Portuguese Creek – includes the Trinity River and Salmon River tributaries.
• S2: Portuguese Creek to Iron Gate Dam – encompasses the highly infectious zone for C. shasta prior to dam removals; includes the Scott River and Shasta River tributaries.
• S3: Iron Gate Dam to Keno Dam – Hydroelectric Reach; will change dramatically in geomorphology, flow and temperature as reservoirs revert to riverine habitat. And in both physical and biological diversity as salmon and other species re-establish.
• S4: Keno Dam upstream – includes the Klamath Project water retention dams, Keno and Link River (which will remain), Upper Klamath Lake and its tributaries. Changes will be driven primarily by shifts in fish distribution and diversity as salmon populations re-establish in the upper tributaries.
5 Predicted effects of fish disease following dam removals
Factors that contribute to alterations in disease risk following dam removals include the abiotic elements discussed in the previous section, and biotic components that include: pathogen virulence and abundance, pathogen replication rate, ability of a pathogen to persist in the environment, how a pathogen is transmitted between hosts, and the presence and overlap of hosts and pathogens in time and space. Progression and severity of pathogen-caused diseases will vary with salmonid species, origin, and time spent in the mainstem Klamath River. We recognize that the Klamath Basin ecosystem is complex, with a multitude of factors that influence host-pathogen disease dynamics. These factors may interact both synergistically and antagonistically. Thus, we have characterized risks qualitatively and offer a prediction of the net change.
This section considers how the pathogens of primary concern (Supplemental Table 1), grouped by their underlying life cycle characters (direct or indirect; Figure 3), will respond to the changes brought about by dam removals. We then make predictions on how fish disease risk from each pathogen will change in each of the four river sections (Figure 1; Table 2). Although our prediction capacity for pathogens other than myxozoans is limited, we felt it important to retain them because future restoration and management could benefit from these limited insights and identification of information gaps.
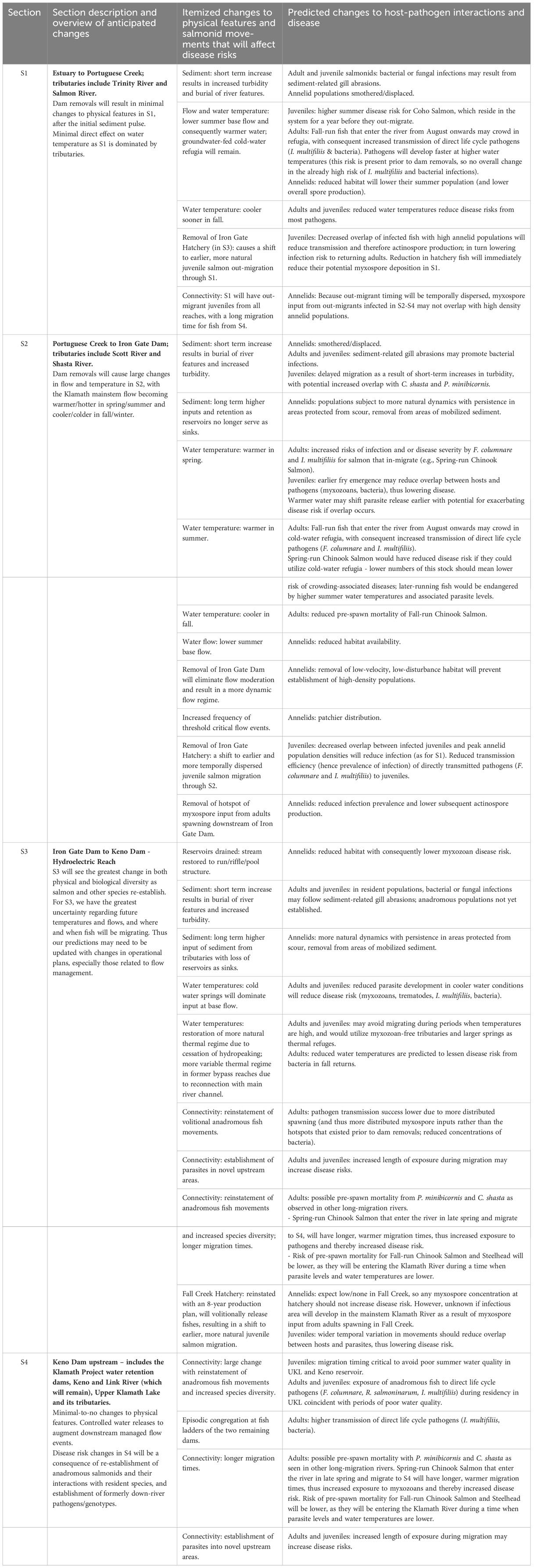
Table 2 Predicted changes to physical features, fish movements and associated host-pathogen interactions and disease for each of the four river sections, after dam removals on the Klamath River. The changes are long term unless specified as short term.
5.1 General responses of pathogens to dam removals
5.1.1 Multi-host parasites
5.1.1.1 Myxozoans
Factors affecting disease risk from myxozoan infections include presence of stable habitats that support annelid hosts, overlap of fish and annelid hosts in time and space, and water temperatures that favor disease. The altered timing of fish movement after dam removals should decrease overlap between out-migrating, infected juvenile salmon and peak annelid population densities, thereby lowering annelid infection prevalence and consequently reducing infection risk for juvenile salmon. But for both C. shasta and P. minibicornis, we expect that the extended migration times (cumulative exposure; Ratliff, 1981) will be associated with the greatest changes in disease risk for juvenile and adult salmon, particularly during periods when water temperatures are warm. Because of the long development of these parasites, actively out-migrating juvenile salmon infected in one section release mature spores in downstream sections of the river. The high prevalence of both myxozoan infections in adult fish suggests that the infection threshold (number of actinospores required to elicit an infection) is likely low in adults (Foott et al., 2016b).
5.1.1.2 Trematodes
Factors likely to affect severity of infections caused by trematodes are the density of snail hosts and overlap of fish, bird and snail hosts in space and time. Changes in snail host habitat and population density will affect abundance of trematode parasites and thus effects on salmonids. Similar to other pathogens, high infection rates are associated with warm water temperatures (Schaaf et al., 2017). However, because few data exist on the distribution of snail hosts in the Klamath River Basin prior to dam removals, and there are significant gaps in our knowledge of snail host habitat tolerance, we have little basis for specific predictions of future disease risk. Therefore, we include considerations for the upper two river sections only. In S3, infection from trematodes may be reduced from current levels if the increased flow variability and habitat changes decrease densities of the snail host, and reduce infection intensities in fish (Field and Irwin, 1994). In S4, the high prevalence and severity of infection with D. pseudospathecum in multiple fish species in UKL could be indicative of the risk to juvenile Spring-run Chinook Salmon in late spring, which will reside longer (and therefore be exposed longer) than Fall-run Chinook Salmon or Steelhead. For trematodes that require a bird in their life cycle, improved habitat in the upper basin could increase both bird and subsequently trematode abundance.
5.1.2 Single-host pathogens
5.1.2.1 I. multifiliis
Factors that we predict are important for future outbreaks are habitats where adult or juvenile fish congregate, high water temperatures and low flows that facilitate rapid transmission and proliferation. This parasite has a broad host range, with other fish species serving as reservoirs for infection (Foott et al., 2016a), thus any post-dam-removal increase in overlap of salmonids with other fishes may increase disease risk when conditions are permissive.
5.1.2.2 Bacteria
Factors affecting disease risk associated with F. columnare in salmonids will be similar to those for I. multifiliis (see above) given that these pathogens are transmitted directly from fish to fish, have a broad host range and are present throughout the basin. Outbreaks of F. columnare infections are associated with areas of high salmonid densities, high water temperature and low flows. In contrast, R. salmoninarum has a narrow host range and is not widely documented in the Klamath River (primarily in S4), but its chronic nature and its ability to transmit both between fish and vertically to their progeny make it difficult to control.
5.2 River section 1 (S1) - Estuary to Portuguese Creek
The effects of dam removals on temperature and flow will be relatively minor in S1 compared to S2 and S3, given the dominance of tributary inputs in this section, and will be most significant in summer months (at baseflow). Lower summer baseflows will mean water temperatures are more sensitive to ambient air temperature, and so will be higher. Increased temperatures in S1 would increase disease risk associated with all pathogens, with fish mortality more likely to result from pathogens with a shorter, more direct life cycle (such as F. columnare or I. multifiliis).
5.2.1 Multi-host parasites
5.2.1.1 Myxozoans
S1 is located downstream from the main infectious zone for both C. shasta and P. minibicornis, but both juvenile and adult salmonids become infected here. Juvenile fishes migrating downriver continue to be exposed as they migrate through S1, and juveniles from S1 tributaries (Trinity and Salmon rivers) become infected upon entering the mainstem Klamath River (Voss et al., 2022). Returning adults become infected by both myxozoans after entering the river (there is no evidence that either of these parasites are present in adult fish prior to their return to freshwater; Slezak, 2009). Adults then move both parasites upriver as they return to their spawning grounds.
5.2.1.1.1 Impacts on annelid hosts
Prior to dam removals, annelid hosts had peak distribution and density in summer and early fall, with prevalence of C. shasta highest in late summer to early fall (JDA unpublished data). Thus, annelids in S1 appear to be important for transmitting C. shasta (and maybe P. minibicornis) to returning adult salmon which in turn transport the parasite upriver, with out-migrating juvenile salmon infected upriver in S2 providing the source of myxospores to infect these annelids the following season (Robinson et al., 2020).
After dam removals, short-term effects on annelids will likely be minimal because the timing of reservoir drawdowns overlap with the period of low population density (population expansion occurs after baseflow in S1). The long-term restoration of a more natural and variable flow regime will likely restrict annelid distribution and reduce densities in S1 because: i) the lower baseflows during summer and fall will reduce habitat availability and co-occur with peak annelid population density in this section; ii) the increased variability will reduce habitat suitability and further restrict annelids to flow refugia; and iii), the restored sediment supply may further prevent the establishment of high-density annelid populations in this section.
5.2.1.1.2 Predictions for juvenile salmon
For juvenile salmon originating in S1, disease risk will continue to be low as they have a short migration time to the ocean and parasite densities in the water are lower than in S2. The disease risk for juvenile salmon migrating from upriver sections following dam removals will primarily depend on factors discussed in those sections. Long-term, disease risk should be decreased for fish migrating through S1, as a result of reduced overlap between infected juvenile salmon releasing myxospores and annelid populations there.
5.2.1.1.3 Predictions for adult salmon
The reduction in annelid habitat in S1, combined with changes to salmon migration timing, may translate into reduced infection risk for returning adult salmon in early fall. Unlike for juvenile salmon, the infectious dose threshold for adults is unknown (but likely low), and thus we expect continued high infection prevalence in adult salmon, particularly Spring-run Chinook Salmon entering the river in late spring and early summer when waterborne parasite densities are usually highest. Consequently, returning adults will continue to contribute myxospores to S2, and provide myxospore inputs to S3-S4. If in-migration co-occurs with warmer temperatures and lower baseflow, infection risk may increase in S1 following dam removals. Pre-spawn mortality associated with either myxozoan has not been observed in S1 and we do not expect this to change because adults will likely move upstream before the parasites have had sufficient time for development and cause disease.
5.2.2 Single-host pathogens
5.2.2.1 I. multifiliis
Prior to dam removals, outbreaks in returning adult Fall-Run Chinook Salmon have occurred in S1. Following dam removals, cooler river temperatures during their fall in-migration will promote both upstream migration beyond this section (and limit congregation and associated bottlenecking) and reduce the concentration of waterborne infectious stages. However, the increase in numbers of Spring-run Chinook Salmon will mean more adult fish entering S1 in spring/summer when water temperatures are high and conditions cause congregation in cool-water refugia that promotes fish-to-fish transmission. Disease risk will likely remain high in S1 but will be affected by each years’ specific environmental conditions and management decisions (e.g., water allocation in the Upper Klamath Basin and major tributaries) that may affect flow.
5.2.2.2 Bacteria
Long term, lower summer flows and higher temperatures in S1 will increase F. columnare proliferation and transmission, and thus disease risk, for salmon that in-migrate or are already present (e.g., returning Spring-run Chinook Salmon or yearling Coho Salmon) when mainstem water temperatures are above 18°C. These conditions will drive increased use of cool-water refugia, and consequently, the likelihood of salmonid congregation. The higher density of fish in refugia will increase F. columnare transmission among fish, but the lower temperature will decrease proliferation, and thus the overall disease risk is difficult to predict.
5.3 River section 2 (S2) - Portuguese Creek to Iron Gate Dam
Prior to dam removals, river flows in S2 were influenced directly by Iron Gate Dam and the Shasta River, with limited sediment coming through the reservoirs. This resulted in low-velocity, low-disturbance habitats in the river downstream. With dams in place, water temperatures in S2 were buffered and temporally lagged. Removal of Iron Gate Reservoir will result in a dynamic flow regime, episodic high flows/floods, increased sediment, and earlier spring warming and earlier fall cooling, plus larger diel fluctuations year-round.
5.3.1 Multi-host parasites
5.3.1.1 Myxozoans
The low-velocity, low-disturbance habitat below Iron Gate Dam supported high densities of the myxozoan annelid host, and an episodic high spore “infectious zone” (Stocking and Bartholomew, 2007; Alexander et al., 2015; Alexander et al., 2016). S2 will be profoundly affected by the removal of the four dams and restoration of a more natural flow regime that will alter myxozoan disease outcomes.
5.3.1.1.1 Impacts on annelid hosts
S2 annelids transmit myxozoan parasites to out-migrating juvenile salmon, which move the parasites downriver where they infect annelids in S1. In low disturbance water years prior to dam removals, S2 annelids were frequently at peak distribution and density year-round, with infection prevalence highest in late winter/early spring. Annelid host distribution and density will change - both immediately after dam removals, and over the long term.
Over the short term in S2, sediment mobilization and deposition will reduce annelid host distribution and densities from a combination of sedimentation/burial and scour, particularly downstream from the location of the former Iron Gate Dam. Habitat complexity may provide refugia from effects of sediment mobilization in some areas, thus annelid populations that persist in these protected areas will expand rapidly, potentially tempering the extent of short-term impacts.
Over the long term, the restoration of more natural and variable flow and sediment regimes will decrease annelid habitat suitability (stability) and prevent establishment of high-density populations (Jordan, 2012; Alexander et al., 2014; Alexander et al., 2016). The predicted increases in frequency of events that disturb substrate and attached periphyton (threshold critical flow events, Curtis et al., 2021) should drive patchier annelid distribution and lower densities overall as a result of mechanical scour and flushing (Alexander et al., 2016). In particular, high density annelid populations within the pre-dam-removal-infectious-zone (within S2), should be reduced greatly after dam removal.
5.3.1.1.2 Predictions for juvenile salmon
In the short term, the mobilization of fine sediments following the first major precipitation event is expected to depress annelid distribution and density resulting in fewer waterborne actinospores. In the long term, the far more dynamic flow regime will decrease stability of microhabitats and prevent establishment of high-density annelid populations (Jordan, 2012; Alexander et al., 2014; Alexander et al., 2016). Consequently, the overlap between annelids and myxospores, and in turn, myxozoan infection and disease risk for migrating juvenile salmon, should be reduced.
Warmer spring temperatures will also alter host-pathogen overlap. Earlier fry emergence (Sykes et al., 2009) and their faster growth will encourage earlier migration downstream (Bartholow et al., 2004; FERC (Federal Energy Regulatory Commission), 2007; Hamilton et al., 2011). Similarly, changes in hatchery operation to volitional releases of juveniles will allow fish to migrate earlier than before dam removals, when they were deliberately released after naturally produced fish had migrated downstream. Although we expect that C. shasta and P. minibicornis actinospore release will occur approximately 2 to 3 weeks earlier in S2 (Chiaramonte, 2013), we expect that many juveniles will migrate before the peak of waterborne spore abundance, similar to migration times of naturally produced fish prior to dam removal. Furthermore, with the expanded release window, fewer fish will be migrating simultaneously and thus overlap of juveniles and peak spore densities, and consequent disease risk, should be reduced. For juvenile salmon migrating in the fall, actinospore levels are expected to be lower as a result of the cooler water temperatures. The lack of temporal overlap between juvenile salmon migration and C. shasta is attributed to why the parasite is not a major cause of juvenile salmon mortality in the Fraser River, British Columbia, Canada (Margolis et al., 1992). Similarly, in the Klamath River it is likely that a greater diversity of salmon life histories will eventually have the opportunity to be expressed, with some of those types more likely to avoid parasite exposure by migrating earlier or overwintering in tributaries and migrating in the fall.
5.3.1.1.3 Predictions for adult salmon
Pre-spawn mortality of Fall-run Chinook Salmon and Steelhead from myxozoan infections was not reported in S2 prior to dam removals. We expect this mortality risk will not increase for these fishes, as spawning in the mainstem Klamath River would occur under cooler water temperatures (CSWRCB (California State Water Resources Control Board), 2018), slowing the proliferation and development of myxozoan parasites. Spring-run Chinook Salmon adults migrating through S2 during summer months may be at risk for pre-spawn mortality because they will encounter pathogens and warmer water temperatures. However, this risk may be mitigated if fish are able to access cold-water refugia and tributaries during migration to the upper basin, or through life history variability (e.g., earlier freshwater migration). Without knowing when peak migration will occur and if it will overlap with actinospore release, more certain predictions cannot yet be made.
5.3.2 Single-host pathogens
5.3.2.1 I. multifiliis
In juvenile fish, infection prevalence of I. multifiliis may be reduced in S2 if there is more dispersal as a result of volitional hatchery releases and warmer water temperatures, which will encourage fish to migrate earlier. In adult salmon, the intensity of I. multifiliis infections lessens typically as they migrate upstream and through S2 (Belchik, 2015) and we do not anticipate this to alter after dam removals.
5.3.2.2 Bacteria
The short-term pulse of reservoir sediments and increased turbidity and suspended sediment may increase risks of infection by F. columnare if river water temperatures are above 15°C in S2 due to gill abrasion. However, over the long term, the infection risk for F. columnare for juvenile salmon is likely to remain similar to the risk prior to dam removals, or decrease, as volitionally released fish will be more dispersed in space and time. For adult Spring-run Chinook Salmon, warmer water temperatures in S2 during their spring migration present some risk. However, for adult salmon returning in fall, reduced water temperatures are predicted to lessen disease risk.
5.4 River section 3 (S3) - Hydroelectric Reach - Iron Gate Dam to Keno Dam
The greatest long-term physical and biological changes to the river will occur in S3 due to the restoration of connectivity and habitats, and changes in flow and temperature regimes. Dam removals will result in access to historically used salmon habitat, and fishes are expected to rapidly (e.g., within 3 to 4 fish generations) reoccupy habitat upstream of Iron Gate Dam (Huntington, 2004; Huntington, 2006; Department of the Interior [DOI], 2007; Cunanan, 2009; Department of the Interior and U. S. Department of Commerce, and National Marine Fisheries Service [NOAA Fisheries], 2013). Restoring connectivity to the upper basin will result in a broader future distribution of Chinook Salmon and alter the abundance and distribution of pathogens that currently occur in S2 (Bartholomew and Foott, 2010; NMFS and USFWS (National Marine Fisheries Service and US Fish and Wildlife Service), 2013; NMFS (National Marine Fisheries Service), 2019).
Fish disease risk in S3 after dam removals is difficult to predict because there will be losses and gains of the habitats that support both the re-establishing salmonids and parasite invertebrate hosts (i.e., annelids, snails). The cessation of hydropeaking operations will affect the former hydropeaking reach below JC Boyle Dam; approximately 27 rkm (25%) of S3. Prior to dam removals these operations resulted in daily flow (3-fold increase/decrease) and temperature fluctuations (up to 10°C). Their cessation will result in a flow regime more similar to that of more natural riverine habitats and temper the diel temperature swings (CSWRCB (California State Water Resources Control Board), 2018), improving habitat suitability for salmonids and invertebrate hosts. Restoration of the reservoirs to riverine sections will affect ~60 rkm (60%) of the mainstem S3 (former reservoir reaches), and will both eliminate inflow environments that supported high annelid densities (Stocking and Bartholomew, 2007) and expose new areas that were previously unsuitable habitats. Consequently, although there will certainly be a marked change in invertebrate host distributions following dam removals, there may be no net change in abundances. Elimination of reservoirs will also result in more variable water temperatures throughout S3. Groundwater spring inputs (~11°C), many of which were previously submerged under reservoirs, will create intermittent cold-water refugia that are expected to provide benefits to migrating salmonids (through reduced parasite dose and proliferation, and stress).
5.4.1 Multi-host parasites
5.4.1.1 Myxozoans
Chinook Salmon and Coho Salmon re-establishing above the former location of Iron Gate Dam will introduce C. shasta genotypes I and II. Predictions on these changes are discussed below. However, for Steelhead, we expect no adverse change in disease risk as infection by genotype 0 rarely causes overt disease.
5.4.1.1.1 Impacts on annelid hosts
The impacts of dam and reservoir removal will be significant for S3 annelids. The greatest short-term changes will occur in reservoir inflows. Reservoir drawdown will desiccate high density populations, the majority of which were distributed throughout reservoir inflow reaches. The cessation of daily hydropeaking operations will increase riverine stability and in turn, habitat suitability. However, over the long term, annelid host re-establishment is not expected to occur to the same extent as under conditions prior to dam removals in S3 because the restoration of reservoirs to riverine habitat will provide less optimal habitat for the annelids and they will not be able to i) redistribute to previously inundated areas (now dry), and ii) re-establish at the same densities due to restored flow regime. Annelids in S3 did not have a role in transmitting C. shasta to anadromous salmon prior to dam removals. Following dam removals, we expect infection dynamics to be similar to those of post-dam S2.
5.4.1.1.2 Predictions for juvenile salmon
Prior to dam removals, moderate densities of P. minibicornis and C. shasta genotypes 0 and II were detected in S3 (Hallett and Bartholomew, 2006; Bartholomew et al., 2007; Atkinson and Bartholomew, 2010b). After dam removals, C. shasta genotype I will be reintroduced to S3 and S4 by returning adult Chinook Salmon infected in S1 and S2, and the lower river biotype of genotype II introduced to S3 by returning Coho Salmon. These introductions will create new infection sources for juvenile Chinook and Coho Salmon. Densities of genotype 0 are likely to remain similar to pre-dam removal levels, and thus risks to Steelhead/Redband are likely to remain low.
Once C. shasta genotype I establishes in S3 and S4, juvenile Chinook Salmon migrating from these sections will have a prolonged exposure (as a result of the extended migration route). Post-dam removals, redistribution of adult salmon to Fall Creek and other tributaries is expected to reduce myxospore input from these fish into the mainstem and eliminate the conditions that drive the infectious zone downstream from Iron Gate Dam (discussed under Adults). Thus we do not expect juvenile Chinook Salmon to encounter high densities of C. shasta genotype I. We predict this will also be true for Coho Salmon and genotype II in S3. This altered exposure regime (reduced densities, prolonged exposure) should enable the fish’s natural resistance to resolve the infection prior to completing their downstream migration, and in turn reduce myxozoan-related mortality in out-migrating juvenile salmonids. Further, while fluctuating temperatures under post-dam conditions will not directly affect myxozoan disease processes in juvenile salmon (Chiaramonte et al., 2016), fish may avoid migrating during periods when temperatures are high, and instead use low infection risk tributaries and the larger springs as temperature refuges. The spring-fed, cold-water refugia in S3 will reduce temperature stress and thus disease risk for both juveniles and adults (Ray et al., 2012).
5.4.1.1.3 Predictions for adult salmon
Adult salmon infected with either myxozoan will benefit from the earlier decrease in temperature in the fall, which will reduce the rate of myxozoan replication, and thus reduce the risk of infection progressing to disease that could otherwise result in pre-spawn mortality. However, the net change in risk is unknown, particularly for Spring-run Chinook Salmon that enter the river in late spring and will experience prolonged exposure to myxozoans coincident with high water temperature. This may increase disease risk and pre-spawn mortality, although some of this risk may be mitigated by residence in cold-water refugia.
The relocation of hatchery operations from downstream of Iron Gate dam to Fall Creek introduces some risk for creating a novel infectious area in S3, below this potential new concentration of spawning adults. However, as Fall Creek Hatchery is located ~2 km upstream from the confluence with the Klamath River, this should reduce dispersal of myxospores into the river mainstem. Adult salmon will also not face a barrier to further migration and so fish not collected by the hatchery for broodstock will be able to migrate further, to spawn either in the Klamath River mainstem or upriver tributaries. However, the emergence of a novel myxozoan “infectious zone” downstream of Fall Creek should be considered in future monitoring programs.
5.4.2 Single-host pathogens
5.4.2.1 I. multifiliis
Because of the coldwater inputs in S3, the risk of infections will be low, especially for out-migrating juvenile fish early in the year. Risks will be greater for adult salmon if there are areas they are congregating and the temperatures and flow are permissive for I. multifiliis proliferation and transmission.
5.4.2.2 Bacteria
In S3, similar to S2, the short-term release of reservoir sediments may increase risks of infection by F. columnare due to gill abrasion if river water temperatures are above 15°C. The infection risk for F. columnare for juvenile salmon will increase as populations re-establish and fish densities increase; however, we expect volitionally released hatchery fish will be dispersed in space and time compared to historical controlled hatchery releases. For adult Spring-run Chinook Salmon, warmer water temperatures in S3 during their spring migration present some risk. For adult salmon returning in fall, cold water spring inputs will lessen disease risk.
5.5 Upstream from Keno Dam to headwater tributaries
Upstream of Keno Dam there will be no alterations to river structure or flow as a result of dam removals, so disease risks in the S4 reservoir and lakes will be a consequence of re-establishment of anadromous salmonids and their interactions with resident species. Adult Spring- and Fall-run Chinook Salmon and Steelhead are expected to migrate through Klamath Lake and into the Williamson River and Sprague River.
5.5.1 Multi-host parasites
5.5.1.1 Myxozoans
Prior to dam removals, P. minibicornis and C. shasta genotypes O and II were well established in the lower 18 rkm of the Williamson River (Hendrickson et al., 1989; Bartholomew et al., 2007; Hurst and Bartholomew, 2012). Neither C. shasta genotype was detected in its main tributaries, the Sprague River and Spring Creek (Hurst and Bartholomew, 2012). The Williamson River supports high densities of annelid hosts in this lower 18 rkm reach between the confluence of the Sprague River, and Williamson River inflow to upper Klamath Lake (Hurst et al., 2012).
5.5.1.1.1 Impacts on annelid hosts
While we do not expect changes in annelid host distribution and density in S4 post dam removals, we do expect changes in infection dynamics. Over the short term, changes in infection prevalence will not be particularly evident because it will take some time for C. shasta (genotype I and Coho Salmon biotype of genotype II) to become established. Over the long term, we expect to observe changes in annelid infection patterns that reflect the spatial and temporal changes associated with the re-establishment of anadromous salmonids.
5.5.1.1.2 Predictions for juvenile salmon
The re-establishment of adult Chinook Salmon spawning in the mainstem Williamson River downstream of the Sprague River confluence will likely result in establishment of genotype I, potentially at densities similar to the current densities of upper river genotype II. Fish that rear here will be exposed to P. minibicornis and to their associated genotypes of C. shasta, and will continue to be exposed throughout their migration. As discussed in S3, we do not know what this extended exposure regime will mean for disease risk in these populations. These disease pressures are likely to be strong selective factors for both migrating adults and juveniles from the UKL. Infection outcomes for juvenile fish migrating from S4 will likely be more dependent on water conditions than for fish originating from downstream sections because of the length of migration.
5.5.1.1.3 Predictions for adult salmon
Spring-run Chinook Salmon that migrate into the Klamath River in spring and migrate upriver to this section are at risk for pre-spawn mortality from prolonged exposure to C. shasta and P. minibicornis (Chapman, 1986; Bradford et al., 2010), the combined effects of pathogens, other temperature-dependent processes (e.g., low dissolved oxygen) and natural senescence (Hinch et al., 2012). Risk of pre-spawn mortality for Fall-run Chinook Salmon and Steelhead will be lower, as they will be entering the Klamath River during a time when parasite levels and water temperatures are lower.
5.5.2 Single-host pathogens
5.5.2.1 I. multifiliis
In S4, I. multifiliis will likely be a risk factor for both juvenile and adult salmon that congregate as they migrate through Keno and Link River dams’ fish ladders, and in UKL and Lake Euwana during periods of poor water quality and high temperatures. Potential areas for bottlenecks like fish ladders (i.e. Keno Dam) will require monitoring for I. multifiliis and F. columnare in both adult and juvenile salmon (upstream and downstream migration).
5.5.2.2 Bacteria
F. columnare is an environmental bacterium that is maintained in non-salmonid fish populations in S4. Risks to both juvenile and adult salmon include crowding during migration through Keno and Link River dams’ fish ladders, and periods of poor water quality and high temperatures in UKL and Lake Euwana. Elevated water temperatures will be an important factor in future F. columnare outbreaks, particularly in UKL where there are reservoir hosts for this bacterium and prolonged fish transit time.
R. salmoninarum is present in S4 in resident, native non-anadromous trout and Kokanee (remnants of stocked non-native populations). Rainbow trout (O. mykiss) are relatively resistant to the effects of disease, however, Spring-run Chinook Salmon are highly susceptible, and this introduces a risk that they have not encountered in the lower river. Because this bacterium is vertically transmitted, infected adults can pass the bacterium to eggs, and the resulting juveniles may be more vulnerable during periods of stress, such as smoltification. Factors that we consider important for future impacts from R. salmoninarum include the likelihood for interaction between susceptible salmonid populations (especially Chinook Salmon) and carriers of the infection.
6 Fish disease management - present and future options
With dams in place, the Klamath River was hydrologically regulated, and mitigation hatcheries were the primary source of endemic salmon. Following the 2002 epizootic, resulting from infections of F. columnare and I. multifiliis, two primary management strategies were implemented to avoid similar outbreaks: prescribed flow events (flushing and dilution flows) and modified release timing of hatchery fishes. A comprehensive fish health monitoring program was implemented in the lower basin, with a focus on these pathogens and others of concern (C. shasta, P. minibicornis). All of these efforts are planned to continue following dam removals, with limitations and modifications.
6.1 Flow manipulation
Discharge in the Klamath River mainstem and also the Trinity River has been increased to mitigate disease associated with C. shasta, I. multifiliis and F. columnare. For C. shasta, three different flow regimes were implemented to reduce enteronecrosis (Hillemeier et al., 2017) and each had a different application, dependent upon their timing (season), magnitude and duration. Two were aimed directly at reducing host annelid populations by moving different sediment types: a “deep flushing flow” of high discharge and short duration in spring every other year, and a “surface flushing flow” of moderate discharge and longer duration each winter. In response to an impending disease outbreak (informed by direct measurement of parasite spore densities in water samples and testing of trapped out-migrating juveniles from the river), an enhanced flow was implemented to reduce exposure dose for out-migrating salmon by diluting parasite spores and reducing exposure time (the increase in discharge, up to a threshold, encourages juveniles to out-migrate) [2013 and 2019 BiOps: NMFS and USFWS (National Marine Fisheries Service and US Fish and Wildlife Service), 2013; NMFS (National Marine Fisheries Service), 2019]. For I. multifiliis, prescribed discharge from the Trinity River (Lewiston Dam) in response to high intensity of infection in returning adults (number of parasites per gill arch) has been utilized to mitigate disease.
After dam removals, options for directly manipulating river flow will be more limited, and the challenge to mimic the natural flow regime while retaining water for agriculture in the upper basin will remain. In general, water availability during dry water years will limit the ability to conduct surface flushing flows. To reach magnitudes similar to those achievable pre-dam removals, any flow action would have to be tied with natural water inputs such as rain on snow events, or timed to coincide with predicted high rainfall. However, bed mobility thresholds will be lower following removals (sediments become armored downstream of dams, requiring more flow to mobilize them), so lower magnitude flushing flows should have relatively more benefit after dam removals.
6.2 Shifting of hatchery release timing
The second management strategy considered after the 2002 epizootic was timing of release of juvenile hatchery salmon to reduce their risk of disease and improve survival to the ocean. This approach was only used once, in 2021, in response to a lack of water available to increase river discharge below the dams (and comply with the BiOp; NMFS (National Marine Fisheries Service), 2019) when C. shasta densities and river temperatures surpassed disease thresholds (unrecoverable disease or mortality). Millions of juvenile Fall-run Chinook Salmon, usually released from Iron Gate Hatchery into S2 in spring, were held into fall until conditions improved. Monitoring (Voss et al., 2022) and modeling (Robinson et al., 2020) support the efficacy of using this approach in dry water years. Following the shift of operations from Iron Gate Hatchery to Fall Creek Hatchery, this approach may warrant consideration under certain water year and disease risk contexts.
6.3 Climate change
Dam removal will not be the sole driver of change in the Klamath Basin; following dam removals, the basin will continue to evolve in an altered climate realm. Shifting patterns for two central abiotic factors, temperature (predicted to increase) and precipitation (timing and form predicted to alter), will have ramifications for host-pathogen dynamics. Aspects of climate change relevant to the Klamath Basin and salmonid disease are well considered for C. shasta (see case study by Ray et al., 2015) and many of those influences will also apply to other pathogens in the Basin; reiterating or delving further into these potential impacts are beyond the scope of this review.
The impacts of dam removals and climate change have been modeled for prevalence of infection in adult salmon in the Klamath Basin (Schakau et al., 2019); however, neither prevalence of disease in this life stage or any aspect of infection in the juvenile life stage were considered. This limited focus is misplaced as infection prevalence in either host does not always correlate with disease (see Voss et al., multiple years) and is an unsuitable predictor of population level impact. It would therefore be more relevant and informative to model the impact of major changes on prevalence of disease, particularly in the juvenile life stage.
6.4 Research and monitoring
Predicting future pathogen abundance and distribution is complex, therefore it is critical that a comprehensive research and monitoring program is in place to capture changes during and after river restoration, and to address information gaps in our knowledge of pathogen transmission and host-pathogen interactions. Before dam removals, pathogen monitoring was multifaceted, and largely focused on S1 and S2, where disease outbreaks in salmon have been observed. Monitoring targets included: C. shasta - molecular quantification of waterborne stages in water samples, molecular tests and microscopy for prevalence of infection and severity of infection in outmigrants and sentinel fishes, and prevalence of infection in, and density of, annelids; P. minibicornis - prevalence of infection in outmigrants, with several years of quantification of waterborne stages in water samples; I. multifiliis - direct, lethal sampling of returning adults; F. columnare - presence of disease signs in juveniles and returning adults. Surveillance of fish in the upper basin has been opportunistic.
In the years immediately before dam removals, existing long-term monitoring efforts were expanded into S3 and S4 to characterize pathogen occurrence prior to reconnection of the upper and lower basin. Additional studies (ODFW; 2022-2024) included experimental releases of tagged (acoustic, radio, and PIT tags) Spring-run Chinook Salmon smolts, which were used to inform reintroduction protocols, and develop and coordinate monitoring programs. Sentinel cage exposures with subsets of these fish were also used to inform their susceptibility and associated disease risk to pathogens present in the Upper Klamath Basin prior to dam removals (ODFW and Oregon State University; 2022-2023). After dam removals in the Klamath Basin, continuation of these monitoring programs to track the distribution and abundance of fish pathogens in water, fishes and invertebrate hosts will be critical to inform the health status of fish during basin restoration efforts.
6.5 Research and monitoring recommendations
- Conduct sentinel fish exposures and collect annelid and water samples to monitor potential emergence of a new C. shasta-infectious zone in the mainstem, below the confluence with Fall Creek and in the former reservoir habitats.
- Conduct sentinel fish exposures and collect annelid and water samples to monitor changes in distribution of myxozoan parasites.
- Sample returning adult salmon, particularly Spring-run Chinook Salmon, to understand prevalence and progression of myxozoan infection and spore development (infection status upon river entry, proportion contributing myxospores, range in production per fish, when sporulation occurs).
- Conduct survey of pre-spawn salmonid mortalities for all target pathogens.
- Conduct survey of adult and juvenile salmon for both I. multifiliis and F. columnare, during upstream and downstream migration at potential areas for bottlenecks such as fish ladders at the remaining dams.
- Monitor R. salmoninarum risk to Spring-run Chinook Salmon in S4.
- Conduct survey for snail hosts of trematodes in the Basin, and monitor for prevalence of trematode disease.
- Conduct surveys to determine which hosts perpetuate C. shasta genotype II in S4.
- Conduct experiments to determine how C. shasta genotype II interacts with Coho Salmon.
- Conduct experiments to determine the susceptibility of Spring-run Chinook Salmon to C. shasta genotype I.
- Conduct experiments to better understand the effects of co-infections in juvenile salmonids.
- Conduct experiments to better understand effects of P. minibicornis infections on juveniles (see Voss et al., 2018).
- Conduct experiments to understand the effects of thiamine deficiency on disease severity associated with infectious agents in juvenile salmonids.
- Conduct experiments that assess the effects of low, but prolonged exposure doses for myxozoans (to better understand the impact of longer migration routes and infection periods).
- Conduct fish disease risk assessments prior to implementing habitat restoration.
- Include fish pathogen distributions as an important factor in risk assessment frameworks (Brenkman et al., 2008).
7 Conclusion
The removal of four dams on the Klamath River will dramatically alter the biotic and abiotic components of the Basin. Dam removals alone will restore a number of physical and ecological processes towards historical, pre-dam conditions. This will result in a more dynamic environment for salmon, trout and their pathogens, reducing the impacts of many of these current stressors (Williams et al., 2018; Bellmore et al., 2019). However, dam removal is only one component of the river restoration story. To meet the project’s overarching goal – the recovery of salmon populations for tribal cultural and subsistence needs – it is imperative that the pathogens that affect these fishes be understood in the context of a re-connected river, which is additionally undergoing significant climatological shifts. Our role as architects of this renewal includes being active in monitoring changes and if necessary intervening to promote fish health, for the benefit of all Basin inhabitants. The predictions we make in this study should assist in achieving these goals.
Author contributions
SH conceived the synthesis theme. SH, JB, JA and SA conceptualized and designed the study and undertook primary writing and editing. JA led sections related to annelid hosts and on physical attributes, JB led sections on pathogens, SH contributed the management section. JB, SA, SH, JF, AV, SB, RH contributed to pathogens, parasites and disease. TS, BM, JA, AG, MB contributed historic and present tribal knowledge. THW, TGW, NS, MH, MB contributed to fish biology and predicted physical changes. SA created the figures. All authors contributed critically to the drafts and approved submission.
Funding
Salmonid pathogen monitoring data that informed this publication were funded, in part or whole, by the Bureau of Reclamation (Reclamation), U.S. Department of Interior. Funding was provided by Reclamation as part of its mission to manage, develop, and protect water and related resources in an environmentally and economically sound manner in the interest of the American public. Funding was provided through Interagency Agreement # R15PG00065 and R19PG00027. The views in this report are the authors’ and do not necessarily represent the views of Reclamation.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. We appreciate the constructive feedback from Dr Miles Daniels (NOAA), and two Frontiers reviewers and the Associate Editor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1245967/full#supplementary-material
Footnotes
- ^ For the purposes of this paper, we combine O. mykiss (anadromous and non-anadromous) and Redband Trout (O. m. newberrii) in the Upper Klamath Basin, since introduced non-native non-anadromous O. mykiss are infected by a different genotype of C. shasta than native O. mykiss and Redband Trout. Historically, Redband Trout were distributed east of the Cascade Range, typically considered Upper Klamath Lake and its tributaries. The dispersal and changes in the distribution (sympatric and allopatric) of anadromous and non-anadromous O. mykiss and Redband Trout, and impacts of C. shasta on these populations following dam removals, will be of great interest (Messmer and Smith, 2007; Currens et al., 2009).
References
Alexander J. D., Hallett S. L., Stocking R. W., Xue L., Bartholomew J. L. (2014). Host and parasite populations after a ten year flood: Manayunkia speciosa and Ceratonova (syn Ceratomyxa) shasta in the Klamath River. Northwest Sci. 88, 219–233. doi: 10.3955/046.088.0305
Alexander J. D., Kerans B. L., El-Matbouli M., Hallett S. L., Stevens L. (2015). Annelid-Myxosporean interactions in “Myxozoan evolution, ecology and development” Vol. 217–234. Eds. Okamura B., Gruhl A., Bartholomew J. (Cham: Springer), 441.
Alexander J. D., Wright K. A., Som N. A., Hetrick N. J., Bartholomew J. L. (2016). Integrating models to predict distribution of the invertebrate host of myxosporean parasites. Freshw. Sci. 35, 1263–1275. doi: 10.1086/688342
Asarian E., Kann J., Walker W. W. (2009). Multi-year nutrient budget dynamics for Iron Gate and Copco Reservoirs, California. Prepared by Riverbend Sciences and Kier Associates, Eureka, California, Aquatic Ecosystem Sciences, LLC, Ashland, Oregon, and William Walker, Concord, Massachusetts for the Karuk Tribe (Orleans, California: Department of Natural Resources).
Atkinson S. D., Bartholomew J. L. (2010a). Disparate infection patterns of Ceratomyxa shasta (Myxozoa) in rainbow trout Oncorhynchus mykiss and Chinook salmon Oncorhynchus tshawytscha correlate with ITS-1 sequence variation in the parasite. Int. J. Parasitol. 40, 599−604. doi: 10.1016/j.ijpara.2009.10.010
Atkinson S. D., Bartholomew J. L. (2010b). Spatial, temporal and host factors structure the Ceratomyxa shasta (Myxozoa) population in the Klamath River Basin. Infect. Genet. Evol. 10, 1019−1026. doi: 10.1016/j.meegid.2010.06.013
Atkinson S. D., Bartholomew J. L., Rouse G. W. (2020). The invertebrate host of salmonid fish parasites Ceratonova shasta and Parvicapsula minibicornis (Cnidaria), is a novel fabriciid annelid, Manayunkia occidentalis sp. nov. (Sabellida: Fabriciidae). Zootaxa 4751, 310−320. doi: 10.11646/zootaxa.4751.2.6
Atkinson S. D., Jones S. R. M., Adlard R. D., Bartholomew J. L. (2011). Geographical and host distribution patterns of Parvicapsula minibicornis (Myxozoa) small subunit ribosomal RNA genetic types. Parasitology 138, 969–977. doi: 10.1017/S0031182011000734
Bartholomew J. L., Alexander J. D., Hallett S. L., Alama-Bermejo G., Atkinson S. D. (2022). Ceratonova shasta: a cnidarian parasite of annelids and salmonids. J. Parasitol. 149, 1862–1875. doi: 10.1017/S0031182022001275
Bartholomew J. L., Atkinson S. D., Hallett S. L. (2006). Involvement of Manayunkia speciosa (Annelida: Polychaeta: Sabellidae) in the life cycle of Parvicapsula minibicornis, a myxozoan parasite of Pacific salmon. J. Parasitol. 92, 742–748. doi: 10.1645/GE-781R.1
Bartholomew J. L., Atkinson S. D., Hallett S. L., Zielinski C. M., Foott J. S. (2007). Distribution and abundance of the salmonid parasite Parvicapsula minibicornis (Myxozoa) in the Klamath River Basin (Oregon-California, USA). Dis. Aquat. Org. 78, 137–146. doi: 10.3354/dao01877
Bartholomew J. L., Foott J. S. (2010). Compilation of information relating to myxozoan disease effects to inform the Klamath basin restoration agreement (Report prepared for Klamath Secretarial Determination Biological Subteam), 53.
Bartholomew J. L., Whipple M. J., Stevens D. G., Fryer J. L. (1997). The life cycle of Ceratomyxa shasta, a myxosporean parasite of salmonids, requires a freshwater polychaete as an alternate host. J. Parasitol. 83, 859–868. doi: 10.2307/3284281
Bartholow J. M., Campbell S. G., Flug M. (2004). Predicting the thermal effects of dam removal on the Klamath River. Environ. Manage. 34, 856–874. doi: 10.1007/s00267-004-0269-5
Belchik M. R. (2015). An outbreak of ichthyophthirius multifiliis in the Klamath and Trinity rivers in 2014 (Yurok Tribal Fisheries Program Data Series Report).
Belchik M., Hillemeier D., Pierce R. M. (2004). The Klamath River fish kill of 2002; analysis of contributing factors (Yurok Tribal Fisheries Program, Final Report February 2004), 42.
Bellmore J. B., Pess G. R., Duda J. J., O’Connor J. E., East A. E., Folley, et al. (2019). Conceptualizing ecological responses to dam removal: if you remove, what’s to come? BioScience 69, 26−39. doi: 10.1093/biosci/biy152
Benda S. E., Naughton G. P., Caudill C. C., Kent M. L., Schreck C. B. (2015). Cool, pathogen-free refuge lowers pathogen-associated prespawn mortality of Willamette River Chinook Salmon. Trans. Am. Fish. Soc 144, 1159–1172. doi: 10.1080/00028487.2015.1073621
Benson S. (2014). Ceratomyxa shasta: timing of myxospore release from juvenile Chinook salmon (Humboldt State University), 58. Master’s thesis.
Bodensteiner L. R., Sheehan R. J., Wills P. S., Brandenburg A. M., Lewis W. M. (2000). Flowing Water: An effective treatment for Ichthyophthiriasis. J. Aquat. Anim. Health 12, 209–219. doi: 10.1577/1548-8667(2000)012<0209:FWAETF>2.0.CO;2
Bradford M. J., Lovy J., Patterson D. A., Speare D. J., Bennett W. R., Stobbart A. R., et al. (2010). Parvicapsula minibicornis infections in gill and kidney and the premature mortality of adult sockeye salmon (Oncorhynchus nerka) from Cultus Lake, British Columbia. Can. J. Fish. Aquat. Sci. 67, 673−683. doi: 10.1139/F10-017
Brenkman S. J., Mumford S. L., House M., Patterson C. (2008). Establishing baseline information on the geographic distribution of fish pathogens endemic in Pacific salmonids prior to dam removal and subsequent recolonization by anadromous fish in the Elwha River, Washington. Northwest Sci. 82, 142–152. doi: 10.3955/0029-344X-82.S.I.142
Burdick S. M., Conway C. M., Elliott D. G., Hoy M. S., Dolan-Caret A., Ostberg C. O. (2017). Health and condition of endangered young-of-the-year Lost River and shortnose suckers relative to water quality in Upper Klamath Lake, Oregon 2014–2015 (U.S. Geological Survey Open-File Report 2017), 40. doi: 10.3133/ofr20171134
CDFW- California Department of Fish and Wildlife- News (2023). Ocean salmon sport fisheries in California closed for April through mid-May. Available at: https://www.google.com/url?q=https://wildlife.ca.gov/News/ocean-salmon-sport-fisheries-in-california-closed-for-april-through-mid-may-2023%23gsc.tab%3D0&sa=D&source=docs&ust=1678915785642735&usg=AOvVaw1bUF8t-bnWvjDw_va_2Mzj (Accessed 13 March 2023).
Chapman P. F. (1986). Occurrence of the non-infective stage of Ceratomyxa shasta in mature summer Chinook Salmon in the South Fork Salmon River, Idaho. Prog. Fish. Cult. 48, 304–306. doi: 10.1577/1548-8640(1986)48<304:OOTNSO>2.0.CO;2
Chen M. F., O’Neill S. M., Carey A. J., Conrad R. H., Stewart B. A., Snekvik K. R., et al. (2018). Infection by Nanophyetus salmincola and Toxic Contaminant Exposure in out-migrating Steelhead from Puget Sound, Washington: implications for early marine survival. J. Aquat. Anim. Health 30, 103−118. doi: 10.1002/aah.10017
Chiaramonte L. V. (2013). Climate warming effects on the life cycle of the parasite Ceratomyxa shasta in salmon of the Pacific Northwest (Corvallis, Oregon: Department of Fisheries and Wildlife). Master’s Thesis.
Chiaramonte L. V., Ray R. A., Corum A., Soto T., Hallett S. L., Bartholomew J. L. (2016). Klamath River thermal refuge provides juvenile salmon reduced exposure to the parasite Ceratonova shasta. Trans. Am. Fish. Soc 145, 810–820. doi: 10.1080/00028487.2016.1159612
Clarke A. H. (1981). The freshwater molluscs of Canada (Ottawa, Canada: National Museum of Natural Sciences, National Museums of Canada), 446.
CSWRCB (California State Water Resources Control Board) (2018). Lower Klamath project license surrender draft environmental impact report (Sacramento, CA: Prepared by State Water Resources Control Board), 1801.
Cunanan M. (2009). Historic anadromous fish habitat estimates for Klamath River mainstem and tributaries under Klamath Hydropower reservoirs (Arcata, California: U.S. Fish and Wildlife Service).
Currens K. P., Schreck C. B., Li H. W. (2009). Evolutionary ecology of redband trout. Trans. Am. Fish. Soc 138, 797–817. doi: 10.1577/T08-120.1
Curtis J., Poitras T., Bond S., Byrd K. (2021). Sediment mobility and river corridor assessment for a 140-kilometer segment of the main-stem Klamath River below Iron Gate Dam, California (U.S. Geological Survey Open-File Report 2020–1141), 38. doi: 10.3133/ofr20201141
Delghandi M. R., El-Matbouli M., Menanteau-Ledouble S. (2020). Renibacterium salmoninarum — The causative agent of Bacterial Kidney Disease in salmonid Fish. Pathogens 9, 845. doi: 10.3390/pathogens9100845
Department of the Interior [DOI] (2007). Modified terms and conditions, and prescriptions for fishways filed pursuant to sections 4(e) and 18 of the Federal Power Act with the Federal Energy Regulatory Commission for the Klamath River Hydroelectric Project No. 2082 (Sacramento, CA: Bureau of Land Management, Bureau of Reclamation, U.S. Fish and Wildlife Service, and National Marine Fisheries Service).
Department of the Interior, U. S. Department of Commerce, and National Marine Fisheries Service [NOAA Fisheries] (2013). Klamath dam removal overview report for the secretary of the interior an assessment of science and technical information, version 1.1. (US Government). Available at: https://klamathrenewal.org/wp-content/uploads/2020/07/A7-Full-SDOR-accessible-022216.pdf.
Doeg T. J., Koehn J. D. (1994). Effects of draining and desilting a small weir on downstream fish and macroinvertebrates. Regul. Rivers Res. Manage. 9, 263–277. doi: 10.1002/rrr.3450090407
Dolan B. P., Fisher K. M., Colvin M. E., Benda S. E., Peterson J. T., Kent M. L., et al. (2016). Innate and adaptive immune responses in migrating spring-run adult Chinook Salmon, Oncorhynchus tshawytscha. Fish Shellfish Immunol. 48, 136–144. doi: 10.1016/j.fsi.2015.11.015
Dunsmoor L. K., Huntington C. W. (2006). Suitability of environmental conditions within Upper Klamath Lake and the migratory corridor downstream for use by anadromous salmonids—Technical memorandum to the Klamath Tribes (Chiloquin, Oregon), 80.
Ebersole J. L., Liss W. J., Frissell C. A. (1997). Restoration of stream habitats in the western United States: Restoration as re-expression of habitat capacity. Environ. Manage. 21, 1–14. doi: 10.1007/s002679900001
Elliott D. G. (2017). “Pathobiology and protection,” in Renibacterium salmoninarum, fish viruses and bacteria. Eds. Woo P. T. K., Cipriano R. C. (Wallingford, U.K: CABI Publishing), 370.
Federal Register (1997). Final Rule. Endangered and threatened species: Threatened status for Southern Oregon/Northern California Coast evolutionarily significant unit (ESU) of coho salmon, Vol. 62. 24588–24609.
FERC (Federal Energy Regulatory Commission) (2007). Final environmental impact statement for hydropower license, Klamath hydroelectric project, FERC project no. 2082-027, FERC/EIS-0201F (Washington, D.C.: Federal Energy Regulatory Commission, Office of Energy Projects, Division of Hydropower Licensing). Available at: https://www.ferc.gov/industries/hydropower/enviro/eis/2007/11-16-07.asp.
Ferguson J. A., Romer J., Sifneos J. C., Madsen L., Schreck C. B., Glynn M., et al. (2012). Impacts of multispecies parasitism on juvenile coho salmon (Oncorhynchus kisutch) in Oregon. Aquaculture 362–363, 184–192. doi: 10.1016/j.aquaculture.2011.07.003
Ferguson J. A., St-Hilaire S., Peterson T. S., Rodnick K. J., Kent M. L. (2011). Survey of parasites in threatened stocks of Coho salmon (Oncorhynchus kisutch) in Oregon by examination of wet tissues and histology. J. Parasitol. 97 (6), 1085–1098. doi: 10.1645/GE-2757.1
Field J. S., Irwin S. W. B. (1994). “The epidemiology, treatment and control of diplostomiasis on a fish farm in Northern Ireland,” in Parasitic diseases of fish. Eds. Pike A. W., Lewis J. W., 87–100.
Foott J. S., Jacobs J., True K., Magneson M., Bland T. (2016a). Prevalence of Ichthyophthirius multifiliis in both resident and sentinel Speckled dace (Rhinichthys osculus) in the Lower Klamath River (Anderson, CA: U.S. Fish and Wildlife Service California – Nevada Fish Health Center). Available at: http://www.fws.gov/canvfhc/reports.asp.
Foott J. S., Stone R., Fogerty R., True K., Bolick A., Bartholomew J. L., et al. (2016b). Production of Ceratonova shasta myxospores from salmon carcasses: carcass removal is not a viable management option. J. Aquat. Anim. Health 28, 75–84. doi: 10.1080/08997659.2015.1103803
Fujiwara M., Mohr M. S., Greenberg A., Foott J. S., Bartholomew J. L. (2011). Effects of ceratomyxosis on population dynamics of Klamath fall-run Chinook salmon. Trans. Am. Fish. Soc 140, 1380–1391. doi: 10.1080/00028487.2011.621811
Greig S. M., Sear D. A., Carling P. A. (2005). The impact of fine sediment accumulation on the survival of incubating salmon progeny: Implications for sediment management. Sci. Total Environ. 344, 241–258. doi: 10.1016/j.scitotenv.2005.02.010
Hallett S. L., Bartholomew J. L. (2006). Application of a real-time PCR assay to detect and quantify the myxozoan parasite Ceratomyxa shasta in river water samples. Dis. Aquat. Org. 71, 109–118. doi: 10.3354/dao071109
Hallett S. L., Ray R. A., Hurst C. N., Holt R. A., Buckles G., Atkinson S. D., et al. (2012). Density of the waterborne parasite, Ceratomyxa shasta, and biological effects on salmon. Appl. Environ. Microb. 78, 3724–3731. doi: 10.1128/AEM.07801-11
Hamilton J. B., Curtis G. L., Snedaker S. M., White D. K. (2005). Distribution of anadromous fishes in the Upper Klamath River watershed prior to hydropower dams—A synthesis of the historical evidence. Fisheries 30, 10–20. doi: 10.1577/1548-8446(2005)30[10:DOAFIT]2.0.CO;2
Hamilton J. B., Rondorf D., Hampton M., Quinones R., Simondet J., Smith T. (2011). Synthesis of the effects to fish species of two management scenarios for the secretarial determination on removal of the lower four dams on the Klamath (Prepared by the Biological Subgroup for the Secretarial Determination Regarding Potential Removal of the Lower Four Dams on the Klamath River. (Technical Report by the Biological Subgroup for the Secretarial Determination). (The USGS). Available at: https://www.usgs.gov/publications/synthesis-effects-fish-species-two-management-scenarios-secretarial-determination.
Hardy T. B., Addley R. C., Saraeva E. (2006). Evaluation of instream flow needs in the lower Klamath River Phase II: Final report to the U.S. Department of the Interior. 247.
Hart D., Johnson T. E., Bushaw-Newton K. L., Horwitz R. J., Bednarek A. T., Charles D. F., et al. (2002). Dam Removal: Challenges and Opportunities for Ecological Research and River Restoration: We develop a risk assessment framework for understanding how potential responses to dam removal vary with dam and watershed characteristics, which can lead to more effective use of this restoration method. BioScience 52, 669–682. doi: 10.1641/0006-3568(2002)052[0669:drcaof]2.0.co;2
Hendrickson G. L., Carleton A., Manzer D. (1989). Geographic and seasonal distribution of the infective stage of Ceratomyxa shasta (Myxozoa) in Northern California. Dis. Aquat. Org. 7, 165–169. doi: 10.3354/dao007165
Hereford M. E., Wise T. G., Gonyaw A. (2021). “Oregon department of fish and wildlife and the Klamath tribes,” in Implementation plan for the reintroduction of anadromous fishes into the Oregon portion of the Upper Klamath Basin, 125. Available at: https://www.dfw.state.or.us/fish/CRP/docs/klamath_reintroduction_plan/ODFW%20and%20The%20Klamath%20Tribes_Upper%20Klamath%20Basin%20anadromous%20reintroduction%20implementation%20plan_Final%202021.pdf.
Higgins P., Dobush S., Fuller D. (1992). Factors in northern California threatening stocks with extinction (Humboldt Chapter, American Fisheries Society), 25.
Hillemeier D., Belchik M., Soto T., Tucker S. C., Ledwin S. (2017). Measures to reduce Ceratonova shasta infection of Klamath River salmonids: a guidance document (Prepared by members of the Disease Technical Advisory Team).
Hinch S. G., Cooke S. J., Farrell A. P., Miller K. M., Lapointe M., Patterson D. A. (2012). Dead fish swimming: a review of research on the early migration and high premature mortality in adult Fraser River Sockeye Salmon Oncorhynchus nerka. J. Fish Biol. 81, 576–599. doi: 10.1111/j.1095-8649.2012.03360.x
Hodge B. W., Wilzbach M. A., Duffy W. G., Quiñones R. M., Hobbs J. A. (2016). Life history diversity in Klamath River steelhead. Trans. Am. Fish. Soc 145, 227–238. doi: 10.1080/00028487.2015.1111257
Holt R. A., Sanders J. E., Zinn J. L., Fryer J. L., Pilcher K. S. (1975). Relation of water temperature to Flexibacter columnaris infection in steelhead trout (Salmo gairdneri), Coho (Oncorhynchus kisutch) and Chinook (O. tshawytscha) salmon. J. Fish. Res. Board Can. 32, 1553−1559. doi: 10.1139/f75-182
Huntington C. W. (2004). Klamath River flows within the J.C. Boyle Bypass and below the J.C. Boyle Powerhouse (Canby, OR: Clearwater BioStudies).
Huntington C. W. (2006). Estimates of anadromous fish runs above the site of Iron Gate Dam (Canby, OR: Clearwater BioStudies, Inc.).
Hurst C. N., Bartholomew J. L. (2012). Ceratomyxa shasta genotypes cause differential mortality in their salmonid hosts. J. Fish Dis. 35, 725–732. doi: 10.1111/j.1365-2761.2012.01407.x
Hurst C. N., Holt R. A., Bartholomew J. L. (2012). Ceratomyxa shasta in the Williamson River, Oregon: Implications for reintroduced salmon. N. Am. J. Fish. Manage. 32, 14–23. doi: 10.1080/02755947.2012.655843
Jacobson K. C., Arkoosh M. R., Kagley A. N., Clemons E. R., Collier T. K., Casillas E. (2003). Cumulative effects of natural and anthropogenic stress on immune function and disease resistance in juvenile Chinook salmon. J. Aquat. Anim. Health 15, 1–12. doi: 10.1577/1548-8667(2003)015<0001:CEONAA>2.0.CO;2
Jacobson K. C., Teel D., Van Doornik D. M., Castillas E. (2008). Parasite-associated mortality of juvenile Pacific salmon caused by the trematode Nanophyetus salmincola during early marine residence. Mar. Ecol. Prog. Ser. 354, 235–244. doi: 10.3354/meps07232
Jones S. R. M., Prosperi-Porta G., Dawe S. C., Barnes D. P. (2003). Distribution, prevalence, and severity of Parvicapsula minibicornis infections among anadromous salmonids in the Fraser River, British Columbia, Canada. Dis. Aquat. Org. 54, 49–52. doi: 10.3354/dao054049
Jordan M. S. (2012). Hydraulic predictors and seasonal distribution of Manayunkia speciosa density in the Klamath River, CA, with implications for ceratomyxosis, a disease of salmon and trout (Corvallis, OR: Oregon State University).
Kent M. L., Soderlund K., Thomann E., Schreck C. B., Sharpton T. J. (2014). Post-mortem sporulation of Ceratomyxa shasta (Myxozoa) after death in adult Chinook salmon. J. Parasitol. 100, 679–683. doi: 10.1645/13-490.1
Klamath River Renewal Corporation and PacifiCorp (2021). United States of America before the federal energy regulatory commission, amended application for surrender of license for major project and removal of project works, exhibit D hatcheries management and operations plan (Lower Klamath Project. FERC Project No. 14803), 642.
LaFrentz B. R., Králová S., Burbick C. R., Alexander T. L., Phillips C. W., Griffin M. J., et al. (2022). The fish pathogen Flavobacterium columnare represents four distinct species: Flavobacterium columnare, Flavobacterium covae sp. nov., Flavobacterium davisii sp. nov. and Flavobacterium oreochromis sp. nov., and emended description of Flavobacterium columnare. Syst. Appl. Microbiol. 45, 126293. doi: 10.1016/j.syapm.2021.126293
Lauringson M., Kahar S., Veevo T., Silm M., Philpott D., Svirgsden R., et al. (2023). Spatial and intra-host distribution of myxozoan parasite Tetracapsuloides bryosalmonae among Baltic sea trout (Salmo trutta). J. Fish Dis. 00, 1–11. doi: 10.1111/jfd.13827
Lehman B. M., Johnson R. C., Adkison M., Burgess O. T., Connon R. E., Fangue N. A., et al. (2020). Disease in Central Valley Salmon: status and lessons from other systems. San Fran. Estuary Watershed Sci. 18, 1–31. doi: 10.15447//sfews.2020v18iss3art2
Levasseur M., Bergeron R. A., Lapointe M. F., Berube F. (2006). Effects of silt and very fine sand dynamics in Atlantic salmon (Salmo salar) redds on embryo hatching success. Can. J. Fish. Aquat. Sci. 63, 1450–1459. doi: 10.1139/f06-050
Margolis L., McDonald T. E., Whitaker D. J. (1992). Assessment of the impact of the Myxosporean parasite Ceratomyxa shasta on survival of seaward migrating juvenile Chinook Salmon, Oncorhynchus tshawytscha, from the Fraser River, British Columbia. Can. J. Fish. Sci. 49, 1883–1889. doi: 10.1139/f92-208
McElroy E., George A., de Buron I. (2015). The muscle dwelling myxozoan, Kudoa inornata, enhances swimming performance in the spotted seatrout, Cynoscion nebulosus. Parasitol. Res. 114, 2451–2457. doi: 10.1007/s00436-015-4441-z
Messmer R. T., Smith R. C. (2007). Adaptive management for Klamath Lake Redband Trout in Redband Trout: Resilience and challenge in a changing landscape: Proceedings of a workshop, Malheur Field Station, Princeton, Oregon. Eds. Schroeder R. K., Hall J. D. (Oregon Chapter of the American Fisheries Society), 92–98.
Min F., Wang J., Liu X., Yuan Y., Guo Y., Zhu K., et al. (2022). Environmental factors affecting freshwater snail intermediate hosts in Shenzhen and Adjacent Region, South China. Trop. Med. Infect. Dis. 7, 426. doi: 10.3390/tropicalmed7120426
National Research Council (2008). Hydrology, ecology, and fishes of the Klamath River basin (Washington, DC: The National Academies Press). doi: 10.17226/12072
NMFS (National Marine Fisheries Service) (2019). National marine fisheries service biological opinions on the endangered species act section 7(a)(2) biological opinion, and magnuson-stevens fishery conservation and management act essential fish habitat response for Klamath project operations from april 1, 2019 through march 31, 2024, NMFS Consultation Number: WCR-2019-11512; WCRO-2019-00113 2019.
NMFS and USFWS (National Marine Fisheries Service and US Fish and Wildlife Service) (2013). “National marine fisheries service Southwest Region Northern California Office, California, and Fish U.S. and wildlife service Pacific Southwest Region Klamath falls fish and wildlife office, Oregon, USA; 2013,” in Biological opinions on the effects of proposed Klamath project operations from may 31, 2013, through march 31, 2023, on five federally listed threatened and endangered species. NMFS file number: SWR-2012-9372; FWS file number: 08EKLA00-2013-F-0014.
North Coast Regional Board (North Coast Regional Water Quality Control Board) (2010). Klamath River total maximum daily loads (TMDLs) addressing temperature, dissolved oxygen, nutrient, and microcystin impairments in California, the proposed site specific dissolved oxygen objectives for the Klamath River in California, and the Klamath River and Lost River implementation plans (Santa Rosa, CA: Final Staff Report with Appendices. North Coast Regional Water Quality Control Board).
Olson A. (1996). Freshwater rearing strategies of spring Chinook salmon (Oncorhynchus tshawytscha) in Salmon River tributaries, Klamath Basin, California (Arcata, CA: Master’s thesis. Humboldt State University).
PacifiCorp (2004). Klamath Hydroelectric Project (FERC project no. 2082): fish resources (Portland, OR: Final technical report Prepared by PacifiCorp).
PacifiCorp (2005). Klamath River water quality model implementation, calibration, and validation, Klamath Hydroelectric Project (FERC Project No. 2082)—Response to November 10, 2005 (Portland, Oregon, PacifiCorp: FERC AIR GN–2). FERC filing: December 16.
PacifiCorp (2018). PacifiCorp Klamath Hydroelectric Settlement Agreement: implementation report (Federal Energy Regulatory Commission Project No. 2082 2018).
Perkins D., Kann J., Scoppettone G. G. (2000). The role of poor water quality and fish kills in the decline of endangered Lost River and shortnose suckers in Upper Klamath Lake (Klamath Falls, OR: U.S. Geological Survey, Biological Resources Division Report Submitted to U.S. Bureau of Reclamation, Klamath Falls Project Office). 97603 - Contract 4-AA-29-12160.
Petts G. E. (1986). Water quality characteristics of regulated rivers. Prog. Phys. Geog. 10, 492–516. doi: 10.1177/030913338601000402
Puget Sound Steelhead Marine Survival Workgroup (2018). Salish Sea Marine Survival Project – Puget Sound Steelhead Marine Survival: 2013-2017 research findings summary (Seattle, WA: Long Live the Kings). Available at: www.marinesurvivalproject.com.
Quiñones R. M., Grantham T. E., Harvey B. N., Kiernan J. D., Klasson M., Wintzer A. P., et al. (2015). Dam removal and anadromous salmonid (Oncorhynchus spp.) conservation in California. Rev. Fish Biol. Fisheries 25, 195–215. doi: 10.1007/s11160-014-9359-5
Ramos M. M., Ward D. M. (2023). Modeling the reestablishment of coho salmon (Oncorhynchus kisutch) in Klamath River tributaries after dam removal. Ecol. Freshw. Fish 32, 133–146. doi: 10.1111/eff.12679
Ratliff D. E. (1981). Ceratomyxa shasta: epizootiology in Chinook salmon of central Oregon. Trans. Am. Fish. Soc 110, 507–513. doi: 10.1577/1548-8659(1981)110<507:CS>2.0.CO;2
Ray R. A., Alexander J. D., De Leenheer P., Bartholomew J. L. (2015). “Myxozoan evolution, ecology and development,” in Modeling the effects of climate change on disease severity: a case study of Ceratonova (syn Ceratomyxa) shasta in the Klamath River (Cham: Springer), 363–378.
Ray R. A., Bartholomew J. L. (2013). Estimation of transmission dynamics of the Ceratomyxa shasta actinospore to the salmonid host. Parasitology 140, 907–916. doi: 10.1017/S0031182013000127
Ray R. A., Holt R. A., Bartholomew J. L. (2012). Relationship between temperature and Ceratomyxa shasta–induced mortality in Klamath River salmonids. J. Parasitol. 98, 520–527. doi: 10.1645/JP-GE-2737.1
Ray R. A., Perry R. W., Som N. A., Bartholomew J. L. (2014). Using cure models for analyzing the influence of pathogens on salmon survival. Trans. Am. Fish. Soc 143, 387–398. doi: 10.1080/00028487.2013.862183
Robinson H. E., Alexander J. D., Hallett S. L., Som N. A. (2020). Prevalence of infection in hatchery-origin Chinook Salmon (Oncorhynchus tshawytscha) correlates with abundance of Ceratonova shasta spores: implications for management and disease risk. N. Am. J. Fish. Manage. 40, 959–972. doi: 10.1002/nafm.10456
Rohovec J. S., Fryer J. L. (1979). “Fish Health management in aquaculture ,” in Aquaculture: a modern fish tail. Ed. Klingeman P. C. ((Oregon State University: Water Resources Research Institute).
Roon S., Alexander J. D., Jacobson K., Bartholomew J. L. (2015). Effect of Nanophyetus salmincola and bacterial co-infection on mortality of juvenile Chinook Salmon. J. Aquat. Anim. Health 27, 209–216. doi: 10.1080/08997659.2015.1094150
Sanders J. E., Pilcher K. S., Fryer J. L. (1978). Relation of water temperature to bacterial kidney disease in coho salmon (Oncorhynchus kisutch), sockeye salmon (O. nerka), and steelhead trout (Salmo gairdneri). J. Fish. Res. Board Can. 35, 8–11. doi: 10.1139/f78-002
Schaaf C. J., Kelson S. J., Nusslé S. C., Carlson S. M. (2017). Black spot infection in juvenile steelhead trout increases with stream temperature in northern California. Environ. Biol. Fish 100, 733–744. doi: 10.1007/s10641-017-0599-9
Schakau V., Hilker F. M., Lewis M. A. (2019). Fish disease dynamics in changing rivers: Salmonid Ceratomyxosis in the Klamath River. Ecol. Complex. 40, 100776. doi: 10.1016/j.ecocom.2019.100776
Slezak R. (2009). Evaluation of Ceratomyxa shasta and Parvicapsula minibicornis infection in returning adult Chinook salmon (Oncorhnychus tshawytscha) throughout the Klamath River basin (Humboldt State University), 53. Master’s thesis.
St-Hilaire S., Boichuk M., Barnes D., Higgins M., Devlin R., Withler R., et al. (2002). Epizootiology of Parvicapsula minibicornis in Fraser River sockeye salmon, Oncorhynchus nerka (Walbaum). J. Fish. Dis. 25, 107–120. doi: 10.1046/j.1365-2761.2002.00344.x
Stillwater Sciences (2008). Klamath River dam removal study: sediment transport DREAM-1 simulation (Oakland, CA: Technical Report. Prepared by Stillwater Sciences, Arcata, California for California Coastal Conservancy).
Stillwater Sciences (2010). Anticipated sediment release from Klamath River dam removal within the context of basin sediment delivery (Oakland, CA: Final Report. Prepared by Stillwater Sciences, Berkeley, California for State Coastal Conservancy).
Stocking R. W., Bartholomew J. L. (2007). Distribution and habitat characteristics of Manayunkia speciosa and infection prevalence with the parasite Ceratomyxa shasta in the Klamath River, Oregon-California. J. Parasitol. 93, 78–89. doi: 10.1645/GE-939R.1
Stocking R. W., Holt R. A., Foott J. S., Bartholomew J. L. (2006). Spatial and temporal occurrence of the salmonid parasite Ceratomyxa shasta in the Oregon–California Klamath River Basin. J. Aquat. Anim. Health 18, 194–202. doi: 10.1577/H05-036.1
Stone R., Foott J. S., Fogerty R. (2008). Comparative susceptibility to infection and disease from Ceratomyxa shasta and Parvicapsula minibicornis in Klamath River basin juvenile Chinook, coho and steelhead populations (Anderson, CA: U.S. Fish and Wildlife Service, California-Nevada Fish Health Center).
Sykes G. E., Johnson C. J., Shrimpton J. M. (2009). Temperature and flow effects on migration timing of Chinook Salmon smolts. Trans. Am. Fish. Soc 138, 1252–1265. doi: 10.1577/T08-180.1
Thorsteinson L., VanderKooi S., Duffy W. (Eds.) (2011). in Proceedings of the Klamath Basin Science Conference, Medford, Oregon, February 1–5, 2010, 312, U.S. Geological Survey Open-File Report 2011-1196. (USGS). Available at: https://pubs.usgs.gov/of/2011/1196/pdf/ofr20111196.pdf.
Traxler G. S., Richard J., McDonald T. E. (1998). Ichthyophthirius multifiliis (Ich) epizootics in spawning sockeye salmon in British Columbia, Canada. J.Aquat. Anim. Health 10, 143–151. doi: 10.1577/1548-8667(1998)010<0143:IMIEIS>2.0.CO;2
U.S. Bureau of Reclamation [USBR] (2011). Appendix E – an analysis of potential suspended sediment effects on anadromous fish in the Klamath Basin (Denver, CO: Prepared for Mid-Pacific Region, Bureau of Reclamation, Technical Service Center), 70.
U.S. Bureau of Reclamation [USBR], California Department of Fish and Game [CDFG] (2012) Klamath facilities removal environmental impact statement/environmental impact report. Volume I and volume II. Available at: https://Klamathrestoration.gov/Draft-EIS-EIR/download-draft-eis-eir.
Voss A., Benson C., Freund S. (2022). Myxosporean Parasite (Ceratonova shasta and Parvicapsula minibicornis) Prevalence of Infection in Klamath River Basin Juvenile Chinook Salmon (Anderson, CA: U.S. Fish and Wildlife Service. California – Nevada Fish Health Center). March – July 2021.
Voss A., Benson C., Freund S. (2023). Myxosporean Parasite (Ceratonova shasta and Parvicapsula minibicornis) prevalence of infection in Klamath River Basin juvenile Chinook Salmon (Anderson, CA: U.S. Fish and Wildlife Service. California – Nevada Fish Health Center). March – August 2022.
Voss A., True K., Foott J. (2018). Myxosporean Parasite (Ceratonova shasta and Parvicapsula minibicornis) Prevalence of Infection in Klamath River Basin Juvenile Chinook Salmon, March-August 2018 (Anderson, CA: U.S. Fish and Wildlife Service California – Nevada Fish Health Center). Available at: http://www.fws.gov/canvfhc/reports.html.
Wagner G. N., Hinch S. G., Kuchel L. J., Lotto A., Jones S. R. M., Patterson D. A., et al. (2005). Metabolic rates and swimming performance of adult Fraser River Sockeye Salmon (Oncorhynchus nerka) after a controlled infection with Parvicapsula minibicornis. Can. J. Fish. Aquat. Sci. 62, 2124–2133. doi: 10.1139/f05-126
Walker R. L., Foott J. S. (1993). Disease survey of Klamath River salmonid smolt populations (USFWS).
Weitkamp D. E., Katz M. (1980). A review of dissolved gas supersaturation literature. Trans. Am. Fish. Soc 109, 659–702. doi: 10.1577/1548-8659(1980)109<659:ARODGS>2.0.CO;2
Williams T. H., Bjorkstedt E. P., Duffy W., Hillemeier D., Kautsky G., Lisle T., et al. (2006). Historical population structure of coho salmon in the Southern Oregon/Northern California Coasts Evolutionarily Significant Unit (NOAA Technical Memorandum NMFS-SWFSC 390: U.S. Dep. Commerce).
Williams T. H., East A. E., Smith D. P., Boughton D. A., Mantua N., Harrison L. R. (2018). Removal of San Clemente Dam: it did more than restore fish passage. Osprey 89 (1), 4–9.
Keywords: Klamath River, salmonids, dam removals, disease, redistribution, pathogens, parasites, Ceratonova shasta
Citation: Bartholomew JL, Alexander JD, Alvarez J, Atkinson SD, Belchik M, Bjork SJ, Foott JS, Gonyaw A, Hereford ME, Holt RA, McCovey B Jr, Som NA, Soto T, Voss A, Williams TH, Wise TG and Hallett SL (2023) Deconstructing dams and disease: predictions for salmon disease risk following Klamath River dam removals. Front. Ecol. Evol. 11:1245967. doi: 10.3389/fevo.2023.1245967
Received: 24 June 2023; Accepted: 08 September 2023;
Published: 27 October 2023.
Edited by:
Jeffrey J. Duda, Western Fisheries Research Center, United StatesReviewed by:
Beth Okamura, Natural History Museum, United KingdomJames Winton, United States Department of the Interior, United States
Copyright © 2023 Bartholomew, Alexander, Alvarez, Atkinson, Belchik, Bjork, Foott, Gonyaw, Hereford, Holt, McCovey, Som, Soto, Voss, Williams, Wise and Hallett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sascha L. Hallett, c2FzY2hhLmhhbGxldHRAb3JlZ29uc3RhdGUuZWR1
 Jerri L. Bartholomew
Jerri L. Bartholomew Julie D. Alexander
Julie D. Alexander Justin Alvarez2
Justin Alvarez2 Stephen D. Atkinson
Stephen D. Atkinson Michael Belchik
Michael Belchik Sarah J. Bjork
Sarah J. Bjork J. Scott Foott
J. Scott Foott Mark E. Hereford
Mark E. Hereford Sascha L. Hallett
Sascha L. Hallett