
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 19 October 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1225998
High promiscuity and low mating partner choosiness in insects can sometimes result in a deviated mating behaviour such as mating with already dead individuals. In this study we investigated the occurrence of necrophilic behaviour in males of the invasive ladybird Harmonia axyridis using two laboratory experiments. For both no-choice and choice experiments, the probability of mating and mating duration were significantly affected by female status (alive, 1-day, 7-day, or 14-day old carcass) and by male mating status (unmated or mated) which was investigated in the no-choice experiment. The presence of chemical cues produced by an alive female did not affect the probability of a male mating with a dead female. In the no-choice experiment, 14-day old female carcasses were significantly less attractive than alive females, and unmated males tended to mate with females of all statuses with significantly higher probability than mated males. In the choice experiment, males showed a significant preference to mate with alive females when compared to 7-day old carcasses but did not distinguish between alive females and 1-day old carcasses. Mating latency (time to the starting of mating) tended to be longer for 14-day old carcasses in the no-choice experiment and was not affected by female status in the choice experiment. Mating duration was negatively affected by age of female carcasses in the no-choice experiment and only 7-day old carcasses were mated for significantly shorter time than alive females in the choice experiment. Mating behaviour was highly consistent for the no-choice and choice experiments. Despite decreasing probability of mating with 7-day and 14-day old carcasses, we observed a surprisingly high frequency of mating with dead conspecifics in the invasive ladybird Harmonia axyridis, which could have serious consequences for transmission of pathogens and affect male fitness even under natural conditions.
Sexual reproduction is the most prevalent evolutionary strategy in Eukaryotes (Otto, 2009). Through sexual reproduction, living organisms generate original combinations of traits in offspring (new generations). Diverse progeny resulting from genetic mixture and consequent phenotypic outcomes can survive under heterogeneous environmental conditions (Forsman, 2014). However, at the same time, many individuals possess traits suboptimal for conditions they are experiencing (Birkhead and Pizzari, 2002; Matthews and Matthews, 2010). Despite being the most common, sexual reproduction is a costly process in many ways and somehow ‘inefficient’ compared to other types of reproduction, and thus strong selection pressure is expected to improve its efficiency (Otto, 2009; Lehtonen et al., 2012).
Specific mating strategies and behaviours can minimise the costs and risks of sexual reproduction (Höglund and Sheldon, 1998; Parker and Birkhead, 2013; Kappeler, 2021; Kappeler et al., 2023). For example, one’s reproductive success and fitness, can be increased with increased mating events and mating partners, potentially resulting in more offspring (e.g., polygyny and polyandry; Brown et al., 1997; Parker and Birkhead, 2013). In insects, sexual promiscuity is one of the most common mating strategies (Birkhead and Pizzari, 2002; Matthews and Matthews, 2010). Yet, this mating strategy carries ‘fecundity costs’, e.g., physiological cost (loss of energy or nutrients) and, ‘survival cost’, e.g., risk of increased predation rate, physical injuries, and exposure to pathogens and parasites (Magnhagen, 1991; Arnqvist and Nilsson, 2000). These costs may conflict with resources otherwise available for somatic growth and survivorship (Schultz and Warner, 1991; Jennings and Philipp, 1992; Kotiaho, 2001; Lehtonen et al., 2012).
In order to minimise costs, and maximise benefits (fitness), male and female insects may optimise their mating behaviour (Arnqvist and Nilsson, 2000; Arnqvist et al., 2004). Mating behaviour typically involves locating and approaching a suitable partner, evaluating the quality of a potential mate, and copulation (Alexander et al., 1997). Mating behaviours are variable among insects, and some are extremely peculiar (Thornhill and Alcock, 1983; Page, 1986; Tatarnic et al., 2014). For example, males of Stylops ovinae (Strepsiptera) pierce the female’s cephalothorax cuticle with their penis during copulation to transfer sperm, an act known as traumatic insemination (Peinert et al., 2016). In odd circumstances, insects can misidentify reproductive partners, due to flawed signals and behaviours. The jewel beetle Julodimorpha bakewelli has been recorded copulating with inanimate things such as beer bottles (Gwynne and Rentz, 1983). Males of the two spotted ladybird Adalia bipunctata have been fooled into copulating with dummies bearing male and female elytra (Hemptinne et al., 1998).
An extreme mating behaviour that sometimes occurs across various animal taxa, is mating with already dead individuals, i.e., necrophilia (Bettaso et al., 2008; Ayres, 2010; Cortés Bedoy et al., 2014). In some arthropods, e.g., cannibalistic mantises or various spider species, killing partner during copulation can be a common part of their mating system (Scardamaglia et al., 2015; Ma et al., 2022). However, in many other cases mating with a dead partner seems to be unintentional. Explanations for this behaviour can be grouped in two major categories: 1) individual sexual deviation, i.e., problem of a given animal and not whole species (Tomita and Iwami, 2015; Swift and Marzluff, 2018; Pettigrew, 2019) and 2) the inability of species to perceive chemical and physical cues that would otherwise distinguish between dead and alive individuals (Goncalves and Biro, 2018). Mating with a dead partner typically reduce fitness and may be maladaptive for a given species or population since copulation does not result in a new generation (Crespi, 2000). However, an interesting example of “necrophilic” behaviour in the small Amazonian frog, Rhinella proboscidea, shows that under specific conditions this behaviour can increase male fitness (Izzo et al., 2012). Mating with a dead partner considered as individual deviation, i.e., necrophilia, has been repeatedly reported for vertebrate species (Tomita and Iwami, 2015; Swift and Marzluff, 2018; Colombo and Mori, 2019; Pintanel et al., 2021). In contrast, in insects, it is quite common that freshly killed individuals may not be recognized as dead by other conspecifics, however, it has been rarely investigated how long this situation persists (Geiselhardt et al., 2009; Buellesbach et al., 2018).
Ladybirds represent a suitable insect group for investigating mating behaviour, as many ladybird species are promiscuous, mate frequently, and mating can last for quite a long time (generally few hours; Hodek et al, 2012). Several studies indicate that ladybird mating behaviour is induced by a combination of visual stimuli (in particular movement) and olfactory cues (semiochemicals; Hemptinne et al., 1998). The ladybird Harmonia axyridis serves as a popular model species because of its invasive success worldwide (Brown et al., 1997; Roy et al., 2016) and because a significant amount of knowledge on its biology and behaviour has been accumulated through extensive studies (Hodek et al, 2012; Awad et al., 2015a; Awad et al., 2017; Knapp and Řeřicha, 2020; Řeřicha et al., 2021). During our own laboratory and field investigations focused on various aspects of ladybird biology (e.g., Knapp et al., 2019; Knapp et al., 2020; Knapp et al., 2022), we repeatedly observed attempts to mate with dead conspecifics in Harmonia axyridis during the growing season and post-overwintering period.
In this study, we investigated several factors that could affect “necrophilic” behaviour in Harmonia axyridis using two laboratory behavioural experiments. We hypothesized that 1) the probability of mating with a carcass will be higher for unmated males compared to sexually experienced ones, 2) the attractiveness of carcasses will decrease with time, and 3) the presence of conspecific chemical cues will increase the probability of mating with carcasses. In addition, we expected that time to the first mating attempt will be higher for carcasses compared to alive individuals and mating duration will be shortened in the case of mating with dead females.
Harmonia axyridis adults used as parents for our experimental beetles were collected in autumn 2018 from three overwintering aggregations across Czech Republic: 1) Ohaře (GPS: 50°10´N, 15°30´E; 200 m a. s. l.), 2) Nučice (GPS: 49°96´N, 14°88´E; 330 m a. s. l.) and 3) Hvozdno (GPS: 49°15´N, 14°56´E; 500 m a. s. l.). Ladybirds were overwintered in Petri dishes in a climatic chamber programmed to a constant mild winter temperature (6°C). In February 2019 several beetles from each population were sexed and 12 parental pairs were established. Each pair was accommodated in a separate Petri dish (9 cm in diameter) containing filter paper strips, which provide a suitable substrate for egg laying. Beetles were exposed to room temperature (23°C), longer-day photoperiod 14L:10D, and provided water and food (Ephestia kuehniella Zeller, 1879 eggs) ad libitum. The following week, 10 out of 12 parental pairs started to lay eggs and their offspring were used for the mating experiment. Presence of new eggs was checked daily, and new clutches were moved to a separate Petri dish. Developing offspring (from eggs to adults) were kept under the same laboratory conditions as parental pairs. To minimize cannibalism between siblings, young 3rd instar larvae were divided into groups of five individuals per Petri dish. Newly hatched adults (1-day old) were sexed. Females were kept in small groups (four individuals per Petri dish), whereas males were strictly separated (one individual per Petri dish) to avoid any mating events among them. All Petri dishes were checked every second day, cleaned when necessary, and water and food was added when missing or exchanged when deteriorated (became mouldy). We kept the parental information of all offspring throughout the experiment to ensure that siblings were not paired in the following experiments.
Adult experimental animals were assigned to ‘mated’ or ‘unmated’ treatment at the age of ca. three weeks. For the ‘mated’ treatment, three males were added to Petri dishes containing four females and were allowed unlimited mating opportunities for the next four weeks. For the ‘unmated’ treatment, all males continued to be isolated individually and females (by that time reared in groups of four) were separated, i.e., placed individually in new Petri dishes, for the next three weeks. Unfortunately, our experimental setting mix together two factors that can affect male mating behaviour: sexual experience (i.e., limited number of sexual partners) and sexual deprivation (i.e., long period without sex). When beetles reached age ca. seven weeks, which represents a period of full sexual activity in ladybirds (Berkvens et al., 2010; Nedvěd and Honěk, 2012), our behavioural experiments started. Note, a subset of females were killed (frozen at -22°C for 6 hours and then accommodated at room temperature in open Eppendorf tubes) either 14 days, 7 days, or 1 day prior to our experiments to create a gradient of female freshness from alive individuals to 14-day old carcasses (for details see Figure 1).
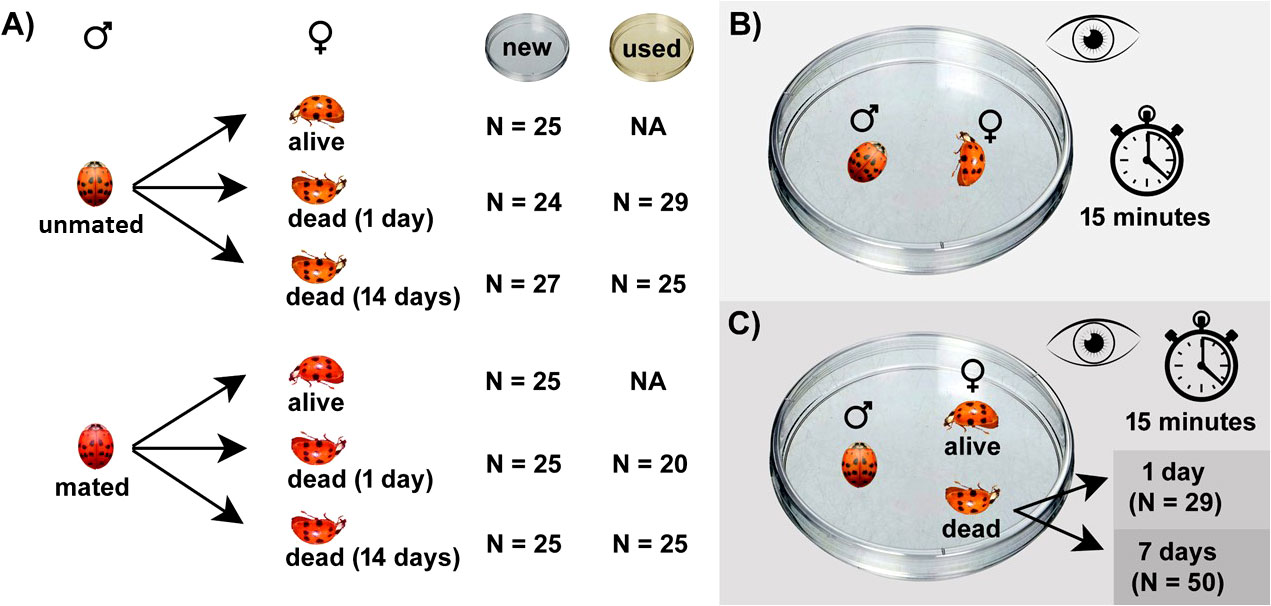
Figure 1 Overview of the experimental setup. (A) Overview of treatment combinations investigated in no-choice experiments including number of replications per treatment combination; (B) trial setting for the no-choice experiment; (C) trial setting for the choice experiment including number of replications per treatment.
In the no-choice experiment, one male (mated or unmated) was paired with one female of the same mating status, i.e., mated males with mated females and unmated males with unmated females. Three different levels of female status (freshness) were tested: 1) alive female, 2) newly dead female (1-day old carcass), and 3) partly deteriorated dead female (14-day old carcass). Note that 14-day old carcasses have marks of decomposition since their colour changed to more brownish and body appendices could be easily separated from the body. Plastic Petri dishes (9 cm in diameter) were used as mating arenas. Two different types of mating arenas were used as treatments: 1) new Petri dishes unpacked from the original packaging immediately before the start of the trial, 2) used Petri dishes with traces of ladybirds (Petri dishes that had groups of females for at least 5 days). Traces of ladybirds has been shown to affect ladybird mating and oviposition behaviour (Magro et al., 2010; Fassotte et al., 2016). Mating assays using alive females were investigated using only new Petri dishes since an alive female fills the arena with their own scent. An overview of the experimental design for the no-choice experiment is shown in Figures 1A, B. Mating behaviour was observed for 15 minutes and mating occurrence was recorded every minute. Note that this period did not cover whole mating event that may last up to several hours in Harmonia axyridis (Obata, 1987). We aimed just to check whether mating with female carcasses will be started later and abrupted sooner or not.
Later we performed the choice experiment in which one unmated male was provided with two females (one alive and one dead). All trials were performed using new Petri dishes. Dead females were newly dead (1-day old carcasses) or 7-day old carcasses. Mating behaviour was observed for 15 minutes and mating occurrence was recorded every minute. The experimental setting for the choice experiment is summarized in Figure 1C). Raw data gathered during both experiments are available in the Supplementary File (Dataset S1).
In the no-choice experiment we analysed three response variables: 1) mating occurrence during the given trial (yes/no), 2) mating latency (time to the starting of mating in minutes) and 3) mating duration (time spent mating out of the trial duration). In the first step we fitted full models including the effect chemical cues for trials using female carcasses (new Petri dish or used Petri dish = with traces of ladybird presence). This effect was not significant in all cases (see results; Figure S1), therefore we fitted the following simplified models to investigate the effects of female status (alive female, 1-day or 14-day old carcass) and male mating status (unmated or mated). In each model for each response variable, we used female status, male mating status, and the interaction between female status and male mating status as fixed effects. Mating occurrence was analysed using a generalized linear model with a binomial distribution (GLM-b). Mating latency and duration were analysed using generalized linear models with a gamma distribution (GLM-g). Suitable error distributions were selected based on data properties and distribution suitability based on the data evaluation using ‘check_distribution’ function from the package ‘performance’ (Lüdecke et al., 2021). Tukey’s honestly significant difference tests were employed for all models to test for significant differences between female status levels (alive, 1-day old or 14-days old carcasses) using the ‘glht’ function from the package ‘multcomp’ (Hothorn et al., 2008).
For the choice experiment, the following response variables were analysed: 1) female preference (alive female vs. 1-day old or 7-day old carcass), 2) mating latency (time to the start of mating with given female in minutes), and 3) mating duration (time spent by mating with given female out of the trial duration). Models similar to the mating experiment (GLM-g) were used to analyse mating latency and mating duration (two separate models). Chi-squared tests (likelihood ratio tests) were used to compare frequency (preference) of mating for alive females versus carcasses (1-day or 7-day old), and to compare the proportion of non-mating males between 1-day and 7-day old carcass treatments.
Finally, we compared values obtained for mating latency and mating duration from the no-choice experiment and the choice experiment using data for alive females and 1-day old carcasses. Analyses were performed using a GLM-g with experiment, female status, and the interaction between experiment and female status as predictors.
All data analyses were performed in R version 4.02 (R Development Core Team, 2022).
The mating behaviour of males was not affected by the presence of chemical cues from alive females when males were provided with female carcasses (new vs. used Petri dishes; GLM-b: χ2 = 0.182; P = 0. 669). Mating latency (GLM-g: χ2 = 0.905; P = 0.236) and mating duration (GLM-g: χ2 = 0.318; P = 0.445) did not differ between new and used Petri dishes (Figures S1A–C)
The probability of mating was significantly affected by female status (alive, 1-day and 14-day old carcass; GLM-b: χ2 = 25.973; P < 0.001) and male mating status (unmated or mated; GLM-b: χ2 = 26.796; P < 0.001). Fourteen-day old female carcasses were significantly less attractive than alive females, and unmated males started copulation with significantly higher probability compared to already mated ones (confirmed for all female treatments; Figure 2). There was no significant interaction between female status and male mating status (GLM-b: χ2 = 1.029; P = 0.597; Figure 2). Pairwise comparisons between treatments (female status) revealed significant differences between 14-day old carcasses and all other female statuses (Tukey’s post hoc test: P < 0.05).
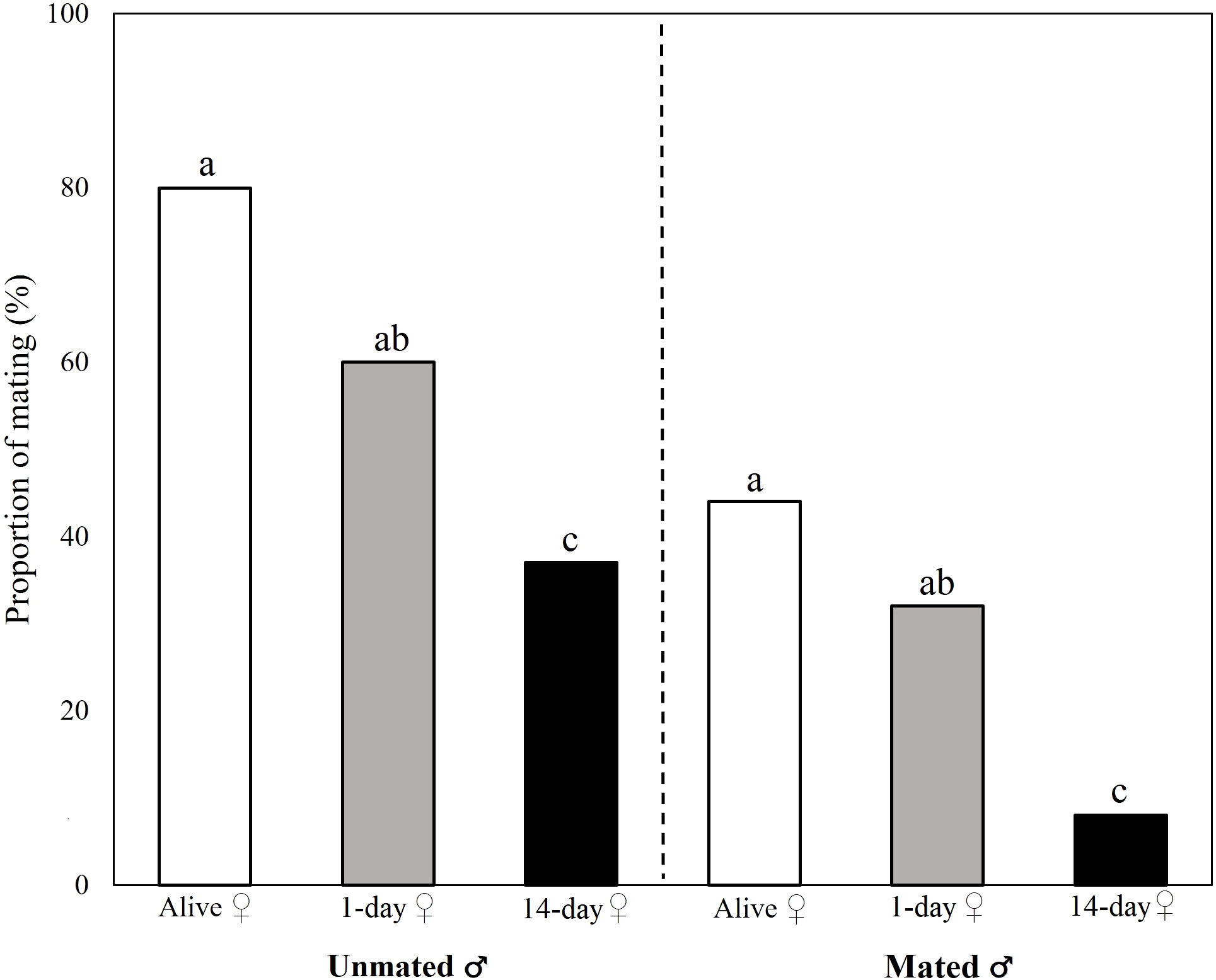
Figure 2 Effects of female status (alive female, 1-day old or 14-day old carcass) and male mating status (unmated or mated) on proportion of mating males. The left panel presents data for unmated males and the right panel presents data for repeatedly mated males in the no-choice experiment. Results of Tukey’s post-hoc tests are used to compare significance of differences between female statuses within a given panel (different letters indicate significant differences between treatments).
Mating latency was marginally affected by female status (GLM-b: χ2 = 3.772; P = 0.052), it took longer to start copulation with 14-day old female carcasses than with alive or freshly dead females (Figure 3). Mating latency was not affected by male mating status (GLM-b: χ2 = 1.097; P = 0.190; Figure 3). Mating duration was significantly affected by male mating status (GLM-g: χ2 = 3.453; P = 0.042) and female status (GLM-g: χ2 = 6.936; P < 0.001). Mating duration was significantly longer for unmated males than mated males, and for alive females compared to 14-day old carcasses, indicating that in many cases males stopped mating with 14-day old carcasses after few minutes, i.e., before the end of 15-minute-long trial (Figure 4).
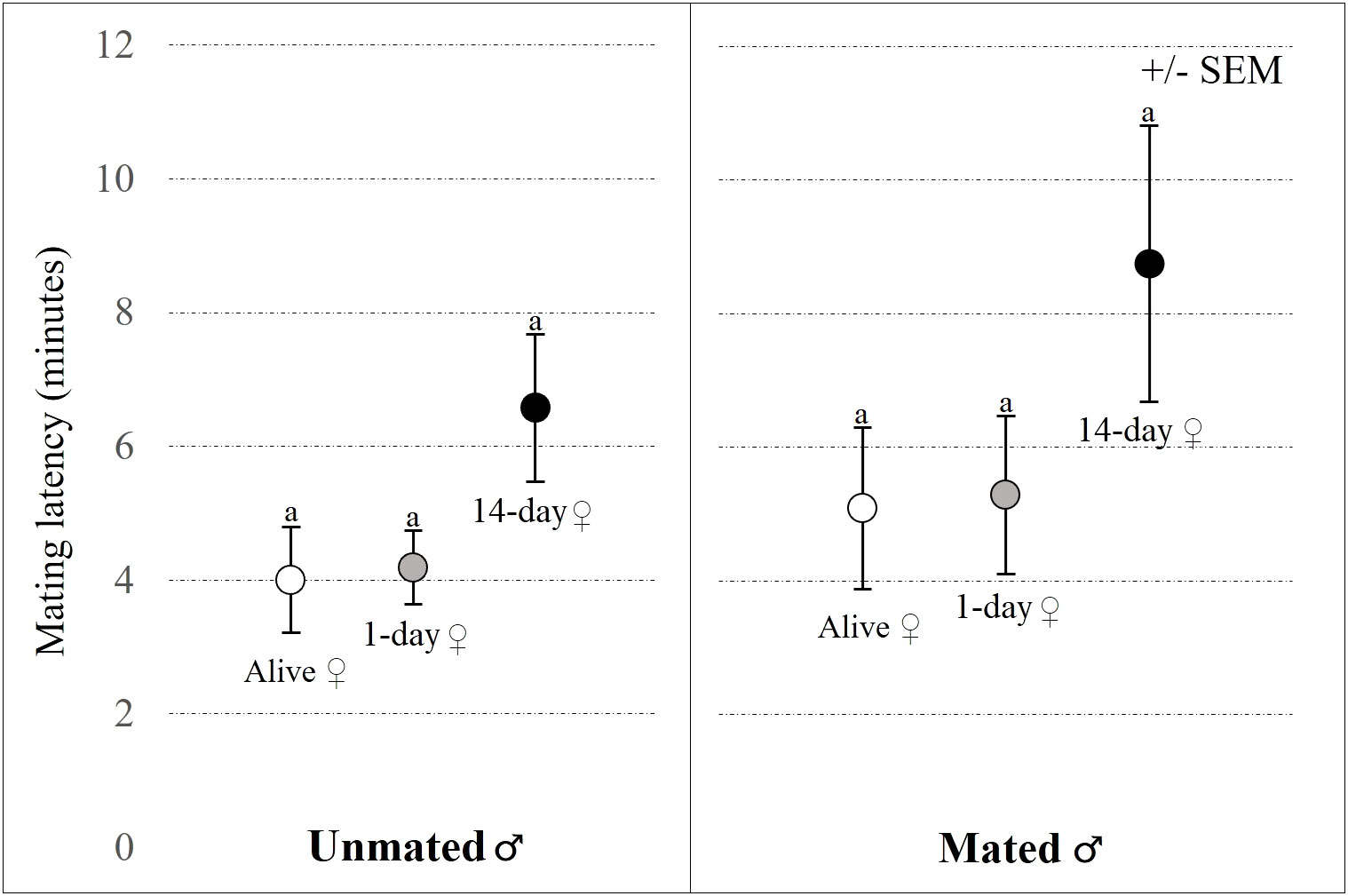
Figure 3 Effects of female status (alive female, 1-day old or 14-day old carcass) and male mating status (unmated or mated) on mating latency. The left panel presents data for unmated males and the right panel presents data for repeatedly mated males in the no-choice experiment. Results of Tukey’s post-hoc tests are used to compare significance of differences between female statuses within a given panel (different letters indicate significant differences between treatments).
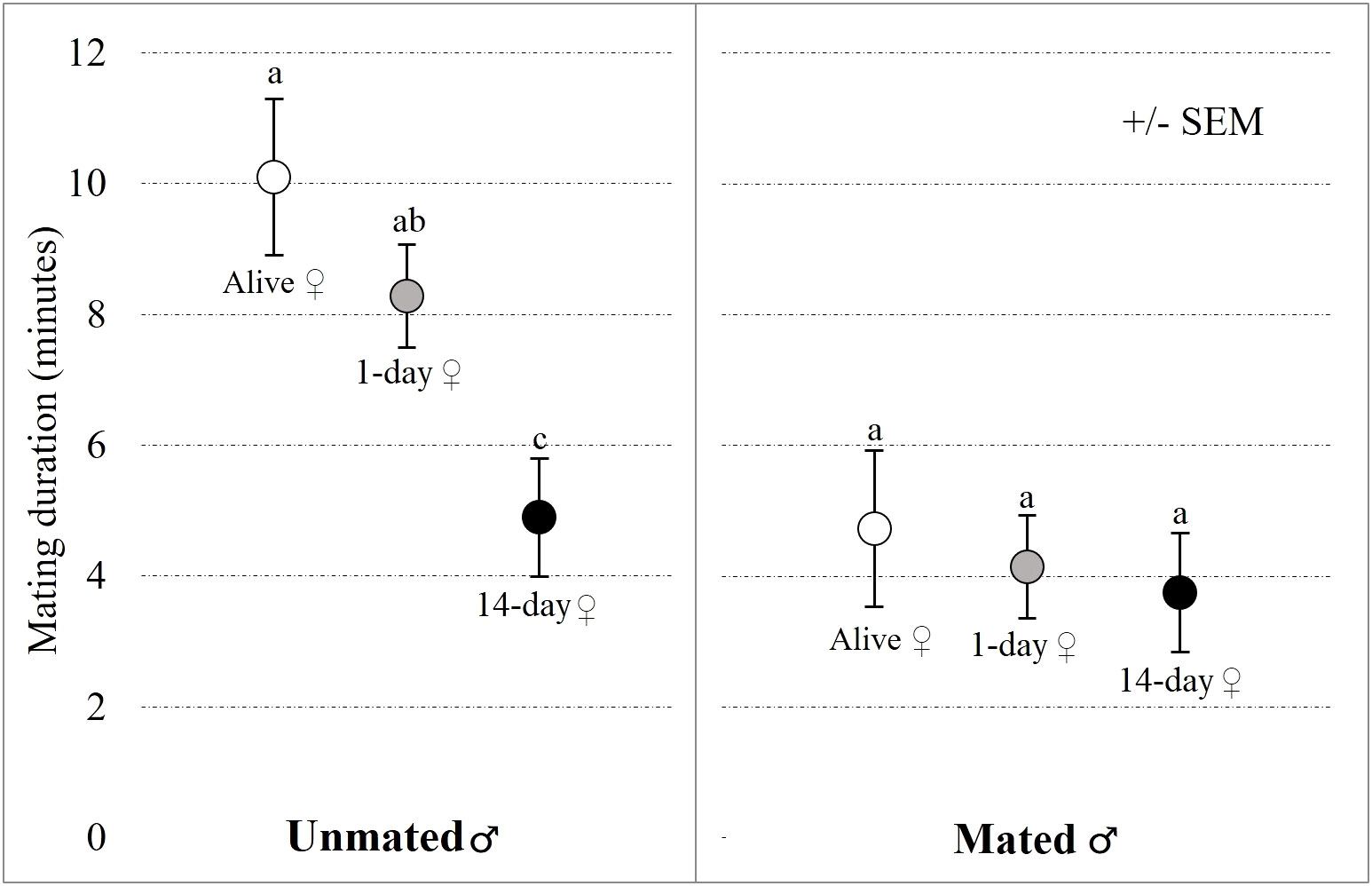
Figure 4 Effects of female status (alive female, 1-day old or 14-day old carcass) and male mating status (unmated or mated) on mating duration. The left panel presents data for unmated males and the right panel presents data for repeatedly mated males in the no-choice experiment. Results of Tukey’s post-hoc tests are used to compare significance of differences between female statuses within a given panel (different letters indicate significant differences between treatments). Note that the maximal mating duration was limited to 15 minutes, i.e., duration of our trial, and mating durations occurring under natural conditions can be much longer.
Males significantly preferred mating with living females in the comparison with 7-day old carcasses (χ2-test: χ2 = 32.491; P < 0.001), but were equally likely to mate with living females or 1-day old carcasses (χ2-test: χ2 = 1.466; P = 0.480). The proportion of males that did not engage in mating was not related to the age of the carcass (χ2-test: χ2 = 0.002; P = 0.961, Figure 5).
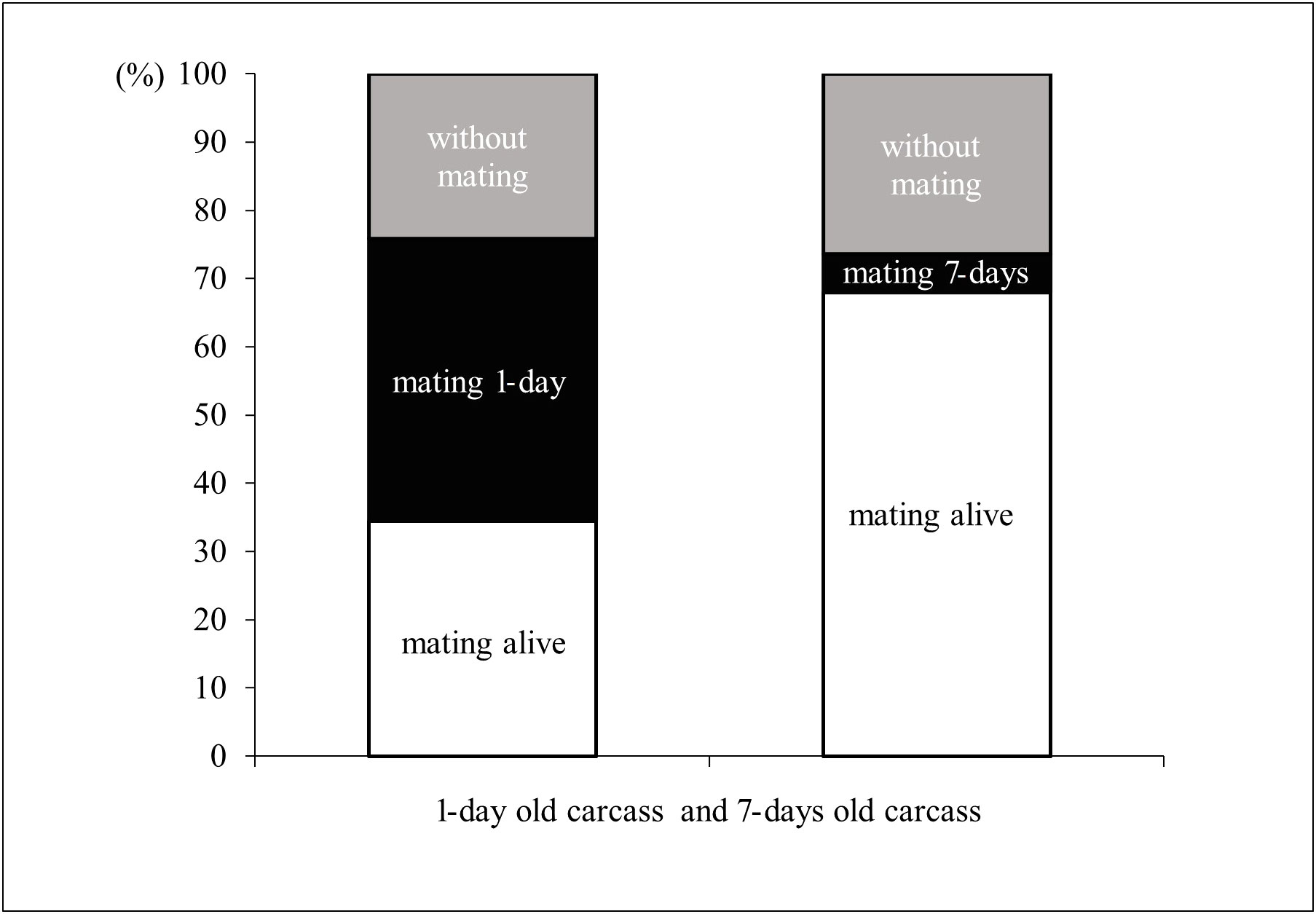
Figure 5 Male’s mating preferences in the choice experiment. The left column represents mating preferences of ladybird males when provided with choice between alive females and 1-day old carcass. The right column represents mating preferences of ladybird males when provided with choice between alive females and 7-day old carcass.
Mating latency was not affected by any of the investigated predictors, even female status had no effect (GLM-g: χ2 = 0.170; P = 0.554; Figure S2). In contrast, mating duration was significantly affected by the interaction between female status (alive or dead) and treatment (1-day or 7-day old carcass; GLM-g: χ2 = 1.583; P = 0.008; Figure 6). Mating durations were significantly shorter with 7-day old carcases than with alive females or 1-day old carcasses.
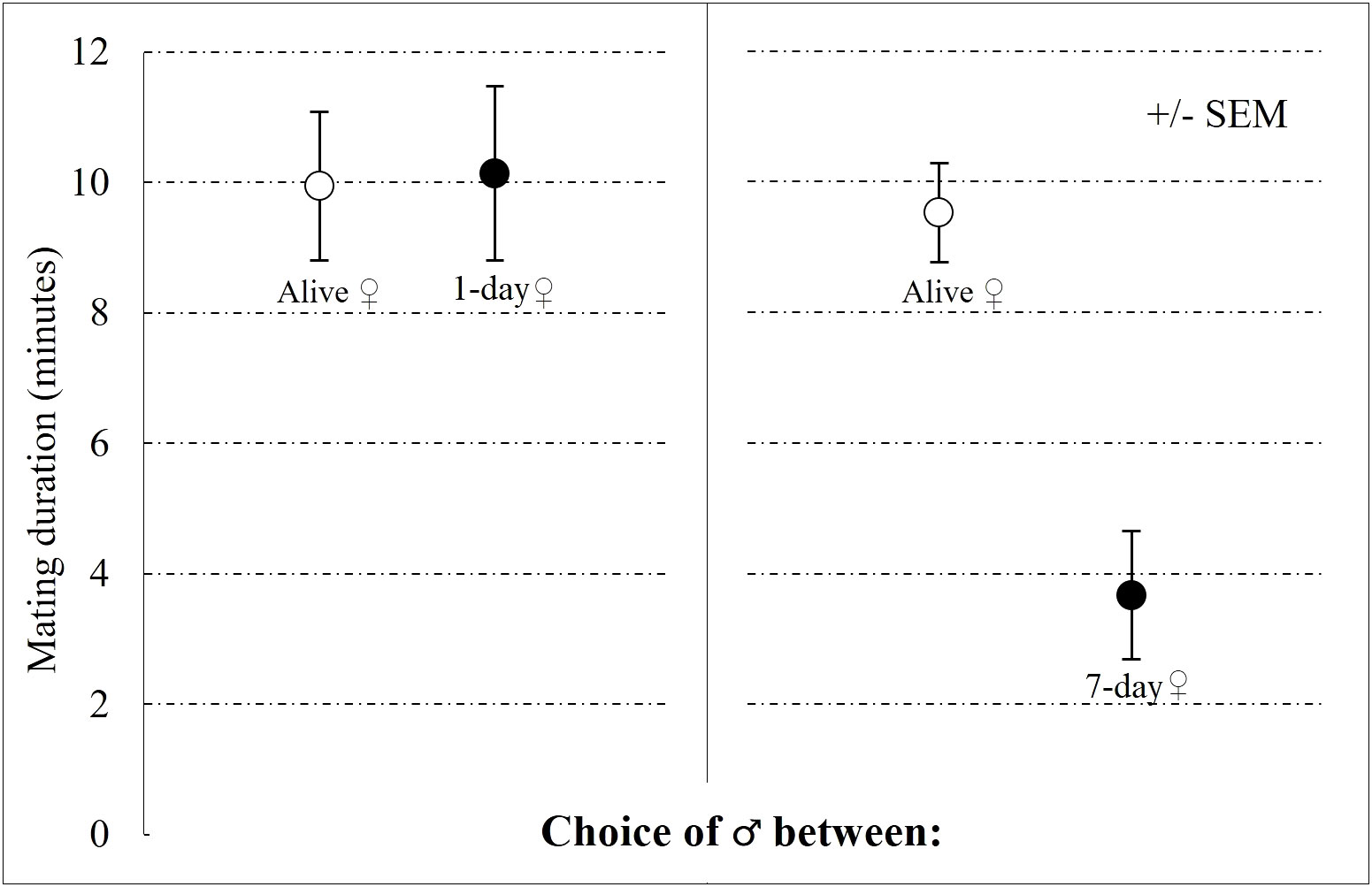
Figure 6 Effects of female status (alive or dead) and carcass age (1-day or 7-days old carcass) on mating duration in the choice experiment. The left panel presents treatment when males choose between alive female and 1-day old carcass. The right panel presents treatment when males choose between alive female and 7-day old carcass.
There was no significant difference between values obtained during no-choice and choice experiments using data for alive females and 1-day old carcasses (mating latency - GLM-g: χ2 = 0.223; P = 0.549; mating duration - GLM-g: χ2 = 0.149; P = 0.455; Figures S3, S4), indicating that experimental setting did not affected ladybird mating behaviour in our study.
Our study showed that mating with a dead conspecific is quite common phenomenon in Harmonia axyridis under laboratory conditions, however its frequency and importance under field conditions should be evaluated in a future study. In general, males are unable to distinguish between alive and freshly dead (1-day old carcass) females. Reduced mating rates were recorded for 7-day old carcasses and ca. 90% of experienced (already mated) males refused to mate with 14-day old carcasses. However, mating probability significantly increased when males were sexually inexperienced or deprived, resulting in ca. 35% of previously unmated males mating with a decaying (14-day old) carcasses. This indicates that despite the ability of Harmonia axyridis males to distinguish low-quality females (14-day old carcasses), extremely low choosiness of sexually-deprived unmated males can result in a surprisingly high occurrence of necrophilic behaviour.
Even though female choice is considered to be more widespread among insects than male choice, accumulated evidence confirmed existence of male mating choice in diverse insects spanning all major orders (Bonduriansky, 2001). Males can evaluate the quality of females, and therefore should be able to distinguish between dead and alive females (Goncalves and Biro, 2018). Ladybirds in general, and specifically Harmonia axyridis, likely have well-developed male mate choice since choosiness is typically favoured when mating is costly (e.g., involving large spermatophores and long copulation duration) and high variability in female quality exists (Bonduriansky, 2001). Harmonia axyridis males can select females based on their age (Osawa, 1994) and colour morph (Awad et al., 2015a). The ability to distinguish between alive and dead females (1-day old or 7-day old carcasses) was confirmed for another large ladybird species Coccinella septempunctata from the same tribe (Coccinellini; Omkar and Srivastava, 2002), and thus our results indicating that Harmonia axyridis males could have a limited ability to recognize dead females is surprising. In addition, mating can be costly for males in some ladybird species when energy requirements of ejaculate production are considered (Perry and Rowe, 2010; Hodek et al., 2012). Therefore, strong selection against wasted mating attempts is expected, especially when mating with a dead female cannot result in any offspring (in contrast to the frog example; Izzo et al., 2012). However, the study by Omkar and Pervez (2005) investigating other ladybird species (Propylea dissecta) showed that 7-day old female carcasses can be attractive to males and 30% of males started mating with such carcasses, which illustrates that Harmonia axyridis is not an exceptional species among ladybirds. Weak ability of Harmonia axyridis to distinguish between alive and dead conspecifics is indicated also by absence of significant differences in mating latency. However, it is possible that this variable is mainly under female control, as it has been previously shown for another ladybird species (Cryptolaemus montrouzieri) that females are able to reduce mating latency when offered with a preferred male (Xie et al., 2014).
It seems that sensorial and cognitive constraints preclude males of various insect species to identify aliveness of their sexual partner (Goncalves and Biro, 2018). In general, insects are largely dependent on chemical cues to identify and evaluate conspecifics. Pheromones and cuticular hydrocarbons have been repeatedly identified as major tool in mate selection (Vaníčková et al., 2012; Gomez-Diaz and Benton, 2013; Chung and Carroll, 2015; Fassotte et al., 2016; Menzel et al., 2019). Ladybird mating behaviour is likely induced by a combination of visual stimuli (in particular movement) and olfactory cues (semiochemicals; Hemptinne et al., 1998), but the importance of sexual semiochemicals is still unclear. Sometimes ladybirds fail even with proper species identification, resulting in repeatedly observed interspecific copulations, and this phenomenon can become more common under laboratory conditions (Majerus, 1997; Mercado, 2023). Existing evidence indicates that the role of cuticular hydrocarbons can be limited in Harmonia axyridis as males are unable to detect differences between mated and unmated females based on cuticular hydrocarbons (Legrand et al., 2019a). Sex pheromones probably play a more important role in Harmonia axyridis (Legrand et al., 2019b), however existing knowledge is surprisingly limited. Potentially limited importance of chemicals for Harmonia axyridis mating behaviour is further supported by this study in which there was no significant difference between new Petri dishes and ones with fresh traces of ladybirds. Such traces were previously shown to be detectable by adult ladybirds and can affect oviposition behaviour (Yasuda et al., 2000; Evans, 2003).
Females that are unresponsive to the mating attempts of a male may be attractive to males in species that typically exhibit female refusing behaviour (Bonduriansky, 2001). Female ladybirds often resist mating attempts by males (Perry et al., 2009), which may decrease males preference for highly active females. In contrary, sometimes female’s inactivity can protect them from intensive harassment. For example, in a dragon fly species, female use inactivity (‘fake death behaviour’) to escape from suffering from intensive harassment in places with extremely high male densities (Khelifa, 2017). However, note that this behaviour essentially makes dragonfly females invisible to males rather than unattractive. This indicates that insect males can have a serious problem to recognize that an immobile female is really dead. Fast recognition of death requires relatively specialized evolutionary novelties as illustrated by social insects that need fast identification of carcasses for their timely transport from the nest (Sun et al., 2018). The Argentine ant (Linepithema humile) produce dolichodial and iridomyrmecin from the pygidial gland and spread it on the cuticle to mask the already present corpse removal stimuli, triglycerides (Choe et al., 2009). This mechanism allow detection of nestmate death in less than one hour. In contrast, detection by traditional necromones resulting from the decomposition process, e.g., fatty acid death cues, can take a few days (Goncalves and Biro, 2018; Sun et al., 2018). Fatty acid death cues are used by a wide range of arthropods, and it is probable that even ladybirds can recognize them (Yao et al., 2009; Goncalves and Biro, 2018).
Males of some insect species can only recognize the quality of a mating partner during copulation and adjust their investment at that time, e.g., reduce copulation duration or amount of ejaculate (Parker, 1983; Gwynne, 1984; Geiselhardt et al., 2009). This may be because some semiochemical, e.g., cuticular hydrocarbons, are relatively stable and can persist in unchanged shape (amount and composition) for many days after a death (Menzel et al., 2019), giving the impression that the potential mate is still alive even days after death. In such cases, the lack of behavioural response from females to a mating attempt may be the most important cue to males as to the status of their potential mate. Despite of a methodological limitation of our study (just 15-minute-long trials), we observed a significant decrease in mating duration with older carcasses for unmated males (no-choice experiment) and specifically for males used in the choice experiment. This suggests that the presence of alternative sexual partners can increase male choosiness when facing low-quality females (7-day old carcasses). At the same time, mating experience increased choosiness of ladybird males, which is in line with the expected costs of mating in Harmonia axyridis. The increased fitness (paternity) that results from repeated matings is outbalanced by physiological costs and an increased risk of contracting sexually transmitted diseases or parasites (Fiedler and Nedvěd, 2019). On the other hand, decreased male choosiness ensures high fitness and can be advantageous under conditions of many unmated females. Such conditions occur in late winter and early spring as Harmonia axyridis overwinter in huge aggregations where many females have empty spermathecas (Awad et al., 2015b). It is the time when we repeatedly observed necrophilic behaviour in Harmonia axyridis in nature (MK and MŘ personal observation - unquantified and unpublished data).
In conclusion, we observed surprisingly high occurrence of mating with dead females in the invasive ladybird Harmonia axyridis under laboratory conditions, even when males were provided with older (7-day or 14-day old) carcasses. Unfortunately, our experiment was not designed to distinguish whether males only initiated mating or completed a mating attempt (transmitted their ejaculate) with dead females, which would confirm an extremely high physiological costs of such behaviour. Nonetheless, mating with already dead conspecifics can exposes ladybird males to an increased risk of disease and parasite transmission. Understanding the mechanisms that would otherwise prevent such behaviour in Harmonia axyridis deserves the attention of researchers and a future research.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
MR and MK developed the main idea and designed laboratory experiments; MR and SM-M performed laboratory experiements; MR and FH analysed data; MR and SM-M wrote draft of the manuscript; all authors contributed to final edits of the manuscript. All authors contributed to the article and approved the submitted version.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic CZ.02.2.69/0.0/0.0/19_073/0016944 (student grant 71/2021).
Many thanks to David N. Awde for language edits and insightful comments on previous version of our manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1225998/full#supplementary-material
Alexander R., Marshall D., Coolet J. (1997). “Evolutionary perspectives on insect mating,” in Mating systems in insects and arachnids. Eds. Choe J. C., Crespi B. J. (Cambridge, UK: Cambridge University Press).
Arnqvist G., Nilsson T. (2000). The evolution of polyandry: multiple mating and female fitness in insects. Ani. Behav. 60, 145–164. doi: 10.1006/anbe.2000.1446
Arnqvist G., Nilsson T., Katvala M. (2004). Mating rate and fitness in female bean weevils. Behav. Ecol. 16, 123–127. doi: 10.1093/beheco/arh119
Awad M., Kalushkov P., Karab̈ÿk F., Nedvěd O. (2015a). Non-random mating activity of colour morphs of ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Acta Soc Zool. Bohem. 79, 11–17.
Awad M., Laugier G. J. M., Loiseau A., Nedvěd O. (2015b). Unbalanced polyandry in wild-caught ladybirds Harmonia axyridis (Coleoptera: Coccinellidae). Appl. Entomol. Zool. 50, 427–434. doi: 10.1007/s13355-015-0348-5
Awad M., Piálek L., Krejčí A., Laugier G., Nedvĕd O. (2017). Paternity following multiple mating in ladybird Harmonia axyridis. Biocontrol 62, 297–307. doi: 10.1007/s10526-017-9806-z
Berkvens N., Bale J. S., Berkvens D., Tirry L., De Clercq P. (2010). Cold tolerance of the harlequin ladybird Harmonia axyridis in Europe. J. Insect Physiol. 56, 438–444. doi: 10.1016/j.jinsphys.2009.11.019
Bettaso J., Haggarty A., Russell E. (2008). Rana boylii (Foothill yellow-legged frog). necrogamy. Herpetol. Rev. 39, 462. doi: 10.2307/1437888
Birkhead T., Pizzari T. (2002). Postcopulatory sexual selection. Nat. Rev. Gen. 3, 262–273. doi: 10.1038/nrg774
Bonduriansky R. (2001). The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76, 305–339. doi: 10.1017/S1464793101005693
Brown W. D., Crespi B. J., Choe J. C. (1997). “Sexual conflict and the evolution of mating systems,” in Mating systems in insects and arachnids. Eds. Choe J. C., Crespi B. J., 352–379. (Cambridge, UK: Cambridge University Press). doi: 10.1007/s10526-011-9379-1
Buellesbach J., Vetter S., Schmitt T. (2018). Differences in the reliance on cuticular hydrocarbons as sexual signaling and species discrimination cues in parasitoid wasps. Front. Zool. 15, 22. doi: 10.1186/s12983-018-0263-z
Choe D. H., Millar J. G., Rust M. K. (2009). Chemical signals associated with life inhibit necrophoresis in Argentine ants. PNAS. 106, 8251–8255. doi: 10.1073/pnas.0901270106
Chung H., Carroll S. B. (2015). Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37, 822–830. doi: 10.1002/bies.201500014
Colombo M., Mori E. (2019). The “corpse bride” strikes again: first report of the Davian behaviour in the Eurasian badger. Mammalia 84, 372–376. doi: 10.1515/mammalia-2019-0039
Cortés Bedoy S., Mantilla-Castaño J. C., Pareja-Márquez I. M. (2014). Necrophiliac and interspecific amplexus in Dendropsophus columbianus (Anura: Hylidae) in the Central Cordillera of Colombia. Herpetol. 7, 515–516.
Crespi B. (2000). The evolution of maladaptation. Heredity 84, 623–629. doi: 10.1046/j.1365-2540.2000.00746.x
Evans E. W. (2003). Searching and reproductive behaviour of female aphidophagous ladybirds (Coleoptera: Coccinellidae): a review. Eur. J. Entomol. 100, 1–10. doi: 10.14411/eje.2003.001
Fassotte B., Francis F., Verheggen F. J. (2016). The scent of love: how important are semiochemicals in the sexual behavior of lady beetles? J. Pest. Sci. 89, 347–358. doi: 10.1007/s10340-016-0735-x
Fiedler L., Nedvěd O. (2019). Fifty shades of the harlequin ladybird and a sexually transmitted fungus. J. Insect Sci. 19, 1–7. doi: 10.1093/jisesa/iez107
Forsman A. (2014). Effects of genotypic and phenotypic variation on establishment are important for conservation, invasion, and infection biology. PNAS 111, 302–307. doi: 10.1073/pnas.1317745111
Geiselhardt S., Otte T., Hilker M. (2009). The role of cuticular hydrocarbons in male mating behavior of the mustard leaf beetle, phaedon cochleariae (F.). J. Chem. Ecol. 35, 1162–1171. doi: 10.1007/s10886-009-9704-7
Gomez-Diaz C., Benton R. (2013). The joy of sex pheromones. EMBO Rep. 14, 874–883. doi: 10.1038%2Fembor.2013.140
Goncalves A., Biro D. (2018). Comparative thanatology, an integrative approach: exploring sensory/cognitive aspects of death recognition in vertebrates and invertebrates. Philos. Trans. R. Soc 373, 20170263. doi: 10.1098/rstb.2017.0263
Gwynne D. T. (1984). Courtship feeding increases female reproductive success in bushcrickets. Nature 307, 361–363. doi: 10.1038/307361a0
Gwynne D. T., Rentz D. C. F. (1983). Beetles on the bottle: Male buprestids mistake stubbies for females (Coleoptera). Aust. J. Entomol. 22, 79–80. doi: 10.1111/j.1440-6055.1983.tb01846.x
Hemptinne J. L., Lognay G., Dixon A. F. G. (1998). Mate recognition in the two-spot ladybird beetle, Adalia bipunctata: role of chemical and behavioural cues. J. Insect Physiol. 44, 1163–1171. doi: 10.1016/S0022-1910(98)00081-X
Hodek I., van Emden H. F., Honěk A. (2012). Ecology and behavior of ladybird beetles (Coccinellidae) (Chichester, UK: Wiley-Blackwell).
Höglund J., Sheldon B. C. (1998). The cost of reproduction and sexual selection. Oikos 83, 478–483. doi: 10.2307/3546675
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biom. J. 50, 346–363. doi: 10.1002/bimj.200810425
Izzo T. J., Rodrigues D. J., Menin M., Magnusson W. E. (2012). Functional necrophilia: a profitable anuran reproductive strategy? J. Nat. Hist. 46, 2961–2967. doi: 10.1080/00222933.2012.724720
Jennings M. J., Philipp D. P. (1992). ). Reproductive investment and somatic growth rates in longear sunfish. Environ. Biol. Fishes 35, 257–271. doi: 10.1007/BF00001892
Kappeler P. M. (2021). Sexual selection: Evolutionary foundations. Anim. Behav. 98, 146–165. doi: 10.1007/978-3-030-82879-0_8
Kappeler P. M., Benhaiem S., Fichtel C., Fromhage L., Höner O. P., Jennions M. D., et al. (2023). Sex roles and sex ratios in animals. Biol. Rev. 98, 462–480. doi: 10.1111/brv.12915
Khelifa R. (2017). Faking death to avoid male coercion: extreme sexual conflict resolution in a dragonfly. Ecology 98, 1724–1726. doi: 10.1002/ecy.1781
Knapp M., Řeřicha M. (2020). Effects of the winter temperature regime on survival, body mass loss and post-winter starvation resistance in laboratory-reared and field-collected ladybirds. Sci. Rep. 10, 4970. doi: 10.1038/s41598-020-61820-7
Knapp M., Řeřicha M., Haelewaters D., González E. (2022). Fungal ectoparasites increase winter mortality of ladybird hosts despite limited effects on their immune system. Proc. R. Soc B 289, 20212538. doi: 10.1098/rspb.2021.2538
Knapp M., Řeřicha M., Maršíková S., Harabiš F., Kadlec T., Nedvěd O., et al. (2019). Invasive host caught up with a native parasitoid: field data reveal high parasitism of Harmonia axyridis by Dinocampus coccinellae in Central Europe. Biol. Invasions 21, 2795–2802. doi: 10.1007/s10530-019-02027-4
Knapp M., Řeřicha M., Židlická D. (2020). Physiological costs of chemical defence: repeated reflex bleeding weakens the immune system and postpones reproduction in a ladybird beetle. Sci. Rep. 10, 9266. doi: 10.1038/s41598-020-66157-9
Kotiaho J. S. (2001). Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. 76, 365–376. doi: 10.1017/s1464793101005711
Legrand P., Vanderplanck M., Lorge S., Maesen P., Lognay G., Vilcinskas A., et al. (2019a). Cuticular hydrocarbon composition does not allow Harmonia axyridis males to identify the mating status of sexual partners. Entomol. Gen. 38, 211–224. doi: 10.1127/entomologia/2019/0552
Legrand P., Vanderplanck M., Verheggen F. J. (2019b). Comparison of the sex pheromone composition of Harmonia axyridis originating from native and invaded areas. Insects 10, 326. doi: 10.3390/insects10100326
Lehtonen J., Jennions M. D., Kokko H. (2012). The many costs of sex. Trends Ecol. Evol. 27, 172–178. doi: 10.1016/j.tree.2011.09.016
Lüdecke D., Ben-Shachar M., Patil I., Waggoner P., Makowski D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Software 6, 3139. doi: 10.21105/joss.03139
Ma Y., Chen Y., Li D., Li H., Li D. (2022). Male opportunistic mating increases with intensity of female sexual cannibalism in 3 web-building spiders. Curr. Zool. 68, 113–119. doi: 10.1093/cz/zoab090
Magnhagen C. (1991). Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186. doi: 10.1016/0169-5347(91)90210-O
Magro A., Lecompte E., Magne F., Hemptinne J. L., Crouau-Roy B. (2010). Phylogeny of ladybirds (Coleoptera: Coccinellidae): are the subfamilies monophyletic? Mol. Phylogenet. Evol. 54, 833–848. doi: 10.1016/j.ympev.2009.10.022
Majerus M. E. N. (1997). Interspecific hybridisation in ladybirds (Col.: Coccinellidae). Entomol. Rec. 109, 11–23.
Menzel F., Morsbach S., Martens J. H., Räder P., Hadjaje S., Poizat M., et al. (2019). Communication versus waterproofing: the physics of insect cuticular hydrocarbons. J. Exp. Biol. 222, jeb210807. doi: 10.1242/jeb.210807
Mercado J. E. (2023). Reproductive interference between native and introduced lady beetles (Coleoptera: coccinellidae) in Puerto Rico. Coleopt. Bull. 77, 348–351. doi: 10.1649/0010-065X-77.3.348
Nedvěd O., Honěk A. (2012). “Life history and development,” in Ecology and behaviour of ladybird beetles (Coccinellidae). Eds. Hodek I., van Emden H. F., Honěk A. (Chichester, UK: Wiley-Blackwell).
Obata S. (1987). Mating behavior and sperm transfer in the ladybird beetle, Harmonia axyridis Pallas: Coleoptera: Coccinellidae. Appl. Entomol. Zool. 22, 434–442. doi: 10.1303/aez.22.434
Omkar, Pervez A. (2005). Mating behavior of an aphidophagous ladybird beetle, Propylea dissecta (Mulsant). Insect Sci. 12, 37–44. doi: 10.1111/j.1672-9609.2005.00006.x
Omkar, Srivastava S. (2002). The reproductive behaviour of an aphidophagous ladybeetle, Coccinella septempunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 99, 465–470. doi: 10.14411/eje.2002.060
Osawa N. (1994). The occurrence of multiple mating in a wild population of the ladybird beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae). J. Ethol. 12, 63–66. doi: 10.1007/BF02350081
Page R. E. (1986). Sperm utilization in social insects. Annu. Rev. Entomol. 31, 297–320. doi: 10.1146/annurev.en.31.010186.001501
Parker G. A. (1983). “Mate quality and mating decisions,” in Mate choice. Eds. Bateson P., Bateson P. P. G. (Cambridge University Press).
Parker G. A., Birkhead T. R. (2013). Polyandry: the history of a revolution. Philos. Trans. R. Soc 368, 20120335. doi: 10.1098/rstb.2012.0335
Peinert M., Wipfler B., Jetschke G., Kleinteich T., Gorb S. N., Beutel R. G., et al. (2016). Traumatic insemination and female counter-adaptation in Strepsiptera (Insecta). Sci. Rep. 6, 25052. doi: 10.1038/srep25052
Perry J. C., Rowe L. (2010). Condition-dependent ejaculate size and composition in a ladybird beetle. Proc. R. Soc B. 277, 3639–3647. doi: 10.1098%2Frspb.2010.0810
Perry J. C., Sharpe D. M., Rowe L. (2009). Condition-dependent female remating resistance generates sexual selection on male size in a ladybird beetle. Anim. Behav. 77, 743–748. doi: 10.1016/j.anbehav.2008.12.013
Pettigrew M. (2019). Fantasy, opportunity, homicide: Testing classifications of necrophilic behaviour. J. Police Crim. Psychol. 34, 14–22. doi: 10.1007/s11896-018-9259-z
Pintanel P., Obando-Moreno G., Merino-Viteri A. (2021). ). Necrophiliac behaviour in the recently described species Scinax tsachila (Anura: Hylidae), with a review of necrophilia in amphibians. Neotrop. Biodivers. 7, 53–56. doi: 10.1080/23766808.2021.1879549
R Development Core Team (2022) A language and environment for statistical computing (Austria: R Foundation for Statistical Computing). Available at: http://www.R-project.org.
Řeřicha M., Dobeš P., Knapp M. (2021). Changes in haemolymph parameters and insect ability to respond to immune challenge during overwintering. Ecol. Evol. 11, 4267–4275. doi: 10.1002/ece3.7323
Roy H. E., Brown P. M. J., Adriaens T., Berkvens N., Borges I., Clusella-Trullas S., et al. (2016). The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol. Invasions 18, 997–1044. doi: 10.1007/s10530-016-1077-6
Scardamaglia R. C., Fosacheca S., Pompilio L. (2015). Sexual conflict in a sexually cannibalistic praying mantid: males prefer low-risk over high-risk females. Front. Zool. 12, 9–14. doi: 10.1016/j.anbehav.2014.10.013
Schultz E. T., Warner R. R. (1991). Phenotypic plasticity in life-history traits of female Thalassoma bifasciatum (Pisces: Labridae): 2. Correlation of fecundity and growth rate in comparative studies. Environ. Biol. Fishes 30, 333–344. doi: 10.1007/BF02028849
Sun Q., Haynes K. F., Zhou X. (2018). Managing the risks and rewards of death in eusocial insects. Philos. Trans. R. Soc 373, 20170258. doi: 10.1098/rstb.2017.0258
Swift K., Marzluff J. M. (2018). Occurrence and variability of tactile interactions between wild American crows and dead conspecifics. Philos. Trans. R. Soc B 373, 20170259. doi: 10.1098/rstb.2017.0259
Tatarnic N. J., Cassis G., Siva-Jothy M. T. (2014). Traumatic insemination in terrestrial arthropods. Annu. Rev. Entomol. 59, 245–261. doi: 10.1146/annurev-ento-011613-162111
Thornhill R., Alcock J. (1983). The evolution of insect mating systems (Cambridge, UK: Harvard University Press). doi: 10.1093/acprof:oso/9780199678020.001.0001
Tomita N., Iwami Y. (2015). What raises the male sex drive? Homosexual necrophilia in the sand martin Riparia riparia. Ornithol. Sci. 15, 95–98. doi: 10.2326/osj.15.95
Vaníčková L., Svatoš A., Kroiss J., Kaltenpoth M., Do Nascimento R. R., Hoskovec M., et al. (2012). Cuticular hydrocarbons of the South American fruit fly Anastrepha fraterculus: variability with sex and age. J. Chem. Ecol. 38, 1133–1142. doi: 10.1007/s10886-012-0177-8
Xie J., Zhang Y., Wu H., Liu P., Deng C., Pang H. (2014). Effects of mating patterns on reproductive performance and offspring fitness in Cryptolaemus montrouzieri. Entomol. Exp. Appl. 153, 20–23. doi: 10.1111/eea.12224
Yao M., Rosenfeld J., Attridge S., Sidhu S., Aksenov V., Rollo C. D. (2009). The ancient chemistry of avoiding risks of predation and disease. Evol. Biol. 36, 267–281. doi: 10.1007/s11692-009-9069-4
Keywords: ethology, Harmonia axyridis, mate choice, mating duration, necromones, necrophilia, sexual promiscuity
Citation: Řeřicha M, Montoya-Molina S, Harabiš F and Knapp M (2023) Mating with dead conspecifics in an invasive ladybird is affected by male sexual fasting and time since the female’s death. Front. Ecol. Evol. 11:1225998. doi: 10.3389/fevo.2023.1225998
Received: 20 May 2023; Accepted: 09 October 2023;
Published: 19 October 2023.
Edited by:
Oldřich Nedvěd, University of South Bohemia in České Budějovice, CzechiaReviewed by:
Ahmad Pervez, Sri Dev Suman Uttarakhand University, IndiaCopyright © 2023 Řeřicha, Montoya-Molina, Harabiš and Knapp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michal Knapp, a25hcHBAZnpwLmN6dS5jeg==
†These authors have equally contributed to this work
‡ORCID: Michal Knapp, orcid.org/0000-0003-4487-7317
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.