- 1Institute of Earth Sciences, University of Lausanne, Lausanne, Switzerland
- 2School of Earth and Environmental Sciences, University of Manchester, Manchester, United Kingdom
Exoskeleton moulting is the process of shedding the old exoskeleton to enable growth, development and repair, representing a crucial recurrent event in the life histories of all euarthropods. The fossil record of moulting allows us to interpret the evolution of this important behaviour and its impact on the evolutionary trajectories of extinct and extant euarthropods. Current knowledge of Palaeozoic euarthropod moulting relates largely to trilobites, with fewer examples known for non-mineralised extinct taxa from early in euarthropod evolutionary history. We describe exuviae from a marrellid marrellomorph found abundantly in the Early Ordovician Fezouata Shale Lagerstätte of Morocco, which allow a novel reconstruction, the second ever, of marrellid moulting behaviours. We identify the moulting suture location, describe preserved moulting assemblages, and suggest how its moulting behaviours are adaptive to its morphology. Several specimens represent complete and nearly complete assemblages and additional disarticulated specimens confirm the suture line location. The suture line is located between the mediolateral and posterolateral spine pairs, dividing the cephalic shield into anterior and posterior parts. The Fezouata marrellid likely exited the exoskeleton during exuviation using posterior and upwards movements, analogous in terms of movement to lobster-like extant arthropods. The suture line is comparable in the closely related marrellid Mimetaster, and distinctive from that of another marrellid, Marrella splendens, which has an exuvial opening at the anterior of the cephalic shield and exited the exoskeleton anteriorly. This difference in moulting behaviour as compared to Marrella is likely adaptive to the greater complexity of the Fezouata marrellid, with upwards rather than forwards movement presumably providing a more favourable angle for the extraction of complex spines. This description of the moulting behaviours and related morphological features of marrellomorphs expands our understanding of this crucial characteristic in extinct euarthropods.
1 Introduction
Moulting is the process of shedding the exoskeleton, which all euarthropods must periodically do to grow and develop. Moulting is of central importance in the life histories of extinct and extant euarthropods, as all individuals must repeat this process multiple times, and they are particularly vulnerable to predation and parasitism during moulting and immediately after (Ewer, 2005). Evidence of moulting has been described for many extinct euarthropod groups, including some of the stratigraphically earliest representatives of the group from the lower Cambrian (see Daley and Drage, 2016, for a review of the pre-2016 literature on the fossil record of moulting). The majority of the fossil record of moulting pertains to crown-group Euarthropoda, with abundant data from the trilobites (e.g., Brandt, 2002; Drage et al., 2018; Drage, 2019; Corrales-García et al., 2020; Zong, 2020; Wang et al., 2021; Zong, 2021; Drage, 2022; Drage et al., 2023), as well as some data from eurypterids (e.g., Tetlie et al., 2008; Brandt, 2021) and other chelicerates (e.g., Selden et al., 1991; McCoy and Brandt, 2009), decapod crustaceans (e.g., Glaessner, 1969; Klompmaker and Fraaije, 2011), ostracods (Olempska, 2012), isopods (e.g., Hyžný et al., 2013), and hexapods (e.g., Kukalova, 1968; Rasnitsyn, 2002). Much less is known about moulting in other euarthropod groups, with clear moult assignments of only a few taxa, including a single marrellomorph specimen (García-Bellido and Collins, 2004), a fuxianhuiid (Yang et al., 2019), and mass moults of Canadaspis and Alalcomenaeus (Haug et al., 2013). These specimens suggest that moulting in stem-group euarthropods was likely comparable in biomechanics to extant crown-group representatives (Daley and Drage, 2016). However, with only sparse knowledge of moulting in euarthropod stem-groups, we have little basis with which to understand the early evolution of moulting as a behaviour, and the impacts this might have had on other evolutionary aspects such as morphology, physiology, ontogeny, and ecology.
The Marrellomorpha are an unusual group of euarthropods known only from the Cambrian to Devonian (c. 390–508 Ma), and best known by the characteristic and highly abundant Marrella splendens from the Burgess Shale (García-Bellido and Collins, 2006). The Marrellomorpha may represent a monophyletic grouping (though this is still in doubt; see Moysiuk et al., 2022), comprising two orders—Marrellida and Acercostraca—with the Marrellida having a cephalic shield supporting large lateral spines, and the Acercostraca having a large cordiform carapace covering more of the body (see Legg, 2015; Legg, 2016 on the Acercostraca). One putative marrellomorph species, the fragmentary Austromarrella klausmuelleri is unresolved within this taxonomic framework (Haug et al., 2012), though there is doubt over its marrellomorph assignment (Legg, 2016). However, the ancestral condition of the Marrellomorpha (spiny cephalic shield or carapace) remains uncertain, as does their phylogenetic position within Euarthropoda as a whole, being variously considered arachnomorphs, stem mandibulates, or stem-lineage euarthropods (e.g., Legg, 2016; Aris et al., 2017; Moysiuk et al., 2022, and references therein).
The Marrellida are currently represented by four genera: Marrella (Cambrian, Canada and China; Whittington, 1971; García-Bellido and Collins, 2006; Liu, 2013), Furca (Upper Ordovician, Czech Republic and suggested for Morocco, see Material and methods section; Van Roy, 2006; Van Roy et al., 2010; Rak et al., 2012), Mimetaster (Lower Ordovician, Argentina, and Lower Devonian, Germany; Kühl and Rust, 2010; Aris et al., 2017), and Tomlinsonus (Late Ordovician, Canada; Moysiuk et al., 2022). Marrella has a comparably simpler cephalic shield morphology, with two pairs of spines and no secondary spinosity (García-Bellido and Collins, 2006; Liu, 2013). Furca, Mimetaster, and potentially Tomlinsonus, all have three pairs of spines on the cephalic shield, and extensive secondary spinosity of the primary cephalic spines.
To date, the only described evidence of moulting in Marrellomorpha is a single specimen of Marrella splendens from the early Cambrian Burgess Shale, Canada (García-Bellido and Collins, 2004), that has been preserved during the act of exuviation (the actual process of exiting the old exoskeleton during moulting), which is sufficiently rare that no other euarthropod fossils have been described as in-the-act. Extant euarthropod representatives spend only minutes to hours (though this is variable between taxa) in the act of exuviation (Ewer, 2005), and therefore the likelihood of an individual being preserved and fossilised during this short period is presumably very low (Drage et al., 2019). This means that, to date, our knowledge of moulting in the Marrellomorpha is restricted to only an exceptional case of a single specimen of an abundant genus, which itself has a simpler cephalic shield morphology than other Marrellida (and differs greatly in morphology to the Acercostraca).
Specimens of a not-yet-formally described marrellid marrellomorph, putatively assigned to the ‘Furca’ genus (Van Roy, 2006; Van Roy et al., 2010; see Material and methods section), are abundantly preserved in the Ordovician Fezouata Shale Lagerstätte, Morocco. The species has a complex cephalic shield morphology, with three pairs of elongate lateral spines and extensive secondary spinosity. Amongst these specimens are complete and nearly complete moult assemblages, as well as disarticulated isolated cephalic shield elements, all of which indicate a consistent moulting suture line location. These specimens allow for the second reconstruction ever of marrellomorph moulting behaviour, and conclusively show that there was variability in moulting within this clade that was likely linked to the complexity of the cephalic shield.
2 Geological setting
All specimens are from the Lower Ordovician Fezouata Shale Konservat-Lagerstätte of the central Anti-Atlas region of Morocco, which is renowned for its exceptional preservation of non-mineralised and lightly-sclerotised inhabitants of a highly diverse marine biota (Van Roy et al., 2010). In the Zagora region, from which all specimens of this study derive, a single unit is designated the Fezouata Shale, comprising a succession from the Tremadocian to Floian, with a regional transgressive contact at the base of the unit (Gutiérrez-Marco and Martin, 2016) and discontinuous, though well-understood, occurrences of exceptional preservation throughout (Martin et al., 2016a; Martin et al., 2016b; Saleh et al., 2021a). The Fezouata Shale is a succession 900 m thick of blue-green to yellow-green siltstones (Destombes et al., 1985).
Several modes of exceptional preservation have been described from the Fezouata Shale Formation, including silica-chlorite concretions preserving radiodonts and trilobites in three-dimensions (Gaines et al., 2012; Van Roy et al., 2015b; Saleh et al., 2021b), and more two-dimensional compressed fossils within claystones preserved initially as carbonaceous films with authigenic minerals such as pyrite, but now found as iron oxide compressions after weathering leached carbon from the fossils (Van Roy et al., 2010; Martin et al., 2016a; Saleh et al., 2020; Pérez-Peris et al., 2021). This second mode of preservation comprises many of the exceptionally preserved fossils of the Fezouata Shale, including those studied herein, and is restricted to two intervals (Martin et al., 2016a). The lower interval is mainly within the Araneograptus murrayi zone and lower parts of the Hunnegraptus copiosus zone, corresponding to the late Tremadocian (Tr3; Gutiérrez-Marco and Martin, 2016; Lehnert et al., 2016; Nowak et al., 2016; Lefebvre et al., 2018). The upper interval is likely of Floian age, within the ? Baltograptus jacksoni zone (Lefebvre et al., 2018). A third interval shows the potential for exceptional preservation (Saleh et al., 2022), corresponding to the upper Floian. All specimens discussed herein originate from the A. murrayi zone of the late Tremadocian.
The depositional environment of the Fezouata Shale in general facilitated rapid burial of autochthonous communities in a shallow open marine environment (Martin et al., 2016a; Saleh et al., 2018; Saleh et al., 2020; Saleh et al., 2021b). This ranged from offshore to foreshore positioning, at a depth of c. 50–150 m (Vaucher et al., 2016). For the exceptionally preserved compressed specimens from the Zagora region, the environment was intermediate to distal open shelf, between the offshore and lower shoreface (Martin et al., 2016a), just below the storm wave base (Saleh et al., 2020).
3 Materials and methods
All specimens examined are from the lower interval of the Fezouata Shale (A. murrayi zone, upper Tremadocian, Lower Ordovician), from the Zagora region of the central Anti-Atlas. Two moult specimens accessioned at the Muséum cantonal des sciences naturelles, département de géologie, Lausanne, Switzerland (MGL; 103019_MGL, 104259_MGL) and one from the Yale Peabody Museum of Natural History, USA (YPM; YPM 532136) were examined. Several disarticulated specimens were also used for the description herein from the MGL and YPM (see Supplementary 1). Finally, complete carcasses from the Université Claude Bernard Lyon 1, France, were contrasted to the moult assemblages and disarticulated specimens (FSL AA.BIZ30.OI.1, FSL AA.BIZ31.OI.39). All specimens in the MGL and YPM collections were collected by authorised Moroccan collector Mohamed ‘Ou Saïd’ Ben Moula and his family during 2009 to 2015 (YPM) and 2015 to 2016 (MGL). These were purchased by the University of Lausanne and the Swiss National Science Foundation (MGL collection), or funds for acquisition of scientific collections of the Yale Peabody Museum (YPM), and in both cases the collections were subjected to export approval from the Ministry of Energy, Mines and the Environment of the federal government of the kingdom of Morocco, before being shipped by sea and land to the MGL and YPM. Export permits and exact GPS coordinates of the localities are curated with the materials. Lyon collection specimens are currently hosted within the Université Claude Bernard Lyon 1, but the Marrakech Collections of the Cadi Ayyad University retains ownership. See also Supplementary 1 for all specimen number and host collections information.
Specimens were photographed using a Canon EOS 800D camera with a Canon macro MP-E 65 mm 1:2.8 1-5X lens. Specimens were lit with long-angle NW lighting, or with full lighting. Photographs of each specimen were stacked in Adobe Photoshop CC to ensure good focus. Resulting images were processed in Adobe CC, in which brightness and contrast were altered to provide the best visibility. Line drawings were made from photographs using Adobe CC.
A morphological and taxonomic description of the Fezouata marrellid is not included herein. This falls outside the scope of this paper, which focuses solely on the novel moulting configuration preserved and relevant suture line location, and will no doubt be addressed independently of this work. Van Roy (2006), as well as subsequent papers to figure specimens of the Fezouata marrellid (Van Roy et al., 2015a; Lefebvre et al., 2016; Martin et al., 2016a; Vaucher et al., 2016; Saleh et al., 2021a), referred to the species as ‘Furca’ mauritanica. However, the species has not been formally described, and we do not wish to further direct the prospective taxonomic assignment of this species without providing a valid description; as such, we refer to the species throughout as the ‘Fezouata marrellid’. The general terminology used follows that of other marrellomorph descriptive literature, including Rak et al. (2012) and Aris et al. (2017).
4 Results
4.1 Suture line location
The Fezouata marrellid moult assemblages (Figure 1B, YPM 532136; Figure 1C, 104259_MGL; Figures 1D–F, 103019_MGL) show the existence of a suture line dividing the cephalic shield into two parts. This suture runs around the cephalic shield, from the posterior edges of the mediolateral spine pair, curving towards the posterior of the individual, and meeting to form a convex curved to squared posterior margin depending on whether it is on the dorsal or ventral side. The suture line thereby divides the cephalic shield into a larger anterior section, supporting the anterolateral and mediolateral pairs of spines, and a smaller posterior section, with the posterolateral pair of spines.
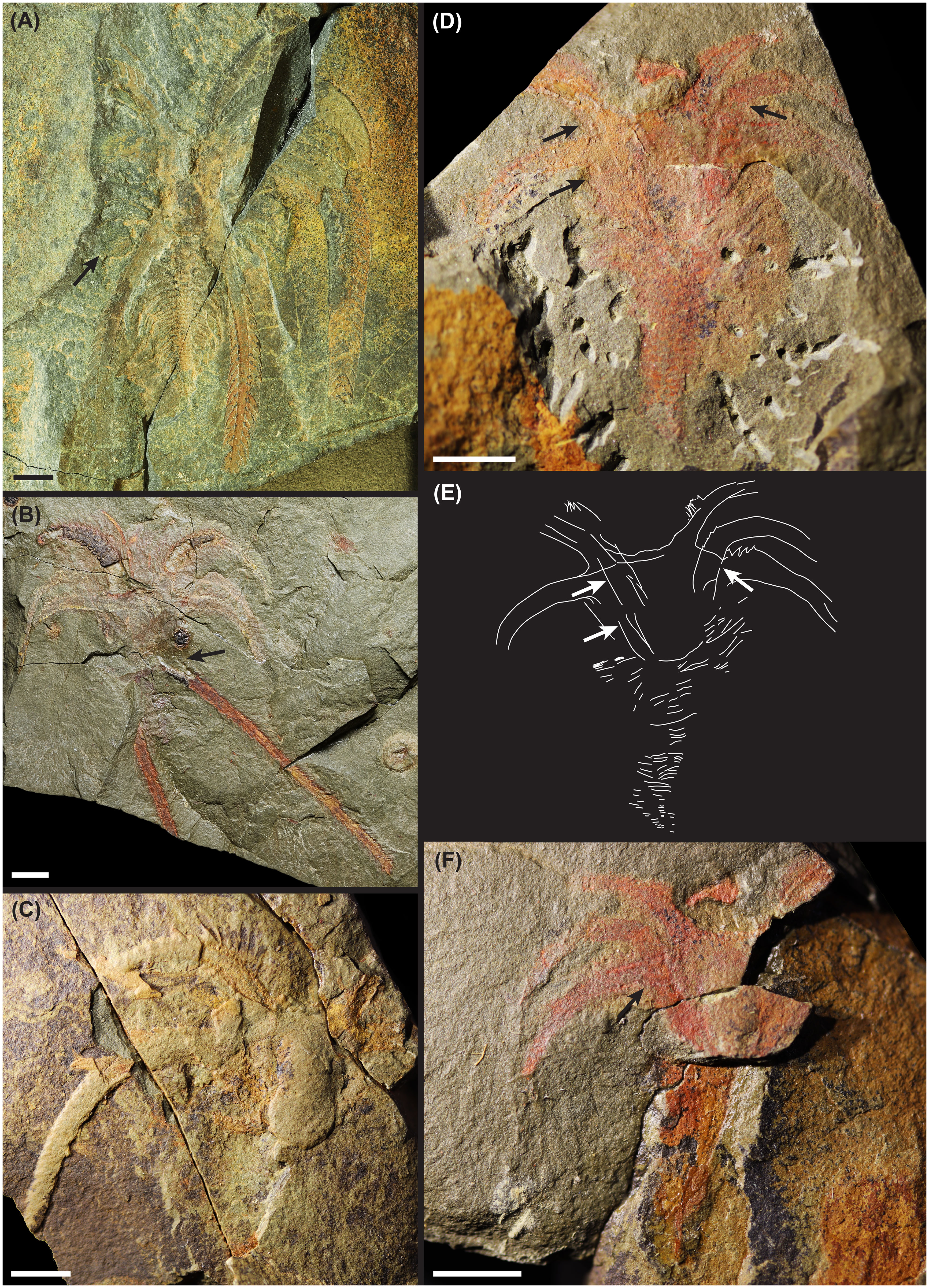
Figure 1 Photographs and drawing of putative moult assemblages of the Fezouata marrellid.: (A) photograph of intact carcass, FSL AA.BIZ.30.OI.1; (B) complete moult assemblage, YPM 532136b (counterpart comparable, not figured); (C) incomplete moult assemblage, 104259_MGL; (D) complete moult assemblage, 103019a_MGL (drawn in E); (E) interpretive drawing of complete moult assemblage, 103019a_MGL (specimen figured in D), drawing not morphologically descriptive; (F), counterpart of (D), 103019b_MGL. Black and white arrows in (B, D–F) point to disarticulated posterior cephalic shield sections, and in (A) to likely impressions of gill material. Scale bars = 5 mm.
The suture line location is further supported by a number of disarticulated specimens, which consist of the anterior or posterior cephalic shield sections in isolation. In particular, 107796_MGL (Figures 2A, B) shows only the posterior section of the cephalic shield, with the posterolateral spine pair intact but no other material in association. This specimen clearly shows a clean disarticulation at the suture line described above, leaving a square-shaped recess into the posterior cephalic shield section and two thin, anteriorly-directed, lateral projections. Additional specimens (103015_MGL, YPM 515771, YPM 517631, YPM 517634, YPM 519694, YPM 519905; e.g., Figures 2C, D), also show comparable isolated posterior sections. Eight specimens (YPM 520988, YPM 521588, YPM 522288, YPM 523536, YPM 525343, 102389_MGL, 104150_MGL, 107769_MGL) appear to represent the disarticulated anterior cephalic shield section (e.g., Figures 2E–G). Specimens YPM 530556 and YPM 530919 also seem to be isolated anterior cephalic shield sections with this suture line location, and are considered to be from juvenile individuals based on their small sizes.
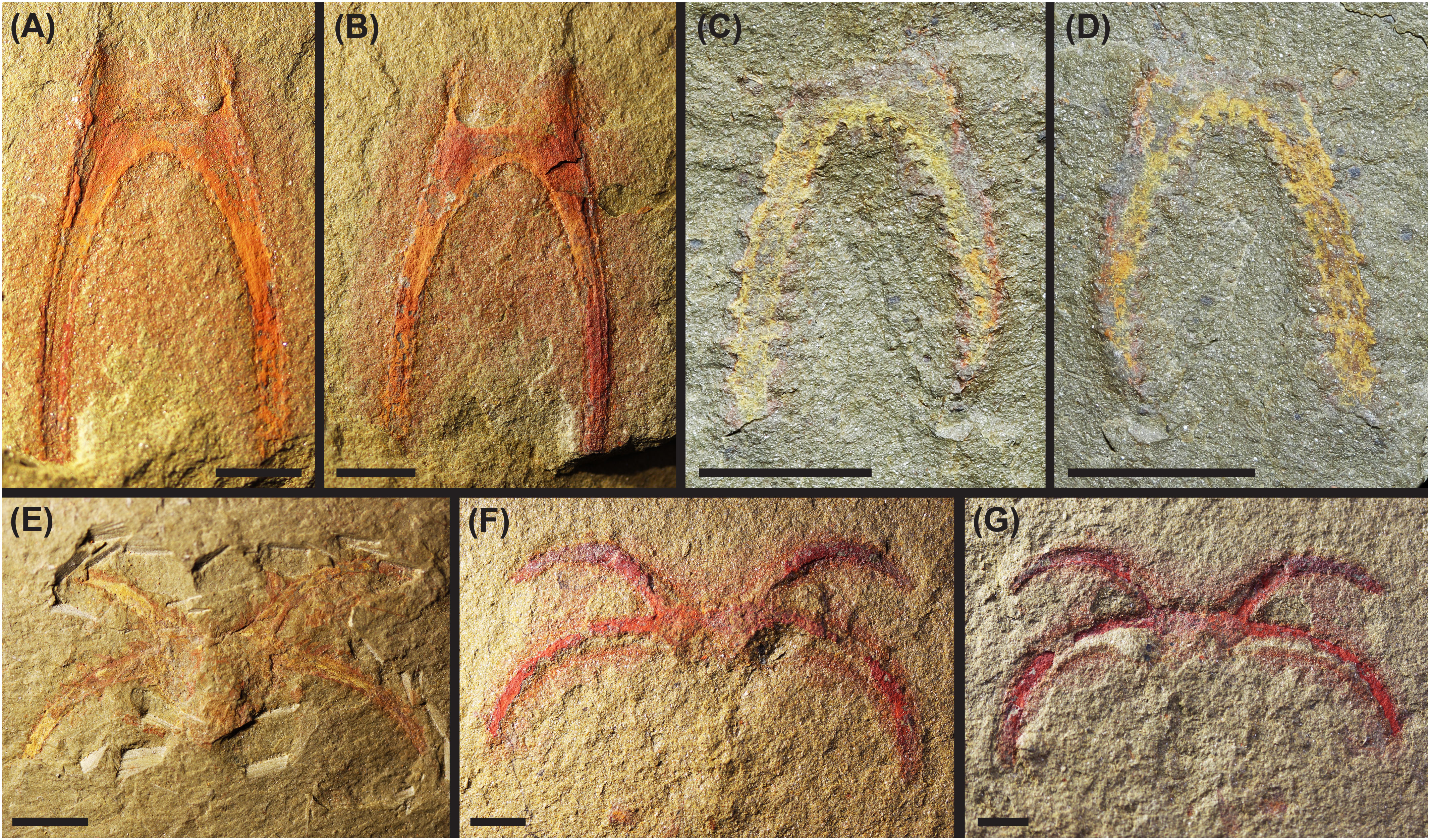
Figure 2 Photographs of disarticulated, isolated anterior and posterior cephalic shield sections of the Fezouata marrellid. (A–D) Posterior cephalic shield sections; (A) 107796b_MGL; (B) counterpart of (A) 107796a_MGL; (C) YPM 515771a; (D) counterpart of (C) YPM 515771b. (E–G) Anterior cephalic shield sections; (E) 104150_MGL; (F) 102389b_MGL; (G) counterpart of (F) 102389a_MGL. Scale bars = 5 mm.
Lastly, the suture line is also observable in many well-preserved complete specimens (presumably carcasses) of the Fezouata marrellid. For example, two large (3–4 cm long) complete specimens from Lyon (FSL AA.BIZ30.OI.1, FSL AA.BIZ31.OI.39) and two YPM specimens (YPM 525233, YPM 530594) show the suture line location particularly well, but it is apparent in many complete specimens in a dorsum-up orientation (e.g., Figures 2A, 3).

Figure 3 Photographs of part (A) with closeup of cephalic shield and suture line (B) and counterpart (C) also with closeup (D) of a complete Fezouata marrellid carcass (YPM 525233); suture line indicated by black arrows. Scale bars = 5 mm.
4.2 Description of moult assemblages
YPM 532136 (Figure 1B) is a complete moult assemblage of an adult individual, with all major exoskeleton parts visible. The anterior cephalic shield section appears mostly in place, but the posterior cephalic shield section is disarticulated, slightly displaced, and rotated approximately 20–30 degrees clockwise. Some anterior trunk tergites are clearly preserved in situ, and several appendages (including the enlarged appendage pair) and the antennae are also visible. Fully soft-tissue internal structures, and other soft-tissue aspects like the gills, are not visible.
Two further specimens represent disarticulated moult assemblages. Specimen 103019_MGL (Figures 1D–F) consists of the anterior cephalic shield section with an almost-complete trunk of tapering tergites, and poorly preserved appendage material. The posterior cephalic shield section is clearly disarticulated but appears to be associated with the rest of the assemblage, as an additional pair of primary spines fully overlaps the anterior cephalic shield section, potentially resulting from an anteriorly pointing posterior section. Specimen 104259_MGL is less well-preserved, and lacking evidence of the posterior cephalic shield section (Figure 1C). However, the anterior section remains with both pairs of primary spines, with some preservation of thoracic and appendage material.
Specimens consisting of only disarticulated anterior or posterior cephalic shield sections may or may not represent moulted exoskeletal material (e.g., 107796_MGL, 102389_MGL; Figure 2). It is impossible to determine whether they are moults without more associated material, as they may simply represent the remains of decayed and disarticulated material, as carcasses would presumably naturally open at the suture line due to it acting as a plane of weakness (see Daley and Drage, 2016). However, for this reason, though impossible to unambiguously assign as moult or decayed carcass remains, these disarticulated cephalic shield fragments are useful in their support of the suture line location and morphology.
5 Discussion and conclusions
5.1 Marrellid suture line locations
The suture line location is alike that suggested for the, presumably carcass, specimen of ‘Mimetaster’ florestaensis described by (Aris et al., 2017, Figure 2). They also suggest that the suture line curves posteriorly from the mediolateral spine pair to divide a posterior section supporting the posterolateral spine pair from the anterior section. However, this is unfortunately based on only one complete cephalic shield. Mimetaster species, which to date include ‘Mi’. florestaensis and Mi. hexagonalis, show similar morphologies to the Fezouata marrellid in terms of the cephalic shield shape and spines, and have been suggested to be closely related to the Fezouata marrellid (Mimetasteridae in Legg, 2016; Aris et al., 2017). ‘Mi’. florestaensis, in particular, is notably similar to the Fezouata marrellid and Furca bohemica, the latter of which also has three pairs of secondary spinose cephalic shield spines, which themselves have a similar length and shape (Rak et al., 2012). However, no suture line has been inferred for Mi. hexagonalis (Stürmer and Bergström, 1976; Kühl and Rust, 2010) or F. bohemica (Rak et al., 2012). In the latter case, a diagrammatic representation infers its possible existence in the same location as the Fezouata marrellid, although only discussed in the text is an ‘inflated cephalic shield’ so this is unclear (Rak et al., 2012, Figure 4A).
Tomlinsonus dimitrii is apparently also closely related to Mimetaster and Furca, as well as the Fezouata marrellid (Moysiuk et al., 2022). Again, no suture line has yet been identified for T. dimitrii, which was described from a single specimen. However, Moysiuk et al. (2022) note that the posterior margins of the cephalic shield have not been observed, resulting in no evidence of a potential posterolateral spine pair (e.g., their Figure 3, 4.1). Based on specimens of the Fezouata marrellid showing the anterior cephalic shield section with in situ trunk, but opened suture and consequently absent posterior section (e.g., Figure 1C), it is possible that the suture line of T. dimitrii is in a comparable location, and the posterior section is disarticulated and missing in the described T. dimitrii specimen, which may therefore represent a moult. Analysis of additional T. dimitrii specimens would be required to confirm or deny this.
Other, probably more distantly related, marrellids must have had very different suture lines to that described here for the Fezouata marrellid. To produce the in-the-act moulting specimen described for Marrella splendens (García-Bellido and Collins, 2004), the suture line must track around the anterior margin of the cephalic shield, allowing for an exuvial gape to open at the anterior-most section of the shield. Thus, the suture line would be impossible to see in the vast majority of M. splendens specimens, which are preserved dorsum-up or ventrum-up rather than obliquely. This means that the actual suture line of M. splendens has not so-far been directly described or figured (neither in Whittington, 1971, nor in García-Bellido and Collins, 2006). Further, there is no apparent sign of a suture line, suggesting a marginal location, in specimens of Marrella sp. described from the Balang Formation, China (Liu, 2013).
The three moult assemblages described here are all presumed to represent adults, based on body size. However, two juvenile anterior cephalic shield sections were also observed (YPM 530556 and YPM 530919), based on their minute size. These juvenile specimens demonstrate that the suture line was seemingly in the same location as for adults (see ontogenetic sequence in Laibl et al., this volume), which suggests that moulting behaviour was also consistent across ontogenetic stages of the Fezouata marrellid. However, to conclusively determine consistent moulting behaviour with development more moult specimens or isolated elements would be required, particularly those at the earliest, most minute, life stages. Further, greater quantities of adult moult specimens would be necessary to determine whether marrellid individuals continued moulting into adulthood or halted following attainment of an adult morphology or certain size.
5.2 Preservation of moult assemblages
All contextual information supports the putative assignments of the three specimens here as moults (see Daley and Drage, 2016; Drage and Daley, 2016), including the completeness of the assemblages (particularly Figures 2C, E), lack of obvious biostratinomic effects (no alignment, no fragmentation of exoskeleton parts), characteristic and consistent disarticulation at the suture line location, and lack of softer external structures (e.g., gills, which are occasionally present in carcasses; see Figure 1A) and internal tissues. The moult assemblages described here thereby greatly differ to similarly well-preserved specimens of marrellid from the Fezouata and other Konservat-Lagerstätten (García-Bellido and Collins, 2006). Internal tissues can be confidently considered to designate specimens as carcasses (Daley and Drage, 2016), though there was significant decay before burial in the Fezouata Shale, often preventing preservation of soft tissue (Saleh et al., 2021a), and so this alone cannot be used to differentiate moults and carcasses from this Konservat-Lagerstätte. Evidence of appendages, and likely antennae, are to be expected in well-preserved moult specimens, as these are exoskeleton components that must necessarily be moulted. Isolated anterior and posterior cephalic shield elements (Figure 2) are impossible to assign as either moults or carcasses, due to the lack of contextual information relayed above; however, they remain important for conclusively demonstrating the suture line location.
5.3 Inferred mode of moulting
The previously described suture line and moult assemblages of the Fezouata marrellid suggest a newly observed mode of moulting behaviour for this group, which is highly divergent from that described for M. splendens (García-Bellido and Collins, 2004). Due to the mediolateral location of the suture, the Fezouata marrellid may have moved upwards and perhaps posteriorly when extracting the body out of the old exoskeleton. Firstly, this direction of movement would have fully opened an exuvial gape at the suture line between the mediolateral and posterolateral spine pairs owing to pressure put on this plane of weakness. The animal would then have moved upwards and/or backwards away from the old exoskeleton, pulling its body free with the large cephalic shield spines coming out afterwards (Figure 4). In comparison, M. splendens, with an exuvial gape at the anterior margin of the cephalic shield, moved forwards to exit the old exoskeleton during moulting. This movement caused severe bending of the cephalic shield spines for M. splendens (see García-Bellido and Collins, 2004, Figure 1). It is worth noting that some extinct arthropod groups, particularly trilobites, show intraspecific variability in the moulting behaviours they employed, even producing exuvial gapes in multiple places, likely due to the specific movements carried out during the traumatic moulting process (e.g., see Drage, 2019; Drage, 2022). It is therefore possible that marrellomorphs also varied in moulting behaviours intraspecifically, though we have no evidence to date that supports this idea.
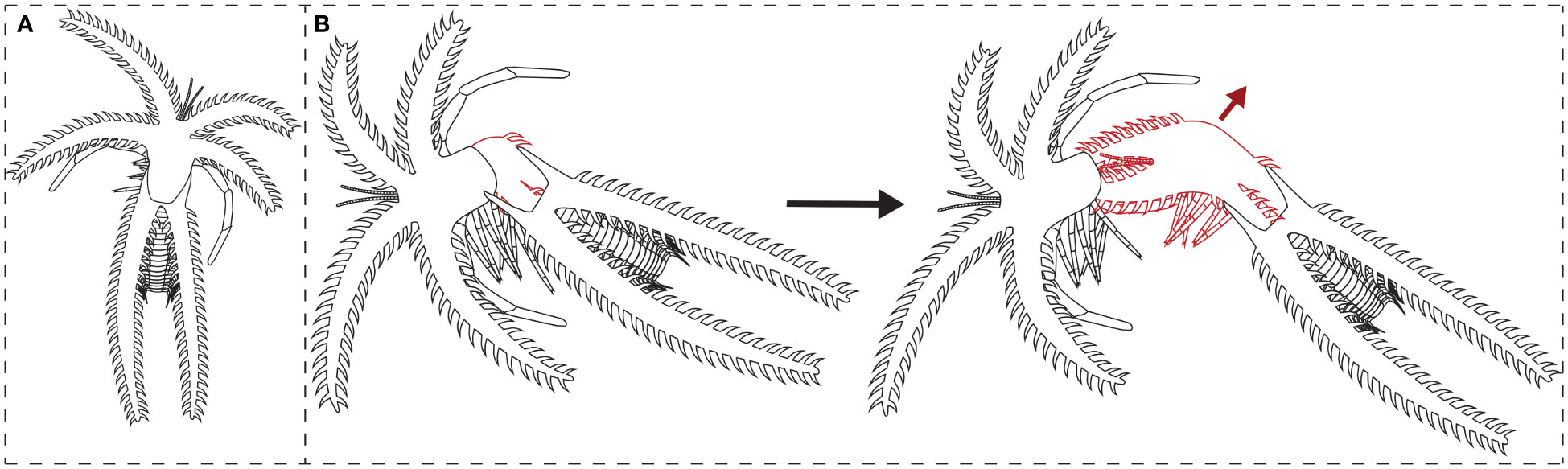
Figure 4 Interpretive drawing of a complete carcass of the Fezouata marrellid, and its suggested moulting process: (A) complete carcass; (B) moulting individual (red lines) opening the exoskeleton at the suture line and withdrawing from the old exoskeleton (black lines) in an upwards and backwards movement (red arrow).
The differences in morphology between the Fezouata marrellid and M. splendens likely explain their dissimilar moulting movements, largely reflecting the greater complexity of the former compared to the latter. The Fezouata marrellid (and Mimetaster and Furca species; Rak et al., 2012; Aris et al., 2017) had an additional pair of cephalic shield spines, all three spine pairs were of greater lengths, and all spines had extensive secondary spinosity, compared to M. splendens. These complex cephalic shield spines may have been correspondingly more difficult to extract during moulting. Perhaps this upwards rather than forwards movement during exuviation provided a more favourable angle for the spines, requiring less bending, and therefore a lower risk of sustaining moulting injuries. This is supported by the angular orientations of the secondary spines; they are pointed distally at a c. 45-degree angle, rather than perpendicular to the primary spine direction. This means that the secondary spines would be at a favourable angle to slide out of the old exoskeleton without much bending (see Figure 4), whereas moulting injuries from the spines bending and catching would be more likely if the secondary spines were perpendicular or proximally orientated. Additionally, the cephalic shield in adult individuals was both physically and proportionally larger in the Fezouata marrellid than M. splendens, which may have also benefitted from a mediolateral exuvial gape the full width of the cephalic shield.
Mechanically, an upwards and/or posterior body movement during moulting for the Fezouata marrellid is more similar to the moulting movements of several other arthropod clades, than those of M. splendens. For example, this movement is overall analogous to the moulting of arthropod clades with lobster-like bodyplans. Lobsters, shrimp, and similar decapods produce a medial split in the dorsal side of the carapace, and the animal then emerges from the old exoskeleton up and backwards, pulling out the appendages after the body (Glaessner, 1969; Daley and Drage, 2016). This produces fossil moults preserved in the characteristic ‘Open Moult Position’. Moulting spiders and crabs also move similarly, disarticulating the dorsal part of the carapace to produce a large exuvial gape, exiting backwards and pulling their appendages after the body (Petrunkevitch, 1942; Glaessner, 1969; Daley and Drage, 2016).
5.4 The marrellomorph moult record
It is remarkable that only one in-the-act-of-moulting specimen (García-Bellido and Collins, 2004) and potentially five isolated cephalic shields (Whittington, 1971) of M. splendens, one of the most abundant animals in the Cambrian Burgess Shale (Whittington, 1971; Conway Morris, 1979; García-Bellido and Collins, 2006), and the three probable moult assemblages of the Fezouata marrellid figured herein, have been described for the entirety of the marrellomorph fossil record. Whittington (1971) notes that almost all M. splendens individuals discovered at that time, numbering thousands of individuals, represent carcasses due to the presence of soft tissue and associated organic material. They consider only five specimens of isolated cephalic shield to be potential moults due to the lack of soft material (Whittington, 1971, plate XVIII, Figure 5) which is a reasonable interpretation based on their notably different preservation to all other individuals from the Burgess Shale. The moult status of the singular M. splendens individual described by García-Bellido and Collins (2004) is indisputable, as are the assignments of several specimens presented here (see discussion above), though it is difficult to consider isolated cephalic shields as moults with absolute certainty and additional moulted exoskeleton material (e.g., of the appendages, thorax) would be expected for well-preserved moult assemblages (see Daley and Drage, 2016). Many researchers have hypothesised that the fossil records of various euarthropod groups are likely to be biased towards more numerous moults than carcasses, based on both arguments of preservation (e.g., moults are less attractive to scavengers) and ontogeny (an individual moults many times during its life, but produces only one carcass) (Braddy, 2001; Daley and Drage, 2016). However, some arthropod groups regularly consume their moults (e.g., in myriapods; Shear and Edgecombe, 2010), or reabsorb materials from the moult, and this may contrastingly lead to a bias towards carcasses in the fossil record, though this is extremely difficult to test in extinct groups and no conclusive evidence of this has so far been advanced for extinct arthropods (Daley and Drage, 2016). On balance, it is almost certain that many more moult assemblages exist in the marrellomorph fossil record, yet await identification and description as such.
Neither empty moults, nor additional in-the-act of moulting specimens have been described for any other marrellomorph (including acercostracans). The paucity of moulting evidence for Marrellomorpha may be related to their preservation potential, which was presumably lower than some other euarthropod groups, like trilobites, because marrellomorphs lacked biomineralised exoskeletons. However, in locations of exceptional preservation, like the Burgess Shale, carcasses of M. splendens seemingly preserve well, given their abundance (see Whittington, 1971), which suggests something else must be responsible for the lack of identified marrellomorph moults. As discussed, it is possible that moulted exoskeletons of marrellids with anterior marginal suture locations, as inferred for Marrella, are more common than realised, and are miscategorised as carcasses because the suture line is almost impossible to observe in the fossil record. This would be a similar scenario to horseshoe crabs and some morphologically-derived trilobites, for example harpetids, both of which have a suture line and exuvial gape around the anterior margin of the cephalon, which tends to mostly close after exuviation, leaving an empty exoskeleton that outwardly appears to be a normal carcass (Babcock et al., 2000; Tetlie et al., 2008; Daley and Drage, 2016; Drage, 2019). However, the exuvial gape described here for the Fezouata marrellid, and potentially Furca, Mimetaster and Tomlinsonus, would not lead to this preservational issue, and would presumably leave recognisable moults, suggesting a reassessment of marrellid museum collections is a worthwhile endeavour. Researchers working on marrellomorphs should aim to distinguish moults from carcasses by looking for specimens lacking obvious soft tissue preservation, with all exoskeleton parts present in association, and disarticulation at described, or potential new, suture line locations. This work may be aided through the application of modern visualisation techniques, such as UV reflected photography and micro-XRF elemental mapping. Through these efforts, we can greatly expand our understanding of moulting in Marrellomorpha, and how their varied behaviours relate to their captivating morphologies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HD: conceptualisation, investigation, writing – original draft, writing – review and editing, visualisation. DL: investigation, writing – review and editing. AD: conceptualisation, writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
HD and AD wish to acknowledge funding from a Swiss National Science Foundation Sinergia grant (198691).
Acknowledgments
B. Lefebvre and E. Robert at the Lyon 1 University, G. Potin and F. Saleh at the University of Lausanne, and J. Utrup, S. Butts and D.E.G. Briggs at the Yale Peabody Museum of Natural History are thanked for their collections access facilitation. We thank two reviewers, the editor Z. Zhang, B. Lieberman, and the managing editors of this special volume for their comments and publishing assistance with this manuscript. We acknowledge P. Van Roy for discussions on marrellomorph moult material during 2015–16. This work would be impossible without the years of intense collecting efforts of all researchers and fossil collectors involved.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1226924/full#supplementary-material
References
Aris M. J., Corronca J. A., Quinteros S., Pardo P. L. (2017). A new marrellomorph euarthropod from the Early Ordovician of Argentina. Acta Palaeontol. Pol. 62, 1–8. doi: 10.4202/app.00240.2016
Babcock L. E., Merriam D. F., West R. R. (2000). Paleolimulus, an early limuline (Xiphosurida), from Pennsylvanian-Permian Lagerstätten of Kansas and taphonomic comparison with modern Limulus. Lethaia 33, 129–141. doi: 10.1080/00241160025100017
Braddy S. J. (2001). Eurypterid palaeoecology: palaeobiological, ichnological and comparative evidence for a ‘mass-moult-mate’ hypothesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 172, 115–132. doi: 10.1016/s0031-0182(01)00274-7
Brandt D. S. (2002). Ecdysial efficiency and evolutionary efficacy among marine arthropods: implications for trilobite survivorship. Alcheringa 26, 399–421. doi: 10.1080/03115510208619264
Brandt D. S. (2021). Eurypterid morphology and implications for ecdysis and evolutionary longevity. Lethaia 54, 711–722. doi: 10.1111/let.12434
Conway Morris S. (1979). The Burgess Shale (middle Cambrian) fauna. Ann. Rev. Ecol. Syst. 10, 327–349. doi: 10.1146/annurev.es.10.110179.001551
Corrales-García A., Esteve J., Zhao Y., Yang X. (2020). Synchonized moulting behaviour in trilobites from the Cambrian Series 2 of South China. Sci. Rep. 10, 14099. doi: 10.1038/s41598-020-70883-5
Daley A. C., Drage H. B. (2016). The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Arthropod Struct. Dev. 45, 71–96. doi: 10.1016/j.asd.2015.09.004
Destombes R. R., Hollard D. E. G., Willefert P. J. (1985). ‘Lower Palaeozoic rocks of Morocco,’ in Lower Palaeozoic rocks of the world 4. Ed. Holland C. H. (Wiley, New York), 91–336.
Drage H. B. (2019). Quantifying intra- and interspecific variability in trilobite moulting behaviour across the Palaeozoic. Palaeontol. Electron. 22.2.34A, 1–39. doi: 10.26879/940
Drage H. B. (2022). Trilobite moulting variability had little association with morphometry. bioRxiv preprint, 1–33. doi: 10.1101/2022.12.12.520015
Drage H. B., Daley A. C. (2016). Recognising moulting behaviour in trilobites by examining morphology, development and preservation: Comment on Błażejowski et al., 2015. BioEssays 38, 981–990. doi: 10.1002/bies.201600027
Drage H. B., Holmes J. D., García-Bellido D. C., Daley A. C. (2018). An exceptional record of Cambrian trilobite moulting behaviour preserved in the Emu Bay Shale, South Australia. Lethaia 51, 473–492. doi: 10.1111/let.12266
Drage H. B., Holmes J. D., García-Bellido D. C., Paterson J. R. (2023). Associations between trilobite intraspecific moulting variability and body proportions: Estaingia bilobata from the Cambrian Emu Bay Shale, Australia. Palaeontology 66, e12651. doi: 10.1111/pala.12651
Drage H. B., Vandenbroucke T. R. A., Van Roy P., Daley A. C. (2019). Sequence of post-moult exoskeleton hardening preserved in a trilobite mass moult assemblage from the Lower Ordovician Fezouata Konservat-Lagerstätte, Morocco. Acta Palaeontol. Pol. 64, 261–273. doi: 10.4202/app.00582.2018
Ewer J. (2005). How the ecdysozoan changed its coat. PloS Biol. 3, e349. doi: 10.1371/journal.pbio.0030349
Gaines R. R., Briggs D. E. G., Orr P. J., Van Roy P (2012). Preservation of giant anomalocaridids in silica-chlorite concretions from the Early Ordovician of Morocco. Palaios 27, 317–325. doi: 10.2110/palo.2011.p11-093r
García-Bellido D. C., Collins D. H. (2004). Moulting arthropod caught in the act. Nature 429, 40. doi: 10.1038/429040a
García-Bellido D. C., Collins D. H. (2006). A new study of Marrella splendens (Arthropoda, Marrellomorpha) from the Middle Cambrian Burgess Shale, British Columbia, Canada. Can. J. Earth Sci. 43, 721–742. doi: 10.1139/e06-012
Glaessner M. F. (1969). ‘Decapoda,’ in Treatise on invertebrate paleontology, vol. Pt R 4. Ed. Moore R. C. (Lawrence: Geological Society of America and University of Kansas Press), R400–R533. doi: 10.17161/dt.v0i0.5629
Gutiérrez-Marco J. C., Martin E. L. O. (2016). Biostratigraphy and palaeoecology of Lower Ordovician graptolites from the Fezouata Shale (Moroccan Anti-Atlas). Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 35–49. doi: 10.1016/j.palaeo.2016.07.026
Haug J. T., Caron J.-B., Haug C. (2013). Demecology in the Cambrian: synchronized molting in arthropods from the Burgess Shale. BMC Biol. 11, 1–10. doi: 10.1186/1741-7007-11-64
Haug J. T., Castellani C., Haug C., Waloszek D., Maas A. (2012). A Marrella-like arthropod from the Cambrian of Australia: A new link between ‘Orsten’-type and Burgess Shale assemblages. Acta Palaeontol. Pol. 58, 629–639. doi: 10.4202/app.2011.0120
Hyžný M., Bruce N. L., Schlögl J. (2013). An appraisal of the fossil record for the Cirolanidae (Malacostraca: Pericarida: Isopoda: Cymothoida), with a description of a new cirolanid isopod crustacean from the early Miocene of the Vienna Basin (western Carpathians). Palaeontology 56, 615–630. doi: 10.1111/pala.12006
Klompmaker A. A., Fraaije R. H. B. (2011). The oldest (Middle Triassic, Anisian) lobsters from the Netherlands: taxonomy, taphonomy, paleoenvironment, and paleoecology. Palaeontol. Electron. 14, 1–15. Available at: https://palaeo-electronica.org/2011_1/220/220.pdf.
Kühl G., Rust J. (2010). Re-investigation of Mimetaster hexagonalis: a marrellomorph arthropod from the Lower Devonian Hunsrück Slate (Germany). Palaeontol. Z. 84, 397–411. doi: 10.1007/s12542-009-0049-x
Lefebvre B., El Hariri K., Lerosey-Aubril R., Servais T., Van Roy P. (2016). The Fezouata Shale (Lower Ordovician, Anti-Atlas, Morocco): A historical review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 7–23. doi: 10.1016/j.palaeo.2015.10.048
Lefebvre B., Gutiérrez-Marco J. C., Lehnert O., Martin E. L. O., Nowak H., Akodad M., et al. (2018). Age calibration of the Lower Ordovician Fezouata Lagerstätte, Morocco. Lethaia 51, 296–311. doi: 10.1111/let.12240
Legg D. A. (2015). The morphology and affinities of Skania fragilis (Arthropoda) from the middle Cambrian Burgess Shale. Bull. Geosci. 90, 509–518. doi: 10.3140/bull.geosci.1532
Legg D. A. (2016). An acercostracan marrellomorph (Euarthropoda) from the Lower Ordovician of Morocco. Sci. Nat. 103, 1–7. doi: 10.1007/s00114-016-1352-5
Lehnert O., Nowak H., Sarmiento G. N., Gutiérrez-Marco J. C., Akodad M., Servais T. (2016). Conodonts from the Lower Ordovician of Morocco – Contributions to age and faunal diversity of the Fezouata Lagerstätte and peri-Gondwana biogeography. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 50–61. doi: 10.1016/j.palaeo.2016.03.023
Liu Q. (2013). The first discovery of Marrella (Arthropoda, Marrellomorpha) from the Balang Formation (Cambrian Series 2) in Hunan, China. J. Paleontol. 87, 391–394. doi: 10.1666/12-118.1
Martin E. L. O., Pittet B., Gutiérrez-Marco J. C., Vannier J., El Hariri K., Lerosey-Aubril R., et al. (2016a). The Lower Ordovician Fezouata Konservat-Lagerstätte from Morocco: Age, environment and evolutionary perspectives. Gondwana Res. 34, 274–283. doi: 10.1016/j.gr.2015.03.009
Martin E. L. O., Vidal M., Vizcaïno D., Vaucher R., Sansjofre P., Lefebvre B., et al. (2016b). Biostratigraphic and palaeoenvironmental controls on the trilobite associations from the Lower Ordovician Fezouata Shale of the central Anti-Atlas, Morocco. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 142–154. doi: 10.1016/j.palaeo.2016.06.003
McCoy V. E., Brandt D. S. (2009). Scorpion taphonomy: criteria for distinguishing fossil scorpion molts and carcasses. J. Arachnol. 37, 312–320. doi: 10.1636/sh09-07.1
Moysiuk J., Izquierdo-López A., Kampouris G. E., Caron J.-B. (2022). A new marrellomorph arthropod from southern Ontario: a rare case of soft-tissue preservation on a Late Ordovician open marine shelf. J. Paleontol. 96, 859–874. doi: 10.1017/jpa.2022.11
Nowak H., Servais T., Pittet B., Vaucher R., Akodad M., Gaines R. R., et al. (2016). Palynomorphs of the Fezouata Shale (Lower Ordovician, Morocco): Age and environmental constraints of the Fezouata Biota. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 62–74. doi: 10.1016/j.palaeo.2016.03.007
Olempska E. (2012). Morphology and affinities of Eridostracina: Palaeozoic ostracods with moult retention. Hydrobiologia 688, 139–165. doi: 10.1007/s10750-011-0659-7
Pérez-Peris F., Laibl L., Vidal M., Daley A. C. (2021). Systematics, morphology, and appendages of an Early Ordovician pilekiine trilobite Anacheirurus from Fezouata Shale and the early diversification of Cheiruridae. Acta Palaeontol. Pol. 66, 857–877. doi: 10.4202/app.00902.2021
Rak S., Ortega-Hernández J., Legg D. A. (2012). A revision of the Late Ordovician marrellomorph arthropod Furca bohemica from Czech Republic. Acta Palaeontol. Pol. 58, 615–528. doi: 10.4202/app.2011.0038
Rasnitsyn A. P. (2002). ‘Introduction to palaeoentomology,’ in History of insects. Eds. Rasnitsyn A. P., Quicke D. L. J. (Dordrecht, The Netherlands: Kluwer Academic Publishers), 19–20.
Saleh F., Candela Y., Harper D. A. T., Polechovà M., Pittet B., Lefebvre B. (2018). Storm-induced community dynamics in the Fezouata Biota (Lower Ordovician, Morocco). Palaios 33, 535–541. doi: 10.2110/palo.2018.055
Saleh F., Pittet B., Sansjofre P., Guériau P., Lalonde S., Perrillat J.-P., et al. (2020). Taphonomic pathway of exceptionally preserved fossils in the Lower Ordovician of Morocco. Geobios 60, 99–115. doi: 10.1016/j.geobios.2020.04.001
Saleh F., Vaucher R., Anticliffe J. B., Daley A. C., El Hariri K., Kouraiss K., et al. (2021a). Insights into soft-part preservation from the Early Ordovician Fezouata Biota. Earth-Sci. Rev. 213, 1–12. doi: 10.1016/j.earscirev.2020.103464
Saleh F., Vaucher R., Vidal M., El Hariri K., Laibl L., Daley A. C., et al. (2022). New fossil assemblages from the early Ordovician Fezouata Biota. Sci. Rep. 12, 20773. doi: 10.1038/s41598-022-25000-z
Saleh F., Vidal M., Laibl L., Sansjofre P., Gueriau P., Pérez-Peris F., et al. (2021b). Large trilobites in a stress-free Early Ordovician environment. Geol. Mag. 158, 261–270. doi: 10.1017/s0016756820000448
Selden P. A., Shear W. A., Bonamo P. M. (1991). A spider and other arachnids from the Devonian of New York, and reinterpretation of Devonian Araneae. Palaeontology 34, 241–281. Available at: http://hdl.handle.net/1808/8336.
Shear W. A., Edgecombe G. D. (2010). The geological record and phylogeny of the Myriapoda. Arthropod Struct. Dev. 39, 174–190. doi: 10.1016/j.asd.2009.11.002
Stürmer W., Bergström J. (1976). The arthropods Mimetaster and Vachonisia from the Devonian Hunsrück Shale. Palaeontol. Z. 50, 78–111. doi: 10.1007/bf03001974
Tetlie O. E., Brandt D. S., Briggs D. E. G. (2008). Ecdysis in sea scorpions (Chelicerata: Eurypterida). Palaeogeogr. Palaeoclimatol. Palaeoecol. 265, 182–194. doi: 10.1016/j.palaeo.2008.05.008
Van Roy P. (2006). Non-trilobite arthropods from the Ordovician of Morocco (Belgium: University of Ghent), 232.
Van Roy P., Briggs D. E. G., Gaines R. R. (2015a). The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician. J. Geol. Soc 172, 541–549. doi: 10.1144/jgs2015-017
Van Roy P., Daley A. C., Briggs D. E. G. (2015b). Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80. doi: 10.1038/nature14256
Van Roy P., Orr P. J., Botting J. P., Muir L. A., Vinther J., Lefebvre B., et al. (2010). Ordovician faunas of Burgess Shale type. Nature 465, 215–218. doi: 10.1038/nature09038
Vaucher R., Martin E. L. O., Hormière H., Pittet B. (2016). A genetic link between Konzentrat- and Konservat-Lagerstätten in the Fezouata Shale (Lower Ordovician, Morocco). Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 24–34. doi: 10.1016/j.palaeo.2016.05.020
Wang Y., Peng J., Wang D., Zhang H., Luo X., Shao Y., et al. (2021). Ontogenetic moulting behavior of the Cambrian oryctocephalid trilobite Arthricocephalites xinzhaiheensis. PeerJ 9, e12217. doi: 10.7717/peerj.12217
Whittington H. B. (1971). Redescription of Marrella splendens (Trilobitoidea) from the Burgess Shale, middle Cambrian, British Columbia. Geol. Sur. Can. Bull. 209, 1–24. doi: 10.4095/102427
Yang J., Ortega-Hernández J., Drage H. B., Du K.-S., Zhang X.-G. (2019). Ecdysis in a stem-group euarthropod from the early Cambrian of China. Sci. Rep. 9:5709, 1–9. doi: 10.1038/s41598-019-41911-w
Zong R. (2020). Coupled exuviae of the Ordovician Ovalocephalis (Pliomeridae, Trilobita) in South China and its behavioral implications. PeerJ 8, e10166. doi: 10.7717/peerj.10166
Keywords: Euarthropoda, exoskeleton, exuviation, Fezouata Shale, Marrellida, moulting, Ordovician, palaeoecology
Citation: Drage HB, Legg DA and Daley AC (2023) Novel marrellomorph moulting behaviour preserved in the Lower Ordovician Fezouata Shale, Morocco. Front. Ecol. Evol. 11:1226924. doi: 10.3389/fevo.2023.1226924
Received: 22 May 2023; Accepted: 11 July 2023;
Published: 21 August 2023.
Edited by:
Zhifei Zhang, Northwest University, ChinaReviewed by:
Thomas Hegna, State University of New York at Fredonia, United StatesLi Tian, China University of Geosciences Wuhan, China
Copyright © 2023 Drage, Legg and Daley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harriet B. Drage, aGFycmlldC5kcmFnZUB1bmlsLmNo
 Harriet B. Drage
Harriet B. Drage David A. Legg2
David A. Legg2 Allison C. Daley
Allison C. Daley