- Department of Entomology, Plant Pathology and Nematology, University of Idaho, Moscow, ID, United States
Chemical signaling underpins behavioral interactions among organisms in the soil. Understanding chemical communication in the soil requires a paradigm shift in methodology and perspectives compared to aboveground ecosystems because olfaction and gustation, accepted modalities of chemosensation aboveground, may not accurately represent chemical communication in the soil. To fully understand chemical communication in the soil, it is essential to consider how soil properties, such as moisture, pH, and adsorption, affect the transport and perception of semiochemicals. De-anthropomorphizing the study of chemosensation can avoid potential biases, particularly in soil systems, where distinctions between olfaction and gustation are confounded by the heterogeneity of the soil environment and its effects on the mobility of chemical signals. In this perspective, we first explore how soil heterogeneity confounds the dichotomy between olfaction and gustation with hypothetical but ecologically relevant examples. Then we examine how anthropomorphic biases in aboveground chemical ecology have influenced soil chemical ecology. Our examples and discussion are prepared primarily in reference to soil arthropods. We conclude by discussing seven future research directions and outstanding questions. The soil is a premier example of a system where investigators should avoid anthropomorphisms when studying behavioral and chemical ecology. Research in soil chemical ecology should further efforts towards developing a unified view of chemosensation that could apply to all environments where chemical communication occurs.
1 Introduction
Olfaction and gustation are two accepted modalities of chemosensation used by animals. In aboveground terrestrial ecosystems, olfaction detects airborne chemicals at a distance from the emitter and these are often referred to as “odors”. In contrast, gustation detects chemical cues, referred to as “tastes”, on the surface or within the emitter during feeding. The distinction between odor and taste, however, is operational, context dependent, and variable depending on environmental conditions.
Recently, there has been a call to avoid the olfaction and gustation dichotomy, which reflects the human chemosensory experience as terrestrial organisms but is not representative of chemosensation in many environments. De-anthropomorphizing the study of chemosensation can avoid potential biases that may impede progress in chemical ecology discovery and application (Mollo et al., 2014; Mollo et al., 2017; Mollo et al., 2022). Avoiding anthropomorphisms has been a tenet of the study of animal behavior (Kennedy, 1992) that should be extended to include chemosensation (Mollo et al., 2022).
In soil matrices, distinctions between olfaction and gustation are confounded by the heterogeneity of the soil environment and its effects on the mobility of chemosensory signals. Following this special collection’s theme, we make the case that de-anthropomorphizing chemical ecology will be especially valuable for understanding chemically mediated interactions in soil systems. We then show how anthropomorphic biases prevalent in aboveground chemical ecology have influenced soil chemical ecology. We conclude with a discussion of future research directions and outstanding questions. Studying chemical communication in ecological environments where humans have no direct experiences, such as the soil matrix, is a crucial component and benefit of de-anthropomorphizing chemosensory science.
2 De-anthropomorphizing chemosensory science: go belowground
Semiochemicals are individual chemical compounds or blends released by organisms that affect the behavior of other individuals. Depending on their mobility in the environment, semiochemicals can function at some distance from the emitter, in its immediate vicinity, or on contact with a receiver. In terrestrial environments, volatile semiochemicals are considered olfactory and function at a distance, while those with low or no volatility are gustatory. In marine or aquatic environments, the reverse tends to be the case (Mollo et al., 2017). In the soil matrix, these distinctions apply variably at fine spatial scales, creating a complex chemosensory environment that must be navigated by soil-dwelling organisms (Figure 1). Olfactory semiochemicals aboveground primarily move through air where temperature, atmospheric pressure, and turbulence can affect compound diffusion and mobility, although solid or liquid substrates (plant surfaces and aerosols) can influence movement of these compounds (Murlis et al., 1992; Vickers, 2006). Adapting to this, terrestrial organisms have developed behaviors that enable them to navigate volatile gradients or heterogeneous plumes (Vickers, 2000). Similarly, in aquatic and marine environments, water soluble compounds move primarily by diffusion or in plumes within the water column, and organisms can respond to this chemosensory milieu (Mathewson and Hodgson, 1972; Stocker and Seymour, 2012). Organisms that respond to these cues benefit by finding resources, thereby improving their fitness. Soil, in contrast to terrestrial, aquatic, and marine systems, is a more heterogeneous environment that includes solid, liquid, and gas phases that change dynamically throughout the soil profile, affecting semiochemical movement and presenting unique challenges to organisms that must navigate this habitat.
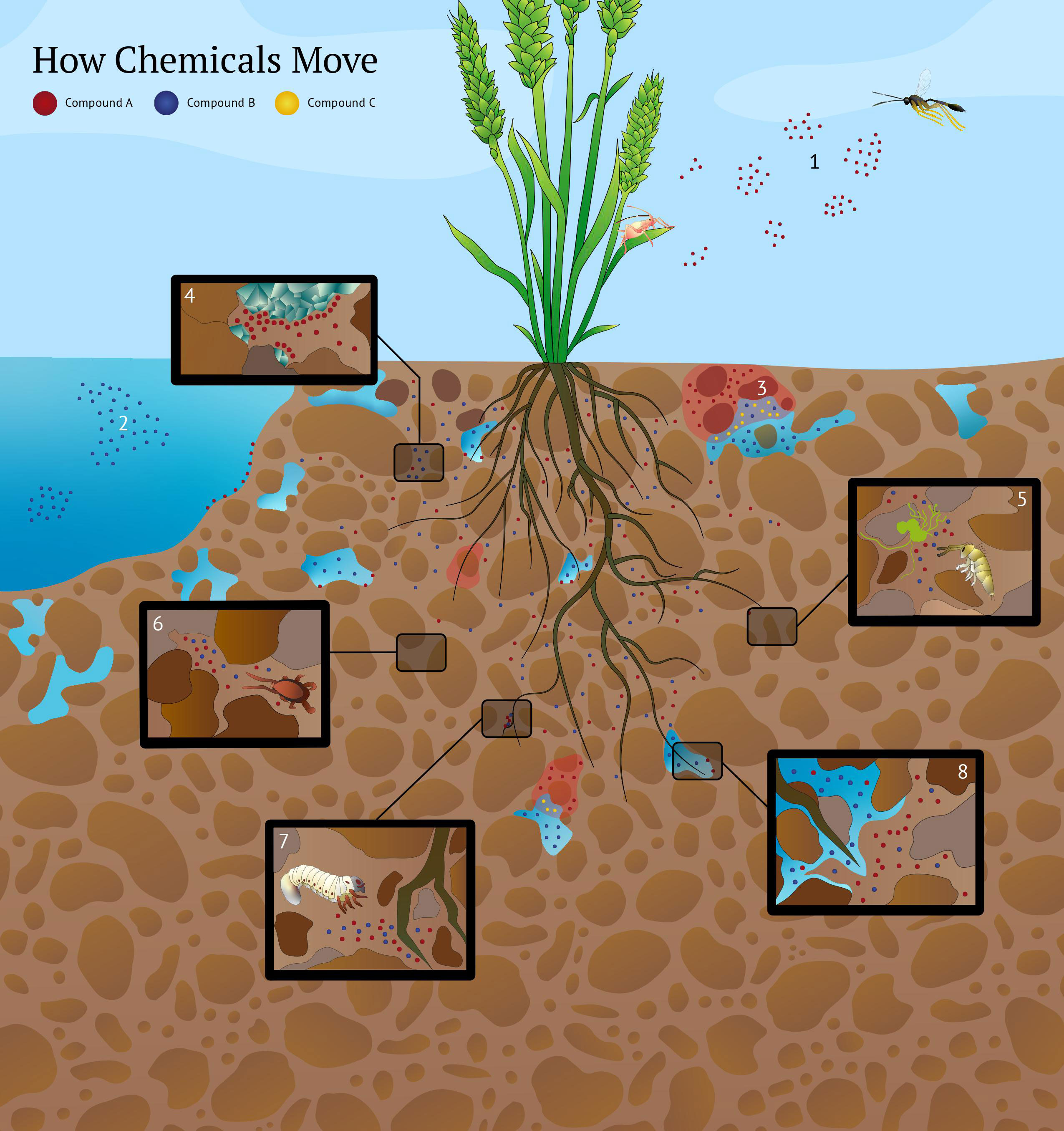
Figure 1 How chemicals move. Compounds A, B and C represent hypothetical semiochemicals. On the continuum of volatility and solubility, compound A is more volatile than water-soluble. Compound B is more water soluble than volatile. Compound C solubility is affected by the pH of water-filled soil pores. Semiochemicals move through heterogeneous soil pores since they are active in gas and liquid phases and capable of re-volatilization after moving through water-filled pores. Eight scenarios are illustrated above. 1) A simplified representation of odor plume packets aboveground. Originating from an herbivore-infested plant, the semiochemical odor packets (patchily distributed pockets of high semiochemical concentrations interspersed by semiochemical-free air) move downwind and elicit a behavioral response from a parasitoid wasp. The movement of a semiochemical in air aboveground is affected by its volatility, air temperature, humidity, wind velocity, and turbulence. 2) Movement of a semiochemical in water is affected by its solubility, temperature, currents, and turbulence. Here, compound B is more water soluble than volatile, enabling it to be transported easily in an aqueous environment. In contrast to air aboveground (scenario 1), compound A is less soluble in water and has limited mobility. The air-filled and water-filled soil pores will impact the movement of semiochemicals A and B differently (scenario 8). 3) Movement of semiochemicals in the soil is affected by soil pH. The acidity of water-filled soil pores can increase the solubility of semiochemicals. For example, sesquiterpenes poorly soluble in water can have their solubility enhanced by low pH in water-filled soil pores. Here, compound C’s diffusion is greater in lower pH areas (represented by red shading). 4) Semiochemical movement in soil is affected by chemical adsorption to soil particles’ mineral and organic fractions. Here, compound A is adsorbed to clay minerals in the soil matrix. Adsorption of semiochemicals can limit the transport of compounds through the soil matrix or generate stable concentration gradients over a distance. Soil organisms may perceive adsorbed semiochemicals through contact-based chemoreception, allowing them to exploit adsorbed semiochemical gradients as distance-based cues. Soil pH can influence adsorption, with lower adsorption in acidic soils. 5) Fungi release semiochemicals differing in solubility that act as kairomones for soil organisms such as Collembola. Collembola exhibit orientation and feeding preferences to certain fungal strains based on their semiochemical profiles (Salmon et al., 2019). Fungal defense chemicals also influence the aggregation behavior of Collembola (Salmon et al., 2019). It remains unknown how changing abiotic soil conditions affect the movement of fungal semiochemicals perceived by Collembola. 6) The tortuosity and physical structure of the soil matrix is important in regulating semiochemical diffusion and perception by soil organisms. Dead-end pores and discontinuities in the soil matrix can limit the transport of semiochemicals and affect soil organism behavior. Predatory mites use semiochemicals from fungi to hunt their Collembola prey (Pfeffer and Filser, 2010). Here, these fungal cues accumulate in a dead-end pore space and disrupt the ability of a predatory mite to locate its prey. The soil’s physical structure can regulate trophic interactions through its effect on semiochemical movement. 7) Plant roots release a variety of semiochemicals that differ in solubility and are used by root-feeding herbivores to locate their host plants. Here, the soil-dwelling larvae of Melolontha melolontha uses semiochemical cues to navigate toward its host plant. M. melolontha possesses gustatory sensilla that can perceive cues that span the volatility–solubility continuum and are challenging to categorize as specifically “olfactory” or “gustatory”. 8) Semiochemicals in the soil transverse through air-filled and water-filled pores and may behave differently depending on their solubility. Here, compound B’s movement is enhanced in water-filled soil pores relative to compound A because it is more water-soluble than volatile. Compound A still moves through the water-filled pore space, albeit at a slower rate. In contrast, the diffusion of compound A is enhanced in air-filled pores relative to compound B because it is more volatile than soluble. But compound B can still move through the air-filled pore space. Solubility and volatility exist on a continuum and are affected by microscale physiochemical conditions in the soil. Therefore, the abiotic conditions (e.g., soil moisture) of the soil present when soil organisms encounter specific semiochemicals could cause the perception of the same chemical compounds to switch from “gustatory” to “olfactory” and vice versa.
This perspective is prepared primarily in reference to soil arthropods, a group that comprises 85% of all described soil-dwelling animal taxa (Decaëns et al., 2006). While nematodes are the most abundant soil animals, they are functionally aquatic and require water to live and move (Neher, 2010). Arthropods, in contrast, experience and must contend with the heterogeneity of the soil matrix and its effects on semiochemical movement.
Semiochemicals move through soil by diffusion through water-filled or gas-filled pores and channels and by advection in the aqueous phase (Minnich and Schumacher, 1993). They also interact with solids in the soil (salts, organic matter, sand, silt, and clay). In contrast to aboveground systems, semiochemical movement in the soil is affected by soil moisture content (Hiltpold and Turlings, 2008; Som et al., 2017; Xavier Chiriboga et al., 2017), soil pH (Som et al., 2017), and the reactivity of soil particles (Insam and Seewald, 2010). Drought and rain events change the water and gaseous phase distributions in soil and in turn the movement patterns of semiochemicals. This may influence the behavior of soil-dwelling organisms, such as root-feeding insects. For example, Diabrotica virgifera virgifera uses [E]-β-caryophyllene (C15H24) to locate maize roots (Robert et al., 2012) and Hylobius abietis uses α-pinene (C10H16) to find pine tree roots (Nordenhem and Nordlander, 1994). Abiotic conditions in the soil influence the movement of these terpene semiochemicals differently. [E]-β-caryophyllene (Log Kow: 6.30) diffuses over a greater distance and more rapidly at low soil moisture levels (Hiltpold and Turlings, 2008) but α-pinene (Log Kow: 4.27) shows an opposite effect (Som et al., 2017), likely reflecting the lower lipophilicity of α-pinene. The soil’s abiotic components influence semiochemical movement and have the potential to modulate the behavior of organisms responding to chemical cues in the soil.
These abiotic factors make the soil a profoundly different medium than air or water alone and one in which the categories “olfaction” or “gustation” become indistinct. For example, volatile organic compounds (VOCs) enable long-distance communication as they diffuse through air-filled pore spaces and tortuous channels in the soil, but upon encountering water-filled soil pores, depending on their physiochemical properties, they are either trapped and prevented from moving or continue to diffuse but at different rates, depending upon their water solubility. All chemical compounds exhibit a continuum of volatility and solubility (Mollo et al., 2017). Depending on the soil environment, root volatiles like hexanal (C6H10O) (Log Kow: 1.80) (Liu et al., 2016) that are volatile but also have relatively high water solubility will traverse the air- and water-filled pores of the soil matrix differently than a compound like methyl jasmonate (C13H20O3) which is volatile but weakly soluble in water (Log Kow: 2.76) (Mollo et al., 2017). The pH of water-filled soil pores can also affect the transport of VOCs. For example, acidic water in soil pores could enhance the solubility of sesquiterpenes via protonation (Matsuoka et al., 2017). Clay–humus complexes may enhance or retard the diffusion of VOCs depending on the chemical interactions at this interface. The adsorption of VOCs by soil particles depends on pH, with higher adsorption in alkaline than in acidic soils (Insam and Seewald, 2010). As a result of these effects and depending on conditions, specific semiochemicals can serve as either reliable long-distance cues, more localized cues, or both. How organisms deal with environmental heterogeneity when responding to chemical cues has been studied in marine environments and could pertain in soil aqueous phases (Stocker and Seymour, 2012). The potentially widely differing behavior of specific compounds depending on soil physiochemical properties and transient levels of saturation blurs the distinction between olfaction (a distance sense) and gustation (a contact sense). Attempts to understand chemical ecology within the soil should discard those notions in favor of approaches that delineate the diverse and dynamic movement of potential chemical cues.
Abiotic conditions in the soil also impact how soil organisms detect and respond behaviorally to cues (Erktan et al., 2020). Consider a soil organism using VOCs as cues to forage or find a mate belowground. If the soil organism encounters VOCs in an air-filled pore space, those VOCs are typically considered olfactory cues. If the soil organism encounters the same VOCs diffusing relatively slowly through a water-filled pore space, these cues might be categorized as localized or gustatory. Regardless of how classified, the adaptive behavioral response to specific cues could differ substantially depending on conditions. The same compound could be perceived both at a distance via olfaction in air-filled pores and by contact via gustation in water-filled pores, depending on the abiotic conditions in the soil. Theoretically, organisms navigating such an environment would be adapted to utilize specific cues optimally depending on context. Responses to a resource associated cue could range from positive orthokinesis and taxis accompanied by reduced klinokineses in a drier soil to reduced orthokinesis and increased klinokinesis or arrestment in response to the same compound in a wetter soil.
Similarly, VOCs behavior could vary when diffusing through air-filled pores bordered by non-reactive substrates versus those bordered by clay particles with adsorptive charged surfaces. Depending on their properties, VOCs could become adsorbed onto soil particles and form a stable chemical gradient towards the emitter (McGechan and Lewis, 2002). Soil organisms would encounter adsorbed cues and perceive them by contact (i.e., gustation) along the gradient. Therefore, gustation could also act as a “distance” sense in heterogenous soil environments where gradients of adsorbed semiochemicals form. These theoretical examples challenge the traditional definitions of olfaction and gustation.
Freed from the notions of olfaction and gustation, the study of soil organism chemical ecology can better incorporate the dynamics inherent in the heterogeneous soil matrix. The examples that follow illustrate how an anthropomorphic view of olfaction and gustation has influenced belowground chemical ecology, potentially preventing understanding of mechanisms in play.
3 Are aboveground conventions already biasing understanding of belowground chemical ecology?
Bioassays that examine organism responses to chemical cues are vital for chemical ecology research. Arena assays and olfactometers are standard bioassay methods to evaluate aboveground arthropod behavioral responses to chemical compounds (Hare, 1998). Arena bioassays typically involve placing an individual organism in a flat arena, such as a Petri dish, containing cues and making qualitative and quantitative measurements of an organism’s behavioral response, presumably to olfactory cues diffusing in the arena volume. Similarly, y-tube and multi-arm olfactometers involve measuring an individual organism’s response to stimuli administered in moving air in different arms of the olfactometer. These methods have advanced knowledge of chemical ecology in aboveground systems but adopting them directly into studies of belowground chemical ecology may be biasing the design and interpretation of experiments.
A key component for a successful belowground bioassay is the medium for organism dispersion and semiochemical diffusion. Several investigations in belowground chemical ecology using entomopathogenic nematodes (EPNs) have successfully used olfactometers or arenas traditionally used in aboveground chemical ecology, adapted for belowground bioassays. These olfactometers and arenas are typically filled with sand (Rasmann et al., 2005; Ali et al., 2010) or field-collected soils (Hiltpold and Turlings, 2008; Xavier Chiriboga et al., 2017). EPNs are placed in the olfactometers or arenas to measure their responses to various cues while navigating the semi-realistic environment that can allow for manipulating abiotic factors, like moisture content or particle size.
In contrast, studies investigating the behavior of soil arthropods rarely use belowground-adapted arenas or olfactometers (Johnson and Gregory, 2006; Erktan et al., 2020). For example, studies evaluating the responses to chemical cues of soil-dwelling mites (Hall and Hedlund, 1999; Aratchige et al., 2004; Brückner et al., 2018) and Collembola (Bengtsson et al., 1988; Bengtsson et al., 1991; Hedlund et al., 1995; Salmon and Ponge, 2001; Nilsson and Bengtsson, 2004; Staaden et al., 2011; Zizzari et al., 2017; Becher et al., 2020) use bioassays that omit the effects of the soil matrix (i.e., arenas and olfactometers adopted from aboveground methods). Most of these studies do not address this limitation, but Aratchige et al. (2004) acknowledged the importance of replicating their results using a belowground-adapted olfactometer. Except for Hall and Hedlund (1999), who discuss whether cues could be entirely olfactory, gustatory, or a mix, all studies cited above categorized the chemosensory responses of their focal soil organisms as olfaction. Notably, the bioassay designs used in the studies cited above were suited to detect olfaction and consider organism responses from an aboveground perspective. They neglected the soil’s impact on semiochemical diffusion and arthropod perception and response (Erktan et al., 2020). Without belowground-adapted bioassays that simulate the soil environment, it is difficult or impossible to decipher when compounds function in an olfactory mode, a gustatory mode, or function dynamically depending upon soil conditions. De-anthropomorphizing soil chemical ecology is a first step to seeing past human biases to understand how these belowground systems function chemically while recognizing possible pitfalls of imposing the olfaction-gustation dichotomy prevalent in aboveground chemical ecology.
Several studies of root-feeding arthropod responses to chemical cues from plant roots used bioassay methods designed to mimic the soil environment (e.g., Hibbard and Bjostad, 1989; Horton and Landolt, 2002; Johnson et al., 2004; Weissteiner et al., 2012; Kojima et al., 2014; Rostas et al., 2015; Liu et al., 2016; Wu and Duncan, 2020). This likely reflects the economic importance of root-feeding arthropods and the necessity to grow plants in soil to conduct meaningful experiments. In contrast, we are aware of only two studies that use belowground-adapted bioassays to investigate how chemical cues mediate foraging behavior by predatory arthropods in the soil, one on soil mites (Pfeffer and Filser, 2010) and one on larvae of Carabidae (Coleoptera) (Thomas et al., 2008). As a result, more is known about the chemically mediated foraging of arthropod herbivores belowground compared to other functional groups, such as predators, microbivores, and detritivores, that inhabit the soil (Johnson and Gregory, 2006; Johnson and Nielsen, 2012).
4 Future research directions
The soil is a unique and challenging environment in which to study chemical communication and one that has been neglected until relatively recently. Importantly, human experiences are largely irrelevant to conceptualizing the processes at play in the soil matrix. Anthropomorphisms are irrelevant or misleading. In addition, the soil environment introduces nested complexities resulting from heterogeneity at multiple scales that must be appreciated to begin deciphering how soil arthropods negotiate these environments. This perspective engenders seven potential future research directions and outstanding questions:
1. Dynamic mobility of chemical cues in the soil. Semiochemicals move differently through the soil depending upon soil characteristics and transient abiotic conditions. Presumably, soil dwelling organisms have evolved adaptations that enable effective responses to cues, facilitating resource location. They exhibit the ability to perceive and respond to specific compounds as either distance or local cues based on the prevailing soil conditions (Ehlers et al., 2020). This may require integration of sensory information about soil conditions with information about the presence of specific chemical cues. Multisensory integration has been studied in terrestrial (Miller et al, 1984) and marine organisms (Gardiner et al., 2014), but this sort of behavioral and sensory plasticity by soil dwelling arthropods appears to be uninvestigated. While integration of visual and olfactory cues, like those used by terrestrial and marine organisms, can make intuitive anthropomorphic sense to human investigators, the types of integration among cues available in the soil will be best studied divested of anthropomorphic intuition.
2. Semiochemical blends in the soil. In terrestrial and marine systems, resource location often depends upon blends of semiochemicals rather than specific compounds (Schoonhoven et al., 2005; Zhang et al., 2011). If blend integrity is as essential for chemical responses belowground as it is aboveground, information could be lost or altered (Som et al., 2017) depending on abiotic soil conditions and how individual blend components behave. For example, if a hypothetical blend of [E]-β-caryophyllene and α-pinene in specific proportions was requisite for a behavioral response in a receiving organism, the moisture content of the soil could disrupt this blend over a distance due to the differing rates of movement of the two compounds. Blend ratios could easily be altered depending on soil moisture, pH, clay content or other conditions. How soil organisms respond to chemical blends that vary spatiotemporally is uninvestigated.
3. Bioassays should capture the complexity of the soil environment. First steps to decipher how soil organisms respond given the complexities outlined in (1) require bioassays that incorporate soil structure, soil moisture, and other abiotic conditions (e.g., clay content or pH). Changing abiotic soil parameters will alter the movement of aqueous and volatile semiochemicals (Som et al., 2017) and affect the behavior of responding organisms (Erktan et al., 2020). Bioassay devices can be modified to simulate soil environmental conditions by filling them with a matrix of sand, soil, peat, or humus, for example, through which the cues and the responding organisms could move. These bioassay designs create a more realistic environment for semiochemicals to move and for soil organisms to respond to tested cues. For example, organisms can move vertically and horizontally and employ thigmotaxis in response to soil properties. Differences in organism responses could be related to differences in the performance of semiochemicals in varying soil environments and improve predictive capabilities. Using bioassay approaches specific to soil systems involves discarding aboveground-based anthropomorphic biases on chemical movement and perception by responding organisms. The term “olfactometer” is problematic in light of the premise of this paper. We caution against the use of this term when the true modality of the response is uncertain.
4. Sensory structures in the soil defy the olfaction-gustation dichotomy. Since specific compounds in soil may be perceived in different ways depending upon conditions, the morphology and sensitivity of chemosensory sensilla of soil dwelling organisms should reflect this. The soil-dwelling larvae of the herbivorous European cockchafer Melolontha melolontha (Coleoptera: Scarabaeidae) possess gustatory sensilla, based on their morphology, that perceive both “olfactory” and “gustatory” cues (Eilers et al., 2012). Similarly, the foretarsal sensory organ present on soil-dwelling mites has multiple sensory functions that are not well understood (Carr and Roe, 2016) and could play a role in chemosensory perception under different environmental conditions. Other soil-dwelling arthropods may have similar variability in the morphology and sensitivity of chemosensory structures appropriate for how specific cues are perceived, but this has yet to be investigated. Knowledge of these adaptations, in turn, could guide studies of how these structures are used for resource location in a heterogenous soil environment. Furthermore, correctly characterizing the differing modalities of the developmental stages of arthropods that are soil-dwelling as larvae and live aboveground as adults can help understand the evolution of the sensory repertoire of these species.
5. Evolutionary implications. The first land animals were arthropods (Little, 1983) and among these colonizing taxa were many groups that are soil dwelling. Challenges to terrestrial life, including support, gas exchange, and prevention of water loss are less severe in the soil, facilitating terrestrial colonization, but sensory challenges of navigating the heterogeneous soil environment would have been substantial. The ability of soil organisms to adapt to this heterogeneity, and specifically to detect and respond to semiochemicals in air-filled pore spaces would have preceded adaptation to terrestrial systems, consistent with the notion that transitioning to terrestrial life did not involve abrupt evolutionary changes to perceive “smells” on land (Mollo et al., 2014; Mollo et al., 2017; Mollo et al., 2022). Rather, odorant-receptors (ORs) that perceive insoluble (or very low solubility) and highly volatile semiochemicals by contact existed for primordial aquatic organisms, and these pre-existing ORs were primed for the detection of airborne distance-based volatile cues during the transition to land. It is possible that olfaction as we know it first evolved in soil organisms, preadapting them for colonization of entirely aboveground habitats. This hypothetical scenario provides a context for studying the chemosensory aspects of terrestrial animal life.
6. Applied belowground chemical ecology. There is growing interest in the use of chemical ecology for optimizing pest control belowground in agricultural systems (Hiltpold and Turlings, 2012; Torto et al., 2018). However, the development and implementation of novel pest control techniques could be impeded if the influence of soil abiotic properties such as pH, moisture, tortuosity, and adsorption on semiochemical movement are ignored. Isotopic labeling could be an effective method to understand how abiotic soil properties affect the movement of chemical signals under field conditions (Johnson et al., 2018). Improved understanding of these factors will allow humans to manipulate the soil system to favor or inhibit beneficial and detrimental interactions. Several agricultural management practices alter soil abiotic properties and may have a measurable and predictable impact on the responses of soil organisms to chemical signals. It is inappropriate to take examples from aboveground, like attract-and-kill (Gregg et al., 2018) or push–pull systems (Cook et al., 2007; Eigenbrode et al., 2015), belowground without considering the influence of soil abiotic properties on chemical communication and resulting behavioral responses.
7. Communication across kingdoms. While this perspective focuses on soil arthropods, the concepts and patterns presented have implications for other organisms that use chemical communication in the soil, such as plants, fungi, and bacteria (reviewed in Effmert et al., 2012; Ehlers et al., 2020). Soil microorganisms also contend with the heterogeneity of the soil and its effects on chemical movement (Schmidt et al., 2019). For example, abiotic properties of the soil matrix can reduce the efficacy of flavonoid signals produced by legumes that attract nitrogen-fixing bacteria (Del Valle et al., 2020). Ideas presented in this paper could be considered in future investigations of chemical communication in other organisms across the several kingdoms of life that inhabit the soil.
5 Conclusion
The soil matrix is a dynamic and complex environment that variously affects the mobility of molecules, thwarting efforts to categorize “olfactory” and “gustatory” semiochemicals. This makes it a premier example of the need to avoid the fallacies of anthropomorphisms when studying chemical ecology and behavior. Such categories have their historic uses but derive from human-centric perspectives that apply to chemosensation aboveground but fail to capture the dynamism and complexity of chemical communication belowground. Research in soil chemical ecology should further the development of a unified view of chemosensation applicable to all environments where chemical communication occurs (Mollo et al., 2017; Mollo et al., 2022).
Author contributions
DE developed the article concept, conducted the research, drafted and edited the manuscript. SE developed the article concept, conducted the research, drafted and edited the manuscript.
Funding
This work was supported by USDA-NIFA predoc #2022-67011-36633 and USDA-NIFA CAP #2017-68002-26819. USDA is an equal opportunity employer and service provider. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Acknowledgments
We thank Delphine Keim, Ashlynn Shoolroy, Megan Schwartz, and Hayley Bowring from the Art and Design Department in the College of Art and Architecture at the University of Idaho for creating Figure 1. We also thank Ed Lewis for helpful comments on an earlier version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali J. G., Alborn H. T., Stelinski L. L. (2010). Subterranean Herbivore-induced Volatiles Released by Citrus Roots upon Feeding by Diaprepes abbreviatus Recruit Entomopathogenic Nematodes. J. Chem. Ecol. 36, 361–368. doi: 10.1007/s10886-010-9773-7
Aratchige N. S., Lesna I., Sabelis M. W. (2004). Below-ground plant parts emit herbivore-induced volatiles: olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp. Appl. Acarol. 33, 21–30. doi: 10.1023/B:APPA.0000030011.66371.3f
Becher P. G., Verschut V., Bibb M. J., Bush M. J., Molnár B. P., Barane E., et al. (2020). Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 5, 821–829. doi: 10.1038/s41564-020-0697-x
Bengtsson G., Erlandsson A., Rundgren S. (1988). Fungal odour attracts soil Collembola. Soil Biol. Biochem. 20 (1), 25–30. doi: 10.1016/0038-0717(88)90122-8
Bengtsson G., Hedlund K., Rundgren S. (1991). Selective odor perception in the soil Collembola Onychiurus armatus. J. Chem. Ecol. 17 (11), 2113–2125. doi: 10.1007/BF00987995
Brückner A., Schuster R., Smit T., Pollierer M. M., Schäffler I., Heethoff M. (2018). Track the snack- olfactory cues shape foraging behaviour of decomposing soil mites (Oribatida). Pedobiologia. 66, 74–80. doi: 10.1016/j.pedobi.2017.10.004
Carr A. L., Roe M. (2016). Acarine attractants: Chemoreception, bioassay, chemistry and control. Pestic. Biochem. Physiol. 131, 60–79. doi: 10.1016/j.pestbp.2015.12.009
Cook S. M., Khan Z. R., Pickett J. A. (2007). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Decaëns T., Jimenez J. J., Gioia C., Measey G. J., Lavelle P. (2006). The values of soil animals for conservation biology. Eur. J. Soil Biol. 42 (1), S23–S38. doi: 10.1016/j.ejsobi.2006.07.001
Del Valle I., Webster T. M., Cheng H., Thies J. E., Kessler A., Miller M. K., et al. (2020). Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci. Adv. 6, eaax8254. doi: 10.1126/sciadv.aax8254
Effmert U., Kalderás J., Warnke R., Piechulla B. (2012). Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 38, 665–703. doi: 10.1007/s10886-012-0135-5
Ehlers B. K., Berg M. P., Staudt M., Holmstrup M., Glasius M., Ellers J., et al. (2020). Plant secondary compounds in soil and their role in belowground species interactions. Trend. Ecol. Evol. 35 (8), 716–730. doi: 10.1016/j.tree.2020.04.001
Eigenbrode S. D., Birch A. N. E., Lindzey S., Meadow R., Snyder W. E. (2015). A mechanistic framework to improve understanding and applications of push-pull systems in pest management. J. App. Ecol. 53, 202–212. doi: 10.1111/1365-2664.12556
Eilers E. J., Talarico G., Hansson B. S., Hilker M., Reinecke A. (2012). Sensing the underground-Ultrastructure and Function of sensory organis in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) Larvae. PloS One 7 (7), e41357. doi: 10.1371/journal.pone.0041357
Erktan A., Or D., Scheu S. (2020). The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol. Biochem. 148, 107876. doi: 10.1016/j.soilbio.2020.107876
Gardiner J. M., Atema J., Hueter R. E., Motta P. J. (2014). Multisensory integration and behavioral plasticity in sharks from different ecological niches. PloS One 9 (4), e93036. doi: 10.1371/journal.pone.0093036
Gregg P. C., Del Socorro A. P., Landolt P. J. (2018). Advances in attract-and-kill for agricultural pests: beyond pheromones. Annu. Rev. Entomol. 63, 453–470. doi: 10.1146/annurev-ento-031616-035040
Hall M., Hedlund K. (1999). The predatory mite Hyoaspis aculeifer is attracted to food of its fungivorous prey. Pedobiologia. 43, 11–17.
Hare J. D. (1998). “Bioassay methods with terrestrial invertebrates,” in Methods in chemical ecology volume 2: bioassay methods. Eds. Haynes K. F., Millar J. G. (Boston, MA: Kluwer Academic Publishers), 212–270.
Hedlund K., Bengtsson G., Rundgren S. (1995). Fungal odour discrimination in two sympatric species of fungivorous collembolans. Funct. Ecol. 9 (6), 869–875. doi: 10.2307/2389984
Hibbard B. E., Bjostad L. B. (1989). Corn semiochemicals and their effects on insecticide efficacy and insecticide repellency toward Western Corn Rootworm larvae (Coleoptera: Chrysomelidae). J. Econ. Entom. 82 (3), 773–781. doi: 10.1093/jee/82.3.773
Hiltpold I., Turlings T. C. J. (2008). Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J. Chem. Ecol. 34, 628–635. doi: 10.1007/s10886-008-9467-6
Hiltpold I., Turlings T. C. J. (2012). Manipulation of chemically mediated interactions in agricultural soils to enhance the control of crop pests and to improve crop yield. J. Chem. Ecol. 38, 641–650. doi: 10.1007/s10886-012-0131-9
Horton D. R., Landolt P. J. (2002). Orientation response of Pacific coast wireworm (Coleoptera: Elateridae) to food baits in laboratory and effectiveness of baits in field. Can. Entomol. 134, 357–367. doi: 10.4039/Ent134357-3
Insam H., Seewald M. S. (2010). Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils .46, 199–213. doi: 10.1007/s00374-010-0442-3
Johnson S. N., Crotty F. V., Ryalis J. M. W., Murray P. J. (2018). “Belowground experimental approaches for exploring aboveground-belowground patterns,” In Aboveground-Belowground community ecology Eds. Ohgushi T., Wurst S., Johnson S. N. (Cham, Switzerland: Springer International Publishing), 47–68.
Johnson S. N., Gregory P. J. (2006). Chemically-mediated host-plant location and selection by root-feeding insects. Phys. Entomol. 31, 1–13. doi: 10.1111/j.1365-3032.2005.00487.x
Johnson S. N., Gregory P. J., Murray P. J., Zhang X., Young I. M. (2004). Host plant recognition by the root feeding clover weevil, Sitona Lepidus (Coleoptera: Curculionidae). Bull. Entomol. Res. 94, 433–439. doi: 10.1079/BER2004317
Johnson S. N., Nielsen U. N. (2012). Foraging in the dark- chemically mediated host plant location by belowground insect herbivores. J. Chem. Ecol. 38, 604–614. doi: 10.1007/s10886-012-0106-x
Kojima W., Ishikawa Y., Takanashi T. (2014). Chemically mediated group formation in soil-dwelling larvae and pupae of the beetle Trypoxylus dichotomus. Naturwissenschaften. 101, 687–695. doi: 10.1007/s00114-014-1199-6
Little C. (1983). The colonisation of land: origins and adaptations of terrestrial animal (New York, NY: Cambridge University Press).
Liu X. F., Chen H. H., Li J. K., Zhang R., Turlings T. C. J., Chen L. (2016). Volatiles released by Chinese liquorice roots mediate host location behavior by neonate Porphyrophora sophorae (Hemiptera: Margodidae). Pest. Mgmt. Sci. 72 (10), 1959–1964. doi: 10.1002/ps.4237
Mathewson R. F., Hodgson E. S. (1972). Klinotaxis and rheotaxis in orientation of sharks toward chemical stimuli. Comp. Biochem. Physiol. Part A: Physiol. 42 (1), 79–82. doi: 10.1016/0300-9629(72)90369-6
Matsuoka K., Sakamoto Y., Hama T., Kajii Y., Enami S. (2017). Reactive uptake of gaseous sesquiterpenes on aqueous surfaces. J. Phys. Chem. A. 121, 4, 810–818. doi: 10.1021/acs.jpca.6b11821
McGechan M. B., Lewis D. R. (2002). Transport of particulate and colloid-sorbed contaminants through soil, part 1: General principles. Biosyst. Eng. 83, 255–273. doi: 10.1006/bioe.2002.0125
Miller J. R., Strickler K. L., Carde R. T. (1984). “Finding and accepting host plants,” in Chemical ecology of insects. Ed. Bell W. J. (New York, NY: Chapman and Hall Ltd), 127–157.
Minnich M., Schumacher B. (1993). Behavior and determination of volatile organic compounds in soil: A literature review (Washington, D. C.: U.S. Environmental Protection Agency EPA), 1–104. 600/R-93/140.
Mollo E., Boero F., Peñuelas J., Fontana A., Garson M. J., Roussis V., et al. (2022). Taste and smell: A unifying chemosensory theory. Q. Rev. Biol. 97, 69–94. doi: 10.1086/720097
Mollo E., Fontana A., Roussis V., Polese G., Amodeo P., Ghiselin M. T. (2014). Sensing marine biomolecules: smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem. 2 (92)16. doi: 10.3389/fchem.2014.00092
Mollo E., Garson M. J., Polese G., Amodeo P., Ghiselin M. T. (2017). Taste and smell in aquatic and terrestrial environments. Nat. Prod. Rep. 34, 496–513. doi: 10.1039/C7NP00008A
Murlis J., Elkinton J. S., Cardé R. T. (1992). Odor plumes and how insects use them. Annu. Rev. Entomol. 37, 505–532. doi: 10.1146/annurev.en.37.010192.002445
Neher D. A. (2010). Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 48, 371–394. doi: 10.1146/annurev-phyto-073009-114439
Nilsson E., Bengtsson G. (2004). Death odour changes movement pattern of a Collembola. Oikos. 104, 509–517. doi: 10.1111/j.0030-1299.2004.12921.x
Nordenhem H., Nordlander G. (1994). Olfactory oriented migration through soil by root-living Hylobius abietis (L.) larvae (Col., Curculionidae). J. Appl. Entomol. 117 (5), 457–462. doi: 10.1111/j.1439-0418.1994.tb00762.x
Pfeffer S. P., Filser J. (2010). Attraction to prey and prey-assocaited odours by the predatory mite Hyoaspis aculeifer in a soil experimental system. Soil Biol. Biochem. 42, 1355–1357. doi: 10.1016/j.soilbio.2010.03.018
Rasmann S., Köllner T. G., Degenhardt J., Hiltpold I., Toepfer S., Kuhlmann U., et al. (2005). Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434, 732–737. doi: 10.1038/nature03451
Robert C. A. M., Erb M., Duployer M., Zwahlen C., Doyen G. R., Turlings T. C. J. (2012). Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 194, 1061–1069. doi: 10.1111/j.1469-8137.2012.04127.x
Rostas M., Cripps M. G., Silcock P. (2015). Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia. 177, 487–497. doi: 10.1007/s00442-014-3104-6
Salmon S., Ponge J. F. (2001). Earthworm excreta attracts soil springtails: laboratory expeirments on Heteromurus nitidus (Collembola: Entomobryidae). Soil Biol. Biochem. 33 (14), 1959–1969. doi: 10.1016/S0038-0717(01)00129-8
Salmon S., Rebuffat S., Prado S., Sablier M., D’Haese C., Sun J., et al. (2019). Chemical communication in springtails: a review of facts and perspectives. Biol. Fertil. Soils. 55, 425–438. doi: 10.1007/s00374-019-01365-8
Schmidt R., Ulanova D., Wick L. Y., Bode H. B., Garbeva P. (2019). Microbe-driven chemical ecology: past, present, and future. ISME J. 13, 2656–2663. doi: 10.1038/s41396-019-0469-x
Schoonhoven L. M., Van Loon B., van Loon J. J., Dicke M. (2005). Insect–plant biology (Oxford, U. K: Oxford University Press).
Som S., Willett D. S., Alborn H. T. (2017). Dynamics of belowground volatile diffusion and degradation. Rhizosphere. 4, 70–74. doi: 10.1016/j.rhisph.2017.07.004
Staaden S., Milcu A., Rohlfs M., Scheu S. (2011). Olfactory cues associated with fungal grazing intensity and secondary metabolite pathway modulate Collembola foraging behaviour. Soil Biol. Biochem. 43, 1411–1416. doi: 10.1016/j.soilbio.2010.10.002
Stocker R., Seymour J. R. (2012). Ecology and physics of bacterial chemotaxis in the ocean. MMBR. 76 (4), 792–812. doi: 10.1128/mmbr.00029-12
Thomas R. S., Glen D. M., Symondson W. O. C. (2008). Prey detection through olfaction by the soil-dwelling larvae of the carabid predator Pterostichus melanarius. Soil Biol. Biochem. 40, 207–216. doi: 10.1016/j.soilbio.2007.08.002
Torto B., Cortada L., Murungi L. K., Haukeland S., Coyne D. L. (2018). Management of cyst and root knot nematodes: A chemical ecology perspective. J. Agric. Food. Chem. 66, 8672–8678. doi: 10.1021/acs.jafc.8b01940
Vickers N. (2000). Mechanisms of animal navigation in odor plumes. Biol. Bull. 198, 203–212. doi: 10.2307/1542524
Vickers N. J. (2006). Winging it: moth flight behavior and responses of olfactory neurons are shaped by pheromone plume dynamics. Chem. Senses. 31 (2), 155–166. doi: 10.1093/chemse/bjj011
Weissteiner S., Huetteroth W., Kollmann M., Weißbecker B., ROmani R., Schachtner J., et al. (2012). Cockchafer larvae smell host root scents in soil. PloS One 7 (10), e45827. doi: 10.1371/journal.pone.0045827
Wu S., Duncan L. W. (2020). Recruitment of an insect and its nematode natural enemy by olfactory cues from a saprophytic fungus. Soil Biol. Biochem. 144, 107781. doi: 10.1016/j.soilbio.2020.107781
Xavier Chiriboga M., Campos-Herrera R., Jaffuel G., Röder G., Turlings T. C. J. (2017). Diffusion of the maize root signal (E)-β-caryophyllene in soils of different textures and the effects on the migration of the entomopathogenic nematode Heterorhabditis megidis. Rhizosphere. 3, 53–59. doi: 10.1016/j.rhisph.2016.12.006
Zhang D., Terschak J. A., Harley M. A., Lin J., Hardege J. D. (2011). Simultaneously hermaphroditic shrimp use lipophilic cuticular hydrocarbons as contact sex pheromones. PloS One 6 (4), e17720. doi: 10.1371/journal.pone.0017720
Keywords: anthropomorphism, chemical ecology, gustation, heterogeneity, olfaction, soil
Citation: Elmquist DC and Eigenbrode SD (2023) Going belowground: burying anthropomorphic biases on gustation and olfaction. Front. Ecol. Evol. 11:1231042. doi: 10.3389/fevo.2023.1231042
Received: 29 May 2023; Accepted: 01 August 2023;
Published: 17 August 2023.
Edited by:
Ernesto Mollo, National Research Council (CNR), ItalyReviewed by:
Giovanni Battista Appendino, University of Eastern Piedmont, ItalyAntonino Cusumano, University of Palermo, Italy
Copyright © 2023 Elmquist and Eigenbrode. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanford D. Eigenbrode, c2FuZm9yZGVAdWlkYWhvLmVkdQ==
 Dane C. Elmquist
Dane C. Elmquist Sanford D. Eigenbrode
Sanford D. Eigenbrode